Abstract
Ischemia-reperfusion injury (IRI) contributes to decreased allograft function and allograft rejection in transplanted kidneys. Thrombospondin-1 is a stress protein typically secreted in response to hypoxia and the ligand activator for the ubiquitously expressed receptor CD47. The function of activated CD47 in IRI remains completely unknown. Here, we found that both CD47 and its ligand thrombospondin-1 were upregulated after renal IRI in mice. CD47-knockout mice were protected against renal dysfunction and tubular damage, suggesting that the development of IRI requires intact CD47 signaling. Chimeric CD47-knockout mice engrafted with wild-type hematopoietic cells had significantly lower serum creatinine and less tubular damage than wild-type controls after IRI, suggesting that CD47 signaling in parenchymal cells predominantly mediates renal damage. Treatment with a CD47-blocking antibody protected mice from renal dysfunction and tubular damage compared with an isotype control. Taken together, these data imply that CD47 on parenchymal cells promotes injury after renal ischemia and reperfusion. Therefore, CD47 blockade may have therapeutic potential to prevent or suppress ischemia-reperfusion–mediated damage.
Renal ischemia-reperfusion injury (IRI) is a major cause of ARF resulting from tubular dysfunction, and contributes to morbidity and mortality. In the context of transplantation, IRI promotes delayed graft function and acute rejection, and subsequently negatively influences long-term allograft outcome.1–3 The cascade of events leading to cellular injury and activation is established by initial oxygen depravation that leads to ATP depletion and mitochondrial dysfunction (reviewed by Murillo et al.4). After reperfusion, proinflammatory conditions and a coordinated release of cytokines, reactive oxygen species (ROS), and adhesion molecules promote the innate immune response to IRI.
Thrombospondin-1 (TSP1) is a matricellular glycoprotein maintained predominantly within platelets and present in nanomolar concentrations in the blood.5 It is secreted by multiple cell types, including inflammatory and vascular cells, typically in response to hypoxia.6 It is also produced by renal tubular epithelial cells (RTECs) after IRI.7 TSP1, through binding the ubiquitously expressed cell surface receptor CD47, regulates the canonical nitric oxide (NO) pathway via limitation of soluble guanylate cyclase activation.8–10 Organs subjected to IRI demonstrate suppression of NO,11 and therapies that supplement NO have been shown to mitigate IRI.12–15
In this study, we tested the hypothesis that renal tissue–expressed CD47 promotes IRI. We demonstrate for the first time that TSP1 and CD47 are significantly induced in IRI. In null mice, absence of activated CD47 leads to profound cytoprotection from renal IRI with significant abrogation of cell death and ROS production, suppressed cytokine production/secretion, and robust reperfusion, as assessed by laser Doppler analysis. This protection is independent of inflammatory cell activation because null mice transplanted with wild-type (WT) bone marrow, and hence bearing CD47+/+ inflammatory cells, continue to experience substantial protection from renal IRI. Furthermore, we show that limiting CD47 activation with an antibody that prevents TSP1 binding mitigates complications of renal IRI in mice, providing a potential, clinically relevant, therapeutic intervention.
Results
CD47 Is Expressed by RTECs and the TSP1-CD47 Signaling Axis Is Upregulated in Cells Exposed to Hypoxia-Reoxygenation
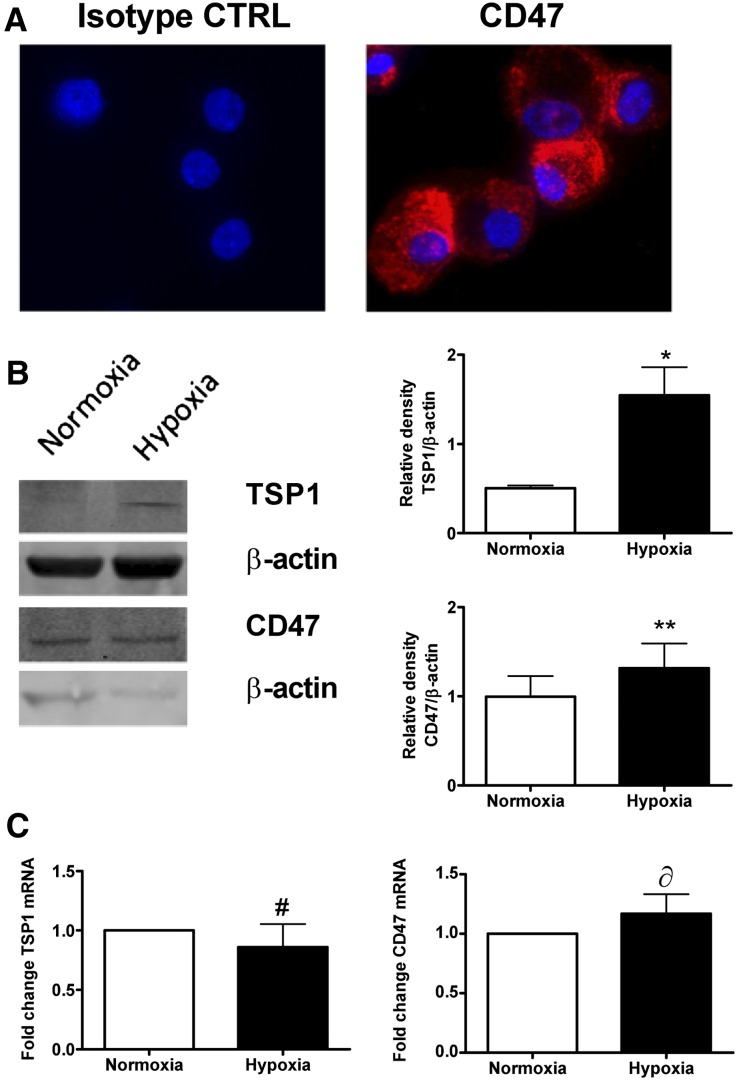
A single previous study reported upregulation of TSP1 protein after renal IRI,7 although expression of cellular TSP1 and CD47 in the kidney has not been defined. Staining cultured human RTECs for CD47 demonstrated significant cell surface expression (Figure 1A). RTECs challenged with hypoxia (FiO2 1%) and then 24 hours of reoxygenation (as a mimic of IRI) showed a significant induction of TSP1 protein coincident with persistence of CD47 protein, and persistence of both TSP1 and CD47 mRNA, compared with cells treated with normoxia (Figure 1, B and C).
Figure 1.
RTECs express CD47 and upregulate TSP1 in response to hypoxia. (A) Human RTECs were stained with CD47 antibody or isotype control (CTRL) and visualized by confocal microscopy. RTECs were exposed to 30-minute hypoxia (FiO2 1%) and then 24-hour reoxygenation or normoxia alone and TSP1 and CD47 (B) protein and (C) mRNA assessed. Data shown are means ± SD, and representative Western blots with relative densities are calculated from n=4 experiments. *P<0.01; **P=0.49; #P=0.88; δP=0.38 normoxia versus hypoxia.
