Abstract
In January of 2004, the Centers for Medicare & Medicaid Services tied provider reimbursement for outpatient hemodialysis services to the number of provider–patient visits per month. We aimed to determine whether greater visit frequency associated with lower mortality and hospitalization rates among incident hemodialysis patients in a large, nationally representative contemporary cohort. Using US Renal Data System data for 130,892 patients who initiated in-center hemodialysis between October 1, 2003 and September 30, 2006, we determined associations between the frequency of provider visits and mortality, first hospitalization, multiple hospitalizations, and cause-specific hospitalizations. Our primary analysis used Cox proportional hazards models, but we also performed time-varying Cox proportional hazards and instrumental variable analyses. In the primary analysis, we did not detect a significance difference in mortality among patients with four provider visits per month compared with those patients with fewer provider visits (adjusted HR=0.98; 95% CI=0.96–1.01), but the risk for first hospitalization was 4% lower among those patients with more frequent visits (adjusted HR=0.96; 95% CI=0.95–0.97). The time-varying Cox analysis produced similar results. The fully adjusted instrumental variable analysis showed a 0.07% higher risk for death that was not statistically significant (P=0.88) but a significant 2.3% lower risk for first hospitalization (P=0.001) for patients with four provider visits per month. In summary, greater frequency of provider visits to hemodialysis patients associates with a small but significant reduction in hospitalizations, but it does not consistently associate with lower risk for death.
The optimal provider–patient visit interval remains unknown in many clinical settings.1–3 Patients with ESRD have a high rate of morbidity and mortality and account for a disproportionate share of healthcare dollars.4 Based on two small observational studies that suggested an association between greater provider–patient interaction and improved patient survival,5,6 increasing the frequency of provider visits with hemodialysis patients has received attention as a potentially useful intervention. Although its efficacy is uncertain, increased visit frequency might modify high morbidity and mortality through rapid identification and treatment of acute conditions and improved management of chronic conditions. In January of 2004, in an attempt to improve patient outcomes, the Centers for Medicare & Medicaid Services (CMS) tied provider reimbursement for outpatient hemodialysis services to the number of times per month providers see their patients.7 Before the reimbursement policy change, providers received a capitated fee per hemodialysis patient-month of care provided, irrespective of the number of monthly visits. Under the new policy, providers could incrementally bill CMS based on the number of visits per month.7 This policy change resulted in increased provider–patient visit frequency8 and created an optimal model for evaluation of the association between provider visit frequency and patient outcomes.
Unlike traditional visit frequency, which is primarily determined by patient indications, visit frequency for dialysis patients is determined by providers based on several factors other than illness severity, including reimbursement and travel distance. After a provider has expended the effort to visit a dialysis facility, all patients at the facility are seen, irrespective of severity of illness. Also, patients choose a dialysis facility based on proximity to their residence and not on severity of illness. Thus, using a model of frequency of provider visits to hemodialysis patients, indication bias is minimized when analyzing the association between provider visit frequency and patient outcomes. We aimed to determine whether greater provider–patient visit frequency was associated with lower mortality and hospitalization rates among incident hemodialysis patients in a large, nationally representative contemporary cohort.
Results
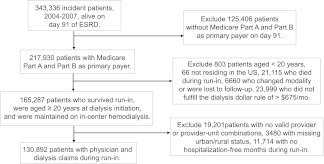
The study cohort included 130,892 patients (Figure 1). During the run-in period, 39.6% and 60.4% of patients were seen by their providers during dialysis less than four and four times per month, respectively. Hemodialysis patients who were seen less frequently (less than four visits per month) by their providers were younger (38.6% versus 36.8% aged <65 years), less likely to be hospitalized during the run-in period (72.0% versus 65.2% with 0 hospital days), and more likely to be white (65.9% versus 64.0%), live in rural areas (32.4% versus 26.6%), have lower socioeconomic status (SES; 49.0% versus 47.5% with SES score below median), and attend fewer dialysis sessions during the run-in period (27.3% versus 16.7% with <38 sessions); absolute differences in some of these measures were small (Table 1).
Figure 1.
Incident cohort flow chart.
Table 1.
