Abstract
Environmental conditions and physical constraints both influence an animal's behavior. We investigate whether behavioral variation among colonies of the black harvester ant, Messor andrei, remains consistent across foraging and disturbance situations and ask whether consistent colony behavior is affected by nest site and weather. We examined variation among colonies in responsiveness to food baits and to disturbance, measured as a change in numbers of active ants, and in the speed with which colonies retrieved food and removed debris. Colonies differed consistently, across foraging and disturbance situations, in both responsiveness and speed. Increased activity in response to food was associated with a smaller decrease in response to alarm. Speed of retrieving food was correlated with speed of removing debris. In all colonies, speed was greater in dry conditions, reducing the amount of time ants spent outside the nest. While a colony occupied a certain nest site, its responsiveness was consistent in both foraging and disturbance situations, suggesting that nest structure influences colony personality.
Keywords: behavioral syndromes, collective behavior, harvester ant, Messor andrei, nest structure, personality, plasticity, social insects, temperament
INTRODUCTION
Behavior changes in response to environmental conditions such as weather (Azcarate et al. 2007), predator abundance (Briffa et al. 2008), food abundance (Johnson et al. 2001), and interactions with other animals (Gordon 2011). Differences among individuals in physiological constraints (Stamps 2007), (Koolhaas et al. 2010), environmental tolerance (Biro et al. 2010; Pruitt et al. 2011), and genetic processes (van Oers and Mueller 2010) lead to consistent individual differences in behavior. The characteristics of an individual's behavior that consistently distinguish that individual from others have been called “personality” (Wilson et al. 1994; Gosling 2001), a “behavioral syndrome” (Sih, Bell, Johnson, and Ziemba 2004), or “temperament” (Reale et al. 2007).
Consistent individual differences in behavior include behavioral plasticity. Individuals may vary in how they adjust to changes in the environment (Dingemanse et al. 2010), which is why it is important to investigate animal personalities in natural environments (Reale et al. 2007; Archard and Braithwaite 2010). Animals change personality in the course of their development (Bell and Stamps 2004; Magnhagen and Staffan 2005; Muller et al. 2010; Hoset et al. 2011) or in response to environmental changes (Biro et al. 2010; Pruitt et al. 2011), which is referred to as “episodic personality” (Pronk et al. 2010). For example, individual coral reef fish differ consistently in both activity and aggression across a range of temperatures (Biro et al. 2010). Here, we examine how harvester ant colonies respond to changing environmental conditions, and whether differences among colonies in behavior remain consistent across situations.
Social insect colonies exhibit consistent individual differences in behavior and respond to changing environmental conditions. The behavior of a social insect colony emerges from the actions of the individual workers, but a colony is the reproductive unit, so selection acts at the colony level. As a result, differences among colonies in behavior may lead to variation in fitness (Gordon et al. 2011; Wray and Seeley 2011; Wray et al. 2011). Colonies adjust their behavior to changing conditions, for example, harvester ant colonies adjust foraging activity in response to weather (Gordon 1991; Brown and Gordon 2000; Azcarate et al. 2007; Cole et al. 2010). Colonies differ in the speed with which they respond to various environmental conditions (Leonard and Herbers 1986). Colonies whose ants move rapidly find food quickly (Pearce-Duvet et al. 2011), and rapid movement may reduce the risk of losing ants due to desiccation (Feener and Lighton 1991) or predation, thus promoting colony survival. Rapid detection and exploitation of a resource can be important in competitive interactions among colonies and among species (Gordon 1984; Hölldobler and Wilson 1990; Jones and Phillips 1990; Gordon and Kulig 1996; Davidson 1998).
A colony's nest morphology affects how it responds to the environment (Tofts 1993; Franks and Tofts 1994) and how workers interact with one another (Pinter-Wollman et al. 2011). For example, brood care (Sendova-Franks and Franks 1995; Powell and Tschinkel 1999; Jandt and Dornhaus 2009) and foraging (Powell and Tschinkel 1999; Mailleux et al. 2011) are affected by the spatial organization of workers inside the nest. Nest structure varies among colonies (Smallwood 1982; Tschinkel 2004). Just as differences among animals in morphological features may lead to consistent individual differences in behavior (Sih, Bell, and Johnson 2004; Sih, Bell, Johnson, and Ziemba 2004; Gyuris et al. 2011), colony nest structure may produce consistent differences in behavior. Here, we examine how colony differences in behavior can be predicted by nest site.
We investigated colony differences in the behavior of the black harvester ant, Messor andrei, in foraging and disturbance situations and under changing environmental conditions. Messor andrei ants forage for seeds from April to October (Brown 1999a; Brown and Gordon 2000) in weather conditions that range in temperature from 6 to 38 °C and in humidity from 16% to 100%. Colonies move among nest sites up to 10 times a year to increase the distance from neighboring colonies (Brown 1999a), allowing us to examine how nest site influences a colony's behavior. We examined variation among colonies in responsiveness, which we measured as the increase in activity in response to food and the decrease in activity on the nest mound in response to alarm stimulus. We also examined variation among colonies in the speed with which they retrieved food and removed debris. We considered the effects of changes in dew point and nest site on colony responsiveness and speed. We ask whether 1) the responsiveness and speed of M. andrei vary among colonies and among trials in both foraging and disturbance situations; 2) the behavior of colonies is correlated in foraging and disturbance situations; 3) colonies adjust their responsiveness and speed to weather changes; and 4) while a colony occupies a certain nest site, its responsiveness and speed are consistent across foraging and disturbance situations.
