Abstract
Long-term graft survival after kidney transplantation remains unsatisfactory and unpredictable. Interstitial fibrosis and tubular atrophy are major contributors to late graft loss; features of tubular cell senescence, such as increased p16INK4a expression, associate with these tubulointerstitial changes, but it is unknown whether the relationship is causal. Here, loss of the INK4a locus in mice, which allows escape from p16INK4a-dependent senescence, significantly reduced interstitial fibrosis and tubular atrophy and associated with improved renal function, conservation of nephron mass, and transplant survival. Compared with wild-type controls, kidneys from INK4a−/− mice developed significantly less interstitial fibrosis and tubular atrophy after ischemia-reperfusion injury. Consistently, mice that received kidney transplants from INK4a/ARF−/− donors had significantly better survival 21 days after life-supporting kidney transplantation and developed less tubulointerstitial changes. This correlated with higher proliferative rates of tubular cells and significantly fewer senescent cells. Taken together, these data suggest a pathogenic role of renal cellular senescence in the development of interstitial fibrosis and tubular atrophy and kidney graft deterioration by preventing the recovery from injury. Inhibiting premature senescence could have therapeutic benefit in kidney transplantation but has to be balanced against the risks of suspending antitumor defenses.
Despite improvements in first-year graft survival, long-term failure of kidney transplants remains an important clinical problem.1 Recent studies have indicated that most transplants fail due to specific causes such as antibody-mediated rejection and recurrent diseases.1,2 Interstitial fibrosis and tubular atrophy occur in response to the injury caused by these processes and reflect the loss of functional nephron mass. Tubulointerstitial changes are a common feature in many chronic renal diseases and eventually are the cause for returning to dialysis after transplantation.3–5 An explanation for the strong predictive value of donor age and transplantation-dependent tissue injury for the development of interstitial fibrosis and tubular atrophy6,7 could be changes in cell fate programming, such as senescence, that lead to the inability of tubular epithelial cells to repair and maintain nephron integrity.8–10
Cellular senescence is reached through two main pathways: telomere-dependent and telomere-independent senescence. Telomere-dependent senescence, also called replicative senescence, refers to telomere shortening and eventually cell cycle arrest with ongoing cell cycling.11–13 Telomere-dependent senescence is circumvented in normal laboratory animals due to the expression of telomerase, an enzyme that maintains telomere length.14 An alternate form of senescence caused by different cellular stresses, like DNA damage, oxidative stress, Ras induction, and epigenetic alterations, is called stress and aberrant signaling-induced senescence arrest or premature senescence.15 The cell cycle regulator and tumor suppressor p16INK4a is associated with this telomere-independent growth arrest that is detectable in humans and rodents.16,17
Several studies have correlated senescence markers, mainly higher expression of p16INK4a and/or shorter telomeres, with the occurrence of interstitial fibrosis and tubular atrophy in CKD or transplant failure.18–22 In experimental models, renal transplantation leads to a transient elevation in p21CIP1/WAF1, sustained increases of p16INK4a, induction of SA-β-GAL expression, and substantial telomere shortening.23 Importantly, old renal allografts show a more rapid emergence of epithelial deterioration that is accompanied by an increased expression of p16INK4a compared with young donor allografts.18 In telomerase-deficient mice, critical telomere shortening in the kidney leads to increased senescence, thereby limiting regenerative capacity.24 Consequently, it has been shown that senescence markers, in particular p16INK4a expression, in renal transplant implantation biopsies are useful to predict transplant outcome.25,26
Here, we evaluated whether loss of the INK4a locus could result in superior morphologic and functional outcome in mice undergoing either renal ischemia-reperfusion injury or receiving a fully MHC-mismatched, vascularized life-supporting kidney transplant.
