Abstract
Prevention of cytomegalovirus (CMV) is essential in organ transplantation. The two main strategies are pre-emptive therapy, in which one screens for and treats asymptomatic CMV viremia, and universal antiviral prophylaxis. We compared these strategies and examined long-term outcomes in a randomized, open-label, single-center trial. We randomly assigned 70 renal transplant recipients (CMV-seropositive recipient or donor) to 3-month prophylaxis with valacyclovir (n=34) or pre-emptive valganciclovir for significant CMV viremia detected at predefined assessments through month 12 (n=36). Among the 55 patients who had a protocol biopsy specimen available at 3 years to allow assessment of the primary outcome, 9 (38%) of 24 patients in the prophylaxis group and 6 (19%) of 31 patients in the pre-emptive therapy group had moderate to severe interstitial fibrosis and tubular atrophy (odds ratio, 2.50; 95% confidence interval, 0.74–8.43; P=0.22). The prophylaxis group had significantly higher intrarenal mRNA expression of genes involved in fibrogenesis. The occurrence of CMV disease was similar in both groups, but pre-emptive therapy improved 4-year graft survival (92% versus 74%; P=0.049) as a result of worse outcomes in patients with late-onset CMV viremia. In conclusion, compared with valacyclovir prophylaxis, pre-emptive valganciclovir therapy may lead to less severe interstitial fibrosis and tubular atrophy and to significantly better graft survival.
Cytomegalovirus (CMV) is the most common opportunistic pathogen in solid organ transplant recipients.1 The main challenge consists of indirect effects associated not only with CMV disease but also with asymptomatic CMV viremia. CMV enhances the immune response to alloantigens, which may result in an increased incidence of acute allograft rejection episodes or interstitial fibrosis and tubular atrophy (IF/TA) after renal transplantation2–5 and, eventually, to impaired graft survival.6 Although the optimal strategy for CMV prevention has the potential to improve the long-term outcomes of transplantation, CMV prevention continues to be controversial. The International Consensus Guidelines admits both main strategies: universal antiviral prophylaxis and pre-emptive therapy.7
Although the efficacy of valganciclovir, ganciclovir, and, in the case of renal transplant recipients, high-dose valacyclovir prophylaxis has been documented in randomized trials, a serious problem yet to be solved is late-onset CMV disease upon prophylaxis completion.8–12 Pre-emptive therapy also helps to reduce the incidence of CMV disease,13–15 but a definite weakness is the demanding logistics of protocols for CMV surveillance monitoring. Moreover, doubts still persist as to whether pre-emptive therapy adequately prevents indirect effects of CMV, given the high incidence of asymptomatic CMV viremia.12,15 The body of data from randomized trials comparing prophylaxis and pre-emptive therapy over long-term follow-up is limited and is affected by the low intensity of CMV monitoring in the pre-emptive therapy group.15 A randomized trial in our renal transplant recipients documented a higher incidence of biopsy-proven acute rejection (BPAR) episodes at 1 year in patients receiving pre-emptive therapy than in those receiving valacyclovir prophylaxis, despite a similar incidence of CMV disease.14 In an effort to identify any potential long-term differences, we followed up our patients for 4 years after transplantation using a detailed analysis of protocol graft biopsy at 3 years, including intrarenal mRNA expression of genes involved in fibrogenesis.
Results
Participants
Seventy patients were included in the study. All but the 12 who died or lost the graft completed the 48-month follow-up (Figure 1). Thirty-six patients and 34 patients were randomly assigned to pre-emptive therapy and valacyclovir prophylaxis, respectively. The two groups did not differ significantly in demographic and immunologic characteristics, CMV serostatus, or initial immunosuppressive therapy (Table 1). Because of the higher number of acute rejection episodes, more patients in the pre-emptive therapy group were switched to tacrolimus-based immunosupppression. By contrast, the higher incidence of gastrointestinal side effects required more frequent withdrawal of mycophenolate mofetil in the pre-emptive therapy group. At 36 months, tacrolimus was given to 25 of 33 (76%) and 13 of 26 (50%) (P=0.056) patients with functioning grafts in the pre-emptive therapy and valacyclovir prophylaxis groups, respectively. The respective figures for mycophenolate mofetil are 25 of 33 (76%) and 25 of 26 (96%) (P=0.064). For a detailed summary of the immunosuppressive therapy used, levels, and doses of immunosuppressives, see Supplemental Table 1.
Figure 1.
Flow of patients through the study.
Table 1.
Patient characteristics of the intention-to-treat population
| Characteristic | Pre-emptive Group (n=36) | Valacyclovir Group (n=34) | P Value |
|---|---|---|---|
| Recipient | |||
| age (yr) | 50±13 | 48±12 | 0.51 |
| men | 28 (78) | 27 (79) | 1.0 |
| previous transplantation | 3 (8) | 4 (12) | 0.71 |
| HLA mismatches (n) | 3.6±1.2 | 3.5±1.2 | 0.77 |
| CMV serostatus | 0.62 | ||
| D+/R− | 6 (17) | 4 (12) | |
| D+/R+ | 23 (64) | 24 (71) | |
| D−/R+ | 7 (19) | 6 (18) | |
| Donor | |||
| age (yr) | 43±16 | 47±14 | 0.25 |
| donor type (deceased) | 34 (94) | 34 (100) | 0.49 |
| expanded-criteria donora | 10 (29) | 11 (32) | 1.0 |
| donation after cardiac death | 2 (6) | 2 (6) | 1.0 |
| Primary immunosuppression | 0.54 | ||
| cyclosporine + mycophenolate mofetil | 25 (69) | 23 (68) | |
| tacrolimus + mycophenolate mofetil | 6 (17) | 5 (15) | |
| sirolimus + mycophenolate mofetil | 5 (14) | 6 (18) | |
| basiliximab | 5 (14) | 7 (21) | |
| rATG induction | 4 (11) | 5 (15) |
For detailed characteristics, see reference 16. Data are number of patients (percentage) or mean ± SD. rATG, rabbit antithymocyte globulin.
According to the United Network for Organ Sharing criteria.
