ABSTRACT
Hypothalamic neurons, which produce the kisspeptin family of peptide hormones (Kp), are critical for initiating puberty and maintaining estrous cyclicity by stimulating gonadotropin-releasing hormone (GnRH) release. Conversely, RFamide-related peptide-3 (RFRP3) neurons inhibit GnRH activity. It has previously been shown that neonatal exposure to bisphenol A (BPA) can alter the timing of female pubertal onset and induce irregular estrous cycles or premature anestrus. Here we tested the hypothesis that disrupted ontogeny of RFamide signaling pathways may be a mechanism underlying advanced puberty. To test this, we used a transgenic strain of Wistar rats whose GnRH neurons express enhanced green fluorescent protein. Pups were exposed by daily subcutaneous injection to vehicle, 17beta-estradiol (E2), 50 μg/kg BPA, or 50 mg/kg BPA, from Postnatal Day (PND) 0 through PND 3, and then cohorts were euthanized on PNDs 17, 21, 24, 28, and 33 (5–8 animals per age per exposure; males were collected on PNDs 21 and 33). Vaginal opening was advanced by E2 and 50 μg/kg BPA. On PND 28, females exposed to E2 and 50 μg/kg BPA had decreased RFRP-3 fiber density and contacts on GnRH neurons. RFRP3 perikarya were also decreased in females exposed to 50 μg/kg BPA. Data suggest that BPA-induced premature puberty results from decreased inhibition of GnRH neurons.
Keywords: endocrine disruptor, kisspeptin, puberty, RFRP
Premature puberty induced by neonatal bisphenol A exposure results from decreased inhibition of GnRH neurons rather than their increased stimulation.
INTRODUCTION
In female rodents, neonatal exposure to bisphenol A (BPA) advances puberty, compromises fertility, and induces irregular estrous cycles or persistent estrus [1–3]. Although these effects are indicative of disruption within the hypothalamic-pituitary-gonadal (HPG) axis, the specific cellular and molecular mechanisms underlying these effects remain poorly understood [4]. Hence, the potential risks associated with human BPA exposure are difficult to evaluate [5, 6]. Hypothalamic neurons which produce the kisspeptin family of peptide hormones (Kp) are critical for initiating puberty and maintaining estrous cyclicity by stimulating gonadotropin-releasing hormone (GnRH) release [7]. Conversely, RFamide-related peptide-3 (RFRP3) neurons inhibit GnRH release [8, 9]; both have been shown to play central but opposing regulatory roles in pubertal maturation, reproductive physiology, and energy balance [8–12]. Thus, the disrupted ontogeny of either could underlie the pubertal advancement and estrous cycle disruption observed in BPA-exposed females. We hypothesized that neonatal BPA exposure would alter the peripubertal ontogeny of RFamide-signaling pathways in the female rat and that this disrupted development would be associated with early onset of puberty. Collectively, the primary goal was to determine whether advanced puberty is accompanied by accelerated maturation of the Kp system or the premature release of RFRP3 inhibition of GnRH neurons.
BPA is a chemical building block of polycarbonate plastics and epoxy resins that readily leaches from food packaging containers into the contents [13–15], resulting in nearly ubiquitous human exposure [16–18]. BPA has been detected in umbilical cord blood and fetal plasma [5, 19], demonstrating that human BPA exposure begins in utero. Developmental exposure is of particular concern because endocrine disruption during this critical period could induce permanent effects [20–22]. BPA has been recognized as having estrogenic activity since the 1930s [23], but its potential for inducing health effects in humans, particularly at low doses, remains controversial. Although it has been hypothesized that exposure to BPA or other endocrine-disrupting compounds (EDCs) may contribute to the rapid advancement of puberty in girls [22, 24], the specific mechanisms underlying this phenomenon remain elusive. Evidence for disruption within RFamide-signaling cascades could give further insight into the cause of premature puberty and related outcomes including irregular cyclicity and metabolic disease [11].
The RFamide protein family is highly conserved across species [9, 10, 25–27], suggesting that effects observed within this system in rodents may be indicative of vulnerability in humans. The Ks proteins (previously called metastins) and their receptor, Kiss1r (formerly known as GPR54), were initially discovered in hypogonadal patients [28] and later characterized in other species, including rodents, sheep, and nonhuman primates [29–31]. RFRP3 is a related RFamide first identified as gonadotropin-inhibiting hormone (GnIH) in birds and now recognized as present in mammals [25, 26, 32, 33]. Kp is critical for the initiation of puberty, as mutations to Kiss1r result in hypogonadism [7, 28], and Kp administration is sufficient to initiate pubertal development in laboratory animals [34–36]. Conversely, RFRP3 administration has been shown to inhibit GnRH synthesis and release [32, 33, 37], but its role on pubertal timing remains controversial due to conflicting data [10, 38]. Generally, it is postulated that Kp, acting as the “accelerator,” and RFRP3, acting as the “brake,” work in tandem to orchestrate the timing of pubertal onset and regulate GnRH activity [8]. Both RFamides are frequently colocalized with steroid hormone receptors in hypothalamic neurons, suggesting that they are hormone-responsive throughout development [10, 39, 40] and thus potentially vulnerable to endocrine disruption.
There are two distinct hypothalamic populations of Kp neurons in the rodents, one within and posterior to the anteroventral periventricular nucleus (AVPV; a region now defined as the rostral periventricular area of the third ventricle [RP3V] [41]) and one in the arcuate nucleus (ARC). Beginning just prior to the second week of life, the RP3V population becomes sexually dimorphic, with females having substantially more Kiss1 expression and Kp-immunoreactive (Kp-ir) neurons and projections than males [40, 42–45]. ARC Kp expression is sexually dimorphic neonatally [46, 47] but not subsequently [44, 48]. As sexual maturity approaches, the number of RP3V Kp neurons increases, as does the density of terminal fibers, making putative contacts on GnRH neurons in the anterior hypothalamus [42, 49]. Although its ontogeny across the pubertal transition appears to be less dynamic, the ARC population hypothetically may also play a role in pubertal development [50]. Thus, we hypothesized that BPA-induced early puberty could result from accelerated maturation of Kp pathways, either in the RP3V or the ARC or both.
