Abstract
With-no-Lysine kinase 4 (WNK4) inhibited ROMK (Kir1.1) channels and the inhibitory effect of WNK4 was abolished by serum-glucocorticoid-induced kinase 1 (SGK1) but restored by c-Src. The aim of the present study is to explore the mechanism by which Src-family tyrosine kinase (SFK) modulates the effect of SGK1 on WNK4 and to test the role of SFK-WNK4-SGK1 interaction in regulating ROMK channels in the kidney. Immunoprecipitation demonstrated that protein phosphatase 1 (PP1) binds to WNK4 at amino acid (aa) residues 695–699 (PP1#1) and at aa 1211–1215 (PP1#2). WNK4−PP1#1 and WNK4−PP1#2, in which the PP1#1 or PP1#2 binding site was deleted or mutated, inhibited ROMK channels as potently as WNK4. However, c-Src restored the inhibitory effect of WNK4 but not WNK4−PP1#1 on ROMK channels in the presence of SGK1. Moreover, expression of c-Src inhibited SGK1-induced phosphorylation of WNK4 but not WNK4−PP1#1 at serine residue 1196 (Ser1196). In contrast, coexpression of c-Src restored the inhibitory effect of WNK4−PP1#2 on ROMK in the presence of SGK1 and diminished SGK1-induced WNK4 phosphorylation at Ser1196 in cells transfected with WNK4−PP1#2. This suggests the possibility that c-Src regulates the interaction between WNK4 and SGK1 through activating PP1 binding to aa 695–9 thereby decreasing WNK4 phosphorylation and restoring the inhibitory effect of WNK4. This mechanism plays a role in suppressing ROMK channel activity during the volume depletion because inhibition of SFK or serine/threonine phosphatases increases ROMK channel activity in the cortical collecting duct of rats on a low-Na diet. We conclude that regulation of phosphatase activity by SFK plays a role in determining the effect of aldosterone on ROMK channels and on renal K secretion.
Keywords: c-Src, SGK1, renal K secretion, collecting duct
with-no-lysine kinase 4 (WNK4) has been shown to inhibit ROMK channels by stimulating clathrin-dependent endocytosis (5, 7, 8). Moreover, the inhibitory effect of WNK4 on ROMK channels is reversed by serum-glucocorticoid-induced kinase 1 (SGK1) which phosphorylates WNK4 at serine residue 1169 (Ser1169) and Ser1196 (18, 19). Because the SGK1-induced WNK4 phosphorylation is expected to change the effect of WNK4 on ROMK channels from the inhibition to the stimulation (9, 18), this amino acid (aa) sequence of WNK4 between Ser1169 and Ser1196 has been named as the switch domain for renal K secretion (9). It is conceivable that SGK1-induced phosphorylation of WNK4 plays a role in modulating renal K secretion in response to different stimuli. The expression of SGK1 is stimulated by mineralocorticoids that are upregulated by both high-K intake and by sodium restriction (volume depletion) (1, 6, 21). However, several studies have shown that Na restriction did not stimulate while a high-K intake increased ROMK channels in the connecting tubule and cortical collecting duct (3, 4, 16). Our previous study demonstrated that Src-family tyrosine kinase (SFK) plays a key role in regulating the effect of SGK1 on WNK4 (33, 34). We showed that SGK1 phosphorylates WNK4 thereby abolishing WNK4-induced inhibition of ROMK only in the absence of but not in the presence of c-Src. The interaction among SFK, WNK4, and SGK1 plays an important role in facilitating K secretion during increasing dietary K intake, which suppresses the expression of SFK, and in preventing K loss during the volume depletion, which maintains a high SFK activity (11, 12, 26). Although the role of SFK in modulating WNK4-SGK1 interaction is established, the molecular mechanism by which c-Src inhibits the effect of SGK1 on WNK4 phosphorylation is not known. Since serine/threonine protein phosphatases play a key role in regulating the protein phosphorylation status (20), the aim of the study is to explore whether serine/threonine phosphatases are involved in modulating the interaction among c-Src, SGK1, and WNK4.
METHODS
Cell culture and transient transfection.
Human embryonic kidney (HEK)293T cells (American Type Culture Collection) were used for transient expression of the proteins including WNK4, SGK1, c-Src, and ROMK channels. The cells were grown in DMEM (Invitrogen) supplemented with 10% FBS (Invitrogen) in 5% CO2-95% air at 37°C. The cells at 50–70% confluence were used for the transfection and the corresponding cDNAs were simultaneously applied to the cells using TurboFect transfection reagent according to the manufacturer's protocol (Fermentas). Briefly, cDNA cocktail (0.4 μg ROMK, 0.4 μg SGK1, 0.4 μg c-Src, and 0.4 μg WNK4) was diluted with 200 μl serum-free DMEM and further mixed with 4 μl Turbofect transfection reagent for the transfection of cells cultured in 35-mm Petri dish. The cells transfected with vector alone were used as the control and their background currents were subtracted from the experimental groups. After a 15-min incubation at room temperature, the mixture of the transfection agents was applied to the cells followed by an additional 24-h incubation before use.
Purify the Flag-WNK4.
The cells cultured in 100-mm Petri dishes were transfected with 10 μg pcDNA3-Flag-WNK4 with TurboFect. After 24-h incubation, the cells were treated with 1 ml 1% Triton X-100 PBS (PBST) lysis buffer with the protease and phosphatase inhibitors and followed by centrifugation at 13,000 rpm for 20 min at 4°C. The supernatant was saved at −80°C for the pull-down experiments in the next step. For harvesting flag-tagged WNK4 in the sample, 80 μl anti-Flag Affinity Gel (Sigma, St. Louis, MO) were washed with PBS and added to the sample while 4 μg IgG-conjugated beads were added to the sample as the control. The sample was gently rocked for 2 h at 4°C and then subjected to centrifuge at 6,000 rpm for 2 min to harvest the WNK4 proteins.
Pull-down experiments.
To detect whether flag-tagged WNK4 is associated with protein phosphatase 1 (PP1) in the mouse kidney, a 1,000-μg tissue lysate of the mouse kidney (cortex and outer medulla) was applied into the sample obtained from the above procedure with 500 μl 1% PBST in the presence of protease and phosphatase inhibitors. The tubes were gently rocked for overnight at 4°C and followed by centrifugation to pellet the resin. The sample was boiled for 5 min and centrifuged to collect the supernatant for the electrophoresis (8% PAGE gel).
Electrophysiology experiment.
Within 24 h after transfection, the cells were treated with trypsin-containing medium (Tryple Ecpresscare; GIBCO) for 10 min to detach the cells. We followed the method described previously to prepare the cells for the patch-clamp experiments (33). We carried out the perforated whole cell patch-clamp experiments at room temperature. The cells were incubated with a bath solution containing 140 mM KCl, 0.5 mM MgCl2, 1.5 mM CaCl2, and 10 mM HEPES (pH 7.4). Fluorescence signal (an indication of positive transfection) was detected with an intensified video imaging system including a SIT 68 camera (Long Island Industries). Borosilicate glass (1.7-mm OD) was used to make the patch-clamp pipettes that were pulled with a Narishege electrode puller. The pipette had a resistance of 2–4 MΩ when filled with 140 mM KCl. The tip of the pipette was filled with pipette solution containing 140 mM KCl, 2 mM MgCl2, 1 mM EGTA, and 5 mM HEPES (pH 7.4). The pipette was then back-filled with amphotericin B (2 μg/0.1 ml) containing pipette solution. After forming a high-resistance seal (>2 GΩ), the membrane capacitance was monitored until the whole cell patch configuration was formed. The cell membrane capacitance was measured and compensated. The K currents were measured by an Axon 200A patch-clamp amplifier. The currents were low-pass filtered at 1 kHz and digitized by an Axon interface (Digidata 1200) and data were stored in an IBM-compatible computer and were analyzed using the pClamp software system 7 (Axon).