CMV Disease and Viremia
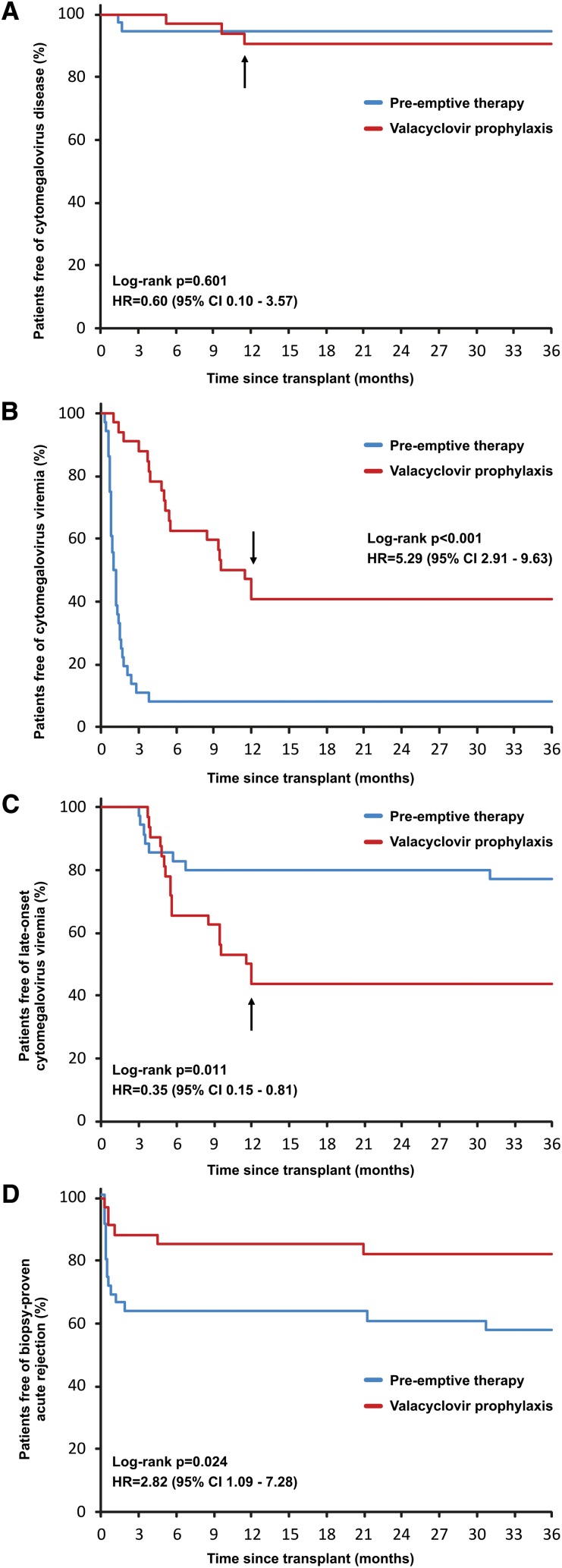
During the 36-month post-transplant period, CMV disease developed in two patients (6%) in the pre-emptive therapy group and three patients (9%) in the valacyclovir prophylaxis group (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.10–3.57; P=0.60) (Figure 2A). No patient in either group developed CMV disease after 12 months. Both patients in the pre-emptive therapy group with CMV disease had donor-positive/recipient-negative (D+/R−) CMV serostatus and at least one episode of CMV disease recurrence. By contrast, all cases of CMV disease in the valacyclovir prophylaxis group involved D+/R+ patients after prophylaxis completion, with no recurrence reported. Detailed characteristics of all CMV disease episodes were published previously.14
Figure 2.
Kaplan-Meier curves for the cumulative probability of freedom from cytomegalovirus (CMV) disease, CMV viremia, late-onset CMV viremia, and biopsy-proven acute rejection. (A) The incidence of CMV disease was similar in both groups. Significantly increased incidence of (B) CMV viremia and (D) acute rejection was observed in the pre-emptive group, whereas (C) late-onset CMV viremia was more common with valacyclovir prophylaxis. Late-onset CMV viremia was defined as the first episode of CMV viremia ≥3 months after transplant and/or recurrent episode of CMV viremia ≥3 months after transplant at least 1 month after clearance of an early CMV viremia episode. Arrow denotes 12 months after transplantation; from this point, CMV disease or viremia were assessed only if clinically indicated.
CMV viremia after 12 months was detected in two (6%) and four (12%) (P=0.42) patients in the pre-emptive therapy and valacyclovir prophylaxis groups, respectively. In none of these patients was the first episode of CMV viremia involved. The cumulative incidence of CMV viremia at 36 months in the pre-emptive therapy group was significantly higher than that in the valacyclovir prophylaxis group (33 of 36 [92%] versus 19 of 34 [59%]; HR, 5.29; 95% CI, 2.91–9.63; P<0.001) (Figure 2B). By contrast, the incidence of late-onset CMV viremia (≥3 months after transplant), including the first episode of viremia or a recurrent episode of viremia after at least 1-month clearance of early CMV viremia, was significantly higher in patients receiving valacyclovir prophylaxis (8 of 36 [23%] versus 18 of 34 [56%]; HR, 0.35; 95% CI, 0.15–0.81; P=0.011) (Figure 2C). Moreover, the first episode of CMV viremia after 3 months in the pre-emptive therapy group occurred in only one patient (CMV load, 300 copies/ml), with spontaneous clearance within 2 weeks. The other episodes were recurrent CMV viremia. By contrast, in the valacyclovir prophylaxis group, late-onset CMV viremia was the first episode of viremia in 15 (44%) patients, including 5 cases with viral load of ≥2000 copies/ml of more than 1 month’s duration.
IF/TA and Gene Expression Analysis on Protocol Biopsy Specimens at 36 Months
Protocol biopsy at 36 months was performed in 31 and 24 patients in the pre-emptive therapy and valacyclovir prophylaxis groups, respectively (Supplemental Table 2). In the pre-emptive therapy group, biopsy was not performed because of graft failure in three patients and for technical reasons in one patient. In one case, sufficient material could not be obtained. In the valacyclovir group, biopsy was not performed in eight patients with graft failure; sufficient material could not be obtained in two patients. Because of insufficient material or failure in RNA extraction, intrarenal mRNA gene expression analysis could be performed in 27 of 31 and 23 of 24 patients in the pre-emptive therapy and valacyclovir prophylaxis groups, respectively. The incidence of moderate to severe IF/TA was numerically higher in the valacyclovir group (9 of 24 [38%] versus 6 of 31 [19%]; odds ratio, 2.50; 95% CI, 0.74–8.43; P=0.22). The increased incidence in the valacyclovir group did not reach statistical significance even when we counted cases of IF/TA with inflammation (10 of 24 [42%] versus 6 of 31 [19%]; odds ratio, 2.98; 95% CI, 0.89–9.93; P=0.08) (Table 2). The results did not significantly change even after inclusion of findings on the last biopsy in patients with graft failure or deceased patients (Supplemental Table 3). The incidence of IF/TA did not differ between tacrolimus- and cyclosporine-treated patients (Supplemental Table 4).