Baseline characteristics by visit frequency
| Characteristics | All n (%) | Exposure Variable (Mean Number of Monthly Visits to Incident Patients During Run-In) | Instrumental Variable (Mean Number of Monthly Visits to Prevalent Patients) | ||||
|---|---|---|---|---|---|---|---|
| <4 | ≥4 | Absolute Difference | <3.7 | ≥3.7 | Absolute Difference | ||
| All | 130,892 (100) | ||||||
| Age (years) | |||||||
| 20–44 | 11,566 (8.8) | 9.3 | 8.5 | 0.8 | 8.9 | 8.8 | 0.1 |
| 45–64 | 37,535 (28.7) | 29.3 | 28.3 | 1.0 | 29.0 | 28.4 | 0.6 |
| 65–74 | 38,708 (29.6) | 29.4 | 29.7 | −0.3 | 29.6 | 29.6 | 0.0 |
| ≥75 | 43,083 (32.9) | 32.0 | 33.5 | −1.5 | 32.6 | 33.3 | −0.7 |
| Women | 61,038 (46.6) | 46.4 | 46.8 | −0.4 | 46.8 | 46.5 | 0.3 |
| Race | |||||||
| white | 84,723 (64.7) | 65.9 | 64.0 | 1.9 | 65.2 | 64.2 | 1.0 |
| African-American | 40,273 (30.8) | 28.8 | 32.1 | −3.3 | 29.6 | 32.0 | −2.4 |
| other | 5896 (4.5) | 5.3 | 4.0 | 1.3 | 5.1 | 3.8 | 1.3 |
| Hispanic | 14,838 (11.3) | 11.5 | 11.3 | 0.2 | 11.4 | 11.3 | 0.1 |
| Primary cause of ESRD | |||||||
| diabetes | 63,484 (48.5) | 47.9 | 48.9 | −1.0 | 48.4 | 48.6 | −0.2 |
| hypertension | 40,607 (31.0) | 30.9 | 31.1 | −0.2 | 31.1 | 31.0 | 0.1 |
| glomerulonephritis | 7514 (5.7) | 5.9 | 5.6 | 0.3 | 5.7 | 5.7 | 0.0 |
| other | 19,287 (14.7) | 15.3 | 14.4 | 0.9 | 14.8 | 14.7 | 0.1 |
| dual eligibilitya | 51,569 (39.4) | 39.3 | 39.5 | −0.2 | 39.2 | 39.6 | −0.4 |
| Comorbidity score | |||||||
| ≤4 | 50,248 (38.4) | 40.9 | 36.7 | 4.2 | 39.0 | 37.8 | 0.02 |
| >4 to ≤8 | 40,975 (31.3) | 31.0 | 31.5 | −0.5 | 31.0 | 31.7 | −0.01 |
| ≥8 | 39,669 (30.3) | 28.1 | 31.8 | −3.7 | 30.1 | 30.6 | −0.01 |
| Comorbid conditions | |||||||
| ASHD | 65,170 (49.8) | 47.8 | 51.1 | −3.3 | 49.5 | 50.1 | −0.6 |
| CHF | 72,212 (55.2) | 53.3 | 56.4 | −3.1 | 54.9 | 55.4 | −0.5 |
| CVA/TIA | 26,704 (20.4) | 19.4 | 21.1 | −1.7 | 20.3 | 20.5 | −0.2 |
| PVD | 48,663 (37.2) | 35.3 | 38.5 | −3.2 | 36.5 | 37.9 | −1.4 |
| Other cardiac | 41,098 (31.4) | 30.2 | 32.2 | −2.0 | 31.6 | 31.2 | 0.4 |
| COPD | 29,697 (22.7) | 21.8 | 23.3 | −1.5 | 22.8 | 22.6 | 0.2 |
| GI bleeding | 9575 (7.3) | 6.9 | 7.6 | −0.7 | 7.3 | 7.4 | −0.1 |
| Liver disease | 11,209 (8.6) | 7.5 | 9.3 | −1.8 | 7.7 | 9.5 | −1.8 |
| Dysrhythmia | 36,722 (28.1) | 26.9 | 28.8 | −1.9 | 28.3 | 27.8 | 0.5 |
| Cancer | 15,161 (11.6) | 11.1 | 11.9 | −0.8 | 11.3 | 11.9 | −0.7 |
| Diabetes | 89,481 (68.4) | 67.4 | 69.0 | −1.6 | 68.3 | 68.5 | 0.2 |
| SES principle component < medianb | 63,001 (48.1) | 49.0 | 47.5 | 1.5 | 49.0 | 47.3 | 1.7 |
| Urban | 93,012 (71.1) | 67.6 | 73.4 | −5.8 | 69.6 | 72.6 | −3.0 |
| Dialysis sessions during run-in | |||||||
| <38 | 27,354 (20.9) | 27.3 | 16.7 | 10.6 | 21.5 | 20.3 | 1.2 |
| ≥38 to <39 | 14,419 (11.0) | 11.6 | 10.6 | 1.0 | 11.0 | 11.1 | −0.1 |
| ≥39 | 89,119 (68.1) | 61.2 | 72.7 | −11.5 | 67.5 | 68.7 | −1.2 |
| Hospital days during run-in | |||||||
| 0 | 88,885 (67.9) | 72.0 | 65.2 | 6.8 | 67.5 | 68.3 | −0.8 |
| >0 to <8 | 21,164 (16.2) | 14.9 | 17.0 | −2.1 | 16.3 | 16.1 | 0.2 |
| ≥8 | 20,843(16.0) | 13.1 | 17.8 | −4.7 | 16.2 | 15.6 | 0.6 |
