Abstract
Novel or changing environments expose animals to diverse stressors that likely require coordinated hormonal and behavioral adaptations. Predicted adaptations to urban environments include attenuated physiological responses to stressors and bolder exploratory behaviors, but few studies to date have evaluated the impact of urban life on codivergence of these hormonal and behavioral traits in natural systems. Here, we demonstrate rapid adaptive shifts in both stress physiology and correlated boldness behaviors in a songbird, the dark-eyed junco, following its colonization of a novel urban environment. We compared elevation in corticosterone (CORT) in response to handling and flight initiation distances in birds from a recently established urban population in San Diego, California to birds from a nearby wildland population in the species' ancestral montane breeding range. We also measured CORT and exploratory behavior in birds raised from early life in a captive common garden study. We found persistent population differences for both reduced CORT responses and bolder exploratory behavior in birds from the colonist population, as well as significant negative covariation between maximum CORT and exploratory behavior. Although early developmental effects cannot be ruled out, these results suggest contemporary adaptive evolution of correlated hormonal and behavioral traits associated with colonization of an urban habitat.
Keywords: adaptation, boldness, corticosterone, evolution, junco, urbanization
INTRODUCTION
Understanding how correlated behavioral and physiological traits respond to new or changing environmental conditions has implications for both the study of basic evolutionary processes and for predicting and managing biological responses to anthropogenic global change (Cockrem 2005; van Oers et al. 2011). For example, exposure to urbanization or climate change likely requires rapid changes in multiple traits in order for populations to persist (Gaston 2010; Møller et al. 2010), yet much remains to be learned about whether preexisting trait correlations act to constrain or facilitate the developmental or genetic responses that enable persistence (Agrawal and Stinchcombe 2009; Ketterson et al. 2009). In particular, few studies have simultaneously evaluated how correlated characters, such as behavioral traits and associated hormonal mechanisms, respond to new environments. Furthermore, it is often unclear the degree to which phenotypic plasticity or genetic evolution may underlie observed differences in behavior or physiology among divergent populations (Diamond 1986; Møller 2008; Angelier et al. 2011), and the interplay of both processes is likely to be significant. In this study, we examined changes in tameness and boldness behaviors associated with the recent establishment of a songbird population in a novel urban environment, as well as changes in the endocrine stress response, a physiological mechanism hypothesized to underlie behavioral variation in personality traits.
Recent research in animal behavior and neuroendocrinology has demonstrated that individual animals vary consistently in suites of behavioral traits called “animal personality” (Wilson et al. 1994; Sih et al. 2004), including those described as “boldness” or “neophobia” behaviors. Boldness can be generally defined as the tendency of individuals to be exploratory and take risks, particularly in novel contexts (Wilson et al. 1994), and there is evidence that underlying hormonal mechanisms play a key role in modulating such behavioral differences (Koolhaas et al. 1999; van Oers et al. 2011). One such personality trait, described as “early exploratory behavior,” generally measures how quickly and/or extensively individuals move through and examine a novel environment. Studies of early exploratory behavior in birds have demonstrated that this trait can be repeatable among individuals (Verbeek et al. 1994), heritable in parent–offspring and sibling studies (Dingemanse et al. 2002), and responsive to artificial selection (Drent et al. 2003; van Oers, Derent, de Goede, et al. 2004). Exploratory behavior has also been shown to be a target of both natural and sexual selection within free-living populations (Dingemanse et al. 2004; van Oers et al. 2008), and its expression can be influenced by both genetic and environmental factors (van Oers, Derent, de Jong, et al. 2004; van Oers et al. 2005).
Collectively, these prior studies indicate that boldness behaviors, such as exploratory and risk-taking behaviors, should diverge among populations occupying environments that favor shy or bold individuals (Fidler et al. 2007), such as wildland versus urban habitats, respectively, but few studies have reported evidence for adaptive evolution of personality traits across populations (but for examples in fish, see Dingemanse et al. 2007; Herczeg et al. 2009). Several studies have provided evidence for divergence of behavioral phenotypes in association with urbanization (e.g., Møller 2008; Evans et al. 2010), but typically not evaluating whether phenotypic plasticity or genetic differences likely underlie it (Diamond 1986; Møller 2008). In this paper, we focus on early exploratory behavior and another behavioral trait, “flight initiation distance” (FID) or “flush distance,” which can also be interpreted in relation to boldness and tameness (Møller 2008). FID also shows consistent individual variation in avian species (Carrete and Tella 2010) and has been associated with adaptation to urbanization in many animals (Møller 2008). We refer to “boldness” generally in our discussion of behavior, as it pertains to both FID and early exploratory behavior (EEB) assays, which were conducted in our field and common garden studies, respectively.
A primary physiological mechanism underlying individual differences in boldness behaviors is the endocrine stress response, particularly that of the hypothalamic–pituitary–adrenal (HPA) axis (Koolhaas et al. 1999; van Oers et al. 2011). Acute elevations in corticosterone (CORT) levels are often associated with adaptive short-term behavioral and physiological survival responses such as self-maintenance behaviors and mobilization of energy reserves, while chronically elevated CORT levels may reduce survival (Breuner et al. 2008). Generally, shyer or more passive behavioral phenotypes are predicted to be associated with more acute HPA axis activity and greater release of glucocorticoids in response to short-term stressors and vice versa for bolder or more assertive behavioral phenotypes (Koolhaas et al. 1999). Results from artificial selection and observational studies support these predictions in birds and mammals (Korte et al. 1997; Lendvai et al. 2011) (but see Martins et al. 2007).
Comparing populations, CORT levels appear to be repeatable, to have a genetic basis, and thus to have the potential to evolve rapidly (Evans et al. 2006; Partecke et al. 2006; Rensel and Schoech 2011). CORT levels vary among subspecies that differ in their life-history (e.g., Angelier et al. 2011) and they have been associated with adaptation to urban environments (e.g., Partecke et al. 2006; Fokidis et al. 2009) and to ecotourism (e.g., Romero and Wikelski 2002).
The research presented here is unique in simultaneously examining population divergence in both a behavioral trait and an associated hormonal mechanism, and in evaluating plasticity versus genetic evolution as possible causes. We sampled behavior and plasma CORT levels in free-living individuals from two recently diverged songbird populations, one montane and the other a costal urban colonist, and in captives from these populations raised in a common garden experiment. These populations diverged following a recent colonization event (∼1983), in which a historically montane forest–breeding songbird species, the dark-eyed junco (Junco hyemalis), colonized a novel urban environment in Southern California, USA. Since establishment, the colonist population has persisted as a small (≈80 pairs) but stable population, and it exists as an effective biogeographic island in the species-atypical urban and coastal habitat, isolated from the ancestral range mountain populations ≈70 km inland to the east. Details of the colonization event and the study system, including previously characterized behavioral and morphological differences in this system, are described below (see MATERIALS AND METHODS).
We tested 1) whether tameness and boldness behaviors and glucocorticoid levels differed in the novel urban habitat when compared with a nearby ancestral range montane forest–breeding population, 2) the degree to which these differences likely represent phenotypic plasticity versus genetic evolution using a common garden approach, and 3) whether boldness behaviors and glucocorticoid levels covary within and among populations. The latter goal allows assessing if the evolutionary responses of these traits are integrated or independent. Because either selection post-colonization or genetic drift could both account for apparent genetic differences inferred between populations, we also further analyzed the possibility that drift alone could explain the observed population divergences in this system. Finally, we examined one possible molecular mechanism underlying behavioral variation (a single-nucleotide polymorphism [SNP 830] in the Drd4 dopamine receptor gene, which has been previously associated with exploratory behavior in a songbird Fidler et al. 2007; Korsten et al. 2010).
MATERIALS AND METHODS
Study system
We collected behavioral data and blood samples, and captured birds for a common garden study from 2 recently diverged dark-eyed junco populations in San Diego County, CA, USA. One of the populations was recently (ca. 1983) established in the novel urban and coastal habitat on the University of California–San Diego (UCSD) campus (elevation 30 m, 32°40′N, 117°10′W), and the other is a nearby ancestral range montane forest–breeding population near Mt Laguna, CA (elevation 1700 m, 32°52′N, 116°25′W).
