SUMMARY
A small-molecule mimetic of Smac/Diablo that specifically counters the apoptosis-inhibiting activity of IAP proteins has been shown to enhance apoptosis induced by cell surface death receptors as well as chemotherapeutic drugs. Survey of a panel of 50 human non-small-cell lung cancer cell lines has revealed, surprisingly, that roughly one-quarter of these lines are sensitive to the treatment of Smac mimetic alone, suggesting that an apoptotic signal has been turned on in these cells and is held in check by IAP proteins. This signal has now been identified as the autocrine-secreted cytokine tumor necrosis factor alpha (TNFα). In response to autocrine TNFα signaling, the Smac mimetic promotes formation of a RIPK1-dependent caspase-8-activating complex, leading to apoptosis.
INTRODUCTION
Cancer is second only to heart disease as the leading cause of death in industrialized countries. Although mortality rates have declined in recent years due to earlier detection and more options in treatment, the outlook for certain cancers remains bleak (Workman and Kaye, 2002). For example, lung cancers, despite treatment advances, have a general long-term survival rate of only ~15%. Conventional cancer chemotherapeutics such as paclitaxel, cisplatin, topotecan, or etoposide have a small therapeutic index between cancer and normal cells. Complicating matters further is the almost inevitable onset of resistance and subsequent relapse.
In recent years, the focus has shifted to less-toxic therapeutics that target specific signaling pathways driving inappropriate cell growth and proliferation. Examples include imatinib and erlotinib, rationally-designed tyrosine kinase inhibitors that block the ATP binding site of the constitutively active BCR-ABL tyrosine kinase translocation mutation found in chronic myeloid leukemia (CML)(Druker, 2002) and the tyrosine kinase domain mutant EGFR found in non-small-cell lung cancers. Monoclonal antibodies such as Herceptin, which targets the HER2/neu (erbB2) receptor that is often amplified in breast cancer, have also been developed (Izumi et al., 2002; Slamon et al., 2001).
In addition to aberrant growth signals, many cancers also have dysfunction in the ability to undergo apoptosis (Danial and Korsmeyer, 2004; Hanahan and Weinberg, 2000). Overexpression of antiapoptotic genes have been correlated with tumorigenesis and resistance to chemotherapy. A classic example is seen in non-Hodgkin’s lymphoma, where constitutive Bcl-2 overexpression blocks apoptosis in the presence of proapoptotic stimuli (Kirkin et al., 2004; Miyashita and Reed, 1993). Additionally, overexpression of various members of the inhibitor of apoptosis (IAP) family of proteins has also been found in cancers able to evade apoptosis (Gordon et al., 2002; Nachmias et al., 2004; Sui et al., 2002). An alternative approach to the treatment of cancer, therefore, would be to reestablish the cell death program and set conditions such that cells can undergo apoptosis given an appropriate stimulus (Fesik, 2005). The potential of such tactics can be seen by the emergence of small-molecule inhibitors targeting various components of the regulatory system of apoptosis. Olterdorf and coworkers recently identified a potent small-molecule inhibitor targeting Bcl-2, Bcl-XL, and Bcl-w that was able to synergize with other chemotherapeutics as well as having single-agent actions against lymphoma and small-cell lung cancer with significant cure rates in animal models (Oltersdorf et al., 2005).
In addition, small-molecule inhibitors of second mitochondria-derived activator of apoptosis (Smac) have recently been developed that promote apoptosis in synergy with other proapoptotic stimuli (Bockbrader et al., 2005; Chauhan et al., 2007; Glover et al., 2003; Li et al., 2004; Mizukawa et al., 2006; Sun et al., 2005, 2006; Wilkinson et al., 2004; Wu et al., 2003; Zobel et al., 2006). Smac is an ideal candidate for small-molecule mimetic design and therapeutic application because of its unique function in regulating apoptosis. Under normal conditions, Smac is sequestered in the mitochondria and is released into the cytosol only upon induction of apoptosis or mitochondrial dysfunction (Chai et al., 2000; Du et al., 2000). Once in the cytosol, Smac is able to bind its targets, which consist of a family of related proteins known as the inhibitors of apoptosis (IAPs) (Liu et al., 2000; Srinivasula et al., 2001; Wu et al., 2000). IAPs function to prevent unregulated activation of the apoptotic cell death program by binding to and inhibiting multiple proteins necessary for apoptosis to occur. For example, XIAP (X chromosome-encoded IAP) binds to activated caspases-3, -7, and -9 and inhibits their activities (Crook et al., 1993; Deveraux et al., 1997; Roy et al., 1997). A key feature of regulated apoptosis is the release of Smac from mitochondria and the subsequent release of caspases from inhibition. A mimetic of Smac is able to relieve caspases from inhibition without need for mitochondrial disruption to promote apoptosis in cancer cells given an additional proapoptotic stimulus.
Previously, our laboratory demonstrated the feasibility of such an approach by preparing a small-molecule mimetic of Smac and showed that it permeates cells readily and acts in a similar fashion as natural Smac in the cell while bypassing mitochondrial regulation (Li et al., 2004). The small-molecule mimetic is a synthetic, two-headed structure designed to resemble the N-terminal amino acid residues (AVPI) of Smac protein that interact with BIR domains of XIAP (Wu et al., 2000). The compound was shown to bind specifically to at least three members of the IAP family: XIAP, cellular IAP 1 (cIAP1), and cIAP2. It was able to promote apoptosis synergistically with proapoptotic stimuli (TRAIL or TNFα) that on their own have no effect on cell death in the cancer cell lines examined (Li et al., 2004).
In addition to inhibiting caspase activation, an ancillary function of both cIAP1 and cIAP2 is that both proteins are implicated in nuclear factor kappa B (NFκB) activation (Chen et al., 2002; Shu et al., 1996) and suppression of caspase-8 activation during TNFα signaling (Deveraux et al., 1998; Wang et al., 1998). TNFα is a pleiotropic ligand of tumor necrosis factor receptor 1 and 2 (TNFR1 and TNFR2) that can signal both cell survival and cell death (Aggarwal, 2003; Bhardwaj and Aggarwal, 2003; Wajant, 2003). The prosurvival and prodeath signaling aspects of TNFα are mediated through two separate protein complexes (Micheau and Tschopp, 2003). The prosurvival complex, in addition to TNFα bound to TNFR1, also contains the adaptor protein TNFR1-associated death domain protein (TRADD), TNF-receptor-associated protein 2 (TRAF2), receptor-interacting protein kinase 1 (RIPK1), and cIAP1 and cIAP2. This complex recruits and activates I kappa kinases (IKK), leading to the activation of NFκB (Devin et al., 2000; Shu et al., 1996). A prodeath complex is also formed, but as long as there is a prosurvival signal being generated, it is unable to induce cell death. The death-inducing complex assembles following internalization of the TNFR1 receptor and consists of TRADD and RIPK1, which then recruit Fas-associated protein with death domain (FADD) and caspase-8 to generate the death inducing signaling complex (DISC) (Micheau and Tschopp, 2003).
