ABSTRACT
Caveolae orchestrate the dominant placental angiogenic growth factor fibroblast growth factor 2 (FGF2) signaling primarily via FGF receptor 1 (FGFR1) in placental artery endothelial cells; however, how the proximal FGF2/FGFR1 signaling is organized in the caveolae is obscure. We have shown in the present study that the FGFR substrate 2alpha (FRS2alpha) is physically associated with FGFR1, and both are targeted to the caveolae via interaction with caveolin-1 in ovine fetoplacental artery endothelial cells. Treatment with FGF2 rapidly stimulated time- and concentration-dependent FRS2alpha tyrosine phosphorylation and recruited the cytosolic growth factor receptor-bound protein 2 (GRB2)-GRB2-associated binding protein 1 (GAB1) complex to the caveolae, where they formed a ternary complex with FRS2alpha. Disruption of caveolae by cholesterol depletion with methyl-beta-cyclodextrin inhibited FGF2-induced FRS2alpha tyrosine phosphorylation, and it blocked the FGF2-induced recruitment of GRB2 and GAB1 to the caveolae and formation of the FRS2alpha-GRB2-GAB1 complex in the caveolae, as well as activation of the PI3K/AKT1 and MAPK1/2 pathways. Thus, these findings have demonstrated that the proximal fibroblast growth factor (FGF2/FGFR1) signaling is compartmentalized in the placental endothelial caveolae via the FGFR substrate 2α that mediates formation of a FRS2α-GRB2-GAB1 complex.
Keywords: caveolae, caveolin-1, FRS2α, placental endothelial cells, proximal FGF2/FGFR1 signaling
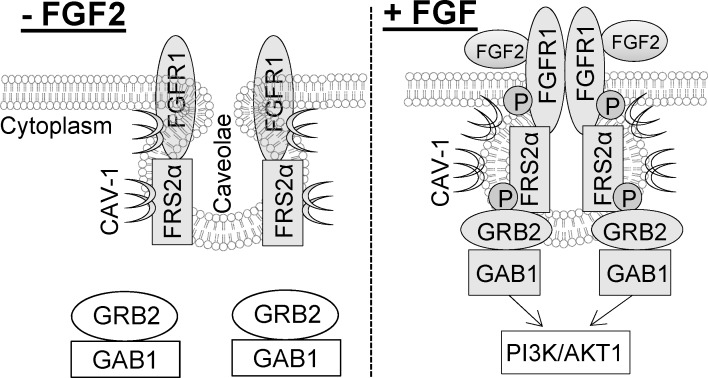
The proximal fibroblast growth factor (FGF2/FGFR1) signaling is compartmentalized in the placental endothelial caveolae via the FGFR substrate 2α that mediates formation of a FRS2α-GRB2-GAB1 complex.
INTRODUCTION
Fibroblast growth factors (FGFs) are a large family of multifunctional peptide growth factors, including 28 distinct members encoded by 22 distinct genes in humans. They play pivotal roles in many different cellular processes, including proliferation, differentiation, migration, and cell survival, during all stages of prenatal and postnatal life [1]. The pleiotropic activities of FGFs are mediated by a family of FGF receptors (FGFRs) that are distinct receptor tyrosine kinases (TRKs; FGFRs 1–4) encoded by distinct genes [2]. The FGF/FGFR signaling is unique in that a specific membrane-linked docking protein FGFR substrate 2α (FRS2α, also called Suc-associated neurotrophic factor-induced tyrosine-phosphorylated target, or SNT) is required [3, 4]. FRS2α is rapidly tyrosine phosphorylated upon FGF stimulation [5] and functions as a “conning center” for the coordinated assembly of multiple signaling proteins for precisely activating specific intracellular signaling pathways, such as mitogen-activated protein kinases 1/2 (MAPK1/2) and phosphoinositide-3-kinase (PI3K)/v-akt murine thymoma viral oncogene homolog 1 (AKT1) pathways. FRS2α contains six tyrosine residues in the C-terminus, with four (Tyr196/306/349/392) binding to an SH2 domain-containing adaptor protein (growth factor receptor-bound protein 2 [GRB2]) [3, 6] and the others (Tyr436/471) binding to an SH2 domain-containing tyrosine phosphatase (SHP2) [7]. FRS2α bridges ligated FGFR1 and adaptor proteins, such as GRB2, Son of sevenless (Sos), and GRB2-associated binding protein 1 (GAB1) [3, 4, 8], unlike the other RTKs, such as vascular endothelial growth factor and epidermal growth factor receptors, which directly interact with GRB2 [9, 10]. Activated Sos leads to Ras activation on the plasma membrane where active RasGTP recruits Raf-1 to the plasma membrane for mediating MEK1-dependent MAPK1/2 activation [11], representing the most characterized RTK-initiated cell signaling for cell survival, proliferation, metabolism, and gene expression [10]. The Ras/MAPK1/2 pathway can be also activated through FRS2α-Shp2 complex [7]. Although FGF2 potently activates the PI3K/AKT pathway, presumptively through the formation of the FRS2α-GRB2-GAB1 complex [8, 12, 13], how this is achieved is much less understood.
Caveolae are the Ω-shaped plasma membrane microdomains enriched in sphingolipids and cholesterol [14–16]. Various growth factor receptors and cell signaling molecules have been found to be compartmentalized in the caveolae, suggesting an important role for caveolae in signal transduction [17]. Caveolin-1 (cav-1) is the principal structural protein of caveolae [18] that are abundantly present in terminally differentiated cells [19], including endothelial cells [20]. Cav-1 is essential for the formation of caveolae, as evidenced by the fact that ectopic expression of caveolin-1 leads to caveolae formation [21] and the loss of caveolae in cav-1−/− mice [22]. Cav-1−/− mice display impaired nitric oxide signaling, uncontrolled proliferation of pulmonary endothelial cells, and dramatic changes in vascular permeability [22], suggesting a critical role for cav-1/caveolae in angiogenesis. Cav-1 functions as a “scaffolding” protein directly interacting with various signaling molecules, and it integrates specific transmembrane signaling pathways activated by various stimuli in the caveolae [23]. Thus, it has been postulated that caveolae functions as a platform for signaling control of cell activity and reactivity [24, 25].