Results
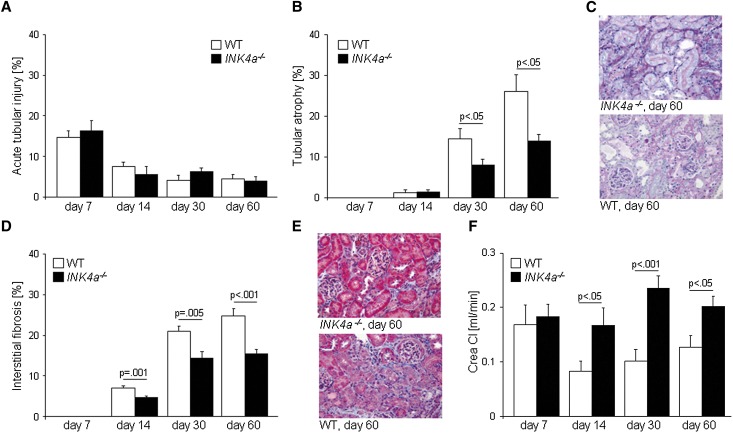
We subjected kidneys from wild-type or INK4a−/− mice to 30 minutes of warm ischemia. Acute tubular damage was similar in both groups, being highest on day 7 after ischemia with a decrease by day 14 (Figure 1A). Tubular atrophy, a marker of unsuccessful epithelial repair, was first observed at day 14 with a significant increase by days 30 and 60 in both groups (Figure 1, B and C). This increase was significantly higher in wild-type kidneys compared with kidneys from INK4a−/− mice. Similarly, interstitial fibrosis was significantly higher in wild-type kidneys (Figure 1, D and E). In wild-type kidneys, ischemia led to a significant increase of p16INK4a expression (Supplemental Figure 1A). Creatinine clearances, as a measure of renal function, showed no difference on day 7 between the two groups but were significantly lower in wild-type mice on days 14, 30, and 60 (Figure 1F).
Figure 1.
Ischemia-reperfusion injury in INK4a−/− and wild-type mice. (A) Acute tubular injury (shown as percentage of affected area) is most pronounced 7 days after injury and decreases thereafter. There are no differences between the groups. (B) Tubular atrophy (percentage of affected area) is first detected 14 days after injury. Significant differences occur 30 and 60 days after injury, with INK4a−/− kidneys developing less tubular atrophy. (C) Representative sections from kidneys 60 days after injury. Tubular atrophy is visualized with the periodic acid–Schiff staining. (D) Interstitial fibrosis (percentage of blue-stained area) reveals a similar picture to tubular atrophy with significantly less interstitial fibrosis seen in INK4a−/− kidneys 14, 30, and 60 days after injury. (E) Representative Masson Trichrome staining of kidneys 60 days after injury. Interstitial fibrosis is visualized as blue-colored collagen fibers. (F) Creatinine clearances are significantly lower in wild-type mice 14, 30, and 60 days after injury. WT, wild-type; Crea Cl, creatine clearance. Original magnification, ×200.
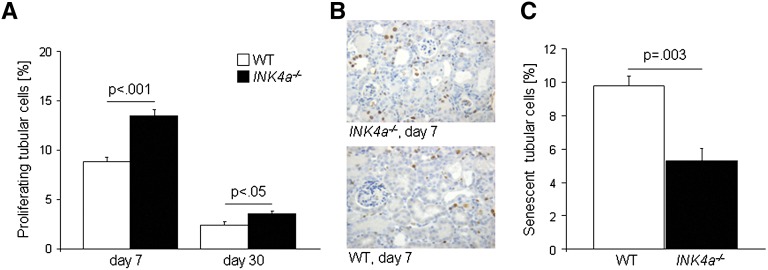
We then sought to determine whether a higher proliferative capacity of renal tubular cells was responsible for the superior outcome of INK4a−/− mice after ischemia-reperfusion injury and whether this would result in fewer senescent tubular cells in their kidneys. The expression of Ki67, a marker strictly associated with cell proliferation (labeling phases S, G2, and M of the cell cycle) was quantified. Proliferation rates were higher on day 7 compared with day 30. The number of proliferating tubular cells was significantly higher in renal sections from INK4a−/− mice on days 7 and 30 compared with wild-type mice (Figure 2, A and B). Senescent cells were detected by γH2A.X/Ki67 co-staining on day 60. The number of senescent tubular cells, defined as Ki67 negative cells with high γH2A.X foci density,27 was significantly higher in wild-type kidneys (Figure 2C and Supplemental Figure 2). Apoptosis measured by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling was low overall in our experimental setting and no differences were seen between the two animal groups (data not shown). Taken together, these data suggest that p16INK4a expression leads to impaired renal repair by reducing the proliferative reserve of tubular epithelial cells. In turn, loss of p16INK4a resulted in a significantly lower amount of senescent cells.
Figure 2.
