Abstract
3-Phosphoinositide-dependent protein kinase 1 (PDK1) is the pivotal element of the phosphatidylinositol 3 kinase (PI3K) signaling pathway because it phosphorylates Akt/PKB through interactions with phosphatidylinositol 3,4,5 phosphate. Recent data indicate that PDK1 is overexpressed in many breast carcinomas and that alterations of PDK1 are critical in the context of oncogenic PI3K activation. However, the role of PDK1 in tumor progression is still controversial. Here, we show that PDK1 is required for anchorage-independent and xenograft growth of breast cancer cells harboring either PI3KCA or KRAS mutations. In fact, PDK1 silencing leads to increased anoikis, reduced soft agar growth, and pronounced apoptosis inside tumors. Interestingly, these phenotypes are reverted by PDK1 wild-type but not kinase-dead mutant, suggesting a relevant role of PDK1 kinase activity, even if PDK1 is not relevant for Akt activation here. Indeed, the expression of constitutively active forms of Akt in PDK1 knockdown cells is unable to rescue the anchorage-independent growth. In addition, Akt down-regulation and pharmacological inhibition do not inhibit the effects of PDK1 overexpression. In summary, these results suggest that PDK1 may contribute to breast cancer, even in the absence of PI3K oncogenic mutations and through both Akt-dependent and Akt-independent mechanisms.
Introduction
The phosphatidylinositol 3 kinase (PI3K) pathway is one of the most important pathways in cancer metabolism and growth [1,2. Class IA PI3Ks, deregulated in cancer, are heterodimers composed of a regulatory (p85) and a catalytic (p110) subunit. Binding of p85 to tyrosine kinase receptors removes the inhibitory effect of p85 on p110, resulting in the full activation of PI3K. The activated kinase catalyzes the phosphorylation of phosphatidylinositol 4,5 biphosphate to phosphatidylinositol 3,4,5 triphosphate (PIP3). PIP3 acts as a docking site for 3-phosphoinositide-dependent kinase 1 (PDK1) and Akt that, in turn, phosphorylates their substrates, including mammalian target of rapamycin and glycogen synthase kinase β (GSK3β) [3.
PDK1 is a cytoplasmic kinase that phosphorylates serine/threonine residues in the activation segment of AGC (cAMP-dependent protein kinases A, cGMP-dependent protein kinases G, and phospholipid-dependent protein kinases C) family protein, initially discovered as the kinase that phosphorylates Akt on threonine 308 upon binding to PIP3 [4–6. In fact, PDK1 is able to recognize the phosphoinositides phosphorylated in position 3 by PI3K, through its C-terminal pleckstrin homology (PH) domain. This event localizes PDK1 to the plasma membrane where it phosphorylates Akt [6. PDK1 substrates lacking the PH domain, such as p70S6K [7, SGK [8, RSK [9, and PKC isoforms [10, require a different mechanism for their activation: PDK1, through its PIF-binding pocket, binds the hydrophobic motif on these substrates, and this leads to their phosphorylation and full activation [4,11. Furthermore, it has been described that PDK1 binds and regulates other substrates through kinase-independent mechanisms. PDK1 has been demonstrated to activate the Ral guanine nucleotide exchange factors through its noncatalytic N-terminal 50 amino acids [12 and found to activate Rho-associated coiled-coil containing protein kinase 1 (ROCK1) by competing against its inhibitor RhoE [13.
The PI3K pathway is often aberrantly activated in breast cancer with mutations occurring in up to one quarter of breast cancers. PIK3CA activating mutations and PTEN loss are the most frequent events in human breast tumors, whereas a significant role for Akt1 mutations is also emerging [14. Moreover, most of the elements of this pathway are found hyperactive or amplified in breast tumors: PIK3CA [15, PIK3CB [16, Akt1 [17, Akt2 [18, PDK1 [19, p70S6 kinase [20, and IKBKE [21. Such alterations strongly correlate with a more aggressive phenotype and a poor prognosis.
Recently, PDK1 was found overexpressed both at the protein and mRNA levels in most human breast cancer with frequent genomic amplifications. Moreover, its Ser-241 phosphorylated form was found enriched in human breast carcinoma versus benign tumors [22,23. Despite this, forced PDK1 expression has been described to be oncogenic only in the Comma-1D murine mammary cell model [24,25, whereas in breast-derived cell lines, it is able to potentiate the oncogenic effects of upstream lesions but not to transform per se [19. In mice, its oncogenic effect seems to function by altering the PI3K pathway because PTEN-driven tumors were severely attenuated in PDK1 knockout and hypomorphic mice. However, results obtained with human cancer cell lines [26 together with the involvement of PDK1 in resistance mechanisms to several anticancer drugs such as gemcitabine, trastuzumab, tamoxifen, and rapamicin suggest that PDK1 regulates others oncogenic signaling pathways [27–30.
Here, we show that PDK1 regulates anchorage-independent growth, resistance to anoikis, and tumor formation in breast cancer cells not only harboring PIK3CA genetic alterations but also in the absence of these lesions.
Materials and Methods
Cell Lines
293T (CRL-11268), MDA-MB-231 (HTB-26), and T-47D (HTB-133) cell lines were obtained from ATCC resource center (http://www.atcc.org). Phoenix-GP was provided by Garry P. Nolan Lab (Stanford, CA). The MDA-MB-231 metastatic variant, LM2-4175, was a gift from Dr. Joan Massagué (Sloan-Kettering Institute, New York, NY) [31. 293T, MDA-MB-231, and Phoenix-GP were cultured in Dulbecco modified Eagle medium (DMEM; catalog no. D5546; Sigma-Aldrich, St Louis, MO), whereas T-47D cells were cultured in RPMI 1640 medium (catalog no. R0883; Sigma-Aldrich). The culture media were supplemented with 10% FBS (catalog no. 10270; Gibco, Life Technologies, Rockville, MD) and 200 U/ml penicillin and 200 µg/ml streptomycin (catalog no. 67513; Sigma-Aldrich).
Soft Agar Colony Formation Assay
One milliliter of bottom layer constituted by 0.7% agar (catalog no. 214220; Becton Dickinson, Franklin Lakes, NJ) in DMEM was spread in each 35-mm-diameter well. A total of 1 x 104 cells were suspended in 3 ml of DMEM-10% FBS 0.35% agar and spread over the bottom layer. A layer of medium (1.5 ml) was added on the gel layers and substituted every 3 to 4 days until the end of the assay (1521 days). For the quantification, colonies grown in soft agar were stained with nitrotetrazolium blue chloride (N-6876; Sigma-Aldrich). High-resolution image acquisitions by ChemiDoc XRS (170-8070; Bio-Rad Laboratories, Hercules, CA) were processed and analyzed using the ImageJ software (http://rsbweb.nih.gov/ij/). Only colonies with diameter bigger than 100 µm were counted.
