Abstract
Regulation of water and urea transport in the inner medullary collecting duct is essential for urine concentration. Aquaporin (AQP)2 water channels and urea transporter (UT)-A1 are inserted into the apical membrane upon phosphorylation of the channels to allow the transcellular movement of water and urea. Since ANG II activates PKC in many cell types, we tested the hypothesis that ANG II-induced regulation of water and urea transport is mediated by PKC. Osmotic minipumps delivered ANG II to wild-type (WT) or PKC-α−/− mice for 7 days. Inner medullas were harvested, and protein abundance was determined by immunoblot. ANG II increased systolic blood pressure to a similar degree in WT and PKC-α−/− mice. ANG II had no effect on the urine output of WT mice but increased that of PKC-α−/− mice. In accordance with observed differences in urine output, AQP2 abundance was unchanged in ANG II-treated WT animals but was decreased in PKC-α−/− mice. No change in membrane accumulation was seen. Phosphorylation of the cAMP-induced transcription factor CREB was decreased in PKC-α−/− mice in response to ANG II with no change in overall CREB abndance. ANG II did not alter the abundance of UT-A1 protein in WT or PKC-α−/− mice. Phosphorylation and overall abundance of tonicity-responsive enhancer-binding protein, a transcription factor that regulates UT-A1, were also unaltered by ANG II in either group. We conclude that PKC-α protects against ANG II-induced decreases in urine concentrating ability by maintaining AQP2 levels through CREB phosphorylation.
Keywords: inner medulla, urea transporter-A1
angiotensin ii (ANG II) was first identified as a paracrine regulator of vascular resistance. More recently, a picture of ANG II as a diverse signaling molecule with varying important effects on almost every cell type in the kidney, including inner medullary collecting duct (IMCD) cells, is beginning to emerge. The primary function of the IMCD is urine concentration. Arginine vasopressin (AVP) increases water reabsorption from the IMCD by two primary mechanisms: 1) increased apical membrane accumulation of the water channel aquaporin (AQP)2 and 2) increased apical membrane accumulation of urea transporter (UT)-A1, which allows for increased urea uptake (17, 25). Though the mechanism by which urea retention promotes the production of concentrated urine is debated (8), mice lacking UT-A1 along with the constitutively basolateral urea transporter UT-A3 produce a very dilute urine, emphasizing the necessity for urea transport in the production of concentrated urine (7). A large amount of data exists implicating synergy between the ANG II and AVP signaling pathways in the regulation of urine output. The effects of AVP on UT-A1 and AQP2 membrane accumulation can be enhanced by ANG II. ANG II increases AVP-mediated urea permeability and UT-A1 phosphorylation in the rat IMCD (13). AVP-mediated increases in AQP2 membrane accumulation and phosphorylation in isolated rat IMCD cells are also lessened with ANG II type 1 (AT1) receptor blockade (19). Furthermore, mice given a low-salt diet show impaired AQP2 and urinary responses to AVP when given an AT1 receptor inhibitor (31), and in murine principal kidney cortical collecting duct cells, ANG II increases AQP2 without the addition of exogenous AVP (20).
ANG II is known to increase cytosolic Ca2+ in intact collecting duct segments (4). Although the exact signaling mechanism whereby this occurs has not been studied in the IMCD, in other cell types, Gq activation of phospholipase C leads to cleavage of phosphatidylinositol 4,5-bisphosphate to form membrane-bound diacylglycerol and soluble inositol 1,4,5-trisphosphate. Diacylglycerol and inositol 1,4,5-trisphosphate work together to activate conventional PKC isoforms, which can, in turn, phosphorylate a variety of proteins. A role for PKC signaling is likely to be important for urine concentration in vivo since mice lacking the PKC α-isoform have impaired urine concentrating ability (34). Much of the synergistic effect seen between ANG II and AVP in the IMCD is impaired by inhibition of PKC (13, 19, 20), and putative PKC phosphorylation sites have been identified on both AQP2 and UT-A1 proteins (26, 30). To further elucidate the role of PKC-α in potentiating the effects of ANG II on the abundance and activity of AQP2 and UT-A1, we tested the effects of a 7-day infusion of ANG II in PKC-α knockout (PKC-α−/−) animals on AQP2 and UT-A1 abundance, membrane accumulation, and phosphorylation.
METHODS
Animals.
The protocols used in this study were approved by the Institutional Animal Care and Use Committee of Emory University and complied with the “Guide for the Care and Use of Laboratory Animals” developed by the Institutional Animal Care and Use Committee. PKC-α−/− mice were initially obtained from Dr. Jeffery Molkentin (Cincinnati Children's Hospital Medical Center) and bred in an in-house breeding facility (5). C57black6 mice [wild type (WT) mice] were used as a control (5). All animals were given standard lab chow and water ad libitum and housed under a 12:12-h light-dark cycle throughout the study. Mice used ranged between 2 mo and 1 yr of age and were of mixed sex.
