Abstract
NADPH is an important component of the antioxidant defense system and a proposed mediator in glucose-stimulated insulin secretion (GSIS) from pancreatic β-cells. An increase in the NADPH/NADP+ ratio has been reported to occur within minutes following the rise in glucose concentration in β-cells. However, 30 min following the increase in glucose, the total NADPH pool also increases through a mechanism not yet characterized. NAD kinase (NADK) catalyzes the de novo formation of NADP+ by phosphorylation of NAD+. NAD kinases have been shown to be essential for redox regulation, oxidative stress defense, and survival in bacteria and yeast. However, studies on NADK in eukaryotic cells are scarce, and the function of this enzyme has not been described in β-cells. We employed INS-1 832/13 cells, an insulin-secreting rat β-cell line, and isolated rodent islets to investigate the role of NADK in β-cell metabolic pathways. Adenoviral-mediated overexpression of NADK resulted in a two- to threefold increase in the total NADPH pool and NADPH/NADP+ ratio, suggesting that NADP+ formed by the NADK-catalyzed reaction is rapidly reduced to NADPH via cytosolic reductases. This increase in the NADPH pool was accompanied by an increase in GSIS in NADK-overexpressing cells. Furthermore, NADK overexpression protected β-cells against oxidative damage by the redox cycling agent menadione and reversed menadione-mediated inhibition of GSIS. Knockdown of NADK via shRNA exerted the opposite effect on all these parameters. These data suggest that NADK kinase regulates intracellular redox and affects insulin secretion and oxidative defense in the β-cell.
Keywords: nicotinamide adenine dinucleotide; reduced nicotinamide adenine dinucleotide phosphate/nicotinamide adenine dinucleotide phosphate+, insulin secretion; β-cells; cytosolic oxidoreductases
insulin secretion, a hallmark feature of pancreatic β-cells, occurs in response to the increase in glucose concentration from basal, nonstimulatory levels (4–7 mM) to stimulatory levels (8–16 mM). Glucose metabolism inside the β-cell leads to an increase in the reduced/oxidized ratio of both NADH and NADPH (4, 29, 49) occurring within minutes following an increase in glucose concentration. NADPH has been suggested to be a coupling factor for insulin secretion (15, 42) based on a study showing that injection of NADPH triggered insulin granule exocytosis from pancreatic β-cells (15). In addition to its putative role as a metabolic mediator of insulin secretion, NADPH is a cofactor for reductive biosynthetic pathways and is essential for oxidative defense, since it replenishes the glutaredoxin and thioredoxin systems (13).
In the β-cell cytosol, NADPH can be formed by the reduction of its oxidized counterpart NADP+ via pyruvate cycling pathways mediated by cytosolic malic enzyme (ME1) and cytosolic isocitrate dehydrogenase (ICDc) (reviewed in Ref. 17) as well as via glucose-6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme of the pentose phosphate shunt (7). Inside the mitochondria, NADPH is regenerated via NADP+-dependent reduction mediated by ME3 and mitochondrial isocitrate dehydrogenase (18, 40), as well as NADH-dependent reduction of NADP+ via nicotinamide nucleotide transhydrogenase (NNT) (16). However, the operation of these pathways changes only the proportion of the reduced/oxidized form of NADPH, without the size of the total (NADPH + NADP+) nicotinamide adenine dinucleotide phosphate pool being affected.
NAD kinase [NADK; ATP: NAD(H) 2′-phosphotransferase] is the only known mammalian enzyme that catalyzes the conversion of NAD+ to NADP+ (reviewed in Ref. 38) and thus regulates the size of the (NADPH + NADP+) pool. NADKs play a crucial role in cell metabolism, survival, and oxidative defense in a variety of organisms, including bacteria, yeasts, and plants (reviewed in Ref. 50). Whereas several isoforms of NADK have been described in yeasts and plants (50), only a single cytosolic isoform exists in mammals (23). Despite the importance of this enzyme for intracellular redox regulation, the existence and function of this enzyme in insulin-secreting cells has not been investigated to date.
In the current study, we demonstrate for the first time the presence of NADK in β-cells and show that this enzyme regulates the size of the NADPH pool, insulin secretion, and β-cell survival. Together, these data suggest that NADK is an integral part of the β-cell redox and metabolic network.
MATERIALS AND METHODS
Materials
Collagenase was from Roche, and fetal calf serum was from Hyclone. All other chemicals were from Sigma-Aldrich unless otherwise specified.
Cell and Islet Preparation and Culture
Clonal INS-1 832/13 cells, provided by Dr. Christopher Newgard (Duke University), were maintained and cultured as described previously (10). Male CD-1 mice and Sprague-Dawley rats (Charles River) were euthanized by halothane. All procedures were performed in accordance with the Institutional Guidelines for Animal Care in compliance with US Public Health Service regulations and were approved by the Institutional Animal Care and Use Committee at the Marine Biological Laboratory. Pancreatic islets were isolated by collagenase digestion (Roche, Indianapolis, IN), as described previously (12). Islets were used after an overnight culture in RPMI supplemented with 10% fetal calf serum (Hyclone), penicillin-streptomycin, and 5 mM glucose.
Construction of Short Hairpin RNA Plasmids, Adenovirus, and Lentivirus
Plasmids containing the green fluorescent protein (GFP) sequence and short hairpin RNA (shRNA)-encoding sequences (cat. no. TG708143) targeted against rat NADK (GenBank accession no. NM_001109678) or GFP and noncoding sequence (scrambled control, cat. no. TR30013) were custom-designed and constructed by OriGene (Rockville, MD). The 29mer shRNA constructs against rat Nadk were GAATCCTGACTCAGCCTCCTACAACTGT and TGACATTTCCAACCAGATAGACTTCATCA.
NADK-overexpressing adenovirus.
Recombinant, replication-deficient type 5 adenovirus (Ad-NADK)-expressing human NADK (OriGene, Rockville, MD) was custom-constructed by Vector BioLabs (Philadelphia, PA). The expression of NADK is under the control of the cytomegalovirus promoter, which also directs the expression of GFP from an internal ribosome entry site. A control virus containing GFP sequence only (Ad-control) was constructed in parallel. Viral titers were determined by the plaque formation assay.
NADK-silencing lentivirus.
The lentiviral plasmid expressing shRNA was from Open Biosystems (cat. no. RMM4431-101290079). The shRNA sequence against mouse/rat Nadk was AGATCCGAGATGCCAGCTT. For control, the scrambled, nonsilencing shRNA sequence (Open Biosystems, cat. no. RHS4346) was used. The lentiviruses were produced according to published methods (20). The pGIPZ vectors containing either shRNA sequence against Nadk or the scrambled sequence were cotransfected with the packaging Δ8.9 and the vesicular stomatitis virus G protein vectors into human embryonic kidney (HEK)-293T cells. Seventy-two hours after transfection, the supernatant was centrifuged for 2 h at 100,000 g. The viral pellet was dissolved in PBS and used as a source of lentivirus.
