Abstract
The caudal gene family (in mice and humans Cdx1, Cdx2, and Cdx4) has been studied extensively during early development as regulators of axial elongation and antero-posterior patterning. In the adult, Cdx1 and Cdx2, but not Cdx4, have been intensively studied for their function in intestinal tissue homeostasis and the pathogenesis of gastrointestinal cancers. Involvement in embryonic hematopoiesis was first demonstrated in zebrafish, where cdx genes render posterior lateral plate mesoderm competent to respond to genes specifying hematopoietic fate, and compound mutations in cdx genes thus result in a bloodless phenotype. Parallel studies performed in zebrafish embryos and murine embryonic stem cells (ESC) delineate conserved pathways between fish and mammals, corroborating a BMP/Wnt-Cdx-Hox axis during blood development that can be employed to augment derivation of blood progenitors from pluripotent stem cells in vitro. The molecular regulation of Cdx genes appears complex, as more recent data suggest involvement of non-Hox–related mechanisms and the existence of auto- and cross-regulatory loops governed by morphogens. Here we will review the role of Cdx genes during hematopoietic development by comparing effects in zebrafish and mice and discuss their participation in malignant blood diseases.
Keywords: Cdx, hematopoiesis, leukemia, Hox, blood development
Introduction
The caudal (Cdx) family of DNA binding proteins was originally identified in Drosophila but homologues with conserved molecular structure and function have been described in several organisms. The three members of the Cdx family in mice and humans, Cdx1, Cdx2, and Cdx4, have been intensively studied for their involvement in axial elongation and early anteroposterior patterning via Hox gene regulation (reviewed by Young and Deschamps1). While Cdx single and compound deficient mice exhibit overt defects in vertebrae and limbs, studies from zebrafish and murine embryonic stem cells (ESC) indicate additional Cdx-driven patterning effects during the development of other mesoderm derivatives such as blood,2–4 kidney5 and cardiac6 cells. Moreover, cdx genes regulate the development of neural (e.g. neural tube and spinal cord) and endodermal tissues (e.g. gut) and play important roles in adult intestinal tissue homeostasis and the pathogenesis of gastrointestinal cancers (reviewed by Guo R. J., E. R. Suh and J. P. Lynch7).
The role of Cdx genes in the blood system is less well elucidated. Data from zebrafish models demonstrate that cdx genes regulate embryonic hematopoiesis through activation of downstream hox genes. While overexpression studies performed with murine ESC confirm these data, corresponding in vivo loss-of-function studies in mice are complicated by functional redundancy among the three Cdx family members. Consistent with the notion that reactivated developmental pathways can contribute to oncogenesis, emerging data indicate expression and functional roles of CDX2 in leukemia. In this review, we discuss the role of Cdx genes during hematopoietic development and their involvement in malignant blood disease.
Insights from knockout mouse models
During early development, Cdx genes follow a similar expression pattern to the developmentally related Hox genes, conferring positional identity to developing mesodermal tissues. In mice, expression is detected in the posterior epiblast and the overlying mesoderm at the posterior end of the primitive streak.1 During their development in the posterior growth zone, anterior trunk tissues are exposed to Cdx genes but, as cells move anteriorly, Cdx transcripts decay.8–10 Persistence of Cdx in the posterior region of the embryo and expression of more posterior Hox genes enable the development of posterior trunk mesoderm and tail tissues. The instructive function of Cdx and Hox genes strongly varies with the developmental stage. As such, overexpression of hox genes in the epiblast alters the contribution of cells to the mesoderm11 and overexpression at the mesoderm stage profoundly impacts morphogenesis of developing tissues such as vertebrae. However, later overexpression in already formed somites shows no effect.12
Cdx mutant mice show posterior body truncations involving the axial skeleton, the neuraxis and caudal uro-rectal structures. The severity of the phenotype depends on the individual Cdx gene and, consistent with the notion of redundancy, is more pronounced in compound gene knockouts.13,14 Studies on triple knockout mice are complicated by the essential role of Cdx2 during placenta development, resulting in lethality of the Cdx2−/− genotype at 3.5 days post coitum (dpc).15 More recently, inactivation of Cdx2 at post-implantation stages by a tamoxifen inducible Cre-system confirmed the axial truncation phenotype and the incomplete uro-rectal septation in Cdx2 deficient animals.16,17 Next to anterior homeotic shifts of the axial skeleton, polyp-like lesions with proximal endoderm have been described in the coecum of Cdx2+/−, suggesting anterior homeotic shifts in the intestinal mucosa.18 Indeed, Cdx1 and Cdx2 are expressed in a second wave starting with day 12.5 p.c. in elements of the developing gut and play important roles not only during gut formation but also in adult tissue homeostasis and carcinogenesis.19 The murine Cdx4 gene appears less potent than Cdx1 and Cdx2. Analyses of Cdx2/Cdx4 compound mutants revealed roles for Cdx4 during placenta development and confirmed redundant roles with Cdx2 during axial elongation. However, Cdx4−/− mice are born healthy and appear morphologically normal.20
In mice, the first hematopoietic cells arise in the yolk sac around 7.5 dpc, representing the primitive wave of hematopoiesis. The second wave of definitive hematopoiesis follows around 9 dpc from hematopoietic stem cells (HSC), which are formed in the aorto-gonado-mesonephros region and then relocate to other anatomic sites including the yolk sac, the fetal liver and, shortly before birth, the bone marrow as the main site of adult hematopoiesis (reviewed by Lengerke and Daley21). Single and compound Cdx deficient mice do not present overt hematopoietic phenotypes. However, careful analysis has revealed subtle defects such as reduced numbers of yolk sac-derived erythroid colonies in Cdx4-deficient versus wild-type mice.22 Functional redundancy between individual Cdx genes may mask effects in single or double knockout mice and targeted triple knockouts have not yet been analyzed.
