Abstract
β-Transducin repeats-containing protein (β-TrCP) is the substrate recognition subunit of the SCF (SKP1, CUL1, and F-box protein)-type E3 ubiquitin ligase complex. SCFβ-TrCP ubiquitinates specifically phosphorylated substrates to promote their subsequent destruction by the 26S proteasome and plays a critical role in various human diseases including tumorigenesis. We and others (Duan S et al. Mol Cell 44: 317–324, 2011; Gao D et al. Mol Cell 44: 290–303, 2011; Zhao Y et al. Mol Cell 44: 304–316, 2011) recently reported that SCFβ-TrCP regulates cell growth and autophagy by controlling the ubiquitination and destruction of DEPTOR, an endogenous mammalian target of rapamycin inhibitor, in a phosphorylation-dependent manner. In this review, we discuss β-TrCP's new downstream substrate, DEPTOR, as well as summarize the novel functional aspects of β-TrCP in controlling cell growth and regulating autophagy, in part through governing the stability of DEPTOR.
Keywords: mammalian target of rapamycin, β-transducin repeats-containing protein, Skp1-Cullin1-F-box protein, cancer
the ubiquitin proteasome system (UPS) plays critical roles in many cellular processes, including cell growth, apoptosis, and cell cycle progression, because UPS regulates the abundance of major regulatory proteins through ubiquitin-mediated degradation by the 26S proteasome (25). It is known that UPS requires three enzymes to exert its functions: a ubiquitin-activating E1 enzyme, a ubiquitin-conjugating E2 enzyme, and a ubiquitin protein E3 ligase (45). The E1 enzyme activates ubiquitin, a 76-amino acid molecule, and the activated ubiquitin is subsequently transferred to the E2 enzyme (45). The E2 enzyme subsequently interacts with an E3 partner and transfers the ubiquitin to the target protein. Thus it is clear that E3 ligases determine substrate specificity for ubiquitination and degradation (45).
Among the identified multiple E3 ligases, the SCF (Skp1-Cullin1-F-box protein) E3 ligase complex is one of the few well-studied examples (16). SCF is composed of three static subunits, namely Skp1 (S-phase kinase-associated protein-1), Cullin 1, Rbx1/Roc1, and a variable subunit denoted as the F-box protein (16). F-box protein serves as the substrate recruitment module of the E3 ligase complex. To date, more than 70 putative F-box proteins that target a wide range of proteins for degradation have been identified in the human genome, which ultimately provides the possible variety for substrate specificity (56). However, the substrates and the functions of most F-box proteins are largely unknown. β-TrCP (β-transducin repeat-containing protein), on the other hand, is one of the earlier-identified F-box proteins; therefore, its physiological functions have been relatively well characterized (16). Recently, we and others found that the mTOR (mammalian target of rapamycin)-dependent phosphorylation-driven pathway is vital for DEPTOR (DEP domain-containing mTOR-interacting protein) destruction via SCFβ-TrCP (14, 19, 65). Hence, in this review, we will summarize the functional aspects of DEPTOR being a novel substrate for SCFβ-TrCP and further discuss how SCF regulates DEPTOR to influence cell decision in cell growth and autophagy.
β-TrCP as the Substrate Recognition Subunit of the SCFβ-TrCP Ubiquitin Ligase
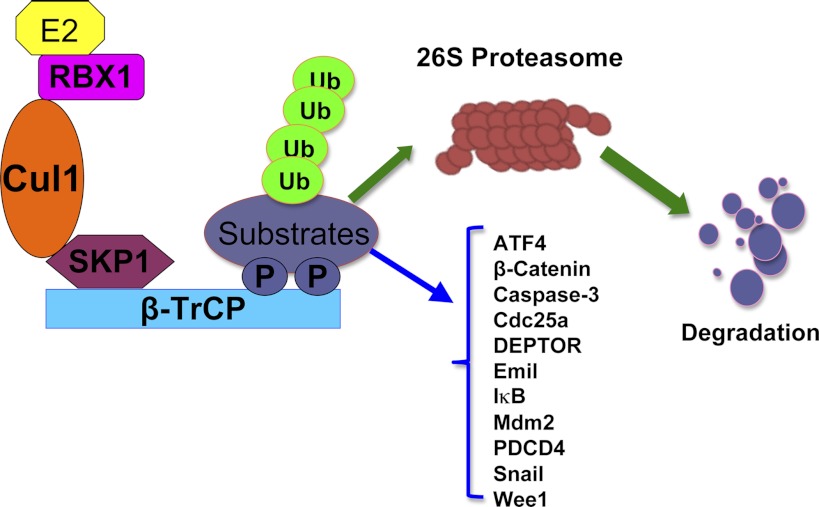
β-TrCP belongs to the F-box family of proteins, which forms a multicomponent SCF-type of E3 ubiquitin ligase complex (Fig. 1). β-TrCP is highly conserved from Drosophila Slimb, Xenopus β-TrCP to mammalian β-TrCP. There are two closely related homologs, termed β-TrCP1 (also called Fbxw1, Fbw1a, or FWD1) and β-TrCP2 (also called Fbxw11, Fbw1b, or HOS) with indistinguishable biochemical properties in promoting the in vitro ubiquitination of substrates (18). It is well known that the consensus sequence DSGXXS degron is favorably recognized by β-TrCP (16). In addition, the phosphorylation of two serines by specific kinases is required for β-TrCP-mediated ubiquitination and degradation (16).
Fig. 1.