Renal IRI Upregulates Cell Receptor CD47 and its Ligand TSP1
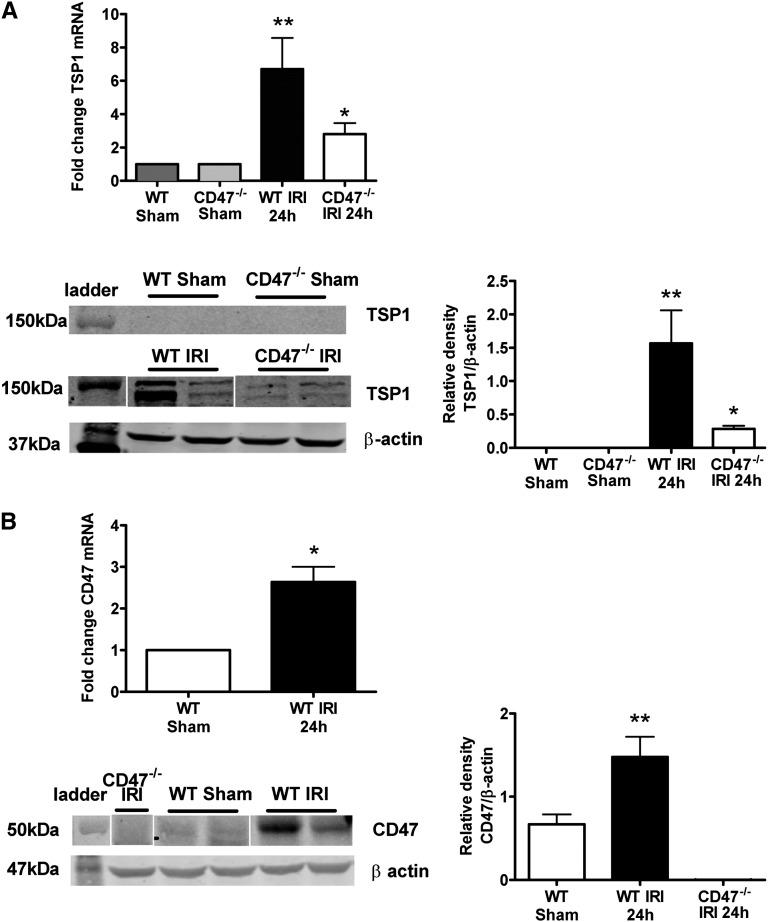
Extending upon results obtained with cultured RTECs, we found robust induction of both TSP1 mRNA and protein expression in WT mice 24 hours after reperfusion, compared with sham-operated animals (Figure 2A). There are no data on expression of its cognate receptor CD47 in response to acute injury, let alone in renal IRI. We now show for the first time concurrent upregulation of CD47 mRNA and protein expression (Figure 2B) in WT mice after IRI. Interestingly, CD47−/− mice showed decreased TSP1 expression compared with WT mice after IRI (Figure 2A).
Figure 2.
Renal IRI upregulates CD47 and its ligand TSP1. Kidney lysate from WT C57BL/6 male mice 24 hours after IRI compared with sham-operated controls was prepared and (A) TSP1 mRNA and protein (TSP1 is detected at 150 kD) or (B) CD47 mRNA and protein expression (CD47 is detected at 47–52 kD) assessed. mRNA data shown are mean ± SD, n=8 mice per group with the WT sham-operated animals as the reference group; representative Western blots with relative densities calculated from n=4 mice per group. *P<0.001 CD47−/− after IRI versus WT after IRI; **P<0.001 WT sham versus WT after IRI.
CD47 Limits Reperfusion after Renal IRI
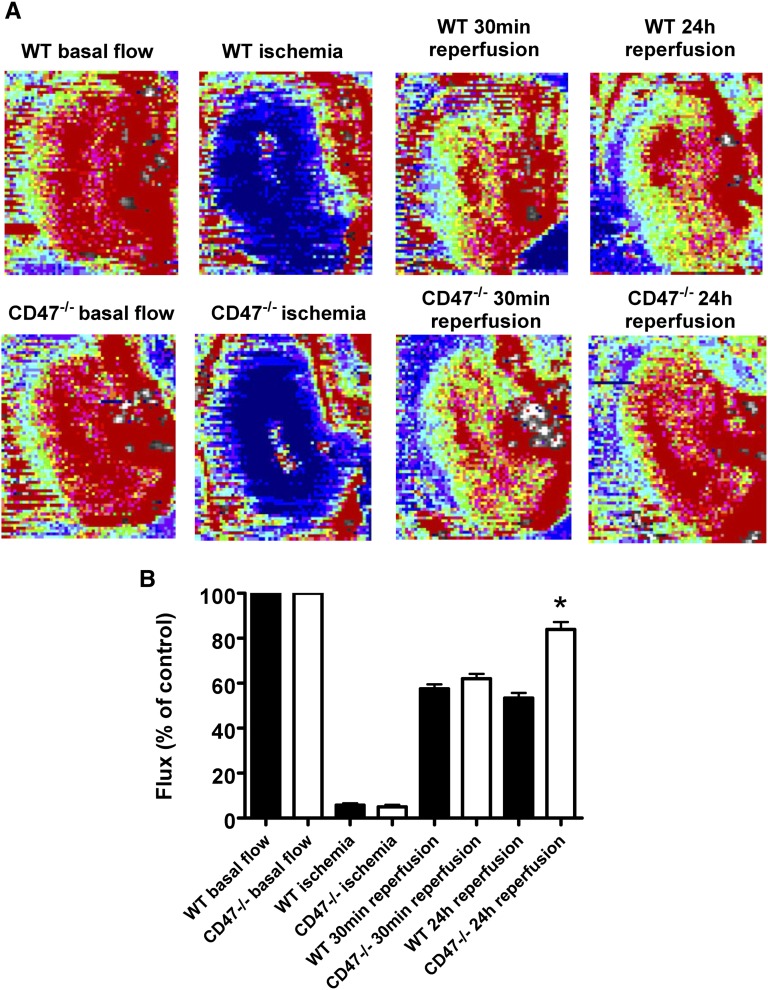
CD47 activation by its ligand TSP1 blocks NO and soluble guanylate cyclase signaling in multiple cell types.9,16,17 NO directly promotes tissue perfusion and deletion of TSP1 dramatically increases blood flow after NO challenge.8 Using real-time laser Doppler analysis of blood flow to the left kidney (Figure 3A), WT and CD47−/− mice demonstrated comparable basal blood flow and subsequently underwent renal IRI. Initial reperfusion (within 30 minutes) was similar between both groups of animals; however, blood flow was significantly greater in CD47−/− mice at 24 hours after IRI (Figure 3B).
Figure 3.
