Abstract
Acute kidney injury (AKI) is the most common kidney disease in hospitalized patients with high mortality. Ischemia and reperfusion (I/R) is one of the major causes of AKI. The combination of α-ketoglutarate+malate (αKG/MAL) showed the ability to reduce hypoxia-induced damage to isolated proximal tubules. The present study utilizes a rat model of I/R-induced AKI accompanied by intensive biomonitoring to examine whether αKG/MAL provides protection in vivo. AKI was induced in male Sprague-Dawley rats by bilateral renal clamping (40 min) followed by reperfusion (240 min). αKG/MAL was infused continuously for 60 min before and 45 min after ischemia. Normoxic and I/R control groups received 0.9% NaCl solution. The effect of αKG/MAL was evaluated by biomonitoring, blood and plasma parameters, histopathology, and immunohistochemical staining for kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), as well as by determination of tissue ATP and nonesterified fatty acid concentrations. Intravenous infusion of αKG/MAL at a cumulative dose of 1 mmol/kg each (146 mg/kg αKG and 134 mg/kg MAL) did not prevent I/R-induced increases in plasma creatinine, histopathological alterations, or cortical ATP depletion. On the contrary, the most notable adverse affect in animals receiving αKG/MAL was the decrease in mean arterial blood pressure, which was also accompanied by a reduction in heart rate. Supplementation with αKG/MAL, which is very protective against hypoxia-induced injury in isolated proximal tubules, does not protect against I/R-induced renal injury in vivo, possibly due to cardiovascular depressive effects.
Keywords: blood pressure
acute kidney injury (AKI) is the most common and most expensive kidney disease in hospitalized patients (20). Despite substantial progress in medical therapy, devastating rates of morbidity and mortality resulting from AKI (4, 15, 22, 25) could not be ameliorated during the last 30 years due to a lack of specific causal-therapeutic treatments (6, 16). Main causes for AKI are a shortage of nutrition and oxygen supply (ischemia) (21). Clinical courses of events show that even after full restoration of nutrition and oxygen supply (reperfusion) kidney function does not recover immediately. Kidney function can be reduced for days or weeks and might arouse a need for renal replacement therapy or progress to end-stage kidney disease (1). In patients undergoing kidney transplantation, AKI causes delayed graft function followed by decreased transplant survival and increased mortality (28).
Proximal tubular damage plays a critical role in the pathogenesis of ischemia-induced AKI. Because of their dependence on aerobic energy metabolism for ATP synthesis (37), proximal tubules are especially susceptible to ischemia-reperfusion (I/R) injury. Freshly isolated proximal tubules subjected to hypoxia in vitro under conditions relevant to ischemia in vivo develop a significant energetic deficit (33) that is not corrected even after full reoxygenation (35, 36). The insufficient recovery of cellular ATP levels during reoxygenation restrains cellular recovery (36). Freshly isolated kidney proximal tubules exposed to hypoxia-reoxygenation (H/R) develop a profound functional mitochondrial deficit characterized by failure of oxidative phosphorylation (respiration) (35), condensation of the mitochondrial matrix, a decrease in cellular pyrindine nucleotides, and only partial recovery of the mitochondrial membrane potential contemporaneous with an intact cellular membrane (8, 36). The hypoxia-induced accumulation of nonesterified fatty acids (NEFA) could be identified as a pivotal reason for the mitochondrial energetic deficit (9).
We and others were able to show in in vitro studies the ability of the citric acid cycle metabolites α-ketoglutarate and malate (αKG/MAL) to considerably reduce hypoxia-induced damage and hypoxia-induced accumulation of NEFAs in isolated proximal tubules (10, 35, 36). Supplementation of αKG/MAL during either hypoxia or reoxygenation ameliorated the mitochondrial functional lesion characterized by energy deficit, respiratory impairment, loss of membrane potential, and mitochondrial matrix condensation. The protective effects result most likely from stimulation of metabolic pathways for anaerobic generation of ATP by substrate-level phosphorylation, with formation of succinate as an end product, and preservation of membrane potential via electron transport in complex I and II (35, 36). A mitigating impact of αKG/MAL on the NEFA-induced uncoupling effect after H/R in proximal tubules by competition for the tricarboxylate carrier is also suggested (10).
The beneficial effect of αKG/MAL supplementation during hypoxia and/or reoxygenation on isolated proximal tubules has been extensively studied (8, 9, 35, 36). However, only few attempts have been made to study the protective effects of αKG/MAL in vivo. Kjellman et al. (18) were able to show that addition of αKG to blood cardioplegic solutions attenuates signs of ischemia in the human heart after surgery. In a study by Jeppsson et al. (14), αKG infusions after coronary operations with cardiopulmonary bypass in patients with preoperative normal kidney function enhanced renal blood flow.
According to our knowledge, studies using αKG/MAL in an experimental in vivo model of renal I/R do not exist. Therefore, we utilized a rat model of ischemia-induced AKI accompanied by intensive biomonitoring to examine whether αKG/MAL provides protection in vivo. The influence of αKG/MAL was evaluated by blood and plasma parameters, histopathology, and immunohistochemical staining for kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), as well as by determination of tissue ATP and NEFA concentrations.