Schematic illustration of an SCF-type of E3 ubiquitin ligase complex. The SCF (Skp1-Cullin1-F-box) complex consists of 4 components: Skp1, Rbx1, Cullin1, and the F-box protein that is responsible for substrate recognition. β-TrCP (β-transducin repeats-containing protein) recognizes the targeted proteins, which are presented close to the E2 enzyme to ensure consequent conjugation of ubiquitin to the targeted substrates. Polyubiquitylated substrates are subsequently targeted for degradation by the 26S proteasome. Known β-TrCP substrates include EMI-1, Wee1, Cdc25A, caspase-3, PDCD4, Snail, IκB, ATF4, p100, p105, Mdm2, and many others. See text for definitions.
There is a growing list of identified downstream β-TrCP ubiquitin substrates, implicating a critical role for β-TrCP in regulating both cell cycle progression and cellular apoptosis (16). Specifically by targeting EMI-1 (early mitotic inhibitor 1) (21, 42), Wee1 (61), and Cdc25A (cell division cycle 25 homolog A) (7, 30) for destruction, β-TrCP plays a pivotal role in regulating the proper cell cycle progression. On the other hand, its function in cellular apoptosis is achieved in part via targeted destruction of the pro-apoptotic protein caspase-3 (59) and PDCD4 (programmed cell death protein-4) (13). Furthermore, β-TrCP was found to regulate cell adhesion and migration through its phosphorylation-dependent regulation of the Snail transcription factor (63, 66). Additional substrates of β-TrCP have been identified, including IκB (inhibitor of κB) (57), ATF4 (activating transcription factor 4) (52), p100 (3) and p105 (11) subunits of NF-κB (nuclear factor-κB), and Mdm2 (murine double minute 2) (28) (Fig. 1). Importantly, we and other groups identified a novel β-TrCP substrate, DEPTOR, that is an endogenous inhibitor of the mTOR pathway (14, 19, 65). Therefore, in the following paragraphs, we will briefly discuss a potential role of β-TrCP in regulating the mTOR pathway.
mTOR Signaling Pathway
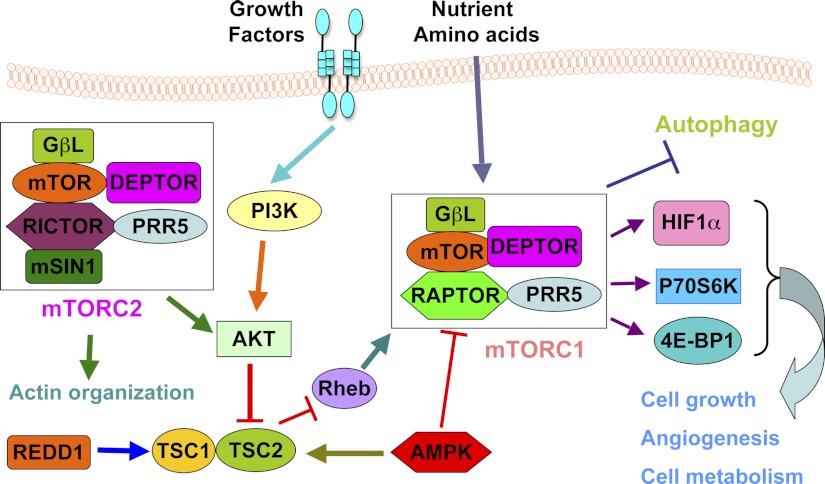
mTOR is an evolutionarily conserved serine/threonine kinase that plays a critical roles in controlling multiple cellular processes such as cell growth, cell division, cell cycle progression, cell metabolism, and autophagy (54). It is well accepted that deregulation of the mTOR pathway occurs in many human diseases including cancer and diabetes (5). This is in part due to the fact that mTOR, one of the family of phosphoinositide 3-kinase (PI3K)-related kinases (PIKKs), could respond to numerous stresses, including genotoxic, nutrient, and metabolic stress, thereby facilitating cell survival under these nonoptimal conditions (62). In doing so, mTOR fulfills its critical function as a sensor for upstream inputs from growth factors, nutrients, and stresses to downstream effector signaling pathways (5). To exert this regulatory function, the mTOR kinase assembles into two distinct multicomponent complexes called mTORC1 (mTOR complex 1) and mTORC2 (mTOR complex 2) (Fig. 2) (46). mTORC1 is composed of mTOR, Raptor (regulatory-associated protein of mTOR), PRAS40 (proline-rich Akt substrate of 40 kDa), mLST8/GβL (mammalian lethal with Sec13 protein 8/G protein β-subunit-like protein) and DEPTOR (8). The two best-characterized targets of mTORC1 are S6K (ribosomal protein p70 S6 kinase) and the eukaryotic translation initiation factor 4E (eIF4E)-binding protein-1 (4E-BP1) (43). Like mTORC1, the mTORC2 complex has multiple components, including mTOR, Rictor (Rptor-independent companion of mTOR), mLST8/GβL, DEPTOR, PROTOR (protein observed with Rictor-1)/PRR5 (proline-rich protein-5) and mSin1 (stress-activated protein kinase interacting protein) (46). mTORC2 phosphorylates the hydrophobic motif of Akt at Ser473 and SGK1 (serum/glucocorticoid-regulated kinase-1) at Ser422, resulting in full kinase activation (46).
Fig. 2.