RFRP3-ir perikarya are primarily confined to the dorsal medial nucleus (DMN) and send efferents throughout the brain, including the anterior hypothalamus [51]. RFRP3 neurons form synapses with GnRH neurons, but specifically how RFRP3 neurons communicate with GnRH neurons is not well characterized. GnRH neurons express the putative RFRP3 receptor (GPR147; also called Npffr1), but it has recently been shown that RFRP3 can also act through GPR74 (also called Npffr2), leaving open the possibility that RFRP3 influences GnRH activity through multiple mechanisms [52–54]. In rodents, RFRP3 mRNA and the number of DMN-ir RFRP3 neurons increase across neonatal development, decline at pubertal onset, and then rise with advanced age [55–57]. RFRP3 neurons play a role in energy balance [58, 59] and neuroendocrine stress responses [12], suggesting that RFRP3 activity could alter the timing of puberty both directly, through action on GnRH neurons, or indirectly, through other influencing pathways. We hypothesized that an accelerated decline in RFRP3 input on GnRH neurons at peripuberty could be associated with BPA-induced advanced puberty.
Collectively, the primary goal of the present studies was to determine whether advanced puberty following early life exposure to BPA was accompanied by accelerated maturation of the Kp system or the premature removal of RFRP3 inhibition on GnRH neurons. Determining the specific mechanisms by which BPA alters the tempo of reproductive development is necessary to help establish whether similar effects are plausible in humans. In addition to exploring a potential new avenue for endocrine disruption in the hypothalamus, the present study contributes important, fundamental information regarding RFRP3 neuroanatomy. Although the peripubertal ontogeny of Kp pathways has been well characterized in numerous species [40, 42–46, 60, 61], comparatively less is known about the ontogeny of RFRP3 pathways in juvenile female rats. Here we addressed these data gap by quantifying RFRP3 cell numbers and the density of efferent projections to preoptic GnRH neurons in peripubertal rats.
MATERIALS AND METHODS
Animals
A transgenic strain of enhanced green fluorescent protein (EGFP)-GnRH Wistar rats was used. These animals, which express EGFP driven by the GnRH promoter were generated and previously characterized by Fujioka et al. [62] and Kato et al. [63] from Nippon Medical School, Tokyo, Japan. Our colony was established from a kind gift from founder Susan Smith at the Oregon National Primate Research Center, Beaverton, OR [64]. Animal care and maintenance were conducted in accordance with the applicable portions of the Animal Welfare Act and the U.S. Department of Health and Human Services publication Guide for the Care and Use of Laboratory Animals and was approved by the North Carolina State University (NCSU) Institutional Animal Care and Use Committee. All litters were born to animals bred in-house. The sire was removed on the day of birth (defined as Postnatal Day zero [PND 0]). Dams (n = 17) were maintained on a 12L:12D light cycle (lights on from 700 to 1900 h) at 23°C and 50% average relative humidity at the Biological Resource Facility at NCSU and maintained on a semipurified, phytoestrogen-free diet ad libitum for the duration of the study (product no. AIN-93G; Test Diet) in thoroughly washed polysulfone caging with glass water bottles (rubber stoppers and metal sippers) and woodchip bedding to minimize exposure to exogenous EDCs [65]. On PND 21, all pups were weaned into same-sex littermate groups of up to 4 and housed under the same conditions as the dams.
Neonatal Exposure and Tissue Collection
All compounds were dissolved in 100% ethanol (EtOH; Pharmaco) and then sesame oil (Sigma) at a 10% EtOH:90% oil ratio as we have done previously [2, 48]. Starting on the day of birth, we injected pups s.c. with 0.05 ml of vehicle (oil), 10 μg of 17β-estradiol (E2; Sigma), and 50 μg/kg body weight (bw) BPA (low BPA; Sigma) or 50 mg/kg bw BPA (high BPA). The high dose corresponds to the lowest observed adverse effect level for chronic oral exposure, and the low dose corresponds to the ‘‘safe,'' or reference dose, for human oral exposure. Injections were administered every 24 h for 4 days (PND 0–3). E2 was used as a positive control, at a dose sufficient to induce hypothalamic masculinization, as we have done previously [66]. Although we recognize that injection does not model a typical human oral exposure, this route was selected for the purposes of these largely mechanistic studies to ensure that every animal received an identical dose. Injection likely results in a higher internal dose than oral exposure [67], although at least one study has shown that this difference is not significant in neonatal mice [68]. Based on that previous work, we estimated that the average plasma concentration over a 24-h period would be approximately 0.3 ng/ml for the low BPA group and 330 ng/ml for the high BPA group [68].
Female pups were euthanized on PNDs 17, 21, 24, 28, or 33 and males on PND 21 or 33 at between 0930 and 1400 h by transcardial perfusion as described previously [48]. To experimentally control for potential litter effects, we used no more than two pups per litter in each age group. Therefore, within each age group, pups came from a minimum of three dams. At the time they were euthanized, females were weighed and checked for vaginal opening, a hallmark of pubertal onset in the rat (occurring on PND 32–36 in unexposed animals) [69]. Vaginal lavage confirmed that none of the animals had started their estrous cycle. Plasma was extracted from trunk blood by centrifugation and stored at −80°C.
Immunohistochemistry
Perfused brains were sliced into 35-μm coronal sections and stored free floating in antifreeze (20% glycerol, 30% ethylene glycol in potassium phosphate buffer solution) at −20°C [70]. Two sets of hypothalamic sections, one containing the organum vasculosum of the lamina terminalis (OVLT) through the caudal border of the medial preoptic area (MPOA), and the other consisting of the rostral borders of the ARC were immunolabeled for Kp, using immunohistochemistry techniques detailed previously [43, 48].
Two more sets of sections (from PND 28 and PND 33 animals only), one containing the OVLT through the caudal border of the MPOA and the other comprising the rostral-to-caudal borders of the DMN, were immunolabeled for RFRP3, using immunohistochemistry techniques similar to those used for Kp labeling. RFRP3 was detected using a rabbit-derived GnIH antibody (1:4000 dilution), a generous gift from Kazuyoshi Tsutsui, Waseda University [26, 71]. Limited quantities of primary antibody required us to restrict our analysis of RFRP3-ir to two time points. We chose PND 28 and 33 because they most closely corresponded to time points at which the low BPA group and the controls underwent vaginal opening, respectively.