Osmotic minipumps.
On day 1, animals were implanted with osmotic pumps set to deliver solution at a rate of 1 μl/min (Alzet) and filled with ANG II in a buffer consisting of 0.15 M NaCl and 1% acetic acid. ANG II was given at a rate of 400 ng·kg−1·min−1 for 7 days. Animals were allowed to recover from surgery for 3 days before the start of blood pressure measurements or placement in metabolic cages. To reduce stress, no animal was subjected to both metabolic cage housing and blood pressure measurement.
Urine analysis.
Mice were housed for 2 days in metabolic mouse cages (Techniplast). Urine was centrifuged at 15,000 rpm for 15 min to separate any soluble substance before analysis. Urine osmolality was measured by a vapor pressure osmometer (Wescor), and urea was determined by colorimetric assay using the Infinity Urea Kit (Thermo Scientific). Urinary Na+ was measured using a Na+-specific electrode (Cole Palmer).
Measurement of blood pressure.
Systolic blood pressure was measured by tail cuff (BP-2000, Visitech Systems). Ten measurements were taken consecutively for each mouse and averaged. An overall average for each treatment group was then obtained.
Western blot analysis.
On day 7, animals were killed and kidneys were harvested. Kidneys were dissected into the cortex, outer medulla (OM), and inner medulla (IM) as identified by morphology. Protein was measured by detergent-compatible protein assay kit (Bio-Rad), and Western blot analysis was performed with SDS-PAGE using in-house antibodies against AQP2 or UT-A1, as previously described (1, 3, 14).
Immunohistochemistry.
Animals were perfused with 4% paraformaldehyde. Kidneys were embedded in Paraplast tissue embedding medium (McCormick Scientific). Immunohistochemistry was performed as previously described (2, 3).
Chemicals and antibodies.
ANG II was purchased from Sigma-Aldrich. In-house antibodies against UT-A1 and AQP2 were used (1, 3, 14). Anti-tonicity-responsive enhancer-binding protein (TonEBP) and anti-phospho-TonEBP were purchased from Calbiochem. Antibodies against CREB and phospho-CREB were purchased from Cell Signaling. For Western blots, 1:2,000 and 1:4,000 dilutions of UT-A1 and AQP2 antibodies were used, respectively.
Data analysis and statistics.
Western blot densitometries were performed using Odyssey software and normalized to the average of the control group. Graphs were created using Microsoft Office software. Statistics were performed as Student's t-tests between control and control + ANG II in all data sets. P values of <0.05 were considered significant. Statistics were performed using GB Stat software.
RESULTS
To study the effects of ANG II on urine production in PKC-α−/− mice, we administered subcutaneous ANG II for 7 days. Baseline systolic blood pressures were similar between strains of mice, with WT mice averaging 110 ± 5 mmHg compared with 119 ± 2 mmHg in PKC-α−/− mice. ANG II increased systolic blood pressure to a similar degree in both strains, with WT mouse blood pressures averaging 147 ± 15 mmHg and mice lacking PKC-α having blood pressures of 151 ± 5 mmHg (Table 1). Urinary Na+ increased significantly in both groups after ANG II administration.
Table 1.
Comparison of physiological parameters between WT and PKC-α−/− mice after 7 days of subcutaneous infusion of ANG II
| WT Mice | WT Mice + ANG II | PKC-α−/− Mice | PKC-α−/− Mice + ANG II | |
|---|---|---|---|---|
| Systolic blood pressure, mmHg | 110 ± 5 | 147 ± 15* | 119 ± 2 | 150 ± 2* |
| Urine output, μl/day | 717 ± 149 | 1,430 ± 497 | 950 ± 182 | 2,800 ± 390* |
| Urine osmolality, mosM | 3,748 ± 505 | 3,656 ± 502 | 3,018 ± 415 | 1,709 ± 220* |
| Urine urea, mM | 2,075 ± 322 | 1,898 ± 291 | 1,480 ± 159 | 787 ± 77* |
| Water intake, ml/day | 6.07 ± 0.7 | 4.06 ± 0.22 | 2.675 ± 0.38 | 7.59 ± 0.69* |
| Urinary Na+, meq/day | 0.25 ± 0.03 | 0.39 ± 0.06* | 0.22 ± 0.02 | 0.37 ± 0.03* |
| Body weight, g | 20 ± 1 | 21 ± 1 | 20 ± 1 | 22 ± 1 |
Values are presented as means ± SE; n ≥ 5 for all groups. WT, wild type; PKC-α−/−, PKC-α knockout.
P < 0.05 compared with untreated animals of the same genotype.
PKC-α−/− mice produce a large volume of dilute urine in response to 7 days of subcutaneous ANG II infusion.
