ABSTRACT
Activin is a well-established modulator of male and female reproduction that stimulates the synthesis and secretion of follicle-stimulating hormone. Nonpituitary effects of activin have also been reported, although the paracrine actions of this growth factor in several reproductive tissues are not well understood. To identify the paracrine functions of activin during mammary gland morphogenesis and tumor progression, we produced transgenic mice that overexpress follistatin (FST), an intrinsic inhibitor of activin, under control of the mouse mammary tumor virus (MMTV) promoter. Although the MMTV-Fst mice were constructed to assess the role of activin in females, expression of the transgene was also observed in the testes and epididymides of males. While all 17 transgenic founder males exhibited copulatory behavior and produced vaginal plugs in females, only one produced live offspring. In contrast, transgenic females were fertile, permitting expansion of transgenic mouse lines. Light and transmission electron microscopic examination of the transgenic testes and epididymides revealed impairment of fluid resorption and sperm transit in the efferent ducts and initial segment of the epididymis, as indicated by accumulation of fluid and sperm stasis. Consequently, a variety of degenerative lesions were observed in the seminiferous epithelium, such as vacuolation and early stages of mineralization and fibrosis. Sperm collected from the caudae epididymidis of MMTV-Fst males had detached heads and were immotile. Together, these data reveal that activin signaling is essential for normal testicular excurrent duct function and that its blockade impairs fertility. These results also suggest that selective inhibitors of activin signaling may provide a useful approach for the development of male contraceptives without compromising androgen synthesis and actions.
Keywords: epididymis, fertility, follistatin, mouse, testis
Follistatin overexpression in the testis and epididymis of transgenic mice causes infertility due to defects in fluid resorption, sperm transit, and morphology in excurrent ducts.
INTRODUCTION
Follistatin (FST) and activin were first isolated in the 1980s as peptides found in ovarian follicular fluids that could either inhibit or activate the production and secretion of follicle-stimulating hormone (FSH) from the pituitary gland, respectively [1, 2]. While activin could feed back at the pituitary gland to activate synthesis and release of FSH, FST was found to modulate FSH release by directly binding to and inhibiting the activity of activin. This duo of proteins was thought to function as an endocrine regulatory loop between the gonads and pituitary gland to modulate reproductive potential. Over the last 30 years, identification of FST and activin subunits in a wide variety of both reproductive and nonreproductive tissues suggests that in addition to regulating FSH, these factors contribute to a plethora of physiological functions including branching morphogenesis of many organs such as the kidney, pancreas and salivary glands, wound repair, inflammation response, and bone remodeling, among others, by acting in both autocrine and paracrine fashions [3–7].
Activins are members of the TGF-β superfamily of ligands that exist as β-subunit dimers, unlike their sister family members, the inhibins, which consist of heterodimers of an α-subunit and one of five β-subunits (βA-βE). Activin A is a homodimer of two βA subunits, while activin AB is a heterodimer of one βA and one βB subunit. Secreted activin binds to either the activin type IIA (ACVR2a) or type IIB (ACVR2b) receptors, both of which are constitutively active. This leads to recruitment and transphosphorylation of ActRIB receptor (ALK4), which in turn phosphorylates the signaling intermediates, Smads 2 and 3. The phosphorylated receptor-regulated Smads then bind the common mediator, Smad4, and the complex translocates to the nucleus, where it binds DNA and modulates transcription of target genes.
FST, a monomeric and highly glycosylated polypeptide, is an endogenous inhibitor of activin. Two secreted FST molecules irreversibly bind an activin subunit dimer with high affinity, thereby blocking the ability of activin to interact with its cognate receptors and activate downstream signaling [8]. FST and activin are produced in many of the same tissues, suggesting that FST provides a finely tuned mechanism for regulating activin activity. While FST binds to activin with high affinity (∼50–450 pM), it has also been shown to bind and inhibit other members of the TGF-β superfamily in mammals including bone morphogenetic proteins (BMPs) 4, 5, 6, 7, 11, 15, and GDF-9 as well as myostatin, albeit with much lower affinity (0.5–40 nM) [8–15]. FST binds and inhibits the activity of BMP2 in Xenopus as well [10, 16]. There are three isoforms of FST that vary in their cellular localization [17]. Two isoforms are splice variants produced from the Fst gene. The FST315 isoform contains all six exons and is found primarily in the systemic circulation. FST288 is the smallest isoform, lacking exon 6, thereby losing the C-terminal tail. This truncated isoform, which displays a higher affinity for heparin sulfate proteoglycans on the cell surface, anchors FST to the cell membrane. The third isoform, FST303, is produced by posttranslational proteolytic cleavage and has a shorter C-terminal tail than FST315. This isoform can bind to the cell surface but with lower affinity than FST288. While all three isoforms bind activin with comparable affinities [17], they vary somewhat in tissue distribution.
The in vivo study of the roles of activin and FST in male reproduction has been hampered due to the essential role of each of these molecules in fetal development. Mice with deletion of the activin-βA subunit, ActRIIB (Acvr2b), ActRIA (Alk2), ActRIB (Alk4), or FST die any time from early gastrulation to shortly after birth [18–21]. Disruption of ActRIIA (Actr2a) in mice also causes ∼22% embryonic mortality; however, male mice that survive are fertile albeit with delayed sexual maturation [18]. In contrast, males with disruption of the activin-βB subunit are viable and fertile, suggesting that activin A has a more profound biological impact than activin-βB. Insertion of activin-βB into the locus of activin-βA locus partially rescued neonatal lethality and craniofacial defects of the activin-βA null mice. However, the seminiferous tubule differentiation was delayed in males, testicular volumes were reduced, and fertility onset was delayed [22]. These data indicate that male reproductive function specifically requires activin-βA. Furthermore, mice with targeted deletion of activin-βA in fetal Leydig cells disrupts testis cord elongation and expansion, culminating in low sperm production and testicular pathology in adulthood [23].
Transgenic mice have also shed light on the role of activin and FST in male reproduction. Overexpression of the activin-βC subunit in Sertoli cells blocks activin A activity and causes male infertility [24]. Overexpression of FST or its related protein follistatin-like 3 (FSTL3) was expected to phenocopy the surviving ActRIIA-deficient male mice. However, adult FST transgenic males display decreased fertility and reduced testicular weight [25]. Similarly, transgenic mice that overexpress FSTL3 under control of the Inha promoter in Sertoli cells are subfertile, with decreased testicular size [26]. These studies confirm that activin signaling in the testes is essential for normal function.
While transgenic mice have been helpful in elucidating the reproductive ramifications of altered activins and FST in the testes, the roles that these two molecules play in excurrent ducts (efferent ducts and epididymis) remain unknown. The epididymis is a highly convoluted duct that connects the rete testis and efferent ducts to the vas deferens and provides a complex environment for the maturation and transport of sperm. The epididymis, along with the efferent ducts, resorbs testicular fluid, differentially endocytosing some luminal fluid-borne proteins while secreting new ones [27–29], thus imparting on sperm the ability to move and fertilize an egg. This sperm maturation [30] occurs before sperm reach the cauda epididymidis, where they are stored until ejaculation. Nearly 90% of this resorption occurs in the efferent ducts, and, on a per sperm basis, the amount of protein present in the central caput epididymidis is less than 15% of that leaving the testis. The resorption of fluid and differential endocytosis in the efferent ducts and along the length of the epididymis are essential for fertility, as perturbation of these processes leads to retention of fluid, elevated pressure in the testis, and impaired spermatogenesis [30–32]. Endogenous expression of FST and activin-β subunits have been documented in the epididymis in humans, primates, pigs, and mice and in some cases is found at levels higher than in the testes [33–35]. While little is known regarding activin receptor localization in the adult epididymis, ActRIIB and phosphorylated Smads 2/3 are present in the Wolffian epithelium, suggesting that activin signaling is operational during epididymal development [36]. Indeed, activin-βA subunit is essential for mouse epididymal coiling, substantiating the importance of activin in excurrent ducts [37].
Herein, we describe a novel murine model that overexpresses follistatin in the testes and epididymides using the mouse mammary tumor virus (MMTV) promoter [38]. Breeding attempts with male founder mice revealed a dramatic infertility phenotype. Characterization of this phenotype unveils an essential role for FST/activin homeostasis in maintaining excurrent ductal function and reproductive performance.
MATERIALS AND METHODS
Generation of MMTV-Follistatin Transgenic Mice
The MMTV-LTR promoter was obtained from Kay-Uwe Wagner [38] and was cloned with HindIII into pBluescript SK+ containing the bovine growth hormone intron/poly A (bGHpolyA). The mouse Fst gene, from Martin Matzuk [25], was digested with XbaI, and the ends were blunted with Klenow and ligated into the MMTV-bGHpolyA-pBluescript vector with EcoRV. The signal peptide of FST located in exon 1 is intact in this construct; hence, an increase in FST secretion is expected from all cells capable of activating the MMTV promoter. The MMTV-Fst transgene was microinjected into fertilized oocytes by the Case Transgenic and Targeting Facility. Mice were genotyped by PCR with primers specific to the Fst gene (forward 5′-CTTAACGCAGTTGCCACCTA-3′) and the 3′ end of the Fst gene overlapping with the bGHpolyA tail (reverse 5′-AAGCAGGAGAGCAGACCGTA-3′). Once MMTV-Fst transgenic males were determined to be infertile, female founders were bred to FVB/N males (Jackson Laboratories) to expand lines. The original male transgenic founders as well as offspring from three independent transgenic lines generated from female founders (designated as lines A, B, and C) were used to study the extent of the infertility in MMTV-Fst males. Age-matched, nontransgenic male littermates were used as wild-type (WT) controls for all experiments. Mice were housed in microisolator cages under pathogen-free conditions with a 12-h light/dark cycle and provided food and water ad libitum. All animal studies were approved by the Case Western Reserve University or Colorado State University Institutional Animal Care and Use Committees.