Renal reperfusion is limited by CD47. Eight-week-old male C57BL/6 WT and CD47−/− mice underwent 22 minutes of left-sided renal pedicle occlusion followed by reperfusion. (A) Representative laser Doppler color images of renal blood flow at baseline, ischemia, 30 minutes, and 24 hours of reperfusion. Red coloration of images indicates maximum blood flow and blue coloration minimum blood flow. (B) Analysis of tissue blood flow was performed. Changes in renal perfusion at indicated time points are presented as flux normalized to baseline values (% control). Results represent the mean ± SD of four measurements at each time point from n=5 WT and n=5 CD47−/− mice. *P<0.001 CD47−/− versus WT at 24 hours of reperfusion.
CD47−/− Mice Experience Less Renal Dysfunction and Acute Tubular Damage in a Model of Severe Renal IRI
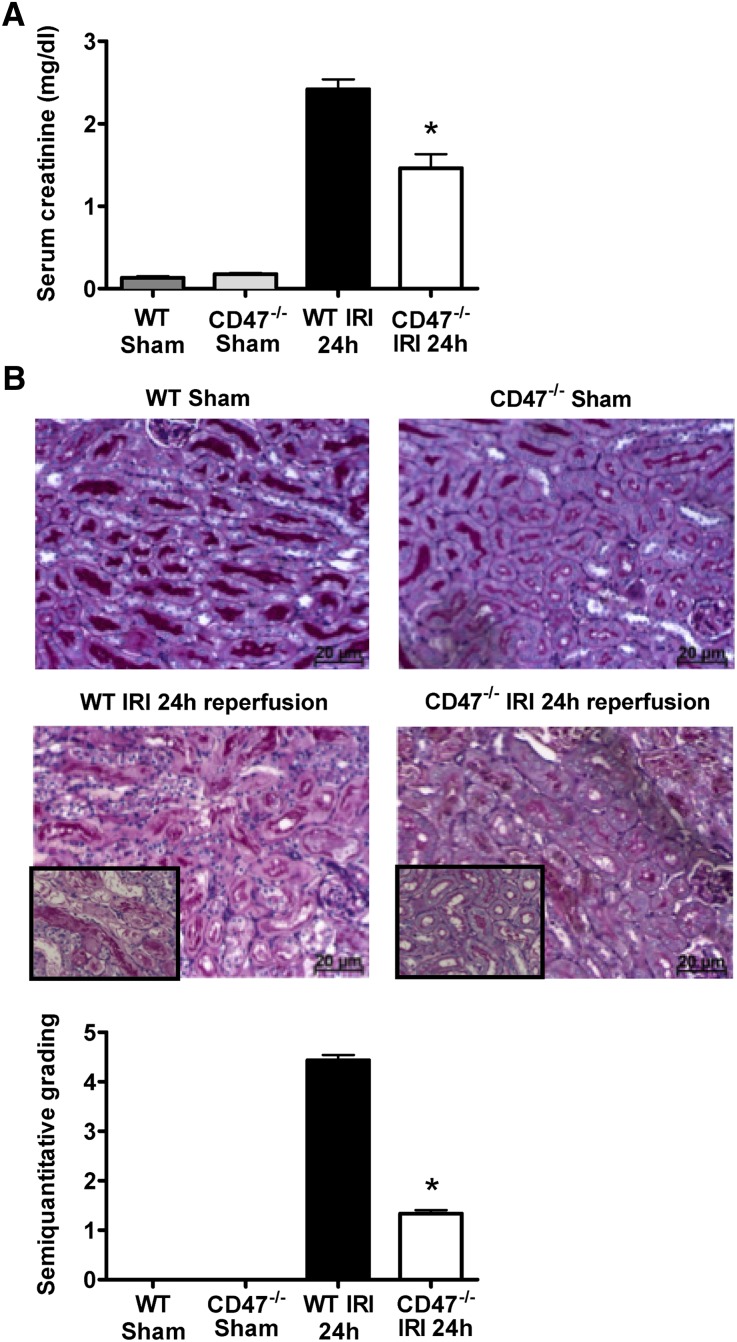
Activation of proinflammatory pathways and oxidative stress are major contributors to tissue injury after IRI.18 Because the TSP1-CD47 axis has been documented to inhibit tissue blood flow, we hypothesized that CD47−/− mice would experience less tissue injury post-IRI. To further test the importance of CD47 in renal IRI, we compared serum creatinine levels in CD47−/− versus congenic WT (CD47+/+) mice. Knockout of CD47 resulted in less functional renal impairment after 24 hours of reperfusion as assessed by serum creatinine levels (Figure 4A), indicating parenchymal cytoprotection. Clinically, WT mice were grossly uremic and failed to survive past 36–48 hours after reperfusion (data not shown). In contrast, CD47−/− mice did not demonstrate these findings and survived long term (up to 7 days; n=4 per group). To further confirm the presence of renal injury in our model, we evaluated renal histology. After 24 hours of reperfusion, kidneys of WT mice showed widespread and substantial tubular necrosis, tubular dilation, and cast formation. The injury, although being predominantly corticomedullary, also typically extended to the renal cortex. In contrast, kidneys from CD47−/− mice displayed significantly less tubular damage 24 hours after IRI (Figure 4B). Thus, CD47 deficiency had a protective effect on renal function and damage after induction of severe IRI.
Figure 4.
CD47−/− mice are protected against severe renal IRI. (A) Quantification of serum creatinine after bilateral renal ischemia and 24 hours reperfusion, in CD47−/− mice (white bars), WT controls (black bars), and sham-operated mice (gray bars). Data are mean ±SD, n=8–10 per group. *P<0.001 CD47−/− versus WT. (B) Representative renal tissue sections and quantitative analysis of tubular damage of corticomedulla from WT and CD47−/− mice 24 hours after sham operation or ischemia-reperfusion (periodic acid–Schiff stained) are shown. Data shown are mean ± SD, n=8–10 per group, *P<0.001 CD47−/− after IRI versus WT after IRI. Original magnification, ×200; inset, ×400 magnification insert.
Inflammatory Cell Influx Is Decreased in CD47−/− Mice after IRI
Leukocyte influx is a hallmark of renal IRI. We found significantly fewer interstitial neutrophils (Figure 5A) and macrophages (Figure 5B) in CD47−/− mice at 24 hours after reperfusion compared with WT mice. The number of infiltrating macrophages (in terms of cell number per high-power field) was also significantly less than reported in other studies.19,20 These data suggest an important role for CD47 in the induction of a cellular inflammatory response during IRI.
Figure 5.
Inflammatory cell infiltration is significantly decreased in CD47−/− mice subjected to renal IRI. Representative photomicrographs and quantitative analysis of kidney tissue sections stained by immunohistochemistry for (A) neutrophils and (B) macrophages (total cell number per 10 hpf for both parameters). Data shown are mean ± SD, n=8–10 per group. *P=0.001 and **P<0.05 CD47−/− after IRI versus WT after IRI. Original magnification, ×400.