MATERIALS AND METHODS
Chemicals/Materials
α-Ketoglutarate, malate, and hematoxylin were obtained from Sigma-Aldrich (Steinheim, Germany). Formalin solution (4.5 and 10%, buffered) and isoflurane (Forene) were from Abbott (Wiesbaden, Germany), ketamine (10%) was from Ceva (Düsseldorf, Germany), lidocaine (Xylocaine, 1%) from AstraZeneca (Wedel, Germany), Ringer's solution Macoflex N was from MacoPharma (Langen, Germany), 0.9% NaCl solution and sterile water (Aqua Ecotainer) were from Braun (Melsungen, Germany), paraffin (Paraplast Tissue Embedding Medium REF 501006) was from McCormick Scientific (St. Louis, MO), medical oxygen was from Air Liquide (Düsseldorf, Germany) and heparin-natrium 25000 from Ratiopharm (Ulm, Germany). Triton X-100 was purchased from AppliChem (Darmstadt, Germany), Tris from Serva Electrophoresis (Heidelberg, Germany), Dulbecco's Phosphate-Buffered Saline (DPBS) was from Invitrogen (Darmstadt, Germany), and EDTA was from Merck (Darmstadt, Germany). Syringe pumps (Perfusor-Secura FT) were from Braun, portex catheters (0.58-mm inner diameter, 0.96-mm outer diameter) were from Smiths Medical International (Hythe, UK), 4-0 Vicryl sutures were from Ethicon (Norderstedt, Germany), 2-ml syringes (Pico50) were from Radiometer Medical (Brønshøj, Denmark), safe-lock tubes (2 ml) were from Eppendorf (Hamburg, Germany), and 15-ml polypropylene tubes (Falcon tubes) were from BD Biosciences (Heidelberg, Germany).
Animals
Male Sprague-Dawley rats (400–470 g) were obtained from Charles River (Sulzfeld, Germany). Animals were kept for at least 1 wk before the experiments in the central animal unit of the University Hospital Essen under standardized conditions of temperature (22 ± 1°C), humidity (55 ± 5%), and 12:12-h light-dark cycles with free access to food (ssniff-Spezialdiäten, Soest, Germany) and water; animals were not fasted before the experimental procedures. All animals received humane care according to the standards of the Federation of European Laboratory Animal Science Association (FELASA). The experimental protocol has been approved based on the local animal protection act.
Anesthesia, Analgesia, and Surgical Procedure
Anesthesia with isoflurane and analgesia with ketamine was performed as described previously (29, 30). A skin-deep incision was made along the thigh of the right hindlimb after application of lidocaine (5 mg/kg sc). Portex catheters were placed in the exposed femoral artery and vein and fixed with 4-0 Vicryl ligatures. Following this procedure, a median abdominal laparotomy was performed along the linea alba, the intestine was carefully evacuated from the abdominal cavity, and both kidneys were localized. The vascular pedicle of each kidney was mobilized. In animals undergoing clamping, both renal pedicles were occluded for 40 min using atraumatic minibulldogs (Aesculap, Tuttlingen, Germany). The intestine was replaced into the abdominal cavity and covered with moistened compresses and aluminum foil to minimize evaporation and cooling. Subsequent to the ischemic period, the microvascular clamps were removed and the kidneys thus reperfused. At the end of the reperfusion period of 240 min, the right kidney was removed. A catheter was placed in the abdominal aorta, and the left kidney perfused at 100 mmHg with 40 ml isotonic NaCl solution containing 1,500 IU heparin-natrium before being resected. Animals remained anesthetized during the whole experiment and were euthanized by cardiac incision under deep isoflurane anesthesia.
Study Groups
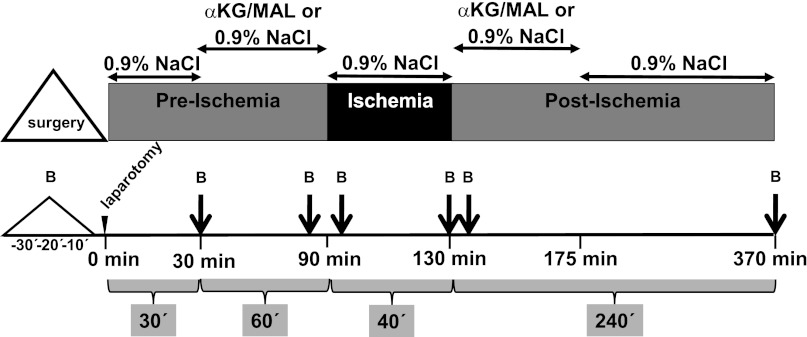
The animal study was performed with six rats/group. αKG and MAL were freshly dissolved in Aqua bidest to obtain an almost isosmolar solution (175.32 mg αKG plus 213.72 mg MAL and 15 ml Aqua bidest). The pH was adjusted to 7.35 with NaOH. Afterward, the solution was filtered through a bacteria-tight filter (Minisart 0.2 μm; Sartorius, Göttingen, Germany) and infused with a syringe pump into the V. femoralis at a rate of 5 ml·kg−1·h−1 according to the following protocol: 0.9% NaCl solution for 30 min and 60 min of αKG/MAL infusion before occlusion of the renal arteries, 0.9% NaCl solution during the ischemic period (40 min), αKG/MAL infusion during 45 min of reperfusion, and 0.9% NaCl solution until harvest of kidneys (Fig. 1). An ischemic control group and a normoxic control group of rats undergoing all surgical procedures except clamping of the renal arteries received only 0.9% NaCl solution during the experimental period. The following experimental groups were compared: group 1, normoxic control group, sham operation, 0.9% NaCl; group 2, I/R control group, 40-min renal clamping, 0.9% NaCl; and group 3, I/R αKG/MAL group, 40-min renal clamping, αKG/MAL 0.0095 mmol·kg−1·min−1 each, cumulative dose in each case 1 mmol/kg (∼146 mg/kg αKG and ∼134 mg/kg MAL).
Fig. 1.
Experimental setup. All infusions were administered at 5 ml·kg−1·h−1. The dose of α-ketoglutarate+malate was 0.0095 mmol·kg−1·min−1. In the normoxic and ischemia-reperfuson (I/R) control group exclusively, 0.9% NaCl solution was infused. Kidney ischemia was induced by atraumatic clamping of both renal vascular pedicles. B, blood sampling. Continous monitoring of blood pressure, heart rate, respiratory rate, core body temperature, and oxygen saturation was performed during the whole experiment.
The cumulative dose of αKG/MAL administered was derived from the optimal concentrations used in experiments with isolated proximal tubules (10, 35, 36) considering the body weight of the rats, distribution within the animal, and duration of the experiment.