Fertility Evaluation
At 8.5 wk of age, 17 MMTV-Fst founder males as well as five WT males were individually housed in cages with one FVB/N female and monitored for copulatory behavior, presence of vaginal plugs, and litters for 4 wk, during which time all WT males produced offspring. FVB/N females were then randomly collected from the breeding cages of the five MMTV-Fst males as well as the five WT males, and the females were monitored for approximately 4 wk to ensure that they were not pregnant. The five females that conceived in the WT breedings, now considered proven female breeders, were placed in the cages with MMTV-Fst transgenic founders and monitored for an additional 4 wk for the presence of litters. The original females from the MMTV-Fst matings were housed with the WT males and also monitored for the generation of litters.
For superovulation studies, 3-wk-old FVB/N females (Jackson Laboratories) were superovulated using a previously described equine chorionic gonadotropin (eCG; Calbiochem) and human chorionic gonadotropin (hCG; Wyeth-Ayerst Laboratories Inc.) regimen [39]. Five MMTV-Fst and three WT males were each housed with one superovulated female on two separate occasions, and breedings were monitored until pups were born.
Serum Hormone Analyses
Whole blood was collected from the retro-orbital sinus of 6- to 10-mo-old mice under avertin anesthesia as well as from cardiac puncture at the end of the study. Blood was clotted overnight at 4°C, centrifuged, and serum collected. Serum was assayed for luteinizing hormone (LH) by sandwich immunoradiometric assay and testosterone and FSH by radioimmunoassay at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Organ Weights and Histology
Mice (aged 6–10 mo) were weighed and killed by CO2 asphyxiation, and blood was collected by cardiac puncture. Testes and seminal vesicles were dissected and weighed. Testis and epididymis from one side were fixed in 4% paraformaldehyde for 4 h and transferred to PBS. Fixed tissue was embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). For FST immunostaining (IHC), sections were deparaffinized and rehydrated, and antigen retrieval was performed in 10 mM sodium citrate buffer, pH 6.0, for 20 min using a decloaking chamber (Biocare Medical). After quenching endogenous peroxidases and blocking in TBS-Tween-20 plus BSA, the primary antibody, goat α-FST (R&D Systems [AF669]) or IgG as a negative control, was applied at 2 μg/ml overnight at 4°C. Bound antibody was detected the following morning with the Vector Elite ABC kit (Vector Laboratories) using 3,3′-diaminobenzidine. Immunostaining for the estrogen receptor (ER; Santa Cruz [MC-20]) was performed as previously described [40]. Sections were counterstained with Gills #3 hematoxylin (Fisher), dehydrated, and mounted in Permount (Fisher). Goat or rabbit IgG (Jackson ImmunoResearch Inc.) was substituted for the primary antibody as a negative control. Images were captured using a Nikon microscope equipped with Zeiss AxioCam HRc imaging system.
RNA Analysis
RNA was isolated from brain, heart, liver, lung, kidney, testes, epididymides, and efferent ducts of 6- to 10-mo-old mice using Trizol Reagent (Invitrogen) and DNase-treated (DNA-Free; Ambion). cDNA was generated using Superscript II and random hexamers (Invitrogen) as per the manufacturer's protocol. Quantitative real-time PCR was performed on an Applied Biosystems Step One Plus Real-Time PCR system using TaqMan Gene Expression Assays for mouse Fst (Mm00514982_m1) and Esr1 (Mm01191130_m1) and normalized to Gapdh (4352932-0804021).
Sperm Evaluation
The left testis and epididymis were removed from anesthetized (ketamine/xylazine) 7-mo-old males after examination of visceral organs. Three FST-expressing and two WT littermates from line A and two FST-expressing and one WT littermate males from line B were used. The epididymis was dissected from the left testis, and individual organs were weighed. The epididymis was placed in 1.0 ml of PBS (300 mOsm; prepared with endotoxin-free H2O), and the cauda was fenestrated with a 25-gauge needle. After a 5-min incubation at 37°C, 5% CO2, a 50-μl epididymal fluid sample was used to assess total motility using a video microscope. An additional 50 μl epididymal fluid sample were fixed in 0.25 ml of 10% buffered formal saline to assess sperm morphology (500× magnification) and sperm counts using a hemocytometer (125× magnification) with a phase contrast microscope. When available, 200 sperm, from each male were classified as morphologically normal or abnormal, with abnormalities categorized as abnormal head shape, the presence of a retained cytoplasmic droplet, reflected midpiece, and detached (tailless) heads [41].
Transmission Electron Microscopy
After removal of the left testis and epididymis for light microscopic evaluation, the anesthetized animals were perfused with 2% glutaraldehyde, and the right testis and epididymis were collected. Four samples of tissue, two from the testes representing the proximal pole adjacent to the efferent ducts and middle portions, one from efferent ducts/initial segment of the epididymis, and one from cauda epididymidis, were postfixed in 1% osmium tetroxide in cacodylate buffer (0.1 M) and embedded in Poly/Bed 812 (Polysciences). A series of 1-μm-thick sections of testes and epididymides were cut (n = ∼30–50 sections/animal), stained with toluidine blue, and evaluated using a Nikon Microphot FXA microscope (equipped with plan apochromatic, bright-field/differential interference contrast optics) interfaced with a computerized imaging system. Images were captured using ImagePro software (Media Cybernetics, Inc.). Thin sections (60–80 nm) were cut from Poly/Bed-embedded tissues and stained with uranyl acetate and lead citrate and examined using a transmission electron microscope (JEOL-1200EX; JEOL USA, Inc.).
Statistical Analyses
The data are expressed as means ± standard deviations. Statistical analyses were performed using a one-way analysis of variance, followed by Student t-test. A minimum P-value of <0.05 was considered statistically significant.
RESULTS
Male MMTV-Fst Transgenic Founders Are Infertile
Thirty-two transgenic founder mice (17 males and 15 females) were generated with the WT mouse Fst gene driven by the MMTV-LTR promoter. All transgenic mice were physically indistinguishable from WT littermates, with similar body sizes and normal hair and tooth development. MMTV-Fst males were initially housed with one FVB/N female to expand lines, but no litters were produced (see breeding schemes in Fig. 1). In contrast, MMTV-Fst founder females were mated with WT FVB/N males and uneventfully produced litters. Hence, transgenic females were used to expand several lines of transgenic mice for additional study. No further fertility studies were performed with the females. A more detailed assessment of the mammary gland phenotypes of MMTV-Fst females is in progress. To further investigate the conspicuous infertility of MMTV-Fst males, all 17 transgenic founder males were mated with WT FVB/N females. As a positive control, five of the WT founder males were also bred to WT FVB/N females. While all five of the WT founder males produced at least one litter of pups, only 1/17 (6%) transgenic founder males yielded any litters despite the observation of vaginal plugs and normal male copulatory behavior (Table 1). To exclude the possibility that the WT females used for this study were infertile, we replaced five of the females housed with the MMTV-Fst founder males with the five proven females that had successfully generated litters when mated with WT males. In addition, five females from the MMTV-Fst breedings were placed with WT males. Again, all five of the WT males produced litters, while 0/5 of the MMTV-Fst males sired offspring (Table 1). To further confirm that the MMTV-Fst male mice were infertile, five MMTV-Fst males and five WT males were mated with superovulated females. Again, no litters resulted from MMTV-Fst males, while all three of the WT males generated litters (Table 1). This superovulation experiment was repeated once with the same outcome. From these data, we conclude that the Fst gene driven by the MMTV-LTR promoter results in a defect that renders male mice infertile. Male offspring of the female founder breedings were also confirmed to be infertile, indicating that the infertility phenotype was heritable and restricted to males (Fig. 1B).
FIG. 1. .
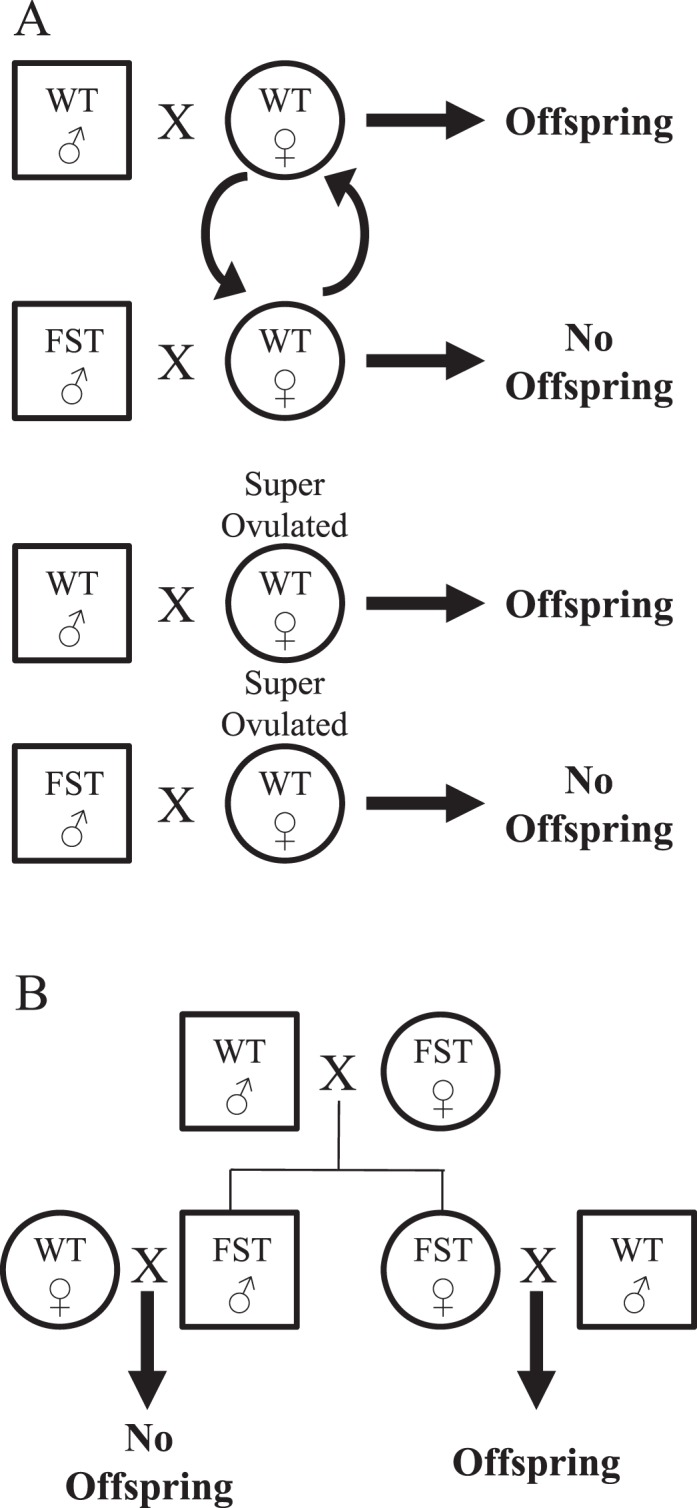
Schematic of the breeding paradigms used to assess infertility in MMTV-Fst males. A) Breeding paradigm used to determine infertility in founder males. Curved arrows indicate that proven females replaced females that had not yielded litters. B) Breeding paradigm used to determine that the male infertility phenotype was heritable and limited to males.