In far southern California, the breeding range of dark-eyed juncos (J. hyemalis thurberi) is confined to higher elevation (e.g., >1500 m) forests and wooded canyons inland from the coast (Miller 1941; Unitt 2005). However, in the early 1980s, a population of juncos became established on the coast of the Pacific Ocean in an urban environment on the campus of UCSD (Walens S, personal communication), most likely established by a flock of wintering migrants staying to breed (Yeh 2004; Yeh and Price 2004). Microsatellite DNA analyses indicate genetic isolation of the San Diego juncos relative to those of the montane breeding range (Rasner et al. 2004), and they suggest that a sizeable number of individuals founded the urban population (e.g., minimum n ≥ 8–20 birds), such that phenotypic divergence due to a founder effect is unlikely (Rasner et al. 2004; Yeh 2004). The San Diego colonist population has remained small but stable at about 80 breeding pairs over the past decade, and it constitutes an effective biogeographic island, since the closest montane forest–breeding ranges lies ≈70 km inland to the east, separated by unsuitable habitat (Unitt 2005).
The environment experienced by the urban colonist population differs in many ways from that in the ancestral range, owing to both its mild coastal climate and urban setting: reduced variation in temperature and rainfall, increased noise and light levels, constant human disturbances (e.g., vehicles and foot traffic), altered acoustic transmission (Slabbekoorn et al. 2007), novel predator communities (Suarez et al. 2005), abundant ornamental vegetation and watered lawns, and novel food and nesting resources (Yeh et al. 2007). Numerous behavioral differences between the colonist and an ancestral range population have been documented, including earlier onset of reproduction (Yeh and Price 2004), loss of migratory behavior (Yeh 2004), reduced male territorial responses (Newman et al. 2006), altered song frequencies (Slabbekoorn et al., 2007; Cardoso and Atwell 2011), and reduced plumage ornamentation (a difference which was shown to be genetic; Yeh 2004). We have also observed that colonist birds are quicker to approach novel objects, such as food bait, walk-in traps, or mist nets (J.W.A., anecdotal observation).
Field and common garden methods
In both populations during 2006–2007, breeding pairs were individually marked with color bands (San Diego: n ≈ 50 pairs; Mt Laguna: n ≈ 30 pairs), and we monitored prebreeding and breeding activities during 1 February–30 July (San Diego) and 15 March–15 July (Mt Laguna). In 2007, we conducted behavioral assays of FID of foraging birds and incubating females, and collected serial blood samples to assess the endocrine stress response (plasma CORT) from nesting females. We also captured juveniles from early life for common garden study that included tests of exploratory behavior, endocrine assays, and genotyping a candidate gene polymorphism for behavioral differences. These are described in turn below.
Flight initiation distance (field)
FID was recorded for both foraging birds and incubating females in the field, using methods similar to prior studies of this measure (Møller 2008). After marking the starting location of the observer by dropping a small weight, the single observer (S.C.N., who wore the same clothes for all trials) directly approached the focal bird or the focal nest, walking at a constant speed of approximately 1.5 m/s. The observer dropped a second small weight to mark the distance at the precise moment when the focal bird took flight and, lastly, marked the exact preflight location of the focal bird or nest. FID and starting distances to the focal bird were then measured with a tape to the nearest centimeter.
For foraging birds, focal individuals (San Diego: n = 30; Mt Laguna: n = 24) were opportunistically chosen throughout both study sites from 7 June to 26 June 2007 between the hours of 0730 and 1400, and we recorded FID, starting distance (range: 5.93–55.55 m; mean: 22.7 m), and flock size (range: 1–6 birds; mean: 1.5 birds). Individuals were identified from color bands when possible (n = 15), and we spatially segregated sampling to avoid testing the same individuals (juncos are territorial during the breeding season).
For incubating females, the observer approached each nest (juncos are ground nesters) from a randomly chosen direction that allowed a path to the nest that was not obviously visually obscured (i.e., to avoid not being perceived by the female). For nests with obvious visual obstructions (e.g., next to a bush), the nest would be approached from a direction of least obstruction. In order to control for possible population differences in nest concealment, we took a digital photograph of each nest from 0.5 m above it and another from 0.5 m in the direction that the nest was most visible, and quantified the exposure of the nest cup or contents in each picture (sum of pixels showing visible parts of the nest or contents using Scion image software, such that higher pixel counts indicated more exposure). We sampled FID from incubating females (San Diego: n = 11; Mt Laguna: n = 17) between 21 May and 14 June 2007 during the hours of 0730 to 1230 and also recorded starting distance (range: 7.00–17.80 m; mean: 13.0 m). Time of day and nest concealment measures did not differ between populations (all |t| < 1.8, all P > 0.1), and these variables did not correlate with FID within either population (all |r| < 0.22, all P > 0.25) and were thus not included in the statistical models described below.
CORT in response to handling (field)
We measured initial plasma CORT levels and the short-term increases in response to handling stress of females during the nesting stage (incubating eggs or feeding nestlings), according to a standardized technique (e.g., see Zysling et al. 2006). Females were caught near their nests with mist nets, and an initial 100 ul blood sample was immediately collected within 0–3 min postcapture. Individuals were placed in a paper holding bag, and 2 additional 50-ul samples were taken at 15 and 30 min postcapture. Samples were stored on ice until plasma was separated by centrifugation and frozen at −20 °C. We sampled females across the respective breeding season in both populations, which was 8 March–27 June 2007 at San Diego (n = 31) and 10 May–25 June 2007 (n = 27) at Mt Laguna.
Common garden, general methods
During June and July 2007, we captured 40 juveniles from both San Diego and Mt Laguna, using mist nets and walk-in traps. We targeted juveniles that had recently become nutritionally independent. The age of juveniles was confirmed through field observations of families and/or measurements of wing and tail length (Nolan et al. 2002). In some cases (n = 12 at Mt Laguna; n = 19 at San Diego), we knew the exact age of the captured juveniles because they were banded as nestlings (mean ± standard error of the mean; San Diego: 40.9 ± 4.3 days; Mt Laguna: 38.2 ± 1.7 days; t 29 = 0.59, P = 0.56). Because junco nestlings fledge at ≈12 days posthatch and remain with and dependent on their parents until ≈25–30 days posthatch (Nolan et al. 2002), the juveniles that we captured had limited early exposure to their natal habitats (on average <15–20 days of life outside the nest).
Capture locations were distributed spatially throughout the study areas to avoid capturing closely related individuals (i.e., siblings), with juveniles captured from more than 13 different locations and on 15 different capture days within each study population across a period of 30 days. Of the subset of captured juveniles that were banded in the nest and thus had known parents (n = 31; see above), we only had 2 siblings from each population that were sampled for this study (San Diego: n = 2 of 31; Mt Laguna: n = 2 of 23).
Juveniles were housed in flocks in temporary outdoor aviaries (2.4 m L × 1.8 m W × 2.4 m H) in a fenced lawn in suburban San Diego, CA until 15 July 2007, when they were shipped via air cargo to the Kent Farm Bird Observatory indoor aviaries at Indiana University. From July 2007 onwards, birds from each population were housed in mixed sex flocks (≈50:50 male:female) in both large (6.4 m L × 3.2 m W × 2.4 m H) and small (2.5 m L × 2.1 m W × 2.4 m H) aviary rooms (henceforth “home aviaries”) with equivalent densities (≈1 bird/m2) and identical housing conditions. Birds were segregated by population, and all aviary rooms had identical exposure to human researchers and animal care staff.
Early exploratory behavior (common garden)
From 25 March to 13 April 2008, we measured how rapidly and how extensively the birds from the common garden explored a novel aviary room, following methods adapted from Verbeek et al. (1994). The test room (2.5 m L × 2.1 m W × 2.4 m H) had not previously been inhabited by any of the birds in this study. There was a small (10 cm × 10 cm) cardboard loading door that allowed us to introduce the bird into the test room with minimal disturbance, and behaviors were observed through a one-way glass window.