| Estimated GFR (ml/min per 1.73 m2) | |||||||
| <10 | 69,641 (53.2) | 52.8 | 53.5 | −0.7 | 52.3 | 54.2 | −1.9 |
| 10 to <15 | 40,884 (31.2) | 31.4 | 31.1 | 0.3 | 31.6 | 30.8 | 0.8 |
| ≥15 | 18,787 (14.4) | 14.6 | 14.2 | 0.4 | 14.8 | 13.9 | 0.9 |
| Missing | 1580 (1.2) | 1.2 | 1.2 | 0.0 | 1.3 | 1.1 | 0.2 |
| Hemoglobin (g/dl) | |||||||
| <11 | 86,461 (66.1) | 66.0 | 66.1 | 0.1 | 66.3 | 65.9 | 0.4 |
| ≥11.0 | 35,354 (27.0) | 26.7 | 27.2 | −0.5 | 26.6 | 27.6 | −1.0 |
| Missing | 9077 (6.9) | 7.4 | 6.7 | 0.3 | 7.2 | 6.7 | 0.5 |
| Serum albumin (g/dl) | |||||||
| <3.4 | 59,174 (45.2) | 44.6 | 45.6 | −1.0 | 44.9 | 45.6 | −0.7 |
| ≥3.4 | 39,768 (30.4) | 30.0 | 30.6 | −0.6 | 29.5 | 31.3 | −1.8 |
| Missing | 31,950 (24.4) | 25.4 | 23.8 | 1.6 | 25.7 | 23.1 | 2.6 |
| BMI (kg/m2) | |||||||
| <18.5 | 4912 (3.8) | 3.8 | 3.7 | 0.1 | 3.8 | 3.7 | 0.1 |
| 18.5 to <25 | 42,962 (32.8) | 33.1 | 32.6 | 0.5 | 32.9 | 32.8 | 0.1 |
| ≥25 | 81,402 (62.2) | 61.9 | 62.4 | −0.5 | 62.1 | 62.3 | −0.2 |
| Missing | 1616 (1.2) | 1.2 | 1.3 | 0.1 | 1.3 | 1.2 | 0.1 |
Unless otherwise indicated, all data are expressed as percentages. ASHD, atherosclerotic heart disease; CHF, congestive heart failure; CVA/TIA, cerebrovascular accident/transient ischemic attack; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal; SES, socioeconomic status; BMI, body mass index.
Medicare and Medicaid.
SES groups based on first principle component.
The mean number of monthly visits that a provider made to his or her prevalent hemodialysis patients in a given dialysis unit was a valid instrumental variable, because the two necessary assumptions (the instrumental variable is associated with the exposure [provider–patient visit frequency] but is independent of the outcome [mortality]) were met. First, the mean number of monthly visits to prevalent patients was significantly associated with provider–patient visit frequency (correlation=0.76; F statistics in the model of instruments for the exposure=36, 100). Second, prognostic characteristics associated with mortality were distributed more similarly across the strata of number of monthly visits to prevalent patients than across the strata of number of monthly visits to incident patients during the run-in period (Table 1), suggesting lack of an association between monthly visits to prevalent patients and risk of mortality.
Provider–Patient Visit Frequency and Survival
In unadjusted analyses, survival at 1 year was 79.8% and 79.0% for hemodialysis patients seen by their providers less than four and four times per month, respectively. In the standard Cox model, the adjusted hazards ratio (AHR; 95% confidence intervals [CIs]) for death was 0.98 (0.96–1.01; P=0.16) for visit frequency of four times per month compared with visit frequency of less than four times per month; the corresponding value for the time-varying Cox model was 0.98 (0.96–1.00; P=0.05) (Table 2). In the instrumental variable analysis, survival of patients with four visits per month did not differ from survival of patients with fewer provider visits (difference in mortality probability=0.07%, P=0.88).
