ABSTRACT
The mechanism(s) by which vitamin D3 regulates female reproduction is minimally understood. We tested the hypothesis that peripubertal vitamin D3 deficiency disrupts hypothalamic-pituitary-ovarian physiology. To test this hypothesis, we used wild-type mice and Cyp27b1 (the rate-limiting enzyme in the synthesis of 1,25-dihydroxyvitamin D3) null mice to study the effect of vitamin D3 deficiency on puberty and reproductive physiology. At the time of weaning, mice were randomized to a vitamin D3-replete or -deficient diet supplemented with calcium. We assessed the age of vaginal opening and first estrus (puberty markers), gonadotropin levels, ovarian histology, ovarian responsiveness to exogenous gonadotropins, and estrous cyclicity. Peripubertal vitamin D3 deficiency significantly delayed vaginal opening without affecting the number of GnRH-immunopositive neurons or estradiol-negative feedback on gonadotropin levels during diestrus. Young adult females maintained on a vitamin D3-deficient diet after puberty had arrested follicular development and prolonged estrous cycles characterized by extended periods of diestrus. Ovaries of vitamin D3-deficient Cyp27b1 null mice responded to exogenous gonadotropins and deposited significantly more oocytes into the oviducts than mice maintained on a vitamin D3-replete diet. Estrous cycles were restored when vitamin D3-deficient Cyp27b1 null young adult females were transferred to a vitamin D3-replete diet. This study is the first to demonstrate that peripubertal vitamin D3 sufficiency is important for an appropriately timed pubertal transition and maintenance of normal female reproductive physiology. These data suggest vitamin D3 is a key regulator of neuroendocrine and ovarian physiology.
Keywords: hypothalamus, nutrition, ovulation, puberty, vitamin D3
Studies in Cyp27b1 null and wild-type mice show that peripubertal vitamin D3 deficiency delays puberty and disrupts ovarian physiology and estrous cycles.
INTRODUCTION
Vitamin D3, a secosteroid hormone, and the vitamin D3 receptor (VDR) have important roles in immune modulation, cellular proliferation, differentiation and survival, and insulin secretion [1–5]. The two main forms of vitamin D3 (cholecalciferol) found in the circulation are 25-hydroxyvitamin D3 [25-(OH)D3; major circulating form] and 1α,25-dihydroxyvitamin D3 [1,25-(OH)2D3; the most active form of vitamin D3]. Vitamin D3 sufficiency is achieved primarily through dietary intake of fatty fish and eggs and endogenous synthesis of cholecalciferol upon ultraviolet B radiation of 7-dehydrocholesterol in dermal fibroblasts and epidermal keratinocytes [6]. Vitamin D3 circulates bound to vitamin D-binding protein until it is converted by 25-hydroxylase to 25-(OH)D3. Synthesis of 1,25-(OH)2D3 takes place in the kidney and other tissues expressing 1α-hydroxylase [Cyp27b1; the rate-limiting enzyme that converts 25-(OH)D3 to 1,25-(OH)2D3].
The effect of 1,25-(OH)2D3 on target cells is achieved primarily by binding to VDR. VDR is a ligand-activated transcription factor that belongs to the nuclear hormone receptor superfamily. It binds 1,25-(OH)2D3 with an affinity in the range of 1–5 nM, compared to 10–50 times lower affinity for 25-(OH)D3 [7–10]. Binding of 1,25-(OH)2D3 to VDR initiates receptor translocation to the nucleus and recruitment and heterodimerization with the 9-cis retinoid receptor (RXR). The liganded VDR/RXR heterodimer forms complexes with steroid receptor coactivators, VDR-interacting protein, and coregulatory proteins before it binds to vitamin D3-response elements located in the promoter region of target genes to allow tissue-specific gene transcription regulation [11]. Alternatively, 1,25-(OH)2D3 may also initiate rapid nongenomic responses by binding to membrane-associated rapid response steroid binding receptors (also known as Erp57/Grp58), mobilizing intracellular calcium stores and activating and modulating second messenger signaling systems such as adenylyl cyclase, protein kinases C and D, mitogen-activated protein kinase, and Raf kinase systems [12].
