Abstract
The present study assessed the mechanisms and time course by which orthographic neighborhood size (ON) influences visual word recognition. ERPs were recorded to words that varied in ON and in word frequency while participants performed a semantic categorization task. ON was measured with the Orthographic Levenshtein Distance (OLD20), a richer metric of orthographic similarity than the traditional Coltheart’s N metric. The N400 effects of ON (260–500 ms) were larger and showed a different scalp distribution for low than for high frequency words, which is consistent with proposals that suggest lateral inhibitory mechanisms at a lexical level. The ERP ON effects had a shorter duration and different scalp distribution than the effects of word frequency (mainly observed between 380–600 ms) suggesting a transient activation of the subset of orthographically similar words in the lexical network compared to the impact of properties of the single words.
Despite a great degree of orthographic similarity across printed words (e.g., mouse-moose), our word-recognition system is able to identify the lexical entry corresponding to a given word within a few hundred milliseconds. Understanding the processes involved in selecting the appropriate candidate from a set of highly confusable “neighbors” is crucial to the study of lexical access during visual word recognition. One shared assumption in studies on lexical access is that orthographically similar word units (“neighbors”) are partially activated during visual word recognition. Most studies of orthographic similarity have defined word neighborhood size (N) as the set of words that can be formed by changing one letter while preserving letter positions (see Coltheart, Davelaar, Jonasson & Besner, 1977). For instance, upon presentation of the stimulus item “house”, not only the word unit corresponding to “house” is partially activated, but also the word units corresponding to orthographically similar words like “mouse”, “rouse”, etc. According to this definition, words with many neighbors like “power” (“poker”, “tower”, “lower”, “poler”, “mower”) would activate a larger candidate set than words with few neighbors like “peace” (“place”, “peach”).
Activation-based models have been proposed that assume that partial activation of orthographic neighbors during visual word recognition is resolved by a lexical level inhibitory mechanism between neighboring word units, whose operation results in the selection of a single word unit (e.g., Multiple Read-Out model, Grainger & Jacobs, 1996). According to these models, the dynamics of word recognition for a given word are determined by the interaction between the characteristics of a word’s orthographic neighborhood (both the number and frequency of the orthographic neighbors) and the characteristics of the stimulus item, such as word-frequency (see Grainger & Jacobs, 1996). These models further assume that units corresponding to more frequent words have higher resting levels than those corresponding to low frequency words, which explains why high-frequency words are identified more rapidly than low-frequency words. With respect to the effect of a word’s orthographic neighborhood, competition at the lexical level would predict inhibitory effects of neighbors, especially for low-frequency words since these are the word units which (because of their resting levels) may be more influenced by other lexical units (e.g., the word unit corresponding to “house” would inhibit its lower frequency neighbor “mouse”). In contrast to this prediction, a number of studies have shown a facilitative effect of neighborhood size on low-frequency words. But Grainger and Jacobs (1996) have argued that this facilitative effect may be due to task-specific strategies. They suggested that a “word” response in a lexical decision task can result not only from the activity of individual word representations (a unique identification criterion), but also from a criterion based on global lexical activity (a fast familiarity-based guess; see Balota & Chumbley, 1984). The idea here is that low frequency words with many neighbors in a lexical decision task would benefit more from the larger non-specific lexical activation that increases the likelihood of “word” responses. Consistent with the argument of Grainger and Jacobs (1996), studies that have used different tasks which rely on retrieval and/or integration of word meaning have indeed found inhibitory effects of orthographic neighborhood (eye-tracking studies; Perea & Pollatsek, 1998; Pollatsek, Perea, & Binder, 1999; Slattery, 2009). However, other studies have obtained mixed results (perceptual identification: Carreiras, Perea, Grainger, 1997; Van Heuven, Dijkstra & Grainger, 1996; Ziegler, Rey & Jacobs, 1998; semantic categorization task; Carreiras, Perea, & Grainger, 1997; Forster & Shen, 1996; Forster & Hector, 2002; Sears, Hino, & Lupker, 1995). Thus, it has yet to be determined whether or not effects of orthographic neighborhood result from competition at the lexical level.
In the present experiment we assessed whether the influence of the size of the orthographic neighborhood on lexical access varies as a function of word frequency -a lexical characteristic of words- in order to evaluate whether the proposed lateral-inhibition mechanism during visual-word recognition can influence processing at a lexical level. Because the lexical decision task is not free from interpretative shortcomings in orthographic neighborhood studies (see above), we employed a semantic categorization task (“Is the word an animal?”) and manipulated neighborhood size and word frequency in a factorial design. We examined the time course of the effects of orthographic neighborhood size and of word frequency during lexical access by recording event-related potentials (ERPs) while participants were reading single words. We used ERPs because they are potentially sensitive to different stages of lexical access. In addition, ERPs are multidimensional, and they vary in amplitude, latency and scalp distribution as a function of experimental manipulations. In our study, we will examine if the ERP-effects of orthographic neighborhood size vary in magnitude as a function of word frequency (ERP amplitude), and whether the onset and duration of the ERP-effects of these two factors differ (ERP latency). We will also examine the topographic distributions of these effects, since different distributions of voltage fluctuations over the scalp are suggestive of (partially) non-overlapping neural sources. Thus, the multidimensional nature of the ERP signal may help to analyze the interplay between properties of a subset of the network (orthographic overlap) and properties of the single word forms (frequency).
Of particular interest to our study is the N400, a negative deflection that reaches its peak amplitude around 400 ms after stimulus onset, and is maximal over centro-parietal electrode sites. Of relevance here is that the N400 has been associated with lexical-semantic processing in studies of visual word recognition, and that modulation of its amplitude may reflect processing costs during the retrieval of properties associated with a word form stored in memory (see Holcomb, Grainger, & O’Rourke, 2002; Kutas & Federmeier, 2000). The amplitude of the N400 is modulated as a function of word frequency (reduced N400 to high- relative to low frequency words: e.g., Barber, Vergara & Carreiras, 2004; Smith & Halgren, 1987; Van Petten & Kutas, 1990), lexicality (reduced N400 to real words relative to pseudowords: e.g., Carreiras, Vergara, & Barber, 2005; Neville, Mills & Lawson, 1992; see also Barber & Kutas, 2007, for a review), and orthographic neighborhood (reduced N400 to words with a small neighborhood relative to those with a large neighborhood: Holcomb et al, 2002; Laszlo & Federmeier, 2009, 2011; Midgley, Holcomb, van Heuven & Grainger, 2008; Müller, Duñabeitia & Carreiras, 2010; for a similar result of syllabic neighborhoods, see Barber et al, 2004). Typically N400 effects of neighborhood have been interpreted to reflect a higher level of activation related to a larger orthographic neighborhood, either at the level of word-form or at the level of lexical-semantic representations (Holcomb et al, 2002).
In the present experiment, orthographic neighborhood will be operationalized with a new measure, the OLD20 (Orthographic Levenshtein Distance). This measure is based on early work by Levenshtein (1966) who calculated the “distance” between letter sequences as a function of the minimum number of substitutions, insertions, and deletions needed to generate one string of elements from another. OLD20 is computed as the mean Levenshtein Distance (LD) from a word to its 20 closest orthographic neighbors (i.e. those words with the minimum number of substitutions, deletions, or additions; see Yarkoni, Balota & Yap, 2008, for a detailed explanation of the selection of these values). OLD20 may provide a better measure of orthographic neighborhood than Coltheart’s classic definition (one letter substitutions, or N) because it overcomes two potential limitations of N. First, the definition of a neighbor is no longer based on one binary measure, since it is not only determined by letter substitution. Second, OLD20 does not require that all neighbors have the same length, but also incorporates longer words. That is, whereas substitution-letter neighbors are position-specific (i.e., “casual” and “causal” are not considered neighbors) and length-dependent (i.e., “hose” and “house” are not considered neighbors), OLD20 neighbors are not. This is important because recent research has shown that words with transposed letters (e.g., causal-casual; Acha & Perea, 2008; Andrews, 1989; Johnson, 2009) and words with addition or deletion of letters (hat-that; Davis & Taft, 2005; Davis, Perea, & Acha, 2009) also affect processing of the stimulus word, which suggests that they are part of a word’s neighborhood as well1. Thus, the OLD20 measure enables a larger range of orthographic variability than the standard N metric. In sum, inclusion of more and longer items in the experimental word set potentially makes OLD20 a better measure for studying orthographic similarity effects in visual-word recognition.