A threefold increase in urine output in response to ANG II infusion in mice lacking PKC-α was observed that was not seen in WT animals (Table 1). To ask whether the increased urine output was due to impaired urine concentration ability, we measured urine osmolality and urea concentration. Whereas WT animals showed no significant change in urine osmolality or urea concentration in response to ANG II infusion, PKC-α−/− mice demonstrated twofold decreases in both. Of note, PKC-α−/− mice also showed a significant increase in water intake over a 24-h period in response to ANG II infusion that was not seen in WT mice. These data indicate that subcutaneous infusion of ANG II impairs urine concentrating ability in PKC-α−/− mice but not in WT mice.
ANG II selectively decreases AQP2 abundance in the IM and OM of PKC-α−/− mice.
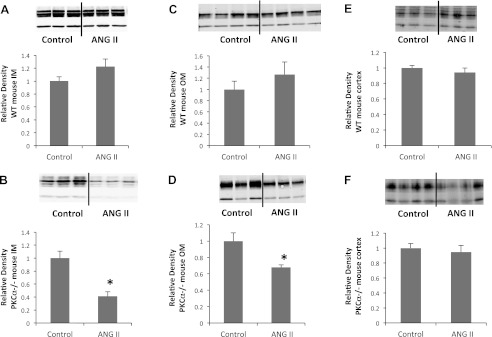
Next, we asked whether changes in renal AQP2 abundance contribute to the large urinary response to ANG II seen in PKC-α−/− mice. AQP2 is expressed at varying levels throughout the kidney (24). Accordingly, we analyzed AQP2 protein abundance separately in the renal cortex, OM, and IM (Fig. 1). Cortical AQP2 protein abundance did not change in WT mice (1.0 ± 0.03 vs. 0.9 ± 0.06, P > 0.3, n = 11 mice/group) or PKC-α−/− mice (1.0 ± 0.06 vs. 0.95 ± 0.09, P > 0.6, n = 10 mice/group). Similarly, there was no change in AQP2 abundance in WT animals in the OM (1.0 ± 0.2 vs. 1.3 ± 0.2, P > 0.3, n = 15 mice/group) or IM (1.0 ± 0.1 vs. 1.2 ± 0.1, P > 0.1, n = 9 mice/group). We observed a significant decrease in AQP2 abundance in the OM (1.0 ± 0.1 vs. 0.7 ± 0.03, P < 0.005, n = 14 mice/group) and IM (1.0 ± 0.1 vs. 0.4 ± 0.07, P < 0.0005, n = 10 mice/group) of PKC-α−/− animals, however. This change in AQP2 is likely responsible for the inability to conserve water seen PKC-α−/− mice after ANG II administration.
Fig. 1.
ANG II decreases the abundance of aquaporin (AQP)2 in the inner medulla (IM) and outer medulla (OM) of PKC-α knockout (PKC-α−/−) mice but has no effect on AQP2 abundance in wild-type (WT) mice. A and B: protein obtained from the IM from WT (A) and PKC-α−/− (B) animals probed with anti-AQP2 antibody. C and D: AQP2 abundance in the OM of WT (C) and PKC-α−/− (D) mice. E and F: lack of change in AQP2 abundance in the cortices of WT (E) and PKC-α−/− (F) mice. Top, representative Western blots from each group probed with AQP2; bottom, average densitometry.
Dilute urine is not the result of alterations in UT-A1 abundance.
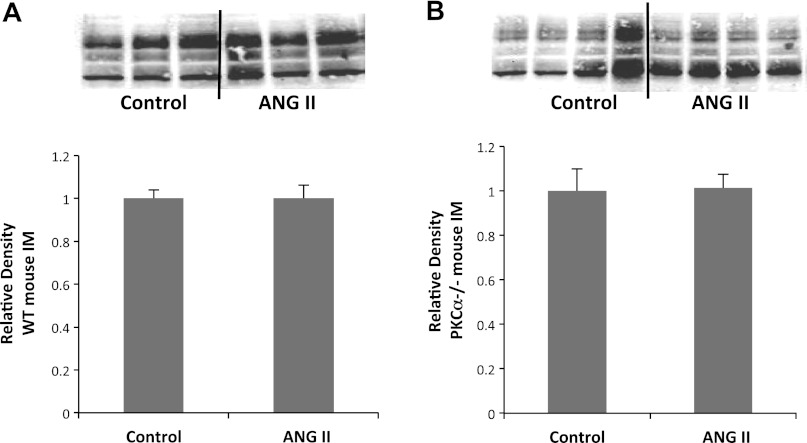
We asked whether the altered abundance of UT-A1 could cause the urine concentration defect seen in PKC-α−/− mice in response to ANG II (Fig. 2). Western blot analysis of whole IMs showed no change in UT-A1 abundance in WT mice (1.0 ± 0.04 vs. 1.0 ± 0.04 average densitometries, P > 0.9, n = 10 mice/group). Similarly, no change was seen in PKC-α−/− animals (1.0 ± 0.09 vs. 1.0 ± 0.08 average densitometries, P > 0.9, n = 10 mice/group).
