Abstract
The contribution of microRNA (miRNA) to the pathogenesis of renal fibrosis is not well understood. Here, we investigated whether miRNA modulates the fibrotic process in Munich Wistar Fromter (MWF) rats, which develop spontaneous progressive nephropathy. We analyzed the expression profile of miRNA in microdissected glomeruli and found that miR-324-3p was the most upregulated. In situ hybridization localized miR-324-3p to glomerular podocytes, parietal cells of Bowman’s capsule, and most abundantly, cortical tubules. A predicted target of miR-324-3p is prolyl endopeptidase (Prep), a serine peptidase involved in the metabolism of angiotensins and the synthesis of the antifibrotic peptide N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP). In cultured tubular cells, transient transfection with a miR-324-3p mimic reduced Prep protein and activity, validating Prep as a target of this miRNA. In MWF rats, upregulation of miR-324-3p associated with markedly reduced expression of Prep in both glomeruli and tubules, low urine Ac-SDKP, and increased deposition of collagen. ACE inhibition downregulated glomerular and tubular miR-324-3p, promoted renal Prep expression, increased plasma and urine Ac-SDKP, and attenuated renal fibrosis. In summary, these results suggest that dysregulation of the miR-324-3p/Prep pathway contributes to the development of fibrosis in progressive nephropathy. The renoprotective effects of ACE inhibitors may result, in part, from modulation of this pathway, suggesting that it may hold other potential therapeutic targets.
Munich Wistar Fromter (MWF) is an inbred rat strain characterized by inborn nephron deficit, glomerular hypertrophy, and high urinary protein excretion.1,2 Males develop hypertension and overt proteinuria with age.3,4 Angiotensin II (Ang II) has a direct role in the pathogenesis of proteinuria and glomerular injury by decreasing the size selectivity of the glomerular capillary barrier against the filtration of plasma proteins5,6 that is followed by development of glomerulosclerosis.4 Treatment with an angiotensin-converting enzyme inhibitor (ACEi) protects MWF rats from kidney damage by both preserving and remodeling the glomerular barrier, thus inducing regression of proteinuria and glomerulosclerosis.7,8 A large body of evidence in this model and other animal models and clinical trials confirms that Ang II is a potential therapeutic target for remission/regression of progressive glomerulopathies.9,10 In advanced nephropathy, chronic tubulointerstitial damage and progressive decline of renal function are invariably associated features,7,11 and they show tight correlation in human glomerulopathies.12 ACEi treatment does ultimately exert antifibrotic effects. Pharmacologic protection against the tubular interstitial component of injury may not yet be complete in either MWF rats or other settings, such as immune glomerulopathy or diabetic nephropathy when treatment is started with delay. Deeper knowledge of activated profibrogenic pathways along the nephron downstream of podocyte injury should allow identification of new therapeutic targets and therefore, ways of fully restoring kidney structure and function, even when the disease is advanced.7,8 In this respect, the MWF rat offers to us a suitable model because of the spontaneous nature of proteinuria and glomerulosclerosis, well characterized pathophysiology and responses to drug treatment, and late tubulointerstitial damage.
Studies have been devoted to elucidating the genetic basis of renal damage in MWF rats. Polygenetic recessive etiology linked to quantitative trait loci was disclosed in this strain, which was unrelated to BP regulation,13,14 but the individual gene and related pathway have not been identified as a single factor that may underlie the pathogenesis of renal fibrosis in the MWF strain. This finding raised interest in studying whether post-transcriptional regulation of gene expression could be responsible for abnormal renal phenotype of disease progression. Discovery of microRNA (miRNA) has given insights into these gene regulatory mechanisms. MiRNAs are a family of short, noncoding, single-stranded RNA molecules of approximately 22 nucleotides in length that acts by inhibiting translation or destabilizing target transcripts.15,16 Specific miRNAs regulate biologic processes and are involved in pathologic states, such as cancer and cardiovascular diseases.17 Key roles for miRNA in kidney function and CKD have been also suggested.18–20
In the present study, we investigated whether post-transcriptional regulation by miRNA along the nephron may have a pathogenetic role in the development of renal fibrosis in MWF rats. We found a regulatory pathway highly perturbed in tubular epithelial cells and shared by glomerular epithelium in disease. We also explored the extent to which renoprotection induced by ACEi could be related to miRNA modulation in this model.
Results
Glomerular miRNA Expression Profiling in Progressive Proteinuric Nephropathy
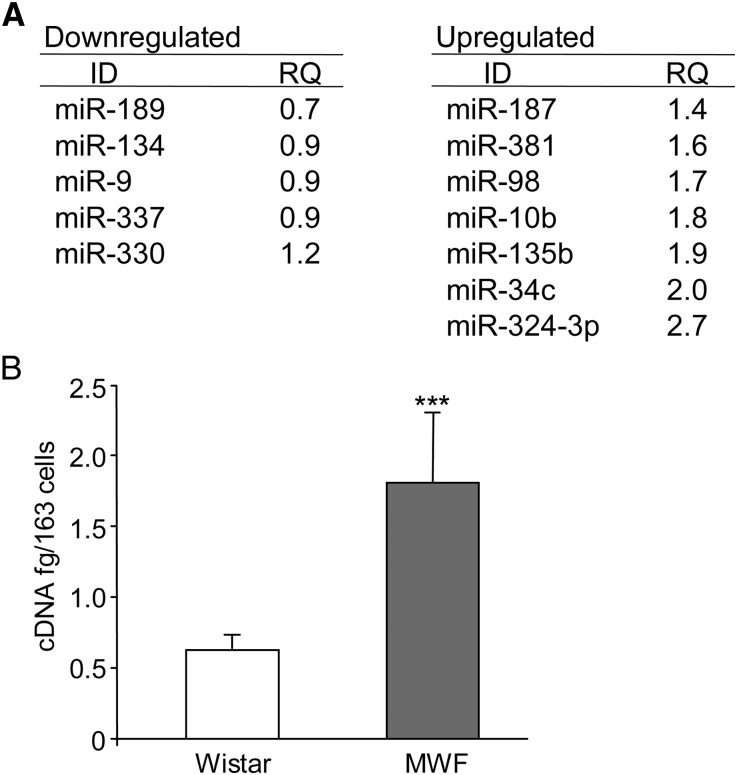
Because glomerular dysfunction is a characteristic feature associated with overt proteinuria and ultimately, glomerulosclerosis in MWF rats, we first investigated miRNA expression profiles in microdissected glomeruli from MWF rats with advanced nephropathy (rats studied for 60 weeks [MWF 60W]) or control Wistar 60W. Expression was altered in 120 of 365 miRNAs examined: 94 miRNAs were upregulated, and 26 miRNAs were downregulated in MWF 60W versus Wistar 60W. Figure 1A shows the most affected miRNAs and relative fold changes. miRNA (miR) -324-3p was the most prominent. Real-time PCR confirmed glomerular miR-324-3p overexpression (Figure 1B).