FGFs are major growth factors of the placenta, with FGF2 as the dominant form [26–28]. FGF2 is expressed by the trophoblast and endothelial cells in the uteri and placentas in ruminants [28–30] and humans [27]. Ovine fetoplacental artery endothelial (oFPAE) cells express FGF2 both in vivo [28, 31] and in vitro [32]. Fetoplacental (cotyledonary) FGF2 mRNA expression in vivo [28] and protein secretion ex vivo are developmentally regulated, greatest at ∼Days 120–130 of ovine pregnancy [28, 31]. Expression of FGF2 changes little in uteroplacental (carunclular) tissues but increases exponentially in fetoplacental tissues in late ovine gestation, implicating that FGF2 functions as a fetal angiogenic factor for branching angiogenesis that occurs mainly in the fetal cotyledonary tissues [28]. In oFPAE cells, we have recently reported that activation of the MAPK and PI3K pathways by FGF2 is mainly mediated by FGFR1, which is compartmentalized in the caveolae and paradoxically regulated by cav-1 [33]. However, how the proximal FGFR1 signaling is regulated in these cells has not been reported. FRS2α protein contains myristyl anchors in their NH2-terminus, which is essential for targeting FRS2α to the plasma membrane and important for FRS2α phosphorylation and subsequent activation of downstream signaling pathways in response to FGF or nerve growth factor stimulation [3]. Although previous studies have shown that FRS2α is spatially present in the caveolae/lipid rafts in human neuroblastoma cells [34, 35], whether FRS2α is compartmentalized in the caveolae in placental endothelial cells remains to be determined. In this study, we hypothesize that the proximal FGF2/FGFR1 signaling via FRS2α is compartmentalized in placental endothelial caveolae via direct interaction with cav-1. We found that FRS2α is stably partitioned in the caveolae via interaction with cav-1. Treatment with FGF2 rapidly recruited GRB2 and GAB1 to the caveolae, where they form a complex with FRS2α. Disrupting caveolae inhibited the FGF2-induced FRS2α phosphorylation, blocked the formation of the FRS2α-GAB1-GRB2 complex, and inhibited downstream PI3K/AKT pathway activation. Thus, these findings suggest that the proximal FGF2/FGFR1 signaling is compartmentalized in the endothelial caveolae.
MATERIALS AND METHODS
Antibodies and Chemicals
Recombinant FGF2 (157 amino acids) and mouse monoclonal antibody (mAb) of FRS2α were from R&D Systems. Rabbit polyclonal antibody (pAb) of FGFR1 was from Zymed. Rabbit pAb against GAB1 and mouse mAb against GRB2 were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Rabbit pAbs against phospho-FRS2αTyr196 (pFRS2α), phosphor-AKTser473 (pAKT1), phosphor-MAPK1/2Thr202/Tyr204 (pMAPK1/2), AKT1, and MAPK1/2 were from Cell Signaling Technology. Horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit antibodies were obtained from Pierce. Caveolin scaffolding domain (Cav-SD; amino acids 82–101) fused with the N-terminus to the antennapedia internalization sequence (amino acids 43–58) and its negative control peptides (Cav-SDX) were from EMD Calbiochem. Cell culture supplies were from Invitrogen/GIBCO. Methyl-β-cyclodextrin (MβCD) and other reagents were from Sigma-Aldrich unless indicated otherwise.
Cell Culture and Preparation of Total Cell Extracts
The oFPAE cells were isolated from four late (Days 120–130, gestation ≈147 days) pregnant ewes as previously described [32]. The animal (sheep) use protocol was approved by the University of California, San Diego, Animal Subjects Committee. Cells were subcultured in MCDB-131 containing 10% fetal bovine serum and were used at passages 7–11. Prior to each experiment, subconfluent (∼70%–80%) cells were serum starved in M-199 containing 1% fetal bovine serum, 0.1% bovine serum albumin, and 25 mM HEPES overnight. Following stimulation, total cellular proteins were harvested in a nondenaturing lysis buffer [36] and protein concentration was measured.
Immunofluorescence Microscopy
Cells were fixed with 3.7% paraformaldehyde in PBS on ice for 20 min. After rinsing with ice-cold PBS three times, cells were permeabilized with 0.1% Triton X-100 in PBS for 20 min on ice. After incubation with 5% bovine serum albumin in PBST (PBS with 0.1% Tween 20) for 1 h at room temperature, the cells were incubated with the primary antibody overnight, followed by incubation with Rhodamine Red-X-conjugated goat anti-rabbit immunoglobulin G (IgG) or Alexa488-conjugated goat anti-mouse IgG (1:500 dilution) for 2 h at room temperature. After washing three times with PBST, cells were mounted in Prolong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). The cells were examined under an inverted Leica fluorescent microscope, and images were captured with a digital camera. Data were analyzed by SimplePCI software (Hamamatsu Corp., Sewickley, PA).
Immunoprecipitation, SDS-PAGE, and Western Blot Analysis
Equal amounts of cellular proteins (500 μg per sample) were precleared by incubation with 50% protein A/G agarose beads (20 μl) for 1 h at 4°C, followed by centrifugation (15 000 × g, 30 sec). The samples were then incubated with specific antibody (1 μg) overnight at 4°C. Protein A/G agarose beads (20 μl) were added to pull down the targeted proteins. After washing three times, the immunoprecipitated samples or total protein extracts were run on SDS-PAGE and analyzed by Western blotting as described previously [36]. The relative density of a protein band was calculated by multiplying the absorbance of the surface areas using the National Institutes of Health ImageJ software.
Competitive Binding Studies
Serum-starved oFPAE cells were treated with or without Cav-SD or Cav-SDX peptides (5 μM) for 2 h at 37°C. The cells were then lysed with a nondenaturing buffer [36]. Total cell extracts (500 μg per sample) were precleared with protein G agarose beads and then used for immunoprecipitation (IP) with rabbit anti-cav-1 pAb (1 μg). The IP samples were analyzed by immunoblotting with the anti-FRS2α and anti-cav-1 antibodies.
Caveolae Isolation
Detergent-free isolation of caveolae membranes was performed exactly as previously described [36]. Briefly, the cells (∼2 × 107) were homogenized and sonicated on ice in 2.5 ml of 0.5 M sodium carbonate buffer (pH 11.0) with protease inhibitors. The homogenates (∼5 mg of proteins) were adjusted to 4 ml of MBS buffer (25 mM MES [pH 6.5], 0.15 M NaCl) containing 45% sucrose and were placed at the bottom of 12.5-ml ultracentrifuge tubes. A 5%–35% discontinuous sucrose gradient in MBS was formed above as 4 ml each. Samples were centrifuged at 39 000 rpm (≈260 000 × g) for 16–20 h in an SW41 rotor (Beckman Instruments). Fractions (1 ml each) were collected from the top and mixed with 5× SDS sample buffer5 immediately for immunoblotting analysis.