Here, we report the finding that various human cancer cell lines undergo apoptosis upon Smac mimetic treatment without the need for exogenous proapoptotic stimuli or cotreatment with chemotherapeutic agents. We have elucidated the molecular mechanism underlying this single-agent sensitivity. Our work reveals a functionality of the Smac mimetic relating specifically to TNFα signaling that is distinct from simple interference with caspase inhibition. The Smac mimetic is able to exploit certain cancer cells that secrete TNFα and usurp this prosurvival signal to promote cell death via formation of a RIPK1-dependent, death-inducing complex.
RESULTS
A Smac Mimetic Induces Cell Death in Human Non-Small-Cell Lung Cancer Cell Lines and Specifically Targets IAPs
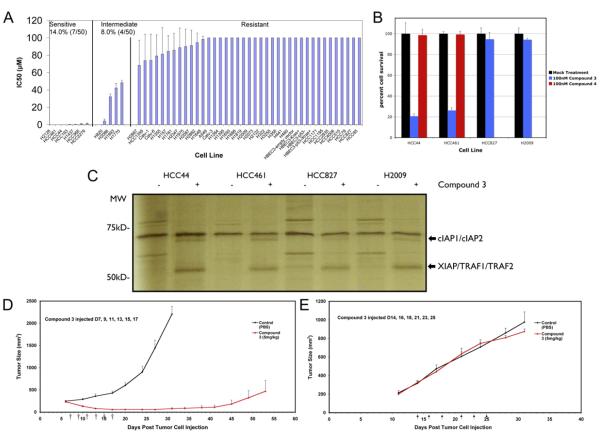
Initial testing of various cell lines to the synergistic effects of Smac mimetic treatment with conventional chemotherapeutics revealed a subset of cells that responded to the Smac mimetic alone (data not shown). A larger panel of non-small-cell lung cancer (NSCL) cell lines was therefore screened for responsiveness to the Smac mimetic (Figure 1A). Of the 50 cell lines examined, 14% responded to low nanomolar concentrations, and an additional 8% had IC50s in the low micromolar range. The majority of cells (78%) were unresponsive to Smac mimetic concentrations up to 100 uM. Closer inspection of two sensitive lung cancer cell lines (HCC44 and HCC461) showed that these cells respond robustly to 100 nM treatment of Smac mimetic, with approximately 80% of the cells killed by the Smac mimetic after 24 hr of treatment (Figure 1B). To ensure that these effects were specific, a compound with its critical alanine group acetylated, and therefore inactivated as a Smac mimetic, was also used (Li et al., 2004). Neither HCC461 nor HCC44 cell lines were sensitive to the treatment of this inactive compound. Two other lung cancer cell lines (H2009 and HCC827) were resistant to 100 nM Smac mimetic treatment (Figure 1B).
Figure 1. Response of a Panel of Lung Cancer Cell Lines to Smac Mimetic In Vitro and In Vivo.
(A) A panel of 50 non-small-carcinoma-cell lung cancer cell lines was tested for responsiveness to a Smac-mimetic treatment alone. IC50s were determined for each cell line based on cell survival as measured by ATP levels in live cells using Cell Titer-glo (Promega). IC50 determination was based on concentrations of compound 3 that yielded half-maximal luminescence relative to untreated cells.
(B) Treatment of selected group of cell lines to 100 nM of compound 3 and to compound 4 (negative control compound with similar structure by differing in function group configuration as described in Li et al., 2004). Smac-mimetic-sensitive cell lines (HCC44 and HCC461) and Smac-mimeticresistant cell lines (HCC827 and H2009) were chosen. Each graphical representation for IC50s and cell survival indicates the mean ± SD of at least three independent testing conditions.
(C) In vitro pull-down utilizing a biotinylated form of compound 3. The biotinylated compound was able to pull down protein bands at around 50 kDa and at around 70 kDa not seen in control lanes (avidin beads only), which were cut out of the gel and analyzed by mass spectroscopy. Proteins identified are indicated.
(D) In vivo response of mouse xenografts of HCC461 cells to compound 3. Harlan athymic nude mice were injected subcutaneously with HCC461 cells in a matrigel randomly separated into treatment groups (n = 5) and given six intravenous injections of compound 3 or saline every other day. Tumors were measured twice perweek until the end of the experiment. In the compound 3 treatment group, 2/5 (40%) remained tumor-free at the end of the experiment.
(E) In vivo response of mouse xenografts of a Smac-mimetic-resistant HCC15 cells to compound 3. Conditions were identical to those for HCC461 xenografts. For tumor size measurements, graphical representations indicate the mean ± SEM of five individual samples per condition.
To ensure that the Smac mimetic was specifically targeting IAPs in these cells, a biotinylated form of the Smac mimetic was synthesized and used for in vitro pull-down to determine what precisely the Smac mimetic was interacting with in these cells. Whole cell lysates were incubated with the biotinylated Smac mimetic and interacting proteins were pulled down with avidin-coated beads and analyzed by SDS-PAGE followed by silver staining (Figure 1C) and mass spectrometry. As compared to the affinity matrix alone, the biotinylated Smac mimetic specifically pulled down two protein bands visualized by silver staining. Mass spectrometric analysis of these bands revealed that the band around the 70 kDa marker contained cIAP1 and cIAP2, while the band near 50 kDa consisted of XIAP, TRAF2, and TRAF1. TRAF1 and TRAF2 were most likely copurified with cIAP1 and cIAP2, since they have been shown previously to interact and form multisubunit complexes involved in NFκB activation (Rothe et al., 1995). It is evident from the above result that the Smac mimetic is highly specific in interacting with IAP proteins. Interestingly, although there was no significant difference in XIAP/TRAF1/TRAF2 pulled down from sensitive (HCC44 and HCC461) or nonsensitive (HCC827 and H2009) cell extracts, there seemed to be more cIAPs from the sensitive cells pulled down by the biotinylated Smac mimetic.