Figure 1.
miRNA expression profiling in microdissected glomeruli. (A) List of fold changes of miRNAs in MWF 60W with respect to Wistar 60W. (B) Quantitative real-time PCR of miR-324-3p expression in microdissected glomeruli from MWF and control Wistar rats. Results are mean ± SD. ***P<0.001 versus Wistar rats.
miR-324-3p Is Upregulated in Glomeruli and Tubules of MWF Kidney
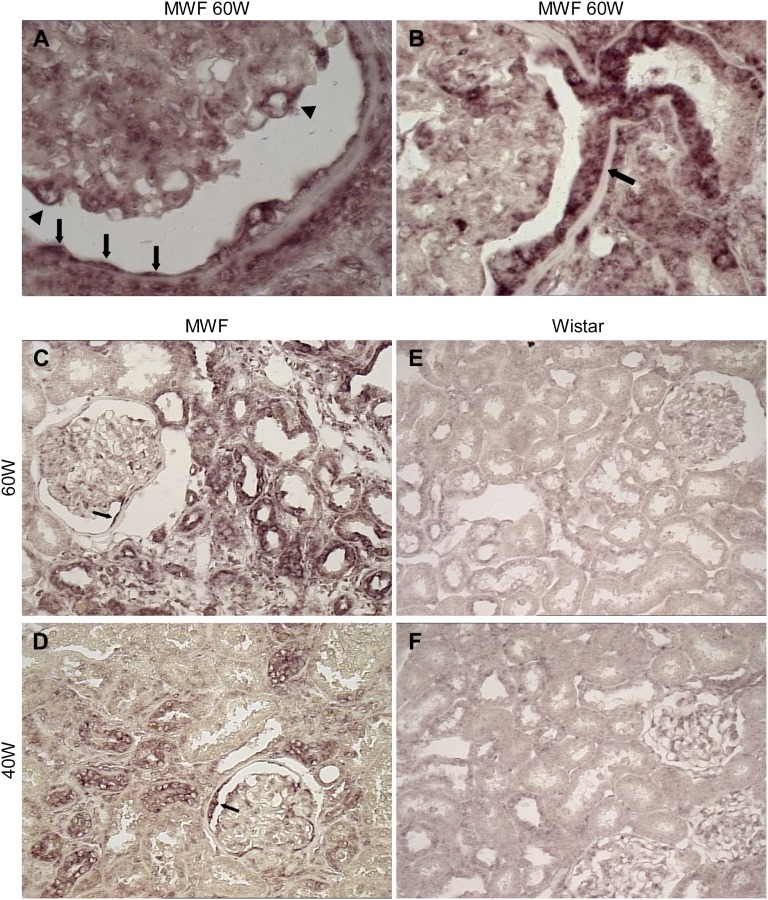
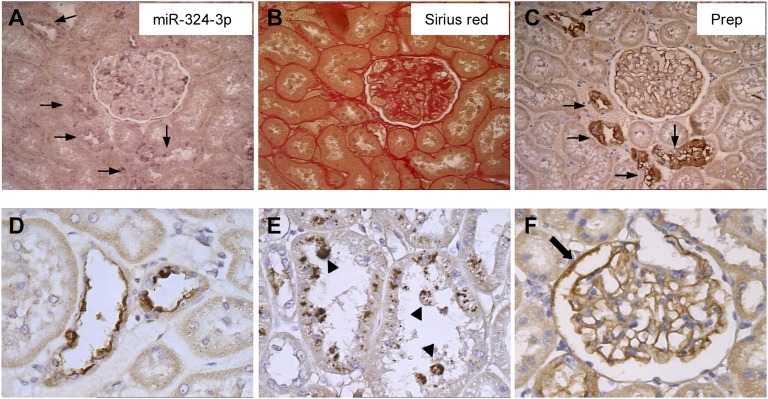
In situ hybridization of miR-324-3p in kidney specimens from MWF 60W revealed glomerular miRNA mainly associated with podocytes and focally, parietal epithelium, including cells at the urinary pole in continuity with tubule (Figure 2, A and B). Importantly, miR-324-3p was highly expressed in cortical tubules (Figure 2C). Positive signal was also observed in tubule clusters and glomeruli of kidneys from MWF rats studied for 40 weeks (40W) (Figure 2D). Wistar 60W and 40W showed more focal and weak glomerular and tubular miR-324-3p signal (Figure 2, E and F).
Figure 2.
miR-324-3p is upregulated in MWF kidney. Representative images are from in situ hybridization of miR-324-3p in kidney cortex from MWF and Wistar rats. (A) In MWF 60W, glomerular miR-324-3p expression was mainly associated with podocytes (arrowheads) and parietal epithelial cells of the Bowman’s capsule (arrows). (B) Strong positive signal was found in parietal cells at the urinary pole in continuity with the proximal tubule (arrow). Comparison of renal miR-324-3p expression between (C and D) MWF rats and (E and F) age-matched Wistar rats. (C) Strong upregulation of miR-324-3p in cortical tubules of MWF 60W. (D) Positive staining of miR-324-3p in both tubular epithelium and glomeruli of MWF 40W. The arrows in C and D mark focal staining of miR-324-3p in the Bowman’s capsule. (E and F) Focal and weak glomerular and tubular miR-324-3p signal in Wistar rats. Magnification, ×1000 in A and B; ×400 in C–F.
In Silico Identification of Potential Targets for miR-324-3p
We used an in silico approach (i.e., database search for targets using bioinformatic tools: microRNA.org, EIMMo miRNA target prediction, miRDB, and the TargetScan 5.1). The EIMMo miRNA target prediction server predicted 95 transcripts in kidney as possible miR-324-3p targets. Because miR-324-3p was markedly expressed both in glomeruli and tubules, we searched for potential targets not strictly related to podocyte injury but also renal scarring/fibrosis. Among miR-324-3p target gene transcripts found in all programs, prolyl endopeptidase (Prep) mRNA ranked within the 10 top-ranked transcripts using microRNA.org (Figure 3A). The translated protein Prep (EC 3.4.21.26) is a serine peptidase belonging to the prolyl oligopeptidase subfamily S9 clan SC, which digests angiotensins and small peptide-like hormones (including neuroactive peptides)21–23 and mediates generation of N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP),24 a tetrapeptide able to suppress the proliferation of cultured renal fibroblasts.25
Figure 3.