Experimental Replication and Statistical Analysis
All experiments were repeated at least four times using cells from different pregnant ewes. Data were presented as means ± SD and analyzed by one-way analysis of variance (ANOVA), followed by Bonferroni test for multiple comparisons using SigmaStat 3.5 (Systat Software Inc.). Student paired t-test was used for comparison of data between two groups. Significance was defined as P < 0.05.
RESULTS
FGF2 Induced FRS2α Tyrosine Phosphorylation in a Time- and Concentration-Dependent Manner
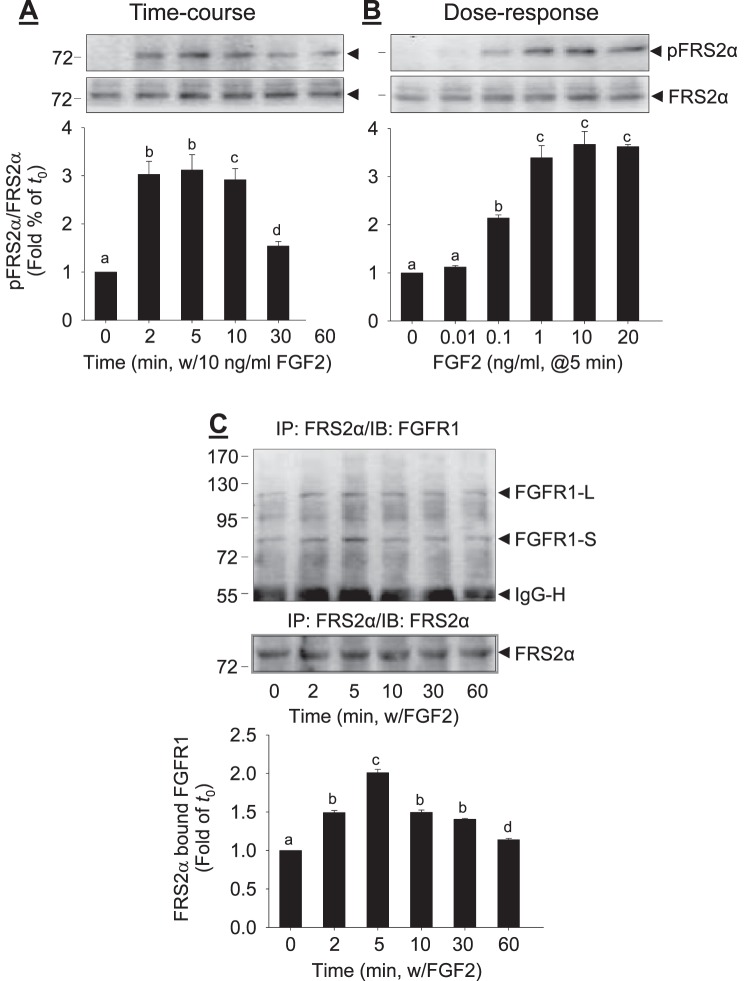
Treatment with FGF2 stimulated rapid tyrosine phosphorylation of FRS2α at Try196 in a time-dependent (Fig. 1A) and dose-dependent (Fig. 1B) fashion in oFPAE cells. When cells were treated with 10 ng/ml FGF2 up to 60 min, FRS2α phosphorylation began within 2 min, reaching a peak level (3.2-fold of control; P < 0.05) within 5–10 min. Thereafter, it declined gradually but did not return to the baseline at 60 min (Fig. 1A). When the cells were treated with increasing concentrations (0.01–100 ng/ml) of FGF2 for 5 min, as little as 0.1 ng/ml FGF2 was able to induce FRS2α phosphorylation. Treatment with 10 ng/ml FGF2 dramatically stimulated FRS2α phosphorylation, consistent with the time-course studies. Higher doses of FGF2 did not further enhance FRS2α phosphorylation. Thus, FGF2-stimulated FRS2α phosphorylation maximizes around 5 min after treatment with 10 ng/ml FGF2.
FIG. 1. .
FGF2-induced FRS2α phosphorylation and binding to FGFR1 in oFPAE cells. The cells were treated with 10 ng/ml FGF2 up to 60 min (A) or with increasing concentrations of FGF2 for 5 min (B). Total cell lysates were subjected to immunoblotting analysis of FRS2α phosphorylation. C) Co-IP of FGFR1 and FRS2α. The oFPAE cells were treated with or without FGF2, and total cell lysates were immunoprecipitated with anti-FRS2α antibody. The immunoprecipitates were subjected to immunoblotting with anti-FGFR1 and anti-FRS2α antibodies. Experiments were repeated four times using cells from different pregnant ewes. Band intensities were quantified and expressed as mean ± SD. Bars with different superscripts differ significantly (P < 0.05). FGFR1-L, FGFR1 long form; FGFR1-S, FGFR1 short form; IgG-H, immunoglobin heavy chain.
FGF2 Increases FGFR1-Bound FRS2α
In oFPAE cells, FRS2α interacted with FGFR1 in the absence and presence of FGF2 (Fig. 1C). However, the amounts of FGFR1-bound FRS2α were increased by treatment with 10 ng/ml FGF2 in a time-dependent manner, reached their maximum (approximately two times that of time zero; P < 0.05) within 2 min after FGF2 treatment, and then declined gradually, but did not return to the baseline at 60 min.