Proliferation and senescence of tubular cells from INK4a−/− and wild-type kidneys after ischemia-reperfusion injury. (A) Tubular cell proliferation (percentage of Ki67 positive tubular cells) measured 7 and 30 days after injury was significantly higher in kidneys from INK4a−/− mice. Proliferation rates declined from day 7 to day 30 in both groups (P<0.05). (B) Representative staining showing Ki67-positive cells in kidneys 7 days after injury. (C) The number of senescent tubular cells at day 60 after injury was significantly lower in kidneys from INK4a−/− mice. WT, wild-type. Original magnification, ×200.
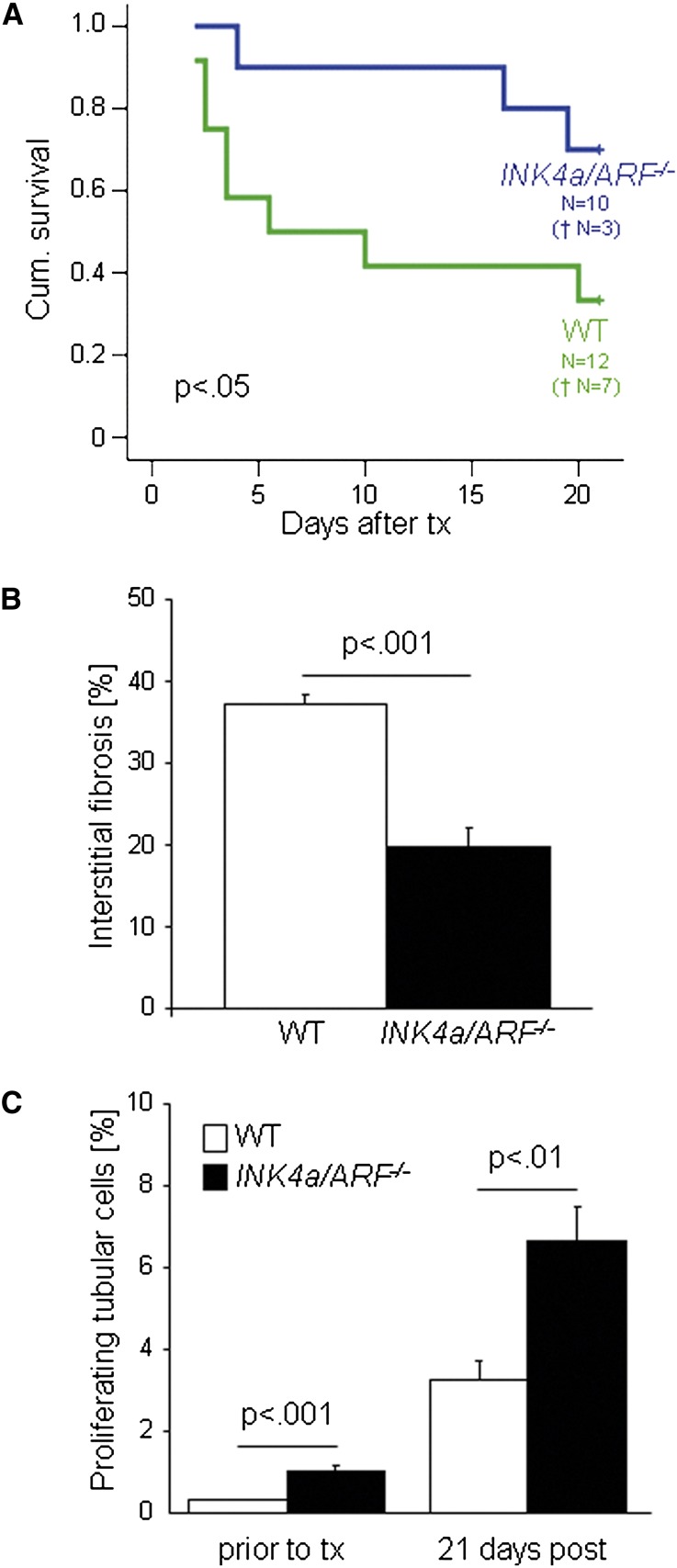
Clinical studies have shown increased p16INK4a expression in transplants with interstitial fibrosis and tubular atrophy or allograft nephropathy, respectively.18,21 To test our findings in the transplant setting, we performed transplants from INK4a/ARF−/− as well as wild-type mice, which are both on a C57BL/6 background, into fully MHC-mismatched wild-type mice on a C3H background. Importantly, the transplant model allowed us to exclude that a reduced immunologic response was the reason for the better outcome of kidneys from INK4a−/− mice after ischemia-reperfusion injury. To assess functional aspects, we used a life-supporting transplant model, in which both recipient kidneys are removed. We found that mice transplanted with INK4a/ARF−/− kidneys had a significantly better survival compared with mice that received a wild-type transplant (Figure 3A).