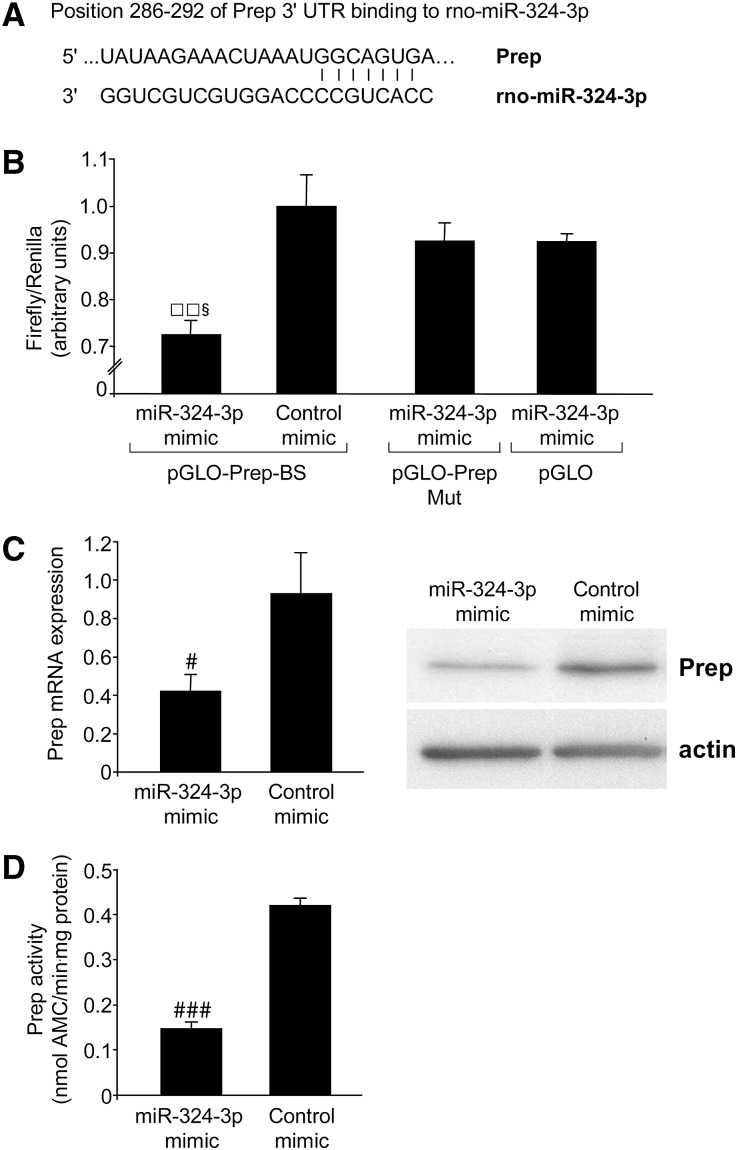
In vitro validation of Prep as a target of miR-324-3p. (A) A possible binding site in Prep 3′ UTR for miR-324-3p as predicted by TargetScan, EIMMo miRNA target prediction, miRDB, and microRNA.org. (B) miR target reporter luciferase assay after miR-324-3p mimic delivery in HEK293 cells. miR-324-3p significantly repressed luciferase activity in HEK293 cells transfected with pGLO-Prep-BS. No significant reduction was observed when cells were transfected with a plasmid containing a mutated binding site (pGLO-Prep-Mut) or an empty plasmid (pGLO). Results are expressed as mean ± SD. □□P<0.01 versus control mimic. §P<0.05 versus pGLO-Prep-Mut and pGLO. (C and D) Validation of the direct binding between miR-324-3p and Prep 3′ UTR in NRK cells naturally expressing Prep. NRK cells transiently transduced with miR-324-3p showed (C) significant reduction of Prep mRNA and protein expression and (D) lower protease activity with respect to control mimic-transfected cells. Results are mean ± SD. #P<0.05 and ###P<0.001 versus control mimic-transfected cells.
In Vitro Validation of Prep as a Target of miR-324-3p
We performed in vitro experiments to assess whether Prep was a target of miR-324-3p as predicted by bioinformatic analysis. We used the pmiRGLO Dual-Luciferase miRNA target expression vector to quantitatively evaluate miRNA activity by inserting miR-324-3p binding site (pGLO-Prep-BS) in the 3′ untranslated region (UTR) of the luciferase gene. Approximately 30% reduction of luciferase activity from HEK293 transfected with pGLO-Prep-BS plasmid and miR-324-3p mimic was observed compared with control mimic-transfected cells. Mutation of the target site prevented downregulation of luciferase activity by miR-324-3p (Figure 3B).
Depending on complementary base pairing, binding of miRNA to 3′ UTR of target mRNA may result in transcript degradation or translational repression. Normal rat kidney (NRK) cells, a rat proximal tubular cell line constitutively expressing Prep as detected by Western blot, were transiently transfected with miR-324-3p mimic. Real-time PCR showed a 55% reduction of Prep mRNA in NRK cells receiving miRNA mimic versus control mimic, indicating reduced mRNA stability after complementation with miRNA mimic (Figure 3C). Consistently, miR-324-3p acted post-transcriptionally to reduce Prep protein levels of 68% in NRK cells receiving miRNA mimic. Changes in Prep protein expression resulted in lower protein activity (Figure 3, C and D).
Reduced Prep Is Associated with miR-324-3p Overexpression in MWF Rats
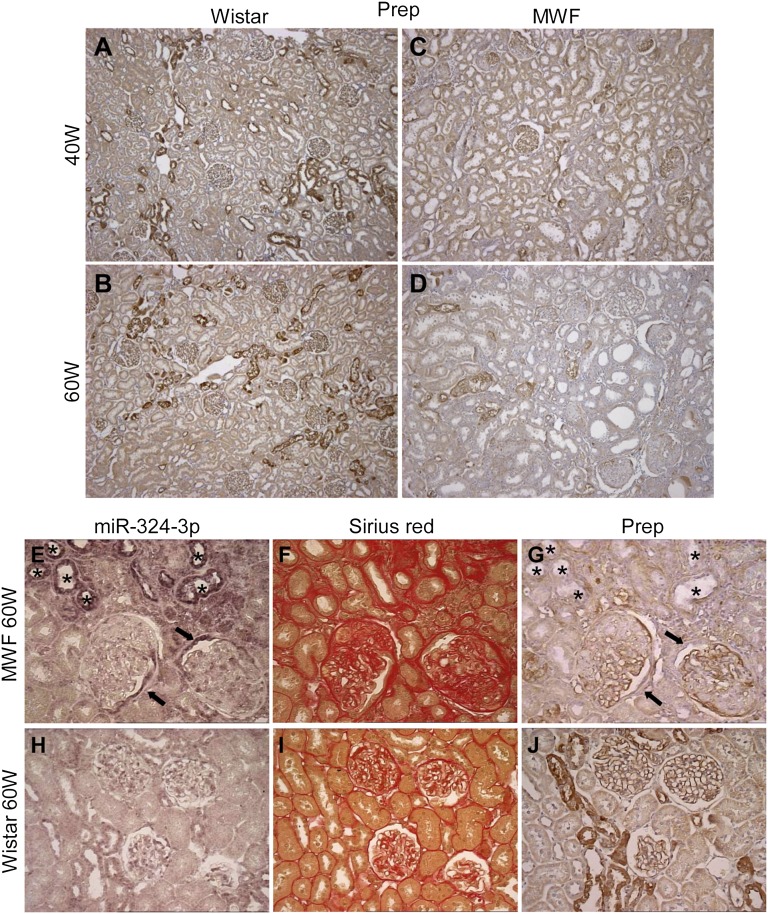
We investigated whether overexpressed miR-324-3p could account for downregulation of Prep in MWF rats. In Wistar 40W and 60W, labeling for Prep was uniformly distributed along glomerular capillary loops and detectable in clusters of cortical tubules (Figure 4, A and B). MWF rats showed reduced glomerular expression of Prep limited to focal areas of the tuft (Figure 4, C and D). Semiquantitative evaluation in nonsclerotic areas of glomeruli of MWF 60W showed a significant reduction of Prep staining compared with Wistar 60W (mean score was 0.81±0.03 versus 1.22±0.16, P<0.05). Moreover, percentages of glomeruli with low immunoreactivity (score=0.5) accounted for 40%±5% and 14%±7% in MWF 60W and Wistar 60W, respectively, whereas no peptidase expression was found in 10%±7% of glomeruli in MWF 60W versus 0 in Wistar 60W (P<0.05).