Overexpression and Knockdown of NADK in INS-1832/13
For NADK overexpression, cells (at 60–70% confluence) were transduced with Ad-NADK or Ad-control at 50 MOI for 12 h; then, viral medium was replaced with the appropriate growth medium. Functional assays were performed 36–48 h posttransduction. For NADK knockdown, cells (at 50–60% confluence) were transfected with shRNA plasmid-Lipofectamine 2000 (Invitrogen) complexes prepared in Optimem medium (Invitrogen). Ten microliters of Lipofectamine with 4 μg/well of plasmid DNA of a six-well plate were used, and amounts were scaled down appropriately for cells grown in 24- or 48-well plates. Cells were incubated with complexes for 6 h, after which complexes were replaced with the appropriate growth medium. Functional assays were performed 72 h posttransduction. Transduction and transfection efficiencies, as determined by the GFP fluorescence, reached >90 and 75%, respectively, under these conditions.
Overexpression and Knockdown of NADK in Islets
For NADK overexpression and knockdown, islets were used immediately following their isolation. Islets were transduced with the adenovirus-carrying NADK sequence or control adenovirus at 50 MOI for 12 h; then, viral medium was replaced with the appropriate growth medium. Functional assays were performed 36–48 h posttransduction. For NADK knockdown, islets were infected with lentivirus-carrying NADK shRNA sequence or control lentivirus-carrying scrambled sequence for 12 h; then, viral medium was replaced with the appropriate growth medium. Functional assays were performed 72–96 h posttransduction. Transduction and transfection efficiencies, as determined by GFP fluorescence, reached >80% under these conditions.
Quantitative RT-PCR
Total RNA was extracted using TriReagent (Sigma, St. Louis, MO) or the RNeasy Micro Kit (Qiagen, Valencia, CA), and RNA was reverse-transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the manufacturers' protocols. Standard curves were generated using serial twofold dilutions from pooled cDNA samples to confirm ≥90% reaction efficiency for each primer set. Real-time PCR was performed using SYBR Green PCR Master Mix (Bio-Rad, Hercules, CA) on a MyiQ2 Real-Time PCR Detection System (Bio-Rad). All PCR primer sequences were generated using PrimerQuest (Integrated DNA Technologies, Coralville, IA). A minimum of three samples was analyzed for each experimental group. Primer sequences are listed in Table 1.
Table 1.
Real-time PCR primer sequences
| Gene | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| Rat | ||
| β-2MG | ACACTGAATTCACACCCACCGAGA | TGATTACATGTCTCGGTCCCAGGT |
| ME1 | CGTGGGTCTTGCATGCCAACAATA | TTCGCTCTCCATCAGTCACCACAA |
| ME2 | AAGAGTTAGCCCAAGGGAGGCTTT | TCATACACATCTGGCAGCAGGGAA |
| ME3 | ACAAACACAACCTGGCCTCCTACT | ATGGCGTCAGACCTTCTGAACACT |
| PC | AGTAATGGTGGCCAACAGAGGTGA | ATGTCTGGAATGTGCAGGTAGGCT |
| ICD1 | TGGGTCGCAGAAAGTGACATACCT | AGCCATTTGGAAGGAACTGTGTGC |
| ICD2 | AGGACCTCATCAGGTTTGCACAGA | AGTCTGTGGTGTTCAGGAAGTGCT |
| NNT | CAACTGGTTGCTGCTTTCCACAGT | AAAGGTGACTCCGCCGATGTAAGT |
| NADK | TATGCTGTCACCAGAAGCCAGGAA | TGGTGATGCTGATGCTGTCTCCAT |
| Human | ||
| NADK | ACCCACACATTCCTCACGTAGCTT | TGAGGACAGCGTCTTGCAGAACAT |
β-2MG, β-2-microglobulin; ME1, -2, and -3, malic enzyme 1, 2, and 3, respectively; PC, pyruvate carboxylase; ICD1 and -2, isocitrate dehydrogenase 1 and 2, respectively; NNT, nicotinamide nucleotide transhydrogenase; NADK, NAD kinase. All sequences are designed for Rattus norvegicus, except as indicated.
INS-1 832/13 Autofluorescence by Two-Photon Excitation of NAD(P)H
Cells cultured on poly-d-lysine-coated coverslips of 35-mm confocal dishes (MatTek) were imaged on a Zeiss LSM510 confocal microscope equipped with a heated stage, using two-photon excitation of NAD(P)H as described previously (12). NAD(P)H was excited by 150-fs pulses of 710 nm light from a Mira laser focused through a ×40 objective. Autofluorescence was collected through a 380-550 custom-made Chroma filter (Bennett BD 1996 JBC), and images were analyzed using Zeiss imaging software.
Determination of Nucleotides
Following alkali extraction, INS-1 832/13 cells (confluent 35-mm dish/experiment) and islets (200 islets/experiment) were vortex-mixed and sonicated for 10 s on ice. Aliquots were heated at 60°C for 20 min to destroy NAD+ and NADP+. Nonheated aliquots were used for determination of total NADH + NAD+ and NADPH + NADP+. ATP/ADP ratio was determined using bioluminescent detection method. All adenine nucleotides were determined using NAD+/NADH, NADP+/NADPH, and ATP/ADP kits (Abcam, Cambridge, MA) according to the manufacturer's protocols.
Determination of Intracellular Oxidative Stress
Cells were preloaded with 10 μM 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) for 60 min and treated with hydrogen peroxide (10 uM) for 3 h. Fluorescence (485 nm excitation, 520 nm emission) was quantified using a SpectraMax M5 plate reader.
Determination of NADK Activity
NADK activity in cell lysates was assayed as described previously (39) in reaction mixture containing 50 mMTris·HCl, pH 7.8, 10 mM MgCl2, 5 mM NAD+, and 10 mM ATP. The amount of NADP+ produced by NADK-dependent phosphorylation was then determined by a cycling assay in the presence of 5 mM of glucose 6-phosphate, NADP-specific yeast G6PD, and 0.5 mM MTT/mPMS. Reduction of MTT was measured at 600 nm. Calibration curve was generated using known amounts of NADP+ standards in the cycling reaction. Reduction of MTT was monitored at 600 nm using a Sunrise spectrophotometer (Tecan). One unit was defined as the amount of enzyme producing 1 μmol of NADP in 1 min at 37°C.
