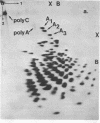
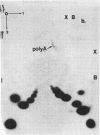
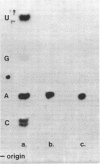
Abstract
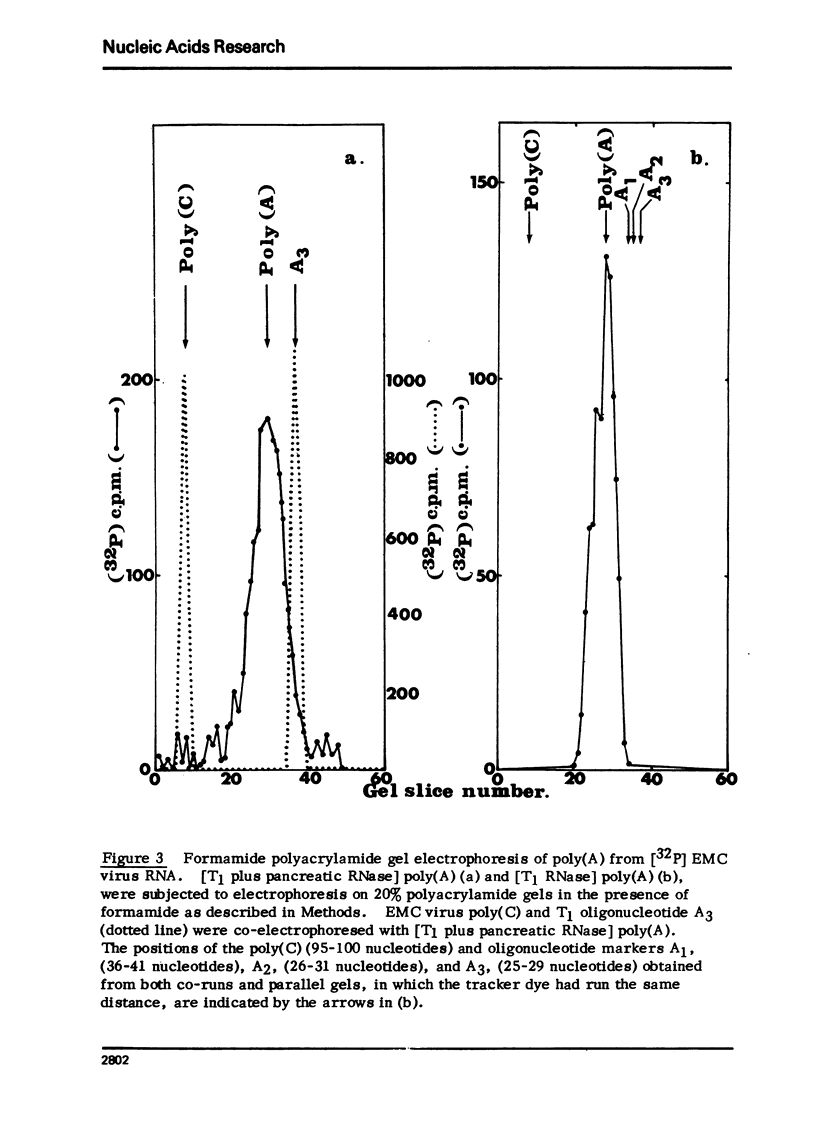
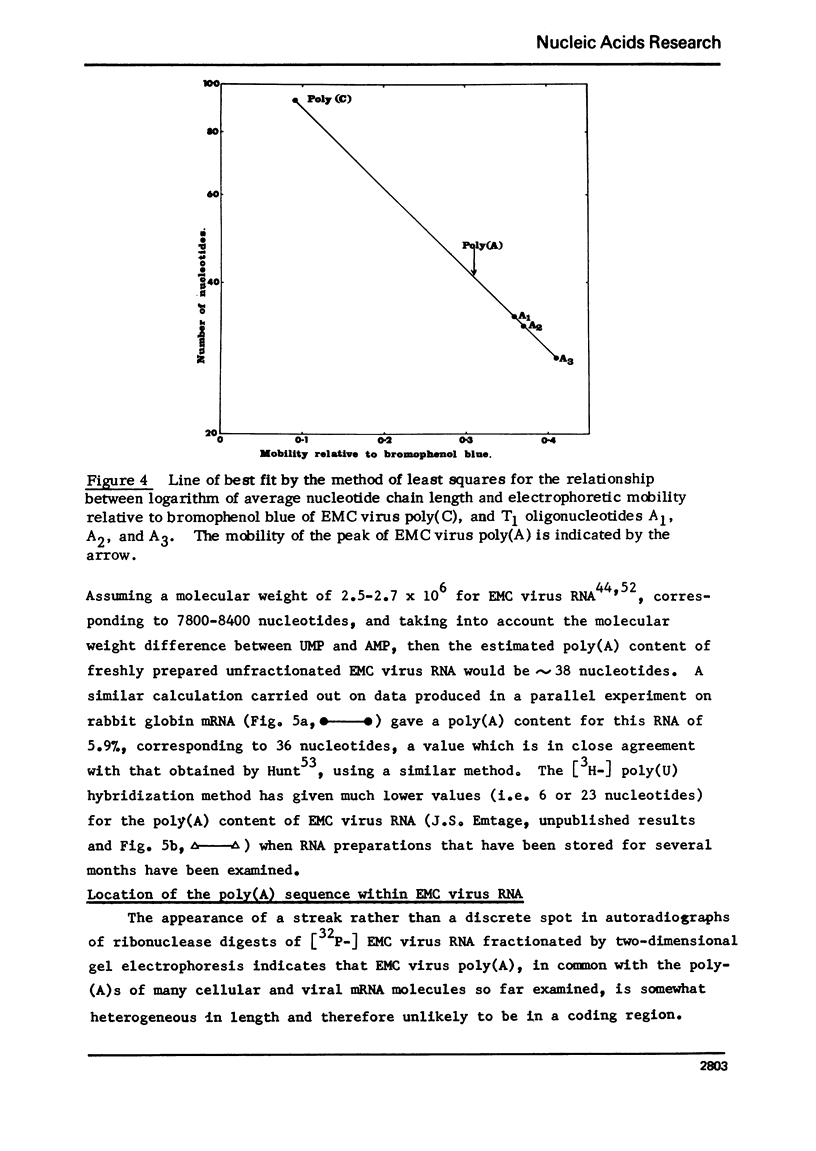
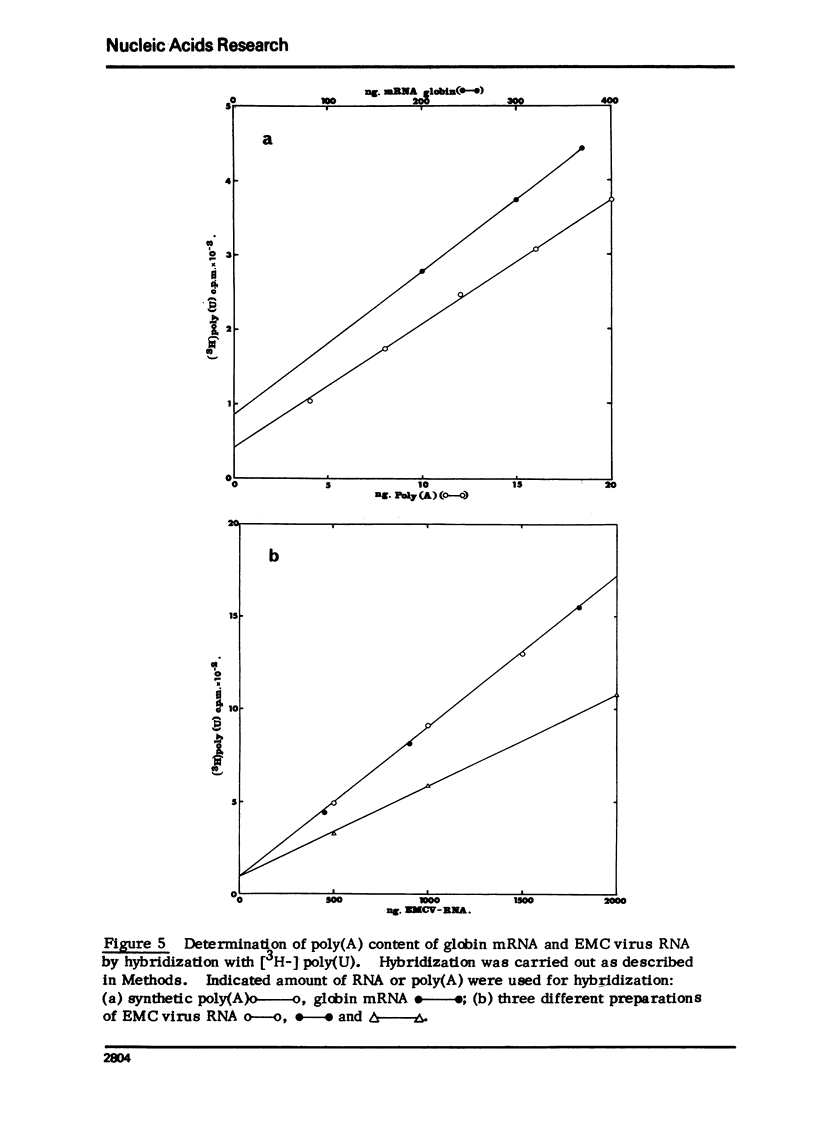
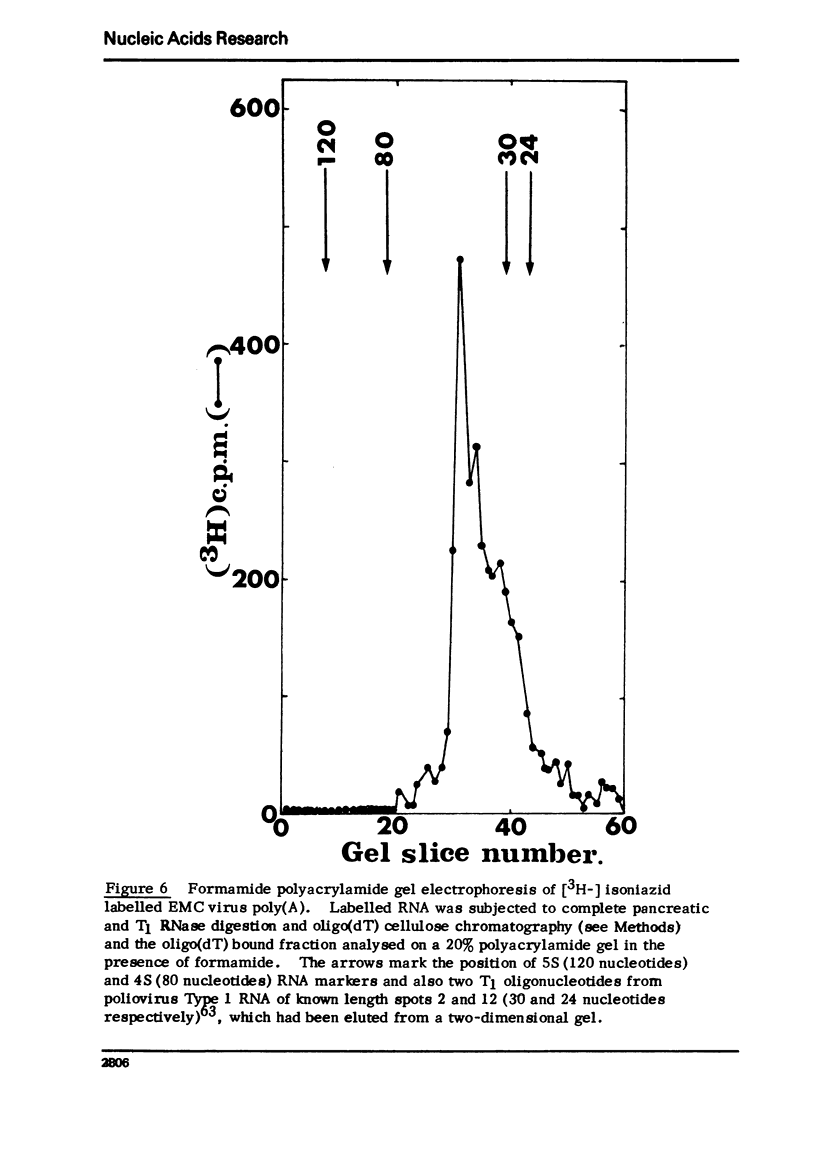
Encephalomyocarditis (EMC) virus RNA contains a covalently bound sequence of polyriboadenylic acid (poly(A). This was determined by two-dimensional gel electrophoresis of complete T1 and pancreatic RNase digests of formamidesucrose gradient-purified RNA and subsequent analysis of the product by alkaline hydrolysis. The size of the EMC virus genomic poly(A) sequence was estimated by formamide-polyacrylamide gel electrophoresis of the RNase-resistant product, or by [3H-]poly(U) hybridization to freshly purified virion RNA, to be, on average, 40 nucleotides in length. The evidence obtained from [3H-]isoniazid labelling and other experiments would indicate that the poly(A) sequence is located at the 3'-terminus of EMC virus RNA.