Schematic illustration of the mTOR signaling network. Signals such as growth factor and nutrients cause a coordinated cellular response through interplaying with mTORC1 and mTORC2. mTORC1 promotes cell growth, angiogenesis, and cell metabolism and inhibits autophagy. mTORC2 promotes actin organization. AMPK, AMP-activated kinase; DEPTOR, DEP domain-containing mTOR-interacting protein; 4E-BP1, eukaryotic initiation factor 4E-binding protein-1; HIF1α, hypoxia-inducible factor 1α; mLST8/GβL, mammalian lethal with Sec13 protein-8/G protein β-subunit-like protein; mSin1, stress-activated protein kinase interacting protein. mTOR, mammalian target of rapamycin; mTORC1, mTOR complex 1; mTORC2, mTOR complex 2; PI3K, phosphatidylinositol 3-kinase; PRAS40, proline-rich Akt substrate of 40 kDa; PROTOR, protein observed with Rictor-1; Raptor, regulatory-associated protein of mTOR; Rheb, Ras homolog enriched in brain; Rictor, Rptor-independent companion of mTOR; S6K, ribosomal p70 S6 kinase. TSC, tuberous sclerosis complex.
The exact function of each component in mTORC1 and mTORC2 remains elusive (4). It has been demonstrated that Raptor regulates mTORC1 assembly and recruits kinase substrates such as 4E-BP1 (24, 32). In addition, Raptor determines the subcellular localization of mTORC1 through regulation of Rag GTPase (55) and in sensing amino acids (24). The role of mLST8 in mTORC1 is largely unknown because mTORC1 activity has no significant changes after specific deletion of mLST8 (29). PRAS40 and DEPTOR, on the other hand, can suppress mTORC1 activity in the dephosphorylated state, whereas phosphorylation of PRAS40 and DEPTOR abolishes their repressive effect toward mTORC1 by reducing their association with mTORC1 complex, thereby promoting the kinase activity of mTORC1 (48). Rictor is mutually exclusive with Raptor for binding to mTOR, and is required for mTORC2's catalytic activity. Rictor and mSin1 provide structural support for mTORC2 through stabilizing each other (17). In a similar situation with mTORC1, DEPTOR binds to the FAT domain of mTOR and inhibits mTORC2's kinase activity (51). Although PROTOR is found to be an mTORC2 interacting protein, deletion of PROTOR minimally affects mTORC2 activity, indicating that PROTOR is not an essential component for the mTORC2 catalytic function (48). Unlike its role in mTORC1, mLST8 is required for mTORC2 catalytic function (29). However, further in-depth investigation is required to determine the exact function of each subunit in both mTORC1 and mTORC2 complexes.
Recent studies reveal that the mTOR signaling pathway occurs in concert with its upstream factors such as Akt and TSC (tuberous sclerous complex) and exerts its functions through several downstream effectors (Fig. 2). Because of its role in activating multiple oncogenic signaling pathways, the mTOR pathway is considered to possess an oncogenic role in human cancers (27). For example, it has been reported that mTOR positively regulates the G1/S transition by inhibiting cyclin D1 turnover and by enhancing degradation of p27 (50, 58). Moreover, mTOR inhibits apoptosis by activation of S6K (38). mTOR is also found to increase the apoptosis-inhibiting protein survivin, simultaneously decreasing the apoptosis-promoting protein PDCD4 (26, 60). Additionally, eIF4E, an important downstream effector of mTOR, has also been shown to function as a positive regulator of cell survival (53). Furthermore, mTOR has been reported to promote angiogenesis through upregulation of HIF-1α (hypoxia-inducible factor 1α) and VEGF (vascular endothelial growth factor) production (67). However, there is also some information in the literature speaking about a possible tumor suppressor-like property for mTOR in some cellular contexts (40, 64). Taken together, further investigation is warranted to determine the exact functions of mTOR signaling pathway in tumorigenesis.
DEPTOR Is an mTOR Inhibitor
A recent study from Dr. Sabatini's group showed that DEPTOR [also known as DEPDC6 (DEP domain-containing protein-6)] is an inhibitor of both mTORC1 and mTORC2 activities (51). This group found that the PDZ domain of DEPTOR mediates its interaction with mTOR to suppress mTOR kinase activity. Moreover, depletion of DEPTOR activates the mTORC1 and mTORC2 substrates S6K1 and Akt, respectively (51). Additionally, depletion of DEPTOR increases cell size and protects cells from undergoing apoptosis (51). Furthermore, both mTORC1 and mTORC2 were found to negatively regulate DEPTOR expression by both transcriptional and posttranslational mechanisms (51). More importantly, DEPTOR is phosphorylated in an mTOR-dependent manner, and furthermore, overexpression of DEPTOR inhibits mTORC1 but activates the PI3K/Akt signaling pathway in part through mTOR1-mediated negative feedback regulation of the IRS-1/PI3K signaling pathway (51). It was further shown that overexpression of DEPTOR is required for Akt activation and cell survival in the multiple myeloma disease setting (51).
More recently, Boyd et al. (6) found that overexpression of DEPTOR is associated with improved survival in patients treated with thalidomide, suggesting that high DEPTOR expression may be a biomarker predictive of therapeutic response to thalidomide in myeloma. Moreover, upregulation of DEPTOR has been found in the paclitaxel-resistant ovarian cancer cell lines, indicating that DEPTOR may play a role in mediating drug resistance in ovarian cancer as well (15). Furthermore, Zhao et al. (65) demonstrated that cancer cells with accumulation of DEPTOR become more resistant to rapamycin and paclitaxel.
Additionally, Pei et al. revealed that DEPTOR is significantly increased in differentiated thyroid carcinoma and is also associated with lymph node status, extrathyroid extension, distant metastasis and recurrence as well as poorer survival, suggesting that DEPTOR might be a novel prognostic marker for thyroid carcinoma (49). Interestingly, DEPTOR has been demonstrated to be involved in the EMT (epithelial-mesenchymal transition) process (10). Specifically, the depletion of DEPTOR decreased expression of the epithelial marker E-cadherin and increased expression of the mesenchymal marker N-cadherin (10). Moreover, Snail protein was increased in the nuclear after DEPTOR depletion, suggesting that downregulation of DEPTOR could induce EMT in cancer cells. Furthermore, depletion of BMK1 (big mitogen-activated protein kinase-1) caused EMT via regulation of the DEPTOR/PI3K/mTORC2/Akt/GSK3β signaling pathways (10). However, the underlying molecular mechanisms remain largely undetermined. Therefore, further investigation is necessary to determine the exact physiological functions of DEPTOR in various human diseases including cancer.