Fig. 2.
Urea transporter (UT)-A1 protein abundance is unchanged by ANG II in both WT mice (A) and those lacking PKC-α (B). Top, representative Western blots of whole IM tissue obtained from control animals or those treated with ANG II for 7 days probed with anti-UT-A1 antibody; bottom, average densitometries from all animals tested (n ≥ 9) normalized to the average of the control group for each Western blot to control for variability between membranes. P > 0.05 for all groups.
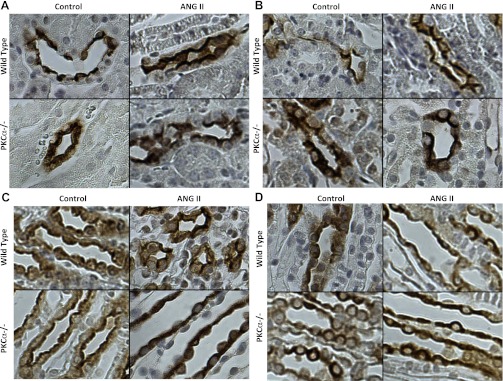
Localization of AQP2 and UT-A1 is unaltered in the kidney in response to ANG II.
To determine whether alterations in membrane localization of AQP2 and/or UT-A1 could contribute to the enhanced urine output seen in PKC-α−/− animals after treatment with ANG II, we performed immunohistochemistry to look for cellular localization of UT-A1. Localization of AQP2 (Fig. 3, A–C) throughout the kidney and UT-A1 (Fig. 3D) in the IM were fairly diffuse in untreated WT and PKC-α−/− animals with some membrane localization. No obvious changes in the cellular distribution of UT-A1 or AQP2 were seen after treatment with ANG II in either strain. Overall, these data suggest that 7-day treatment with ANG II does not result in a redistribution of AQP2 or UT-A1 in mice and that lack of PKC-α does not change this response.
Fig. 3.
UT-A1 and AQP2 membrane localization in the IM is unchanged by ANG II treatment in WT and PKC-α−/− animals. Immunohistochemistry was performed using antibodies against AQP2 or UT-A1 stained with diaminobenzidine (brown). Nuclei were counterstained with hematoxylin (blue). Images are representative of a minimum of 3 animals/group and are presented at ×400 magnification. AQP2 was examined in the cortex (A), OM (B), and IM (C), whereas UT-A1 was examined in the IM only (D).
Phosphorylation of the transcription factor CREB is selectively decreased in the IM of PKC-α−/− mice after ANG II administration.
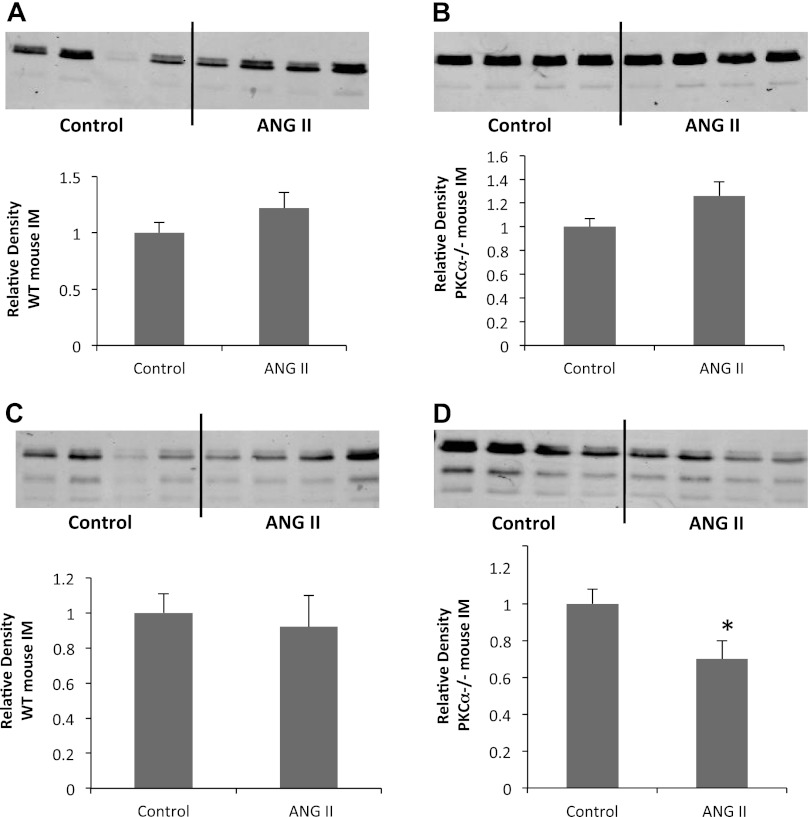
AQP2 transcription is regulated by the cAMP-dependent transcription factor CREB (11, 22). We analyzed the abundance and phosphorylation of CREB by Western blot analysis (Fig. 4). Total CREB protein abundance was unaltered by ANG II administration in WT mice (1.0 ± 0.08 vs. 1.2 ± 0.1, P > 0.1, n = 12 mice/group) or PKC-α−/− mice (1.0 ± 0.08 vs. 1.3 ± 0.1, P > 0.09, n = 12 mice/group). Whereas phosphorylation of CREB did not change in WT animals in response to ANG II infusion (1.0 ± 0.1 vs. 0.9 ± 0.2, P > 0.1, n = 12 mice/group), PKC-α−/− animals exhibited a significant decrease in CREB phosphorylation after 7-day administration of ANG II (1.0 ± 0.8 vs. 0.7 ± 0.1, P < 0.04, n = 12 mice/group). These data indicate that the decreased abundance of AQP2 that selectively occurs in PKC-α−/− mice in response to ANG II is likely the result of decreased gene transcription downstream of decreased phosphorylation of the transcription factor CREB.