Full text
PDF





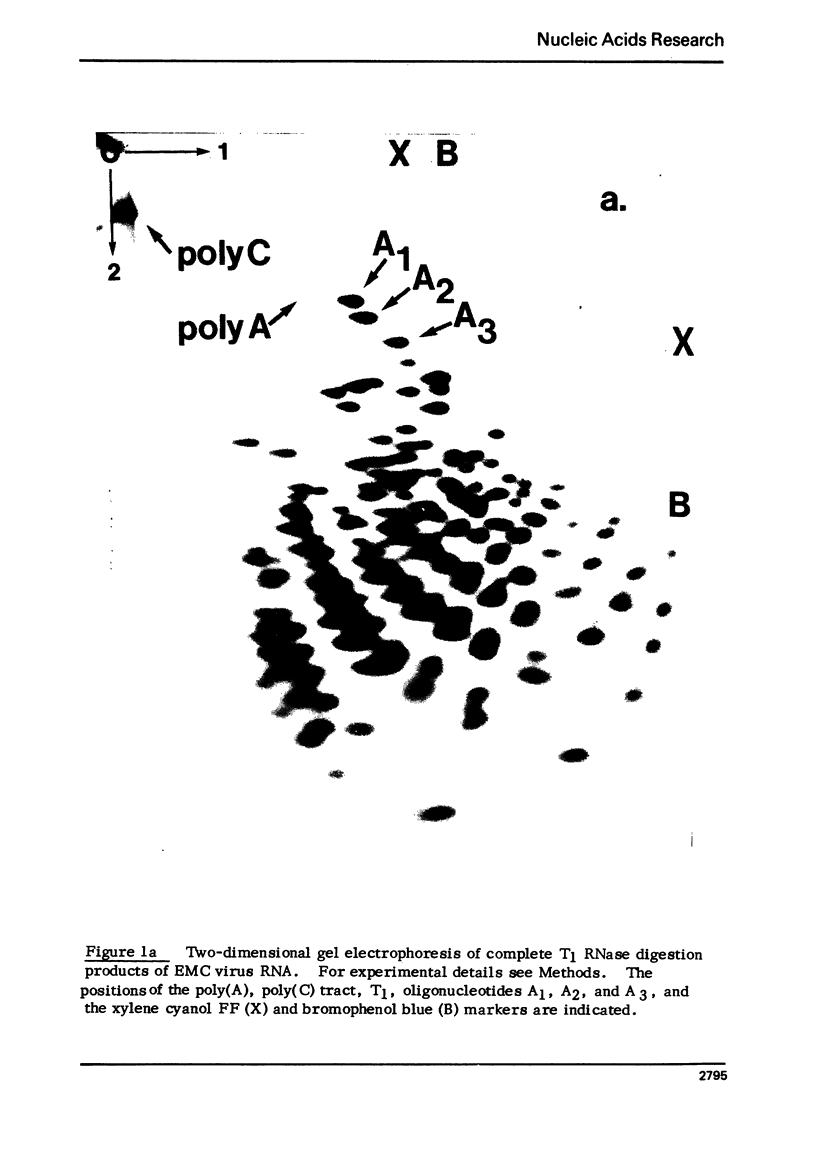
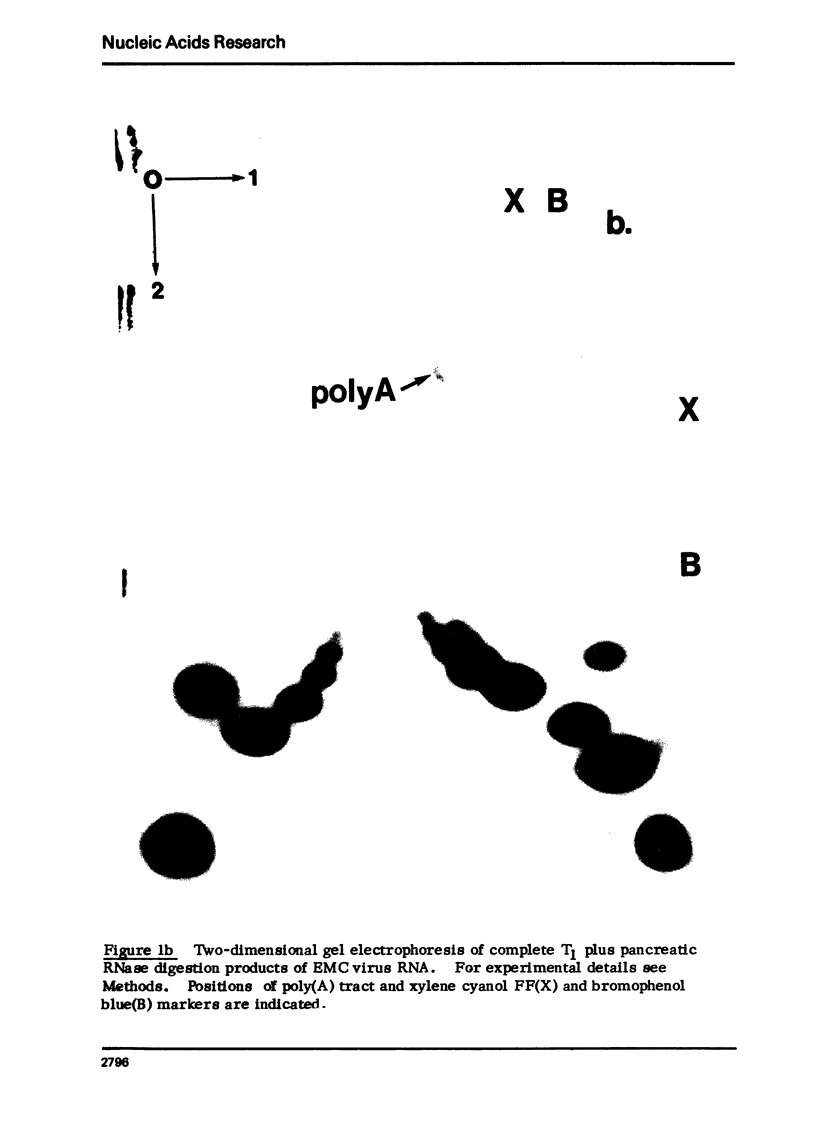
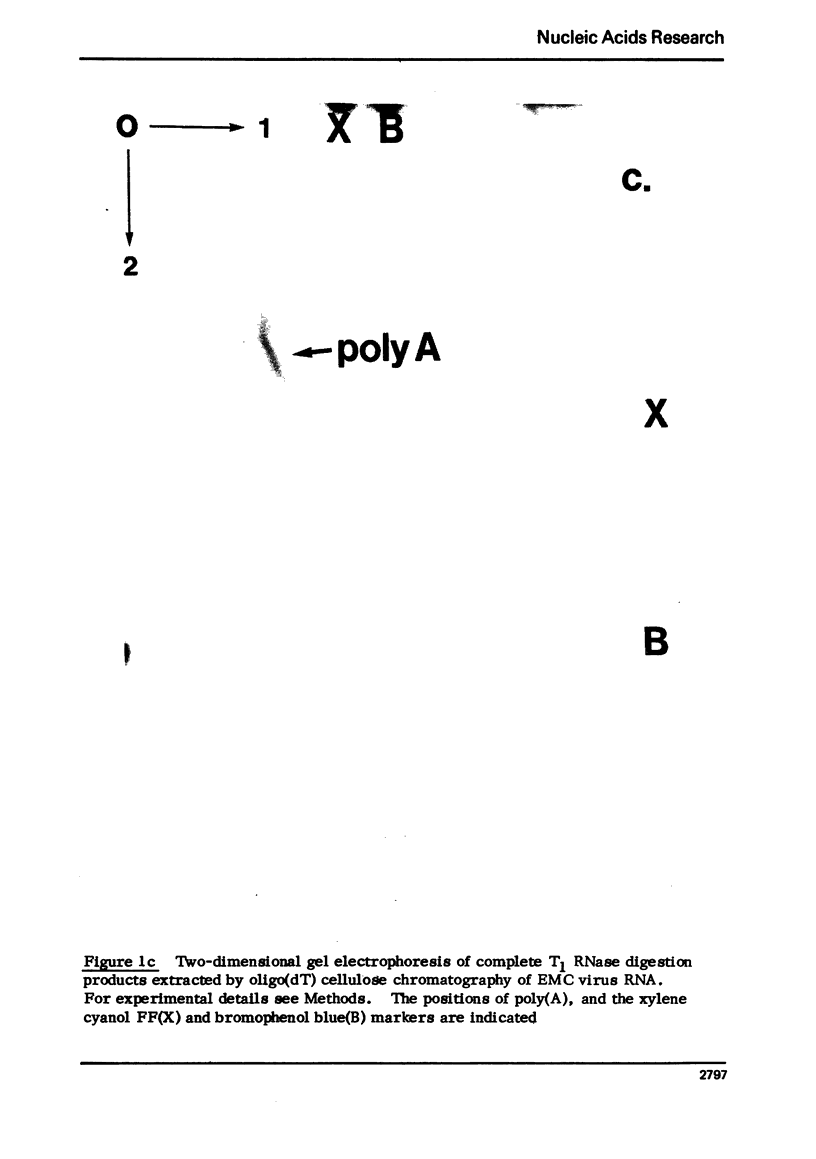

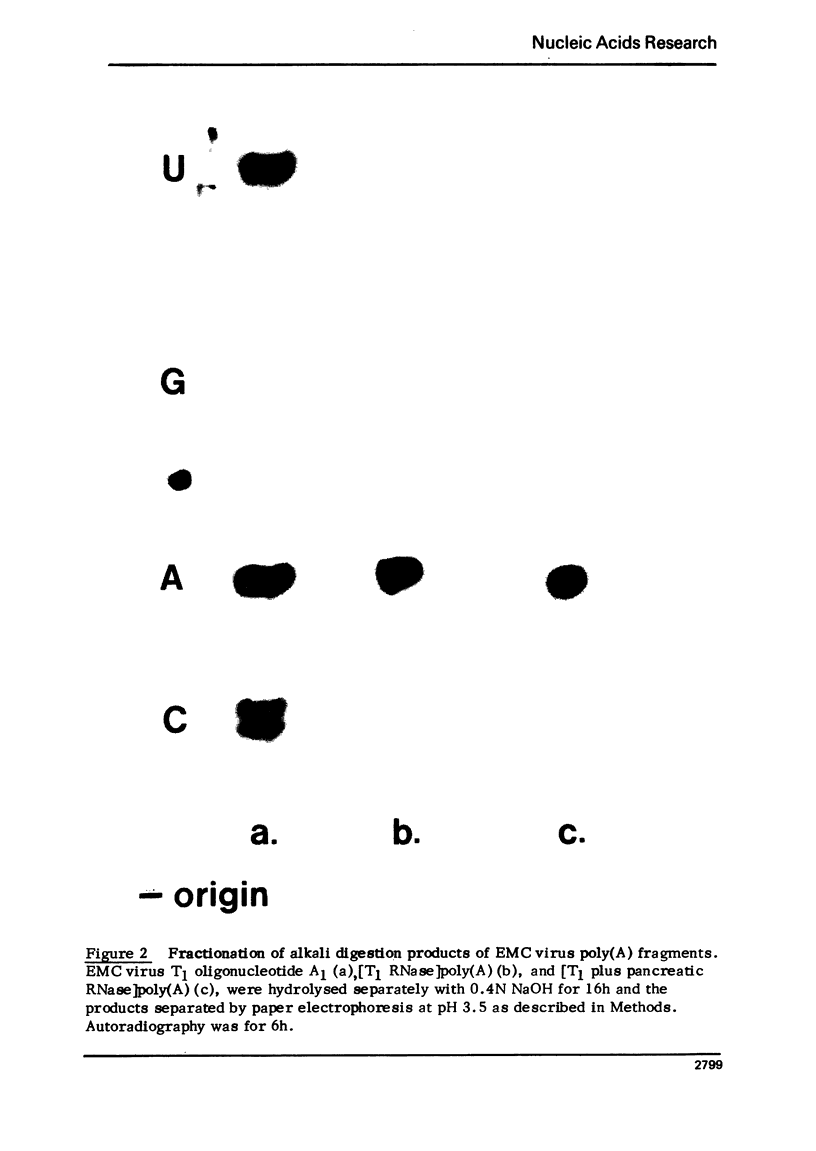
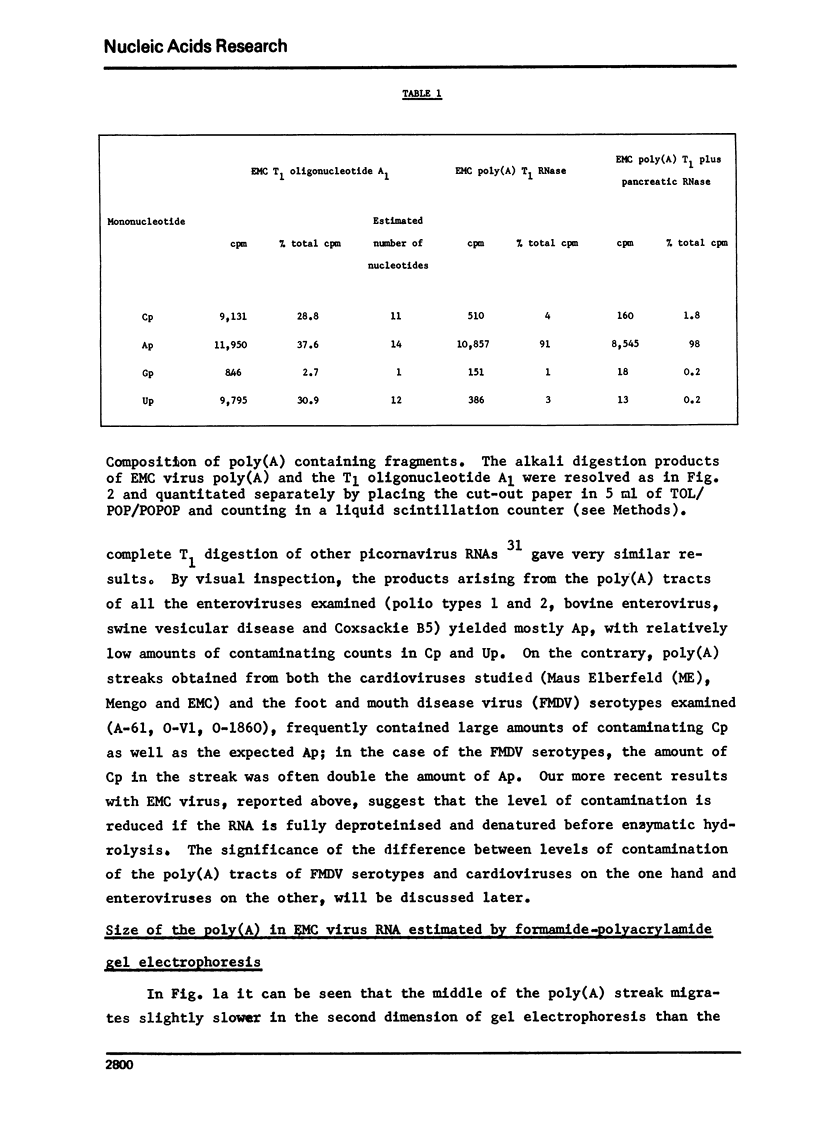






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Edmonds M., Nakazato H., Phillips B. A., Vaughn M. H. Polyadenylic acid sequences in the virion RNA of poliovirus and Eastern Equine Encephalitis virus. Science. 1972 May 5;176(4034):526–528. doi: 10.1126/science.176.4034.526. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr Hydrolysis of polyadenylic acid by pancreatic ribonuclease. J Biol Chem. 1960 Aug;235:2393–2398. [PubMed] [Google Scholar]
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971 Sep;35(3):235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Eukaryotic messenger RNA. Annu Rev Biochem. 1974;43(0):621–642. doi: 10.1146/annurev.bi.43.070174.003201. [DOI] [PubMed] [Google Scholar]
- Brown F., Newman J., Stott J., Porter A., Frisby D., Newton C., Carey N., Fellner P. Poly(C) in animal viral RNAs. Nature. 1974 Sep 27;251(5473):342–344. doi: 10.1038/251342a0. [DOI] [PubMed] [Google Scholar]
- Burness A. T., Pardoe I. U., Goldstein N. O. Overestimates of the size of poly(A) segments. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1408–1414. doi: 10.1016/0006-291x(75)90183-7. [DOI] [PubMed] [Google Scholar]
- Burness A. T. Purification of Encephalomyocarditis virus. J Gen Virol. 1969 Sep;5(2):291–303. doi: 10.1099/0022-1317-5-2-291. [DOI] [PubMed] [Google Scholar]
- Burness A. T. Ribonucleic acid content of encephalomyocarditis virus. J Gen Virol. 1970 Mar;6(3):373–380. doi: 10.1099/0022-1317-6-3-373. [DOI] [PubMed] [Google Scholar]
- Content J. Cell-free translation of influenza virus mRNA. J Virol. 1976 May;18(2):604–618. doi: 10.1128/jvi.18.2.604-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Yogo Y., Wimmer E. Replication of picornaviruses. I. Evidence from in vitro RNA synthesis that poly(A) of the poliovirus genome is genetically coded. J Virol. 1975 Dec;16(6):1512–1517. doi: 10.1128/jvi.16.6.1512-1517.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Influenza viral messenger RNA. Virology. 1974 Nov;62(1):38–45. doi: 10.1016/0042-6822(74)90301-8. [DOI] [PubMed] [Google Scholar]