Anoikis and Apoptosis Assay
For the anoikis assay, 4 x 105 MDA-MB-231 or T-47D were seeded in 35-mm dishes coated with poly-hydroxy-ethyl-methacrylate (catalog no. P3942; Sigma-Aldrich) in medium with 10% FBS. For the apoptosis assay, 4 x 105 MDA-MB-231 or T-47D were seeded in 35-mm dishes in the absence of FBS. After 2 days, the percentage of apoptotic cells was evaluated by FACS analysis using M30 Cyto-DEATH (12-140-349-001; Roche Applied Science, Indianapolis, IN), or alternatively, the rate of apoptosis was evaluated using Cell Death Detection ELISAplus (11-774-425-001; Roche).
Xenograft Assay
MDA-MB-231 cells were inoculated subcutaneously in nude athymic mice (5 x 106 cells) or in NOD/SCID mice (3 x 106 cells). After 30 days, mice were killed, and tumor weight was evaluated. The tumors were cryopreserved by OCT embedding at -80°C. Cryosections of 15 µm thickness were stained with In Situ Cell Death Detection Kit, TMR red (12-156-792-910; Roche) for the evaluation of apoptotic cells.
Statistical Analysis
Data were compared using a Student's t test. Results were expressed as mean and SD of at least three independent experiments each in triplicate. The EC50 of log[inhibitor-versus-response curves was calculated with the nonlinear regression tool of the GraphPad 5 Prism software (GraphPad, La Jolla, CA).
PDK1, Akt, and PI3K Inhibitors
BX-795 (catalog no. 1390; Axon Medchem, Groningen, the Netherlands), OSU-03012 (catalog no. 1-800-364-9897; Cayman Chemical, Ann Arbor, MI), LY294002 (catalog no. 9901; Cell Signaling, Danvers, MA), and Akt inhibitor VIII (catalog no. 124017; Calbiochem, Darmstadt, Germany) were reconstituted in DMSO at 10 mM. All the inhibitors were stored in small aliquots at -20°C and thawed at the time of use.
PDK1 Mutants and Cloning into pCCL Lentiviral Vector
Myc-tagged PDK1, PDK1-KD (K110N), PDK1-ΔPH, and PDK1-K465E previously cloned into PINCO retroviral vector [32 were subcloned into a third-generation lentiviral vector pCCL sin.cPPT.PGK.GFP.WPRE [33 with In-Fusion 2.0-CF Dry-Down-PCR Cloning-Kit (catalog no. 639607; Clontech Laboratories, Mountain View, CA). For cloning, the following primers were designed: “FW-rec-pCCL” (GGGGATCC CCCGGGCTGCAGATGGAGCAGAAGCTGATCAGCGAGGAGG), “RE-rec-pCCL” (GAGGTTGATTGTCGACTCACTGCACAGCGGCGTCCGGG), and “ΔPH-RE-rec-pCCL” (GAGGTTGATTGTCGACTCACTGGTGCCAAGGGTTTCCGCCAG). The acceptor plasmid pCCL sin.cPPT.PGK.GFP.WPRE was digested in PstI and SalI sites. During cloning, two punctiform and silent substitutions were added to PDK1 coding sequence to make it resistant to the shPDK1#79 short hairpin RNA (shRNA) (TRCN0000039779) by using the following primers: “RE-mut” (GCTTCTCCAACAACAAGCGCTTCTCATCTTCGG) and “FW-mut” primers (CCGAAGATGAGAAGCGCTTGTTGTTGGAGAAGC).
Akt T308D S473D Cloning into pBABE-puro Retroviral Vector
The bovine coding sequence of phosphomimetic Akt1 was cloned from “HA PKB T308D S473D pcDNA3” (a gift from Jim Woodgett; Addgene plasmid no. 14751, Cambridge, MA). The cloning was obtained by recombination using the In-Fusion 2.0-CF Dry-Down-PCR Cloning-Kit. The acceptor plasmid pBABE-puro was digested with BamHI and EcoRI, and the two primers used are as follows: “FW” GGCGCCGGCCGAATCCATGTACCCATACGATGTTCCAG and “RE”CTGTGCTGGCGAATTCTCAGGCCGTCGCGC.
Lentiviral Vector Production and Infection
For PDK1 stable silencing, two pLKO.1 lentiviral vectors carrying PDK1 targeting shRNA called shPDK1#79 (TRCN0000039779; Sigma-Aldrich) and shPDK1#81 (TRCN0000039781) were used, respectively. For Akt1 and Akt2 the following vectors were used: shAkt1#86 (TRCN0000039968), shAkt1#97 (TRCN0000039797), shAkt2#17 (TRCN0000255917), and shAkt2#68 (TRCN0000039968). A vector leading the expression of a scrambled not targeting shRNA, called shScr (Sigma-Aldrich), and a vector targeting the green fluorescent protein (GFP) construct (EGFP control vector; Sigma-Aldrich) were used as negative controls. For the expression of PDK1 constructs, the pCCL sin.cPPT.PGK.GFP.WPRE lentiviral vector [33 was used, leading the expression, through a bidirectional promoter, of both PDK1 constructs and GFP. As a negative control, a plasmid expressing only GFP was used (empty vector). All viruses were produced as described in the TRC shRNA guidelines. Infection of cells was performed with a multiplicity of infection equal to 1 for pLKO.1 and multiplicity of infection equal to 3 for pCCL sin.cPPT.PGK.GFP.WPRE in the presence of 8 µg/ml Polybrene (H-9268; Sigma-Aldrich). Cells infected with pLKO.1 lentiviral vectors were selected with 2.5 µg/ml puromycin for 2 days, and the surviving cell population was used for the experiments.
Retroviral Vector Production and Infection
For Akt1 or Akt2 expression, the following retroviral vectors were used: “pBABE puro” negative control vector (a gift from Bob Weinberg; Addgene plasmid no. 1764); “pBABE myr-Akt1” (a gift from William Hahn, Addgene plasmid no. 15294); “pBABE Akt1,” “pBABE myr-Akt2,” “pLNCX Akt1,” and “pLNCX myr-Akt1,” “pLNCX myr-Akt1 K179M” (gifts from William Sellers; Addgene plasmid nos. 9011, 9018, 9004, 9005, and 9006, respectively); and “pBABE Akt1 T308D S473D” (see cloning). For retroviral particles production, Phoenix-GP cells were transfected with retroviral vector plasmid and pMD2.G vector, expressing the VSV-G envelope. Collection and infection of retroviral particles were performed as described [32. Infected cells were selected using 2.5 µg/ml of puromycin (P7255; Sigma-Aldrich) for pBABE vector vectors and 1 mg/ml Geneticin (catalog no. 10131-027; Gibco) for pLNCX series vectors.
Immunoblot Analysis
Immunoblot analysis was performed as described [32. The following primary antibodies were used: PDK1 (no. 3062), pS241PDK1 (no. 3061), Akt1 (no. 2967), Akt2 (no. 2964), pT308Akt (no. 4056), pS473Akt (no. 4060), pS9GSK3β (no. 9323), pFoxO1(Thr24)/FoxO3a(Thr32)/FoxO4(Thr28) (no. 2599) from Cell Signaling and γ-tubulin (sc-17787) and β-actin (sc-1616) from Santa Cruz Biotechnology (Santa Cruz, CA).