Smac Mimetic Alone Induces Tumor Regression in HCC461 Xenograft Model
Observing the robustness of HCC461 cell death induced by the Smac mimetic alone in cell culture, we decided to examine whether such a property remained unchanged in a xenograft mouse tumor model. HCC461 cells were grown in matrigel and injected subcutaneously into athymic nude mice. Tumors were allowed to grow for 7 days (average tumor size: 200-300 mm3). Tumor-bearing mice were thereafter given six separate intravenous injections of either Smac mimetic or saline over the following 11 days (D7, 9, 11, 13, 15, 17). As shown in Figure 1D, mice treated with saline did not survive past day 30 (average tumor size: 2200 mm3), while mice treated with the Smac mimetic showed marked and sustained tumor regression (average tumor size: < 50 mm3). At day 40, tumors began to gradually reappear, but these were still significantly smaller at day 50 (average tumor size: 500 mm3) than control tumors at day 30. Of the mice treated with the Smac mimetic, 2 out of 5 (40%) were tumor free at the end of the experiment. This result is quite different than that obtained from a breast cancer xenograft mouse model treated with the same Smac mimetic compound at the same doses (5 mg/kg) and dosing schedule. In the breast cancer model, Smac alone had little effect, and the mice needed to be dosed together with Trail to achieve similar therapeutic effect (data not shown). To ensure that the xenograft response was specific to cell lines that showed sensitivity in cell culture, an additional resistant NSCL cancer cell line, HCC15, was also tested for responsiveness to the Smac mimetic alone. As shown in Figure 1E, this cell line that was resistant to Smac mimetic treatment in cell culture remained so in vivo under identical conditions as were used to test HCC461.
Smac-Mimetic-Induced Apoptosis
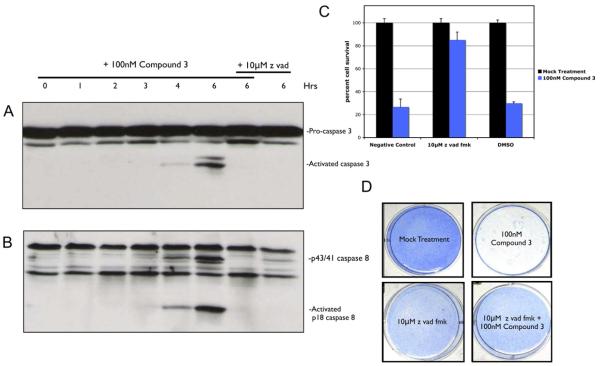
HCC461 was examined in greater detail to determine how exactly the Smac mimetic caused cell death in cell culture. Time-course measurements of caspase activation revealed that apoptosis was the mechanism of cell death and that within 6 hr of Smac mimetic treatment, the executioner caspase-3 was activated (Figure 2A). Prior to that, the initiator caspase-8 (Figure 2B) and caspase-9 (data not shown) were activated at approximately 4 hr. Furthermore, inhibition of caspase activation by a pancaspase inhibitor, z vad fmk (z vad), prevented cleavage of caspases-3, -8 and -9 following 6 hr of Smac mimetic treatment (Figures 2A and 2B and data not shown, respectively). Caspase inhibition was also correlated with response to Smac mimetic, where a 1 hr z vad pretreatment was able to rescue cells from cell death following 24 hr of Smac mimetic treatment (Figure 2C). The effects of z vad were also tested for long-term survival in the presence of Smac mimetic. Cells were treated with Smac mimetic, z vad, or both for 5 days to ensure that rescue by z vad was permanent and that rescue was not a transient artifact of z vad exposure. As shown in Figure 2D, cells treated with Smac mimetic plus z vad were still viable after 5 days, whereas cells treated with the Smac mimetic alone were killed.
Figure 2. Smac-Mimetic-Mediated Cell Death Displays Caspase Activation, which Can Be Blocked by Caspase Inhibition.
Analysis of caspase activation in HCC461 cell lysates following a time course of 100 nM compound 3 treatments. For caspase inhibition, 10 μM z vad fmk (Sigma) was added 1 hr prior to compound 3.
(A) Western blot analysis of caspase-3 activation.
(B) Western blot analysis of caspase-8 activation.
(C) Rescue of Smac mimetic cell death by 10 μM z vad.
(D) Long-term survival of cells treated with z vad plus compound 3. Cells were treated as indicated for 5 days to ensure that rescue from cell death was not a transient artifact of z vad treatment. Cell viability was determined using methylene blue staining. Each graphical representation indicates the mean ± SD of at least three independent testing conditions.
Smac-Mimetic-Induced Apoptosis Occurs through Engagement of TNFR1 by TNFα
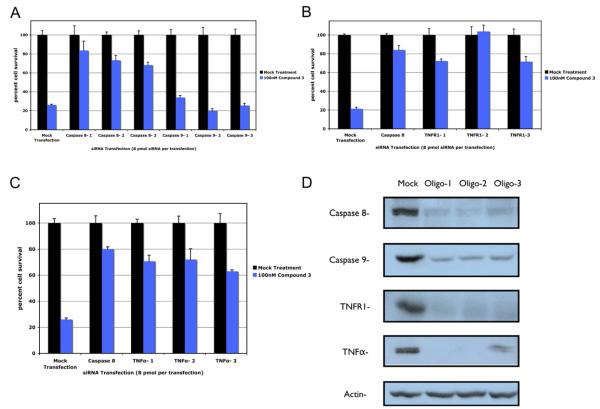
Because both caspase-8 and caspase-9 are activated in the same time frame, a candidate short-interfering RNA (siRNA) screen was used in HCC461 to determine which caspase was the initiator of apoptosis and ultimately what signaling pathways mediates Smac-mimeticinduced apoptosis. Experiments using siRNAs to knockdown caspase-8 or caspase-9 revealed that transient knockdown of caspase-8 rescued cells from cell death, while knockdown of caspase-9 had no effect on cell viability (Figure 3A). Given that caspase-8 activation is most likely caused by certain members of the TNF super-family of receptors, further screening of those receptors was conducted. The canonical members of the death receptor family are TNFR1, TRAIL receptor 1 (TRAIL-R1/DR4), TRAIL receptor 2 (TRAIL-R2/DR5), and Fas/CD95 (Aggarwal, 2003). siRNA knockdown of TNFR1 rescued cells from Smac-mimetic-mediated cell death, whereas knockdown of the other receptors had little or no effect (Figure 3B and data not shown). Further knockdown of the ligands of TNFα, TRAIL, and FasL confirmed that TNFR1 is a critical component, since knockdown of TNFα was also able to rescue cells from apoptosis (Figure 3C and data not shown). Knockdown efficiencies of the siRNAs used were determined by western blotting utilizing three individual siRNA oligos (Figure 3D).
