Abstract
Type 1 diabetes (T1D) develops as a consequence of a progressive autoimmune response that destroys insulin-producing β-cells in pancreatic islets. Because of their role(s) in controlling immune responses, considerable effort has been directed towards resolving whether regulatory T cells (Tregs) offer a clinical treatment to restore tolerance in T1D. We previously reported that in vitro induced adaptive Treg cells (aTregs) can reverse T1D and persist as protective memory cells in the NOD mouse model. In the current study, we investigated mechanisms that regulate aTregs. We found that these FoxP3+ aTregs expressed high levels of the IL-7 receptor, IL-7Rα, without the high affinity receptor for IL-2, CD25, which is found on natural Treg cells (nTregs). IL-7Rα expression was mirrored by the dependency of aTregs on IL-7 for persistence. IL-10 and TGF-β, effector cytokines of aTregs, were not essential for their maintenance at the level of systemic antibody blocking. Nevertheless, IL-10 modulated cytokine production by aTregs and TGF-β was critical for protection. aTregs were found to infiltrate islets and the expression of integrin-β7 was required for their localization in the pancreas. Furthermore, blocking aTreg entry into the pancreas prevented their control of diabetogenic effector T cells, implying the need for local control of the autoimmune response. The distinct homeostatic regulation of aTregs independently of a response to IL-2, which is defective in T1D patients, suggests that these cells represent a translatable candidate to control the autoimmune response.
Keywords: Diabetes, Regulatory T cell, NOD mouse, IL-7, Integrin-β7
1. Introduction
Type 1 diabetes mellitus (T1D) results from the destruction of pancreatic islet β-cells that is largely orchestrated by CD4+ T cells. Although both genetic and environmental factors contribute to the autoimmune etiology of T1D, the detailed mechanisms remain largely unknown. Thus, while T1D can be predicted in human patients by the expression of antibodies specific to some autoantigens, such as insulin, GAD65 (glutamic acid decarboxylase), IA-2 (insolenoma antigen), and ZnT8 (islet zinc transporter) [1], there is no known way to prevent the development of T1D, nor is there a proven treatment that can control the anti-β-cell autoimmune response after the diagnosis of diabetes. Nonetheless, the gradual loss of self-tolerance to islet β-cell antigens in human patients and animal models, especially the Non-Obese Diabetic (NOD) mouse model, is one of the most important contributing factors to T1D development. Regulatory T cells (Treg), a CD4+ T cell lineage that expresses the transcription factor FoxP3 and the high affinity receptor for IL-2, CD25, play a central role in maintaining self-tolerance [2]. Thus, Treg cells have been considered as a potential cell-based treatment to restore self-tolerance in T1D. FoxP3+CD25+ Treg cells comprise two major subsets in the immune system: naturally occurring Treg cells (nTreg) that develop in the thymus and adaptive Treg cells (aTreg) that develop in the periphery from conventional naïve CD4+ T cells [3]. However, there is increasing controversy as to the degree of plasticity of these cells and their capacity to lose FoxP3 along with regulatory function in the context of an autoimmune response, providing a strong rationale to study other subsets of Tregs that can be induced in vitro or in vivo in various experimental systems in the context of T1D [4].
A concerted effort over the past decade to test the feasibility of using FoxP3+CD25+ Treg cells as an efficacious clinical intervention and treatment for T1D has revealed key challenges that currently limit translation to a human therapy. Despite promising adoptive transfer studies of in vitro expanded nTreg cells in the NOD mouse model [5], it has been difficult to unequivocally identify and isolate these cells from human patients, as well as to expand populations that retain FoxP3. In mouse models, under inflammatory conditions in vivo, nTreg cells can convert into effector cells [6, 7]. This conversion has also been clearly demonstrated in the NOD model in which nTreg cells lose FoxP3 and become pathogenic T cells that can transfer diabetes to adoptive hosts [8]. The critical dependence of nTregs on IL-2 signaling for homeostatic maintenance and functional capacity [4, 9] is also problematic for T1D[10]. In both human T1D patients and NOD mice, there is a clear genetic linkage between defects in the IL-2 pathway and the disease [11, 12]. Therefore, as immunotherapeutic agents to treat T1D, Tregs with a more stable, long-lasting, and IL-2 independent phenotype that can be easily generated would have the necessary attributes for clinical translation.
The studies of our lab and many others have focused on FoxP3+ aTreg cells that can be generated from naïve phenotype CD4+FoxP3−CD25− T cells from NOD mice through TCR stimulation in the presence of TGF-β [13, 14]. Our studies showed that these cells could also be induced from naïve phenotype CD4+ T cells from diabetic mice. Furthermore, a recent study showed that similar aTreg cells can be elicited from naïve CD4+ T cells from human diabetic patients [15]. Of critical importance, we found that aTreg cells generated not only from antigen-specific, TCR transgenic (Tg) T cells, but also from polyclonal CD4+ T cells, conferred protection against diabetes in prediabetic recipient NOD mice [13, 16]. Furthermore, these cells could reverse and thereafter control diabetes as functional memory cells [13, 16]. Interestingly, despite maintaining FoxP3 expression, these cells persisted as CD25− in the recipients. We therefore sought to determine the mechanisms by which these aTreg cells are regulated that could be advantageous for the treatment of T1D. Our results show that these aTreg cells expressed high levels of the IL-7 receptor, IL-7Rα (CD127), without CD25, and that IL-7 was essential for their regulation. Although the cells expressed IL-10 and TGF-β, these cytokines were dispensable for their maintenance. However, IL-10 dampened the production of proinflammatory cytokines by aTreg cells. TGF-β was critical for protection against T1D and local response of aTregs in the pancreas, while the expression of integrin-α4β7 on aTreg cells was necessary for their entry into the target organ. Since we have previously shown that polyclonal aTreg cells become selected by antigen in vivo after transfusion [16], this unique regulation suggests that such aTreg cells are possible candidates for a cell-based treatment for T1D.
2. Materials and Methods
2.1. Mice
NOD, NOD.Scid, NOD.Thy1.1, NOD.CD45.2, NOD.Integrin-β7−/− mice, and B6.CD45.1 were obtained from the Jackson Laboratory (Bar Harbor, Maine). NOD.BDC2.5, NOD.FoxP3-GFP, and NOD.IL-10−/− mice were acquired from the JDRF Center on Immunological Tolerance in Type 1 Diabetes at Harvard Medical School (Boston, MA). IL-7−/− and IL-7Rα−/− mice were obtained from Dr. Charles Surh (The Scripps Research Institute, La Jolla, CA). NOD.BDC2.5 mice were bred to NOD.Thy1.1 mice. All animals were bred in a specific pathogen free (SPF) facility at Sanford-Burnham Medical Research Institute. Only female mice were used in the experiments. All experiments in this study were approved by the Institutional Animal Care And Use Committee (IACUC).
2.2. Differentiation of aTreg cells in vitro
Adaptive Treg cells were differentiated as previously described [13, 16]. Briefly, naïve CD4+ T cells were isolated from the lymphoid tissues of 6–8 week old mice by negtive selection with EasySep kits (StemCell Technologies, Vancouver, Canada) according to the manuafacturer’s instructions, except that biotin-conjugated anti-CD25 antibody was included to deplete nTreg cells. In some experiments, naïve CD4+ T cells were purified by sorting CD4+CD25−GFP− cells from NOD.FoxP3-GFP reporter mice on a FACS Aria cell sorter (BD Biosciences, San Jose, CA) in the core facility. Purified CD4+CD25− T cells were cultured in 6-well plates coated with anti-CD3 (clone 145.2c11, Biolegend, San Diego, CA) (10–25μg/ml) with complete RPMI-1640 medium for 5 days. The cultures were supplemented with 10μg/ml anti-IFN-γ (clones XMG1.2 or R46A2, purfied from hybridoma culture supernatant in house), 200units/ml rIL-2 (NCI Biological Resource Branch), and 10ng/ml rTGF-β1 (Biolegend). To rest these cells, after the 5-day differentiation, cells were harvested and cultured with or without 10ng/ml rIL-7 (NCI Biological Resource Branch) without any other stimulation for indicated periods of time before analysis or cell transfer.