Proliferation Assay
The proliferation assay was performed as previously described [34.
Immunofluorescence
Cryosection from experimental tumors were fixed in 3.7% para-formaldehyde pH 7.4 for 1 hour, washed three times with PBS, and permeabilized for 1 hour in PBS 0.5% Triton X-100 (T8294; Sigma-Aldrich) and 10% donkey serum (D9663; Sigma-Aldrich). The primary antibodies were left on the slices overnight in PBS 10% donkey serum at 1:100 dilution at 4°C. The secondary staining was performed at 25°C for 1 hour with fluorescent dye-conjugated antibodies (Alexa Fluor series; Invitrogen, Carlsbad, CA). The images were acquired with a confocal laser scanning microscope (TCS SP2 with DM IRE2; Leica Microsystems, Wetzlar, Germany) equipped with 20x, 40x, and 63x/1.40 HCX Plan-Apochromat oil immersion objective. Confocal images are the maximum-intensity projections of the complete z section. The immunostaining signal was quantified using the ImageJ Software. The primary antibodies used are as follows: anti-Ki-67 (MAB4190; Millipore), anti-CD31 (553370; BD, Franklin Lakes, NJ), anti-Akt1 (no. 2967; Cell signaling), antipT308Akt (no. 4056; Cell Signaling), anti-PDK1 (611070; BD), and anti-pS241PDK1 (no. 3061; Cell Signaling).
Results
PDK1 Is Required for Anchorage-Independent Growth in Breast Cancer Cells
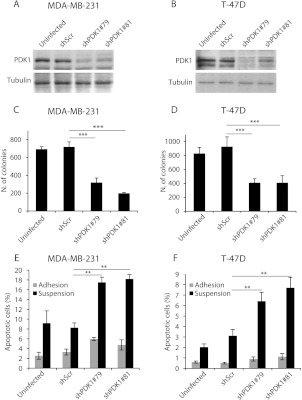
To evaluate the role of PDK1 in breast cancer, we stably down-regulated it in human mammary tumor cell lines harboring different genetic lesions. MDA-MB-231 cells are mutated for KRAS (G13D), whereas T-47D cells harbor a mutation in the PI3K (H1047R) catalytic domain. Specifically, we transduced MDA-MB-231 and T-47D cells with shRNAs for PDK1 (shPDK1#79 and shPDK1#81) by a lentiviral-mediated based approach. PDK1 knockdown cells exhibited low levels of PDK1 compared to cells transduced with a nontargeting construct (shScr) and uninfected cells (Figure 1, A and B). Apparently, the reduced level of PDK1 did not modify the ability of both MDA-MB-231 and T-47D to the growth on plastic culture dishes (Figure W1, A and B). However, when grown in soft agar, the PDK1-silenced cell lines exhibited reduced anchorage-independent growth ability (Figure 1, C and D). Interestingly, both cell lines require PDK1 to grow in the absence of anchorage irrespective of their different origin and genetic lesions.
Figure 1.
PDK1 down-regulation reduces anchorage-independent growth and increases apoptosis. MDA-MB-231 and T-47D cells were transduced with a scramble shRNA (shScr), two separate PDK1-targeting shRNAs (shPDK1#79 and shPDK1#81), or untransduced (uninfected) and were used for the indicated investigations: (A and B) Protein lysates were obtained and used in immunoblot analysis for PDK1. Tubulin was used as a loading control. (C and D) Soft agar colony formation assay. Only colonies larger than 100 µm diameter were counted. (E and F) Apoptosis assay of cells cultured for 24 hours on plastic (gray bar) or maintained in suspension (black). The number of apoptotic cells was evaluated by using the anti-cytokeratin 18 fragment antibody. *P < .05, **P < .01, ***P < .001.
PDK1 Down-regulation Increases Sensitivity to Anoikis and Serum Deprivation
A common feature of malignant transformation is the ability to evade apoptotic cell death signals, such as lack of growth factors. Furthermore, tumor cells are often resistant to anoikis, the process of apoptosis induced by cell matrix detachment. T-47D and MDA-MB-231 are particularly resistant to anoikis; in fact, the number of apoptotic cells after 48 hours of growth in suspension is less than 4% and 10%, respectively. PDK1 silencing strongly increased the cells' susceptibility to apoptosis in the absence of anchorage, evaluated both as caspase 3 activation (Figure 1, E and F) and as number of oligonucleosomes (Figure W1, C and D). PDK1 down-modulation also increased apoptosis induced by serum deprivation in adherent cells, which was particularly evident in MDA-MB-231 cells (Figure W1E) compared with T-47D (Figure W1F).
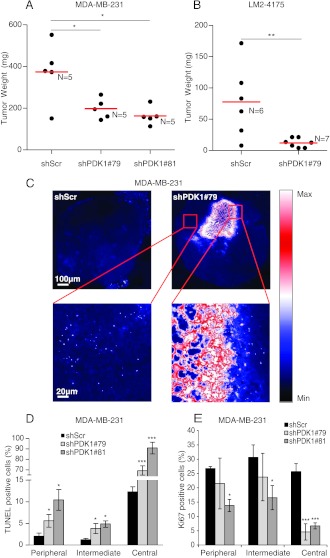
In Vivo Tumor Growth Is Reduced by PDK1 Knockdown
To further analyze the role of PDK1 in tumorigenesis, we injected PDK1 knockdown and control MDA-MB-231 cells into immunodeficient mice. ShPDK1#79-and shPDK1#81-expressing tumors grew significantly slower than did control tumors expressing shScr (Figure 2A). We performed similar experiments with a more aggressive variant of MDA-MB-231—the LM2-4175 cells [31. Tumors formed with PDK1 knockdown LM2-4175 cells exhibited an impairment of growth compared to LM2-4175 cells transduced with shScr, and interestingly, the difference in tumor volume was more pronounced than in MDA-MB-231 wild-type cells (Figure 2B).
Figure 2.
PDK1 knockdown impairs tumor formation. (A) MDA-MB-231 or (B) LM2-4175 cells transduced with PDK1-targeting shRNAs (shPDK1#79 and shPDK1#81) or with a scramble sequence (shScr) were injected subcutaneously in immunodeficient mice. Tumor weight was evaluated 4 weeks after injection and expressed in milligrams. Red line indicates the average tumor weight. N = number of mice. (C) Apoptosis within tumors originating from MDA-MB-231 cells, transduced as in A, was evaluated using TUNEL assay, and representative images are shown in false colors: black/blue, low fluorescence intensity; white, high intensity. Quantification of apoptosis (D) and proliferating Ki-67-positive cells (E) in three different regions of tumors as in A. The percentage of apoptotic and proliferating cells was evaluated in the peripheral, intermediate, and central regions of the tumors. *P < .05, **P < .01, ***P < .001.