TABLE 1. .
Detailed summary of mating experiments and outcomes.
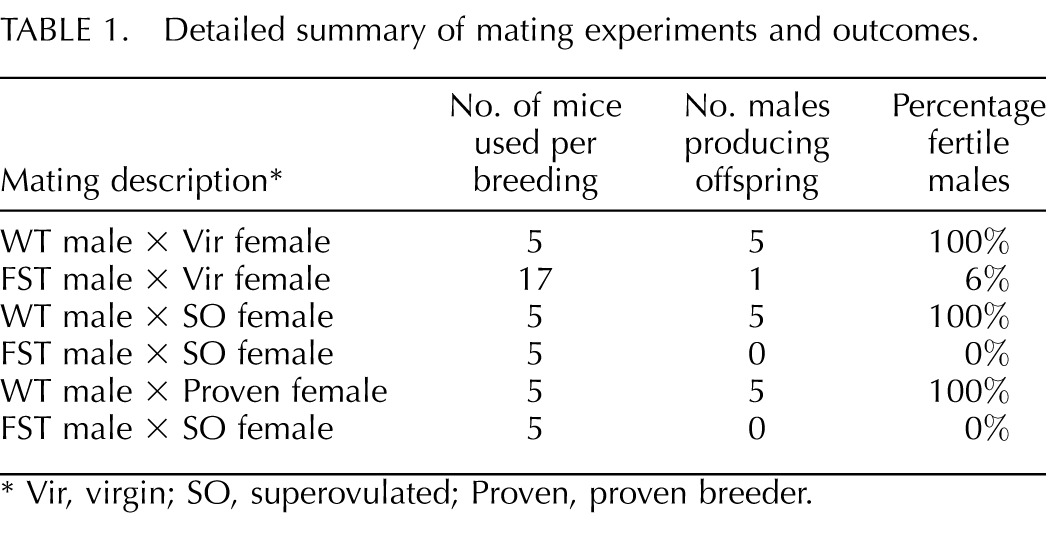
Vir, virgin; SO, superovulated; Proven, proven breeder.
Infertility in MMTV-Fst Males Is Not Due to Systemic Disruption of Pituitary Gonadotropins or Testosterone
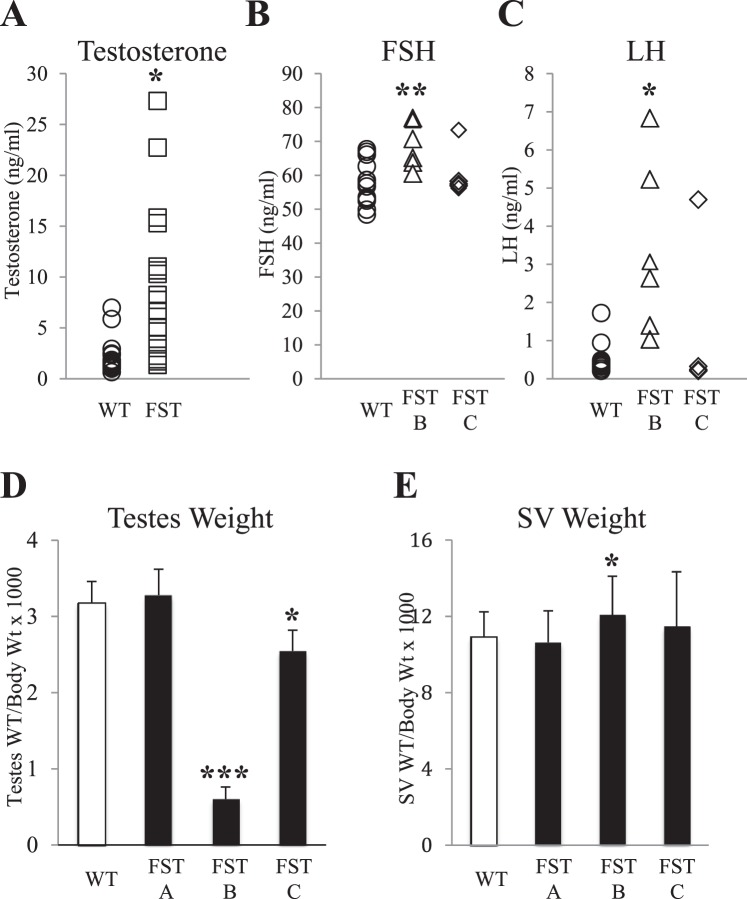
Activin plays an essential role in regulating the synthesis and release of FSH from the pituitary gland and subsequent steroidogenesis. Serum testosterone and FSH were quantified in adult MMTV-Fst founders or F1 progeny from female founders (lines FST-A and FST-B) and their WT littermates (Fig. 2A). While some of the MMTV-Fst males exhibited elevated levels of testosterone, many of the founders had levels within the normal range but were infertile. Similarly, circulating FSH levels in infertile F1 males were only modestly elevated, and most males were within the normal range (Fig. 2B). Moreover, while LH levels were elevated to a greater extent in some MMTV-Fst mice, several infertile males also maintained normal levels (Fig. 2C). The changes in serum gonadotropins or testosterone were not consistently altered in infertile males, indicating that endocrine dysfunction was not the primary cause of infertility.
FIG. 2. .
Many infertile MMTV-Fst males have serum hormone levels and testes and seminal vesicle weights that are identical to WT males. A) Serum testosterone levels in WT (n = 16) and MMTV-Fst (n = 17) male founder mice (*P < 0.05). B) Serum FSH in WT and MMTV-Fst F1 offspring from transgenic lines B and C. WT littermates from both transgenic lines are combined (n = 14), FST B (n = 6; **P < 0.01), FST C (n = 4). C) Serum LH in WT and FST F1 offspring from transgenic lines B and C. WT littermates from lines B and C are combined (n = 14), FST B (n = 6; *P < 0.05), FST C (n = 4). D) Testes weight relative to body weight in FST lines A (n = 3), B (n = 5; ***P < 0.001) and C (n = 6; *P < 0.05) and WT littermates combined from all three lines (n = 20). E) Seminal vesicle weight relative to body weight in FST lines A (n = 3), B (n = 5; *P < 0.05) and C (n = 6) and WT littermates combined from all three lines (n = 20).
Infertility Is Not Consistently Associated with a Decrease in Testis Weight
Previous studies have shown that disruption of activin signaling via ligand manipulation or enhanced FST or FSTL3 expression within the testes impacts testes weights [24–26]. The testes and seminal vesicles in adult WT and MMTV-Fst transgenic mice were weighed, and, consistent with serum hormone levels, organ weights were variable both within and between infertile transgenic lines (Fig. 2, D and E). No differences in body weights were observed between WT and transgenic mice in any line examined (data not shown). Testes weights were decreased by as much as 80% in line B, not changed in line A, and intermediate in line C (decreased 17%), even though all mice examined from these lines were infertile. It is expected that a mere accumulation of testicular fluid might initially increase the organ weight, whereas the degenerative changes in the seminiferous epithelium (which lead to fibrosis over time) consequent to impediment of fluid resorption and transit might ultimately decrease the weight. Interestingly, while reports have shown inverse correlations between testicular FST transgene expression and testicular weights [25, 26], this did not occur with targeted expression of FST driven by the MMTV promoter (Fig. 3A). This may simply be a matter of the time at which the parameter is measured relative to the time line of the progression of testicular lesions.
FIG. 3. .
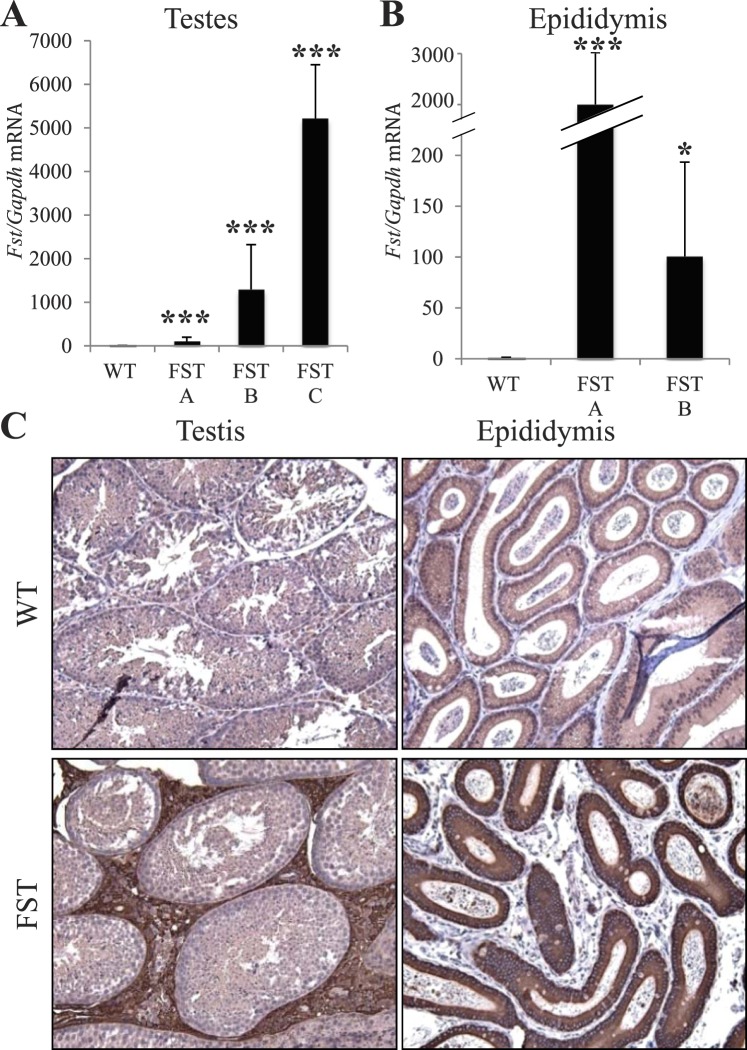
FST overexpression occurs in the Leydig cells of the testes and principal cells of the epididymis of MMTV-Fst mice. A) Quantitative real-time PCR from RNA isolated from whole testes comparing WT (n = 13) and FST offspring from lines A (n = 4), B (n = 4), and C (n = 4). ***P < 0.001 for all three lines compared to WT controls. B) Quantitative real-time PCR of RNA isolated from epididymides comparing littermate WT (n = 6) and FST Lines A (n = 3; ***P < 0.01) and B (n = 3; *P < 0.05). C) Immunohistochemistry utilizing anti-FST antibody reveals FST overexpression (brown stain) is localized to Leydig cells of the testes and principal cells of the epididymis. Original magnification for all images ×200.