Absence of Activated CD47 Reduces Proinflammatory Cytokine and Chemokine Expression after Renal IRI
We next investigated whether CD47 deficiency also influenced expression of cytokines and chemokines critical to the inflammatory response after IRI. mRNA transcript profiles of IL-6, TNFα, IL-1β, monocyte chemoattractant protein-1 (CCL2), and macrophage inflammatory protein-1 (CXCL2) were examined in kidneys from WT and CD47−/− mice after sham operation or IRI (Figure 6). mRNA expression in WT mice subjected to renal IRI was uniformly increased compared with sham-operated controls. Overall, cytokine expression increased to a much lesser extent in CD47−/− mice and both TNFα and IL-1β transcript levels were not elevated above baseline values.
Figure 6.
Proinflammatory cytokine and chemokine mRNA profiles are reduced in CD47−/− mice subjected to renal IRI. mRNA expression of proinflammatory cytokines IL-6, TNFα, and IL-1β and CCL2 and CXCL2 in kidneys from WT and CD47−/− mice subjected to IRI. Results have been normalized to the housekeeping gene (HPRT1) and WT sham-operated animals used as the reference group. Data shown are means ± SD, n=6–10 per group. *P<0.001 CD47−/− after IRI versus WT after IRI; #P=0.01 CD47−/− after IRI versus WT after IRI.
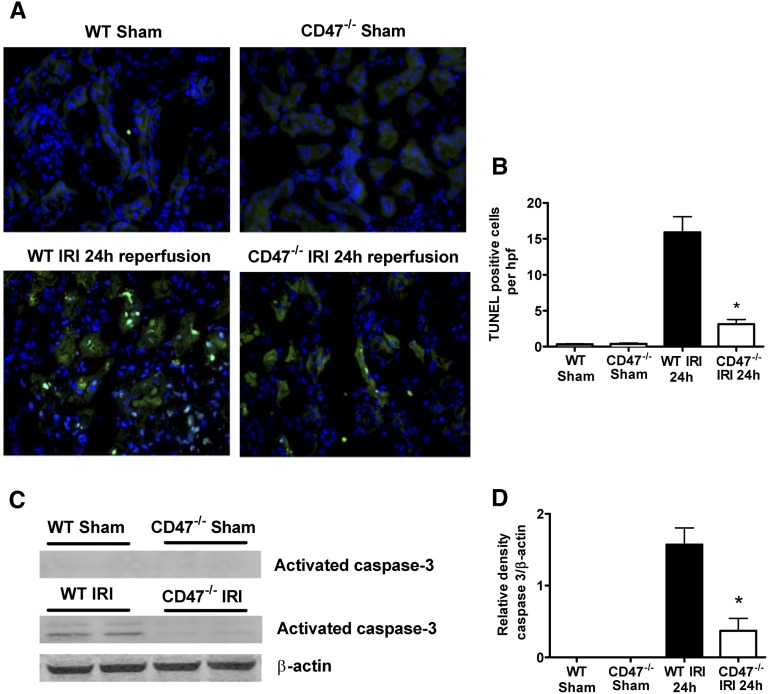
CD47−/− Mice Show Reduced Evidence of Programmed Renal Cell Death after IRI
Renal IRI is also characterized by RTEC apoptosis.21 CD47 ligation by TSP1 regulates cellular apoptotic machinery by modulating Fas signaling22 and has been linked through Bcl-2 homology 3-only protein 19 kD interacting protein-3 (BNIP3) activation to programmed cell death.23 We assessed tubular cell apoptosis by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) staining and expression of caspase-3, a transducer of programmed cell death. Compared with sham-operated animals, WT mice subjected to IRI demonstrate significant RTEC apoptosis at 24 hours (Figure 7, A and B). This correlated with detection of activated caspase-3 on Western blot (Figure 7, C and D). Notably, the number of apoptotic cells in kidneys and caspase-3 expression were markedly lower in CD47−/− mice at 24 hours after reperfusion.
Figure 7.
Programmed cell death and apoptosis are reduced in the kidney in CD47−/− mice after IRI. (A) Representative renal tissue sections from IRI and sham-operated mice (n=6 per group) were stained by TUNEL assay and visualized by confocal microscopy. (B) Apoptotic cells/hpf were counted in 10 successive fields in CD47−/− mice (white bars) and WT mice (black bars). *P<0.001 CD47−/− after IRI versus wild-type after IRI. (C) Kidney tissue lysate was prepared from WT or CD47−/− mice subjected to sham operation or IRI, resolved by SDS-PAGE and probed for caspase-3 (activated caspase-3 is detected at 17 and 19 kD). Densitometry represents mean ±SD of n=6 samples per group (D). *P<0.001 CD47−/− after IRI versus WT after IRI. Original magnification, ×200.
Absence of Activated CD47 Mitigates Renal IRI through a Reduction in ROS
IRI is associated with increased production of ROS, particularly superoxide (O2−), which is a major mediator of cellular injury.24 To assess whether the cytoprotective effect of a lack of CD47 expression could be attributed to a reduction in the production of ROS, intracellular generation of the ROS moiety O2− was visualized with the fluoroprobe dihydroethidium (DHE). Using confocal microscopy, sections of kidney from WT mice showed a widespread and marked increase in DHE fluorescence compared with sham-operated controls (Figure 8A), and this signal was dramatically abrogated in CD47−/− mice at 24 hours of reperfusion.
Figure 8.
Renal oxidative stress is reduced in CD47−/− mice after IRI. (A) Representative renal tissue sections from IRI and sham-operated mice (n=6–8 per group) stained with DHE and visualized by confocal microscopy. Intensity of staining was quantified using Image J. *P<0.001 CD47−/− after IRI versus WT after IRI. (B) Kidney tissue lysate was prepared from WT or CD47−/− mice subjected to sham operation or IRI, resolved by SDS-PAGE, and probed for 3-nitrotyrosine (3NT). Densitometry represents mean ± SD of n=4 samples per group. *P<0.001 CD47−/− after IRI versus WT after IRI. (C) mRNA expression profile of iNOS in renal tissue in WT and CD47−/− mice subjected to IRI or sham operation. Results have been normalized to the housekeeping gene (HPRT1) and WT sham-operated animals used as the reference group. Data shown are mean ± SD, n=6–10 per group. *P<0.001 CD47−/− after IRI versus WT after IRI. Original magnification, ×200.
The combination of O2− with resident NO forms peroxynitrite (ONOO−), which is capable of aggravating cellular damage by nitration of protein tyrosine residues.25 Western blot analysis of kidney tissue indicated the presence of nitrated protein bands at 27 and approximately 47 kD (only bands at 47 kD are shown in Figure 8B) in WT mice after 24-hour reperfusion, which was reduced in CD47−/− mice. Interestingly, basal tissue nitration trended lower in CD47−/− kidneys compared with WT. Oxidative stress also induces nitric oxide synthase (iNOS) expression, which aggravates tubular injury by supplying additional NO for ONOO− formation in the presence of excess ROS.26,27 mRNA expression for renal iNOS was increased in WT mice but significantly attenuated in CD47−/− mice (Figure 8C).