To test whether PDK1-dependent inhibition of MDA-MB-231 xenograft growth in vivo was associated with reduced cell proliferation and/or increased apoptosis, tumors were stained with an antibody for Ki-67 and were subjected to TUNEL assays. Because histologic analyses showed that tumors formed from PDK1-depleted MDA-MB-231 cells had a larger central necrotic area compared with controls (Figure 2C), characterized by high levels of apoptosis, we considered and quantified the peripheral and intermediate regions of the tumor. The percentage of apoptotic cells, measured by TUNEL assay, was significantly higher in tumor silenced for PDK1 compared to those formed by shScr cells (Figure 2D). Moreover, Ki-67 immunostaining indicated a decrease in cell proliferation in tumors with reduced PDK1 levels in comparison to MDA-MB-231 cells infected with shScr (Figures 2E and W2A). Apparently, the antiapoptotic effect of PDK1 did not depend on the ability to attract new vessels because the tumor vascularization level was similar in both tumor types without any significant decrease in vessel volume and diameter (Figure W2, B and C).
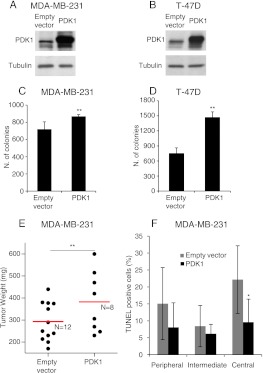
Increased PDK1 Potentiates Soft Agar and Tumor Growth
Because it has been shown that PDK1 protein and mRNA are overexpressed in a majority of human breast cancers, we assessed the tumorigenic effect of PDK1 overexpression in both MDA-MB-231 (Figure 3A) and T-47D (Figure 3B). The addition of exogenous PDK1 significantly increased the number of colonies grown in the soft agar (Figure 3, C and D). We next determined whether this in vitro-enhanced tumorigenicity resulted in a tumor growth increase. PDK1-overexpressing MDA-MB-231 cells, subcutaneously injected in mice, formed tumors with a significantly larger volume than those of cells transduced with the empty vector (Figure 3E). Accordingly, tumors originating from PDK1-overexpressing cells displayed a reduced number of apoptotic cells and an increase in proliferating cells, statistically significant only in the central region of the tumors (Figures 3F and W3, A–C).
Figure 3.
PDK1 overexpression increases anchorage-independent growth and tumor formation. (A and B) Expression of PDK1 was evaluated by immunoblot in MDA-MB-231 or T-47D cells transduced with PDK1 or with an empty vector. Tubulin was used as loading control. (C and D) Soft agar colony formation assay of MDA-MB-231 and T-47D cells transduced as indicated in A and B. Only colonies larger than 100 µm diameter were counted. (E) MDA-MB-231 cells transduced as in A were injected subcutaneously in immunodeficient mice. Tumor weight was evaluated 4 weeks after injection and expressed in milligrams (mg). Red line indicates the average tumor weight. N = number of mice. (F) Quantification of apoptosis in three different regions of the tumors referring to E. The percentage of apoptotic cells was evaluated in the peripheral, intermediate, and central regions of the tumors. *P <.05, **P <.01, ***P <.001.
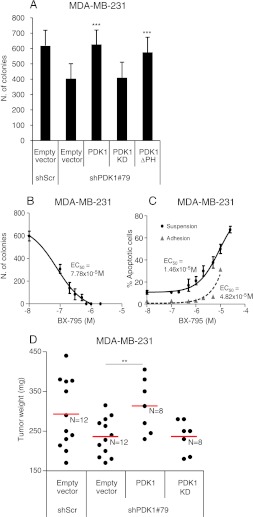
The Kinase Activity of PDK1 Is Required to Regulate Tumor Growth
To understand the molecular mechanism activated by PDK1 during anchorage-independent and tumor growth, we investigated which activity of PDK1 is required for this function. To achieve this purpose, cells, downregulated for PDK1, were transduced with lentiviral vectors expressing PDK1 mutants that are insensitive to gene silencing. The following cDNAs were expressed in MDA-MB-231: PDK1 wild-type (PDK1), K110N mutant that abolishes kinase activity (PDK1-KD), and PH domain-deleted mutant that impedes binding to PIP3 at the membrane (PDK1-ΔPH and PDK1-K465E) (Figures W4A and W5A). The introduction of PDK1 into silenced cells was able to recover the ability to grow in soft agar, whereas the PDK1-KD was unable to rescue the phenotype, suggesting that kinase activity is required for tumorigenesis. On the contrary, PDK1 mutant in the PH domain was able to rescue the anchorage-independent growth (Figures 4A and W5B). To further support the involvement of PDK1 kinase activity in soft agar growth and anoikis, we used two kinase inhibitors of PDK1: BX-795 and OSU-03012. BX-795 inhibited soft agar growth very effectively (EC50 = 7.78 x 10-5 M; Figure 4B) and promoted anoikis (EC50 = 1.46 x 10-5 M; Figure 4C). Notably, BX-795 was much more effective in inducing apoptosis when cells were grown in the absence of adhesion than when they were plated on plastic (EC50 = 4.82 x 10-5 M; Figure 4C). Similar results were obtained with OSU-03012 (Figure W6, A and B). Although these chemical compounds are not specific inhibitors for PDK1, their EC50 concentration was sensitive to PDK1 expression levels. In fact, PDK1 silencing sensitized apoptosis induced by BX-795, by reducing the EC50 to 3.80 x 10-6 M, whereas PDK1 overexpression made them more resistant with EC50 = 4.30 x 10-5 M (Figure W6C). To assess whether the PKD1 kinase activity was also required for tumor growth, we subcutaneously injected silenced cells transduced with PDK1 or PDK1-KD. The reintroduction of PDK1 induced the formation of tumors similar to controls, whereas the expression of PDK1-KD mutant was totally unable to rescue the phenotype (Figure 4D). Furthermore, PDK1 reexpression restored the percentage of Ki-67-positive cells in the central region of the tumor (Figure W4B), whereas it reduced the number of apoptotic cells (Figure W4C).
Figure 4.
PDK1 regulates anchorage-independent and tumor growth by a kinase-dependent mechanism. (A) MDA-MB-231 PDK1 knockdown cells (shPDK1#79) as in Figure 1 were transduced with vectors carrying PDK1 wild-type (PDK1), PDK1 kinase-dead (PDK1 KD), PDK1 PH-domain-deleted (PDK1 ΔPH), or empty vectors. PDK1 expression levels are shown in Figure W4. Soft agar colony formation assay was performed with the various cell lines and only colonies larger than 100 µm diameter were counted. (B) Soft agar colony formation assay with MDA-MB-231 cells was measured in the presence of different concentrations of the PDK1 inhibitor BX-795. EC50 is indicated. (C) Apoptosis assay with MDA-MB-231 cultured on plastic (gray bar) or maintained in suspension (black) for 24 hours and treated with different concentrations (M) of the PDK1 inhibitor BX-795. The number of apoptotic cells was measured considering the cytokeratin 18 fragment-positive cells and the reported EC50. (D) MDA-MB-231 cells transduced with scramble shRNA (shScr) or with PDK1-targeting shRNAs (shPDK1#79) and retransduced with PDK1 wild-type (PDK1), PDK1 kinase-dead (PDK1-KD), or empty vector were injected subcutaneously in immunodeficient mice. Tumor weight was evaluated 4 weeks after injection and expressed in milligrams (mg). Red line indicates the average tumor weight. *P <.05, **P < .01, ***P < .001.
