Abstract
Dietary potassium (K+) restriction and hypokalemia have been reported to change the abundance of most renal Na+ and K+ transporters and aquaporin-2 isoform, but results have not been consistent. The aim of this study was to reexamine Na+, K+ and H2O transporters' pool size regulation in response to removing K+ from a diet containing 0.74% NaCl, as well as from a diet containing 2% NaCl (as found in American diets) to blunt reducing total diet electrolytes. Sprague-Dawley rats (n = 5–6) were fed for 6 days with one of these diets: 2% KCl, 0.74% NaCl (2K1Na, control chow) compared with 0.03% KCl, 0.74% NaCl (0K1Na); or 2% KCl, 2%NaCl (2K2Na) compared with 0.03% KCl, 2% NaCl (0K2Na, Na+ replete). In both 0K1Na and 0K2Na there were significant decreases in: 1) plasma [K+] (<2.5 mM); 2) urinary K+ excretion (<5% of control); 3) urine osmolality and plasma [aldosterone], as well as 4) an increase in urine volume and medullary hypertrophy. The 0K2Na group had the lowest [aldosterone] (172.0 ± 17.4 pg/ml) and lower blood pressure (93.2 ± 4.9 vs. 112.0 ± 3.1 mmHg in 2K2Na). Transporter pool size regulation was determined by quantitative immunoblotting of renal cortex and medulla homogenates. The only differences measured in both 0K1Na and 0K2Na groups were a 20–30% decrease in cortical β-ENaC, 30–40% increases in kidney-specific Ste20/SPS1-related proline/alanine-rich kinase, and a 40% increase in medullary sodium pump abundance. The following proteins were not significantly changed in both the 0 K groups: Na+/H+ exchanger isoform 3; Na+-K+-Cl− cotransporter; Na+-Cl− cotransporter, oxidative stress response kinase-1; renal outer medullary K+ channel; autosomal recessive hypercholesterolemia; c-Src, aquaporin 2 isoform; or renin. Thus, despite profound hypokalemia and renal K+ conservation, we did not confirm many of the changes that were previously reported. We predict that changes in transporter distribution and activity are likely more important for conserving K+ than changes in total abundance.
Keywords: aldosterone, ROMK, Na transporters, blood pressure
plasma potassium must be closely controlled because it is a key determinant of membrane potential and excitability. This is critical for both cardiac action potential and central nervous system excitability (36). Among the major electrolytes, K+ has the highest ratio of daily intake to extracellular pool size (i.e., turnover), that is, the most significant homeostatic challenge (56). To meet this challenge, the kidneys are very efficient at clearing K+ in proportion to K+ intake, and the kidneys and muscles are efficient at maintaining extracellular fluid [K+] between meals. During prolonged fasting or a K+-deficient diet, urinary K+ excretion falls dramatically, which is evidence of renal adaptation. Eventually, plasma K+ falls, indicating that muscle K+ stores cannot fully compensate when K+ output chronically exceeds K+ input (46). In this setting, renal adaptations and compensations are key to reducing K+ excretion to prevent K+ losses from the body.
Many studies have investigated the renal adaptations to conserve K+ when intake is less than output. Along the nephron, reports include reduced glomerular filtration rate, increased fractional reabsorption of K+ in the early nephron, decreased K+ delivery to the distal nephron (50), and polyuria. Abundance of the K+ secretion pathway in the distal nephron principal cell, the renal outer medullary K+ channel (ROMK), has been reported to both decrease (12, 32) and to not change (49), yet ROMK has been reported to redistribute out of the apical membranes secondary to an increase in protein tyrosine kinase and c-Src mediated phosphorylation of ROMK (51). Abundance of intercalated cell H+-K+-ATPases has been reported to increase to facilitate active K+ reabsorption (6, 22). Many other effects of chronically removing K+ from the diet and hypokalemia have also been reported, including decreased weight gain and marked kidney hypertrophy (19), multiple changes in Na+ transporters including: a fourfold increase in Na+/H+ exchanger isoform 3 (NHE3) abundance along with profound decreases in Na+-K+-2Cl− cotransporter (NKCC2), Na+-Cl− cotransporter (NCC), and epithelial sodium channel (ENaC) (7), as well as no change in NCC (12, 48), increased Na+-K+-ATPase abundance in the distal nephron (30), decreased aquaporin 2 isoform (AQP2) abundance in cortex and medulla (28), and recruitment of renin along the afferent arterioles (40).
Previous studies of the effects of K+ deficiency on transporter abundance utilized a variety of starting samples from homogenates to fractionated membranes. The first aim of this study was to measure the pool size of Na+, K+, and H2O transporters in unfractionated homogenates prepared from renal cortex and medulla in hypokalemic vs. control rats using quantitative immunoblotting. Previous studies compared control vivarium chow (∼2% KCl, 0.74% NaCl) to KCl-deficient chow (< 0.03% KCl, 0.74% NaCl), which yields a diet with low overall electrolyte content. A second aim of this study was to test the effect of elevating NaCl to partially compensate for the decrease in KCl to rule out this potential complication. Specifically, we compared the effects of normal dietary KCl vs. K+ deficiency in diets that contained either control dietary NaCl (0.74%) or NaCl increased to 2%, which is the amount of salt found in the typical American diet (47). A third aim was to carefully control regular vs. K+-deficient diet composition by starting with a single powdered diet and varying only the KCl and NaCl content. Thus, we aimed to examine the effects of hypokalemia per se on physiologic parameters, as well as the total pool size of Na+, K+, and H2O transporters. We discovered that while most physiologic parameters previously reported were replicated in this study, only ENaC-β and medullary sodium pump abundance were significantly altered during hypokalemia in both the 0 K diet-fed groups.
MATERIALS AND METHODS
Animal protocols.
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Keck School of Medicine of the University of Southern California and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were performed on male Sprague-Dawley rats (225–250 g wt) purchased from Harlan Laboratories (San Diego, CA).
Animals were divided into four groups (n = 5–6 each) and fed with diets prepared from potassium-deficient powdered rat chow (cat. no. TD 88239; Harlan-Teklad, Madison, WI), which was supplemented with KCl and/or NaCl to the following percentages (in dry wt): 0.03% KCl and 0.74% NaCl (0K1Na); 2% KCl and 0.74% NaCl (2K1Na); 0.03% KCl and 2% NaCl (0K2Na); and 2% KCl and 2% NaCl (2K2Na). To gel the diets, 25 g of Difco Agar Noble was dissolved by heating in 835 ml of deionized water and added to 500 g of dry diet. Diet was stored at −20°C in meal size blocks until use. Rats were provided with 60–70 g of gelled diets per rat per day and free access to water for 6 days. To increase ENaC and Ste20/SPS1-related proline/alanine-rich kinase (SPAK) expression, a subset of rats was fed a pelleted sodium-deficient diet for 6 days (cat. no. TD 90228; Harlan-Teklad, Madison, WI).
Physiologic measurements.
At the end of the 6-day dietary treatment period, urine was collected in metabolic cages (Techniplast) overnight (16–18 h), and animals were weighed. Rats were anesthetized intraperitoneally with Inactin (100 mg/kg; Sigma), body temperature was maintained thermostatically at 37°C, and cannulas were inserted in the jugular for fluid infusion (0.9% NaCl + 4% BSA, 50 μl/min) and into the carotid artery for blood pressure measurement. After blood pressure was stabilized and recorded, blood samples were collected, plasma was prepared, and kidneys were removed and weighed.
Urine volume was recorded in graduated cylinders, urine and plasma [Na+] and [K+] were measured by flame photometry (Radiometer FLM3), and osmolality was measured with an osmometer (Precision Systems μOsmette). Plasma aldosterone levels were determined by 125I -radioimmunoassay (Coat-A-Count, TKAL kit; Siemens Healthcare Diagnostics).
Homogenate preparation.
Cortex and medulla (outer and inner) from both kidneys of each rat were dissected, diced, and suspended separately: cortex in 5 ml and medulla in 3 ml of isolation buffer [5% sorbitol, 0.5 mM disodium EDTA, and 5 mM histidine-imidazole buffer, pH = 7.5, with the addition of 0.2 mM PMSF, 9 μg/ml aprotinin, and 5 μl/ml phosphatase inhibitor cocktail (Sigma)]. Each sample was homogenized for 5 min at a low-speed setting with an Ultra-Turrax T25 (IKA-Labortechnik) and then centrifuged at 2,000 g for 10 min. Supernatants were retained, and the cortex (not medulla) pellets were rehomogenized in another 5 ml of isolation buffer, recentrifuged, and pooled with the first supernatants. The 2,000 g supernatant (So) protein concentrations were determined using the Pierce BCA kit (Thermo Scientific). The samples were aliquoted and stored at −80°C. So protein concentrations were ∼10 mg/ml for cortex and ∼3 mg/ml for medulla. The low-speed pellets, assayed by immunoblot, contained only negligible amounts of NHE3 and NCC (not shown) and were discarded.
Differential fractionation of intracellular membranes vs. plasma membranes.
In a subset of samples, intracellular (ICM) and plasma membranes (PM) were enriched as described by Sachs et. al. (43). In brief, the 2,000 g supernatant, prepared as above, was spun at 17,000 g; the resultant 17,000-g pellet, enriched in PM, was resuspended in isolation buffer (see Homogenate preparation). The 17,000 g supernatant were spun at 150,000 g for 80 min, and the pellet, enriched in ICM, was resuspended in isolation buffer. Aliquots of the PM and ICM fractions were frozen at −80°C pending assay.
Quantitative immunoblotting.