The cdx-hox axis regulates embryonic hematopoiesis
The first link between cdx genes and developmental hematopoiesis was made in zebrafish. Homozygous zebrafish embryos carrying the autosomal recessive mutation kugelig (kgg), initially identified because of their tail defect were found to die early during development (day 5 to 10 post fertilization) and to exhibit pronounced anemia. In 2003, Davidson et al. identified loss of function mutations in the cdx4 gene as the causative mutation for the hematopoietic phenotype of the kgg zebrafish embryo.2 Zebrafish cdx4 expression occurs in the early gastrula and becomes restricted to the posterior-most cells during gastrulation and early somitogenesis, preceding the expression of hematopoietic markers.2 In vivo injection of cdx4 mRNA rescues hematopoiesis in kgg mutants and induces a “posteriorized phenotype” with ectopic expression of hematopoietic markers in wildtype fish. During development, hematopoietic cells share common progenitors with endothelial cells and arise from so-called scl+ hemangioblasts. Up to the 5 somite stage, cdx4 co-expresses with scl in the posterior blood islands, suggesting a role for cdx4 in regulation of scl.2 However, while in wild-type zebrafish embryos hematopoietic cells in the posterior lateral plate mesoderm were expanded by scl overexpression,23 no rescue of hematopoiesis occurred in kgg embryo injected with scl-mRNA, indicating that cdx4 is not a direct inducer of scl, or that other co-factors are needed to compensate for the loss of cdx4. Moreover, cdx4 mutations induce a posterior shift in the boundary between anteriorly localized scl+ cells giving rise to endothelial cells, and the more posterior population of cells, which display hemangioblastic properties. Thus, cdx4 disruption inhibits the formation of blood but not endothelial cells.2
Several developmental studies demonstrate that Cdx genes act as master regulators of Hox genes.13,24,25 Consistently, kgg mutants display profound alterations in hox expression domains with almost complete absence of intermediate and more posterior hox genes such as hoxb6b and hoxa9a, while ectopic cdx4 can restore hox expression patterns. Moreover, overexpression of individual target hox genes (e.g. hoxb7a, hoxa9a) also rescue the formation of gata1+ hematopoietic cells in kgg mutants, corroborating the role of a cdx-hox axis during blood development. Notably, disruption of the developmental blood program cannot be achieved by targeted inhibition of single hox genes, indicating redundant functions of target hox genes during blood development.2 Redundancy has also been observed between cdx genes. In zebrafish, the cdx4 mutation causes a severe but not complete loss of embryonic blood formation. Additional suppression of cdx1a in kgg mutants however induces a complete failure to specify blood and enhances the severity of hox gene deregulation.3
Shared upstream and downstream regulatory pathways
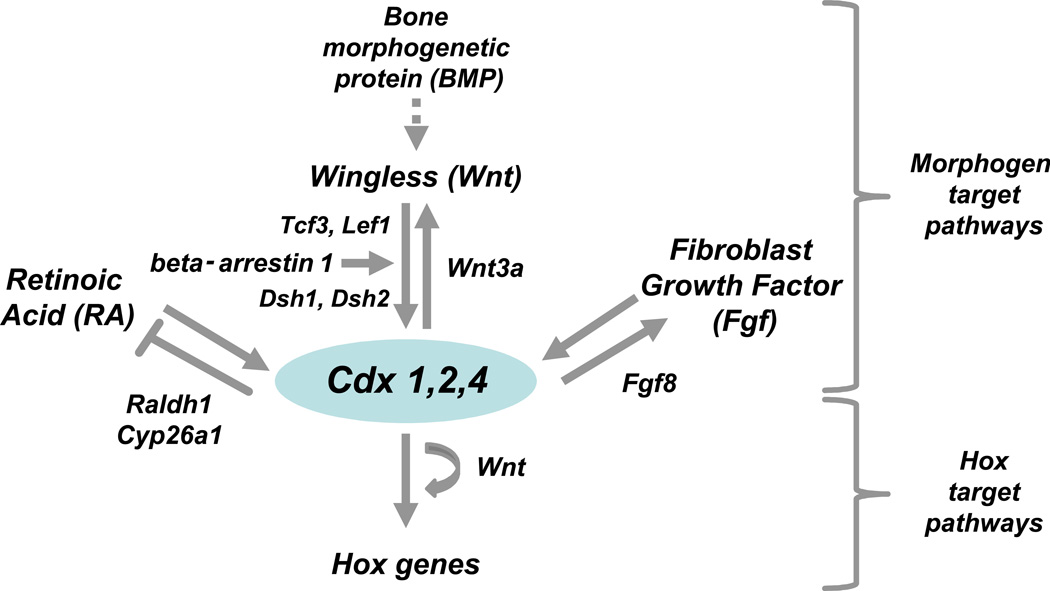
Individual Cdx genes display not only high amounts of functional redundancy but also share auto- and cross-regulatory molecular loops through direct binding to promoter sites26 or via regulation of molecular pathways that can act both up- and downstream (e.g. Wnt signaling).4,14,27
Interactions with the retinoic acid pathway
Classically, retinoic acid (RA) has been demonstrated as an upstream regulator of Cdx1 expression.28,29 Cdx1 responsiveness to excess RA has been documented in vivo and functional RA-responsive elements have been identified in the Cdx1 gene.8,28 In the zebrafish embryo, RA modulates the formation of gata1+ hematopoietic cells.30 Interestingly, treatment with RA inhibitors rescues hematopoiesis in cdx mutants, suggesting that cdx proteins act by modulating the RA pathway.30 During kidney development, cdx-dependent modulation of expression boundaries of the RA synthesizing and degrading raldh2 and cyp26a1 regulate the formation of distal tubule segments.5 In the posterior growth zone, concomitant regulation of cyp26a1 expression restraining RA signaling has been demonstrated to participate in the Cdx-Hox–orchestrated trunk-to-tail transition.14 Furthermore, cdx inhibition expands the formation of tbx5a+ anterior lateral plate cardiogenic mesoderm but effective differentiation of tbx5a+ cells into nkx2.5+ cardiac precursor cells requires simultaneous suppression of the retinoic acid pathway, supporting the notion that the cdx and RA pathways closely interact during development.6
