Abstract
Mesangial and circulating IgA1 with aberrantly glycosylated hinge region O-glycans characterize IgA nephropathy (IgAN). Unlike healthy individuals, some IgA1 is galactose deficient in patients with IgAN, leaving terminal N-acetylgalactosamine residues in the hinge region exposed. Circulating autoantibodies that recognize such galactose-deficient IgA1 as an autoantigen, or the levels of the autoantigen itself, may allow prediction of disease progression. Here, we analyzed serum samples obtained at diagnosis for autoantigen and autoantibodies from 97 patients with IgAN selected from our prospective cohort according to their absolute renal risk for progression to dialysis or death (0, very low; 1, low; 2, high; 3, very high). We also analyzed samples from controls comprising 30 healthy volunteers and 30 patients with non-IgAN disease. The mean follow-up was 13.8 years. We found that mean serum levels of total autoantigen, normalized IgG autoantibody, and total IgA autoantibody were significantly higher in patients than in the combined controls (all P≤0.01). Furthermore, increasing levels correlated with worse clinical outcomes. In Cox regression and Kaplan–Meier analyses, IgG autoantibody levels ≥1.33 predicted dialysis or death (both P≤0.01). In conclusion, these data suggest that serum levels of IgG and IgA autoantibodies strongly associate with the progression of IgAN nephropathy.
Berger and Hinglais first described IgA nephropathy (IgAN) in 1968.1,2 This renal disease is characterized by predominant deposits of IgA in the renal mesangium and exclusively of the IgA1 subclass.3 This finding has important implications because the molecular structures of the two IgA subclasses, IgA1 and IgA2, differ. IgA1 has a unique hinge region between the first and second constant-region domains of its heavy chain. This segment is rich in serine and threonine to which usually 3–6 O-glycans may be attached. Synthesis of IgA1 O-glycans is initiated by attachment of N-acetylgalactosamine (GalNAc). The structure may be extended by connecting galactose to GalNAc and is completed by adding sialic acid to galactose, GalNAc, or both.
Patients with IgAN have increased serum levels of IgA1 with truncated galactose-deficient hinge region O-glycans (Gd-IgA1)4–14 and IgA1 eluted from the glomeruli in renal tissue of patients with IgAN has the same glycosylation aberrancy.9,15 In the absence of galactose, terminal GalNAc residues are exposed. Consequently, such IgA1 molecules are presented as autoantigens,16 and IgG or IgA1 glycan-specific autoantibodies recognize Gd-IgA1 to form immune complexes17 either in the circulation or in situ in the glomerular mesangium, and renal injury ensues. It is now possible to measure serum levels of the autoantigen, Gd-IgA1, and of the IgG and IgA autoantibodies specific for Gd-IgA1.
In the clinic, it remains difficult to predict at time of diagnosis the long-term clinical outcome for patients with IgAN.18 In a large cohort followed prospectively, we recently used proteinuria, hypertension, and light microscopic features on the renal biopsy to estimate the absolute renal risk (ARR) for ultimate dialysis or death.19
In the present study, for patients with IgAN at varying degrees of risk for progression to dialysis or death, we measured serum levels of autoantigen, Gd-IgA1, and autoantibodies (IgG and IgA isotypes) at time of the diagnostic biopsy to assess their respective effect on the long-term clinical course.
Results
Clinical Demographics
Our goal was to randomly select 25 patients with IgAN from each ARR category; however, only 22 patients with an ARR of 1 met the selection criteria. The 97 IgAN patients included 73 men (75%). Demographic and clinical characteristics of the IgAN patients are listed in Table 1. The mean age at diagnosis was 43.6 years (SD 14.2), with a median of 45.0 years (range, 18.2–78.5). The mean observation intervals were 13.8 years from onset of clinical disease to final event (dialysis/death) or last follow-up visit and 7.3 years from diagnosis by biopsy to final event or last follow-up visit.
Table 1.
Characteristics of the selected IgAN patients (n=97) at time of diagnosis and last follow-up and according to the ARR of dialysis or death
| Characteristic | IgAN ARR = 0 | IgAN ARR = 1 | IgAN ARR = 2 | IgAN ARR = 3 | Statistics | IgAN All Patients |
|---|---|---|---|---|---|---|
| Patients (n) | 25 | 22 | 25 | 25 | 97 | |
| Men | 16 (64) | 16 (73) | 18 (72) | 23 (92) | NS | 73 (75) |
| At diagnosis | ||||||
| age (yr) | 37.8 (12.1) | 40.2 (12.4) | 45.7 (12.9) | 50.0 (16.1) | 43.6 (14.2) | |
| proteinuria (g/d) | 0.16 (0.22) | 0.90 (0.93) | 1.58 (1.04) | 3.56 (2.27) | 1.59 (1.87) | |
| class 0: <0.30 | 19 (76) | 6 (27) | 2 (8) | 0 (0) | 27 (28) | |
| class 1: 0.30–0.99 | 6 (24) | 8 (36) | 6 (24) | 0 (0) | χ2=79.03 | 20 (21) |
| class 2: 1.00–2.99 | 0 (0) | 7 (32) | 14 (56) | 11 (44) | P<0.0001 | 32 (33) |
| class 3: ≥3.00 | 0 (0) | 1 (4) | 3 (12) | 14 (56) | 18 (19) | |
| proteinuria ≥1 g/d | 0 (0) | 8 (36) | 16 (64) | 25 (100) | χ2=53.59 | 49 (50) |
| P<0.0001 | ||||||
| hypertension | 0 (0) | 10 (46) | 15 (60) | 25 (100) | χ2=51.54 | 50 (52) |
| P<0.0001 | ||||||
| GOS | 5.1 (1.2) | 6.0 (2.0) | 8.7 (1.9) | 13.3 (2.9) | 8.3 (3.8) | |
| GOS ≥8 U | 0 (0) | 4 (18) | 19 (76) | 25 (100) | χ2=65.67 | 48 (50) |
| P<0.0001 | ||||||
| eGFR (ml/min per 1.73 m2) | 82.0 (14.9) | 80.4 (22.3) | 64.2 (29.4) | 28.3 (25.7) | 63.2 (32.0) | |
| stage 1 | 7 (28) | 6 (27) | 6 (24) | 1 (4) | 20 (21) | |
| stage 2 | 17 (68) | 12 (54) | 9 (36) | 2 (8) | χ2=61.30 | 40 (41) |
| stage 3 | 1 (4) | 4 (18) | 5 (20) | 6 (24) | P<0.0001 | 16 (16) |
| stage 4 | 0 (0) | 0 (0) | 5 (20) | 5 (20) | 10 (10) | |
| stage 5 | 0 (0) | 0 (0) | 0 (0) | 11 (44) | 11 (11) | |
| At last follow-up: | ||||||
| exposure time (yr) | 13.8 (8.2) | 10.3 (6.1) | 15.4 (11.2) | 15.1 (12.1) | 13.8 (9.8) | |
| eGFR (ml/min per 1.73 m2) | 81.3 (17.7) | 72.9 (29.3) | 59.9 (35.6) | 16.9 (18.4) | 57.3 (36.1) | |
| stage 1 | 7 (28) | 8 (36) | 6 (24) | 0 (0) | 21 (22) | |
| stage 2 | 16 (64) | 9 (41) | 8 (32) | 1 (4) | 34 (35) | |
| stage 3 | 2 (8) | 3 (14) | 6 (24) | 4 (16) | χ2=58.33 | 15 (16) |
| stage 4 | 0 (0) | 0 (0) | 1 (4) | 3 (12) | P<0.0001 | 4 (4) |
| stage 5 | 0 (0) | 2 (9) | 4 (16) | 17 (68) | 23 (24) | |
| dialysis | 0 (0) | 2 (9) | 3 (12) | 16 (64) | χ2=36.76 | 21 (22) |
| P<0.0001 | ||||||
| death | 2 (8) | 0 (0) | 2 (8) | 1 (4) | NS | 5 (5.2) |
| D/D event | 2 (8) | 2 (9) | 5 (20) | 17 (68) | χ2=30.24 | 26 (27) |
| P<0.0001 |
Data are n (%) or mean (SD). D/D, outcome of dialysis/death; NS, not statistically significant.