Cortical and medullary homogenates were denatured in SDS-PAGE sample buffer for 20 min at 60°C and then resolved on SDS-polyacrylamide gels (24). For each sample, one-half of the amount of protein was loaded adjacent to the full amount of protein to verify linearity of the detection system, as evident in the figures. Additionally, loading gels were run and stained with Coomassie blue, and random bands were quantified to verify that total protein loading was uniform. Gels were transferred to polyvinylidene difluoride membranes (Immobilon-FL; Millipore, Temecula, CA), blocked (bløk-FL; Millipore), and then probed with one of the following antibodies (diluted in TBST: 1% BSA, 15 mM NaN3): polyclonal anti-NHE3 (1:2,000; Millipore); monoclonal anti-NHE3 phosphorylated at Ser552 [NHE3pS552, 1:1,000; (21)]; anti- Na-phosphate cotransporter type-2 [anti-NaPi2; 1:1,000; McDonough lab (54)]; monoclonal anti-NKCC2 [1:1,000; C. Lytle, Univ. of California, Riverside, CA (27)]; polyclonal anti-NKCC2 phosphorylated at Thr96, Thr101 [NKCC2pT96T101, 1:2,000; B. Forbush, Yale Univ. (10)]; anti-NCC [1:1,000; D. Ellison, Oregon Health and Science University (3)]; anti-NCC phosphorylated at Ser71 [NCCpS71, 1:1,500; S. Bachmann lab (34)]; anti-α-ENaC, anti-β-ENaC, and anti-γ-ENaC [all 1:1,000; L. Palmer, Weill Cornell University (8)]; anti-ROMK (1:2,000; Alomone Labs, Israel); anti-c-Src (1:500; Santa Cruz Biotechnology, Santa Cruz, CA); anti-AQP2 (H-40) (1:1,000; Santa Cruz Biotechnology); monoclonal anti-NKAα1 (464.6, 1:200; M. Kashgarian, Yale University, New Haven, CT); anti-NKAβ1 (1:500; McDonough lab); anti-renin (1:2,000; AnaSpec, San Jose, CA); anti-autosomal recessive hypercholesterolemia (anti-ARH; 1:2,000; Abcam, Cambridge, MA); anti-SPAK [1:1,000; E. Delpire, (38)]; and anti-oxidative stress response kinase-1 (1:1,000; Division of Signal Transduction Therapy, Dundee, UK). Primary antibodies were recognized by either Alexa Fluor 680 (Invitrogen)- or IRDye 800 (LI-COR, Lincoln, NE)-labeled goat anti-rabbit, goat anti-mouse, or donkey anti-sheep secondary antibodies. Signals were detected with Odyssey Infrared Imaging System (LI-COR) and quantitated by accompanying software.
Quantitation and statistical analysis.
The range for linearity of signal intensity with sample loading was established for each protein and on each blot by loading one and one-half amounts of each sample side by side to verify doubling of signal intensity with doubling of sample volume. Absorbance values of 0 K diet groups were normalized to mean intensity of paired 2 K diet groups defined as 1.0 (0K1Na vs. 2K1Na and 0K2Na vs. 2K2Na). The normalized values for the one and one-half protein loading lanes were averaged. Difference in total abundance and phosphorylation of transporters and their associated proteins was assessed by unpaired two-tailed Student's t-test, not assuming equal variance. Data were expressed as means ± SE. Differences were regarded significant at P < 0.05.
RESULTS
Effects of K+-deficient diets with 0.74% NaCl (0K1Na) and with 2% NaCl (0K2Na) on physiological parameters.
To test whether the overall level of dietary electrolytes influences the responses to K+ deficiency, Sprague-Dawley rats (n = 5–6/group) were fed one of the following diets for 6 days: 1) K+-deficient diet with the sodium level typically found in control chow [0.03% KCl, 0.74% NaCl (0K1Na)]; 2) diet with Na+ and K+ levels typically found in control chow [2% KCl, 0.74% NaCl (2K1Na)]; 3) K+-deficient diet with NaCl level raised to 2% of that found in the typical American diet [0.03% KCl, 2% NaCl (0K2Na)]; and 4) diet with control level of KCl and NaCl elevated to 2% [2% KCl, 2% NaCl (2K2Na)]. Six days on the K+-deficient diets reduced plasma [K+] from control levels of 3.6 to 3.8 mM to 2.5 ± 0.1 mM in the 0K1Na group and 2.4 ± 0.1 mM in the 0K2Na group (Table 1). Plasma [Na+] was unchanged, remaining at 137–140 mM. Similar effects of a K+-deficient diet have been previously reported (7, 19). Recently, Abu Hossain et al. (1) reported that a 6-day K+-deficient diet did not significantly reduce plasma [K+]; [K+] was 5.1 meq vs. 4.9 meq in control vs. K+-deficient diet-fed rats. It is not obvious whether diet, method of anesthesia or death, collection, or measurement accounts for this difference. Plasma aldosterone, which is increased by both high-K+ diets and low-Na+ diets, was predictably highest in the group fed with 2K1Na (831 ± 88 pg/ml), lower in the groups fed 2K2Na and 0K1Na (417 ± 19 and 413 ± 21 pg/ml, respectively), and lowest in the group fed 0K2Na (172 ± 17 pg/ml). Interestingly, the 0K2Na group with the lowest concentration of aldosterone had significantly lower blood pressure, measured directly at the carotid under Inactin anesthesia [93.2 ± 4.9 mmHg in 0K2Na vs. 112.0 ± 3.1 mmHg in 2K2Na (P < 0.05)], while no significant difference in blood pressure was detected in the groups fed 0K1Na vs. 2K1Na. Urine was collected overnight before death. Urine volume was doubled in the 0K1Na group vs. 2K1Na group (1.37 ± 0.24 vs. 0.69 ± 0.13 ml/h, P < 0.05), as previously reported (2, 19, 28). The volumes excreted in the 0K2Na vs. 2K2Na groups followed the same pattern (1.56 ± 0.22 vs. 1.01 ± 0.11 ml/h) but the difference did not reach statistical significance. Removing K+ from the diet did not alter Na+ excretion; Na+ excreted in the 0K2Na and 2K2Na groups was roughly twofold higher than Na+ excreted in the 0K1Na and 2K1Na groups, indicating that NaCl output corresponded to intake. Similarly, urinary K+ excretion reflected the amount of KCl in the diets. The 0K1Na and 0K2Na groups had K+ excretion rates of 3.0 ± 0.7 and 9.3 ± 0.6 μmol/h, respectively, while the 2K1Na and 2K2Na groups had excretion rates of 127.7 ± 12.4 and 183.8 ± 18.6 μmol/h, respectively.
Table 1.
Effects of K+-deficient diets with 0.74% NaCl and 2% NaCl compared with their 2% KCl controls on physiological parameters
| Parameter | 0.74% NaCl | 2% NaCl | 2% KCl controls |
|
|---|---|---|---|---|
| 2K1Na, n = 6 | 0K1Na, n = 6 | 2K2Na, n = 5 | 0K2Na, n = 5 | |
| Diet %KCl/%NaCl, dry wt | 2.0:0.74 | 0.05:0.74 | 2.0:2.0 | 0.05:2.0 |
| PK, mM | 3.6 ± 0.2 | 2.5 ± 0.1* | 3.8 ± 0.1 | 2.4 ± 0.1* |
| PNa, mM | 133.9 ± 3.1 | 139.4 ± 3.5 | 145.5 ± 1.6 | 137.2 ± 3.9 |
| Paldosterone, pg/ml | 831.2 ± 88.1 | 4,13.2 ± 20.8* | 417.4 ± 18.9 | 172.0 ± 17.4* |
| MAP, mmHg | 117.5 ± 8.6 | 109.8 ± 8.4 | 112.0 ± 3.1 | 93.2 ± 4.9* |
| UV, ml/h | 0.69 ± 0.13 | 1.37 ± 0.24* | 1.01 ± 0.11 | 1.56 ± 0.22 |
| UNaV, μmol/h | 127.8 ± 18.7 | 137.4 ± 35.9 | 212.0 ± 16.3 | 218.2 ± 12.7 |
| UKV, μmol /h | 127.7 ± 12.4 | 3.0 ± 0.7* | 183.8 ± 18.6 | 9.3 ± 0.6* |
| Uosm, mosmol/kgH2O | 1,159.9 ± 149.4 | 331.8 ± 43.3* | 1,020.8 ± 52.0 | 507.0 ± 81.9* |
| UosmV, μmosmol·kg−1·h−1 | 705.3 ± 71.0 | 413.3 ± 34.7* | 1,014.0 ± 79.0 | 722.0 ± 26.1* |
| Initial body wt, g | 240.7 ± 2.3 | 239.8 ± 4.9 | 224.2 ± 3.8 | 252.6 ± 10.2* |
| Body wt at sacrifice, g | 275.7 ± 2.1 | 251.3 ± 4.2* | 276.0 ± 4.7 | 287.2 ± 5.9 |
| Body wt gain per day, g | 5.83 ± 0.39 | 1.92 ± 0.48* | 8.63 ± 0.65 | 5.77 ± 0.86* |
| Kidney wt/100 g body wt | 0.40 ± 0.01 | 0.49 ± 0.03* | 0.42 ± 0.01 | 0.48 ± 0.001† |
Values represent means ± SE. PK, plasma K; PNa, plasma Na; Paldosterone, plasma aldosterone; UV, urine volume; UNaV, urinary Na+ excretion rate; UKV, urinary K+ excretion rate; Uosm, urine osmolality; UosmV, urine osmolality excretion rate.
P< 0.05 vs. respective controls, assessed by unpaired two-tailed Student's t-test, assuming unequal variance;
measurements on 2 rats.
K+ depletion has been reported to significantly lower urine osmolality (2, 7, 19), which was confirmed in this study. Urine osmolality was lower in 0K1Na vs. 2K1Na (331.8 ± 43.3 vs. 1,159.9 ± 149.4 mosmol/kg) and in 0K2Na vs. 2K2Na (507.0 ± 81.9 vs. 1,020.8 ± 52.0 mosmol/kg) (Table 1). When corrected for volume, the UosmV values were also significantly lower in both 0 K-fed groups, and higher in the 2 Na vs. the 1 Na groups. Overall, the amount of solutes excreted per hour correlated to the total salt intake (Na+ + K+) with the highest excretion rate in 2K2Na and the lowest in 0K1Na group.