Suboptimal vitamin D3 intake and reduced sun exposure have resulted in near-epidemic levels of vitamin D3 insufficiency and deficiency [13–15]. Importantly, populations with the greatest physiological need for vitamin D3, pregnant women [15, 16], neonates [17], children, and adolescents [17–19], are at highest risk for vitamin D3 deficiency. Recent studies in humans and rodents suggest that vitamin D3 may be important for normal reproductive physiology [20–26], but the mechanisms by which vitamin D3 deficiency adversely affect female fertility and reproductive physiology are not understood [21, 22, 26–28]. Cyp27b1 and VDR are found in the gonads, hypothalamus, and pituitary, suggesting that the reproductive axis may be regulated by paracrine and/or autocrine activities of 1,25-(OH)2D3 [29–34]. Female rats with dietary vitamin D3 deficiency and hypocalcemia exhibit severely compromised fertility characterized by a 45%–70% reduction in probability of becoming pregnant, a 67%–100% reduction in the number of viable pups, and a 0%–33% probability of rearing normal sized and healthy litters [21, 22]. Vitamin D3 deficiency-associated hypocalcemia or VDR mutations in male rodents cause subfertility and infertility by impairing spermatogenesis, capacitation, and acrosome reaction [35–37]. Although hypocalcemia accounts for reproductive dysfunction in males, restoring calcium homeostasis in females does not routinely reverse female reproductive abnormalities [22, 27, 36].
Phenotypes in female VDR and Cyp27b1 null mice include hypogonadism, arrested ovarian follicular development, and hypoplastic uteri [26, 38, 39]. Uterine responsiveness to exogenous steroids is intact in VDR knockout (KO) mice, suggesting that vitamin D3 deficiency impairs one or more components of the hypothalamic-pituitary-ovarian axis [38]. Interestingly, vitamin D3 receptor expression peaks in the hypothalamus during the peripubertal period in male rats, suggesting that central vitamin D3 signaling may be important for pubertal transition [40]. We used transgenic Cyp27b1 null and wild-type (WT) littermate mice supplemented with calcium to test the hypothesis that vitamin D3 deficiency acts on the hypothalamic-pituitary-ovarian axis to disrupt the pubertal transition and establishment of normal estrous cyclicity.
MATERIALS AND METHODS
Animals, Housing, and Diet
All experiments were carried out in WT (Cyp27b1+/+) and Cyp27b1 null (KO; Cyp27b1−/–) mice generated from a mixed genetic background with contributions from C57BL/6J and BALB/c mice, generously provided by the laboratory of David Goltzman (Department of Medicine, McGill University) [39]. WT (control) and Cyp27b1 null mice were maintained by breeding phenotypically normal heterozygous pairs (Cyp27b1+/–). Mice were fed ad libitum with either mouse chow fortified with vitamin D3 (Vit D3+; 0.81% calcium, 0.63% phosphorus, 2.2 IU/g vitamin D3; Lab Diet formula 5053; Purina Mills, Richmond, IN) or vitamin D3-deficient diet (Vit D3−; 0.81% calcium, 0.63% phosphorus, 0 IU/g vitamin D3; Lab Diet formula AIN-93M; Purina Mills) supplemented with water containing 1.5% calcium gluconate to maintain calcium homeostasis [41]. The colony was maintained at a controlled temperature (25°C) with a 14L:10D schedule (lights on at 0600 h). All procedures followed the National Institutes of Health guide for the care and use of laboratory rodents and were approved by the Institutional Animal Care and Use Committee at Albert Einstein College of Medicine.
Experiment 1: Pubertal Onset and Characterization of Estrous Cycles in WT and Cyp27b1 KO Mice
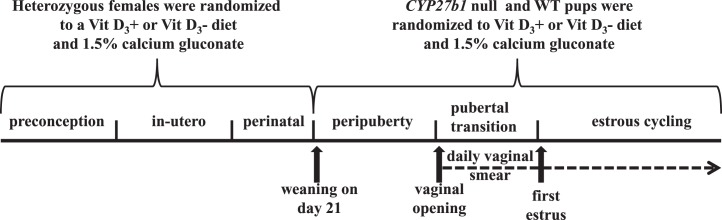
Pups were weighed weekly from birth through puberty and genotyped before weaning by PCR with DNA extracted from either toe or tail biopsy. All pups were born to dams fed a Vit D3+ or Vit D3− diet prior to conception, during pregnancy, and until weaning. All mice were weaned on Postnatal Day 21 and randomized to a Vit D3+ or Vit D3− diet (Fig. 1). Reproductive phenotypes were assessed according to genotype and diet. All WT littermates born from heterozygous matings were considered controls.