Fig. 4.
CREB phosphorylation is decreased by ANG II in mice lacking PKC-α. A and B: total CREB abundance was assayed in the whole IM of WT (A) or PKC-α−/− (B) animals by Western blot analysis with an anti-CREB antibody and remained unchanged by ANG II in both groups. CREB phosphorylation was assayed by anti-phospho-CREB antibody. Top, representative Western blots; bottom, average densitometries. n = 12 animals/group. *P < 0.05.
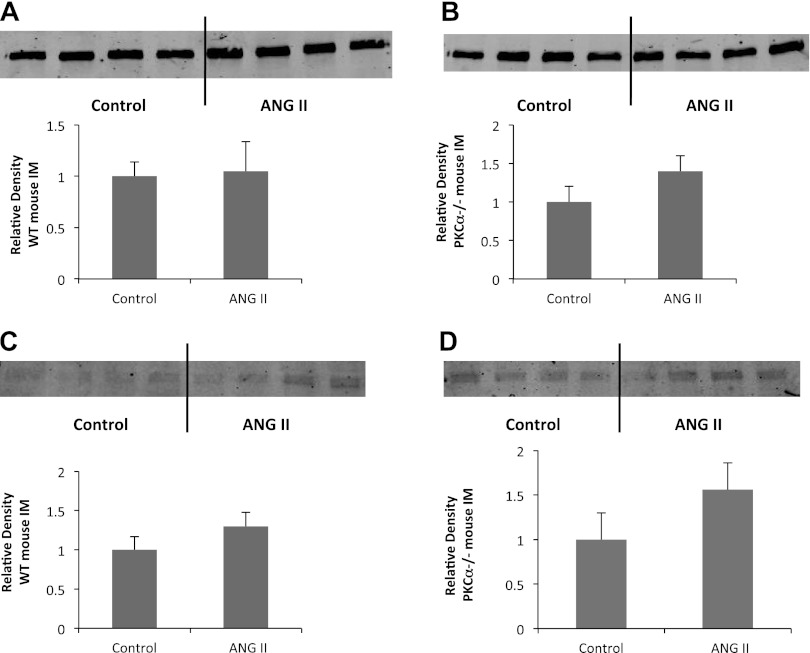
Phosphorylation and transcription of the transcription factor TonEBP are unaltered after ANG II infusion in WT and PKC-α−/− mice.
TonEBP is regulated in the IMCD in response to changes in interstitial osmolality and stimulates transcription of UT-A1 and, to a lesser extent, AQP2 DNA (18). To determine whether alterations in TonEBP abundance or activity mediate the urine concentrating defect observed after ANG II administration in mice lacking PKC-α, we compared the effect of ANG II on TonEBP abundance and phosphorylation between WT and PKC-α−/− mice (Fig. 5). ANG II did not alter TonEBP abundance (1.0 ± 0.14 vs 1.0 ± 0.3, P > 0.8, n = 12 mice/group) or phosphorylation (1.0 ± 0.2 vs. 1.3 ± 0.2, P > 0.05, n = 12 mice/group) in WT animals. No effect of ANG II was seen on TonEBP abundance (1.0 ± 0.2 vs. 1.4 ± 0.2, P > 0.05, n = 12 mice/group) or phosphorylation (1.0 ± 0.3 vs. 1.5 ± 0.3, P > 0.05, n = 12 mice/group) in PKC-α−/− mice. The changes in urine output in response to ANG II were not the result of TonEBP-mediated changes in gene transcription.
Fig. 5.
Tonicity-responsive enhancer-binding protein (TonEBP) abundance and phosphorylation are unchanged by ANG II in WT mice and those lacking PKC-α. A and B: total abundance of TonEBP was compared between WT (A) and PKC-α−/− (B) mice. C and D: phosphorylation of TonEBP was detected in WT mice (C) and those lacking PKC-α (D). Top, representative Western blots; bottom, average densitometries. n = 12 animals/group. P > 0.05 for all groups.
