Abstract
In spike timing-dependent plasticity (STDP), the order and precise temporal interval between presynaptic and postsynaptic spikes determine the sign and magnitude of long-term potentiation (LTP) or depression (LTD). STDP is widely utilized in models of circuit-level plasticity, development, and learning. However, spike timing is just one of several factors (including firing rate, synaptic cooperativity, and depolarization) that govern plasticity induction, and its relative importance varies across synapses and activity regimes. This review summarizes the forms, cellular mechanisms, and prevalence of STDP, and evaluates the evidence that spike timing is an important determinant of plasticity in vivo.
Keywords: synapse, Hebbian, anti-Hebbian, LTP, LTD, learning rule
In associative synaptic plasticity, simultaneous or rapid sequential activation of two synaptically connected neurons leads to a change in the strength of synapses between them. This type of plasticity has been proposed as a basis for learning and memory since the late 19th century (James, 1890). In his famous implementation of this rule, Hebb proposed that when cell A reliably contributes to spiking of postsynaptic cell B, the functional strength of the synapse from A to B is increased (Hebb, 1949). Others amended this idea to include weakening of ineffective synapses (Stent, 1973; von der Malsburg, 1973; Sejnowski, 1977; Bienenstock et al., 1982). It is now clear that associative synapse strengthening and weakening are implemented at many synapses by long-term potentiation (LTP) and depression (LTD).
Understanding the rules governing LTP and LTD induction is essential for understanding their function. Early work showed that high-frequency presynaptic firing drove LTP, while low-frequency firing drove LTD (e.g., Bliss and Lømo, 1973). The critical requirement at most synapses was found to be temporally correlated presynaptic spiking and postsynaptic depolarization, with strong depolarization leading to LTP, and weaker, more sustained depolarization leading to LTD (Wigström et al., 1986; Lisman, 1989; Artola et al., 1990). This reflects the molecular properties of postsynaptic NMDA receptors, which provide calcium to trigger LTP and LTD. While most early studies suggested a correlation requirement of about ± 100 ms for plasticity (Baranyi and Fehér, 1981; Gustafsson et al., 1987), a few studies noted an effect of spike order, with LTP occurring when presynaptic inputs led or were synchronous with postsynaptic spikes (evoked by a second pathway or by current injection), and LTD occurring when presynaptic input followed postsynaptic spikes (Levy and Steward, 1983; Debanne et al., 1994; Debanne et al., 1997). Precise timing- and order-dependent plasticity was predicted by Gerstner (1996) to explain development of phase locking in sound localization. In 1997, Markram et al. controlled pre- and postsynaptic spike timing using dual whole-cell recording, and discovered that the sign and magnitude of LTP and LTD indeed depended on the order and timing of pre- and postsynaptic spikes on the 10-ms time scale (Markram et al., 1997). This dependence was characterized in detail by Bi and Poo (1998) and named “spike timing-dependent plasticity” (STDP) by Song et al. (2000).
In canonical STDP, LTP occurs when presynaptic spikes (and associated EPSPs) lead postsynaptic spikes by up to ~20 ms, and LTD occurs when postsynaptic spikes lead presynaptic spikes and EPSPs by up to 20–100 ms, with a sharp (1–5 ms) transition between LTP and LTD (Markram et al., 1997; Bi and Poo, 1998; Celikel et al., 2004) (Fig. 1). Plasticity requires multiple (typically, 60–100) pre-post spike pairs. This is termed ‘Hebbian’ STDP because it strengthens synaptic inputs that lead (and therefore contribute to) postsynaptic firing, and depresses inputs that are uncorrelated with postsynaptic spikes. Not all STDP is alike, however. LTD in a cerebellum-like structure in the electric fish was also discovered in 1997 to be tightly spike timing-dependent, but in this case pre-leading-post spike order drove LTD (Bell et al., 1997), similar to anti-Hebbian LTD at the parallel fiber-Purkinje cell synapse in mammalian cerebellum. Thus, spike timing governs multiple forms of plasticity.
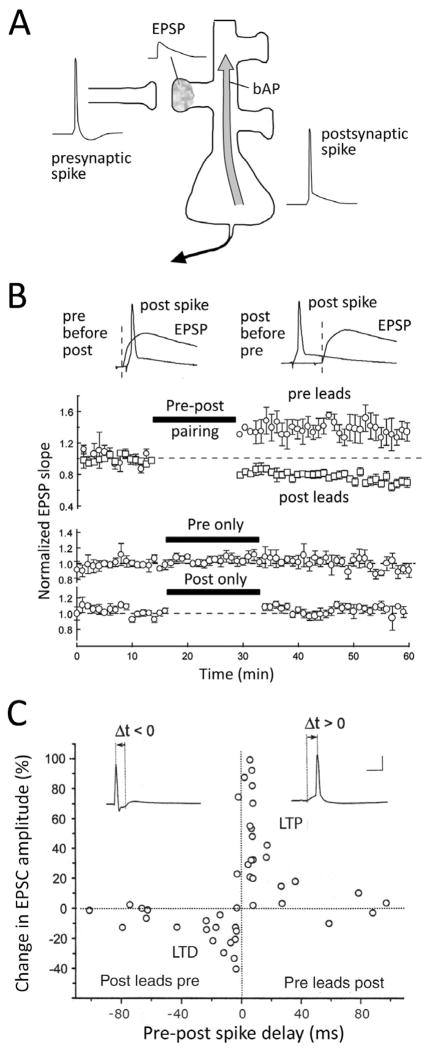
Figure 1. Spike timing-dependent plasticity.
A, Induction of STDP by pairing presynaptic spikes and associated EPSPs with postsynaptic spikes. bAP, back-propagating spike. B, Pre-leading-post spiking drives LTP, while post-leading-pre spiking drives LTD. Pre- or postsynaptic spikes alone do not alter synapse strength. From Feldman, 2000. C, STDP in hippocampal cell culture. Each symbol is one neuron. From Bi and Poo, 1998.
STDP has now been observed at > 20 different types of synapses from insects to mammals, and from striatum to neocortex. Its cellular basis is increasingly understood. It is widely utilized in computational models of neural network plasticity and learning, and its apparent simplicity has led some to propose that it is a universal “first rule” or kernel for associative plasticity. However, this view is oversimplified. Early studies recognized that spike timing is only one of several factors, including firing rate and dendritic depolarization, within a multi-factor plasticity rule (Markram et al., 1997; Sjöström et al., 2001). The relevance of spike timing varies across synapses, with strong spike timing-dependence (i.e., classical STDP) being restricted to specific dendritic zones and activity regimes. This review summarizes our understanding of STDP, and evaluates in detail the relative importance of spike timing vs. other factors for plasticity in vitro and in vivo. Many excellent reviews have been published on STDP (e.g., Abbott and Nelson, 2000; Dan and Poo, 2006; Letzkus et al., 2007; Caporale and Dan, 2008; Sjöström et al., 2008; Froemke et al., 2010a), including a comprehensive history (Markram et al., 2010).
Definition and forms of STDP
Canonical STDP is bidirectional and order-dependent, with pre-leading-post spiking driving LTP, and post-leading-pre spiking driving LTD. It also has precise temporal windows for LTP and LTD (10 to ~ 100 ms time scale) (Markram et al., 1997; Bi and Poo, 1998). This original definition has expanded to include other plasticity that depends on spike timing, but is not bidirectional or order-dependent (e.g., that contains only LTD). Several basic forms of STDP exist at different synapses (Fig. 2). Substantial variation exists within each form, presumably reflecting both synapse specialization and variation in experimental conditions.
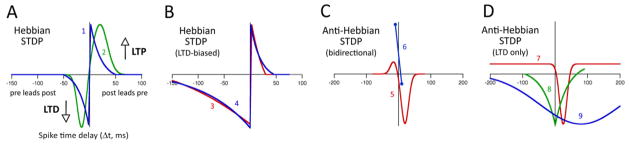
Figure 2. STDP exists in different forms.
Selected examples illustrating each form are shown schematically. A, Hebbian STDP that is equally balanced between LTP and LTD. 1, Froemke et al., 2002. 2, Fino et al. 2008. B, Hebbian STDP that is biased towards LTD. 3, Celikel et al., 2004. 4, Froemke et al., 2002. C, Anti-Hebbian STDP that contains both LTP and LTD. 5, Fino and Venance, 2005. 6, Letzkus et al. 2006. D, Anti-Hebbian STDP that contains only LTD (anti-Hebbian LTD). 6, Han et al., 2000. 7, Lu et al., 2007. 8. Safo and Regehr, 2008.
Hebbian STDP
In Hebbian STDP, LTP occurs when presynaptic spikes precede postsynaptic spikes by ~0 to 20 ms (defined as positive Δt), while LTD is induced when post leads pre by ~0 to 20–100 ms (negative Δt) (Fig. 2A, B). It is prevalent at excitatory synapses onto neocortical (Markram et al., 1997; Feldman, 2000; Sjöström et al., 2001; Nevian and Sakmann, 2006) and hippocampal pyramidal neurons (Bi and Poo, 1998; Nishiyama et al., 2000; Wittenberg and Wang, 2006), excitatory neurons in auditory brainstem (Tzounopoulos et al., 2004), parvalbumin-expressing fast-spiking striatal interneurons (Fino et al., 2008; 2009), and striatal medium spiny neurons in the presence of dopamine (Pawlak and Kerr, 2008; Shen et al., 2008). Some synapses exhibit long LTD windows producing a net bias toward LTD (Debanne et al., 1998; Feldman, 2000; Sjöström et al., 2001; Froemke et al., 2005). Hebbian STDP implements Hebb’s postulate by strengthening synapses whose activity is causal for postsynaptic spiking and weakening non-causal synapses (Abbott and Nelson, 2000). It can also occur at inhibitory synapses (Haas, 2006).
Anti-Hebbian STDP
In anti-Hebbian STDP, pre-leading-post spike order drives LTD. In a few cases, post-leading-pre spiking also drives LTP, resulting in bidirectional STDP opposite to Hebbian STDP (Fig. 2C). This has been observed at excitatory synapses onto striatal medium spiny neurons (Fino et al., 2005) and cholinergic interneurons (Fino et al., 2008), and can occur when EPSPs are paired with spike bursts at distal L2/3 synapses onto L5 pyramids in somatosensory cortex (Letzkus et al., 2006).
In most cases, however, anti-Hebbian STDP contains only the LTD component, and is often referred to simply as anti-Hebbian LTD (Han et al., 2000; Zhao and Tzounopolous, 2011; Requarth and Sawtell, 2011). This is often temporally asymmetric, with stronger LTD for pre-leading-post spike order (Fig. 2D). It occurs at excitatory inputs onto fast-spiking GABAergic interneurons in neocortex (Lu et al., 2007) and GABAergic cartwheel neurons in the dorsal cochlear nucleus (Tzounopoulos et al., 2004), as well as onto spiny stellate cells in somatosensory cortex (Egger et al., 1999). It also occurs at parallel fiber synapses onto Purkinje-like neurons in the electrosensory lobe of the electric fish, where it co-occurs with timing-independent LTP (Bell et al., 1997; Han et al., 2000). Classical parallel fiber-Purkinje cell LTD in cerebellum is anti-Hebbian, with maximal LTD when parallel fiber stimulation precedes postsynaptic spiking by 80–150 ms (Safo and Regehr, 2008; Wang et al., 2000). Anti-Hebbian LTD is prominent in distal dendrites of L2/3 and L5 cortical pyramids when synaptic cooperativity is minimal (Birtoli and Ulrich, 2004; Sjöström and Häusser, 2006).