Male fertility is directly linked to Sertoli cell number, which is established during postnatal development [42]. Together with FSH, activin stimulates the initial wave of Sertoli cell proliferation between Postnatal Days 4 and 9 in rodent testes [43–46]. To determine if FST overexpression in the testes could abrogate the FSH/activin-induced initial wave of Sertoli cell proliferation and lead to a smaller testicular weight, we harvested testes from Postnatal Days 5 and 8 of WT and MMTV-Fst pups and evaluated transgene expression and relative testicular size. FST protein was below the level of detection by IHC in the postnatal testes of transgenic mice, and the size of the transgenic testes was indistinguishable from WT mice at both days examined (data not shown). This indicates that the transgene is inactive at these days and that early suppression of Sertoli cell number is unlikely to be the underlying cause of the reduced testicular weights or the infertility of adult MMTV-Fst male mice. Indeed, smaller testicular size and reduced sperm counts have been documented in FSH-β and ACTRIIA null mice, while both mouse models remain fertile [18, 47]. Seminal vesicle weights were also compared between adult WT and MMTV-Fst mice and surprisingly are only modestly elevated in one of the three transgenic lines examined (line B, +30%) despite an overall elevation in serum testosterone levels (Fig. 2E). As observed for testes weight, serum gonadotropin levels, and testosterone levels, seminal vesicle weights for many infertile mice were similar to those of WT mice.
FST Is Overexpressed in the Testes, Efferent Ducts, and Epididymides of Juvenile and Adult Transgenic Mice
We next evaluated the level and localization of transgene activity in the male reproductive tract. F1 MMTV-Fst transgenic males had elevated levels of Fst mRNA in the testes compared to WT male littermates (Fig. 3A). Fst mRNA was also increased in the epididymides (Fig. 3B) and in the efferent ducts (Supplemental Figure S1; all Supplemental Data are available online at www.biolreprod.org). Immunohistochemical staining revealed that FST protein was similarly elevated in both the testes and the epididymides (Fig. 3C). Specifically in the testes, FST overexpression was localized to the Leydig cells, in agreement with previous reports of MMTV-LTR promoter activity [48]. In the epididymides, the MMTV promoter targeted overexpression to the principal epithelial cells. In addition, we observed a range of FST expression in other organs of the adult male transgenics from line FST C, including brain, heart, liver, lung, and kidney (Supplemental Figure S2). Given that the FST transgene was not active in neonatal testes (data not shown), we determined if FST was overexpressed in the testes and epididymides of juvenile males at Postnatal Days 20 and 24. Fst mRNA was elevated at both time points in the testes, efferent ducts, and epididymides of juvenile transgenic mice (Supplemental Figure S3), suggesting that FST overexpression during postnatal development may precipitate a cascade of aberrations culminating in the infertility phenotype observed in adults.
FST Overexpression Causes Fluid and Sperm Accumulation in the Testes
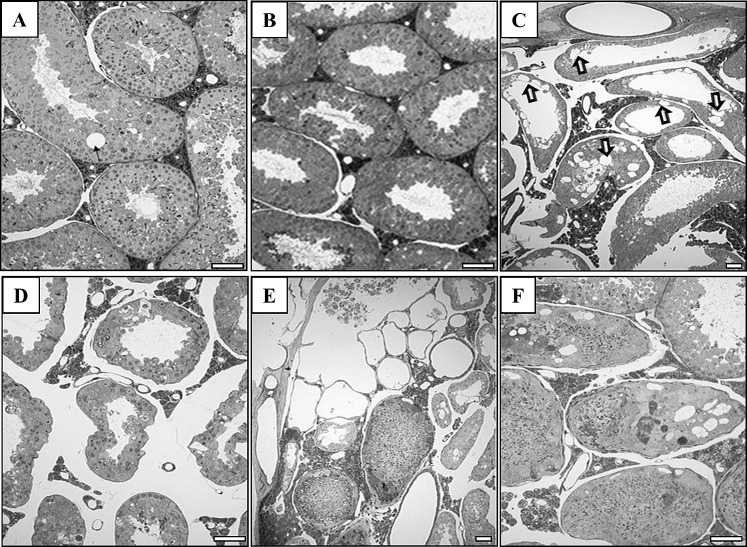
To further characterize the effects of elevated FST in the testes and epididymides, we examined the histological features of both organs. H&E-stained sections of WT testes exhibited normal spermatogenesis in the seminiferous epithelium except for focal vacuolation, which is not uncommon even in a healthy tubule (Fig. 4A). In contrast, while normal spermatogenesis was evident in some seminiferous tubules of MMTV-Fst transgenic mice (Fig. 4B), a spectrum of testicular lesions were evident. These included extensive germinal cell death and desquamation, seminiferous epithelial vacuolation, and interstitial edema causing large spaces between the seminiferous tubules (Fig. 4, C and D). Other tubules with evidence of degeneration manifested sperm stasis and mineralization (Fig. 4, E and F). Engorged (fluid-filled) rete tubules, some containing denuded cells, were evident (Fig. 4E). Together, these observations indicate that overexpression of FST induces an accumulation of fluid that leads to the pressure buildup and consequent degeneration of seminiferous epithelium. Importantly, FST overexpression does not appear to disrupt spermatogenesis as a result of a global hormonal defect because all stages of spermatogenesis were observed in some regions of the testes.
FIG. 4. .
FST overexpression leads to a spectrum of testicular lesions consistent with a fluid reabsorption defect in the excurrent ducts. A and B) Representative 1-μm-thick testicular sections from WT (A) and MMTV-Fst (B) mice displaying normal morphology. C–F) A variety of lesions are evident in the epithelium of seminiferous tubules and rete testis of transgenic MMTV-Fst mice. Extensive vacuolation in seminiferous epithelium (C; arrows), interstitial edema (D), fluid-engorged rete testis with denuded seminiferous epithelial elements in two rete tubules and sperm stasis in adjacent seminiferous tubule cross sections (E), and seminiferous epithelial vacuolation, sperm stasis, and mineralization (F). Toluidine blue staining. Bars = 40 μm (A, B, D, and F) and 20 μm (C and E).
Epididymal Sperm Transit and Fluid Reabsorption Are Impaired in MMTV-Fst Transgenic Mice
A primary function of the rete testis, efferent ducts, and the initial segment of the epididymis is resorption and transport of luminal fluids exiting the testes. We postulated that the excessive fluid accumulation in the testes of MMTV-Fst males was the result of aberrant function of either fluid resorption or contractility in proximal excurrent ducts, predominantly the efferent ducts. Hence, we examined H&E-stained sections of the excurrent ducts of MMTV-Fst and WT littermate mice by light microscopy. In WT mice, there was no evidence of fluid accumulation or sperm stasis in the efferent ducts (Fig. 5A). In addition, WT mice had normal spermiation and sperm transit, as reflected by the normal luminal contents of the cauda epididymidis (Fig. 5C). In contrast, accumulation of sperm in the efferent ducts was evident in MMTV-Fst mice (Fig. 5B), indicating impaired fluid resorption and transit or contractile function. Reflecting the degenerative changes in the testes of MMTV-Fst mice, degenerate prematurely shed germ cells were evident in the lumina of the cauda epididymidis (Fig. 5D) and distal corpus/proximal cauda (Fig. 5, E and F). The scarcity of tail-intact sperm was apparent in these epididymal regions of MMTV-Fst mice (Fig. 5, D–F). There was also an outpouching, or cystic formation, in the epithelial lining of the efferent ducts (arrow in Fig. 5B) in one of the transgenic mice examined. Collectively, this histopathological profile indicates that overexpression of FST leads to impaired fluid resorption and/or contractility of the efferent ducts and initial epididymal segment, resulting in fluid accumulation, cystic outpouchings in excurrent ducts, and seminiferous epithelial degeneration.
FIG. 5. .
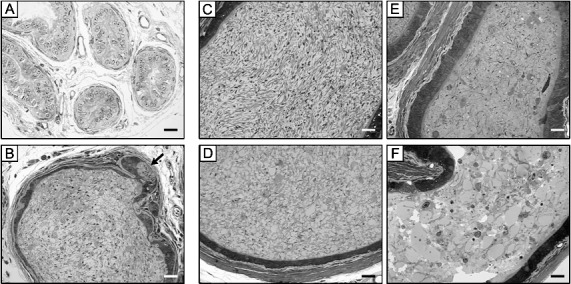
Fluid resorption and sperm transit are impaired in the testicular excurrent ducts of MMTV-Fst mice. Representative 1-μm-thick sections of the efferent ducts (A and B), caudae epididymidis (C and D) and distal corpora/proximal caudae epididymidis (E and F). A and C: WT. B and D–F: MMTV-Fst. In contrast to normal morphological features of efferent ducts (A) and cauda epididymidis (C) in the WT mice, accumulation of sperm in the efferent ducts is evident in MMTV-Fst mice (B); degenerate prematurely shed germ cells are seen both in the efferent duct (B) and in the lumina of cauda epididymidis (D) and the distal corpus/proximal cauda (E and F). Tail-intact sperm are sparse in these epididymal regions (D–F) of MMTV-Fst mice. An adenomyotic outpouching of epithelial lining, or cystic formation (arrow in B), is seen in this section of efferent duct from a MMTV-Fst mouse. Toluidine blue staining. Bar = 20 μm.