Figure 4.
Reduced Prep is associated with miR-324-3p overexpression in MWF rats. (A–D) Representative images of immunostaining for Prep in Wistar rats at 40 and 60 weeks of age show a strong positive signal in both glomeruli and tubule clusters that was markedly reduced in MWF rats. Staining for miR-324-3p, collagen, and Prep in adjacent kidney sections from (E–G) MWF 60W and (H–J) Wistar 60W. In MWF rats, (E) renal tubules strongly positive for miR-324-3p (asterisks) were (F) located in discrete fibrotic regions and (G) negative for Prep. (E and G) Focal areas of the Bowman’s capsule positive for miR-324-3p were negative for Prep (arrows). Magnification, ×100 in A–D; ×400 in E–J.
Prep staining in cortical tubules of MWF rats showed an overall focal distribution pattern associated with cell cytoplasm, which was also found in Wistar rats. At variance with the latter, some degree of intracellular granular staining was also found in the tubular epithelium. The volume density (Vv) of Prep-positive tubules in MWF 60W was halved compared with Wistar 60W (2.6%±0.7% versus 5.9%±1.6%, P<0.05). A reduction in Prep intensity was also observed (mean score was 0.55±0.01 versus 1.03±0.12, P<0.01). Staining of adjacent sections clearly showed that, in MWF 60W, tubular profiles highly expressing miR-324-3p were located in discrete fibrotic regions and invariably negative for Prep (Figure 4, E–G).
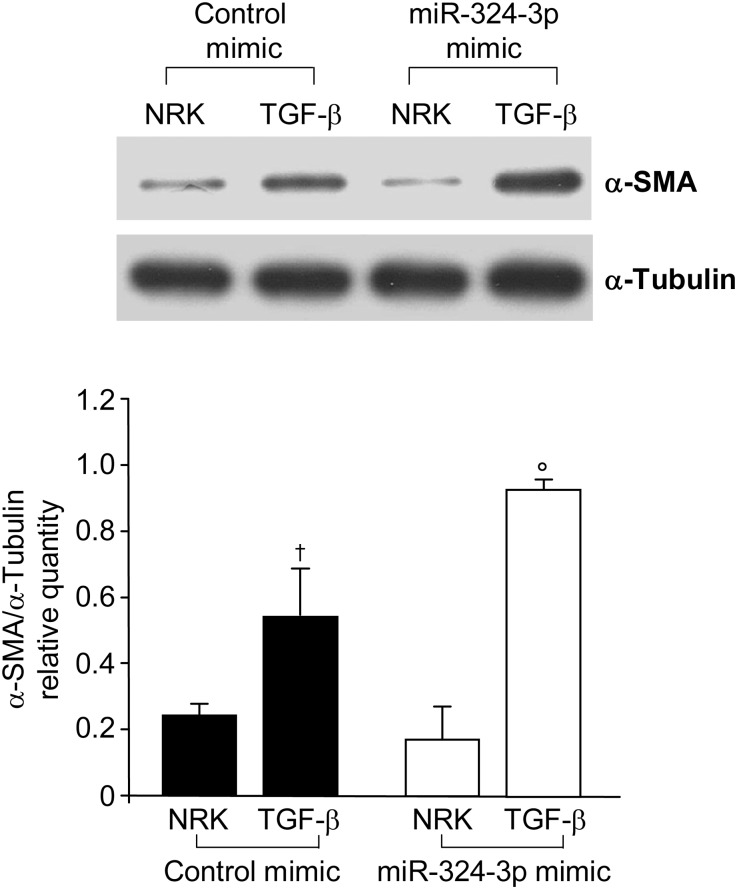
To clarify whether a decrease of Prep could render NRK cells more susceptible to a profibrotic environment, we evaluated the effect of dysregulation of miR-324-3p/Prep on the expression of α-smooth muscle actin (α-SMA; an index of fibrosis induction) and its change on TGF-β stimulation. Transfection of NRK cells with miR-324-3p did not affect α-SMA expression (Figure 5). TGF-β–treated NRK cells showed α-SMA upregulation, which was further significantly increased in the presence of miR-324-3p–dependent Prep downregulation (Figure 5).
Figure 5.
Effect of miR-324-3p on NRK cells stimulated with TGF-β. Western blot analysis and quantification of α-SMA on cell extracts from NRK cells transfected with control mimic or miR-324-3p in the absence or presence of TGF-β. †P<0.05 versus NRK + control mimic. °P<0.05 versus NRK + TGF-β + control mimic.
Effect of ACE Inhibition on miR-324-3p and Its Target Prep
Compared with MWF 60W, lisinopril-treated MWF rats showed lower miR-324-3p and higher Prep expression (mean score was 0.80±0.08 versus 0.55±0.01, P<0.05) in cortical tubules (Figure 6, A–C), although Vv of Prep-positive tubules did not significantly change (3%±0.5% versus 2.6%±0.7%). Prep protein expression pattern in tubules was also different; other than preserved diffuse cytoplasmic staining (Figure 6C), the epithelium showed Prep reactivity concentrated in the apical region (Figure 6D) and more evident granular structures within cells and tubular lumens (Figure 6E).
Figure 6.
Effect of ACE inhibition on miR-324-3p and its target Prep. (A–C) Staining for miR-324-3p, collagen, and Prep in adjacent kidney sections from lisinopril-treated MWF rats. (A) Renal tubules with mild miR-324-3p staining (arrows) within (B) renal parenchyma with no collagen deposition (C) expressed strong Prep signal. (D and E) Heterogeneous pattern of Prep protein expression in renal tubules of ACEi-treated MWF rats: Prep immune reactivity in the (D) apical region and (E) granular structures within cells and tubular lumens (arrowhead). (F) Prep staining in the glomerular capillary tuft and focal areas of the Bowman’s capsule (arrow). Magnification, ×400 in A–C and F; ×1000 in D and E.
ACEi reduced the percentage of glomeruli with miR-324-3p–positive Bowman’s capsules (from 68%±17% to 16%±11%, P<0.05 versus MWF 60W). All glomeruli from lisinopril-treated MWF rats were positive for Prep (mean score was 1.02±0.24 versus 0.81±0.03). Moreover, Prep staining became detectable in Bowman’s capsule (Figure 6F).