Figure 3. Sirna Candidate Screen Implicates TNFα Signaling as a Requirement for Smac-Mimetic-Mediated Apoptosis.
Cells were assayed for the ability of particular transiently transfected individual siRNAs (Dharmacon) to produce a rescue phenotype in HCC461 cells following 100 nM compound 3 treatments. Cell viability was determined by measuring total ATP levels. Each siRNA treatment and corresponding compound treatment (n = 4, per transfection and compound 3 treatment) was normalized to the mock-transfected control to account for cytotoxicity.
(A) Caspase-8 and -9 siRNA transfection.
(B) TNFR1 siRNA transfection.
(C) TNFα siRNA transfection.
D) Efficiency of total protein level knockdown per siRNA was determined by western blot. Caspase-8-positive controls in (B) and (3) utilized Dharmacon’s siGENOME SMARTpool predesigned pools of four oligos. Each graphical representation indicates the mean ± SD of at least three independent testing conditions.
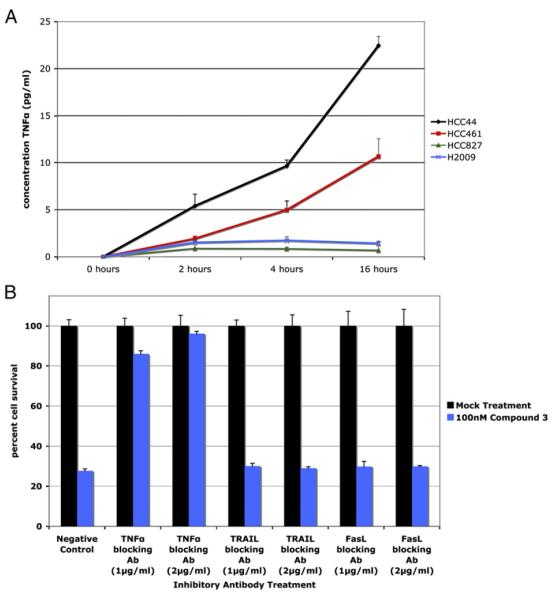
Since TNFR1-TNFα signaling appears to be a requirement for the Smac-mimetic-induced apoptosis, we reasoned that these cells might be secreting TNFα into the culture medium. Elisa analysis of the conditional media from these cells revealed that sensitive cell lines (HCC44 and HCC461) were secreting TNFα into the culture medium over time, whereas resistant cell lines (HCC827 and H2009) were not (Figure 4A). To validate that TNFα secretion was critical for the Smac-mimetic-induced cell death, HCC461 cells were pretreated with a neutralizing TNFα antibody (1–2 μg/ml) (Oettinger et al., 1999) and then treated with the Smac mimetic. As shown in Figure 4B, the TNFα neutralizing antibody completely rescued cells from apoptosis, whereas neutralizing antibodies against TRAIL and FasL (1–2 μg/ml) had no rescuing effect.
Figure 4. Autocrine TNFα Signaling Is Required for Smac-Mimetic-Mediated Apoptosis.
(A) Smac-mimetic-sensitive cell lines (HCC44 and HCC461) and Smac-mimetic-resistant cell lines (HCC827 and H2009) were tested for the presence of TNFα in conditioned cell culture media for each cell line. Samples were removed at the indicated time points and used for quantitative sandwich enzyme immunoassay analysis (R&D Systems) to determine the concentration of TNFα present, as described in the Experimental Procedures section. Sensitive cells were secreting low levels of TNFα in the cell culture medium, while there was no detectable TNFα levels present in resistant cell lines.
(B) Pretreatment (1 hr) of neutralizing antibodies (1–2 ug/mL) against TNFα (R&D Systems), TRAIL (Biolegend), and FasL/CD95 (Biolegend) prior to 100 nM compound 3 treatments. Cell viability was determines as previously described. Each graphical representation indicates the mean ± SD of at least three independent testing conditions.
Smac-Mimetic-Mediated Cell Death Is Dependent on RIPK1
Although the Smac mimetic alone requires endogenous TNFα signaling to induce cell death, there is a seeming incongruity between the fact that the Smac mimetic interferes with IAP-caspase interactions and that under normal conditions, TNFα is a prosurvival signal that upregulates antiapoptotic genes including cIAP1 and cIAP2 (Wang et al., 1998). Indeed, given cIAP1 and cIAP2’s purported roles in NFκB signaling, there is no apparent disruption of basal NFκB activation, which is a key mediator of survival signaling, upon Smac mimetic treatment in these cells (Li et al., 2004). However, the Smac mimetic is still able to initiate apoptosis even under conditions of constitutively active TNFα signaling by autocrine secretion. Therefore, to determine how the Smac mimetic is able to initiate apoptosis under such conditions, experiments using siRNAs to target components of the TNFα signaling pathway were carried out to determine which components were necessary for cell death to occur.
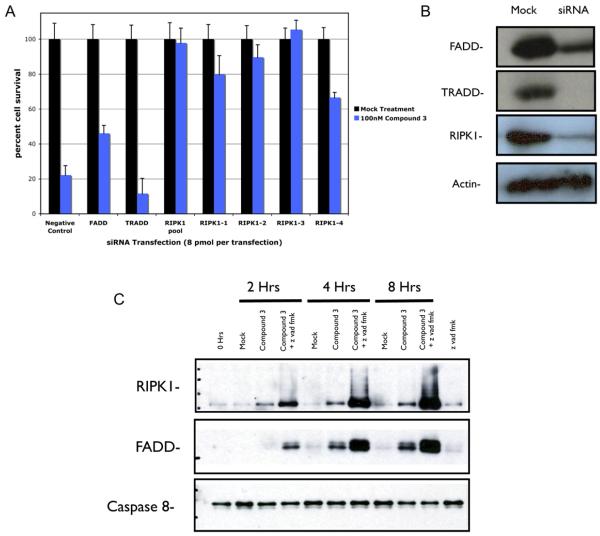
Targeting of components known to be part of the TNFα signaling pathway revealed that caspase-8 activation and subsequent cell death were RIPK1 dependent. siRNA knockdown of RIPK1 completely rescued cells from apoptosis, while knockdown of the adaptor protein TRADD seemed to enhance cell death, and knockdown of FADD had only a partial rescue effect (Figure 5A). The effect of RIPK1 knockdown was verified by four different siRNA oligos that target different segments of the mRNA. RIPK1 dependence in Smac-mimetic-mediated cell death was unexpected, given the traditional view that RIPK1 is involved more in NFκB survival signaling than in signaling apoptosis. This finding is consistent with a recent report showing that shRNA knockdown of RIPK1 prevented TNFα-induced apoptosis in human tumor cells (Jin and El-Deiry, 2006). However, the precise function of RIPK1 in survival and in cell death is not yet clear. Overexpression of RIPK1 was shown to be able to sensitize 293 cells to apoptosis by TNFα treatment (Hsu et al., 1996), whereas RIPK1-deficient Jurkat T cells do not activate NFκB when treated with TNFα and are sensitized to TNFα-induced apoptosis (Kelliher et al., 1998; Ting et al., 1996). Knockdown efficiencies for RIPK1, FADD, and TRADD were examined by western blot (Figure 5B).