Insulin Secretion
INS-1 832/13 (48-well plates) and isolated islets (15 islets/tube) were preincubated for 2 h in the presence of 2 mM (INS-1 832/13 cells) or 4 mM (islets) glucose in Krebs-Ringer bicarbonate buffer. The amount of released insulin was determined after 60 min of static incubation, using an ELISA kit (Alpco Diagnostics, Salem, NH). Data were normalized for protein content determined by the Micro-BCA Protein Assay Kit (Pierce, Rockford, IL).
Statistical Analysis
Data are expressed as means ± SE. Significance was determined for multiple comparisons using one-way analysis of variance. A P value of <0.05 was considered significant.
RESULTS
NADK Expression in β-Cells and Rodent Islets
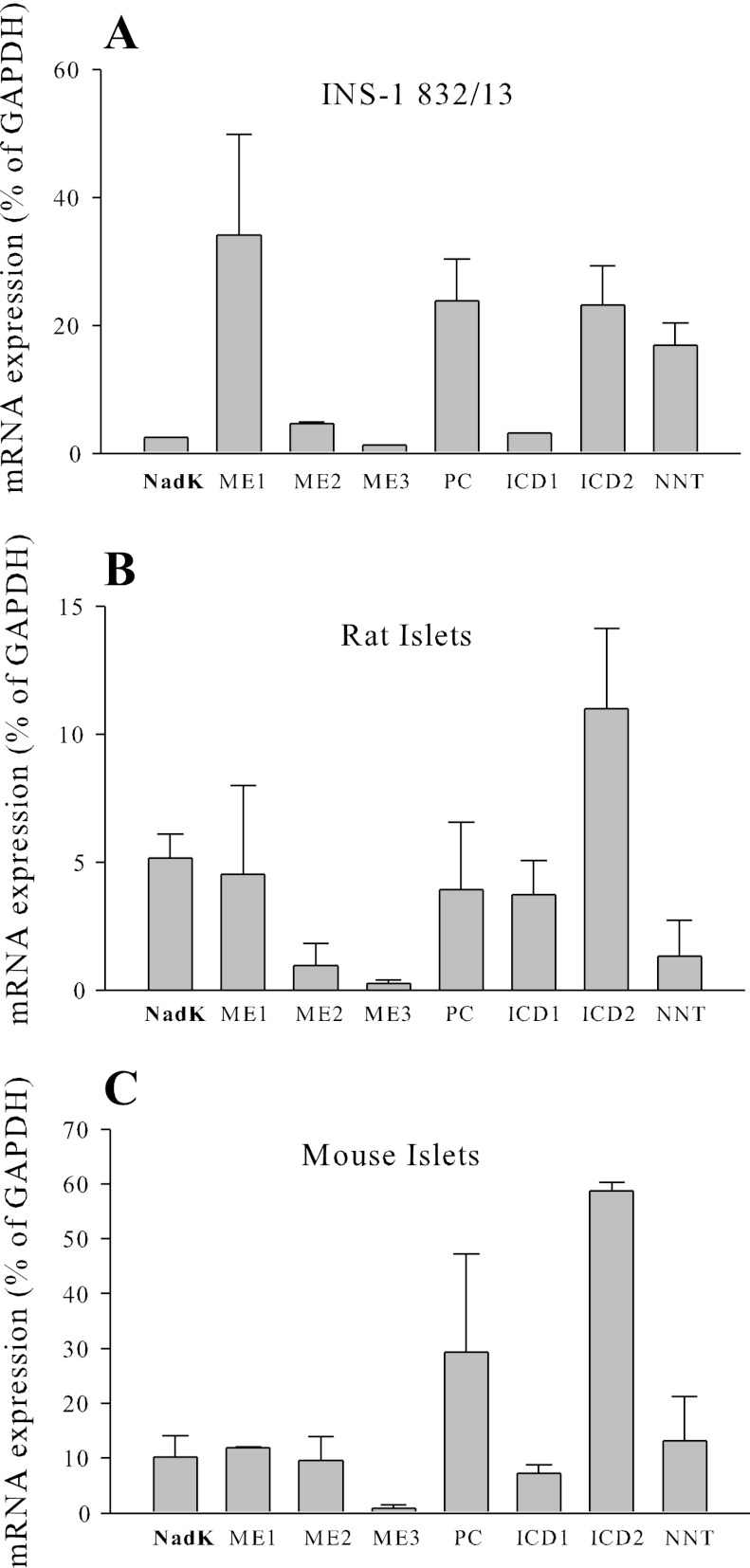
Relative expression of mRNA in INS-1 832/13 cells and rat and mouse islets was determined using quantitative real-time PCR (Fig. 1). Real-time-PCR primer sequences are listed in Table 1. Cell and islet mRNA expression levels were normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase. The mRNA levels of NADK were found to be similar to the mRNA levels of ME2 and ME3, mitochondrial NAD+ and NADP+-dependent malic enzymes in both INS-1 832/13 cells and mouse islets (Fig. 1). Expression of NADK in INS-1 832/13 cells was not different following 48 h treatment with basal or stimulatory glucose levels (data not shown).
Fig. 1.
Relative abundance of NAD kinase (NADK) mRNA in INS-1 832/13 cells (A) and rat (B) and mouse (C) islets. ME1, -2, and -3, malic enzymes 1, 2, and 3; PC, pyruvate carboxylase; ICD1 and -2, isocitrate dehydrogenase 1 and 2; NNT, nicotinamide nucleotide transhydrogenase.