Table 2.
Incidence of IF/TA and other histologic findings on protocol biopsy at 36 months after transplant
| Variable | Pre-emptive Group (n=31) | Valacyclovir Group (n=24) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Glomeruli per biopsy (n) | 10.9±8.9 | 10.1±6.2 | 0.69 | |
| Arteries per biopsy (n) | 1.7±1.7 | 1.5±1.1 | 0.95 | |
| Moderate to severe IF/TAa | 6 (19) | 9 (38) | 2.50 (0.74–8.43) | 0.22 |
| IF/TA with inflammationb | 6 (19) | 10 (42) | 2.98 (0.89–9.93) | 0.08 |
| IF/TA (all grades) | 13 (42) | 13 (54) | 1.64 (0.56–4.79) | 0.42 |
| Chronic “ci + ct” score | 1.48±1.50 | 2.00±1.82 | 0.33 | |
| Change of “ci + ct” score from month 3 to 36c | 0.95±1.71 | 1.29±1.82 | 0.48 | |
| Transplant glomerulopathy | 4 (13) | 3 (13) | 1.0 | |
| Chronic T cell–mediated rejection | 0 (0) | 1 (4) | 1.0 | |
| Calcineurin inhibitor toxicity | 1 (3) | 1 (4) | 1.0 | |
| Vascular nephrosclerosis | 4 (13) | 1 (4) | 0.37 | |
| GN recurrence | 1 (3) | 0 (0) | 1.0 |
Data are number of patients (percentage) or mean ± SD. ci, interstitial fibrosis score; ct, tubular atrophy score.
Grade II or III according to the Banff 05 classification.
IF/TA grade ≥1 with inflammation “i” score ≥1.
Findings on protocol biopsy at 3 months after transplant were used for comparison.
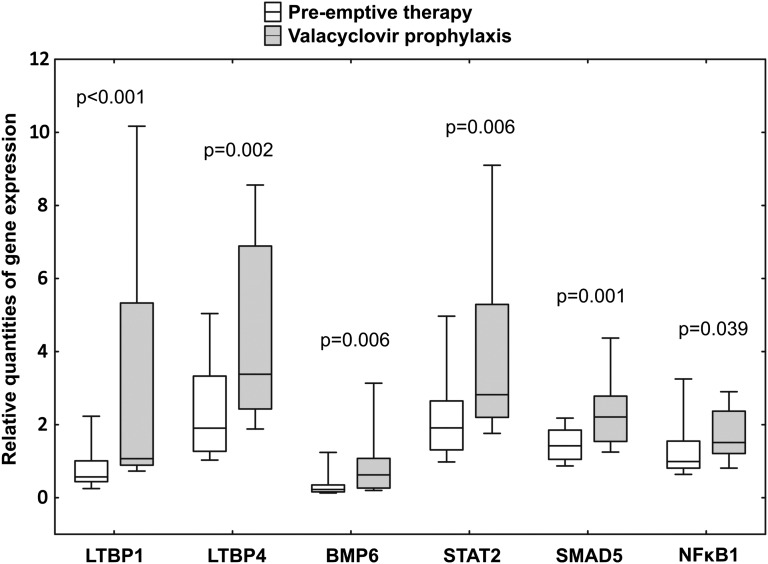
Consistent with the trend toward a higher proportion of the more severe forms of IF/TA, our data revealed significantly increased intrarenal mRNA expression of many genes involved in fibrogenesis in the valacyclovir group. These included genes belonging to the TGFβ superfamily, transcription factors and regulators, and SMAD family members (Figure 3 and Table 3). Overexpression of these genes was also documented in patients found to have moderate to severe IF/TA (Supplemental Table 5) and in patients with late-onset CMV viremia (Supplemental Table 6). Increased expression of a number of genes was proved in patients treated with cyclosporine compared with those receiving tacrolimus. Although tacrolimus was more often used in the pre-emptive therapy group, we analyzed mRNA expression of selected genes in tacrolimus-treated patients only. Statistical power of such an analysis was limited, but many of the genes remained significantly overexpressed in the valacyclovir prophylaxis group compared with the pre-emptive therapy group; other genes showed similar trend (Supplemental Tables 7 and 8).
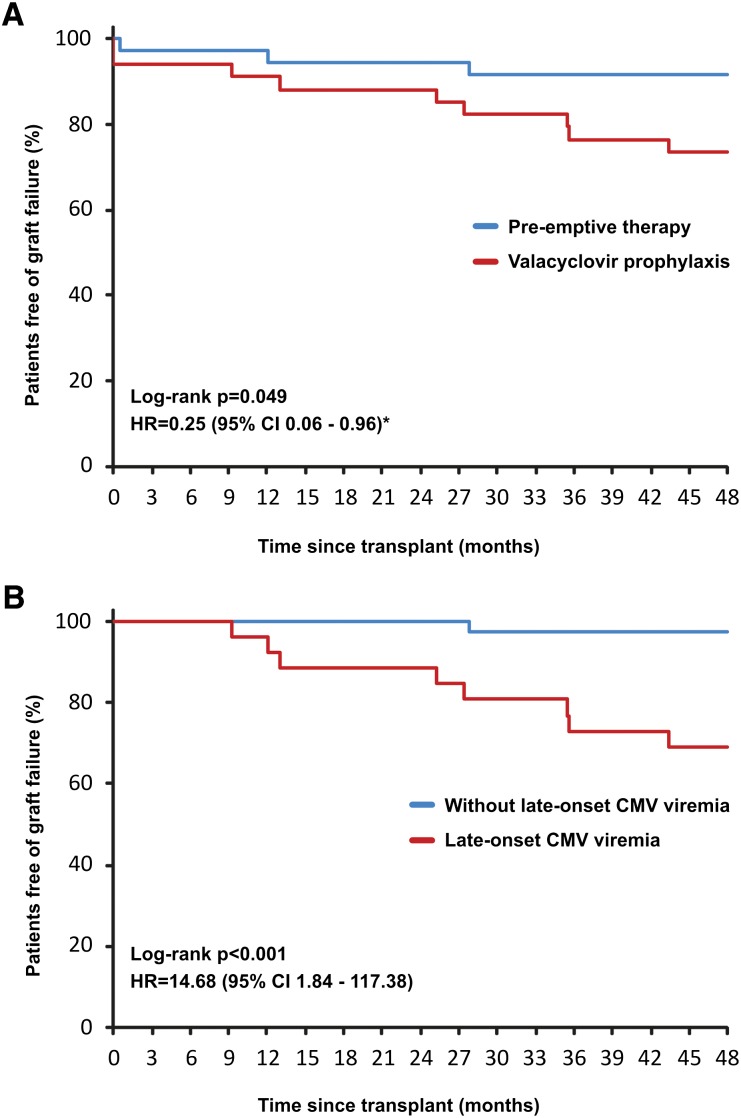
Figure 4.