At time of diagnosis (by renal biopsy) the major risk factors were distributed as follows: hypertension in 51.5% of patients (50 of 97), proteinuria ≥1 g/d in 50.5% (49 of 97), and a global optical score (GOS) ≥8 in 49.5% (48 of 97). Additional light-microscopy histologic features included obsolescent glomeruli ≥10% in 53% of patients, focal/segmental hyalinized glomeruli ≥10% in 53%, and crescentic glomeruli ≥10% in 11%. The immunofluorescent deposits were mesangial, with 100% IgA, by definition, and 72% C3, 35% IgM, 2% fibrin; however, there was 0% mesangial IgG. In addition, we observed 60% arteriolar C3 and 19% subendothelial IgM.
At last follow-up, 56 patients (58%) were taking an angiotensin-converting enzyme inhibitor or angiotensin II receptor type 1 blocker prescribed for hypertension and/or proteinuria ≥1 g/d (corresponding to 74% of all patients with these risk factors, but 6 patients progressed in a few months to dialysis and thus received only brief treatment). Thus, the fractions of patients treated by an angiotensin-converting enzyme inhibitor or an angiotensin II receptor type 1 blocker were 8%, 64%, 76%, and 84% for those with an ARR 0, 1, 2, or 3, respectively. In addition, 38 patients had received substantial prednisolone treatment (cumulative dose of 7 g for a 70-kg patient) prescribed for a GOS ≥8 (corresponding to 81% of all patients with this risk factor) and the fractions of patients treated according to an of ARR 0, 1, 2, or 3 were 2%, 32%, 56%, and 64%, respectively. No patient had received fish oil. The ARR scoring distribution was worse in male patients and in older patients. The final primary event, dialysis or death before dialysis, was reached in 8%, 9%, 20%, and 68% of our selected patients in ARR category 0, 1, 2, or 3, respectively.
The alternative definition for progressive IgAN based on decrement in estimated GFR (eGFR) sorted 39 patients as progressors and 58 patients as nonprogressors.
Serum Levels of IgA, Normalized and Total Autoantigen, IgG, Normalized and Total IgG Autoantibody, and Normalized and Total IgA Autoantibody in Controls and in IgAN Patients
Mean and median values for IgA, total autoantigen (U/ml), normalized IgG autoantibody (OD/0.5 µg IgG), and total IgA autoantibody (U/ml) were significantly higher in IgAN patients than in the combined controls (Table 2); however, normalized autoantigen (% of Helix aspersa [HAA]), total IgG autoantibody (U/ml), and normalized IgA autoantibody (OD/1 µg IgA) were poorly discriminatory.
Table 2.
Serum levels of IgA, normalized and total autoantigen (Gd-IgA1), IgG, normalized and total IgG autoantibody, and normalized and total IgA autoantibody in controls (healthy, diseased, and combined) and in IgAN patients
| Healthy Controls | Diseased Controls | P Value(t and U Tests) | Combined Controls | All IgAN Patients | P Value (t and U Tests) | |
|---|---|---|---|---|---|---|
| Patients (n) | 30 | 30 | 60 | 97 | ||
| IgA (mg/ml) | 2.20 (0.76) | 2.01 (0.81) | NS | 2.10 (0.78) | 2.88 (1.38) | 0.0001 |
| 2.09 (0.75–4.12) | 1.98 (0.79–3.85) | NS | 2.03 (0.75–4.12) | 2.68 (0.49–8.01) | 0.0003 | |
| Gd-IgA1 (% HAA) | 31.3 (9.1) | 36.1 (15.3) | NS | 33.7 (12.7) | 33.1 (12.4) | NS |
| 29.5 (15.4–57.0) | 31.3 (15.9–86.5) | NS | 30.8 (15.4–86.5) | 31.5 (10.0–76.5) | NS | |
| Gd-IgA1 (U/ml) | 68.2 (26.0) | 71.5 (41.1) | NS | 69.9 (34.1) | 92.6 (49.4) | 0.002 |
| 65.4 (16.7–112.3) | 62.0 (25.3–184.3) | NS | 63.4 (16.7–184.3) | 84.4 (10.0–267.5) | 0.003 | |
| IgG (mg/ml) | 11.01 (2.64) | 9.94 (4.05) | NS | 10.47 (3.43) | 10.32 (3.79) | NS |
| 10.54 (6.69–18.41) | 9.81 (4.88–21.58) | NS | 9.92 (4.88–21.58) | 10.00 (1.33–25.77) | NS | |
| IgG autoantibody (OD/0.5 µg) | 1.172 (0.242) | 1.300 (0.304) | NS (0.08) | 1.236 (0.280) | 1.483 (0.643) | 0.006 |
| 1.195 (0.85–1.54) | 1.272 (0.76–2.00) | NS | 1.255 (0.76–2.00) | 1.490 (0.63–3.01) | 0.07 (NS) | |
| IgG autoantibody (U/ml) | 25.60 (7.28) | 25.16 (10.58) | NS | 25.38 (9.01) | 30.79 (19.04) | 0.04 |
| 24.34 (14.88– 41.96) | 24.55 (12.04–60.97) | NS | 24.40 (12.04–60.97) | 26.03 (5.72–91.62) | NS | |
| IgA autoantibody (OD/1 µg) | 0.549 (0.234) | 0.770 (0.638) | NS (0.08) | 0.660 (0.489) | 0.748 (0.451) | NS |
| 0.458 (0.29–1.13) | 0.556 (0.24–2.84) | NS | 0.493 (0.24–2.84) | 0.589 (0.21–2.31) | NS (0.06) | |
| IgA autoantibody (U/ml) | 1.29 (0.91) | 1.55 (1.37) | NS | 1.42 (1.16) | 2.33 (2.02) | 0.002 |
| 0.93 (0.28–3.45) | 0.90 (0.19–5.99) | NS | 0.91 (0.19–5.99) | 1.78 (0.12–10.63) | 0.002 |
Data are mean (SD) or median (range). NS, not statistically significant.