MATERIALS AND METHODS
Study site
The research was conducted at a 1-ha site in serpentine grassland at Jasper Ridge Biological Preserve, Stanford University, CA (122°12W, 36°25N). In April 2010, we conducted 1 m–wide transects throughout the hectare to locate all M. andrei colonies on the site. We located 24 colonies and tagged them using distinctive color-coded bamboo sticks. Behavioral observations were conducted in the spring (April 30th–June 19th, 2010) and fall (September 6th–October 12th, 2010). We monitored the location of all colonies twice weekly from April to October, using the methods of Brown (1999a). An M. andrei colony moves at most twice a month among nest sites (Brown 1999a). Because adjacent colonies rarely moved simultaneously and all colonies in the study plot were tagged, we were able to determine the identity of each colony that moved even when the move itself was not observed. All 24 colonies were observed in the spring 2010 observation period. Only 18 were observed in the fall 2010 observation period because 4 colonies moved outside the study site between June and September and an additional 2 adjacent colonies moved simultaneously so their identity could not be reliably determined.
Behavioral observations
We used experimental stimuli to examine colony differences in response to foraging and disturbance situations. To measure a change in colony activity, that is, responsiveness, we presented food bait and a disturbance, blowing air into the nest, the response to which is referred to as alarm hereafter. To measure how quickly colonies move objects, we presented seeds, which were brought into the nest, and toothpicks, which were removed as debris. A diagram of the experimental setup is in Supplementary Figure S1.
Responsiveness
We counted the number of ants on the nest mound before and after presenting a stimulus. The nest mound, which varied in size among colonies, was defined as the area around the nest entrance that was covered by seed chaff, mound areas ranged from 0.1 to 1 m2. We defined responsiveness as the difference in the number of ants on the nest mound before and after the presentation of a stimulus. The mean number of ants on the mound before a stimulus was presented was 57 ants.
Food bait
To measure changes in activity in response to food, we placed a piece of about 1 cm3 of apple on the nest mound, 15–20 cm from the nest entrance and from all existing foraging trails. We counted the numbers of ants on the nest mound before placing the piece of apple on the mound and the numbers of ants on the mound and on the piece of apple 20 min later. Preliminary observations indicated that the number of ants on the apple tended to remain about the same 10–15 min after the apple was placed on the nest mound.
Alarm
To measure changes in activity in response to a disturbance, we blew air 3 times, for 3 s each, at intervals of about 5 min, into the nest entrance using a plastic tube wrapped with mesh. The mesh-covered tube was then left at the nest entrance for 20 s to allow attacking ants to climb and latch on it. Attacking ants did not go on the nest mound but merely climbed the tube. In the interval between bouts of blowing into the nest, the ants that had climbed on the mesh were removed and placed into a plastic box to simulate removal of ants by a predator. Natural predators of M. andrei include spiders, assassin bugs (Brown M, personal communication), wasps, and army ants, which spend from 20 min and up to 2 days at a nest (Pinter-Wollman N, personal observation). We counted the number of ants on the mound before and after the 16–20 min it took to induce the alarm response. Air was blown into the nest by a single observer who always ate the same breakfast. The tube was cleaned with ethanol after each trial.
Speed
We measured how quickly (centimeters per minute) colonies brought seeds to the nest or removed debris that obstructed the nest entrance and could disrupt the movement of foragers in and out of the nest. During speed trials, we counted the number of ants on the nest mound before and after each trial (as described above for responsiveness) to determine whether speed trials also elicited recruitment of ants from the nest.
Seed retrieval
To measure colony speed, we measured the speed of seed retrieval (Azcarate et al. 2007). We placed a pod of French broom (Genista monspessulana), of average length 2cm, on an active foraging trail 30–70 cm from the nest entrance. The seedpod spanned the width of the active foraging trail (see Supplementary Figure S1), ensuring that any forager walking on the trail would encounter it. French broom is a nonnative plant that is not found in serpentine grassland, so all colonies were equally unfamiliar with this plant. We measured the distance of the pod from its starting position every minute for 20 min. The speed of seedpod retrieval, distance moved (cm) per time unit (min), was calculated from the minute the ants started moving the seedpod until either they brought it to the nest entrance or 20 min had elapsed. There was no relationship between the speed at which seedpods were retrieved and the distance from the nest entrance at which they were placed (Pearson's correlation: R 2 = 0.03, P value = 0.09).
Debris removal
To determine how quickly colonies removed debris that obstructed the nest entrance, we placed 6 toothpicks, 3 cm long, at the nest entrance (as in Gordon 1989a). This number of toothpicks was chosen to ensure that the nest entrance was sufficiently obstructed, and ants would respond to the stimulus. The distance of each toothpick from the nest entrance was measured every 2 min for 20 min. The speed of debris removal, distance moved (centimeters) per time unit (minutes), was calculated from the minute ants started moving the toothpick until they abandoned it on the nest mound or until 20 min had elapsed. Speeds for moving the 6 toothpicks in each trial were averaged.
To examine how colony behavior responded to changes in dew point, an absolute measure of humidity, we conducted 3 trials for each of 24 colonies in the spring observation period (April–June) and 3 trials for each of 18 colonies in the fall (September–October). During each trial, a colony was presented with one stimulus a day at intervals of 1–3 days. By the end of each trial, all colonies had been presented with each of the 4 stimuli. Each trial lasted 14 days in the spring for the 24 colonies observed and 10 days in the fall for the 18 colonies observed. Within each spring or fall observation period, 2–3 days elapsed between trials. To control for sequence effects, the stimuli were presented in a randomized order using a Latin square design (Sokal and Rohlf 1995). On each day at least 4 colonies were tested so that each stimulus was presented every day and each colony was assigned to one of 6 possible orders in which stimuli were presented. There was no significant effect of presentation order on response to the 4 stimuli, determined by using an analysis of variance (ANOVA) test. A diagram of the experimental timeline is in Supplementary Figure S2.
Nest site
Nest relocations occurred throughout the spring, summer, and fall. A given colony occupied from 1 to 6 unique nest sites and moved up to 8 times during the 6 months of the study and up to 3 times during the spring or fall observation period. From April to October, the 24 colonies occupied 64 unique nest sites. Only 2 sites were ever occupied by 2 different colonies in the course of our study. However, these sites were not occupied for long enough to allow us to gather sufficient data on their behavior to conduct further comparison.