In summary, while orthographic neighborhood has been assumed to tap initially into sublexical processes (mainly the activation of similar words overlapping in orthography), the selection of the lexical candidate may emerge as the result of competition among similarly activated representations at the lexical level. In order to assess whether neighborhood influences competition at a lexical level, we manipulated the size of the neighborhood and the frequency of words. We predicted larger ERP effects of the size of the orthographic neighborhood on low than high frequency words because lateral inhibitory connections from low frequency words are weaker than those from high frequency words. Previous studies indicate that the N400 effect is sensitive to the “stabilization” in activation at the level of whole-word form (lexical level) representations (Massol, Grainger, Dufau & Holcomb, 2010). Therefore, if N400 effects of orthographic neighborhood are modulated by word frequency in our study, then this would be supportive of a lateral inhibition mechanism at the lexical level. Finally, a direct comparison of the time-course and scalp distribution of the ERP effects of orthographic neighborhood and word frequency may help to disentangle the interplay between sublexical factors (that mainly reflect the activation of multiple candidates overlapping in orthography) and lexical factors (that mainly reflect the resting–level activation of single word form representations) during visual word recognition.
Method
Participants
Twenty-two (14 women) undergraduate students of UC Davis participated in the experiment in exchange for course credit. All of them were native English speakers with no history of neurological or psychiatric impairment, and with normal or corrected-to-normal vision. Ages ranged from 18 to 23 years (mean=19.1 years). All participants were right-handed, as assessed with an abridged version of the Edinburgh Handedness Inventory (Oldfield, 1971). Participants gave informed consent prior to the experiment.
Materials
A list of 132 English words of four to six letters (4-letter words: 36, 5-letter words: 28, 6-letter words: 68) was selected from the English Lexicon Project database (ELP: http://elexicon.wustl.edu/). These words were distributed across four conditions in a 2 (Word Frequency: Low, High) × 2 (OLD20 Orthographic Neighborhood: Low, High) within-subjects design. Thirty-three words were used in each condition. Table 1 shows that words differed on Log of Frequency (LFr): F(1,128)=296.98, p < .001; and on OLD20: F(1,128)=117.98, p < .001. LFr was defined as the mean log-frequency of a word according to the Hyperspace Analogue to Language (HAL) frequency norms (Lund & Burgess, 1996), which are derived from approximately 131 million words gathered from Usenet newsgroups. OLD20 is computed as the mean Levenshtein Distance (LD) from a word to its 20 closest orthographic neighbors (i.e. those words with the minimum number of substitutions, deletions, or additions; see Yarkoni, Balota & Yap, 2008, for a detailed explanation of the selection of these values). Although the difference between conditions in terms of OLD20 may seem small (2.15 in the low neighborhood condition vs. 1.15 in the high neighborhood condition), it is actually quite large when one takes into account that the minimum OLD20 value is 1, and the maximum OLD20 values for 4, 5 and 6 letter words are 2.1, 2.8 and 3 respectively (ELP lexicon). For example, a word with low orthographic neighborhood will have a high OLD20 value (“veil”; OLD20: 1.8) based on the number of changes needed to create similar words like “boil” (2), “cell” (2), “feel” (2), etc. A word with high orthographic neighborhood will have a low OLD20 value (“lime”; OLD20: 1.2) based on the number of changes needed to create words such as “lie” (1), “dime” (1), “limb” (1), etc. Thus, OLD20 values are smaller for words with dense neighborhoods, and larger for words with small neighborhoods. This may be confusing relative to the classic measure of neighborhood of Coltheart et al., where a greater value indicated a dense neighborhood (i.e., the number of words that can be created with one substitution).
TABLE 1.
Mean values of Psycholinguistic Characteristics of words across conditions (SDs in Italics). Word frequency is provided both as the Log frequency according to HAL (Log_F) and as in Francis & Kucěra (1982) data base (F_KF). Orthographic neighborhood is provided both as OLD20 (according to the Levenshtein Distance definition) and as N (according to Coltheart’s definition).
| Freq. | OS | Log_F | F_KF | OLD20 | ON | Letters | Phonemes | Syllables | OLDE | BG_Freq | Concreteness | Imageability |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | High | 9.67 | 49.67 | 1.53 | 6.55 | 5.24 | 4.00 | 1.36 | 7.71 | 1463.90 | 499.06 | 526.64 |
| 0.65 | 41.87 | 0.22 | 3.91 | 0.81 | 0.87 | 0.49 | 0.53 | 629.72 | 107.01 | 80.21 | ||
| Low | 9.51 | 41.81 | 2.06 | 1.64 | 5.24 | 4.18 | 1.58 | 7.70 | 1521.10 | 501.24 | 518.91 | |
| 0.68 | 37.19 | 0.34 | 1.75 | 0.87 | 0.92 | 0.61 | 0.59 | 471.60 | 103.95 | 78.73 | ||
| Low | High | 7.42 | 7.55 | 1.50 | 6.94 | 5.24 | 4.00 | 1.42 | 7.32 | 1290.80 | 530.15 | 529.58 |
| 0.76 | 6.29 | 0.22 | 4.07 | 0.87 | 0.83 | 0.50 | 0.89 | 375.68 | 74.80 | 56.64 | ||
| Low | 7.28 | 8.07 | 2.15 | 1.67 | 5.24 | 4.39 | 1.64 | 7.62 | 1270 | 541.70 | 533.27 | |
| 0.87 | 8.29 | 0.42 | 1.71 | 0.87 | 1.00 | 0.55 | 0.56 | 509.84 | 79.86 | 66.67 | ||
Words were matched for the following parameters across the 2 × 2 design (see table 1): word length (in terms of graphemes and syllables), OLD20 frequency (defined as the mean log-frequency of a word’s orthographic neighbors according to HAL frequency norms; the target word frequency value is not included), bigram frequency (calculated as the summed bigram frequencies by position), concreteness and imageability (both derived from a merging of the Paivio, Colorado, and Gilhooly-Logie norms; MRC Psycholinguistic Database User Manual, Coltheart, 1981). Although words in the different conditions were not initially matched regarding the number of neighbors of higher frequency (according to the OLD20 operativization of neighborhood), there was no significant correlation between this value and OLD20 values (Pearson coefficient -.10, p=.23). No inflected forms were included. A list of 35 animal names, proportionally equated for frequency and length according to the experimental word set, served as probe items in a go/no-go semantic categorization task.
Procedure
Participants were seated comfortably in a dimly lit, electrically shielded and sound-attenuated chamber. All stimuli were presented on a high-resolution monitor that was positioned at eye level 100 cm in front of the participant. The words were displayed in white lowercase Courier 24 font against a dark-gray background. The animal names served as probe items in a go/no-go semantic categorization task in which participants were instructed to press a single button as soon as they detected an animal name. Importantly, critical stimuli did not require an overt response. Response hand was counterbalanced between subjects.
The sequence of events in each trial was as follows: First, a fixation cross (“+”) appeared in the center of the screen for 1000 ms. This was followed by a 200 ms blank screen which was replaced by the target word presented in lowercase letters, which remained on the screen for 400 ms. Each word was followed by a 1500 ms blank screen, after which a picture of a smiley face was presented on the screen for another 1500 ms. The appearance of the smiley face signaled to the subjects that they could move or blink their eyes. They were asked to refrain from blinking and eye-movements from the onset of the fixation cross to the onset of the smiley face. Each participant saw the stimuli in a different random order. The session started with twelve warm-up trials (including 4 animal names), which were not further analyzed. These trials were repeated if necessary. Participants were instructed to read the words and to press the button when they saw an animal name.