- Frisby D. P., Newton C., Carey N. H., Fellner P., Newman J. F., Harris T. J., Brown F. Oligonucleotide mapping of picornavirus RNAs by two-dimensional electrophoresis. Virology. 1976 Jun;71(2):379–388. doi: 10.1016/0042-6822(76)90365-2. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Takemoto K., Robert M., Gallo R. C. Polyadenylic acid in Visna virus RNA. Science. 1973 Mar 30;179(4080):1328–1330. doi: 10.1126/science.179.4080.1328. [DOI] [PubMed] [Google Scholar]
- Glass S. E., McGeoch D., Barry R. D. Characterization of the mRNA of influenza virus. J Virol. 1975 Dec;16(6):1435–1443. doi: 10.1128/jvi.16.6.1435-1443.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein N. O., Pardoe I. U., Burness A. T. Requirement of an adenylic acid-rich segment for the infectivity of encephalomyocarditis virus RNA. J Gen Virol. 1976 May;31(2):271–276. doi: 10.1099/0022-1317-31-2-271. [DOI] [PubMed] [Google Scholar]
- HUNT J. A. TERMINAL-SEQUENCE STUDIES OF HIGH-MOLECULAR-WEIGHT RIBONUCLEIC. THE REACTION OF PERIODATE-OXIDIZED RIBONUCLEOSIDES , 5'-RIBONUCLEOTIDES AND RIBONUCLEIC ACID WITH ISONIAZID. Biochem J. 1965 May;95:541–551. doi: 10.1042/bj0950541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. A. Interaction between polyuridylic acid and rabbit globin messenger ribonucleic acid. Biochem J. 1973 Feb;131(2):327–333. doi: 10.1042/bj1310327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. E., Bose H. R. Correlation of messenger RNA function with adenylate-rich segments in the genomes of single-stranded RNA viruses. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1514–1516. doi: 10.1073/pnas.69.