Statistical analysis
To determine variation among colonies in behavior, and whether behavior was consistent over time, that is, whether there were differences among trials and among days, we used a nested ANOVA. The dependent variable was one of the 4 behavioral measures: responsiveness to food bait, responsiveness to alarm, speed of moving a seedpod, or speed of moving toothpicks. The independent variables were colony, trial, and day nested within trial, all treated as fixed categorical factors (Sokal and Rohlf 1995).
To test whether, within each trial, response to food bait was correlated with response to alarm and whether speed of retrieving a seedpod was correlated with speed of removing toothpicks, we used Pearson's correlation test.
To examine how behavioral variation among trials could be explained by changes in the weather, we used Pearson's correlation to test whether the behavioral response to each of the 4 stimuli was correlated with temperature, dew point, or relative humidity. To investigate how a colony's consistent response to the experimental stimuli was affected by weather, we used a principal component analysis to create a linear combination of responsiveness and of speed. The higher the values of the component combining responsiveness to food bait and responsiveness to alarm, the more ants recruited to food bait and the fewer ants came out of the nest in response to alarm. The higher the values of the component combining speed of moving toothpicks and speed of moving seed pods, the more rapidly the colony moved the objects. To test whether speed or responsiveness was correlated with dew point, we used Pearson's correlation. To examine if weather varied more among or within trials, we used an ANOVA.
To examine whether colony behavior was consistent while occupying a certain nest site, we first averaged the behavioral responses of each colony to a certain stimulus over all the trials in which it occupied a certain nest site. Then, to test whether within each nest site, response to food bait is correlated with response to alarm, and speed of retrieving a seed pod is correlated with speed of removing toothpicks, we used Pearson's correlation test. We removed 2 observations of 1 colony for responsiveness and 4 observations of 2 other colonies for speed because the colonies moved before both stimuli were presented at the same nest site. Some colonies moved back and forth between nest sites, so averaging the data by nest site sometimes included nonconsecutive trials. We tested whether the number of ants on the mound changed in response to each of the 4 stimuli using a Z-test. To examine whether variation in colony behavior was greater among or within nest sites, we used an ANOVA. The dependent variable was 1 of the 4 behavioral measures: responsiveness to food bait, responsiveness to alarm, speed of moving a seedpod, or speed of moving toothpicks. The independent variable was nest site, treated as a fixed categorical factor.
To meet the normality assumptions of all statistical tests used, both measures of speed, moving a seedpod and moving toothpicks, were normalized using a logarithmic transformation after adding 0.5 to include colonies that did not respond to our stimuli (Sokal and Rohlf 1995). Data for one colony were removed from the analysis of response to alarm and from any analyses that required a comparison with response to alarm because the counts of ants on the nest mound at the start of 2 of its alarm response observations were not reliable. Statistical analyses were carried out in R version 2.11.1.
RESULTS
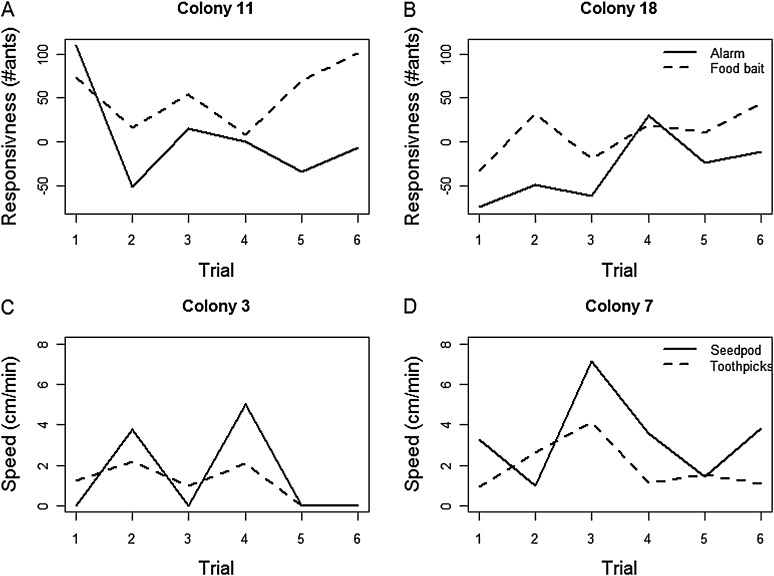
Colonies differed in their response to the 4 stimuli (Figure 1). There was a significant effect of colony (Table 1), for response to food bait, response to alarm, and the speed of removing toothpicks. The behavior of all colonies was not consistent among trials (Figure 1 and Table 1) but was consistent among days within a trial (Table 1). The nested ANOVA, including colony, trial, and day nested within trial explained most of the variation among colonies in responsiveness to food bait and speed of moving toothpicks (Table 1). The overall model approached significance for the response to alarm but was not a good fit to the speed of moving a seedpod, possibly due to other factors that were not measured or due to low variation in speed of moving a seedpod.
Figure 1.
Examples of behavioral variation among colonies and trials and of consistent behavior across disturbance and foraging situations. Each panel shows the behavior of a single colony in response to 2 stimuli during the 6 trials: responsiveness (A and B) to alarm (solid line) and to food bait (dashed line); speed (C and D) of moving a seedpod (solid line) and toothpicks (dashed line).
Table 1.
ANOVA results for the effects of colony, trial, and day nested in trial on each of the 4 behaviors
| Behavior | Overall model statistics | Effect statistics | ||||||||||
| Colony | Trial | Day (nested in trial) | ||||||||||
| Adjusted R | F | P | df | F | P | df | F | P | df | F | P | |
| Response to food bait | 0.42 | 2.16 | <0.01 | 22 | 1.97 | 0.03 | 5 | 6.39 | <0.001 | 46 | 1.86 | 0.02 |
| Response to alarm | 0.23 | 1.49 | 0.07 | 22 | 2.6 | <0.01 | 5 | 4.28 | 0.01 | 45 | 1.01 | 0.48 |
| Speed of moving seedpod | 0.16 | 1.32 | 0.15 | 23 | 1.19 | 0.29 | 5 | 2.76 | 0.03 | 48 | 1.12 | 0.35 |
| Speed of moving toothpicks | 0.33 | 1.81 | 0.01 | 23 | 1.89 | 0.03 | 5 | 5.99 | <0.001 | 47 | 1.23 | 0.24 |
Values in bold are statistically significant.