FST Overexpression Causes Detachment of Sperm Heads and Nuclear Defects
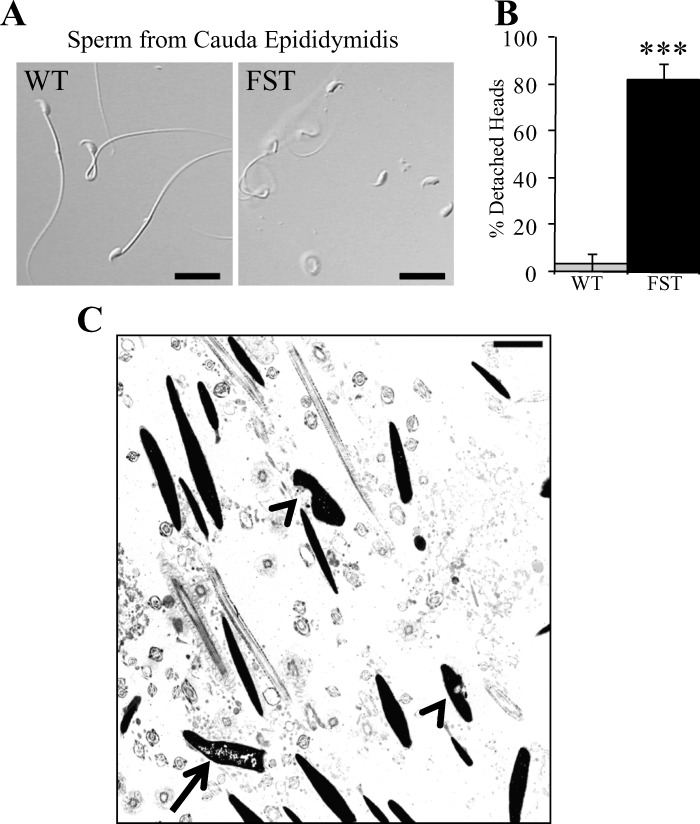
Modulation of activin ligand availability and receptors or alteration of FST within the testes leads to defects in spermatogenesis, sperm numbers, and sperm motility [24–26]. As indicated above, normal spermatogenesis was evident in some seminiferous tubules of MMTV-Fst transgenic mice (Fig. 4B). This indicates that spermatogenesis can proceed despite the locally elevated FST in the Leydig cells and that FST inhibition of activin or BMP ligands does not directly impact spermatogenesis. However, H&E sections of the caudae epididymidis revealed marked sperm degeneration (Fig. 5, D–F). To characterize the extent of sperm abnormalities, sperm was harvested from the caudae epididymidis of WT and transgenic mice and examined for motility and morphology. Sperm were present in all but one MMTV-Fst male that had only a single, intact, but immotile sperm in the sample examined. Less than 1% of the sperm from the MMTV-Fst males were motile, whereas the WT males demonstrated normal (45–75%) motility. The percentage of morphologically normal sperm were also significantly lower in the MMTV-Fst samples as compared to WT samples (3.4% vs. 24.5%, respectively), the predominant abnormality being a significant increase in the incidence of detached heads (Fig. 6, A and B). Because of the high frequency of detached (tailless) heads, it was not possible to determine the incidence of retained cytoplasmic droplets and reflected midpieces in MMTV-Fst males as compared to WT males. In addition, transmission electron microscopy of cauda epididymal sperm revealed that a few sperm nuclei in MMTV-Fst mice had impaired chromatin condensation and nuclear craters (Fig. 6C). While it is possible that FST has a role in spermiogenesis, this defect in sperm chromatin condensation along with the majority of sperm defects (i.e., detachment of heads and tails) observed in the FST transgenic mice are consistent with blockade of efferent ducts and consequent degeneration of seminiferous epithelium.
FIG. 6. .
FST overexpression results in decreased sperm motility consequent to detachment of sperm heads and tails and also causes ultrastructural defects in sperm nuclei. A) Differential interference contrast images of sperm harvested from caudae epididymidis from WT (left) and MMTV-Fst (right) mice. Bars = 20 μm. B) Graph showing incidence of detached sperm heads (***P < 0.001). C) Transmission electron micrograph of sperm showing defects in chromatin condensation (arrow) and nuclear craters (arrowheads). Bars = 20 μm (A) and 2 μm (C).
Epididymal Fluid Resorption and Sperm Transit Are Impeded in FST-Overexpressing Mice
Proximal excurrent ducts (efferent ducts and initial segment of the epididymis) were examined using transmission electron microscopy (Fig. 7). In efferent ducts of WT mice, both ciliated and nonciliated cells had normal apical specializations: junctional complexes between both cell types, cilia in ciliated cells, microvilli and elaborate endocytotic apparatus with coated pits, and tubulovesicular system in the nonciliated cells (Fig. 7, A and B). In addition, the lumen was clear, with the presence of an occasional sperm profile, as sperm transit in this region is normally very swift. Tight junctions between epithelial cells were indicative of epithelial integrity. In contrast, sperm had accumulated in the lumina of efferent ducts of MMTV-Fst transgenic mice (Fig. 7, D and E). The pressure resulting from the accumulated luminal contents caused the microvilli to be compressed and flattened. The compacted epithelial cytoplasm provided further evidence of increased ductal pressure. In addition, active phagocytosis of cellular debris (presumably fragmented sperm nuclei) was evident in the form of phagosomes. Endocytotic pits and vesicles were loaded, indicating that the transcytosis pathway was saturated with material. On the basal aspect of the transgenic efferent ducts (Fig. 7F), the lamina lucida of the basal lamina (also called lamina rara; the zone closest to the epithelium that contains laminin, heparan sulfate, and cell surface integrins) was abnormally thickened with inclusions compared to the same component in the WT mice (Fig. 7C). Likewise, the lamina densa underlying the lamina lucida was also thickened, indicating ineffective basal transcytosis and fluid uptake by the lymphatics and blood vessels. Together these observations demonstrate that overexpression of FST in the efferent ducts causes sperm stasis due to impaired fluid resorption and/or contractility.
FIG. 7. .
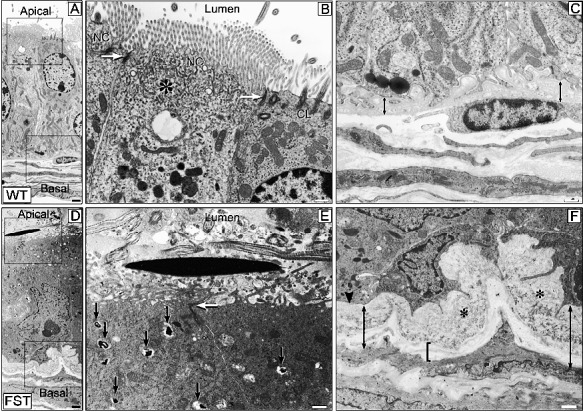
Transmission electron micrographs of efferent ducts in WT and MMTV-Fst mice. WT (A–C), MMTV-Fst (D–F). A and D) In contrast to the normal nonciliated (NC) and ciliated (CL) epithelial cells in the WT efferent duct, the ultrastructural organelles of these cells in the FST mouse appear to be compacted and abnormal even at this low magnification. The boxed areas of representing apical and basal aspects of the epithelium are magnified in B and C and E and F, respectively. Whereas the lumen is clear and apical specializations—microvilli and cilia—appear freely protruding into the lumen in the WT (A), these structures are compressed by the accumulated luminal contents in the FST efferent duct (D); a longitudinal section of a sperm head is seen. B and E) An elaborate endocytotic apparatus consisting of coated pits and tubulovesicular system (asterisk) is seen in the WT (B), whereas this apparatus is loaded with cellular debris and phagosomes are clearly seen (arrows) in the FST duct (E). The apical tight junctions between the epithelial cells (arrow) do not appear to be compromised in the FST mouse. C and F) On the basal aspect, the basal laminae are abnormally thickened in FST (F) compared to WT (C). In the lamina lucida, the zone closest to the epithelium (double-headed arrow), FST mice exhibit abnormal inclusions (asterisks). The lamina densa underlying the lamina lucida (bracket) is also thickened in FST, whereas it is barely discernible in the WT duct. These abnormally thickened basal laminae impede transcytosis of luminal fluid toward the lymphatics and microcirculation in the lamina propria. Bars = 1 μm (A and D) and 500 nm (B, C, E, and F).
Estrogen Receptor-α Is Present in the Testes and Epididymides of FST Transgenic Mice
Estrogen receptor-α (ER-α)-null mice exhibit morphological defects in the efferent ductules similar to those observed in the FST transgenic mice [49–51]. To determine if ER expression was maintained in the presence of elevated FST, we performed IHC for ER in both the testes and the epididymides of WT and MMTV-Fst mice. ER-α was abundantly present in the Leydig cells of the testes of FST transgenic mice (Fig. 8A). While ER was also distinctly present in the epididymis of MMTV-Fst and WT mice, levels were highly variable. In addition, the qualitative nature of IHC precluded determining if the level of expression was altered in various regions of the epididymis between the mouse strains. To determine if the level of Esr1 mRNA was altered in the entire epididymis of transgenic males, quantitative real-timePCR of total mRNA was used. This revealed that Esr1 mRNA levels were diminished by twofold in the two transgenic lines examined (Fig. 8B). In addition, Esr1 mRNA was downregulated in the efferent ducts of adult FST overexpressing mice (Supplemental Figure S4A) despite being unchanged or slightly elevated in juvenile mice at Postnatal Days 20 and 24 (Supplemental Figure S4, B and C) These data suggest that ER-α is suppressed by FST overexpression in the principal cells of the epididymis and efferent ducts and that its dysregulation may, in part, contribute to infertility of FST transgenic males.
FIG. 8. .