Modulation of Ac-SDKP Contributes to Reduction of Interstitial Fibrosis by ACEi
In addition to being generated by Prep-mediated enzymatic activity, Ac-SDKP is a substrate of the N-terminal active domain of ACE that, by hydrolyzing the tetrapeptide, contributes to its metabolic clearance.26,27 We, therefore, asked whether MWF rats could exhibit changes in antifibrotic Ac-SDKP levels that may reflect altered Prep-dependent generation and/or ACE-mediated clearance of the peptide, and also, we asked whether they could be modulated as part of the renoprotective action of ACEi.
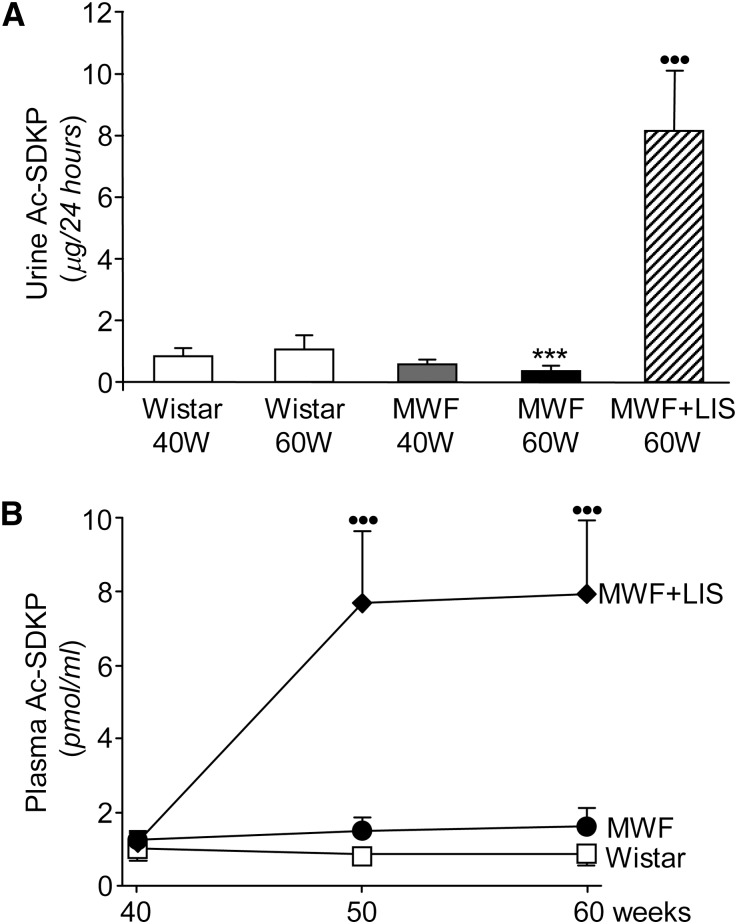
MWF 60W showed a 67% reduction in urinary Ac-SDKP excretion compared with Wistar 60W (Figure 7A) and developed severe interstitial fibrosis, which was documented by deposition of Sirius red-positive collagen (Supplemental Figure 1C). Conversely, renal levels of Ac-SDKP were higher than controls in MWF 40W and did not further increase significantly at 60W (MWF 40W and 60W were 251±59 and 286±80 pg/mg kidney cortex, respectively, and Wistar 40W and 60W were 113±15 and 143±19 pg/mg kidney cortex, respectively), suggesting unbalanced generation/clearance of peptide engaged in antifibrotic pathway. Lisinopril-treated MWF rats at 60W did show a marked increase of urinary Ac-SDKP concentration (23-fold) (Figure 7A) and partial normalization of renal Ac-SDKP (183±55 pg/mg kidney cortex). Notably, plasma Ac-SDKP did not change over time either in Wistar or MWF (Figure 7B) but was markedly increased by ACEi (Figure 7B), which was paralleled by significantly reduced interstitial fibrosis (Supplemental Figure 1D).
Figure 7.
Effect of ACE inhibition on Ac-SDKP in MWF rats. (A) Urine and (B) plasma concentrations of Ac-SDKP in Wistar rats and untreated or lisinopril-treated MWF rats. Untreated MWF 60W showed urine Ac-SDKP excretion lower than Wistar 60W. ACE inhibition significantly raised urine and plasma concentration of Ac-SDKP in MWF rats. Results are mean ± SD. ***P<0.001 versus Wistar 60W. ••••••P<0.001 versus untreated MWF rats and Wistar rats.
Discussion
In the present study, analysis of miRNA expression profile in kidneys of MWF rats with advanced nephropathy has shown that, among differentially expressed miRNAs, miR-324-3p is the most upregulated miRNA within glomeruli. Importantly, in situ hybridization analysis revealed abnormal expression of miR-324-3p in both glomeruli and tubular epithelium along individual nephrons, highlighting the activation of a miRNA-dependent regulatory pathway that may play a role in the development of chronic parenchymal lesions of MWF rats. Previous reports have shown the functional importance of miRNAs in glomerular injury.28,29 Mice with podocyte-specific deletion of Dicer, a RNAase III required for production of mature miRNAs, developed proteinuria and glomerulosclerosis.28,29 Podocyte-specific deletion of Drosha (i.e., another RNAase III required for production of pre-miRNA within nucleus) resulted in a similar phenotype, confirming the role for miRNAs in the maintenance of normal function of mature podocytes that is disrupted in podocytopathies.30 Despite the recognized role of the miR-30 family in podocyte homeostasis,28,29 the finding here that glomerular levels of miR-30 family were unchanged rules out its likely involvement in an intranephron pathway underlying propagation of glomerular injury in MWF rats. Similarly, upregulation of miR-192 was previously reported in both diabetic glomeruli and whole kidney of animals with nondiabetic proteinuric nephropathies31,32 possibly explaining TGF-β–mediated injury. However, comparable glomerular miRNA-192 expression here in MWF and controls excluded miR-192 from additional analysis in the present study.
In glomeruli, miR-324-3p was expressed in podocytes and parietal cells of Bowman’s capsule. The latter cells, which include progenitor cells, proliferate abnormally and acquire a migratory phenotype, leading to crescent formation and subsequent sclerosis during progression of disease in MWF rats.33 Whether upregulated miR-324-3p might modulate targets involved in podocyte dysfunction and abnormal behavior of parietal epithelial cells deserves ad hoc investigation. Here, strong expression of miR-324-3p in cortical tubules of MWF rats suggests a wide range of modulation of renal target gene transcripts not restricted to the glomerular compartment.
Tubulointerstitial deposition of extracellular matrix proteins, tubular atrophy, and tubular dilatation are common findings in chronic proteinuric nephropathies, including advanced disease of MWF rats.7 Highly upregulated miR-324-3p prompted us to investigate whether miR-324-3p may play a role in the fibrotic process. Given the remarkably antifibrotic effect afforded by ACEi in the MWF strain, we also focused on miR-324-3p targets possibly involved in the metabolism of small peptides, including angiotensins and/or related pro- or antifibrogenic factors. Bioinformatic algorithms indicated that the serine peptidase Prep was a miR-324-3p target involved in formation of both Ang (1–7) and the antifibrotic peptide Ac-SDKP. Our data, which showed markedly reduced Prep expression and activity in NRK cells transiently transduced with miR-324-3p mimic, validated this prediction, establishing a causal link between miR-324-3p and its target Prep function in renal tubular cells.