NADK Overexpression and its Effect on NADPH levels, the NADPH/NADP+ Ratio, and Insulin Secretion
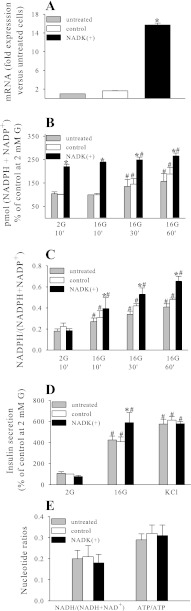
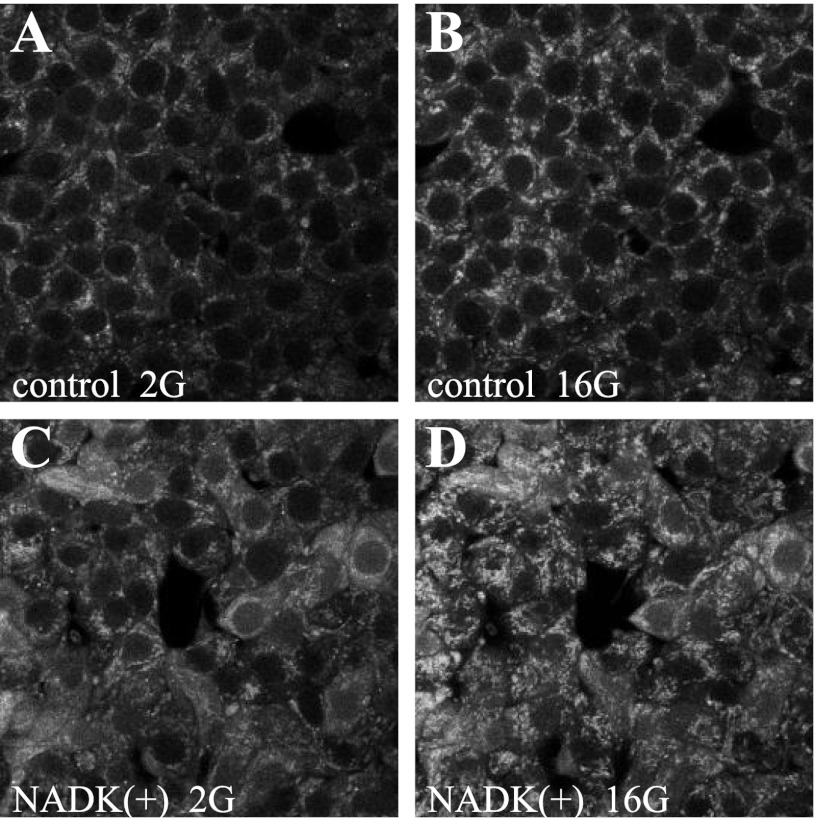
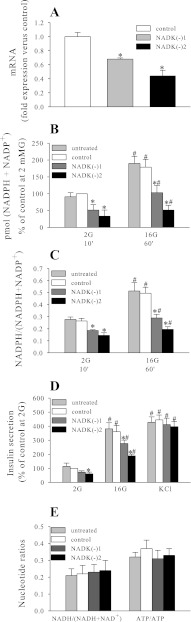
Adenoviral-mediated overexpression of NADK resulted in a >10-fold increase in NADK mRNA (Fig. 2A) and NADK enzymatic activity (Table 2). The total NADPH pool (NADPH and NADP+) and the NADPH/NADP+ ratio were measured following 10, 30, and 60 min of 2 or 4 and 16 mM glucose exposure. In control (control virus treated) and untreated cells and islets, an increase in the total NADPH pool, in addition to an increase in the ratio, was noted following 30 and 60 min of exposure to 16 mM glucose (Figs. 2, B and C, and 5A), and these effects were enhanced in the NADK-overexpressing cells (Figs. 2B and 5A). Upon analysis, it was found that the increase in the size of the total NADPH pool was due mainly to the increase in NADPH but not NADP+. This was illustrated further by the two-photon imaging of live NAD(P)H autofluorescence, demonstrating a 245 ± 35% increase in autofluorescence in NADK-overexpressing [NADK(+)] cells (Fig. 3). Since only the reduced form of adenine nucleotides (NADPH and NADH) displays autofluorescence, this increase in signal was due to the increase in NADPH, since NADK overexpression did not cause significant changes in the NADH + NAD+ pool or in the NADH/NAD+ ratio (Fig. 2E). This is not surprising considering that the size of the total (NADH + NAD+) pool is far greater than the size of the (NADPH + NADP+) pool (12) and that alternate synthetic pathways might serve to replenish any NAD+ phosphorylated by NADK (43).
Fig. 2.
Effect of NADK overexpression on NADK mRNA (A), total (NADPH + NADP+) levels (B), NADPH/(NADPH + NADP+) ratio (C), insulin secretion (D), and NADH/(NADH + NAD+) and ATP/ADP ratio (E) in INS-1 832/13 cells. Nucleotides were determined using NADPH/NADP+, NADH/NAD+, and ATP/ADP kits (Abcam, Cambridge, MA) according to the manufacturer's protocol. Insulin secretion was determined by ELISA (Alpco, Salem, NH). Data are means ± SE from 3–5 independent experiments performed in duplicate or triplicate measurements. 2G, 2 mM glucose; 16G, 16 mM glucose. *P < 0.05 NADK(+) vs. NADK control; #P < 0.05 2G vs. 16G.
Table 2.
Effect of NADK overexpression (+) and knockdown (−) on NAD+ kinase activity
| INS-1 832/13 | Mouse Islets | |
|---|---|---|
| NADK(+) control | 0.76 ± 0.06 | 0.91 ± 0.2 |
| NADK(+) | 121.20 ± 10.4 | 79.20 ± 14.4 |
| NADK (−) control | 0.50 ± 0.04 | 0.76 ± 0.11 |
| NADK (−)1 | 0.09 ± 0.03 | 0.18 ± 0.04 |
| NADK (−)2 | 0.060 ± 0.01 |
Data are means ± SE of 2–3 experiments performed in duplicate measurements and are presented as units/g protein. NAD+ kinase activity was assayed as described in materials and methods. One unit is defined as 1 μmol/min NADP produced.
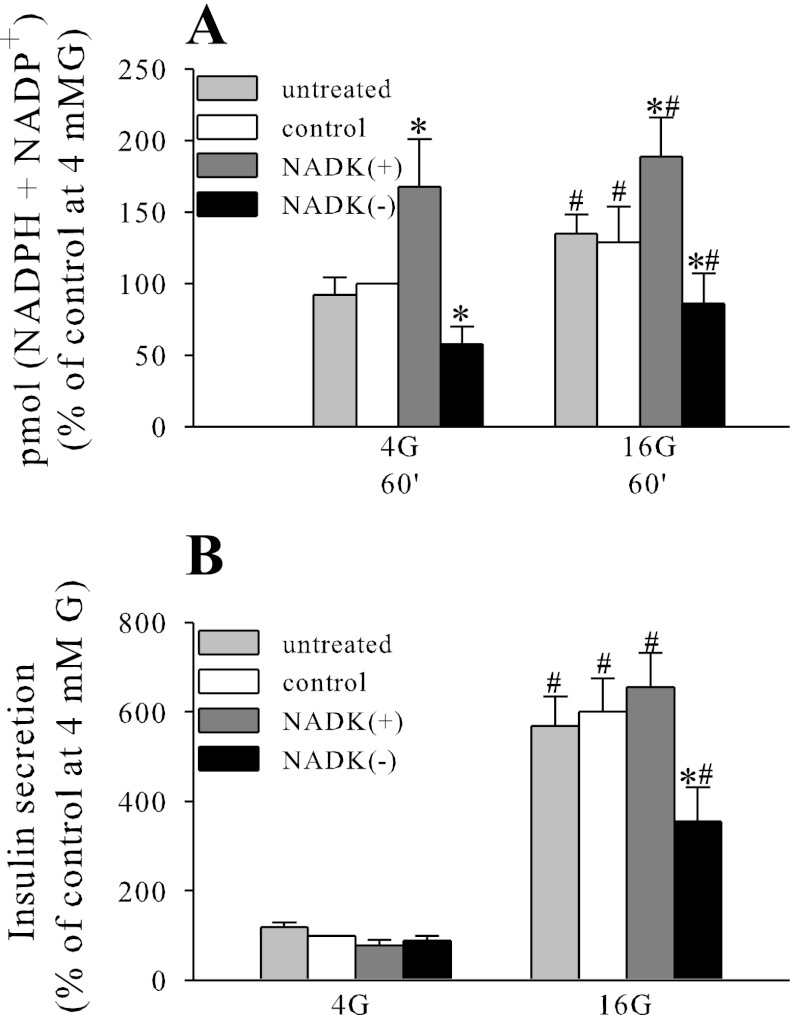
Fig. 5.