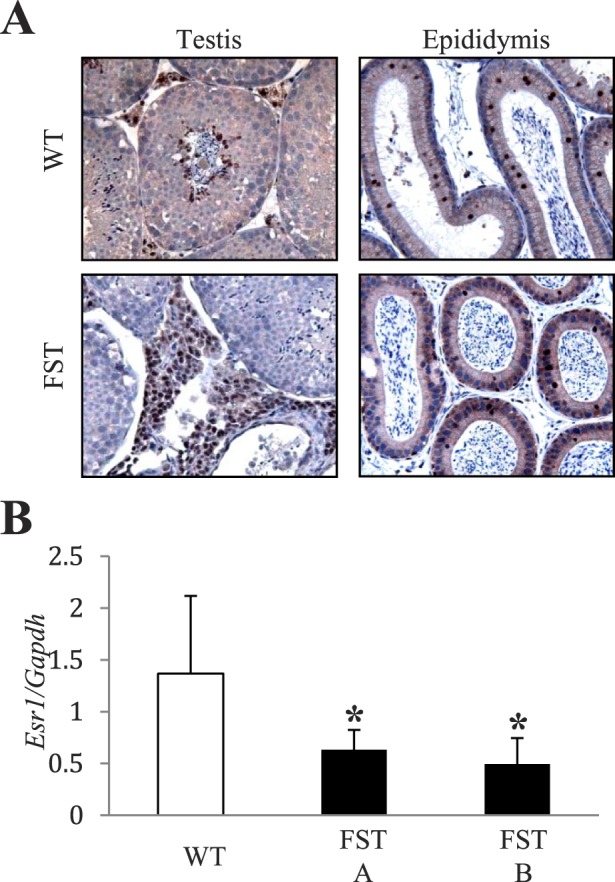
ER-α expression is downregulated in the epididymis as a consequence of FST overexpression. A) Representative images of immunohistochemistry utilizing antibody against ER on sections from the testes and epididymides of WT and MMTV-Fst mice. Original magnification of images ×200. B) Quantitative real-time PCR from RNA isolated from total epididymides of WT (n = 7) and FST line A (n = 3, *P < 0.05 compared to WT) and line B (n = 4, *P < 0.05 compared to WT).
DISCUSSION
While overexpression of FST or FSTL3 in the testes leads to testicular dysfunction and infertility, there are no studies to date examining the functional role of FST or its preferred target, activin, in the epididymis. The MMTV-Fst mice described herein have elevated FST in the testis and epididymis and the lesions noted in these mice suggest either: 1) a disruption of resorption of fluid entering the efferent ductules from the rete testis, 2) an impairment of rhythmic segmental contractions beginning in the initial segment, or 3) a distal blockage of sperm transit. The ultrastructural changes in the efferent ducts as well as the diminished expression of Esr1 indicate a disruption of fluid resorption in the efferent ducts. To our knowledge, this is the first demonstration that FST regulates fluid resorption in the efferent ducts of mice.
Not surprisingly, male mice with overexpression of FST driven by the metallothionin promoter (herein referred to as MT1-Fst) yielded a slightly different phenotype than the MMTV-Fst mice described here [25]. MT1-Fst mice were smaller and had shiny, irregular fur, while the MMTV-Fst mice were indistinguishable from WT littermates. Both MMTV-Fst and MT1-Fst males had reduced testicular weights in some of the lines, although only one of the five MT1-Fst transgenic lines had 100% infertility of male offspring. In addition, FSH levels were only slightly dampened in one of the MT1-Fst lines, while FSH levels were in the normal-to-elevated range in the MMTV-Fst mice. These discrepencies can be explained largely by the pattern of expression of the different promoters that were used to create each of these models. MT1-Fst mice not only have FST overexpression in the Sertoli cells but also have robust expression in the liver and skin. High systemic levels of FST were indicated by the smaller size and fur defects of these mice. In contrast, MMTV-Fst mice targeted transgene expression to the Leydig cells but also in the efferent ducts and epididymides. Although the transgene also resulted in high expression of FST in additional tissues, no changes in weight or fur were observed, indicating that the FST expression did not have broad-ranging effects on the mice. It is possible (but unknown) that the MT1-Fst mice also had overexpression in the epididymides. If so, this may have also contributed to the testicular degeneration described in those mice. Similar testes and epididymal phenotypes have been described in mice null for full-length Esr1 as well as truncated and mutated forms of its associated protein [49, 50, 52–54]. These mice are also infertile, have similar testicular lesions, and retain fluid in the efferent ducts. Expression of ER-α begins around Embryonic Day 16 in the epididymis and is expressed throughout adulthood to varying degrees with its highest expression in the efferent ducts [55]. Tracer studies conducted in Esr1 knockout mice demonstrated that the epithelial cells were capable of endocytosing luminal contents of the efferent ducts; however, the total area of endocytotic apparatus was significantly reduced [56]. Although there is no reported evidence for direct regulation of ER-α by FST, activin does induce expression of both the Esr1 and the Esr2 (ER-β) genes in granulosa cells, and this is reversed by treatment with FST [57]. In this context, activin A activation of the Esr1 promoter is dependent on Smads 2 and 3. Conversely, the same dose of activin A causes a decrease in ESR1 expression in MCF-7 breast cancer cells at 24 h [58], implying that activin regulation of ER transcription may be cell-type dependent. While ER-α is present in the epididymis of the FST mice, its expression is reduced. This reduction may impact expression of solute or fluid transporter target genes, such as SLc9a3 or Aquaporins (AQP) 1, 5, and 9, all of which contribute to fluid homeostasis in the epididymis [59–61] and have been proposed to be direct or indirect targets of ER-α. In addition, FST inhibits AQP5 expression in organ cultures of the submandibular gland, and AQP5 is upregulated by estradiol treatment of the efferent ducts [59, 62, 63]. Whether AQP5 is downregulated in the Esr1 knockout mice has not yet been reported. Of note, AQP1 and AQP9 are expressed on either the apical or the basolateral surface of epididymal epithelium, while AQP5 is colocalized to endosomal membranes [64]. Future studies are necessary to determine if FST regulates expression of AQP5 in the epididymis and whether such regulation is modulated by ER.
While we expect that the reduction in ER levels observed in FST mice is due to specific inhibition by FST, it is also possible that ER is subject to autoregulation. Pharmacological levels (75 μg/ml) of systemic estradiol in castrated mice can decrease ER-α to undetectable levels in the efferent ducts, while exogenous administration of testosterone or dihydrotestosterone has no effect [65]. Although we did not determine the circulating estradiol levels in FST mice, it is unlikely that estradiol is elevated to this level to achieve ER-α downregulation. If estradiol levels were within this pharmacological range, additional disruptions of the hormonal milieu would be anticipated. Similarly, if FST were significantly elevated in systemic circulation, disruption of the hypothalamic/pituitary/testis axis should occur because of systemic inhibition of activin. However, we found that most of the FST transgenic mice displayed hormonal profiles similar to WT mice. This suggests that the consistent infertility observed in these animals is unlikely to have resulted from a systemic disruption of the hormonal milieu. Further supporting this notion, the MMTV-Fst mice displayed no overt abnormalities in tooth development or irregularities in the hair, which has been reported for other models in which systemic overexpression of FST was achieved [25, 66]. While not directly assessed in this study, it is also possible that FST increases intratesticular levels of estradiol without increasing systemic levels, and this, in turn, results in high local concentrations of estradiol in the epididymides.
The propensity for FST to bind and inhibit activin suggests that activin may be critical for normal epididymal function. However, FST can also bind and inhibit myostatin and several BMP family members. Myostatin expression is restricted primarily to skeletal muscle and adipose tissue in adults, and homozygous null mice are viable and fertile [67]. Hence, myostatin is unlikely to be involved in the infertility defect observed in the MMTV-Fst mice. Of the many BMP ligands that FST has been shown to block, only BMP7 is found in the epididymis, and its expression is restricted to the initial segment in the mouse after puberty [68, 69]. BMP7 null mice die perinatally, and heterozygous male mice are fertile, with no obvious gonadal lesions [70, 71]. Therefore, it is also unlikely that FST blockade of BMP7 alone would cause male infertility. While FST has not been shown to block the activity of BMP8a, it is one of the members of the 60A subgroup of the BMP family along with BMPs 5, 6, and 7, and many members of this subgroup can be inhibited by FST [72]. BMP8a null mice do have a moderate disruption in the epididymal epithelium. However, all homozygous mutant males were fertile initially, with only 2 of 16 becoming infertile with increasing age [68]. Hence, as with BMP7, FST inhibition of BMP8a alone would not yield the extreme infertility phenotype that is observed in the MMTV-Fst mice. Additional disruption of one allele of Bmp7 within BMP8a null mice results in infertility in approximately 75% of the mice [69]. The compound mutants display a similar phenotype to MMTV-Fst mice with degeneration of the epididymal epithelium along with an accumulation of sperm and subsequent obstruction of the lumen of the initial segment of the epididymis. Interestingly, the infertility phenotype in compound mutants is dependent on the C57BL/6 genetic background and does not occur in mice within a mixed genetic milieu. The MMTV-Fst founder mice described herein are in a combined genetic background of C57BL/6 and SJL mouse strains, while the F1 generation has genetic contributions from the C57BL/6, SJL, and FVB/N strains. The penetrance of the infertility phenotype remains constant in MMTV-Fst males despite the differences in genetics between the founder and F1 generation. This underscores the importance of FST regulation of fluid resorption in the efferent ducts independently of genetic predisposition and supports the notion that inhibition of all signaling from activin in addition to BMP7 and BMP8a may underlie the profound phenotype in MMTV-Fst males.
While FST has not been demonstrated to bind to mammalian BMP8b and BMP4, both factors also impact the epididymis. BMP8b is expressed only in the testis, yet inactivation of the Bmp8b gene also has a functional consequence in the initial segment of the epididymis, suggesting that BMPs as well as activins may serve as luminal fluid cytokines to maintain function of the rete testes and excurrent ducts [68]. BMP4 is expressed ubiquitously in the epididymis throughout postnatal development, and while homozygous null mice die in utero, heterozygous male mice are subfertile/infertile when bred into the C57BL/6 background [73]. These mice have reduced sperm counts and motility along with compromised epithelial integrity of the corpus epididymis. It is unclear if FST can bind and inhibit the function of BMP4 in mammals [10, 74, 75]; however, in Xenopus, the antagonistic affects of FST on BMP4 have been well established [9, 75]. Although disrupting the expression of individual BMPs or activin from the epididymis leads to mild to moderate infertility in mice, the fact that FST overexpression in a mixed genetic background leads to a catastrophic loss of fertility and epididymal epithelial integrity suggests that FST may also inhibit additional BMP function in the efferent ducts.