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S. J., Gross K. W. Quantitation and size determination of poly(A) by hybridization to (3H)poly(dT). Biochim Biophys Acta. 1974 Jun 27;353(2):133–145. doi: 10.1016/0005-2787(74)90180-4. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Wimmer E. "Fingerprinting" high molecular weight RNA by two-dimensional gel electrophoresis: application to poliovirus RNA. Nucleic Acids Res. 1976 Jul;3(7):1647–1658. doi: 10.1093/nar/3.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod M. C. Uncertainty in the determination of the molecular weight of poly(A)-containing RNA. Anal Biochem. 1975 Sep;68(1):299–310. doi: 10.1016/0003-2697(75)90708-3. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Plagemann P. G. Purification of mengovirus and identification of an A-rich segment in its ribonucleic acid. J Gen Virol. 1972 Dec;17(3):349–353. doi: 10.1099/0022-1317-17-3-349. [DOI] [PubMed] [Google Scholar]
- Nair C. N., Owens M. J. Preliminary observations pertaining to polyadenylation of rhinovirus RNA. J Virol. 1974 Feb;13(2):535–537. doi: 10.1128/jvi.13.2.535-537.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J. F., Brown F. Absence of poly (A) from the infective RNA of Nodamura virus. J Gen Virol. 1976 Jan;30(1):137–140. doi: 10.1099/0022-1317-30-1-137. [DOI] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips L. A., Park J. J., Hollis V. W., Jr Polyriboadenylate sequences at the 3'-termini of ribonucleic acid obtained from mammalian leukemia and sarcoma viruses. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4366–4370. doi: 10.1073/pnas.71.11.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder J. C., Gratzer W. B. Gel electrophoretic analysis of poly(riboadenylic acid). Biochim Biophys Acta. 1974 Apr 27;349(1):47–52. doi: 10.1016/0005-2787(74)90007-0. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Staynov D. Z., Gratzer W. B. Electrophoresis of RNA in formamide. Biochemistry. 1974 Dec 17;13(26):5373–5378. doi: 10.1021/bi00723a019. [DOI] [PubMed] [Google Scholar]