EEG recording and analyses
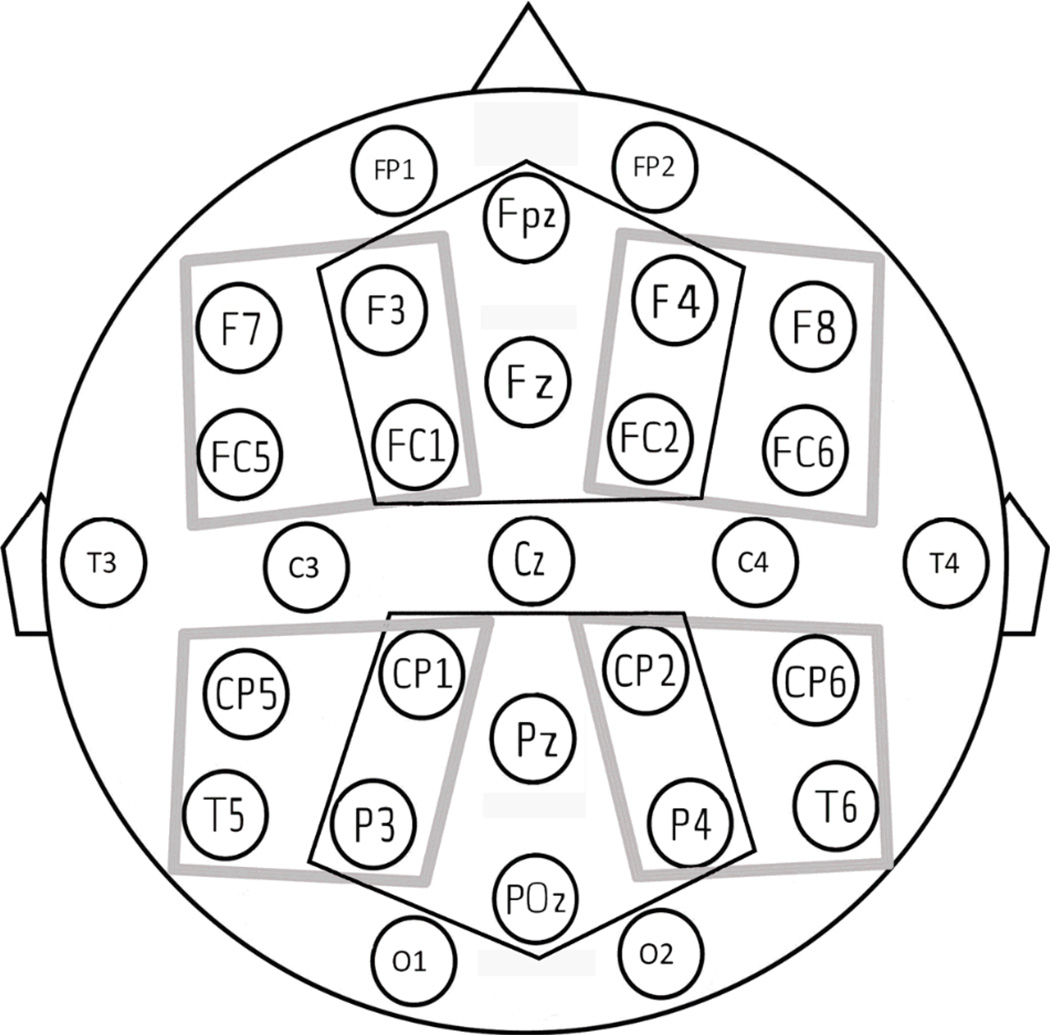
The electroencephalogram was recorded from 29 electrodes mounted in an elastic cap, referenced to the right mastoid (except for the electrodes that were used to measure potential blinks and eye-movements: one electrode placed beneath the left eye was referenced to FP1 and two placed at the outer canthi of both eyes were referenced to each other). The EEG recording was re-referenced off-line to an average of the left and right mastoids. Impedances were kept below 5 kΩ. All single-trial waveforms were screened offline for amplifier blocking, drift, muscle artifacts, eye movements, and blinks. This was done for a 900 ms epoch with a 200 ms pre-stimulus baseline; trials containing artifacts were not included in the average ERPs or in the statistical analyses. This led to a rejection rate of 7% over all trials, with no statistical difference in the number of rejections across conditions (F<1). The EEG signal was band-pass filtered between 0.01 and 30 Hz and sampled at 250 Hz. ERPs were averaged separately for each of the subjects, each of the electrode sites, and each of the conditions. In order to capture topographic differences in the ERP effects over anterior and posterior regions of the scalp, we performed ANOVAs that included the factor Anterior-Posterior (AP) over ventral and dorsal electrode sites. In both the ventral and dorsal analyses we calculated the averaged amplitude values across a number of electrodes in different representative scalp areas. In the “Ventral Analysis”, four representative scalp areas resulted from the factorial combination of the factors Laterality (left, right) and the Anterior-Posterior (AP) distribution (anterior, posterior): left-anterior (F7, F3, FC5, FC1), left-posterior (CP5, CP1, T5, P3), right-anterior (F4, F8, FC2, FC6), and right-posterior (CP2, CP6, P4, T6) (see Figure 1). In the “Dorsal Analysis”, two representative scalp areas were computed out of six electrodes (see Figure 1, areas enclosed by black lines): anterior (F3, AFz, F4, FC1, Fz and FC2), posterior (CP1, CPz, CP2, P3, POz, and P4).
Figure 1.
Schematic flat representation of the 29 electrode positions from which EEG activity was recorded (front of head is at top). The electrodes contributing to the analyses are those grouped in the four critical regions (Ventral analysis: grey lines), and in the two critical regions (Dorsal analysis: black lines).
Since we were particularly interested in the time course of the interplay between orthographic neighborhood and word frequency, mean amplitudes were calculated for three time windows of analysis around the typical N400 time window: 260–380 ms, 380–500 ms, and 500–600 ms. The onset of these time windows was statistically determined by the first latency at which the difference between waveforms was significant using a series of point-by-point T-tests. Modulation of average ERPs across these time windows was examined through two analyses of variance (ANOVAs) for repeated measures that included the factors Orthographic Neighborhood (Low vs. High), Word Frequency (Low vs. High), AP distribution (anterior, posterior) and latency (first epoch: 260–280 ms, second epoch: 380–500 ms, third epoch: 500–600 ms). The factor Laterality (left, right) was only included in the “Ventral Analysis”. When appropriate, p-values are reported using the Greenhouse-Geisser (1959) correction. Effects for the topographic factors will only be reported when they interact with the experimental manipulations.
Behavioral results
The behavioral results from the go trials showed that participants correctly categorized more than 97% of the animal target words.
ERP results
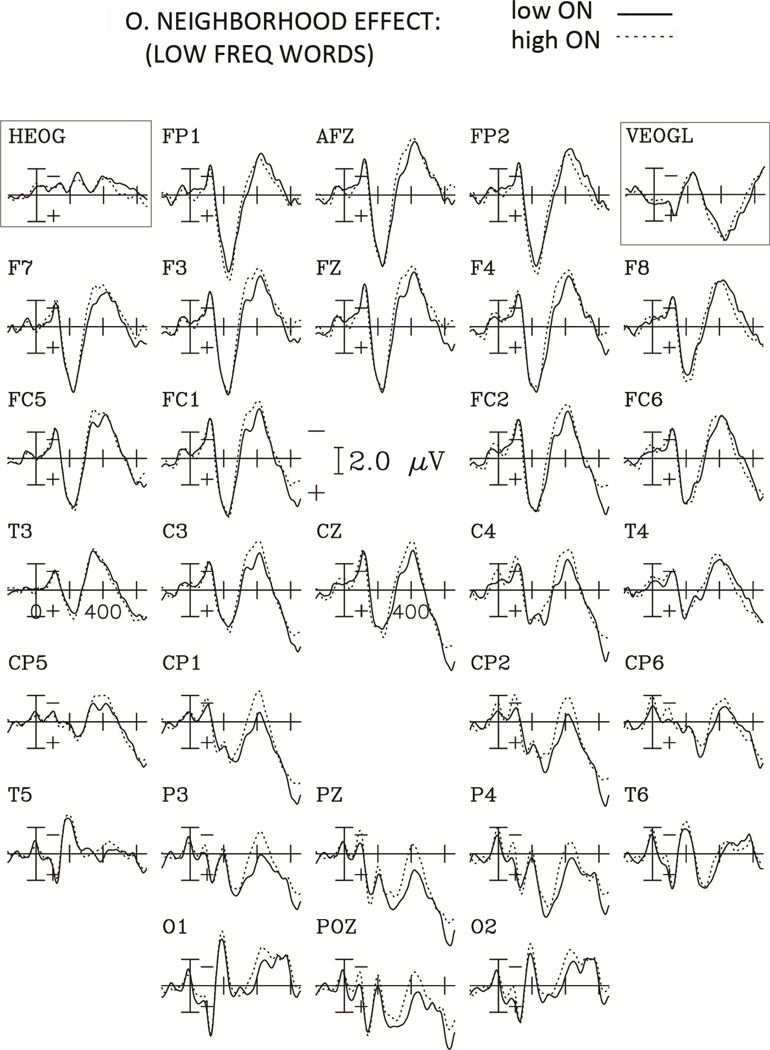
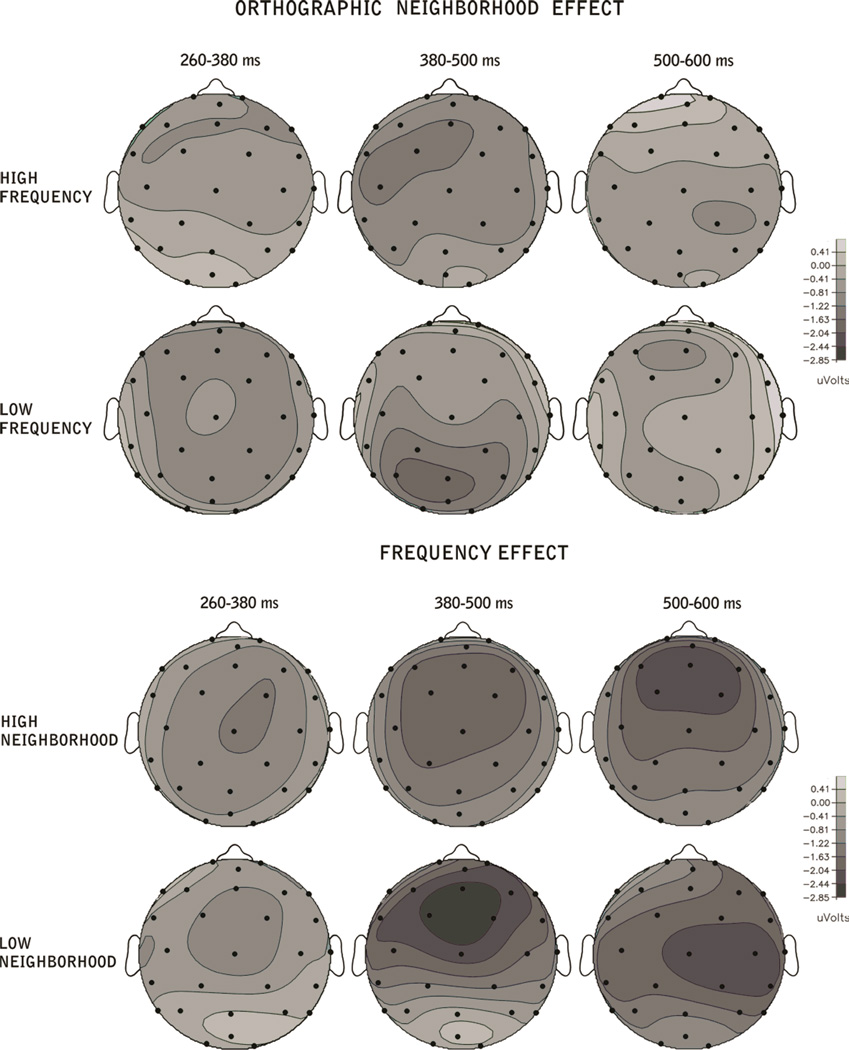
ERP grand averages time-locked to the onset of the target words for the Orthographic Neighborhood (ON) and Word Frequency comparisons are presented in Figures 2 through 5. As can be seen in these figures, the ERPs show a negative potential which is maximal around 100 ms, followed by a bipolar component that is maximal around 200 ms with a positive polarity over frontal regions but a negative polarity over posterior regions. Following these early deflections, a negativity starting around 250 ms and peaking around 400 ms (N400) post-stimulus onset is observed for the orthographic neighborhood comparison (figures 2 and 3) with a more posterior distribution for low frequency words (see figure 6).