Regulation by Wnt, BMP, and FGF
In murine embryoid bodies, BMP and Wnt are necessary for patterning hematopoietic fate from mesoderm. In detail, activation of BMP signaling induces Wnt3a and the canonical Wnt pathway, thereby activating the Cdx-Hox pathway.4 Consistently, mice engineered for loss of responsive elements in the Wnt effector Lef display phenotypic effects resembling Cdx1−/− mice29 and Wnt3a hypomorph mutants share phenotype with Cdx2/4 mutants. On the molecular level, direct interactions between Lef1 and Cdx1/4 have been demonstrated,4,31,32 but more complex molecular mechanisms also take place. As such, Wnt proteins have been recently shown to stimulate phosphorylation of the T cell factor (TCF) family member 3 (TCF3), thereby inducing its dissociation from the Cdx4 promoter and relieving inhibitory effects on Cdx4 expression.33,34 Surprisingly, in Cdx mutants, Cdx expression can be restored and phenotypic posterior defects corrected by posterior gain of function of Lef1,16 suggesting that Wnt signaling also acts downstream of Cdx proteins. These effects were confirmed by studies in ESC, where overexpression of Cdx1 and Cdx4 was shown to induce Wnt3a.4
Next to RA and Wnt, the Fgf pathway has been involved in activation of Cdx genes during development.35 More recently, Fgf molecules have also been implicated as downstream targets of Cdx. For example Fgf8, next to Wnt3a, T and Cyp26a1, is downregulated in Cdx2 conditional knockout mice and directly responds to Cdx2.17
Intersection with other transcriptional pathways regulating developmental hematopoiesis
More recently, the signal transduction protein and nuclear transcription regulator beta-arrestin 1 has been shown to regulate zebrafish hematopoiesis by binding and sequestration of the suppressive polycomb group recruiter YY1. Interestingly, overexpression of cdx4, hoxa9a, or hoxb4a was able to rescue hematopoiesis in beta-arrestin 1 suppressed fish, suggesting that beta-arrestin 1- YY1 effects are mediated by the cdx-hox axis.36 Arrestins are known mediators of several developmental pathways important for developmental hematopoiesis such as Wnt, Hedgehog, Notch, and TGF-β.37 In detail, beta-arrestin1 has been shown to interact with phosphorylated dishevelled proteins and thereby to modulate LEF-mediated transcriptional activity.38
Other data from zebrafish models suggest a linear activation of cdx4 driven by the TATA-box-binding-protein (TBP)-related factor 3, trf3 (or tbp2) through binding of mespa. Inhibition of trf3 during zebrafish development induces multiple defects, including depletion of cdx4 and a failure to undergo hematopoiesis, which can be rescued by mespa expression. Molecularly, mespa is a direct target of trf3 and itself directly activates cdx4. Consistently, ectopic mespa can rescue the developmental defects of trf3 suppressed zebrafish embryo, suggesting an ordered trf3-mespa-cdx4 molecular axis during zebrafish blood development.39 In mice, the Mesp1 gene has been reported as one of the earliest markers of cardiac development,40 but its involvement in hematopoiesis remains unclear. Interestingly, augmentation of Mesp1 levels can be achieved in differentiating mouse ESC by supplementation with high-dose BMP4, a condition that is also associated with enhanced blood formation (Grauer and Lengerke, unpublished). The molecular interactions between trf3 and classical morphogen pathways reported to induce cdx expression (Wnt, RA, and Fgf) are to our knowledge largely unknown.