STDP rules are synapse specific, but also malleable
Different forms of STDP are often intermixed in a seemingly synapse-specific manner. For example, parallel fiber synapses onto fusiform cells in the dorsal cochlear nucleus exhibit Hebbian STDP, while those onto cartwheel neurons show anti-Hebbian LTD (Tzounopoulos et al., 2004). STDP rules also vary by postsynaptic cell type in striatum (Fino et al., 2008; 2009). However, STDP is also dramatically shaped by dendritic depolarization and neuromodulation. For example, anti-Hebbian LTD on cortical pyramidal cells is converted into Hebbian STDP by manipulations that depolarize dendrites or promote the spread of back-propagating action potentials (bAPs) (Sjöström and Häusser, 2006; Letzkus et al., 2006; Zilberter et al., 2009), and dopamine alters the sign of STDP in the hippocampus (Zhang et al., 2009). The combination of synapse specificity and modulation may be useful in specializing different synapses for different types of information storage, while providing dynamic control over plasticity.
Spike timing as part of a multi-factor plasticity rule
STDP depends not only on spike timing, but also on firing rate, synaptic cooperativity, and postsynaptic voltage (Markram et al., 1997; Sjöström et al., 2001). Cooperativity refers to the need for multiple coactive synaptic inputs to generate sufficient depolarization (or spiking) to drive LTP in classical hippocampal experiments (McNaughton et al., 1978). In slice experiments, unitary connections (which lack cooperativity and generate only modest dendritic depolarization) exhibit Hebbian STDP only when pre- and postsynaptic spikes occur at moderate firing rates (10–20 Hz). Higher firing rates (>30 Hz) induce LTP independent of spike timing, and lower firing rates (<10 Hz) generate only LTD for pre-leading- post spike intervals (Markram et al., 1997; Sjöström et al., 2001; Wittenberg and Wang, 2006; Zilberter et al., 2009). Thus, Hebbian STDP operates primarily in a permissive middle range of firing frequency, superimposed on a standard Bienenstock, Cooper & Munro (BCM) plasticity function in which high firing rates drive LTP, and low firing rates drive LTD (Bienenstock et al., 1982) (Figure 3A, B). The underlying constraint is that LTP requires additional postsynaptic depolarization beyond a pre- and postsynaptic spike. This depolarization can also be provided by cooperative activation of multiple nearby synapses, which allows Hebbian STDP to be induced at lower frequency (Sjöström et al., 2001; Stuart and Häusser, 2001; Sjöström and Häusser, 2006) (Figure 3C). The firing rate and depolarization requirements demonstrate that a single postsynaptic somatic spike is not a sufficient signal for associative plasticity, nor the basis for cooperativity – multiple spikes are required, and these must interact with local dendritic depolarization produced in part by spatial summation of local synaptic potentials.
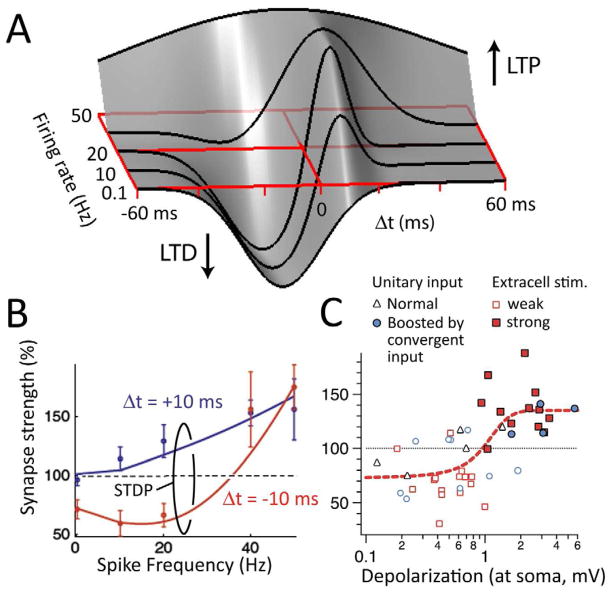
Figure 3. Plasticity is interdependent on spike timing, firing rate, and depolarization.
A, STDP at L5-L5 pyramid unitary synapses as a function of firing rate. Based on Sjöström et al., 2001. B, Joint firing rate-and timing-dependence for this same synapse modeled using a phenomenological multi-factor STDP rule (points show data, Sjöström et al., 2001; lines show model, Clopath et al., 2010). C, The LTP component of Hebbian STDP requires dendritic depolarization provided by synaptic cooperativity. Data are from distal synapses on L5 pyramidal cells (Δt= +10 ms) (Sjöström and Häusser, 2006). Open and filled symbols show inputs with weak and strong cooperativity, respectively.
Other factors governing STDP include the need for multiple spike pairings, and nonlinear summation of plasticity across spike pairs within natural spike trains (e.g., Froemke and Dan, 2002; Wang et al., 2005; Wittenberg and Wang, 2006). Though consistent rules for summation have not emerged across synapses, short-timescale nonlinearities predominate (Pfister and Gerstner, 2006; Clopath et al., 2010; Froemke et al., 2010b). Why STDP requires multiple pairings remains unclear. STDP also depends importantly on baseline synaptic weight (Bi and Poo, 1998; Sjöström et al., 2001; Morrison et al., 2008) and on neuromodulators, which can shape STDP both during and after spike pairing (Seol et al., 2007; Pawlak & Kerr, 2008; Shen et al., 2008; Cassenaer & Laurent, 2012).
These findings indicate that spike timing is not the sole or principal factor governing plasticity, but is one of several factors within a multi-factor rule. In this view, what is measured experimentally as STDP is not a distinct plasticity process, but is the spike timing-dependent component of a common process that also mediates rate- and depolarization-dependent LTP and LTD. This spike timing dependence varies across synapses and activity regimes, suggesting that spike timing will be a major determinant of plasticity in some instances, but a minor or negligible factor in others. This graded view of spike timing dependence differs from the concept of STDP as a fundamental kernel underlying rate-dependent plasticity (Froemke et al., 2002; Wang et al., 2005), or the idea that different synapses either express STDP or lack it.
Theoretical properties of STDP
The computational properties of Hebbian STDP have been reviewed in detail elsewhere (Abbott and Nelson, 2000; Morrison et al., 2008; Clopath et al., 2010). Briefly, Hebbian STDP implements the exact causal nature of Hebb’s postulate by strengthening synapses whose activity leads postsynaptic spikes, and weakening synapses whose activity lags postsynaptic spikes, which represent ineffective synapses onto otherwise active neurons (Abbott and Nelson, 2000; Song et al., 2000; van Rossum et al., 2000; Song and Abbott, 2001). Hebbian STDP that is biased toward LTD (e.g., Debanne et al., 1998; Feldman, 2000; Sjöström et al., 2001; Froemke et al., 2005) powerfully depresses inputs that are uncorrelated with postsynaptic spiking by this mechanism (Feldman, 2000).
In development, Hebbian STDP is appropriate to build topographic maps and receptive fields based on temporal correlations in input activity (Song et al., 2000; Song and Abbott, 2001; Gütig et al., 2003; Clopath et al., 2010), and implements competition between convergent inputs (Zhang et al., 1998; Kempter et al., 1999; Abbott and Nelson, 2000; Song et al., 2000). Some implementations of STDP can also reduce positive feedback instability of synapse strength and network activity that occur commonly with Hebbian learning rules (Song et al., 2000; van Rossum et al., 2000; Kempter et al., 2001; Song and Abbott, 2001).
In mature networks, Hebbian STDP supports learning of temporal sequences (Blum and Abbott, 1996; Rao and Sejnowski, 2001). This occurs because sequential activation of neurons in a recurrent network drives LTP at synapses in the forward direction but LTD in the reverse, thus creating directional connections (Clopath et al., 2010). The result is tuning for learned sequences, direction-selective visual responses, spontaneous repeated spike sequences for motor patterning, and the ability to predict future events from past stimuli (e.g., Mehta et al., 2000; Buchs and Senn, 2002; Engert et al., 2002; Fiete et al., 2010). STDP also enforces synchronous spiking during signal propagation in feedforward networks, which is a common feature in vivo. To understand this, consider a feedforward network in which neurons exhibit a range of spike latencies to a synchronous network input. With STDP, feedforward synapses onto neurons that spike earliest are weakened, thereby increasing spike latency, while synapses onto neurons that spike later are strengthened, reducing their spike latency (Gerstner et al., 1996; Suri and Sejnowski, 2002). This has been directly observed in the insect olfactory system (Cassenaer and Laurent, 2007). STDP can also mediate temporal difference learning (Rao and Sejnowski, 2003) and reinforcement learning (Farries and Fairhall, 2007; Izhikevich, 2007; Cassenaer and Laurent, 2012), and can tune neurons for temporal features of input (Masquelier et al., 2009).
For anti-Hebbian STDP, fewer computational properties are understood. In the cerebellum-like electrosensory lobe of electric fish, the LTD component of this plasticity (anti-Hebbian LTD) stores negative images of predicted sensory input, so that novel (unexpected) sensory inputs can be better represented (Roberts and Bell, 2000; Requarth and Sawtell, 2011). Anti-Hebbian LTD at parallel fiber-Purkinje cell synapses in mammalian cerebellum may perform a similar computation. Anti-Hebbian STDP is also prominent in distal dendrites of pyramidal cells (Sjöström and Häusser, 2006; Letzkus et al., 2006). This may serve to strengthen late-spiking distal (layer 1) inputs which would have been weakened under Hebbian STDP (Rumsey and Abbott, 2004). Alternatively, anti-Hebbian LTD may keep distal synapses weak, thereby requiring greater firing synchrony for effective transmission and specializing distal vs. proximal synapses for different computations (Sjöström and Häusser, 2006).
Theory has also shed light on the basis and functional properties of multi-factor STDP. In an early study, the firing rate- and timing-dependence of plasticity was predicted from dynamic activation and calcium-dependent inactivation of NMDA receptors during pre- and postsynaptic spike trains (Senn et al., 2000). More recent biophysically realistic models of NMDA receptors, AMPA receptors, and cannabinoid signaling support and extend this unified model of plasticity (Shouval et al., 2002; Badoual et al., 2006; Rachmuth et al., 2011; Graupner and Brunel, 2012). Functional consequences within large networks have been investigated with simpler phenomenological models. One such model (Clopath et al., 2010, built on earlier work by Pfister and Gerstner, 2006) is based on interaction of presynaptic spikes with instantaneous and time-filtered postsynaptic membrane potential. At the synapse level, the model predicts the timing, rate and voltage-dependence of plasticity. On the network level, this learning rule stores information about both slow input correlations and rapid spatiotemporal sequences, depending on the structure of spike train input, thus capturing functional aspects of rate-dependent plasticity and STDP (Clopath et al 2010).
Cellular machinery for STDP
Hebbian STDP at glutamatergic synapses is mediated by the same three signaling pathways that mediate most classical, correlation-dependent LTP and LTD. These are: 1) NMDA receptor (NMDAR)- dependent LTP and 2) NMDAR-dependent LTD, in which correlated presynaptic release and postsynaptic depolarization trigger calcium influx through postsynaptic NMDARs (and voltage-sensitive calcium channels, VSCCs). LTP vs. LTD induction is determined by the magnitude and time course of calcium flux, with brief, high calcium generating LTP, sustained moderate calcium generating LTD, and low calcium inducing no plasticity (Lisman, 1989; Yang et al., 1999). The primary expression mechanisms are postsynaptic, via addition or removal of postsynaptic AMPA receptors (AMPARs) and changes in single-channel conductance (Malinow and Malenka, 2002), though presynaptic expression can also occur. 3) Metabotropic glutamate receptor (mGluR)-dependent and/or cannabinoid type 1 receptor (CB1R)-dependent LTD, in which postsynaptic NMDARs are not involved, and LTD is expressed via a decrease in presynaptic transmitter release probability. This form is heterogeneous. In CB1Rdependent LTD, which is linked most strongly to STDP, postsynaptic calcium and mGluR activation trigger dendritic synthesis of endocannabinoids, which diffuse retrogradely to activate CB1Rs on the presynaptic terminal and drive a long-lasting decrease in release probability (Chevaleyre et al., 2006). Other forms of mGluR-LTD are CB1R-independent and postsynaptically expressed, but are less linked to STDP.