Interplay Between E3 Ligase and mTOR Pathway
Recent studies have begun to reveal a close relationship between the mTOR complexes and ubiquitin-dependent proteolysis. For example, mTOR was found to be targeted for ubiquitination and degradation by FBW7 (41). Ghosh et al. (20) found that the function of 26S proteasome is required for mTORC1-mediated signaling events because of inhibition of 26S proteasome, which caused rapid inhibition of phosphorylation of S6K and 4E-BP1. Moreover, they discovered that Raptor and mLST8 bind the CUL4-DDB1 (Cullin4 DNA damage-binding protein-1) E3 ubiquitin ligase and regulate the mTORC1-mediated signaling pathway (20). Moreover, it has been recently found that REDD1 (regulated in development and DNA damage responses 1), an inhibitor of mTORC1 signaling during hypoxic stress conditions, is regulated by the CUL4-DDB1 ubiquitin ligase and through the activity of GSK3β (glycogen synthase kinase-3β) (31). Guo et al. (22) reported that Rictor is associated with FBW7, an F-box protein in SCF, to form an E3 complex participating in regulating c-Myc and cyclin E protein ubiquitination and degradation. Han et al. (23) also found that Pam functions as an E3 ubiquitin Ligase toward tuberin and subsequently regulates mTOR signaling, leading to regulation of cell growth as well as neuronal function. In addition, it has also been found that ubiquitin ligase ROC1 could regulate S6K ubiquitination and subsequent proteasomal degradation (47). Specifically, overexpression of ROC1 leads to an increase in S6K1 ubiquitination, whereas downregulation of ROC1 promotes stabilization of S6K1 protein (47).
Recently, Kume et al. (36) found that E3 ubiquitin ligases UBR1 (ubiquitin-protein ligase E3 componenent N-recognin 1) and UBR2 are negative regulators of the leucine-mTOR signaling pathway. Specifically, overexpression of UBR1 or UBR2 led to a reduction in mTOR-dependent S6K1 phosphorylation, whereas knockdown of UBR1 or UBR2 increased S6K1 phosphorylation (36). Additionally, it has been reported that SMER3 (small-molecule enhancers of rapamycin 3) enhances rapamycin's effect and also inhibits SCFMet30 ubiquitin ligase, suggesting a connection between the TOR and SCFMet30 pathways (1). Moreover, mTORC1 is inhibited by REDD1, and REDD1 upregulation is VHL (Von Hippel-Lindau) E3 ubiquitin ligase dependent, suggesting the connection between mTOR pathway and VHL E3 ubiquitin ligase (35). Liu et al. demonstrated that depletion of MID1, an E3 ubiquitin ligase, leads to disruption of the mTOR/Raptor complex and downregulates mTORC1 signaling (39), suggesting that mTORC1 signaling could be a downstream pathway regulated by the MID1. However, there is no characterized role for β-TrCP in mTOR signaling regulation. To this end, we and other groups recently expanded the current understanding of the physiological function of β-TrCP by pinpointing its critical role in regulating mTOR activity (14, 19, 65). In the following section, we will discuss the role of β-TrCP in mTOR signaling regulation.
Role of β-TrCP in mTOR Pathway
Recently, three groups reported that the stability of DEPTOR is controlled by the SCFβ-TrCP E3 ubiquitin ligase (14, 19, 65). Specifically, depletion of β-TrCP leads to impaired DEPTOR destruction and ensuing suppression of mTOR activity (14, 19, 65). Moreover, DEPTOR is phosphorylated by mTOR in vitro on multiple sites that promote interaction with β-TrCP, and casein kinase-1 (CK1) cooperates with mTOR in vivo to promote timely DEPTOR turnover (14, 19, 65). Furthermore, mutations in DEPTOR that block its phosphorylation by mTOR led to mTOR inhibition, reduced S6K activity, activation of autophagy, and alterations in the metabolic state of the cell, which collectively reduce cell growth (14, 19, 65). mTOR therefore functions as a central component of a positive feedback loop that ensures rapid induction of mTOR activity in response to growth stimulatory signals (14, 19, 65).
β-TrCP controls DEPTOR degradation.
It is well accepted that there are two required conditions for β-TrCP to fully execute its molecular functions by promoting the degradation of its ubquitin substrates. First, β-TrCP substrates typically contain the DSGxxS degron (16). Second, proper phosphorylation of the substrate is required for β-TrCP to efficiently recognize and target its substrate for ubiquitination (16). However, unlike other well-characterized β-TrCP substrates, including Cdc25A, PDCD4, or claspin (16), DEPTOR does not contain a canonical DpSGxxpS degron that could be recognized by SCFβ-TrCP. DEPTOR contains the similar variants, such as pS/TpSGxxpS that is seen in other reported β-TRCP substrates, including Period1 and Bim1. To directly address whether the ubiquitination and destruction of DEPTOR is mediated by β-TrCP, we performed mass spectrometry analysis to identify DEPTOR-interacting protein(s). In this effort, we identified β-TrCP as a specific DEPTOR-interacting protein (19). The endogenous interaction between DEPTOR and β-TrCP1 was further confirmed by coimmunoprecipitation analysis (19). Furthermore, we observed a sharp decrease in interaction between DEPTOR and a mutant form of β-TrCP1, whose substrate interacting site was inactivated, suggesting that SCFβ-TrCP might be the upstream E3 ubiquitin ligase controlling DEPTOR stability (19). Moreover, increased DEPTOR expression in response to depletion of β-TrCP subsequently results in suppression of both mTORC1 and mTORC2 kinase activities, as evidenced by the decreases in both p-Thr389-S6K1 and p-Ser473-Akt1 (19).
