Abstract
We recently reported that necrotic renal proximal tubular cells (RPTC) can induce the death of renal interstitial fibroblasts. Since autophagy plays either cytoprotective or cytodestructive roles depending on the experimental condition, the present study was carried out to investigate whether necrotic RPTC would induce autophagy of renal interstitial fibroblasts and, if so, whether autophagy would contribute to cell death or exert a protective effect. Exposure of necrotic RPTC supernatant (RPTC-Sup) induced autophagy in renal interstitial fibroblast cells (NRK-49F) in a time- and dose-dependent manner, and its induction was earlier than caspase-3 activation. Inhibition of autophagy with 3-methyladenine (3-MA) or knockdown of Beclin-1, a molecule involved in the initiation of autophagosome formation, with small interference RNA (siRNA) significantly enhanced necrotic RPTC-Sup-induced cell death. Necrotic RPTC-Sup induced phosphorylation of extracellular signal-regulated kinases (ERK1/2), p38, c-Jun NH2-terminal kinases (JNKs), and AKT. Treatment with an ERK1/2 pathway inhibitor, but not with specific inhibitors for p38, JNKs, or AKT pathways, blocked NRK-49F autophagy and cell death upon exposure to necrotic RPTC-Sup. Furthermore, knockdown of MEK1 with siRNA also reduced autophagy along with cell death in NRK-49F exposed to necrotic RPTC-Sup. In contrast, overexpression of MEK1/2 increased RPTC-Sup-induced fibroblast cell death without enhancing autophagy. Collectively, this study demonstrates that necrotic RPTC induce both autophagy and cell death and that autophagy plays a cytoprotective or prosurvival role in renal fibroblasts. Furthermore, necrotic RPTC-induced autophagy and cell death in renal fibroblasts is mediated by the activation of the MEK1-ERK1/2 signaling pathway.
Keywords: necrosis, apoptosis, extracellular signal-regulated kinases, caspase-3
autophagy is a normal cellular process that is responsible for removal and recycling of bulk cytoplasmic constituents, misfolded proteins, and damaged intracellular organelles to maintain cellular homeostasis. Generally, cells exhibit a low basal rate of autophagy, but autophagy is upregulated in response to nutrient or growth factor deprivation in order to replenish amino acids and glucose for cellular function (3, 7, 23). Autophagy is also a self-adaptive process that protects cells during periods of profound cellular stress like prolonged starvation, hypoxia, endoplasmic reticulum stress, and pathogen infection. Typically, autophagy fulfills a prosurvival function, but this rescue function turns deleterious under certain circumstances (7, 9).
Autophagy is a tightly controlled multistep process. It is initiated by the formation of phagophore (also called the isolation membrane), which engulfs the targets in cytoplasm and then transforms into autophagosome. During autophagy, the formation of autophagosomes requires redistribution of LC3 from the cytosol to autophagic vesicles by conversion of cytosolic LC3 I to the phospholipid conjugate form, LC3 II. In addition, autophagy involves Beclin-1, a core component of initiation, and a ubiquitin-like system composed of autophagy-related gene (ATG) proteins, the Atg12-Atg5 conjugation system (7, 18). Expression of Atg12-Atg5 and conversion of LC3 I to LC3 II are widely used to monitor autophagy.
It is generally accepted that specific stimuli may trigger unique signaling pathways that are then integrated into the core regulatory mechanisms to induce autophagy. Among the numerous signaling pathways involved in this process, the mitogen-activated protein kinase (MAPK) family of protein kinases has been shown to play an important role at different checkpoints of the autophagy process. This family is composed of three pathways: extracellular signal-regulated kinase (ERK)1/2, c-Jun NH2-terminal kinase (JNK), and p38 (5, 8, 13, 16). ERK1/2 and p38 pathways are reported to be involved in maturation of autophagosome (8, 13), and JNK leads to autophagy through upregulation of Beclin-1 and LC3 expression (16, 22).
Autophagy has been implicated in various diseases, including urinary unilateral obstruction (UUO)-induced renal fibrosis (14), glomerular diseases (25), polycystic kidney disease (1), and acute kidney injury (AKI) (11, 12, 15, 27). During the early stage of AKI, damaged or injured cells release various cytokines and cellular contents into the intercellular environment, where they may induce death of interstitial cells like peritubular fibroblasts. This could be related to the reduced number of peritubular fibroblasts in the injured kidney, as the result of a variety of insults (i.e., ischemia, ureteric obstruction, and focal needle stick injury), mostly in the area adjacent to the damaged tubular epithelium (17). Using a necrotic renal proximal tubular cell (RPTC) and renal interstitial fibroblast coculture system, we have provided evidence that necrotic RPTC can directly induce death of renal interstitial fibroblasts through an ERK-dependent mechanism (20, 21). However, it remains unknown whether necrotic RPTC would cause autophagy of renal fibroblasts and, if so, whether it regulates death of this cell type. In this study, we addressed these issues.
MATERIALS AND METHODS
Chemicals and antibodies.
LY-294002 was obtained from Biomol (Plymouth Meeting, PA). U-0126, SB-203580, and SP-600125 were purchased from Calbiochem (San Diego, CA). The small interference RNAs (siRNAs) specific for rat P2X7, mitogen-activated ERK kinase 1 (MEK1), and Beclin-1 were purchased from Invitrogen (Carlsbad, CA). Antibodies to Atg12, P2X7, and GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All other antibodies used in this study were purchased from Cell Signaling Technology (Danvers, MA). α-Tubulin and all other chemicals were purchased from Sigma (St. Louis, MO).
Cell culture.
Rat renal interstitial fibroblasts (NRK-49F) and immortalized mouse RPTC were used in this study. Both of them were cultured in Dulbecco's modified Eagle's medium-nutrient F-12 (DMEM-F-12; Sigma-Aldrich, St. Louis, MO) containing 5% fetal bovine serum (FBS), penicillin, and streptomycin in an atmosphere of 5% CO2-95% air at 37°C. NRK-49F were confluent when used for various treatments. When necessary, various inhibitors were directly added to the culture and then incubated for the desired time as indicated in Figs. 2 and 4.