STDP is mediated by these three mechanisms, with postsynaptic spikes providing a critical component of postsynaptic depolarization for plasticity. There are two major, biochemically distinct forms of Hebbian STDP. One is composed of NMDAR-dependent LTP and NMDAR-dependent LTD (Fig. 4A, left). This occurs at CA3-CA1 hippocampal synapses and some synapses on neocortical L2/3 pyramidal cells (Nishiyama et al., 2000; Froemke et al., 2005). Here, the magnitude of the NMDAR calcium signal determines the sign of plasticity (along with calcium from VSCCs) (Lisman, 1989). With pre-leading-post spike order, the EPSP coincides with the bAP to produce a strong supralinear NMDAR calcium signal, while a post-leading-pre spike order triggers a weaker, sublinear calcium signal (Magee and Johnston, 1997; Koester and Sakmann, 1998; Nevian and Sakmann, 2006). This timing dependence is achieved by several mechanisms. Brief pre-leading-post spike intervals drive maximal calcium signals because (i) EPSPs activate voltage-gated sodium channels and/or inactivate A-type K+ channels, generating a brief temporal window in which bAPs—and therefore NMDAR currents—are boosted in dendritic branches whose activity was causal for postsynaptic spikes (Hoffman et al., 1997; Stuart and Häusser, 2001), (ii) the non-instantaneous kinetics of Mg++ unblock of NMDARs causes maximal NMDAR current when glutamate binding leads depolarization by a short interval (Kampa et al., 2004), and (iii) perhaps most importantly, AMPAR-mediated EPSPs provide local depolarization that critically boosts the supralinear interaction between NMDAR current and the bAP, so that LTP is induced when the AMPA-EPSP and bAP coincide (Fuenzalida et al., 2010; Holbro et al., 2010). Post-leading-pre spike order generates weaker calcium signals because (i) the EPSP coincides not with the bAP itself, but with the modest afterdepolarization following the bAP, generating NMDAR currents only modestly greater than would occur at Vrest (Karmarkar and Buonomano, 2002; Shouval et al., 2002), and (ii) at some synapses, calcium influx during the bAP causes calcium-dependent inactivation of NMDARs, so that presynaptic release evokes even less NMDAR current (Rosenmund et al., 1995; Tong et al., 1995; Froemke et al., 2005).
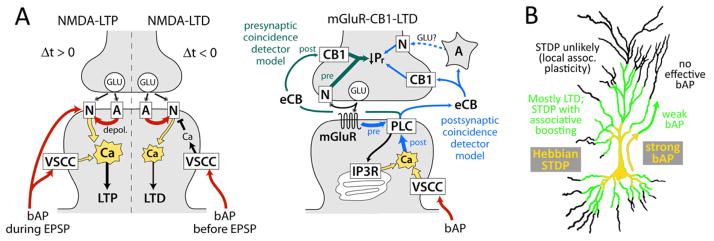
Figure 4. Cellular mechanisms for timing dependence of plasticity.
A, Biochemical signaling pathways for major forms of STDP. N and A, NMDA and AMPA receptors. Red, depolarization. For mGluR-CB1-LTD, the proposed presynaptic coincidence detector is in green, and the postsynaptic coincidence detector is in blue. A, astrocyte. Signals conveying pre- and postsynaptic spike timing in each model are labeled. B, Dendritic plasticity zones based on efficiency of bAP propagation through the dendrites.
A second form of Hebbian STDP is composed of NMDAR-dependent LTP and mGluR- and/or CB1R-dependent LTD (Fig. 4A, right). This occurs at several synapses in L2/3 and L5 of somatosensory and visual cortex, and at cortical synapses onto striatal medium spiny neurons. Here, postsynaptic NMDARs are required for spike timing-dependent LTP, but not LTD (Sjöström et al., 2003; Bender et al., 2006b; Nevian and Sakmann, 2006; Corlew et al., 2007; Rodríguez-Moreno and Paulsen, 2008; Fino et al., 2010). LTD instead requires postsynaptic group I mGluRs, their effector phospholipase C, low-threshold T-, R- or L-type VSCCs, and calcium release from IP3 receptor-gated internal stores (Bi and Poo, 1998; Nishiyama et al., 2000; Bender et al., 2006b; Nevian and Sakmann, 2006; Seol et al., 2007; Fino et al., 2010). Coincident activation of mGluRs and VSCCs synergistically activates PLC (Hashimotodani et al., 2005), leading to generation and release of the endocannabinoid (eCB) transmitter 2-arachidonoyl glycerol (2-AG) (Nakamura et al., 1999). Retrograde eCB signaling leads to activation of presynaptic CB1Rs, and LTD expression occurs by a decrease in presynaptic transmitter release probability (Sjöström et al., 2003; Bender et al., 2006b; Nevian and Sakmann, 2006; Rodríguez-Moreno and Paulsen, 2008; Shen et al., 2008; Fino et al., 2010). Thus, this form of STDP involves two separate coincidence detectors: NMDARs detect pre-leading-post spike intervals and exclusively trigger LTP, whereas a separate mechanism within the mGluR-VSCC-PLC-CB1 pathway detects post-leading-pre spike intervals and exclusively triggers LTD (Bender et al., 2006b; Nevian and Sakmann, 2006; Fino et al., 2010).
The mGluR-CB1R-dependent form of LTD is independent of postsynaptic NMDARs but often depends on presynaptic NMDARs (preNMDARs) (Sjöström et al., 2003; Bender et al., 2006b; Corlew et al., 2007; Rodríguez-Moreno and Paulsen, 2008). At synapses with this form of STDP, loading the NMDAR blocker MK-801 into the presynaptic neuron blocks only LTD, while MK-801 in the postsynaptic neuron blocks only LTP (Rodríguez-Moreno and Paulsen, 2008). PreNMDARs contain NR2B, NR2C/D, and/or NR3A subunits, and STDP-LTD is selectively blocked by NR2B and NR2C/D antagonists and in NR3 knockouts (Sjöström et al., 2003; Bender et al., 2006b; Banerjee et al., 2009; Larsen et al., 2011). In cerebral cortex, preNMDAR-dependent LTD is prominent in juveniles, and then declines in parallel with preNMDARs themselves (Corlew et al., 2007; Banerjee et al., 2009).
How does spike timing dependence arise for mGluR-CB1R-preNMDAR-LTD? In the presynaptic coincidence detector model, each postsynaptic spike evokes a brief eCB signal that activates presynaptic CB1Rs, each presynaptic spike supplies glutamate and depolarization to activate preNMDARs, and precise co-activation of CB1Rs and preNMDARs is required to drive LTD (Sjöström et al., 2003; Duguid and Sjöström, 2006). In the postsynaptic coincidence detector model, postsynaptic spikes activate VSCCs while presynaptic spikes activate mGluRs, and post-pre spike timing is computed postsynaptically by integration of mGluR and VSCC-derived calcium signals (Bender et al., 2006b, Nevian and Sakmann, 2006). The likely coincidence detector is PLC, which is a known molecular coincidence detector that responds synergistically to mGluR activation and cytosolic calcium, and which drives production of 2-AG (Hashimotodani et al., 2005). As a result, 2-AG synthesis and release occur only in response to appropriately timed pre- and postsynaptic spikes (Chevaleyre et al., 2006). The eCB signal then diffuses retrogradely to reduce release probability either by activating CB1Rs on presynaptic terminals (Bender et al., 2006b) or by activating CB1Rs on astrocytes which in turn signal to presynaptic terminals, perhaps via preNMDARs (Min and Nevian, 2012). Importantly, eCB activation of astrocytes is only observed during post-leading-pre spike pairing, and extracellular eCB accumulates slowly during the multiple spike pairings required for LTD induction. These observations suggest both coincidence detectors may contribute to LTD: the postsynaptic coincidence detector detects pre-post spike timing to generate a slow retrograde signal, while the presynaptic coincidence detector may restrict LTD to active presynaptic terminals, thus mediating synapse specificity.
Anti-Hebbian LTD is heterogeneous and involves several different CB1R-dependent and mGluR-dependent mechanisms. For example, anti-Hebbian LTD at excitatory synapses onto inhibitory cartwheel cells in the dorsal cochlear nucleus is presynaptic and CB1R-dependent. Higher stimulation frequencies evoke postsynaptic NMDAR-dependent LTP, echoing the coexistence of these mechanisms in Hebbian STDP in pyramidal cells (Tzounopoulos et al., 2007). Anti-Hebbian LTD in the electrosensory lobe of electric fish is also presynaptically expressed (Han et al., 2000). Anti-Hebbian LTD at cerebellar parallel fiber-Purkinje cell synapses involves postsynaptic mGluRs, VSCCs, IP3Rs, and presynaptic CB1R activation, but is expressed postsynaptically by AMPAR internalization (Safo and Regehr, 2005; Steinberg et al., 2006). Strong evidence suggests that the order-dependent coincidence detector is the IP3 receptor, which is co-activated by PLC-produced IP3 and VSCC-derived cytosolic calcium (Nakamura et al., 1999; Wang et al., 2000; Sarkisov and Wang, 2008). At other synapses, anti-Hebbian LTD involves postsynaptic mGluR signaling and sometimes IP3R signaling (Egger et al., 1999; Birtoli and Ulrich, 2004; Lu et al., 2007).
Thus, the timing dependence of plasticity emerges, in part, from well-known molecular coincidence detectors within classical LTP and LTD signaling pathways, including NMDARs, PLC, and IP3Rs. This is consistent with spike timing as one factor within a multi-factor plasticity process that is also driven by firing rate and depolarization. A second major source of precise time dependence is the dynamics of electrical signaling in dendrites, including interactions between AMPA-EPSPs, NMDARs, and bAPs.
Dendritic excitability and location dependence of STDP
In STDP, somatic action potentials backpropagate from the axonal initiation site to the dendrites, where they provide a key part of the associative signal for STDP induction (Magee and Johnston, 1997). However, bAPs are brief and propagate decrementally, typically losing 50% of amplitude within several hundred microns of the soma, and failing completely in the most distal branches (Spruston, 2008). This results in postsynaptic depolarization that is sufficient for LTD, but not for LTP, particularly at distal synapses. Full STDP requires enhancement of bAP propagation and/or additional sources of depolarization (Sjöström et al., 2001; Sjöström and Häusser, 2006). In L5 pyramidal cell distal dendrites, EPSPs occurring < 10 ms prior to the bAP enhance bAP amplitude 3-fold via recruitment of dendritic sodium channels (Stuart and Häusser, 2001). This enhancement is highly localized and is greater for larger EPSPs. This likely contributes to the time window and cooperativity requirement for spike timing-dependent LTP. In CA1 pyramidal cells, bAP enhancement also promotes LTP, but enhancement occurs by inactivation of A-type potassium currents (Watanabe et al., 2002).
bAPs must also interact with, and recruit, additional sources of depolarization for STDP. An obligate source is the AMPA-EPSP, which provides critical synapse-specific depolarization that summates with the bAP to activate NMDARs sufficiently for STDP-LTP (Holbro et al., 2010). In other cases, bAPs prime the dendrite to produce synaptically evoked calcium spikes which mediate STDP-LTP (Zhou et al., 2005; Kampa et al., 2006) For more on dendritic excitability and STDP, see Sjöström et al., 2008.