As previously described by us and others (40, 46), the 0K1Na group had a weight gain per day of only one-third of that measured in the 2K1Na group (1.92 ± 0.48 vs. 5.83 ± 0.39 g/day, P < 0.05, Table 1). Elevating dietary Na+ to 2% increased the rate of weight gain in both of the 2 Na groups, and the 0K2Na group had a weight gain that was two-thirds of that in the 2K2Na group (5.77 ± 0.86 vs. 8.63 ± 0.65 g/day, P < 0.05). Kidney weight per 100 g body wt was also increased in both groups of 0 K-fed rats as previously reported (30, 40), indicating that K+ deficiency induces renal hypertrophy, independent of dietary NaCl content.
Effects of K+ depletion on proximal tubule sodium transporters.
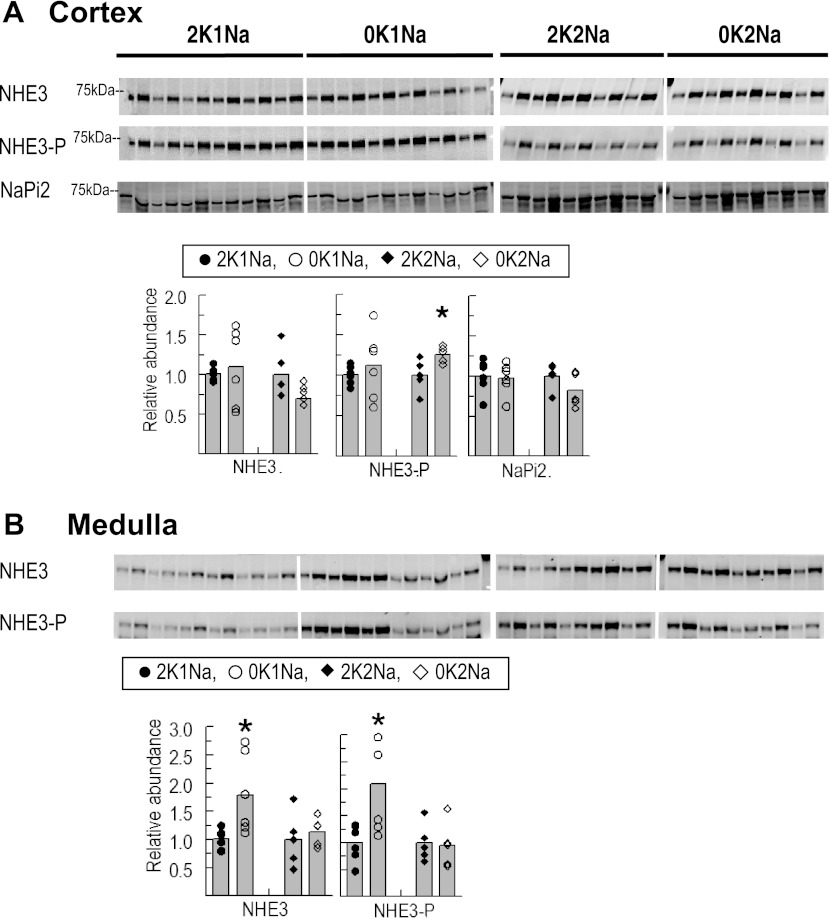
A previous study reported a sevenfold increase in NHE3 in a pooled sample of cortex plus outer medulla (proximal tubule plus cortical thick ascending limbs) in rats fed K+-deficient chow for 4 days (7). To address whether the total abundance and phosphorylation of NHE3 were altered under the condition of K+ depletion ± Na+ supplementation, kidney cortex and medulla So samples were immunoblotted with antibodies against NHE3 total and NHE3pS552 on the same blot (Fig. 1). An increase in NHE3pS552 correlates with a less active NHE3 located at the base of the microvilli (20). 0K1Na changed neither NHE3 nor NHE3pS552 abundance compared with 2K1Na. However, in the rats fed 0K2Na, NHE3pS552 was increased slightly (1.26 ± 0.05) vs. 2K2Na (1.00 ± 0.09, P < 0.05), and the NHE3pS552-to-NHE3 total ratio was increased 50% (to 1.57 ± 0.09, P < 0.05) suggesting an increase in NHE3 localized to the base of the proximal tubule microvilli in the 0K2Na group compared with the 2K2Na group. Abundance of medullary, i.e., thick ascending limb, NHE3, and NHE3pS552 increased significantly with 0K1Na diet compared with the 2K1Na diet (1.79 ± 0.29, 2.09 ± 0.37 and 1.00 ± 0.08, 1.00 ± 0.14, respectively) without a change in NHE3pS552-to-NHE3 total ratio, in agreement with a previous study reporting increased medullary NHE3 (7). However, in rats fed 0K2Na, there was neither increase in medullary NHE3 nor NHE3pS552 compared with 2K2Na, despite a similar degree of hypokalemia, indicating that the increases observed in the 0K1Na group were not driven by hypokalemia per se. Thus, this study does not support the previous report that K+ deficiency resulting in hypokalemia provokes increases in NHE3 in cortex or medulla.
Fig. 1.
Abundance of total and phosphorylated Na+/H+ exchanger isoform 3 (NHE3) and Na+/phosphate cotransporter type 2 (NaPi2) in renal cortex (A) and medulla (B) of rats fed K+-deficient diets with either 0.74% NaCl (0K1Na) (n = 6) or 2% NaCl (0K2Na) (n = 5), compared with 2% KCl-fed controls (2K1Na and 2K2Na, respectively). Immunoblots of homogenate samples are shown. To ensure linearity of the detection system, 1.0 and 0.5 amounts of each sample are loaded in adjacent lanes for each animal: 30, 15 μg for cortex and 34, 17 μg for medulla NHE3 and NHE3pS552; 30, 15 μg for cortex NaPi2. Density values were normalized to mean density of control K+ groups (1.00). Values are displayed as dot plots over a bar at the mean value. *P < 0.05 vs. respective 2 K group control.
Decreased sodium-phosphate cotransporter activity and increased urinary phosphate excretion have also been reported during dietary K+ deficiency accompanied by an increase in brush-border membrane NaPi2 total abundance (57). In the present study, neither 0K1Na nor 0K2Na diets altered total NaPi2 abundance (Fig. 1A) compared with their respective controls. We did not assay trafficking of NaPi2 between microvilli and endocytic vesicles that could explain phosphaturia.
Effects of K+ depletion on loop of Henle and distal convoluted tubule sodium transporters and their regulatory kinases.
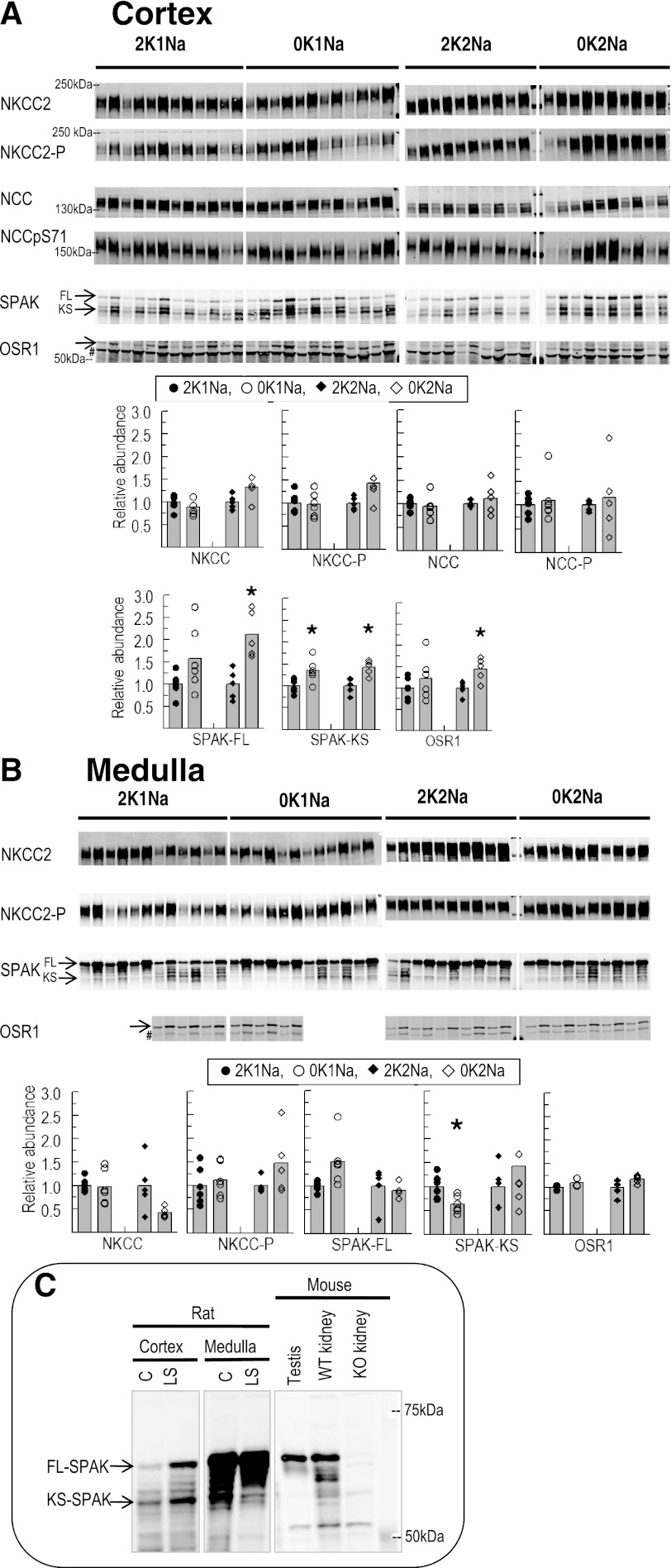
Overall, we would predict that increasing sodium reabsorption upstream from the region where K+ secretion is coupled to Na+ reabsorption in the distal nephron (i.e., by increasing NKCC, NCC abundance, and/or phosphorylation) would promote K+ conservation. However, previous reports have concluded that K+ deficiency either decreases expression of NKCC and NCC by ∼50% (7, 32) or has no effect on NCC abundance (12, 48). In this study, homogenates were subjected to immunoblotting with both anti-total NKCC2 and anti-NKCC2pT96T101 antibodies on the same blot. Phosphorylation of NKCC2 at these sites is associated with increased transport activity (10, 42). In cortex, neither 0K1Na nor 0K2Na altered the total abundance or phosphorylation of NKCC2 (Fig. 2A). No significant decrease in medullary NKCC2 abundance or phosphorylation was observed with the 0K1Na diet (Fig. 2B). However, in the animals fed 0K2Na diet, the NKCC2 appeared to decrease > 50%, which could contribute to the lower blood pressure in these animals, but this did not reach statistical significance. However, the NKCC2pT96T101-to-NKCC2 total ratio (measured on the same blot) increased nearly threefold (2.87 ± 0.58 vs. 2K2Na 1.00 ± 0.29, P < 0.05). Whether overall NKCC transport activity is elevated by this increased phosphorylation was not determined; however, increased Na+ reabsorption in this region would reduce Na+ delivery downstream to the region where K+ is secreted.