Preparation of the cortical collecting ducts.
Male Sprague-Dawley rats (5–6 wk, <90 g) were used in the experiments (Taconic Farms, Germantown, NY) and they were fed with control diet (0.4% Na and 1% KCl), low-Na diet (Na <0.0001%), or a high-K diet (2.5% KCl; Harlan Laboratory) for 7 days. Animals were euthanized by cervical dislocation and kidneys were removed immediately for dissection of the cortical collecting duct (CCD). The isolated split-open CCD was placed on a 5 × 5-mm coverglass coated with polylysine and then transferred to a chamber (1,000 μl) mounted on an inverted Nikon microscope. The protocol was approved by New York Medical College's Institutional Animal Care and Use Committee. The method for conducting the patch-clamp experiments in the split-open CCD has been described previously (27).
Preparation of protein samples.
The cells were placed in a lysis buffer containing 150 mM NaCl, 50 mM Tris·HCl, 1% Nonidet P-40 (pH 8.0), and protease inhibitor mixture (1%; Sigma) was added to the lysis buffer. The tissues were then homogenized and allowed to sit on ice for an additional 30 min. The sample was subjected to centrifugation at 13,000 rpm for 8 min at 4°C, and protein concentrations were measured in duplicate using a Bio-Rad Dc protein assay kit.
Immunoprecipitation and Western blot analysis.
The corresponding antibody was added to the protein samples (500 μg) harvested from cell cultures with a ratio of 4 μg/1 mg protein. The mixture was gently rotated at 4°C overnight, followed by incubation with 25 μl protein A/G plus agarose (Santa Cruz Biotechnology) for an additional 2 h at 4°C. The tube containing the mixture was centrifuged at 3,000 rpm and the agarose bead pellets were mixed with 25 μl 2× SDS sample buffer containing 4% SDS, 100 mM Tris·HCl (pH 6.8), 20% glycerol, 200 mM DTT, and 0.2% bromophenol blue. After the sample was boiled for 5 min, the proteins were resolved by electrophoresis on 8% SDS-polyacrylamide gels followed by transferring them to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS), rinsed, and washed with 0.05% Tween 20-TBS buffer. The membranes were washed three times with PBS and scanned by Odyssey infrared imaging system (LI-COR) at a wave-length of 680 or 800 nM.
Experimental materials and statistics.
Antibodies for c-Src, P-c-SrcY416, and tyrosine phosphorylation (PY20) were purchased from Santa Cruz Biotechnology. Antibodies for SGK1, Flag, and HA were obtained from Sigma. The antibody for PP1 phosphatase and WNK4 was obtained from Bethyl (Montgomery, TX) and from Abcam (Cambridge, MA), respectively. We also used WNK4 antibody generated by Yan-Hua Chen (East Carolina University). Anti-WNK4S1196 phospho antibody was provided by Richard Lifton and Jesse Rinehart. Calyculin A and Okadaic acid were purchased from Sigma. The data are presented as means ± SE. We used a one-way ANOVA test to determine the statistical significance. P < 0.05 was considered to be significant.
RESULTS
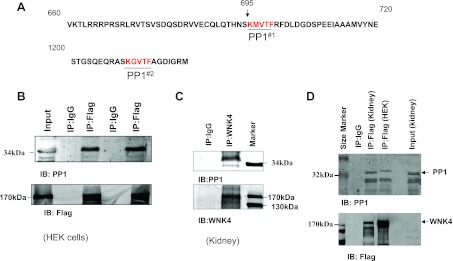
PP1 is associated with WNK4.
It has been shown that a protein with the (R/K)(X) (V)X(F/W) motif (x, any amino acid) could potentially interact with PP1 phosphatase and serves as a PP1 regulatory subunit (20). WNK4 has two putative PP1 binding sites located from aa 695 to 699 (KMVTF) and from aa 1211 to 1215 (KGVTF), respectively (Fig. 1A). To test whether WNK4 interacts with PP1, we carried out coimmunoprecipitation (CO-IP) with lysate of HEK cells transfected with flag-tagged-WNK4. From inspection of Fig. 1B, it is apparent that endogenous PP1 was coimmunuprecipitated with flag-tagged WNK4. To further examine whether the association between PP1 and WNK4 also takes place in the native tissue, the tissue lysates from mouse cortex and outer medulla were used for CO-IP with WNK4 antibody. Figure 1C is a Western blot showing that the endogenous PP1 of the mouse kidney was coimmunoprecipitated by WNK4 antibody. The notion that PP1 is associated with WNK4 in the kidney was also suggested by CO-IP of the lysates of the mouse kidney with flag-tagged WNK4 expressed in HEK cells and enriched with anti-Flag affinity Gel. From inspection of Fig. 1D, it is apparent that endogenous PP1 from the mouse kidney was pulled down by flag-tagged WNK4.
Fig. 1.
Protein phosphatase 1 (PP1) is associated with With-no-Lysine kinase 4 (WNK4). A: two partial amino acid (aa) sequences of WNK4 show the putative PP1 phosphatase binding motifs (PP1#1 and PP1#2) in the mouse WNK4. B: coimmunoprecipitation (Co-IP) shows that the endogenous PP1 phosphatase was associated with WNK4 in human embryonic kidney (HEK) cells transfected with flag-tagged WNK4 (n = 5). C: Western blot demonstrates that PP1 was pulled down by CO-IP of tissue lysate of mouse kidney with WNK4 antibody (n = 5). D: Western blot shows that the endogenous PP1 of mouse kidney was pulled down by CO-IP with flag-tagged WNK4 expressed in HEK cells (n = 5). The flag-tagged WNK4 was enriched with anti-flag affinity gel and detected with flag antibody (bottom).
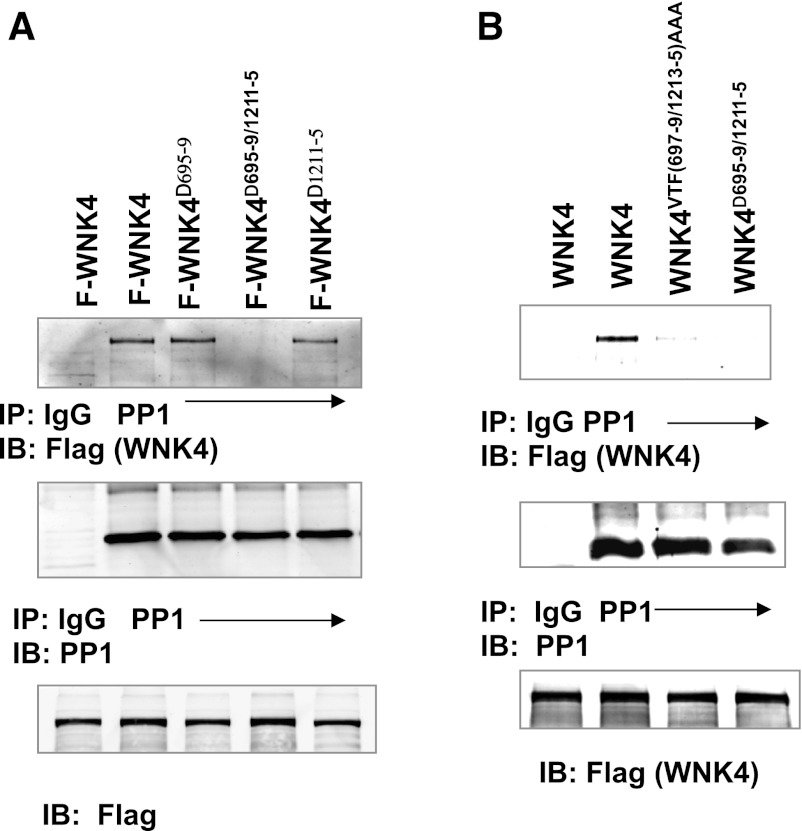
PP1 binds to WNK4 at both sites because the association between WNK4 and PP1 is still observed in HEK cells transfected with either flag-tagged-WNK4Δ695–9 or WNK4Δ1211–5 in which the putative PP1#1 (aa 695–699) or PP1#2 binding site (aa 1211–1215) was deleted (Fig. 2A). Similar results were observed in cells transfected with WNK4VTF(697–9)AAA or WNK4VTF(1213–5)AAA in which three aa (VTF) were mutated to triple alanines. However, either mutating or deleting both PP1 binding sites in WNK4 [WNK4VTF(697–9/1213–5)AAA or WNK4Δ695–9/1211–5] eliminated the association between WNK4 and PP1 (Fig. 2, A and B). Because WNK4 mutants with either deletion of or mutation of PP1 binding site had the same inhibitory effect on ROMK and affected WNK4-PP1 association in the same way, we pooled data. Also, we referred to WNK4VTF(697–9)AAA/WNK4Δ695–9 as WNK4−PP1#1 and WNK4VTF(1213–5)AAA/WNK4Δ1211–5 as WNK4−PP1#2 , respectively.