The decremental propagation of bAPs creates a profound spatial gradient of STDP in neurons. In L5 pyramidal cells in neocortex, brief pre- and postsynaptic spike trains evoke Hebbian STDP at proximal synapses (< 100 μm from soma), but progressively less LTP at more distal synapses. The most distal synapses (> 500 μm) show only anti-Hebbian LTD in response to pre-leading-post pairing. Distal LTD can be converted to LTP by supplying sufficient dendritic depolarization to either enhance bAP propagation (Sjöström and Häusser, 2006) or convert the single bAP into a dendritic-somatic spike burst (Letzkus et al., 2006). Smaller L2/3 pyramidal cells exhibit a similar trend in which distal synapses express less STDP and a broader LTD window than proximal synapses (Froemke et al., 2005).
Thus, decremental bAP propagation creates distinct dendritic plasticity zones in which different rules for synapse modification exist (Fig. 4B) (Kampa et al., 2007; Spruston, 2008). In general, the most proximal synapses experience the strongest bAPs and are expected to exhibit Hebbian STDP with minimal requirements for synaptic convergence and firing rate. More distal synapses will exhibit LTDbiased Hebbian STDP (Froemke et al., 2005) or anti-Hebbian LTD (Sjöström and Häusser, 2006) and will require high firing rates or strong synaptic convergence for Hebbian STDP. These synapses can exhibit anti-Hebbian STDP, if post-leading-pre firing drives synaptically evoked calcium spikes (Kampa et al., 2006; Letzkus et al., 2006). Very distal synapses may be largely outside the influence of bAPs, so that STDP is absent and plasticity is induced by cooperative firing of neighboring inputs that evokes dendritic sodium or calcium spikes or regenerative NMDA spikes (Golding et al., 2002; Gordon et al., 2006). The existence of different plasticity rules within dendritic regions may contribute to activity-dependent stabilization of different functional classes of synapses in these regions (Froemke et al., 2005). Modulation of dendritic excitability will regulate both the shape of STDP rules and the spatial extent of dendritic plasticity zones, including increasing or decreasing the prevalence of STDP relative to local, associative forms of plasticity.
Neuromodulation and STDP
Neuromodulation has robust effects on the spike timing dependence of plasticity. This includes gating of STDP, as in adult visual cortex slices, where exogenous activation of receptors coupled to adenylate cyclase (e.g., β-adrenergic receptors) and PLC (e.g., muscarinic acetylcholine receptors) are necessary for LTP and LTD, respectively, within Hebbian STDP (Seol et al., 2007). Dopamine gates Hebbian STDP at several synapses (e.g., Bissiere et al., 2003; Pawlak and Kerr, 2008; Shen et al., 2008). Neuromodulation can also alter the shape of STDP rules, including converting Hebbian STDP into anti-Hebbian LTD (Shen et al., 2008; Zhang et al., 2009, Zhao and Tzounopoulos, 2011). Remarkably, neuromodulation occurring up to several seconds after spike pairing can alter the sign of STDP in the insect olfactory system (Cassenaer and Laurent, 2012), providing a potential basis for reward-based learning via STDP (Izhikevich, 2007). These results suggest that neuromodulation should be considered an additional explicit factor in some STDP rules. For detailed review, see Pawlak et al., 2010.
Objections to STDP
Explicit objections have been raised to STDP. These derive from concerns that postsynaptic spikes and spike timing are relatively minor factors for plasticity under natural network conditions, and therefore that STDP is not a particularly accurate or useful description of natural plasticity (Lisman and Spruston, 2005; Lisman and Spruston, 2010; Shouval et al., 2010). These are summarized and addressed here.
-
The textbook model of STDP depends only on the timing of the bAP relative to the EPSP. However, bAPs are too brief and small to be sufficient for STDP. STDP depends strongly on other sources of depolarization, leading to dependence on firing rate and cooperativity. Thus, spike timing is not the primary determinant of plasticity.
While bAPs do not provide sufficient depolarization for STDP, they can control plasticity by interacting with or recruiting other forms of depolarization (e.g., AMPA-EPSPs, dendritic calcium spikes). Within multi-factor STDP rules, bAPs and spike timing are important factors determining the sign of plasticity over a relatively broad operating regime of firing rate (10–30 Hz in brief bursts, as low as 0.1 Hz at some synapses) and dendritic depolarization (2–10 mV) (Markram et al., 1997; Feldman, 2000; Sjöström et al., 2001). This dendritic depolarization could result from cooperative activation of as few as 2–10 inputs (assuming 0.2–1 mV unitary EPSP). Thus, while timing is not everything, it is one important thing for plasticity.
-
LTP and LTD induction protocols that use only synaptic stimulation, rather than direct current injection, to evoke a postsynaptic spike, do not require sodium spikes or bAPs. Instead, synaptic input evokes local dendritic calcium or NMDA spikes, and these induce plasticity. This indicates that STDP is not the basis of natural plasticity.
STDP cannot be claimed as a universal basis of plasticity. For example, distal synapses outside the range of bAP propagation exhibit LTP via local calcium spikes, not via STDP (Golding et al. 2002). The same is true for proximal synapses under conditions of especially strong convergence or when somatic spikes are suppressed (Golding et al., 2002; Gordon et al., 2006). This plasticity is computationally distinct from STDP because it implements associative plasticity between nearby synapses within individual dendritic branches or compartments, but not between spatially distant synapses (Hardie and Spruston, 2009) or between one synapse and somatic spiking that reflects overall synaptic drive. Classical single-pathway LTP and LTD experiments at Schaffer collateral-CA1 synapses only measure local associative plasticity, because inputs are spatially clustered, so that focal dendritic depolarization is the main determinant of plasticity.
The question is therefore whether pairing of two distant synaptic inputs (e.g. onto apical vs. basal dendrites) drives STDP using somatic spikes as part of the associative signal. Hebbian STDP has been induced in vivo in this manner in both hippocampus and Xenopus tectum, by activating a weak synaptic input at varying times relative to a strong synaptic input that evokes postsynaptic spikes (Levy and Steward, 1983, Zhang et al., 1998; Mu and Poo, 2006). However, it has not been proven that bAPs contribute to the associative signal for synaptically induced STDP. The multi-factor learning rule would suggest that within an appropriate firing rate and depolarization regime, the relative timing of EPSPs and bAPs is one factor controlling plasticity for synapses within effective bAP propagation range.
-
STDP is not likely to be relevant in vivo, because spontaneous synaptic activity and inhibition reduce bAP propagation even further than in brain slices.
In vivo, inhibition and increased dendritic conductance will reduce action potential back-propagation, further limiting STDP induction (Spruston, 2008). However, while the spatial range of the bAP will be less in vivo, evidence suggests that spike timing is still relevant. STDP can be induced in anesthetized animals with inhibition intact by pairing sensory stimulation with intracellularly- or extracellularly-evoked somatic spikes (Schuett et al., 2001; Meliza and Dan, 2006; Mu and Poo, 2006; Vislay-Meltzer et al., 2006; Jacob et al., 2007; Sawtell et al., 2007), or by pairing synaptic stimulation of one weak and one strong (spike-eliciting) pathway (Levy and Steward, 1983; Zhang et al., 1998; Mu and Poo, 2006). In addition, stimulus timing-dependent plasticity in awake animals and humans suggests indirectly that an STDP-like process is at work (e.g., Yao and Dan, 2001; Fu and Dan, 2002; McMahon and Leopold, 2012). Thus, while in vivo conditions are expected to reduce the prevalence of STDP, empirical measurements suggest that it remains relevant at least for some synapses.
-
STDP is not as computationally elegant as it may seem, because (1) the prevalence of pre- and postsynaptic spikes makes stored information too vulnerable to erasure, and (2) information can’t be read out without modifying stored information.
While this is true for the textbook STDP model, the firing rate dependence of STDP provides a simple solution, by implementing a higher activity threshold for plasticity. When firing rate is high, associative learning occurs. When firing rate is low, erasure is minimized, and information can be read out with single spikes without modifying synapses by inducing further STDP. Additional solutions may be found in the requirement for multiple pairings, the gating of STDP by neuromodulators, or the requirement for additional signals to achieve late-phase LTP.
In summary, while spike timing is clearly not the only factor governing LTP and LTD, it is one important factor at many synapses, at least under controlled conditions in vitro. It is therefore an empirical question whether spike timing is a major, minor, or negligible factor for plasticity under natural conditions in vivo. This evidence is summarized below.
Spike timing dependence of plasticity in vivo
Multiple classes of experiments support a role for spike timing in plasticity in vivo. In sensory-spike pairing, STDP is induced by presenting a sensory stimulus at a specific time delay relative to spikes in a single neuron, evoked by direct current injection. In stimulus timing-dependent plasticity, presentation of two precisely timed sensory stimuli alters sensory tuning with time- and order-dependence consistent with STDP. In psychophysical experiments, this same conditioning protocol alters sensory perception with STDP-like time- and order-dependence. Additional evidence for the importance of spike timing is found in development of visual motion tuning in Xenopus, sensory prediction in electric fish, map plasticity in sensory cortex, and olfactory learning in insects.
Sensory-spike pairing in vivo
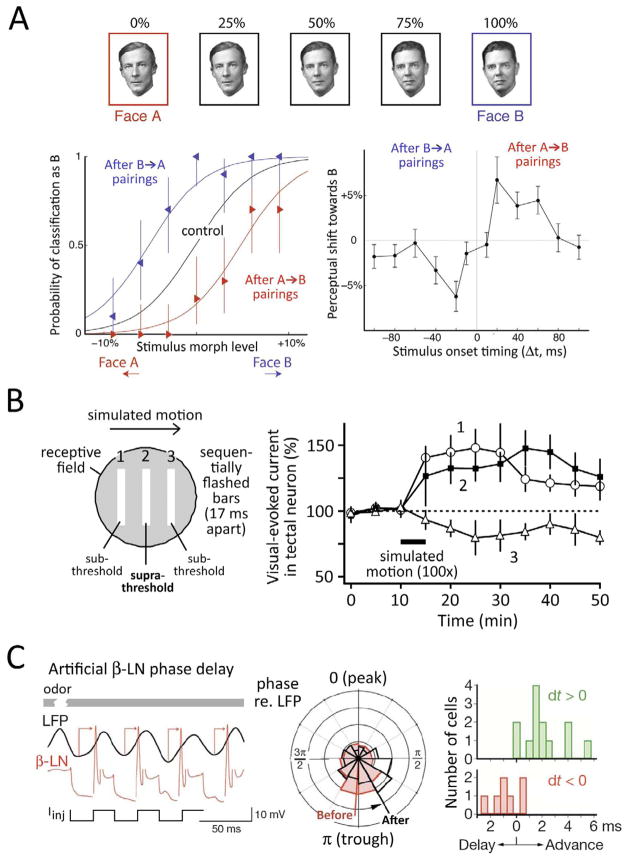
In the Xenopus visual system, spikes in retinal ganglion cells evoke EPSCs in tectal neurons. When a subthreshold retinal input is stimulated before a second, suprathreshold input that evokes a postsynaptic spike, the subthreshold response is potentiated (0 < Δt < 20 ms). When order is reversed, the subthreshold input is weakened (−20 < Δt < 0 ms) in a Hebbian STDP rule (Zhang et al., 1998). Identical STDP of visual-evoked synaptic currents occurs after pairing visual stimuli at precise times relative to postsynaptic spikes elicited by intracellular current injection (Mu and Poo, 2006). Such sensory-spike pairing within specific receptive field subregions increases or decreases visual responses to those subregions as predicted by STDP, thereby shifting tectal neuron receptive fields in vivo (Vislay-Meltzer et al., 2006). STDP is also observed with single, suprathreshold visual stimuli, which naturally elicit pre-leading-post spiking in tectal neurons, thus driving LTP of visual responses (Zhang et al., 2000).