2.3. Adoptive transfer
In vitro differentiated aTreg cells were transferred into NOD or NOD.Scid recipient mice via i.v. injection in a dose of 2×106 unless otherwise indicated. Anti-TGF-β1,2,3 (clone 1D11), anti-IL-10 (clone JES-2A5), or anti-IL-7 (clone M25), all purfied from hybridoma culture supernatants in house, anti-IL-10R (clone 1B1.3a, Biolegend) or control rat or mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) were injected at indicated doses and times. In some experiments, diabetes was accelerated by transferring total splenocytes from diabetic donor mice in a dose that contained 4×106 CD3+ cells. Diabetes incidence was monitored by weekly blood glucose testing using Bayer’s Countour meters. A reading of >250mg/dl was indicative of loss of glycemic control; two consecutive readings of higher than 300mg/dl were considered indictive of diabetes. To detect in vivo division of donor cells, donor cells were labeled with CFSE (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions, or recipients were given BrdU (Sigma-Aldrich, St. Louis, MO) in the drinking water as previously described [17].
2.4. Flow cytometry
Most fluorochrome-conjugated antibodies for FACS analysis were purchased from Biolegend (San Diego, CA) with exceptions as noted. For intracellular cytokine staining, cells were restimulated with 50ng/ml PMA (Sigma-Aldrich) and 1μg/ml Ionomycin (Sigma-Aldrich) with 10μg/ml Brefeldin A (Sigma-Aldrich) for 4 hours. Cells were stained for surface markers first; after fixation and permeablization with Cytofix/Cytoperm buffer (BD Biosciences), the cells were then stained with anti-cytokine antibodies. For FoxP3 staining, cells were stained for surface markers first; after fixation and permeabliztion, the cells were stained with PE-conjugated anti-mouse FoxP3 (clone FJK-16S, eBioscience, San Diego, CA). For BrdU detection, a BrdU staining kit (BD Biosciences) was used according to manufacturer’s instructions. FACS data were collected on FACS Calibur or Canto with CellQuest or Diva softwares (BD Biosciences) and were analyzed with FlowJo program (Tree Star, Ashland, OR).
2.5. Cytokine assays
BDC2.5 aTreg donor cells that were recovered from adoptive transfer recipients were stimulated in culture with a BDC2.5 TCR-specific peptide (RTRPLWVRME) [18]. Culture supernatants were collected after 24 hours of stimulation. Cytokines were assayed by Luminex cytokine assay with multiplex reagent kits from Biolegend, according to manufacturer’s instructions.
2.6. Histology
Pancreata from NOD.Scid mice that received NOD.BDC2.5.Thy1.1 aTreg cells 10 days previously were embedded in Frozen Section Compound (Surgipath Medical Industries, Richmond, IL). Frozen sections were stained with biotin-anti-Thy1.1 (BD Bioscience) and rabbit-anti-Insulin (Santa Cruz Biotechnology, Santa Cruz, CA). Binding of antibodies was detected by Alexa Fluor 568 labeled strepavidin (Invitrogen) and Alexa Fluor 488 labeled goat-anti-rabbit antibody (Invitrogen), respectively. Images were acquired at a magnification of 40X with a SPOT Flex digital camera (Diagnostic Instruments Inc, Sterling Heights, MI) attached to an Olympus BX50 fluorescent microscope (Center Valley, PA).
3. Results
3.1. aTreg cells can reverse T1D in NOD recipients and express low or undetectable levels of CD25
There are several ways to induce aTreg cells in vitro or in vivo (for review, see reference [14]). In our studies, we have used optimal TCR stimulation through CD3 on naïve CD4+CD25− cells from NOD mice in cultures supplemented with IL-2 and TGF-β1, as we reported previously [13, 16]. We observe a greater expansion and development of FoxP3+ cells with this protocol (up to 10x expansion) than with lower doses of anti-CD3 (<10 μg/ml, data not shown). Thus, an advantage of this method is a high yield of usable cells, thereby offering the potential for clinical translation. When these cells were transferred into spontaneously hyperglycemic NOD mice, the majority of which became diabetic by the time of aTreg cell injection, blood glucose levels were reduced in most recipients. Within about 4 weeks after cell transfer, 75% of recipients became normoglycemic (Fig. 1A). We obtained similar results with an accelerated diabetes model, where the disease was induced by transferring total splenocytes from diabetic NOD mice into prediabetic NOD or NOD.Scid recipients. This induced/accelerated diabetes can be prevented or reversed by aTreg cells (Fig. 5F and 7D). These results corroborate our previous studies [13, 16], and demonstrate that in vitro differentiated polyclonal aTreg cells restore normal blood glucose levels quite rapidly, supporting the idea that β-cell function can be restored when the autoimmune response is under control.
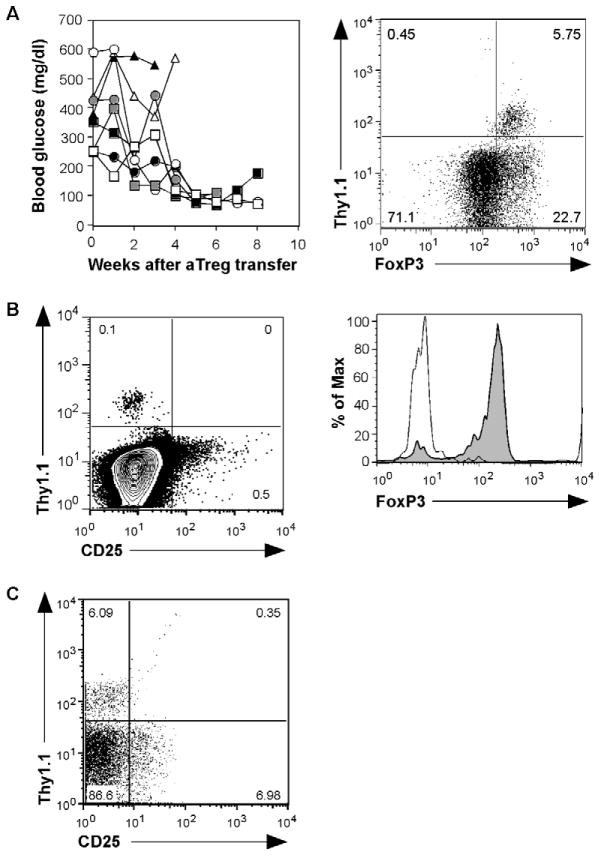
Fig. 1. aTreg cells that reverse T1D persist as CD25− memory cells.
(A) Polyclonal (NOD.Thy1.1) aTreg cells were injected into spontaneously diabetic NOD mice (Thy1.2) (n=8) at 1 week following a blood glucose reading >250 mg/dl. Blood glucose levels were monitored weekly thereafter (left). FoxP3 intracellular staining on CD4+ cells recovered from pancreata of recipient mice 3 months after diabetes reversal (right). (B) Donor aTreg cells were identified by Thy1.1+ staining from lymph nodes in a 2-year-old diabetes-free recipient that received aTreg transfer at 3 weeks of age (left panel). FoxP3 expression was examined on the CD4+Thy1.1+ donor population (right panel). (C) Spontaneously diabetic NOD mice received aTreg cell transfer as in (A). Three months after diabetes reversal, CD4+ cells from the pancreas were analyzed for aTreg cells (Thy1.1+) and CD25 expression. Data shown is from a representative recipient from 5 experiments.