Effect of NADK overexpression knockdown on total (NADPH + NADP+) levels (A) and insulin secretion (B) in mouse islets. Nucleotide determination and insulin secretion were performed as described in the legend to Fig. 2. Data are means ± SE from 2–3 independent experiments performed in duplicate or triplicate measurements. *P < 0.05 NADK(+) or NADK(−) vs. NADK control; #P < 0.05 4G vs. 16G.
Fig. 3.
Effect of NADK overexpression on autofluorescence of NAD(P)H. NAD(P)H autofluorescence was monitored in live control (A and B) or NADK(+) (C and D) INS-1 832/13 cells in the presence of 2 or 16 mM glucose (2G or 16G) by 2-photon excitation of NAD(P)H, as described previously (12).
In INS-1 832/13, NADK overexpression caused an ∼30% increase in glucose-stimulated insulin secretion (GSIS), whereas insulin secretion initiated by the depolarizing agent KCl was not affected significantly (Fig. 2D). However, in rodent islets, NADK overexpression elicited only a modest increase in GSIS, which was not statistically significant (Fig. 5B).
NADK Knockdown and its Effect on NADPH, the NADPH/NADP+ Ratio, and Insulin Secretion
Transfection of INS-1 832/13 cells with NADK-shRNA plasmids decreased NADK mRNA expression (Fig. 4A) and NADK activity (Table 2). Similarly, NADK activity was decreased in isolated islets infected with shRNA lentivirus (Table 2). Nonspecific shRNA (scrambled) plasmid and control lentivirus had no significant effect on NADK activity, as determined by comparison of NADK activity in untreated native cells vs. cells and islets treated with scrambled shRNA and control shRNA lentivirus (Table 2).
Fig. 4.
Effect of NADK knockdown on NADK mRNA (A), total (NADPH + NADP+) levels (B), the NADPH/(NADPH + NADP+) ratio (C), insulin secretion (D), and NADH/(NADH + NAD+) and ATP/ADP ratio (E) in INS-1 832/13 cells. Nucleotide determination and insulin secretion were performed as described in the legend to Fig. 2. Data are means ± SE from 3–5 independent experiments performed in duplicate or triplicate measurements. *P < 0.05 NADK(−) vs. NADK control; #P < 0.05 2G vs. 16G.
NADK knockdown caused decreases in both the total (NADPH + NADP+) pool (Figs. 4B and 5A) and the NADPH/NADP+ ratio (Fig. 4C). The incremental increase in the total (NADPH + NADP+) pool observed following 60 min of exposure to 16 mM glucose in control cells and islets was virtually abolished by NADK knockdown (Figs. 4B and 5A), suggesting that NADK kinase is indeed responsible for de novo synthesis of NADPH and NADP+ following prolonged exposure to 16 mM glucose.
Similarly, GSIS was inhibited significantly by NADK knockdown in both INS-1 832/13 cells and mouse islets (Fig. 4D and 5B), without significant effect on KCl-mediated secretion (Fig. 4D), suggesting that NADK manipulation affects intracellular metabolic pathways proximal to plasma membrane depolarization.
Effect of NADK on the β-Cell Oxidative Defense
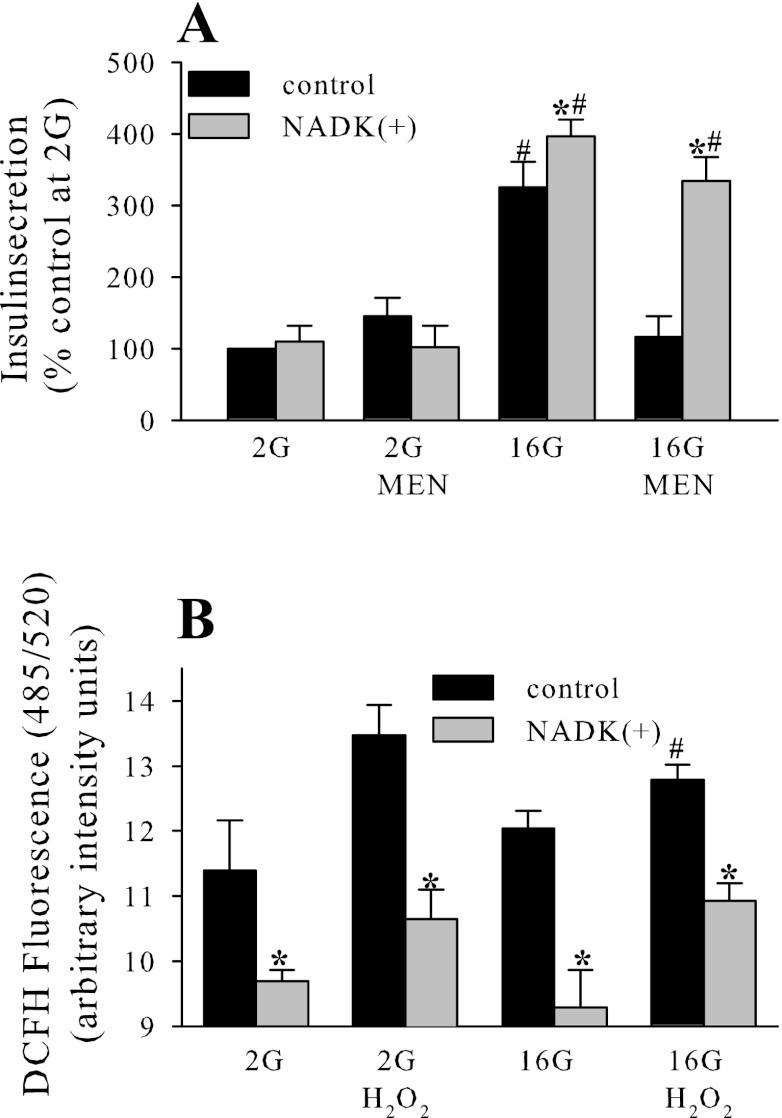
Compared with other tissues, β-cells have unusually low levels of classical antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase (reviewed in Ref. 37), leaving the possibility that NADPH-dependent systems, such as the thioredoxin or glutaredoxin systems, might play a more important role in the antioxidant defense of these cells. Since we have demonstrated that NADK regulates NADPH levels in β-cells, we tested whether NADK overexpression affected their capacity to resist oxidative stress. Menadione, a redox cycling agent that produces hydrogen peroxide (H2O2), was shown at a dose of 10 μM to inhibit GSIS in INS-1 832/13 cells, and this inhibitory action of menadione on GSIS was reversed by the overexpression of NADK (Fig. 6A). Menadione redox cycling produces H2O2 (3), and the application of high and toxic doses of H2O2 (50 μM) was previously shown to inhibit insulin secretion (47). To determine whether NADK-dependent removal of H2O2 is the mechanism behind its protection against toxic menadione doses, H2O2 levels following the application of 50 μM H2O2 were measured in control and NADK-overexpressing cells (Fig. 6B). NADK overexpression decreased H2O2 levels, consistent with the NADPH-dependent maintenance of the glutaredoxin system and the role of glutathione reductase in destruction/removal of H2O2 (5).