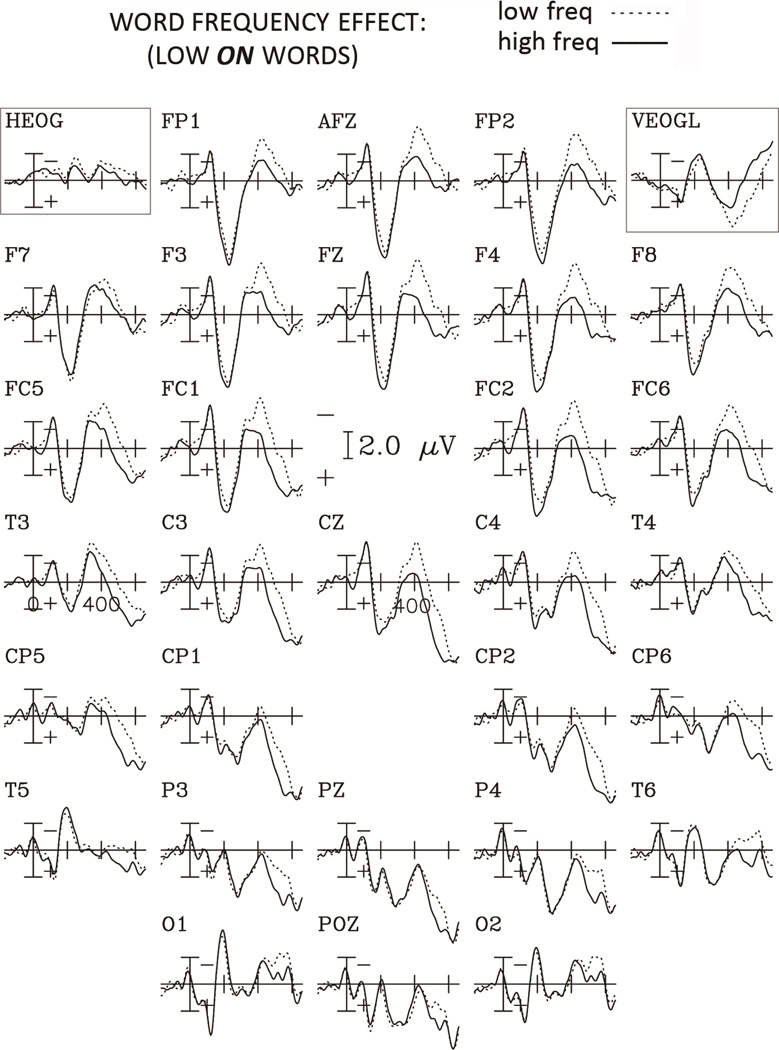
Figure 2.
Grand average ERPs to words with high and low orthographic neighborhood for the Low Frequency condition for the 29 electrodes, and the horizontal and vertical EOG channels. Negative potentials are plotted upwards and each tick mark on the horizontal axis represents 200 ms.
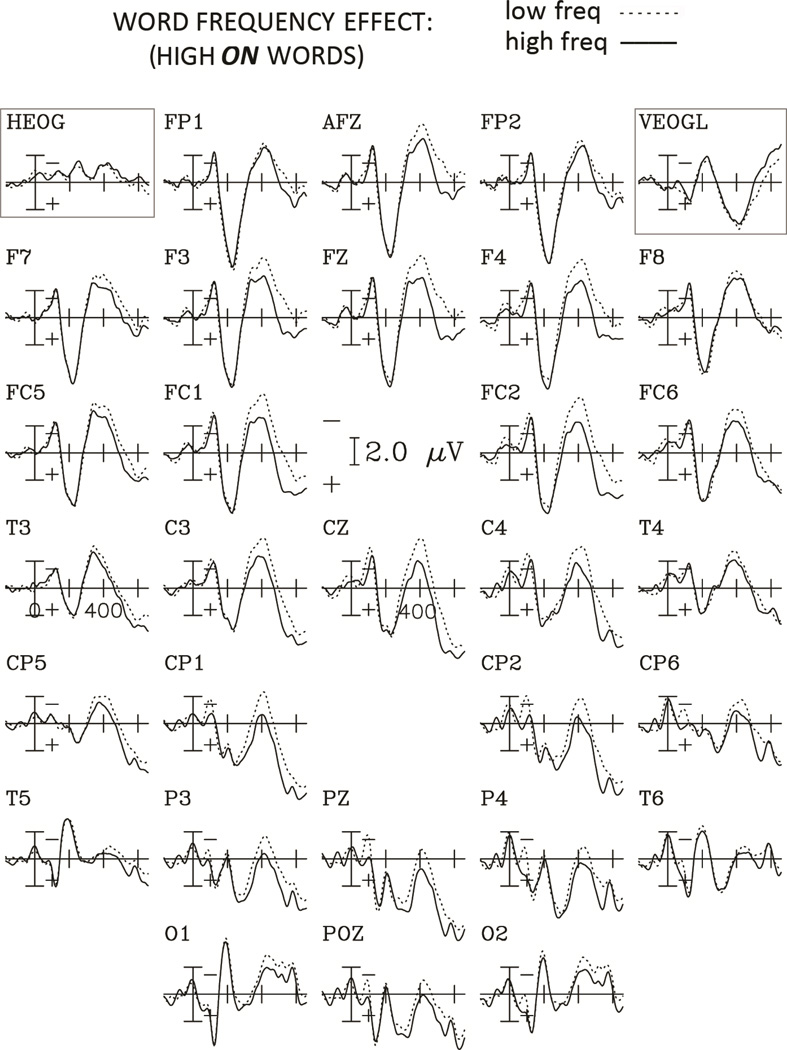
Figure 5.
Grand average ERPs to words with high and low frequency for the High O. Neighborhood condition for the 29 electrodes, and the horizontal and vertical EOG channels. Negative potentials are plotted upwards and each tick mark on the horizontal axis represents 200 ms.