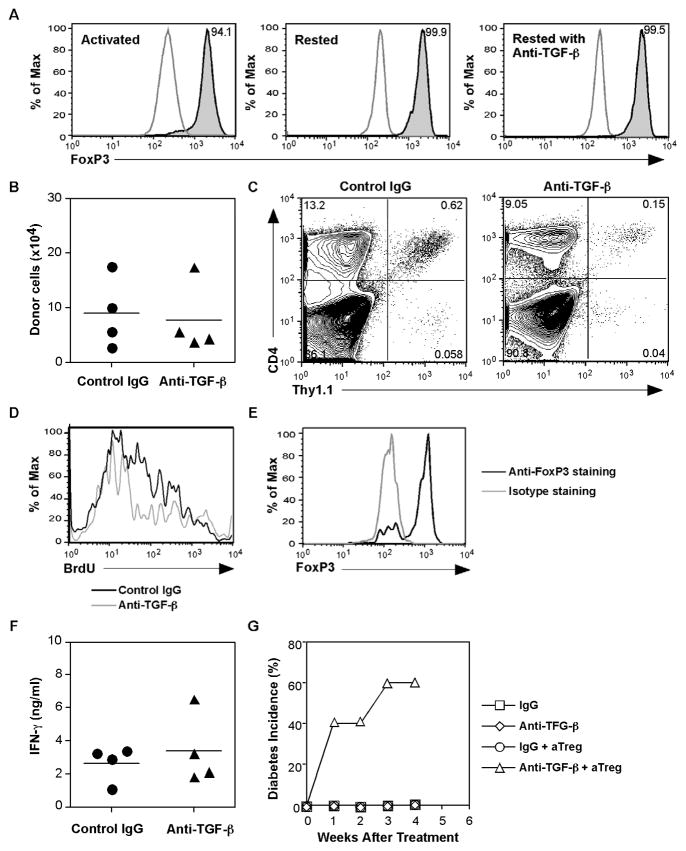
Fig. 5. aTreg cells require TGF-ββ β for their response in the pancreas and protection against T1D.
(A) aTreg cells were differentiated from BDC2.5Thy1.1+ CD4+CD25− T cells and stained for expression of FoxP3 before (left panel) and after resting in medium for 3 days (middle panel) or in rIL-7-containing medium (10ng/ml) with anti-TGF-β (10μg/ml) for 7 days (right panel). Shown are histograms gated on CD4+ T cells; the stained cells (shaded) are compared to isotype controls (open). (B - F) BDC2.5 Thy1.1+ naïve CD4+ T cells were used to generate aTreg cells. Cells were injected into NOD mice (n = 4/group). At the time of cell transfer, the mice were then treated with rat IgG or anti-TGF-β, 300ug/injection, two times/week for two weeks. One week after cell transfer, the mice were treated with BrdU in the drinking water for 1 week. (B) The recovery of donor cells from the spleens was calculated (mean ± SEM). (C) The lymphocytes pooled from the pancreata of recipients were analyzed for CD4+Thy1.1+ cells. (D) BrdU staining was analyzed after gating on the Thy1.1+ CD4+ donor populations shown in (C). (E) FoxP3 expression was analyzed after gating on the donor populations shown in (C). (F) Recovered cells from the spleens were stimulated in vitro with a BDC2.5 TCR-specific peptide for 24 hours. IFN-γ secretion into culture supernatants was measured by a Luminex cytokine assay (Mean ± SD). (G) Young prediabetic NOD mice were injected with spleen cells from diabetic donors and with aTreg cells. The mice were then treated with control IgG or anti-TGFβ (300μg/injection) as indicated for 4 weeks (n = 5 group). Blood glucose levels and diabetes incidence were monitored.
By using aTreg cells generated from naïve CD4+ T cells isolated from NOD.Thy1.1 mice for adoptive transfer, it was possible to distinguish the aTreg donor cells from the endogenous Thy1.2+/+ cells of the recipients. Importantly, almost all of the cells retained FoxP3 expression (Fig. 1B), suggesting that the preexisting proinflammatory conditions did not cause instability of the population. The recipients were able to survive several months after cell transfer, suggesting that the control on the anti-β-cell autoimmune response is long lasting. Indeed, when we transferred these polyclonal aTreg cells into prediabetic NOD mice, recipients were able to survive for up to two years without the development of diabetes, and most of the recovered donor cells expressed high levels of FoxP3 (Fig. 1C). These results indicate that aTreg cells can persist in recipients as memory cells under long-term homeostatic regulation. Interestingly, we consistently found that the recovered cells expressed very low or undetectable levels of CD25 (Fig. 1C), although all cells upregulated CD25 expression after in vitro differentiation (data not shown, and Fig. 2B). This is in stark contrast to the nTreg cells, which express high levels of CD25 and require IL-2 for their maintenance [10]. These results suggest that signaling via IL-2 is not essential for aTreg cell survival and homeostasis.
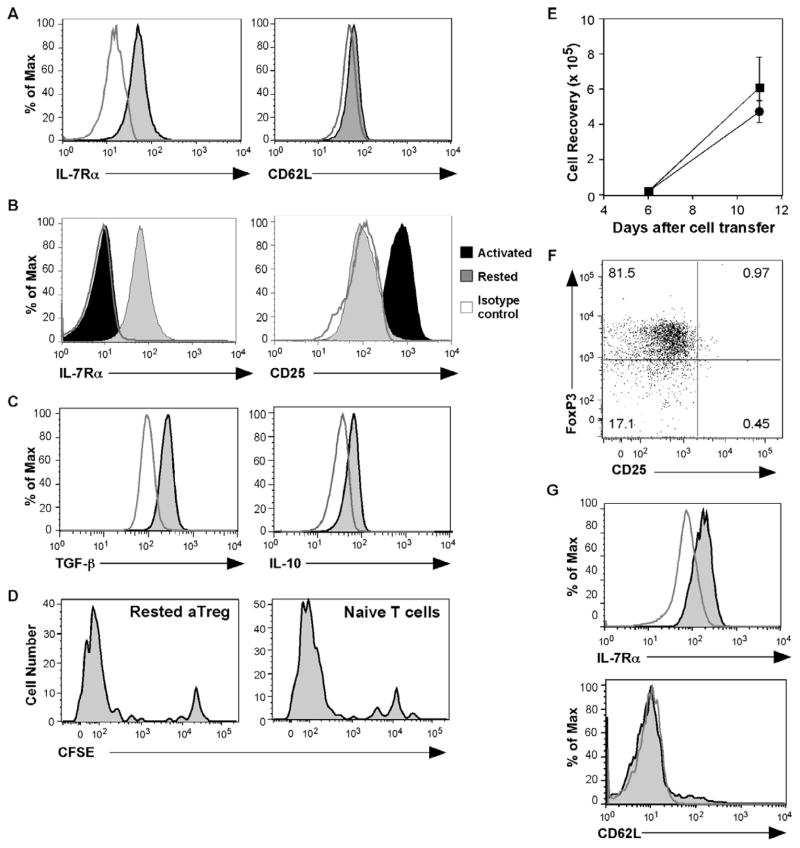
Fig. 2. IL-7Rαα α expression with the development of memory aTregs.
(A) IL-7Rα and CD62L expression were examined at 9 months after aTreg cell transfer and diabetes reversal. Shown are histograms of gated CD4+Thy1.1+ donor cells; the stained cells (shaded) are compared to isotype controls (open). (B) aTreg cells rested in the absence of rIL-7 for 3 days (gray histograms) were compared to aTreg cells immediately after differentiation (black histograms) for expression of IL-7Rα and CD25. The open histograms show isotype staining controls. (C) The rested cells were restimulated with PMA and ionomycin, and stained for intracellular TGF-β and IL-10. Shown are histograms of CD4+ gated T cells; the stained cells (shaded) are compared to isotype controls (open). (D – G) aTreg cells were differentiated and rested in the presence of rIL-7 (10ng/ml). The cells were then CFSE-labeled and transferred into NOD.Scid mice (n = 5). For comparison, naïve CD4 cells were also transferred (n = 4). (D) Eleven days after cell transfer, the cells were tested for proliferation by CFSE dilution in comparison to naïve CD4+ T cells. Shown are representative histograms after gating on CD4+Thy1.1+ donor cells from the spleen. (E) The recovered naïve donor cells (squares) or aTreg donor cells (circles) from the spleens were enumerated (mean ± SEM). (F) The expression of FoxP3 and CD25 is shown on a representative dot plot on gated CD4+Thy1.1+ donor cells. (G) IL-7Rα and CD62L were examined after gating on CD4+Thy1.1+ cells. Shown are representative histograms of antibody staining (shaded) compared to isotype control staining (open) on gated CD4+Thy1.1+ donor cells.