Fig. 6.
NADK overexpression in INS-1 832/13 protects against menadione (MEN)-dependent inhibition of glucose-stimulated insulin secretion (A) and decreases H2O2 levels (B) in INS-1 832/13 cells. MEN and H2O2 were applied at 10 and 50 μM, respectively. Insulin secretion was performed as described in the legend to Fig. 2. H2O2 was determined by 2′,7′-dichlorodihydrofluorescein (DCFH) fluorescence. Data are means ± SE from 2–3 independent experiments performed in duplicate or triplicate measurements. *P < 0.05 NADK(+) vs. NADK control; #P < 0.05 2G vs. 16G.
DISCUSSION
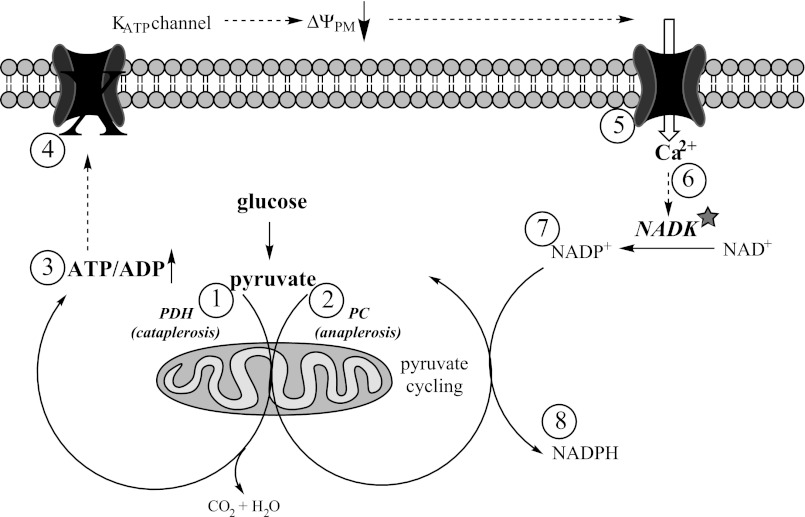
The glucose-dependent increase in the β-cell NADPH/NADP+ ratio has been reported to occur as early as a few minutes after the elevation of glucose concentration from basal to stimulatory levels (12). However, the effect of stimulatory glucose on the total (reduced plus oxidized) pool of NADPH (NADPH + NADP+) in β-cells has not been addressed directly. Data in the literature regarding changes in the total pool of NADPH are byproducts of studies related to different aspects of β-cell metabolism and yield conflicting results. In some studies, the total NADPH pool (NADPH + NADP+) was found to remain roughly the same following 2 h of stimulatory glucose exposure (14, 19), where the glucose-dependent increase in NADPH was compensated for completely by the corresponding decrease in the NADP+. However, other studies reported that an increase in the NADPH pool (a net rise in NADPH with no converse decrease in the pool of NADP+) occurred after 40 min and longer of stimulatory glucose exposure (1, 35). In line with these latter observations, we also found an increase in the total NADPH pool following 1-h exposure of INS-1 832/13 cells or islets to stimulatory glucose (Figs. 2B and 5A). Our data support the hypothesis that NADK is responsible for this increase in the total NADPH pool, since knockdown of NADK blunted this increase (Fig. 4B), and further suggest that NADK kinase activity increases under elevated glucose levels. NADK activity has been shown to be sensitive to free Ca2+ levels, with half-maximal activity observed at ∼400 nM (57), and an increase in glucose from basal (2–5 mM) to stimulatory levels (10–20 mM) is known to elicit a rise in the free cytosolic Ca2+ from <100 to ∼500 nM (32, 48). Thus, the glucose-stimulated increase in Ca2+ may activate NADK in β-cells (Fig. 7).
Fig. 7.
Role of NADK in the context of β-cell metabolic pathways. The glucose-dependent increase in pyruvate decarboxylation (1) leads to the rise in the ATP/ADP ratio (3), closure of the KATP channels (4), plasma membrane depolarization, and Ca2+ influx (5). The rise in cytosolic Ca2+ activates NADK (6), which phosphorylates NAD+ to produce NADP+ (7). The net increase in the NADP+ pool serves as a substrate for pyruvate cycling pathways (activated by the rise in glucose via an increase in pyruvate cycling; 2) and leads to the NADK-dependent net increase in the NADPH pool (8). PDH, pyruvate dehydrogenase; PC, pyruvate carboxylase.
Although mammalian NADK kinase can utilize either NADH or NAD+ as a substrate, it has a strong preference for the latter (39). Despite this preference, our data demonstrate that the NADK-dependent increase in the (NADPH + NADP) pool is due mainly to the increase in NADPH and not so much NADP+. This suggests that the NADP+, formed by NADK, is rapidly converted to NADPH via NADP+-dependent enzymes. Our findings are in agreement with reported data in HEK-293 cells, where overexpression of NADK resulted in a similar increase in the NADPH rather than NADP+ pool (39). Indeed, mammalian cells typically maintain an elevated NADPH/NADP+ ratio to support reductive biosynthesis and to protect the cells from oxidative stress (56). Because mammalian NADK is located in the cytosol (31, 39), several NADP+-dependent, NADPH generating cytosolic enzymes are potential acceptors of NADP+ formed via NADK in β-cells. These include enzymes of the pyruvate-cycling pathways: ME1, ICDc, and the pentose phosphate pathway. Although the pentose phosphate pathway has been suggested to not be significantly active in β-cells (reviewed in Ref. 26), its rate-limiting enzyme G6PD has received recent attention as a critical determinant of antioxidant defense in pancreatic β-cells (reviewed in Ref. 58). However, mRNA expression of these enzymes was not affected by NADK overexpression (data not shown). This suggests that, similar to findings reported in HEK-293 cells (39), the existing level of these enzymes has sufficient capacity to accommodate the reduction of additional NADP+ generated following NADK overexpression.