Recent studies have also revealed that most β-TrCP downstream ubiquitin targets are modified by phosphorylation events by kinases prior to their recognition and subsequent ubiquitination by SCFβ-TrCP (16). Therefore, it is critical to further understand the upstream modifying enzyme(s) that trigger DEPTOR destruction by β-TrCP. To pinpoint the upstream signaling pathways that control DEPTOR stability, we used a panel of kinase inhibitors to evaluate how inhibition of a specific kinase pathway affects DEPTOR expression (19). Kinase inhibitor experiments revealed that inhibition of CK1 leads to a significant upregulation of DEPTOR, indicating a possible role of CK1 in DEPTOR stability control (19). Moreover, we identified phosphopeptides containing either p-Ser286 or p-Ser287 in CK1-treated DEPTOR samples (19). Furthermore, we also found that mTOR could phosphorylate DEPTOR in p-Ser265, p-Ser286, p-Ser293, p-Thr295, and p-Ser299 (19). Another group, led by Dr. Pagano, reported similar results (14). The Pagano group identified that phosphorylation of all three serine residues (Ser286, Ser287, and Ser291) in the DEPTOR is required for the interaction with β-TrCP (14). Additionally, phosphorylation of Ser293 and Ser299 by mTOR functions to prime the phosphorylation of three serine residues by CK1α (14). Interestingly, study from Dr. Sun's laboratory demonstrated that DEPTOR can be phosphorylated by S6K1 and RSK1 (ribosomal protein S6 kinase-1) on the degron serine residues upon growth factor stimulation (65). Taken together, results from three independent groups consistently showed that β-TrCP controls DEPTOR degradation.
DEPTOR accumulation increases cell survival in part through activation of the akt oncoprotein.
It has been reported that DEPTOR could regulate cell survival through interacting with mTOR (51, 65). A study led by Dr. David Sabatini showed that overexpression of DEPTOR represses mTORC1, thereby leading to reduced suppression of IRS-1 by S6K, which subsequently activates PI3K/Akt signaling, leading to increased cell survival in multiple myeloma cells (51). This function seems as rapamycin that inhibits mTOR but induces transactivation of the EGFR and activates ERK1/2, resulted in promotion of cell survival (9). Recently, Zhao et al. (65) found that cancer cells after β-TrCP knockdown become more resistant to rapamycin, which is an allosteric inhibitor of mTOR and is considered as an anticancer drug. DEPTOR has been also reported to play a critical role in mediating resistance to paclitaxel, a mitotic inhibitor used in ovarian cancer chemotherapy, since significant upregulation of DEPTOR is found in paclitaxel-resistant cells (15). Consistent with this notion, under β-TrCP knockdown condition, multiple cancer cells are also more resistant to paclitaxel, whereas blockage of mTORC1/2 and PI3K/Akt pathways abrogated paclitaxel resistance (65). Moreover, DEPTOR accumulation as a result of β-TrCP knockdown inhibited paclitaxel-mediated cell death, while simultaneous DEPTOR knockdown abrogated the induction of cell death by paclitaxel (65). Furthermore, the research group led by Dr. Sun demonstrated that DEPTOR plays a survival role against paclitaxel-induced cell death through activation of Akt and inhibition of PARP cleavage (65). Interestingly, Duan et al. (14) found that cells expressing mutant DEPTOR displayed decreased cell size compared with cells expressing wild-type DEPTOR.
β-TrCP-mediated DEPTOR destruction participates in the regulation of autophagy.
It has been well documented that autophagy is a conserved cellular degradation pathway that governs intracellular homeostasis via degrading proteins and cellular organelles (44). Different from ubiquitin-mediated protein destruction, autophagy could clear damaged organelles and superfluous and long-lived proteins as well as invading microorganisms from cells (44). Autophagy also serves as an alternative energy source due to recycling of intracellular constituents, leading to providing nutrients and energy in response to certain stresses such as glucose or serum starvation to maintain homeostasis and viability (44). It has been well known that mTOR is a negative regulator of the autophagy process. Energy depletion including starvation induces autophagy by inhibition of mTOR via AMPK/TSC2 (12) or GAPDH (glyceraldehyde-3-phosphate dehydrogenase)-Rheb (Ras homolog enriched in brain) signaling pathways (37). Specifically, mTORC1 inhibits autophagosome formation, while mTORC2 suppresses the expression of autophagy-related genes and regulators (2). In line with this concept, rapamycin as an inhibitor of mTOR is used by many laboratories to induce autophagy (34). However, the role of DEPTOR in regulation of autophagy as an endogenous mTOR inhibor remains largely unaddressed.