Figure 3.
Life-supporting allogeneic renal transplantation of INK4a/ARF−/− and wild-type kidneys. (A) Cumulative survival was significantly better in mice that received a transplant from a INK4a/ARF−/− mouse. (B) Interstitial fibrosis (percentage of blue-stained area) was significantly higher in wild-type transplants. (C) Tubular cell proliferation (percentage of Ki67-positive tubular cells) was significantly higher in INK4a/ARF−/− transplants, before and 21 days after transplantation. WT, wild-type; tx, transplant.
Human transplant biopsy studies have shown that peri- and post-transplantation stresses (such as ischemia-reperfusion injury,24,28,29 acute rejection,30 or hypertension31) lead to an increased p16INK4a expression. Our results were in agreement, and we found that experimental transplantation led to a significant increase in p16INK4a expression in wild-type kidneys (Supplemental Figure 1B). INK4a/ARF−/− transplants showed less chronic pathologic changes with a significant difference in the amount of interstitial fibrosis detected at day 21 (Figure 3B), which was comparable with what was seen after ischemia-reperfusion injury. The better functional integrity was not due to differences in the inflammatory response of the recipient: the amount of infiltrating cells as measured by the number of infiltrating CD3-positive lymphocytes and the typical lesions of tubulitis were similar in both groups (Supplemental Figure 3 and Supplemental Table 1). However, the proliferation rate of renal tubular cells was significantly greater in INK4a/ARF−/− kidneys before and after transplantation (Figure 3C). These observations provide evidence that loss of a major senescence pathway leads to increased allograft regenerative capacity and protects from the development of interstitial fibrosis and tubular atrophy.
Discussion
Our data are consistent with the hypothesis that p16INK4a is crucially implicated in the mechanism of regenerative loss of renal aging. Irreversible parenchymal changes such as interstitial fibrosis and tubular atrophy were less common in either of the two transgenic mouse strains. INK4a deletion led to improved renal function and resulted in superior recipient survival in a life-supporting transplant model. We were able to confirm our results in two independent transgenic mouse strains. Obviously, these two mouse strains are not equivalent to each other because of the additional deletion of the ARF transcript in one of the strains and p19ARF being an additional potent cell cycle inhibitor. The confirmatory nature of both experimental settings strongly argues for the INK4a locus as the driving force for the observed phenotype, even though the contribution of the ARF transcript was not further explored.
The utilization of different models was also important in light of a recent renal study that showed an aggravating effect of deleting INK4a in a model of obstructive uropathy.32 The authors concluded from their results that p16INK4a limits inflammation and prevents fibrosis, whereas deletion of the gene increases tissue injury. Although it cannot be excluded that the apparent discrepancy to our study might be related to a difference in the strain of knockout mice, it seems likely that it reflects the heterogeneous effect of p16INK4a expression depending on the biologic context and on the affected cell type. While models of ischemic injury require a burst of epithelial cell proliferation as an instrumental part of their repair mechanism,33 epithelial and/or interstitial cell proliferation might be detrimental in fibrosis models such as obstructive uropathy.34
Our data clearly show that the potentially promitogenic INK4A deletion does not necessarily induce a profibrogenic phenotype in renal fibroblasts. Instead, the primary problem in the postischemic kidney consists in the failure of epithelial repair, which is more successful in INK4A deleted mice with a better proliferative reserve. The development of interstitial fibrosis is a secondary process that results from defective epithelial repair and can be regarded as a default mechanism of inadequate regeneration. In this context, our findings might extend previous data on the association of epithelial cell cycle arrest at G2/M and fibrotic outcome35 by showing a similar phenotype if epithelial cells are arrested at the G1 state of cellular senescence. Tubular cells that are arrested in G2/M after acute injury secrete profibrogenic factors such as TGF-β1 and connective tissue growth factor.35 On the basis of data from other cell types, it is likely that senescent renal tubular cells also secrete specific factors.36 However, these might not overlap with the secretome of G2/M arrested cells. Further studies are needed to define effector molecules of senescent renal tubular cells in order to better understand the switch from defective epithelial repair to promotion of interstitial fibrosis.