DISCUSSION
Our results demonstrate, for the first time, a role for PKC-α in the maintenance of urine concentrating ability during ANG II-induced hypertension. Mice lacking PKC-α present with a mild urine concentrating defect (34), which, as we show here, is exacerbated by ANG II infusion. PKC-α−/− animals responded to ANG II with increased urine output, decreased urine osmolality, and decreased urine urea, whereas none of these parameters were altered in WT mice after ANG II. Systolic blood pressure increased to a similar degree in both strains of animals, indicating that the cardiovascular effects of ANG II did not differ between strains. For this reason, we analyzed the abundance of proteins in the renal IM commonly involved in urine concentration. This analysis revealed a highly significant decrease in AQP2 abundance in the IM and OM of PKC-α−/− mice in response to ANG II that was not seen in WT animals. Immunohistochemistry revealed normal membrane recruitment of AQP2 throughout the kidney of all groups of animals. AQP2 is typically transcriptionally regulated by AVP-induced production of cAMP, an effect mediated by phosphorylation of the cAMP-dependent transcription factor CREB (29, 35). While other transcription factors, such as AP1 and TonEBP, have been shown to regulate AQP2, it is generally agreed that CREB is the primary regulator of AQP2 in renal tubules (29). In the present study, we discovered decreased CREB phosphorylation despite no significant change in total CREB abundance in response to ANG II in PKC-α−/− animals, an effect which was not seen in WT animals. Overall, our data suggest a role for PKC-α in the regulation of CREB phosphorylation in the presence of ANG II.
Urine concentration by the IMCD is primarily the result of changes in AVP and/or IM osmolality. Mice lacking PKC-α have increased basal urine output and decreased osmolality compared with controls under basal conditions or during water deprivation but are indistinguishable from controls during water loading or vasopressin type 2 receptor blockade, indicating that these mice have an inability to concentrate urine in response to AVP (34). Previous data from our laboratory have indicated a decrease in basal IM abundance of UT-A1 (15) as well as a 36% decrease in AQP2 abundance (data not shown; n = 3, P < 0.05), suggesting that there are likely differences in interstitial osmolality between strains. PKC inhibition leads to altered urea transport in the IMCD (32) in response to a combination of AVP and hyperosmolality. Molecularly, membrane abundance and phosphorylation of UT-A1 are regulated by PKC in response to hyperosmolality (15). PKC isoforms have also been shown to augment AQP2 trafficking (20). Together, these data implicate PKC isoforms, including PKC-α, in most aspects of urine concentration. Our data add to this body of knowledge by showing that mice lacking PKC-α have a different urinary response to ANG II infusion. Despite the body of evidence demonstrating the importance of PKC isoforms in mediating urea transport in the IMCD, we found no difference in UT-A1 abundance or membrane localization in response to ANG II between PKC-α−/− and WT mice. It may be that ANG II is less important in the regulation of UT-A1 than AQP2 in mice, but whether this is the case remains to be determined.
The selective decrease in phospho-CREB, AQP2, and urine concentrating ability seen in PKC-α−/− mice leads us to wonder about possible signaling mechanisms underlying these effects. It is clear from our results that PKC-α participates in the maintenance of AQP2 levels during ANG II-induced hypertension. We found that phosphorylation of the transcription factor CREB was attenuated accompanying the decrease in AQP2 abundance. These data suggest that direct or indirect regulation of CREB by PKC-α is occurring downstream of ANG II. This is not surprising given the large amount of literature implicating PKCs downstream of ANG II (28). If ANG II infusion led to phosphorylation of CREB under normal conditions, we should see increased CREB phosphorylation in WT animals, which was not the case. This may indicate either inhibition of a phosphatase that recognizes CREB by PKC-α or phosphorylation of CREB by PKC-α that is typically masked by concurrent dephosphorylation by a separate mechanism. Either case is possible given the current literature investigating the relationship between CREB and conventional PKCs. Although CREB is traditionally known to be activated by phosphorylation by PKA downstream of cAMP, conventional PKCs can also phosphorylate CREB at multiple sites (12). Phosphorylation by PKC may be mediated by MAPK or may occur directly by PKC. Phosphorylation of CREB by PKCs may result in the activation of CREB by dimerization (27). In this case, the question remains as to why ANG II did not stimulate PKC to increase phosphorylation of CREB in WT animals. It is possible that the stimulation of PKC-induced phosphorylation of CREB is masked by parallel activation of phosphatases to dephosphorylate CREB. ANG II is known to increase intracellular Ca2+, which can activate a variety of phosphatases, including calcineurin, that can act on CREB (10). PKC-α may inhibit one of these phosphatases. This would result in decreased phosphorylation of CREB, a parallel decrease in AQP2 abundance, and a urine concentrating defect. Elucidation of the role of Ca2+ phosphatases and/or MAPK-dependent phosphorylation of CREB in this model remains to be achieved and may be an interesting topic for future studies.