Sensory-spike pairing also induces Hebbian STDP in cortical pyramidal cells in anesthetized rats. In primary visual cortex (V1), visual-evoked EPSCs recorded in L2/3 pyramidal cells are potentiated by pairing visual responses prior to intracellularly evoked postsynaptic spikes (0 < Δt < 20 ms), and are depressed by pairing after evoked spikes (−50 < Δt < 0 ms). For temporally extended visual responses, sensory-spike pairing potentiates components of the response occurring prior to the postsynaptic spike, and depresses components after the spike, consistent with STDP (Meliza and Dan, 2006). Orientation tuning can be modified by STDP, as shown by repeatedly pairing an oriented visual stimulus with extracellularly evoked spikes in V1 neurons. When visual responses precede spikes (Δt ≈ 20 ms), orientation tuning shifts toward the paired stimulus, but when the order is reversed (Δt ≈ −10 ms), tuning shifts away from the paired orientation, consistent with Hebbian STDP at intracortical synapses (Schuett et al., 2001).
Similar plasticity occurs in L2/3 pyramidal cells in rat somatosensory cortex. Pairing whiske-revoked postsynaptic potentials (wPSPs) following intracellularly evoked postsynaptic spikes (−30 ms < Δt < 0 ms) weakens wPSPs, but evokes no depression, and sometimes potentiation, when wPSPs lead spikes (Δt ≈ 20 ms) (Jacob et al 2007). This is reminiscent of Hebbian STDP at L4-L2/3 synapses in vitro, but with reduced LTP (Feldman, 2000). Significant LTP has been observed with this pairing protocol in older mice (Gambino and Holtmaat, SFN Abstract 2011). Pairing of spontaneous postsynaptic spikes prior to whisker deflections (−20 < Δt < 0 ms) also drives depression of whisker-evoked responses during extracellular recording (Jacob et al 2007).
STDP can also be induced in vivo in the locust olfactory system, at synapses from Kenyon cells (KCs) onto β-lobe neurons (β-LN). Associative strengthening of KC → β-LN synapses occurs when a subthreshold KC input precedes a second, suprathreshold KC input that evokes a spike in the β-LN. Pairing single KC inputs with a suprathreshold current pulse in the β-LN induces synapse-specific, Hebbian STDP of the KC synapse, with LTP occurring for pre-leading-post spike pairings (0 < Δt < 20 ms), and LTD for post-leading-pre pairings (−20 < Δt < 0 ms) (Cassenaer and Laurent, 2007).
Thus, sensory-spike pairing evokes STDP in vivo that can be directly observed at the synapse level. STDP in vivo is often smaller, briefer and more variable compared to in vitro brain slices, and the LTP component is less prominent (Feldman, 2000; Froemke and Dan, 2002; Meliza and Dan, 2006; Jacob et al., 2007). This may reflect reduced bAP propagation in vivo, or involvement of more distal synapses that show less STDP.
Stimulus timing-dependent plasticity
Two different visual stimuli that are sequentially flashed at a brief delay evoke spikes in two corresponding neuronal populations at the flashed interval (Fu et al., 2002; Yao and Dan, 2001). This may induce STDP at synapses between these populations. This was first tested in V1 of adult cats using extracellular single unit recording. The orientation tuning of a neuron was measured, followed by a conditioning period in which a non-optimal oriented stimulus (the “conditioned orientation”) was flashed just before (after) a preferred orientation stimulus. After 1600–3200 stimulus pairings, the neuron’s orientation tuning shifted toward (away) from the conditioned orientation, but only for pairing delays of < 20 ms, not 42 ms (Yao and Dan, 2001; Yao et al., 2004). This temporal order and timing dependence is consistent with Hebbian STDP at horizontal projections between neurons tuned to the trained orientations. Similarly, repeated sequential presentation of two neighboring retinotopic stimuli (< 50 ms delay, 800–1200 pairings) causes the spatial location of V1 receptive fields to shift toward the location activated first, consistent with Hebbian STDP at intracortical connections between nearby retinotopic loci in V1. Cross-correlation analysis confirmed that connections from early- to late-activated neurons functionally strengthen, while those in the opposite direction weaken, consistent with Hebbian STDP (Fu et al., 2002). Similar stimulus timing-dependent plasticity also occurs for frequency tuning in ferret primary auditory cortex (Dahmen et al., 2008). However, the magnitude of these plasticity effects is quite small (2° change in preferred orientation, < 2% shift in retinotopic position), and direct evidence that they represent STDP is lacking.
Psychophysical experiments show that stimulus timing-dependent plasticity alters visual perception in humans, also as predicted by Hebbian STDP. Conditioning with 300 pairs of oriented gratings (Δt < 20 ms) shifted perception of visual orientation toward the second orientation in the pair, which is consistent with standard population decoding models of the single-cell orientation tuning shifts in V1. This perceptual shift has the same order- and interval-dependence as STDP (Yao and Dan, 2001). Similar stimulus timing-dependent plasticity was observed for perception of retinotopic position (Fu et al., 2002). This phenomenon also occurs for high-level vision: in a face perception experiment, rapid serial presentation of two faces (100 pairings over ~ 2 min) biases face perception toward the second face presented, but only for pairing delays < 60 ms (McMahon and Leopold, 2012) (Fig. 5A). These findings argue that STDP-like plasticity occurs in the intact, attentive brain, and influences human visual perception, but again direct evidence that STDP is the causal cellular process is lacking.
Figure 5. Recent evidence for STDP in vivo.
A, Stimulus-timing dependent plasticity of face perception in humans. Subjects classified a series of morphed face images as being “more like face A” or “more like face B”. Sequential A→B or B→A pairing (Δt = 20 ms) biased perception toward the earlier-presented face, with a dependence on Δt similar to Hebbian STDP. From McMahon and Leopold, 2012. B, STDP induced by visual motion stimuli in Xenopus optic tectum. Simulated motion consisting of three rapidly flashed bars was presented within the receptive field of a tectal neuron (Δt = 17 ms between bars). Bars 1 and 3 were adjusted to evoke subthreshold PSPs, while bar 2 evoked spikes. Simulated motion training caused bar 1 and 2-evoked synaptic currents to increase, but bar 3-evoked synaptic currents to decrease, consistent with Hebbian STDP. No plasticity occurred when bar 2 did not evoke spikes (not shown). From Mu and Poo, 2006. C, STDP synchronizes β-LN firing in the locust olfactory system. Odors normally evoke β-LN spikes synchronized with the trough of the local field potential (LFP). Injecting current in a β-LN to phase-delay spikes (left) induces LTP at Kenyon cell→β-LN synapses, thus phase-advancing future odor-evoked spikes (middle). Spike phase shifts bidirectionally depending on Δt during conditioning, consistent with STDP (right). From Cassenaer and Laurent, 2007.
STDP in emergence of direction selectivity in Xenopus
Computationally, STDP can store information about spatiotemporal patterns of input activity (Blum and Abbott, 1996; Rao and Sejnowski, 2001; Clopath et al., 2010). A highly relevant spatiotemporal pattern is visual motion, and many neurons in adults are selective (tuned) for visual motion direction. Strong evidence links STDP to development of direction selectivity in Xenopus tectum.
In young Xenopus tadpoles, tectal neurons lack selectivity for visual motion direction. When a bar is repeatedly moved in a consistent direction across a young neuron’s receptive field, excitatory synaptic responses evoked by the trained movement direction are selectively increased, causing tectal neurons to become tuned for the trained direction (Engert et al., 2002). Several lines of evidence show that this is due to STDP at retinotectal synapses. First, retinotectal synapses exhibit robust Hebbian STDP in vivo, by pairing either electrically or visually evoked presynaptic spikes with postsynaptic spikes (Zhang et al., 1998; Zhang et al., 2000). Second, successful motion training occurs only when visual motion stimuli elicit postsynaptic spikes. Third, training causes retinal inputs active before evoked tectal spikes to be potentiated, while inputs active after tectal spikes are depressed, which is the hallmark of Hebbian STDP (Engert et al., 2002; Mu and Poo, 2006). The mechanics of this process have been determined using three sequentially flashed bars at different spatial positions to simulate visual motion (Fig. 5B). When sequentially flashed bars are paired with postsynaptic spikes that occur just after the center bar stimulus (either evoked by this stimulus or by current injection), responses to the first and second bars are increased, while responses to the third bar are decreased, as predicted by Hebbian STDP. Moreover, training with both real and simulated motion increases visual responses to flashed stimuli at spatial locations that are active prior to the receptive field center. This asymmetrically expands the receptive field toward earlier-activated spatial locations (Engert et al., 2002). In a computational model, STDP at retinotectal synapses explained these findings (Honda et al., 2011). These results strongly suggest that natural motion stimuli drive emergence of motion direction tuning via STDP.
Whether STDP drives development of motion direction selectivity in mammalian V1 is unclear. Motion direction tuning is absent in V1 at eye opening, and develops as a result of visual experience (White and Fitzpatrick, 2007). Training with visual motion stimuli immediately after eye opening induces motion direction tuning in young ferrets (Li et al 2008), as predicted by STDP (Buchs and Senn, 2002). However, whether STDP is the causal mechanism is not known. Some support for this hypothesis derives from a careful analysis of motion-selective properties of receptive fields in V1 in adult cats (Fu et al., 2004). Fu et al. found that complex cells received stronger rightward (leftward) motion input from visual field locations to the left (right) of receptive field center. This anisotropy in intracortical circuits is exactly as predicted by STDP driven by natural visual motion, and suggests that STDP was active during development of circuits for motion direction tuning (Fu et al., 2004).
Sensory map plasticity in neocortex
Experience and deprivation drive robust plasticity of cortical sensory maps that involves LTP and LTD at multiple synaptic loci. A major feature of plasticity is the active weakening of deprived inputs via LTD-like processes (Feldman, 2009). In rodent somatosensory (S1) cortex, STDP appears to be one mechanism driving synapse weakening. S1 contains a somatotopic map of the whiskers, with one cortical column per whisker. Deflection of a single whisker drives spikes in L4 followed by L2/3 of its corresponding column, due to feedforward intracolumnar excitatory projections from thalamus to L4 to L2/3. In addition, whisker deflection drives weaker responses in neighboring columns via horizontal cross-columnar projections. In juvenile rats, trimming or plucking a subset of whiskers weakens and shrinks the representation of deprived whiskers in L2/3, mediated in part by weakening of L4-L2/3 excitatory synapses within deprived columns (Feldman and Brecht, 2005). This weakening appears to represent CB1-LTD induced in vivo by sensory deprivation, because it occludes subsequent CB1-LTD, is expressed presynaptically by reduced release probability, and is prevented by CB1 antagonist treatment in vivo during whisker deprivation (Bender et al., 2006a; Feldman, 2009; Li et al., 2009).
In S1, L4-L2/3 synapses exhibit LTD-biased Hebbian STDP consisting of NMDAR-dependent LTP and CB1-LTD (Feldman, 2000; Bender et al., 2006b; Nevian and Sakmann, 2006). This STDP rule drives net LTD in response to either uncorrelated spiking or systematic post-leading-pre spiking (Feldman, 2000). Deprivation is likely to drive LTD in vivo via STDP, because whisker deprivation acutely alters mean L4 and L2/3 firing rate in S1 of awake rats only modestly, but powerfully alters L4-L2/3 spike timing. This was shown in anesthetized animals, where simultaneous deflection of all whiskers (to mimic normal whisking) evokes L4 spikes reliably before L2/3 spikes, whereas deflection of all but one whisker (to mimic acute whisker deprivation) immediately causes L4-L2/3 firing in the deprived column to decorrelate and firing order to reverse (Celikel et al., 2004). These findings suggest that STDP may be the primary mode for induction of LTD at L4-L2/3 synapses during deprivation-induced plasticity.