To identify additional pathways interacting with cdx4 during primitive hematopoiesis, Paik et al. conducted a chemical screen for compounds that increase gata1 expression in cdx4 mutant zebrafish.41 Only two of 2640 compounds performed a rescue, both belonging to the psoralen family and showing anteroposterior patterning effects similar to DEAB, a known inhibitor of Raldh enzymes. Further analyses are required to discern the molecular mechanisms by which psoralens impact embryonic hematopoiesis, and whether they act downstream or parallel to the cdx4-hox pathway.
Cdx proteins direct blood development from pluripotent stem cells
In vitro differentiating pluripotent stem cells (PSC) model early stages of development21,42 and insights from developmental models can be used to modulate their efficient differentiation into tissues of interest. After exit from the undifferentiated stage, PSC initiate gastrulation and form primitive-streak like cells. During this process, AP-patterning can be imposed by exogenous supplementation with morphogens. For example, BMP4 can stimulate the generation of posterior mesoderm cells and afterwards direct their differentiation into blood cells by activating a Wnt-Cdx-Hox pathway.4 Accordingly, Cdx genes are expressed in waves, peaking during the developmental window of hemangioblast specification in murine and human differentiating PSC.43 In detail, BMP4 has been shown to induce Wnt3a and activate the Cdx-Hox pathway through the Wnt effector molecule Lef1.4 Overexpression of Cdx1 or Cdx4 fully rescues hematopoiesis in the presence of BMP and Wnt inhibition, while overexpression of Hoxa9 exhibits a partial rescue.4 Taken together, these data indicate the conserved functions of the BMP-Wnt-Cdx-Hox pathway during blood formation in vertebrates, from zebrafish to mammals.4
Single Cdx gene deficient mice present surprisingly modest hematopoietic phenotypes. Since overexpression studies indicate redundancy among Cdx genes, loss-of-function studies have been performed on not only single but also on compound Cdx-deficient ESC. As expected, in vitro differentiation of Cdx4−/−, Cdx1−/− and Cdx2−/− ESC reveal only subtle reductions in numbers of blood progenitors obtained in colony forming assays, primarily involving erythroid and mixed progenitor colonies, and a slight decrease in hematopoietic expansion in OP9-coculture assays. However, more profound suppression of hematopoiesis is achieved by knockdown of Cdx1 and/or Cdx2 by RNA interference in the background of Cdx4−/− ESC, culminating in an almost bloodless phenotype in Cdx4−/− cells treated with both Cdx2 and Cdx1 inhibitory RNAs.22 Molecularly, these changes are associated with decreased levels of posterior HoxA cluster genes. Confirming the notion of Cdx gene redundancy, ectopic Cdx4 can compensate not only for itself but also for Cdx compound deficiency. Notably, chimera studies performed with Cdx2−/− and wildtype cells show that the Cdx effect is cell autonomous, since no rescue is observed in Cdx2−/− cells exposed to a wild-type environment.22
To further dissect individual and redundant effects of Cdx family members, doxycycline-inducible Cdx1, Cdx2, and Cdx4 murine ESC have been analyzed in parallel. As previously reported, overexpression of Cdx1 and Cdx4 during their endogenous expression window strongly enhances the generation of hematopoietic progenitor cells. 4,44,45 Moreover, transient Cdx4 overexpression enhances lymphoid engraftment from transplanted ESC-derived hematopoietic progenitors, suggesting inductive patterning effects on definitive hematopoiesis and the generation of hematopoietic stem cells.22 Interestingly, induction of Cdx2 during embryoid body development shows suppressive effects on hematopoiesis, possibly mediated by induction of additional (anterior) Hox genes.44 Differential impacts of Cdx1, Cdx2, and Cdx4 are also detected in pre-formed CD41+ ckit+ blood progenitors isolated from embryoid bodies. All three Cdx genes strongly regulate the hematopoietic potential of CD41+ c-kit+ cells. However, while Cdx4 expand hematopoietic colonies, Cdx1 and Cdx2 have inhibitory effects, possibly by inhibiting differentiation. Notably, ectopic Cdx is unable to re-specify CD41− cells to hematopoietic fate.44
Together, these data demonstrate important roles for Cdx proteins in the specification of hematopoietic progenitor cells. The co-existence of both redundant and non-redundant Cdx-effects, which has been documented also in the gut,46 may explain the differential impact of the three Cdx genes on adult hematopoiesis and leukemia, which will be discussed below.