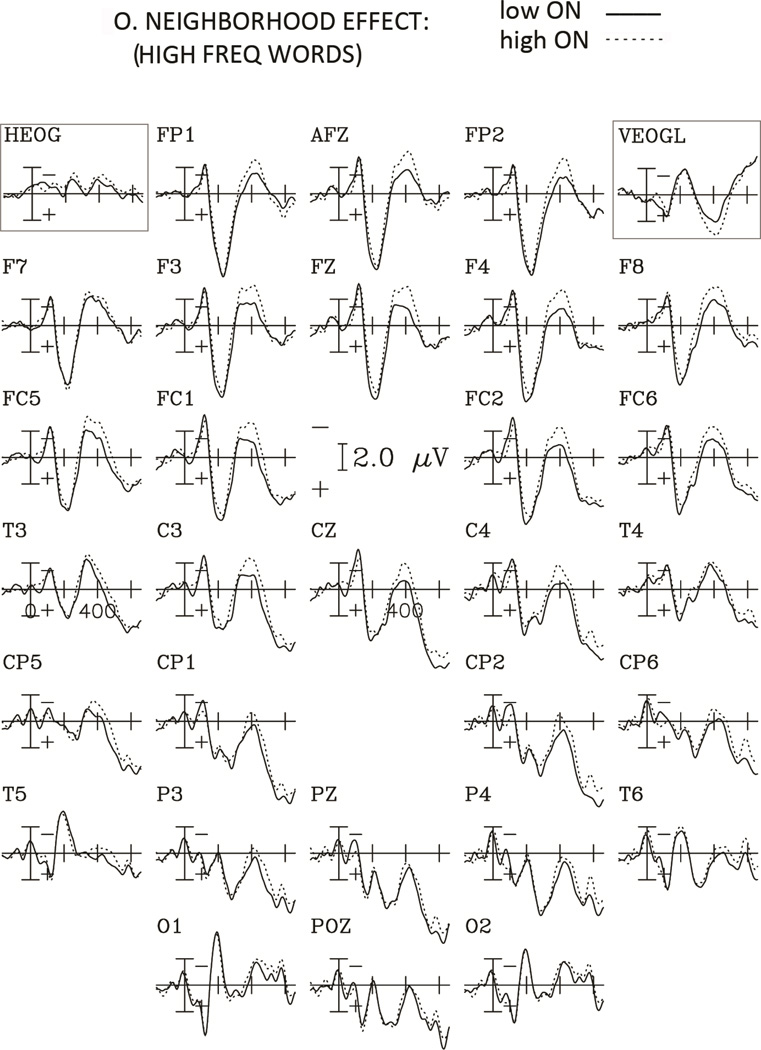
Figure 3.
Grand average ERPs to words with high and low orthographic neighborhood for the High Frequency condition for the 29 electrodes, and the horizontal and vertical EOG channels. Negative potentials are plotted upwards and each tick mark on the horizontal axis represents 200 ms.
Figure 6.
Topographic distribution of the effects of orthographic neighborhood (calculated as the difference in voltage amplitude between the ERP responses to Low vs. High Orthographic Neighborhood conditions, for High and Low Frequency words), and of word frequency (calculated as the difference in voltage amplitude between the ERP responses to High vs. Low Frequency conditions, for High vs. Low Orthographic neighborhood words) for the three time windows.
Large orthographic neighborhoods elicited larger negativities than small orthographic neighborhoods. For the frequency comparison (figures 4 and 5), this negativity lasted longer (beyond 600 ms) and was more broadly distributed over the scalp, with larger amplitudes for low compared to high frequency words (see figure 6).
Figure 4.
Grand average ERPs to words with high and low frequency for the Low O. Neighborhood condition for the 29 electrodes, and the horizontal and vertical EOG channels. Negative potentials are plotted upwards and each tick mark on the horizontal axis represents 200 ms.
Ventral Analysis
The overall ANOVA showed a marginally significant effect of ON [F(1,21)=3.50, p=0.07] and a significant effect of Frequency [F(1,21)=20.5, p<0.01]. These effects were modulated by interactions with Latency and AP distribution [ON × Latency × AP: F(2,42)=4.52, p=0.03; Freq. × Latency: F(2,42)=14.7, p<0.01; ON × Freq. × Latency × AP: F(2,42)=4.02, p=0.03]. To further examine the source of these interactions paired comparisons were performed to assess the effect of ON at each level of Frequency, and the effect of Frequency at each level of ON across each epoch and region separately (see table 2). No interaction with Laterality was observed.
TABLE 2.
Ventral Analysis: Summary of the F values for the pairwise comparisons of Neighborhood and Word frequency over the critical regions and at the three time epochs (260–380 ms, 380–500 ms and 500–600 ms). None of the effects interacted with the factor Laterality, and the F values reported in the table result from collapsing the results over all ventral electrode sites.
| O.NEIGH | REGION: | ||
|---|---|---|---|
| ANT. | POST. | ||
| 260–380 | Low Freq | 7.15** | 1.56 |
| High Freq | 5.08* | F<1 | |
| 380–500 | Low Freq | F<1 | 3.25 |
| High Freq | 3.57 | 4.83* | |
| 500–600 | Low Freq | F<1 | F<1 |
| High Freq | F<1 | 3.65 | |
| W.FREQ | REGION: | ||
|---|---|---|---|
| ANT. | POST. | ||
| 260–380 | Low ON | 2.01 | F<1 |
| High ON | 1.39 | 3.77 | |
| 380–500 | Low ON | 9.48** | 4.22* |
| High ON | 5.84* | 5.33* | |
| 500–600 | Low ON | 7.30* | 26.8*** |
| High ON | 7.21** | 8.37** | |
: p<.001;
:p<.01;
:p<.05;
df of comparisons: 1,21;
Orthographic Neighborhood
Significant effects of ON were mainly observed in the first epoch (260–380 ms) and over anterior regions of the scalp (see Table 2). ON effects in the second epoch (380–500 ms) were only significant in posterior regions and marginally significant in anterior regions for high frequency words. For low frequency words, effects of ON were only marginally significant over posterior regions. ON effects were absent in the third epoch (500–600 ms).
Word Frequency
As can be seen in Table 2, frequency effects were absent in the first epoch (260–380 ms). However, significant effects of Frequency were consistently observed in the second and in the third epochs for both high and low ON (380–500 ms and 500–600 ms).
Summary
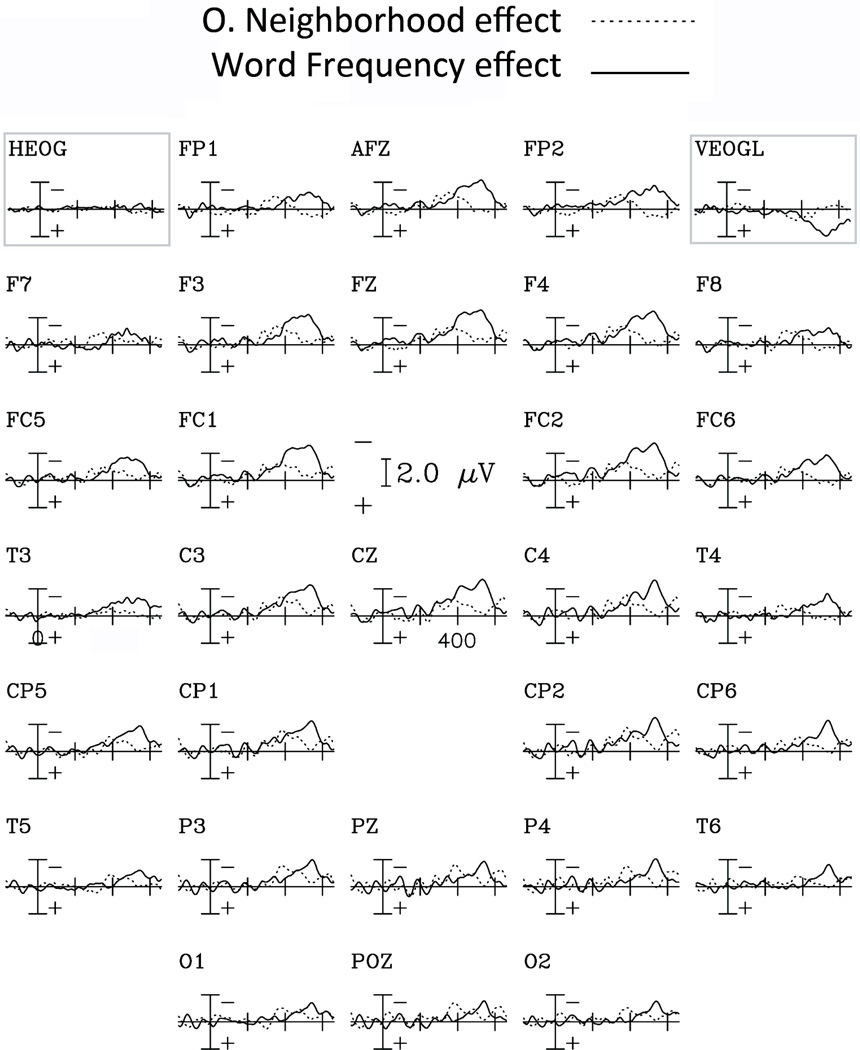
ON and Frequency effects showed a different time-course and scalp distribution. Effects of ON were found in the early 260–380 ms epoch and were maximal over anterior scalp regions; in the 380–500 ms epoch a marginally significant effect of ON was found for low frequency words over posterior regions. In contrast, effects of Word Frequency had a later onset, lasted longer (between 380 and 600 ms) and were widely distributed over the scalp (see figures 6 and 7).
Figure 7.
Difference waveforms comparing the effects of Orthographic Neighborhood and Word Frequency effects for all 29 electrodes’ sites. O. Neighborhood effect is calculated as the difference in voltage amplitude between the ERP responses to Low vs. High Orthographic Neighborhood conditions (collapsed over High and Low frequency words). Word Frequency effect is calculated as the difference in voltage amplitude between the ERP responses to High vs. Low Frequency conditions (collapsed over High and Low Neighborhood words).