Biomonitoring
Parameters of biomonitoring were assessed every 10 min starting with the beginning of the surgical procedure (Fig. 1). Systolic, diastolic, and mean arterial blood pressure (MAP) were continuously recorded via the femoral artery catheter that was connected to a pressure transducer (MX 960; Medex Medical, Rossendale, UK). An infusion bag containing Ringer solution delivered 3 ml/h to keep the catheter functional. At a systolic arterial blood pressure below 70 mmHg for >5 min, bolus injections of 0.5 ml 0.9% NaCl solution were administered repetitively through the femoral artery catheter up to a maximum volume of 5 ml·kg−1·h−1. Rat heart rates were determined from systolic blood pressure spikes. The core body temperature of the rats was continuously monitored using a rectal sensor and maintained at 37.5 ± 0.2°C by means of an underlying thermostat operating table and by coverage with aluminum foil. The oxygen saturation was recorded continuously using a pulse oximeter (OxiCliq A; Nellcor, Boulder, CO) placed at the catheter-free left hindlimb. The breathing rate was determined based on ventilation movements in 10-min intervals.
Assessment of Blood and Plasma Parameters
Using a 2-ml syringe containing 80 IU electrolyte-balanced heparin, blood samples (0.5 ml) were taken from the femoral artery catheter immediately after its insertion, after 30 min, immediately before ischemia, 5 min after beginning of ischemia, after 40 min of ischemia, 5 min after beginning of reperfusion, and after 240 min of reperfusion (Fig. 1). For each blood sample, animals were substituted with 0.5 ml 0.9% NaCl solution via the femoral artery. For determination of arterial Po2 and Pco2, oxygen saturation, pH, acid-base status, hemoglobin concentration and hematocrit, electrolytes (Na+, K+, Cl−, Ca2+), metabolic parameters (lactate, glucose), and osmolality, a blood-gas analyzer equipped with additional electrodes was used (ABL 715; Radiometer, Copenhagen, Denmark).
Blood plasma was obtained by centrifugation (3,000 g for 15 min at 25°C) and stored at 4°C until use (within 4 h). The plasma activity of lactate dehydrogenase (LDH) served as a general indicator of cell injury, creatine kinase (CK) was determined to assess the influence of muscle cell injury due to the operative procedures, while plasma creatinine (pCrea) was measured as an indicator for AKI. Plasma levels were determined spectrophotometrically by a fully automated clinical chemistry analyzer (Vitalab Selectra E; VWR International, Darmstadt, Germany).
Histopathological Evaluation of I/R Injury of the Kidney
For histological examination, the left blood-free and the right blood-containing kidney were sliced in half and fixed in formalin (10%, neutral buffered) for 24–48 h. Afterward, they were embedded in paraffin and cut on a rotary microtome in serial sections of 2-μm thickness. Tissue sections were mounted on slides and stained with hematoxylin-eosin. Histopathological changes were evaluated in a blinded fashion based on the following criteria: 1) blood content in the inner medulla, outer medulla, and cortex; 2) detachment of tubular basal membranes in the inner medulla, outer medulla, and cortex; and 3) loss of brush border.
Immunohistohemical Staining of KIM-1 and NGAL
KIM-1 is a sensitive and specific marker of proximal tubular injury (3) in humans and rodents that has been reported to be elevated in urine as early as within 3 h after renal I/R in rats (32). NGAL is a biomarker that can be detected in plasma and urine. It is highly expressed in kidney tubules and becomes detectable in urine within 3 h after renal ischemia in rats (26). For both KIM-1 and NGAL staining, sections (2 μm) of the left kidney were deparaffinated, permeabilized with 0.25% Triton X-100 in DPBS and washed with Tris-buffered saline (50 mM Tris·HCl, 150 mM NaCl, pH 7.4–7.6).
KIM-1.
To block endogenous biotin, sections were incubated with avidin- and biotin-blocking solutions (Sigma-Aldrich). Nonspecific hydrophobic interactions between the antibodies and the tissue were diminished with 5% normal donkey serum (Jackson ImmunoResearch, Suffolk, UK) in DPBS (incubation for 60 min at room temperature). Sections were then incubated with the primary antibody overnight at 4°C (AF3689 polyclonal goat antibody, R&D Systems, Wiesbaden-Nordenstadt, Germany). To quench endogenous peroxidase activity, samples were incubated with 3% H2O2 (AppliChem) in DPBS for 15 min and washed with DPBS thereafter. Sections were then incubated with biotinylated donkey anti-goat antibody (Jackson ImmunoResearch) for 30 min at room temperature (1:250 dilution in 5% normal donkey serum). Afterward, samples were incubated with streptavidin-horseradish peroxidase-conjugate (diluted 1:250 in DPBS) for 20 min at room temperature, washed with DPBS, and finally incubated with diaminobenzidine chromogen substrate solution (Zytomed Systems, Berlin, Germany) for 2 min.
NGAL (lipocalin 2).
To reduce nonspecific hydrophobic interactions between the antibodies and the tissue, sections were blocked in a solution containing 5% normal goat serum (Jackson ImmunoResearch), 1% bovine serum albumin Fraction V in DPBS, and 0.025% Tween 20 (at room temperature for 60 min). Sections were then incubated with the primary antibody overnight at 4°C (AB41105 polyclonal rabbit antibody, Abcam, Cambridge, UK). To quench endogenous peroxidase activity, samples were incubated in 3% H2O2 in Tris-buffered saline for 15 min and washed in DPBS. Slides were then incubated with streptavidin-horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch) for 30 min at room temperature (1:250 dilution in blocking solution). After washing with DPBS, sections were incubated with diaminobenzidine chromogen solution for 8–10 min.