Cdx genes in adult hematopoiesis and hematopoietic malignancies
Several genes required during hematopoietic development, such as Scl/Tal-1 and AML/Runx1, have also been shown to regulate adult blood cell homeostasis and malignant transformation.47 The effect of the three murine Cdx genes on CD41+c-kit+ ESC derived blood progenitor cells strongly suggests an impact on hematopoietic cell biology beyond early developmental specification. However, in adult whole bone marrow samples of mice and humans, Cdx1 and Cdx4 can be detected in only low levels, and Cdx2 is absent. Lack of Cdx2 expression in healthy blood cells has been confirmed in analyses of sorted human CD34+ stem and progenitor cells, CD19+ B− and CD3+ T cells and in murine hematopoietic stem cells (HSC, Lin− Sca1+ c-kit+), common myeloid progenitors (CMP), granulocyte-macrophage progenitors (GMP), and megakaryocyte-erythroid progenitors (MEP). Interestingly, aberrant activation of CDX2 can be observed in most cases of acute myeloid and lymphoid leukemia, suggesting a contribution to oncogenic transformation of blood cells. In support of this hypothesis, retroviral overexpression of Cdx2 in murine whole bone marrow has been shown to enhance in vitro serial replating activity and to robustly induce acute myeloid leukemia in mice.48,49 Molecularly, induction of downstream Hox genes such as Hoxa10 and Hoxb8 is observed. Thus, acquisition of aberrant CDX2 expression has been proposed as the mechanism for the deregulated Hox expression observed in several leukemias.48,49 In support of this hypothesis, deletion of the Cdx2 N-terminal domain abrogated its ability to perturb Hox genes as well as its leukemogenic activity in mice.52 Additionally, expression analysis performed on 115 patients with AML showed a correlation between CDX2 and HOX gene levels.52
The mechanism by which CDX2 is reactivated in leukemia remains unclear. In AML, expression of CDX2 has been shown to be predominantly monoallelic.48 Amplifications at the 13q12.3 locus of the human CDX2 gene have been observed in only 3 out of 170 AML patients, all three belonging to the complex karyotype group and showing high CDX2 transcripts. Despite its first description in a t(12;13)(p13;q12) positive AML,53 CDX2 expression levels analyzed in samples from a cohort of 170 patients with AML showed highest expression associated with t(9;11)(p22;p23) translocations, followed by those with normal karyotype, t(15;17)(q22;q11–21), t(8;21)(q22;22), inv(16)(p13q22) or other chromosomal aberrations and complex karyotype, defined as 3 or more cytogenetic abnormalities in the absence of t(8;21), inv(16), t(15;17), or t(11q23). CDX2 expression levels correlate with disease burden and response to therapy, suggesting possible use of CDX2 as a marker of minimal residual disease in AML. CDX2 expression has also been detected in subgroups of patients with myelodysplasia and chronic myeloproliferative leukemia, where, in a low number of analyzed patients, increased CDX2 expression was associated with transit into secondary AML and respectively with blast and accelerated phase.48
As previously mentioned, the downstream pathways regulated by CDX2 in leukemia are likely to involve HOX genes. However, to our knowledge, no data is available showing that modulation of human CDX2 expression levels affect HOX gene expression patterns in human leukemic cells. Analyses available on CDX2 in human AML are limited to the correlative expression study mentioned above, showing association between CDX2 and HOX expression levels, and data from AML cell lines showing reduced in vitro growth and colony forming capacity after CDX2 suppression. No functional or molecular data exists in acute lymphoblastic leukemia (ALL), where CDX2 expression is also a common event.54,55 Unlike in AML, recent data in pediatric ALL suggest that HOXA9 expression correlates with better prognosis (P = 0.03, n = 61 pediatric ALL samples56), while CDX2 is associated with negative prognosis in adult ALL.55 Intriguingly, CDX2 expression was not found to correlate with specific HOX expression deregulation in pediatric ALL, which may explain why no associations between CDX2 expression and MLL rearrangements previously associated with HOX deregulation have been yet reported in leukemia.57,58 Moreover, CDX2 has been directly implicated in the up-regulation of Bcl-2in t(14;18) positive lymphoid cells,59 partially by interaction with C/EBP.60 Furthermore, miR-125b is involved as a CDX2 target regulating hematopoietic cell differentiation through repression of the core binding factor in hematopoietic malignancies.61 Finally, as reviewed above, more recent data from developmental studies indicates that major morphogen pathways, which also impact leukemogenesis, are downstream regulators of Cdx.
The roles of Cdx1 and Cdx4 in adult hematopoiesis and leukemia are less well defined. Expression of CDX1 and CDX4 has been noted in subgroups of AML in one report,62 but could not be confirmed in another.52 Recent data document low levels of CDX1 but not of CDX4 in a subgroup of pediatric ALL samples (Grauer and Lengerke, unpublished). In murine models, Cdx4 has also been shown to activate Hox genes and induce myeloid leukemia.62,63 However, leukemia occurs with a long latency of almost nine months, suggesting that Cdx4 by itself is insufficient to drive leukemogenesis.62 More robust contribution of Cdx4 to leukemia has been shown in combination with other genetic events such as MLL-AF964and Meis1a.62 Interestingly, Cdx4 has also proven to be a downstream target of the leukemogenic HoxA10.65 Confirming these data and the studies on embryonic hematopoiesis, no significant effect on during adult hematopoiesis is observed in single knockout Cdx4 mice.64
In summary, these data suggest that CDX2 is an important co-regulator of malignant transformation in blood cells, but its function and molecular regulation remain poorly understood. The role of CDX2 may be different in myeloid versus lymphoid neoplasia and its molecular effectors in leukemia likely include non-HOX targets. The role of CDX1 and CDX4 in adult blood cell homeostasis remain to be defined, however, low or negative expression levels in human leukemia samples suggest modest roles in leukemogenesis when compared to CDX2.