In vivo, overexpression of miR-324-3p was associated with reduced Prep expression in both glomeruli and cortical tubules in MWF rats, in fibrotic areas, and after the initial stages of tubulointerstitial damage. Findings that NRK cells transfected with miR-324-3p downregulated Prep expression, resulting in higher susceptibility to developing a fibrogenic phenotype in response to TGF-β, directly document a feedback loop-sustaining fibrosis.
The kidney shows high Prep mRNA expression compared with other organs and also displays abundant protein34 and high enzyme activity in both rats and humans.35,36 Prep cleaves the peptide bond at the C-terminal side of an internal proline (-Pro-Xaa, where Xaa is any amino acid other than proline) of short 30-mer peptides. Peptides larger than this size do not gain access to the active site of the enzyme and are spared from proteolysis.22,23 A role for Prep in the regulation of BP37 has been proposed, because substrates of Prep include Ang I and II, which are metabolized by Prep to Ang (1–7) and counteract the pressor, proliferative, profibrotic, and prothrombotic effects of Ang II.38
Prep mediates generation of Ac-SDKP from the thymosin β4 precursor24 that plays a homeostatic role in collagen balance, which is shown by evidence that low endogenous levels of Ac-SDKP in the heart and kidneys promote and/or accelerate organ fibrosis in the presence of profibrotic stimuli, such as Ang II.39 In parallel, from our findings, decreased urinary excretion of Ac-SDKP was associated with renal fibrosis in MWF rats, and importantly, the underlying profibrogenic stimulation is attributed to miR-324-3p–dependent downregulation of Prep, which was documented here in NRK cells and with the use of combined in situ hybridization and immunohistochemical analysis in fibrotic areas.
Here, renal upregulation of miR-324-3p and reduced Prep expression in MWF rats were accompanied by increased renal Ac-SDKP compared with Wistar rats. It is tempting to speculate that Ac-SDKP levels can be modulated by the surrounding profibrotic environment as a compensatory mechanism accounting for unbalanced generation/clearance of peptide. We cannot exclude the importance of other peptidases. However, Prep downregulation greatly limits the full activation of an effective Ac-SDKP antifibrotic pathway in MWF kidney.
Additional support for the role of miR-324-3p in the development of interstitial fibrosis in MWF rats is the finding that the antifibrotic effect of ACEi was accompanied by combined downregulation of miR-324-3p, increased Prep expression in tubular epithelium, and partial normalization of Ac-SDKP in kidney cortex. Prep-positive granular structures in tubular lumens of lisinopril-treated MWF rats could vehicle Prep release into the urine,40 which is in line with Prep activity detectable in tubular fluid collected from proximal and distal portions.41 Lisinopril-treated MWF rats did show dramatic increases in urinary and, to a lesser extent, plasma Ac-SDKP attributable to the action of ACEi of reducing upstream miR-324-3p overexpression at the first step of the Prep-mediated pathway in the tubule. Thus, ACEi (by this action) may exert antifibrotic effects complementary to Ang II inhibition. Consistently, previous evidence showed attenuation of antifibrotic and anti-inflammatory effects of ACEi by anti–Ac-SDKP antibody in aldosterone salt-induced hypertension.42
In summary, dysregulation of miR-324-3p/Prep complex contributes to the progression of experimental proteinuric nephropathy. Reduced Prep expression impairs pathways underlying Ang I/II metabolism and Ac-SDKP synthesis and may sensitize MWF rats to Ang II and TGF-β, which favor fibrosis. Our findings suggest that miR-324-3p is a potential target of both ACE inhibition and future drug approaches to fully halt fibrogenic events by post-transcriptional regulation of Prep and modulation of downstream pathways.
Concise Methods
Additional details regarding the materials and procedures are provided in Supplemental Material.
Study Design
Twenty male MWF rats from our colony and eight male Wistar rats (Harlan Nossan, Udine, Italy) were used in this study. MWF rats were divided into five groups. Group 1 (n=5) consisted of untreated male MWF 40W rats. Group 2 (n=7) was untreated MWF 60W rats. Group 3 (n=8) was treated with a high dose of ACEi lisinopril in drinking water (80 mg/L) from 40 to 60 weeks of age. Wistar rats were either studied at 40 weeks of age (group 4; n=4) or followed from 40 to 60 weeks of age (group 5; n=4) and used as controls (Wistar 40W and Wistar 60W, respectively). Systolic BP, urinary protein excretion, and serum creatinine were measured by conventional methods.8 Systolic BP and kidney functional parameters are reported in Table 1.
Table 1.
BP and kidney functional parameters
| Age (wk) | MWF (n=7) | MWF + Lisinoprila (n=8) | Wistar (n=4) |
|---|---|---|---|
| SBP (mmHg) | |||
| 40 | 197±13 | 186±14 | 135±7b |
| 50 | 206±15 | 126±9c | 124±7c |
| 60 | 213±11d | 126±8c | 136±5c |
| UPE (mg/24 h) | |||
| 40 | 504±56 | 463±64 | 17±3b |
| 50 | 610±112 | 250±80cd | 19±4b |
| 60 | 663±91 | 249±88cd | 16±6b |
| SCrea (mg/dl) | |||
| 40 | 0.57±0.12 | 0.57±0.32 | 0.33±0.06 |
| 50 | 0.77±0.30d | 0.66±0.14 | 0.34±0.04 |
| 60 | 1.78±1.38de | 0.61±0.08c | 0.32±0.07 |
Data are mean ± SD. MWF, Munich Wistar Fromter rats; SBP, systolic BP; UPE, urinary protein excretion; SCrea, serum creatinine.
SBP, UPE, and SCrea in MWF + Lisinopril at 40 weeks of age are basal pretreatment values.
P<0.01 versus MWF and MWF + Lisinopril at the same age.
P<0.01 versus MWF at the same age.
P<0.01 versus 40 weeks of age within the same groups.
P<0.05 versus 50 weeks of age within the same groups.
All animals were maintained in a temperature-controlled room regulated with a 12-hour light/dark cycle, and they had free access to water and food. Animal care and treatment conformed to the institutional guidelines that comply with national and international laws and policies. After the animal was euthanized, one kidney was perfused under anesthesia with PBS, and midcoronal sections were taken and appropriately processed for morphologic and biochemical determinations (see below). The other kidney was rapidly frozen in liquid nitrogen.
Sirius Red for Collagen Staining
Kidney sections were stained with picro-sirius red, and the Vv value of interstitial collagen in the cortex was estimated using computer-based analysis, which is described in detail in Supplemental Material.