Subsequent to the staining of KIM-1 or NGAL, sections were thoroughly rinsed with Aqua bidest, counterstained with hematoxylin for 2 min, dehydrated, and finally mounted with Eukitt Mounting medium (Fluka Analytics, Darmstadt, Germany). Staining was analyzed densitometrically using a Zeiss microscope and AxioVision software (Carl Zeiss MicroImaging, Göttingen, Germany). The KIM-1- or NGAL-positive area was evaluated in percentage per field of vision. Average values per section were calculated from two representative fields of vision of the border of the cortex and medulla and from another two within the medulla.
Determination of Tissue ATP and NEFA
The poles of both kidneys were cut off, stored in safe-lock tubes (2 ml) containing 1 M perchloric acid in an extracellular buffer for deproteinization (10), mixed vigorously, and immediately frozen in liquid nitrogen. Samples were kept frozen at −80°C. Tissue ATP concentration was measured using a luciferase-driven bioluminescence assay (ATP Bioluminescence Assay Kit CLS II, Roche, Mannheim, Germany). After thawing, samples were diluted in buffer containing 100 mM Tris and 4 mM EDTA (pH 7.75) and mixed immediately with luciferase reagent. Light emission was detected at 550 nm by a luminometer (Berthold Detection Systems, Pforzheim, Germany).
The tissue protein content was determined according to Lowry (23).
To determine the tissue concentration of NEFA, the cortex of the half right kidney was cut off, stored in 15-ml polypropylene tubes containing an extracellular buffer. and immediately frozen in liquid nitrogen. Samples were kept frozen at −20°C until NEFA concentration was detected as described previously (10). The tissue was homogenized using a glass homogenizer. The tissue homogenate (3 ml) was mixed with an ice-cold solution of 7.5 ml methanol and 3.75 ml chloroform by vortexing. After 15 min including several additional mixing steps, a further 3.75 ml of ice-cold chloroform were added, vigorously mixed, followed by addition of 3.75 ml ice-cold 2 M KCl. The final suspension was centrifuged (1,000 g, 4°C for 10 min; Hettich Rotina 3512, Hettich, Tuttlingen, Germany) to separate two layers. The top layer was discarded, while the bottom chloroform layer containing the extracted lipids was carefully removed, dried down under N2, and stored at −20°C until assayed. The NEFA concentration of the kidney cortex was determined using a quantitative colorimetric assay [NEFA-HR (2), Wako Chemicals, Neuss, Germany (10)].
Statistics
Experiments were performed with six animals/experimental group. Biochemical assays were run in duplicate unless stated otherwise. Data are expressed as means ± SE. Comparisons among multiple groups were performed using ANOVA either for nonrecurring or for repeated measures (analysis over time) followed by Tukey's or Games-Howell's post hoc analysis. A P value <0.05 was considered significant.
RESULTS
Effects of αKG/MAL on Blood Pressure and Other Vital Parameters
Baseline values of MAP of all animals were ∼80 mmHg at the start of the experimental procedure (Fig. 2). In the normoxic control group, MAP slightly increased following intra-abdominal manipulation for sham renal clamping, but otherwise remained stable during the entire experiment. In the I/R control group, blood pressure increased upon renal clamping and subsequently decreased, but these alterations during kidney ischemia were not significantly different from MAP in the normoxic control group. During early reperfusion, MAP was temporarily lower compared with the normoxic control group, but the values of both groups approached in the course of the experiment. Compared with the I/R control group the infusion of αKG/MAL had no effect on the preischemic MAP, but led to a strong decrease in blood pressure to 50.5 ± 4.3 mmHg during the ischemic period. Upon reperfusion, values gradually normalized to those of the normoxic control group, but tended to be lower than in the I/R control group during early reperfusion (30 min). In line with the adverse effects of intravenous αKG/MAL on MAP during kidney ischemia, animals of the I/R αKG/MAL group received significantly more bolus injections of 0.9% NaCl solution during both ischemia and early reperfusion (6.7 ± 1.4 ml) than rats of the normoxic and I/R control groups (1.7 ± 0.8 ml and 2.7 ± 1.3 ml).
Fig. 2.
Effect of αKG/MAL on the mean arterial blood pressure (MAP) during kidney I/R in rats. Anesthetized rats were subjected to bilateral kidney ischemia (I; 40 min) and subsequent reperfusion (R; 240 min). During the periods indicated (gray areas), αKG/MAL was infused at 0.0095 mmol·kg−1·min−1 each. Normoxic and I/R control group animals received only 0.9% NaCl solution (5 ml·kg−1·h−1). Values are means ± SE of 6 rats/group. For experimental details see Fig. 1. *P < 0.05 vs. normoxic control group. **P < 0.05 vs. I/R control group.
The heart rate of normoxic and I/R control animals remained constant at ∼286 beats/min during the whole experiment. The infusion of αKG/MAL resulted in a significant decrease in heart rate during early ischemia (I/R control group: 280 ± 10 beats/min; I/R αKG/MAL group: 244 ± 10 beats/min). The respiratory rate, peripheral oxygen saturation as assessed by pulse oximetry, and the core body temperature were not significantly changed by kidney I/R in the absence or presence of αKG/MAL.
Effects of αKG/MAL on Blood pH, Pco2, Base Excess, Electrolytes, Osmolality, and Metabolic Parameters
In animals of the normoxic control group, all parameters remained fairly stable during the course of the experiment (Table 1). Kidney I/R resulted in a decreased base excess, increased K+ levels, and a lower blood glucose concentration. Blood pH was lowered after I/R but did not reach significance (P = 0.3; Table 1). The other electrolytes, Pco2, osmolality, and blood lactate concentration did not change significantly during kidney I/R. The infusion of αKG/MAL neither affected the preischemic values of all parameters (data not shown) nor did it change the values related to kidney I/R as obtained in the I/R control group (Table 1).
Table 1.