Conclusion
Cdx genes are major regulators of embryonic hematopoiesis in zebrafish and conserved roles have been demonstrated in murine pluripotent stem cells, where ectopic Cdx can be used to direct blood specification via activation of Hox genes. While functional redundancy among the individual Cdx family members and compensation during development make it difficult to discern loss-of-function phenotypes in single gene mutant mice, gene specific effects have been revealed by extensive in vitro analysis of differentiating pluripotent stem cells. These may be due to differential activation of Hox-gene combinations, or to other downstream targets such as morphogens or miRNAs. Non-Hox-related effects may explain the activity of Cdx2 in some leukemia types, especially in MLL-negative ALL and other leukemia in which the role of Hox genes is less prominent.
Figure 1.
Schematic view of the molecular interactions between Cdx and morphogen pathways.
Acknowledgments
C.L. is supported by grants from Deutsche Krebshilfe (Max-Eder program), the Deutsche Forschungsgemeinschaft (SFB773), the University of Tübingen (Fortüne program) and the Böhringer Ingelheim Foundation (Exploration Grant program). G.Q.D. is an investigator of the Howard Hughes Medical Institute and supported by grants from the NIH (R24DK092760, UO1-HL100001, RC4-DK090913, P50HG005550, and spezial funds from the ARRA stimulus package- RC2-HL102815), the Roche Foundation for Anemia Research, Alex’s Lemonade Stand, Ellison Medical Foundation, Doris Duke Medical Foundation, and the Harvard Stem Cell Institute. G.Q.D. is an affiliated member of the Broad Institute and a senior scientist of the Manton Center for Orphan Disease Research.
References
- 1.Young T, Deschamps J. Hox, Cdx, and anteroposterior patterning in the mouse embryo. Curr Top Dev Biol. 2009;88:235–255. doi: 10.1016/S0070-2153(09)88008-3. [DOI] [PubMed] [Google Scholar]
- 2.Davidson AJ, et al. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature. 2003;425:300–306. doi: 10.1038/nature01973. [DOI] [PubMed] [Google Scholar]
- 3.Davidson AJ, Zon LI. The caudal-related homeobox genes cdx1a and cdx4 act redundantly to regulate hox gene expression and the formation of putative hematopoietic stem cells during zebrafish embryogenesis. Dev Biol. 2006;292:506–518. doi: 10.1016/j.ydbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Lengerke C, et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Wingert RA, et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3:1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lengerke C, et al. Interactions between Cdx genes and retinoic acid modulate early cardiogenesis. Dev Biol. 2011;354:134–142. doi: 10.1016/j.ydbio.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 8.Gaunt SJ, Drage D, Cockley A. Vertebrate caudal gene expression gradients investigated by use of chick cdx-A/lacZ and mouse cdx-1/lacZ reporters in transgenic mouse embryos: evidence for an intron enhancer. Mech Dev. 2003;120:573–586. doi: 10.1016/s0925-4773(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 9.Gaunt SJ, Cockley A, Drage D. Additional enhancer copies, with intact cdx binding sites, anteriorize Hoxa-7/lacZ expression in mouse embryos: evidence in keeping with an instructional cdx gradient. Int J Dev Biol. 2004;48:613–622. doi: 10.1387/ijdb.041829sg. [DOI] [PubMed] [Google Scholar]
- 10.Gaunt SJ, Drage D, Trubshaw RC. cdx4/lacZ and cdx2/lacZ protein gradients formed by decay during gastrulation in the mouse. Int J Dev Biol. 2005;49:901–908. doi: 10.1387/ijdb.052021sg. [DOI] [PubMed] [Google Scholar]
- 11.Iimura T, Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 2006;442:568–571. doi: 10.1038/nature04838. [DOI] [PubMed] [Google Scholar]
- 12.Carapuco M, et al. Hox genes specify vertebral types in the presomitic mesoderm. Genes Dev. 2005;19:2116–2121. doi: 10.1101/gad.338705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian V, Meyer BI, Gruss P. Disruption of the murine homeobox gene Cdx1 affects axial skeletal identities by altering the mesodermal expression domains of Hox genes. Cell. 1995;83:641–653. doi: 10.1016/0092-8674(95)90104-3. [DOI] [PubMed] [Google Scholar]
- 14.Young T, et al. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev Cell. 2009;17:516–526. doi: 10.1016/j.devcel.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Strumpf D, et al. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 16.van de Ven C, et al. Concerted involvement of Cdx/Hox genes and Wnt signaling in morphogenesis of the caudal neural tube and cloacal derivatives from the posterior growth zone. Development. 2011;138:3451–3462. doi: 10.1242/dev.066118. [DOI] [PubMed] [Google Scholar]
- 17.Savory JG, et al. Cdx2 regulation of posterior development through non-Hox targets. Development. 2009;136:4099–4110. doi: 10.1242/dev.041582. [DOI] [PubMed] [Google Scholar]