Fig. 2.
Abundance of total and phosphorylated Na+-K+-2Cl− cotransporter type 2 (NKCC2), Na+-Cl− cotransporter (NCC), and abundance of their regulatory kinases Ste20/SPS1-related proline/alanine-rich kinase (SPAK), oxidative stress response kinase-1 (OSR1) in renal cortex (A) and medulla (B) of rats fed K+-deficient diets with either 0.74% NaCl (0K1Na) (n = 6) or 2% NaCl (0K2Na) (n = 5), compared with control 2% KCl-fed controls (2K1Na and 2K2Na, respectively). Immunoblots of homogenate samples are shown. To ensure linearity of the detection system, 1.0 and 0.5 amounts of each sample are loaded in adjacent lanes for each animal (in cortex: 15, 7.5 μg for NKCC2, NKCC2pT96T101, and SPAK; 30, 15 μg for NCCpS71 and OSR1; 60 and 30 μg for NCC; and in medulla: 10 and 5 μg for NKCC2 and NKCC2pT96T101 and SPAK; and 6 and 3 μg for OSR1). #Anti-OSR1 antibody recognizes tubulin (39). Density values were normalized to mean density of control K+ groups (1.00). Values are displayed as dot plots over a bar at the mean value. *P < 0.05 vs. respective 2 K group control. C: immunoblots demonstrating the detection of SPAK in: cortex (30 μg) and medulla (15 μg) of rats fed with either control (C) or low-salt (LS) diet, in mouse testis homogenate (1 μg), in wild-type (WT), and SPAK knockout [KO (14)] total kidney homogenates (10 μg); FL-SPAK, full-length SPAK; KS-SPAK, kidney-specific SPAK.
We did not observe a significant change in NCC abundance with either 0K1Na or 0K2Na, compared with their respective control groups in agreement with other recent studies (12, 48) that failed to reproduce the decreases previously reported with K+-deficient diets (7). Phosphorylated NCC is localized to the apical membrane alone (37) and associated with increased transporter activity (33). In the present study, we did not detect a significant change in NCCpS71 in either 0K1Na or 0K2Na diets, implying no significant change in NCC transporter activity during 0 K diets. This is in contrast to a recent report of a significant increase in renal cortex NCCpS71 after K+-deficient diet feeding (48). This difference may be due to the use of a membrane fraction vs. the total homogenate used in this study.
To determine whether kinases responsible for activating NKCC2 and NCC were increased by K+-deficient diets, we analyzed total abundance of Ste20/SPS1-related proline/alanine-rich kinase (SPAK) and oxidative stress response kinase-1 (OSR1) (Fig. 2, A and B) (41, 42). McCormick et al. (29) recently reported that, in addition to full-length SPAK (FL-SPAK), a kidney-specific SPAK isoform (KS-SPAK), which lacks the kinase domain and inhibits the effects of FL-SPAK to phosphorylate NCC and NKCC2, is highly expressed along the thick ascending limb (TAL), and less so in the distal convoluted tubule (DCT). These authors also found that FL- and KS-SPAK are differentially regulated in response to changes in extracellular fluid volume (29). To verify the mobility of the SPAK isoforms on SDS-PAGE, Fig. 2C demonstrates detection of FL- and KS-SPAK in rats fed with control and low-salt diet for 6 days using mouse testis as a positive control for FL-SPAK and SPAK knockout kidney homogenates (14) as a negative control. FL-SPAK abundance increased in both cortex and medulla of rats fed with a low-salt diet (Fig. 2C), confirming the role of SPAK in activating NKCC2 and NCC under low extracellular fluid volume condition (4). KS-SPAK decreased in the medullas of rats on the low-salt diet, as reported (29), consistent with the hypothesis that there is less inhibition of FL-SPAK by KS-SPAK in the TAL and DCT with low-salt diet.
In cortex, K+ depletion increased FL-SPAK abundance in 0K2Na and tended to increase in 0K1Na (P = 0.05). In both 0K1Na and 0K2Na groups, KS-SPAK also significantly increased. Using the FL-SPAK-to-KS-SPAK ratio as an indication of activity, the ratio increased in the 0K2Na group, consistent with higher SPAK activity, despite the lowest aldosterone levels in this group; the ratio was not significantly increased in the 0K1Na diet group. In the medulla, FL-SPAK was not significantly changed but tended to increase in 0K1Na. We were unable to clearly identify medullary KS-SPAK. Medullary OSR1 abundance was unchanged by K+ depletion, while cortical OSR1 was increased in the 0K2Na but not significantly in the 0K1Na group, which indicates that the response was not due to hypokalemia per se. Whether there is physiological significance to the changes in SPAK and OSR1 expression is not readily apparent, as there was no accompanying increase in NCC and/or NKCC phosphorylation.
Effects of K+ depletion on ENaC subunits' abundance (α-, β-, and γ-ENaC).
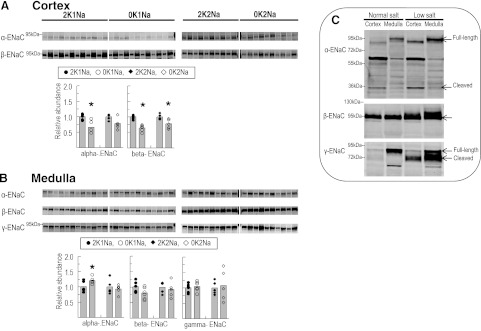
ENaC activity is one of the most important determinants of K+ secretion because, by reabsorbing a Na+ from the tubular fluid without removing an anion, it provides a driving force for K+ secretion into the tubular fluid. A decrease in ENaC activity, mediated by decreasing abundance or proteolytic processing, therefore, decreases the driving force for K+ secretion in the distal nephron and promotes K+ conservation. Cortical and medullary homogenates were probed for the three subunits of ENaC as previously described (8) (Fig. 3, A and B). To illustrate the ability to use the antibodies to detect processing and proteolysis of all three subunits, we analyzed samples of cortex and medulla from Sprague-Dawley rats fed with a low-salt diet to activate ENaC (8) vs. normal diet-fed rats (2K1Na). As previously illustrated, activation of ENaC under salt depletion is associated with an increase in full-length and putative-cleaved forms of α-ENaC, increased total β-ENaC expression and β hyperglycosylation, and increased γ-ENaC cleavage (Fig. 3C) (8).
Fig. 3.
Total abundance of ENaC subunits in renal cortex (A) and medulla (B) of rats fed K+-deficient diets with either 0.74% NaCl (0K1Na) (n = 6) or 2% NaCl (0K2Na) (n = 5), compared with 2% KCl-fed controls (2K1Na and 2K2Na, respectively). Immunoblots of homogenate samples are shown. To ensure linearity of the detection system, 1.0 and 0.5 amounts of each sample are loaded in adjacent lanes for each animal: 60 and 30 μg for cortex α-ENaC; 30 and 15 μg for cortex β-ENaC, (cortex γ-ENaC signal too low to quantitate); 34 and 17 μg for medulla α-, β-, and γ-ENaC. Density values were normalized to mean density of control K+ groups (1.00). Values are displayed as dot plots over a bar at the mean value. *P < 0.05 vs. respective 2 K group control. C: immunoblots demonstrating the detection of ENaC subunits in the cortex and medulla of rats fed control (C) vs. low-salt (LS) diets (60 μg of kidney homogenate/lane).
In the cortex of 0K1Na-fed animals both α- and β-ENaC were significantly decreased vs. 2K1Na diet-fed animals (0.66 ± 0.07 vs. 1.00 ± 0.04; and 0.65 ± 0.04 vs. 1.00 ± 0.02, respectively) (Fig. 3A). Likewise, in cortex of 0K2Na-fed animals, β-ENaC abundance decreased (0.78 ± 0.07 vs. 2K2Na 1.00 ± 0.04), and there was a similar, albeit insignificant, decrease in mean α-ENaC abundance. These findings confirmed previous reports (7, 12). The abundance of γ-ENaC in cortex and the cleaved form of α-ENaC (30-kDa band) in cortex and medulla were too low to be detected in homogenates, as evident in Fig. 3C as well. Unlike cortex, in medulla, neither K+-deficient diets reduced ENaC protein levels; in fact, α-ENaC abundance was slightly increased in 0K1Na (Fig. 3B), indicating differential regulation of ENaC in cortex compared with medulla during K+ deficiency. Overall, the results are consistent with the concept that reduced ENaC activity in cortical collecting duct during K+ deficiency will contribute to K+ conservation.
Effects of K+-deficient diets on the abundance of the secretory K+ channel (ROMK), c-Src and ARH.