Effects of αKG/MAL on blood pH, base excess, Pco2, electrolytes, osmolality, and metabolic parameters after kidney ischemia-reperfusion in rats
| Normoxic Control Group |
I/R Control Group |
I/R αKG/MAL Group |
||
|---|---|---|---|---|
| Baseline Values | End values | |||
| pH | 7.30 ± 0.02 | 7.30 ± 0.06 | 7.17 ± 0.04 | 7.24 ± 0.03 |
| Base excess, mmol/l | 2.8 ± 0.5 | −0.9 ± 0.3 | −5.6 ± 0.8* | −4.3 ± 0.3* |
| Pco2, mmHg | 60.1 ± 2.4 | 56.8 ± 12.5 | 64.3 ± 6.8 | 54.7 ± 5.2 |
| K+, mmol/l | 5.02 ± 0.14 | 4.65 ± 0.41 | 6.85 ± 0.22* | 6.50 ± 0.17* |
| CI−, mmol/l | 108.8 ± 1.8 | 113.3 ± 1.5 | 115.7 ± 1.7 | 114.0 ± 0.7 |
| Na+, mmol/l | 138.0 ± 0.8 | 138.5 ± 0.9 | 139.7 ± 1.0 | 142.0 ± 0.0* |
| Ca2+, mmol/l | 1.40 ± 0.03 | 1.36 ± 0.03 | 1.37 ± 0.02 | 1.33 ± 0.03 |
| Osmolality, mmol/kgH2O | 285.1 ± 1.9 | 285.3 ± 1.9 | 285.0 ± 2.0 | 289.2 ± 0.5 |
| Glucose, mg/dl | 174.0 ± 3.4 | 148.8 ± 7.86 | 100.3 ± 11.2* | 101.5 ± 10.0 |
| Lactate, mmol/l | 0.67 ± 0.12 | 0.87 ± 0.87 | 1.05 ± 0.12 | 1.12 ± 0.1* |
Values are means ± SE; n = 6/group.
I/R, ischemia-reperfusion; αKG/MAL, α-ketoglutarate+malate.
Baseline values were determined from blood from the normoxic control group obtained from the first blood sampling; these values were not significantly different from the baseline values of the other experimental groups. The other values were assessed from the last blood sampling. For experimental details, see Fig. 1.
P < 0.05 vs. normoxic control group at the final blood sampling.
Effects of αKG/MAL on Markers of Organ Injury, Kidney ATP Content, and Accumulation of NEFAs
In the normoxic control group, the plasma LDH concentration increased only slightly during the experiment (Table 2). CK levels were elevated immediately after the (preischemic) surgical procedures and did not further increase significantly. The pCrea concentration remained stable (Table 2).
Table 2.
Effects of αKG/MAL on markers of organ injury and accumulation of NEFAs
| Baseline Values | Normoxic Control Group | I/R Control Group | I/R αKG/MAL Group | |
|---|---|---|---|---|
| LDH, U/l | 111.6 ± 23.7 | 275.8 ± 73.2 | 808.1 ± 112.6* | 2,239.0 ± 512.0* |
| CK, U/l | 268.3 ± 65.4 | |||
| After 30 min | 538.3 ± 61.0 | 481.7 ± 75.6 | 525.0 ± 61.2 | |
| Postischemia | 357.5 ± 38.6 | 393.3 ± 91.4 | 484.3 ± 77.3 | |
| End | 703.0 ± 336.6 | 572.3 ± 94.5 | 1,248.8 ± 278.2† | |
| pCrea, mg/dl | 0.56 ± 0.05 | |||
| Preischemia | 0.48 ± 0.08 | 0.62 ± 0.05 | 0.55 ± 0.04 | |
| 5-min Ischemia | 0.62 ± 0.05 | 0.55 ± 0.08 | 0.64 ± 0.05 | |
| Postischemia | 0.49 ± 0.08 | 0.74 ± 0.03* | 0.82 ± 0.03* | |
| 5-min Reperfusion | 0.59 ± 0.03 | 0.85 ± 0.05* | 0.85 ± 0.03* | |
| End | 0.70 ± 0.12 | 1.47 ± 0.05* | 1.43 ± 0.02* | |
| ATP, nmol/mg protein | 11.1 ± 1.4 | 6.7 ± 0.6* | 4.7 ± 0.7* | |
| NEFA, nmol/mg protein | 5.9 ± 0.5 | 7.0 ± 0.9 | 7.5 ± 0.7 |
Values are means ± SE; n = 6/group. Baseline values were determined from blood from the normoxic control group obtained from the first blood sampling; these values were not significantly different from the baseline values of the other experimental groups. The other values were assessed from the last blood sampling or at the time points indicated. For experimental details see Fig. 1.
LDH, lactate dehydrogenase; CK, creatine kinase; pCrea, plasma creatinine; NEFA, nonesterified fatty acids.
P < 0.05 vs. normoxic control group.
P < 0.05 vs. I/R control group.
Kidney I/R had no affect on CK levels but resulted in a strong increase in plasma LDH activity and pCrea concentration (Table 2). Kidney ATP levels were significantly decreased after I/R compared with the normoxic control group (Table 2); the NEFA content of the kidney determined after 4-h reperfusion was not altered (Table 2).
The infusion of αKG/MAL had no effect on the preischemic values of all the parameters (data not shown) but tended to increase the plasma LDH level (P = 0.1, not significant vs. I/R control group) and strongly elevated the activity of CK at the end of the reperfusion phase (Table 2). Intravenous αKG/MAL had no effect on I/R-induced alterations in pCrea (Table 2) and kidney ATP content (Table 2). Kidney NEFA levels in the I/R αKG/MAL group were not different from those in the normoxic and I/R control group (Table 2).