The floor of the test room was visually divided by tape markings into 4 quadrants, and 5 plastic food containers were positioned around the floor. The small (10-cm) plastic food dishes were identical to the ones used in the home aviaries but contained no food. Instead, we placed wood shavings (which covered the floors of the home aviaries) inside the food dishes, which thus provided no incentive for the birds to remain with the first dish they visited. We also positioned 5 artificial trees made of wooden rods in the test room to provide perches and additional spaces that birds could explore.
The target individual was captured from its home aviary after darkening the lights and then immediately (<60 s) introduced into the darkened test room. The lights of the test room were then turned on and a single observer (K.W.R.), blind to the bird's population of origin, recorded 8 behaviors for a period of 20 min: latency to make first movement (0–1200 s), number of floor quadrants visited (1–4), latency to visit 4/4 floor quadrants (0–1200 s), number of food dishes visited (0–5), latency to visit 4/5 food dishes (0–1200 s), number of trees visited (0–5), latency to visit 4/5 trees (0–1200 s), and total number of movements (≥0). Movements were defined as any flights or hops in which a bird crossed quadrant boundaries.
CORT in response to handling (common garden)
We collected blood samples to measure both initial CORT (0 min) and stress-induced CORT at 15 and 30 min postcapture for a subset of individuals (n = 10 per population per sex; n = 40 total) in the common garden using methods analogous to those described above for field studies. Focal birds were captured from home aviary rooms within 30 s of dimming the aviary lights, in order to minimize stress during capture. This was conducted during 11–13 July 2008 for females and 24–27 July 2009 for males, which were 12 and 24 months following establishment of the common garden study, respectively.
Radioimmunoassay for CORT
Plasma CORT concentrations were measured using direct radioimmunoassay (RIA) methods that are described elsewhere (e.g., Schoech et al. 1998). In brief, ≈20-ul samples were allowed to equilibrate overnight with 2000 cpm of radiolabeled CORT. Samples were then extracted with 4.0 ml of diethyl ether anhydrous and reconstituted with phosphate buffered saline with gelatin. The competitive binding assay was run in duplicate and calculations corrected for variation in plasma volume and individual recoveries. We ran a total of 6 assays (4 for field studies and 2 for common garden studies), and individual samples were randomized across assays. We included 3 standards of known concentration in each assay, and these were used to calculate intraassay variation (4–28%), interassay variation (25%), and assay correction factors (assay standard mean/global standard mean) that were multiplied to standardize values across assays.
Statistical analyses
We tested for population differences in FID, exploratory behaviors and plasma CORT levels with General Linear Models (GLMs) that included factors and covariates associated with our a priori hypotheses. The full GLM models, including β, F, and P values for all factors and covariates, are reported in Supplementary Tables S1–S5; test statistics most relevant to our hypotheses appear in RESULTS and figures of the main text. We used principal components analysis to reduce correlated behavioral variables into composite boldness scores for the early exploratory behavior assay (Table 1). We also compared populations (by sex) for the individual behavioral variables in the exploratory behavior assay using Mann–Whitney U-tests (Supplementary Table S6). With respect to CORT, we analyzed 1) Initial (baseline) CORT (“0 min”), 2) maximum CORT (at 15 or 30 min postcapture), and 3) CORT Response, which was determined by subtracting the Initial CORT from all 3 time points and calculating the area under the resulting curve. This integrated approach considers both the CORT increase and clearance over the 30 min of restraint (Breuner et al. 1999). All analyses were made with SPSS v.18 (SPSS 2010), and all reported P values are two-tailed.
Table 1.
Trait loadings and eigenvalues in principal components analysis (n = 54)
| Variablea | PC1 | PC2 |
| Floor quadrants visited (of 4) | 0.63 | 0.39 |
| Latency to visit 4 quadrants | −0.87 | −0.24 |
| Food dishes visited (of 5) | 0.89 | 0.33 |
| Latency to visit 4/5 dishes | −0.66 | −0.41 |
| Trees visited (of 5) | 0.58 | −0.70 |
| Latency to visit 4/5 trees | −0.40 | 0.83 |
| Number of hops + flights | 0.79 | −0.25 |
| Latency to first hop/flight | −0.64 | 0.14 |
| Eigenvalue | 3.93 | 1.73 |
| Percentage of variance | 49.1 | 21.7 |
All eight variables loaded significantly for PC1 and PC2
Evaluating drift
Using methods similar to Yeh (2004), we examined whether drift alone could explain the observed population differences reported here. We used the following equation from Lande (1976):where N* is the maximum population size that would allow drift to account for an observed difference in trait values between 2 populations over time (P < 0.05) and in the total absence of selection. Observed effective population sizes greater than N* indicate that drift alone is unlikely to account for population differences in trait values. h2 denotes heritability of the trait, t is time in number of generations, x 1 is the trait value at time 1, x 2 is the trait value at time 2, and σ is the standard deviation of the trait (Lande 1976; Yeh 2004).
For exploratory behavior and plasma CORT levels, we used published heritability estimates from prior parent–offspring, sibling, or artificial selection studies of other songbird species: 0.22–0.54 reported range for exploratory behavior (Dingemanse et al. 2002; Drent et al. 2003) and 0.08–0.27 for CORT (Evans et al. 2006). We were unable to find reported heritability values for FIDs, but we did find a published estimate of individual repeatability (0.88, see Carrete and Tella 2010), which can be interpreted as a reasonable upper bound of heritability (Falconer and Mackay 1996). For estimating the number of generations (t) since colonization, we used methods following Yeh (2004), based on the time since population establishment (≈27 years) divided by the average age of adults in the San Diego population (2.17) to arrive at a conservative estimate of 12 generations.
Sequencing of SNP 830 region
We sequenced the SNP 830 region of the Drd4 dopamine receptor gene, which has been found to correlate with exploratory behavior in both captive and free-living populations of another passerine species, Parus major (Fidler et al. 2007; Korsten et al. 2010). Genomic DNA was isolated from blood samples using standard phenol–chloroform extraction procedures. We amplified a 152-bp region of the Drd4 gene using primers designed for P. major: SNP830 forward, 5′-AAGCTGAGAGGCTGCATCTATGG-3′ and SNP830 reverse, 5′-ATCCCACTGTTCATCCCACACTC-3′ (Fidler et al. 2007). This region was amplified in a 20-ul reaction containing 2 mM MgCl2 and 1 U Taq, using standard thermocycling conditions with an annealing temperature of 61 °C.
To test whether these primers amplify the same region in J. hyemalis as in P. major, we first sequenced 12 juncos and compared the sequences to a published P. major dopamine receptor D4 mRNA sequence (Genbank accession DQ006801; Figure 1a). All of these individuals but one were homozogyous for a single allele; one individual was a heterozygote with an allele that differed at a single site (basepair 119 in the alignment in Supplementary Figure S1a). These sequences are very similar to a region of the P. major sequence, with a 3-bp deletion corresponding to a single amino acid at basepairs 100–102 and 2 substitutions, both transversions, at sites 39 (synonymous) and 93 (nonsynonymous) (Supplementary Figures S1a,b).
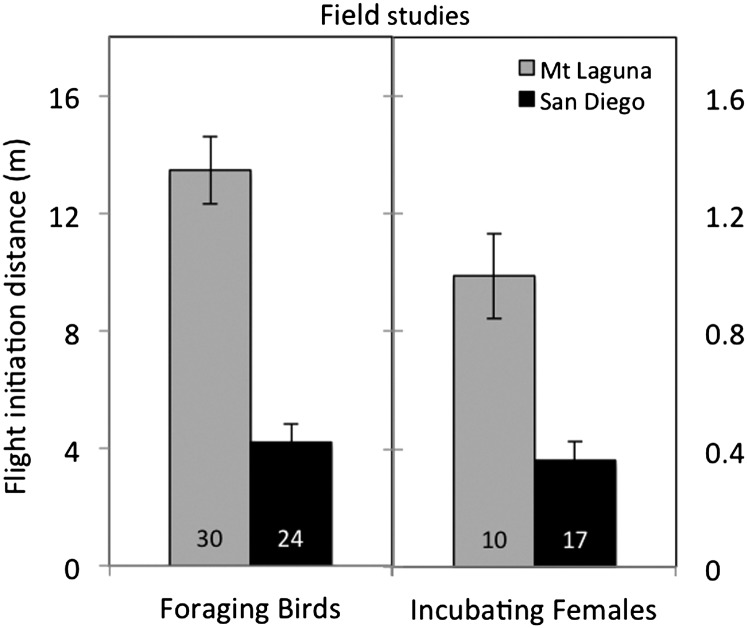
Figure 1.