Akt Phosphorylation Is Not Affected by PDK1 Down-regulation
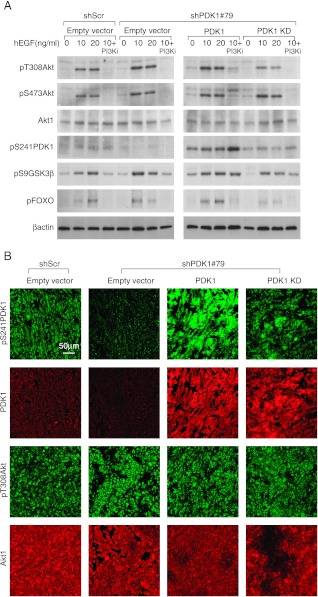
To further evaluate PDK1 kinase activity arising from reintroduction of PDK1 mutants, we analyzed Akt1 phosphorylation on Thr308 after stimulation with hEGF. Unexpectedly, the low levels of PDK1 remaining after gene silencing were still sufficient to phosphorylate Akt at the same extent of control cells (Figure 5A). However, PDK1 reexpression, which actually increased PDK1 expression above its physiological levels, led to an increase in Akt Thr308 phosphorylation, which was prevented by inactivating mutations in the PDK1 kinase domain (Figure 5A). Similar effects were observed on phospho-Ser473 Akt. The Akt phosphorylation trend was paralleled by the phosphorylation of Akt downstream effectors. PDK1 knockdown was unable to impair the phosphorylation of both GSK3β and FOXO, and PDK1 overexpression caused an increased phosphorylation, which was not observed in cells expressing PDK1 kinase dead (Figure 5A). The addition of PI3K inhibitor, before the hEGF stimulation, completely abolished both FOXO and Akt phosphorylation, whereas it was ineffective in inhibiting PDK1 and GSK3β phosphorylation.
Figure 5.
PDK1 knockdown does not affect Akt phosphorylation. (A) Immunoblot analysis with the indicated antibodies was performed on lysates of MDA-MB-231 cells transduced with scramble shRNA (shScr) or PDK1-targeting shRNAs (shPDK1#79) and retransduced with PDK1 wild-type (PDK1), PDK1 kinase-dead (PDK1-KD), or empty vectors. Cells were treated or not with PI3K inhibitor then stimulated with different concentrations of hEGF. (B) Immunostaining and confocal microscopy analysis of tumor sections originating from MDA-MB-231 transduced as indicated in A. The images are representatives of four tumors and were acquired with the same confocal parameters and magnification. Top panels are stained with anti-phosphoPDK1 (pS241PDK1; red) and anti-PDK1 (PDK1; green). Bottom panels are stained with anti-phosphoAkt (pT308Akt; red) and anti-Akt1 (Akt1; green).
Then, we extended the Akt phosphorylation analysis in tumors of MDA-MB-231 cells. The confocal microscopy analysis revealed that phosphorylation of Thr308 of Akt was unchanged on PDK1 silencing. In this case, PDK1 reexpression was unable to increase Akt phosphorylation in tumors (Figure 5B). However, levels of PDK1 and phospho-Ser241 PDK1 were modest in shPDK1#79 compared with those in shScr tumors, whereas levels were more evident in tumors in which PDK1 was reexpressed. In contrast, PDK1-KD tumors exhibited low levels of PDK1 phosphorylation on Ser241, as expected in the case of autophosphorylation (Figure 5B) [35.
PDK1 Tumorigenesis Is Akt Independent
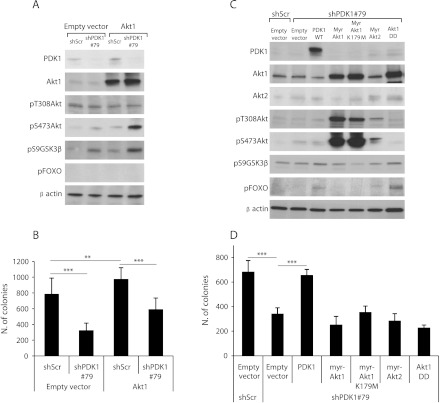
Given that PDK1 kinase activity was essential for both cell anchorage-independent and tumor growth, although its main substrate, Akt, was not differentially phosphorylated in PDK1 knockdown cells, we decided to unravel the functional role of Akt in PDK1-mediated tumorigenesis. The overexpression of Akt1 in MDA-MB-231 did not increase the fraction of Akt1 phosphorylated on Thr308 both in PDK1-silenced and control cells. Interestingly, cells with reduced levels of PDK1 and overexpressing Akt1 showed enhanced Ser473 Akt phosphorylation. Moreover, the phosphorylation of GSK3β was increased in PDK1-silenced cells, whereas phospho-FOXO was undetectable. Despite these biochemical results, the overexpression of Akt1 (Figure 6A) increased the number of colonies grown in soft agar, but it was not sufficient to overcome the effect of PDK1 silencing (Figure 6B). These results suggest that PDK1 and Akt control tumorigenesis independently, although the phosphorylation of Thr308 of Akt by PDK1 has been indicated by several pieces of evidence as the critical event for Akt activation [4.
Figure 6.
Akt overexpression does not rescue anchorage-independent growth of PDK1 knockdown cells. (A) Immunoblot analysis for the indicated antibodies was performed on lysates of MDA-MB-231 cells transduced with scramble shRNA (shScr) or with PDK1-targeting shRNAs (shPDK1#79) and retransduced with Akt1 or empty vectors. β-Actin was used as loading control. (B) Soft agar colony formation assay performed on MDA-MB-231 cells transduced as indicated. Only colonies larger than 100 µm diameter were counted. (C) Immunoblot analysis of lysates of MDA-MB-231 cells transduced with scramble shRNA (shScr) or with PDK1-targeting shRNAs (shPDK1#79) and retransduced with PDK1 wild-type (PDK1), different active mutants of Akt (myr-Akt1, myr-Akt2, Akt1DD), inactive mutant of Akt (myr-Akt1 K179M), or empty vectors. (D) Soft agar colony formation assay performed on MDA-MB-231 cells transduced as indicated. Colonies larger than 100 µm diameter were counted. ***P <.001.