Figure 5. Smac-Mimetic-Mediated Apoptosis Is RIPK1 Dependent and Promotes the Formation of a RIPK1-FADD-Caspase 8 Complex in HCC461 Cells.
(A) siRNAs targeting known components of the TNF signaling pathway (FADD, TRADD, and RIPK1). RIPK1 dependence was verified by the use of single siRNA oligos targeting different regions of the mRNA. siRNA transfection and viability assays were done as previously described.
(B) Western blot analysis of protein levels following siRNA transfection, as described previously.
(C) RIPK1, FADD, caspase-8 complex coimmunoprecipitation using caspase-8 antibody (Santa Cruz, SC-6136). Cells were treated in the indicated manner and coimmunoprecipitations were done as described in the Experimental Procedures. Z vad was added as a means of capturing caspase-8 in complex with RIPK1 and FADD by preventing full activation and subsequent dissociation of the complex from caspase-8. Each graphical representation indicates the mean ± SD of at least three independent testing conditions.
Given the apparent role of RIPK1 in Smac-mimetic-mediated cell death, it might be possible the Smac mimetic is somehow able to allow the formation of a RIPK1 containing signaling complex that activates caspase-8, hence overriding the otherwise prosurvival signal of TNFα. Immunoprecipitation of caspase-8 following Smac mimetic treatment revealed the formation of a RIPK1-FADD-caspase-8 complex that did not contain TRADD or TRAF2 (Figure 5C). Formation of the complex was enhanced by pretreatment with z vad, likely due to the fact that z vad prevents full activation of caspase-8 and is able to trap caspase-8 into the RIPK1-FADD-caspase-8 complex, which would normally disassociate following full caspase-8 activation. Similar coimmunoprecipitation experiments of caspase-8 in cells that are unable to respond to the Smac mimetic (HCC827) showed that they do not readily form a RIPK1-FADD-caspase-8 complex when the Smac mimetic and TNFα are added (data not shown), indicating that formation of this complex is a requirement for cell death to occur.
Single-Agent Sensitivity to the Smac Mimetic in Other Cell Lines Is Similarly Due to TNFα Signaling
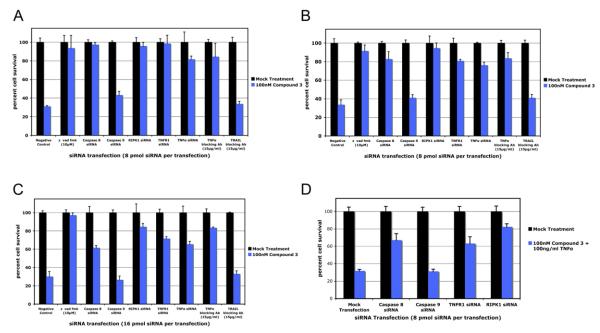
In addition to HCC461 cells as described above, other cell lines including another lung cancer line (HCC44), a breast cancer line MDA-MB-231, and a melanoma SK MEI-5 cell line, undergo Smac mimetic-induced apoptosis in a TNFα and RIPK1 dependendent fashion. siRNA knockdown of caspase-8, TNFR1, TNFα, and RIPK1 as well as addition of neutralizing TNFα antibody to the culture media rescued these cell lines from cell death (Figures 6A–6C). Additionally, in the case of cell lines that are resistant to Smac mimetic alone but do respond to costimulation by TNFα (H2009 and T98G), siRNA knockdown of caspase-8, TNFR1, and RIPK1 revealed that in cases where exogenous TNFα is added, the mechanism of response is the same as that for cells able to secrete TNFα (Figure 6D and data not shown).
Figure 6. Other Smac-Mimetic-Sensitive Cells Respond in a Similar Fashion as HCC461 Cells.
Cell lines HCC44, MDA-MB-231, and SK MEL-5 were tested for rescue by siRNA transfection and neutralizing antibody pretreatments. siRNAs targeting caspase-8, TNFR1, TNFα, and RIPK1 (Dharmacon siGENOME SMARTpool predesigned pools of four oligos), as well as neutralizing antibody against TNFα (R&D Systems), were able to rescue these cell lines from Smac-mimetic-mediated apoptosis, while caspase-9 siRNA transfection (Dharmacon siGENOME SMARTpool) and pretreatment with TRAIL (Biolegend) neutralizing antibody had no rescue effects.
(A) HCC44.
(B) MDA-MB-231.
(C) SK MEL-5.
(D) H2009 cells were shown to respond to 100 nM compound 3 and 100 ng/ml TNFα. siRNA knockdown of caspase-8, TNFR1, and RIPK1 were able to rescue these cells from compound 3/TNFα cotreatment. siRNA transfection and cell viability assays were done as previously described. Each graphical representation indicates the mean ± SD of at least three independent testing conditions.
DISCUSSION
The perennial challenge in cancer drug discovery is specificity; namely, how to eradicate cancer cells without harming normal tissues. Most currently used therapeutics indiscriminately target fast growing cells regardless of origin. Among the hallmark characteristics that cancer cells display are the ability to evade apoptotic signals such as oncogene transformation, resulting in unregulated growth and proliferation, hypoxia, lack of proper growth factor stimulation or inhibition, and genomic instability, which will normally induce apoptosis. As knowledge of the selective molecular mechanisms by which cancer cells are able to grow, proliferate, and evade apoptosis is being gained, therapeutically viable targets present themselves. The now classic example is Gleevec. Selective growth advantages that the constitutively activated BCR-ABL kinase translocation mutation provides CML also presents an ideal single-agent target that if successfully blocked, would restore the proper balance of signaling to the cell. Similarly, many cancers have also been shown to upregulate antiapoptotic genes, which when overexpressed confer resistance to apoptosis, making such targets ideal candidates for therapeutic intervention. Here, we identify autocrine TNFα production as another potential means by which cancer cells gain selective growth advantage and resistance to apoptosis and a means by which a single-agent Smac mimetic can exploit that signal to restore sensitivity to apoptosis.