In V1, whether STDP contributes to deprivation-induced plasticity is unclear. In a focal retinal lesion model of plasticity, neurons in a visually deprived region of V1 acquire novel visual receptive fields via functional and anatomical reorganization of intracortical horizontal connections (Yamahachi et al., 2009). A computational study found that the pattern of acquired receptive fields was consistent with STDP at intracortical synapses, but not with classical correlation-dependent plasticity (Young et al., 2007). An STDP model of ocular dominance plasticity has been proposed in which monocular deprivation alters the precise temporal patterning of V1 spikes, thus inducing STDP in deprived-eye or open-eye pathways (Hensch, 2005; Hofer et al., 2006). Direct evidence for STDP is lacking, but the dynamics of plasticity in fast-spiking interneurons may be consistent with STDP (Yazaki-Sugiyama et al., 2009).
Place cells and sequence learning in hippocampus
Hebb predicted that the temporally asymmetric nature of synapse strengthening drives learning of sequences. Blum and Abbott (1996) modeled temporally asymmetric LTP in hippocampus, and showed that it learns sequences of spatial positions (i.e., spatial paths). They predicted that place fields will shift backward along well-learned paths due to LTP at synapses from earlier- to later-activated place cells. This shift was observed experimentally by Mehta et al. (1999), and was shown to be consistent with both simple Hebbian STDP (Mehta et al., 2000) and with a biophysically inspired, unified model of rate-and timing-dependent plasticity (Yu et al., 2006). Recently, Bush et al. (2010) showed that a rate- and timing-dependent plasticity model explains both learning of spatial sequences and increased functional connectivity between neurons with overlapping place fields. Thus, STDP is an appropriate candidate to mediate learning within the hippocampal cognitive map.
Sensory image cancellation in electric fish
Sensory systems must distinguish true external sensory stimuli from behaviorally irrelevant, self-generated sensory signals. Anti-Hebbian LTD plays a major role in this process, which has been studied in electrosensation in fish (for review, see Requarth and Sawtell, 2011). Weakly electric fish emit electric currents, and detect nearby objects by sensing object-induced distortions in the electric field via body surface electroreceptors. Self-motion (e.g., swimming) produces large changes in the electric field which could obscure external signals. Cerebellum-like circuits in the fish’s electrosensory lobe use anti-Hebbian LTD to generate a representation of predictable electrosensory input arising from motor commands, and to cancel self-generated electrosensory input. Purkinje-like medium ganglion (MG) cells receive strong electrosensory input at their basal dendrites, and a self-movement related input (corollary discharge and proprioceptive information) via sparse, parallel fiber inputs on their apical dendrites. Parallel fiber synapses exhibit anti-Hebbian LTD (Bell et al., 1997; Han et al., 2000). When a specific self-movement signal consistently precedes a spike-eliciting electrosensory input, those parallel fiber synapses weaken, thus generating a negative image of predicted electrosensory input in MG cell activation. This learned negative image summates with the total electrosensory input arriving at the basal dendrites, so that predicted electrosensory signals are canceled, and MG cell spiking reflects only unexpected stimuli.
The specific form of the anti-Hebbian LTD rule is consistent with this role: the narrow temporal window increases the accuracy of the negative image, and is broader in species that lack precisely timed corollary discharge signals (Harvey-Girard et al., 2010). The temporal asymmetry causes only self-motion inputs that immediately precede electrosensory input to be weakened, thus emphasizing causal relationships. A computational model of anti-Hebbian LTD predicts the formation of negative images as observed in vivo (Roberts and Bell, 2000).
This same circuit and anti-Hebbian LTD rule exist in other species, including in skates, where it cancels self-generated electrical signals associated with respiration during passive electrosensation. In mammals, a remarkably similar circuit exists in the dorsal cochlear nucleus, with anti-Hebbian LTD at parallel fiber synapses onto Purkinje-like cartwheel cells (Tzounopoulos et al., 2004). Function of this circuit is not well understood, but it may adaptively adjust for ear position during sound localization, or more speculatively may cancel self-generated auditory signals associated with chewing, respiration, or vocalization (Requarth and Sawtell, 2011).
Olfactory processing and learning in insects
The insect mushroom body contains hundreds of thousands of Kenyon cells (KCs) and is critical for associative olfactory learning. KCs sparsely encode olfactory input and make strong, convergent synapses on GABAergic β-lobe neurons (β-LNs) that provide a major inhibitory output to higher brain centers. During odor presentation, KC inputs evoke β-LN spikes that are highly synchronous across neurons, which is thought to facilitate feedforward information flow through olfactory circuits. KC→β-LN synapses exhibit robust Hebbian STDP, which enforces synchronous bLN spiking. This occurs because KC inputs onto late-spiking β-LNs undergo LTP, which phase-advances future KC-evoked spikes, while inputs onto early-spiking β-LNs undergo LTD, which phase-delays future spikes (Cassenaer and Laurent, 2007) (Fig. 5C). Enforcement of synchrony in feedforward networks is a basic property of Hebbian STDP (Suri and Sejnowski, 2002).
Recent work in this system focuses on a potential role of STDP in associative olfactory learning, in which presenting an appetitive reward just after a specific odor induces conditioned responses to the trained odor. During training, odor-evoked spikes in KCs precede reward delivery by several seconds, indicating that STDP between odor-evoked KC spikes and reward-related signals cannot mediate learning (Ito et al., 2008). The solution may be in the effects of octopamine, the putative positive reinforcement signal, on KC→β-LN STDP (Cassenaer and Laurent, 2012). Presentation of the training odor evokes a pre-leading-post spike sequence at corresponding KC→β-LN synapses. Normally, this would induce LTP via Hebbian STDP. However, octopamine (delivered up to tens of seconds after odor presentation) causes synapses that had experienced pre-post spike pairing to instead undergo anti-Hebbian LTD. Thus, octopamine is a third factor in the STDP rule that can act seconds after pre-post pairing to determine the sign of plasticity. (This suggests that spike pairing doesn’t directly induce LTP or LTD, but instead deposits a persistent synaptic tag that will drive plasticity upon later reinforcement, similar to Frey and Morris (1997).) The result is that octopamine selectively weakens KC outputs that represent the trained odor onto inhibitory β-LN output cells, which could be a potential trigger for odor-evoked conditioned behavior (Cassenaer and Laurent, 2012). Thus, neuromodulation of recently triggered STDP can solve the distal reward problem for reinforcement learning, as proposed computationally (Izhikevich, 2007).
STDP in human cortex
Evidence for STDP in humans is, by necessity, indirect. As discussed above, stimulus timing-dependent plasticity alters some aspects of low-level visual perception, including orientation and spatial position judgments, with order- and timing-sensitivity similar to STDP (Yao and Dan, 2001; Fu et al., 2002). A similar effect has also been observed in high-level vision for face perception (McMahon and Leopold, 2012).
Paired stimulation of somatosensory afferents in the median nerve and transcranial magnetic stimulation (TMS) of cerebral cortex also suggests timing-dependent plasticity in awake humans. When TMS is repeatedly applied to somatosensory cortex 10–20 ms prior to the median nerve-evoked potential, a long-lasting decrease in median nerve-evoked potentials results, while TMS within ± 5 ms of the evoked potential peak causes a long-lasting increase in evoked potential. This is interpreted to reflect Hebbian STDP in cortical circuits by pairing of median nerve-evoked EPSPs with TMS-evoked postsynaptic spiking, and is associated with changes in two-point discrimination threshold (Wolters et al., 2005; Litvak et al., 2007). In motor cortex, similar pairing bidirectionally alters the amplitude of motor-evoked potentials (Wolters et al., 2003). While these phenomena exhibit timing-dependence similar to STDP, whether they represent STDP induced at cortical synapses is unknown.
Conclusions and new questions
Fifteen years after the discovery of STDP, it is clear that spike timing is an important factor governing LTP and LTD induction at many synapses. However, STDP is neither the fundamental kernel of all plasticity, nor a distinct plasticity process from classical rate- or correlation-dependent plasticity. Instead, what is measured as STDP is the spike timing-dependent component of a multi-factor plasticity process that depends jointly on firing rate, spike timing, dendritic depolarization and synaptic cooperativity. The magnitude and shape of spike timing dependence varies across synapse classes, dendritic locations, and activity regimes, with the basic forms shown in Figure 2. Thus, spike timing is one important factor for plasticity, but is not universal or even always dominant. Theory suggests unique benefits of spike timing dependence, including network stability, competition, sequence learning and prediction. These benefits may present when even a subpopulation of synapses shows timing-dependent plasticity. The computational effects of dendritic STDP gradients remain incompletely understood.
Spike timing dependence originates in both molecular coincidence detection within classical LTP/LTD pathways (e.g., by NMDA receptors) and the temporal requirements for dendritic electrogenesis (e.g., transient boosting of bAPs by EPSPs). Important mechanistic questions remain. What is the mGluR- and VSCC-dependent coincidence detection mechanism that drives eCB release for spike timing-dependent, CB1-dependent LTD? How do presynaptic NMDARs function in plasticity? How do neuromodulators change the sign of STDP when delivered minutes after spike pairing?
Functionally, is spike timing is a major factor governing plasticity under natural conditions in vivo (Lisman & Spruston, 2010)? Evidence suggests that it is, for some forms of plasticity. The strongest direct evidence for STDP induced purely by natural stimuli is in development of motion direction selectivity in Xenopus (Engert et al., 2002; Mu and Poo, 2006). STDP can also be induced by spiking of two convergent synaptic pathways in vivo (Levy and Steward, 1983; Zhang et al., 1998), suggesting broad relevance, but this needs to be tested further. A prediction is that associative plasticity between distant synapses requires STDP, while that between nearby synapses is based on local dendritic signals rather than somatic spikes or their timing. Copious other evidence implies a role for spike timing in natural plasticity, but is only correlative. This includes stimulus timing-dependent plasticity in sensory cortex, which bears strong resemblance to Hebbian STDP, experience-dependent shifts in hippocampal place fields, plasticity of odor responses during insect olfactory learning, and deprivation-induced map plasticity in cortex. In cerebellum-like circuits in fish, anti-Hebbian LTD is beautifully suited to explain sensory cancellation, but causal evidence is again lacking. Proof will not come from selective blockade of STDP (which lacks unique cellular plasticity mechanisms), so clever strategies must be developed. One strategy is already apparent but is rarely used: to measure the precise temporal patterns of spiking associated with learning in vivo, to see if they are consistent with STDP. Another approach may be to use optogenetic manipulations to edit spike timing during natural learning.