In MMTV-Fst seminiferous tubules that retain normal morphology, spermatogenesis appears to proceed normally, even in the presence of overexpressed FST in the testes. Similar results were seen in the testes of transgenic mice with FST driven by the metallothionin promoter [25]. This suggests that FST overexpression does not directly cause sperm abnormalities in these mice. While the data presented herein support the postulate that increased pressure/tension in the epididymal lumen leads to mechanical shear forces during sperm transport, there are other factors that could contribute to the observed sperm defects. Sperm chromatin abnormalities can be induced by increasing the temperature of the scrotum to 38°C, and sperm is altogether eliminated in the cauda epididymis if internal temperatures reach 42°C [76]. In addition, luminal acidification is necessary for sperm maturation and maintains immature sperm in an immotile, quiescent state. On admixture with the more alkaline prostatic fluid, motility is restored to achieve maximum mobility at ejaculation [77–79]. Elevation of the luminal pH of the epididymis results in male infertility due to the inability of sperm to reach the female genital tract [80]. In the Esr1 knockout mice, sperm defects were attributed to an increase in pH in addition to altered osmolality of the lumen, which resulted in reduced motility and severe flagellar coiling (also known as the Dag defect) [81, 82]. However, the primary sperm defect in MMTV-Fst mice is not the result of immotility due to premature exit from quiescence, but rather separation of the head from the tail. Mice with FST, FSTL3, or activin C overexpressed in the Sertoli cells have reduced sperm numbers and sperm motility. In contrast, we found that MMTV-Fst mice overexpress FST in the Leydig cells. This permits normal spermatogenesis in the testes, and sperm defects are secondary to the efferent duct blockade [24–26]. Further studies are necessary to elucidate whether the sperm defects in the MMTV-Fst mice are restricted to detachment of heads consequent to fluid buildup resulting in degeneration or if the sperm also suffer from additional deficits not apparent in the assays performed herein.
The ability of FST to induce sperm decapitation and infertility raises the possibility that FST could serve as a male contraceptive. The guiding axiom of current hormonal contraceptive research is to abrogate LH and FSH to inhibit spermatogenesis while at the same time supplementing with testosterone to maintain libido [83]. MMTV-Fst males manifest infertility while testosterone levels and copulatory behavior are maintained, suggesting no impact on male libido, making FST an ideal candidate for male contraception. Further studies are necessary to determine the threshold and timing of epididymal FST that is necessary to induce infertility as well as the reversibility of its effects.
In summary, we show for the first time that male reproductive fitness is dependent on a balance of activin and/or BMP signaling that is modulated by FST in the efferent ductules of the epididymis. Dysregulation of this equilibrium leads to fluid retention and ensuing pathologies of the seminiferous epithelia that culminate in abnormalities of sperm transport and chromatin condensation. This ultimately causes sperm decapitation and complete infertility. FST inhibition of either activin, BMP, or both may be critical for proper epithelial integrity, contractility, and vesicular trafficking of the epididymis. Whether FST alters Esr1 expression through activin or BMP inhibition in the epididymis is still unclear. Further analysis of the contributions of ER-α downregulation to the infertility phenotype of MMTV-Fst mice is necessary to determine if this is the primary mechanism by which FST causes infertility.
ACKNOWLEDGMENTS
The excellent technical expertise of Kristen Lozada in mouse husbandry and Carol Moeller and Jennifer Palmer in transmission electron microscopy are gratefully acknowledged. We also recognize Gina Bernardo for assistance in editing the manuscript. The Case Transgenic and Targeting Facility generated MMTV-Fst transgenic mice and the Tissue Procurement, Histology, and Immunohistochemistry core facility of the Case Comprehensive Cancer Center (P30 CA043703) and ARBL Morphological Services at Colorado State University provided technical services associated with histopathology and microscopy.
Footnotes
Supported by the National Institutes of Health (R01-CA090398) to R.A.K.
REFERENCES
- Ling N, Ying S-Y, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. Pituitary FSH is released by a heterodimer of the [beta]-subunits from the two forms of inhibin. Nature 1986; 321: 779 782 [DOI] [PubMed] [Google Scholar]
- Ueno N, Ling N, Ying SY, Esch F, Shimasaki S, Guillemin R. Isolation and partial characterization of follistatin: a single-chain Mr 35,000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proc Natl Acad Sci U S A 1987; 84: 8282 8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med 2002; 227: 724 752 [DOI] [PubMed] [Google Scholar]
- Davies JA, Davey MG. Collecting duct morphogenesis. Pediatr Nephrol 1999; 13: 535 541 [DOI] [PubMed] [Google Scholar]
- Ball EMA, Risbridger GP. Activins as regulators of branching morphogenesis. Dev Biol 2001; 238: 1 12 [DOI] [PubMed] [Google Scholar]
- Ritvos O, Tuuri T, Eramaa M, Sainio K, Hilden K, Saxen L, Gilbert SF. Activin disrupts epithelial branching morphogenesis in developing glandular organs of the mouse. Mech Dev 1995; 50: 229 245 [DOI] [PubMed] [Google Scholar]
- Cancilla B, Jarred RA, Wang H, Mellor SL, Cunha GR, Risbridger GP. Regulation of prostate branching morphogenesis by activin a and follistatin. Dev Biol 2001; 237: 145 158 [DOI] [PubMed] [Google Scholar]
- Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS. The structure of the follistatin: activin complex reveals antagonism of both type i and type ii receptor binding. Dev Cell 2005; 9: 535 543 [DOI] [PubMed] [Google Scholar]
- Iemura S, Yamamoto TS, Takagi C, Uchiyama H, Natsume T, Shimasaki S, Sugino H, Ueno N. Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc Natl Acad Sci U S A 1998; 95: 9337 9342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology 2006; 147: 3586 3597 [DOI] [PubMed] [Google Scholar]
- Schneyer AL, Sidis Y, Gulati A, Sun JL, Keutmann H, Krasney PA. Differential Antagonism of activin, myostatin and growth and differentiation factor 11 by wild-type and mutant follistatin. Endocrinology 2008; 149: 4589 4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F, Moore RK, Iemura S-I, Ueno N, Shimasaki S. Follistatin inhibits the function of the oocyte-derived factor BMP-15. Biochem Biophys Res Comm 2001; 289: 961 966 [DOI] [PubMed] [Google Scholar]
- Beck H, Drahushuk K, Jacoby D, Higgins D, Lein P. Bone morphogenetic protein-5 (BMP-5) promotes dendritic growth in cultured sympathetic neurons. BMC Neurosci 2001; 2: 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto O, Kawasaki N, Tsuchida K, Shimasaki S, Hayakawa T, Sugino H. Difference between follistatin isoforms in the inhibition of activin signalling: activin neutralizing activity of follistatin isoforms is dependent on their affinity for activin. Cell Signal 2000; 12: 565 571 [DOI] [PubMed] [Google Scholar]
- Glister C, Kemp CF, Knight PG. Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction 2004; 127: 239 254 [DOI] [PubMed] [Google Scholar]
- Yamamoto TSIS, Takagi C, Shimasaki S, Ueno N. Characterization of follistatin isoforms in early Xenopus embryogenesis. Int J Dev Biol 2000; 44: 341 348 [PubMed] [Google Scholar]
- Sugino K, Kurosawa N, Nakamura T, Takio K, Shimasaki S, Ling N, Titani K, Sugino H. Molecular heterogeneity of follistatin, an activin-binding protein. Higher affinity of the carboxyl-terminal truncated forms for heparan sulfate proteoglycans on the ovarian granulosa cell. J Biol Chem 1993; 268: 15579 15587 [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature 1995; 374: 356 360 [DOI] [PubMed] [Google Scholar]
- Oh SP, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev 1997; 11: 1812 1826 [DOI] [PubMed] [Google Scholar]
- Gu Z, Nomura M, Simpson BB, Lei H, Feijen A. van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I activin receptor ActRIB is required for egg cylinder organization and gastrulation in the mouse. Genes Dev 1998; 12: 844 857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT. van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development 1999; 126: 2551 2561 [DOI] [PubMed] [Google Scholar]
- Brown CW, Houston-Hawkins DE, Woodruff TK, Matzuk MM. Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet 2000; 25: 453 457 [DOI] [PubMed] [Google Scholar]
- Archambeault DR, Yao HH-C, Activin A. a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc Natl Acad Sci 2010; 107: 10526 10531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold E, Jetly N, O'Bryan MK, Meachem S, Srinivasan D, Behuria S, Sanchez-Partida LG, Woodruff T, Hedwards S, Wang H, McDougall H, Casey V. et al. Activin C antagonizes activin A in vitro and overexpression leads to pathologies in vivo. Am J Pathol 2009; 174: 184 195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Kumar TR, Woodruff T, Hadsell LA, DeMayo FJ, Matzuk MM. Overexpression of mouse follistatin causes reproductive defects in transgenic mice. Mol Endocrinol 1998; 12: 96 106 [DOI] [PubMed] [Google Scholar]
- Xia Y, Sidis Y, Schneyer A. Overexpression of follistatin-like 3 in gonads causes defects in gonadal development and function in transgenic mice. Mol Endocrinol 2004; 18: 979 994 [DOI] [PubMed] [Google Scholar]
- Rao Veeramachaneni DN, Amann RP. Oxytocin in the ovine ductuli efferentes and caput epididymidis: immunolocalization and endocytosis from the luminal fluid. Endocrinology 1990; 126: 1156 1164 [DOI] [PubMed] [Google Scholar]
- Veeramachaneni DN, Amann RP. Endocytosis of androgen-binding protein, clusterin, and transferrin in the efferent ducts and epididymis of the ram. J Androl 1991; 12: 288 294 [PubMed] [Google Scholar]
- Veeramachaneni DN, Amann RP, Palmer JS, Hinton BT. Proteins in luminal fluid of the ram excurrent ducts: changes in composition and evidence for differential endocytosis. J Androl 1990; 11: 140 154 [PubMed] [Google Scholar]