- 18.Chawengsaksophak K, et al. Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci U S A. 2004;101:7641–7645. doi: 10.1073/pnas.0401654101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chawengsaksophak K, et al. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- 20.van Nes J, et al. The Cdx4 mutation affects axial development and reveals an essential role of Cdx genes in the ontogenesis of the placental labyrinth in mice. Development. 2006;133:419–428. doi: 10.1242/dev.02216. [DOI] [PubMed] [Google Scholar]
- 21.Lengerke C, Daley GQ. Patterning definitive hematopoietic stem cells from embryonic stem cells. Exp Hematol. 2005;33:971–979. doi: 10.1016/j.exphem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, et al. Cdx gene deficiency compromises embryonic hematopoiesis in the mouse. Proc Natl Acad Sci U S A. 2008;105:7756–7761. doi: 10.1073/pnas.0708951105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gering M, et al. The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 1998;17:4029–4045. doi: 10.1093/emboj/17.14.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charite J, et al. Transducing positional information to the Hox genes: critical interaction of cdx gene products with position-sensitive regulatory elements. Development. 1998;125:4349–4358. doi: 10.1242/dev.125.22.4349. [DOI] [PubMed] [Google Scholar]
- 25.Hunter CP, et al. Hox gene expression in a single Caenorhabditis elegans cell is regulated by a caudal homolog and intercellular signals that inhibit wnt signaling. Development. 1999;126:805–814. doi: 10.1242/dev.126.4.805. [DOI] [PubMed] [Google Scholar]
- 26.Savory JG, et al. Cdx4 is a Cdx2 target gene. Mech Dev. 2011;128:41–48. doi: 10.1016/j.mod.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Beland M, et al. Cdx1 autoregulation is governed by a novel Cdx1-LEF1 transcription complex. Mol Cell Biol. 2004;24:5028–5038. doi: 10.1128/MCB.24.11.5028-5038.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houle M, et al. Retinoic acid regulation of Cdx1: an indirect mechanism for retinoids and vertebral specification. Mol Cell Biol. 2000;20:6579–6586. doi: 10.1128/mcb.20.17.6579-6586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houle M, Sylvestre JR, Lohnes D. Retinoic acid regulates a subset of Cdx1 function in vivo. Development. 2003;130:6555–6567. doi: 10.1242/dev.00889. [DOI] [PubMed] [Google Scholar]
- 30.de Jong JL, et al. Interaction of retinoic acid and scl controls primitive blood development. Blood. 2010;116:201–209. doi: 10.1182/blood-2009-10-249557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilon N, et al. Wnt signaling is a key mediator of Cdx1 expression in vivo. Development. 2007;134:2315–2323. doi: 10.1242/dev.001206. [DOI] [PubMed] [Google Scholar]
- 32.Pilon N, et al. Cdx4 is a direct target of the canonical Wnt pathway. Dev Biol. 2006;289:55–63. doi: 10.1016/j.ydbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Hikasa H, et al. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell. 2010;19:521–532. doi: 10.1016/j.devcel.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ro H, Dawid IB. Modulation of Tcf3 repressor complex composition regulates cdx4 expression in zebrafish. EMBO J. 2011;30:2894–2907. doi: 10.1038/emboj.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keenan ID, Sharrard RM, Isaacs HV. FGF signal transduction and the regulation of Cdx gene expression. Dev Biol. 2006;299:478–488. doi: 10.1016/j.ydbio.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 36.Yue R, et al. Beta-arrestin1 regulates zebrafish hematopoiesis through binding to YY1 and relieving polycomb group repression. Cell. 2009;139:535–546. doi: 10.1016/j.cell.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs JJ, et al. Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell. 2009;17:443–4458. doi: 10.1016/j.devcel.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W, et al. beta-Arrestin1 modulates lymphoid enhancer factor transcriptional activity through interaction with phosphorylated dishevelled proteins. Proc Natl Acad Sci USA. 2001;98:14889–14894. doi: 10.1073/pnas.211572798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart DO, et al. Initiation of zebrafish haematopoiesis by the TATA-box-binding protein-related factor Trf3. Nature. 2007;450:1082–1085. doi: 10.1038/nature06349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.David R, et al. MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat Cell Biol. 2008;10:338–345. doi: 10.1038/ncb1696. [DOI] [PubMed] [Google Scholar]