Acknowledgments
Supported by NSF grant #SBE-0542013 to the Temporal Dynamics Of Learning Center, and NIH R01 073912. I thank Daniel Shulz, Vincent Jacob, Vanessa Bender and Kevin Bender for many discussions. I apologize for omitting important studies due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl):1178–83. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- Artola A, Bröcher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Meredith RM, Rodríguez-Moreno A, Mierau SB, Auberson YP, Paulsen O. Double dissociation of spike timing-dependent potentiation and depression by subunit-preferring NMDA receptor antagonists in mouse barrel cortex. Cereb Cortex. 2009;19:2959–69. doi: 10.1093/cercor/bhp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badoual M, Zou Q, Davison AP, Rudolph M, Bal T, Frégnac Y, Destexhe A. Biophysical and phenomenological models of multiple spike interactions in spike-timing dependent plasticity. Int J Neural Syst. 2006;16:79–97. doi: 10.1142/S0129065706000524. [DOI] [PubMed] [Google Scholar]
- Baranyi A, Fehér O. Synaptic facilitation requires paired activation of convergent pathways in the neocortex. Nature. 1981;290:413–5. doi: 10.1038/290413a0. [DOI] [PubMed] [Google Scholar]
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–81. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- Bender KJ, Allen CB, Bender VA, Feldman DE. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci. 2006a;26:4155–65. doi: 10.1523/JNEUROSCI.0175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006b;26:4166–77. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–72. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtoli B, Ulrich D. Firing mode-dependent synaptic plasticity in rat neocortical pyramidal neurons. J Neurosci. 2004;24:4935–40. doi: 10.1523/JNEUROSCI.0795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum KI, Abbott LF. A model of spatial map formation in the hippocampus of the rat. Neural Comput. 1996;8:85–93. doi: 10.1162/neco.1996.8.1.85. [DOI] [PubMed] [Google Scholar]
- Buchs NJ, Senn W. Spike-based synaptic plasticity and the emergence of direction selective simple cells: simulation results. J Comput Neurosci. 2002;13:167–86. doi: 10.1023/a:1020210230751. [DOI] [PubMed] [Google Scholar]
- Bush D, Philippides A, Husbands P, O’Shea M. Spike-timing dependent plasticity and the cognitive map. Front Comput Neurosci. 2010;4:142. doi: 10.3389/fncom.2010.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike Timing-Dependent Plasticity: A Hebbian Learning Rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Cassenaer S, Laurent G. Hebbian STDP in mushroom bodies facilitates the synchronous flow of olfactory information in locusts. Nature. 2007;448:709–13. doi: 10.1038/nature05973. [DOI] [PubMed] [Google Scholar]
- Cassenaer S, Laurent G. Conditional modulation of spike-timing-dependent plasticity for olfactory learning. Nature. 2012;482:47–52. doi: 10.1038/nature10776. [DOI] [PubMed] [Google Scholar]
- Celikel T, Szostak VA, Feldman DE. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat Neurosci. 2004;7:534–41. doi: 10.1038/nn1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Clopath C, Büsing L, Vasilaki E, Gerstner W. Connectivity reflects coding: a model of voltage-based STDP with homeostasis. Nat Neurosci. 2010;13:344–52. doi: 10.1038/nn.2479. [DOI] [PubMed] [Google Scholar]
- Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–45. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen JC, Hartley DE, King AJ. Stimulus-timing-dependent plasticity of cortical frequency representation. J Neurosci. 2008;28:13629–39. doi: 10.1523/JNEUROSCI.4429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–48. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM. Asynchronous pre- and postsynaptic activity induces associative long-term depression in area CA1 of the rat hippocampus in vitro. Proc Natl Acad Sci U S A. 1994;91:1148–52. doi: 10.1073/pnas.91.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM. Bidirectional associative plasticity of unitary CA3-CA1 EPSPs in the rat hippocampus in vitro. J Neurophysiol. 1997;77:2851–5. doi: 10.1152/jn.1997.77.5.2851. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gähwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol. 1998;507 (Pt 1):237–47. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid I, Sjöström PJ. Novel presynaptic mechanisms for coincidence detection in synaptic plasticity. Curr Opin Neurobiol. 2006;16:312–22. doi: 10.1016/j.conb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Egger V, Feldmeyer D, Sakmann B. Coincidence detection and changes of synaptic efficacy in spiny stellate neurons in rat barrel cortex. Nat Neurosci. 1999;2:1098–105. doi: 10.1038/16026. [DOI] [PubMed] [Google Scholar]
- Engert F, Tao HW, Zhang LI, Poo MM. Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature. 2002;419:470–5. doi: 10.1038/nature00988. [DOI] [PubMed] [Google Scholar]
- Farries MA, Fairhall AL. Reinforcement learning with modulated spike timing dependent synaptic plasticity. J Neurophysiol. 2007;98:3648–65. doi: 10.1152/jn.00364.2007. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron. 2000;27:45–56. doi: 10.1016/s0896-6273(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–5. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Fiete IR, Senn W, Wang CZ, Hahnloser RH. Spike-time-dependent plasticity and heterosynaptic competition organize networks to produce long scale-free sequences of neural activity. Neuron. 2010;65:563–76. doi: 10.1016/j.neuron.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Fino E, Deniau JM, Venance L. Cell-specific spike-timing-dependent plasticity in GABAergic and cholinergic interneurons in corticostriatal rat brain slices. J Physiol. 2008;586:265–82. doi: 10.1113/jphysiol.2007.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci. 2005;25:11279–87. doi: 10.1523/JNEUROSCI.4476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Paille V, Cui Y, Morera-Herreras T, Deniau JM, Venance L. Distinct coincidence detectors govern the corticostriatal spike timing-dependent plasticity. J Physiol. 2010;588:3045–62. doi: 10.1113/jphysiol.2010.188466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Paille V, Deniau JM, Venance L. Asymmetric spike-timing dependent plasticity of striatal nitric oxide-synthase interneurons. Neuroscience. 2009;160:744–54. doi: 10.1016/j.neuroscience.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–6. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Dan Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature. 2002;416:433–8. doi: 10.1038/416433a. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–5. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Debanne D, Bi GQ. Temporal modulation of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010b;2:19. doi: 10.3389/fnsyn.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Letzkus JJ, Kampa BM, Hang GB, Stuart GJ. Dendritic synapse location and neocortical spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010a;2:29. doi: 10.3389/fnsyn.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Djupsund K, Gao H, Hayden B, Shen K, Dan Y. Temporal specificity in the cortical plasticity of visual space representation. Science. 2002;296:1999–2003. doi: 10.1126/science.1070521. [DOI] [PubMed] [Google Scholar]
- Fu YX, Shen Y, Gao H, Dan Y. Asymmetry in visual cortical circuits underlying motion-induced perceptual mislocalization. J Neurosci. 2004;24:2165–71. doi: 10.1523/JNEUROSCI.5145-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuenzalida M, Fernández de Sevilla D, Couve A, Buño W. Role of AMPA and NMDA receptors and back-propagating action potentials in spike timing-dependent plasticity. J Neurophysiol. 2010;103:47–54. doi: 10.1152/jn.00416.2009. [DOI] [PubMed] [Google Scholar]
- Gambino F, Holtmaat A. Society for Neuroscience Abstracts 74.13. 2011. A compound mechanism for correlation-based plasticity in the mouse barrel cortex in vivo. [Google Scholar]
- Gerstner W, Kempter R, van Hemmen JL, Wagner H. A neuronal learning rule for sub-millisecond temporal coding. Nature. 1996;383:76–81. doi: 10.1038/383076a0. [DOI] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–31. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Gordon U, Polsky A, Schiller J. Plasticity compartments in basal dendrites of neocortical pyramidal neurons. J Neurosci. 2006;26:12717–26. doi: 10.1523/JNEUROSCI.3502-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupner M, Brunel N. Calcium-based plasticity model explains sensitivity of synaptic changes to spike pattern, rate, and dendritic location. Proc Natl Acad Sci USA. 2012;109:3991–6. doi: 10.1073/pnas.1109359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Wigström H, Abraham WC, Huang YY. Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J Neurosci. 1987;7:774–80. doi: 10.1523/JNEUROSCI.07-03-00774.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gütig R, Aharonov R, Rotter S, Sompolinsky H. Learning input correlations through nonlinear temporally asymmetric Hebbian plasticity. J Neurosci. 2003;23:3697–714. doi: 10.1523/JNEUROSCI.23-09-03697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JS, Nowotny T, Abarbanel HD. Spike-timing-dependent plasticity of inhibitory synapses in the entorhinal cortex. J Neurophysiol. 2006;96:3305–13. doi: 10.1152/jn.00551.2006. [DOI] [PubMed] [Google Scholar]
- Han VZ, Grant K, Bell CC. Reversible associative depression and nonassociative potentiation at a parallel fiber synapse. Neuron. 2000;27:611–22. doi: 10.1016/s0896-6273(00)00070-2. [DOI] [PubMed] [Google Scholar]
- Hardie J, Spruston N. Synaptic depolarization is more effective than back-propagating action potentials during induction of associative long-term potentiation in hippocampal pyramidal neurons. J Neurosci. 2009;29:3233–41. doi: 10.1523/JNEUROSCI.6000-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey-Girard E, Lewis J, Maler L. Burst-induced anti-Hebbian depression acts through short-term synaptic dynamics to cancel redundant sensory signals. J Neurosci. 2010;30:6152–69. doi: 10.1523/JNEUROSCI.0303-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, et al. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–68. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Lifelong learning: ocular dominance plasticity in mouse visual cortex. Curr Opin Neurobiol. 2006;16:451–9. doi: 10.1016/j.conb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18:3521–8. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–75. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Holbro N, Grunditz A, Wiegert JS, Oertner TG. AMPA receptors gate spine Ca(2+) transients and spike-timing-dependent potentiation. Proc Natl Acad Sci U S A. 2010;107:15975–80. doi: 10.1073/pnas.1004562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Urakubo H, Tanaka K, Kuroda S. Analysis of Development of Direction Selectivity in Retinotectum by a Neural Circuit Model with Spike Timing-Dependent Plasticity. J Neurosci. 2011;31:1516–27. doi: 10.1523/JNEUROSCI.3811-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito I, Ong RC, Raman B, Stopfer M. Olfactory learning and spike timing dependent plasticity. Commun Integr Biol. 2008;1:170–1. doi: 10.4161/cib.1.2.7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM. Solving the distal reward problem through linkage of STDP and dopamine signaling. Cereb Cortex. 2007;17:2443–52. doi: 10.1093/cercor/bhl152. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM, Desai NS. Relating STDP to BCM. Neural Comput. 2003;15:1511–23. doi: 10.1162/089976603321891783. [DOI] [PubMed] [Google Scholar]
- Jacob V, Brasier DJ, Erchova I, Feldman D, Shulz DE. Spike timing-dependent synaptic depression in the in vivo barrel cortex of the rat. J Neurosci. 2007;27:1271–84. doi: 10.1523/JNEUROSCI.4264-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The principles of psychology. New York: Henry Holt & Co; 1890. [Google Scholar]
- Kampa BM, Clements J, Jonas P, Stuart GJ. Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity. J Physiol. 2004;556:337–45. doi: 10.1113/jphysiol.2003.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity. J Physiol. 2006;574:283–90. doi: 10.1113/jphysiol.2006.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Dendritic mechanisms controlling spike-timing-dependent synaptic plasticity. Trends Neurosci. 2007;30:456–63. doi: 10.1016/j.tins.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. A model of spike-timing dependent plasticity: one or two coincidence detectors? J Neurophysiol. 2002;88:507–13. doi: 10.1152/jn.2002.88.1.507. [DOI] [PubMed] [Google Scholar]
- Kempter R, Gerstner W, Van Hemmen JL. Hebbian learning and spiking neurons. Phys Rev E Stat Nonlin Soft Matter Phys. 1999;59:4498–514. doi: 10.1103/PhysRevE.65.051915. [DOI] [PubMed] [Google Scholar]
- Kempter R, Gerstner W, van Hemmen JL. Intrinsic stabilization of output rates by spike-based Hebbian learning. Neural Comput. 2001;13:2709–41. doi: 10.1162/089976601317098501. [DOI] [PubMed] [Google Scholar]
- Koester HJ, Sakmann B. Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc Natl Acad Sci U S A. 1998;95:9596–601. doi: 10.1073/pnas.95.16.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RS, Corlew RJ, Henson MA, Roberts AC, Mishina M, et al. NR3A-containing NMDARs promote neurotransmitter release and spike timing-dependent plasticity. Nat Neurosci. 2011;14:338–44. doi: 10.1038/nn.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J Neurosci. 2006;26:10420–9. doi: 10.1523/JNEUROSCI.2650-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Does spike timing-dependent synaptic plasticity underlie memory formation? Clin Exp Pharmacol Physiol. 2007;34:1070–6. doi: 10.1111/j.1440-1681.2007.04724.x. [DOI] [PubMed] [Google Scholar]