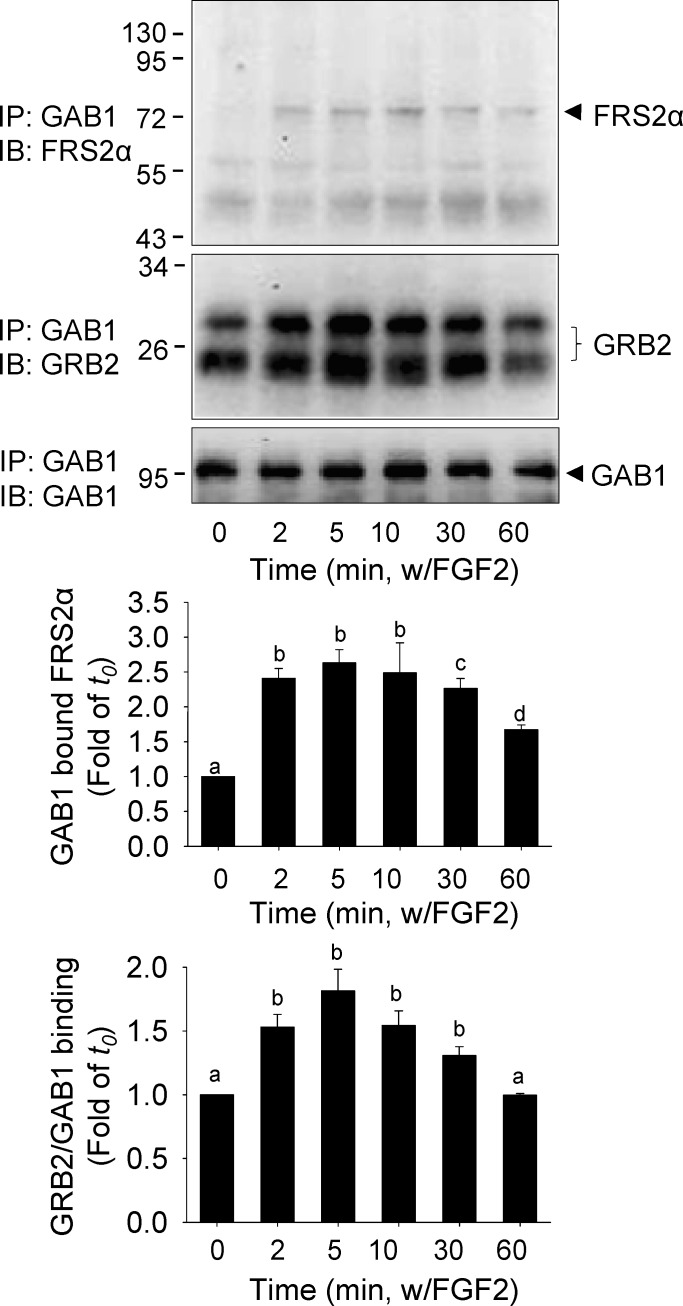
FGF2 Induced the Formation of an FRS2α-GRB2-GAB1 Complex
The proximal FGF/FGFR signaling is mediated by the adaptor protein GRB2 binding to FRS2α via the SH2 domain and to GAB1 and other signaling molecules via the SH3 domain [3]. In oFPAE cells, very low levels of GAB1 were found to be bound to FRS2α without FGF2 stimulation (Fig. 2). However, treatment with 10 ng/ml FGF2 rapidly stimulated the amounts of FRS2α-bound GAB1, maximizing (up to about 2.6 times of that of the time zero; P < 0.05) within 2 min and declining after 30 min of FGF2 stimulation. In resting cells, GAB1 was found to be associated with GRB2, and the binding was significantly enhanced by treatment with 10 ng/ml FGF2 (up to about 1.8 times of that of the time zero, P < 0.05; Fig. 2). Thus, these results suggest assembly of a ternary complex containing FRS2α, GRB2, and GAB1 following FGF2 stimulation.
FIG. 2. .
Effects of FGF2 on the formation of FRS2α-GAB1-GRB2 complex in oFPAE cells. The cells were treated with 10 ng/ml FGF2 up to 60 min. Total cell lysates were prepared for IP with anti-GAB1 antibody. The immunoprecipitates were subjected to immunoblotting with anti-GAB1, anti-GRB2, and anti-FRS2α antibodies. Experiments were repeated four times using cells from different pregnant ewes, and band intensities were quantified and expressed as mean ± SD. Bars with different superscripts differ significantly (P < 0.05).
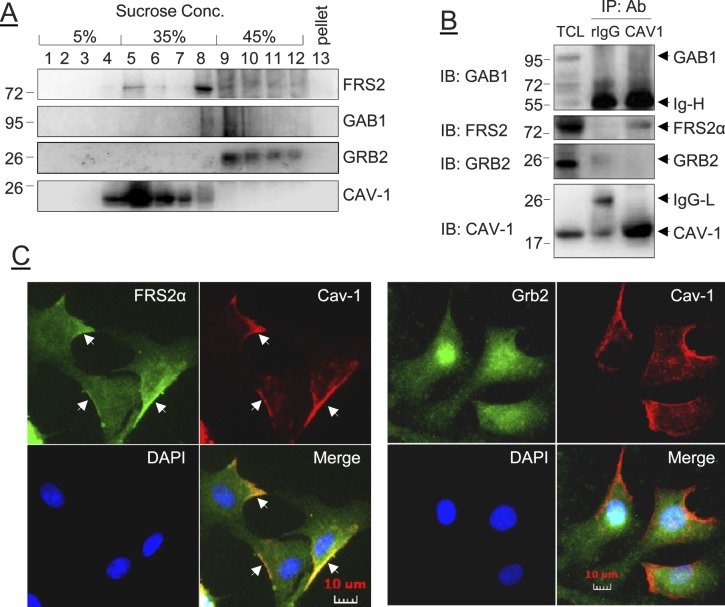
FRS2α, but Not GRB2 or GAB1, Was Physically Bound to Cav-1 in Resting Endothelial Cell Caveolae
FRS2α is targeted to the plasma membrane via myristylation [3]. In human neuroblastoma cell LAN-1 cells, FRS2 is located in the plasma membrane caveolaelike microdomain [34, 35]. By using the well-established discontinuous sucrose gradient isolation procedure [36, 37], FRS2α was found in the purified caveolae membranes coexisting with cav-1, although most FRS2α was found in the noncaveolae fractions; neither GAB1 nor GRB2 was detected in the caveolae fractions in the resting cells (Fig. 3A). We used co-IP studies to verify these findings. As shown in Figure 3B, only FRS2α, but not GRB2 or GAB1, was associated with cav-1 in the resting cells. We also preformed double immunofluorescence labeling experiments to localize FRS2α, GRB2, and cav-1 in the cells. As shown in Figure 3C, FRS2α and GRB2 (in green fluorescence) were found to be localized throughout the cells; however, only FRS2α, but not GRB2, was localized in the plasma membrane. Cav-1 (in red) was mainly present at the leading edges of cells. In the merged micrographs, FRS2α, but not GRB2, was found to be colocalized with cav-1 along the plasma membrane.