CD47-Mediated Kidney IRI Requires Functional CD47 Signaling on Renal Parenchymal Cells
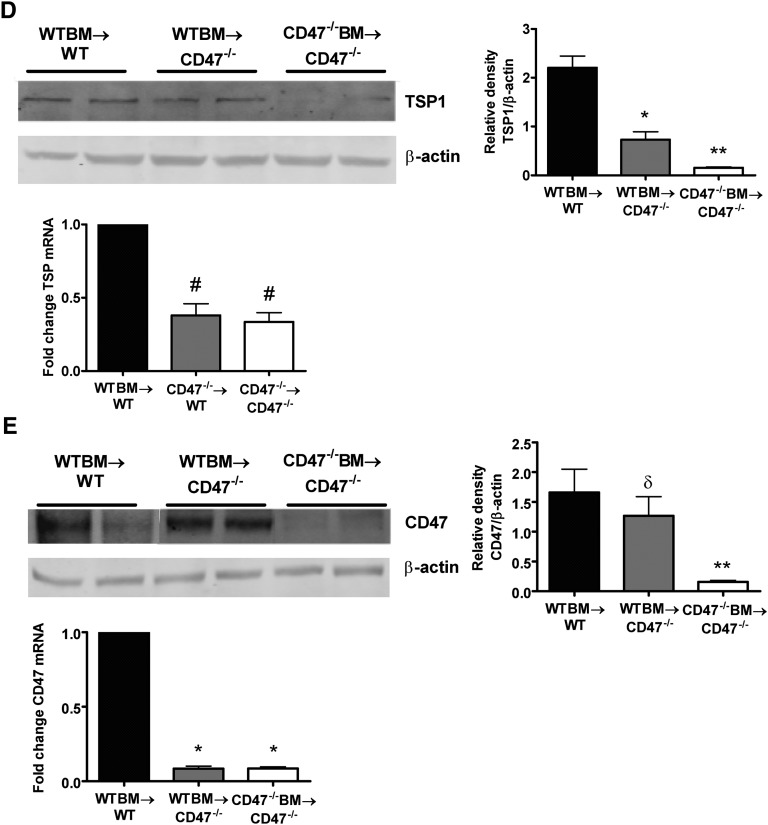
CD47 is expressed on all circulating cells, including leukocytes, platelets, and red blood cells.28 Thus, it is not clear if results in CD47−/− mice are secondary to decreased signaling among tissue parenchymal cells or circulating and interstitial leukocytes. To test this, we used bone marrow (BM) transplantation in irradiated CD47+/+ (WT) and CD47−/− mice to generate chimeric mice expressing CD47 on renal-resident leukocytes but not on renal parenchymal cells (WTBM-CD47−/−), as well as appropriate control animals. The inability of CD47−/− BM to engraft WT mice has been documented previously,29 putatively from augmented recipient phagocytosis of donor BM cells in vivo. Recapitulating this observation, we were not able to generate chimeric mice expressing an absence of leukocyte CD47 (CD47−/−BM-WT) because all mice (n=5) died in the early post-transplant period (median survival time 19 days). In other BM chimeras, we determined the expression of CD47 on whole blood by flow cytometric analysis 8 weeks after transplantation, demonstrating complete engraftment in all animals (n=7) (Figure 9A). WTBM-WT mice showed significant kidney dysfunction and injury 24 hours after IRI, whereas CD47−/−BM-CD47−/− chimeric mice were substantially protected from kidney IRI as measured by serum creatinine and tubular damage (Figure 9, B and C, respectively). The difference in serum creatinine levels and tubular injury scores recapitulated the results seen previously (see Figure 3). In addition, WTBM-CD47−/− chimeras were protected from renal IRI to the same degree as CD47−/−BM-CD47−/− mice. These results suggest that functional CD47 on renal parenchymal cells makes the greatest contribution to tissue damage.
Figure 9.
Expression of parenchymal activated CD47 determines kidney damage after IRI. (A) Donor recipient chimerism of the hematopoietic system 8 weeks after BM transplantation in wild-type and CD47−/− mice. Whole blood collected from CD47−/− mice transplanted with WT BM were subjected to FACS analysis of CD47 expression and compared with relevant control groups. Analysis of (B) serum creatinine and (C) tubular damage in respective BM chimeric mice at 24 hours reperfusion after bilateral renal ischemia. Data are mean ± SEM, n=6–7 per group. *P<0.001 WTBM→WT versus WTBM→CD47−/− and CD47−/−BM→CD47−/−. Kidney tissue lysate was prepared from chimeric mice and (D) TSP1 and (E) CD47 protein and mRNA assessed. Data are mean ± SEM, n=6 per group. *P<0.01 WTBM→WT versus WTBM→CD47−/−; **P=0.001 WTBM→CD47−/− versus CD47−/−BM→CD47−/−; #P<0.0001 compared with WTBM→WT; δP=0.45 WTBM→WT versus WTBM→CD47−/−.
Interestingly, TSP1 protein expression was decreased after IRI in WTBM- CD47−/− chimeras and even further suppressed in CD47−/−BM-CD47−/− mice (Figure 9D) suggesting that activated leukocytes are a significant source of tissue TSP1 after injury. Conversely, CD47 protein expression was significantly decreased in CD47−/−BM-CD47−/− mice after IRI (Figure 9E). Renal mRNA levels of TSP1 and CD47 were suppressed in CD47−/− mice after IRI regardless of BM genotype.
Blockade of CD47 Using mAb In Vivo Abrogates Renal IRI
We have reported soft tissue protection in mice treated with a CD47 blocking antibody.30 However, it was not clear if systemic treatment results in significant tissue localization of the CD47 antibody. To test this, we treated uninjured animals with a CD47 mAb (0.4 µg/g body weight in sterile saline) via a single intraperitoneal injection. Analysis of tissue sections obtained from animals 90 minutes after treatment demonstrated the presence of CD47 antibody on RTECs (Figure 10A). Extending these studies, we evaluated whether blockade of CD47 activation could influence clinical outcome of renal IRI, treating mice with the same dose of the CD47 blocking antibody as above 90 minutes before induction of IRI. Western blot analysis of renal tissue demonstrates downregulation of both CD47 and TSP1 protein expression in WT mice receiving the CD47 blocking antibody compared with mice that received an isotype control antibody (Figure 10B). Mice treated with a CD47 blocking antibody had markedly improved renal function at 24 hours of reperfusion, as reflected by lower serum creatinine levels and decreased tubular damage by light microscopy assessment of tissue sections (Figure 10, C and D), with results recapitulating those achieved in CD47−/− mice after IRI. Moreover, CD47 blockade resulted in global suppression of inflammation after IRI, with significantly fewer tissue neutrophils observed via immunohistology and markedly lower mRNA levels of renal IL-1β, IL-6, CCL2, CXCL2, and iNOS observed on quantitative PCR (Figure 10, E and F). Interestingly, TNF-α mRNA levels were not decreased in CD47 antibody-treated mice after IRI.
Figure 10.