- Porter A., Carey N., Fellner P. Presence of a large poly(rC) tract within the RNA of encephalomyocarditis virus. Nature. 1974 Apr 19;248(5450):675–678. doi: 10.1038/248675a0. [DOI] [PubMed] [Google Scholar]
- Quade K., Smith R. E., Nichols J. L. Poly(riboadenylic acid) and adjacent nucleotides in Rous sarcoma virus RNA. Virology. 1974 Nov;62(1):60–70. doi: 10.1016/0042-6822(74)90303-1. [DOI] [PubMed] [Google Scholar]
- Rho H. M., Green M. The homopolyadenylate and adjacent nucleotides at the 3'-terminus of 30-40s RNA subunits in the genome of murine sarcoma-leukemia virus. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2386–2390. doi: 10.1073/pnas.71.6.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Animal RNA viruses: genome structure and function. Annu Rev Biochem. 1974;43(0):643–665. doi: 10.1146/annurev.bi.43.070174.003235. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Kates J., Kelley D. E., Perry R. P. Polyadenylic acid sequences on 3' termini of vaccinia messenger ribonucleic acid and mammalian nuclear and messenger ribonucleic acid. Biochemistry. 1972 Sep 26;11(20):3829–3834. doi: 10.1021/bi00770a023. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Stavrianopoulos J. G., Schutz G., Feigelson P. Translational properties of rabbit globin mRNA after specific removal of poly(A) with ribonuclease H. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4635–4639. doi: 10.1073/pnas.71.11.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater D. W., Slater I., Gillespie D. Post-fertilization synthesis of polyadenylic acid in sea urchin embryos. Nature. 1972 Dec 8;240(5380):333–337. doi: 10.1038/240333a0. [DOI] [PubMed] [Google Scholar]
- Slater I., Gillespie D., Slater D. W. Cytoplasmic adenylylation and processing of maternal RNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):406–411. doi: 10.1073/pnas.70.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Poly(A) on mengovirus RNA. J Virol. 1975 Oct;16(4):1081–1084. doi: 10.1128/jvi.16.4.1081-1084.