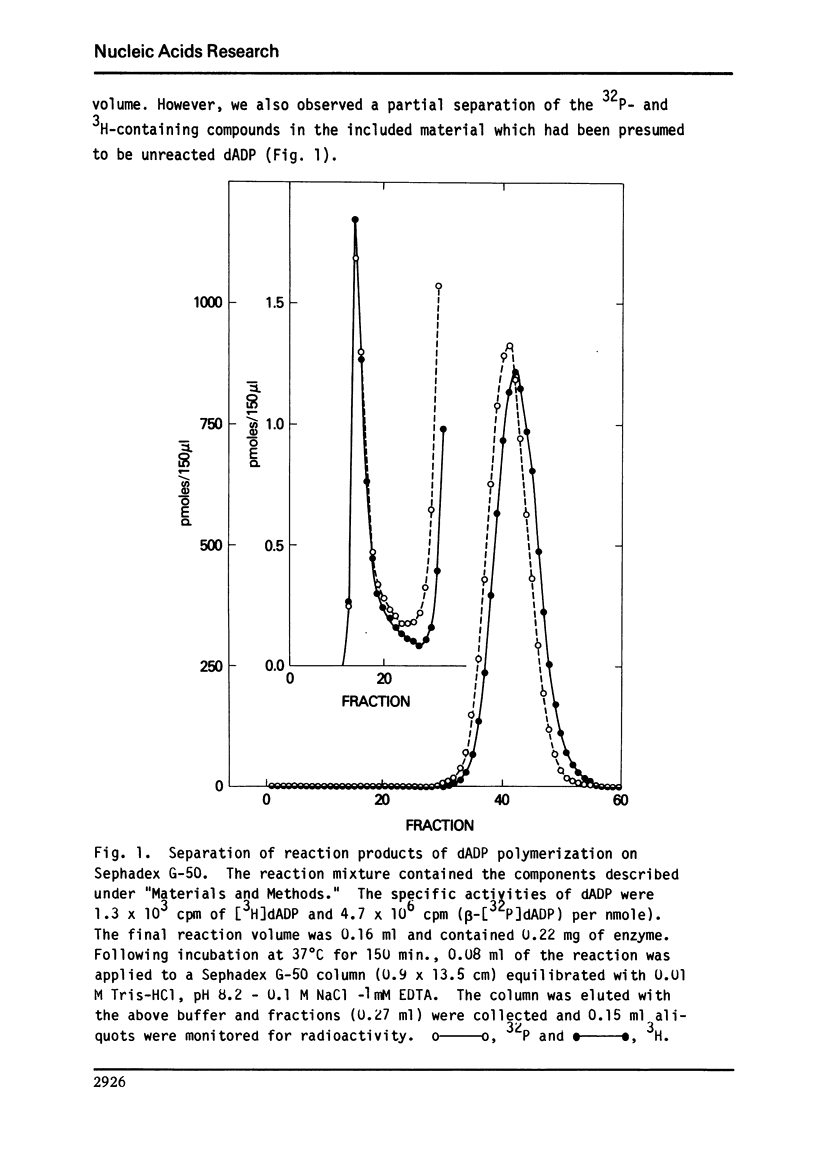
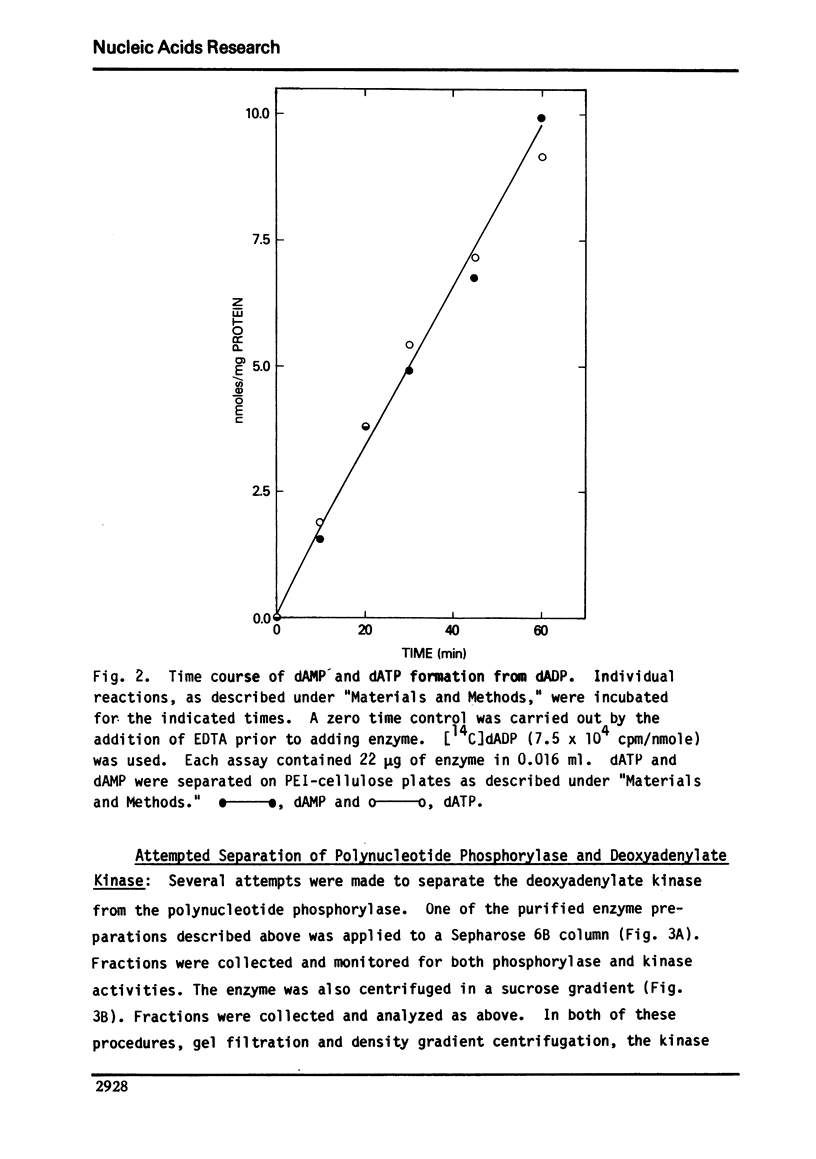
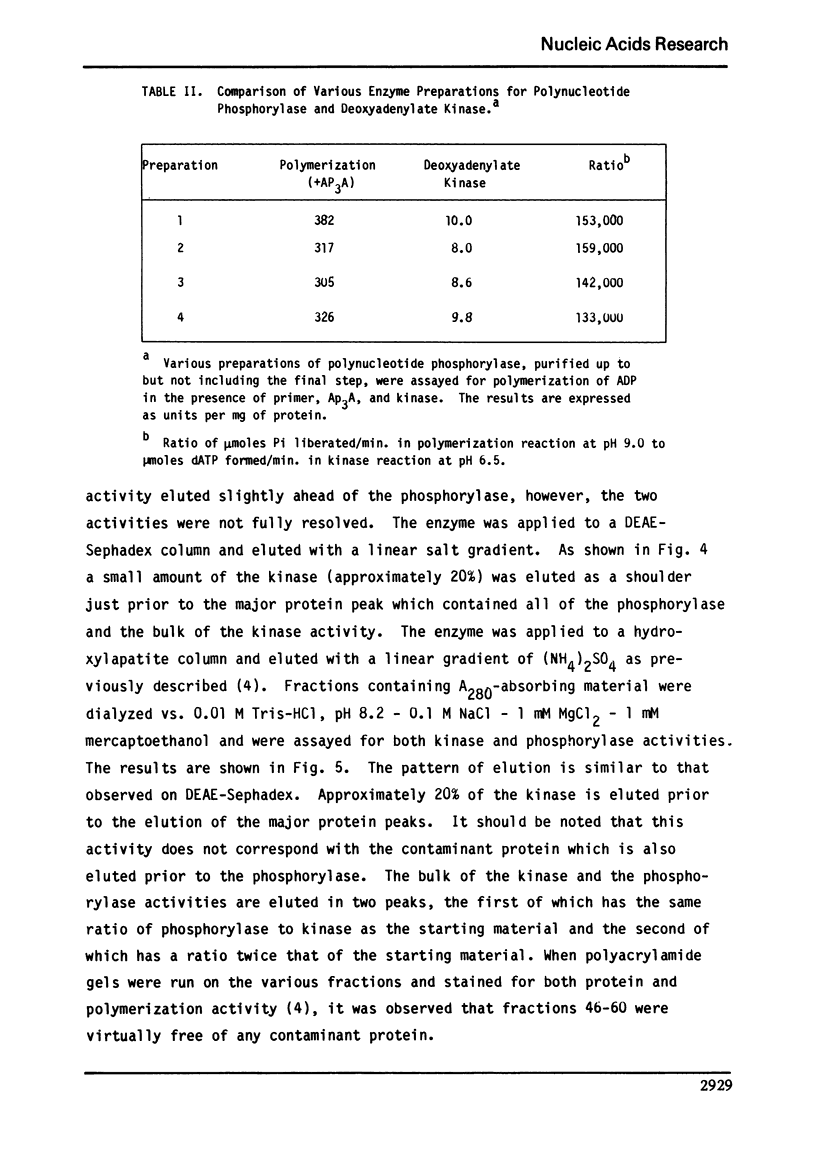
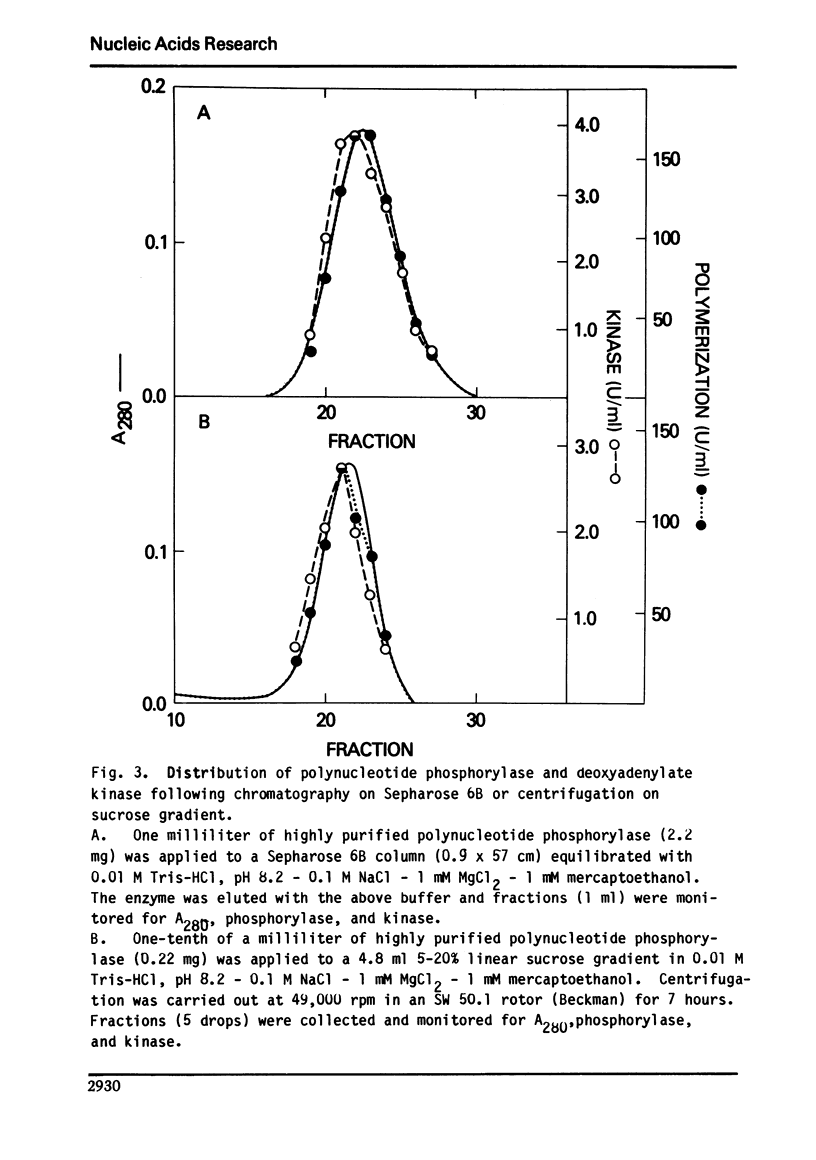
Abstract
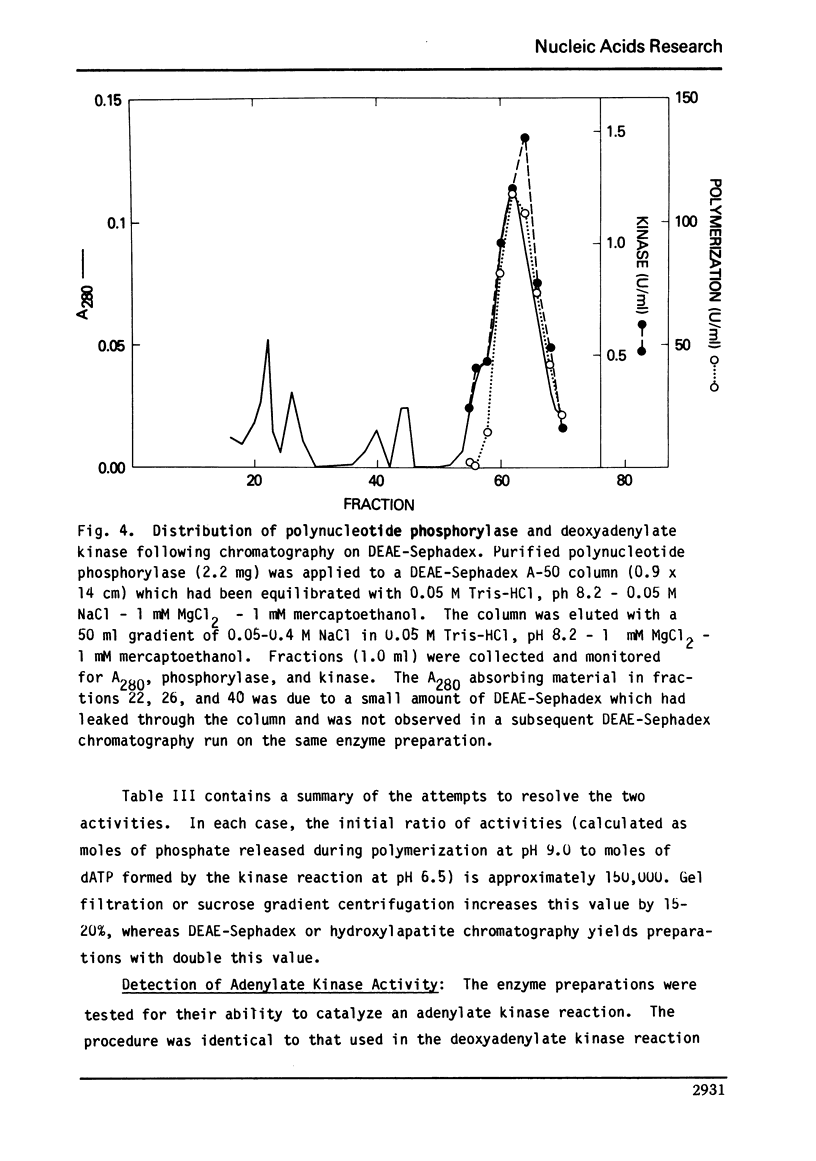
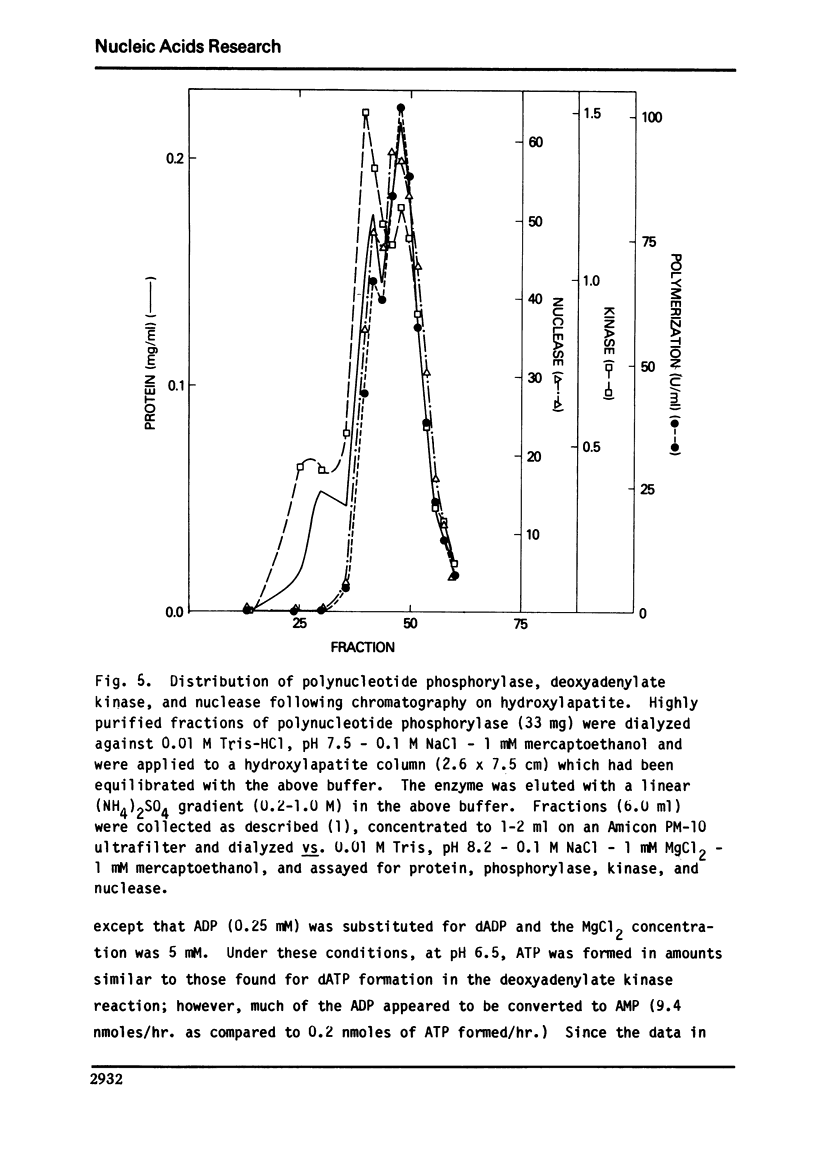
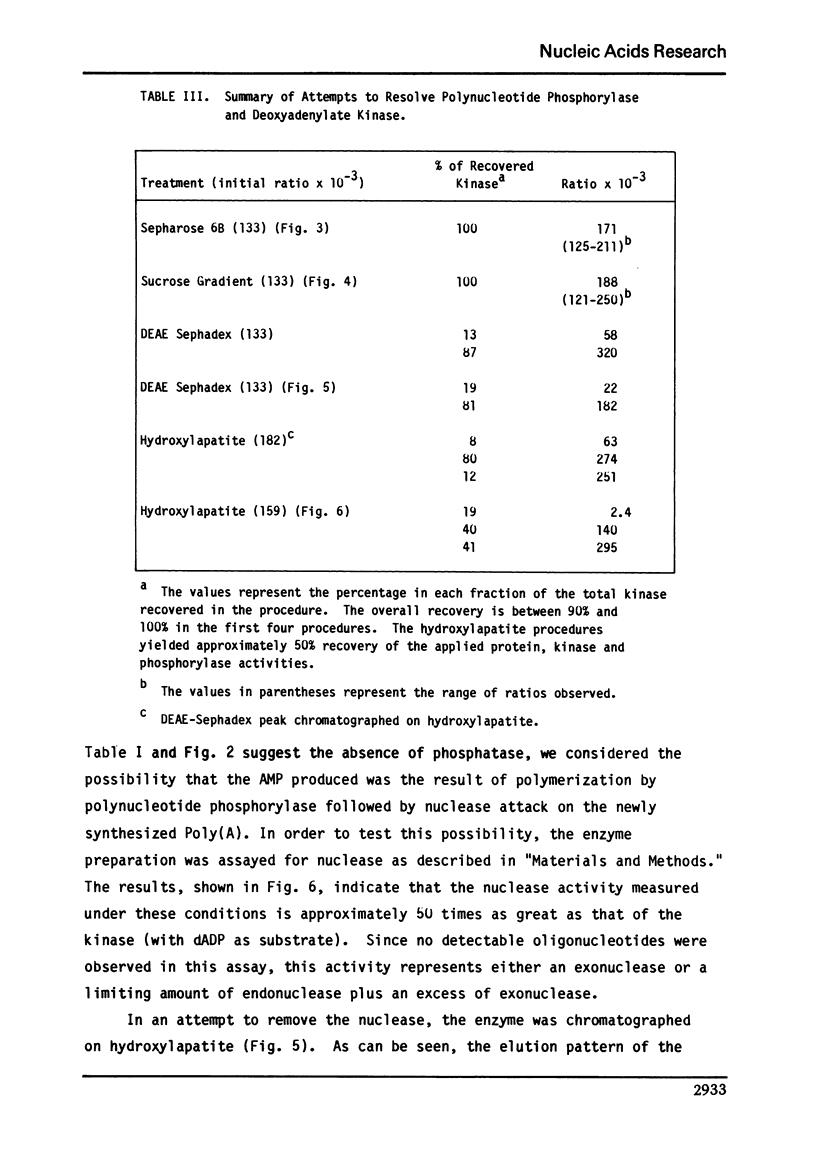
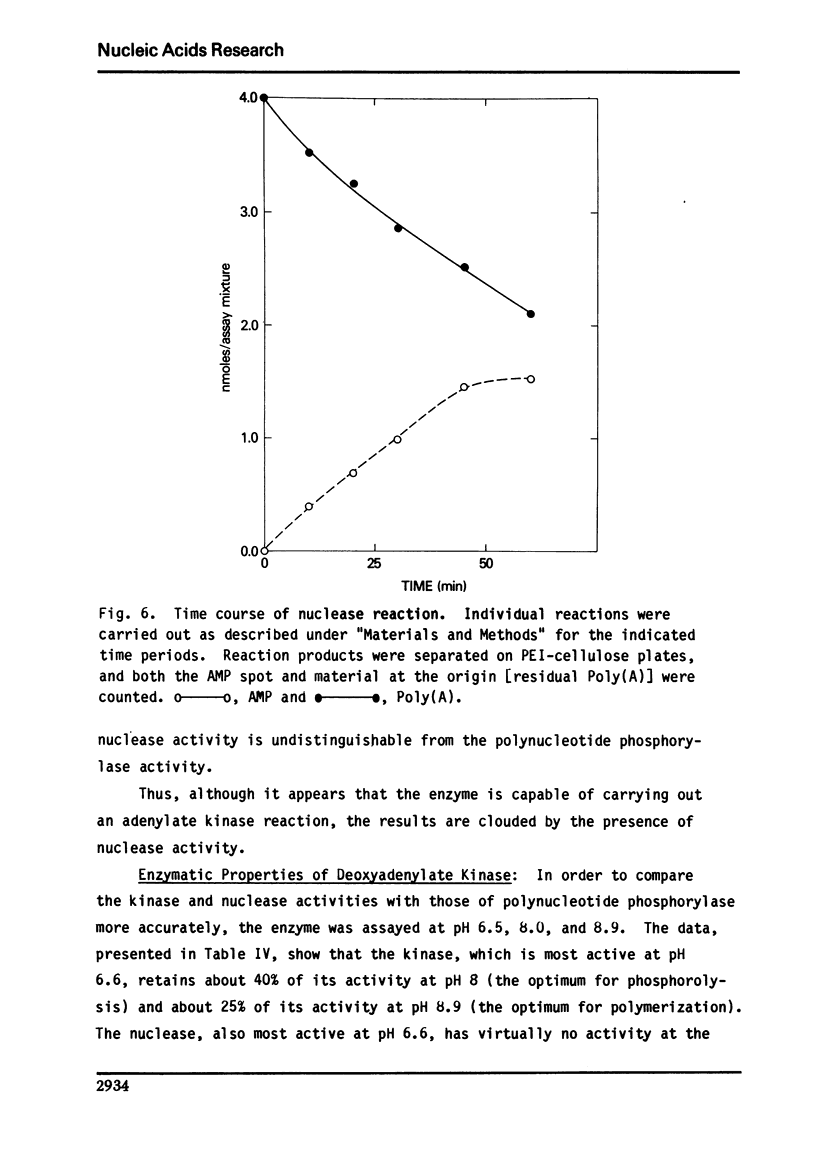