Laser Capture Microdissection and miRNA Expression Profiling
Frozen renal tissue specimens were subjected to laser capture microdissection with the Palm-Axiovert 200 System (Zeiss, Jena, Germany) according to the manufacturer’s suggestions. Briefly, serial 10-μm-thick cut sections were mounted on a polyethylene naphthalane membrane slide (Palm Membrane Slides), fixed in 70% ice cold ethanol, and counterstained with Mayer’s Hematoxylin solution and Eosin Y. Whole glomerular structures corresponding to a total number of 30,000 cells (8×106 mm2) were microdissected from MWF 60W (n=3) and Wistar 60W (n=3), respectively. Microdissected glomeruli were collected for RNA extraction using the TaqMan microRNA Cells-to-Ct kit (Ambion, Huntingdon, United Kingdom), and then, for analysis of miRNA expression, the TaqMan Low-Density array microRNA panel 1 was performed43; 5000 cells were used for each 384-well microfluid card that contains primers and probes for different miRNAs (Applied Biosystems, Warrington, United Kingdom). Data analysis was performed by using RQ Manager software version 1.2 (Applied Biosystems) to define miRNAs that were differentially expressed between MWF and control Wistar rats.
Real-Time PCR
Levels of the miR-324-3p, normalized to endogenous control U6, were assessed with a specific TaqMan miRNA assay (Applied Biosystems) and quantitatively analyzed by comparing experimental levels with standard curves generated with serial dilutions of the same positive sample. Taq-Man RT-PCR was performed as described previously.44
In Situ Hybridization
The expression of individual miRNAs was confirmed by locked nucleic acid (LNA) -based in situ hybridization; 5′-dig–labeled LNA probes with approximately 30% LNA base insertions (Exiqon) were used to detect mature miRNAs (Supplemental Material). U6 and scramble probes were used as positive and negative controls, respectively.
In Silico Predicted miR-324-3p Targets
Four algorithms were used to computationally predict targets of differentially expressed miRNAs. They included TargetScan 5.1 (http://www.targetscan.org/), the EIMMo miRNA target prediction server (http://www.mirz.unibas.ch/ElMMo3/), the miRDB (http://mirdb.org/miRDB/), and microRNA.org (http://www.microrna.org).
miR Target Reporter Luciferase Assay
pGLO-Prep-BS was cloned in the 3′ UTR of the firefly luciferase gene in the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega). HEK293 cells were grown in DMEM supplemented with 10% (vol/vol) heat-inactivated FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin; 1 day before transfection, cells were seeded in a 24-well plate at 8×104 cells/well (∼90% confluence). Cells were cotransfected with 100 ng respective plasmid and 100 nmol/L miR-324-3p or control mimic using Lipofectamine 2000 (Invitrogen, Life Technologies, Milan, Italy). Cells were lysed after 48 hours, and luciferase activity was determined using the dual-luciferase reporter assay system (Promega). To confirm the specificity of the inhibition, the reporter assay was carried out using the pmiRGLO containing a mutated binding site of miR-324-3p (pGLO-Prep-Mut) or an empty vector (pGLO). Data are presented as the ratio of firefly to renilla luciferase activity.
Cell Culture and Transfection of miRNA Mimic Precursor
NRK cells, a rat proximal tubular epithelial cell line (DSMZ, Braunschweig, Germany), were cultured in a humidified chamber at 37°C and 5% CO2 in DMEM containing 4.5 g/L glucose supplemented with 10% FBS, 2 mmol/L l-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin; 1 day before transfection, NRK cells were seeded in a six-well plate at 3×105 cells/well. Lipofectamine 2000 transfection reagent (Invitrogen) was employed to transfect cells with syn-rno-miR-324-3p mimic (100 nmol/L, MSY0000554; Qiagen S.r.l., Milan, Italy) in OPTIMEM Medium. Control cells were transfected with Silencer Negative Control siRNA (Control mimic, 100 nmol/L; Ambion). Samples were harvested 48 hours later for real-time PCR of Prep mRNA expression, and samples were harvested 72 hours later for Western blot analysis of Prep protein (Supplemental Material) and activity assay. For specific experiments, TGF-β was added at the concentration of 10 ng/ml.
Immunohistochemistry
Immunostaining for Prep was performed on kidney sections using a goat anti-Prep antibody (Santa Cruz Biotechnology, Santa Cruz, CA) as the primary antibody. The Vv of Prep-positive tubules was estimated by point counting according to the work by Weibel.45 A semiquantitative analysis of Prep staining was assessed in both tubules and glomeruli by giving a score of zero to three. Methodological details are in Supplemental Material.
Prep Activity
Prep activity in NRK cells transfected with miR-324-3p mimic or control mimic was assayed using the fluorogenic substrate Suc-Gly-Pro-amino methylcoumarin.46 Data are expressed as nanomoles of amino methylcoumarin per minute × milligram protein.
Ac-SDKP Assay
Ac-SDKP in the kidney cortex, plasma, and urine was measured using a commercially available enzyme immunoassay kit (Bertin Pharma, Artigues Pres Bordeaux, France).
Statistical Analyses
Results are expressed as mean ± SD. Data analysis was performed using the computer software Prism (GraphPad Software, Inc., San Diego, CA). Comparisons were made by ANOVA with Bonferroni posthoc test or unpaired t test as appropriate. Kruskal–Wallis test was used for nonparametric data (Prep score). Statistical significance was defined as P<0.05.
Disclosures
None.
Acknowledgments
We thank Michela Cunietti and Daniela Cavallotti for the excellent technical assistance and Andrea Remuzzi for helpful discussion.
P.T. and P.R. are recipients of a fellowship from Fondazione Aiuti per la Ricerca sulle Malattie Rare, Bergamo, Italy. This study was supported in part by the Commission of the European Communities within EuReGene Project LSHG-CT-2004-005085.
Part of this work was presented at the American Society of Nephrology Kidney Week 2011 Annual Meeting held November 8–13 in Philadelphia, PA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Angiotensin Converting Enzyme Inhibitor–Modulated MicroRNAs Targeting Renal Fibrosis,” on pages 1441–1443.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011121144/-/DCSupplemental.