The above results suggest that targeting of the IAPs via Smac mimetic is a promising strategy for the treatment of cancer. Specifically, we show that targeting the IAPs through the use of a small-molecule mimetic of Smac had the unintended but fortuitous effect of also altering the functional state of the TNFα receptor from one that signals through the NFκB survival pathway to one that also promotes the formation of a RIPK1-dependent, death-inducing complex that is able to override the prosurvival signal and initiate apoptosis. Furthermore, the Smac mimetic is able to do this by exploiting the tumor cell’s own pool of TNFα, in the form of autocrine secretion. It seems likely that cells that produce autocrine TNFα might rely on such a signal for survival, particularly to activate prosurvival and antiapoptotic genes regulated by NFκB, and that such dependence might be the difference between Smac-mimetic sensitivity and Smac-mimetic resistance. Namely, because IAPs play potentially significant and unique roles in TNF-mediated activation of NFκB, that such cells would be particularly sensitive to the Smac mimetic. Other cell lines may rely on other cytokines and growth factors for such signaling and be resistant because IAPs do not play a significant role in signaling in these other systems. Given the identification of cancer cells that secrete TNFα and that rely on TNFα for survival signaling, it is possible that cancers fitting such a profile would be ideal targets of single-agent, smac-mimetic therapy.
In addition, Smac-mimetic-alone treatment of human tumor (HCC461) xenografted into mice reduced the size of tumors over an extended period of time. Tumors treated with Smac mimetic showed immediate response by reducing in size to nearly undetectable levels and staying that way for an extended period of time. Only 30 days after the final treatment did the tumors start to reemerge, and within the treatment group, 40% remained tumor free at the end of the experiment. This experiment suggests that the single-agent sensitivity observed in cultured cells maintained this property in animals. Unfortunately, although the secreted TNFα was readily detectable with a simple Elisa method in the culture media when these lating TNFα could be detected in the sera from HCC461 tumor-bearing mice using the same method (data not shown). Consistently, admission of the TNFα neutralizing antibody to HCC461 tumor-bearing mice also did not block tumor shrinkage after Smac-mimetic treatment (data not shown). It is possible that too little TNFα got into the circulation and/or that most of it may act locally at the tumor site. A more sensitive method able to detect low levels of TNFa in sera and in the tumor will be needed if this feature is going to be potentially developed into a biomarker for single-agent sensitivity in human patients. Another key aspect of the treatment was the observation that animals treated with the Smac mimetic showed no signs of associated cytotoxicity and were in general good health.
While only 22% of non-small-cell lung cancer cell lines examined responded to Smac-mimetic treatment alone, some portion of the other 78% of cell lines that have no response should respond to combined Smac mimetic/TNFα (as shown for H2009) treatment, while another portion will not respond at all (as in the case of HCC827 [data not shown]). Why some cells respond and others do not is a key issue facing cancer therapy in general. Unique differences in the nature of survival signaling and the mechanism of evasion of apoptosis make this a remarkably difficult task. It seems likely that as the ability of profiling a unique tumor’s sensitivity to a variety of agents expands, the utilization of various combination therapies should specifically resolve this issue.
EXPERIMENTAL PROCEDURES
Reagents
Compound 3 and compound 4 were synthesized as previously described (Li et al., 2004), and the biotinylated variant used for affinity purifications was identical to that reported earlier, except the linker connecting the biotin motif to the Smac mimetic was five carbons longer [synthesized using commercial (+)-biotinamidohexanoic acid N-hydroxysuccinimide ester; see supporting information for Li et al., 2004]. The compound was diluted to 100 uM stocks. Lung cancer cell lines were obtained from the laboratory of Dr. John Minna (UT Southwestern). Antibodies used: caspase-3 (Cell Signaling, 9662), caspase-8 (Cell Signaling, 9746), TNFR1 (Abcam, ab19139), TNFα (Cell Signaling, 3707), FADD (Cell Signaling, 2782), RIPK1 (BD PharMingen, 551041), TRADD (Cell Signaling, 3684), TNFα (R&D, MAB210), FasL (Biolegend, 306408), and TRAIL (Biolegend, 308207).
Cell Culture
Cell lines HCC44, HCC461, HCC827, H2009, and MDA-MB-231 were cultured in HyQ RPMI-1640 medium (Hyclone) supplemented with 5% fetal bovine serum (FBS, Hyclone) and 100 units/ml penicillin/streptomycin (GIBCO). SK MEL-5 cells were cultured in Minimum Essential Medium (GIBCO) supplemented with 10% FBS, 100 units/ml penicillin/streptomycin, and 2 μg/ml L-glutamine (GIBCO).
Cell Survival Assay
Cells were plated onto 96 well-assay plates (white with clear bottom [3610], Corning Costar) at different cell densities, depending on cell type, in 100 μl media per well. Cells were allowed to grow to near confluence and treated with compound 3 or vehicle (H20) by adding 100 μl media with compound 3, diluted to 2× the desired final concentration, to each well. Cells were incubated overnight and assayed the following day utilizing the Cell Titer-glo Luminescent Cell Viability Assay (Promega), which measures cell viability based on ATP levels present in live cells. As per manufacturer’s protocol, media was aspirated from each well and 100 μl fresh media added. Cells were allowed to equilibrate to room temperature when 100 μl of the Cell Titer-glo reagent was added. Cells were placed on an orbital shaker for 2 min and then were incubated for an additional 10 min. Luminescent measurements were done on a Tecan SPECTRAFluor Plus 96-well plate reader. For IC50 determination, half-maximal luminescent readings, relative to the vehicle-treated cells, were considered to be representative of the IC50 for each cell line tested. For assays measuring rescue effects, all values were normalized to the mock-treated or mock-transfected conditions to account for variability in the cytotoxicity of transfecting siRNA into cells and for possible cytotoxic effects that knockdown of the particular gene used might have. All values are represented graphically as mean ± SD for three independent samples.