3.2. aTreg cells express high levels of IL-7Rα in vivo and in vitro
We then sought to determine the cytokine-dependent mechanisms that regulate homeostasis and function of aTreg cells as memory cells. An obvious focus was on IL-7, another common γ chain (γc) cytokine that is critical for T cell development and homeostasis. In addition, our previous studies demonstrated that IL-7 is the essential γc cytokine for the persistence of memory CD4+ T cells [19]. We examined IL-7Rα (CD127) expression on donor cells that were recovered after diabetes reversal by flow cytometry and found that recovered aTreg cells expressed high levels of IL-7Rα (Figure 2A). In addition, the expression of CD62L was downregulated on these cells (Fig. 2A). These results suggest that aTreg cells have an effector memory phenotype in recipient mice, and that they may depend on IL-7 signaling for survival/homeostatic regulation. To understand the kinetics and relationship of the expression of CD25 and IL-7Rα, we differentiated aTreg cells and cultured them in media without further stimulation (resting). Consistent with our previous studies of rested effector CD4+ T cells [19], after only 3 days of resting, the expression level of IL-7Rα was greatly upregulated. During the same period the expression level of CD25 was reduced to an almost undetectable level (Fig. 2B). The reciprocal expression pattern between CD25 and IL-7Rα suggests that CD25 identifies activated aTreg cells, whereas IL-7Rα distinguishes cells undergoing the transition to memory. As expected, the in vitro resting did not affect the expression of aTreg effector cytokines, IL-10 and TGF-β (Fig. 2C). Moreover, the FoxP3 expression levels were not changed (Fig. 5A, and not depicted), suggesting that the transition to memory does not have an effect on regulatory function in the short term.
Although we have shown that FoxP3 expression in aTreg cells is maintained over an extended period of time in vivo (Fig. 1B), it was recently reported that aTreg cells could downregulate FoxP3 and lose regulatory function as a consequence of lymphopenia driven proliferation [20]. To further address the stability of aTreg cells, we differentiated and rested aTreg cells from naïve BDC2.5 CD4+ T cells, labeled them with CFSE, and transferred the cells into NOD.Scid recipients. After 11 days, aTreg cells and naïve CD4+ T cells showed similar proliferation in lymphopenic hosts (Fig. 2D); the recovery of donor cells were not significantly different (Fig. 2E). Importantly, after extensive lymphopenia driven proliferation, the expression level of FoxP3 and its frequency in donor aTreg cells that were recovered from spleen, pancreatic lymph nodes, and pancreas of recipients of aTreg cells were not diminished (Fig. 2F, and not depicted). Furthermore, as seen with aTreg cells recovered after diabetes reversal, after lymphopenia driven proliferation, the cells remained CD25−, IL-7Rα+, and CD62L− (Fig. 2F and 2G).
3.3. aTreg cells depend on IL-7
To determine whether the consistently high expression of IL-7Rα confers IL-7 dependency of aTreg cells in vivo, we differentiated aTreg cells and transferred them into NOD.Scid recipients that also received an anti-IL-7 neutralizing antibody or control mouse IgG injections. After 10 days, the recovered donor cells were analyzed. As shown in Fig. 3A, treatment with anti-IL-7 antibody resulted in a significantly reduced recovery of donor cells, indicating a loss of donor aTreg cells.
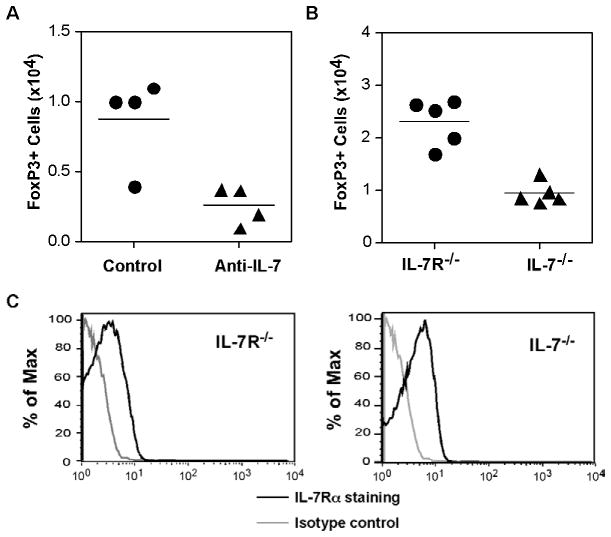
Fig. 3. aTreg cells are dependent on IL-7 in vivo.
(A) Polyclonal aTreg cells were differentiated from CD4+CD25−GFP− cells from NOD.FoxP3-GFP reporter mice. The cells were injected into NOD.Scid recipients and treated with control mouse IgG or anti-IL-7 neutralizing antibody at the time of cell transfer and on days 3 and 7, in a dose of 300μg/injection. Ten days after cell transfer, the cells from the spleen were analyzed for the presence of GFP+ cells (n = 5/group, mean ± SEM). (B) aTreg cells were differentiated from CD4+CD25− cells from B6.CD45.1 mice. The cells were transferred into IL-7−/− or IL-7R−/− recipients in a dose of 3 × 106/recipient. The recipient mice were evaluated on day 14 after cell transfer. The recovery of FoxP3+ donor cells (left) was calculated, and IL-7Rα expression (right) was analyzed by flow cytometry after gating on FoxP3+ donor cells from the spleen (n = 5/group).
Since treatment with functional blocking antibodies may not be completely efficient, to further test the dependence of aTreg cells on IL-7, we sought to evaluate aTreg maintenance in NOD.IL-7−/− recipients. However, the backcross of the IL-7−/− line (B6 background) onto the NOD background has not been successful. Therefore, we performed the experiments on the B6 background. In this experiment, aTreg cells were differentiated from naïve CD4+CD25− T cells from B6 donor mice (CD45.1), and were transferred into IL-7−/− recipients or IL-7Rα−/− recipients (CD45.2). Two weeks after transfer, the cells from recipient mice were analyzed. Similar to the antibody-depletion treatment, the recovery of donor cells from IL-7−/− recipients was dramatically lower than that from IL-7Rα−/− recipients. In addition, the recovered donor cells expressed IL-7Rα, even in the absence of IL-7 (Fig. 3B), where receptor maintenance could be regulated by signals from an alternative ligand, TSLP [21]. These data indicate that in vitro differentiated aTreg cells indeed depend on IL-7 for their in vivo survival/maintenance. Both IL-7−/− and IL-7Rα−/− recipients have defective lymphocyte development resulting in lymphopenia, which can induce proliferation of transferred T cells. Therefore, in our model, we cannot rule out the possibility that the low recovery of donor aTreg cells from IL-7 deficient recipients was in part due to lower lymphopenic-induced proliferation in these mice. Significantly, since there is no known defect in IL-7 expression in T1D patients and NOD mice, the IL-7 dependency affords a distinct advantage for these cells in T1D therapy.