FIG. 1. .
Timeline of experimental events. All experiments were carried out with mice born from heterozygote matings in which the dams received a Vit D3+ or Vit D3− diet. WT and Cyp27b1 null mice female pups were weaned at 21 days and randomized to either a Vit D3+ or a Vit D3− diet supplemented with calcium gluconate. WT (+/+) mice that received a Vit D3+ diet throughout gestation, lactation, and weaning served as controls. All mice were inspected daily for vaginal opening. After vaginal opening, vaginal lavage was performed daily at 1500 h for a minimum of 7 wk, and estrous cycle length and estrous stage frequency were determined with vaginal smears.
Pubertal transition was assessed by recording the age at vaginal opening and first estrus [42]. First estrus and estrous staging were determined with daily vaginal lavage performed at approximately 1500 h postvaginal opening (Fig. 1). Estrous stages were defined as proestrus (80%–100% epithelial cells), estrus (100% cornified epithelial cells), diestrus I (∼50% cornified epithelial cells and 50% leukocytes), and diestrus II (80%–100% leukocytes) [42]. Estrous cycle length was defined by the number of days required for a mouse to transition from one proestrus event to the next. To determine whether the effect of vitamin D3 deficiency on estrous cyclicity was reversible, the Vit D3−/calcium gluconate diet of adult Cyp27b1 null females (n = 6) exhibiting irregular estrous cycles was replaced with a Vit D3+ diet, and estrous cycle length and percentage of time mice spent in each stage of the estrous cycle per 5 days (the average length of an estrous cycle) were determined for a minimum of 4 additional weeks.
Experiment 2: Ovarian Superovulation
Seven- to to nine-week-old Vit D3-sufficient and -deficient mice were superovulated with 0.1 cc i.p. injections of equine chorionic gonadotropin (eCG, 5 IU; Sigma-Aldrich, St. Louis, MO) or with saline at 0900 h, followed by 0.1 cc of human chorionic gonadotropin (hCG; 5 IU; Sigma-Aldrich) or saline 48 h later [43]. Mice superovulated with exogenous gonadotropins were killed 16 h after hCG, oviducts harvested, and oocytes that were deposited into the oviducts were quantified. The number of oocytes present within the oviducts was counted by an individual blinded to diet status and genotype. Ovaries were collected from mice injected with saline, or exogenous gonadotropins were weighed, fixed overnight in 4% paraformaldehyde, dehydrated, and paraffin embedded. Paraffin-embedded ovaries were stored until sectioned and stained with hematoxylin and eosin for gross histologic evaluation.
Experiment 3: Determination of Serum Gonadotropins
Reproduction-aged mice in diestrus were killed by anesthetic overdose and exsanguinated. We assessed gonadotropin levels during diestrus because we wanted to use intact mice, and as intact Vit D3-exposed mice spend most of their time in diestrus, we used diestrus WT control mice for comparison. Blood was stored at 4°C overnight, and serum was separated with centrifugation (7000 × g for 15 min at 7°C) the next day. Serum follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels were determined in duplicate using Milliplex Map rat pituitary panel (Millipore, Billerica, MA) [44]. The lower limit of detection was 4.9 pg/ml and 47.7 pg/ml for LH and FSH, respectively. Intra-assay coefficients of variation were 13.46% and 4.25% for LH and FSH, respectively.
Experiment 4: Expression of VDR by GT1-7 Neurons
GT1-7 cells (immortalized GnRH neurons, generously provide by Dr. Pamela Mellon) and mouse kidney tissue were probed for VDR expression by Western blotting. GT1-7 cells were cultured as described by Chu et al. [45]. One hundred micrograms of protein from GT1-7 cells (40 μl) and 40 μg of kidney (40 μl) tissue lysates were loaded onto 8% polyacrylamide gels and subjected to electrophoresis. Proteins were transferred to nitrocellulose membranes for immunoblotting with VDR rat monoclonal antibody (1:150 dilution; product code sc124548; Santa Cruz Biotechnology, Santa Cruz, CA). Immunoblots were probed with anti-VDR overnight at 4°C in tris-buffered saline with 5% milk, washed, and stripped. Protein bands were detected after 2 h of incubation with peroxidase donkey secondary antibody (1:5000 dilution) and visualized with enhanced chemiluminescent reagent (Jackson Immunoresearch Laboratories, West Grove, PA). Kidney lysate served as the positive control. Kidney and GT1-7 lysates without primary VDR antibody served as the negative controls.