Fig. 2.
c-Src modulates the association of PP1 with WNK4. A: Western blot shows the association between PP1 and WNK4Δ695–9, WNK4Δ1211–5, or WNK4Δ1211–5 in which aa residues from 695 to 699 (KMVTF), from 1211 to 1215 (KGVTF), or both were deleted (n = 5) in HEK293 cells transfected with flag-tagged-WNK4 (F-WNK4) or flag-tagged WNK4 mutants. The cells were lysed and subjected to IP with PP1 antibody or IgG (negative control) and immunoblot (IB) with flag (WNK4) or PP1 antibody, respectively. B: Western blot shows that PP1 was CO-IP with WNK4 but the association was almost absent in the cells transfected with flag-tagged-WNK4VTF697–9/1213–5AAA or WNK4Δ697–9/1213–5, in which the VTF at 697–699 and VTF at 1213–1215 were mutated to AAA (n = 5).
PP1 is involved in modulating the interaction among c-Src, SGK1, and WNK4.
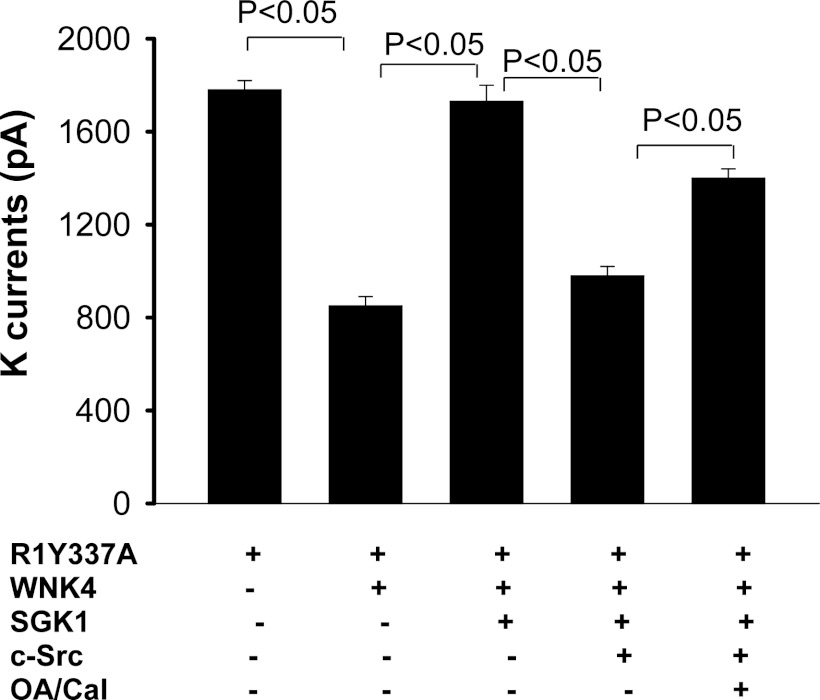
After demonstrating that PP1 was associated with WNK4, we explored the role of PP1 phosphatase in regulating the interaction among c-Src, SGK1, and WNK4 with the perforated whole cell recording in HEK cells transfected with R1Y337A. R1Y337A has similar biophysical properties as ROMK1 but is not directly regulated by c-Src because SFK-phosphorylation site, tyrosine residue 337, is mutated to alanine (11, 22). The Ba2+-sensitive K currents were normalized by measuring the membrane capacitance such that K currents were presented as per cell with 25-pF capacitance. Expression of WNK4 decreased K currents from 1,780 ± 40 to 850 ± 40 pA (n = 7) and coexpression of SGK1 abolished the effect of WNK4 (K currents, 1,730 ± 70 pA). The stimulatory effect of SGK1was reversed by coexpression of c-Src which decreased K currents to 980 ± 40 pA (n = 6; Fig. 3). However, treatment of the cells with 2 nM calyculin A or 1 μM okadaic acid (OA), inhibitors of PP1 and PP2A phosphatases, significantly increased K currents to 1,400 ± 40 pA (n = 5) in HEK cells transfected with R1Y337A, WNK4, SGK1, and c-Src (Fig. 3). This suggests the role of serine/threonine phosphatase in modulating the interaction among c-Src, SGK1, and WNK4. But, the inhibition of phosphatase did not completely abolish the effect of c-Src, suggesting that PP1 phosphatases associated with WNK4 might have a function other than inhibiting SGK1-induced stimulation of ROMK channels.
Fig. 3.
Inhibition of serine/threonine phosphatase partially abolishes the inhibition of ROMK by WNK4. K currents were measured with the perforated whole cell recording technique at −100 mV in HEK cells transfected with GFP-tagged ROMK1Y337A (R1Y337A) in which putative Tyr phosphorylation site (Tyr337) was mutated to Ala. K currents were normalized by measuring cell membrane capacitance and presented as pA/per cell with 25-pF capacitance. The experiments were performed 24 h after transfection and some measurements were performed in the cells treated with 2 nM Calyculin A (Cal) or 1 μM okadaic acid (OA) for 30–60 min. The cells were bathed with a bath solution containing 140 mM KCl, 0.5 mM MgCl2, 1.5 mM CaCl2, and 10 mM HEPES (pH 7.4). The pipette solution contains 140 mM KCl, 2 mM MgCl2, 1 mM EGTA, and 5 mM HEPES (pH 7.4).
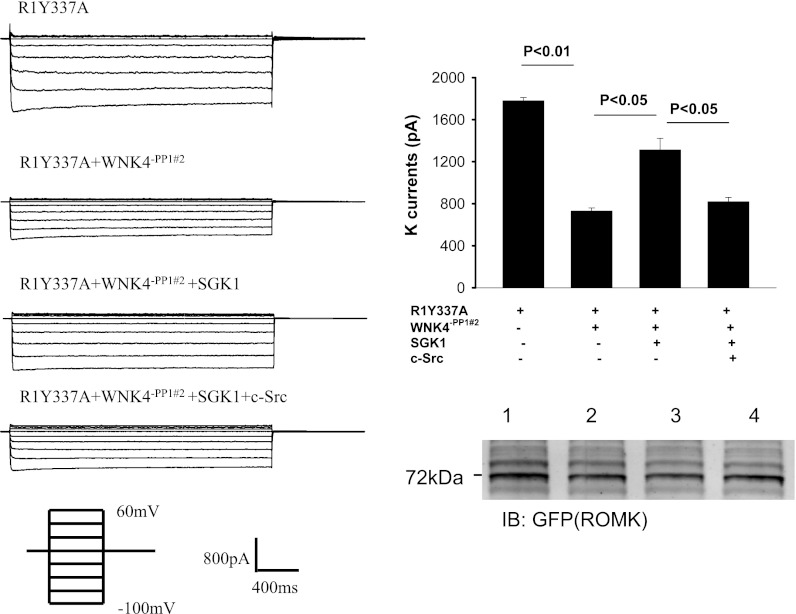
PP1#1 binding site is required for the effect of c-Src on SGK1-WNK4 interaction.