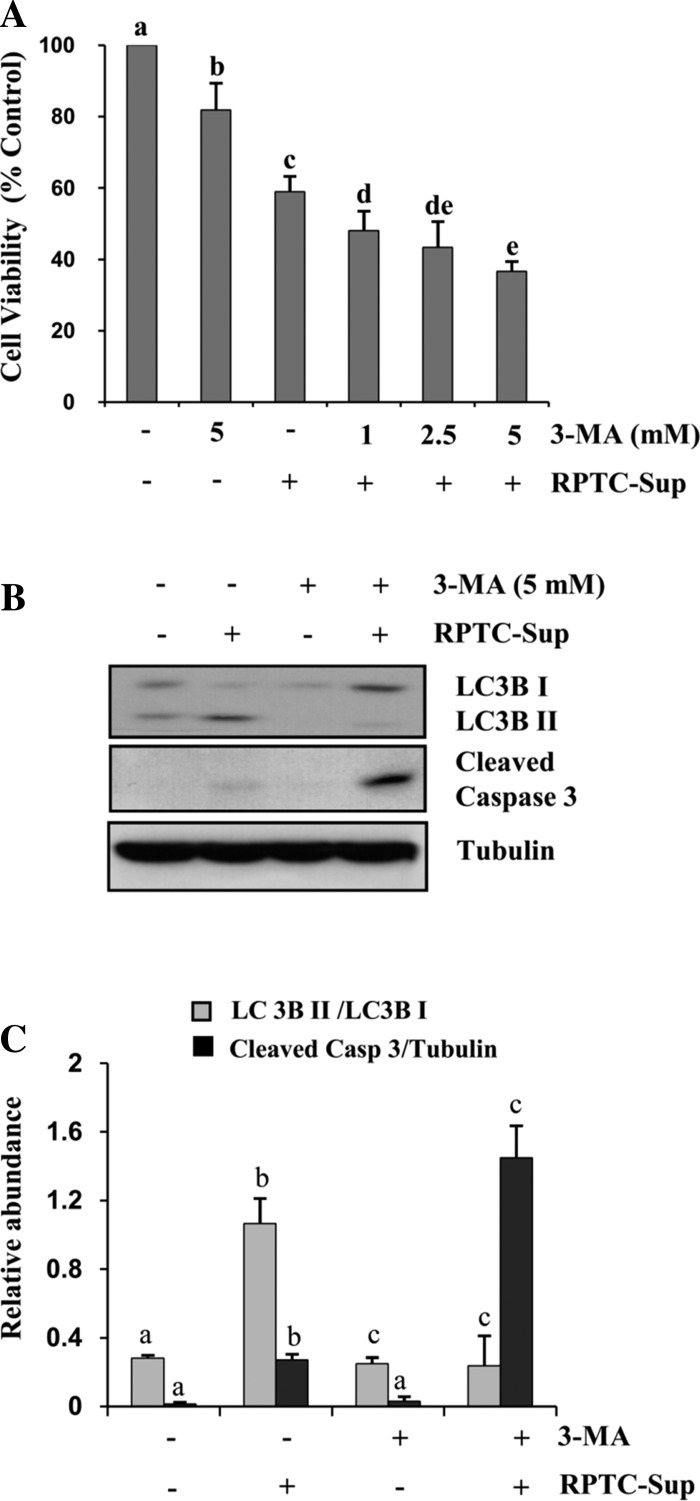
Fig. 2.
Effect of 3-methyladenine (3-MA) on necrotic RPTC-induced cell death in NRK-49F. A: NRK-49F were pretreated with different concentrations of 3-MA for 1 h and then incubated with necrotic RPTC-Sup for 24 h, and cell viability was determined by MTT assay. B: cells were pretreated with 5 mM of 3-MA for 1 h and then incubated with necrotic RPTC-Sup for 24 h. Cell lysates were prepared and subjected to immunoblot analysis with specific antibodies against LC3B I and II, cleaved caspase-3, or α-tubulin. Representative immunoblots from 3 experiments are shown. C: levels of LC3B I and II were quantified by densitometry analysis, and the ratio of LC3B II to LC3B I was calculated. The level of cleaved caspase-3 was quantified by densitometry and normalized with α-tubulin. Values are means ± SD of 3 independent experiments. Bars with different superscript letters are significantly different from one another (P < 0.01).
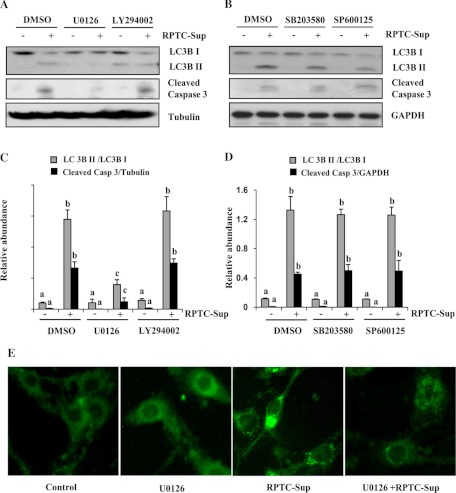
Fig. 4.
Effect of inhibition of ERK1/2, AKT, p38 and JNK on necrotic RPTC induced autophagy in renal fibroblasts. A–D: NRK-49F cells were pretreated with U-0126 (20 μM), LY-294002 (20 μM), SB-203580 (10 μM), or SP-600125 (10 μM) for 1 h and then incubated with necrotic RPTC-Sup for 24 h. A and B: cells were harvested, and cell lysates were subjected to immunoblot analysis with specific antibodies against LC3B I and II, cleaved caspase-3, GAPDH, or α-tubulin. Levels of LC3B I and II were quantified by densitometry analysis, and the ratio of LC3B II to LC3B I was calculated. C and D: level of cleaved caspase-3 was quantified by densitometry and normalized with α-tubulin or GAPDH. Data are represented as means ± SD. Bars with different superscript letters are significantly different from one another (P < 0.01). Representative immunoblots from 3 experiments are shown. E: NRK-49F cells grown in antibiotic-free medium were transfected with LC3B-GFP and then treated with RPTC-Sup for 24 h in the presence or absence of MEK1 inhibitor (U-0126). Cells were photographed with a fluorescent microscope.
Preparation of necrotic RPTC cell lysate and treatment.
RPTC were harvested and washed twice with sterile PBS and then reconstituted to a cell number of 2 × 106/ml in complete culture medium. These cells were immediately used for cell lysate preparation by repetitive (5 cycles) freezing at −80°C and thawing at 37°C. Cell lysate was then centrifuged at 15,000 rpm for 20 min to obtain supernatant (RPTC-Sup). For the experiments, one-third of the volume (330 μl per 1 ml) of medium was removed and 330 μl of RPTC-Sup was added to the culture. When we replaced 330 μl per 1 ml of medium with RPTC-Sup, the final concentration of cell lysate was equal to RPTC-Sup from 6.6 × 105 cells/ml. As a control, the same volume of complete medium (5 cycles of freeze and thaw) was added.
Transfection of siRNA into cells.
siRNA oligonucleotides targeted specifically to rat Beclin-1, MEK1, and P2X7 were used in this experiment. Cells were seeded to 60–70% confluence in antibiotic-free medium, and 24 h later siRNA (750 pmol) was transfected into NRK-49F with Lipofectamine 2000 (Invitrogen). In parallel, 750 pmol of scrambled siRNA was used to control for off-target changes in NRK-49F. After transfection, cells were cultured in antibiotic-free DMEM-F-12 for 24 h before being used for the experiments.