Effects of αKG/MAL on Histopathological Changes and Immunohistochemical Stainings of the Kidney
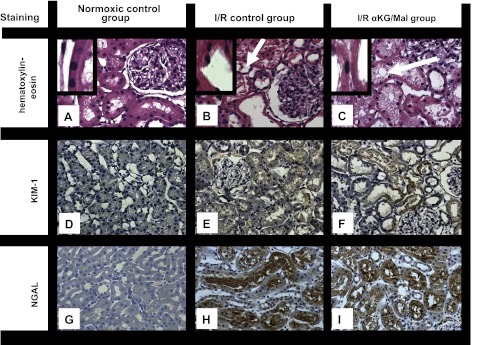
Light microscopy (hematoxylin-eosin staining) revealed normal kidney histology in the normoxic control group (Fig. 3A). In kidneys exposed to I/R (Fig. 3B), a loss of the brush border and a general dilatation or collapse of the tubular lumen became apparent with a trend to be more concentrated in the outer medulla. There were no differences in the histological architecture of kidneys from the I/R control group and the I/R αKG/MAL group (Fig. 3, B and C).
Fig. 3.
Effect of αKG/MAL on kidney histopathology (hematoxylin-eosin staining, A–C), immunohistochemical staining of kidney injury molecule-1 (KIM-1; D–F), and neutrophil gelatinase-associated lipocalin (NGAL; G–I) following kidney I/R. Anesthetized rats were subjected to bilateral kidney ischemia (I; 40 min) and subsequent reperfusion (R; 240 min). αKG/MAL was infused at 0.0095 mmol·kg−1·min−1 each. Normoxic and I/R control group animals received only 0.9% NaCl solution (5 ml·kg−1·h−1). Representative pictures at 400-fold magnification are shown. A, D, and G: normoxic control group. B, E, and H: I/R control group. C, F, and I: I/R αKG/MAL group. Stainings were performed at the end of the experimental procedure. Arrows in B and C indicate vacuolization. Insets: damage to brush border.
KIM-1 was hardly detectable by quantitative immunohistochemistry in kidneys of the normoxic control group (0.1 ± 0.1%/field of vision) (Fig. 3D). In the I/R control group, KIM-1 staining was significantly increased (9.4 ± 0.6%) (Fig. 3E). The infusion of αKG/MAL had no effect on KIM-1 staining (11.8 ± 3.6%) (Fig. 3F).
For NGAL, immunohistochemical staining was low in the normoxic control group (5.8 ± 1.3%) (Fig. 3G). It was severely increased in the I/R control group (28.0 ± 2.1%) (Fig. 3H). Significantly less NGAL was detectable in kidneys of the I/R αKG/MAL group (17.6 ± 3.5%) (Fig. 3I).
DISCUSSION
Intravenous infusion of αKG/MAL at a cumulative dose of 1 mmol/kg each (146 mg/kg αKG and 134 mg/kg MAL) in a rat model of AKI (40-min ischemia, 240-min reperfusion) did not provide protection from I/R-induced renal injury, but resulted in adverse effects primarily on MAP, heart rate, and plasma CK levels.
Protective effects of the citric acid cycle metabolites αKG/MAL against renal I/R injury and underlying mechanisms have only been reported for in vitro studies on isolated proximal tubules (8–10, 35, 36). Because of their enzymatic equipment restricting glycolysis (13), proximal renal tubules are almost exclusively dependent on mitochondrial function for energy production. The mechanism of action for salvage of isolated proximal tubules exposed to I/R can be explained by the support of two interacting pathways: substrate-level phosphorylation and anaerobic respiration in complexes I and II of the mitochondrial respiratory chain (36). During inadequate oxygen supply, αKG is metabolized to succinate via succinyl-coenzyme A. During this process, GTP is produced, which then transphosphorylates ADP to ATP. In addition, under hypoxic conditions malate is dehydrated to fumarate, which is reduced to succinate. The reduction of fumarate to succinate leads to oxidation of reduced ubiquinone via a flavine adenine dinucleotide-coupled process in mitochondrial complex II. The consequential re-reduction of ubiquinone forces proton extrusion by complex I, which stabilizes the mitochondrial membrane potential and offers energy for ATP generation by mitochondrial ATP synthase. In addition, NADH, which accumulates during the metabolism of αKG, is anaerobically oxidized to NAD+ and therefore encourages further substrate-level phosphorylation. Maintenance of an essentially low level of energy content and mitochondrial membrane potential prevents accumulation of NEFA, which is a major reason for mitochondrial damage and deficiency of tubular ATP production. Therefore, αKG/MAL, by maintaining energy content, enables isolated proximal tubules to recover from ischemia-induced injury. This is reflected by a significant amelioration of renal microscopic changes, mitochondrial membrane potential, and ATP levels (9, 35, 36).
In the present study, we tried to transfer the promising results of the previous in vitro studies to an in vivo rat model of AKI. Compared with the normoxic control group, injury was evident in all animals undergoing I/R. Derangement of the systemic acid-base equilibrium and K+ levels as well as an increased pCrea in both the I/R and I/R αKG/MAL group are consistent with impaired renal clearance function. Decreased tissue ATP levels could reflect impairment of intrinsic cellular capacity for ATP production, compromised reperfusion, or both. In our study, there was no significant difference in renal NEFA content after I/R measured at the end of 240 min of reperfusion. Increases in NEFA during both ischemia in vivo and hypoxia of isolated tubules are well documented (9, 24, 38). NEFA levels decreased during 60 min of reoxygenation in vitro (9) and 120 min of reperfusion in vivo (24), but remained well above normal and were shown to impair mitochondrial function recovery in vitro. The present results at 240 min of reperfusion indicate further recovery of NEFA at that point, at least as averaged over the entire cortex, which might also be influenced by washout during continued reperfusion or binding of NEFA by blood albumin.
As indicated by all vital parameters and those of injury, except for renal NGAL staining, rats exposed to I/R and receiving a cumulative dose of 1 mmol/kg αKG/MAL were not protected, and for some parameters were even more severely affected compared with those of the I/R control group receiving a 0.9% NaCl solution. In particular, animals receiving αKG/MAL had decreases in MAP during clamping of the renal vascular pedicle, which was also accompanied by a reduction in heart rate (Fig. 2).