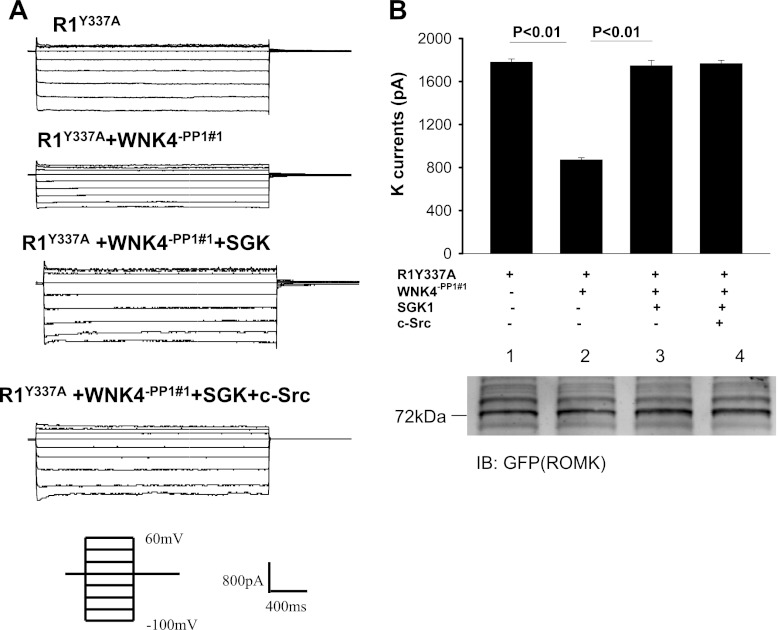
To examine the role of PP1#1 binding site in modulating the interaction among c-Src, SGK1, and WNK4, we used perforated whole cell recording to measure the Ba2+-sensitive K currents in HEK cells transfected with R1Y337A, R1Y337A+WNK4−PP1#1, R1Y337A+WNK4−PP1#1+SGK1, and R1Y337A+WNK4−PP1#1+SGK1+c-Src. Figure 4A is a recording showing that expression of WNK4−PP1#1 decreased Ba2+-sensitive K currents from 1,780 ± 30 to 870 ± 25 pA (n = 6) while the total ROMK expression was unchanged (Fig. 4B, bottom). Moreover, SGK1 reversed the inhibitory effect of WNK4 (K currents, 1,750 ± 50 pA). However, c-Src failed to restore the inhibitory effect of WNK4 (K currents, 1,760 ± 30 pA; n = 6) in the cells transfected with WNK4−PP1#1 (Fig. 4B). This is in a sharp contrast to those of intact WNK4 in which expression of c-Src restored the inhibitory effect of WNK4 (Fig. 3). The notion that the effect of c-Src on SGK1-WNK4 interaction requires PP1 binding to aa 695–9 is also suggested by experiments in which the effect of WNK4 on ROMK channels was examined in HEK cells transfected with R1Y337A, R1Y337A+WNK4−PP1#2, R1Y337A+WNK4−PP1#2+SGK1, and R1Y337A+WNK4−PP1#2+SGK1+c-Src (Fig. 5). As WNK4−PP1#1, WNK4−PP1#2 inhibited R1Y337A and decreased K currents from 1,790 ± 30 to 730 ± 30 pA (Fig. 5A; n = 6) without altering the expression of ROMK channels (Fig. 5B). Coexpression of SGK1 partially reversed the inhibitory effect of WNK4−PP1#2 and increased K currents to 1,320 ± 110 pA (n = 6; Fig. 5, A and B). However, expression of c-Src restored the inhibitory effect of WNK4−PP1#2 and decreased K currents to 820 ± 40 pA (n = 6). Taken together, results indicate that PP1#1 but not PP1#2 binding site in WNK4 plays a role in c-Src-induced restoration of WNK4's inhibition of ROMK.
Fig. 4.
Elimination of PP1#1 binding site abolishes the effect of c-Src on WNK4-serum-glucocorticoid-induced kinase 1 (SGK1) interaction. A: whole cell recording showing K currents in HEK cells transfected with R1Y337A and cotransfected with WNK4−PP1#1 [WNK4VTF(697–9)AAA or WNK4Δ695–9], SGK1, and c-Src. The K currents were measured at −100 to 60 mV with 20-mV increments. B: bar graph summarizes experiments in which Ba2+-sensitive K currents were measured with the perforated patch at −100 mV in HEK cells transfected with R1Y337A, WNK4−PP1#1, SGK1, and cSrc. A Western blot (bottom) demonstrates the expression of ROMK in HEK cells transfected with R1Y337A (group 1), R1Y337A + WNK4−PP1#1 (group 2), SGK1 + R1Y337A + WNK4−PP1#1 (group 3), and c-Src + SGK1 + R1Y337A + WNK4−PP1#1 (group 4).
Fig. 5.
Elimination of PP1#2 binding site does not affect the c-Src-WNK4-SGK1 interaction. Left: whole cell recording showing K currents in HEK cells transfected with R1Y337A and cotransfected with WNK4−PP1#2 [WNK4VTF(1213–5)AAA or WNK4Δ1211–5], SGK1, and c-Src. The K currents were measured at −100 to 60 mV with 20-mV increments. Right: bar graph summarizes experiments in which Ba2+-sensitive K currents were measured with the perforated patch at −100 mV in HEK cells transfected with R1Y337A, WNK4−PP1#2, SGK1, and cSrc. A Western blot (bottom) demonstrates the expression of ROMK in HEK cells transfected with R1Y337A (group 1), R1Y337A + WNK4−PP1#2 (group 2), SGK1 + R1Y337A + WNK4−PP1#2 (group 3), and c-Src + SGK1 + R1Y337A + WNK4−PP1#2 (group 4).
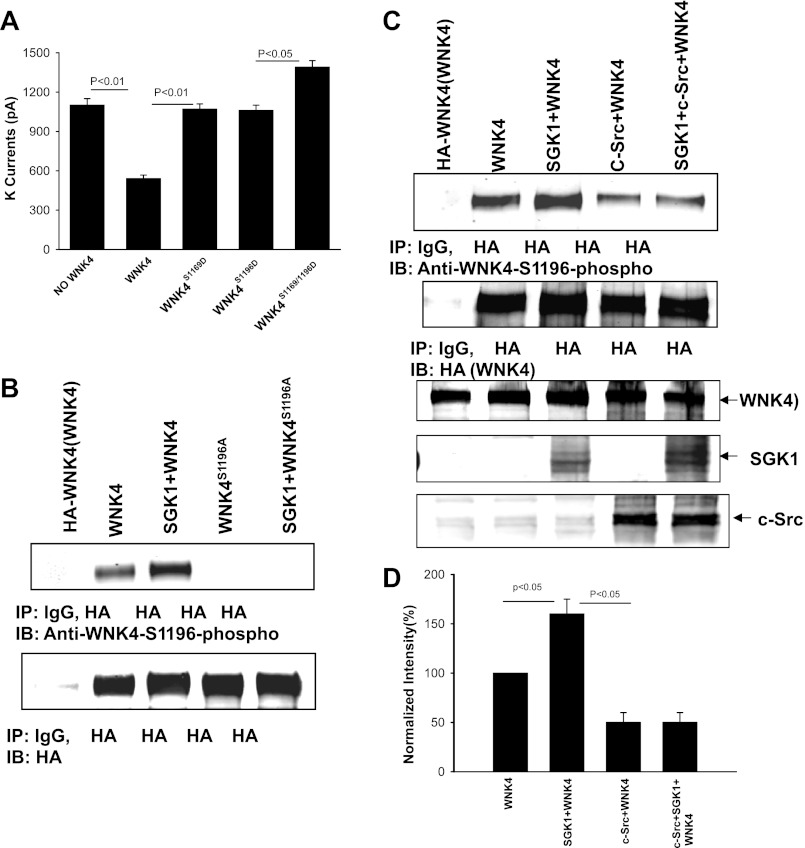
PP1#1 binding site is required for the effect of c-Src on SGK1-induced Ser1196 phosphorylation.