3.4. IL-10 is not required for aTreg cell survival but modulates their function
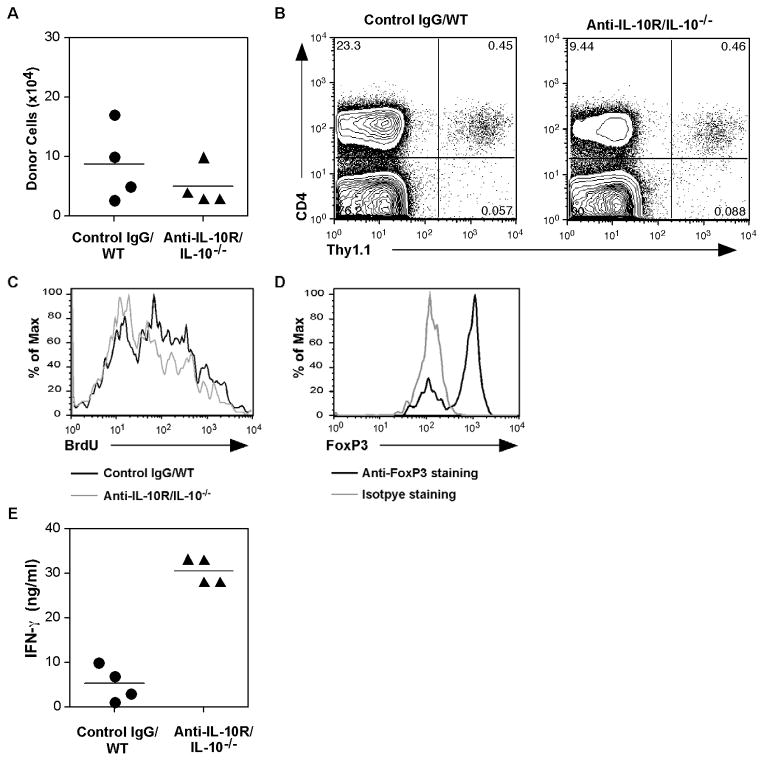
One effector cytokine that the aTreg cells produce is IL-10 (not shown and Fig. 2C). Our gene expression array analysis also showed IL-10R expression on these cells (unpublished data). A recent report showed that IL-10 was needed for FoxP3+ Treg cell maintenance and function in a colitis model [24], suggesting that IL-10 and IL-10R may form a positive feedback loop for survival and/or function of aTreg cells. To test this hypothesis, we differentiated aTreg cells from naïve BDC2.5 Thy1.1 CD4+ T cells and transferred the cells into NOD.IL-10−/− recipient mice, which were then treated with an anti-IL-10R neutralizing antibody. As a control, aTreg cells were transferred into wild type NOD mice that received control rat IgG. When the donor cells were analyzed two weeks later, no significant change was observed in their recovery from the peripheral lymphoid compartment (Fig. 4A) or in the pancreas (Fig. 4B), suggesting that blocking IL-10 does not result in an aTreg cell loss that would suggest an IL-10-dependence for survival. In addition, when the recipients were treated with BrdU to follow cell turnover, no significant difference in BrdU uptake was found in control versus IL-10-deficient recipients (Fig. 4C). In additional experiments in which we transferred aTreg cells into NOD.Scid recipients with anti-IL-10 or anti-IL-10R antibody treatments, we obtained similar results (not depicted). Importantly, FoxP3 expression was maintained on donor aTreg cells in NOD.IL-10−/− recipients that also received anti-IL-10R treatment (Fig. 4D).
Fig. 4. IL-10 blocking does not alter the survival of Treg cells.
BDC2.5 Thy1.1+ naïve CD4+ T cells were used to differentiate aTreg cells. The cells were injected into NOD or NOD.IL-10−/− mice (n = 4/group). The mice were then treated with control rat IgG or anti-IL-10R, 300μg/injection, 2 times/week for 2 weeks. One week after the start of the injections, the mice were treated with BrdU in the drinking water for 1 week. (A) The recovery of CD4+Thy1.1+ donor cells from the spleens was calculated (mean ± SEM). (B) The recovery of donor cells from the pooled pancreata of recipients treated with control IgG or anti-IL-10. (C) BrdU staining after gating on the donor populations shown in (B). (D) FoxP3 expression after gating on the donor populations shown in (B). (E) Recovered cells from the spleens were stimulated in vitro with a BDC2.5 TCR-specific peptide for 24 hours. IFN-γ secretion into culture supernatants was measured by Luminex cytokine assay (Mean + SD, 4 replicate cultures).
These results indicate that IL-10 is not required for aTreg cell homeostasis in adoptive transfer recipients. However, when the recovered cells were restimulated in culture with a BDC2.5 TCR-specific mimetope peptide, aTreg cells recovered from NOD.IL-10−/− recipients produced a much higher amount of IFN-γ than cells recovered from control recipients (Fig. 4E). These latter results suggest that IL-10 can modulate cytokine expression of aTreg cells. However, since the results were from in vitro stimulation, it is not clear whether the cells could produce IFN-γ or other proinflammatory cytokines in vivo under IL-10 blocking conditions.
3.5. TGF-β is needed for local response in the pancreas and protection from diabetes by aTreg cells
Since TGF-β is a critical cytokine for the induction of FoxP3+ aTregs in vitro and is essential for nTreg cell homeostasis [25, 26], we asked if TGF-β was required for aTreg cell maintenance and function. We first tested whether TGF-β was needed to maintain FoxP3 expression in aTreg cells in vitro. Thus, differentiated aTreg cells were rested for 7 days in medium containing rIL-7, with or without neutralizing anti-TGF-β antibody. As shown in Fig. 5A, blocking TGF-β did not reduce FoxP3 expression in induced aTreg cells in vitro. Next, induced aTreg cells were transferred into NOD recipient mice that were then treated with neutralizing anti-TGF-β antibody or control rat IgG. Two weeks after cells transfer, the recovery of donor cells from different organs of recipient mice was analyzed. As shown in Fig. 5B, aTreg recovery from the peripheral lymphoid compartment of anti-TGF-β treated or control IgG treated recipients was not significantly different, suggesting that TGF-β is not critical for aTreg cell homeostasis and survival. However, when we analyzed aTreg cell recovery from the pancreas, we found reduced numbers of donor cells in the anti-TGF-β treated hosts (Fig. 5C). Furthermore, when aTreg cell turnover in hosts was tested by BrdU incorporation, cells recovered from anti-TGF-β treated hosts showed fewer proliferating cells when compared to the controls (Fig. 5D), suggesting a role for TGF-β in supporting a local immune response by aTregs, which could indicate a requirement for migration, local survival, and/or expansion. When the recovered cells were tested for cytokine production upon restimulation in culture, cells from anti-TGF-β treated recipients did not produce a significantly increased amount of IFN-γ (Fig. 5F) and the recovered donor cells also maintained their FoxP3 expression (Fig. 5E). These data suggest that TGF-β is not needed for regulation of the T1D effector cytokine, IFN-γ, or for the maintenance of FoxP3 by aTregs.
To further explore the role of TGF-β in the regulatory function of aTreg cells in vivo, total splenocytes from diabetic mice along with in vitro differentiated aTreg cells were transferred into prediabetic NOD recipients. The recipients were treated with anti-TGF-β neutralizing antibody or control IgG for 1 month, and blood glucose levels were monitored. Indeed, the anti-TGF-β treatment abolished protection of aTreg cells against pathogenic cells from diabetic mice (Fig. 5G). These results suggest that although TGF-β may not be essential for aTreg cell survival, it is required either directly or indirectly for these cells to mediate their regulatory function.
3.6. Integrin-β7 is needed for aTreg cell localization in target organs
Our data demonstrate that in vitro differentiated aTreg cells can control T1D and thereafter provide long-term protection in adoptive recipients. Our previous studies suggested that these effects may be in part due to apoptosis of pathogenic CD4+ T cells triggered by aTreg cells in the pancreatic LN [13]. However, we have not yet addressed whether protection requires the migration and maintenance of aTreg cells in the pancreas. To address this question, we first examined whether aTregs have the capacity to infiltrate islets. aTregs were induced from naïve CD4+ T cells from BDC2.5 Thy1.1+ donors and injected into NOD.Scid recipients. One week later, the pancreata were analyzed for the presence of donor aTregs. As shown in Fig. 6A, we observed aTregs that were infiltrating islets in a polarized fashion adjacent to insulin-staining β-cells (left panel) and aTregs that were distributed within islets (right panel). Although the majority of islets were associated with infiltrating aTregs, we also observed islets with no infiltrates. The data demonstrate that aTregs can enter the islets even when there is no preexisting autoimmune response.