Kaplan-Meier curves for the cumulative probability of graft loss uncensored for death. Significantly better graft survival was observed in patients managed by pre-emptive therapy compared with valacyclovir prophylaxis (A) and in patients without late-onset CMV viremia (B). Late-onset CMV viremia was defined as the first episode of CMV viremia ≥3 months after transplant or recurrent episode of CMV viremia ≥3 months after transplant at least 1 month after clearance of an early CMV viremia episode. Effect of late-onset CMV viremia was analyzed only in patients with functioning graft at 3 months after transplantation. *Adjusted for expanded-criteria donor, delayed graft function, and biopsy-proven acute rejection. Reasons for graft loss in the pre-emptive therapy group: two cases of antibody-mediated rejection, one case of IF/TA; reasons in the valacyclovir prophylaxis group: three cases of antibody-mediated rejection (in combination with T cell–mediated rejection in one case), three cases of interstitial fibrosis and tubular atrophy, one case of primary nonfunction, one case of recurrence of IgA nephropathy, and one death with function.
Table 3.
Summary of intrarenal mRNA gene expression on protocol biopsy at 36 months after transplant
| Gene | Pre-emptive Group (n=26) | Valacyclovir Group (n=23) | P Value |
|---|---|---|---|
| BMP6 | 0.23 (0.16–0.35) | 0.63 (0.27–1.08) | 0.006 |
| CXCL12 | 1.77 (1.15–3.06) | 3.29 (2.16–7.33) | 0.005 |
| CD86 | 2.39 (1.42–7.47) | 5.03 (3.78–12.34) | 0.05 |
| COL1A1 | 1.36 (0.71–3.13) | 3.07 (1.74–8.47) | 0.01 |
| COL1A2 | 0.71 (0.47–1.45) | 1.88 (1.04–3.36) | 0.005 |
| COL3A1 | 1.04 (0.62–1.99) | 3.49 (1.51–4.51) | 0.002 |
| FOS | 0.08 (0.05–0.15) | 0.11 (0.08–0.33) | 0.05 |
| GDF5 | 1.74 (1.33–2.62) | 2.81 (2.00–6.86) | 0.04 |
| IFNγR1 | 0.51 (0.42–0.73) | 0.75 (0.57–1.11) | 0.005 |
| INHβA | 1.74 (0.76–3.86) | 2.52 (1.59–7.52) | 0.03 |
| JUNB | 1.74 (1.33–2.62) | 2.81 (2.00–6.86) | 0.04 |
| LTBP1 | 0.54 (0.44–1.01) | 1.07 (0.90–4.83) | <0.001 |
| LTBP2 | 2.27 (1.60–3.79) | 5.28 (2.43–7.85) | 0.02 |
| LTBP4 | 1.91 (1.27–3.33) | 3.38 (2.43–6.89) | 0.002 |
| NFκB1 | 0.99 (0.82–1.53) | 1.51 (1.21–2.28) | 0.04 |
| PDGFβ | 1.32 (0.96–1.84) | 1.86 (1.42–2.89) | 0.02 |
| SMAD3 | 1.44 (1.26–2.00) | 1.89 (1.58–2.24) | 0.02 |
| SMAD4 | 1.01 (0.74–1.44) | 1.42 (0.99–2.03) | 0.02 |
| SMAD5 | 1.42 (1.06–1.84) | 2.21 (1.56–2.78) | 0.001 |
| SMAD9 | 2.08 (1.51–2.67) | 3.33 (1.91–4.67) | 0.03 |
| STAT1 | 1.04 (0.85–2.77) | 2.52 (1.23–4.11) | 0.04 |
| STAT2 | 1.91 (1.28–2.83) | 2.82 (1.24–5.11) | 0.006 |
| STAT3 | 0.39 (0.30–0.52) | 0.55 (0.37–1.05) | 0.02 |
| STAT4 | 2.08 (1.62–6.99) | 5.60 (3.39–9.55) | 0.04 |
| STAT5B | 1.16 (0.98–1.40) | 1.48 (1.11–1.92) | 0.03 |
| TGFβ1 | 1.31 (0.91–2.86) | 2.37 (1.32–3.70) | 0.05 |
| TGFβ2 | 1.07 (0.83–1.72) | 2.44 (1.30–3.99) | 0.004 |
| TP53 | 1.43 (1.07–2.12) | 2.58 (1.86–3.28) | 0.005 |
All genes with a statistically significant difference are shown. Data are median and interquartile range. Results are expressed as the ratio of the gene of interest to the housekeeping glyceraldehyde-3-phosphate dehydrogenase gene. For complete names and functional characteristics of genes, see Supplemental Table 12.
Figure 3.
Quantitative intrarenal mRNA gene expression on protocol biopsy at 36 months after transplant. Results are expressed as the ratio of the gene of interest to the housekeeping glyceraldehyde-3-phosphate dehydrogenase gene. The box plots show 25th and 75th (boundaries of boxes), 50th (median), and 10th and 90th (error bars) percentile values. Mann-Whitney U test was used for comparison between pre-emptive therapy and valacyclovir prophylaxis groups.