NGAL is a product of multiple sources. Systemic events accompanying AKI can also trigger expression of NGAL in organs other than the kidney, including the liver and lung (7, 12). While kidney NGAL originates from the distal tubules, systemic NGAL is reabsorbed and possibly degraded in proximal tubular cells (27). Additional contributions to the systemic pool during AKI may derive from the fact that NGAL is an acute-phase reactant in rodents and may be released from neutrophils or epithelial cells during inflammation (19). Assessment of αKG/MAL-induced changes in other organs leading to decreased immunohistochemical staining of NGAL in the kidney were not included in our model of AKI. The αKG/MAL-induced decrease in renal NGAL staining in contrast to the other markers of renal damage (pCrea and KIM-1) could possibly be caused by extrarenal inhibition of NGAL production or by anti-inflammatory effects of αKG/MAL.
In a rat model testing oral application of αKG with interest in its antidotal behavior in cyanide poisoning, significant decreases in MAP accompanied by a trend toward bradycardia were examined in animals receiving 4 g αKG/kg (∼27 mmol/kg), but not in animals receiving half the dose (2). An absorption rate of 70% for enteral administration of αKG in rats (11) taken into account, the lower dose (2 g/kg) was still ∼10 times the dose in our experiments. It appears possible that the stress induced by kidney I/R increases the vulnerability to cardiovascular-depressive effects that rarely appear in otherwise healthy animals. The infusion of αKG/MAL had no effect on the preischemic MAP in our experiment, but the absence of the sharp increase in MAP following abdominal manipulation in the I/R αKG/MAL group emphasizes the increased susceptibility for cardiovascular-depressive effects directly following preischemic αKG/MAL infusion.
Kjellman et al. (17, 18) performed human studies using high concentrations of αKG added to blood cardioplegic solution during coronary surgery. αKG provided myocardial protection indicated by the reduced appearance of ischemic markers such as CK-MB and troponin T, possibly secondary to enhanced myocardial oxidative capacity. Adverse effects on blood pressure following the procedure were not detected (17). However, transferability to our study is limited, because administration of αKG was accompanied by the use of a heart-lung machine, which conceals direct effects on circulatory parameters. In another study, infusion of 20 g αKG over 40 min in a low-risk cardiopulmonary bypass population increased renal blood flow significantly, but within the range of renal autoregulation (14). Increased renal blood flow did not lead to an increased glomerular filtration rate, because of its association with a decreased filtration fraction. Therefore, a positive appraisal of αKG could not be concluded.
Although the major decrease in blood pressure in our study occurred during ischemia when the kidneys were not perfused, pressure tended to recover more slowly during early reperfusion, which might be crucial for the outcome in this type of model (34). Thus the cardiovascular-depressive effect of αKG/MAL may have prolonged and aggravated the effect of the initial renal damage. The higher CK levels detected in the I/R αKG/MAL group suggest a generally more ischemic situation in the whole rat due to decreased blood pressure. A possible explanation for the absence of transferability of protective effects detected in isolated tubules to in vivo conditions may be that the cardiovascular-depressive effects dominate over preservation of mitochondrial function. Furthermore, the addition of αKG/MAL might increase filtration work through the basolateral membrane into the cells of the proximal tubules, which is ATP dependent and characterized by high affinity and low capacity (5). Therefore, by increasing the workload, αKG/MAL supplementation would result in an energy-consuming strain in times of low oxygen supply. This increased cellular effort of substrate uptake cannot be assessed from our earlier work with isolated cells. Multiple citric acid cycle metabolites can restore metabolic function in isolated tubules (36), including citrate, which is commonly used in the intensive care unit setting for anticoagulation during continuous renal replacement therapy and circulates at high levels (31). αKG/MAL was used for the current studies because of its particularly favorable effect profile in the isolated tubules (36), but clearly this was not depicted in vivo due to its systemic effects. Whether other substrates or substrate combinations would be more favorable is unknown.
In conclusion, supplementation with αKG/MAL, which is very protective against I/R injury in isolated tubules, did not protect the kidney from I/R injury in our rat model, possibly due to adverse effects on cardiovascular function. Nevertheless, our rat model of renal I/R injury accompanied by extensive biomonitoring has proven to be very useful for assessment of protective methods against renal damage in vivo and offers valuable possibilities for studying the critical early phases of renal I/R injury that are often incompletely assessed.
GRANTS
This study was supported by a Junior Excellence Research Group grant funded by the Dr. Werner Jackstädt-Foundation (T. Feldkamp). A. Bienholz received a research grant from the Dr. Werner Jackstädt-Foundation. J. M. Weinberg was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-34275.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.B., J.M.W., and T.F. provided conception and design of research; A.B. and T.F. analyzed data; A.B., J.M.W., and T.F. interpreted results of experiments; A.B. prepared figures; A.B. drafted manuscript; A.B., F.P., J.M.W., O.W., A.K., H.d.G., and T.F. edited and revised manuscript; A.B., F.P., P.W., P.I., J.M.W., O.W., A.K., H.d.G., and T.F. approved final version of manuscript; P.W. and P.I. performed experiments.
ACKNOWLEDGMENTS
We thank Tanja Hinkeldein for excellent technical assistance. We also thank Anika Weber for establishing and modifying renal NGAL and KIM-1 stainings.