Although our results suggest that inhibiting premature senescence could have therapeutic benefits, it is important to keep potential risks in mind. Not only is cellular senescence an integral part of the physiologic antitumor barrier37,38 but it might also be tissue protective under specific circumstances senescence.
In summary, our results demonstrate that the development of premature senescence via p16INK4a expression is a critical regulator of cell repair and can contribute to postischemic interstitial fibrosis and tubular atrophy development. Interference with p16INK4a leads to increased regenerative capacity and may thus represent a novel strategy in improving the outcome of kidney transplantation. This will be of particular importance for aged donor kidneys that have to be increasingly utilized to overcome the shortage in available transplant organs.
Concise Methods
Animals
All procedures performed on animals were done in accordance with institutional guidelines for animal research and were approved by the local government authorities.
Mice
INK4a−/− mice on a 129/Ola-C57BL/6J background that carry a point mutation specifically affecting p16INK4a but not p19ARF expression39 were provided by Dr. A. Berns (The Netherlands Cancer Institute, Amsterdam) and bred at the animal facility of the University of Heidelberg. INK4a/ARF−/− mice on a C57BL/6J background17 used for the transplantation experiments came from the stock of Dr. M. Serrano.
Ischemia-Reperfusion Injury
Ischemia-reperfusion surgery was performed according to current standard protocols in age-matched male INK4a−/− and wild-type mice that were 3–4 months old. Briefly, mice were anesthetized with inhalational isoflurane and were kept on a heating pad to maintain body temperature during surgery. A midline laparotomy was made. The bowel was gently placed aside by retractors. The renal pedicles were exposed and adjacent fat tissue was removed carefully. The left renal pedicle (artery and vein) was clamped for 30 minutes, using nontraumatic microsurgical vascular clips (Aesculap, Tuttlingen, Germany). Occlusion of blood flow was confirmed by visual inspection of the kidneys. The right kidney was left in situ. After removal of the clip, the kidneys were observed for approximately 5 minutes to ensure blood reflow, and then fascia and skin were sutured in two layers. All animals received the same volume of warm saline instilled in the peritoneal cavity during the surgical procedure and were allowed to recover with ad libitum access to food and water. Mice were sacrificed after 7 (wild-type, n=8; INK4a−/−, n=8), 14 (wild-type, n=7; INK4a−/−, n=7), 30 (wild-type, n=9; INK4a−/−, n=9), or 60 (wild-type, n=9; INK4a−/−, n=9) days, respectively.
After sacrifice, the kidneys were excised, adjacent tissue was carefully removed, kidneys were decapsulated, and weight was determined. Part of each kidney was immediately snap-frozen in liquid nitrogen for later RNA extraction; a second part was fixed in 4% buffered formalin and embedded in paraffin.
Renal Transplantation
Donor mice (INK4a/ARF−/− mice on a C57BL/6J background) were anesthetized and the abdomen was opened through a midline incision. The right kidney was excised, flushed, and preserved in cold saline solution for 30 minutes. The host mice (C3H background purchased from Charles River Laboratories, Sulzfeld, Germany) were similarly anesthetized and both native kidneys were excised (life-supporting kidney transplantation). The donor kidney was anastomosed heterotopically to the inferior aorta, vena cava, and bladder. The mice were allowed to recover and were followed for 21 days. Mice that had not died within the observational period were killed by cervical dislocation at day 21. None of the transplanted hosts received immunosuppressive therapy. Mice with technical complications or pyelonephritis were removed from the study.
We studied 10 transplants from INK4a/ARF−/− mice and 12 transplants from wild-type mice.
Creatinine Clearance Determination
Mice (wild-type and INK4a−/−) were kept in metabolic cages for the last 24 hours before sacrifice. Twenty-four–hour urine was collected, urine volume was recorded, and aliquots were stored at –80°C for subsequent analysis. Blood was obtained by cardiac puncture under deep anesthesia right before sacrifice. Plasma and urinary creatinine were determined using an enzymatic method that has been validated in rodents.40 Creatinine clearance (ml/min) was derived from the following formula: urinary creatinine × urine volume × 1440 min–1 × plasma creatinine–1.