Table 2.
Adjusted mortality hazards ratios associated with provider visit frequency
| Visit Frequency | Cox Proportional Hazards Modela | Time-Variable Cox Proportional Hazards Modelb | Instrumental Variable Analysisc | |||
|---|---|---|---|---|---|---|
| AHR (95% CI) | P | AHR (95% CI) | P | Probability Difference (%)d | P | |
| <4 visits/mo | 1 | 1 | ||||
| 4 visits/mo | 0.98 (0.96–1.01) | 0.16 | 0.98 (0.96–1.00) | 0.05 | 0.07 | 0.88 |
AHR, adjusted hazard ratio; CI, confidence interval.
Adjusted for age, sex, race, ethnicity, primary cause of ESRD, year of dialysis initiation, comorbidity score, socioeconomic status, dual eligibility status, urban status, number of dialysis sessions during run-in, hospitalization days during run-in, body mass index, estimated GFR, hemoglobin, and albumin at dialysis initiation (1 year follow-up).
Exposure variable updated every 3 months. Adjusted for age, sex, race, ethnicity, primary cause of ESRD, year of dialysis initiation, comorbidity score, socioeconomic status, urban status, number of dialysis sessions during run-in, hospitalization days during run-in, body mass index, estimated GFR, hemoglobin, and albumin at dialysis initiation. Comorbidity score, number of dialysis sessions (compliance), and number of hospitalization days were updated every 3 months. Follow-up until December 31, 2009.
Bivariate probit models for different outcomes adjusted for age, sex, race, ethnicity, primary cause of ESRD, year of dialysis initiation, comorbidity score, socioeconomic status, dual eligibility status, urban status, number of dialysis sessions during run-in, hospitalization days during run-in, body mass index, estimated GFR, hemoglobin, and albumin at dialysis initiation.
Probability difference of corresponding event when there are at least four visits in the run-in period compared with less than four visits given other covariates evaluated at the mean values.
Provider–Patient Visit Frequency and Hospitalizations
Over 12 months of follow-up, 67.33% of hemodialysis patients seen less than four and 67.32% of patients seen four times per month had been hospitalized. Overall, 85,071 patients had been hospitalized at least one time during follow-up. After adjustment for patient characteristics, the mean number of provider–patient visits was consistently associated with a small but statistically significant decrease in the risk of first hospitalization in all models. AHRs (95% CIs) of first hospitalization for visit frequency of four per month compared with less than four were 0.96 (0.95–0.97) from the standard Cox model, 0.97 (0.96–0.98) from the time-varying Cox model that evaluated risks of first hospitalization (Table 3), and 0.97 (0.96–0.98) from the time-varying Cox model that evaluated risk of recurrent hospitalizations. Similarly, the instrumental variable analysis revealed a 2.28% decrease in probability of first hospitalization (P=0.001) for patients seen four times per month compared with patients seen less frequently.
Table 3.
Adjusted hazards ratios of first hospitalization associated with provider visit frequency
| Visit Frequency | Cox Proportional Hazards Modela | Time-Variable Cox Proportional Hazards Modelb | Instrumental Variable Analysisc | |||
|---|---|---|---|---|---|---|
| AHR (95% CI) | P | AHR (95% CI) | P | Probability Difference (%)d | P | |
| Any hospitalization | ||||||
| <4 visits/mo | 1 | 1 | ||||
| 4 visits/mo | 0.96 (0.95–0.97) | <0.001 | 0.97 (0.96–0.98) | <0.001 | −2.28 | 0.001 |
| Hospitalization for CHF | ||||||
| <4 visits/mo | 1 | 1 | ||||
| 4 visits/mo | 0.96 (0.93–0.99) | 0.01 | 0.96 (0.94–0.99) | 0.005 | −1.02 | 0.007 |
| Hospitalization for CVD | ||||||
| <4 visits/mo | 1 | 1 | ||||
| 4 visits/mo | 0.96 (0.95–0.98) | 0.001 | 0.97 (0.95–0.99) | 0.001 | −1.61 | 0.005 |
| Hospitalizations for infection | ||||||
| <4 visits/mo | 1 | 1 | ||||
| 4 visits/mo | 0.94 (0.92–0.97) | <0.001 | 0.96 (0.94–0.98) | <0.001 | −2.76 | <0.001 |
| Hospitalizations related to vascular access | ||||||
| <4 visits/mo | 1 | 1 | ||||
| 4 visits/mo | 0.94 (0.91–0.96) | <0.001 | 0.95 (0.93–0.98) | <0.001 | −2.26 | <0.001 |
AHR, adjusted hazard ratio; CI, confidence interval; CHF, congestive heart failure; CVD, cardiovascular disease.