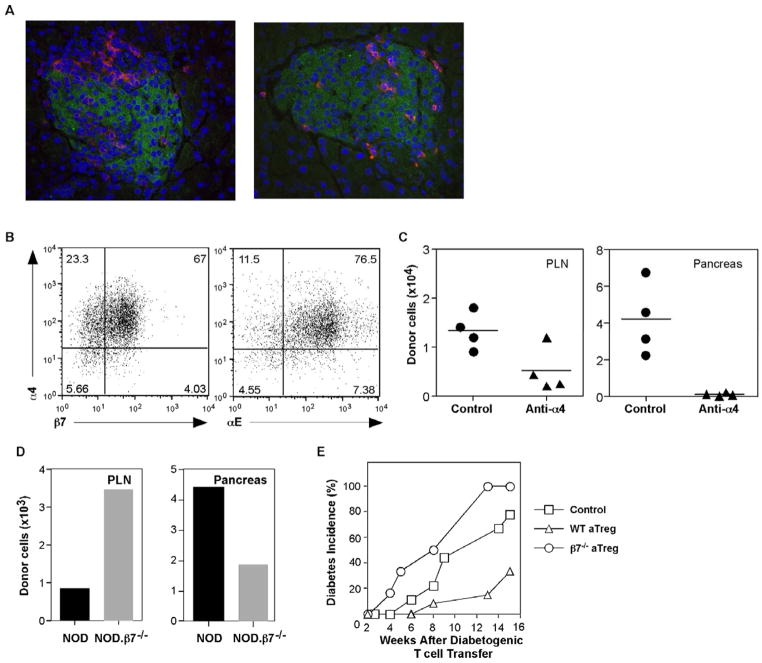
Fig. 6. Integrin-α4β7 dependent localization in the pancreas is required for protection by aTreg cells.
(A) NOD.Scid mice were injected with Thy1.1+ BDC2.5 aTreg cells. Ten days after cell transfer, pancreata from recipients were analyzed for islet localization of transferred aTreg cells by staining with Thy1.1-(red) and insulin-(green) specific antibodies. Representative images were shown at a magnification of 40X. (B) BDC2.5 Thy1.1+ aTreg cells were tested at the time of harvest for expression of the integrin chains, α4, αE, and β7. (C) NOD.Scid mice were injected with Thy1.1+ BDC2.5 aTreg cells and treated at the time of cell transfer with either anti-integrin-α4 antibody or control IgG in a dose of 200μg/recipient (n = 4/group). The mice were treated again on day 3, and the tissues were harvested and analyzed on day 7 for the recovery of CD4+Thy1.1+ donor cells (mean ± SEM). (D) Polyclonal aTreg cells were differentiated from CD4+CD25− cells from NOD and NOD.β7−/− mice and injected into NOD.Scid recipients. Two months later, the indicated tissues were harvested, pooled, and analyzed for the presence of CD4+Thy1.1+ donor cells. (E) Young prediabetic NOD mice were injected with splenic cells from diabetic donors. Two weeks later, these mice were given polyclonal aTreg cells differentiated from WT NOD (n = 12), NOD.integrin-β7−/− (n = 6), or were not treated with aTreg cells (control, n = 9). Blood glucose levels and diabetes incidence were monitored weekly.
To further study the requirement for localization of aTregs in the pancreas for their protective function in the context of an ongoing autoimmune response, we analyzed aTregs for adhesion receptors that have been implicated in T1D. It has been shown previously that the expression of the mucosal addressin cell adhesion molecule, MAdCAM-1, is highly upregulated in inflamed islets in NOD mice [27, 28]. MAdCAM-1 is the ligand for integrin-α4β7, which is expressed on a subset of effector T cells. Furthermore, both integrin-α4 and β7 are important for infiltration of diabetogenic T cells into islets [29, 30]. Since the in vitro differentiated aTreg cells display effector memory phenotype, we hypothesized that these cells may use similar mechanisms to migrate into the pancreatic islets. To test this, we first analyzed integrin expression on a Treg cells. Indeed, the majority of in vitro induced Treg cells expressed both α4 and β7 integrin subunits (Fig. 6B). Interestingly, most integrin-α4 expressing cells also co-expressed integrin-αE (Fig. 6B), a marker of a subset of nTreg cells with effector/memory phenotype [31]. This expression pattern was not changed after a 5-day in vitro resting period (not depicted). To test if either the α4 or β7 integrin subunits are required for aTreg cells to localize in the pancreas, in vitro differentiated aTreg cells were transferred into NOD.Scid recipient mice that were then treated with anti-integrin-α4 blocking antibody or isotype IgG control. Seven days after cell transfer, donor cells were analyzed in the pancreatic LN (PLN) and pancreata of recipients. As shown in Fig. 6C, antibody treatment significantly reduced donor cell recovery from the PLN. Moreover, very few donor cells could be recovered from the pancreas after antibody treatment. These results indicate that integrin-α4 is needed for pancreatic homing of aTreg cells.
To assess a potential role for integrin-β7 in the regulation of homing, aTreg cells were differentiated from naïve CD4+ T cells from NOD integrin-β7−/− donor mice and transferred into NOD.Scid recipients. As a control, WT NOD aTreg cells were transferred into another group of NOD.Scid recipients. As shown in Fig. 6D, the recovery of integrin-β7 deficient donor cells from the pancreata of the recipients was significantly reduced compared to the recovery of the WT control aTreg cells. However, the recovery of integrin-β7 deficient donor cells in the PLN was slightly increased than that of WT controls (Fig. 6C). These results suggest that integrin-β7 is also important to pancreatic localization of aTreg cells, which may derive from the PLN.
To distinguish if localization in the pancreas is necessary for the protective function of aTreg cells, we tested whether integrin-β7−/− aTregs could prevent onset of diabetes. For these experiments, total splenocytes from diabetic NOD mice were transferred into a group of young normoglycemic NOD mice to accelerate the development of diabetes. Two weeks later, these mice received integrin-β7−/− or WT NOD aTreg cells, and were monitored for blood glucose levels. The integrin-β7 deficient aTreg cells were not able to protect recipients from diabetes induced by diabetogenic splenocytes (Fig. 6E). Overall, these data support the conclusion that aTreg cells use integrin-α4β7 to migrate into the pancreas, and that they exert their protective function(s) against T1D in the local microenvironment of the islets.
4. Discussion
We have previously reported that in vitro differentiated aTreg cells can prevent development of T1D and can also reverse diabetes after its onset in the NOD mouse model. We have shown that both islet antigen-specific (BDC2.5 TCR transgenic) and polyclonal (wild type NOD) CD4+ T cells function similarly after aTreg induction [13, 16]. In this study, we investigated regulation of the homeostasis and function of these cells in adoptive hosts. We show here that aTreg cells induced by anti-CD3 stimulation in the presence of IL-2 and TGF-β express high levels of IL-7Rα without CD25 after adoptive transfer and depend on IL-7 for maintenance in vivo. In this regard, aTreg cells are regulated similarly to typical memory CD4 T cells [19]. Moreover, the results demonstrate that aTreg cells are regulated by mechanisms that are distinct from nTreg cells, which are IL-7Rαlow and CD25hi, and are completely dependent on IL-2 for their homeostasis as well as the maintenance of FoxP3 and regulatory function [32].
It is noteworthy that treatment with aTreg cells does not reverse diabetes in all recipients. For instance, some recipients did not respond to the treatment and some recipients that had initial decreases in blood glucose levels reverted to hyperglycemia. These results may, at least partially, reflect a temporal requirement for the treatment with respect to the status of disease progression. It is possible that only with earlier diabetes onset can the control of the autoimmune response by transferred aTregs lead to β-cell mass recovery by regranulation, proliferation, or possibly neogenesis [33]. In recipients that display a longer time course to development of diabetes, the extent of remaining β-cell mass or function may be less and/or the potential for regeneration could be lower such that recovery does not occur. Another possibility is that pathogenic effector T cells in some recipients may not respond as well to transferred Treg cells when the development of diabetes is protracted. While this aspect of our results will require further investigation, it is important clinically since β-cell proliferation has been shown in pancreata from recent T1D onset human patients [34]. Thus, further studies will be needed to determine optimal timing of aTreg treatment with respect to recovery of β-cell mass.