NADPH has been suggested to be a coupling mediator for GSIS (15). Overexpression and knockdown of NADK increased and decreased, respectively, the level of NADPH. Whereas NADK knockdown inhibited GSIS significantly in both clonal INS-1 832/13 cells and isolated islets, overexpression of NADK had only a modest effect on GSIS. This suggests that the existing level of NADPH is sufficient to support GSIS-dependent pathways. The defect in GSIS following NADK knockdown supports the role of NADPH as a coupling mediator, or it may suggest that a decrease in NADPH can, in general, negatively affect pathways involved in the regulation of GSIS. Further studies are needed to fully elucidate this topic.
NADPH serves as a reducing cofactor for glutaredoxin and thioredoxin, cytosolic defense systems involved in protection from oxidative stress that reduce inappropriate disulfide bonds, restoring cysteinyl sulfhydryl residues and eliminating hydroperoxides (13). NADPH-dependent oxidative defense might be particularly important in β-cells since they have unusually low levels of classical antioxidant enzymes (reviewed in Ref. 37) and might rely on other defense systems to combat oxidant challenge. Depending on their dose, reactive oxygen intermediates (ROI) can either be detrimental to β-cell function or serve a positive role as signaling messengers (reviewed in Ref. 11). This is in agreement with the notion that low and physiological levels of ROI occur as a natural part of metabolism under physiological conditions and serve a signaling function in various cell types (8, 9, 27, 28, 44–46, 52). In agreement with the latter, several studies reported glucose-dependent elevation of ROI content in β-cells (2, 22, 36, 53), whereas others demonstrated the opposite effect (21, 30, 41). Since careful time course analysis of the rise and fall of ROI has not been performed in any of these studies, the timing of ROI measurement may be of the essence and explain these contradictory results. We hypothesize that glucose-dependent activation of NADK via activation of the glutaredoxin and thioredoxin systems will ensure the prompt removal of ROIs after they have performed their signaling function.
Overexpression of NADK counteracted inhibition of GSIS induced by toxic levels of ROI produced by the redox cycling of high doses of menadione in INS-1 832/13 cells (Fig. 6A). This NADK-dependent action was accompanied by a decrease in intracellular ROI in both untreated cells and cells treated with H2O2 (Fig. 6B) at a concentration (50 μM) previously shown to inhibit insulin secretion (47). Similar results were found in HEK-293 cells overexpressing NADK (39), which also have less H2O2-induced intracellular oxidative stress and cell toxicity than controls. These data suggest that NADK-mediated production of NADP+, and subsequently NADPH, mediates removal of excess of H2O2 and thus plays a protective role in the face of oxidant overload.
The fact that NADK overexpression counteracted the inhibitory effect of menadione (whose redox cycling produces H2O2) on GSIS and at the same time enhanced GSIS seems to be somewhat contradictory, since low levels of ROI and H2O2 are necessary to mediate GSIS (36). However, NADK, via production of NADPH, may also stimulate the activity of the NADPH oxidases (NOX), which generate ROI. NADPH oxidases, originally discovered to be important for oxidative burst in neutrophils (reviewed in Ref. 25), have been shown to be involved in endothelial cell signaling by localized ROI production at the plasma membrane and endoplasmic reticulum (6, 51), and NADPH oxidase activity in endothelial cells was associated with the cytoskeletal fractions (24). Recently, NADPH oxidase has been shown to be present in islets (54) and was implicated in the regulation of insulin secretion (33, 34, 53). Therefore, NADK, by regulating the de novo formation of NADPH, might play a dual role in β-cells by supporting functionally and compartmentally distinct systems, i.e., glutaredoxin and thioredoxin systems in the cytosol by increasing the reduced/oxidized ratio of glutathione and thioredoxin while simultaneously providing NADPH that would activate NADPH oxidase and provide low physiological levels of ROI at the plasma and other cellular membranes to support glucose-mediated signaling under stimulatory glucose levels. Thus, NADK-dependent activation of the glutaredoxin/thioredoxin system in the cytosol and NADPH oxidase in the membranes might result in complementary functions (antioxidant vs. pro-oxidant) enabled by the compartmentalization of these two components (reviewed in Ref. 55). Future studies are underway to evaluate role of NADK in these pathways. Altogether, we have demonstrated that via its control of the level of NADPH, NADK regulates insulin secretion and protects β-cells from oxidative stress.
GRANTS
This work was supported by American Diabetes Association Grant 7-08-JF-18 and National Institute of Diabetes and Digestive and Kidney Diseases Grant R56-NIDDK-088093 (E. A. Heart), the Alexander Trust Fund, the James A. and Faith Miller Memorial Fund, and the Elisabet Samuelsson Fund (J. P. Gray). J. P. Gray is a professor at the US Coast Guard Academy. The views presented here are those of J. P. Gray and not necessarily those of the US Coast Guard Academy or other branches of the US government.
DISCLOSURES
The authors report no competing interests, financial or otherwise.
AUTHOR CONTRIBUTIONS
J.P.G., K.N.A., E.A.J., and E.A.H. performed the experiments; J.P.G. and E.A.H. analyzed the data; J.P.G. and E.A.H. interpreted the results of the experiments; J.P.G. prepared the figures; J.P.G. edited and revised the manuscript; K.N.A. and E.A.H. did the conception and design of the research; K.N.A., E.A.J., and E.A.H. approved the final version of the manuscript; E.A.H. drafted the manuscript.
ACKNOWLEDGMENTS
We thank M. Meow for helpful comments and support in the preparation of the manuscript.