Transfection of green fluorescent protein-tagged LC3B.
NRK-49F cells were seeded to 50–60% confluence on glass coverslips placed inside a 35-mm dish and allowed to grow for 24 h in antibiotic-free medium. Green fluorescent protein-tagged LC3B (LC3B-GFP; from Dr. Zheng Dong, Georgia Health Science University, Augusta, GA) was transfected into NRK-49F by using Lipofectamine 2000 (Invitrogen). After transfection, cells were cultured in antibiotic-free DMEM-F-12 for at least 24 h before being used for the experiments. At the end of the experimental period, cells were washed twice with PBS and fixed with 4% paraformaldehyde for 20 min. Photographs of 10–15 random cortical fields (×400) from each sample were taken with a SPOT camera attached to a fluorescent microscope.
Transfection of adenoviral vector with MEK1.
NRK-49F cells were seeded to 60% confluence in complete medium on six-well culture plates. Infection of recombinant adenovirus containing constitutively active MEK1 (Ad-MEK1) was performed as described elsewhere. A recombinant adenovirus vector encoding β-galactosidase (Ad-LacZ) was used as a negative control. Briefly, cells were infected with Ad-LacZ or Ad-MEK1 at a multiplicity of infection (MOI) of 100 plaque-forming units for 24 h at 37°C in a humidified 5% CO2 incubator. Afterward, the culture medium was changed and allowed to grow for an additional 24 h. Cells were then treated with RPTC-Sup as described in Fig. 6.
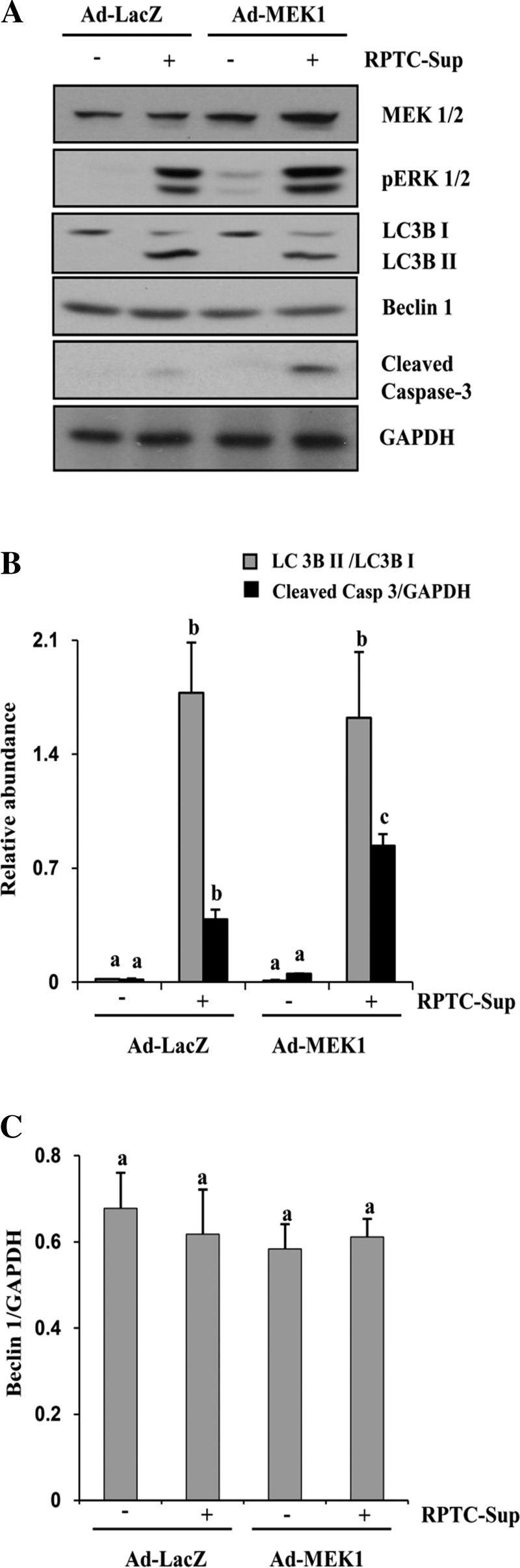
Fig. 6.
Effect of overexpression of wild-type MEK1 on autophagy and cell death induced by necrotic RPTC-Sup. NRK-49F cells were infected with Ad-LacZ or Ad-wild-type MEK1 as described in materials and methods and then treated with necrotic RPTC for 24 h. After treatment, cells were harvested and subjected to immunoblot analysis for MEK1, pERK1/2, LC3B I and II, Beclin-1, cleaved caspase-3, or GAPDH (A). The level of LC3B I and II were quantified by densitometry analysis, and the ratio of LC3B II to LC3B I was calculated. The levels of cleaved caspase-3 (B) and Beclin-1 (C) were quantified by densitometry and normalized with GAPDH. Data are represented as means ± SD. Bars with different superscript letters are significantly different from one another (P < 0.01). Representative immunoblots from 3 experiments are shown.
Determination of cell viability by MTT assay.
Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After treatment, MTT was added (final concentration 0.5 mg/ml) and incubated for 1 h. Tetrazolium formed in viable cells was released by the addition of DMSO, and optical density was determined with a spectrophotometer at 570 nm (Molecular Devices, Sunnyvale, CA).
Immunoblot analysis.
After various treatments, cells were washed once with ice-cold PBS and harvested in a cell lysis buffer. Proteins (20 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with 5% skim milk for 1 h at room temperature, membranes were incubated with a primary antibody for overnight at 4°C and then incubated with appropriate horseradish peroxidase-conjugated secondary antibody for 1 h in room temperature. Bound antibodies were visualized by chemiluminescence detection.
Densitometry.
The quantitative analysis of different proteins was carried out with ImageJ software developed at the National Institutes of Health (NIH). The quantification is based on the intensity (density) of the band, which is calculated by area and pixel value of the band. The quantification data are given as a ratio between target protein and loading control (housekeeping protein).
Statistical analysis.
Data are presented as means ± SD and were subjected to one-way analysis of variance. Multiple means were compared with Tukey's test, and differences between two groups were determined by Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
Necrotic RPTC supernatant induces autophagy in renal interstitial fibroblasts.