Experiment 5: GnRH Neuron Immunohistochemistry and Quantification GnRH Neurons
Animals were perfused with 4% paraformaldehyde in phosphate buffer (pH 6.8) between 1200 and 1400 h. Brains were postfixed in 4% paraformaldehyde overnight at 4°C and then placed in 30% sucrose until they sank. Six sets of coronal sections (30 μm) starting at the level of the organum vasculosum of lamina terminalis (Bregma +0.62 mm) and continuing through the preoptic area (POA; Bregma −0.10 mm) were collected from each animal, with each set containing every sixth section. Sections were stored in cryoprotectant at −20°C until processed for immunolabeling [46, 47].
As previously described [48], cryoprotectant was removed by rinsing hypothalamic sections with potassium phosphate buffered saline (KPBS; 0.05 M, pH 7.4). Endogenous peroxidase activity was blocked with a 10-min incubation in 3% H2O2. Sections were subsequently incubated in KPBS plus 0.04% Triton-X 100 (KPBS-Tx) and 1% bovine serum albumin (BSA) for 1 h at room temperature before incubation in rabbit-anti GnRH antiserum in KPBS-Tx and 1% BSA (1:5000 dilution; LR-5, a generous gift from Dr. R. Benoit, McGill University, Montreal, Canada) for 24 h at 4°C. Sections were next incubated in biotinylated anti-rabbit immunoglobulin G (IgG; 1:600 dilution; Vector Laboratories, Burlingame, CA) in KPBS-Tx for 1 h at room temperature, rinsed, and incubated for 1 h in avidin-biotin complex (Elite ABC kit; Vector Laboratories). After sections were rinsed in KPBS and Tris (0.05 M, pH 7.2; Sigma-Aldrich), they were stained with a mixture of H2O2 and diaminobenzidine–HCl in Tris for 10 min to yield a brown staining in cytoplasm. Sections were mounted onto Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) after a final rinse in Tris and KPBS. After drying overnight, sections were dehydrated with ascending alcohol concentrations, cleared with xylene, and cover slips were added. To assess antibody specificity, we treated a set of tissue sections identically except primary antibody was eliminated.
To quantify GnRH-immunoreactive (ir) neurons, five sections of POA in the 1-in-6 series were viewed under a microscope (Zeiss Axioversion; Carl Zeiss, Thornwood, NY) [48]. Hypothalamic sections reviewed corresponded to plates 25–32 of the Paxinos and Watson mouse atlas [49]. GnRH-ir cells were counted when the cell body was clearly identified and if they had brown cytoplasmic staining. The average number of GnRH neurons counted in each of the five sections is reported (n = 3 mice per group) for WT mice on a Vit D3+ diet and Cyp27b1 null mice exposed to a peripubertal Vit D3− diet. A Student t-test was used to determine statistical differences between GnRH neuron counts.
PCR
PCR with Southern blot analysis was performed using the primers and conditions previously described [39]. PCR assays were carried out using the MyCycler thermal cycler (Bio-Rad, Hercules, CA) and AccuPrime TaqDNA polymerase High Fidelity (Invitrogen, Carlsbad, CA) in a final volume of 25 μl. Thirty cycles were used to amplify samples with denaturation at 94°C for 1 min; annealing at 58°C for 1 min; and extension at 72°C for 1 min. Primer sequence for 1α-hydroxylase sense was 5′-AGACTGCACTCCACTCTGAG-3′, and 5′-GTTTCCTACACGGATGTCTC-3′ for antisense; and 5′-ACAACAGACAATCGGCTGCTC-3′ for neomycin sense and 5′-CCATGGGTCACGACGAGATC-3′ for antisense (Integrated DNA Technologies, Coralville, IA). PCR products were electrophoresed in 1.5% agarose gels and stained with ethidium bromide solution for 45 min, and bands were visualized under ultraviolet light.