Between months 12 and 36, late-onset BPAR occurred in only two patients (6%) in the pre-emptive therapy group and in one (3%) nonadherent patient in the valacyclovir prophylaxis group. Because of the higher incidence of BPAR over the first 12 months,14 the cumulative incidence of BPAR remained significantly increased in the pre-emptive therapy group at 36 months (15 of 36 [42%] versus 6 of 34 [18%]; P=0.024; HR, 2.82; 95% CI, 1.09–7.28) (Figure 2D).
Patient and Graft Survival
Cumulative patient survival at 48 months after transplant was similar in both groups (P=0.14). Two patients died during the study (both in the valacyclovir group): one of septic shock 1 month after transplant and the other of metastatic renal cell carcinoma at 36 months. By contrast, graft survival was significantly better in the pre-emptive therapy group (33 of 36 [92%]) than in the valacyclovir prophylaxis group (25 of 34 [74%]) (P=0.049 by log-rank test) (Figure 4A). After adjustment for expanded-criteria donor, delayed graft function, and BPAR, the relative risk for graft loss in the pre-emptive group was 75% lower than in patients receiving valacyclovir prophylaxis (HR, 0.25; 95% CI, 0.06–0.96; P=0.04 by Cox hazard model). In the D+/R− subgroup, graft failure occurred in one of six patients in the pre-emptive therapy group and in one of four in the valacyclovir prophylaxis group. Graft survival in patients with CMV viremia was similar to that of patients without CMV viremia (42 of 52 [81%] versus 16 of 18 [89%]; HR, 1.70; 95% CI, 0.37–7.74; P=0.49). However, in patients with late-onset CMV viremia, graft survival was dramatically deteriorated (18 of 26 [69%] versus 40 of 41 [98%]; HR, 14.68; 95% CI, 1.84–117.38; P<0.001) (Figure 4B). The results were not influenced by HLA DR matching (Supplemental Table 9).
Safety
Renal function was similar in both groups (Table 4). Serum creatinine at 36 months did not differ in patients treated with cyclosporine or tacrolimus (mean ± SD, 136±43 versus 156±70 µmol/L; P=0.42). Just as in the first post-transplant year,14 the incidence of other types of infection did not differ between the groups between 12 and 36 months (Supplemental Table 10). The groups did not differ in the cumulative incidence of cardiovascular events (9 of 36 [25%] versus 11 of 34 [32%]; P=0.599) or new-onset diabetes (4 of 30 [13%] versus 7 of 30 [30%]; P=0.34). Side effects and basic laboratory data are summarized in Supplemental Table 11.
Table 4.
Renal function variables
| Variable | Pre-emptive Group (n=36) | Valacyclovir Group (n=34) | P Value |
|---|---|---|---|
| Serum creatinine (μmol/L) | |||
| week 4 | 195±133 | 194±106 | 0.94 |
| week 12 | 148±33 | 150±40 | 0.86 |
| month 12 | 146±59 | 167±88 | 0.07 |
| month 24 | 147±61 | 168±76 | 0.06 |
| month 36 | 143±58 | 162±65 | 0.07 |
| month 48 | 145±54 | 155±51 | 0.14 |
| Estimated GFR (ml/min)a | |||
| week 4 | 38±13 | 41±18 | 0.54 |
| week 12 | 52±16 | 53±18 | 0.95 |
| month 12 | 56±20 | 51±18 | 0.26 |
| month 24 | 55±16 | 51±23 | 0.38 |
| month 36 | 58±20 | 51±24 | 0.25 |
| month 48 | 56±18 | 52±19 | 0.39 |
Data are mean ± SD.
According to the Modification of Diet in Renal Disease 7 formula.
Discussion
Although the short-term outcomes of this study documented a decrease in BPAR in patients receiving valacyclovir prophylaxis,14 4-year follow-up did not confirm any advantages of prophylaxis over pre-emptive therapy. Quite unexpectedly, there was a trend toward a higher incidence of moderate to severe IF/TA at 3 years in the prophylaxis group. This finding was supported by the significantly increased intragraft mRNA expression of genes involved in fibrogenesis. Another important finding was the improved 4-year graft survival in patients managed by pre-emptive therapy. The main reason for this were the poor outcomes in patients with late-onset CMV viremia that developed in more than half of patients upon valacyclovir prophylaxis completion. To our knowledge, this randomized trial is the first to show that use of pre-emptive therapy with frequent monitoring and high adherence rates after renal transplantation can result in outcomes superior to those obtained with universal antiviral prophylaxis.
IF/TA is a frequent histologic finding, particularly in late renal graft biopsy specimens.16–19 Moderate to severe IF/TA, and particularly IF/TA combined with inflammation, is associated with a several times higher risk for graft loss.17,18 In this context, the more than double incidence of moderate to severe IF/TA or IF/TA with inflammation in patients with anti-CMV prophylaxis is a clinically serious finding. It should be noted that the differences did not reach statistical significance. The reason for this was overestimation of the anticipated incidence of IF/TA reported in earlier studies.16 Despite the overwhelming predominance of deceased donors and routine use of expanded-criteria donors, the incidence of moderate to severe IF/TA was as low as 27% in our study, a figure consistent with recently reported data.19 Moreover, the statistical power of our study was limited by the fact that protocol biopsy could be performed in fewer patients than anticipated, mainly because of the higher graft failure rates in the valacyclovir prophylaxis group.