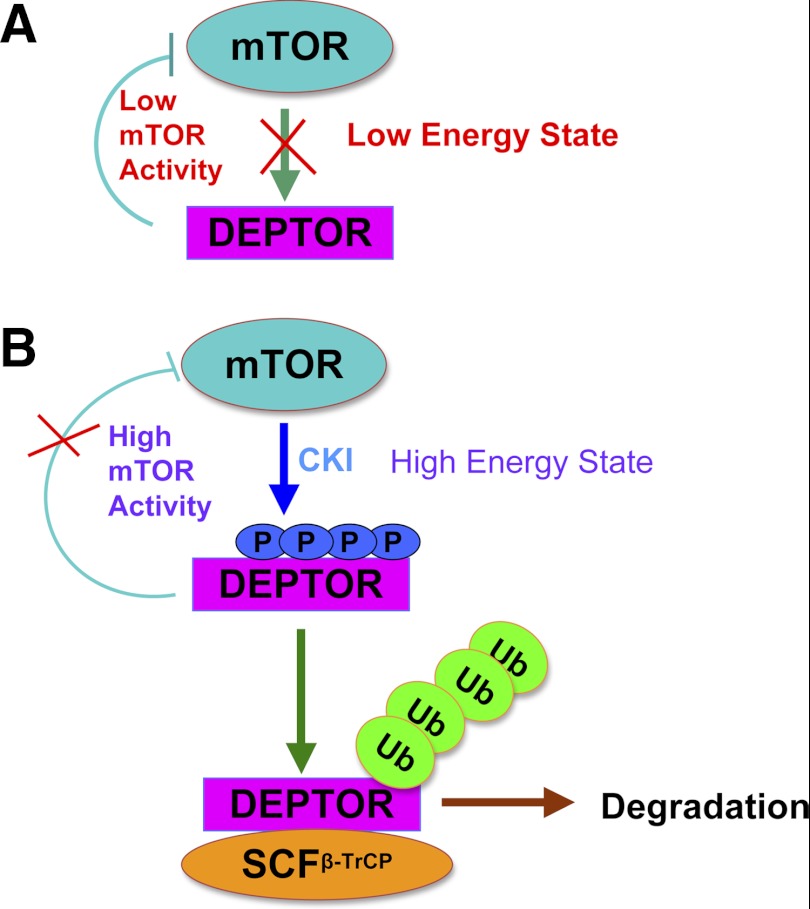
To this end, two groups including our own laboratory recently reported that DEPTOR is a positive regulator of the autophagy pathway (19, 65). We found that DEPTOR is induced in response to serum starvation, leading to suppression of the mTOR signaling pathway and induction of autophagy, as indicated by the increased species of the LC3B (light-chain protein-3B) autophagy marker (19). Specifically, in a low-energy state, DEPTOR inhibits mTOR activity. However, in a high-energy state, mTOR is rapidly switched from the low-activity state to the high-activity state through mTOR and CK1-dependent DEPTOR phosphorylation and β-TrCP-mediated DEPTOR destruction (Fig. 3). Our study therefore indicates interplay among β-TrCP, DEPTOR, and mTOR pathways to allow cells to respond rapidly and accurately to environmental changes. Meantime, a study led by Dr. Sun also demonstrated that upon glucose deprivation DEPTOR accumulates with corresponding induction of autophagy (65). These studies coherently suggest that DEPTOR mediated an elegant cellular protective mechanism in part through induction of autophagy in energy deprivation conditions. It is known that several molecules, including ULK1 and AMPK, play a critical role in autophagy. In a high-nutrient state, mTOR phosphorylates the ULK1 complex, thereby repressing its kinase activity, leading to inhibition of autophagy (33, 44). Upon nutrient depletion, AMPK represses mTORC1 and also phosphorylates ULK1, which causes enhanced catalytic activity (33, 44). Due to DEPTOR as an mTOR inhibitor, DEPTOR could be involved in this network among AMPK, ULK1, and mTOR that regulates autophagy. However, further studies are required to provide the experimental evidence to validate this interesting concept.
Fig. 3.
Proposed model for the ubiquitination and destruction of DEPTOR by SCFβ-TrCP. In low-energy state, DEPTOR inhibits mTOR activity. However, in high-energy state, mTOR as the priming kinase phosphorylates DEPTOR to trigger subsequent casein kinase-1 (CK1)-mediated phosphorylation of DEPTOR. Phosphorylation of DEPTOR by both mTOR and CK1 triggers SCFβ-TrCP-mediated ubiquitination of DEPTOR.
Conclusions
In conclusion, DEPTOR, an mTOR inhibitor, is a substrate of the SCFβ-TrCP E3 ubiquitin ligase, which could regulate cell survival and autophagy by modulating mTOR activity. The regulatory loop involves a dual repressive mechanism between mTOR and DEPTOR, which leads to a timely activation of mTOR signaling in response to growth stimulation signals. Studies from three independent research groups expanded the current understanding of the physiological function of β-TrCP by pinpointing its critical role in regulating the mTOR activity, thus participating in governing cellular metabolism and nutrient sensing. Due to the critical role of DEPTOR in regulating mTOR activity, DEPTOR expression level might be used as a biomarker to direct anti-cancer therapies or to predict therapeutic response, especially for those patients with aberrant mTOR kinase activities. Furthermore, DEPTOR was recently found to be involved in drug resistance (19, 65), suggesting that targeting DEPTOR may be a novel strategy for efficient anti-cancer treatment. However, it should be recognized that further in-depth investigations are required to fully determine the exact function of DEPTOR in human diseases including cancer, which will provide molecular insights for its therapeutic implications in the future.
GRANTS
The work cited in this review was funded by grants from the National Institute of General Medicines, NIH (GM-089763 and GM-09477) to W. Wei, and Massachusetts Life Science Center New Investigator award (W. Wei), and Department of Defense Prostate New Investigator award to W. Wei.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.W. and W.W. drafted manuscript; Z.W., J.Z., D.G., H.I., P.L., and W.W. edited and revised manuscript; Z.W., J.Z., D.G., H.I., P.L., and W.W. approved final version of manuscript; J.Z. prepared figures.