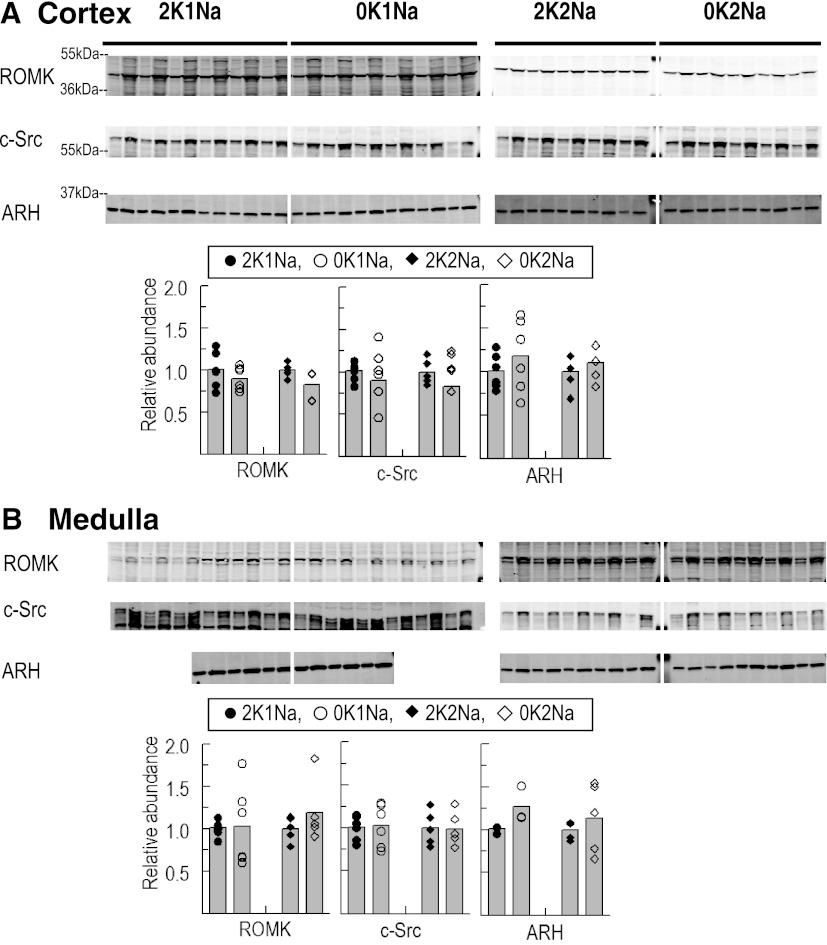
K+ restriction has been reported to decrease distal nephron principal cell apical ROMK expression by provoking retraction from the apical membrane leading to a reduction in K+ secretion and excretion (51). Whether K+ deficiency also provokes a decrease in ROMK abundance is unclear: decreased abundance has been reported in some studies (12, 32) and not in others (49). In the present study, ROMK abundance was not significantly depressed by either 0 K diet in either cortex or medulla (Fig. 4, A and B). The ROMK regulator c-Src has been reported to increase during K+-deficient diets, phosphorylating ROMK and provoking its internalization (52). In the present study, we did not observe any change in total homogenate c-Src expression during 0 K diets in cortex or medulla (Fig. 4, A and B). The clathrin adaptor molecule ARH has been reported to interact with ROMK and facilitate its internalization via clathrin-coated pits. It has also been reported in mice that in the transition between high-K+ (10% K+) diet and K+-deficient diet, ARH abundance increases ∼70% (9). In this study, ARH total abundance was not changed in either cortex or medulla in the transition between control K+ and 0 K diet (Fig. 4, A and B). This leads us to speculate that the increase in ARH abundance measured in the transition between high- and low-K+-fed mice (9) is due to an increase in ARH abundance in the transition between high- and control-K+ diet, not an increase in the transition from control to 0 K diet-fed rats. These results indicate that changes in cell surface expression and activity of ROMK are central to K+ conservation and that changes in ROMK, c-Src, and ARH abundance are not required for K+ conservation.
Fig. 4.
Total abundance of renal outer medullary K+ channel (ROMK), Src family protein tyrosine kinase (c-Src), and clathrin adaptor molecule autosomal recessive hypercholesterolemia (ARH) in renal cortex (A) and medulla (B) of rats fed K+-deficient diets with either 0.74% NaCl (0K1Na) (n = 6) or 2% NaCl (0K2Na) (n = 5), compared with control 2% KCl-fed controls (2K1Na and 2K2Na, respectively). Immunoblots of homogenate samples are shown. To ensure linearity of the detection system, 1.0 and 0.5 amounts of each sample are loaded in adjacent lanes for each animal: in cortex: 30 and 15 μg for ROMK and c-Src; 5 and 2.5 μg for ARH; in medulla: 34 and 17 μg for ROMK; 15 and 7.5 μg for c-Src; 6 and 3 μg for ARH; Density values were normalized to mean density of control K+ groups (1.00). Values are displayed as dot plots over a bar at the mean value.
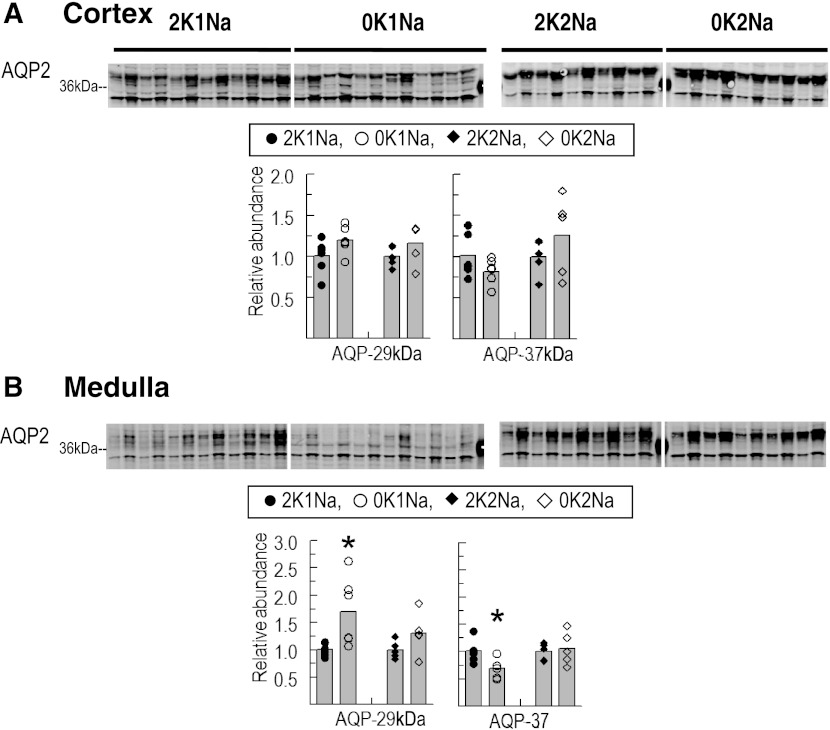
Effects of K+-deficient diets on aquaporin-2 water channel abundance.
It has been previously reported that the polyuria observed during K+ deficiency is associated with a > 50% decrease in abundance of the apical water channel aquaporin-2 (AQP2) detected in total membranes prepared from either renal cortex or medulla (2, 28). In this study, urine volume doubled (P < 0.05) in the animals fed 0K1Na vs. 2K1Na and tended to increase (insignificant 50% increase) in the 0K2Na vs. 2K2Na-fed rats. AQP2, detected by immunoblot after SDS-PAGE, runs as two major bands at 29- and 37-kDa (2, 28). In renal cortex homogenates, there was no change in either major band in the 0 K-fed groups vs. the control 2 K-fed groups (Fig. 5), in contrast to previous reports. In renal medulla homogenates, the 37-kDa band decreased 30% in the 0K1Na but not the 0K2Na group, and the 29-kDa band increased in the 0K1Na group (Fig. 5). In this study, the length of time on the K+-deficient diets as well as the method of sample preparation (homogenates vs. membranes) are different from the previous studies (2, 28). Nonetheless, this study replicates a decrease in the mature glycosylated form of AQP2 in medulla associated with polyuria in the 0K1Na-fed animals, as previously reported. It appears that the change in AQP2, and perhaps the polyuria, are blunted in animals fed additional NaCl (0K2Na) despite profound hypokalemia.
Fig. 5.
Total abundance of water channel aquaporin-2 (AQP2) in renal cortex (A) and medulla (B) of rats fed K+-deficient diets with either 0.74% NaCl (0K1Na) (n = 6)- or 2% NaCl (0K2Na) (n = 5)-fed, compared with control 2% KCl-fed controls (2K1Na and 2K2Na, respectively). Immunoblots of homogenate samples are shown. To ensure linearity of the detection system, 1.0 and 0.5 amounts of each sample are loaded in adjacent lanes for each animal: 75 and 37.5 μg for cortex and 34 and 17 μg for medulla. Both 37-kDa and 29-kDa bands were quantified. Density values were normalized to mean density of control K+ groups (1.00). Values are displayed as dot plots over a bar at the mean value. *P < 0.05 vs. respective 2 K group control.
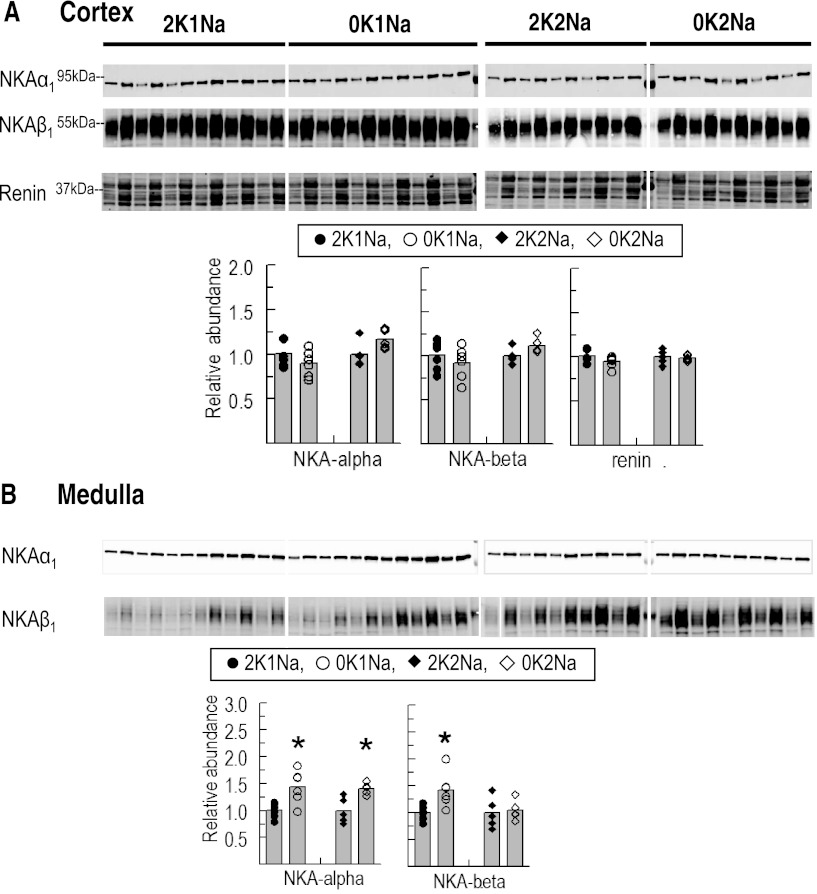
Effects of K+-deficient diets on abundance of Na+-K+-ATPase α1- and β1- subunits.