Quantification of Kp-ir Fiber Density in the AVPV and ARC
In the rat, RP3V Kp perikarya are not labeled without colchicine administration [72], a procedure that would have compromised these studies. Thus, we quantified Kp-ir localized within extended lengths of fibers throughout the RP3V, as previously described [43, 73]. We have shown that sex difference in RP3V Kp-ir fiber density emerges at approximately the same age and with similar magnitude as Kiss1 mRNA expression [48]. Three anatomically matched AVPV sections per animal, consisting of the caudal, medial, and rostral regions, were selected. Fiber density was also quantified in the ARC as we have done previously [43, 73]. Here the perikarya were labeled, but the dense plexus of fibers made them difficult to resolve and quantify [42, 45, 48]. In the peripubertal rat, the fiber plexus is sexually dimorphic (and masculinized by neonatal E2 administration), but cell numbers and mRNA expression are not [40, 43, 44, 73]. Thus, we quantified fiber density, hypothesizing that only the sexually dimorphic feature of ARC Kp-ir would be vulnerable to endocrine disruption. Three sections per animal, encompassing the caudal, medial, and rostral ARC regions, were selected and quantified.
The tissue was visualized with a Leica model TCS SPE confocal microscope using a 40× corrective objective lens. Image stacks (z-step distance of 1 μm) were analyzed using Image J software (National Institutes of Health, Bethesda, MD), as detailed previously [43, 73], to obtain the average number of immunoreactive voxels within the region of interest. The quantification process was completed independently by two people, both blind to the exposure groups. The values obtained by each were then averaged to create the data set used for analysis [43, 73].
Quantification of RFRP3-ir Perikarya and Fiber Density
To quantify RFRP3-ir perikarya, 1–2 sections containing the DMN were selected from PND 28 females and PND 33 males and females. A set of serial image planes (z-step distance of 3.5 μm) was collected with confocal microscopy at a magnification of 40×. Image stacks were visualized using ImageJ software, and the number of RFRP3-ir perikarya were manually counted by two people, and results were averaged across observers. RFRP3-ir fibers were quantified in PND 28 females, and PND 33 males and females, using the same methods applied for Kp-ir fiber density (detailed above). For this analysis, 2–4 sections containing the OVLT were selected based on the presence of EGFP-positive GnRH neurons in the area of interest.
Quantification of Putative RFamide Contacts on GnRH Neurons
Putative contacts on OVLT GnRH neurons by Kp-ir fibers (PND 33 animals) and RFRP3-ir fibers (PND 28 and 33 animals) were evaluated using confocal microscopy. For every animal, 5–6 GnRH neurons were scanned at ×40 magnification, using a 2.5× digital zoom and a 1.5-μm-step distance, and were assessed for close appositions of RFamide fibers. The percentage of GnRH neurons with contacts was calculated for every group and recorded.
Assessment of Serum Hormone Levels
Serum luteinizing hormone (LH) levels for PND 28 and 33 females (n = 4–7 per group) were assessed in collaboration with Jerome Goldman and Ashley Murr at the U.S. Environmental Protection Agency. LH levels were determined using iodination preparation I-10, reference preparation RP-3, and antisera S-11, all supplied by the National Institute of Diabetes and Digestive and Kidney Diseases. Iodination materials were radiolabeled with iodine-125 (125I; New England Nuclear), using the chloramine-T method [74], and labeled hormone was separated from unreacted iodide by using a P-60 gel column. Assays were carried out according to recommendations provided in the kit, with an increase in sensitivity due to a 24-h incubation of sample and first antibody prior to the addition of 125I-labeled tracer. Goat anti-rabbit gamma globulin (Calbiochem) was used as the second antibody [75]. Assay sensitivity was 0.115 ng/tube, and all samples were run in duplicate with an intra-assay coefficient of variation of 7.75%. Serum levels of total E2 for PND 17, 21, 24, 28, and 33 females (n = 4–6) were quantified in-house by radioimmunoassay using the Coat-A-Count estradiol kit (Siemens). All samples were run in duplicate, and the intra-assay coefficient of variation was 6.50%. An insufficient volume of blood was available from PND 28 vehicle control females, so they were not included in the analysis.
Statistical Analysis
Statistical analysis followed established guidelines for low-dose endocrine disruptor exposure [76]. Generally, AVOVA was used to compare effects between groups of females, and t-tests were used to explore sex differences. Bodyweight, estrogen levels, and Kp-ir fiber densities in the AVPV and ARC were analyzed first by three-way ANOVA with litter, age, and exposure as factors. No main effects of litter nor any significant interactions with litter were identified, hence, data were subsequently analyzed by two-way ANOVA with age and exposure as factors. This was followed with a one-way ANOVA for each age examined and Fisher least significant difference (LSD) post hoc test when ANOVA was significant. On PND 21 and 33, a t-test comparing males to control females was conducted to confirm the expected sex difference. RFRP3 fiber density was analyzed at PND 28 and PND 33 by one-way ANOVA (for PND 33, males were included because it was not known whether a sex difference existed) and then followed with a Fisher LSD post hoc test. LH levels were analyzed by one-way ANOVA for each age group, as only two ages were examined. Percentages of GnRH neurons with Kp contacts, RFRP3 cell numbers, and percentages of GnRH neurons with RFRP3 contacts were analyzed with a hypothesis-driven (based on fiber density results) one-tailed Dunnett test [76]. We hypothesized that Kp contacts on GnRH neurons would be upregulated, while RFRP3 cell numbers and contacts on GnRH neurons would be downregulated.
RESULTS
Vaginal Opening, Body Weight, and Circulating Hormone Levels
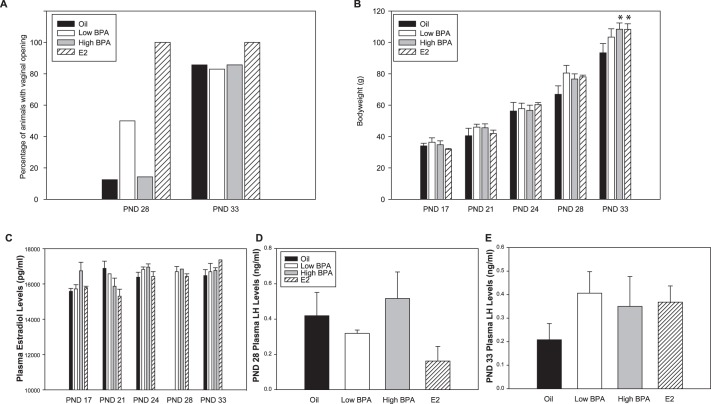
Consistent with our previous observations [2] and those of others [1, 3, 77–79], vaginal opening occurred earlier in the low BPA and E2 groups than in controls. All E2-exposed females and 50% of the low BPA females displayed vaginal opening on PND 28, compared to 14% of the high BPA females and 12% of the vehicle controls. By PND 33, 85% of the low BPA animals, high BPA animals, and vehicle control females were open (Fig. 1A). Advanced puberty was not associated with increased bw (Fig. 1B). A significant effect of exposure on bw was present only on PND 33 (F[3,123] = 3.220; P ≤ 0.03). At this age, high BPA and E2-exposed animals were significantly heavier than controls (n = 7, P ≤ 0.01; and n = 5, P ≤ 0.02, respectively). No significant effect of exposure or age on serum E2 (Fig. 1C) or LH level was observed (Fig. 1, D and E).