Accuracy parameters and concordant statistics showed good discrimination between IgAN patients and the combined controls for total autoantigen (U/ml) (area under the curve [AUC], 0.64; 95% confidence interval [95% CI], 0.55–0.73); P=0.003), normalized IgG autoantibody (OD/0.5 µg) (AUC, 0.63; 95% CI, 0.56–0.72; P=0.01), and total IgA autoantibody (U/ml) (AUC, 0.65; 95% CI, 0.56–0.74; P=0.002). The respective optimal derived cut-off values were 69.0 (U/ml) for Gd-IgA1, 1.33 (OD) for the normalized IgG autoantibody level, and 1.12 (U/ml) for total IgA autoantibody (Supplemental Table 1). The net reclassification index (NRI) failed to demonstrate any significant additional effect of these parameters on the basal serum IgA and among each other.
The combination of an elevated normalized IgG autoantibody level (≥1.33) and an elevated total IgA autoantibody level (≥1.12) improved sensitivity to 0.77, but decreased specificity to 0.38.
Serum Levels of IgA, Normalized and Total Autoantigen, IgG, Normalized and Total IgG Autoantibody, and Normalized and Total IgA Autoantibody in Subgroups of IgAN Patients Based on ARR of Dialysis/Death
The serum levels of total autoantigen (Gd-IgA1), normalized IgG autoantibody, and total IgA autoantibody in IgAN patient subgroups with an ARR of 2 or 3 were significantly increased compared with IgAN patients with an ARR of 0 and the combined controls (Table 3 and Figures 1–3). For the total IgG autoantibody, the results were less significant, but this was explained by a step-wise decrease of serum total IgG from an ARR of 0 to an ARR of 3; patients with an ARR of 3 had significantly less IgG (Table 3). The parameters normalized autoantigen (% HAA) and normalized IgA autoantibody were not discriminatory.
Table 3.
Serum levels of IgA, normalized and total autoantigen (Gd-IgA1), IgG, normalized and total IgG autoantibody, and normalized and total IgA autoantibody in subgroups of IgAN patients according to the ARR of dialysis/death
| Comparisons | IgAN ARR = 0 | IgAN ARR = 1 | IgAN ARR = 2 | IgAN ARR = 3 | |
|---|---|---|---|---|---|
| IgA (mg/ml) | Mean (SD) | 2.17 (0.99) | 2.87 (1.10) | 3.28 (1.52) | 3.21 (1.58) |
| Median (range) | 2.25 (0.49–4.23) | 2.85 (1.24–5.13) | 3.15 (1.36–7.62) | 2.95 (1.43–8.01) | |
| t test | Versus ARR = 0 | — | 0.02 | 0.003 | 0.01 |
| Versus all controls | NS | 0.001 | <0.0001 | <0.0001 | |
| Gd-IgA1 (% HAA) | Mean (SD) | 33.7 (13.5) | 32.6 (11.6) | 32.1 (14.6) | 34.2 (9.9) |
| Median (range) | 31.8 (17.1–73.1) | 32.0 (10.0–50.9) | 27.7 (13.4–76.5) | 32.0 (15.5–65.3) | |
| t test | Versus ARR = 0 | — | NS | NS | NS |
| Versus all controls | NS | NS | NS | NS | |
| Gd-IgA1 (U/ml) | Mean (SD) | 71.4 (37.5) | 90.8 (40.9) | 100.2 (54.6) | 107.8 (56.2) |
| Median (range) | 68.1 (10.0–155.3) | 93.7 (26.8–167.2) | 84.3 (24.3–211.0) | 87.0 (39.0–267.5) | |
| t test | Versus ARR = 0 | — | NS (0.10) | 0.03 | 0.01 |
| Versus all controls | NS | 0.02 | 0.003 | 0.0003 | |
| IgG (mg/ml) | Mean (SD) | 11.40 (2.69) | 10.94 (4.84) | 10.55 (3.79) | 8.47 (3.16) |
| Median (range) | 11.32 (5.62–16.08) | 9.75 (4.72–25.74) | 9.23 (6.71–21.72) | 8.65 (1.33–14.50) | |
| t test | Versus ARR = 0 | — | NS | NS | <0.001 |
| Versus all controls | NS | NS | NS | 0.01 | |
| IgG autoantibody (OD/0.5 µg) | Mean (SD) | 1.08 (0.36) | 1.23 (0.51) | 1.55 (0.71) | 2.05 (0.48) |
| Median (range) | 0.91 (0.63–1.73) | 0.95 (0.69–2.36) | 1.55 (0.68–3.01) | 2.13 (1.37–2.97) | |
| t test | Versus ARR = 0 | — | NS | 0.01 | <0.0001 |
| Versus all controls | 0.04 | NS | 0.01 | <0.0001 | |
| IgG autoantibody (U/ml) | Mean (SD) | 25.06 (11.45) | 28.38 (20.90) | 34.25 (23.33) | 35.18 (17.88) |
| Median (range) | 20.87 (9.73–48.26) | 23.28 (8.15–87.52) | 29.14 (10.15–91.72) | 30.84 (5.72–76.88) | |
| t test | Versus ARR = 0 | — | NS | NS (0.08) | 0.02 |
| Versus all controls | NS | NS | 0.01 | 0.001 | |
| IgA autoantibody (OD/1 µg) | Mean (SD) | 0.68 (0.50) | 0.69 (0.44) | 0.79 (0.47) | 0.83 (0.40) |
| Median (range) | 0.56 (0.23–2.19) | 0.56 (0.28–2,26) | 0.63 (0.21–2.31) | 0.69 (0.27–1.63) | |
| t test | Versus ARR = 0 | — | NS | NS | NS |
| Versus all controls | NS | NS | NS | NS | |
| IgA autoantibody (U/ml) | Mean (SD | 1.59 (1.37) | 2.19 (1.88) | 2.66 (2.02) | 2.88 (2.49) |
| Median (range) (OD/1µg) | 1.21 (0.12–1.37) | 1.77 (0.46–8.60) | 1.96 (0.29–9.50) | 2.14 (0.71–10.63) | |
| t test | Versus ARR = 0 | — | NS | 0.03 | 0.03 |
| Versus all controls | NS | 0.03 | 0.001 | 0.0004 |
NS, not statistically significant.