We report here the presence of two enzymatic activities associated with highly purified preparations of polynucleotide phosphorylase from Micrococcus luteus. The first, a nuclease activity, which is not separated from the phosphorylase on hydroxylapatite, may be due to substitution of H2O for phosphate in the phosphorolysis reaction. The second ac tivity, a deoxyadenylate kinase, the bulk of which is not resolved from the phosphorylase using gel filtration, sucrose density gradient centrifugation, DEAE-Sephadex, or hydroxylapatite chromatography, may represent a new activity of polynucleotide phosphorylase or be due to an enzyme which is tightly bound to the phosphorylase. Several properties of the kinase are described and its possible significance with respect to the overall enzyme mechanism is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou J. Y., Singer M. F. Deoxyadenosine diphosphate as a substrate and inhibitor of polynucleotide phosphorylase of Micrococcus luteus. I. Deoxyadenosine diphosphate as a substrate for polymerization and the exchange reaction with inorganic 32 P. J Biol Chem. 1971 Dec 25;246(24):7486–7496. [PubMed] [Google Scholar]
- Harvey R. A., Grunberg-Manago M. Identification of the nucleoside monophosphate end-group on the product of the polynucleotide phosphorylase reaction. Biochem Biophys Res Commun. 1966 May 25;23(4):448–452. doi: 10.1016/0006-291x(66)90748-0. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Suit J. L., Plate C. A. Initiation of transcription is temperature-dependent in an E. coli mutant with ts adenylate kinase. Biochem Biophys Res Commun. 1975 Nov 3;67(1):353–358. doi: 10.1016/0006-291x(75)90323-x. [DOI] [PubMed] [Google Scholar]
- Miller L. K., Wells R. D. Nucleoside diphosphokinase activity associated with DNA polymerases. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2298–2302. doi: 10.1073/pnas.68.9.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Singer M. F. The processive degradation of individual polyribonucleotide chains. I. Escherichia coli ribonuclease II. J Biol Chem. 1968 Mar 10;243(5):913–922. [PubMed] [Google Scholar]
- Wunderli W., Hüttler R., Staehelin M., Wehrli W. Poly(A) synthesis in T2L phage-infected Escherichia coli. A combination of polynucleotide phosphorylase and ATPase. Eur J Biochem. 1975 Oct 1;58(1):87–94. doi: 10.1111/j.1432-1033.1975.tb02352.x. [DOI] [PubMed] [Google Scholar]