We previously demonstrated that expression of c-Src inhibited SGK1-induced phosphorylation of WNK4 (33). Two studies have reported that SGK1 phosphorylates WNK4 at Ser1169 and Ser1196 (18, 19). To determine which phosphorylation site is important for modulating the inhibitory effect of WNK4 on ROMK channels, we used the perforated whole cell recording to examine the effect of WNK4 on ROMK channels in the HEK cells transfected with WNK4, WNK4S1169D, WNK4S1196D, or WNK4S1169/1196D. The results are summarized in Fig. 6A showing that mutation of either Ser1169 or Ser1196 to aspartate abolished the inhibitory effect of WNK4 on ROMK channels. Moreover, expression of WNK4S1169/1196D slightly increased K currents. After demonstrating that phosphorylation of WNK4 at either site has equivalent effect on the WNK4's inhibition of ROMK channels, we examined the effect of c-Src on WNK4 phosphorylation on Ser1196 with specific anti-WNK4S1196 phosphorylation antibody (p-WNK4S1196). HEK cells were transfected with HA-tagged WNK4 or the WNK4 mutant, WNK4S1196A, in which serine was mutated to alanine (Ala). Figure 6B is a Western blot demonstrating that expression of SGK1 increased phosphorylation of WNK4 at Ser1196. The p-WNK4S1196 was specific because no reaction was observed in cells transfected with WNK4S1196A. We then examined whether expression of c-Src diminished the SGK1-induced phopshorylation of WNK4 on Ser1196. The HEK cells were transfected with HA-WNK4, SGK1+WNK4, c-Src+WNK4, and SGK1+c-Src+WNK4 and WNK4 protein was harvested by IP with HA antibody. Figure 6C is a Western blot demonstrating that expression of c-Src not only decreased the basal level of Ser1196 phosphorylation by 50 ± 10% (n = 5; Fig. 6D) but it also abolished the effect of SGK1 on Ser1196 phosphorylation while the WNK4 expression was the same among four groups. Therefore, c-Src decreased the SGK1-induced phosphorylation of WNK4 at Ser1196.
Fig. 6.
Coexpression of c-Src inhibits SGK1-induced phosphorylation of WNK4. A: bar graph summarizes experiments in which Ba2+-sensitive K currents were measured with the perforated patch at −100 mV in HEK cells transfected with ROMK1, WNK4, or WNK4 mutants. B: Western blot demonstrates that phosphor-WNK4S1196 antibody is specific for recognizing the phosphorylated WNK4 at Ser1196. SGK1 stimulates the phosphorylation of WNK4 but not WNK4S1196A in which Ser1196 was mutated to Ala. C: Western blot showing that expression of c-Src abolished the stimulatory effect of SGK1 on WNK4 phosphorylation at Ser1196 in HEK cells transfected with HA-tagged WNK4 and other constructs as indicated in the figure. D: bar graph summarizes the results from 4 experiments similar to those shown in C. The ratio of phosphorylated WNK4 to total WNK4 protein was calculated to normalize the changes in WNK4 phosphorylation.
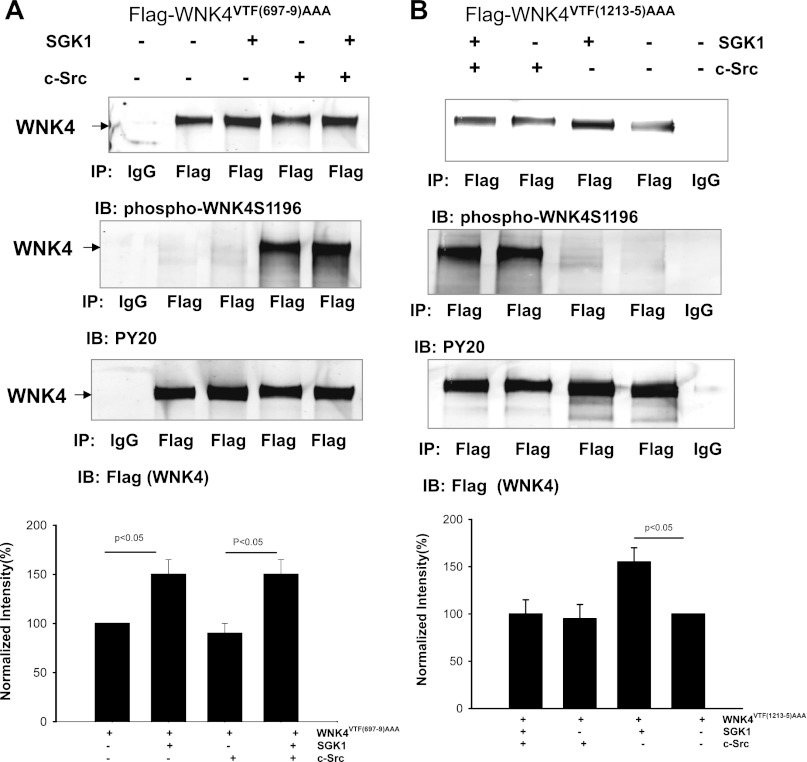
We then examined the role of PP1#1 and PP1#2 binding sites in modulating the effect of c-Src on SGK1-induced phosphorylation of WNK4 with p-WNK4S1196 antibody in HEK cells transfected with flag-tagged-WNK4−PP1#1 or WNK4−PP1#2. From inspection of Fig. 7A, it is apparent that expression of SGK1 increased Ser1196 phosphorylation of WNK4VTF(697–9)AAA. However, in contrast to intact WNK4 whose phosphorylation at Ser1196 by SGK1 was inhibited by c-Src (Fig. 6C), removal of PP1#1 binding site abolished the inhibitory effect of c-Src on the SGK1-induced Ser1196 phosphorylation. In the presence of c-Src, SGK1 augmented Ser1196 phopshorylation by 50 ± 10% (n = 4; Fig. 7A, bottom). Similar results were observed in the cells transfected with WNK4Δ695–9 (data not shown). The view that c-Src-induced inhibition of Ser1196 phosphorylation of WNK4 requires an intact PP1#1 binding site was also supported by the finding that c-Src inhibited SGK1-mediated phosphorylation of WNK4 at Ser1196 in the cells transfected with WNK4−PP1#2. From inspection of Fig. 7B, it is apparent that SGK1 failed to increase WNK4−PP1#2 phosphorylation at Ser1196 in the presence of c-Src.
Fig. 7.
Inhibition of SGK1-induced phosphorylation of WNK4 by c-Src requires PP1 binding to WNK4 at aa 695–9. A Western blot showing the effect of SGK1 on WNK4 phosphorylation with phosphor-WNK4S1196 antibody in the presence or in the absence of c-Src in HEK293 cells transfected with WNK4VTF(697–9)AAA (A) and WNK4VTF(1213–5)AAA (B). Results shown in A and B are summarized in a bar graph at the bottom of the corresponding panel. The ratio of phosphorylated WNK4 to total WNK4 protein was calculated to normalize the changes in WNK4 phosphorylation.
Inhibition of phosphatase and SFK increases ROMK channels in the CCD.