Therefore, we tried to rescue the effect of PDK1 silencing with active Akt mutants, which are independent from the upstream activators PI3K and PDK1. PDK1-silenced MDA-MB-231 cells were transduced with retroviruses expressing the constitutive active and membrane-anchored mutants of Akt1 and Akt2 (myr-Akt1 and myr-Akt2), the constitutive active mutants in which Thr308 and Ser473 are substituted by Asp mimicking the phosphate required for Akt full activation (Akt1-DD) and, as control, the kinase-inactive form of membrane-anchored Akt1 (myr-Akt1-KD; Figure 6C). Surprisingly, myr-Akt1 and myr-Akt1-KD did not regulate either GSK3β or FOXO, although they showed elevated levels of phosphorylation both on Thr308 and on Ser473. Moreover, the down-regulation of PDK1 did not affect the levels of myr-Akt1 phosphorylation, suggesting that low levels of PDK1 were not limiting for Akt1 activation. The myr-Akt2 expression gave similar results despite the low expression levels we obtained. Instead, Akt1-DD was able to phosphorylate FOXO but not GSK3β, indicating a substrate selectivity for different Akt1 mutants. The expression of both myr-Akt1 and myr-Akt2 was not able to rescue the anchorage-independent growth after PDK1 silencing. Unexpectedly, the Akt1-DD mutant, as well, was not able to compensate the reduced PDK1 activity, although it was able to phosphorylate FOXO at a level comparable to PDK1 reexpression (Figure 6D).
In contrast, the expression of myr-Akt1 and myr-Akt2 in PDK1-silenced T-47D cells increased the phosphorylation of GSK3β and rescued the ability to grow in soft agar (Figure W7).
Differential Effects of Akt and PDK1 Inhibition on PDK1-Overexpressing Cells
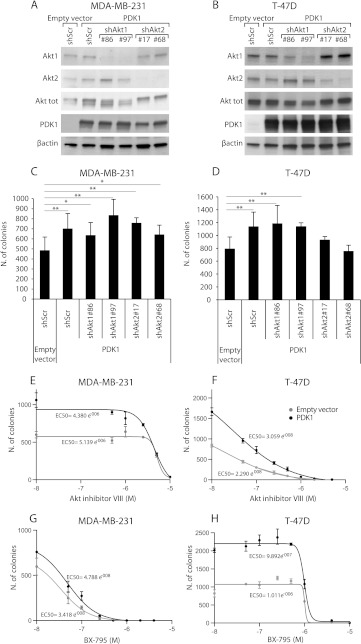
It has been recently demonstrated that PDK1 is overexpressed in a large proportion of human breast cancers [19. Therefore, we investigated the role of Akt in regulating the effects of PDK1 over-expression in anchorage-independent growth of MDA-MB-231 and T-47D cells. We stably silenced Akt1 and Akt2 using two different constructs per gene in cells overexpressing wild-type PDK1 (Figure 7, A and B). Down-regulation of both Akt1 and Akt2 did not halt the soft agar growth of MDA-MB-231 cells (Figure 7C). However, although Akt1 knockdown was ineffective, the Akt2 silencing inhibited the colony formation of PDK1-overexpressing T-47D cells (Figure 7D).
Figure 7.
Akt down-regulation and inhibition does not reduce anchorage-independent growth of PDK1-overexpressing MDA-MB-231. (A and B) PDK1-overexpressing MDA-MB-231 and T-47D cells were transduced with a scramble shRNA (shScr), two different Akt1 (shAkt1#86 and shAkt1#97) or Akt2 (shAkt2#17 and shAkt2#68) targeting shRNAs, lysed, and analyzed by immunoblot with the indicated antibodies. (C and D) Soft agar colony formation assay performed on MDA-MB-231 and T-47D cells transduced as indicated above. Only colonies larger than 100 µm diameter were counted. (E and F) Soft agar colony formation assay of MDA-MB-231 and T-47D cells transduced with PDK1 or empty vectors and treated with different concentrations of the Akt inhibitor VIII, PDK1 inhibitor, BX-795. EC50 is indicated. (G and H) Soft agar colony formation assay of MDA-MB-231 and T-47D cells transduced with PDK1 or empty vectors and treated with different concentrations of PDK1 inhibitor, BX-795. EC50 is indicated. *P <.05, **P <.01, ***P <.001.
Interestingly, treatment with an Akt inhibitor was almost completely ineffective in blocking the soft agar growth of MDA-MB-231, in a range of concentration compatible with the reported efficacy (Figure 7E), whereas it inhibited T-47D at lower concentrations (Figure 7F). In contrast, both T-47D and MDA-MB-231 cells were sensitive to the PDK1 inhibitor BX-795, but the former responded to lower concentrations (Figure 7, G and H). Overexpression of PDK1 shifted the dose-response curve increasing the EC50 in cells treated with BX-795. These data suggested that MDA-MB-231 are more sensitive to PDK1 inhibition than T-47D, and this effect is not superimposed to that of Akt inhibition.
Discussion
Although only sporadic PDK1 mutations have been found in tumors till now [36, PDK1 has been frequently suggested as a critical component of the oncogenic PI3K signaling in cancer progression [19,37–40. In this study, we demonstrate that PDK1 is required for anchorage-independent growth of breast cancer cells and tumor formation in mice. The reduction of PDK1 activity by shRNA and chemical inhibitors impairs the soft agar cell growth and sensitizes to apoptosis, particularly when induced by the absence of anchorage (anoikis). Nevertheless, the proliferation of adhering breast cancer cells is not regulated by PDK1. This suggests that PDK1 is involved in the antiapoptotic signaling rather than in the mitogenic pathway, in agreement with previous studies reporting a specific role of PDK1 in cell motility and invasion but not in proliferation [13,26,32,41.
Other studies have found PDK1 to be involved in the anchorage-independent growth of cells carrying PIK3CA mutations [19,40. However, our results demonstrate that breast cancer cells, independent of their PIK3CA mutational status, are as well dependent on PDK1 for in vitro tumorigenesis. Indeed, MDA-MB-231 cells, carrying K-RAS and p53 mutations, are more sensitive to PDK1 inhibition than breast cancer cells harboring PIK3CA mutation, such as T-47D. In contrast, the inhibition of Akt activity is poorly effective in blocking anchorage-independent growth of MDA-MB-231, whereas T-47D cells exhibit an elevated sensitivity to Akt inhibition. Consistently, Akt phosphorylation in MDA-MB-231 cells becomes clearly detectable only on acute stimulation with EGF but not under normal culture conditions, and notably, it does not change after PDK1 silencing both in cultured cells and in xenograft tumors.
Although the kinase activity of PDK1 has been considered the unique activity of this enzyme, recent publications spread light to different mechanisms that are independent from its kinase activity. PDK1 activates both ROCK1 [13 and Ral-GEF [12 through two different mechanisms that do not require kinase activity. Nevertheless, in our experimental model, we demonstrate that kinase activity of PDK1 is required for both anchorage-independent growth and in vivo tumor formation. The role of kinase domain is further supported by the results obtained with PDK1 inhibitors that, although lacking complete specificity for PDK1, inhibit soft agar growth and sensitize cells to anoikis.