- Amann RP, Hammerstedt RH, Veeramachaneni DN. The epididymis and sperm maturation: a perspective. Reprod Fertil Dev 1993; 5: 361 381 [DOI] [PubMed] [Google Scholar]
- Robaire B, Hermo L. Efferent ducts, epididymis, and vas deferens: structure, functions, and their regulation. : Knobil E, Neill J. (eds.), Physiology of Reproduction, vol. 1, 3rd ed. New York: Raven Press; 1988: 999 1080 [Google Scholar]
- Hess R. The efferent ductules: structure and function : Robaire B, Hinton B. (eds.), The Epididymis: From Molecules to Clinical Practice: A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. New York: Plenum Publishing Corporation; 2002: 49 80 [Google Scholar]
- Bahathiq AO, Stewart RL, Baxter L, Wells M, Moore HD, Ledger WL. Tissue immunoexpression and messenger ribonucleic acid localization of inhibin/activin subunit in human epididymis. Fertil Steril 2005; 83: 78 85 [DOI] [PubMed] [Google Scholar]
- Zhang T, Guo CX, Hu ZY, Liu YX. Localization of plasminogen activator and inhibitor, LH and androgen receptors and inhibin subunits in monkey epididymis. Mol Hum Reprod 1997; 3: 945 952 [DOI] [PubMed] [Google Scholar]
- Schneider O, Nau R, Michel U. Comparative analysis of follistatin-, activin beta A- and activin beta B-mRNA steady-state levels in diverse porcine tissues by multiplex S1 nuclease analysis. Eur J Endocrinol 2000; 142: 537 544 [DOI] [PubMed] [Google Scholar]
- Esquela AF, Se-J Lee. Regulation of metanephric kidney development by growth/differentiation factor 11. Dev Biol 2003; 257: 356 370 [DOI] [PubMed] [Google Scholar]
- Tomaszewski J, Joseph A, Archambeault D, Yao HH-C. Essential roles of inhibin beta A in mouse epididymal coiling. Proc Natl Acad Sci U S A 2007; 104: 11322 11327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K-U, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res 1997; 25: 4323 4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugh R. The Mouse: Its Reproduction and Development. New York: Oxford University Press; 1990. [Google Scholar]
- Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, Godwin AK, Korach KS, Visvader JE, Kaestner KH, Abdul-Karim FW, Montano MM. et al. FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development 2010; 137: 2045 2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Hata T, Suzuki O, Matsuda J. The relationship between sperm morphology and in vitro fertilization ability in mice. J Reprod Dev 2006; 52: 561 568 [DOI] [PubMed] [Google Scholar]
- Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 1988; 122: 787 794 [DOI] [PubMed] [Google Scholar]
- Boitani C, Stefanini M, Fragale A, Morena AR. Activin stimulates Sertoli cell proliferation in a defined period of rat testis development. Endocrinology 1995; 136: 5438 5444 [DOI] [PubMed] [Google Scholar]
- Buzzard JJ, Farnworth PG, de Kretser DM, O'Connor AE, Wreford NG, Morrison JR. Proliferative phase Sertoli cells display a developmentally regulated response to activin in vitro. Endocrinology 2003; 144: 474 483 [DOI] [PubMed] [Google Scholar]
- Wreford NG. Rajendra Kumar T, Matzuk MM, de Kretser DM. Analysis of the testicular phenotype of the follicle-stimulating hormone {beta}-subunit knockout and the activin type II receptor knockout mice by stereological analysis. Endocrinology 2001; 142: 2916 2920 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature 1995; 374: 356 360 [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 1997; 15: 201 204 [DOI] [PubMed] [Google Scholar]
- Wagner K-U, Ward T, Davis B, Wiseman R, Hennighausen L. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res 2001; 10: 545 553 [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphologic changes in efferent ductules and epididymis in estrogen receptor-alpha knockout mice. J Androl 2000; 21: 107 121 [PubMed] [Google Scholar]
- Lee K-H, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol Reprod 2000; 63: 1873 1880 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A 1993; 90: 11162 11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding EH, Hewitt SC, Nakamura N, Hamilton K, Korach KS, Eddy EM. Ex3alphaERKO male infertility phenotype recapitulates the alpha ERKO male phenotype. J Endocrinol 2010; 207: 281 288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 2000; 127: 4277 4291 [DOI] [PubMed] [Google Scholar]
- Chen M, Hsu I, Wolfe A, Radovick S, Huang K, Yu S, Chang C, Messing EM, Yeh S. Defects of prostate development and reproductive system in the estrogen receptor-{alpha} null male mice. Endocrinology 2009; 150: 251 259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Shur BD, Hess RA. Estrogen, efferent ductules, and the epididymis. Biol Reprod 2011; 84: 207 217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M, Bouma J, Nie R, Zhou Q, Carnes K, Lubahn DB, Hess RA. Morphological analysis of endocytosis in efferent ductules of estrogen receptor-α knockout male mouse. Anat Rec 2001; 263: 10 18 [DOI] [PubMed] [Google Scholar]
- Kipp JL, Kilen SM, Woodruff TK, Mayo KE. Activin regulates estrogen receptor gene expression in the mouse ovary. J Biol Chem 2007; 282: 36755 36765 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Otsuka F, Miyoshi T, Otani H, Goto J, Yamashita M, Ogura T, Makino H, Doihara H. Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 mitogen-activated protein kinase activation. J Endocrinol 2008; 199: 445 455 [DOI] [PubMed] [Google Scholar]
- Snyder EM, Small CL, Li Y, Griswold MD. Regulation of gene expression by estrogen and testosterone in the proximal mouse reproductive tract. Biol Reprod 2009; 81: 707 716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai L-W, Lien Y-HH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci U S A 2001; 98: 14132 14137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CA, Carnes K, França LR, Hermo L, Hess RA. Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol Cell 2005; 97: 385 395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu T, Azlina A, Purwanti N, Karabasil MR, Hasegawa T, Yao C, Hosoi K. Inhibition and transcriptional silencing of a subtilisin-like proprotein convertase, PACE4/SPC4, reduces the branching morphogenesis of and AQP5 expression in rat embryonic submandibular gland. Dev Biol 2009; 325: 434 443 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takahashi E, Miyagawa S-I, Watanabe H, Iguchi T. Chromatin immunoprecipitation-mediated target identification proved aquaporin 5 is regulated directly by estrogen in the uterus. Genes Cells 2006; 11: 1133 1143 [DOI] [PubMed] [Google Scholar]
- Hermo L, Schellenberg M, Liu LY, Dayanandan B, Zhang T, Mandato CA, Smith CE. Membrane domain specificity in the spatial distribution of aquaporins 5, 7, 9, and 11 in efferent ducts and epididymis of rats. J Histochem Cytochem 2008; 56: 1121 1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CA, Mahecha GAB, Carnes K, Prins GS, Saunders PTK, Franca LR, Hess RA. Differential hormonal regulation of estrogen receptors ER{alpha} and ER{beta} and androgen receptor expression in rat efferent ductules. Reproduction 2004; 128: 73 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-P, Suomalainen M, Jorgez CJ, Matzuk MM, Werner S, Thesleff I. Follistatin regulates enamel patterning in mouse incisors by asymmetrically inhibiting BMP signaling and ameloblast differentiation. Dev Cell 2004; 7: 719 730 [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee S-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 1997; 387: 83 90 [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Liaw L, Hogan BL. Bone morphogenetic protein 8A plays a role in the maintenance of spermatogenesis and the integrity of the epididymis. Development 1998; 125: 1103 1112 [DOI] [PubMed] [Google Scholar]
- Zhao G-Q, Chen Y-X, Liu X-M, Xu Z, Qi X. Mutation in Bmp7 exacerbates the phenotype of Bmp8a mutants in spermatogenesis and epididymis. Dev Biol 2001; 240: 212 222 [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn 1997; 208: 349 362 [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 1995; 9: 2808 2820 [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 2004; 25: 72 101 [DOI] [PubMed] [Google Scholar]
- Hu J, Chen Y-X, Wang D, Qi X, Li T-G, Hao J, Mishina Y, Garbers DL, Zhao G-Q. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev Biol 2004; 276: 158 171 [DOI] [PubMed] [Google Scholar]
- Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation 2007; 116: 1258 1266 [DOI] [PubMed] [Google Scholar]
- Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, Pillemer G, Steinbeisser H, Blum M. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev 1997; 63: 39 50 [DOI] [PubMed] [Google Scholar]
- Sailer BL, Sarkar LJ, Bjordahl JA, Jost LK, Evenson DP. Effects of heat stress on mouse testicular cells and sperm chromatin structure. J Androl 1997; 18: 294 301 [PubMed] [Google Scholar]
- Turner TT, Reich GW. Cauda epididymal sperm motility: a comparison among five species. Biol Reprod 1985; 32: 120 128 [DOI] [PubMed] [Google Scholar]
- Caflisch CR, DuBose TD. Direct evaluation of acidification by rat testis and epididymis: role of carbonic anhydrase. Am J Physiol 1990; 258: E143 E150 [DOI] [PubMed] [Google Scholar]
- Carr DW, Usselman MC, Acott TS. Effects of pH, lactate, and viscoelastic drag on sperm motility: a species comparison. Biol Reprod 1985; 33: 588 595 [DOI] [PubMed] [Google Scholar]
- Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J 2006; 25: 4131 4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Hess RA, Schaeffer DJ, Ko C, Hudgin-Spivey S, Chambon P, Shur BD. Absence of estrogen receptor alpha leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biol Reprod 2010; 82: 948 957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Shur BD, Ko C, Chambon P, Hess RA. Epididymal hypo-osmolality induces abnormal sperm morphology and function in the estrogen receptor alpha knockout mouse. Biol Reprod 2010; 82: 958 967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieschlag E. The struggle for male hormonal contraception. Best Pract Res Clin Endocrinol Metab 2011; 25: 369 375 [DOI] [PubMed] [Google Scholar]