Histopathology of Kidney
Tissue sections were cut at 3-µm sections with a Leica RM 2165 microtome (Leica Instruments, Nussloch, Germany) and stained with hematoxylin and eosin, periodic acid–Schiff , or Masson Trichrome, respectively. All analyses were performed using a Leica DM LB2 digitizing microscope and a Leica DFC 320 camera with QWin V3 software (all from Leica Microsystems GmbH, Wetzlar, Germany).
Acute and chronic tubular damage were quantified as the ratio of damaged tubules to total tubular area. Acute damage was mainly reflected by tubular necrosis, whereas chronic damage consisted of tubular deterioration including reduced tubular diameter, thickened tubular basement membrane, and loss of tubular nuclei. The degree of renal interstitial fibrosis was measured using Masson Trichrome stainings and a semiquantitative scoring system. The area of blue-stained interstitial fibrosis was detected using the QWin V3 software package and related to total tubulointerstitial area. Both analyses were based on the evaluation of 20 high-power fields (HPFs) (×200 magnification) for each animal.
Real-Time RT-PCR
Total RNA was extracted from murine renal tissue samples using Trizol reagent (Invitrogen, Karlsruhe, Germany), RNA integrity verified by agarose gel electrophoresis, and cDNA obtained by reverse transcription of 1 µg total RNA using Moloney murine leukemia virus reverse transcriptase and random primers (Invitrogen, Karlsruhe, Germany). The following intron-spanning primers and probes were used for quantitative PCR of p16INK4a: forward, 5′GGGCACTGCTGGAAGCC 3′; reverse, 5′AACGTTGCCCATCATCATC 3′; and probe, 5′CCGAACTCTTTCGGTCGTA 3′.
Immunohistochemistry for Ki67 and CD3
Immunoperoxidase staining for Ki67 and CD3 was performed on paraffin-embedded tissue. Antigen retrieval was performed in citrate buffer, pH 6.0, using a pressurized heating chamber (Pascal Pressurized Heating Chamber; Dako, Hamburg, Germany). The sections were immersed in 3% H2O2 in methanol, blocked with 1× Universal Blocking Reagent (BioGenex, San Ramon, CA), and then incubated at room temperature with the primary antibody for Ki67 (MIB-9, 190 mg/ml, at a dilution of 1:25; Dako) or for CD3 (CD3–12, 1 mg/ml, at a dilution of 1:50; Serotec, Martinsried, Germany) or with the appropriate isotype control antibodies, and rinsed with PBS. After 30 minutes of incubation with the Envision Monoclonal System (Dako) for Ki67 or with a biotinylated goat-anti-rat (Southern Biotech, Birmingham, AL), sections were washed again in PBS. In case of CD3 staining, 30 minutes of incubation with ABC reagent (Vectastain; Vector Laboratories, Burlingame, CA) was performed and rinsed with PBS. Visualization was performed using the DAB substrate kit (Dako). The slides were counterstained with hematoxylin for Ki67 or methylene blue for CD3.
Analysis for Ki67 was done by counting 10 randomly photographed HPFs (×200 magnification) within the cortex by a blinded observer. The percentage of positively stained tubular nuclei was related to the total number of nuclei. For Ki67, a subgroup of animals for days 7 (wild-type, n=8; INK4a−/−, n=8) and 30 (wild-type, n=9; INK4a−/−, n=9) were evaluated.
CD3-positive cells were assessed only in INK4a/ARF−/− transplants on 10 HPFs (×200 magnification) by a blinded observer. Positive cells were attributed either to the interstitial, tubular, or glomerular compartment. In addition, the number of infiltrating CD3-positive cells per tubular cross-section was assessed.