Western Blot Analysis of Caspase Activation and of siRNA Knockdown Efficiency
HCC461 cells were plated onto 6-well cell culture dished (Corning Costar) at differing cell densities, depending on the application, in 2 ml media. For caspase-activation determination, cells were plated to near confluence and allowed to attach overnight. Cells were then treated as indicated in Figure 2A. Cells were treated to a final concentration of 100 nM compound 3 at each time point. For cells treated with z vad fmk (Sigma), 10 mM z vad was added 1 hr prior to compound 3. For siRNA knockdown efficiency, HCC461 cells were plated in 2 ml antibiotic-free media at a density of 8 × 104 cells per well and allowed to attach overnight. siRNAs were then transfected as described below. Cells were lysed in lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 10% glycerol, 0.5 mM DDT, and Complete protease inhibitor [Roche]) and were then incubated on ice for 20 min and spun down at 10,000 RPM for 10 min. The soluble fraction was kept and protein concentration determined by Bradford assay. Protein concentrations were normalized to 50 μg total and SDS-PAGE was done followed by western blotting of target antibody.
Biotinylated Smac Mimetic Pull-Down
Cells were plated on 15 cm dishes and grown to confluence. Cells were harvested and lysed as previously described. Biotinylated Smac mimetic (100 nM) was preincubated with 20 μl (bead volume) streptavidin dynabeads (Invitrogen) for 1 hr at 4°C. Beads were then washed two times in lysis buffer. Smac mimetic bound dynabeads were then incubated with cell lysates overnight at 4°C. The next day, beads where washed four times with cell lysis buffer, and SDS-loading buffer was added. Samples were run on SDS-PAGE and silver stained with Bio-Rad silver stain plus kit. Bands for each lane were cut out for mass spectrometry.
siRNA Transfection
siRNA transfections were done in both 6-well and 96-well dish formats. For 6-well dishes, the day prior to transfection, cells were plated at a density of 8 × 104 cells per well in antibiotic-free media. The next day, Lipofectamine 2000 was used to transfect cells, as per manufactures protocol. Briefly, 3 μl Lipofectamine 2000 was combined with 120 pmol (6 μl of a 20 μM stock) siRNA in a volume of 500 μl Opti-mem media (GIBCO) and incubated for 20 min; the complexes of Lipofectamine 2000 and siRNAs were then added directly to each well, and the cells were incubated until nearly confluent, approximately 48–72 hr later depending on growth conditions. For 96-well dishes, the day prior to transfection, cells were plated at a density of 1.2 × 103 cells per well in antibiotic-free media. Lipofectamine 2000 was used, as above, by mixing 0.2 μl Lipofectamine 2000 and 8 pmol (0.4 μl of a 20 μM stock) siRNA in a total volume of 50 μl. All siRNAs were purchased from Dharmacon. For caspase-8 and caspase-9, individual oligos were designed and tested (caspase-8-1 target sequence, 5-UGAAGAUAAUCAACGA CUAUU-3; caspase-8-2 target sequence, 5-UGGAUUUGCUGAUUA CCUAUU-3; caspase-8-3, obtained from Dharmacon [J-003466-14], caspase-9-1 target sequence, 5-GAUGCCUGGUUGCUUUAAUUU; caspase-9-2, obtained from Dharmacon [J-003309-05]; caspase-9-3, obtained from Dharmacon [J-003309-06]). For TNFR1 and TNFα, individual oligos were purchased from Dharmacon (TNFR1: TNFR1-1, J-005187-05; TNFR1-2, J-005197-06; TNFR1-3, J-005197-08) (TNFα: TNFa-1, J-010546-09; TNFα-2, J-010546-10, TNFα-3, J-010546-12). For all pooled siRNAs, Dharmacon’s siGENOME SMARTpool predesigned pools of four oligos were used and validated by western blot. These included siRNAs for caspase-8, TNFR1, TNFα, FADD, TRADD, and RIPK1.
Methylene Blue Viability Assay
Cells were plated onto 6-well dishes at low density and grown to 25% confluence. Cells were then treated as indicated in Figure 2D. Five days following treatment, cells were washed two times with cold PBS, after which 2 ml methylene blue reagent was added (2% methylene blue [w/v] in 50% ethanol) for 15 min. Cells were then washed with water until all excess dye was removed. The plate was then photographed.
Elisa Analysis of Autocrine TNFα Secretion
Cells were plated onto 6-well dishes and allowed to grow to near confluence (approximately 80%). Media was then aspirated and the cells were washed two times with cold PBS. Fresh media (1 ml) was added and 100 μl aliquots were removed at each time point (three independent wells were tested at each time point). Samples were kept at −20°C until ready for use. Elisa analysis was performed using a quantitative sandwich enzyme immunoassay from R&D Systems (TNFα Quanti-glo Chemiluminescent Elisa, QTA00B), as per manufacturer’s instructions.
Caspase-8 Antibody Immunoprecipitation
Cells were grown on 15 cm plates, treated as indicated, and harvested in 5× volume lysis buffer (as previously described). Cells were left on ice for 20 min and centrifuged at 20000× g for 20 min. Twenty microliters (bead volume) protein A agarose beads were coupled to 2 μg of caspase-8 antibody (Santa Cruz, SC-6136) in 250 μl PBS (supplemented with 5 mg/ml BSA). After 2 hr incubation at room temperature, the beads were washed two times in lysis buffer, whereupon 2 mg cell lysates (2mg/ml) were added and incubated over night at 4°C. The following day, beads were washed four times with lysis buffer and protein eluted off the beads using low pH elution buffer (Pierce 21004). Elution buffer was neutralized by adding 1:20 1 M Tris-HCl (pH 9.4). Samples were then analyzed by SDS-PAGE followed by western blot.
In Vivo Matrigel Model of HCC461 and HCC15 Tumor Model into Nude Mice
Harlan Athymic Nude-Foxnlnu 5- to 6-week-old mice were injected subcutaneously with 1 × 107 HCC461 or HCC15 tumor cells in the left flank. Seven days later, when tumors had reached 200–300 mm3 in size, mice were randomized into treatment groups of five mice per group. Compound 3 was first dissolved in H2O at 20 mg/ml as the stock solution and then diluted in PBS to 5 mg/kg based on the weight of individual mice. The compound and the vehicle (saline) were administered intravenously in a total volume of 0.2 ml every other day for six treatments total (q2d × 6). Mice were weighed and tumors measured using vernier calipers two times per week. Tumor volumes were calculated according to (length × width2)/2. All animal experiments were performed in the vivarium of Joyant Pharmacology Department. The animal protocol was approved by IACUC, which is valid until May 1, 2009. All animal experiments performed in Joyant conform to the relevant regulatory standards. All values are represented graphically as the mean ± SEM.