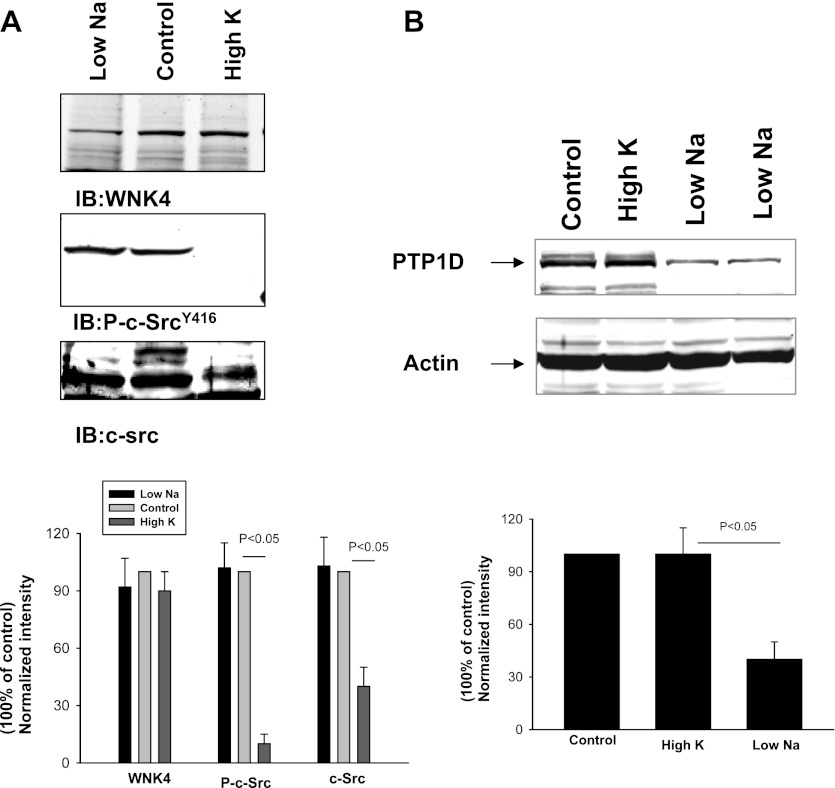
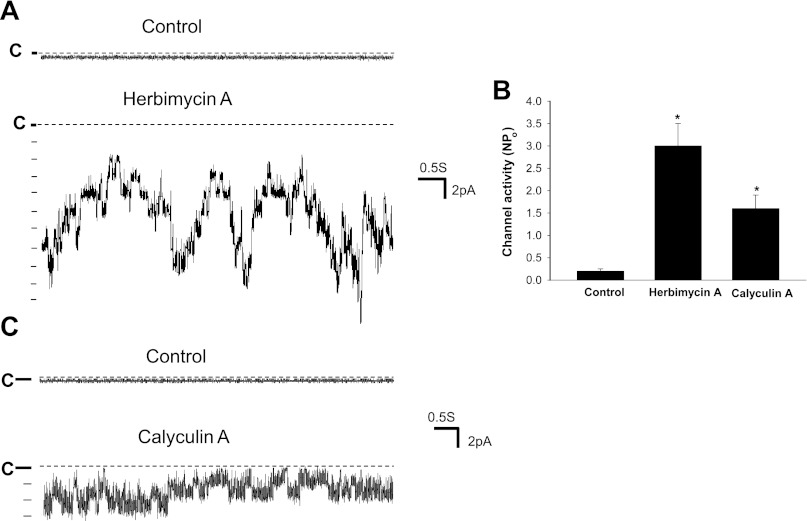
After establishing the role of c-Src and serine/threonine phosphatases in modulating WNK4's inhibition of ROMK channels, we explored the physiological role of SFK and serine/threonine phosphatases in regulating ROMK channels during volume depletion. Previous studies demonstrated that low-Na intake or aldosterone infusion did not increase ROMK channel activity in the CCD despite a high aldosterone level (17). We speculated that unlike high-K intake, Na depletion enhances SFK activity and therefore ROMK channel activity is not increased by the volume depletion. This hypothesis was tested by examining the effect of high-K intake and low-Na intake on the expression of WNK4, c-Src and phosphorylated-c-SrcY416 (P-c-SrcY416), an indication of c-Src activation. Figure 8A is a Western blot demonstrating that a high-K intake significantly decreased c-Src expression by 60 ± 10% and P-c-SrcY416 level by 90 ± 15% but had no effect on WNK4 expression in the kidney (a mixture of cortex and outer medulla). In contrast, low-Na intake had no effect on c-Src expression and P-c-SrcY416 level (n = 3 rats) compared with the control animals. Moreover, low-Na intake significantly decreased protein tyrosine phosphatase (PTP) expression. Figure 8B is a Western blot demonstrating that the expression of PTP1D was 60 ± 10% lower in rats on a low-Na diet than those on a control diet (n = 3 rats). Thus, although low-Na intake did not change the expression of c-Src, a decrease in PTP1D expression would enhance the activity of SFK. To test the role of SFK and serine/threonine phosphatases in suppressing ROMK channel activity, we used the patch-clamp technique to examine the effect of inhibiting SFK and phosphatases on ROMK channels in the CCD of rats on a low-Na diet. We observed ROMK channel activity in 2 patches of total 11 patches with mean NPo, 0.27 ± 0.2, in the CCD of rats on a low-Na diet for 7 days. In contrast, we observed ROMK channel activity in 6 patches from total 11 patches with mean NPo, 2.2 ± 0.6, in the CCD of rats on a high-K diet (2.5% K). However, inhibition of SFK significantly increased ROMK channel activity in the CCD of rats on a low-Na diet. Figure 9A is a channel recording showing that inhibition of SFK with herbimycin A (1 μM) activated ROMK channels, which is confirmed by measuring the channel conductance (∼35 pS). We observed that inhibition of SFK activated ROMK channels in 7 patches of total 10 experiments and increased NPo to 3.0 ± 0.5 (n = 10; Fig. 9B). In contrast, we confirmed the previous observation that inhibition of SFK had no effect on ROMK channels in the CCD of rats on a high-K diet (25). Although the stimulatory effect of herbimycin A on ROMK channels may in part be the result of decreasing ROMK channel tyrosine phosphorylation, modulation of PP1 activity through inhibiting WNK4's tyrosine phosphorylation may be partially responsible for increasing ROMK channel activity. This notion was supported by the experiments in which inhibition of serine/threonine phosphatases increased ROMK channel activity in the CCD of rats on a low-Na diet. Figure 9C is a channel recording showing that calyculin A (2 nM) increased ROMK channel activity in the CCD of rats fed a low-Na diet. In a total of seven experiments, inhibition of the phosphatase increased ROMK channel activity in six patches and augmented NPo from 0.2 ± 0.05 to 1.6 ± 0.3 (Fig. 9B). We postulated that stimulatory effect of calyculin A on ROMK channels in the CCD was the result of inhibition of PP1 binding to WNK4 thereby enhancing the effect of SGK1 on WNK4. Therefore, the results suggest the role of serine/thereonine phosphatase in modulating the effect of aldosterone/SGK1 on ROMK channels during volume depletion.
Fig. 8.
High-K intake suppresses c-Src while low-Na intake decreases protein tyrosine phosphatase (PTP)1D. A: Western blot demonstrates the expression of WNK4, P-c-SrcY416, and c-Src in the rat renal cortex and outer medulla (mixture). B: Western blot shows the effect of low Na and high K on PTP1D expression. Animals were fed with control (0.4% Na + 1% KCl), low-Na (<0.001% Na + 1% KCl), and high-K (2.5% KCl) diet for 7 days, respectively. A bar graph summarizes the results from 4 experiments shown in A and B at the bottom of the corresponding panel. The band intensity was normalized compared with the control value.
Fig. 9.