FIG. 3. .
Effects of FGF2 on the subcellular localization of FRS2α, GRB2, and GAB1 in oFPAE cells. A) The resting cells were homogenized and fractioned by sucrose-gradient ultracentrifugation. Fractions were subjected to immunoblotting with antibodies against FRS2α, GAB1, GRB2, and cav-1. B) Co-IP of FRS2α, GRB2, and GAB1. The cells were treated with or without FGF2, and total cell lysates were immunoprecipitated with anti-cav-1 antibody. The immunoprecipitates were immunoblotted with antibodies against FRS2α, GRB2, GAB1, and cav-1. Blots of a typical experiment are shown representing similar data from four experiments using cells from different pregnant ewes. TCL, total cell lysate; Ab, antibody; rIgG, rabbit immunoglobin; IgG-H, immunoglobin heavy chain; IgG-L, immunoglobin light chain. C) Cells grown on glass coverslips were fixed and subjected to double-immunofluorescence labeling with anti-FRS2α or anti-GRB2 paired with anti-cav-1 antibodies, followed by corresponding Rhodamine Red-X-conjugated goat anti-rabbit IgG or Alexa Fluor488-conjugated goat anti-mouse IgG secondary antibodies. Cells were mounted in Prolong Gold antifade reagent with DAPI and examined under an inverted Leica fluorescence microscope for image acquisition by a digital camera. Arrows indicate plasma membrane FRS2α and Cav1 and their colocalization.
FGF2 Regulates FRS2α Interaction with Cav-1
Targeting signaling molecules to caveolae is mainly mediated via the so-called “caveolin binding motif,” defined as φXφXXXXφ or φXXXXφXXφ (where φ is one of the aromatic amino acids Trp, Phe, or Tyr [38]), which can directly interact with the caveolin scaffolding domain of caveolin proteins. Amino acid sequence analysis revealed that FRS2α protein possesses one caveolin-binding motif (65YGYDSNLF72). We used synthetic Cav-SD peptides to verify whether FRS2α binds to cav-1 in oFPAE cells. As shown in Figure 4A, pretreatment with the Cav-SD, but not Cav-SDX, peptides reduced the amounts of cav-1-bound FRS2α to one third of that in resting oFPAE cells (P < 0.05). In addition, time-course studies showed that FGF2 dynamically regulated FRS2α and cav-1 interaction. Treatment with 10 ng/ml FGF2 rapidly decreased the amount of cav-1-bound FRS2α to less than half of that in the resting cells within 5–10 min; this inhibition was lost after 60 min after FGF2 treatment (Fig. 4B).
FIG. 4. .
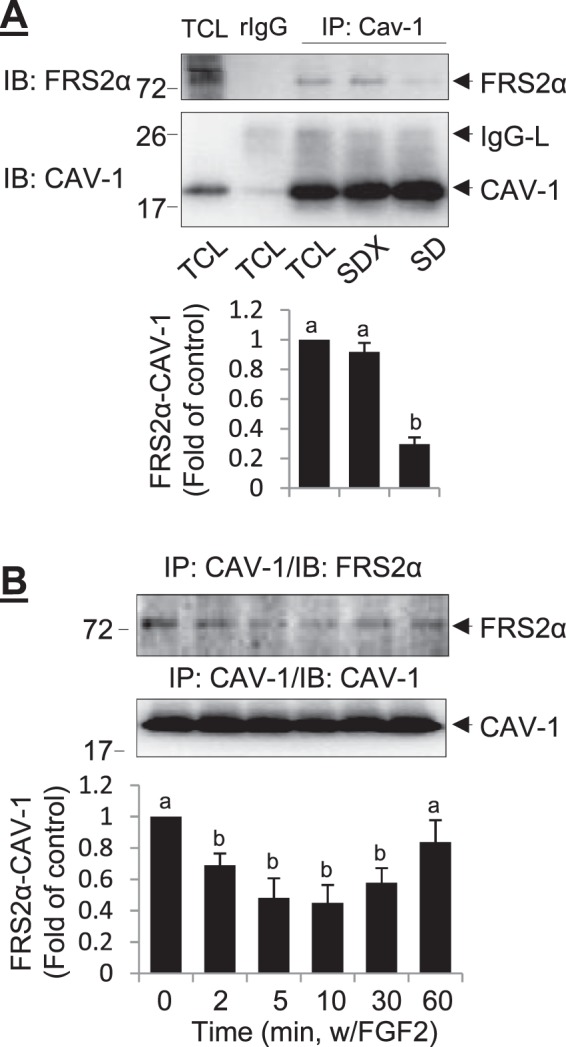
Effects of FGF2 on FRS2α interaction with cav-1 in oFPAE cells. A) Caveolin scaffolding domain (SD) peptide competes off cav-1 binding to FRS2α. The cells were pretreated with 5 μM Cav-SD or Cav-SDX (control) peptides, and total cell lysates were prepared for immunoprecipitation with anti-cav-1 antibody. The immunoprecipitates were immunoblotted with anti-FRS2α and anti-cav-1 antibodies. B and C) The oFPAE cells were treated with or without 10 ng/ml FGF2 up to 60 min. Total cellular proteins were immunoprecipitated with anti-cav-1 antibody followed by immunoblotting with anti-FRS2α and anti-cav-1 antibodies. Experiments were repeated four times using cells from different pregnant ewes, and band intensities were quantified and expressed as mean ± SD. Bars with different superscripts differ significantly (P < 0.05). TCL, total cell lysate; rIgG, rabbit immunoglobin; IgG-H, immunoglobin heavy chain; IgG-L, immunoglobin light chain.