References
- 1.Rovira-Halbach G, Alt JM, Brunkhorst R, Frei U, Kühn K, Stolte H: Single nephron hyperfiltration and proteinuria in a newly selected rat strain with superficial glomeruli. Ren Physiol 9: 317–325, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Fassi A, Sangalli F, Maffi R, Colombi F, Mohamed EI, Brenner BM, Remuzzi G, Remuzzi A: Progressive glomerular injury in the MWF rat is predicted by inborn nephron deficit. J Am Soc Nephrol 9: 1399–1406, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Remuzzi A, Puntorieri S, Mazzoleni A, Remuzzi G: Sex related differences in glomerular ultrafiltration and proteinuria in Munich-Wistar rats. Kidney Int 34: 481–486, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Remuzzi A, Puntorieri S, Alfano M, Macconi D, Abbate M, Bertani T, Remuzzi G: Pathophysiologic implications of proteinuria in a rat model of progressive glomerular injury. Lab Invest 67: 572–579, 1992 [PubMed] [Google Scholar]
- 5.Macconi D, Abbate M, Morigi M, Angioletti S, Mister M, Buelli S, Bonomelli M, Mundel P, Endlich K, Remuzzi A, Remuzzi G: Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am J Pathol 168: 1073–1085, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapinski R, Perico N, Remuzzi A, Sangalli F, Benigni A, Remuzzi G: Angiotensin II modulates glomerular capillary permselectivity in rat isolated perfused kidney. J Am Soc Nephrol 7: 653–660, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Remuzzi A, Gagliardini E, Sangalli F, Bonomelli M, Piccinelli M, Benigni A, Remuzzi G: ACE inhibition reduces glomerulosclerosis and regenerates glomerular tissue in a model of progressive renal disease. Kidney Int 69: 1124–1130, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A: Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol 174: 797–807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remuzzi G, Benigni A, Remuzzi A: Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116: 288–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macconi D: Targeting the renin angiotensin system for remission/regression of chronic kidney disease. Histol Histopathol 25: 655–668, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Remuzzi A, Gagliardini E, Donadoni C, Fassi A, Sangalli F, Lepre MS, Remuzzi G, Benigni A: Effect of angiotensin II antagonism on the regression of kidney disease in the rat. Kidney Int 62: 885–894, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB: Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol 22: 176–186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz A, Standke D, Kovacevic L, Mostler M, Kossmehl P, Stoll M, Kreutz R: A major gene locus links early onset albuminuria with renal interstitial fibrosis in the MWF rat with polygenetic albuminuria. J Am Soc Nephrol 14: 3081–3089, 2003 [DOI] [PubMed] [Google Scholar]
- 14.van Es N, Schulz A, Ijpelaar D, van der Wal A, Kuhn K, Schütten S, Kossmehl P, Nyengaard JR, de Heer E, Kreutz R: Elimination of severe albuminuria in aging hypertensive rats by exchange of 2 chromosomes in double-consomic rats. Hypertension 58: 219–224, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Pillai RS, Bhattacharyya SN, Filipowicz W: Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell Biol 17: 118–126, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Nilsen TW: Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet 23: 243–249, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kloosterman WP, Plasterk RH: The diverse functions of microRNAs in animal development and disease. Dev Cell 11: 441–450, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kato M, Arce L, Natarajan R: MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol 4: 1255–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oba S, Kumano S, Suzuki E, Nishimatsu H, Takahashi M, Takamori H, Kasuya M, Ogawa Y, Sato K, Kimura K, Homma Y, Hirata Y, Fujita T: miR-200b precursor can ameliorate renal tubulointerstitial fibrosis. PLoS One 5: e13614, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorenzen JM, Haller H, Thum T: MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nat Rev Nephrol 7: 286–294, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Rea D, Fülöp V: Structure-function properties of prolyl oligopeptidase family enzymes. Cell Biochem Biophys 44: 349–365, 2006 [DOI] [PubMed] [Google Scholar]
- 22.García-Horsman JA, Männistö PT, Venäläinen JI: On the role of prolyl oligopeptidase in health and disease. Neuropeptides 41: 1–24, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Szeltner Z, Polgár L: Structure, function and biological relevance of prolyl oligopeptidase. Curr Protein Pept Sci 9: 96–107, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Cavasin MA, Rhaleb NE, Yang XP, Carretero OA: Prolyl oligopeptidase is involved in release of the antifibrotic peptide Ac-SDKP. Hypertension 43: 1140–1145, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwamoto N, Xano HJ, Yoshioka T, Shiraga H, Nitta K, Muraki T, Ito K: Acetyl-seryl-aspartyl-lysyl-proline is a novel natural cell cycle regulator of renal cells. Life Sci 66: PL221–PL226, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Rousseau A, Michaud A, Chauvet MT, Lenfant M, Corvol P: The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J Biol Chem 270: 3656–3661, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Fuchs S, Xiao HD, Cole JM, Adams JW, Frenzel K, Michaud A, Zhao H, Keshelava G, Capecchi MR, Corvol P, Bernstein KE: Role of the N-terminal catalytic domain of angiotensin-converting enzyme investigated by targeted inactivation in mice. J Biol Chem 279: 15946–15953, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Shi S, Yu L, Chiu C, Sun Y, Chen J, Khitrov G, Merkenschlager M, Holzman LB, Zhang W, Mundel P, Bottinger EP: Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol 19: 2159–2169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH: Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol 19: 2150–2158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhdanova O, Srivastava S, Di L, Li Z, Tchelebi L, Dworkin S, Johnstone DB, Zavadil J, Chong MM, Littman DR, Holzman LB, Barisoni L, Skolnik EY: The inducible deletion of Drosha and microRNAs in mature podocytes results in a collapsing glomerulopathy. Kidney Int 80: 719–730, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R: MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A 104: 3432–3437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung AC, Huang XR, Meng X, Lan HY: miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol 21: 1317–1325, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, Ghezzi S, Remuzzi A, Remuzzi G: Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol 179: 628–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myöhänen TT, García-Horsman JA, Tenorio-Laranga J, Männistö PT: Issues about the physiological functions of prolyl oligopeptidase based on its discordant spatial association with substrates and inconsistencies among mRNA, protein levels, and enzymatic activity. J Histochem Cytochem 57: 831–848, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuse Y, Polk DH, Lam RW, Reviczky AL, Fisher DA: Distribution and ontogeny of thyrotropin-releasing hormone degrading enzymes in rats. Am J Physiol 259: E787–E791, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Goossens F, De Meester I, Vanhoof G, Scharpé S: Distribution of prolyl oligopeptidase in human peripheral tissues and body fluids. Eur J Clin Chem Clin Biochem 34: 17–22, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Welches WR, Brosnihan KB, Ferrario CM: A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: Angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci 52: 1461–1480, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Dilauro M, Burns KD: Angiotensin-(1-7) and its effects in the kidney. ScientificWorldJournal 9: 522–535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavasin MA, Liao TD, Yang XP, Yang JJ, Carretero OA: Decreased endogenous levels of Ac-SDKP promote organ fibrosis. Hypertension 50: 130–136, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Casarini DE, Alves KB, Araújo MS, Stella RC: Endopeptidase and carboxypeptidase activities in human urine which hydrolyze bradykinin. Braz J Med Biol Res 25: 219–229, 1992 [PubMed] [Google Scholar]
- 41.Casarini DE, Boim MA, Stella RC, Schor N: Endopeptidases (kininases) are able to hydrolyze kinins in tubular fluid along the rat nephron. Am J Physiol 277: F66–F74, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Peng H, Carretero OA, Liao TD, Peterson EL, Rhaleb NE: Role of N-acetyl-seryl-aspartyl-lysyl-proline in the antifibrotic and anti-inflammatory effects of the angiotensin-converting enzyme inhibitor captopril in hypertension. Hypertension 49: 695–703, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anglicheau D, Sharma VK, Ding R, Hummel A, Snopkowski C, Dadhania D, Seshan SV, Suthanthiran M: MicroRNA expression profiles predictive of human renal allograft status. Proc Natl Acad Sci U S A 106: 5330–5335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P: An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 197: 1537–1549, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weibel ER: Practical Methods for Biological Morphometry. Stereological Methods, London, Academic Press, 1979 [Google Scholar]
- 46.Myöhänen TT, Venäläinen JI, García-Horsman JA, Piltonen M, Männistö PT: Distribution of prolyl oligopeptidase in the mouse whole-body sections and peripheral tissues. Histochem Cell Biol 130: 993–1003, 2008 [DOI] [PubMed] [Google Scholar]