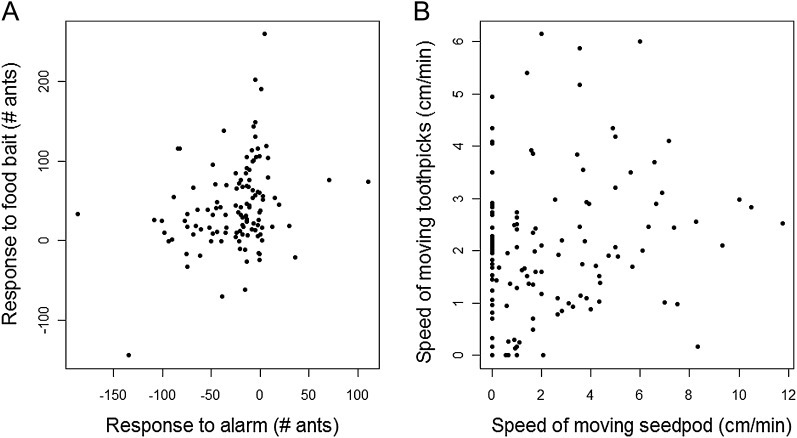
All colonies responded consistently across foraging and disturbance situations (Figures 1 and 2). Within each trial, there was a significant correlation between the response to alarm and response to food bait (Pearson's correlation: R = 0.28, P value = 0.002; Figures 1A,B and 2A) and between the speed of moving toothpicks and speed of moving a seedpod (Pearson's correlation: R = 0.18, P value = 0.03; Figures 1C,D and 2B).
Figure 2.
Consistent behavior across foraging and disturbance situations within each trial. (A) Responsiveness—difference in number of ants on mound (Pearson's correlation: R = 0.28, P value = 0.002); (B) speed—centimeters per minute (Pearson's correlation: R = 0.18, P value = 0.03).
All colonies responded to dew point, the temperature at which water vapor condenses into water, which is an absolute measure of humidity, the higher the dew point, the higher the humidity at high temperature. Each of the 4 types of behavior measured, and the 2 linear combinations that represent consistent colony behavior across situations, changed in relation to dew point. Ants were slower at high dew point, when it was humid at high temperatures: both the speed of moving seedpods and the speed of moving toothpicks were each negatively correlated with dew point (Pearson's correlation: speed of moving seedpod: R = −0.17, P value = 0.05; toothpicks: R = −0.26, P value = 0.004). Ants retreated into the nest in response to an alarm stimulus, and fewer ants recruited to food bait when it was dry at high temperatures: response to an alarm stimulus was positively correlated with dew point (Pearson's correlation: R = 0.18, P value = 0.05) and responsiveness to food bait approached a significant positive correlation with dew point (Pearson's correlation: R = 0.17, P value = 0.069). Both linear combinations of responsiveness and of speed varied among trials (responsiveness: ANOVA: F = 5.72, degrees of freedom [df] = 5, P value < 0.0001; speed: ANOVA: F = 4.82, df = 5, P value < 0.001). This variation among trials in responsiveness and speed was explained by changes in dew point: responsiveness was greater when it was humid (Pearson's correlation: R = 0.23, P value = 0.01) and colonies were faster when conditions were drier (Pearson's correlation: R = −0.29, P value = 0.001). Dew point varied more among than within trials (ANOVA: F = 9.7, df = 5, P value < 0.0001). Temperature and relative humidity were not significantly associated with responsiveness or speed.
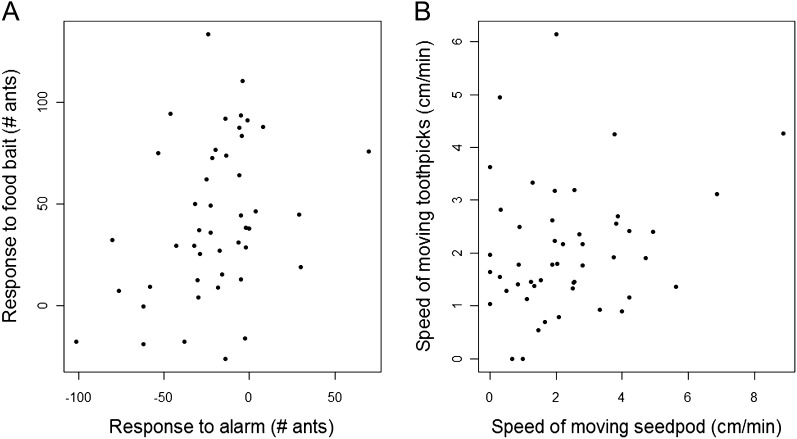
Nest site influenced the responsiveness, but not the speed, of all colonies (Figure 3). When a colony was in a certain nest site its response to food bait was significantly correlated with its response to alarm (Pearson's correlation: R = 0.35, P value = 0.01; Figure 3A). However, the speed at which a colony moved seedpods was not correlated with its speed of moving toothpicks while it occupied a certain nest site (Pearson's correlation: R = 0.16, P value = 0.27; Figure 3B). Changes in numbers of ants on the nest mound were observed only in response to food bait and alarm (Z-test: response to food bait: Z = 0.06, P value < 0.0001; response to alarm: Z = −7.35, P value < 0.001) but not in response to seedpods and toothpicks (Z-test: speed of moving seedpod: Z = −0.46, P value = 0.64; speed of moving toothpicks: Z = −1.69, P value = 0.09). We found that colonies differed more among than within nest sites in their alarm response (ANOVA: F = 1.73, df = 44, P value = 0.02) and in their speed of moving toothpicks (ANOVA: F = 1.58, df = 48, P value = 0.04). However, colonies did not move toothpicks faster when occupying a nest in which more ants retreated from an alarm stimulus: response to alarm was not significantly correlated with speed of moving toothpicks while a colony occupied a certain nest site (Pearson's correlation: R = −0.03, P value = 0.86).