FIG. 1. .
A) Percentage of females displaying vaginal opening on PNDs 28 and 33. Low BPA and E2 animals opened earlier than control or high BPA females. B) Female bw at time of death. Weight was higher in high BPA and E2-exposed animals than control females by PND 33. C) Plasma E2 levels at time of death revealed no significant effect of exposure. An insufficient volume of blood was available for the PND 28 oil females, so they were excluded from analysis. D–E) Plasma LH levels at time of death on PNDs 28 and 33 were not significantly altered by exposure. Data are means ± SEM; *P ≤ 0.05 compared to oil controls.
Peripubertal Kp-ir Fiber Density and GnRH Contacts
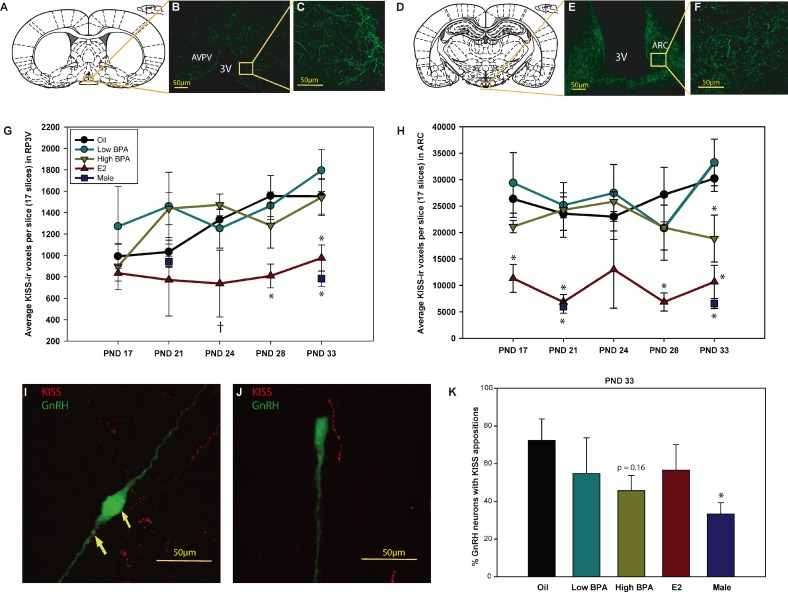
Main effects of exposure and age on female AVPV Kp-ir fiber density were identified (F[3,103] = 6.403, P ≤ 0.001; and F[4,103] = 2.565, P ≤ 0.04, respectively). As anticipated, Kp-ir fiber density increased with age in the vehicle control group, becoming sexually dimorphic by PND 33 (Fig. 2, A and G) [42, 45, 48]. Kp-ir fiber density in the E2-exposed animals, however, remained relatively low and flat and thus more reminiscent of the male pattern (Fig. 2G). No significant effect of BPA exposure was detected.
FIG. 2. .
Diagrammatic (A) and representative (D) confocal images (single image planes) show Kp-ir labeling in the female AVPV (B and C) and ARC (E and F). Labeling was readily observed within extended lengths of fibers in both regions. G) AVPV Kp-ir fiber density was significantly lower in E2-exposed females than in control females on PNDs 28 and 33 and was equivalent to male levels. H) Kp-ir fiber density in the ARC was significantly lower in E2-exposed females at all time points except PND 24. High BPA exposure resulted in significantly reduced Kp-ir fiber density in the ARC by PND 33. These levels were statistically between those of control females and E2-exposed females or males. I) Confocal image shows Kp-ir (red) appositions on an EFGP-labeled GnRH neuron (green). J) Confocal image shows a Kp-ir fiber in close proximity to but not making contact with a GnRH neuron. K) Percentage of GnRH neurons with Kp-ir appositions was sexually dimorphic, with males having fewer than females. This dimorphism was not significantly altered by exposure. Data are means ± SEM; †P ≤ 0.07, *P ≤ 0.05. 3V, third ventricle. Bar = 50 μm.
There was a significant effect of exposure (F[3, 113] = 14.977; P ≤ 0.001) but not age, on Kp-ir fiber density in the ARC (Fig. 2, D and H). Consistent with our what we have reported previously [48], Kp-ir fiber density in the ARC was sexually dimorphic (P ≤ 0.001), with females having a denser plexus (Fig. 2H). A significant effect of exposure was present on all 5 days examined (P ≤ 0.05). E2-exposed females had fewer Kp-ir fibers than vehicle controls (P ≤ 0.05 on all days except PND 24, where P ≤ 0.07), with levels remaining relatively low and flat across peripuberty (Fig. 2H) and statistically indistinct from male levels. No significant effect of low BPA exposure was observed. By PND 33, high BPA-exposed females had Kp-ir levels in between those of control females (P ≤ 0.05) and males (P ≤ 0.03) (Fig. 2H). Prior work from our laboratory demonstrated that this effect persists into adulthood [43].
Kp appositions on GnRH neurons (Fig. 2I) were readily distinguishable from situations where the two were in close proximity but not actually in contact (Fig. 2J). As expected, the percentage of GnRH neurons in close apposition to Kp-ir fibers was sexually dimorphic on PND 33, with males having fewer contacts than females (P ≤ 0.02) [42]. Results of the Dunnett test comparing control females to exposed females revealed no significant effect of exposure (Fig. 2K), demonstrating that this aspect of the Kp/GnRH signaling pathway is resistant to disruption by BPA but also by E2.