- 41.Paik EJ, et al. A chemical genetic screen in zebrafish for pathways interacting with cdx4 in primitive hematopoiesis. Zebrafish. 2010;7:61–68. doi: 10.1089/zeb.2009.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Lengerke C, et al. Hematopoietic development from human induced pluripotent stem cells. Ann N Y Acad Sci. 2009;1176:219–227. doi: 10.1111/j.1749-6632.2009.04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKinney-Freeman SL, et al. Modulation of murine embryonic stem cell-derived CD41+c-kit+ hematopoietic progenitors by ectopic expression of Cdx genes. Blood. 2008;111:4944–4953. doi: 10.1182/blood-2007-11-124644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lengerke C, et al. The cdx-hox pathway in hematopoietic stem cell formation from embryonic stem cells. Ann N Y Acad Sci. 2007;1106:197–208. doi: 10.1196/annals.1392.006. [DOI] [PubMed] [Google Scholar]
- 46.Calon A, et al. Different effects of the Cdx1 and Cdx2 homeobox genes in a murine model of intestinal inflammation. Gut. 2007;56:1688–1695. doi: 10.1136/gut.2007.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izraeli S. Leukaemia -- a developmental perspective. Br J Haematol. 2004;126:3–10. doi: 10.1111/j.1365-2141.2004.04986.x. [DOI] [PubMed] [Google Scholar]
- 48.Scholl C, et al. The homeobox gene CDX2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis. J Clin Invest. 2007;117:1037–1048. doi: 10.1172/JCI30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rawat VP, et al. Ectopic expression of the homeobox gene Cdx2 is the transforming event in a mouse model of t(12;13)(p13;q12) acute myeloid leukemia. Proc Natl Acad Sci U S A. 2004;101:817–822. doi: 10.1073/pnas.0305555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rice KL, Licht JD. HOX deregulation in acute myeloid leukemia. J Clin Invest. 2007;117:865–868. doi: 10.1172/JCI31861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frohling S, et al. HOX gene regulation in acute myeloid leukemia: CDX marks the spot? Cell Cycle. 2007;6:2241–2245. doi: 10.4161/cc.6.18.4656. [DOI] [PubMed] [Google Scholar]
- 52.Rawat VP, et al. Overexpression of CDX2 perturbs HOX gene expression in murine progenitors depending on its N-terminal domain and is closely correlated with deregulated HOX gene expression in human acute myeloid leukemia. Blood. 2008;111:309–319. doi: 10.1182/blood-2007-04-085407. [DOI] [PubMed] [Google Scholar]
- 53.Chase A, et al. Fusion of ETV6 to the caudal-related homeobox gene CDX2 in acute myeloid leukemia with the t(12;13)(p13;q12) Blood. 1999;93:1025–1031. [PubMed] [Google Scholar]
- 54.Riedt T, et al. Aberrant expression of the homeobox gene CDX2 in pediatric acute lymphoblastic leukemia. Blood. 2009;113:4049–4051. doi: 10.1182/blood-2008-12-196634. [DOI] [PubMed] [Google Scholar]
- 55.Thoene S, et al. The homeobox gene CDX2 is aberrantly expressed and associated with an inferior prognosis in patients with acute lymphoblastic leukemia. Leukemia. 2009;23:649–655. doi: 10.1038/leu.2008.355. [DOI] [PubMed] [Google Scholar]
- 56.Starkova J, et al. HOX gene expression in phenotypic and genotypic subgroups and low HOXA gene expression as an adverse prognostic factor in pediatric ALL. Pediatr Blood Cancer. 2010;55:1072–1082. doi: 10.1002/pbc.22749. [DOI] [PubMed] [Google Scholar]
- 57.Muntean AG, Hess JL. The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol. 2012;7:283–301. doi: 10.1146/annurev-pathol-011811-132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ernst P, et al. An Mll-dependent Hox program drives hematopoietic progenitor expansion. Curr Biol. 2004;14:2063–2069. doi: 10.1016/j.cub.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Heckman CA, et al. A-Myb up-regulates Bcl-2 through a Cdx binding site in t(14;18) lymphoma cells. J Biol Chem. 2000;275:6499–6508. doi: 10.1074/jbc.275.9.6499. [DOI] [PubMed] [Google Scholar]
- 60.Heckman CA, Wheeler MA, Boxer LM. Regulation of Bcl-2 expression by C/EBP in t(14;18) lymphoma cells. Oncogene. 2003;22:7891–7899. doi: 10.1038/sj.onc.1206639. [DOI] [PubMed] [Google Scholar]
- 61.Lin KY, et al. miR-125b, a target of CDX2, regulates cell differentiation through repression of the core binding factor in hematopoietic malignancies. J Biol Chem. 2011;286:38253–38263. doi: 10.1074/jbc.M111.269670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bansal D, et al. Cdx4 dysregulates Hox gene expression and generates acute myeloid leukemia alone and in cooperation with Meis1a in a murine model. Proc Natl Acad Sci U S A. 2006;103:16924–16929. doi: 10.1073/pnas.0604579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan J, et al. Cdx4 and menin co-regulate Hoxa9 expression in hematopoietic cells. PLoS One. 2006;1:e47. doi: 10.1371/journal.pone.0000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koo S, et al. Cdx4 is dispensable for murine adult hematopoietic stem cells but promotes MLL-AF9-mediated leukemogenesis. Haematologica. 2010;95:1642–1650. doi: 10.3324/haematol.2010.023168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bei L, et al. HoxA10 activates CDX4 transcription and Cdx4 activates HOXA10 transcription in myeloid cells. J Biol Chem. 2011;286:19047–19064. doi: 10.1074/jbc.M110.213983. [DOI] [PMC free article] [PubMed] [Google Scholar]