A number of previous studies, including our own, have reported that hypokalemia provokes hypertrophy of the outer medulla (40) and an increase in Na+-K+-ATPase activity and α1- and β1-subunit abundance in that region (7, 30). In this study, neither subunit was increased in cortex during K+-deficient diets, and medullary α1 was increased 40% in animals fed with either 0 K diet (Fig. 6, A and B), confirming previous studies and providing evidence of a response to K+ deficiency per se or secondary to K+ deficiency-provoked renal hypertrophy. Medullary β1 was also increased in the 0K1Na group, but not in the 0K2Na group, despite hypokalemia and renal hypertrophy (Fig. 6, A and B).
Fig. 6.
Total abundance of Na+-K+-ATPase (NKA) α1- and β1-subunits in renal cortex (A) and medulla (B) and cortex renin (A) of rats fed K+-deficient diets with either 0.74% NaCl (0K1Na) (n = 6) or 2% NaCl (0K2Na) (n = 5), compared with control 2% KCl-fed controls (2K1Na and 2K2Na, respectively). Immunoblots of homogenate samples are shown. To ensure linearity of the detection system, 1.0 and 0.5 amounts of each sample are loaded in adjacent lanes for each animal: 1.0 and 0.5 μg for NKAα1; 10 and 5 μg for NKAβ1; and 60 and 30 μg for renin. For renin, all the bands in the box were quantified together. Density values were normalized to mean density of control K+ groups (1.00). Values are displayed as dot plots over a bar at the mean value. *P < 0.05 vs. respective 2 K group control.
Effect of K+-deficient diets on abundance of renal cortical renin.
We hypothesized that the previous report of increased renin expression along afferent arterioles in 0 K-fed rats, detected by immunohistochemistry (40), might be a consequence of a diet low in overall electrolyte content stimulating the renin-angiotensin system. In the present study, we measured total abundance of renin in cortical homogenates (Fig. 6A). In a preliminary study of mice with a clip around one renal artery to raise renin abundance, we found that a set of bands migrating around 37-kDa were markedly increased compared with controls (not shown). We quantitated the bands migrating at the same mobility in the rats fed 0 K vs. 2 K diets (Fig. 6A) but did not observe a difference in renal cortical renin.
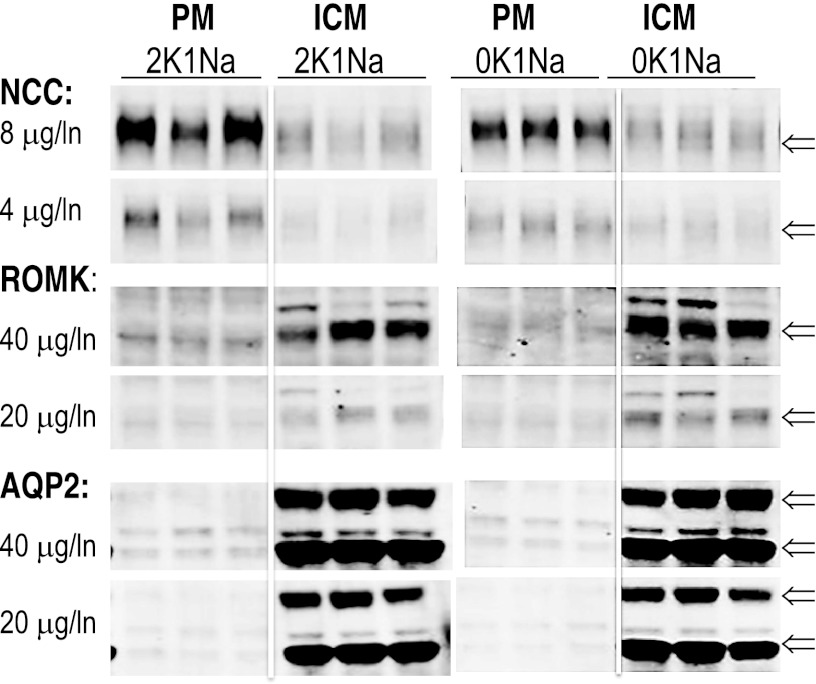
Subcellular distribution of NCC, ROMK, and AQP2.
The analysis of transporter abundance along the nephron in response to K+-deficient diets (Figs. 1–6) arrives at the prediction that changes in transporter distribution and activity are likely more important for conserving K+ than changes in total abundance of transporters. To illustrate the importance of subcellular distribution of renal transporters, three each of the 2K1Na and 0K1Na homogenates were fractionated into plasma membranes (PM) and intracellular membranes (ICM) using a simple differential centrifugation protocol developed to study AQP2 trafficking (43). Equal protein amounts of PM and ICM were analyzed by immunoblot (Fig. 7). NCC was enriched approximately twofold in PM vs. ICM, while ROMK was enriched two- to threefold in ICM vs. PM, and AQP2 was enriched ∼10-fold in ICM vs. PM. These distribution patterns are not unexpected in rats that have been provided with water but no food for a couple of hours before they are anesthetized and infused with saline for an hour: 1) hours after feeding, even in the normal K-fed rats, ROMK would likely retract to ICM to conserve K+; 2) AQP2 would likely retract to the ICM to allow urine volume flow rate to compensate for volume infusion; and 3) NCC resides predominantly in the PM, and its activity is regulated by phosphorylation (34, 44, 55). These findings indicate that there are very large intracellular pools of all three transporters, and indicate that assessing the effect of K+ deplete vs. replete diets on transporter distribution would best be analyzed in postprandial rats without prolonged anesthesia or infusions.
Fig. 7.
Subcellular distribution of NCC, ROMK, and AQP2 determined by differential centrifugation of 2K1Na and 0K1Na homogenates (n = 3 each) at 17,000 g to pellet-enriched plasma membranes (PM) and centrifugation of the 17,000 g supernatants at 150,000 g to pellet-enriched intracellular membranes (ICM) (43). Equal protein amounts of PM and ICM, as indicated, were analyzed by immunoblot, and the results illustrate significant differences in the PM-to-ICM ratio. Arrows indicate specific bands of interest.
DISCUSSION
Hypokalemia is a common electrolyte disturbance that can be life threatening when severe ([K+] < 2.5 meq). Acute hypokalemia can develop as a consequence of redistribution of K+ from the small extracellular pool to the much larger intracellular K+ pool in response to insulin or adrenergic receptor stimulation (5). Chronic hypokalemia develops as a consequence of K+ excretion rising above K+ intake. Common causes include inappropriate renal loss (diuretics) or gastrointestinal loss (diarrhea). Fasting, famine, and anorexia, if prolonged, can lead to hypokalemia even though the kidneys have the capacity to greatly reduce K+ excretion when K+ intake is reduced, as evident in Table 1. It has been estimated that > 20% of hospitalized patients develop hypokalemia. Since severe hypokalemia can provoke cardiac dysfunction, it is important to understand not only why it develops but also the homeostatic mechanisms that help to minimize the fall in extracellular K+ (56).
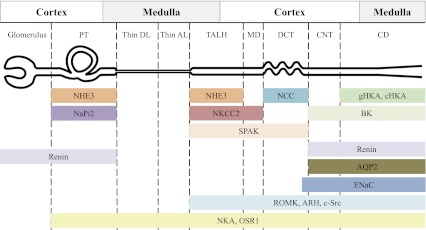
We aimed to specifically determine whether hypokalemia changed the total pool size of Na+, K+, and H2O transporters along the nephron. During a K+-deficient diet, the kidney adapts to reduce urinary K+ to match the fall in intake (Table 1). This occurs without a noticeable change in Na+ excretion. Nonetheless, Na+ transport along the nephron can impact the renal K+ conservation response because Na+ reabsorption via ENaC provides a driving force for K+ secretion via ROMK in the principal cells of the connecting segment (CNT) and cortical collecting ducts (CCD) just past the DCT (11, 53). Inhibiting Na+ reabsorption in the loop of Henle or DCT with diuretics not only increases Na+ excretion but also increases Na+ delivery and reabsorption in the CNT and CCD, thus, increasing K+ secretion to the point that these diuretics can provoke hypokalemia. Extending this logic, decreasing Na+ delivery to the CNT and CCD, for example, by increasing Na+ reabsorption proximal to this region, would decrease the driving force for K+ secretion and promote K+ conservation without affecting Na+ balance. The subunit abundance and activity of ENaCs in the CNT and CCD has a direct impact on Na+ reabsorbed, and thus, K+ secreted. For these reasons, we measured the total abundance of ENaC subunits; ROMK and associated regulators; abundance and phosphorylation status of NHE3, NKCC, and NCC; and Na+-K+-ATPase subunits (Fig. 8).
Fig. 8.
Schematic representation of transporter and regulator distributions along the rat nephron. PT, proximal tubule; Thin DL, thin descending limb; Thin AL, thin ascending limb; TALH, thick ascending limb of Henle's loop; MD, macula densa; DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct; gHKA, gastric H+-K+-ATPase; cHKA, colonic H+-K+-ATPase (16); BK, large conductance, calcium activated K+ channel (18); SPAK (39); renin (35); ENaC, epithelial Na+ channel; ROMK (13); ARH (9); c-Src (26); NKA, Na+-K+-ATPase; OSR1 (39).