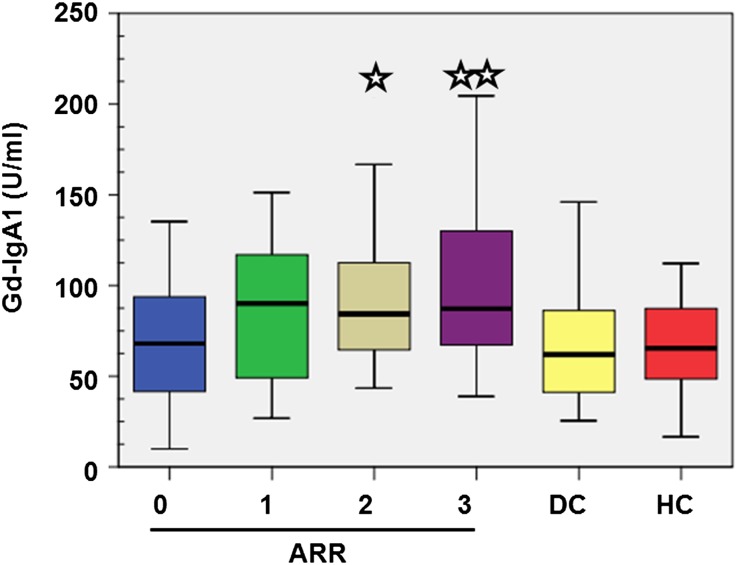
Figure 1.
Box plots of serum level of total autoantigen Gd-IgA1 (U/ml) according to the ARR of dialysis/death (ARR = 0, 1, 2, or 3) evaluated at diagnosis, in both IgAN patients and diseased (DC) and healthy (HC) controls. The comparisons were made against both ARR = 0 and combined controls. *P<0.01; **P<0.001.
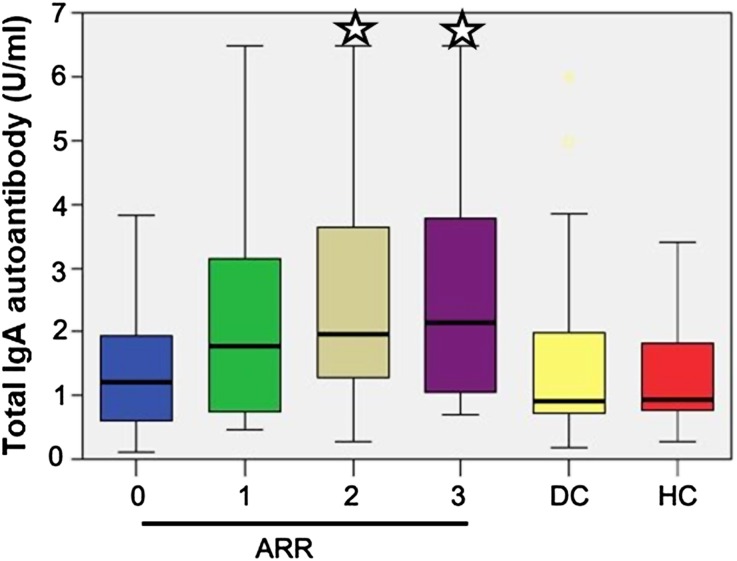
Figure 3.
Box plots of serum levels of total IgA autoantibody (U/ml) according to the ARR evaluated at diagnosis, in both IgAN patients and in diseased controls (DC) and healthy controls (HC). The comparisons were made against both ARR = 0 and combined controls. *P<0.01.
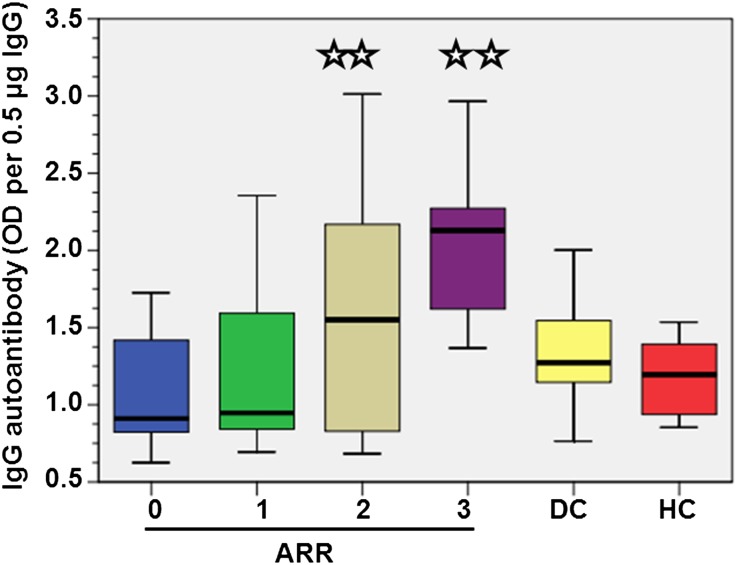
Figure 2.
Box plots of serum levels of normalized IgG autoantibody (OD/0.5 µg IgG) according to the ARR evaluated at diagnosis, in both IgAN patients and in diseased (DC) and healthy (HC) controls. The comparisons were made against both ARR = 0 and combined controls. **P<0.001.