ACKNOWLEDGMENTS
In this review article, we sincerely apologize to all those colleagues whose important work was not cited here due to space limitations. W. Wei is an American Cancer Society Scholar. Z. Wang is supported by a National Institutes of Health NRSA fellowship.
REFERENCES
- 1. Aghajan M, Jonai N, Flick K, Fu F, Luo M, Cai X, Ouni I, Pierce N, Tang X, Lomenick B, Damoiseaux R, Hao R, Del Moral PM, Verma R, Li Y, Li C, Houk KN, Jung ME, Zheng N, Huang L, Deshaies RJ, Kaiser P, Huang J. Chemical genetics screen for enhancers of rapamycin identifies a specific inhibitor of an SCF family E3 ubiquitin ligase. Nat Biotechnol 28: 738–742, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32: 2–11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amir RE, Haecker H, Karin M, Ciechanover A. Mechanism of processing of the NF-kappa B2 p100 precursor: identification of the specific polyubiquitin chain-anchoring lysine residue and analysis of the role of NEDD8-modification on the SCF(beta-TrCP) ubiquitin ligase. Oncogene 23: 2540–2547, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov 10: 868–880, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 4: 335–348, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Boyd KD, Walker BA, Wardell CP, Ross FM, Gregory WM, Davies FE, Morgan GJ. High expression levels of the mammalian target of rapamycin inhibitor DEPTOR are predictive of response to thalidomide in myeloma. Leuk Lymphoma 51: 2126–2129, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Busino L, Donzelli M, Chiesa M, Guardavaccaro D, Ganoth D, Dorrello NV, Hershko A, Pagano M, Draetta GF. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature 426: 87–91, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Cam H, Houghton PJ. Regulation of mammalian target of rapamycin complex 1 (mTORC1) by hypoxia: causes and consequences. Target Oncol 6: 95–102, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Chaturvedi D, Gao X, Cohen MS, Taunton J, Patel TB. Rapamycin induces transactivation of the EGFR and increases cell survival. Oncogene 28: 1187–1196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen R, Yang Q, Lee JD. BMK1 kinase suppresses epithelial-mesenchymal transition through the Akt/GSK3beta signaling pathway. Cancer Res 72: 1579–1587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ciechanover A, Gonen H, Bercovich B, Cohen S, Fajerman I, Israel A, Mercurio F, Kahana C, Schwartz AL, Iwai K, Orian A. Mechanisms of ubiquitin-mediated, limited processing of the NF-kappaB1 precursor protein p105. Biochimie 83: 341–349, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev 18: 1533–1538, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314: 467–471, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Duan S, Skaar JR, Kuchay S, Toschi A, Kanarek N, Ben-Neriah Y, Pagano M. mTOR generates an auto-amplification loop by triggering the betaTrCP- and CK1alpha-dependent degradation of DEPTOR. Mol Cell 44: 317–324, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foster H, Coley HM, Goumenou A, Pados G, Harvey A, Karteris E. Differential expression of mTOR signalling components in drug resistance in ovarian cancer. Anticancer Res 30: 3529–3534, 2010 [PubMed] [Google Scholar]
- 16. Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 8: 438–449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol 16: 1865–1870, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Fuchs SY, Spiegelman VS, Kumar KG. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene 23: 2028–2036, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Gao D, Inuzuka H, Tan MK, Fukushima H, Locasale JW, Liu P, Wan L, Zhai B, Chin YR, Shaik S, Lyssiotis CA, Gygi SP, Toker A, Cantley LC, Asara JM, Harper JW, Wei W. mTOR drives its own activation via SCF(betaTrCP)-dependent degradation of the mTOR inhibitor DEPTOR. Mol Cell 44: 290–303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghosh P, Wu M, Zhang H, Sun H. mTORC1 signaling requires proteasomal function and the involvement of CUL4-DDB1 ubiquitin E3 ligase. Cell Cycle 7: 373–381, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Guardavaccaro D, Kudo Y, Boulaire J, Barchi M, Busino L, Donzelli M, Margottin-Goguet F, Jackson PK, Yamasaki L, Pagano M. Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev Cell 4: 799–812, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Guo Z, Zhou Y, Evers BM, Wang Q. Rictor regulates FBXW7-dependent c-Myc and cyclin E degradation in colorectal cancer cells. Biochem Biophys Res Commun 418: 426–432, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han S, Witt RM, Santos TM, Polizzano C, Sabatini BL, Ramesh V. Pam (protein associated with Myc) functions as an E3 ubiquitin ligase and regulates TSC/mTOR signaling. Cell Signal 20: 1084–1091, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Hoeller D, Hecker CM, Dikic I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat Rev Cancer 6: 776–788, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Hwang SK, Minai-Tehrani A, Lim HT, Shin JY, An GH, Lee KH, Park KR, Kim YS, Beck GR, Jr, Yang HS, Cho MH. Decreased level of PDCD4 (programmed cell death 4) protein activated cell proliferation in the lung of A/J mouse. J Aerosol Med Pulm Drug Deliv 23: 285–293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 37: 19–24, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Inuzuka H, Tseng A, Gao D, Zhai B, Zhang Q, Shaik S, Wan L, Ang XL, Mock C, Yin H, Stommel JM, Gygi S, Lahav G, Asara J, Xiao ZX, Kaelin WG, Jr, Harper JW, Wei W. Phosphorylation by casein kinase I promotes the turnover of the Mdm2 oncoprotein via the SCF(beta-TRCP) ubiquitin ligase. Cancer Cell 18: 147–159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6: 1122–1128, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Jin J, Shirogane T, Xu L, Nalepa G, Qin J, Elledge SJ, Harper JW. SCFbeta-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev 17: 3062–3074, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Katiyar S, Liu E, Knutzen CA, Lang ES, Lombardo CR, Sankar S, Toth JI, Petroski MD, Ronai Z, Chiang GG. REDD1, an