Our study was also designed to test whether removal of K+, the primary salt in the diet, would lead to changes provoked by low dietary electrolyte content per se, separate from effects provoked by dietary K+ deficiency. We compared a 0 K diet containing 0.74% NaCl (0K1Na) to a 0 K diet containing 2% NaCl (0K2Na), the amount of NaCl found in the average American diet, which partially compensated for the removal of the KCl found in control diet. We postulated that any effects due to K+ deficiency per se would be evident in both of the 0 K-fed groups, while effects due to low electrolytes would be evident in the 0K1Na but not 0K2Na-fed group. By 6 days, plasma [K+] fell from control levels of 3.6–3.8 mM to 2.5 mM in both 0K1Na and 0K2Na groups, and the rate of weight gain was less in both 0 K groups vs. 2 K groups, suggesting that dietary K+ is rate limiting for growth. Weight gain per day was higher in the 0K2Na group than the 0K1Na group, suggesting that higher electrolyte content may also play a role in weight gain; however, this parameter may be influenced by the initial body weight, which was ∼15% higher in the 0K2Na group. We postulated that effects that did not appear by 6 days were unlikely to be primary responses to hypokalemia. Plasma aldosterone levels were reduced to one-half both when dietary Na+ was increased to 2% (2 Na vs. 1 Na groups) and when K+ was removed from the diet (0 K vs. 2 K groups) (Table 1). Low-Na+ intake (via activation of renin-angiotensin system) and high-K+ intake independently stimulate the transcription and translation of enzymes in the aldosterone synthesis pathway in the zona glomerulosa of the adrenal glands (17). It was difficult for us to locate previous studies addressing how low-K+ intake changes aldosterone production. In this study, removing K+ from the diet reduced aldosterone levels ∼50%, whether NaCl in the diet was 0.74% or 2% (Table 1). While evidence from both clinical and animal studies indicate that dietary K+ restriction significantly increases blood pressure (23, 40), in this study the 0K2Na group had the lowest blood pressure. We postulate that combined effects of reduced renin-angiotensin system with the 2% NaCl and reduced aldosterone with 0 K might reduce Na+ reabsorption along the nephron and reduce blood pressure set point over this 6-day time course.
Dietary K+ restriction has been the most common protocol implemented to study responses to hypokalemia in experimental animals (2, 7, 19, 22, 28, 30, 32, 40, 48, 49). Our study did not replicate most of the previously reported effects of 0 K diets, including increased NHE3, decreased NKCC and NCC (7), decreased ROMK (32), increased c-Src (52), increased ARH (9), and decreased AQP2 (28). The changes we observed in both the 0 K diet groups were 1) decreased cortical β-ENaC and α-ENaC [decrease in 0K1Na (P < 0.05), marginal decrease in α-ENaC in 0K2Na (P = 0.05)]; 2) increased medullary Na+-K+-ATPase; 3) increased cortical KS-SPAK; and perhaps 4) FL-SPAK. Assuming reduced α- and β-ENaC leads to less ENaC in the apical membranes of the CNT and CCD, this response could decrease Na+ reabsorption in this region and the driving force for stimulation of K+ secretion. Frindt et al. (12) reported that low-K+ diet decreased α-, β-, and γ(cleaved)-ENaC subunits in membranes prepared from the superficial cortex . Our results also indicate a decrease in ENaC subunits in the cortex, especially in the 2K1Na vs. 0K1Na groups (Fig. 3A). In contrast, medullary α-ENaC increased in the 0K1Na vs. 2K1Na groups, suggesting differential regulation in cortical vs. medullary collecting ducts. Perhaps lower aldosterone levels (Table 1) contribute to the reductions in cortical ENaC. Whether there is an analogous decrease in principal cell Na+-K+-ATPase activity cannot be determined in cortical homogenates due to much higher levels of Na+-K+-ATPase in other cortical tubules, such as proximal tubule and DCT (30).
The increase in medullary sodium pump has been reported previously by us and others (7, 30). It is difficult to provide a physiologic rationale for the increase that is associated with hypertrophy of the outer medulla (40). Likewise, it is difficult to assign a physiologic rationale for an increase in FL- and KS-SPAK expression during 0 K diets except to speculate that elevated SPAK activity might increase apical expression of NCC and NKCC (independent of transporter phosphorylation) and reduce Na+ delivery to the CNT and CCD where Na+ reabsorption drives K+ secretion or that the changes in SPAK may indirectly facilitate ROMK retraction from the apical surface. These are important questions for further analysis.
Preserving the abundance of key renal transporters, perhaps by retracting to intracellular pools, would help to maintain potassium homeostasis over highly varied levels of intake. Consider a carnivorous mammal in the wild punctuating long periods of fast with feasts after the animal successfully hunts down and consumes another K+-rich mammal. The homeostatic challenge immediately switches from conserving K+ to secreting a large K+ load. The switch would be sluggish if it depended on changing transporter biosynthetic rates and optimized by simply activating existing transporters by redistribution or covalent modification. Likewise, changes in K+ dietary status may alter AQP2 subcellular distribution, rather than abundance as previously reported (28), contributing to the lower urinary osmolality apparent in this and other previous studies.
A number of potential explanations could account for the differences reported in this study vs. previous studies of the effect of 0 K diets. The past studies discussed were conducted in both Sprague-Dawley (2, 22, 30, 32, 40, 57) and Wistar (7, 19, 28) rats as well as in mice (31, 48, 49); thus, species and strain differences should be taken into account. Another potential explanation is that the composition of the control vs. 0 K diets may not be closely matched between studies. In the present study, normal rodent chow is 2% KCl and 0.74% NaCl, but these percentages vary slightly between control chows depending on vendor. In past studies, either a commercial K+-deficient diet was paired to the normal vivarium chow or two similar diets were purchased from a single vendor. We could identify only one lab, before this present study, to substitute NaCl for the KCl removed (57). To eliminate unidentified differences between the control and K+-deficient diets as experimental variables, we purchased a single, powdered synthetic diet and manipulated only the potassium and sodium salts. Finally, and likely more significant than strain differences or diet, sample preparation varies between studies. Transporter activity can be regulated by changing total abundance, subcellular distribution between plasma membranes and intracellular membranes, covalent modification (e.g., phosphorylation) or protein-protein association. Previous investigations of transporter abundance regulation during K+ deficiency have utilized a number of preparations, such as Triton-SDS extracted membranes (9, 49), brush-border membranes (57), total membranes pelleted at 150–200,000 g (2, 7, 32), or membranes pelleted at 16–18,000 g (12, 48), which would enrich for plasma membranes over intracellular membranes (43). We chose to study unfractionated renal homogenates after a low-speed spin (2,000 g, 10 min) to remove unhomogenized materials, nuclei, and mitochondria. Since we determined that the low-speed pellet contained only insignificant amounts of NHE3 or NCC (not shown), our starting sample contains virtually all the transporters and eliminates concern about differential recoveries. Third, we paid careful attention to establishing the linearity of the signal with the abundance. Linearity was also aided by the use of a stable detection system rather than a chemiluminescence detection system in which the signal is transient. Stable detection systems also allow probing a single blot with multiple secondary antibodies conjugated to fluorescent probes emitting at distinct wavelengths (e.g., Alexa Fluor 680 and IRDye 800). Thus, we were able to probe for total and phosphorylated forms of a transporter on the same blot to assess changes in the phosphorylation status.
Supplementing NaCl in 0 K diet to 2% (0K2Na) increased NHE3 phosphorylation in the cortex. NHE3 phosphorylation does not change NHE3 transport activity, but it is a marker for redistribution of NHE3 to the base of the microvilli where activity is reduced (20). Reduced proximal tubule Na+ reabsorption could contribute to the lower blood pressure observed in the 0K2Na group (Table 1); the increased NHE3 phosphorylation-to-total ratio appears to be independent of hypokalemia because it is not seen in 0K1Na group.
We did not assess all the known transporters key to K+ homeostasis for lack of antibody probes that could reproducibly detect their targets in renal homogenates. Intercalated cells of the distal nephron express BK channels that facilitate flow induced K+ secretion, important for secreting a K+ load (18); there is no suggestion that they would be regulated in response to K+ deprivation. Intercalated cells also express H+-K+-ATPase (HKA) isoforms that are postulated to participate in K+ conservation by actively reabsorbing K+ from tubular fluid (6, 22). Two isoforms of HKA are expressed in kidney: the gastric isoform (gHKA) expressed from CCD to IMCD, and the colonic isoform (cHKA) expressed from TAL to IMCD (16). During dietary K+ restriction or hypokalemia, it has been reported that cHKA increases in whole kidney (22) or selectively in medulla (6). We were unable to identify antibodies that could clearly discriminate between the HKA isoforms in renal homogenates. However, previous studies suggest that HKA isoforms may not be central to K+ conservation during K+ deficiency. First, cHKA−/− mice exhibit the same profound drop in urinary K+ excretion with K+-deficient diets (and with the same time course) as cHKA+/+ mice (31). Second, gHKA protein appears to be expressed constitutively along the collecting duct (45) and regulation has not been evaluated as thoroughly as the colonic isoform (16), however, there is no notable renal phenotype reported for the gHKA−/− mice. Third, recent analysis of cHKA, gHKA double knockout mice indicate that they have significantly higher plasma [K+] compared with wild-type mice (15). All three studies are consistent with the lack of a significant role of HKA in renal K+ conservation.