RFRP3 Fiber Density, Cell Numbers, and GnRH Contacts
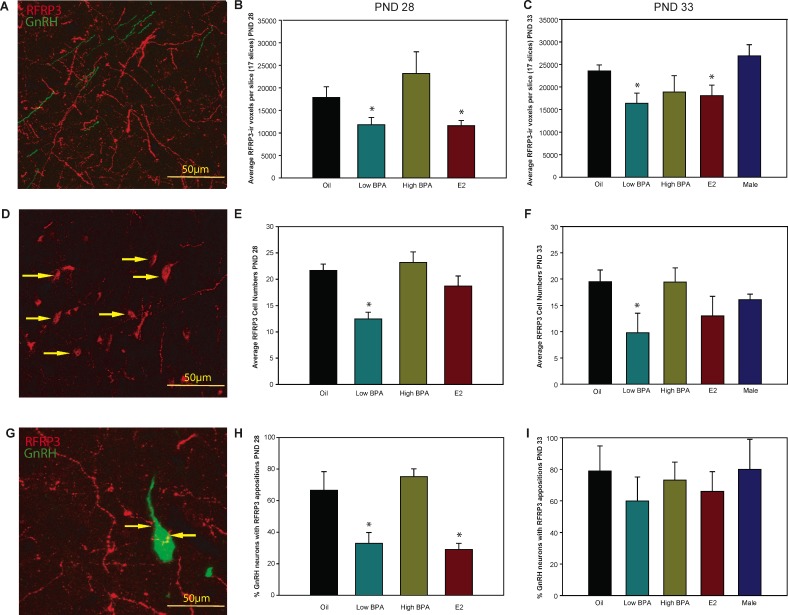
In females, a main effect of exposure on RFRP3 fiber density was detected on PNDs 28 and 33 (F[3,17] = 4.976, P ≤ 0.02; and F[4,16] = 3.447, P ≤ 0.03, respectively) (Fig 3, A–C) with low BPA and E2-exposed animals having significantly fewer fibers than controls (P ≤ 0.05). Similarly, low BPA-exposed animals had significantly fewer RFRP3-ir cells on PNDs 28 and 33 (P ≤ 0.001 and P ≤ 0.05, respectively) (Fig. 3, D–F) than unexposed controls. On PND 28, a main effect of exposure on the percentage of GnRH with putative RFRP3 contacts was detected (F[3,15] = 7.464, P ≤ 0.003) (Fig. 3, G and H), but this did not persist to PND 33 (Fig. 3I). The percenage of GnRH neurons with RFRP3 appositions on PND 28 was significantly lower in the low BPA and E2-exposed animals than the control females (P ≤ 0.03 and P ≤ 0.01, respectively). None of these features was sexually dimorphic in peripuberty (Fig. 3, C, F, and I).
FIG. 3. .
A, D, and G) Representative confocal images show the plexus of OVLT RFRP3-ir fibers, RFRP3-ir perikarya in the DMN, and RFRP3-ir appositions on EGFP-labeled GnRH neurons. Bar = 50 μm. B and C) RFRP3-ir fiber density was significantly lower in low BPA and E2-exposed females compared to oil controls on PND 28 and 33. E and F) RFRP3-ir neuron numbers were significantly decreased in the low BPA group on PND 28 and 33. H) The percentage of GnRH neurons with RFRP3-ir appositions was significantly decreased in low BPA and E2-exposed females compared to that in oil controls on PND 28. I) The percentage of GnRH neurons with RFRP3-ir appositions was not sexually dimorphic or significantly affected by exposure on PND 33. Data are means ± SEM; *P ≤ 0.05.
DISCUSSION
Advanced vaginal opening and compromised estrous cyclicity following estrogen or low-dose perinatal BPA exposure has been reported numerously by our group and others [1–3, 77–79], but the mechanisms underlying these effects remain elusive. Collectively, our data indicate that accelerated female pubertal onset following neonatal exposure to low dose BPA may result from decreased inhibition of GnRH activity by RFRP3 neurons. The alternative hypothesis stating that neonatal BPA exposure accelerates the ontogeny of Kp projections to GnRH neurons was not supported by the data. Instead, evidence for masculinization at the high dose of BPA was observed in the ARC, an effect which may contribute to irregular or persistent estrus. These data provide evidence that low dose BPA can alter the organizational tempo and sexual differentiation of RFamide pathways in the female rat and suggest a novel mechanism by which the timing of pubertal onset and female reproductive physiology may be altered by early life exposure to EDCs.
The nonmonotonic effect on pubertal maturation by BPA reported here replicates our prior observation [2] and is typical for BPA and other endocrine disruptors [5, 22, 80]. Low dose BPA has been shown to advance vaginal opening [1, 79], while higher doses have no effect or delay it [81]. Estrogen can also generate biphasic effects in hormone-sensitive tissues. For example, nonmonotonic effects of estradiol on terminal end bud formation in the mammary gland and prostate hyperplasia have been described previously [82, 83]. A definitive mechanism to explain this phenomenon is lacking, but several hypotheses have been suggested, including the overlap of two distinct mechanisms of action and the downregulation of hormone receptors at high doses [80, 84].
Although neonatal exposure to low BPA resulted in fewer RFRP3 cell numbers on PND 28 and 33, neonatal E2 exposure did not. One possible explanation is that vaginal opening occurred a few days earlier in the E2-exposed group, and thus appreciable differences in cell numbers occurred at a younger age. Prior studies have found conflicting effects of E2, depending on the timing and context of administration. For example, E2 has been shown to downregulate prepro-RFRP mRNA expression in ovariectomized adult mice [85], but a recent RT-PCR study revealed that peripubertal E2 administration increases RFRP3 mRNA levels within the whole hypothalamus of female rats [56]. We surmise that the sensitivity of RFRP3 neurons to E2 and, by extension, EDCs depends on dose, exposure window, and duration of exposure. This differential sensitivity could underlie the biphasic effect of BPA on RFRP3 cell numbers and the density of OVLT efferents. An alternative possibility is that the BPA effect is not classically “estrogenic” and that two different mechanisms drive early vaginal opening in BPA and E2-exposed females. In addition to a change in cell numbers, we also found that neonatal low BPA exposure resulted in a decreased percentage of GnRH neurons with RFRP3 appositions on PND 28, a difference that was recapitulated by E2 exposure. By PND 33, this difference was no longer statistically significant, suggesting that it is a transient effect accompanying vaginal opening. These results could reflect a lower density of RFRP3 projections to GnRH neurons or reduced protein content within these projections at the time of vaginal opening. Collectively, these data reveal a novel mechanism by which EDC exposure may modulate the tempo of reproductive development.