γH2A.X/Ki67 Co-Staining
Staining was performed on paraffin-embedded kidney sections. Antigen retrieval was performed in citrate buffer, pH 6.0, using a pressurized heating chamber (Pascal Pressurized Heating Chamber; Dako). The sections were incubated in blocking buffer (5% skim milk) for 60 minutes at room temperature. Primary antibodies for γH2A.X (JBW301, 1:200; Millipore) and Ki67 (Clone SP6, 1:200; Thermo Scientific) were applied overnight at 4°C. Slides were then washed with PBS-Tween and incubated for 1 hour at room temperature with secondary antibodies for γH2A.X (anti-mouse Alexa-555, A21422; Invitrogen) and for Ki67 (anti-rabbit Alexa-488, A21206; Invitrogen). Sections were counterstained with 4',6-diamidino-2-phenylindole and mounted with 1,4-diazabicyclo[2.2.2]octane antifade mounting medium.
Analysis was performed using 15 HPFs (×400 magnification) within the cortex. The percentage of Ki67-negative and γH2A.X-positive (≥4 foci) tubular nuclei was related to total number of nuclei. For γH2A.X/Ki67-co-staining, a subgroup of animals for day 60 (wild-type, n=5; INK4a−/−, n=5) was evaluated.
Statistical Analyses
Means among different treatment groups were compared using ANOVA, and t tests with Bonferroni correction were applied for post hoc analysis. Data were evaluated using the SPSS 16.0 statistical software package (SPSS Inc, Chicago, IL). All data are shown as mean ± SEM, unless otherwise indicated.
Disclosures
None.
Acknowledgments
We thank Margit Überheide and Julian Lünig for their technical assistance.
This study was supported by grants from the Roche Organ Transplantation Research Foundation to A.M. and from the German Research Foundation (DFG) Collaborative Research Center 738 (project C8) to A.M. and R.S.
Footnotes
H.B. and B.M.W.S. contributed equally to this work.
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011100967/-/DCSupplemental.
References
- 1.Lamb KE, Lodhi S, Meier-Kriesche HU: Long-term renal allograft survival in the United States: A critical reappraisal. Am J Transplant 11: 450–462, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF: Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9: 2520–2531, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Nakorchevsky A, Hewel JA, Kurian SM, Mondala TS, Campbell D, Head SR, Marsh CL, Yates JR, 3rd, Salomon DR: Molecular mechanisms of chronic kidney transplant rejection via large-scale proteogenomic analysis of tissue biopsies. J Am Soc Nephrol 21: 362–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terasaki PI, Cecka JM, Gjertson DW: Impact analysis: A method for evaluating the impact of factors in clinical renal transplantation. In: Clinical Transplants, edited by Cecka JM, Terasaki PI, Los Angeles, CA, UCLA Tissue Typing Laboratory, 1998, pp 437–441 [PubMed] [Google Scholar]
- 7.Gourishankar S, Hunsicker LG, Jhangri GS, Cockfield SM, Halloran PF: The stability of the glomerular filtration rate after renal transplantation is improving. J Am Soc Nephrol 14: 2387–2394, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Melk A: Senescence of renal cells: Molecular basis and clinical implications. Nephrol Dial Transplant 18: 2474–2478, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Melk A, Halloran PF: Cell senescence and its implications for nephrology. J Am Soc Nephrol 12: 385–393, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Halloran PF, Melk A, Barth C: Rethinking chronic allograft nephropathy: The concept of accelerated senescence. J Am Soc Nephrol 10: 167–181, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Hayflick L, Moorhead PS: The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621, 1961 [DOI] [PubMed] [Google Scholar]
- 12.Harley CB, Futcher AB, Greider CW: Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Martens UM, Chavez EA, Poon SSS, Schmoor C, Lansdorp PM: Accumulation of short telomeres in human fibroblasts prior to replicative senescence. Exp Cell Res 256: 291–299, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu C-P, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE: Extension of life-span by introduction of telomerase into normal human cells. Science 279: 349–352, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Wright WE, Shay JW: Telomere dynamics in cancer progression and prevention: Fundamental differences in human and mouse telomere biology. Nat Med 6: 849–851, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Weinberg RA: The retinoblastoma protein and cell cycle control. Cell 81: 323–330, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Serrano M, Lee HW, Chin L, Cordon-Cardo C, Beach D, DePinho RA: Role of the INK4a locus in tumor suppression and cell mortality. Cell 85: 27–37, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Melk A, Schmidt BMW, Vongwiwatana A, Rayner DC, Halloran PF: Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am