FID (means ± standard error of the mean) were shorter in field studies of both foraging birds (GLM, P < 0.001) and incubating females (GLM, P = 0.003) in colonist (San Diego) versus ancestral range (Mt Laguna) populations.
The J. hyemalis sequences did not show the same polymorphism as in P. major (SNP830C/T); however, another polymorphism appeared to affect a neighboring amino acid. G is replaced with A, a nonsynonymous substitution changing glycine to aspartic acid. This polymorphism also affects a NaeI restriction enzyme site, as in P. major, by resulting in the presence (5′-GCCGGC-3′) or the absence (5′-GCCGAC-3′) of the restriction enzyme cleavage site (also see Fidler et al. 2007).
We used the NaeI restriction enzyme to screen the captive birds for this polymorphism. We amplified the region with a fluorescently labeled forward SNP830 primer, incubated the product with the NaeI restriction enzyme following standard protocols, and analyzed the resulting fragments with the ABI 3730XL automated sequencer and the Genescan 4.0 software. The “normal” allele was cut by the restriction enzyme and the resulting fragment was 110-bp long; the “mutant” allele was not cut and the fragment was 147 bp. Individuals that were found to have one or two 147 bp alleles were then sequenced to verify the presence of the mutant allele, and thus to exclude the possibility of incomplete digestion by the restriction enzyme reaction.
RESULTS
Flight initiation distances—field study
For both foraging birds and incubating females, FID were significantly shorter in the San Diego population (foraging birds: F 1,53 = 30.1, P < 0.001; incubating females: F 1,27 = 10.4, P = 0.003) (Figure 1). For foraging birds, the flock size and the starting distance of the observer both had significant effects in the GLM model and were positively correlated with FID (flock size: F 1,53 = 6.1, P = 0.017; starting distance: F 1,53 = 9.4, P = 0.004). We also tested for interactions between starting distance and population and flock size and population for the foraging birds, however, neither interaction term was significant and both were removed from the final model (population × flock size: F 1,53 = 1.05, P = 0.31; population × starting distance: F 1,53 = 0.95, P = 0.36). There was no significant effect of starting distance on FID for incubating females (F 1,27 = 0.1, P = 0.75).
CORT—field study
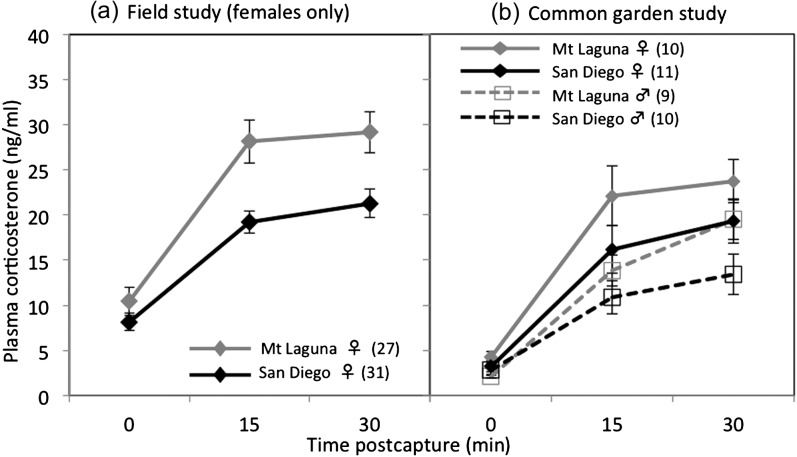
For initial (baseline) CORT levels, we found no significant difference between females from the San Diego versus Mt Laguna populations (F 1,52 = 0.42, P = 0.518), and there was no significant effect of body mass (F 1,27 = 0.1, P = 0.75) or date (F 1,27 = 0.1, P = 0.75) (Figure 2a). However, birds from the San Diego population exhibited attenuated plasma CORT elevations in response to handling, as both maximum CORT and overall CORT response (area) were significantly lower in the San Diego population (max CORT: F 1,52 = 10.8, P = 0.002; CORT response: F 1,52 = 4.4, P = 0.042) (Figure 2a).
Figure 2.
Initial (baseline) and stress-induced plasma corticosterone (CORT; means 6 standard error of the mean) in colonist (San Diego) and ancestral range populations are shown from (a) field study of freeliving nesting females and (b) a captive common garden study of birds raised from early life under identical aviary conditions. We found significant population differences in both maximum CORT and CORT responsiveness (area) in both field and common garden studies, and there were also sex differences in the common garden (see RESULTS).
Exploratory behavior—common garden study
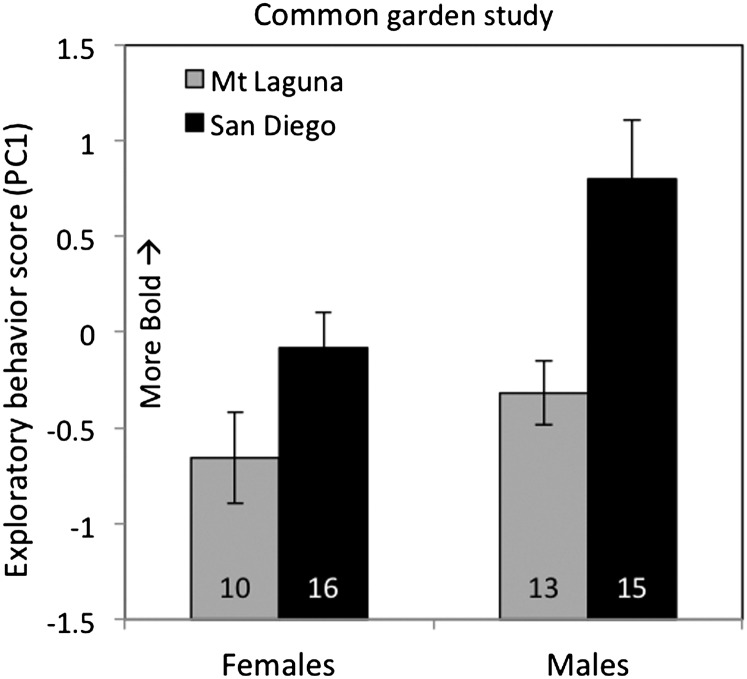
The 8 behavioral variables of the exploratory behavior assay were highly correlated with one another, and a PCA returned 2 principal components with eigenvalues larger than 1 (Table 1). The first component (PC1, eigenvalue = 3.93, 49.1% variation) was clearly interpretable as positively associated with faster and more extensive (i.e., bolder) exploratory behavior (Table 1). Specifically, birds that scored highly for PC1 made their initial movement sooner, visited more floor quadrants, food dishes, and trees more quickly, and made more total movements (Table 1). PC2 (eigenvalue = 1.73, 21.7% of variance) was less readily interpretable; it mainly predicted whether individuals, regardless of their level of exploration, tended to remain on the ground or visit trees (strongest trait loadings were fewer trees visited and higher latency to visit trees, Table 1). PC1 is the focus of our subsequent analysis, because it quantifies the speed and extent of exploratory behavior, but we also analyzed PC2 because it explained a considerable proportion of variation in behavior.