Information about the neuroanatomical organization of female peripubertal RFRP3 signaling pathways is sparse but rapidly emerging, and the observations reported here are consistent with those of prior work revealing the fact that these pathways are dynamic and hormone sensitive but not sexually dimorphic in peripuberty [57]. A recent study reported that in female rats, hypothalamic RFRP3 expression increased with age but remained relatively flat between PNDs 28 and 35 [56]. Similarly, hypothalamic expression levels of the putative RFRP3 receptor GPR147 also increased as puberty approached and then declined. Because these assessments were made using the whole hypothalamus, it is unclear whether a similar expression pattern occurs specifically in GnRH neurons. In the present study, RFRP3 fiber density in the vicinity of GnRH neurons rose with age, an effect that is consistent with RFRP3 expression data. A notable caveat, however, is that the fiber density data obtained from the two time points cannot be statistically compared because they were not processed simultaneously.
Here we report for the first time that 60%–80% of GnRH neurons have RFRP3 appositions as early as PND 28 in female rats, an observation which suggests that most GnRH neurons in this region respond to RFRP3. This finding conflicts with that of a prior study reporting that only 15% of GnRH neurons express GPR147 [57], a discrepancy that could simply be age-dependent. The present study focused on juveniles, while the prior study characterized expression only in adults. GnRH neuron responsiveness to RFRP3 appears to be age, dose, and cycle dependent [37, 86], suggesting that GPR147 mRNA expression varies with age and hormonal milieu. Moreover, it has recently been shown that RFRP3 can also act through GPR74 (also called Npffr2), leaving open the possibility that RFRP3 influences GnRH activity via multiple mechanisms [52–54]. It is not yet known how the relative expression of each putative RFRP3 receptor in GnRH neurons varies with age or sex.
In contrast to RFRP3, BPA exposure had only minimal effects on Kp-ir fiber density in either of the examined regions. As expected, neonatal E2 exposure had a masculinizing effect, resulting in RP3V and ARC Kp-ir levels more typical of males [43, 48, 73, 87]. The percentage of OVLT GnRH neurons with Kp fiber appositions was sexually dimorphic on PND 33, with females having more than males, an observation consistent with prior work in mice [42]. This difference was not altered by neonatal exposure to BPA or, unexpectedly, by exposure to E2. Either the neonatal window is not the critical period for masculinization of this aspect of the pathway or the system is able to compensate and the percentage of GnRH neurons receiving Kp-ir appositions remains unaltered, even though there are fewer total Kp-ir fibers.
In the ARC, the highest dose of BPA resulted in decreased ARC Kp-ir fiber density by PND 33. This difference persists into adulthood, is associated with irregular estrous cycles [87], and is indicative of masculinization (rather than an accelerated tempo of development). In contrast to the AVPV population, sex differences in ARC fiber density are not accompanied by sexually dimorphic Kiss1 expression or cell numbers, either perinatally [48] or in adulthood [44]. Most likely, this sex difference in ARC Kp-ir fiber density results from sex-specific differences in the density of projections originating from the RP3V. It has recently been shown that the RP3V population sends numerous efferents to the ARC and, ultimately, the median eminence (ME), where they form synapses with GnRH dendrites [88, 89]. These ME fibers may be critical regulators of GnRH release, but the peripubertal ontogeny of this synaptic network has not yet been delineated. Thus, we hypothesize that the decreased density of ARC Kp-ir fibers following E2 or high BPA exposure is likely indicative of decreased efferents from the RP3V population of Kp neurons projecting to the ARC and, ultimately, the ME. Future studies quantifying the density of Kp contacts on GnRH terminals in this region are needed to determine if there are sex differences and/or vulnerabilities to EDC exposure.
Circulating LH and E2 levels did not significantly differ between groups, an observation consistent with previous work showing that serum gonadotropins remain largely unchanged prior to the first preovulatory surge [90]. LH levels on PND 33 were slightly higher in the two groups displaying early vaginal opening, an effect which is in accord with prior work showing that neonatal BPA exposure advances puberty and alters GnRH pulsatility [91]. Although single time point LH assessments may not give a comprehensive picture of LH release amplitude and frequency [69], collectively these data support the hypothesis that exposure to E2 or low dose BPA can release GnRH neurons from RFRP3 inhibition thereby resulting in enhanced LH release. Equivalent endogenous estrogen levels across exposure groups, however, suggest that LH levels have not increased to the degree required to appreciably elevate ovarian steroid hormone production. Early vaginal opening, however, may be initiated by more localized factors including increased sensitivity to estrogen and increased aromatase expression [90, 92].
Finally, it has been suggested that increased bw could be a contributing factor for early puberty in humans and rodents [1, 93, 94], but it does not appear to have played a significant role here. Only the high dose of BPA resulted in significantly increased bw, and this effect did not manifest until PND 33. While this suggests that increased bw is not driving advanced vaginal opening, it could indicate that energy balance is disrupted. It has previously been shown that RFamides, including Kp and RFRP3, play an important role in energy balance and metabolism [58, 59]. In addition to the effects of RFamides on GnRH release, they also have opposing effects on pro-opiomelanocortin (POMC) cells, with Kp being stimulatory and RFRP3 being inhibitory on these anorexigenic neurons [95]. Therefore, disruption of these signaling pathways could be a contributing factor for the observed changes in energy balance and bw following EDC exposure.
Overall, data indicate that early vaginal opening following neonatal low dose BPA exposure results from the premature release of GnRH neurons from RFRP3 inhibition rather than from premature stimulation from Kp neurons. Considering that the RFamide system is highly conserved across species [96], these findings may give further insight into the neuroendocrine systems that coordinate pubertal timing and maintenance of normal estrous cyclicity in all mammalian species, including humans. Numerous xenoestrogens have been reported to affect pubertal timing [65, 97–99]. Understanding the mechanisms by which these compounds can alter the tempo of pubertal development is fundamental for making predications about how exposure to these compounds may impact human reproductive health.
ACKNOWLEDGMENT
The authors thank Barbara Welker, Kay Coole, and Linda Hester for their assistance with animal husbandry. We also appreciate the help of Linwood Joyner, an undergraduate student and NCSU Park Scholar, with cell counting. We gratefully acknowledge Susan Smith at Oregon Health and Science University for providing us with EGFP-positive GnRH rats to start our colony and Jerome Goldman and Ashley Murr at the U.S. Environmental Protection Agency (Research Triangle Park, NC) for analyzing serum LH levels. Thanks also to Emily Sluzas for critical reading of the manuscript.
Footnotes
Supported by National Institute of Environmental Health Sciences (NIEHS) grant R01 ES016001 to H.B.P. Portions of this work were presented at the 44th Annual Meeting of the Society for the Study of Reproduction, Portland, OR, July 31–August 4, 2011.