Adjusted for age, sex, race, ethnicity, primary cause of ESRD, year of dialysis initiation, comorbidity score, socioeconomic status, dual eligibility status, urban status, number of dialysis sessions during run-in, hospitalization days during run-in, body mass index, estimated GFR, hemoglobin, and albumin at dialysis initiation (1 year follow-up).
Exposure variable updated every 3 months. Adjusted for age, sex, race, ethnicity, primary cause of ESRD, year of dialysis initiation, comorbidity score, socioeconomic status, urban status, number of dialysis sessions during run-in, hospitalization days during run-in, body mass index, estimated GFR, hemoglobin, and albumin at dialysis initiation. Comorbidity score, number of dialysis sessions (compliance), and number of hospitalization days were updated every 3 months. Follow-up until December 31, 2009.
Bivariate probit models for different outcomes adjusted for age, sex, race, ethnicity, primary cause of ESRD, year of dialysis initiation, comorbidity score, socioeconomic status, dual eligibility status, urban status, number of dialysis sessions during run-in, hospitalization days during run-in, body mass index, estimated GFR, hemoglobin, and albumin at dialysis initiation.
Probability difference of corresponding event when there are at least four visits in the run-in period compared with less than four visits given other covariates evaluated at the mean values.
Greater provider–patient visit frequency was associated with statistically significant lower risk of first hospitalization because of congestive heart failure, cardiovascular causes, infectious etiologies, and causes related to vascular access complications (Table 3). The instrumental variable analysis revealed that probability of selected cause-specific hospitalizations was decreased for patients seen four times per month as follows: 1.02% for congestive heart failure (P=0.007), 1.61% for cardiovascular causes (P=0.005), 2.76% for infections (P<0.001), and 2.26% for vascular access complications (P<0.001).
Discussion
In this national cohort of incident dialysis patients, we found no consistent association between frequency of provider visits to hemodialysis patients and patient risk of death. In a time-varying Cox proportional hazards model, we found a 2% relative reduction in mortality in patients seen four times per month compared with patients seen less frequently. Frequency of four visits per month was consistently and independently associated with a small but statistically significant reduction in risk of first hospitalization, recurrent hospitalizations, and cause-specific hospitalizations. Our study is the first to evaluate the association between frequency of provider visits determined at the patient level and patient outcomes in a national cohort of hemodialysis patients.
In most analyses, we did not find an association between provider–patient visit frequency during hemodialysis and risk of patient death. The observed association of greater visit frequency with lower mortality was present in only one model with a specific categorization of visit frequency, and it was of borderline statistical significance in the presence of multiple testing; therefore, it should be interpreted cautiously. Despite this limitation, the time-varying Cox model, which produced the same AHR as the standard Cox model with 1 year follow-up, included longer follow-up time and better power to detect the small relative risk difference. Results of prior studies have been inconsistent; a study of incident dialysis patients did not find an association between provider visit frequency and patient outcomes,9 whereas a study of prevalent dialysis patients did find an association.5 If present, the association between greater provider visit frequency and lower patient mortality is likely to be very small in magnitude.
Our study found an association between greater frequency of provider visits and a small reduction in risk of first and recurrent hospitalizations. Smaller studies, one in incident9,10 and one in prevalent8 dialysis patients, found an association between higher provider visit frequency and greater achievement of clinical performance measures, such as higher albumin10 and achieved dialysis dose8,10 and lower serum phosphorus8 as well as fewer missed dialysis sessions.8 These studies did not find an association between physician visit frequency and hospitalizations.8,9 Only one study has examined the effect of the Medicare reimbursement policy change on provider practice patterns and health outcomes.8 This study’s findings suggest that, although provider–patient visit frequency increased significantly with the policy change (1.52 mean visits per month before the policy change to 3.14 mean visits per month after the change), neither patient satisfaction nor hospitalization rates improved.8 Our study found a consistently small reduction in risk of both first and recurrent hospitalizations among hemodialysis patients seen four times per month compared with patients seen less frequently. Although the mechanisms underlying these associations are uncertain, more frequent visits might modify hospitalization risk through improved chronic disease management,8,10 fewer missed dialysis sessions,8 or rapid identification and treatment of acute conditions.