Birds from the San Diego population had significantly higher boldness (PC1) scores than birds from Mt Laguna (F 1,53 = 13.3, P = 0.001, Figure 3). The effect of sex was also highly significant (F 1,53 = 7.3, P = 0.009), with males showing higher PC1 scores than females in both populations (Figure 3). For PC2, there were no significant effects of population, sex, or body mass (all P > 0.25), but we did detect a significant interaction of population × sex (P = 0.05) (Supplementary Table S3). Population comparisons (by sex) for individual behavioral variables are summarized in Supplementary Table S1 and are consistent with the conclusions of the main test of PC1, with faster and more extensive exploratory behavior observed in birds originating from the urban colonist population (Supplementary Table S1).
Figure 3.
Exploratory behavior (PC1, means 6 standard error of the mean) scores from a common garden study are shown by population of origin and sex. The effects of both population (GLM, P = 0.001) and sex (GLM, P = 0.009) were significant.
CORT—common garden study
In the common garden study, we found that males had lower initial (baseline) CORT than females (F 1,39 = 4.42, P = 0.043), but we did not detect significant population differences for initial CORT (F 1,39 = 0.3, P = 0.59) (Figure 2b). For maximum CORT, there was a trend toward lower values in the San Diego population (F 1,39 = 3.10, P = 0.089), and males also had lower maximum CORT compared with females (F 1,39 = 6.63, P = 0.017) (Figure 2b). For CORT responsiveness (area), a measure that controls for initial CORT levels, we found a marginally significant effect of population (F 1,39 = 4.10, P = 0.052), but not for sex (F 1,39 = 1.99, P = 0.17), with birds originating from San Diego exhibiting an attenuated CORT response (Figure 2b). There were no significant effects of body mass or the interaction of sex × population on any of the CORT measures in the common garden study (all P > 0.1; Supplementary Table S4).
Covariation between CORT and exploratory behavior—common garden study
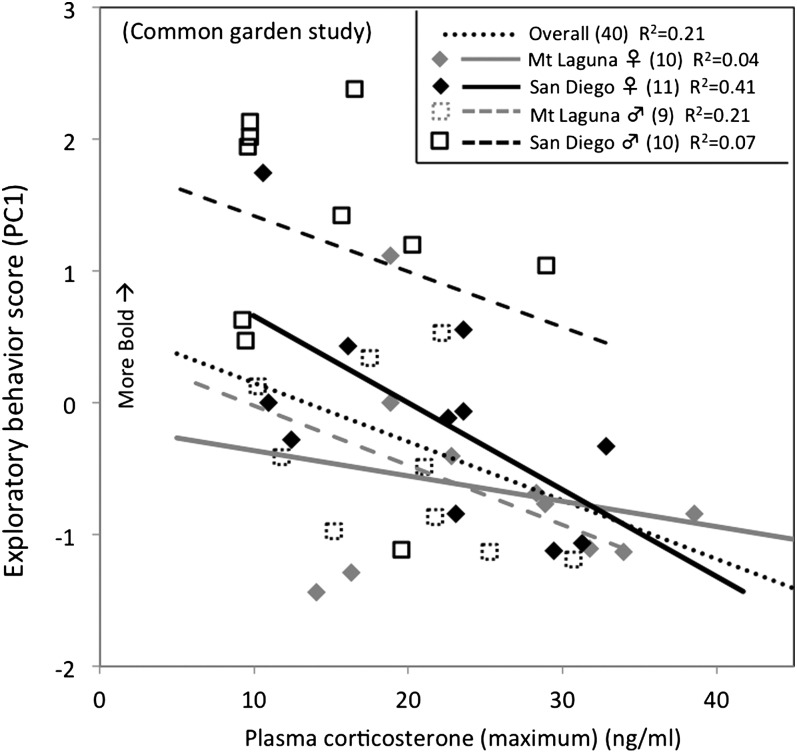
In the common garden study, the same individuals were sampled for exploratory behavior and plasma CORT, allowing us to test for covariation between behavioral and hormonal measures. While controlling for variation attributed to population, sex, and body mass differences in exploratory behavior (Supplementary Table S5; Figure 4), we found that maximum plasma CORT negatively predicted exploratory behavior (F 1,39 = 5.84, P = 0.021) (Figure 4). Negative covariation between maximum CORT and exploratory behavior was similar for both sexes within both populations (Figure 4), and thus there was no detectable interaction effect of population × maximum CORT (F 1,39 = 2.11, P = 0.16). Neither initial (baseline) CORT nor CORT response (area) predicted exploratory behavior (P > 0.1; Supplementary Table S5).
Figure 4.
Individual variation for maximum CORT and exploratory behavior from the common garden study. Max CORT predicted individual exploratory behavior scores (P = 0.021) in a GLM model the also included significant effects of population (P = 0.015) and sex (P = 0.049).
Drift
We estimated the maximum effective population size (N*) at which drift alone, in the absence of selection, could likely explain the observed population differences in the field and the common garden, given the time since population establishment (≈12 generations) and heritability estimates from the literature. These N* values ranged from 1 to 23 (average 3–13) and are shown in Table 2. Since 1998, when systematic observations were begun, the population has remained stable at ≈160 birds (Yeh and Price 2004; Atwell J, unpublished data). Thus, given typical models of population growth, it is unlikely that the observed population differences could be due to drift alone in the absence of selection. Similarly, an explanation based on a founder effect would require that the maximum contributing number of founders was limited to fewer than ≈8 individuals. Microsatellite diversity in the colonist population suggests a minimum of ≈8–20 individuals established the population (Rasner et al. 2004).
Table 2.
Estimates of effective population above which drift would be unlikely to explain divergence
| Population difference | Heritabiltyc | Generations | Max N* |
| Exploratory behavior ♀ | 0.22–0.54 | 12 | 4–23 |
| Exploratory behavior ♂ | 0.22–0.54 | 12 | 1–8 |
| Max corticosteronea ♀ | 0.08–0.24 | 12 | 1–16 |
| CORT responsea ♀ | 0.08–0.24 | 12 | 2–23 |
| Max corticosteronea ♂ | 0.08–0.24 | 12 | 1–7 |
| CORT reponse (area)a ♂ | 0.08–0.24 | 12 | 1–4 |
| Flight distance (foraging)b | 0.88 | 12 | 9 |
| Flight distance (incubating)b ♀ | 0.88 | 12 | 15 |
| Max CORTb ♀ | 0.08–0.24 | 12 | 1–15 |
| CORT response (area)b ♀ | 0.08–0.24 | 12 | 1–15 |
Measured in common garden study.
Measured in field study.
Estimated from published studies, see MATERIALS AND METHODS.
Variation at Drd4 locus
As described above (see MATERIALS AND METHODS), the J. hyemalis sequences did not show the same polymorphism as in P. major (SNP830C/T); however, another polymorphism appeared to affect a neighboring amino acid. Only 3 individuals (of 59 screened) were found to be heterozygous at the new polymorphic locus, and none was homozygous for the mutant allele. Two of the heterozygotes were from the Mt Laguna population, and one from San Diego. Thus, the lack of variation found at these loci precluded any further analyses of the relationships between sequence variation and behavior.
DISCUSSION
Birds colonizing an urban environment are required to use novel food, water, and nesting resources, and they must interact with constant anthropogenic disturbances and highly diverse stimuli and stressors, including vehicles, humans, pets, lights, and noises associated with their urban environment. We found that free-living birds in a recently established (∼1983) urban colonist population exhibited reduced FID and attenuated endocrine (CORT) responses to handling. In a common garden study, in which birds from both populations were raised from early life under identical aviary conditions, we found faster and more extensive exploratory behavior in the colonist urban population, as well as persistent population differences in CORT levels. Importantly, in the common garden study, birds with higher maximum CORT levels were less bold, even after controlling for variation attributed to sex and population. This may shed light on both the hormonal basis of boldness and the potential that hormones may facilitate rapid evolution of correlated traits. Together, to the extent that common garden data can be used to draw inferences about genetic divergence, these findings provide unique evidence for rapid adaptive genetic evolution of behavior and a correlated hormonal mechanism after colonization of an urban environment.