To date, our study has been the only one to systematically assess late protocol biopsy findings on top of intrarenal mRNA gene expression analysis in the long term. The combination of standard histology with gene expression in renal graft lesions provides more accurate information about the pathologic features of grafts.20 Our study population showed upregulation of a variety of genes involved in fibrogenesis in the group receiving valacyclovir prophylaxis. TGFβ1 upregulation in patients with IF/TA has been shown to predict subsequent deterioration of graft function.21
The presence and severity of IF/TA reflect the cumulative burden of injury.20 Although multifactorial in etiology, both acute rejection and subclinical rejection are associated with an increased incidence of IF/TA.16 Several studies have documented an increased risk for acute rejection episode after previous CMV disease or asymptomatic CMV viremia.3 Valacyclovir prophylaxis resulted in a reduced incidence of the mild forms of early T cell–mediated BPAR.14 Early CMV viremia with viral load of ≥2000 copies/ml was associated with an increased risk for IF/TA on protocol biopsy at 3 months after transplant.4 However, our study has shown that the preceding benefits of prophylaxis are only short-term ones. Long-term follow-up, during which the effects of late-onset CMV disease or viremia may be seen to play an adverse role, helps identify the potential advantages of pre-emptive therapy with a minimal risk for late-onset CMV.13,14 In a pediatric population with valganciclovir prophylaxis, asymptomatic late-onset CMV or Epstein-Barr virus viremia was associated with more than a four-fold risk for development of moderate to severe IF/TA on protocol biopsy at 2 years after transplant and with decreased renal function.5 Consistent with this, graft survival was significantly worse in our patients with late-onset CMV viremia; although we could not demonstrate its adverse effect on the incidence of IF/TA in protocol biopsies, many fibrogenic genes were upregulated in patients with late-onset CMV viremia. A true limitation of our study is the absence of systematic PCR monitoring for CMV after the first year. Likewise, the relatively low frequency of monitoring after 6 months may have resulted in our overlooking some asymptomatic episodes of CMV viremia at a later time.
The improved graft survival with pre-emptive therapy compared with valacyclovir prophylaxis is consistent with the trend toward improved renal function and an increase in IF/TA. Still, this finding should be considered with caution. Graft survival was not a primary end point, and the number of enrolled patients is small. Our data do not completely explain the cause of better results with pre-emptive therapy. The dramatically worse graft survival in patients with previous late-onset CMV viremia implies the adverse effect of uncontrolled late CMV viremia episodes. It is assumed that CMV affects alloimmunity by activating the local inflammatory response through activation of NFκB, which plays a key role in the regulation of genes of many cytokines and adhesion molecules.2 Although the overall incidence of CMV viremia was higher in the pre-emptive therapy group, our results do not seem to suggest that the episodes of CMV viremia in the early post-transplant period controlled by pre-emptive therapy had an adverse effect on long-term outcomes. This is the key difference from the studies not using pre-emptive therapy, which showed negative consequences of uncontrolled early-onset CMV viremia for graft survival and mortality.6 According to our hypothesis, the beneficial effect of early reconstitution of CMV-specific T cell immune response is likely to prevail subsequent to antigen stimuli during controlled CMV viremia. Adequate CMV-specific T cell immunity is associated not only with protection against late-onset CMV disease but also with reduced alloreactivity and improved renal function.22,23
Our results are inconsistent with data from an earlier study in which pre-emptive therapy compared with ganciclovir prophylaxis failed to prevent CMV disease and was associated with deteriorated graft survival.15 However, our studies differed completely in the conduct of pre-emptive therapy. In our study, in line with the International Consensus Guidelines,7 once-a-week CMV monitoring with PCR was performed during the first 4 months after transplantation; compliance with monitoring was excellent (99%), as was the time to instituting pre-emptive therapy (1 day). By contrast, the above study used a lower frequency of monitoring and quantitative PCR from plasma with a higher detection limit. The authors did not mention compliance.15 Unsatisfactory results were also reported in a recent study with similarly insufficient monitoring.24 Managing the logistics of surveillance monitoring protocols is critical for pre-emptive therapy to be successful. The only randomized trial with adequate monitoring and compliance documented similar outcomes for pre-emptive therapy and valganciclovir prophylaxis in the first post-transplant year.13 Long-term retrospective follow-up documented at least similar results in the incidence of CMV indirect effects.25 Consistent with this, our study failed to document, in patients receiving pre-emptive therapy, higher rates of cardiovascular events, new-onset diabetes, and other infections.
In addition to the limited sample size and single-center design, other limitations of this study should be mentioned. The proportion of D+/R− patients at highest risk was small, as seen in other randomized trials.13,15 Our results thus cannot be extrapolated to the D+/R– population. Recent retrospective studies have suggested unsatisfactory efficacy and a higher incidence of ganciclovir resistance in D+/R– patients receiving pre-emptive therapy.26 On the other hand, the incidence of late-onset CMV disease after antiviral prophylaxis is also the highest in D+/R– patients. Extending prophylaxis to 6 months had only a partial effect, and a hybrid approach (pre-emptive therapy after prophylaxis completion) has yielded disappointing results to date.27,28 Consistent with the International Consensus Guidelines, prophylaxis in our patients was provided with valacyclovir,7 but the most commonly used agent is valganciclovir. Valacyclovir efficacy has been demonstrated in a large randomized trial.9 Compared with oral ganciclovir, the efficacy of valacyclovir was equivalent.10 However, a head-to-head comparison of valganciclovir with valacyclovir prophylaxis has not been published to date. In summary, although efficacy of valacyclovir seems acceptable, it is not possible to rule out that the use of valganciclovir prophylaxis may have changed our findings.
This study, with its detailed clinical and histologic monitoring, has demonstrated excellent efficacy of pre-emptive therapy in preventing CMV disease as well as indirect effects of CMV. Use of high-sensitivity diagnostic techniques to detect CMV activity, frequent monitoring, and effective oral valganciclovir therapy allow management of the logistics of pre-emptive therapy. If high compliance rates are maintained, pre-emptive therapy may be associated with lower incidence rates of IF/TA and better graft survival compared with universal antiviral prophylaxis with valacyclovir. Our results support the conduct of a large multicenter study to compare pre-emptive therapy versus prophylaxis in centers with experience with pre-emptive therapy.
Concise Methods
Patients
Patients were recruited from a single transplant center at the Charles University Teaching Hospital, Pilsen, Czech Republic. From October 2003 through August 2006, all adult renal transplant recipients with R+ or donor D+ CMV serologic status were eligible for inclusion. The main exclusion criterion was D−/R− serostatus. Other inclusion and exclusion criteria were published previously.14 The study was approved by the local ethics committee and conducted in compliance with the Declaration of Helsinki. Written informed consent was obtained before enrollment.
Randomization and Masking
Eligible patients were randomly assigned by transplant physician using a random-numbers table, at a 1:1 ratio, to pre-emptive therapy with valganciclovir or to valacyclovir prophylaxis. Randomization was stratified by D/R CMV serostatus. Sequentially numbered sealed envelopes were used for allocation concealment. The study was not blinded (except for physicians assessing PCR results for CMV DNA and renal graft histopathology).