The aTreg cells generated in this study do not require either IL-10 or TGF-β for their maintenance or FoxP3 expression. However, their regulatory function requires TGF-β as well as localization in the pancreas. TGF-β was required for response in this site, which could support integrin-dependent migration in addition to local cell survival and or/ expansion. nTreg cells ultimately lose the ability to control the autoimmune response in T1D, and because of a defective IL-2 response, they can convert to pathogenic CD4+ T effector cells in the pancreas [8, 35]. The apparent stability of aTreg cells described herein even under conditions of lymphopenia-driven expansion suggests that they fulfill criteria necessary for the induction and maintenance of tolerance that will ultimately be required for the success of treatments to restore islet β-cells.
The aTreg cells differentiated in vitro by our protocol exhibit similarities to nTreg cells with respect to FoxP3 expression, production of the effector cytokines TGF-β as well as IL-10, and, as shown herein, their ability to migrate into the pancreas and control diabetes by a local response. However, they display additional distinct attributes as well. In contrast to nTreg cells and Treg cells induced with TGF-β that were reported by other groups [36], we have shown that these cells do not display an anergic phenotype and produce IL-2 [13]. Thus, one mechanism by which they could contribute to the restoration and maintenance of tolerance is to sustain the expression of FoxP3 and regulatory function by local nTreg cells and inhibit their conversion to pathogenic cells.
Although there is considerable controversy over the stability of FoxP3+CD25+ nTreg cells, in T1D after downregulation of FoxP3, nTreg cells can transfer diabetes to nondiabetic recipients [8]. Moreover, we have observed a loss of FoxP3 by nTreg cells when sorted GFP+ cells from FoxP3-GFP NOD reporter mice were transferred into diabetic recipients (unpublished data). This “reprogramming” of nTreg could contribute to the pathogenesis in T1D, even though this outcome may normally represent a beneficial response in host defense [7, 37]. As we have shown here and previously, in vitro differentiated aTreg cells are maintained as a stable memory population in adoptive hosts for extended periods of time, indicating that aTreg cells and perhaps other CD4+ T cell subsets that do not express FoxP3, such as the IL-10- producing Tr-1 cells and the TGF-β-producing TH3 cells, as well as some CD8+ T cells, which can provide regulatory functions in T1D [38], should be considered for use as cell-based therapies for autoimmune disorders in which nTreg cells are defective.
A unique feature of the homeostatic regulation of the aTreg cells used in our studies is the reciprocal expression pattern of CD25 (IL-2Rα) versus CD127 (IL-7Rα). Upon activation/ differentiation, the naïve CD4+ T cells are induced to express high levels of CD25, consistent with many other reports [36, 39, 40], and downregulate IL-7Rα. Similar to other subsets of in vitro generated CD4+ T effectors, withdrawal of TCR stimulation and cytokines is sufficient to initiate the transition to the CD25− and IL-7Rαhigh phenotype that characterizes memory cells [41], which we showed are IL-7 dependent for their persistence [19]. Since aTreg cells develop into memory cells upon transfer, and lymphopenia-induced homeostatic proliferation of memory CD4+ T cells does not depend on IL-7 [22, 23], our results most likely reflect dependence of aTreg cells on IL-7 for survival. However, an effect of IL-7 on lymphopenia driven turnover cannot be ruled out. Irrespective of the mechanism, the results confirm that the expression of IL-7Rα on aTreg cells engenders IL-7-dependence for their maintenance in adoptive hosts. We do not know CD25 downregulation could have occurred in other studies of aTregs generated in vitro with TGF-β as few publications have reported CD25 expression levels after adoptive transfer. Nonetheless, this expression pattern is of functional significance since the high levels of surface IL-7Rα allows for aTreg homeostasis as memory CD4+ T cells in the absence of strong IL-2 signals, although IL-2 is needed for their differentiation in vitro to overcome the anti-proliferative effects of TGF-β. IL-7, in addition to IL-2, participates in nTreg thymic development [42, 43], but it is dispensable for their peripheral maintenance [21, 44, 45]. Thus, it is possible that IL-7 is sufficient to maintain FoxP3 in the aTreg cells generated in our model.
Although the mechanism by which CD25 is lost on aTregs generated in our studies is not clear, the ability to downregulate its expression may be a consequence of the conditions we use with strong anti-CD3 stimulation and minimal anti-CD28 costimulation. CD28 signaling has been reported to inhibit aTreg differentiation, most likely through the PI3K-mTOR pathway [14, 46]. In a separate study, using low anti-CD3 stimulation and strong anti-CD28 costimulation, we were able to generate aTreg cells with high CD25 expression, low IL-7Rα expression, and an anergic phenotype, similar to nTreg cells immediately ex vivo. However, the cell yield with that protocol was much lower, and a majority of cells lost FoxP3 expression after transfer into NOD.Scid recipients (unpublished observations). One possible implication of these results is that high levels of TCR stimulation in the presence of TGF-β drives naïve CD4+ T cells to a more differentiated and stable effector state.
In our effort to determine if a local response in the pancreas was necessary for aTreg cell function, we examined the expression of adhesion receptors that mediate cell migration. Consistent with the finding that TGF-β can directly upregulate the expression of integrin-β7 [47], we found that aTreg cells express integrin-α4β7, and that this integrin is necessary for trafficking of aTreg cells into the pancreas. This requirement is consistent with the increased expression levels of MAdCAM-1, the ligand for integrin-α4β7, in inflamed islets in NOD mice, which also drives the migration of pathogenic effector T cells into islets [27–30, 48]. The integrin-β7 deficient mice allowed us to determine that, although aTreg cells can control pathogenic effector responses in the draining PLN [13], their local response in the pancreas, which depends upon the availability of TGF-β, is essential for protection. It is noteworthy that aTreg cells also express integrin-αE (CD103), which is regulated by TGF-β and can also pair with integrin3 β7 [49]. On nTregs, CD103 marks an effector/memory population [31, 50], but adhesion mechanisms governing their migration into the pancreas have not been reported. Although CD103 was shown to be unnecessary for the protective functions of nTregs in an inflammatory bowel disease model [51] or for the migration of T cells from blood into tissue [52], integrin-αEβ7 could play an important role in retaining aTreg cells in pancreatic islets, which express the ligand E-cadherin [53]. Taken together with our observation that aTreg cells are CD62L−, we conclude that they maintain an effector memory phenotype indefinitely and control pathogenic T cells within the islets where they are likely to be engaged by islet antigens to mediate their effector function [54].
Although the physiological relevance of aTreg cells that can be generated in normal individuals has been questioned [4, 14] and the mechanisms that control their development, maintenance, and functions in vivo have not been elucidated, aTreg cells differentiated from naïve CD4+ T cells in vitro have promising therapeutic potential to confer long-term tolerance for autoimmune disorders and transplantation [55]. Our finding that such cells can be regulated and function as bona fide protective memory CD4+ T cells that can be maintained by IL-7 in the context of T1D brings further impetus for clinical translation of these cells to control the autoimmune response which reemerges after pancreas transplantation [56] and which will confound efforts to achieve long-term restoration of β-cells by differentiation, expansion, or transplantation.
Although ex vivo expanded nTreg cells can control autoimmune responses in the NOD model with remarkable success [5, 39], it is impossible to unequivocally isolate cells with regulatory function from human peripheral blood on the basis of the currently used markers, high levels of CD25 combined with low levels of IL-7Rα expression on CD4+ T cells, as these are shared by effector cells [57, 58]. Moreover, nTreg cells must be greatly expanded and repetitive in vitro stimulation of human nTreg cells can result in the loss of FoxP3 expression [6]. The ability to use the much more numerous naïve CD4+ T cell population to generate aTreg cells, combined with their stable phenotype, and the apparent lack of dependence on IL-2 for homeostatic maintenance as inferred from the absence of CD25 on persisting aTreg memory cells, indicates that they may have significant potential for treatment of T1D. This is particularly true because of the genetic polymorphisms that result in suboptimal IL-2 signaling in both human T1D patients and NOD mice [11, 12]. Indeed, nTreg cells from T1D patients downregulate FOXP3 levels due to defective IL-2R signaling [59]. It is important to note that FOXP3+ cells with in vitro regulatory function have been differentiated from naïve CD4+ T cells from T1D patients [15]. Furthermore, it was possible to use candidate autoantigens associated with particular HLA-DR haplotypes for the selection and expansion of antigen-specific regulatory T cells, either FOXP3+ or FOXP3−, in vitro [15, 60]. On the basis of our findings, further studies on aTreg cells generated from T1D patients are warranted.