Dorsal Analysis
Significant effects were found for both ON [F(1,21)=5, p=0.03] and Frequency [F(1,21)=20.4, p<0.01]. These effects were modulated by interactions with Latency and AP distribution [Freq. × Latency: F(2,42)=9.35, p<0.01; ON × Freq. × Latency × AP distribution: F(2,42)=5.14, p=0.02]. Again, we further examined these interactions in pairwise comparisons of the effects of ON at each level of Frequency, and the effects of Frequency at each level of ON across each epoch and electrode region separately (see Table 3).
TABLE 3.
Dorsal Analysis: Summary of the F values for the pairwise comparisons of Neighborhood and Word frequency over the critical regions and at the three time epochs (260–380 ms, 380–500 ms and 500–600 ms). None of the effects interacted with the factor Laterality, and the F values reported in the table result from collapsing the results over all ventral electrode sites.
| O.NEIGH | REGION: | ||
|---|---|---|---|
| ANT. | POST. | ||
| 260–380 | Low Freq | 7.02** | 5.13* |
| High Freq | 3.01 | F<1 | |
| 380–500 | Low Freq | F<1 | 5.78* |
| High Freq | 3.48 | 3.16 | |
| 500–600 | Low Freq | F<1 | F<1 |
| High Freq | F<1 | 2.22 | |
| W.FREQ | REGION: | ||
|---|---|---|---|
| ANT. | POST. | ||
| 260–380 | Low ON | 2.59 | F<1 |
| High ON | 2.93 | 5.82* | |
| 380–500 | Low ON | 10.9** | 1.54 |
| High ON | 7.49** | 5.62* | |
| 500–600 | Low ON | 6.22* | 14.5** |
| High ON | 10.5** | 6.06* | |
: p<.001;
:p<.01;
:p<.05;
df of comparisons: 1,21;
Orthographic Neighborhood
As in the previous analysis, significant effects of ON were observed in the first epoch (260–380 ms) but mainly over low frequency words and over both anterior and posterior regions of the scalp (see Table 3). Importantly, ON effects in the second epoch (380–500 ms) were only significant in the posterior region for low frequency words. ON effects were absent in the third epoch (500–600 ms).
Word Frequency
In the first epoch (260–380 ms) Frequency effects were only observed for high ON words and over the posterior region. In the second epoch (380–500 ms) significant effects of Frequency were present in both anterior and posterior regions for high ON words, while for low ON words, they were present over the anterior region only. The Frequency effect was significant for all comparisons in the third epoch (500–600 ms).
Summary
The results of the ANOVAs over ventral and dorsal electrode sites showed significant Anterior-Posterior differences. Figure 7 shows that the ON effect for high frequency words is not as consistent (regarding scalp distribution) as for low frequency words (epoch 1). In fact, both the ventral and the dorsal analyses systematically showed effects for low frequency words over anterior areas. In the 350–500 epoch, the ON effect was only significant for the posterior region. With respect to the Frequency effect (starting in the 350–500 ms epoch) both analyses systematically showed a large and localized effect (anterior) for low ON words while for high ON words this effect was observed over both anterior and posterior regions (see tables 2 & 3, and figure 7). In the 500–600 ms epoch, both low and high ON words showed a frequency effect over both anterior and posterior regions. Interestingly, as can be seen in figures 6 and 7 and table 3, significant effects of both ON and Word Frequency were found in the 380–500 ms time-window, but with different scalp distributions.
Discussion
The aim of the present ERP study was to investigate whether the selection of a lexical candidate during visual word processing is driven by a lateral inhibitory mechanism that operates at the lexical level. In order to test this, we assessed the interplay of the effects of orthographic neighborhood and of word frequency on visual word recognition by measuring ERPs during single word reading in a semantic categorization task where these factors were manipulated in a factorial design. In the following we will discuss the main findings of the study.
On the Transient Effects of a Word’s Orthographic Neighbors
Early and broadly distributed effects of orthographic neighborhood were observed in the 260–380 ms epoch. Effects of neighborhood were also found in the 380–500 ms epoch for both high and low frequency words, but whereas this effect remained broadly distributed for the high frequency words, it was now maximal over posterior sites for the low frequency words. This change in distribution of the effects of orthographic neighborhood on low frequency words may be interpreted as the transition from sublexical activation driven by the orthographic properties of the words (observed for low and high frequency during early stages of processing) to between-word inhibitory processes (observed mainly for low frequency words during later stages of lexical processing). This explanation is in line with previous studies on the functional dissociation of sublexical and lexical processes in lexical access (Carreiras, Vergara & Barber, 2005), where effects of syllabic manipulations were found earlier (on the P200) and later on the N400 especially for low frequency words, whereas lexical variables only affected the N400. Further support for this distinction was obtained by two subsequent fMRI studies that revealed robust and dissociable brain activation for sublexical and lexical effects (Carreiras, Mechelli & Price, 2006; Carreiras, Riba, Vergara et al, 2009). Importantly, effects of the orthographic manipulation were not observed in the 500–600 ms time window. This may indicate that any transient activation of lexical candidates as a function of orthographic similarity between words has been efficiently counteracted by the domination of the input word target. We interpret the earlier neighborhood ERP effects in terms of multiple activations of orthographically similar representations. Nevertheless, according to interactive models of processing, the mapping of prelexical orthographic representations onto whole-word representations involves the transfer of feedforward and feedback activation between letter and whole word representations. Thus these earlier negativities could instead reflect top-down feedback (larger for words with many neighbors) that would feed excitation back to the letter level, and thus would in turn speed the activation of the target word (Whitney, 2004, has proposed this explanatory mechanism for the facilitative –behavioral- effects of orthographic neighborhood). We believe that further research will clarify this important issue regarding the mechanisms underlying these early ERP negativities.
Consistent with the early onset of the effects of orthographic neighborhood in the present results are the results of a number of previous ERP studies in which orthographic similarity was manipulated as a function of substitution only (the classical Coltheart measure). These studies also found effects of orthographic neighborhood that had an early onset-latency (Holcomb et al, 2002; Müller et al, 2009). However, in these studies the effects of neighborhood lasted much longer than in the present study, until 800 ms after word presentation. These longer lasting negativities may have resulted from prolonged competition between co-activated whole-word representations from high frequency neighbors (neighborhood frequency was not controlled in previous studies). Another explanation of the discrepancy in the duration of the effects of orthographic neighborhood in our study (short) and in the previous studies (long) may be related to our use of the OLD20 measure, and the use of Coltheart’s N in these previous studies. As discussed in the introduction, the OLD20 measure reflects the number of segments shared by the 20 words that are most similar to the target, maximizing differences at a sublexical level. In contrast, Coltheart’s N is a measure of the number of words overlapping in all but one grapheme from the target word, which does not maximize differences at the sublexical level. Grainger and Ziegler (2011; Grainger and Holcomb’s 2009) dual-route interactive activation model could account for differences in the duration of the effects of orthographic neighborhood driven by the different emphases on sublexical level features of these measures. This model uses the dual route model of reading aloud as a starting point (see also Coltheart et al., 1993; Ellis & Young, 1988; 2001) and assumes the existence of two distinct routes for orthographic coding: the coarse-grained route and the fine-grained route. The distinction between these routes is motivated by two specific phenomena: the need to precisely code letter position during print-to-sound conversion, and the empirical evidence for a more flexible and imprecise sublexical orthographic code at the early stages of lexical access. In their dual-route interactive activation model visual features are integrated into final word forms through these distinct coarse-grained and fine-grained routes of orthographic coding. The coarse-grained route uses a code that processes ordered pairs of contiguous and non-contiguous letters (in terms of open bigrams; see Grainger & van Heuven, 2003; Grainger & Whitney, 2004; Whitney, 2001). This route is primarily governed by bottom-up processing, and encodes combinations of the most visible letters that best constrain word identity. The second route, on the other hand, uses a fine-grained code to process grapheme identities and their specific position in the string (beginning-to-end position coding). In the coarse-grained pathway, partial orthographic overlap would be enough to trigger multiple activations of similar word-forms. However, in the fine-grained pathway, not only partial overlap between grapheme identities but also their relative position in the word is needed to trigger the activation of multiple candidates. Our results suggest that the OLD20 measure captures multiple activations according to a coarse-grained code. The short duration of the OLD20 effect could be interpreted as a fast counteraction from lateral-inhibitory mechanisms onto activated representations that partially overlap in orthography. Prolonged effects of Coltheart’s N during visual word recognition suggest multiple activation of similar candidates in both the coarse- and the fine-grained routes. A larger overlap among word forms at sublexical and lexical levels accumulates into larger activation of semantic representations, which together may underlie the long-lasting ERP effects found with the N metric in previous experiments. Further research on the differences between these two measures may shed light on the flexibility of early orthographic coding.