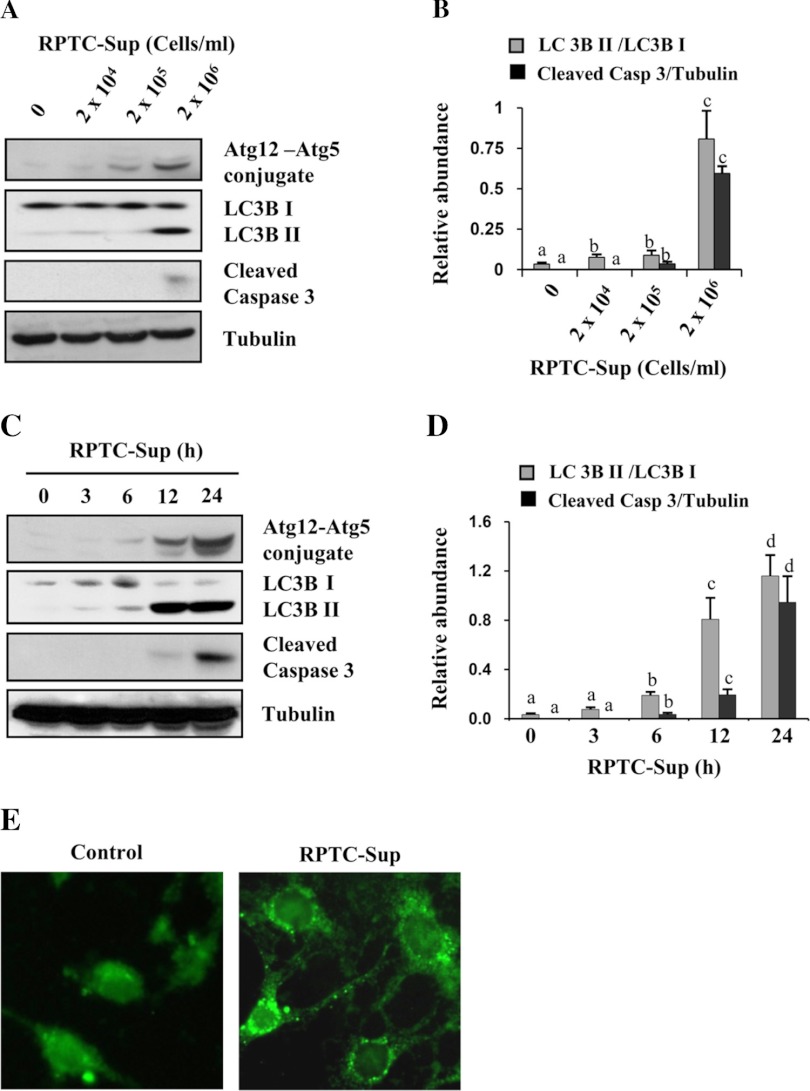
It has been reported that autophagy and apoptosis can be simultaneously induced in cells exposed to various stimuli (3, 7). Recently, we observed that necrotic RPTC induce renal fibroblast cell death in a coculture system (21). To address whether necrotic RPTC would be able to induce autophagy, we examined the effect of necrotic RPTC-Sup on the expression of autophagy markers LC3B II and Atg12-Atg5 complex in renal fibroblasts. As shown in Fig. 1, exposure of renal fibroblasts to RPTC-Sup for 24 h at a concentration of 2 × 106 cells/ml caused caspase-3 cleavage and also resulted in increased expression of Atg12-Atg5 complex and LC3B II levels, the markers of autophagy, whereas the nonlethal concentrations of necrotic RPTC-Sup (2 × 104 and 2 × 105 cells/ml) did not increase the level of these markers (Fig. 1, A and B). A slight increase of LC3B II conversion was also observed in control cells. This may be due to the role of autophagy in cellular homeostasis under growth factor deprivation conditions (3).
Fig. 1.
Necrotic renal proximal tubular cells (RPTC) induce autophagy in NRK-49F. Cultured NRK-49F cells were treated for 24 h with necrotic RPTC supernatant (RPTC-Sup) prepared from the indicated densities of cells in cell lysate (A) or treated with RPTC-Sup prepared from 2 × 106 cells/ml for the indicated times (3–24 h) (C). After treatment, cell lysates were prepared and subjected to immunoblot analysis with antibodies for LC3B I and II, Atg12-Atg5 conjugate, cleaved caspase-3, or α-tubulin (A and C). Representative immunoblots from 3 experiments are shown. The levels of LC3B I and II were quantified by densitometry analysis, and the ratio of LC3B II to LC3B I was calculated. The level of cleaved caspase-3 was quantified by densitometry and normalized with α-tubulin (B and D). Values are means ± SD of 3 independent experiments. Bars with different superscript letters are significantly different from one another (P < 0.01). NRK-49F cells grown in antibiotic-free medium were transfected with green fluorescent protein-tagged LC3B (LC3B-GFP) and then treated with RPTC-Sup for 24 h. Cells were photographed with fluorescent microscope (E).
A time course study demonstrated that Atg12-Atg5 complex and LC3B II levels in renal fibroblasts were remarkably increased within 6 h after exposure of NRK-49F cells to necrotic RPTC-Sup, rapidly increased after 12 h, and further increased up to 30-fold at 24 h. Compared with autophagy induction, necrotic RPTC-Sup-induced cleavage of caspase-3 was detectable only at 12 h after RPTC-Sup exposure and further enhanced up to 24 h (Fig. 1, C and D).
To confirm necrotic RPTC-Sup-induced autophagy, we conducted the fluorescent microscopic analysis in renal fibroblasts transfected with LC3B-GFP. As shown in Fig. 1E, autophagy vesicles were clearly detected as small bright dots in the cytoplasm of NRK-49F cells exposed to necrotic RPTC-Sup. This change was barely detectable in control cells. Overall, these data suggest that necrotic RPTC can induce both autophagy and apoptosis, and that autophagy occurs earlier than apoptosis.
Early inhibition of autophagy enhances RPTC-Sup-induced cell death.
Autophagy is involved in cell survival or cell death, depending on intracellular circumstances (3, 7). To examine the role of autophagy in renal fibroblasts after necrotic RPTC exposure, we used both chemical inhibition and a gene silencing approach to inhibit autophagy. Pretreatment with 3-methyladenine (3-MA), a chemical inhibitor of autophagy, significantly enhanced necrotic RPTC-Sup-induced cell death in a dose-dependent manner, with the maximum effect at a concentration of 5 mM (Fig. 2A). 3-MA significantly reduced (up to 6-fold) the formation of autophagy vesicles in RPTC-Sup-treated cells as indicated by the level of conversion of LC3B I into LC3B II. In contrast, the level of active caspase-3 remarkably increased (up to 5-fold) in 3-MA with RPTC-Sup-treated cells compared with RPTC-Sup alone-treated cells (Fig. 2, B and C).