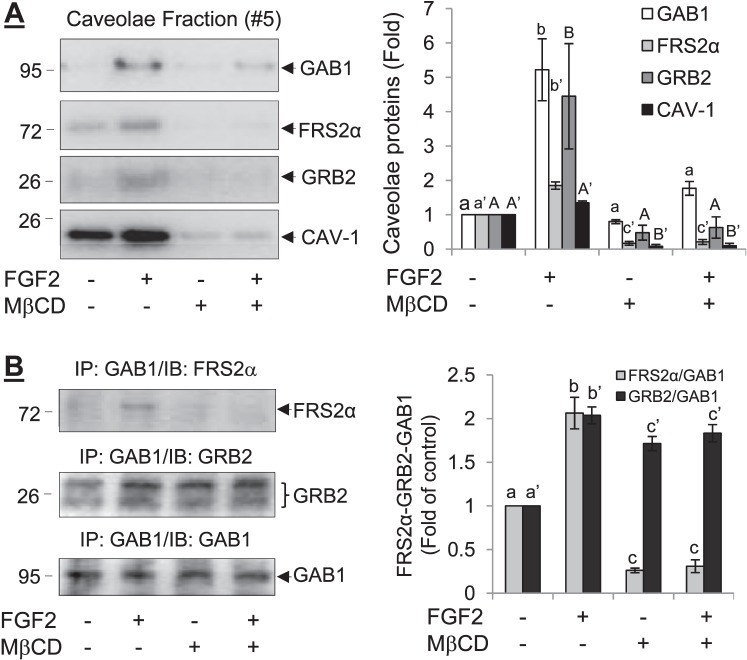
Integral Caveolae Is Essential for the Formation of Caveolar FRS2α-GRB2-GAB1 Complex in Response to FGF2 Stimulation
Cholesterol depletion with MβCD has been widely used as a means for studying the role of integral caveolae in signal transduction [39]. In oFPAE cells, treatment with 10 mM MβCD for 1 h resulted in loss of caveolae [36]. Treatment with FGF2 (10 ng/ml, 5 min) resulted in the recruitment of GRB2 and GAB1 to the caveolae in oFPAE cells without MβCD pretreatment (Fig. 5). However, when the cells were pretreated with MβCD to disrupt integral caveolae, FGF2 was unable to recruit GRB2 and GAB1 to the caveolae (Fig. 5A). Furthermore, without MβCD pretreatment FGF2 (10 ng/ml, 5 min) was able to induce the formation of the FRS2α-GRB2-GAB1 complex in the oFPAE cell caveolae. However, with MβCD pretreatment FGF2 was unable to do so (Fig. 5B).
FIG. 5. .
Effects of caveolae disruption with MβCD on FGF2-induced FRS2α-GRB2-GAB1 formation in oFPAE cells. A) The cells were pretreated with or without 10 mM MβCD for 1 h, followed by stimulation with 10 ng/ml FGF2 for 5 min. Cells were lysed to isolate the caveolae membranes by sucrose-gradient fractionation. The caveolae membrane fractions were analyzed by immunoblotting with antibodies for FRS2α, GRB2, GAB1, and cav-1. B) The cells were pretreated with or without 10 mM MβCD for 1 h, followed by stimulation with 10 ng/ml FGF2 for 5 min. Total cellular proteins were prepared for IP with anti-GAB1 antibody, followed by immunoblotting with antibodies for FRS2α, GRB2, and GAB1. Experiments were repeated four times using cells from different pregnant ewes, and band intensities were quantified and expressed as mean ± SD. Bars with different superscripts differ significantly (P < 0.05).
Integral Caveolae Is Required for the Full Activation of FGF2 Signaling
We also investigated the effects of caveolae disruption with MβCD on the FGF2-induced FRS2α phosphorylation and activation of MAPK1/2 and AKT. As summarized in Figure 6, treatment with FGF2 (10 ng/ml, 5 min) potently increased the levels of phosphorylated FRS2α, MAPK1/2, and AKT1 in oFPAE cells. However, pretreatment with 10 mM MβCD for 1 h inhibited FGF2-induced FRS2α phosphorylation and activation of MAPK1/2 and AKT1.
FIG. 6. .
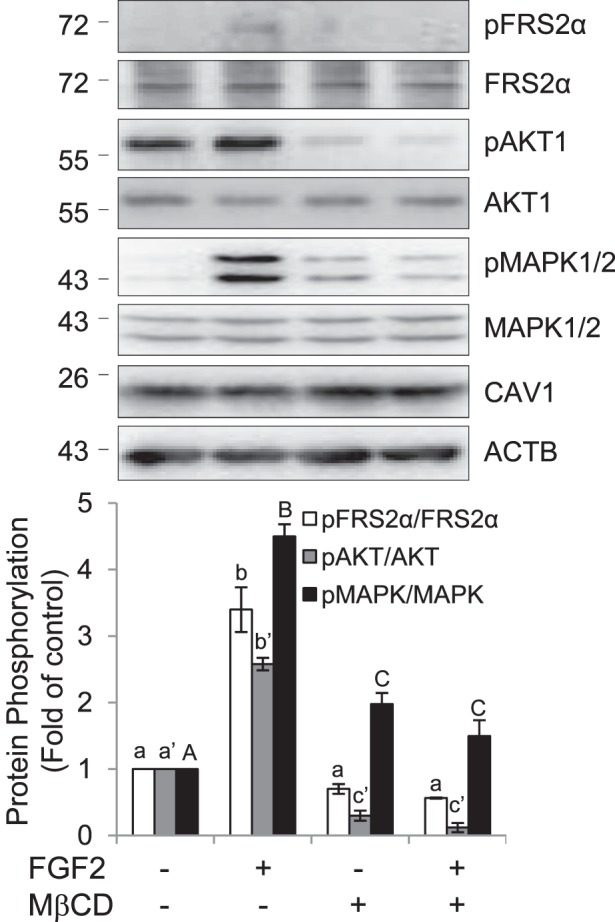
Effects of caveolae disruption with MβCD on FGF2-induced FRS2α phosphorylation and activation of AKT1 and MAPK1/2 in oFPAE cells. The cells were pretreated with or without 10 mM MβCD for 1 h, followed by stimulation with 10 ng/ml FGF2 for 5 min. Total cellular proteins were prepared for analyzing phosphorylation of FRS2α, AKT1, and MAPK1/2 by immunoblotting with specific antibodies. Total cav-1 and ACTB were measured by immunoblotting for a loading control. Experiments were repeated four times using cells from different pregnant ewes, and band intensities were quantified and expressed as mean ± SD. Bars with different superscripts differ significantly (P < 0.05).