Of note, hemodialysis patient hospitalization rates rose nationally from 1997 to 1998 (1.91 per person-year) to 2003–2004 (2.01 per person-year), but they have consistently fallen since then to 1.92 admissions per person-year in 2007–2008.4 The Medicare reimbursement policy change might be at least partially responsible for this observation. Because dialysis patient hospitalizations are common, a small reduction in hospitalization risk is likely to confer large cost savings in this patient population. In 2008, Medicare spent $8.7 billion on inpatient costs for dialysis patients; a 3% reduction in hospitalizations for patients currently seen less than four times per month would result in savings of approximately $104 million per year.4 Whether this benefit outweighs increased outpatient hemodialysis provider costs remains to be determined. More importantly, preventing need for hospitalizations is likely associated with improved patient quality of life.11
Our results support a benefit of more frequent provider visits to dialysis patients in reducing risk of hospitalization and provide some evidence for policy-makers. Formal cost–benefit analysis will provide additional much-needed evidence regarding the merits of more frequent dialysis patient visits by providers. Although reimbursement policy change resulted in increased provider visit frequency, 40% of dialysis patients are seen less than four times a month. Limited numbers of renal providers and growing burdens of CKD and ESRD make implementation of more frequent visits difficult, particularly in remote areas. Novel techniques, such as increased involvement of physician extenders and telemonitoring, can be implemented to increase the frequency of provider–patient interaction.
Our study has several strengths. It is highly generalizable to incident hemodialysis patients in the United States. It is large and well powered to detect small differences. Provider visit frequency was determined on an individual patient level. The study employed multiple analytic methods to reduce possible observational biases, and results were mostly consistent across analytic methods. Despite these strengths, the study is limited in several ways, including possible lack of generalizability to patients who die during the first 6 months on dialysis or without Medicare as the primary insurance source, lack of information pertaining to duration and nature of the provider–patient visits, and potential residual bias. We have no data on the number of provider–patient visits preceding implementation of the new policy, and therefore, we cannot perform a before and after analysis. Although we used number of dialysis sessions attended during the run-in period as a proxy for patient compliance, we could not determine whether sessions were missed or lower dialysis frequency was prescribed. In addition, we could not determine whether care by other providers, such as primary care physicians, altered the association between nephrology visit frequency and patient outcomes. However, in the absence of randomized controlled trials, this study provides the best evidence to date that increased provider–patient visit frequency is associated with decreased risk of hospitalizations in incident dialysis patients. Additional research is needed to evaluate mediators of the benefit, provide formal cost–benefit analyses, evaluate the influence of provider visit frequency on patient quality of life, and determine the best implementation strategy. Future policies with potential effects on clinical practice should be implemented in a staged manner to allow for evaluation of their effects on outcomes before widespread adoption.
Overall, our study suggests that greater frequency of provider visits to hemodialysis patients is associated with a small but significant reduction in all-cause and cause-specific hospitalizations and recurrent hospitalizations. We found no consistent evidence that increased visit frequency was associated with a subsequent reduction in mortality. How these observations apply to other healthcare settings remains to be determined.
Concise Methods
The study cohort consisted of incident in-center hemodialysis patients who initiated dialysis between October 1, 2003 and September 30, 2006 and had Medicare as primary payer for both Part A and Part B at day 91 after dialysis initiation; were aged 20 years or older and resided in the United States; survived and continued in-center hemodialysis for the first 6 months of ESRD; and were not hospitalized during at least 1 month of months 4–6 of ESRD. We excluded patients who had undergone previous kidney transplant. Patients for whom the instrumental variable could not be defined were also excluded (see the instrumental variable definition below).
Data were from the US Renal Data System, including all Medicare claims (Part A institutional and Part B physician/supplier), Medicare enrollment data, ESRD Medical Evidence Report (CMS-2728) data, and ESRD Death Notification form (CMS-2746) data.