REFERENCES
- 1. Ammon HP, Bacher M, Brandle WF, Waheed A, Roenfeldt M, el-Sayed ME, Ahmed AA, Wahl MA. Effect of forty-eight-hour glucose infusion into rats on islet ion fluxes, ATP/ADP ratio and redox ratios of pyridine nucleotides. J Endocrinol 156: 583–590, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem 278: 9796–9801, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol 13: 135–160, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Capito K, Hedeskov CJ, Landt J, Thams P. Pancreatic islet metabolism and redox state during stimulation of insulin secretion with glucose and fructose. Acta Diabetol Lat 21: 365–374, 1984 [DOI] [PubMed] [Google Scholar]
- 5. Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605, 1979 [DOI] [PubMed] [Google Scholar]
- 6. Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giroix MH, Sener A, Malaisse WJ. Pentose cycle pathway in normal and tumoral islet cells. FEBS Lett 185: 1–3, 1985 [DOI] [PubMed] [Google Scholar]
- 8. Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes 54: 311–321, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal 7: 1021–1031, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gray JP, Eisen T, Cline GW, Smith PJ, Heart E. Plasma membrane electron transport in pancreatic β-cells is mediated in part by NQO1. Am J Physiol Endocrinol Metab 301: E113–E121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gray JP, Heart E. Usurping the mitochondrial supremacy: extramitochondrial sources of reactive oxygen intermediates and their role in beta cell metabolism and insulin secretion. Toxicol Mech Methods 20: 167–174, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Heart E, Yaney GC, Corkey RF, Schultz V, Luc E, Liu L, Deeney JT, Shirihai O, Tornheim K, Smith PJ, Corkey BE. Ca2+, NAD(P)H and membrane potential changes in pancreatic beta-cells by methyl succinate: comparison with glucose. Biochem J 403: 197–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holmgren A, Johansson C, Berndt C, Lonn ME, Hudemann C, Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans 33: 1375–1377, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Huypens P, Pillai R, Sheinin T, Schaefer S, Huang M, Odegaard ML, Ronnebaum SM, Wettig SD, Joseph JW. The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia 54: 135–145, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, in 't Veld P, Renström E, Schuit FC. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54: 2132–2142, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Jackson JB. Proton translocation by transhydrogenase. FEBS Lett 545: 18–24, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 295: E1287–E1297, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 53: 1019–1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joseph JW, Jensen MV, Ilkayeva O, Palmieri F, Alarcon C, Rhodes CJ, Newgard CB. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem 281: 35624–35632, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Komai S, Licznerski P, Cetin A, Waters J, Denk W, Brecht M, Osten P. Postsynaptic excitability is necessary for strengthening of cortical sensory responses during experience-dependent development. Nat Neurosci 9: 1125–1133, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Lacraz G, Figeac F, Movassat J, Kassis N, Coulaud J, Galinier A, Leloup C, Bailbe D, Homo-Delarche F, Portha B. Diabetic beta-cells can achieve self-protection against oxidative stress through an adaptive up-regulation of their antioxidant defenses. PloS One 4: e6500, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, Pénicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 58: 673–681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lerner F, Niere M, Ludwig A, Ziegler M. Structural and functional characterization of human NAD kinase. Biochem Biophys Res Commun 288: 69–74, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem 277: 19952–19960, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Li JM, Shah AM. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol 14: S221–S226, 2003 [DOI] [PubMed] [Google Scholar]
- 26. MacDonald MJ. Differences between mouse and rat pancreatic islets: succinate responsiveness, malic enzyme, and anaplerosis. Am J Physiol Endocrinol Metab 283: E302–E310, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol 24: 1844–1854, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem 276: 21938–21942, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Malaisse WJ, Hutton JC, Kawazu S, Herchuelz A, Valverde I, Sener A. The stimulus-secretion coupling of glucose-induced insulin release. XXXV. The links between metabolic and cationic events. Diabetologia 16: 331–341, 1979 [DOI] [PubMed] [Google Scholar]
- 30. Martens GA, Cai Y, Hinke S, Stange G, Van de Casteele M, Pipeleers D. Glucose suppresses superoxide generation in metabolically responsive pancreatic beta cells. J Biol Chem 280: 20389–20396, 2005 [DOI] [PubMed] [Google Scholar]
- 31. McGuinness ET, Butler JR. NAD+ kinase—a review. Int J Biochem 17: 1–11, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Misler S, Barnett DW, Gillis KD, Pressel DM. Electrophysiology of stimulus-secretion coupling in human beta-cells. Diabetes 41: 1221–1228, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Morgan D, Rebelato E, Abdulkader F, Graciano MF, Oliveira-Emilio HR, Hirata AE, Rocha MS, Bordin S, Curi R, Carpinelli AR. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology 150: 2197–2201, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Newsholme P, Morgan D, Rebelato E, Oliveira-Emilio HC, Procopio J, Curi R, Carpinelli A. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 52: 2489–2498, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Panten U, Rustenbeck I. Fuel-induced amplification of insulin secretion in mouse pancreatic islets exposed to a high sulfonylurea concentration: role of the NADPH/NADP+ ratio. Diabetologia 51: 101–109, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56: 1783–1791, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol 244: 77–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pollak N, Dolle C, Ziegler M. The power to reduce: pyridine nucleotides—small molecules with a multitude of functions. Biochem J 402: 205–218, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pollak N, Niere M, Ziegler M. NAD kinase levels control the NADPH concentration in human cells. J Biol Chem 282: 33562–33571, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Pongratz RL, Kibbey RG, Shulman GI, Cline GW. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J Biol Chem 282: 200–207, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Rebelato E, Abdulkader F, Curi R, Carpinelli AR. Control of the intracellular redox state by glucose participates in the insulin secretion mechanism. PloS One 6: e24507, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reinbothe TM, Ivarsson R, Li DQ, Niazi O, Jing X, Zhang E, Stenson L, Bryborn U, Renstrom E. Glutaredoxin-1 mediates NADPH-dependent stimulation of calcium-dependent insulin secretion. Mol Endocrinol 23: 893–900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab 6: 363–375, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science 312: 1882–1883, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol 14: S211–S215, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 17: 183–189, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Sakai K, Matsumoto K, Nishikawa T, Suefuji M, Nakamaru K, Hirashima Y, Kawashima J, Shirotani T, Ichinose K, Brownlee M, Araki E. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun 300: 216–222, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Satin LS. Localized calcium influx in pancreatic beta-cells: its significance for Ca2+-dependent insulin secretion from the islets of Langerhans. Endocrine 13: 251–262, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Sener A, Malaisse WJ. The coupling of metabolic to secretory events in pancreatic islets: comparison between insulin release and cytosolic redox state. Biochem Int 14: 897–902, 1987 [PubMed] [Google Scholar]
- 50. Shi F, Li Y, Li Y, Wang X. Molecular properties, functions, and potential applications of NAD kinases. Acta Biochim Biophys Sin (Shanghai) 41: 352–361, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Smith KR, Klei LR, Barchowsky A. Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 280: L442–L449, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8: 243–270, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Syed I, Kyathanahalli CN, Kowluru A. Phagocyte-like NADPH oxidase generates ROS in INS 832/13 cells and rat islets: role of protein prenylation. Am J Physiol Regul Integr Comp Physiol 300: R756–R762, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Uchizono Y, Takeya R, Iwase M, Sasaki N, Oku M, Imoto H, Iida M, Sumimoto H. Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sci 80: 133–139, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 11: 1289–1299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Veech RL, Eggleston LV, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem J 115: 609–619, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Williams MB, Jones HP. Calmodulin-dependent NAD kinase of human neutrophils. Arch Biochem Biophys 237: 80–87, 1985 [DOI] [PubMed] [Google Scholar]
- 58. Zhang Z, Liew CW, Handy DE, Zhang Y, Leopold JA, Hu J, Guo L, Kulkarni RN, Loscalzo J, Stanton RC. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and beta-cell apoptosis. FASEB J 24: 1497–1505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]