J Transplant 5: 1375–1382, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Sis B, Tasanarong A, Khoshjou F, Dadras F, Solez K, Halloran PF: Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int 71: 218–226, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Ferlicot S, Durrbach A, Bâ N, Desvaux D, Bedossa P, Paradis V: The role of replicative senescence in chronic allograft nephropathy. Hum Pathol 34: 924–928, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Chkhotua AB, Gabusi E, Altimari A, D’Errico A, Yakubovich M, Vienken J, Stefoni S, Chieco P, Yussim A, Grigioni WF: Increased expression of p16(INK4a) and p27(Kip1) cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am J Kidney Dis 41: 1303–1313, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Szeto CC, Poon PY, Lai FM, Chow KM, Szeto CY, Li PK: Chromosomal telomere shortening of kidney cells in IgA nephropathy by the measurement of DNA in urinary sediment. Clin Nephrol 64: 337–342, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Joosten SA, van Ham V, Nolan CE, Borrias MC, Jardine AG, Shiels PG, van Kooten C, Paul LC: Telomere shortening and cellular senescence in a model of chronic renal allograft rejection. Am J Pathol 162: 1305–1312, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westhoff JH, Schildhorn C, Jacobi C, Hömme M, Hartner A, Braun H, Kryzer C, Wang C, von Zglinicki T, Kränzlin B, Gretz N, Melk A: Telomere shortening reduces regenerative capacity after acute kidney injury. J Am Soc Nephrol 21: 327–336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koppelstaetter C, Schratzberger G, Perco P, Hofer J, Mark W, Ollinger R, Oberbauer R, Schwarz C, Mitterbauer C, Kainz A, Karkoszka H, Wiecek A, Mayer B, Mayer G: Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation. Aging Cell 7: 491–497, 2008 [DOI] [PubMed] [Google Scholar]
- 26.McGlynn LM, Stevenson K, Lamb K, Zino S, Brown M, Prina A, Kingsmore D, Shiels PG: Cellular senescence in pretransplant renal biopsies predicts postoperative organ function. Aging Cell 8: 45–51, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Lawless C, Wang C, Jurk D, Merz A, Zglinicki T, Passos JF: Quantitative assessment of markers for cell senescence. Exp Gerontol 45: 772–778, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Hochegger K, Koppelstaetter C, Tagwerker A, Huber JM, Heininger D, Mayer G, Rosenkranz AR: p21 and mTERT are novel markers for determining different ischemic time periods in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 292: F762–F768, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Chkhotua AB, Abendroth D, Froeba G, Schelzig H: Up-regulation of cell cycle regulatory genes after renal ischemia/reperfusion: Differential expression of p16(INK4a), p21(WAF1/CIP1) and p27(Kip1) cyclin-dependent kinase inhibitor genes depending on reperfusion time. Transpl Int 19: 72–77, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Melk A, Schmidt BM, Braun H, Vongwiwatana A, Urmson J, Zhu LF, Rayner D, Halloran PF: Effects of donor age and cell senescence on kidney allograft survival. Am J Transplant 9: 114–123, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Westhoff JH, Hilgers KF, Steinbach MP, Hartner A, Klanke B, Amann K, Melk A: Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension 52: 123–129, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Wolstein JM, Lee DH, Michaud J, Buot V, Stefanchik B, Plotkin MD: INK4a knockout mice exhibit increased fibrosis under normal conditions and in response to unilateral ureteral obstruction. Am J Physiol Renal Physiol 299: F1486–F1495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K: Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 120: 4040–4054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010 [DOI] [PMC free article] [PubMed]
- 36.Campisi J, Andersen JK, Kapahi P, Melov S: Cellular senescence: A link between cancer and age-related degenerative disease? Semin Cancer Biol 21: 354–359, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS: The essence of senescence. Genes Dev 24: 2463–2479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitiyage GN, Slijepcevic P, Gabrani A, Chianea YG, Lim KP, Prime SS, Tilakaratne WM, Fortune F, Parkinson EK: Senescent mesenchymal cells accumulate in human fibrosis by a telomere-independent mechanism and ameliorate fibrosis through matrix metalloproteinases. J Pathol 223: 604–617, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Krimpenfort P, Quon KC, Mooi WJ, Loonstra A, Berns A: Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413: 83–86, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Keppler A, Gretz N, Schmidt R, Kloetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J: Plasma creatinine determination in mice and rats: An enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int 71: 74–78, 2007 [DOI] [PubMed] [Google Scholar]