Most dialysis patients are eligible for Medicare coverage 3 months after dialysis initiation. We used a 3-month run-in period, consisting of months 4–6, to define exposure variables and patient characteristics, including hospital days, number of dialysis sessions attended, and comorbid conditions. To define a short but meaningful run-in period in which provider–patient visit frequency could be determined, we calculated the correlation in visit frequency between providers and their patients over periods of 3 and 12 months. Correlation between the mean monthly provider visit frequency at 3 months of follow-up and the mean monthly visit frequency at 12 months was high (0.86), and we chose the 3-month run-in period. We used calendar months to define the run-in period, because providers’ bills for dialysis visits occur on the calendar cycle. The start date for patient follow-up was the first day after the run-in period.
We chose provider–patient visit frequency as the primary exposure variable. Visit frequency was calculated as the mean number of visits per month by a dialysis provider to each patient during the 3-month run-in period. Visit frequency information was obtained using Medicare Part B claims; the billing code in each claim denotes the number of visits a provider made to a patient over 1 month of care. For our calculations, we counted the code for one visit (G0319) as one visit, the code for two to three visits (G0318) as 2.5 visits, and the code for four or more visits (G0317) as four visits. For patients hospitalized for any portion of 1 month during the run-in period, that month’s provider visit frequency was counted as missing, because the patient was not present in the dialysis unit to be seen, potentially biasing our estimation of visit frequency. The mean visit frequency variable was expressed as clinical categories (less than four and greater than or equal to four visits per month).
The primary outcome measure was mortality. Death information was obtained from the US Renal Data System, which obtains and reconciles death dates from several sources, including the CMS Enrollment Database, CMS Form 2746 (ESRD Death Notification), the ESRD Network Database, and the Social Security Death Master File.4 Secondary outcome measures were time to first hospitalization and cause-specific hospitalizations for cardiovascular events, congestive heart failure, infections, and vascular access complications. We also evaluated the odds of recurrent hospitalizations. Hospitalization information was obtained from Part A Medicare inpatient claims. Cause of hospitalization was defined based on the primary diagnostic code (Supplemental Appendix).
Information on demographics, comorbid conditions, and laboratory values at dialysis initiation was obtained from the Medical Evidence Report.12 Comorbid conditions were defined based on the Medical Evidence Report and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes from Medicare claims incurred during days 0–180 of ESRD.12 The comorbidity index for dialysis patients was calculated based on 11 comorbid conditions.13 An SES score was determined for each patient based on home zip code using 2000 census data.14 We used dual eligibility for Medicaid and Medicare as another surrogate for low SES. Rural and urban definitions were adopted from the US Census Bureau based on zip code.15
Patient characteristics were tabulated and stratified by provider–patient visit frequency and the instrumental variable categories.
We used several statistical methods to determine the association between provider–patient visit frequency and outcomes. We conducted unadjusted analyses for time to mortality and first hospitalization between visit groups using the Kaplan–Meier method. A Cox proportional hazard regression model was used to assess the association of provider–patient visit frequency and time to death or first hospitalization (all-cause and cause-specific) adjusting for the variables listed in Table 1. For mortality analyses, patients were followed until death, change of renal replacement modality, recovery of kidney function, or 1 year. For the hospitalization outcome, patients were additionally censored if they changed insurance type. To incorporate changes in provider–patient visit frequency over time, we also conducted analyses using a time-varying Cox proportional hazards regression model for mortality, first hospitalization, and recurrent hospitalizations. In time-varying analyses, provider–patient visit frequency, comorbid conditions, hospital days, and number of dialysis sessions were updated every 3 months, and patients were followed until December 31, 2008.
Instrumental variable analysis is a method used to attempt to remove the effects of hidden bias in observational studies.16–18 To be successful, an instrumental variable must meet two assumptions: it must be highly correlated with the exposure variable (provider–patient visit frequency) and not affect the outcome variables independently of the exposure variable or through other variables.17,18 For this analysis, we defined the instrumental variable as the mean number of monthly visits that a provider made to his or her prevalent hemodialysis patients in a given dialysis unit over a calendar 1 year. We hypothesized that frequency of provider visits to incident hemodialysis patients is determined by the effort that the provider makes to visit the dialysis unit. Therefore, the number of times a provider visits his or her prevalent patients in the same unit influences the number of times that an incident patient is seen in 1 month. Because patients choose dialysis units mainly based on proximity to their homes, we made an assumption that the number of times that a provider sees prevalent patients in a given dialysis unit should not be associated with the characteristics or health status of the incident patients in that unit. To calculate the mean number of monthly visits that a provider made to prevalent patients in a given dialysis unit, we identified providers caring for patients at various units using the Unique Provider Identification Number. For each provider–unit pair, we determined the mean number of visits per month to prevalent patients, calculated over 1 year, divided at the median. We checked the above assumptions for the selected instrumental variable and present them above.