Figure 3.
Consistent behavior across foraging and disturbance situations while a colony occupies a certain nest site. (A) Responsiveness—difference in number of ants on mound (Pearson's correlation: R = 0.35, P value = 0.01); (B) speed—centimeters per minute (Pearson's correlation: R = 0.16, P value = 0.27).
DISCUSSION
Colonies of M. andrei differed in their behavior. Variation among individuals in behavior is common in many animals (Lott 1991). In social insects, variation can occur at both worker and colony levels. Behavioral variation among workers of social insects is ubiquitous (Wilson 1976; Jaisson et al. 1988). Because the colony is the unit of reproduction, natural selection acts on variation among colonies. Such variation has been documented in several species: ant colonies of Rhytidoponera confuse vary in aggression (Crosland 1990); Temnothorax albipennis colonies vary in speed of finding new nest sites (Franks et al. 2006); and harvester ant colonies, both Pogonomyrmex barbatus and P. occidentalis, vary in foraging behavior (Gordon 1991; Gordon et al. 2008; Cole et al. 2010; Gordon et al. 2011). We found that M. andrei colonies vary in recruitment to food bait, response to alarm, and speed of debris removal. The only behavior that did not vary significantly among colonies was how fast they moved a seedpod of a novel plant, even though other studies of individual differences in behavior find variation among individuals in response to novel stimuli (Reale et al. 2007). It is possible that the seeds were not perceived as novel because they were similar in odor to local plants (Lanza et al. 1992; Pfeiffer et al. 2010). As in other Messor species (Azcarate et al. 2007), environmental conditions may have a stronger influence than colony variation on the speed of seed retrieval.
We found that behavioral variation of M. andrei colonies was consistent across foraging and disturbance situations. It is unlikely that this is due to consistent behavior of individual workers because foraging and disturbance stimuli were presented on different days, and each ant does not necessarily work every day (Gordon et al. 2005). In addition, foraging and response to disturbance were probably performed by ants of different task groups, for example, seedpod retrieval by foragers and toothpick removal by nest maintenance workers (Gordon 1989a). Two other recent studies have shown consistent behavior of social insect colonies across situations: colonies of the red harvester ant, P. barbatus, vary consistently in the tempo of patrolling activity and in the regulation of foraging (Gordon et al. 2011) and colonies of the honeybee, Apis mellifera, behave consistently across defense and foraging situations (Wray et al. 2011). Here, we show that consistent colony behavior is influenced by its nest site, that is, morphology.
Colony behavior that required interaction among ants inside the nest was influenced by nest site, whereas behavior that occurred outside the nest was not. Responses to the 2 stimuli, food bait and alarm, that affected ant activity on the nest mound, were consistent across situations while a colony occupied a certain nest. The initiation and regulation of foraging activity in red harvester ants depend on the rate of interaction among ants inside the nest (Schafer et al. 2006; Greene and Gordon 2007; Gordon et al. 2008). Nest structure may determine where ants interact. For example, interaction hot spots occur where ants exit from tunnels into nest chambers (Pinter-Wollman et al. 2011). Nest structure also influences how quickly ants move; for example, the presence of an obstruction near the nest entrance slows ant traffic flow (Burd et al. 2010). Thus, nest structure that facilitates many interactions or rapid ant movement may expedite a colony's response to alarm or increase the numbers and walking speed of ants recruited to food bait. Just as morphology constrains an animal's behavior and variation in morphology may lead to consistent variation across situations (Sih, Bell, and Johnson 2004; Sih, Bell, Johnson, and Ziemba 2004; Gyuris et al. 2011), a colony's nest structure is a physical attribute (Tschinkel 2004) that may play an important role in determining colony personality. In contrast, behaviors, which were performed solely outside the nest and did not change ant activity on the mound, that is, seed retrieval and debris removal, were not consistent across situations while a colony occupied a certain nest. How fast an ant moves items outside the nest in various situations does not depend on nest site and may vary in response to changes in weather (Azcarate et al. 2007).
A colony can modify the structure of its nest and choose which nest site it occupies, actively manipulating its environment, but it cannot control other environmental conditions, such as weather. Nest structure emerges from the collective behavior of the workers (Theraulaz et al. 2003) and varies among species (Tschinkel 2011b) and colonies due to age (Tschinkel 2011a) and worker composition (Tschinkel 2005). So worker activity determines the structure of their nest. However, not all species excavate nests all the time, some occupy existing structures built by other colonies, for example, M. andrei, or move among naturally occurring crevices, e.g., Temnothorax. Niche-picking, choosing where to live, often produces consistent behavioral responses (Stamps and Groothuis 2010). Many ant species relocate among nest sites (Smallwood 1982) thus manipulating their physical environment. Nest relocation results in changes to colony demography (Herbers 1986), increased distance from conspecifics (Brown 1999a), and improved nest quality (Dornhaus et al. 2004). A Messor colony's decision to relocate to a new nest may result in changes to its foraging success because nest sites may vary in structure and therefore, in how they influence colony behavior. Environmental conditions other than nest site, such as weather, are beyond a colony's control and they too may influence how a colony behaves.
Colonies of M. andrei adjusted their behavior in response to dew point, as it changes over time. Dew point is an absolute measure of humidity, the higher the dew point, the higher the humidity at high temperatures. Desiccation has an important cost to ants while outside the nest because water is lost through the ants' permeable cuticle (Lighton and Feener 1989). We found that at low dew point, when the air is drier, ants move faster while retrieving seeds and removing debris and thus spend less time outside. In dry conditions, colonies also recruited fewer ants to food bait and recalled more ants into the nest in response to an alarm. A previous study at the same site showed that ants of M. andrei forage further from the nest in the fall, when dew point is higher, than in the spring (Brown and Gordon 2000). As in other animals, environmental constraints link behavioral responses across various situations (Biro et al. 2010; Pruitt et al. 2011).