Inhibition of Src-family tyrosine kinase (SFK) and serine/threonine phosphatase activates ROMK in the cortical collecting duct (CCD). A: channel recording demonstrating the effect of herbimycin A (1 μM) on ROMK channel activity in the CCD of rat on a low-Na diet. The experiment was performed in a cell-attached patch and the ROMK channel activity was continuously monitored before (control) and after herbimycin A treatment (20 min). The filter rate was 200 Hz. B: bar graph summarizes the effect of herbimycin A (1 μM) and calyculin A (2 nM) on ROMK channels in the CCD. *Significance compared with the control value. C: channel recording showing the effect of calyculin A (2 nM) on ROMK channels in the CCD of rats fed a low-Na diet. The experiment was performed in a cell-attached patch. The channel closed level is indicated by “C.” The filterrate was 1 kHz. The pipette solution contains (in mM) 140 KCl, 1.8 MgCl2, and 10 HEPES (pH 7.4). The composition of the buffer solution is (in mM) 140 NaCl, 5 KCl, 1.5 MgCl2, 1.8 CaCl2, and 10 HEPES (pH 7.4). The holding potential was 0 mV.
DISCUSSION
WNK4 is expressed in the aldosterone-sensitive distal nephron including the connecting tubule and the CCD and it plays an important role in modulating renal K secretion and Na absorption (7, 9, 18, 23, 28, 29). The effect of WNK4 on K secretion is achieved by two mechanisms: 1) WNK4 regulates the activity of NaCl cotransporter (NCC) and epithelial Na channels thereby affecting the driving force for K secretion (7, 29–31); 2) WNK4 and other WNK family kinases have been shown to inhibit ROMK channel activity by stimulating clathrin-mediated endocytosis (5, 7, 33). Lost-function mutations of WNK4 increased NCC activity in the distal convoluted tubule and decreased ROMK channel activity in the connecting tubule and CCD thereby causing hyperkalemia and hypertension, type II pseudohypoaldosteronism syndrome (PHAII) (28). Although hyperkalemia induced by WNK4's PHAII mutations is, at least in part, the result of decreasing Na delivery to the distal nephron, PHAII mutations enhance WNK4's inhibition of ROMK channels that may also play a role in causing hyperkalemia. Therefore, modulation of WNK4's inhibition of ROMK channels is essential for the regulation of renal K secretion in response to a variety of physiological stimuli. In this regard, previous studies demonstrated that SGK1 and SFK play a role in modulating the inhibitory effect of WNK4 on ROMK channels (18, 33).
SGK1 has been reported to stimulate ROMK channels by facilitating the phosphorylation of ROMK channels at the NH2 terminus thereby enhancing the export of ROMK channels from the endoplasmic reticulum to the plasma membrane (14, 32), an effect that requires the involvement of type II Na/H exchanger regulating factor (35). Moreover, SGK1 also suppresses the effect of WNK4 on ROMK channels through the phosphorylation of WNK4 at Ser1169 (18) and Ser1196 (19). In contrast to SGK1, SFK inhibits ROMK channels by directly phosphorylating ROMK channels (11) thereby facilitating internalization of ROMK channels (12) and by inhibiting the SGK1-mediated phosphorylation of WNK4 (33). Consequently, SFK restores the WNK4-mediated inhibition of ROMK channels despite the presence of SGK1. Three lines of evidence suggest that the effect of c-Src, SGK1, and PP1 on ROMK channels was the result of their interaction rather than the result of an independent action. First, SGK1 reversed the inhibitory effect of WNK4 on R1Y337A but did not stimulate ROMK channels in the cells transfected with WNK4S1169/1196D (Wang Z and Wang WH, unpublished observations), suggesting that the effect of SGK1 on the ROMK channels in the presence of WNK4 was due to phosphorylation of WNK4; 2) c-Src did not have an effect on R1Y337A in the presence of or in the absence of SGK1 in cells transfected with WNK4S1169/96D (Wang WH, unpublished observations), suggesting that the effect of c-Src on R1Y337A was the result of modulating SGK1-induced WNK4 phosphorylation; 3) inhibition of phosphatases with OA or calyculin A had no effect on R1Y337A in the cells transfected with WNK4−pp1#1, suggesting that the effect was due to inhibition of phosphatase binding to WNK4.
The interaction among SFK, WNK4, and SGK1 could play a role in preventing K waste during the volume depletion and in stimulating K secretion during increasing dietary K intake. In the present study, we delineate an integrated mechanism by which SFK regulates the effect of SGK1 on WNK4's inhibition of ROMK channels. The notion that serine/threonine phosphatases play a role in modulating the interaction among c-Src, WNK4, and SGK1 is supported by the finding that inhibition of serine/threonine phosphatases diminished the effect of c-Src on restoration of WNK4's inhibition of ROMK channels in the presence of SGK1.
WNK4 has two PP1 phosphatase-binding sites located at aa 695–699 and at aa 1211–1215. However, two lines of evidence suggest that the PP1 phosphatase binding to aa 695–699 is directly responsible for the effect of c-Src on SGK1-WNK4 interaction. First, eliminating PP1#1 binding site in WNK4 completely abolished the c-Src-induced restoration of WNK4's inhibition of ROMK channels in the presence of SGK1. In contrast, c-Src still restored the WNK4-induced inhibition of ROMK channels in the presence of SGK1 in the cells transfected with WNK4−PP1#2. Second, c-Src failed to abolish the SGK1-induced Ser1196 phosphorylation of WNK4 in the cells transfected with WNK4−PP1#1 but inhibited the SGK1-induced WNK4's phosphorylation at Ser1196 in the cells transfected with WNK4−PP1#2. These results indicate that phosphatase binding to aa 695–699 is directly responsible for dephosphorylation of Ser1196 and, possibly, SGK1-mediated phosphorylation of WNK4's switch domain.
While the phosphatase binding to PP1#1 of WNK4 is directly involved in removal of SGK1-mediated phosphorylation of WNK4, the role of phosphatases binding to PP1#2 of WNK4 is not clear. We speculate that the phosphatases binding to PP1#2 may be responsible for dephosphorylating WNK4 at particular serine/threonine residues whose phosphorylation enhanced WNK4's inhibition of ROMK channels. In this regard, it has been observed that WNK1 was able to phosphorylate WNK4 thereby enhancing WNK4's inhibition of ROMK channels (Rinehart J, unpublished observations). This possibility is also suggested by the observation that inhibition of serine/threonine phosphatase failed to fully restore ROMK channel activity in cells transfected with WNK4, SGK1, and c-Src (see Fig. 3). Several studies have demonstrated that WNK1 inhibited ROMK channels and that the inhibitory effect of WNK1 was attenuated by a kidney-specific splice form of WNK1 (KS-WNK1) (2, 10, 13, 24), in which an alternative 5′-exon replaces the first four exons of WNK1 (15). We need further experiments to explore whether the phosphatases binding to the PP1#2 site are involved in modulating WNK1-WNK4 interaction.