One thing that needs to be considered during systemic infusion of ANG II is that ANG II can work on many parts of the body. In particular, when considering urine concentrating effects, one needs to consider central effects of ANG II. For example, ANG II can stimulate AVP release from the hypothalamus (23). If this release were dependent on PKC-α, it would follow that PKC-α−/− animals treated with ANG II would show a urine concentrating defect and decreased CREB-induced transcription of AQP2. If this were the case, however, membrane recruitment of AQP2 and UT-A1 would be impaired since AVP promotes movement of both transporters to the apical membrane to increase water and urea transport (25). ANG II has a powerful effect on the stimulation of thirst (9). If PKC-α was working to inhibit the actions of ANG II on the thirst mechanism, then in the absence of PKC-α it is possible that increased water intake would lead to a compensatory downregulation of AQP2. Indeed, in our study, we observed increased water intake in PKC-α−/− animals after ANG II treatment. An impaired ability to concentrate the urine can, in itself, result in enhanced thirst due to the decrease in blood volume and increase in circulating AVP in an attempt to compensate for the loss of water. In our present study, we were unable to determine whether the increased thirst causes the tubular defect or whether a tubular defect alters thirst.
We were also unable, from our study, to determine whether the differing effects of ANG II on urine output and channel abundance are due to ANG II directly or secondary to a rise in blood pressure. Hypertension alone can alter baroreceptor signaling and subsequent alterations in AQP2 abundance. Previous experiments in rats showed a similar change in AQP2 and UT-A1 abundance after ANG II- or norepinephrine-induced hypertension (16), but based on our present findings, the exact cause of the change in AQP2 and the selectivity for PKC-α−/− mice are unknown. Future experiments should be aimed at analyzing the response of PKC-α−/− mice to other forms of hypertension.
It is important to point out that AQP2 is not the only downstream target of CREB. A wide variety of genes are regulated by cAMP-induced activation of CREB (6, 33), and it is inevitable that some of these genes were altered in our study. We chose to limit our study to two important mediators of urine concentration: AQP2 and UT-A1. Interesting future studies may be aimed at determining whether PKC-α knockout affects other CREB targets in the kidney as well as in other organs.
In the present study, we found no change in AQP2 or UT-A1 abundance after 7 days of subcutaneous ANG II infusion in WT mice. We also observed no change in the abundance of Na+-K+-2Cl− cotransporter 2, another protein involved in the urine concentrating mechanism (data not shown). Previous work from our group has shown a decrease in the abundance of these three proteins in response to 7- or 14-day administration of the same dose of ANG II in Sprague-Dawley rats (16). It is interesting that the WT mice used in our study did not respond to ANG II in the same manner as Sprague-Dawley rats. This interesting species-specific difference in urinary response to ANG II should be taken into consideration in the planning of future studies.
We found that PKC-α−/− animals responded to ANG II by producing a large amount of dilute urine. While this agrees with studies in Sprague-Dawley rats, mice lacking the AT1a receptor are unable to concentrate urine (21), and, accordingly, cultured mouse renal collecting duct principal cells respond to acute ANG II administration by increasing AQP2 abundance (20), suggesting that ANG II should result in increased AQP2 abundance and water conservation. This discrepancy may be reflective of differences between models. As the first study to examine the effects of 7-day treatment of ANG II on AQP2 expression in mice, our study should not be directly compared with previous studies in rats or cells.
Overall, we conclude that PKC-α participates in CREB phosphorylation, either directly or indirectly, after 7-day treatment with ANG II. To our knowledge, we are the first group to implicate a role for PKC-α downstream of ANG II in vivo and the first to demonstrate the severe effects of ANG II on urine concentrating ability in PKC-α−/− mice. Future studies should be done to determine the role of PKC-α downstream of ANG II in WT animals and the signaling pathways involved.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants T32-DK-007771, RO1-DK-41707, and RO1-DK-89828.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank the laboratory of Dr. Susan Wall for technical assistance.