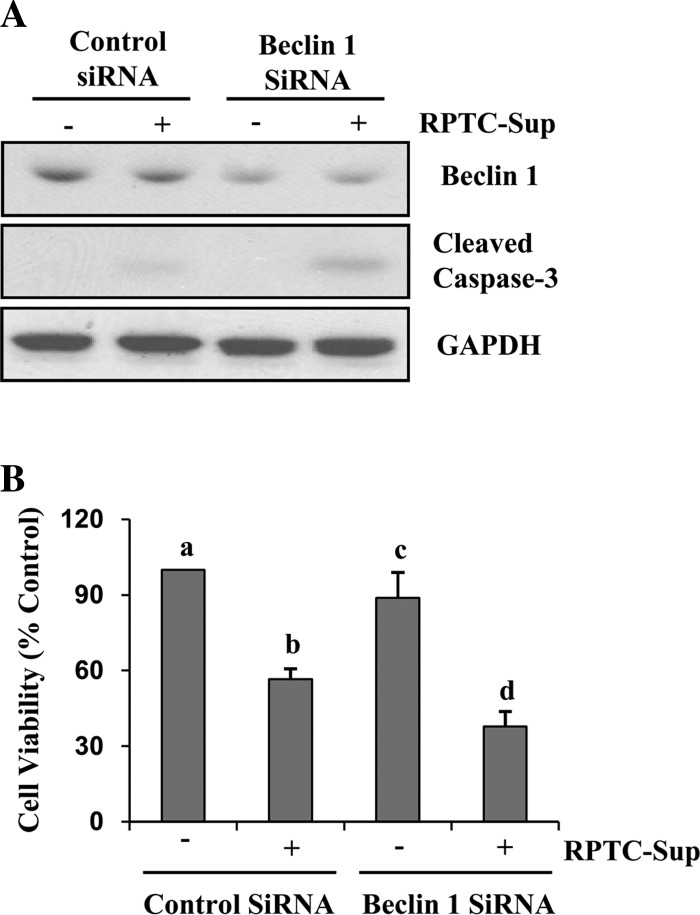
To confirm the above results, we did a further study using rat-specific siRNA for Beclin-1, a key protein involved in initiation of autophagosome formation. NRK-49F cells were transfected with Beclin-1 siRNA, and then the cells were treated with necrotic RPTC. As shown in Fig. 3, Beclin-1 expression was reduced >50%, and downregulation of Beclin-1 enhanced necrotic RPTC-Sup-induced activation of caspase-3 (Fig. 3A). In addition, the MTT cell viability assay showed that RPTC-Sup-induced cell death in renal fibroblasts was further increased in Beclin-1 siRNA-transfected cells (Fig. 3B). These data further suggest that autophagy plays a protective role in renal fibroblast cell death induced by necrotic RPTC.
Fig. 3.
Knockdown of Beclin-1 increases necrotic RPTC induced renal fibroblast cell death. NRK-49F cells were transfected with small interference RNA (siRNA) targeting Beclin-1 or scrambled (control) siRNA, and 24 h after transfection, cells were treated with RPTC-Sup for 24 h. A: cell lysates were prepared and subjected to immunoblot analysis with antibodies for Beclin-1, cleaved caspase-3, or GAPDH. Representative immunoblots from 3 experiments are shown. B: cell viability was determined by MTT assay. Values are means ± SD of 3 independent experiments conducted in triplicate and expressed as % of control. Bars with different superscript letters are significantly different from one another (P < 0.01).
Activation of ERK is required for both autophagy and cell death induction.
Previously, we showed that exposure of fibroblast cells to RPTC-Sup induced phosphorylation of AKT, ERK1/2, p38, and JNK. Among these molecules, ERK1/2 plays a major role in RPTC-Sup-induced cell death in fibroblasts, but other molecules did not have any effect on RPTC-Sup-induced cell death (21). However, all these signaling pathways have been reported to regulate autophagy activation and processing pathways (8, 16, 22). To examine the role of these molecules in RPTC-Sup-induced autophagy, NRK-49F cells were treated with RPTC-Sup in the presence or absence of inhibitors specific for p38 (SB-203580), JNK (SP-600125), AKT (LY-294002), and MEK1, a direct upstream activator of ERK1/2 (U-0126). Interestingly, U-0126 completely blocked the conversion of LC3B I into LC3B II (reduced up to 4-fold) as well as RPTC-Sup-induced cell death as indicated by blockade of caspase-3 cleavage (Fig. 4, A and C), but the inhibitors for AKT, p38, and JNK did not affect either autophagy or cell death (as shown by caspase-3 cleavage) induced by RPTC-Sup (Fig. 4, A–D). Furthermore, NRK-49F cells were transfected with LC3B-GFP and then treated with necrotic RPTC-Sup in the presence of U-0126. Fluorescent microscopic observations showed that necrotic RPTC-Sup-induced formation of autophagic vesicles was completely inhibited by U-0126 (Fig. 4E), which indicates the role of ERK1/2 in both induction of autophagy and apoptosis.
Silencing of MEK1 suppresses RPTC-Sup-induced autophagy and cell death of renal fibroblasts.
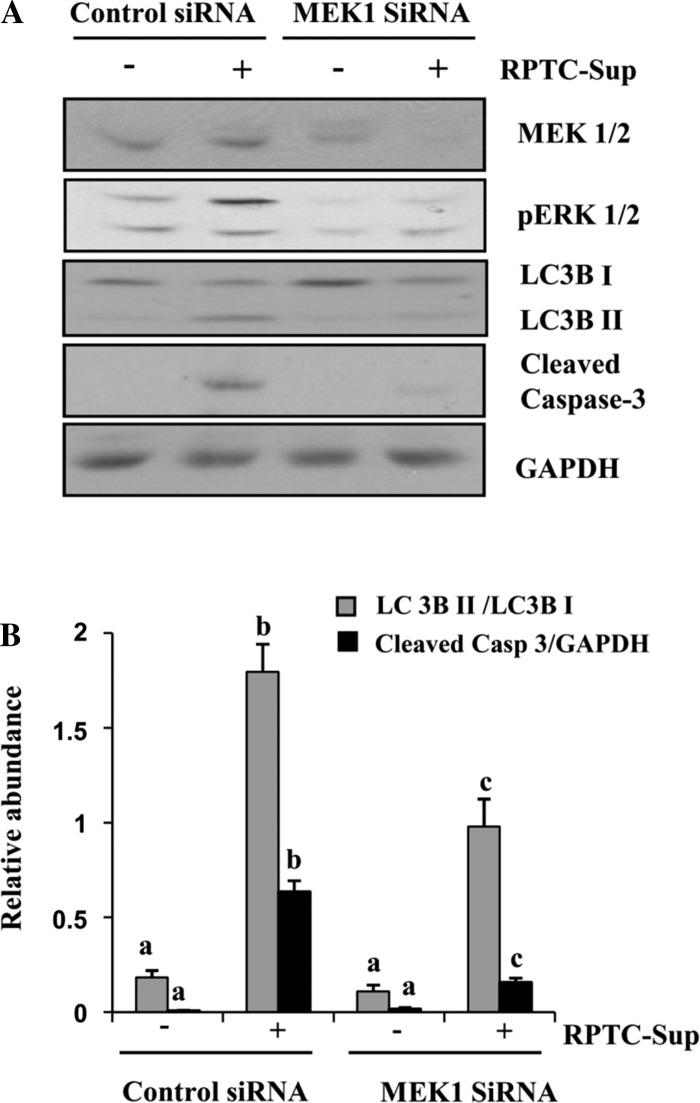
MEK1 is the upstream kinase of ERK1/2. Hence, we used MEK1 siRNA to confirm the dual role of MEK1-ERK signaling in induction of autophagy and cell death in renal fibroblasts. NRK-49F cells were transfected with rat-specific siRNA for MEK1 and then exposed to RPTC-Sup for 24 h. As shown in Fig. 5A, the expression level of total MEK1 was reduced up to 75% and the level of ERK1/2 phosphorylation remarkably decreased in the cells transfected with siRNA for MEK1. In addition, RPTC-Sup-induced conversion of LC3B I into LC3B II decreased significantly (up to 2-fold) along with blockage of caspase-3 cleavage in fibroblast cells transfected with MEK1 siRNA (Fig. 5, A and B). These results suggest that MEK1-ERK1/2 plays an important role in stimulation of autophagy and cell death in renal fibroblasts.
