Abstract
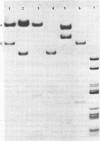
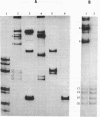
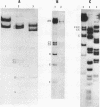
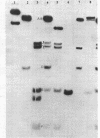
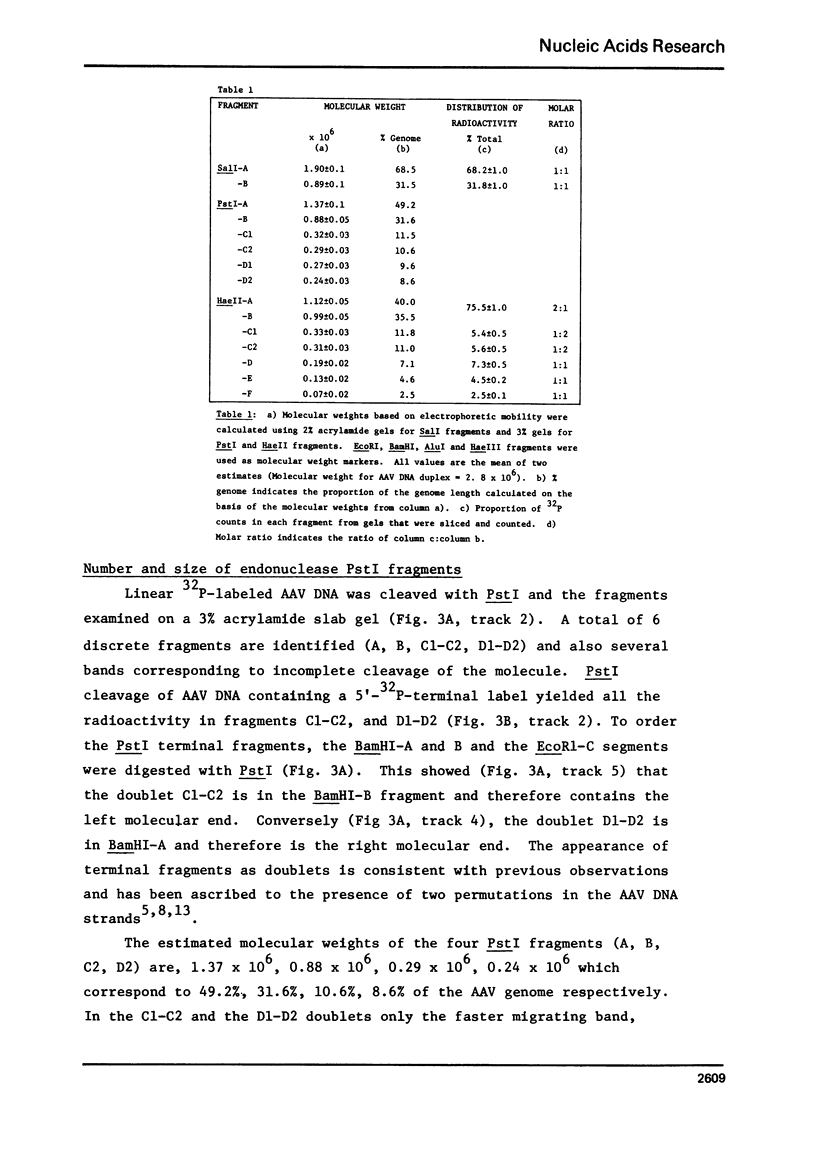
Duplex AAV-2 DNA was digested with SalI, PstI or HaeII restriction endonucleases and the cleavage sites were mapped. SalI cleaves AAV DNA at 0.310 map units, PstI at 0.106, 0.422 and 0.914 and the five HaeII sites were mapped at 0.110. 0.156, 0.181, 0.536 and 0.600 map units. These cleavage products will be useful for the isolation of specific regions from the AAV DNA, located outside of the stably transcribed region of the genome, and will also help to map more complex restriction enzyme cleavages.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Kort J., Fife K. H., Grogan E. W., Spear I. Study of the fine structure of adeno-associated virus DNA with bacterial restriction endonucleases. J Virol. 1975 Sep;16(3):712–719. doi: 10.1128/jvi.16.3.712-719.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Khoury G., Denhardt D. T. Physical map and strand polarity of specific fragments of adenovirus-associated virus DNA produced by endonuclease R-EcoRI. J Virol. 1975 Sep;16(3):559–568. doi: 10.1128/jvi.16.3.559-568.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Khoury G. Specific cleavage of adenovirus-associated virus DNA by restriction endonuclease R-EcoRI--characterization of cleavage products. Virology. 1975 Feb;63(2):523–538. doi: 10.1016/0042-6822(75)90325-6. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Eisenberg S., Bartok K., Carter B. J. Multiple structures of adeno-associated virus DNA: analysis of terminally labeled molecules with endonuclease R-Hae III. J Virol. 1976 May;18(2):672–684. doi: 10.1128/jvi.18.2.672-684.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. I., Blattner F. R., Davies J. The isolation and partial characterization of a new restriction endonuclease from Providencia stuartii. Nucleic Acids Res. 1976 Feb;3(2):343–353. doi: 10.1093/nar/3.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Live T. R., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. V. End group labeling and analysis of deoxyribonucleic acid containing single straned breaks. J Biol Chem. 1968 Sep 10;243(17):4530–4542. [PubMed] [Google Scholar]