Interventions
Patients in the pre-emptive therapy group were followed up using quantitative real-time PCR from whole blood for CMV DNA (sensitivity of 50 copies/ml) and received valganciclovir (Valcyte; Hoffman-La Roche, Grenzach-Wyhlen, Germany) at a dosage of 900 mg twice daily in those with detected CMV viremia of ≥2000 copies/mL until they reached clearance of CMV viremia (for at least 14 days). PCR was performed at 1-week intervals for the first 4 months and at 5, 6, 9, and 12 months thereafter. Patients managed by prophylaxis received valacyclovir (Valtrex; Glaxo Wellcome, Dartford, United Kingdom) at a dosage of 2 g four times daily for 3 months, beginning on day 7 after transplant at the latest. PCR was performed at 2-week intervals for the first 4 months and then identically to the protocol in the pre-emptive therapy group. After 12 months, PCR was performed only if clinically required. In both groups, antiviral drugs doses were tapered according to renal function and side effects.14 Asymptomatic CMV viremia in the prophylaxis group was not treated. Treatment of CMV disease, other antimicrobial prophylactic regimens, and the immunosuppressive protocols have been described previously.14
Study Outcomes and Follow-up
The primary end point in long-term follow-up was the incidence of IF/TA assessed on protocol biopsy specimens at 36 months after transplant. This included assessment of IF/TA severity using a standard histology and intrarenal mRNA gene expression analysis. Secondary end points included the cumulative incidence of CMV disease, defined as CMV syndrome or tissue invasive disease,14,29 patient and graft survival rates (not censored for patients death), renal graft function (assessed as estimated GFR calculated using the Modification of Diet in Renal Disease 7 equation), chronic T cell–mediated or antibody-mediated rejection on protocol biopsy specimens, and incidence of other infections. In addition, other potential indirect effects of CMV, such as cardiovascular events or new-onset diabetes mellitus, malignancy, and findings on routine laboratory testing, were recorded prospectively. All patients remained on follow-up for a minimum of 4 years after transplant or until death. Patient and graft survival and renal function were assessed at 4 years, with the other secondary end points by the end of year 3.
Biopsy Sample Processing
In patients with functioning grafts, protocol biopsy was performed at 36 months using an 18-gauge needle (biopsy gun). At least two cores were obtained. Tissues for light microscopy were fixed in 4% formaldehyde and embedded in paraffin using routine procedure. Sections 3 μm thick were cut from tissue blocks and stained with hematoxylin and eosin, blue trichrome, silver staining, and Congo red staining. Immunohistochemical analysis was performed in the Ventana automated slide stainer without manual antigen retrieval and was detected using a Ventana ultraView universal DAB detection kit (Ventana-Roche, Tucson, AZ) as recommended by manufacturer. The primary antibodies against C4d (Venatana-Roche) and SV40 (Ventana-Roche), p53 (Ventana-Roche), and CMV (Dako, Glostrup, Denmark) were used. Appropriate positive controls were employed. For all biopsies, fresh tissue samples were examined using immunofluorescence staining for C4d (Biomedica Gruppe, Vienna, Austria) and for C3 (Dako) depositions in peritubular capillaries in parallel. All biopsy specimens were evaluated according to the Banff 05 classification.30 Small portions of renal tissue from the cortical or juxtamedullary zone were immediately stored in a preserving solution (RNA later; Qiagen, Hilden, Germany) for expression analysis. For RNA isolation and gene expression analysis, the renal tissue was homogenized; total RNA was extracted using RNeasy Fibrous Tissue Mini Kit (Qiagen) automated on the QIAcube device and reverse-transcribed into cDNA using SuperScript II Reverse transcription (Invitrogen, Carlsbad, CA). Samples of cDNA from each biopsy specimen were analyzed on TaqMan Low Density Array cards containing primers and probe sets for 95 targets in duplicates for each sample by 7900HT Fast Real Time PCR System (Applied Biosystems, Foster City, CA). The set of targets (chemokines and receptors, interferons, TGFβ pathway, genes for extracellular matrix, and transcription factors) was chosen on the basis of potential relevance to the study according to the existing literature data (Supplemental Table 12). Specific gene expression was calculated relative to that of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase and the calibrator sample (FirstChoice Human Kidney Total RNA; Ambion, Life Technologies, Carlsbad, CA) by comparative threshold cycle method (2-ΔΔCT) using RQ Manager 1.2 software (Applied Biosystems) for automated data analysis and was expressed as relative quantity.
Sample Size and Statistical Analyses
Because of the relatively old age of donors and the significant proportion of expanded-criteria donors, we expected a 60% incidence of moderate to severe (Banff grade II or III) IF/TA, similar to results of protocol biopsy at 3 years obtained in groups of younger donors.16 Given the well known association between acute rejection and the development of IF/TA16 and the approximately 3.8-fold higher risk of developing IF/TA in patients with CMV viremia,4 we assumed a 66% reduction in the relative risk for moderate to severe IF/TA in patients treated with valacyclovir prophylaxis. After consideration of all the above facts, it was necessary to enroll at least 56 patients to ensure 80% power for detection of a treatment difference with type 1 error of 0.05. Given the anticipated number of patients to be lost to follow-up, a total of 70 patients were enrolled. Quantitative parametric data were compared between the groups using a t test and the Mann-Whitney U test in nonparametric distribution. Qualitative data were analyzed using the Fisher exact test. Patient and graft survival, incidence of CMV disease and viremia, and incidence of acute rejection episodes were calculated using Kaplan-Meier curves, with the log-rank test used for comparison. The Cox proportional hazard model adjusting for acute rejection, delayed graft function, and expanded-criteria donor was used to calculate the HR and 95% CI for graft loss in the pre-emptive group compared with the prophylaxis group. Data were analyzed according to the intention-to-treat principle. Statistical calculations were made using SPSS (SPSS Inc., Chicago, IL) and Statistica 9.0 (StatSoft, Tulsa, OK) software. P values < 0.05 were considered to represent statistically significant findings. This trial is registered with ANZCTR (ACTRN12610000015044).
Disclosures
None.