Fig. 5.
Effect of downregulation of MEK1 on necrotic RPTC-Sup-induced autophagy. NRK-49F cells were grown in antibiotic-free medium, transfected with scrambled siRNA or MEK1-specific siRNA, and treated with RPTC-Sup for 24 h. Cells were then harvested and subjected to immunoblot analysis for MEK1, pERK1/2, LC3B I and II, Beclin-1, cleaved caspase-3, or GAPDH (A). The level of LC3B I and II were quantified by densitometry analysis, and the ratio of LC3B II to LC3B I was calculated. The level of cleaved caspase-3 was quantified by densitometry and normalized with GAPDH (B). Data are represented as means ± SD. Bars with different superscript letters are significantly different from one another (P < 0.01). Representative immunoblots from 3 experiments are shown.
Overexpression of MEK1 enhances cell death but not autophagy of renal fibroblasts.
To further confirm the role of ERK1/2 in autophagy induction and cell death, we examined the effect of overexpression of MEK1 on RPTC-Sup-induced autophagy in NRK-49F. As shown in Fig. 6A, MEK1 expression level increased up to twofold in Ad-MEK1-infected cells compared with Ad-LacZ. In addition, RPTC-Sup-induced phosphorylation of ERK1/2 was significantly enhanced in cells overexpressing MEK1. Upon exposure of fibroblasts overexpressing MEK1 to RPTC-Sup, a significant increase in cleaved caspase-3 was observed compared with that of RPTC-Sup-treated control NRK-49F (Fig. 6A). However, the level of RPTC-Sup-induced conversion of LC3B I into LC3B II was not significantly altered in NRK-49F overexpressing Ad-MEK1 (Fig. 6, A and B). In addition, RPTC-Sup did not alter the level of Beclin-1 in fibroblasts overexpressing MEK1 (Fig. 6, A and C). These results suggest that increased MEK1 levels enhanced necrotic RPTC-Sup-induced death of renal fibroblasts and that the endogenous level of active ERK1/2 may be sufficient to induce autophagy in renal fibroblasts.
Silencing of P2X7 decreases autophagy of renal fibroblasts induced by necrotic RPTC supernatant.
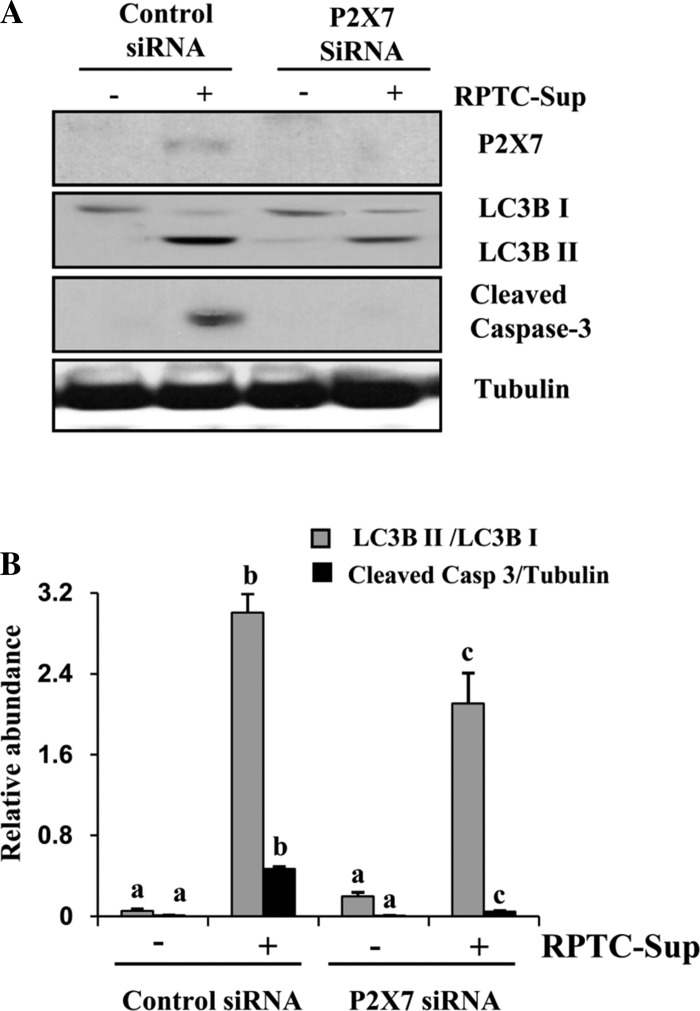
Our recent studies have shown that P2X7 mediates necrotic RPTC-Sup-induced death of renal fibroblasts and the ERK pathway plays a key role in regulating expression of P2X7 (20). Therefore, we further examined the possible role of P2X7 in regulating autophagy of renal fibroblasts. In line with our previous observation, silencing of P2X7 by siRNA inhibited RPTC-Sup-induced apoptosis as indicated by blockage of caspase-3 cleavage. However, the RPTC-Sup-induced conversion of LC3B I into LC3B II was only slightly decreased in P2X7 siRNA-transfected renal fibroblasts (Fig. 7, A and B). This result suggests that P2X7 only plays a partial role in regulating autophagy of renal fibroblasts in response to necrotic RPTC-Sup.
Fig. 7.
Effect of downregulation of P2X7 on necrotic RPTC-Sup-induced autophagy. NRK-49F cells were grown in antibiotic-free medium, transfected with scrambled siRNA or P2X7-specific siRNA, and treated with necrotic RPTC-Sup for 24 h. Cells were then harvested and subjected to immunoblot analysis for P2X7, LC3B I and II, cleaved caspase-3, or α-tubulin (A). The levels of LC3B I and II were quantified by densitometry analysis, and the ratio of LC3B II to LC3B I was calculated. The level of cleaved caspase-3 was quantified by densitometry and normalized with α-tubulin (B). Data are represented as means ± SD. Bars with different superscript letters are significantly different from one another (P < 0.01). Representative immunoblots from 3 experiments are shown.