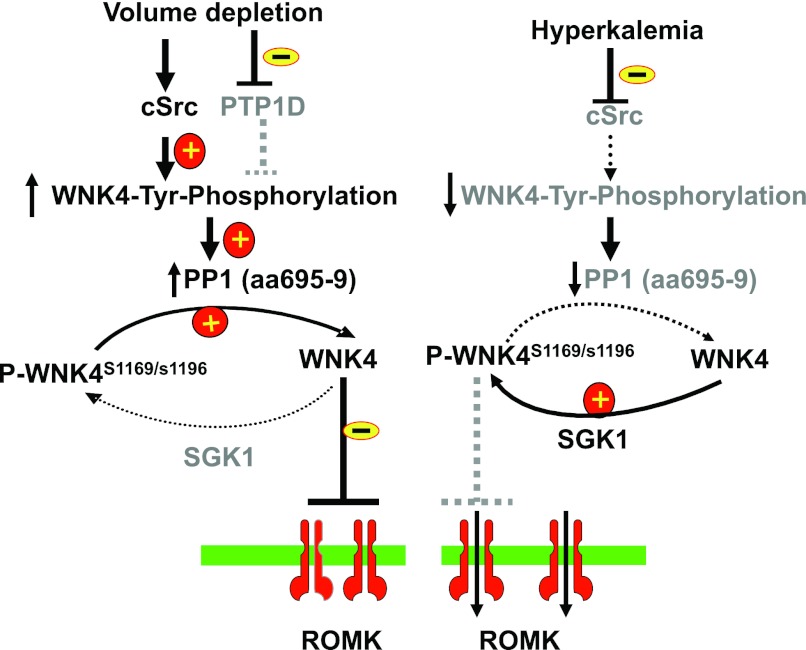
Figure 10 is a scheme illustrating the mechanism by which the effect of aldosterone on renal K secretion is modulated through interaction among c-Src, WNK4, and SGK1. We postulate that stimulation of SFK increases WNK4's tyrosine phosphorylation thereby activating phosphatase at the PP1#1 site. Activation of the phosphatase at PP1#1 site dephosphorylates WNK4 in the switch domain thereby restoring the WNK4-induced inhibition of ROMK channels. Therefore, although SGK1 level is upregulated by both high-K intake and volume depletion, increased SGK1 level does not stimulate K secretion during volume depletion because of a high-SFK activity. On the other hand, since a high-K diet suppresses SFK activity (12), the phosphatase binding to WNK4 at aa 695–9 is expected to be inactivated. Thus, increased SGK1 should enhance WNK4 phosphorylation at the switch domain thereby activating ROMK channels and increasing K secretion during hyperkalemia. Hence, the interaction among SFK, SGK1, and WNK4 modulates the effect of aldosterone on ROMK channels. We conclude that regulation of phosphatase activity by SFK plays a role in determining the effect of aldosterone on ROMK channels and on renal K secretion.
Fig. 10.
Scheme illustrates the role of serine/threonine phosphatase 1 (PP1) binding to WNK4 at aa 695–699 in mediating the effect of c-Src on the SGK1-WNK4 interaction. A solid line and dotted line represent an enhanced and a diminished effect, respectively. The gray fonts mean that the particular signaling pathway is inactivated.
GRANTS
This work was supported by National Institutes of Health Grant DK54983 (to W. H. Wang) and an American Heart Association Scientific Development grant (to D. H. Lin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.-H.L. and W.-H.W. conception and design of research; D.-H.L., P.Y., P.S., and Z.W. performed experiments; D.-H.L., P.Y., P.S., Z.W., and W.-H.W. analyzed data; D.-H.L., P.Y., and W.-H.W. prepared figures; D.-H.L., P.Y., J.R., P.S., Z.W., R.P.L., and W.-H.W. approved final version of manuscript; J.R., R.P.L., and W.-H.W. interpreted results of experiments; J.R., R.P.L., and W.-H.W. edited and revised manuscript; W.-H.W. drafted manuscript.
REFERENCES
- 1. Bhargava A, Fullerton MJ, Myles K, Purdy TM, Funder JW, Pearce D, Cole TJ. The serum- and glucocorticoid-induced kinase is a physiological mediator of aldosterone action. Endocrinology 142: 1587–1594, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Cope G, Murthy M, Golbang AP, Hamad A, Liu CH, Cuthbert AW, O'Shaughnessy KM. WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol 17: 1867–1874, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Frindt G, Shah A, Edvinsson J, Palmer LG. Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol 296: F347–F354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol 274: F817–F833, 1998 [DOI] [PubMed] [Google Scholar]
- 5. He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinase to endocytosis of ROMK1. J Clin Invest 117: 1078–1087, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hou J, Speirs HJL, Seckl JR, Brown RW. Sgk1 gene expression in kidney and its regulation by aldosterone: spatio-temporal heterogeneity and quantitative analysis. J Am Soc Nephrol 13: 1190–1198, 2002 [PubMed] [Google Scholar]
- 7. Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Kahle KT, Wilson FH, Lifton RP. Regulation of diverse ion transport pathways by WNK4 kinase: a novel molecular switch. Trends Endocrinol Metab 16: 98–103, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol 70: 329–355, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin DH, Sterling H, Lerea KM, Welling P, Jin L, Giebisch G, Wang WH. K depletion increases the protein tyrosine-mediated phosphorylation of ROMK. Am J Physiol Renal Physiol 283: F671–F677, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol 286: F881–F892, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Z, Wang HR, Huang CL. Regulation of ROMK channel and K+ homeostasis by kidney-specific WNK1 kinase. J Biol Chem 284: 12198–12206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Connell AD, Leng Q, Dong K, MacGregor GG, Giebisch G, Hebert SC. Phosphorylation-regulated endoplasmic reticulum retention signal in the renal outer medullary K+ channel (ROMK). Proc Natl Acad Sci USA 102: 9954–9959, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Reilly M, Marshall E, Speirs HJL, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol 14: 2447–2456, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Palmer LG. Potassium secretion and the regulation of distal nephron K channels. Am J Physiol Renal Physiol 277: F821–F825, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA 104: 4025–4029, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yong-Feng Y, David LL, Xiaoman Z, Chao-Ling Y, Ellison DH. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest 119: 2601–2612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell 139: 468–484, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Shigaev A, Asher C, Latter H, Garty H, Reuveny E. Regulation of sgk by aldosterone and its effects on the epithelial Na+ channel. Am J Physiol Renal Physiol 278: F613–F619, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Sterling H, Lin DH, Gu RM, Dong K, Hebert SC, Wang WH. Inhibition of protein-tyrosine phosphatase stimulates the dynamin-dependent endocytosis of ROMK1. J Biol Chem 277: 4317–4323, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Subramanya AR, Yang CL, McCormick JA, Ellison DH. WNK kinases regulate sodium chloride and potassium transport by the aldosterone-sensitive distal nephron. Kidney Int 70: 630–634, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Wade JB, Fang L, Liu J, Li D, Yang CL, Subramanya AR, Maouyo D, Mason A, Ellison DH, Welling PA. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci USA 103: 8558–8563, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang W, Lerea KM, Chan M, Giebisch G. Protein tyrosine kinase regulates the number of renal secretory K channels. Am J Physiol Renal Physiol 278: F165–F171, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Wei Y, Bloom P, Lin DH, Gu RM, Wang WH. Effect of dietary K intake on the apical small-conductance K channel in the CCD: role of protein tyrosine kinase. Am J Physiol Renal Physiol 281: F206–F212, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small-conductance K channel in renal cortical collecting duct during dietary K restriction. J Biol Chem 282: 6455–6462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100: 680–684, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang CL, Zhou X, Wang Z, Subramanya AR, Ellison DH. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J Clin Invest 115: 1379–1387, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoo D, Kim BY, Campo C, Nance L, King A, Maouyo D, Welling PA. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem 278: 23066–23075, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Yue P, Lin DH, Pan CY, Leng Q, Giebisch G, Lifton RP, Wang WH. Src family protein tyrosine kinase (PTK) modulates the effect of SGK1 and WNK4 on ROMK channels. Proc Natl Acad Sci USA 106: 15061–15066, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yue P, Sun P, Lin DH, Pan C, Xing W, Wang W. Angiotensin II diminishes the effect of SGK1 on the WNK4-mediated inhibition of ROMK1 channels. Kidney Int 79: 423–431, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yun CC, Palmada M, Embark HM, Fedorenko O, Feng Y, Henke G, Setiawan I, Boehmer C, Weinman EJ, Sandrasagra S, Korbmacher C, Cohen P, Pearce D, Lang F. The serum and glucocorticoid-inducible kinase SGK1 and the Na+/H+ exchange regulating factor NHERF2 synergize to stimulate the renal outer medullary K+ channel ROMK1. J Am Soc Nephrol 13: 2823–2830, 2002 [DOI] [PubMed] [Google Scholar]