Acknowledgments
The authors thank Milada Cadova for her assistance in data collection.
The study was supported by Research Project no. MSM0021620819, “Replacement of and Support to Some Vital Organs,” awarded by the Ministry of Education, Youth, and Physical Training of the Czech Republic, and by project CZ.1.05/2.1.00/03.0076 from the European Regional Development Fund.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Randomized Trial of Pre-Emptive or Prophylactic Valganciclovir Therapy for Prevention of Cytomegalovirus Infection in Renal Transplantation,” on pages 1446–1448.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012010100/-/DCSupplemental.
References
- 1.Fishman JA: Infection in solid-organ transplant recipients. N Engl J Med 357: 2601–2614, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Reischig T: Cytomegalovirus-associated renal allograft rejection: New challenges for antiviral preventive strategies. Expert Rev Anti Infect Ther 8: 903–910, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Sagedal S, Nordal KP, Hartmann A, Sund S, Scott H, Degré M, Foss A, Leivestad T, Osnes K, Fauchald P, Rollag H: The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant 2: 850–856, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Reischig T, Jindra P, Hes O, Bouda M, Kormunda S, Treska V: Effect of cytomegalovirus viremia on subclinical rejection or interstitial fibrosis and tubular atrophy in protocol biopsy at 3 months in renal allograft recipients managed by preemptive therapy or antiviral prophylaxis. Transplantation 87: 436–444, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Smith JM, Corey L, Bittner R, Finn LS, Healey PJ, Davis CL, McDonald RA: Subclinical viremia increases risk for chronic allograft injury in pediatric renal transplantation. J Am Soc Nephrol 21: 1579–1586, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagedal S, Hartmann A, Nordal KP, Osnes K, Leivestad T, Foss A, Degré M, Fauchald P, Rollag H: Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int 66: 329–337, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Snydman DR, Allen U, Humar A, Transplantation Society International CMV Consensus Group : International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 89: 779–795, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Pescovitz MD, Valganciclovir Solid Organ Transplant Study Group : Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 4: 611–620, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Lowance D, Neumayer HH, Legendre CM, Squifflet JP, Kovarik J, Brennan PJ, Norman D, Mendez R, Keating MR, Coggon GL, Crisp A, Lee IC, International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group : Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. N Engl J Med 340: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Reischig T, Jindra P, Mares J, Cechura M, Svecová M, Hes O, Opatrný K, Jr, Treska V: Valacyclovir for cytomegalovirus prophylaxis reduces the risk of acute renal allograft rejection. Transplantation 79: 317–324, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Hodson EM, Jones CA, Webster AC, Strippoli GF, Barclay PG, Kable K, Vimalachandra D, Craig JC: Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: A systematic review of randomised controlled trials. Lancet 365: 2105–2115, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG: Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med 143: 870–880, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, Lockwood M, Gaudreault-Keener M, Koch MJ, Miller BW, Hardinger KL, Schnitzler MA, Brennan DC: Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant 6: 2134–2143, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Reischig T, Jindra P, Hes O, Svecová M, Klaboch J, Treska V: Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant 8: 69–77, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F: Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: Results of a randomized clinical trial. Am J Transplant 8: 975–983, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Cosio FG, Grande JP, Wadei H, Larson TS, Griffin MD, Stegall MD: Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant 5: 2464–2472, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Mannon RB, Matas AJ, Grande J, Leduc R, Connett J, Kasiske B, Cecka JM, Gaston RS, Cosio F, Gourishankar S, Halloran PF, Hunsicker L, Rush D, DeKAF Investigators : Inflammation in areas of tubular atrophy in kidney allograft biopsies: A potent predictor of allograft failure. Am J Transplant 10: 2066–2073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stegall MD, Park WD, Larson TS, Gloor JM, Cornell LD, Sethi S, Dean PG, Prieto M, Amer H, Textor S, Schwab T, Cosio FG: The histology of solitary renal allografts at 1 and 5 years after transplantation. Am J Transplant 11: 698–707, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Halloran PF, de Freitas DG, Einecke G, Famulski KS, Hidalgo LG, MengeL M, Reeve J, Sellares J, Sis B: An integrated view of molecular changes, histopathology and outcomes in kidney transplants. Am J Transplant 10: 2223–2230, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Pribylova-Hribova P, Kotsch K, Lodererova A, Viklicky O, Vitko S, Volk HD, Lacha J: TGF-beta1 mRNA upregulation influences chronic renal allograft dysfunction. Kidney Int 69: 1872–1879, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Benmarzouk-Hidalgo OJ, Cisneros JM, Cordero E, Martín-Peña A, Sanchez B, Martin-Gandul C, Gentil MA, Gomez-Bravo MA, Lage E, Perez-Romero P: Therapeutic effect of the acquisition of cytomegalovirus-specific immune response during preemptive treatment. Transplantation 91: 927–933, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Nickel P, Bold G, Presber F, Biti D, Babel N, Kreutzer S, Pratschke J, Schönemann C, Kern F, Volk HD, Reinke P: High levels of CMV-IE-1-specific memory T cells are associated with less alloimmunity and improved renal allograft function. Transpl Immunol 20: 238–242, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Witzke O, Hauser IA, Bartels M, Wolf G, Wolters H, Nitschke M, VIPP Study Group : Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 93: 61–68, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Spinner ML, Saab G, Casabar E, Bowman LJ, Storch GA, Brennan DC: Impact of prophylactic versus preemptive valganciclovir on long-term renal allograft outcomes. Transplantation 90: 412–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couzi L, Helou S, Bachelet T, Moreau K, Martin S, Morel D, Lafon ME, Boyer B, Alain S, Garrigue I, Merville P: High incidence of anticytomegalovirus drug resistance among D+R- kidney transplant recipients receiving preemptive therapy. Am J Transplant 12: 202–209, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, Abramowicz D, Jardine AG, Voulgari AT, Ives J, Hauser IA, Peeters P: The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant 10: 1228–1237, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Lisboa LF, Preiksaitis JK, Humar A, Kumar D: Clinical utility of molecular surveillance for cytomegalovirus after antiviral prophylaxis in high-risk solid organ transplant recipients. Transplantation 92: 1063–1068, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Preiksaitis JK, Brennan DC, Fishman J, Allen U: Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant 5: 218–227, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff ’05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]