Antibody blockade of CD47 significantly ameliorates renal IRI. WT mice were treated with CD47 blocking antibody (αCD47) or isotype IgG control (CTRL) antibody (0.4 μg/g body weight) 90 minutes before induction of IRI. (A) Sham-operated kidneys were sectioned, stained with anti-rat AlexaFluor-555, and visualized by confocal microscopy. Representative images are shown. (B) Western blots of kidney tissue lysates were probed for TSP1 and CD47. Densitometry represents mean ± SD of n=4 samples per group. *P<0.001 WT + αCD47 antibody after IRI versus WT + CTRL antibody after IRI. (C) Levels of serum creatinine, and (D) quantitative analysis of tubular damage with representative renal tissue sections of corticomedulla from treatment groups 24 hours after ischemia-reperfusion are shown. Data are mean ± SD, n=10–12 per group. *P<0.001 and **P<0.0001 WT + αCD47 antibody after IRI versus WT + CTRL antibody after IRI. (E) Representative photomicrographs and quantitative analysis of kidney tissue sections stained by immunohistochemistry for neutrophils (total cell number per 10 hpf). Data shown are mean ±SD, n=6 per group and six independent fields assessed. (F) mRNA expression of proinflammatory cytokines IL-6, TNFα, and IL-1β, chemokines CCL2 and CXCL2, and iNOS in kidneys from WT mice pretreated with CTRL or αCD47 antibody and subjected to IRI. Results have been normalized to the housekeeping gene (HPRT1) and WT CTRL antibody animals used as the referent control. Data shown are mean ± SD, n=7 per group. **P<0.0001; ∞P=0.90; #P=0.02. Original magnification, ×400 in A and E; ×200 in C.
Discussion
This study has several major findings . First, we provide evidence of strong expression of cell receptor CD47 on human RTECs. In response to the IRI-like conditions of hypoxia and reoxygenation, RTECs demonstrate persistent upregulation of the TSP1-CD47 signaling axis. Results obtained with cultured RTECs were reproduced by results in animals challenged with renal IRI. In WT mice there is rapid upregulation of activated CD47 after renal IRI, providing the first in vivo evidence of injury-mediated CD47 induction. Further support for an instigating role for CD47 in the pathophysiology of renal IRI is provided by studies in CD47−/− mice that are protected from severe renal IRI. Although we have reported increased tissue survival after injury in CD47 null mice,30–32 the exact mechanism involved has remained unclear. We herein demonstrate that, in renal IRI, cytoprotection is conferred predominantly by absence of CD47 expression on RTECs and identify RTECs as major targets of and responders to CD47-mediated IRI. Finally, in translational studies we show that therapeutic blockade of CD47 activation in the kidney decreases renal dysfunction after IRI. Together, these data suggest an important role for tissue-resident activated CD47 in the pathophysiology of renal IRI.
TSP1 is a secreted glycoprotein made by vascular cells, inflammatory cells, and platelets in response to injury, although it is detectable at low concentrations in plasma of healthy individuals.5 In contrast, cell membrane CD47 is expressed ubiquitously6 and TSP1 binding to and activation of CD47 serves an important physiologic function in terms of regulating cardiovascular responses.33 However, nothing is known about the role of CD47 in renal disease. We are the first to show increased CD47 mRNA and protein expression in kidneys subjected to IRI, which occurs concurrently with upregulation of TSP1. Intriguingly, data from CD47−/− and chimeric mice demonstrated that, in the absence of parenchymal CD47, IRI-mediated gene induction of both TSP1 and CD47 did not occur, and are in agreement with our recent report of linked TSP1-CD47 gene regulation in hypoxic pulmonary endothelial cells.34 These data suggest an active role of the CD47 receptor in affecting pathophysiology in acute renal injury and the possible existence of a common pathway controlling the gene expression of these proteins.
Experimental data suggest that IRI rapidly activates multiple pathways involving renal hemodynamics, proinflammatory mediators, oxidative stress, and the innate immune system (reviewed in Eltzschig and Eckle35 and Snoeijs et al.36). From our data, it is clear that an absence of CD47, despite the presence of its soluble ligand TSP1, significantly abrogates the induction of many of these pathophysiological pathways. WT mice demonstrate substantial ongoing renal perfusion defects at 24 hours after IRI, whereas CD47−/− mice have enhanced blood flow recovery approaching baseline. Improved blood flow may be part of the reparative repertoire seen in CD47−/− mice and reflect significant normalization of renal dysfunction (measured by serum creatinine) and abrogation of tissue damage (preservation of tubular integrity assessed by light microscopy) compared with WT mice.
Programmed cell death remains an important feature of IRI and both apoptosis, assessed by TUNEL staining, and expression of activated caspase-3 were elevated in WT compared with CD47−/− mice after 24 hours of reperfusion. The difference between WT and knockout mice may have been greater if observed at an earlier time point (3–6 hours of reperfusion) that reflects the early phase development of apoptosis in IRI,37 but apoptotic figures are typically evident at 24 hours of reperfusion.38,39 The role of CD47 in programmed cell death has been defined in T cells through an interaction with BNIP3.23 The involvement of CD47 in promoting apoptosis in other cell types has not been investigated, but the decreased RTEC apoptosis observed in CD47−/− mice may be explained by the proapoptotic function of CD47.
Recruitment of leukocytes, particularly neutrophils and macrophages, is a hallmark of IRI40–42 and both cell types are actively involved in tissue damage in ischemia-related ARF.41,43 The impaired leukocyte influx seen in kidneys from both CD47−/− mice (and WT mice treated with a CD47 blocking antibody) may be in part as a consequence of reduced proinflammatory cytokine and chemokine levels (particularly CXCL2), and may account for the lower degree of renal dysfunction and tissue damage compared with WT controls.
Arguing against a primary role for inflammatory cells in CD47 promotion of renal IRI are results obtained in chimera mice. Mice with CD47−/− parenchymal cells, but CD47+/+ circulating leukocytes, were protected from renal dysfunction and damage to the same degree as fully CD47−/− mice, suggesting that the absence of CD47 on tissue-resident cells is most protective against injury. Due to the inability to engraft CD47−/− leukocytes into WT mice, this model remains incomplete and further work is required to fully characterize the contribution of CD47−/− myeloid cells after renal IRI.
Lastly, to assess the clinical implications of these findings, we tested whether CD47 blockade could mimic the improved outcome seen in null mice after renal IRI. Immunofluorescent histology provided preliminary evidence that treating via systemic injection with CD47 mAb results in renal cellular and tissue-specific localization of the antibody. These data are important in that the results were obtained in the absence of renal IRI, and suggest the potential to obtain adequate receptor targeting as a pretreatment to renal transplant. WT mice treated with a CD47 antibody that blocks its activation30,44 demonstrated downregulation of tissue expression of both cell receptor CD47 and ligand TSP1, concurrent with decreased renal damage and pan-suppression of inflammatory cell response and cytokine mRNA after IRI. Results for a single intraperitoneal dose of antibody recapitulated the null phenotypic response after renal IRI and suggest that this therapeutic approach may be efficacious as a mitigant for transplant IRI.