In addition, accuracy parameters and concordant statistics confirmed good discrimination between the high/very high risk IgAN patients (grouping of ARRs of 2 and 3) versus low/very low risk patients (grouping of ARRs of 0 and 1), for total autoantigen (U/ml) (AUC, 0.62; 95% CI, 0.50–0.73; P=0.04), normalized IgG autoantibody (OD/0.5 µg) (AUC, 0.77; 95% CI, 0.67–0.86; P=0.0001), and total IgA autoantibody (U/ml) (AUC, 0.64; 95% CI, 0.53–0.75; P=0.02). The respective optimal derived cut-off values were 92.0 (U/ml) for total autoantigen, 1.33 (OD) for normalized IgG autoantibody, and 1.79 (U/ml) for total IgA autoantibody (Supplemental Table 2). The NRI was highly significant only for normalized IgG autoantibody compared with basal serum IgA (+89%) or to total IgA autoantibody (+93%), indicating its better discrimination power and its significant added value over the ARR classification.
The combination of an elevated IgG autoantibody level (≥1.33) and/or an elevated IgA autoantibody level (≥1.79) improved sensitivity to 0.65, but decreased specificity slightly to 0.49.
Influence of These Serum Biomarkers on Prospective Survival (without a Dialysis/Death Event) Assessed by Cox Regression Analysis and Kaplan–Meier Survival (Time Zero Set at Diagnosis)
By univariate Cox regression analysis, only two parameters were significant: total IgA autoantibody (U/ml) (β/SE=+2.20; χ2=4.84; relative risk, 1.22; 95% CI, 1.02–1.44; P=0.03) and normalized IgG autoantibody (OD) (β/SE=+3.60; χ2=12.95; relative risk, 2.77; 95% CI, 1.59–4.81; P=0.0003).
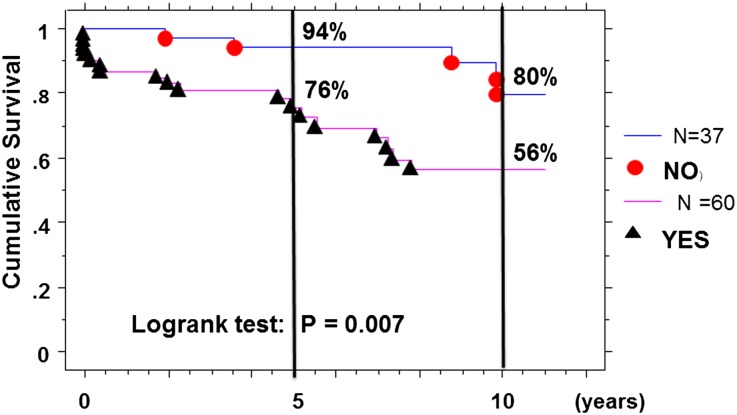
Using the Kaplan–Meier survival method with the optimal derived cut-off values, we confirmed a worse survival rate only in IgAN patients with serum IgG autoantibody ≥1.33 (OD) of 76% and 56%, respectively, at 5 and 10 years postdiagnosis compared with 94% and 80% in the others (log-rank test: χ2=7.42; P=0.01) (Figure 4).
Figure 4.
Kaplan–Meier survival curves without dialysis/death event, with time zero set at diagnosis and serum level of IgG autoantibody (≤ or >1.33 OD) at diagnosis in IgAN patients.
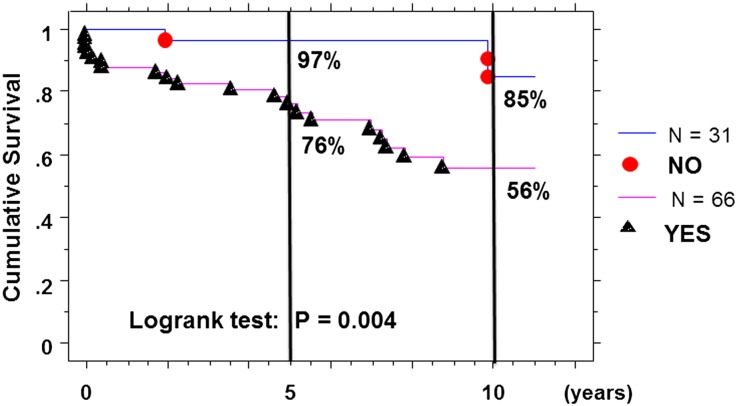
Using the Kaplan–Meier survival method, patients with an elevated serum autoantibody level (IgG ≥1.33 and/or IgA ≥1.79) had a worse survival rate of 76% and 56%, respectively, at 5 and 10 years postdiagnosis compared with 97% and 85% for the others (log-rank test: χ2=8.28; P=0.004) (Figure 5).
Figure 5.
Kaplan–Meier survival curves without dialysis/death event, with time zero set at diagnosis and elevated serum levels of autoantibodies (IgG >1.33 OD and/or IgA >1.79 U/ml) at diagnosis in IgAN patients.
Using the alternative definition for progressive IgAN based on eGFR reduction, only the normalized IgG autoantibody (OD) discriminated the progressors from the nonprogressors, with a mean of 1.76 (SD 0.67) versus a mean of 1.30 (SD 0.56) (t test=−3.62; P=0.0004).
In addition, there was no correlation between sex or age at diagnosis/sampling and any of these serum biomarkers.
Discussion
The autoantigen (Gd-IgA1) plays a central role in the pathogenesis of IgAN. Patients with IgAN have increased serum levels of IgA1 with galactose-deficient hinge region O-glycans.11,20–22 IgA1 eluted from renal tissue of patients has the same unusual biochemical feature,9,15 raising the possibility that the glomerular IgA1 is derived from the circulation. Serum Gd-IgA1 is almost exclusively within immune complexes, bound by IgG or IgA antibodies specific for the aberrantly glycosylated IgA1.12,16,17,23 Because Gd-IgA1 is present in serum samples of normal individuals, albeit in lower concentrations, the difference in patients with IgAN is consistent with an autoimmune mechanism of disease, with the aberrantly glycosylated IgA1 as autoantigen and glycan-specific IgG or IgA as autoantibodies.
In this study, we confirmed a significantly increased serum total autoantigen level (U/ml) in IgAN patients as has been demonstrated in ethnically diverse populations, including North Americans11,20 as well as Japanese21 and Chinese individuals.22 Whereas the serum Gd-IgA1 level has shown potential as a diagnostic test for IgAN,11 it also has value as a marker for the clinical severity of IgAN. In this study of European patients with IgAN, we found that patients with a high or very high risk of progression to dialysis/death (ARR of 2 and 3) had significantly higher serum total autoantigen levels at time of diagnosis by renal biopsy. Furthermore, a steady increase in serum Gd-IgA1 levels was associated with a greater risk of clinical progression. However, the normalized autoantigen (% HAA) did not differ among the study groups (healthy controls, diseased controls, and IgAN patients) or subgroups of patients with IgAN (ARR of 0, 1, 2, or 3). The difference in serum total autoantigen level was due to higher serum IgA levels in the patients with poor clinical outcomes. In another study, an increased serum level of aberrantly glycosylated IgA1 correlated with worse proteinuria.24 Furthermore, in that study, the combination of a high serum Gd-IgA1 level and a high circulating level of advanced oxidation protein products correlated with a faster decline in eGFR, suggesting that oxidative stress may modulate the nephrotoxicity of aberrantly glycosylated IgA1.