Energetic requirements, colony composition, and growth probably influence the behavior of M. andrei colonies. Winged reproductives are more costly to produce than are workers (Mackay 1985; Smith 2007). Colonies of M. andrei produce reproductives during the weeks before the mating flight in June and July (Brown 1999b). This may account for the more rapid seed retrieval, which could result in higher food and energy intake per time unit that we observed in the spring, while colonies are producing reproductives. Individual variation among workers in activity, within each task, determines the behavior of the colony as a whole, as the behavior of other social groups as a whole is determined by the distribution of the various personalities of the individuals comprising them (Crosland 1990; Sih and Watters 2005; Paleolog 2009; Kurvers et al. 2010; Modlmeier and Foitzika 2011; Pruitt and Riechert 2011). Variation among colonies in the distribution of worker activity levels results in behavioral variation among colonies (Crosland 1990; Paleolog 2009; Pinter-Wollman et al. 2011; Pinter-Wollman 2012). Workers move from one task to another as they age (Calderone 1995; Gordon et al. 2005; Seid and Traniello 2006; Camargo et al. 2007) and in response to environmental perturbations (Gordon 1989a, 1996). Such temporal changes in the distribution of worker activities determine the colony's response to its environment. For example, when more ants are allocated to foraging, the colony collects more food (Gordon 1989b). These natural changes in the group composition of social insect colonies provide ample opportunities for studying how consistent behaviors change over the course of an individual's lifetime (Stamps and Groothuis 2010).
We showed that consistent individual variation among ant colonies is affected by their nest sites and by their response to humidity. Such individual variation at the colony level may have important consequences for colony fitness. Because the reproductive unit of social insects, the colony, comprised many modular subunits, workers, social insects provide unique opportunities to investigate the mechanisms underlying consistent individual variation. Understanding what produces consistent behavior in social insects may shed light on the causes and consequences of consistent individual differences in behavior of other animals.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at http://www.beheco.oxfordjournals.org/.
FUNDING
National Science Foundation Biological Informatics Postdoctoral Fellowship to N.P.-W.; National Institutes of Health (5-R01GM086884 to S.H.).
Supplementary Material
Acknowledgments
We would like to thank Alec Mill and Marguerite Stevens for help collecting data and Mira Parekh, Dan Quinn, and Anne Rosenthal for their help tracking colony relocations over the summer. We thank Mark Brown for his helpful advice during the initial stages of this work and for his constructive comments on the manuscript, Judy Stamps for valuable advice, and Andrew Merrell and Maxine Zylberberg for helpful comments on the manuscript.
References
- Archard GA, Braithwaite VA. The importance of wild populations in studies of animal temperament. J Zool. 2010;281:149–160. [Google Scholar]
- Azcarate FM, Kovacs E, Peco B. Microclimatic conditions regulate surface activity in harvester ants Messor barbarus . J Insect Behav. 2007;20:315–329. [Google Scholar]
- Bell AM, Stamps JA. Development of behavioural differences between individuals and populations of sticklebacks, Gasterosteus aculeatus . Anim Behav. 2004;68:1339–1348. [Google Scholar]
- Biro PA, Beckmann C, Stamps JA. Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc R Soc Lond B Biol Sci. 2010;277:71–77. doi: 10.1098/rspb.2009.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa M, Rundle SD, Fryer A. Comparing the strength of behavioural plasticity and consistency across situations: animal personalities in the hermit crab Pagurus bernhardus . Proc R Soc B Biol Sci. 2008;275:1305–1311. doi: 10.1098/rspb.2008.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJF. Nest relocation and encounters between colonies of the seed-harvesting ant Messor andrei . Insectes Soc. 1999a;46:66–70. [Google Scholar]
- Brown MJF. Semi-claustral founding and worker behaviour in gynes of Messor andrei . Insectes Soc. 1999b;46:194–195. [Google Scholar]
- Brown MJF, Gordon DM. How resources and encounters affect the distribution of foraging activity in a seed-harvesting ant. Behav Ecol Sociobiol. 2000;47:195–203. [Google Scholar]
- Burd M, Shiwakoti N, Sarvi M, Rose G. Nest architecture and traffic flow: large potential effects from small structural features. Ecol Entomol. 2010;35:464–468. [Google Scholar]
- Calderone NW. Temporal division-of-labor in the honey-bee, Apis mellifera—a developmental process or the result of environmental influences. Can J Zool. 1995;73:1410–1416. [Google Scholar]
- Camargo RS, Forti LC, Lopes JFS, Andrade APP, Ottati ALT. Age polyethism in the leaf-cutting ant Acromyrmex subterraneus brunneus Forel, 1911 (Hym., Formicidae) J Appl Entomol. 2007;131:139–145. [Google Scholar]
- Cole BJ, Smith AA, Huber ZJ, Wiernasz DC. The structure of foraging activity in colonies of the harvester ant, Pogonomyrmex occidentalis . Behav Ecol. 2010;21:337–342. [Google Scholar]
- Crosland MWJ. Variation in ant aggression and kin discrimination ability within and between colonies. J Insect Behav. 1990;3:359–379. [Google Scholar]
- Davidson DW. Resource discovery versus resource domination in ants: a functional mechanism for breaking the trade-off. Ecol Entomol. 1998;23:484–490. [Google Scholar]