Although we did not study natural or sexual selection gradients in the colonist versus ancestral range habitats, the differences in boldness behaviors and CORT profiles in the colonist population likely represent adaptive phenotypic changes that have allowed the colonist population to persist in this species-atypical urban environment. We acknowledge that one drawback of our study system is that by comparing only two populations there are limitations on the inferences that can be made—both with respect to the generality of the observed phenomena as well as the causality of which specific ecological factors may underlie population differences (Garland and Adolph 1994). However, our interpretation of the joint differences in behavior and CORT levels of the urban population are based on a priori predictions supported by studies in other systems that found differences either in one or the other trait (behavior: Møller 2008; Evans et al. 2010; CORT levels: Romero and Wikelski 2002; Partecke et al. 2006) associated with human-disturbed habitats. Colonization events such as that presented here provide rare opportunities to study evolution, and it is only by building up results from multiple studies that we can assess the generality of findings.
In most similar studies, it is unclear whether population differences are underlain by genetic change or phenotypic plasticity (e.g., habituation to frequent human disturbance). Persistence of character differences in a common garden provides strong evidence that the likely explanation for the current population differences in behavior and CORT is not plasticity. Accordingly, earlier molecular studies confirmed that the colonist population is genetically distinct from ancestral range montane populations at microsatellite loci (Rasner et al. 2004), and a previous common garden study also found persistent differences between these 2 populations in morphological characters (Rasner et al. 2004; Yeh 2004).
Based on our results, we propose that novel selective forces associated with the urban environment have likely led to rapid evolution of increased boldness and an attenuated endocrine stress response postcolonization, and that the population differences we found reflect divergent behavioral and physiological fitness optima in the colonist versus ancestral range habitats. Given the negative correlation between maximum CORT and exploratory behavior, it is plausible that these traits have evolved in concert, either in response to selection on boldness behavior or selection on CORT responsiveness via its modulation of other traits. It is also possible that correlational selection acted directly on hormone–phenotype coexpression. To test these hypotheses, future work should focus on the shape of natural or social selection gradients, and there is evidence from other systems that both exploratory behavior (Dingemanse et al. 2004) and CORT profiles (Breuner et al. 2008) are subject to selection in free-living songbird populations.
An alternative explanation is that the apparent genetic differences reported here are the result of drift. For example, it is possible that a founder population of individuals with tamer or bolder behavioral phenotypes and corresponding attenuations of the endocrine stress response may have been “preadapted” to establish an urban colonist population. Consistent with this alternative hypothesis, some studies on other species have found bolder or more exploratory individuals to disperse more or over longer distances (e.g., Dingemanse et al. 2003), but negative correlations between boldness and dispersal have also been reported (reviewed in Cote et al. 2010). We calculated that in our study system, the effective population size would have had to remain unrealistically small for several generations for any type of drift (including a founder effect) to explain the observed divergences in boldness behaviors and CORT observed in our studies (Lande 1976; Yeh 2004). Nevertheless, a nonrandom founder event in which only a small proportion of preadapted individuals became the sole genetic contributors to the small and relatively isolated colonist population cannot be ruled out—a scenario that invokes aspects of both selection and drift and has been described previously as “immigrant selection” (Brown and Lomolino 1998, p. 436).
Whether via selection or drift, this study provides some of the first evidence for rapid adaptive evolution of boldness behavior across natural populations. We are aware of at least 2 studies providing evidence for adaptive evolution of personality traits across populations of stickleback fish, apparently in response to varying predation pressures, but on much longer time scales than reported here (e.g. Dingemanse et al. 2007; and Herczeg et al. 2009). Another common garden study of birds reported that house sparrows (Passer domesticus) approached and consumed novel foods more quickly if they were members of an actively invading 28-year-old population as compared with members of a 150-year-old resident population, but the sparrows were adults when captured and the behavioral assays were substantially different from the ones employed here (Martin and Fitzgerald 2005).
Similarly, studies of population or subspecific variation in hormonal systems are rare, particularly those that evaluate developmental versus genetic underpinnings. In an exception and a system similar to ours, Partecke et al. (2006) found that urban blackbirds raised in a common garden showed attenuated CORT response to handling stress when compared with their wildland counterparts. Thus, our findings further extend the generalization that attenuated HPA responsiveness and reduced neophobia are associated with adaptation to urbanization.
Another common garden study also recently concluded that variation in the CORT response among closely related songbird species with varying life histories also likely has a genetic basis (Angelier et al. 2011). One particularly relevant life-history difference that has been hypothesized to underlie variation in glucocorticoid responses is the value of current versus future reproduction and survival opportunities, and comparative data across species support the idea that breeding season length correlates positively with HPA responsiveness to short-term stressors (Bokony et al. 2009). This would predict weaker not stronger response to stressors in the urban junco population which has an unusually long breeding season length (February–August) as compared with the species-typical May–July breeding season observed at Mt Laguna. This contrasting result to the usual among-species pattern suggests that in our case, the selective factors associated with urbanization are likely more important than those associated with length of the breeding season.
Although our findings suggest a genetic basis for behavioral and hormonal differences, environmental effects or early developmental effects could also play a role. The magnitude of the difference in CORT profiles between populations was not as strong in the common garden as in the field (although sample sizes were also smaller and absolute levels were generally lower in captivity), and reported heritabilities in other systems for exploratory behavior and CORT levels are moderate (≈0.2–0.4) (Dingemanse et al. 2002; van Oers et al. 2005; Evans et al. 2006), which leaves room for a substantial environmental contribution to the behavioral and hormonal phenotype. For example, further reduced CORT response in the urban population due to habituation to frequent stressors may be a likely explanation for the larger difference in CORT profiles in the field than in the common garden study.
Maternal effects are another type of developmental effects that could influence behavior in adulthood. For example, differential maternal allocation of egg yolk hormones can alter behavioral development in ways that persist into adulthood (Groothuis et al. 2005). For example, 9-month-old zebra finches (Taeniopygia gutatta) hatched from testosterone-treated eggs habituated faster in a test of neophobia (Tobler and Sandell 2007). Therefore, if junco females in the urban population deposited more androgens into their egg yolks, this might contribute to the faster exploratory behavior of San Diego birds. But most of the phenotypic differences between the San Diego and Laguna Mountain populations (e.g., breeding season length, male territoriality, plumage ornamentation) have been associated with lower testosterone not higher, which would predict lower androgen levels in the San Diego population during development (Yeh 2004; Newman et al. 2006). Thus differences in yolk androgens are unlikely to explain the population differences in behavior reported here. Exposure to elevated maternal and postnatal CORT and developmental stress treatments is also known to alter boldness behaviors and glucocorticoid levels even into adulthood (reviewed in Schoech et al. 2011), but the direction of the effects and diverse methodologies make generalizing the results of these studies challenging.
We also cannot rule out the possibility that early learning of exploratory behaviors could have taken place between hatching and capture for the common garden study. The period during which the juveniles were foraging independently (≤10–15 days on average) was far briefer than the time spent developing in the common environment prior to behavioral testing (>8 months), but little is known about critical periods for development of exploratory behavior. Notwithstanding that maternal or early developmental effects cannot be completely ruled out, we suggest that genetic evolution is the most likely cause for the persistent behavioral and hormonal differences in the colonist population.
Differences in exploratory behavior were not explained by allelic variation at the SNP830 region of the dopamine receptor gene (Drd4), as this region was found to be mostly invariant in our sample. A polymorphism at the SNP830 locus correlates with exploratory behavior in some populations of great tits (P. major), both captive and free-living, and this polymorphism responded to artificial selection for fast or slow exploratory behavior (Fidler et al. 2007; Korsten et al. 2010). However, many other loci with small effects are also expected to contribute to differences in boldness among individuals (van Oers, Drent, de Jong, et al. 2004; van Oers et al. 2005), especially since the allelic variation at SNP830 only explained 4.5–6.0% of the variation in behavior in the P. major study (Fidler et al. 2007; Korsten et al. 2010). The lack of variation at the Drd4 SNP830 in the junco suggests that the divergence in the urban population may have been achieved via other genes or alternative mutations within the larger Drd4 gene. This is unsurprising given that studies of 3 additional P. major populations found no clear association between SNP830 and behavior, suggesting this mutation was population specific (Korsten et al. 2010). The SNP830 region of the Drd4 gene was highly conserved in dark-eyed juncos, which enhances the prospects for future sequencing of other regions of the Drd4 gene, and across avian taxa, several other indels and SNP within Drd4 are being found (Abe et al. 2011).