Acknowledgments
The authors would like to thank Jennifer Nguyen and Jed Bassein for technical assistance, and Yoav Altman and Amy Cortez for cell sorting. This study was supported by grants from NIH (AI081238) and from Juvenile Diabetes Research Foundation (JDRF) (31-2008-353) to LMB.
Abbreviations
- T1D
Type 1 diabetes mellitus
- NOD
Non-Obese Diabetic
- PLN
Pancreatic lymph node
- B6
C57BL/6J
- GAD
glutamic acid decarboxylase
- IA
insolenoma antigen
- ZnT8
islet zinc transporter
- Treg
Regulatory T cells
- nTreg
Naturally arising (occurring) Treg cells
- aTreg
Adaptive (induced) Treg cells
- γc
common γ chain
Footnotes
Disclosure:
The authors claim no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stadinski B, Kappler J, Eisenbarth GS. Molecular targeting of islet autoantigens. Immunity. 2010;32:446–56. doi: 10.1016/j.immuni.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 4.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–25. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–97. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 7.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–86. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dendrou CA, Wicker LS. The IL-2/CD25 pathway determines susceptibility to T1D in humans and NOD mice. J Clin Immunol. 2008;28:685–96. doi: 10.1007/s10875-008-9237-9. [DOI] [PubMed] [Google Scholar]
- 12.Wicker LS, Clark J, Fraser HI, Garner VE, Gonzalez-Munoz A, Healy B, et al. Type 1 diabetes genes and pathways shared by humans and NOD mice. J Autoimmun. 2005;25 (Suppl):29–33. doi: 10.1016/j.jaut.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Weber SE, Harbertson J, Godebu E, Mros GA, Padrick RC, Carson BD, et al. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J Immunol. 2006;176:4730–9. doi: 10.4049/jimmunol.176.8.4730. [DOI] [PubMed] [Google Scholar]
- 14.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–95. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 15.Long SA, Walker MR, Rieck M, James E, Kwok WW, Sanda S, et al. Functional islet-specific Treg can be generated from CD4+CD25- T cells of healthy and type 1 diabetic subjects. Eur J Immunol. 2009;39:612–20. doi: 10.1002/eji.200838819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godebu E, Summers-Torres D, Lin MM, Baaten BJ, Bradley LM. Polyclonal adaptive regulatory CD4 cells that can reverse type I diabetes become oligoclonal long-term protective memory cells. J Immunol. 2008;181:1798–805. doi: 10.4049/jimmunol.181.3.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–35. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2. 5 nonobese diabetic mice. J Immunol. 2001;166:908–17. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 19.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koenecke C, Czeloth N, Bubke A, Schmitz S, Kissenpfennig A, Malissen B, et al. Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur J Immunol. 2009;39:3091–6. doi: 10.1002/eji.200939432. [DOI] [PubMed] [Google Scholar]
- 21.Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, et al. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008;112:3283–92. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–32. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Leeuwen EM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T Cells. Curr Opin Immunol. 2009;21:167–72. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–84. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan YY, Flavell RA. Regulatory T cells, transforming growth factor-beta, and immune suppression. Proc Am Thorac Soc. 2007;4:271–6. doi: 10.1513/pats.200701-020AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanninen A, Taylor C, Streeter PR, Stark LS, Sarte JM, Shizuru JA, et al. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Invest. 1993;92:2509–15. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faveeuw C, Gagnerault MC, Lepault F. Expression of homing and adhesion molecules in infiltrated islets of Langerhans and salivary glands of nonobese diabetic mice. J Immunol. 1994;152:5969–78. [PubMed] [Google Scholar]
- 29.Yang XD, Michie SA, Tisch R, Karin N, Steinman L, McDevitt HO. A predominant role of integrin alpha 4 in the spontaneous development of autoimmune diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 1994;91:12604–8. doi: 10.1073/pnas.91.26.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XD, Sytwu HK, McDevitt HO, Michie SA. Involvement of beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in obese diabetic mice. Diabetes. 1997;46:1542–7. doi: 10.2337/diacare.46.10.1542. [DOI] [PubMed] [Google Scholar]
- 31.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–13. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–35. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Chung CH, Levine F. Adult pancreatic alpha-cells: a new source of cells for beta-cell regeneration. Rev Diabet Stud. 2010;7:124–31. doi: 10.1900/RDS.2010.7.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Evidence of increased islet cell proliferation in patients with recent-onset type 1 diabetes. Diabetologia. 2010;53:2020–8. doi: 10.1007/s00125-010-1817-6. [DOI] [PubMed] [Google Scholar]
- 35.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–97. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, et al. Reprogrammed Foxp3(+) Regulatory T Cells Provide Essential Help to Support Cross-presentation and CD8(+) T Cell Priming in Naive Mice. Immunity. 2010;33:942–54. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ablamunits V, Bisikirska BC, Herold KC. Human regulatory CD8 T cells. Ann N Y Acad Sci. 2008;1150:234–8. doi: 10.1196/annals.1447.000. [DOI] [PubMed] [Google Scholar]
- 39.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–77. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo X, Tarbell KV, Yang H, Pothoven K, Bailey SL, Ding R, et al. Dendritic cells with TGF-beta1 differentiate naive CD4+CD25- T cells into islet-protective Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:2821–6. doi: 10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harbertson J, Biederman E, Bennett KE, Kondrack RM, Bradley LM. Withdrawal of stimulation may initiate the transition of effector to memory CD4 cells. J Immunol. 2002;168:1095–102. doi: 10.4049/jimmunol.168.3.1095. [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 44.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–34. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–90. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merkenschlager M, von Boehmer H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J Exp Med. 2010;207:1347–50. doi: 10.1084/jem.20101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim SP, Leung E, Krissansen GW. The beta7 integrin gene (Itgb-7) promoter is responsive to TGF-beta1: defining control regions. Immunogenetics. 1998;48:184–95. doi: 10.1007/s002510050422. [DOI] [PubMed] [Google Scholar]
- 48.Hanninen A, Salmi M, Simell O, Jalkanen S. Endothelial cell-binding properties of lymphocytes infiltrated into human diabetic pancreas. Implications for pathogenesis of IDDM. Diabetes. 1993;42:1656–62. doi: 10.2337/diab.42.11.1656. [DOI] [PubMed] [Google Scholar]
- 49.Hadley GA, Rostapshova EA, Gomolka DM, Taylor BM, Bartlett ST, Drachenberg CI, et al. Regulation of the epithelial cell-specific integrin, CD103, by human CD8+ cytolytic T lymphocytes. Transplantation. 1999;67:1418–25. doi: 10.1097/00007890-199906150-00005. [DOI] [PubMed] [Google Scholar]
- 50.Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A, et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–61. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng Y, Wang D, Yuan R, Parker CM, Farber DL, Hadley GA. CD103 expression is required for destruction of pancreatic islet allografts by CD8(+) T cells. J Exp Med. 2002;196:877–86. doi: 10.1084/jem.20020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kilshaw PJ, Higgins JM. Alpha E: no more rejection? J Exp Med. 2002;196:873–5. doi: 10.1084/jem.20021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tonkin DR, He J, Barbour G, Haskins K. Regulatory T cells prevent transfer of type 1 diabetes in NOD mice only when their antigen is present in vivo. J Immunol. 2008;181:4516–22. doi: 10.4049/jimmunol.181.7.4516. [DOI] [PubMed] [Google Scholar]
- 55.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–65. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59:947–57. doi: 10.2337/db09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010;59:407–15. doi: 10.2337/db09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dromey JA, Lee BH, Yu H, Young HE, Thearle DJ, Jensen KP, et al. Generation and expansion of regulatory human CD4(+) T-cell clones specific for pancreatic islet autoantigens. J Autoimmun. 2011;36:47–55. doi: 10.1016/j.jaut.2010.10.005. [DOI] [PubMed] [Google Scholar]