On Word Frequency Effects
With respect to the effect of word-frequency on the N400, our results are consistent with previous research (Barber et al, 2004; Neville, Mills & Lawson, 1992; Smith & Halgren, 1987; Van Petten & Kutas, 1990) and can be interpreted as a reflection of lexical-semantic access processes. Low and high frequency words differ with regard to the strength of memory traces at lexical and semantic levels of representation with processing consequences extending beyond 500 ms after word presentation. Interactive-activation models of visual word recognition (IA model: McClelland & Rummelhart, 1981; MROM: Grainger & Jacobs, 1996; DRC model: Coltheart, Rastle, Perry, Langdon & Ziegler, 2001) would accommodate the early effects of frequency in terms of facilitatory feedback from lexical to sublexical representations, which is enhanced for high compared to low frequency words (however, for earlier latency of word frequency effects see Dambacher, Kliegl, Hofmann, & Jacobs, 2006; Hauk & Pulvermüller, 2004; Proverbio, Vecchi, & Zani, 2004; Sereno, Rayner & Posner, 1998; Sereno, Brewer, & O’Donell, 2003). Our N400 effect of word frequency was somewhat more frontally distributed than the canonical centro-parietal distribution that is typical of the N400. This may in part have resulted from the fact that all the words that were used in the present study were highly concrete, with an average concreteness value that was greater than 500 (range 100–700, see methods section). Previous studies have shown that concrete words and pictures elicit an N400 that has a more frontal distribution (e.g., Ganis, Kutas & Sereno, 1996; Kounios & Holcomb, 1994; Swaab, Baynes & Knight, 2002). Thus, semantic processing may be supported by partially different sets of neural sources for different types of stimuli (see Kutas & Federmeier, 2009). Pairwise comparisons revealed a larger effect of frequency for low neighborhood compared to high neighborhood words. We suggest that effects of word frequency are enhanced when the process of competition is minimized (as is the case of words with few neighbors). However, when competition between lexical candidates increases (as in the case of words with many neighbors), any advantage in processing of high over low frequency words may be mitigated by early activation of (and thus, confusability with) similar word-forms (Goh, Suárez, Yap & Tan, 2009).
We believe that the evidence presented here provides further support for a functional distinction between properties of a subset of the network (orthographic overlap) and properties of the single word forms (frequency). The effects of automatic activation from similarly spelled words are observed around 250 ms after stimulus presentation and spread from lexical to semantic representations in a feed-forward and feedback manner. However, if the neighborhood effects were merely a reflection of lexical-semantic activation, they would not be modulated by frequency. Instead, we did observe modulation of the effects of orthographic neighborhood as a function of word frequency. We therefore suggest that our data provide further support for a lateral-inhibitory mechanism that exerts its influence at a lexical level of processing. A recent masked priming study by Massol et al (2010) presented a fine-grained temporal analysis of the inhibitory effect of primes that are high-frequency orthographic neighbors of target words, and found effects of neighborhood on the N400. This was also interpreted as further support for a lateral inhibition mechanism operating at a lexical level. Masked priming is an excellent technique to unveil the effects of prime stimuli on a target word, however we must keep in mind that priming experiments focus on the relationship between prime and target and not on the specific timing of the processing of the critical stimulus itself. In addition, whereas results from masked priming experiments may be difficult to generalize to normal reading processing, the findings of our present experiment, which were obtained in a semantic categorization task on single word presentation, clearly support this type of explanatory mechanism.
In sum, the present study has revealed an important dissociation in the time course of the effects of orthographic neighborhood and word frequency during visual word recognition. Specifically, our results suggest that the effects of orthographic neighborhood start and end earlier than the effects of word frequency. With respect to the earlier onset and shorter duration effects of ON, note that the OLD20 is a measure of orthographic similarity that enables a larger range of variability than the standard N metric, as it maximizes the ability to measure differences at a sublexical level (it reflects the number of segments shared by the 20 words that are most similar to a target word) and because it is free from the letter position requirement which is implicit in the definition of the classic Coltheart’s N measure. In addition, we also found overlap of the N400 effects of orthographic neighborhood and word frequency in a 380–500 ms time-window. This can be interpreted as a reflection of competitive interactions at the lexical level between a subset of the activated lexical network driven by orthographic similarity and the representation that matched the actual input. These findings are consistent with interactive-activation based models of visual word recognition.
Acknowledgments
Marta Vergara-Martínez was supported by a postdoctoral contract under the Spanish Fulbright–MEC Commission and a Postdoctoral contract at UC Davis. Tamara Swaab was supported by grants from NIMH (1R24MH081807) and NSF (BCS-1024003).
Footnotes
Indeed, studies of auditory word recognition already define phonological neighbors as word representations that differ from the candidate word as a function of substitution, addition, and deletion (Luce & Pisoni, 1998).
References
- Acha J, Perea M. The effect of neighborhood frequency in reading: Evidence with transposed-letter neighbors. Cognition. 2008;108:290–300. doi: 10.1016/j.cognition.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Andrews S. Frequency and neighborhood effects on lexical access: Activation or search? Journal of Experimental Psychology: Learning, Memory and Cognition. 1989;15:802–814. [Google Scholar]
- Andrews S. Frequency and neighborhood effects on lexical access: Lexical similarity or orthographic redundancy? Journal of Experimental Psychology: Learning Memory and Cognition. 1992;18:234–254. [Google Scholar]
- Andrews S. The effect of orthographic similarity on lexical retrieval: Resolving neighborhood conflicts. Psychonomic Bulletin & Review. 1997;4:439–461. [Google Scholar]
- Balota DA, Chumbley JI. Are lexical decisions a good measure of lexical access? The role of word frequency in the neglected decision stage. Journal of Experimental Psychology: Human Perception and Performance. 1984;10:340–357. doi: 10.1037//0096-1523.10.3.340. [DOI] [PubMed] [Google Scholar]
- Barber HA, Kutas M. Interplay between computational models and cognitive electrophysiology in visual word recognition. Brain Research Reviews. 2007;53:98–123. doi: 10.1016/j.brainresrev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Barber HA, Vergara M, Carreiras M. Syllable-frequency effects in visual word recognition: evidence from ERPs. Neuroreport. 2004;15:545–548. doi: 10.1097/00001756-200403010-00032. [DOI] [PubMed] [Google Scholar]
- Bentin S, McCarthy G, Wood CC. Event-related potentials, lexical decision and semantic priming. Electroencephalography and Clinical Neurophysiology. 1985;60:343–355. doi: 10.1016/0013-4694(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Buchwald A, Rapp B. Consonants and vowels in orthographic-representations. Cognitive Neuropsychology. 2006;23:308–337. doi: 10.1080/02643290442000527. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Perea M, Grainger J. Effects of orthographic neighborhood in visual word recognition: Cross-task comparisons. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1997;23:857–871. doi: 10.1037//0278-7393.23.4.857. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Vergara M, Barber H. Early Event-related Potential Effects of Syllabic Processing during Visual Word Recognition. Journal of Cognitive Neuroscience. 2005;17:1803–1817. doi: 10.1162/089892905774589217. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Riba J, Vergara M, Heldmann M, Münte TF. Syllable congruency and word frequency effects on brain activation. Human Brain Mapping. 2009;30:3079–3088. doi: 10.1002/hbm.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M, Mechelli A, Price C. The effect of word and syllable frequency on activation during lexical decision and reading aloud. Human Brain Mapping. 2006;27:863–972. doi: 10.1002/hbm.20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A Dual Route Cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. Quarterly Journal of Experimental Psychology, Section A: Human Experimental Psychology. 1981;33:497–505. [Google Scholar]
- Coltheart M, Davelaar E, Jonasson JE, Besner D. Access to the internal lexicon. In: Dornio S, editor. Attention and performance. VI. Hillsdale, NJ: Erlbaum; 1977. pp. 535–555. [Google Scholar]
- Dambacher M, Kliegl R, Hofmann M, Jacobs AM. Frequency and predictability effects on event-related potentials during reading. Brain Research. 2006;1084:89–103. doi: 10.1016/j.brainres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Taft M. More words in the neighborhood: Interference in lexical decision due to deletion neighbors. Psychonomic Bulletin & Review. 2005:904–910. doi: 10.3758/bf03196784. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Perea M, Acha J. Re(de)fining the orthographic neighborhood: the role of addition and deletion neighbors in lexical decision and reading. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:1550–1570. doi: 10.1037/a0014253. [DOI] [PubMed] [Google Scholar]