In sum, our data show an integrated pattern of adaptive population divergence for a behavioral trait and a putative causal hormonal mechanism that persisted in a common environment, suggesting genetic evolution. The differences between populations lie in the direction predicted by within-population hormone-phenotype relationships, suggesting correlated evolution along lines of least resistance (Schluter 1996), rather than changes in reactions norms to adjust behavior independently of hormonal levels. Maintenance of individual covariation among CORT and boldness behaviors within divergent populations underscores the point that there is likely no single optimum hormone–behavior phenotypic strategy in a population, but rather a range of successful individual strategies maintained by functional trade-offs or opposing or variable selective factors (Koolhaas et al. 1999; Schoech et al. 2011).
Although evolutionary conservation of trait correlations, including hormone–phenotype associations, has most generally been considered evidence of a likely constraint on evolutionary diversification (Schluter 1996; Hau 2007), functionally sensible trait correlations could also facilitate adaptation to novel environments (Agrawal and Stinchcombe 2009; Ketterson et al. 2009). This may be the case in our study system, where changes in an integrating hormonal mechanism (the HPA endocrine axis) may underlie divergence for a suite of adaptive behavioral and physiological characteristics, allowing for more rapid adaptation (evolutionary or developmental) to novel environments. Additional studies of the neuroendocrine and genetic mechanisms linking hormonal and behavioral phenotypes are needed to assess the evolutionary significance of these trait associations. Future research should thus continue to focus both on identifying the mechanistic sources of variance underlying trait correlations and evaluating how integrated phenotypes respond to novel and changing environments.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://www.beheco.oxfordjournals.org/.
FUNDING
National Science Foundation (Grant #BSC05-19211 to E.D.K.; 0808284 to J.W.A. and E.D.K.); Indiana University Faculty Research Support Program and Graduate Student and Professional Organization; the Center for the Integrative Study of Animal Behaviour at Indiana University, the Indiana Academy of Sciences, the Explorer ' s Club, Sigma Xi, the Society for Integrative and Comparative Biology, and the Animal Behaviour Society.
Supplementary Material
Acknowledgments
We would like to thank the Descanso Ranger District of the Cleveland National Forest and the Ecology, Behavior & Evolution Division of Biology at University of California–San Diego (UCSD) for logistical support and permission to work in the study areas. K. Marchetti and R. Lande at UCSD, T. Price at University of Chicago, and the Al Bahr Shrine Camp on Mt Laguna also provided logistical support. Amanda Brothers, Eric Snajdr, Kim Roth, Sarah Puckett, Angela Kemsley, Allison Miller, and Russell Nichols assisted with fieldwork. Ediri Metitiri assisted with lab work. J.W.A. was supported by a Graduate Research Fellowship from the National Science Foundation and the Training Grant from National Institute of Health (NICHD: T32HD049336) and G.C.C. by a postdoctoral fellowship from the Fundação para a Ciência e a Tecnologia. This research was conducted with the approval of the Indiana University Animal Care and Use Committee (Study #06-242) and with permits from the US Fish and Wildlife Service, the California Department of Fish and Game, and the US Forest Service.
References
- Abe H, Ito S, Inoue-Murayama M. Polymorphisms in the extracellular region of dopamine receptor D4 within and among avian orders. J Mol Evol. 2011;72:253–264. doi: 10.1007/s00239-011-9432-9. [DOI] [PubMed] [Google Scholar]
- Agrawal AF, Stinchcombe JR. How much do genetic covariances alter the rate of adaptation? Proc R Soc Lond B Biol Sci. 2009;276:1183–1191. doi: 10.1098/rspb.2008.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelier F, Ballentine B, Holberton RL, Marra PP, Greenberg R. What drives variation in the corticosterone stress response between subspecies? A common garden experiment of swamp sparrows (Melospiza georgiana) J Evol Biol. 2011;24:1274–1283. doi: 10.1111/j.1420-9101.2011.02260.x. [DOI] [PubMed] [Google Scholar]
- Bokony V, Lendvai AZ, Liker A, Angelier F, Wingfield JC, Chastel O. Stress response and the value of reproduction: are birds prudent parents? Am Nat. 2009;173:589–598. doi: 10.1086/597610. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Patterson SH, Hahn TP. In search of relationships between the acute adrenocortical response and fitness. Gen Comp Endocrinol. 2008;157:288–295. doi: 10.1016/j.ygcen.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Wingfield JC, Romero LM. Diel rhythms of basal and stress-induced corticosterone in a wild, seasonal vertebrate, Gambel's white-crowned sparrow. J Exp Zool. 1999;284:334–342. [PubMed] [Google Scholar]
- Brown JH, Lomolino MV. Biogeography. 2nd ed. Sunderland (MA): Sinauer; 1998. [Google Scholar]
- Cardoso GC, Atwell JW. Directional cultural change by modification and replacement of memes. Evolution. 2011;65:295–300. doi: 10.1111/j.1558-5646.2010.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrete M, Tella JL. Individual consistency in flight initiation distances in burrowing owls: a new hypothesis on disturbance-induced habitat selection. Biol Lett. 2010;6:167–170. doi: 10.1098/rsbl.2009.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrem JF. Conservation and behavioral neuroendocrinology. Horm Behav. 2005;48:492–501. doi: 10.1016/j.yhbeh.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cote J, Clobert J, Brodin T, Fogarty S, Sih A. Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos Trans R Soc Lond B Biol Sci. 2010;365:4065–4076. doi: 10.1098/rstb.2010.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JM. Rapid evolution of urban birds. Nature. 1986;324:107–108. [Google Scholar]
- Dingemanse NJ, Both C, Drent PJ, Tinbergen JM. Fitness consequences of avian personalities in a fluctuating environment. Proc R Soc Lond B Biol Sci. 2004;271:847–852. doi: 10.1098/rspb.2004.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Both C, Drent PJ, Van Oers K, Van Noordwijk AJ. Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim Behav. 2002;64:929–938. [Google Scholar]
- Dingemanse NJ, Both C, van Noordwijk AJ, Rutten AL, Drent PJ. Natal dispersal and personalities in great tits (Parus major) Proc R Soc Lond B Biol Sci. 2003;270:741–747. doi: 10.1098/rspb.2002.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R, Dawnay N. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol. 2007;76:1128–1138. doi: 10.1111/j.1365-2656.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- Drent PJ, van Oers K, van Noordwijk AJ. Realized heritability of personalities in the great tit (Parus major) Proc R Soc Lond B Biol Sci. 2003;270:45–51. doi: 10.1098/rspb.2002.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Boudreau K, Hyman J. Behavioural syndromes in urban and rural populations of song sparrows. Ethology. 2010;116:588–595. [Google Scholar]
- Evans MR, Roberts ML, Buchanan KL, Goldsmith AR. Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. J Evol Biol. 2006;19:343–352. doi: 10.1111/j.1420-9101.2005.01034.x. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. Upper Saddle River (NJ): Longman; 1996. [Google Scholar]