- Dingemanse NJ, Kazem AJN, Reale D, Wright J. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol Evol. 2010;25:81–89. doi: 10.1016/j.tree.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Dornhaus A, Franks NR, Hawkins RM, Shere HNS. Ants move to improve: colonies of Leptothorax albipennis emigrate whenever they find a superior nest site. Anim Behav. 2004;67:959–963. [Google Scholar]
- Feener DH, Lighton JRB. Is foraging in the desert ant, Messor-pergandei (Hymenoptera, Formicidae), limited by water. Ecol Entomol. 1991;16:183–191. [Google Scholar]
- Franks NR, Dornhaus A, Best CS, Jones EL. Decision making by small and large house-hunting ant colonies: one size fits all. Anim Behav. 2006;72:611–616. [Google Scholar]
- Franks NR, Tofts C. Foraging for work—how tasks allocate workers. Anim Behav. 1994;48:470–472. [Google Scholar]
- Gordon DM. Species-specific patterns in the social activities of harvester ant colonies (Pogonomyrmex) Insectes Soc. 1984;31:74–86. [Google Scholar]
- Gordon DM. Dynamics of task switching in harvester ants. Anim Behav. 1989a;38:194–204. [Google Scholar]
- Gordon DM. Caste and change in social insects. In: Harvey PH, Partridge L, editors. Oxford surveys in evolutionary biology. Oxford: Oxford University Press; 1989b. pp. 56–72. [Google Scholar]
- Gordon DM. Behavioral flexibility and the foraging ecology of seed-eating ants. Am Nat. 1991;138:379–411. [Google Scholar]
- Gordon DM. The organization of work in social insect colonies. Nature. 1996;380:121–124. [Google Scholar]
- Gordon DM. The fusion of behavioral ecology and ecology. Behav Ecol. 2011;22:225–230. [Google Scholar]
- Gordon DM, Chu J, Lillie A, Tissot M, Pinter N. Variation in the transition from inside to outside work in the red harvester ant Pogonomyrmex barbatus . Insectes Soc. 2005;52:212–217. [Google Scholar]
- Gordon DM, Guetz A, Greene MJ, Holmes S. Colony variation in the collective regulation of foraging by harvester ants. Behav Ecol. 2011;22:429–435. doi: 10.1093/beheco/arq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM, Holmes S, Nacu S. The short-term regulation of foraging in harvester ants. Behav Ecol. 2008;19:217–222. doi: 10.1093/beheco/arq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM, Kulig AW. Founding, foraging, and fighting: colony size and the spatial distribution of harvester ant nests. Ecology. 1996;77:2393–2409. [Google Scholar]
- Gosling SD. From mice to men: what can we learn about personality from animal research? Psychol Bull. 2001;127:45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- Greene MJ, Gordon DM. Interaction rate informs harvester ant task decisions. Behav Ecol. 2007;18:451–455. [Google Scholar]
- Gyuris E, Fero O, Tartally A, Barta Z. Individual behaviour in firebugs (Pyrrhocoris apterus) Proc R Soc Lond B Biol Sci. 2011;278:628–633. doi: 10.1098/rspb.2010.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers JM. Nest site limitation and facultative polygyny in the ant Leptothorax longispinosus . Behav Ecol Sociobiol. 1986;19:115–122. [Google Scholar]
- Hölldobler B, Wilson EO. The ants. Harvard (MA): Harvard University Press; 1990. [Google Scholar]
- Hoset KS, Ferchaud AL, Dufour F, Mersch D, Cote J, Le Galliard JF. Natal dispersal correlates with behavioral traits that are not consistent across early life stages. Behav Ecol. 2011;22:176–183. [Google Scholar]
- Jaisson P, Fresneau D, Lachaud J-P. Individual traits of social behaviour in ants. In: Jeanne RL, editor. Interindividual behavioral variability in social insects. Boulder (CO): Westview Press; 1988. pp. 1–51. [Google Scholar]
- Jandt JM, Dornhaus A. Spatial organization and division of labour in the bumblebee Bombus impatiens . Anim Behav. 2009;77:641–651. [Google Scholar]
- Johnson CJ, Parker KL, Heard DC. Foraging across a variable landscape: behavioral decisions made by woodland caribou at multiple spatial scales. Oecologia. 2001;127:590–602. doi: 10.1007/s004420000573. [DOI] [PubMed] [Google Scholar]
- Jones SR, Phillips SA. Resource collecting abilities of Solenopsis invicta (Hymenoptera, Formicidae) compared with those of 3 sympatric Texas ants. Southwest Nat. 1990;35:416–422. [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31:307–321. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kurvers R, Prins HHT, van Wieren SE, van Oers K, Nolet BA, Ydenberg RC. The effect of personality on social foraging: shy barnacle geese scrounge more. Proc R Soc B Biol Sci. 2010;277:601–608. doi: 10.1098/rspb.2009.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza J, Schmitt MA, Awad AB. Comparative chemistry of elaiosomes of 3 species of Trillium. J Chem Ecol. 1992;18:209–221. doi: 10.1007/BF00993754. [DOI] [PubMed] [Google Scholar]
- Leonard JG, Herbers JM. Foraging tempo in 2 woodland ant species. Anim Behav. 1986;34:1172–1181. [Google Scholar]
- Lighton JRB, Feener DH. Water-loss rate and cuticular permeability in foragers of the desert ant Pogonomyrmex rugosus . Physiol Zool. 1989;62:1232–1256. [Google Scholar]
- Lott DF. Intraspecific variation in the social systems of wild vertebrates. Cambridge (MA): Cambridge University Press; 1991. [Google Scholar]
- Mackay WP. A comparison of the energy budgets of 3 species of Pogonomyrmex harvester ants (Hymenoptera, Formicidae) Oecologia. 1985;66:484–494. doi: 10.1007/BF00379338. [DOI] [PubMed] [Google Scholar]
- Magnhagen C, Staffan F. Is boldness affected by group composition in young-of-the-year perch (Perca fluviatilis)? Behav Ecol Sociobiol. 2005;57:295–303. [Google Scholar]
- Mailleux AC, Sempo G, Depickere S, Detrain C, Deneubourg JL. How does starvation affect spatial organization within nests in Lasius niger? Insectes Soc. 2011;58:219–225. [Google Scholar]
- Modlmeier AP, Foitzika S. Productivity increases with variation in aggression among group members in Temnothorax ants. Behav Ecol. 2011 doi: 10.1093/beheco/arr086. [Google Scholar]