In summary, our results document a new and important role for activated CD47 in mediating RTEC injury in IRI and that inhibition of this pathway is central to cytoprotection from inflammatory and oxidative stress. Significantly, renal IRI appears to be mediated predominantly by tissue-expressed CD47. Elimination of activated CD47 signaling, whether in knockout animals or via antibody blockade, provided robust protection from renal IRI, suggesting a role for CD47 blockade strategies to improve outcomes after procedures and disease processes that effect organ perfusion.
Concise Methods
A detailed methods section can be found in the Supplemental Material.
Animals
Male C57BL/6 WT and CD47−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All studies were performed using protocols approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and in accordance with National Institutes of Health guidelines.
Cell-Based Experiments
Human RTECs (Lonza, Basel, Switzerland) were grown in appropriate media. Cells were serum starved at 80% confluence, subjected to 30-minute hypoxia (FiO2 1%) and then 24-hour reoxygenation and collected for RNA or protein (as described below). For immunofluorescence, cells were subjected to cytospin, stained with αCD47 antibody (clone B6H12; Santa Cruz Biotechnology, Santa Cruz, CA) or IgG1 isotype control, and then AlexaFluor 555 (Molecular Probes, Eugene, OR) and 4′,6-diamidino-2-phenylindole (Molecular Probes).
IRI
Mice were anesthetized using isoflurane and oxygen titrated to effect, and body temperature maintained at 37°C. Microaneurysm clips were placed to occlude both renal pedicles for 22 minutes. The abdomen was closed with 5/0 monofilament suture. Mice were euthanized 24 hours after reperfusion; blood was collected and kidney tissue snap frozen, placed in RNAlater, embedded in optimal cutting temperature compound (OCT; Sakura Finetek, Torrance, CA), or fixed in 10% neutral buffered formalin.
Laser Doppler Blood Flow Analysis
Renal perfusion was measured using laser Doppler imaging (MoorLDI-2%; Moor Instruments, Devon, UK). Briefly, animals were anesthetized and core temperature maintained at 37°C. Renal blood flow was assessed at baseline, in response to ischemia and reperfusion at 30 minutes and 24 hours. Results are expressed as the percent change from baseline control of the region of interest.
CD47 Antibody Treatment
WT C57BL/6 mice were randomized to receive either anti-mouse CD47 mAb (clone 301, Santa Cruz Biotechnology; 0.4 µg/g intraperitoneally in 100 µl sterile PBS) or an IgG2a isotype-matched control antibody (Santa Cruz Biotechnology) injected 90 minutes before surgery. Measurement of creatinine and histology are as described below. Control mice received antibody treatment but did not undergo IRI, and kidneys were embedded in optimal cutting temperature compund. Sections were fixed in ethanol and subsequently stained with AF-555 and 4’,6-diamidino-2-phenylindole.
Assessment of Renal Function after IRI
Renal function was assessed by measurement of serum creatinine at 24 hours after IRI using a Jaffe creatinine picric acid reaction (OSR 6178; Beckman Coulter, Brea, CA) analyzed on an Olympus AU640 analyzer (Beckman Coulter).
Kidney Histology
Kidneys embedded in paraffin were sectioned at 3 µm and stained with periodic acid–Schiff stain by standard methods. Markers of tubular damage (tubular dilation, cell necrosis, infarction, and cast formation) were scored by calculation of the percentage of tubules in the corticomedullary junction that displayed such features. The scores were as follows: 0, none; 1, 1%–10%; 2, 11%–25%; 3, 26%–45%; 4, 46%–75%; and 5, >75%. Histologic examination was performed blinded on six randomly selected corticomedullary fields (magnification, ×200).
Immunostaining
Formalin-fixed, paraffin-embedded sections of 4 µm thickness were deparaffinized and boiled for 30 minutes in 10 mM sodium citrate buffer (pH 6.0). Immunohistochemistry was performed using the following primary antibodies: rat anti-mouse neutrophil (clone Ly6B.2) and macrophage (clone F4/80, both AbDSerotec, Oxford, UK). Sections were exposed to 3% H2O2 in methanol to quench endogenous peroxidases and blocked with 10% normal goat serum and Avidin/Biotin block (Vector Laboratories, Burlingame, CA). The primary antibody was incubated for 2 hours at room temperature, sections were washed with PBS, and then biotinylated goat anti-rat secondary antibody was added in combination with a Vectastain ABC kit (Vector Laboratories) and a metal enhanced 3,3′ diaminobenzidine substrate kit (Thermo Scientific, Waltham, MA). Sections were counterstained for hematoxylin, dehydrated, and covered. Quantification of the cellular infiltrate was performed in a blinded manner by assessing 20 consecutive high-power fields (magnification, ×400).
Renal Parenchymal Cellular Apoptosis
TUNEL staining was used to detect apoptosis with a commercially available in situ cell death detection kit (Roche, Basel, Switzerland), performed according to the manufacturer’s instructions.
Renal Oxidative Stress
DHE (Molecular Probes) was used to evaluate in situ production of superoxide. DHE (10 µM) was applied to unfixed frozen sections, incubated in a light-protected humidified chamber at 37°C for 30 minutes, washed with PBS, and mounted with fluorescent mounting medium (DAKO). Images were quantified using Image J software (http://rsbweb.nih.gov/ij/).
RNA Extraction and Quantification by Real-Time PCR
Total RNA was extracted using Qiagen RNeasy Mini Kits (Qiagen, Hilden, Germany) as per the manufacturer’s instructions. RNA was quantified using the Take3 Gen5 spectrophotometer (BioTek, Winooski, VT). One microgram of RNA was treated with DNase I (amplification grade; Invitrogen) and then reverse-transcribed using the Superscript III First Strand Synthesis SuperMix (Invitrogen). cDNA was amplified using Platinum Quantitative PCR SuperMix-UDG (Invitrogen) in 20-µl volumes in quadruplicate with gene-specific primers and probe on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA), according to the manufacturer’s instructions. Thermal cycling conditions were 50°C for 2 minutes, 95°C for 2 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Data were analyzed using the ΔΔCt method with expression normalized to the housekeeping gene and WT sham-operated animals used as the referent control.
Western Blotting
Kidney tissue was homogenized in ice-cold lysis buffer. Supernatants were collected and lysates quantified using a Bradford assay (BioRad, Hercules, CA). Thirty micrograms of total protein was resolved by SDS-PAGE and transferred onto nitrocellulose (BioRad). In blots for CD47, nonreducing Laemmli buffer was used with 8% SDS-PAGE. Blots were probed with primary antibody to the respective proteins and visualized on an Odyssey Imaging System (Licor, Lincoln, NE). The intensity of the bands was quantified using Image J.
Generation of BM Chimeric Mice
BM was collected from WT or CD47−/− mice by flushing femurs and tibiae with HBSS. Recipient mice were lethally irradiated with two doses of 5 Gy from a 137Cs source using a Mark1 irradiator (JL Shepherd & Associates, San Fernando, CA). Six hours after irradiation, recipient mice were given 1×107 BM cells retro-orbitally. The animals were allowed to recover for 8 weeks to ensure stable engraftment before being subjected to renal IRI. Full chimerism of each mouse was confirmed by flow cytometry of whole blood.