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Requirement of 3'-terminal poly(adenylic acid) for the infectivity of poliovirus RNA. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2983–2987. doi: 10.1073/pnas.71.8.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. L., Scott J. F., Zamecnik P. C. Evidence that the polyadenylic acid segment of "35S" RNA of avian myeloblastosis virus is located at the 3'-OH terminus. Biochem Biophys Res Commun. 1973 Nov 1;55(1):8–16. doi: 10.1016/s0006-291x(73)80052-x. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Banerjee A. K. Two oligonucleotide classes of single-stranded ribopolymers in reovirus A-rich RNA. Arch Biochem Biophys. 1972 Oct;152(2):733–743. doi: 10.1016/0003-9861(72)90269-x. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Shatkin A. J., Banerjee A. K. Absence of polyadenylic acid from reovirus messenger ribonucleic acid. J Biol Chem. 1973 Dec 10;248(23):7993–7998. [PubMed] [Google Scholar]
- Verma D. P., Nash D. T., Schulman H. M. Isolation and in vitro translation of soybean leghaemoglobin mRNA. Nature. 1974 Sep 6;251(5470):74–77. doi: 10.1038/251074a0. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R., Banerjee A. K., LaFiandra A., Shatkin A. J. Reovirus-specific ribonucleic acid from polysomes of infected L cells. J Virol. 1972 Jan;9(1):61–69. doi: 10.1128/jvi.9.1.61-69.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. Nuclear RNA metabolism. Annu Rev Biochem. 1973;42:329–354. doi: 10.1146/annurev.bi.42.070173.001553. [DOI] [PubMed] [Google Scholar]
- Woo S. L., Rosen J. M., Liarakos C. D., Choi Y. C., Busch H., Means A. R., O'Malley Physical and chemical characterization of purified ovalbumin messenger RNA. J Biol Chem. 1975 Sep 10;250(17):7027–7039. [PubMed] [Google Scholar]
- Yogo Y., Teng M. H., Wimmer E. Poly(U) in poliovirus minus RNA is 5'-terminal. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1101–1109. doi: 10.1016/s0006-291x(74)80397-9. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]