- Muller H, Grossmann H, Chittka L. ‘Personality’ in bumblebees: individual consistency in responses to novel colours? Anim Behav. 2010;80:1065–1074. [Google Scholar]
- van Oers K, Mueller JC. Evolutionary genomics of animal personality. Philos Trans R Soc B Biol Sci. 2010;365:3991–4000. doi: 10.1098/rstb.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleolog J. Behavioural characteristics of honey bee (Apis mellifera) colonies containing mix of workers of divergent behavioural traits. Anim Sci Pap Rep. 2009;27:237–248. [Google Scholar]
- Pearce-Duvet JMC, Elemans CPH, Feener DH. Walking the line: search behavior and foraging success in ant species. Behav Ecol. 2011;22:501–509. [Google Scholar]
- Pfeiffer M, Huttenlocher H, Ayasse M. Myrmecochorous plants use chemical mimicry to cheat seed-dispersing ants. Funct Ecol. 2010;24:545–555. [Google Scholar]
- Pinter-Wollman N. Personality in social insects: how does worker personality determine colony personality? Curr Zool. Forthcoming 2012 [Google Scholar]
- Pinter-Wollman N, Wollman R, Guetz A, Holmes S, Gordon DM. The effect of individual variation on the structure and function of interaction networks in harvester ants. J R Soc Interface. 2011;8:1562–1573. doi: 10.1098/rsif.2011.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S, Tschinkel WR. Ritualized conflict in Odontomachus brunneus and the generation of interaction-based task allocation: a new organizational mechanism in ants. Anim Behav. 1999;58:965–972. doi: 10.1006/anbe.1999.1238. [DOI] [PubMed] [Google Scholar]
- Pronk R, Wilson DR, Harcourt R. Video playback demonstrates episodic personality in the gloomy octopus. J Exp Biol. 2010;213:1035–1041. doi: 10.1242/jeb.040675. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Demes KW, Dittrich-Reed DR. Temperature mediates shifts in individual aggressiveness, activity level, and social behavior in a spider. Ethology. 2011;117:318–325. [Google Scholar]
- Pruitt JN, Riechert SE. How within-group behavioural variation and task efficiency enhance fitness in a social group. Proc R Soc B Biol Sci. 2011;278:1209–1215. doi: 10.1098/rspb.2010.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol Rev. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Holmes S, Gordon DM. Forager activation and food availability in harvester ants. Anim Behav. 2006;71:815–822. doi: 10.1016/j.anbehav.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seid MA, Traniello JFA. Age-related repertoire expansion and division of labor in Pheidole dentata (Hymenoptera: Formicidae): a new perspective on temporal polyethism and behavioral plasticity in ants. Behav Ecol Sociobiol. 2006;60:631–644. [Google Scholar]
- Sendova-Franks AB, Franks NR. Spatial relationships within nests of the ant Leptothorax unifasciatus (Latr.) and their implications for the division of labour. Anim Behav. 1995;50:121–136. [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE. Behavioral syndromes: an integrative overview. Q Rev Biol. 2004;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- Sih A, Watters JV. The mix matters: behavioural types and group dynamics in water striders. Behaviour. 2005;142:1417–1431. [Google Scholar]
- Smallwood J. Nest relocation in ants. Insectes Soc. 1982;29:138–147. [Google Scholar]
- Smith CR. Energy use and allocation in the Florida harvester ant, Pogonomyrmex badius: are stored seeds a buffer? Behav Ecol Sociobiol. 2007;61:1479–1487. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York: W.H. Freeman and Company; 1995. [Google Scholar]
- Stamps JA. Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol Lett. 2007;10:355–363. doi: 10.1111/j.1461-0248.2007.01034.x. [DOI] [PubMed] [Google Scholar]
- Stamps JA, Groothuis TGG. The development of animal personality: relevance, concepts and perspectives. Biol Rev. 2010;85:301–325. doi: 10.1111/j.1469-185X.2009.00103.x. [DOI] [PubMed] [Google Scholar]
- Theraulaz G, Gautrais J, Camazine S, Deneubourg JL. The formation of spatial patterns in social insects: from simple behaviours to complex structures. Philos Trans R Soc Lond A. 2003;361:1263–1282. doi: 10.1098/rsta.2003.1198. [DOI] [PubMed] [Google Scholar]
- Tofts C. Algorithms for task allocation in ants—(a study of temporal polyethism—theory) Bull Math Biol. 1993;55:891–918. [Google Scholar]
- Tschinkel W. The nest architecture of the Florida harvester ant, Pogonomyrmex badius . J Insect Sci. 2004;21:1–19. doi: 10.1093/jis/4.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschinkel WR. The nest architecture of the ant, Camponotus socius . J Insect Sci. 2005;5:9–27. doi: 10.1093/jis/5.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschinkel WR. Back to basics: sociometry and sociogenesis of ant societies (Hymenoptera: Formicidae) Myrmecol News. 2011a;14:49–54. [Google Scholar]
- Tschinkel WR. The nest architecture of three species of north Florida Aphaenogaster ants. J Insect Sci. 2011b;11:1–30. doi: 10.1673/031.011.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS, Clark AB, Coleman K, Dearstyne T. Shyness and boldness in humans and other animals. Trends Ecol Evol. 1994;9:442–446. doi: 10.1016/0169-5347(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Wilson EO. Behavioral discretization and the number of castes in an ant species. Behav Ecol Sociobiol. 1976;1:141–154. [Google Scholar]
- Wray MK, Mattila HR, Seeley TD. Collective personalities in honeybee colonies are linked to colony fitness. Anim Behav. 2011;81:559–568. [Google Scholar]
- Wray MK, Seeley TD. Consistent personality differences in house-hunting behavior but not decision speed in swarms of honey bees (Apis mellifera) Behav Ecol Sociobiol. 2011;65:2061–2070. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.