Statistical Analyses
The data are presented as mean ± SD unless otherwise stated. Data were analyzed with the t test (parametric variables) or Mann–Whitney U test (nonparametric variables) for means between two groups or ANOVA between multiple (>2) groups using STATA software (version 11.0; STATA Corporation, College Station, TX). In all cases, P<0.05 was deemed significant.
Disclosures
J.S.I. is chair of the scientific advisory board of Vasculox Inc. (St. Louis, MO) and Radiation Control Technologies Inc. (Rockville, MD).
Acknowledgments
This work was supported by the National Heart Lung and Blood Institute (R01 HL-108954 to J.S.I.), the National Institutes of Health (1P01HL103455-01), the American Heart Association (11BGIA7210001 to J.S.I.), the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania (to J.S.I.), and the Australian National Health and Medical Research Council (APP1016276 C.J. Martin Award to N.M.R.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012020137/-/DCSupplemental.
References
- 1.Halloran PF, Homik J, Goes N, Lui SL, Urmson J, Ramassar V, Cockfield SM: The “injury response”: A concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc 29: 79–81, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Hetzel GR, Klein B, Brause M, Westhoff A, Willers R, Sandmann W, Grabensee B: Risk factors for delayed graft function after renal transplantation and their significance for long-term clinical outcome. Transpl Int 15: 10–16, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Shoskes DA, Halloran PF: Delayed graft function in renal transplantation: Etiology, management and long-term significance. J Urol 155: 1831–1840, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Murillo D, Kamga C, Mo L, Shiva S: Nitrite as a mediator of ischemic preconditioning and cytoprotection. Nitric Oxide 25: 70–80, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smadja DM, d’Audigier C, Bièche I, Evrard S, Mauge L, Dias JV, Labreuche J, Laurendeau I, Marsac B, Dizier B, Wagner-Ballon O, Boisson-Vidal C, Morandi V, Duong-Van-Huyen JP, Bruneval P, Dignat-George F, Emmerich J, Gaussem P: Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol 31: 551–559, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS: The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol 31: 162–169, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M: Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest 115: 3451–3459, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD: Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood 109: 1945–1952, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD: Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A 102: 13141–13146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD: CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem 281: 26069–26080, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Phillips L, Toledo AH, Lopez-Neblina F, Anaya-Prado R, Toledo-Pereyra LH: Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg 22: 46–55, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Huang Y, Pokreisz P, Vermeersch P, Marsboom G, Swinnen M, Verbeken E, Santos J, Pellens M, Gillijns H, Van de Werf F, Bloch KD, Janssens S: Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. J Am Coll Cardiol 50: 808–817, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Peña A, Garcia-Criado FJ, Eleno N, Arevalo M, Lopez-Novoa JM: Intrarenal administration of molsidomine, a molecule releasing nitric oxide, reduces renal ischemia-reperfusion injury in rats. Am J Transplant 4: 1605–1613, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Mier G, Toledo-Pereyra LH, Bussell S, Gauvin J, Vercruysse G, Arab A, Harkema JR, Jordan JA, Ward PA: Nitric oxide diminishes apoptosis and p53 gene expression after renal ischemia and reperfusion injury. Transplantation 70: 1431–1437, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Lang JD, Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP: Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest 117: 2583–2591, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD: Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood 111: 613–623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenberg JS, Wink DA, Roberts DD: Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res 71: 785–793, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Rogers NM, Matthews TJ, Kausman JY, Kitching AR, Coates PT: Review article: Kidney dendritic cells: Their role in homeostasis, inflammation and transplantation. Nephrology (Carlton) 14: 625–635, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ: TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S: Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gobé G, Willgoss D, Hogg N, Schoch E, Endre Z: Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int 56: 1299–1304, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Manna PP, Dimitry J, Oldenborg PA, Frazier WA: CD47 augments Fas/CD95-mediated apoptosis. J Biol Chem 280: 29637–29644, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Lamy L, Ticchioni M, Rouquette-Jazdanian AK, Samson M, Deckert M, Greenberg AH, Bernard A: CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis. J Biol Chem 278: 23915–23921, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M, Flaherty JT: Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268: 18532–18541, 1993 [PubMed] [Google Scholar]
- 25.Chiao H, Kohda Y, McLeroy P, Craig L, Housini I, Star RA: Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J Clin Invest 99: 1165–1172, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noiri E, Peresleni T, Miller F, Goligorsky MS: In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clin Invest 97: 2377–2383, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wildhirt SM, Weismueller S, Schulze C, Conrad N, Kornberg A, Reichart B: Inducible nitric oxide synthase activation after ischemia/reperfusion contributes to myocardial dysfunction and extent of infarct size in rabbits: Evidence for a late phase of nitric oxide-mediated reperfusion injury. Cardiovasc Res 43: 698–711, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP: Role of CD47 as a marker of self on red blood cells. Science 288: 2051–2054, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, Iizuka K, Yokoyama WM, Taylor PA: CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med 194: 541–549, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD: Increasing survival of ischemic tissue by targeting CD47. Circ Res 100: 712–720, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD: Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plast Reconstr Surg 124: 1880–1889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, Wink DA, Isenberg JS, Roberts DD: Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med 1: ra7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS: Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 88: 471–481, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS: Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res 93: 682–693, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eltzschig HK, Eckle T: Ischemia and reperfusion—from mechanism to translation. Nat Med 17: 1391–1401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snoeijs MG, van Heurn LW, Buurman WA: Biological modulation of renal ischemia-reperfusion injury. Curr Opin Organ Transplant 15: 190–199, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Daemen MA, van ’t Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA: Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest 104: 541–549, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nogae S, Miyazaki M, Kobayashi N, Saito T, Abe K, Saito H, Nakane PK, Nakanishi Y, Koji T: Induction of apoptosis in ischemia-reperfusion model of mouse kidney: Possible involvement of Fas. J Am Soc Nephrol 9: 620–631, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Rogers NM, Stephenson MD, Kitching AR, Horowitz JD, Coates PT: Amelioration of renal ischaemia-reperfusion injury by liposomal delivery of curcumin to renal tubular epithelial and antigen-presenting cells. Br J Pharmacol 166: 194–209, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awad AS, Rouse M, Huang L, Vergis AL, Reutershan J, Cathro HP, Linden J, Okusa MD: Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int 75: 689–698, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK: Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 21: 1231–1239, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, Lobo PI, Okusa MD: The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthijsen RA, Huugen D, Hoebers NT, de Vries B, Peutz-Kootstra CJ, Aratani Y, Daha MR, Tervaert JW, Buurman WA, Heeringa P: Myeloperoxidase is critically involved in the induction of organ damage after renal ischemia reperfusion. Am J Pathol 171: 1743–1752, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD: Treatment of liver ischemia-reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery 144: 752–761, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]