DISCUSSION
Renal tissue has the ability to bring out protective mechanisms in response to an injury in order to minimize or tolerate tissue damage. Autophagy is one of those mechanisms, which protects renal cells from toxic injury and acute insults (2, 19). Recently, autophagy has gained attention in renal epithelial cell survival under various pathological conditions, especially during AKI (11, 12, 15, 27). It has been reported that autophagy protects against renal tubular cell death after a short-duration ischemia-reperfusion injury (12, 15) but promotes cell death in the kidney following long-duration ischemia-reperfusion (27). Severe and prolonged ischemic injury can induce both apoptosis and necrosis of renal tubular cells. Release of necrotic material from dead renal tubular cells may affect the fate of cells surrounding tubules such as renal interstitial fibroblasts. Recently, we found that exposure of cultured renal interstitial fibroblasts to necrotic RPTC-Sup results in cell death. Here, we have further demonstrated that necrotic RPTC-Sup can also induce autophagy of renal interstitial fibroblasts.
To our knowledge this is the first study demonstrating that necrotic RPTC induce autophagy of renal fibroblasts. Autophagy was initiated in a short time period (6 h) after necrotic RPTC-Sup exposure and escalated over time. This was clearly indicated by conversion of LC3 I to LC3B II and upregulation of Atg12-Atg5 complex, which are the hallmarks of autophagy. Furthermore, the large number of fluorescent bright dots observed in LC3B-GFP-transfected cells denote autophagic vesicles, which also enlighten the induction of autophagy by necrotic RPTC. In contrast, induction of apoptosis as indicated by caspase-3 cleavage was not detectable until 12 h in renal fibroblasts after RPTC-Sup exposure. Since the formation of these markers is irreversible during autophagy and apoptosis, their accumulation at different time points suggests that the occurrence of autophagy in renal fibroblasts is an early response to necrotic RPTC stimulation.
Autophagy can exert both cytoprotective and detrimental effects, depending on cellular contexts (11, 14, 15, 27). Given the fact that necrotic RPTC-Sup induced both autophagy and apoptosis in renal fibroblasts and that autophagy occurs earlier than apoptosis, we examined the relationship between these two events. The results showed that inhibition of autophagy by either 3-MA or Beclin-1 siRNA increased caspase-3 cleavage and enhanced fibroblast death. Thus these findings support the notion that the early activation of autophagy prevents necrotic RPTC-Sup-induced renal fibroblast death. It should be noted that autophagy-mediated cellular protection is not complete since active caspase-3 accumulated along with autophagosomes in cells exposed to necrotic RPTC-Sup for 24 h. These findings are consistent with recent reports showing that autophagy is one of the initial responses for cell survival and if the stress is severe and sustained, the cells overwhelm the cell survival response, leading to cell death (2, 6, 28).
The MAPK and phosphatidylinositol 3-kinase (PI3-kinase)/Akt pathways have been reported to be involved in regulation of autophagy (8, 13, 16, 22). Our previous studies have indicated that necrotic RPTC-Sup induces activation of the PI3-kinase/Akt and all three MAPK (ERK1/2, p38, and JNK) pathways in renal fibroblasts (20); hence we assessed the inhibition of individual pathways on induction of renal fibroblast autophagy after exposure to necrotic RPTC-Sup. Surprisingly, inhibition of ERK1/2, but not other pathways, blocked autophagy. Furthermore, knockdown of MEK1 by its siRNA also reduced autophagy in renal fibroblasts exposed to RPTC-Sup. These data suggest a critical role of ERK pathway in mediating autophagy activation. Consistent with our previous observations, blockade of this pathway by both pharmacological and genetic approaches decreased the expression of active caspase-3 to basal levels. Thus these data indicate that the ERK1/2 pathway plays a regulating role in processes of both autophagy and apoptosis (Fig. 8).
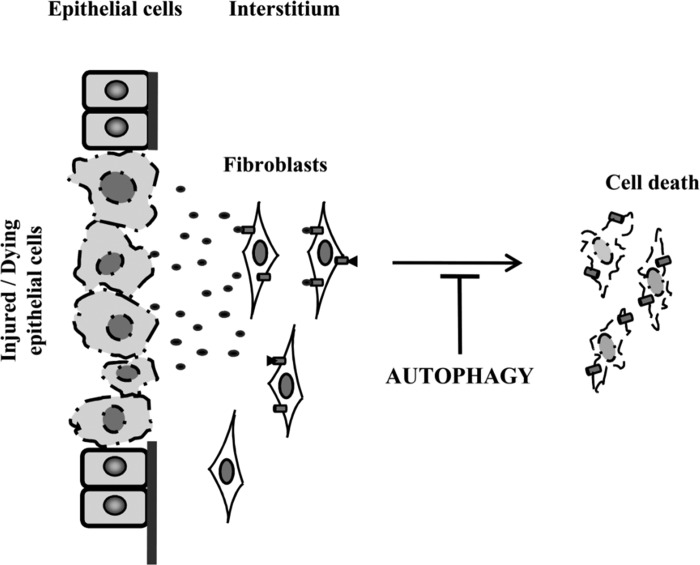
Fig. 8.
Scheme of ERK pathway-mediated autophagy and cell death in renal fibroblasts. Necrotic RPTC induces autophagy at earlier stage, but continuous activation of autophagy mediates cell death in renal fibroblasts.
Our previous studies showed that inactivation of the ERK1/2 pathway can totally block necrotic RPTC-induced death of renal fibroblasts. This result, in conjunction with the role of ERK1/2 in inhibiting autophagy, suggests that ERK1/2-mediated autophagy may be associated with induction of apoptosis. This concept is supported by the observation that consecutive activation of ERK1/2 induces the formation of a large amount of autophagic vesicles and this massive vacuolization leads to cell death (4, 8, 24, 26). In our study, we observed that the active state of ERK1/2 was sustained up to 24 h and overexpression of MEK1 did not enhance necrotic RPTC-Sup-induced autophagy but increased the activation of caspase-3. These findings suggest that either the exaggeration of cell death by overexpression of MEK1 may reduce the autophagy or the endogenous level of active ERK1/2 is sufficient for LC3B conversion. Moreover, the autophagy inhibitor data and ERK inhibition data reveal that initially autophagy upregulation is a cell rescue phenomenon but at later stages aggregation of autophagic vesicles due to prolonged activation of ERK1/2 may promote cell death. In addition, we found that although ERK1/2 is required for induction of P2X7 expression and P2X7 plays a role in mediating the death of renal fibroblasts after necrotic RPTC-Sup exposure, silencing of P2X7 by siRNA only partially suppressed autophagy in renal fibroblasts. These data suggest that the ERK1/2 pathway regulates renal fibroblast autophagy through a mechanism involving not only P2X7 but also other downstream mediators. Future studies are needed to identify additional molecules that mediate ERK1/2-mediated regulation of autophagy in renal fibroblasts.