Surprisingly, the PDK1 PH domain, which interact with PIP3 [6,42, is not involved in soft agar growth. Because PDK1 binding to PIP3 is required for Akt activation [43, these data suggest that Akt is not involved in PDK1-mediated tumorigenesis. Accordingly, we found that constitutive active mutants of Akt are not able to rescue the effects of PDK1 down-regulation on anchorage-independent growth. Moreover, we show that PDK1 is not a limiting factor for the phosphorylation of both wild-type and constitutive active Akt mutants. Actually, residual PDK1 is sufficient to support normal levels of Thr308 Akt phosphorylation in EGF-stimulated cells, in agreement with previously published results reporting normal Akt activation in PDK1-hypomorphic and RNAi-mediated PDK1 knockdown mice [44,45.
We can conclude that partial inhibition of PDK1 is sufficient to reduce breast cancer cell soft agar growth even when Akt is normally activated. Directly related to this conclusion are the results obtained by PDK1 overexpression. A large fraction of human mammary tumors have been described to have increased expression of PDK1 caused by gene copy number alteration or epigenetic modulations [19. However, it is largely unknown which mechanisms involved in cancer progression are activated by PDK1. Our results suggest that Akt is not the main substrate activated in this process because the effects of PDK1 overexpression are not affected by Akt knockdown or enzymatic inhibition. Currently, the nature of PDK1 substrate involved in the tumorigenic process remains elusive and requires further studies focused on its identification.
Several studies suggest PDK1 as an oncology target; however, they do not provide a definitive assessment of the targeting efficacy of PDK1. The in vivo pharmacological inhibition of PDK1 remains a challenge for the poor selectivity of existing drugs [26,46,47. Instead, the genetic approaches produced strong evidence about the role of PDK1 in PTEN-driven tumor progression. PDK1 hypomorphic mice, which express low levels of PDK1, when crossed to PTEN+/- mice suppress PTEN-driven tumorigenesis [37. Unexpectedly, a recent report demonstrated a lack of antitumor efficacy by RNAi-mediated long-term PDK1 knockdown in different mouse models of PTEN-deficient cancer [45. Notably, all these results have been obtained in tumor models dependent on PTEN deficiency. Here, we show that PDK1 is required for experimental tumor formation in the absence of any alteration of PI3K pathway. Both MDA-MB-231 parental breast cancer cells and their highly metastatic variant, LM2-4175 [31, are dependent on PDK1 for tumor growth in mouse. Therefore, the common idea of PDK1 as a potential therapeutic target in tumors with altered regulation of PI3K signaling should be overcome. Consistently, reduced levels of PDK1 are still sufficient to phosphorylate Akt in our experimental tumors, suggesting its involvement in other signaling pathways. This hypothesis is also supported by recent results reporting that the inhibition of PDK1 abrogates the rapamycin resistance of colon cancer in a PI3K and Akt-independent manner but anyhow dependent on its kinase activity [30. Notably, by reexpression of kinase-dead mutants, we clearly demonstrate that the phosphorylation ability of PDK1 is required for experimental tumor formation. Then, our results strongly support the efforts to discover specific PDK1 inhibitors and to develop the existing ones for preclinical studies in tumor models [48–50.
Supplementary Material
Abbreviations
- PDK1
3-phosphoinositide-dependent protein kinase 1
- PI3K
phosphatidylinositol 3 kinase
- PIP3
phosphatidylinositol 3,4,5 triphosphate
- PH
pleckstrin homology
- shRNA
short hairpin RNA
Footnotes
This work was supported in part by grants of Italian Association for Cancer Research (to F.B. and L.P.), Intramural Grant “5 xmille” 2008 Fondazione Piemontese per la Ricerca sul Cancro ONLUS (L.P.), Regione Piemonte (Ricerca Tecnologie Convergenti 2007, Grant PHOENICS, Piattaforme Tecnologiche per le Biotecnologie Grant Druidi, Industrial Research 2009 Grants BANP) and eLab Italian Fondazione CRT-Torino (F.B.), and Fondo per gli Investimenti della Ricerca di Base Grant RBAP11BYNP NEWTON (L.P. and F.B.), Compagnia San Paolo (to D.T.).
This article refers to supplementary materials, which are designated by Figures W1 to W7 and are available online at www.neoplasia.com.
References
- 1.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(suppl 1):12–19. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- 2.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 3.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 4.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 5.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 6.Currie RA, Walker KS, Gray A, Deak M, Casamayor A, Downes CP, Cohen P, Alessi DR, Lucocq J. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337(pt 3):575–583. [PMC free article] [PubMed] [Google Scholar]
- 7.Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Cohen P. Activation of serum-and glucocorticoidregulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339(pt 2):319–328. [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen CJ, Buch MB, Krag TO, Hemmings BA, Gammeltoft S, Frodin M. 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 1999;274:27168–27176. doi: 10.1074/jbc.274.38.27168. [DOI] [PubMed] [Google Scholar]
- 10.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 11.Biondi RM, Kieloch A, Currie RA, Deak M, Alessi DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 2001;20:4380–4390. doi: 10.1093/emboj/20.16.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian X, Rusanescu G, Hou W, Schaffhausen B, Feig LA. PDK1 mediates growth factor-induced Ral-GEF activation by a kinase-independent mechanism. EMBO J. 2002;21:1327–1338. doi: 10.1093/emboj/21.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10:127–137. doi: 10.1038/ncb1675. [DOI] [PubMed] [Google Scholar]
- 14.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G, Xing M, Mambo E, Huang X, Liu J, Guo Z, Chatterjee A, Goldenberg D, Gollin SM, Sukumar S, et al. Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Res. 2005;7:R609–R616. doi: 10.1186/bcr1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho S, Milanezi F, Costa JL, Amendoeira I, Schmitt F. PIKing the right isoform: the emergent role of the p110β subunit in breast cancer. Virchows Arch. 2010;456:235–243. doi: 10.1007/s00428-010-0881-0. [DOI] [PubMed] [Google Scholar]
- 17.Sun M, Wang G, Paciga JE, Feldman RI, Yuan ZQ, Ma XL, Shelley SA, Jove R, Tsichlis PN, Nicosia SV, et al. AKT1/PKBα kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159:431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacus SS, Altomare DA, Lyass L, Chin DM, Farrell MP, Gurova K, Gudkov A, Testa JR. AKT2 is frequently upregulated in HER-2/neu-positive breast cancers and may contribute to tumor aggressiveness by enhancing cell survival. Oncogene. 2002;21:3532–3540. doi: 10.1038/sj.onc.1205438. [DOI] [PubMed] [Google Scholar]