DISCUSSION
We have demonstrated in the present study that the proximal FGF2/FGFR1 signaling is compartmentalized in the caveolae via FRS2α interactions with cav-1 in placental endothelial cells. This conclusion is drawn based on the following findings. First, treatment with FGF2 rapidly stimulates tyrosine phosphorylation of FRS2α that is physically associated with FGFR1 in the caveolae [33]. Second, FRS2α, but neither GAB1 nor GRB2, is found in the caveolae, where it is associated with cav-1 in resting endothelial cells. Third, GRB2 is associated with GAB1 but neither is associated with FRS2α in resting endothelial cells; however, treatment with FGF2 stimulated the formation of a ternary complex containing FRS2α, GRB2, and GAB1 in the caveolae. Fourth, disruption of integral caveolae with MβCD blocks the formation of the FRS2α-GRB2-GAB1 complex and significantly inhibits the activation of the downstream MAPK1/2 and AKT1. Together with our most recent report demonstrating a paradoxical role for cav-1/caveolae in regulating the FGF2/FGFR1 signaling control of angiogenic responses via the MEK/ERK2/1 and PI3K/AKT pathways in oFPAE cells [33], our current findings have further strengthened the concept that caveolae functions as a focal center for orchestrating FGF2/FGFR1 signaling control of placental angiogenesis.
A major finding in our present study is that of caveolar targeting of the proximal FGF2/FGFR1 signaling via the formation of the FRS2α-GRB2-GAB1 complex. This is because although GAB1 has been considered a major player for mediating the activation of the PI3K/AKT1 pathway by FGF via FGFRs [8, 12, 13], little is known regarding the proximal FGF/FGFR signaling mechanism that controls activation of the PI3K/AKT1 pathway. Our findings suggest that caveolae functions as a focal center for organizing the proximal FGF2/FGFR1 signaling via FRS2α in placental endothelial cells because: 1) FRS2α has a caveolin binding motif (65YGYDSNLF72, aromatic amino acids underlined) [38] that is highly conserved among different species [40–42]; 2) FRS2α cofractionates with cav-1 in the caveolae membranes; 3) FRS2α colocalizes with cav-1 at the plasma membrane; and 4) FRS2α coimmunoprecipitates with cav-1, which can be competed off with caveolin scaffolding domain peptide. Moreover, treatment with FGF2 rapidly reduces the levels of cav-1-bound FRS2α and then returns to baseline in a time-dependent fashion, suggesting that FGF2 temporally regulates the dissociation of FRS2α from cav-1. Consistently, we have shown more recently that FGF2 regulates a similar time-dependent change in FGFR1 association/dissociation with cav-1 in oFPAE cells [33]. Thus, these findings suggest that binding to cav-1 renders FGFR1 and FRS2α in an inactive state; upon FGF2 treatment, dissociation from cav-1 might be required for FGFR1 and FRS2α activation. From this standpoint, cav-1 seems to act as an intrinsic switch to the spatiotemporal control of the proximal FGF/FGFR signaling via FRS2α in the caveolae.
It is noteworthy that, contrasting with a previous report suggesting cofractionation of GRB2 and GAB1 with cav-1 in the caveolae membranes in other cell types [43], our current results do not support these two important proximal FGF/FGFR signaling proteins to be caveolar proteins. First, they are found to be mainly present in the noncaveolar compartments in resting oFPAE cells. Second, the two proteins cannot be immunoprecipitated with the anti-cav-1 antibody. Third, they are constitutively associated with each other without binding to FRS2α in resting cells. Fourth, they do not have the caveolin binding domain that provides a structural base for being a caveolar protein according to amino acid sequence analysis. However, our data suggest that GRB2 and GAB1 can be rapidly recruited to the caveolae, where they form a ternary complex with FRS2α upon FGF2 stimulation. This notion is supported by the findings showing that disruption of integral caveolae with MβCD reduces the FGF2-induced FRS2α phosphorylation and blocks the FGF2-induced FRS2α-GRB2-GAB1 ternary complex formation and AKT1 activation, without altering GRB2 association with GAB1 in oFPAE cells (Fig. 5). In keeping with the fact that GAB1 is responsible for mediating PI3K/AKT1 activation via FGF/FGFR signaling [8, 12, 13], our findings allow us to conclude that caveolae functions as a platform for organizing the proximal FGF2/FGFR1 signaling via formation of the FRS2α-GRB2-GAB1 ternary complex, thereby mediating the downstream activation of the PI3K/AKT1 signaling pathway in placental endothelial cells. In parallel, caveolae disruption also inhibited MAPK1/2 activation (as shown above and in Feng et al. [33]), suggesting a critical role for caveolae in regulating the ERK2/1 pathway in placental endothelial cells. However, how the proximal FGF2/FGFR1 signaling to the MAPK1/2 pathway, likely via FRS2α-GRB2-Sos [3, 4, 8], is organized in the caveolae is waiting for further investigation.
Together, we have demonstrated in the present study that the proximal FGF2/FGFR1 signaling via FRS2α is compartmentalized in placental endothelial caveolae. In resting conditions, binding to cav-1 sequesters FGFR1 and FRS2α in an inactive form. Upon FGF2 stimulation, FGFR1 and FRS2α are temporally dissociated from cav-1, which facilitates their phosphorylation; the cytosolic GRB2-GAB1 complex is then recruited to the caveolae, where it forms a ternary complex with FRS2α for mediating downstream signaling transduction cascades (Fig. 7).
FIG. 7. .
Caveolae targeting of the proximal FGF2/FGFR1 signaling in oFPAE cells via FRS2α.
Footnotes
Supported in part by National Institutes of Health grants R01 HL74947, R01 HL70562, and R21 HL98746 to D.b.C. H.h.Z. was supported by an American Heart Association Postdoctoral Fellowship (AHA11Post7610115).