SIGNIFICANCE.
The use of small-molecule mimetics of Smac offers significant potential in the treatment of many cancers, given that the Smac protein functions to uninhibit apoptosis by relieving IAP inhibition of caspases. The intended application of such a compound was to complement the effectiveness of other chemotherapeutic agents; hence, the apparent ability of a Smac mimetic, in one-quarter of cell lines tested, to induce cell death as a single agent was quite remarkable. Not only is single-agent Smac mimetic treatment highly effective at inducing cell death in these cell lines as well as in xenografts, but it offers the possibility of highly specific and relatively nontoxic future therapeutic treatments by exploiting certain cancer cells’ own production of TNFα.
ACKNOWLEDGMENTS
This work is also supported by a program project grant from the National Cancer Institute (NCI) (PO1 CA 95471). J.D.M. is supported by grants (CA84971 and CA70907) from NCI. X.W. and P.H. are the cofounders of Joyant Pharmaceuticals, Inc.
REFERENCES
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Aggarwal BB. Receptor-mediated choreography of life and death. J. Clin. Immunol. 2003;23:317–332. doi: 10.1023/a:1025319031417. [DOI] [PubMed] [Google Scholar]
- Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24:7381–7388. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature. 2000;406:855–862. doi: 10.1038/35022514. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Neri P, Velankar M, Podar K, Hideshima T, Fulciniti M, Tassone P, Raje N, Mitsiades C, Mitsiades N, et al. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM) Blood. 2007;109:1220–1227. doi: 10.1182/blood-2006-04-015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol. Cell. 2002;9:401–410. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J. Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- Druker BJ. STI571 (Gleevec) as a paradigm for cancer therapy. Trends Mol. Med. 2002;8:S14–S18. doi: 10.1016/s1471-4914(02)02305-5. [DOI] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- Glover CJ, Hite K, DeLosh R, Scudiero DA, Fivash MJ, Smith LR, Fisher RJ, Wu JW, Shi Y, Kipp RA, et al. A highthroughput screen for identification of molecular mimics of Smac/ DIABLO utilizing a fluorescence polarization assay. Anal. Biochem. 2003;320:157–169. doi: 10.1016/s0003-2697(03)00389-0. [DOI] [PubMed] [Google Scholar]
- Gordon GJ, Appasani K, Parcells JP, Mukhopadhyay NK, Jaklitsch MT, Richards WG, Sugarbaker DJ, Bueno R. Inhibitor of apoptosis protein-1 promotes tumor cell survival in mesothelioma. Carcinogenesis. 2002;23:1017–1024. doi: 10.1093/carcin/23.6.1017. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- Jin Z, El-Deiry WS. Distinct signaling pathways in TRAIL-versus tumor necrosis factor-induced apoptosis. Mol. Cell. Biol. 2006;26:8136–8148. doi: 10.1128/MCB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- Kirkin V, Joos S, Zornig M. The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta. 2004;1644:229–249. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- Liu Z, Sun C, Olejniczak ET, Meadows RP, Betz SF, Oost T, Herrmann J, Wu JC, Fesik SW. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–1008. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. BcI-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151–157. [PubMed] [Google Scholar]
- Mizukawa K, Kawamura A, Sasayama T, Tanaka K, Kamei M, Sasaki M, Kohmura E. Synthetic Smac peptide enhances the effect of etoposide-induced apoptosis in human glioblastoma cell lines. J. Neurooncol. 2006;77:247–255. doi: 10.1007/s11060-005-9045-5. [DOI] [PubMed] [Google Scholar]
- Nachmias B, Ashhab Y, Ben-Yehuda D. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin. Cancer Biol. 2004;14:231–243. doi: 10.1016/j.semcancer.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Oettinger CW, D’Souza M, Milton GV. Targeting macrophages with microspheres containing cytokine-neutralizing antibodies prevents lethality in gram-negative peritonitis. J. Interferon Cytokine Res. 1999;19:33–40. doi: 10.1089/107999099314397. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of BcI-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HB, Takeuchi M, Goeddel DV. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc. Natl. Acad. Sci. USA. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. Int. J. Oncol. 2002;21:315–320. [PubMed] [Google Scholar]
- Sun H, Nikolovska-Coleska Z, Chen J, Yang CY, Tomita Y, Pan H, Yoshioka Y, Krajewski K, Roller PP, Wang S. Structure-based design, synthesis and biochemical testing of novel and potent Smac peptido-mimetics. Bioorg. Med. Chem. Lett. 2005;15:793–797. doi: 10.1016/j.bmcl.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Sun H, Nikolovska-Coleska Z, Lu J, Qiu S, Yang CY, Gao W, Meagher J, Stuckey J, Wang S. Design, synthesis, and evaluation of a potent, cell-permeable, conformationally constrained second mitochondria derived activator of caspase (Smac) mimetic. J. Med. Chem. 2006;49:7916–7920. doi: 10.1021/jm061108d. [DOI] [PubMed] [Google Scholar]
- Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- Wajant H. Death receptors. Essays Biochem. 2003;39:53–71. doi: 10.1042/bse0390053. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Wilkinson JC, Wilkinson AS, Scott FL, Csomos RA, Salvesen GS, Duckett CS. Neutralization of Smac/Diablo by inhibitors of apoptosis (IAPs) A caspase-independent mechanism for apoptotic inhibition. J. Biol. Chem. 2004;279:51082–51090. doi: 10.1074/jbc.M408655200. [DOI] [PubMed] [Google Scholar]
- Workman P, Kaye SB. Translating basic cancer research into new cancer therapeutics. Trends Mol. Med. 2002;8:S1–S9. doi: 10.1016/s1471-4914(02)02319-5. [DOI] [PubMed] [Google Scholar]
- Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- Wu TY, Wagner KW, Bursulaya B, Schultz PG, Deveraux QL. Development and characterization of nonpeptidic small molecule inhibitors of the XIAP/caspase-3 interaction. Chem. Biol. 2003;10:759–767. doi: 10.1016/s1074-5521(03)00157-1. [DOI] [PubMed] [Google Scholar]
- Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJ, Okawa DC, Flygare JA, Vucic D, Fairbrother WJ, Deshayes K. Design, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACS Chem. Biol. 2006;1:525–533. doi: 10.1021/cb600276q. [DOI] [PubMed] [Google Scholar]