To our knowledge, this is the first report that the serum levels of IgG or IgA autoantibodies in IgAN patients associate with the ARR scoring, and the first presentation of serum IgA autoantibody measurements in a significant cohort of IgAN patients versus controls. This finding is consistent with a multi-hit hypothesis for the disease mechanism of IgAN, wherein an increased serum level of autoantigen alone is not sufficient to induce renal injury25–27; it must combine with autoantibodies either in the circulation to form immune complexes that deposit in the glomerular mesangium or in situ with Gd-IgA1 already in the mesangium. Based on the physical and biologic characteristics of the immune complexes, such as composition and size,17,28 mesangial cells may be activated to proliferate and overproduce components of mesangial matrix, chemokines, and cytokines.29,30 Overall, the serum levels of normalized IgG autoantibody and total IgA autoantibody steadily increased with the ARR level (ARR=3 > ARR=2 > ARR=0). However, the discriminating power for “high/very high” risk of progression to dialysis/death is greater for IgG autoantibody compared with IgA autoantibody, with a NRI of +93%. It is also noteworthy that the optimal cut-off values for normalized IgG autoantibody are strictly similar for discrimination between patients with IgAN and controls (1.33) and between the very high/high risk and very low/low risk subgroups of the patients with IgAN (1.33). These findings are consistent with our earlier observation that serum levels of IgG autoantibody at the time of biopsy correlated with magnitude of proteinuria.16
The antigen used in this study for ELISA analyses of autoantibodies was Fab fragment prepared from galactose-deficient IgA1 myeloma protein (Ste). Its use as antigen for IgA1 autoantibody detection has been described previously.17 The Fab fragment was generated by cleavage of the myeloma protein with bacterial IgA-specific protease from Haemophilus influenzae HK50 followed by isolation of the Fab fragment. Because one of the main sites on IgA1 from patients with IgAN that exhibit galactose deficiency31 is included on this Fab fragment, such preparation represents a suitable autoantigen material and it allows detection of both IgG and IgA1 autoantibodies. However, it does not cover overall heterogeneity of O-glycosylation in the IgA1 hinge region, because the C-terminal portion of IgA1 hinge region is missing. Thus, future studies will be needed to identify O-glycosylation patterns on the pathogenic galactose-deficient IgA1 in patients with IgAN and the corresponding autoantibodies recognizing these pathogenic IgA1 molecules.
We found no correlation between the serum level of IgG autoantibody and the mesangial co-deposition of IgG because none of our selected patients had mesangial IgG. The absence of such staining in a cohort of 97 patients with IgAN is somewhat surprising. However, the frequency of IgG co-deposits was 20% in the Oxford extension study32 and about 10%–15% in our other cohorts (retrospective and prospective). There was a trend for more IgA deposits (2+ and 3+) in patients with elevated normalized IgG autoantibody (>1.33). We found a significant linear correlation between the glomerular index of our GOS score and the normalized IgG autoantibody serum level (R=0.50; P<0.0001) and the serum total IgA autoantibody level (R=0.28; P=0.01). To clarify the apparent discrepancy, we believe that future studies are needed for a better understanding of the autoantibody specificities.
Patients with IgAN may develop autoantibodies (IgG and/or IgA isotype) after exposure to O-linked GalNAc on the cell surfaces of commonly encountered pathogens (molecular mimicry), including streptococci and some viruses.16 Such glycan-specific antibodies may then bind to Gd-IgA1 to form immune complexes either in the circulation or in the renal mesangium that eventually induce renal injury.
The serum level of Gd-IgA1 has been shown to exhibit significant inheritability, with about one-half of first-degree relatives affected.25 However, most of these relatives do not manifest renal disease, even after extended periods of observation. The level of circulating autoantibodies (IgG or IgA) specific for Gd-IgA1 has not been examined in that setting. Thus, variation in the capacity to synthesize such antibodies or differences in the biologic characteristics of the resulting immune complexes may determine the renal consequences of an elevated serum level of Gd-IgA1.
The IgAN patients studied in this report have the same characteristics as those in the original cohort, IgAN-STET-CO,19 from which they were extracted; in particular, the ARR score at diagnosis still predicted the ultimate outcome of dialysis/death by Cox regression analysis (χ2=11.8; P=0.01) and Kaplan–Meier survival (log-rank: χ2=14.1; P=0.003), despite much smaller numbers of patients.
However, our study has two important limitations. We cannot infer a direct causal relationship between the serum levels of either autoantigen or autoantibodies and disease progression. Here, we demonstrated only a significant association. To evaluate a possible causative link, a longitudinal study with serial measurements of these biomarkers in a diverse cohort of IgAN patients with close monitoring of the clinical course will be required. Another limitation stems from the variation in the length of the intervals between clinical onset and diagnosis by biopsy with a greater interval for IgAN subgroups with an ARR of 2 or 3, indicating that the diagnosis was established by biopsy likely later in the clinical course; however, we homogeneously handled the “immortal time,” with time zero set at either onset of the disease or at diagnosis in the different regression analyses.