REFERENCES
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 2009; 8: 235 253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res 1993; 60: 1 41 [DOI] [PubMed] [Google Scholar]
- Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 1997; 89: 693 702 [DOI] [PubMed] [Google Scholar]
- Hadari YR, Gotoh N, Kouhara H, Lax I, Schlessinger J. Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc Natl Acad Sci U S A 2001; 98: 8578 8583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N. Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci 2008; 99: 1319 1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ. et al. SH2 domains recognize specific phosphopeptide sequences. Cell 1993; 72: 767 778 [DOI] [PubMed] [Google Scholar]
- Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol 1998; 18: 3966 3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamothe B, Yamada M, Schaeper U, Birchmeier W, Lax I, Schlessinger J. The docking protein Gab1 is an essential component of an indirect mechanism for fibroblast growth factor stimulation of the phosphatidylinositol 3-kinase/Akt antiapoptotic pathway. Mol Cell Biol 2004; 24: 5657 5666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000; 103: 211 225 [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science 2004; 306: 1506 1507 [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 1995; 80: 225 236 [DOI] [PubMed] [Google Scholar]
- Mattoon DR, Lamothe B, Lax I, Schlessinger J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol 2004; 2: 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SH, Hadari YR, Gotoh N, Guy GR, Schlessinger J, Lax I. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc Natl Acad Sci U S A 2001; 98: 6074 6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RR, Palade GE. Studies on blood capillaries. II. Transport of ferritin molecules across the wall of muscle capillaries. J Cell Biol 1968; 37: 277 299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RR, Palade GE. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol 1968; 37: 244 276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE, Bruns RR. Structural modulations of plasmalemmal vesicles. J Cell Biol 1968; 37: 633 649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laurentiis A, Donovan L, Arcaro A. Lipid rafts and caveolae in signaling by growth factor receptors. Open Biochem J 2007; 1: 12 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell 1992; 68: 673 682 [DOI] [PubMed] [Google Scholar]
- Predescu D, Palade GE. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am J Physiol 1993; 265: H725 H733 [DOI] [PubMed] [Google Scholar]
- Minshall RD, Tiruppathi C, Vogel SM, Malik AB. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem Cell Biol 2002; 117: 105 112 [DOI] [PubMed] [Google Scholar]
- Glenney JR, Jr, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci U S A 1992; 89: 10517 10521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H. et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001; 293: 2449 2452 [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 1998; 273: 5419 5422 [DOI] [PubMed] [Google Scholar]
- Isshiki M, Ando J, Yamamoto K, Fujita T, Ying Y, Anderson RG. Sites of Ca(2+) wave initiation move with caveolae to the trailing edge of migrating cells. J Cell Sci 2002; 115: 475 484 [DOI] [PubMed] [Google Scholar]
- Isshiki M, Ying YS, Fujita T, Anderson RG. A molecular sensor detects signal transduction from caveolae in living cells. J Biol Chem 2002; 277: 43389 43398 [DOI] [PubMed] [Google Scholar]
- Biswas SB, Hammond RW, Anderson LD. Fibroblast growth factors from bovine pituitary and human placenta and their functions in the maturation of porcine granulosa cells in vitro. Endocrinology 1988; 123: 559 566 [DOI] [PubMed] [Google Scholar]
- Arany E, Hill DJ. Fibroblast growth factor-2 and fibroblast growth factor receptor-1 mRNA expression and peptide localization in placentae from normal and diabetic pregnancies. Placenta 1998; 19: 133 142 [DOI] [PubMed] [Google Scholar]
- Borowicz PP, Arnold DR, Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Placental growth throughout the last two thirds of pregnancy in sheep: vascular development and angiogenic factor expression. Biol Reprod 2007; 76: 259 267 [DOI] [PubMed] [Google Scholar]
- Pfarrer C, Weise S, Berisha B, Schams D, Leiser R, Hoffmann B, Schuler G. Fibroblast growth factor (FGF)-1, FGF2, FGF7 and FGF receptors are uniformly expressed in trophoblast giant cells during restricted trophoblast invasion in cows. Placenta 2006; 27: 758 770 [DOI] [PubMed] [Google Scholar]
- Cooke FN, Pennington KA, Yang Q, Ealy AD. Several fibroblast growth factors are expressed during pre-attachment bovine conceptus development and regulate interferon-tau expression from trophectoderm. Reproduction 2009; 137: 259 269 [DOI] [PubMed] [Google Scholar]
- Zheng J, Vagnoni KE, Bird IM, Magness RR. Expression of basic fibroblast growth factor, endothelial mitogenic activity, and angiotensin II type-1 receptors in the ovine placenta during the third trimester of pregnancy. Biol Reprod 1997; 56: 1189 1197 [DOI] [PubMed] [Google Scholar]
- Zheng J, Bird IM, Melsaether AN, Magness RR. Activation of the mitogen-activated protein kinase cascade is necessary but not sufficient for basic fibroblast growth factor- and epidermal growth factor-stimulated expression of endothelial nitric oxide synthase in ovine fetoplacental artery endothelial cells. Endocrinology 1999; 140: 1399 1407 [DOI] [PubMed] [Google Scholar]
- Feng L, Liao WX, Luo Q, Zhang HH, Wang W, Zheng J, Chen DB. Caveolin-1 orchestrates fibroblast growth factor 2 signaling control of angiogenesis in placental artery endothelial cell caveolae. J Cell Physiol 2012; 227: 2480 2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Feuerstein C, Robbins SM. Signaling within a caveolae-like membrane microdomain in human neuroblastoma cells in response to fibroblast growth factor. J Neurochem 2000; 74: 676 683 [DOI] [PubMed] [Google Scholar]
- Ridyard MS, Robbins SM. Fibroblast growth factor-2-induced signaling through lipid raft-associated fibroblast growth factor receptor substrate 2 (FRS2). J Biol Chem 2003; 278: 13803 13809 [DOI] [PubMed] [Google Scholar]
- Liao WX, Feng L, Zhang H, Zheng J, Moore TR, Chen DB. Compartmentalizing VEGF-induced ERK2/1 signaling in placental artery endothelial cell caveolae: a paradoxical role of caveolin-1 in placental angiogenesis in vitro. Mol Endocrinol 2009; 23: 1428 1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 1996; 271: 15160 15165 [DOI] [PubMed] [Google Scholar]
- Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem 1997; 272: 6525 6533 [DOI] [PubMed] [Google Scholar]
- Parpal S, Karlsson M, Thorn H, Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J Biol Chem 2001; 276: 9670 9678 [DOI] [PubMed] [Google Scholar]
- Lu F, Ogawa R, Nguyen DT, Chen B, Guo D, Helm DL, Zhan Q, Murphy GF, Orgill DP. Microdeformation of three-dimensional cultured fibroblasts induces gene expression and morphological changes. Ann Plast Surg 2011; 66: 296 300 [DOI] [PubMed] [Google Scholar]
- Sims-Lucas S, Cusack B, Eswarakumar VP, Zhang J, Wang F, Bates CM. Independent roles of Fgfr2 and Frs2alpha in ureteric epithelium. Development 2011; 138: 1275 1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman PE, Sanz-Ezquerro J, Overton IM, Burt DW, Bosch E, Fong WT, Tickle C, Brown WR, Wilson SA, Hubbard SJ. A comprehensive collection of chicken cDNAs. Curr Biol 2002; 12: 1965 1969 [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol 1994; 126: 111 126 [DOI] [PMC free article] [PubMed] [Google Scholar]