inhibitor of mTOR signalling, is regulated by the CUL4A-DDB1 ubiquitin ligase. EMBO Rep 10: 866–872, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Kimmelman AC. The dynamic nature of autophagy in cancer. Genes Dev 25: 1999–2010, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5: 726–734, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Kucejova B, Pena-Llopis S, Yamasaki T, Sivanand S, Tran TA, Alexander S, Wolff NC, Lotan Y, Xie XJ, Kabbani W, Kapur P, Brugarolas J. Interplay between pVHL and mTORC1 pathways in clear-cell renal cell carcinoma. Mol Cancer Res 9: 1255–1265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kume K, Iizumi Y, Shimada M, Ito Y, Kishi T, Yamaguchi Y, Handa H. Role of N-end rule ubiquitin ligases UBR1 and UBR2 in regulating the leucine-mTOR signaling pathway. Genes Cells 15: 339–349, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Lee MN, Ha SH, Kim J, Koh A, Lee CS, Kim JH, Jeon H, Kim DH, Suh PG, Ryu SH. Glycolytic flux signals to mTOR through glyceraldehyde-3-phosphate dehydrogenase-mediated regulation of Rheb. Mol Cell Biol 29: 3991–4001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li B, Desai SA, MacCorkle-Chosnek RA, Fan L, Spencer DM. A novel conditional Akt “survival switch” reversibly protects cells from apoptosis. Gene Ther 9: 233–244, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Liu E, Knutzen CA, Krauss S, Schweiger S, Chiang GG. Control of mTORC1 signaling by the Opitz syndrome protein MID1. Proc Natl Acad Sci USA 108: 8680–8685, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manning BD, Logsdon MN, Lipovsky AI, Abbott D, Kwiatkowski DJ, Cantley LC. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev 19: 1773–1778, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science 321: 1499–1502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Margottin-Goguet F, Hsu JY, Loktev A, Hsieh HM, Reimann JD, Jackson PK. Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev Cell 4: 813–826, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway—beyond rapalogs. Oncotarget 1: 530–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer 7: 961–967, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer 6: 369–381, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle 10: 2305–2316, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Panasyuk G, Nemazanyy I, Filonenko V, Gout I. Ribosomal protein S6 kinase 1 interacts with and is ubiquitinated by ubiquitin ligase ROC1. Biochem Biophys Res Commun 369: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Pearce LR, Huang X, Boudeau J, Pawlowski R, Wullschleger S, Deak M, Ibrahim AF, Gourlay R, Magnuson MA, Alessi DR. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J 405: 513–522, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pei L, Xie P, Zhou E, Yang Q, Luo Y, Tang Z. Overexpression of DEP domain containing mTOR-interacting protein correlates with poor prognosis in differentiated thyroid carcinoma. Mol Med Report 4: 817–823, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F, Lacombe C, Bouscary D. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene 21: 6587–6597, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137: 873–886, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pons J, Evrard-Todeschi N, Bertho G, Gharbi-Benarous J, Tanchou V, Benarous R, Girault JP. Transfer-NMR and docking studies identify the binding of the peptide derived from activating transcription factor 4 to protein ubiquitin ligase beta-TrCP. Competition STD-NMR with beta-catenin. Biochemistry 47: 14–29, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Proud CG. The eukaryotic initiation factor 4E-binding proteins and apoptosis. Cell Death Differ 12: 541–546, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 6: 729–734, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Skaar JR, Pagano M. Control of cell growth by the SCF and APC/C ubiquitin ligases. Curr Opin Cell Biol 21: 816–824, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Strack P, Caligiuri M, Pelletier M, Boisclair M, Theodoras A, Beer-Romero P, Glass S, Parsons T, Copeland RA, Auger KR, Benfield P, Brizuela L, Rolfe M. SCF(beta-TRCP) and phosphorylation dependent ubiquitinationof I kappa B alpha catalyzed by Ubc3 and Ubc4. Oncogene 19: 3529–3536, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Takuwa N, Fukui Y, Takuwa Y. Cyclin D1 expression mediated by phosphatidylinositol 3-kinase through mTOR-p70(S6K)-independent signaling in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol 19: 1346–1358, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tan M, Gallegos JR, Gu Q, Huang Y, Li J, Jin Y, Lu H, Sun Y. SAG/ROC-SCF beta-TrCP E3 ubiquitin ligase promotes pro-caspase-3 degradation as a mechanism of apoptosis protection. Neoplasia 8: 1042–1054, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vaira V, Lee CW, Goel HL, Bosari S, Languino LR, Altieri DC. Regulation of survivin expression by IGF-1/mTOR signaling. Oncogene 26: 2678–2684, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Watanabe N, Arai H, Nishihara Y, Taniguchi M, Hunter T, Osada H. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc Natl Acad Sci USA 101: 4419–4424, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer 8: 851–864, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Xu Y, Lee SH, Kim HS, Kim NH, Piao S, Park SH, Jung YS, Yook JI, Park BJ, Ha NC. Role of CK1 in GSK3beta-mediated phosphorylation and degradation of snail. Oncogene 29: 3124–3133, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, Griffin JD, Kwiatkowski DJ. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest 117: 730–738, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhao Y, Xiong X, Sun Y. DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF(betaTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol Cell 44: 304–316, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou BP, Hung MC. Wnt, hedgehog and snail: sister pathways that control by GSK-3beta and beta-Trcp in the regulation of metastasis. Cell Cycle 4: 772–776, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]