In summary, in patients with IgAN the serum levels of IgG and IgA autoantibodies (specific for galactose-deficient O-glycans) at the time of diagnostic biopsy were significantly associated with clinical progression toward dialysis/death. In this setting, the discriminating power of the IgG autoantibody was superior to that for the IgA autoantibody. These findings strongly support a predominant role of an autoimmune mechanism in the pathogenesis of IgAN, which remains partly unsolved.33
Concise Methods
Patients and Controls
This study was conducted in full adherence to the Declaration of Helsinki, and was approved by the internal review board at the University Hospital of Saint-Etienne, France. All participants provided written informed consent. Patients with IgAN were selected from a cohort from the Saint-Etienne area who had participated in the IGAN-STET-CO study. The initial cohort included 332 patients (237 men),18,19 with biopsy performed between January 1, 1990 and December 31, 1999. Disease onset was defined as the first occurrence/discovery of macroscopic hematuria, microscopic hematuria and proteinuria, hypertension, or chronic renal insufficiency. Hypertension was defined according to World Health Organization criteria as a BP >140/90 mmHg or treatment with antihypertensive medication. Proteinuria was measured in a 24-hour collection. The eGFR was calculated using the four-variable Modified Diet in Renal Disease formula. Light microscopic glomerular, vascular, tubular, and interstitial changes in the biopsy specimen were assessed using the GOS.34 The maximal score was 20; the optimal cut-off value of ≥8 (obtained from the receiver-operator-characteristic [ROC] curve) was used as a dichotomous variable.19 Calculation of the ARR for dialysis/death incorporated three major, commonly accepted, risk factors assessed at the time of diagnosis by biopsy: hypertension, proteinuria ≥1 g/d, and severe light-microscopy pathologic lesions (GOS ≥8). Each factor was assessed as a dichotomous variable as present (1) or absent (0); ARR was the sum of these scores. After the diagnostic biopsy, all patients had been optimally treated, including possible use of an angiotensin-converting enzyme inhibitor or an angiotensin receptor type 1 blocker when indicated to treat hypertension to a target BP of 130/80 mmHg, to reduce proteinuria if excretion was ≥1 g/d, or both. Most patients with a GOS ≥8 had received glucocorticoid therapy. We selected IgAN patients for this study who met the following three criteria: ARR score (0, 1, 2, or 3) at diagnosis, sex (to reflect the male predominance in each ARR category), and availability of aliquoted and archived serum samples (stored at −80°C) obtained at time of diagnosis by biopsy.
The 60 controls, all Caucasian, included 30 normal healthy controls, selected from healthy persons who had been occasional voluntary blood donors (20 men and 10 women; mean age 45.7 years [SD 2.3] at time of blood sampling) and 30 patients with non-IgAN kidney disease (20 men and 10 women; mean age 37.0 years [SD 6.0] at time of diagnosis and blood sampling), including 15 patients with biopsy-proven membranous nephropathy and 15 with biopsy-proven nephro-arteriolosclerosis in the absence of hypertension (also termed C3 afferent-arterial disease).
To define progressive disease, we applied two approaches. The first used the final event as need for dialysis (or death before dialysis) and the second was based on final eGFR. For the latter, patients were grouped by the following subsections of the CKD stages: 1, 2A, 2B, 3A, 3B, 4, and 5 (evidenced by a step-wise decrement in eGFR of 15 ml/min per 1.73 m2). All patients with an eGFR at CKD stage 4 and 5 at last observation were considered as progressors. Patients with CKD stage 3A or 3B who had shown a decline in CKD stage subsection or decrease in eGFR more than 15 ml/min per 1.73 m2 from diagnosis to last follow-up were also defined as progressors. Furthermore, patients whose last eGFR was in stage 2A or 2B were considered progressors if the decrement in eGFR exceeded 30 ml/min per 1.73 m2. All other patients were defined by this approach as nonprogressors.
Measurement of Serum IgA and Gd-IgA1
Serum IgA (mg/ml) was measured by ELISA.11,12 Gd-IgA1 (autoantigen) levels were measured using lectin ELISA with GalNAc-specific lectin from HAA (Sigma-Aldrich, St. Louis, MO, USA).5,11,31 HAA reactivity of IgA1 in each sample was calculated as OD units per 1 μg IgA. Naturally galactose-deficient polymeric IgA1 (Ale) myeloma protein12 was used as the standard. For comparisons of HAA binding, the OD units (490 nm) per 50 ng standard galactose-deficient IgA1 (Ale) were assigned a value of 100% and the OD units of a sample (100 ng IgA) were expressed relative to that (% HAA).12 This value was then multiplied by IgA concentration in serum and expressed as serum levels of total autoantigen (U/ml).
Measurement of Serum IgG and Gd-IgA1–Specific IgG Autoantibody
Serum IgG was measured by ELISA,16 with a pool of normal human sera calibrated for all Ig isotypes as standard (Binding Site, San Diego, CA).
Serum levels of IgG autoantibody specific for Gd-IgA1 were determined by ELISA using plates coated with 1 μg/ml Fab fragment of galactose-deficient IgA1 (Ste) myeloma protein (Fab-IgA1).16,17 Results were expressed as OD units per 0.5 μg of total IgG and termed “normalized” IgG autoantibody. Serum total IgG autoantibody level (U/ml) was calculated by multiplying the above value (OD units per 0.5 μg total IgG) by the serum total IgG concentration.
Measurement of Serum Gd-IgA1–Specific IgA Autoantibody
Serum levels of IgA autoantibody specific for Gd-IgA1 were determined by ELISA using plates coated 4 hours at room temperature with 1.0 μg/ml solution of the Fab-IgA1 generated using an IgA-specific protease from H. influenzae HK50. Plates were blocked 4 hours at room temperature or overnight at 4°C with 2% BSA (Sigma-Aldrich) in 0.05% PBS-T. Samples were diluted in 0.05% PBS-T, added to each well, and incubated 4 hours at room temperature or overnight at 4°C. Captured antibodies were detected by treatment for 2 hours at 37°C with mouse mAb to human IgA (Fc-specific) (Applied Biologic Materials Inc, Richmond, British Columbia, Canada) at a concentration of 0.5 μg/ml and developed after 2 hours at 37°C with 1:20,000 diluted peroxidase-conjugated AffiniPure Goat Anti-Mouse IgG (H+L) (Jackson ImmunoResearch Laboratories Inc, West Grove, PA). Results were expressed as OD units per 1 µg of total IgA and termed “normalized” IgA autoantibody. Serum total IgA autoantibody level (U/ml) was calculated by multiplying the above value by the serum total IgA concentration.
Statistical Analyses
Descriptive statistics included mean (SD) and median (with range values). We compared results for continuous variables by unpaired t test for normally distributed data or nonparametric tests (Mann–Whitney U test) when the distribution was not Gaussian.
To set the cut-off values between patients and controls, and within the cohort of IgAN patients grouped as progressive cases versus nonprogressive cases, we used the ROC curve analyses to find the best compromise value between sensitivity and specificity; we also calculated all accuracy parameters and concordant c statistics (area under the ROC) with 95% CIs and P values using Statview 5.0 (SAS institute Inc, Cary, NC) and IBM-SPSS Statistics 19.1 software (IBM-SPSS Inc, Armonk, NY). The comparative discrimination power of the different serum biomarkers was refined by the NRI recently described35,36 and that estimates the real added value.