In summary, the only consistent change in transporter abundance that could contribute to a decrease in K+ secretion was a decrease in ENaC abundance. Replacing K+ with additional Na+ reduced aldosterone levels and blood pressure, but otherwise, did not significantly affect the responses to hypokalemia. Our findings suggest that previous studies measuring large differences in transporter abundance (cited above) may have been influenced by assay of membrane fractions rather than unfractionated homogenates, thus, reflecting distribution in apical membranes rather than total homogenates. There is already clear evidence for retraction of ROMK from the apical membranes of the CNT and CCD during K+-deficient diets (25) and the simple subcellular fractionation results in this study (Fig. 7) suggest most of the ROMK is in intracellular pools hours after feeding even in normalkalemic animals. Reducing Na+ delivery to the CNT and CCD could further reduce K+ secretion. Since we did not measure increases in total abundance or phosphorylation of Na+ transporters proximal to this region, we postulate that any decrease in Na+ delivery to CNT and CCD would involve subcellular redistribution or covalent modification of transporters. We previously determined that high-Na+ diets provoke retraction of Na+ transporters along the nephron from low-density apical membranes into the base of the microvilli or into subapical vesicles (55). Determining whether redistribution of Na+ transporters into apical membranes occurs during 0 K diets will require similar subcellular fractionation assays.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-083785, an ASN Scherbenske Award, and a grant from the University Kidney Research Organization (to AA McDonough); National Institute of General Medical Sciences GM74771 (to E. Delpire), National Institute of Diabetes and Digestive and Kidney Diseases Grant K08-DK-072075–02 (to H. Kocinsky), and Deutsche Forschungsgemeinschaft Grant FOR667 (to S. Bachmann).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.T.X.N., L.E.Y., D.H.L., H.S.K., and A.A.M. conception and design of research; M.T.X.N., L.E.Y., N.K.F., D.H.L., and A.A.M. performed experiments; M.T.X.N., L.E.Y., N.K.F., D.H.L., H.S.K., S.B., E.D., and A.A.M. analyzed data; M.T.X.N., L.E.Y., N.K.F., D.H.L., H.S.K., S.B., E.D., and A.A.M. interpreted results of experiments; M.T.X.N., N.K.F., and A.A.M. prepared figures; M.T.X.N. drafted manuscript; M.T.X.N., L.E.Y., H.S.K., S.B., E.D., and A.A.M. edited and revised manuscript; M.T.X.N., L.E.Y., N.K.F., D.H.L., H.S.K., S.B., E.D., and A.A.M. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1. Abu Hossain S, Chaudhry FA, Zahedi KA, Siddiqui F, Amlal H. Cellular and molecular basis of increased ammoniagenesis in potassium deprivation. Am J Physiol Renal Physiol 301: (5) F969–F978, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Amlal H, Krane CM, Chen Q, Soleimani M. Early polyuria and urinary concentrating defect in potassium deprivation. Am J Physiol Renal Physiol 279: F655–F663, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bostanjoglo M, Reeves WB, Reilly RF, Velazquez H, Robertson N, Litwack G, Morsing P, Dorup J, Bachmann S, Ellison DH. 11β-Hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J Am Soc Nephrol 9: 1347–1358, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403–1409, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Clausen T. Hormonal and pharmacological modification of plasma potassium homeostasis. Fundam Clin Pharmacol 24: 595–605, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Codina J, Delmas-Mata JT, DuBose TD., Jr Expression of HKα2 protein is increased selectively in renal medulla by chronic hypokalemia. Am J Physiol Renal Physiol 275: F433–F440, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Elkjaer ML, Kwon TH, Wang W, Nielsen J, Knepper MA, Frokiaer J, Nielsen S. Altered expression of renal NHE3, TSC, BSC-1, and ENaC subunits in potassium-depleted rats. Am J Physiol Renal Physiol 283: F1376–F1388, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol 291: F683–F693, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Fang L, Garuti R, Kim BY, Wade JB, Welling PA. The ARH adaptor protein regulates endocytosis of the ROMK potassium secretory channel in mouse kidney. J Clin Invest 119: 3278–3289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl cotransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem 277: 37551–37558, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Frindt G, Houde V, Palmer LG. Conservation of Na+ vs. K+ by the rat cortical collecting duct. Am J Physiol Renal Physiol 301: F14–F20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frindt G, Palmer LG. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frindt G, Shah A, Edvinsson J, Palmer LG. Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol 296: F347–F354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geng Y, Hoke A, Delpire E. The Ste20 kinases Ste20-related proline-alanine-rich kinase and oxidative-stress response 1 regulate NKCC1 function in sensory neurons. J Biol Chem 284: 14020–14028, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenlee MM, Lynch IJ, Gumz ML, Cain BD, Wingo CS. Mineralocorticoids stimulate the activity and expression of renal H+,K+-ATPases. J Am Soc Nephrol 22: 49–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gumz ML, Lynch IJ, Greenlee MM, Cain BD, Wingo CS. The renal-ATPases: physiology, regulation, and structure. Am J Physiol Renal Physiol 298: F12–F21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol 350: 151–62, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holtzclaw JD, Grimm PR, Sansom SC. Role of BK channels in hypertension and potassium secretion. Curr Opin Nephrol Hypertens 20: 512–517, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imbert-Teboul M, Doucet A, Marsy S, Siaume-Perez S. Alterations of enzymatic activities along rat collecting tubule in potassium depletion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F408–F417, 1987 [DOI] [PubMed] [Google Scholar]
- 20. Kocinsky HS, Dynia DW, Wang T, Aronson PS. NHE3 phosphorylation at serines 552 and 605 does not directly affect NHE3 activity. Am J Physiol Renal Physiol 293: F212–F218, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol 289: F249–F258, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kraut JA, Hiura J, Besancon M, Smolka A, Sachs G, Scott D. Effect of hypokalemia on the abundance of HKα1 and HKα2 protein in the rat kidney. Am J Physiol Renal Physiol 272: F744–F750, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Krishna GG, Miller E, Kapoor S. Increased blood pressure during potassium depletion in normotensive men. N Engl J Med 320: 1177–1182, 1989 [DOI] [PubMed] [Google Scholar]
- 24. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 25. Lin DH, Sterling H, Wang WH. The protein tyrosine kinase-dependent pathway mediates the effect of K intake on renal K secretion. Physiology (Bethesda) 20: 140–146, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol 286: F881–F892, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lytle C, Xu JC, Biemesderfer D, Forbush B., 3rd Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies. Am J Physiol Cell Physiol 269: C1496–C1505, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Marples D, Frokiaer J, Dorup J, Knepper MA, Nielsen S. Hypokalemia-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla and cortex. J Clin Invest 97: 1960–1968, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McDonough AA, Magyar CE, Komatsu Y. Expression of Na+-K+-ATPase α- and β-subunits along rat nephron: isoform specificity and response to hypokalemia. Am J Physiol Cell Physiol 267: C901–C908, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Meneton P, Schultheis PJ, Greeb J, Nieman ML, Liu LH, Clarke LL, Duffy JJ, Doetschman T, Lorenz JN, Shull GE. Increased sensitivity to K+ deprivation in colonic H,K-ATPase-deficient mice. J Clin Invest 101: 536–542, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mennitt PA, Frindt G, Silver RB, Palmer LG. Potassium restriction downregulates ROMK expression in rat kidney. Am J Physiol Renal Physiol 278: F916–F924, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Mercier-Zuber A, O'Shaughnessy KM. Role of SPAK and OSR1 signalling in the regulation of NaCl cotransporters. Curr Opin Nephrol Hypertens 20: 534–540, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Mutig K, Saritas T, Uchida S, Kahl T, Borowski T, Paliege A, Bohlick A, Bleich M, Shan Q, Bachmann S. Short-term stimulation of the thiazide-sensitive Na+-Cl− cotransporter by vasopressin involves phosphorylation and membrane translocation. Am J Physiol Renal Physiol 298: F502–F509, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol 11: 180–186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Packer M. Potential role of potassium as a determinant of morbidity and mortality in patients with systemic hypertension and congestive heart failure. Am J Cardiol 65: 45E–51E, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA. Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O'Shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med 2: 63–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ray PE, Suga S, Liu XH, Huang X, Johnson RJ. Chronic potassium depletion induces renal injury, salt sensitivity, and hypertension in young rats. Kidney Int 59: 1850–1858, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Richardson C, Sakamoto K, de Los Heros P, Deak M, Campbell DG, Prescott AR, Alessi DR. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci 124: 789–800, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sachs AN, Pisitkun T, Hoffert JD, Yu MJ, Knepper MA. LC-MS/MS analysis of differential centrifugation fractions from native inner medullary collecting duct of rat. Am J Physiol Renal Physiol 295: F1799–F1806, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sandberg MB, Maunsbach AB, McDonough AA. Redistribution of distal tubule Na+-Cl− cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol 291: F503–F508, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Silver RB, Breton S, Brown D. Potassium depletion increases proton pump (H+-ATPase) activity in intercalated cells of cortical collecting duct. Am J Physiol Renal Physiol 279: F195–F202, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Thompson CB, McDonough AA. Skeletal muscle Na,K-ATPase α and β subunit protein levels respond to hypokalemic challenge with isoform and muscle type specificity. J Biol Chem 271: 32653–32658, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Tobian L. Dietary sodium chloride and potassium have effects on the pathophysiology of hypertension in humans and animals. Am J Clin Nutr 65: 606S–611S, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol 300: F1385–F1393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walter SJ, Shore AC, Shirley DG. Effect of potassium depletion on renal tubular function in the rat. Clin Sci (Lond) 75: 621–628, 1988 [DOI] [PubMed] [Google Scholar]
- 51. Wang WH. Regulation of ROMK (Kir1.1) channels: new mechanisms and aspects. Am J Physiol Renal Physiol 290: F14–F19, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical small-conductance K channel in CCD: role of protein tyrosine kinase. Am J Physiol Renal Physiol 281: F206–F212, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Weinstein AM. A mathematical model of rat distal convoluted tubule. II. Potassium secretion along the connecting segment. Am J Physiol Renal Physiol 289: F721–F741, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol 287: F896–F906, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Yang LE, Sandberg MB, Can AD, Pihakaski-Maunsbach K, McDonough AA. Effects of dietary salt on renal Na+ transporters' subcellular distribution, abundance, and phosphorylation status. Am J Physiol Renal Physiol 295: F1003–F1016, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol 71: 381–401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zajicek HK, Wang H, Puttaparthi K, Halaihel N, Markovich D, Shayman J, Beliveau R, Wilson P, Rogers T, Levi M. Glycosphingolipids modulate renal phosphate transport in potassium deficiency. Kidney Int 60: 694–704, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.