REFERENCES
- Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature 1999; 401: 763 764 [DOI] [PubMed] [Google Scholar]
- Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod 2009; 81: 690 699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, Gadbois JL, Tharp AP, Whitt GS, Sonnenschein C, Soto AM. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect 2011; 119: 547 552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci 2009; 3: 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007; 24: 139 177 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 2009; 30: 75 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev 2009; 30: 713 743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ. Driving reproduction: RFamide peptides behind the wheel. Horm Behav 2006; 50: 655 666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Kriegsfeld LJ, Osugi T, Seong JY, Vaudry H. Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinol 2010; 22: 716 727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Gibson EM, Williams WP, 3rd, Zhao S, Mason AO, Bentley GE, Tsutsui K. The roles of RFamide-related peptide-3 in mammalian reproductive function and behaviour. J Neuroendocrinol 2010; 22: 692 700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Mikkelsen JD, Tena-Sempere M. Kisspeptins: bridging energy homeostasis and reproduction. Brain Res 2010; 1364: 129 138 [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Onaka T. Roles of prolactin-releasing peptide and RFamide related peptides in the control of stress and food intake. FEBS J 2010; 277: 4998 5005 [DOI] [PubMed] [Google Scholar]
- Cooper JE, Kendig EL, Belcher SM. Assessment of bisphenol A released from reusable plastic, aluminum and stainless steel water bottles. Chemosphere 2011; 85: 943 947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam 2003; 20: 684 689 [DOI] [PubMed] [Google Scholar]
- Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect 2009; 117: 1368 1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 2005; 113: 391 395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K, Hauser R. Exposure to Bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 2009; 117: 639 644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 2008; 116: 39 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod 2002; 17: 2839 2841 [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 2002; 25: 507 536 [DOI] [PubMed] [Google Scholar]
- Heindel JJ. The fetal basis of adult disease: Role of environmental exposures–introduction. Birth Defects Res A Clin Mol Teratol 2005; 73: 131 132 [DOI] [PubMed] [Google Scholar]
- Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM. et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril 2008; 90: 911 940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds E, Lawson W. Synthetic oestrogenic agents without the phenanthrene nucleus. Nature 1936; 137: 996 [Google Scholar]
- Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics 2009; 123: e932 e939 [DOI] [PubMed] [Google Scholar]
- Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS One 2009; 4: e8400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun 2000; 275: 661 667 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li S, Liu Y, Lu D, Chen H, Huang X, Liu X, Meng Z, Lin H, Cheng CH. Structural diversity of the GnIH/GnIH receptor system in teleost: its involvement in early development and the negative control of LH release. Peptides 2010; 31: 1034 1043 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J. et al. The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349: 1614 1627 [DOI] [PubMed] [Google Scholar]
- Smith JT. Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Rev 2008; 57: 288 298 [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A 2005; 102: 2129 2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompolo S, Pereira A, Estrada KM, Clarke IJ. Colocalization of kisspeptin and gonadotropin-releasing hormone in the ovine brain. Endocrinology 2006; 147: 804 810 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A 2006; 103: 2410 2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Yin H, Ubuka T, Chowdhury VS, Osugi T, Ukena K, Sharp PJ, Wingfield JC, Bentley GE. A new key neurohormone controlling reproduction, gonadotrophin-inhibitory hormone in birds: discovery, progress and prospects. J Neuroendocrinol 2009; 21: 271 275 [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A. et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A 2005; 102: 1761 1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C. et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 2005; 146: 3917 3925 [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 2005; 25: 11349 11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology 2009; 150: 2799 2804 [DOI] [PubMed] [Google Scholar]
- Johnson MA, Fraley GS. Rat RFRP-3 alters hypothalamic GHRH expression and growth hormone secretion but does not affect KiSS-1 gene expression or the onset of puberty in male rats. Neuroendocrinology 2008; 88: 305 315 [DOI] [PubMed] [Google Scholar]
- Poling MC, Kim J, Dhamija S, Kauffman AS. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology 2012; 153: 1827 1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS. Sexual differentiation and the Kiss1 system: hormonal and developmental considerations. Peptides 2009; 30: 83 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 2008; 57: 277 287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006; 147: 5817 5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Todd KL, Mickens JA, Adewale HB. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology 2009; 30: 350 357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007; 148: 1774 1783 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 2009; 150: 3214 3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and kiss1 in neonatal male and female rats. J Comp Neurol 2011; 519: 2954 2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology 2012; 153: 782 793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa SM, Todd KL, Sullivan AW, Cao J, Mickens JA, Patisaul HB. Neonatal exposure to genistein adversely impacts the ontogeny of hypothalamic kisspeptin signaling pathways and ovarian development in the peripubertal female rat. Reprod Toxicol 2011; 31: 280 289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 2004; 145: 4565 4574 [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 2009; 297: E1212 E1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Ukena K. Hypothalamic LPXRF-amide peptides in vertebrates: identification, localization and hypophysiotropic activity. Peptides 2006; 27: 1121 1129 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Habata Y, Hosoya M, Kawamata Y, Kitada C, Hinuma S. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim Biophys Acta 2003; 1593: 151 157 [DOI] [PubMed] [Google Scholar]
- Talmont F, Mouledous L, Piedra-Garcia L, Schmitt M, Bihel F, Bourguignon JJ, Zajac JM, Mollereau C. Pharmacological characterization of the mouse NPFF2 receptor. Peptides 2010; 31: 215 220 [DOI] [PubMed] [Google Scholar]
- Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, Tsutsui K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology 2012; 153: 373 385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Tsutsui K, Chaturvedi CM. Age-dependent variation in the RFRP-3 neurons is inversely correlated with gonadal activity of mice. Gen Comp Endocrinol 2010; 168: 326 332 [DOI] [PubMed] [Google Scholar]