Disclosures
None.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (DK078244, DK082753, DK083663, DK075868, and GM098539). We also thank A.S.S.E.T.A.R. (Saint-Etienne, France) for its financial support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012010053/-/DCSupplemental.
References
- 1.Berger J, Hinglais N: [Intercapillary deposits of IgA-IgG]. J Urol Nephrol (Paris) 74: 694–695, 1968 [PubMed] [Google Scholar]
- 2.Berger J: IgA glomerular deposits in renal disease. Transplant Proc 1: 939–944, 1969 [PubMed] [Google Scholar]
- 3.Conley ME, Cooper MD, Michael AF: Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylactoid purpura nephritis, and systemic lupus erythematosus. J Clin Invest 66: 1432–1436, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen AC, Harper SJ, Feehally J: Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol 100: 470–474, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J: Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int 52: 509–516, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Hiki Y, Tanaka A, Kokubo T, Iwase H, Nishikido J, Hotta K, Kobayashi Y: Analyses of IgA1 hinge glycopeptides in IgA nephropathy by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Am Soc Nephrol 9: 577–582, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Hiki Y, Kokubo T, Iwase H, Masaki Y, Sano T, Tanaka A, Toma K, Hotta K, Kobayashi Y: Underglycosylation of IgA1 hinge plays a certain role for its glomerular deposition in IgA nephropathy. J Am Soc Nephrol 10: 760–769, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Allen AC, Bailey EM, Barratt J, Buck KS, Feehally J: Analysis of IgA1 O-glycans in IgA nephropathy by fluorophore-assisted carbohydrate electrophoresis. J Am Soc Nephrol 10: 1763–1771, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, Shinzato T, Kobayashi Y, Maeda K: Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 59: 1077–1085, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Renfrow MB, Cooper HJ, Tomana M, Kulhavy R, Hiki Y, Toma K, Emmett MR, Mestecky J, Marshall AG, Novak J: Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation Fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem 280: 19136–19145, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, Huang W-Q, Anreddy SR, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J: Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71: 1148–1154, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, Alarcón GS, Kimberly RP, Tomino Y, Mestecky J, Novak J: IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 118: 629–639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes MM, Suzuki H, Brooks MT, Tomana M, Moldoveanu Z, Mestecky J, Julian BA, Novak J, Herr AB: Recognition of galactose-deficient O-glycans in the hinge region of IgA1 by N-acetylgalactosamine-specific snail lectins: A comparative binding study. Biochemistry 49: 5671–5682, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odani H, Yamamoto K, Iwayama S, Iwase H, Takasaki A, Takahashi K, Fujita Y, Sugiyama S, Hiki Y: Evaluation of the specific structures of IgA1 hinge glycopeptide in 30 IgA nephropathy patients by mass spectrometry. J Nephrol 23: 70–76, 2010 [PubMed] [Google Scholar]
- 15.Allen AC, Bailey EM, Brenchley PE, Buck KS, Barratt J, Feehally J: Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int 60: 969–973, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J: Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 104: 73–81, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berthoux FC, Mohey H, Afiani A: Natural history of primary IgA nephropathy. Semin Nephrol 28: 4–9, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L: Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 22: 752–761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastings MC, Moldoveanu Z, Julian BA, Novak J, Sanders JT, McGlothan KR, Gharavi AG, Wyatt RJ: Galactose-deficient IgA1 in African Americans with IgA nephropathy: Serum levels and heritability. Clin J Am Soc Nephrol 5: 2069–2074, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimozato S, Hiki Y, Odani H, Takahashi K, Yamamoto K, Sugiyama S: Serum under-galactosylated IgA1 is increased in Japanese patients with IgA nephropathy. Nephrol Dial Transplant 23: 1931–1939, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Ding J, Zhu L, Shi S, Jiang L, Zhao M, Zhang H: Aberrant galactosylation of IgA1 is involved in the genetic susceptibility of Chinese patients with IgA nephropathy. Nephrol Dial Transplant 24: 3372–3375, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Novak J, Julian BA, Tomana M, Mestecky J: IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol 28: 78–87, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilla R, Suzuki H, Daprà V, Loiacono E, Peruzzi L, Amore A, Ghiggeri GM, Mazzucco G, Scolari F, Gharavi AG, Appel GB, Troyanov S, Novak J, Julian BA, Coppo R: Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin J Am Soc Nephrol 6: 1903–1911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA: Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol 19: 1008–1014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiryluk K, Moldoveanu Z, Sanders JT, Eison TM, Suzuki H, Julian BA, Novak J, Gharavi AG, Wyatt RJ: Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int 80: 79–87, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak J, Tomana M, Matousovic K, Brown R, Hall S, Novak L, Julian BA, Wyatt RJ, Mestecky J: IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int 67: 504–513, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Coppo R, Fonsato V, Balegno S, Ricotti E, Loiacono E, Camilla R, Peruzzi L, Amore A, Bussolati B, Camussi G: Aberrantly glycosylated IgA1 induces mesangial cells to produce platelet-activating factor that mediates nephrin loss in cultured podocytes. Kidney Int 77: 417–427, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Novak J, Raskova Kafkova L, Suzuki H, Tomana M, Matousovic K, Brown R, Hall S, Sanders JT, Eison TM, Moldoveanu Z, Novak L, Novak Z, Mayne R, Julian BA, Mestecky J, Wyatt RJ: IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured human mesangial cells. Nephrol Dial Transplant 26: 3451–3457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore JS, Kulhavy R, Tomana M, Moldoveanu Z, Suzuki H, Brown R, Hall S, Kilian M, Poulsen K, Mestecky J, Julian BA, Novak J: Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol 44: 2598–2604, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellur SS, Troyanov S, Cook HT, Roberts IS, Working Group of International IgA Nephropathy Network and Renal Pathology Society : Immunostaining findings in IgA nephropathy: Correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant 26: 2533–2536, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Boyd JK, Cheung CK, Molyneux K, Feehally J, Barratt J: An update on the pathogenesis and treatment of IgA nephropathy. Kidney Int 81: 833–843, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F: Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analyses. Am J Kidney Dis 18: 12–19, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Demler OV, Pencina MJ, D’Agostino RB, Sr: Equivalence of improvement in area under ROC curve and linear discriminant analysis coefficient under assumption of normality. Stat Med 30: 1410–1418, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Pencina MJ, D’Agostino RB, Sr, Demler OV: Novel metrics for evaluating improvement in discrimination: Net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med 31: 101–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]