- Levy WB, Steward O. Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience. 1983;8:791–7. doi: 10.1016/0306-4522(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Li L, Bender KJ, Drew PJ, Jadhav SP, Sylwestrak E, Feldman DE. Endocannabinoid signaling is required for development and critical period plasticity of the whisker map in somatosensory cortex. Neuron. 2009;64:537–49. doi: 10.1016/j.neuron.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Van Hooser SD, Mazurek M, White LE, Fitzpatrick D. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature. 2008;456:952–6. doi: 10.1038/nature07417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989;86:9574–8. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Spruston N. Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity. Nat Neurosci. 2005;8:839–41. doi: 10.1038/nn0705-839. [DOI] [PubMed] [Google Scholar]
- Lisman J, Spruston N. Questions about STDP as a General Model of Synaptic Plasticity. Front Synaptic Neurosci. 2010;2:140. doi: 10.3389/fnsyn.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Zeller D, Oostenveld R, Maris E, Cohen A, et al. LTP-like changes induced by paired associative stimulation of the primary somatosensory cortex in humans: source analysis and associated changes in behaviour. Eur J Neurosci. 2007;25:2862–74. doi: 10.1111/j.1460-9568.2007.05531.x. [DOI] [PubMed] [Google Scholar]
- Lu JT, Li CY, Zhao JP, Poo MM, Zhang XH. Spike-timing-dependent plasticity of neocortical excitatory synapses on inhibitory interneurons depends on target cell type. J Neurosci. 2007;27:9711–20. doi: 10.1523/JNEUROSCI.2513-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–13. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–5. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Markram H, Gerstner W, Sjöström PJ. A history of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010;3:4. doi: 10.3389/fnsyn.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masquelier T, Guyonneau R, Thorpe SJ. Competitive STDP-based spike pattern learning. Neural Comput. 2009;21:1259–76. doi: 10.1162/neco.2008.06-08-804. [DOI] [PubMed] [Google Scholar]
- Mehta MR, Barnes CA, McNaughton BL. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc Natl Acad Sci USA. 1997;94:8918–21. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MR, Quirk MC, Wilson MA. Experience-dependent asymmetric shape of hippocampal receptive fields. Neuron. 2000;25:707–15. doi: 10.1016/s0896-6273(00)81072-7. [DOI] [PubMed] [Google Scholar]
- Meliza CD, Dan Y. Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron. 2006;49:183–9. doi: 10.1016/j.neuron.2005.12.009. [DOI] [PubMed] [Google Scholar]
- McMahon DB, Leopold DA. Stimulus timing-dependent plasticity in high-level vision. Curr Biol. 2012;22:332–7. doi: 10.1016/j.cub.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci. 2012;15:746–53. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- Morrison A, Diesmann M, Gerstner W. Phenomenological models of synaptic plasticity based on spike timing. Biol Cybern. 2008;98:459–78. doi: 10.1007/s00422-008-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Poo MM. Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron. 2006;50:115–25. doi: 10.1016/j.neuron.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Barbara JG, Nakamura K, Ross WN. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with back-propagating action potentials. Neuron. 1999;24:727–37. doi: 10.1016/s0896-6273(00)81125-3. [DOI] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–13. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–8. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–46. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak V, Wickens JR, Kirkwood A, Kerr JN. Timing is not everything: neuromodulation opens the STDP gate. Frontiers in Synaptic Neuroscience. 2010;2:1–14. doi: 10.3389/fnsyn.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister JP, Gerstner W. Triplets of spikes in a model of spike timing-dependent plasticity. J Neurosci. 2006;26:9673–82. doi: 10.1523/JNEUROSCI.1425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP, Sejnowski TJ. Predictive learning of temporal sequences in recurrent neocortical circuits. Novartis Found Symp. 2001;239:208–29. doi: 10.1002/0470846674.ch16. discussion 29–40. [DOI] [PubMed] [Google Scholar]
- Rao RP, Sejnowski TJ. Self-organizing neural systems based on predictive learning. Philos Transact A Math Phys Eng Sci. 2003;361:1149–75. doi: 10.1098/rsta.2003.1190. [DOI] [PubMed] [Google Scholar]
- Requarth T, Sawtell NB. Neural mechanisms for filtering self-generated sensory signals in cerebellum-like circuits. Curr Opin Neurobiol. 2011;21:602–8. doi: 10.1016/j.conb.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Roberts PD, Bell CC. Computational consequences of temporally asymmetric learning rules: II. Sensory image cancellation. J Comput Neurosci. 2000;9:67–83. doi: 10.1023/a:1008938428112. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Paulsen O. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat Neurosci. 2008;11:744–5. doi: 10.1038/nn.2125. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Feltz A, Westbrook GL. Calcium-dependent inactivation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol. 1995;73:427–30. doi: 10.1152/jn.1995.73.1.427. [DOI] [PubMed] [Google Scholar]
- Rumsey CC, Abbott LF. Equalization of synaptic efficacy by activity- and timing-dependent synaptic plasticity. J Neurophysiol. 2004;91:2273–80. doi: 10.1152/jn.00900.2003. [DOI] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–59. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Safo P, Regehr WG. Timing dependence of the induction of cerebellar LTD. Neuropharmacology. 2008;54:213–8. doi: 10.1016/j.neuropharm.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisov DV, Wang SS. Order-dependent coincidence detection in cerebellar Purkinje neurons at the inositol trisphosphate receptor. J Neurosci. 2008;28:133–42. doi: 10.1523/JNEUROSCI.1729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB, Williams A, Bell CC. Central control of dendritic spikes shapes the responses of Purkinje-like cells through spike timing-dependent synaptic plasticity. J Neurosci. 2007;27:1552–65. doi: 10.1523/JNEUROSCI.5302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuett S, Bonhoeffer T, Hübener M. Pairing-induced changes of orientation maps in cat visual cortex. Neuron. 2001;32:325–37. doi: 10.1016/s0896-6273(01)00472-x. [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ. Storing covariance with nonlinearly interacting neurons. J Math Biol. 1977;4:303–21. doi: 10.1007/BF00275079. [DOI] [PubMed] [Google Scholar]
- Senn W, Markram H, Tsodyks M. An algorithm for modifying neurotransmitter release probability based on pre- and postsynaptic spike timing. Neural Comput. 2001;13:35–67. doi: 10.1162/089976601300014628. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, et al. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–29. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–51. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval HZ, Bear MF, Cooper LN. A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc Natl Acad Sci U S A. 2002;99:10831–6. doi: 10.1073/pnas.152343099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouval HZ, Wang SS, Wittenberg GM. Spike timing dependent plasticity: a consequence of more fundamental learning rules. Front Comput Neurosci. 2010;4:19. doi: 10.3389/fncom.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Häusser M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron. 2006;51:227–38. doi: 10.1016/j.neuron.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Rancz EA, Roth A, Häusser M. Dendritic excitability and synaptic plasticity. Physiol Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–64. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–54. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Song S, Abbott LF. Cortical development and remapping through spike timing-dependent plasticity. Neuron. 2001;32:339–50. doi: 10.1016/s0896-6273(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Song S, Miller KD, Abbott LF. Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat Neurosci. 2000;3:919–26. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–21. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, et al. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–60. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Stent GS. A physiological mechanism for Hebb’s postulate of learning. Proc Natl Acad Sci U S A. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Häusser M. Dendritic coincidence detection of EPSPs and action potentials. Nat Neurosci. 2001;4:63–71. doi: 10.1038/82910. [DOI] [PubMed] [Google Scholar]
- Suri RE, Sejnowski TJ. Spike propagation synchronized by temporally asymmetric Hebbian learning. Biol Cybern. 2002;87:440–5. doi: 10.1007/s00422-002-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–2. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–25. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum MC, Bi GQ, Turrigiano GG. Stable Hebbian learning from spike timing-dependent plasticity. J Neurosci. 2000;20:8812–21. doi: 10.1523/JNEUROSCI.20-23-08812.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vislay-Meltzer RL, Kampff AR, Engert F. Spatiotemporal specificity of neuronal activity directs the modification of receptive fields in the developing retinotectal system. Neuron. 2006;50:101–14. doi: 10.1016/j.neuron.2006.02.016. [DOI] [PubMed] [Google Scholar]
- von der Malsburg C. Self-organization of orientation sensitive cells in the striate cortex. Kybernetik. 1973;14:85–100. doi: 10.1007/BF00288907. [DOI] [PubMed] [Google Scholar]
- Wang HX, Gerkin RC, Nauen DW, Bi GQ. Coactivation and timing-dependent integration of synaptic potentiation and depression. Nat Neurosci. 2005;8:187–93. doi: 10.1038/nn1387. [DOI] [PubMed] [Google Scholar]
- Wang SS, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–73. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 2002;99:8366–71. doi: 10.1073/pnas.122210599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LE, Fitzpatrick D. Vision and cortical map development. Neuron. 2007;56:327–38. doi: 10.1016/j.neuron.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Wigström H, Gustafsson B, Huang YY, Abraham WC. Hippocampal long-term potentiation is induced by pairing single afferent volleys with intracellularly injected depolarizing current pulses. Acta Physiol Scand. 1986;126:317–9. doi: 10.1111/j.1748-1716.1986.tb07822.x. [DOI] [PubMed] [Google Scholar]
- Wittenberg GM, Wang SS. Malleability of spike-timing-dependent plasticity at the CA3-CA1 synapse. J Neurosci. 2006;26:6610–7. doi: 10.1523/JNEUROSCI.5388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, et al. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–45. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, et al. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–52. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahachi H, Marik SA, McManus JN, Denk W, Gilbert CD. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron. 2009;64:719–29. doi: 10.1016/j.neuron.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol. 1999;81:781–7. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Yao H, Dan Y. Stimulus timing-dependent plasticity in cortical processing of orientation. Neuron. 2001;32:315–23. doi: 10.1016/s0896-6273(01)00460-3. [DOI] [PubMed] [Google Scholar]
- Yao H, Shen Y, Dan Y. Intracortical mechanism of stimulus-timing-dependent plasticity in visual cortical orientation tuning. Proc Natl Acad Sci U S A. 2004;101:5081–6. doi: 10.1073/pnas.0302510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Kang S, Cateau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature. 2009;462:218–21. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]
- Young JM, Waleszczyk WJ, Wang C, Calford MB, Dreher B, Obermayer K. Cortical reorganization consistent with spike timing-but not correlation-dependent plasticity. Nat Neurosci. 2007;10:887–95. doi: 10.1038/nn1913. [DOI] [PubMed] [Google Scholar]
- Yu X, Shouval HZ, Knierim JJ. A biophysical model of synaptic plasticity and metaplasticity can account for the dynamics of the backward shift of hippocampal place fields. J Neurophysiol. 2008;100:983–92. doi: 10.1152/jn.01256.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Lau PM, Bi GQ. Gain in sensitivity and loss in temporal contrast of STDP by dopaminergic modulation at hippocampal synapses. Proc Natl Acad Sci USA. 2009;106:13028–33. doi: 10.1073/pnas.0900546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Holt CE, Harris WA, Poo M. A critical window for cooperation and competition among developing retinotectal synapses. Nature. 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Poo M. Visual input induces long-term potentiation of developing retinotectal synapses. Nat Neurosci. 2000;3:708–15. doi: 10.1038/76665. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tzounopoulos T. Physiological activation of cholinergic inputs controls associative synaptic plasticity via modulation of endocannabinoid signaling. J Neurosci. 2011;31:3158–68. doi: 10.1523/JNEUROSCI.5303-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YD, Acker CD, Netoff TI, Sen K, White JA. Increasing Ca2+ transients by broadening postsynaptic action potentials enhances timing-dependent synaptic depression. Proc Natl Acad Sci U S A. 2005;102:19121–5. doi: 10.1073/pnas.0509856103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter M, Holmgren C, Shemer I, Silberberg G, Grillner S, et al. Input specificity and dependence of spike timing-dependent plasticity on preceding postsynaptic activity at unitary connections between neocortical layer 2/3 pyramidal cells. Cereb Cortex. 2009;19:2308–20. doi: 10.1093/cercor/bhn247. [DOI] [PMC free article] [PubMed] [Google Scholar]