A reduced number of peritubular fibroblasts in the area adjacent to the damaged tubular epithelium in the injured kidney has been reported (17), suggesting a possible deleterious cross talk between renal epithelial cells and fibroblasts. To date, there is still no published report indicating whether renal fibroblast autophagy occurs in vivo after acute injury. Since the damaged tubular cells after acute injury are expected to release their contents into the intercellular environment and our present studies indicate that necrotic RPTC-Sup can induce both apoptosis and autophagy of renal fibroblasts, it is possible that autophagy may occur in peritubular fibroblasts as well in vivo after AKI. In this context, it was reported that within acutely injured human liver tissue autophagy occurs in hepatic stellate cells, a cell type that has the features of fibroblasts and is located in the perisinusoidal space (10). Thus it is worthwhile to examine autophagy of renal fibroblasts in the kidney after acute injury in the future.
Our study demonstrates that induction of autophagy in renal fibroblasts is an intracellular rescue process that maintains the cell homeostasis at earlier time points but overaccumulation of autophagic microvesicles due to reduction of its turnover may provoke cell death at later stages. Moreover, unrelenting activation of ERK1/2 pathway involved in activation of autophagy, in addition to its role in cell death induction, reveals the dual role of ERK1/2 under certain circumstances. Overall, our findings unveil the responsiveness of cells residing in interstitial space of kidney tissue when there is a massive release of tubular contents, which is a common event during AKI.
GRANTS
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-071997 and DK-085065) and the Brown Kidney Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.P. and S.Z. conception and design of research; M.P. and N.L. performed experiments; M.P., H.M., and S.Z. analyzed data; M.P., T.C.Z., and S.Z. interpreted results of experiments; M.P. drafted manuscript; R.S. and S.Z. edited and revised manuscript; S.Z. approved final version of manuscript.
REFERENCES
- 1. Belibi F, Zafar I, Ravichandran K, Segvic AB, Jani A, Ljubanovic DG, Edelstein CL. Hypoxia-inducible factor-1alpha (HIF-1alpha) and autophagy in polycystic kidney disease (PKD). Am J Physiol Renal Physiol 300: F1235–F1243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, Agarwal A. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol 21: 1702–1712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bursch W, Karwan A, Mayer M, Dornetshuber J, Frohwein U, Schulte-Hermann R, Fazi B, Di Sano F, Piredda L, Piacentini M, Petrovski G, Fesus L, Gerner C. Cell death and autophagy: cytokines, drugs, and nutritional factors. Toxicology 254: 147–157, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J 277: 2–21, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Cheng Y, Qiu F, Tashiro S, Onodera S, Ikejima T. ERK and JNK mediate TNFalpha-induced p53 activation in apoptotic and autophagic L929 cell death. Biochem Biophys Res Commun 376: 483–488, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett 274: 95–100, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 12, Suppl 2: 1509–1518, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Corcelle E, Djerbi N, Mari M, Nebout M, Fiorini C, Fenichel P, Hofman P, Poujeol P, Mograbi B. Control of the autophagy maturation step by the MAPK ERK and p38: lessons from environmental carcinogens. Autophagy 3: 57–59, 2007 [DOI] [PubMed] [Google Scholar]
- 9. He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43: 67–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernández-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, Czaja MJ, Friedman SL. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 142: 938–946, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inoue K, Kuwana H, Shimamura Y, Ogata K, Taniguchi Y, Kagawa T, Horino T, Takao T, Morita T, Sasaki S, Mizushima N, Terada Y. Cisplatin-induced macroautophagy occurs prior to apoptosis in proximal tubules in vivo. Clin Exp Nephrol 14: 112–122, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol 176: 1181–1192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawakami T, Inagi R, Takano H, Sato S, Ingelfinger JR, Fujita T, Nangaku M. Endoplasmic reticulum stress induces autophagy in renal proximal tubular cells. Nephrol Dial Transplant 24: 2665–2672, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Kim WY, Nam SA, Song HC, Ko JS, Park SH, Kim HL, Choi EJ, Kim YS, Kim J, Kim YK. The role of autophagy in unilateral ureteral obstruction rat model. Nephrology (Carlton) 17: 148–159, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T, Soga T, Rakugi H, Isaka Y. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22: 902–913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li DD, Wang LL, Deng R, Tang J, Shen Y, Guo JF, Wang Y, Xia LP, Feng GK, Liu QQ, Huang WL, Zeng YX, Zhu XF. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene 28: 886–898, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Maxwell PH, Ferguson DJ, Nicholls LG, Johnson MH, Ratcliffe PJ. The interstitial response to renal injury: fibroblast-like cells show phenotypic changes and have reduced potential for erythropoietin gene expression. Kidney Int 52: 715–724, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Noda T, Fujita N, Yoshimori T. The late stages of autophagy: how does the end begin? Cell Death Differ 16: 984–990, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int 74: 631–640, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Ponnusamy M, Liu N, Gong R, Yan H, Zhuang S. ERK pathway mediates P2X7 expression and cell death in renal interstitial fibroblasts exposed to necrotic renal epithelial cells. Am J Physiol Renal Physiol 301: F650–F659, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ponnusamy M, Ma L, Gong R, Pang M, Chin YE, Zhuang S. P2X7 receptors mediate deleterious renal epithelial-fibroblast cross talk. Am J Physiol Renal Physiol 300: F62–F70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun T, Li D, Wang L, Xia L, Ma J, Guan Z, Feng G, Zhu X. c-Jun NH2-terminal kinase activation is essential for up-regulation of LC3 during ceramide-induced autophagy in human nasopharyngeal carcinoma cells. J Transl Med 9: 161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy 4: 947–948, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Wang J, Whiteman MW, Lian H, Wang G, Singh A, Huang D, Denmark T. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J Biol Chem 284: 21412–21424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weide T, Huber TB. Implications of autophagy for glomerular aging and disease. Cell Tissue Res 343: 467–473, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Wong CH, Iskandar KB, Yadav SK, Hirpara JL, Loh T, Pervaiz S. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PLos One 5: e9996, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu HH, Hsiao TY, Chien CT, Lai MK. Ischemic conditioning by short periods of reperfusion attenuates renal ischemia/reperfusion induced apoptosis and autophagy in the rat. J Biomed Sci 16: 19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu Y, Zhao L, Liu L, Gao P, Tian W, Wang X, Jin H, Xu H, Chen Q. Beclin 1 cleavage by caspase-3 inactivates autophagy and promotes apoptosis. Protein Cell 1: 468–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]