REFERENCES
- 1. Bhandari S, Turney J. Survivors of acute renal failure who do not recover renal function. QJM 89: 415–421, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Bhattacharya R, Kumar D, Sugendran K, Pant S, Tulsawani R, Vijayaraghavan R. Acute toxicity studies of α-ketoglutarate: a promising antidote for cyanide poisoning. J Appl Toxicol 21: 495–499, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bonventre J. Kidney injury molecule-1 (KIM-1): a specific and sensitive biomarker of kidney injury. Scand J Clin Lab Invest 68: 78–83, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Chertow G, Burdick E, Honour M, Bonventre J, Bates D. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Edwards R, Stack E, Trizna W. α-Ketoglutarate transport in rat renal brush-border and basolateral membrane vesicles. J Pharmacol Exp Ther 281: 1059–1064, 1997 [PubMed] [Google Scholar]
- 6. Esson M, Schrier R. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med 137: 744–752, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Feldkamp T, Bienholz A, Kribben A. Urinary neutrophil gelatinase-associated lipocalin (NGAL) for the detection of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant 26: 1717–1723, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Feldkamp T, Kribben A, Roeser N, Senter R, Kemner S, Venkatachalam M, Nissim I, Weinberg J. Preservation of complex I function during hypoxia-reoxygenation-induced mitochondrial injury in proximal tubules. Am J Physiol Renal Physiol 286: F749–F759, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Feldkamp T, Kribben A, Roeser N, Senter R, Weinberg J. Accumulation of nonesterified fatty acids causes the sustained energetic deficit in kidney proximal tubules after hypoxia-reoxygenation. Am J Physiol Renal Physiol 290: F465–F477, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Feldkamp T, Weinberg J, Hörbelt M, Kropff CV, Witzke O, Nürnberger J, Kribben A. Evidence for involvement of nonesterified fatty acid-induced protonophoric uncoupling during mitochondrial dysfunction caused by hypoxia and reoxygenation. Nephrol Dial Transplant 24: 43–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Filip R, Pierzynowski S. The absorption, tissue distribution and excretion of enteraly administered α-ketoglutarate in rats. J Anim Physiol Anim Nutr 92: 182–189, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Grigoryev DL, M, Hassoun H, Cheadle C, Barnes K, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol 19: 547–558, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guder W, Ross B. Enzyme distribution along the nephron. Kidney Int 26: 101–111, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Jeppsson A, Ekroth R, Friberg P, Kirnö K, Milocco I, Nilsson F, Svensson S, Wernerman J. Renal effects of α-ketoglutarate early after coronary operations. Ann Thorac Surg 65: 684–690, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Joannidis M, Druml W, Forni L, Groeneveld A, Honore P, Straaten Hv Ronco C, Schetz M, Woittiez A. Prevention of acute kidney injury and protection of renal function in the intensive care unit: expert opinion of the working group nephrology, ESICM. Intensive Care Med 36: 392–411, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Kelly K, Molitoris B. Acute renal failure in the new millenium: time to consider combination therapy. Semin Nephrol 20: 4–19, 2000 [PubMed] [Google Scholar]
- 17. Kjellman U, Björk K, Ekroth R, Karlsson H, Jagenburg R, Nilsson F, Svensson G, Wernerman J. Addition of alpha-ketoglutarate to blood cardioplegia improves cardioprotection. Ann Thorac Surg 63: 1625–1633, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Kjellman U, Björk K, Ekroth R, Karlsson H, Jagenburg R, Nilsson F, Svensson G, Wernerman J. Alpha-ketoglutarate for myocardial protection in heart surgery. Lancet 345: 552–553, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Klausen P, Niemann C, Cowland J, Krabbe K, Borregaard N. On mouse and man: neutrophil gelatinase associated lipocalin is not involved in apoptosis or acute response. Euro J Haematol 75: 332–340, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kribben A, Herget-Rosenthal S, Pietruck F, Philipp T. Acute renal failure—an review. Dtsch Med Wochenschr 2003 128: 1231–1236, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Lameire N, Biesen Wv, Vanholder R. Acute renal failure. Lancet 365: 417–430, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Lassnigg A, Schmid E, Hiesmayr M, Falk C, Druml W, Bauer P, Schmidlin D. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med 36: 1129–1137, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 24. Matthys E, Patel Y, Kreisberg J, Stewart J, Venkatachalam M. Lipid alterations induced by renal ischemia: pathogenic factor in membrane damage. Kidney Int 26: 153–161, 1984 [DOI] [PubMed] [Google Scholar]
- 25. Mehta R, McDonald B, Gabbai F, Pahl M, Pascual M, Farkas A, Kaplan R. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int 60: 1154–1163, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Paragas N, Qiu A, Zhang Q, Samstein B, Deng S, Schmidt-Ott K, Viltard M, Yu W, Forster C, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner A, Devarajan P, Landry D, D'Agati V, Lin C, Barasch J. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 17: 216–222, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peeters P, Terryn W, Vanholder R, Lameire N. Delayed graft function in renal transplantation. Curr Opin Crit Care 10: 489–498, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Petrat F, de Groot H. Protection against severe intestinal ischemia/reperfusion injury in rats by intravenous resveratrol. J Surg Res 167: e145–e155, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Petrat F, Rönn T, de Groot H. Protection by pyruvate infusion in a rat model of severe intestinal ischemia-reperfusion injury. J Surg Res 167: e93–e101, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Saner F, Treckmann J, Geis A, Lösch C, Witzke O, Canbay A, Herget-Rosenthal S, Kribben A, Paul A, Feldkamp T. Efficacy and safety of regional citrate anticoagulation in liver transplant patients requiring post-operative renal replacement therapy. Nephrol Dial Transplant 27: 1651–1657, 2012 [DOI] [PubMed] [Google Scholar]
- 32. Vaiday V, Bonventre J. Mechanistic biomarkers for cytotoxic acute kidney injury. Expert Opin Drug Metab Toxicol 2: 697–713, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Weinberg J, Roeser N, Davis J, Venkatachalam M. Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int 52: 140–151, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Weinberg J, Venkatachalam M. Preserving postischemic reperfusion in the kidney: a role for extracellular adenosine. J Clin Invest 122: 493–496, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weinberg J, Venkatachalam M, Roeser N, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci USA 97: 2826–2831, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weinberg J, Venkatachalam M, Roeser N, Saikumar P, Dong Z, Senter R, Nissim I. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Renal Physiol 279: F927–F943, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wirthensohn G, Guder W. Metabolism of isolated kidney tubule segments. Methods Enzymol 191: 325–340, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Zager R, Gmur D, Bredl C, Eng M. Temperature effects on ischemic and hypoxic renal proximal tubular injury. Lab Invest 64: 766–776, 1991 [PubMed] [Google Scholar]