REFERENCES
- 1. Blessing NW, Blount MA, Sands JM, Martin CF, Klein JD. Urea transporters UT-A1 and UT-A3 accumulate in the plasma membrane in response to increased hypertonicity. Am J Physiol Renal Physiol 295: F1336–F1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Blount MA, Sim JH, Zhou R, Martin CF, Lu W, Sands JM, Klein JD. Expression of transporters involved in urine concentration recovers differently after cessation of lithium treatment. Am J Physiol Renal Physiol 298: F601–F608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouby N, Hus-Citharel A, Marchetti J, Bankir L, Corvol P, Llorens-Cortes C. Expression of type 1 angiotensin II receptor subtypes and angiotensin II-induced calcium mobilization along the rat nephron. J Am Soc Nephrol 8: 1658–1667, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-α regulates cardiac contractility and propensity toward heart failure. Nat Med 10: 248–254, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Conkright MD, Guzmán E, Flechner L, Su AI, Hogenesch JB, Montminy M. Genome-wide analysis of CREB target genes reveals a core promoter requirement for cAMP responsiveness. Mol Cell 11: 1101–1108, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA. Renal rhenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol 16: 1583–1592, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev 78: 583–686, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Grossmann C, Wuttke M, Ruhs S, Seiferth A, Mildenberger S, Rabe S, Schwerdt G, Gekle M. Mineralocorticoid receptor inhibits CREB signaling by calcineurin activation. FASEB J 24: 2010–2019, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Hozawa S, Holtzman EJ, Ausiello DA. cAMP motifs regulating transcription in the aquaporin 2 gene. Am J Physiol Cell Physiol 270: C1695–C1702, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal 16: 1211–1227, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Kato A, Klein JD, Zhang C, Sands JM. Angiotensin II increases vasopressin-stimulated facilitated urea permeability in rat terminal IMCDs. Am J Physiol Renal Physiol 279: F835–F840, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Klein JD, Blount MA, Fröhlich O, Denson CE, Tan X, Sim JH, Martin CF, Sands JM. Phosphorylation of UT-A1 on serine 486 correlates with membrane accumulation and urea transport activity in both rat IMCDs and cultured cells. Am J Physiol Renal Physiol 298: F935–F940, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein JD, Martin CF, Kent KJ, Sands JM. Protein kinase C-α mediates hypertonicity-stimulated increase in urea transporter phosphorylation in the inner medullary collecting duct. Am J Physiol Renal Physiol 302: F1098–F1103, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klein JD, Murrell BP, Tucker S, Kim YH, Sands JM. Urea transporter UT-A1 and aquaporin-2 proteins decrease in response to angiotensin II or norepinephrine-induced acute hypertension. Am J Physiol Renal Physiol 291: F952–F959, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol Renal Physiol 272: F3–F12, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Lam AKM, Ko BCB, Tam S, Morris R, Yang JY, Chung SK, Chung SSM. Osmotic response element-binding protein (OREBP) is an essential regulator of the urine concentrating mechanism. J Biol Chem 279: 48048–48054, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Lee YJ, Song IK, Jang KJ, Nielsen J, Frøkiær J, Nielsen S, Kwon TH. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT1 receptor. Am J Physiol Renal Physiol 292: F340–F350, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Li C, Wang W, Rivard CJ, Lanaspa MA, Summer S, Schrier RW. Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking. Am J Physiol Renal Physiol 300: F1255–F1261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li XC, Shao Y, Zhuo JL. AT1a receptor knockout in mice impairs urine concentration by reducing basal vasopressin levels and its receptor signaling proteins in the inner medulla. Kidney Int 76: 169–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8: 861–867, 1997 [DOI] [PubMed] [Google Scholar]
- 23. McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 and AT2 receptors in the brain. Clin Exp Pharmacol Physiol Suppl 3: S99–S104, 1996 [PubMed] [Google Scholar]
- 24. Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA 90: 11663–11667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sands JM. Molecular mechanisms of urea transport. J Membr Biol 191: 149–163, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Shayakul C, Hediger MA. The SLC14 gene family of urea transporters. Pflügers Arch 447: 603–609, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68: 821–861, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Touyz RM, Berry C. Recent advances in angiotensin II signaling. Braz J Med Biol Res 35: 1001–1015, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Umenishi F, Narikiyo T, Vandewalle A, Schrier RW. cAMP regulates vasopressin-induced AQP2 expression via protein kinase A-independent pathway. Biochim Biophys Acta 1758: 1100–1105, 2006 [DOI] [PubMed] [Google Scholar]
- 30. van Balkom BW, Savelkoul PJM, Markovich D, Hofman E, Nielsen S, van der Sluijs P, Deen PM. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem 277: 41473–41479, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Wang W, Li C, Summer S, Falk S, Schrier RW. Interaction between vasopressin and angiotensin II in vivo and in vitro: effect on aquaporins and urine concentration. Am J Physiol Renal Physiol 299: F577–F584, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Klein JD, Liedtke CM, Sands JM. Protein kinase C regulates urea permeability in the rat inner medullary collecting duct. Am J Physiol Renal Physiol 299: F1401–F1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu W, Kasper LH, Lerach S, Jeevan T, Brindle PK. Individual CREB-target genes dictate usage of distinct cAMP-responsive coactivation mechanisms. EMBO J 26: 2890–2903, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yao L, Huang DY, Pfaff IL, Nie X, Leitges M, Vallon V. Evidence for a role of protein kinase C-α in urine concentration. Am J Physiol Renal Physiol 287: F299–F304, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Yasui M, Zelenin SM, Celsi G, Aperia A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol Renal Physiol 272: F443–F450, 1997 [DOI] [PubMed] [Google Scholar]