We used the bivariate probit model to jointly model provider–patient visit frequency and outcomes. The model specification and estimation of marginal effect are detailed elsewhere.18 All models controlled for covariates are presented in Table 1.
All analyses were performed using 9.1 SAS software except the instrumental variable analyses, for which LIMDEP software was used. Estimated P values are reported without adjustment for multiple comparisons.
Disclosures
None.
Acknowledgments
The authors thank Shane Nygaard and Nan Booth, MSW, ELS, of the Chronic Disease Research Group, for manuscript preparation and manuscript editing, respectively.
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health Grant R01DK082415.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012010051/-/DCSupplemental.
References
- 1.Morrison F, Shubina M, Turchin A: Encounter frequency and serum glucose level, blood pressure, and cholesterol level control in patients with diabetes mellitus. Arch Intern Med 171: 1542–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schatz M, Rodriguez E, Falkoff R, Zeiger RS: The relationship of frequency of follow-up visits to asthma outcomes in patients with moderate persistent asthma. J Asthma 40: 49–53, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Weinberger M, Oddone EZ, Henderson WG: Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med 334: 1441–1447, 1996 [DOI] [PubMed] [Google Scholar]
- 4.US Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States, 2010 Ed., Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 5.McClellan WM, Soucie JM, Flanders WD: Mortality in end-stage renal disease is associated with facility-to-facility differences in adequacy of hemodialysis. J Am Soc Nephrol 9: 1940–1947, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Pifer TB, Satayathum S, Dykstra DM, Mapes DL, Goodkin DA, Canaud B, Bommer J, Kurokawa K, Held PJ, Pisoni RL, Wolfe RA, Young EW: Hemodialysis (HD) staffing and patient outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 13: 425A, 2002 [Google Scholar]
- 7.Centers for Medicare & Medicaid Services (CMS), HHS : Medicare program; revisions to payment policies under the physician fee schedule for calendar year 2004. Final rule with comment period. Fed Regist 68: 63195–63395, 2003 [PubMed] [Google Scholar]
- 8.Mentari EK, DeOreo PB, O’Connor AS, Love TE, Ricanati ES, Sehgal AR: Changes in Medicare reimbursement and patient-nephrologist visits, quality of care, and health-related quality of life. Am J Kidney Dis 46: 621–627, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Plantinga LC, Fink NE, Sadler JH, Levey AS, Levin NW, Rubin HR, Coresh J, Klag MJ, Powe NR: Frequency of patient-physician contact and patient outcomes in hemodialysis care. J Am Soc Nephrol 15: 210–218, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Plantinga LC, Jaar BG, Fink NE, Sadler JH, Levin NW, Coresh J, Klag MJ, Powe NR: Frequency of patient-physician contact in chronic kidney disease care and achievement of clinical performance targets. Int J Qual Health Care 17: 115–121, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Rosenberger J, van Dijk JP, Nagyova I, Roland R, Geckova AM, van den Heuvel WJ, Groothoff JW: Do dialysis- and transplantation-related medical factors affect perceived health status? Nephrol Dial Transplant 20: 2153–2158, 2005 [DOI] [PubMed] [Google Scholar]
- 12.US Renal Data System: Researcher's Guide to the USRDS Database: 2009 ADR Edition. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Available at: http://www.usrds.org/2009/fb/index_rg.html Accessed May 25, 2012.
- 13.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ: An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 77: 141–151, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Ward MM: Socioeconomic status and the incidence of ESRD. Am J Kidney Dis 51: 563–572, 2008 [DOI] [PubMed] [Google Scholar]
- 15.US Census Bureau: US Census Bureau Data Set 2000. Available at: http://factfinder.census.gov/servlet/DownloadDatasetServlet?_lang=en Accessed May 25, 2012.
- 16.McClellan M, McNeil BJ, Newhouse JP: Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA 272: 859–866, 1994 [PubMed] [Google Scholar]
- 17.Newhouse JP, McClellan M: Econometrics in outcomes research: The use of instrumental variables. Annu Rev Public Health 19: 17–34, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Suying Li, Jiannong Liu, Gilbertson D, McBean M, Dowd B, Collins A: An instrumental variable analysis of the impact of practice guidelines on improving quality of care and diabetes-related outcomes in the elderly Medicare population. Am J Med Qual 23: 222–230, 2008 [DOI] [PubMed] [Google Scholar]