- Fidler AE, van Oers K, Drent PJ, Kuhn S, Mueller JC, Kempenaers B. Drd4 gene polymorphisms are associated with personality variation in a passerine bird. Proc R Soc Lond B Biol Sci. 2007;274:1685–1691. doi: 10.1098/rspb.2007.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokidis HB, Orchinik M, Deviche P. Corticosterone and corticosteroid binding globulin in birds: relation to urbanization in a desert city. Gen Comp Endocrinol. 2009;160:259–270. doi: 10.1016/j.ygcen.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Garland T, Adolph SC. Why not to do 2-species comparative-studies—limitations on inferring adaptation. Physiol Zool. 1994;67:797–828. [Google Scholar]
- Gaston KJ. Urban ecology. Cambridge (UK): Cambridge University Press; 2010. [Google Scholar]
- Groothuis TGG, Muller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays. 2007;29:133–144. doi: 10.1002/bies.20524. [DOI] [PubMed] [Google Scholar]
- Herczeg G, Gonda A, Merila J. Predation mediated population divergence in complex behaviour of nine-spined stickleback (Pungitius pungitius) J Evol Biol. 2009;22:544–552. doi: 10.1111/j.1420-9101.2008.01674.x. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Atwell JW, McGlothlin JW. Phenotypic integration and independence: hormones, performance, and response to environmental change. Integr Comp Biol. 2009;49:365–379. doi: 10.1093/icb/icp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Korsten P, Mueller JC, Hermannstadter C, Bouwman KM, Dingemanse NJ, Drent PJ, Liedvogel M, Matthysen E, van Oers K, van Overveld T, et al. Association between Drd4 gene polymorphism and personality variation in great tits: a test across four wild populations. Mol Ecol. 2010;19:832–843. doi: 10.1111/j.1365-294X.2009.04518.x. [DOI] [PubMed] [Google Scholar]
- Korte SM, Beuving G, Ruesink W, Blokhuis HJ. Plasma catecholamine and corticosterone levels during manual restraint in chicks from a high and low feather pecking line of laying hens. Physiol Behav. 1997;62:437–441. doi: 10.1016/s0031-9384(97)00149-2. [DOI] [PubMed] [Google Scholar]
- Lande R. Natural-selection and random genetic drift in phenotypic evolution. Evolution. 1976;30:314–334. doi: 10.1111/j.1558-5646.1976.tb00911.x. [DOI] [PubMed] [Google Scholar]
- Lendvai AZ, Bokony V, Chastel O. Coping with novelty and stress in free-living house sparrows. J Exp Biol. 2011;214:821–828. doi: 10.1242/jeb.047712. [DOI] [PubMed] [Google Scholar]
- Martin LB, Fitzgerald L. A taste for novelty in invading house sparrows, Passer domesticus. Behavioral Ecology. 2005;16:702–707. doi: 10.1093/beheco/ari044. [Google Scholar]
- Martins TLF, Roberts ML, Giblin I, Huxham R, Evans MR. Speed of exploration and risk-taking behavior are linked to corticosterone titres in zebra finches. Horm Behav. 2007;52:445–453. doi: 10.1016/j.yhbeh.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Miller AH. Speciation in the avian genus Junco . Univ Calif Publ Zool. 1941;44:173–434. [Google Scholar]
- Møller AP. Flight distance of urban birds, predation, and selection for urban life. Behav Ecol Sociobiol. 2008;63:63–75. [Google Scholar]
- Møller AP, Fiedler W, Berthold P. Effects of climate change on birds. Oxford: Oxford University Press; 2010. [Google Scholar]
- Newman MM, Yeh PJ, Price TD. Reduced territorial responses in dark-eyed juncos following population establishment in a climatically mild environment. Anim Behav. 2006;71:893–899. [Google Scholar]
- Nolan V, Ketterson ED, Cristol DA, Rogers CM, Clotfelter ED, Titus RC, Schoech SJ, Snajdr E. Dark-eyed junco (Junco hyemalis). No. 716. In: Poole A, Gill F, editors. The birds of North America. Philadelphia (PA) and Washington (DC): The American Ornithologists Union; 2002. [Google Scholar]
- van Oers K, Buchanan KL, Thomas TE, Drent PJ. Correlated response to selection of testosterone levels and immunocompetence in lines selected for avian personality. Anim Behav. 2011;81:1055–1061. [Google Scholar]
- van Oers K, Drent PJ, de Goede P, van Noordwijk AJ. Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proc R Soc Lond B Biol Sci. 2004;271:65–73. doi: 10.1098/rspb.2003.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers K, Drent PJ, Dingemanse NJ, Kempenaers B. Personality is associated with extrapair paternity in great tits, Parus major. Anim Behav. 2008;76:555–563. [Google Scholar]
- van Oers K, Drent PJ, de Jong G, van Noordwijk AJ. Additive and nonadditive genetic variation in avian personality traits. Heredity. 2004;93:496–503. doi: 10.1038/sj.hdy.6800530. [DOI] [PubMed] [Google Scholar]
- van Oers K, de Jong G, van Noordwijk AJ, Kempenaers B, Drent PJ. Contribution of genetics to the study of animal personalities: a review of case studies. 2005;142:1185–1206. [Google Scholar]
- Partecke J, Schwabl I, Gwinner E. Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology. 2006;87:1945–1952. doi: 10.1890/0012-9658(2006)87[1945:satcua]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rasner CA, Yeh P, Eggert LS, Hunt KE, Woodruff DS, Price TD. Genetic and morphological evolution following a founder event in the dark-eyed junco, Junco hyemalis thurberi. Mol Ecol. 2004;13:671–681. doi: 10.1046/j.1365-294x.2004.02104.x. [DOI] [PubMed] [Google Scholar]
- Rensel MA, Schoech SJ. Repeatability of baseline and stress-induced corticosterone levels across early life stages in the Florida scrub-jay (Aphelocoma coerulescens) Horm Behav. 2011;59:497–502. doi: 10.1016/j.yhbeh.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Romero LM, Wikelski M. Exposure to tourism reduces stress-induced corticosterone levels in Galápagos marine iguanas. Biol Conserv. 2002;108:371–374. [Google Scholar]
- Schluter D. Adaptive radiation along genetic lines of least resistance. Evolution. 1996;50:1766–1774. doi: 10.1111/j.1558-5646.1996.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Ketterson ED, Nolan V, Sharp PJ, Buntin JD. The effect of exogenous testosterone on parental behavior, plasma prolactin, and prolactin binding sites in dark-eyed juncos. Horm Behav. 1998;34:1–10. doi: 10.1006/hbeh.1998.1455. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Heiss RS. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr Zool. 2011;57:514–530. [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Yeh P, Hunt K. Sound transmission and song divergence: a comparison of urban and forest acoustics. Condor. 2007;109:67–78. [Google Scholar]
- SPSS. Version 18.0. Chicago (IL): SPSS, Inc; 2010. [Google Scholar]
- Suarez AV, Yeh P, Case TJ. Impacts of Argentine ants on avian nesting success. Insectes Soc. 2005;52:378–382. [Google Scholar]
- Tobler M, Sandell MI. Yolk testosterone modulates persistence of neophobic responses in adult zebra finches, Taeniopygia guttata. Horm Behav. 2007;52:640–645. doi: 10.1016/j.yhbeh.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Unitt P. San Diego county bird atlas. San Diego (CA): San Diego Natural History Museum; 2005. [Google Scholar]
- Verbeek MEM, Drent PJ, Wiepkema PR. Consistent individual-differences in early exploratory-behavior of male great tits. Anim Behav. 1994;48:1113–1121. [Google Scholar]
- Wilson DS, Clark AB, Coleman K, Dearstyne T. Shyness and boldness in humans and other animals. Trends Ecol Evol. 1994;9:442–446. doi: 10.1016/0169-5347(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Yeh PJ. Rapid evolution of a sexually selected trait following population establishment in a novel habitat. Evolution. 2004;58:166–174. doi: 10.1111/j.0014-3820.2004.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Yeh PJ, Hauber ME, Price TD. Alternative nesting behaviours following colonisation of a novel environment by a passerine bird. Oikos. 2007;116:1473–1480. [Google Scholar]
- Yeh PJ, Price TD. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am Nat. 2004;164:531–542. doi: 10.1086/423825. [DOI] [PubMed] [Google Scholar]
- Zysling DA, Greives TJ, Breuner CW, Casto JM, Dernas GE, Ketterson ED. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis) Hormones and Behavior. 2006;50:200–207. doi: 10.1016/j.yhbeh.2006.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.