- 19.Maurer M, Su T, Saal LH, Koujak S, Hopkins BD, Barkley CR, Wu J, Nandula S, Dutta B, Xie Y, et al. 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma. Cancer Res. 2009;69:6299–6306. doi: 10.1158/0008-5472.CAN-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Hage JA, van den Broek LJ, Legrand C, Clahsen PC, Bosch CJ, Robanus-Maandag EC, van de Velde CJ, van de Vijver MJ. Overexpression of p70 S6 kinase protein is associated with increased risk of locoregional recurrence in node-negative premenopausal early breast cancer patients. Br J Cancer. 2004;90:1543–1550. doi: 10.1038/sj.bjc.6601741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, Sjostrom SK, Garraway LA, Weremowicz S, Richardson AL, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 22.Xie Z, Yuan H, Yin Y, Zeng X, Bai R, Glazer RI. 3-Phosphoinositide-dependent protein kinase-1 (PDK1) promotes invasion and activation of matrix metalloproteinases. BMC Cancer. 2006;6:77. doi: 10.1186/1471-2407-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin HJ, Hsieh FC, Song H, Lin J. Elevated phosphorylation and activation of PDK-1/AKT pathway in human breast cancer. Br J Cancer. 2005;93:1372–1381. doi: 10.1038/sj.bjc.6602862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Z, Zeng X, Waldman T, Glazer RI. Transformation of mammary epithelial cells by 3-phosphoinositide-dependent protein kinase-1 activates β-catenin and c-Myc, and down-regulates caveolin-1. Cancer Res. 2003;63:5370–5375. [PubMed] [Google Scholar]
- 25.Zeng X, Xu H, Glazer RI. Transformation of mammary epithelial cells by 3-phosphoinositide-dependent protein kinase-1 (PDK1) is associated with the induction of protein kinase Cα. Cancer Res. 2002;62:3538–3543. [PubMed] [Google Scholar]
- 26.Nagashima K, Shumway SD, Sathyanarayanan S, Chen AH, Dolinski B, Xu Y, Keilhack H, Nguyen T, Wiznerowicz M, Li L, et al. Genetic and pharmacological inhibition of PDK1 in cancer cells: characterization of a selective allosteric kinase inhibitor. J Biol Chem. 2011;286:6433–6448. doi: 10.1074/jbc.M110.156463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang K, Lu Y, Li X, Zeng X, Glazer RI, Mills GB, Fan Z. Differential roles of phosphoinositide-dependent protein kinase-1 and Akt1 expression and phosphorylation in breast cancer cell resistance to paclitaxel, doxorubicin, and gemcitabine. Mol Pharmacol. 2006;70:1045–1052. doi: 10.1124/mol.106.023333. [DOI] [PubMed] [Google Scholar]
- 28.Tseng PH, Wang YC, Weng SC, Weng JR, Chen CS, Brueggemeier RW, Shapiro CL, Chen CY, Dunn SE, Pollak M. Overcoming trastuzumab resistance in HER2-overexpressing breast cancer cells by using a novel celecoxibderived phosphoinositide-dependent kinase-1 inhibitor. Mol Pharmacol. 2006;70:1534–1541. doi: 10.1124/mol.106.023911. [DOI] [PubMed] [Google Scholar]
- 29.Iorns E, Lord CJ, Ashworth A. Parallel RNAi and compound screens identify the PDK1 pathway as a target for tamoxifen sensitization. Biochem J. 2009;417:361–370. doi: 10.1042/BJ20081682. [DOI] [PubMed] [Google Scholar]
- 30.Tan J, Lee PL, Li Z, Jiang X, Lim YC, Hooi SC, Yu Q. B55β-associated PP2A complex controls PDK1-directed Myc signaling and modulates rapamycin sensitivity in colorectal cancer. Cancer Cell. 2010;18:459–471. doi: 10.1016/j.ccr.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Primo L, di Blasio L, Roca C, Droetto S, Piva R, Schaffhausen B, Bussolino F. Essential role of PDK1 in regulating endothelial cell migration. J Cell Biol. 2007;176:1035–1047. doi: 10.1083/jcb.200607053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 34.Primo L, Roca C, Ferrandi C, Lanfrancone L, Bussolino F. Human endothelial cells expressing polyoma middle T induce tumors. Oncogene. 2000;19:3632–3641. doi: 10.1038/sj.onc.1203708. [DOI] [PubMed] [Google Scholar]
- 35.Casamayor A, Morrice NA, Alessi DR. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem J. 1999;342(pt 2):287–292. [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 37.Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(+/?) mice. Curr Biol. 2005;15:1839–1846. doi: 10.1016/j.cub.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 38.Finlay DK, Sinclair LV, Feijoo C, Waugh CM, Hagenbeek TJ, Spits H, Cantrell DA. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. J Exp Med. 2009;206:2441–2454. doi: 10.1084/jem.20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raimondi C, Chikh A, Wheeler AP, Maffucci T, Falasca M. A novel regulatory mechanism links PLCγ1 to PDK1. J Cell Sci. 2012 doi: 10.1242/jcs.100511. E-pub ahead of print May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biondi RM, Cheung PC, Casamayor A, Deak M, Currie RA, Alessi DR. Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA. EMBO J. 2000;19:979–988. doi: 10.1093/emboj/19.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McManus EJ, Collins BJ, Ashby PR, Prescott AR, Murray-Tait V, Armit LJ, Arthur JS, Alessi DR. The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J. 2004;23:2071–2082. doi: 10.1038/sj.emboj.7600218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawlor MA, Mora A, Ashby PR, Williams MR, Murray-Tait V, Malone L, Prescott AR, Lucocq JM, Alessi DR. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21:3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellwood-Yen K, Keilhack H, Kunii K, Dolinski B, Connor Y, Hu K, Nagashima K, O'Hare E, Erkul Y, Di Bacco A, et al. PDK1 attenuation fails to prevent tumor formation in PTEN-deficient transgenic mouse models. Cancer Res. 2011;71:3052–3065. doi: 10.1158/0008-5472.CAN-10-2282. [DOI] [PubMed] [Google Scholar]
- 46.Feldman RI, Wu JM, Polokoff MA, Kochanny MJ, Dinter H, Zhu D, Biroc SL, Alicke B, Bryant J, Yuan S, et al. Novel small molecule inhibitors of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2005;280:19867–19874. doi: 10.1074/jbc.M501367200. [DOI] [PubMed] [Google Scholar]
- 47.Arico S, Pattingre S, Bauvy C, Gane P, Barbat A, Codogno P, Ogier-Denis E. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J Biol Chem. 2002;277:27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 48.Najafov A, Sommer EM, Axten JM, Deyoung MP, Alessi DR. Characterization of GSK2334470, a novel and highly specific inhibitor of PDK1. Biochem J. 2011;433:357–369. doi: 10.1042/BJ20101732. [DOI] [PubMed] [Google Scholar]
- 49.Falasca M, Chiozzotto D, Godage HY, Mazzoletti M, Riley AM, Previdi S, Potter BV, Broggini M, Maffucci T. A novel inhibitor of the PI3K/Akt pathway based on the structure of inositol 1,3,4,5,6-pentakisphosphate. Br J Cancer. 2010;102:104–114. doi: 10.1038/sj.bjc.6605408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medina JR, Becker CJ, Blackledge CW, Duquenne C, Feng Y, Grant SW, Heerding D, Li WH, Miller WH, Romeril SP, et al. Structure-based design of potent and selective 3-phosphoinositide-dependent kinase-1 (PDK1) inhibitors. J Med Chem. 2011;54:1871–1895. doi: 10.1021/jm101527u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.