- Iwasa T, Matsuzaki T, Murakami M, Kinouchi R, Osugi T, Gereltsetseg G, Yoshida S, Irahara M, Tsutsui K. Developmental changes in the mammalian gonadotropin-inhibitory hormone (GnIH) ortholog RFamide-related peptide (RFRP) and its cognate receptor GPR147 in the rat hypothalamus. Int J Dev Neurosci 2012; 30: 31 37 [DOI] [PubMed] [Google Scholar]
- Poling MC, Kim J, Dhamija S, Kauffman AS. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology 2012; 153: 1827 1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Oldfield BJ, Clarke IJ. Projections of RFamide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J Neuroendocrinol 2009; 21: 690 697 [DOI] [PubMed] [Google Scholar]
- Bentley GE, Ubuka T, McGuire NL, Calisi R, Perfito N, Kriegsfeld LJ, Wingfield JC, Tsutsui K. Gonadotrophin-inhibitory hormone: a multifunctional neuropeptide. J Neuroendocrinol 2009; 21: 276 281 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004; 145: 4073 4077 [DOI] [PubMed] [Google Scholar]
- Guerriero KA, Keen KL, Terasawa E. Developmental Increase in Kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta). Endocrinology 2012; 153: 1887 1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka H, Suzuki M, Yamanouchi K, Ohta A, Nagashima H, Kato M, Nishihara M. Generation of transgenic rats expressing enhanced green fluorescent protein in gonadotropin-releasing hormone neurons. J Reprod Dev 2003; 49: 523 529 [DOI] [PubMed] [Google Scholar]
- Kato M, Ui-Tei K, Watanabe M, Sakuma Y. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology 2003; 144: 5118 5125 [DOI] [PubMed] [Google Scholar]
- Xu J, Kirigiti MA, Cowley MA, Grove KL, Smith MS. Suppression of basal spontaneous gonadotropin-releasing hormone neuronal activity during lactation: role of inhibitory effects of neuropeptide Y. Endocrinology 2009; 150: 333 340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, Kissling GE, Grant MG, Forsythe DB. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ Health Perspect 2007; 115: 1717 1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol 2006; 28: 111 118 [DOI] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol Appl Pharmacol 2010; 247: 158 165 [DOI] [PubMed] [Google Scholar]
- Taylor JA, Welshons WV. Vom Saal FS. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reprod Toxicol 2008; 25: 169 176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Wheaton JE, Jameson HE, McCann SM. The onset of puberty in the female rat: changes in plasma prolactin, gonadotropins, luteinizing hormone-releasing hormone (LHRH), and hypothalamic LHRH content. Endocrinology 1976; 98: 630 638 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Le WW. Just cool it! Cryoprotectant anti-freeze in immunocytochemistry and in situ hybridization. Peptides 2004; 25: 425 431 [DOI] [PubMed] [Google Scholar]
- Ukena K, Ubuka T, Tsutsui K. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res 2003; 312: 73 79 [DOI] [PubMed] [Google Scholar]
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K. et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367 378 [DOI] [PubMed] [Google Scholar]
- Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology 2008; 29: 988 997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood FC, Hunter WM, Glover JS. The preparation of I-131-labelled human growth hormone of high specific radioactivity. Biochem J 1963; 89: 114 123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Cooper RL, Rehnberg GL, Hein JF, McElroy WK, Gray LE., Jr Effects of low subchronic doses of methoxychlor on the rat hypothalamic-pituitary reproductive axis. Toxicol Appl Pharmacol 1986; 86: 474 483 [DOI] [PubMed] [Google Scholar]
- Haseman JK, Bailer AJ, Kodell RL, Morris R, Portier K. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol Sci 2001; 61: 201 210 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Jr, Hauser R, Heindel JJ, Ho SM, Hunt PA, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol 2007; 24: 131 138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Ota T, Furuhashi T, Ohta Y, Iguchi T. Changes in reproductive organs of female rats treated with bisphenol A during the neonatal period. Reprod Toxicol 2003; 17: 283 288 [DOI] [PubMed] [Google Scholar]
- Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol 2002; 16: 117 122 [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM. Vom Saal FS, Welshons WV, Zoeller RT, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 2012; 33: 378 455 [DOI] [PMC free article] [PubMed]
- Nikaido Y, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, Tsubura A. Effects of prepubertal exposure to xenoestrogen on development of estrogen target organs in female CD-1 mice. In Vivo 2005; 19: 487 494 [PubMed] [Google Scholar]
- Vandenberg LN, Wadia PR, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM. The mammary gland response to estradiol: monotonic at the cellular level, non-monotonic at the tissue-level of organization? J Steroid Biochem Mol Biol 2006; 101: 263 274 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A 1997; 94: 2056 2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 2003; 111: 994 1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar CS, Kallo I, Liposits Z, Hrabovszky E. Estradiol down-regulates RF-amide-related peptide (RFRP) expression in the mouse hypothalamus. Endocrinology 2011; 152: 1684 1690 [DOI] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology 2008; 149: 4958 4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewale HB, Todd KL, Mickens JA, Patisaul HB. The impact of neonatal bisphenol–a exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology 2011; 32: 38 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol 2010; 324: 45 50 [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 2010; 166: 680 697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Advis JP, Andrews WW. Neuroendocrine control of the onset of puberty in the rat. Fed Proc 1980; 39: 2365 2371 [PubMed] [Google Scholar]
- Fernandez M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect 2009; 117: 757 762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke W, Dohler KD, Gelato M. Oestrogens and prolactin as possible regulators of puberty. J Endocrinol 1976; 68: 391 396 [DOI] [PubMed] [Google Scholar]
- Foster PM, McIntyre BS. Endocrine active agents: implications of adverse and non-adverse changes. Toxicol Pathol 2002; 30: 59 65 [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Pan H, Zhang DX, Wu QY, Zhang K, Li M, Gong FY, Wu XY, Deng JY, Shi YF. Effect of bodyweight on the onset of puberty of female children and adolescents [in Chinese]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2010; 32: 25 28 [DOI] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci 2010; 30: 10205 10219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Osugi T. Evolutionary origin and divergence of GnIH and its homologous peptides. Gen Comp Endocrinol 2009; 161: 30 33 [DOI] [PubMed] [Google Scholar]
- Davis LK, Murr AS, Best DS, Fraites MJ, Zorrilla LM, Narotsky MG, Stoker TE, Goldman JM, Cooper RL. The effects of prenatal exposure to atrazine on pubertal and postnatal reproductive indices in the female rat. Reprod Toxicol 2011; 32: 43 51 [DOI] [PubMed] [Google Scholar]
- Rollerova E, Wsolova L, Urbancikova M. Neonatal exposure to herbicide acetochlor alters pubertal development in female Wistar rats. Toxicol Mech Methods 2011; 21: 406 417 [DOI] [PubMed] [Google Scholar]
- Zorrilla LM, Gibson EK, Stoker TE. The effects of simazine, a chlorotriazine herbicide, on pubertal development in the female Wistar rat. Reprod Toxicol 2010; 29: 393 400 [DOI] [PubMed] [Google Scholar]