- Forster KI, Shen D. No enemies in the neighborhood: Absence of inhibitory neighborhood effects in lexical decision and semantic categorization. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1996;22:696–713. doi: 10.1037//0278-7393.22.3.696. [DOI] [PubMed] [Google Scholar]
- Forster KI, Hector J. Cascaded versus noncascaded models of lexical and semantic processing the turple effect. Memory & Cognition. 2002;30:1106–1116. doi: 10.3758/bf03194328. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency Analysis of English Usage: Lexicon and Grammar. Houghton Mifflin: Boston, Mass; 1982. [Google Scholar]
- Ganis G, Kutas M, Sereno MI. The search for the "common sense”: an electrophysiological study of the comprehension of words. Journal of Cognitive Neuroscience. 1996;8:89–106. doi: 10.1162/jocn.1996.8.2.89. [DOI] [PubMed] [Google Scholar]
- Goh WD, Suárez L, Yap MJ, Tan SH. Distributional analyses in auditory lexical decision: Neighborhood density and word frequency effects. Psychonomic Bulletin & Review. 2009;16:882–887. doi: 10.3758/PBR.16.5.882. [DOI] [PubMed] [Google Scholar]
- Grainger J, Jacobs AM. Orthographic processing in visual word recognition: a multiple read-out model. Psychological Review. 1996;103:518–565. doi: 10.1037/0033-295x.103.3.518. [DOI] [PubMed] [Google Scholar]
- Grainger J, Van Heuven W. Modeling letter position coding in printed word perception. In: Bonin P, editor. The Mental Lexicon. New York: Nova Science Publishers; 2003. pp. 1–24. [Google Scholar]
- Grainger J, Whitney C. Does the huamn mind raed wrods as a wlohe? Trends in Cognitive Sciences. 2004;8:58–59. doi: 10.1016/j.tics.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Grainger J, Holcomb PJ. Watching the Word Go by: On the Time-course of Component Processes in Visual Word Recognition. Language and Linguistics Compass. 2009;3:128–156. doi: 10.1111/j.1749-818X.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Grainger J, Ziegler JC. A dual-route approach to orthographic processing. Frontiers in Language Sciences. 2011;2(54):1–13. doi: 10.3389/fpsyg.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J, Holcomb PJ. Watching the word go by: On the time-course of component processes in visual word recognition. Language and Linguistic Compass. 2009;3(1):128–156. doi: 10.1111/j.1749-818X.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J, van Heuven W. Modeling letter position coding in printed word perception. In: Bonin P, editor. The Mental Lexicon. New York: Nova Science Publishers; 2003. pp. 1–24. [Google Scholar]
- Holcomb PJ, Grainger J, O'Rourke T. An Electrophysiological Study of the Effects of Orthographic Neighborhood Size on Printed Word Perception. Journal of Cognitive Neuroscience. 2002;14:938–950. doi: 10.1162/089892902760191153. [DOI] [PubMed] [Google Scholar]
- Johnson R. The Quiet Clam Is Quite Calm: Transposed-Letter Neighborhood Effects on Eye Movements During Reading. Journal of Experimental Psychology: Learning, Memory and Cognition. 2009;35:943–969. doi: 10.1037/a0015572. [DOI] [PubMed] [Google Scholar]
- Hauk O, Pulvermüller F. Effects of word length and frequency on the human event-related potential. Clinical Neurophysiology. 2004;115(5):1090–1103. doi: 10.1016/j.clinph.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Kounios J, Holcomb PJ. Concreteness effects in semantic processing: ERP evidence supporting dual-coding theory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20(4):804–823. doi: 10.1037//0278-7393.20.4.804. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Sciences. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Scholarpedia. 2009;4(10):7790. [Google Scholar]
- Laszlo S, Federmeier KD. The N400 as a snapshot of interactive processing:Evidence from regression analyses of orthographic neighbor and lexical associate effects. Psychophysiology. 2011;48:176–186. doi: 10.1111/j.1469-8986.2010.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo S, Federmeier KD. A beautiful day in the neighborhood: An event-related potential study of lexical relationships and prediction in context. Journal of Memory and Language. 2009;61(3):326–338. doi: 10.1016/j.jml.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenshtein VI. Binary codes capable of correcting deletions, insertions, and reversals. Soviet Physics Doklady. 1966;10:707–710. [Google Scholar]
- Luce PA, Pisoni DB. Recognizing spoken words: The neighborhood activation model. Ear and Hearing. 1998;19:1–36. doi: 10.1097/00003446-199802000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund K, Burgess C. Producing high-dimensional semantic spaces from lexical co-occurrence. Behavior Research Methods, Instruments & Computers. 1996;28:203–208. [Google Scholar]
- Massol S, Grainger J, Dufau S, Holcomb P. Masked priming from Orthographic Neighbors: An ERP Investigation. Journal of Experimental Psychology: Human Perception and Performance. 2010;36(1):162–174. doi: 10.1037/a0017614. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rumelhart DE. An interactive activation model of context effects in letter perception: I. An account of basic findings. Psychological Review. 1981;88:375–407. [PubMed] [Google Scholar]
- Midgley K, Holcomb PJ, van Heuven WJB, Grainger J. An electrophysiological investigation of cross-language effects of orthographic neighborhood. Brain Research. 2008;1246:123–135. doi: 10.1016/j.brainres.2008.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller O, Duñabeitia JA, Carreiras M. Orthographic and associative neighborhood density effects: what is shared, what is different? Psychophysiology. 2010;47:455–466. doi: 10.1111/j.1469-8986.2009.00960.x. [DOI] [PubMed] [Google Scholar]
- Neville H, Mills DL, Lawson DS. Fractionating Language: Different Neural Subsystems with Different Sensitive Periods. Cerebral Cortex. 1992;2:244–258. doi: 10.1093/cercor/2.3.244. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perea M, Pollatsek A. The effects of neighborhood frequency in reading and lexical decision. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:767–779. doi: 10.1037//0096-1523.24.3.767. [DOI] [PubMed] [Google Scholar]
- Pollatsek A, Perea M, Binder KS. The effects of neighborhood size in reading and lexical decision. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1142–1158. [PubMed] [Google Scholar]
- Proverbio AM, Vecchi L, Zani A. From orthography to phonetics: ERP Measures of grapheme-to-phoneme conversion mechanisms in reading. Journal of Cognitive Neuroscience. 2004;16:301–317. doi: 10.1162/089892904322984580. [DOI] [PubMed] [Google Scholar]
- Sears CR, Hino Y, Lupker SJ. Neighborhood size and neighborhood frequency effects in visual word recognition. Journal of Experimental Psychology: Human Perception & Performance. 1995;21:876–900. [Google Scholar]
- Sereno SC, Rayner K, Posner MI. Establishing a time-line of word recognition: evidence from eye movements and event-related potentials. Neuroreport. 1998;9:2195–2200. doi: 10.1097/00001756-199807130-00009. [DOI] [PubMed] [Google Scholar]
- Sereno SC, Brewer CC, O'Donnell PJ. Context effects in word recognition: evidence for early interactive processing. Psychological Science. 2003;14:328–333. doi: 10.1111/1467-9280.14471. [DOI] [PubMed] [Google Scholar]
- Slattery TJ. Word misperception, the neighbor frequency effect, and the role of sentence context: Evidence from eye movements. Journal of Experimental Psychology: Human Perception and Performance. 2009;35(6):1969–1975. doi: 10.1037/a0016894. [DOI] [PubMed] [Google Scholar]
- Smith ME, Halgren E. Event-related potentials during lexical decision: effects of repetition, word frequency, pronounceability, concreteness. Electroencephalography and Clinical Neurophysiology Supplement. 1987;40:417–421. [PubMed] [Google Scholar]
- Swaab TY, Baynes K, Knight RT. Separable effects of priming and imageability on word processing: an ERP study. Cognitive Brain Research. 2002;15:99–103. doi: 10.1016/s0926-6410(02)00219-7. [DOI] [PubMed] [Google Scholar]
- Van Heuven WJB, Dijkstra T, Grainger J. Orthographic neighborhood effects in bilingual word recognition. Journal of Memory and Language. 1998;39:458–483. [Google Scholar]
- Van Petten C, Kutas M. Interactions between sentence context and word frequency in event-related brain potentials. Memory and Cognition. 1990;18(4):380–393. doi: 10.3758/bf03197127. [DOI] [PubMed] [Google Scholar]
- Vergara-Martínez M, Perea M, Marín A, Carreiras M. The processing of consonants and vowels during letter identity and letter position assignment in visual-word recognition: An ERP study. Brain and Language. 2011;118(3):105–117. doi: 10.1016/j.bandl.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Balota D, Yap M. Moving beyond Coltheart's N: a new measure of orthographic similarity. Psychonomic Bulletin & Review. 2008;15:971–979. doi: 10.3758/PBR.15.5.971. [DOI] [PubMed] [Google Scholar]
- Whitney C. How the brain encodes the order of letters in a printed word: The SERIOL model and selective literature review. Psychonomic Bulletin & Review. 2001;8:221–243. doi: 10.3758/bf03196158. [DOI] [PubMed] [Google Scholar]
- Whitney C. Hemisphere-specific effects in word recognition do not require hemisphere-specific modes of access. Brain and Language. 2004;88:279–293. doi: 10.1016/S0093-934X(03)00160-3. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Rey A, Jacobs AM. Simulating individual word identification thresholds and errors in the fragmentation task. Memory and Cognition. 1998;26:490–550. doi: 10.3758/bf03201158. [DOI] [PubMed] [Google Scholar]