Statistical Analysis
All data were analyzed using Prism software (GraphPad software Inc., La Jolla, CA). Repeated measures ANOVA was used to determine the effect of diet and genotype on growth. Two-way ANOVA (diet × genotype) was used to determine the effect of diet on vaginal opening, first estrus, and percentage of time spent in the different stages of the estrous cycle. One-way ANOVA was used to determine the effect of diet on the number of oocytes collected after superovulation with exogenous gonadotropins. Bonferroni post hoc analyses were used throughout to determine individual group differences following significant main effects or effect interactions. A Wilcoxon matched t-test was used to determine the effect of vitamin D3 replacement on estrous cycle length and frequency of estrous cycle stage. A Student t-test was used to assess the effect of diet on the number of GnRH neurons. All data are means ± SEM. A P value <0.05 was considered significantly different.
RESULTS
Experiment 1: Vitamin D3 Deficiency Delays Puberty and Reversibly Disrupts the Estrous Cycle
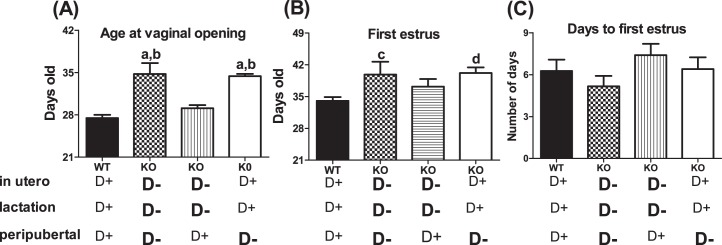
To determine whether vitamin D3 deficiency affected pubertal transition, we assessed the age at vaginal opening and first estrus in WT and Cyp27b1 null female offspring born from heterozygous matings, with dams maintained on either Vit D3+ or Vit D3− diet throughout pregnancy and lactation and then randomly weaned onto a Vit D3+ or Vit D3− diet (n = 8–15) (Fig. 2). Compared to WT control females (maintained on Vit D3+ diet throughout pregnancy, lactation, and puberty), delayed vaginal opening (27.5 ± 0.5 vs. 34.8 ± 1.5 days; F = 20.85; P < 0.0001) and delayed first estrus (34 ± 0.8 vs. 39 ± 2.8 days; F = 5.2; P = 0.005) were observed in females born to dams maintained on a Vit D3− diet throughout pregnancy and lactation and then weaned onto a Vit D3− diet (Fig. 2, A and B). In contrast, neither vaginal opening nor first estrus was significantly delayed in female mice (n = 10) born from dams maintained on a Vit D3− diet in utero and during lactation but then weaned onto a Vit D3+ diet (Fig. 2, A and B). Cyp27b1 null females (n = 10) born to dams maintained on a Vit D3+ diet and then weaned onto a Vit D3− diet had significantly delayed vaginal opening (27.5 ± 0.5 vs. 34.4 ± 0.4; F = 20.85; P < 0.0001) and first estrus (34.5 ± 0.8 vs. 40.2 ± 1.2; F = 5.2; P = 0.005) compared to those of WT females (Fig. 2, A and B). Vitamin D3 deficiency did not affect the time between first estrus and vaginal opening for any group (Fig. 2C), suggesting that delayed vaginal opening caused the delayed first estrus.
FIG. 2. .
Peripubertal 1,25-(OH)2 vitamin D3 deficiency delays puberty. A) Age at vaginal opening (VO) in WT (Cyp27b1+/+) mice fed a Vit D3+ diet throughout pregnancy (in utero) and lactation (perinatal period) and peripubertal transition and in Cyp27b1 null female mice subjected to a Vit D3− diet in utero and perinatal period and peripubertal transition, or in utero and perinatal period, or only during the peripubertal transition. B) Age at first estrus in WT mice fed a D+ diet in utero and perinatal period and peripubertal transition and in Cyp27b1 null female mice subjected to D− diet in utero and perinatal period and peripubertal transition or in utero and perinatal period, or only during the peripubertal transition. C) Number of days between VO and first estrus in WT mice fed a D+ diet in utero and perinatal period and peripubertal transition and in Cyp27b1 null female mice subjected to D− diet in utero and perinatal period and peripubertal transition, or in utero and perinatal period, or only during the peripubertal transition (n = 8–15); aP < 0.0001 vs. WT; bP < 0.0001 vs. in utero and lactation; cP < 0.05 vs. WT; dP < 0.01 vs. WT.
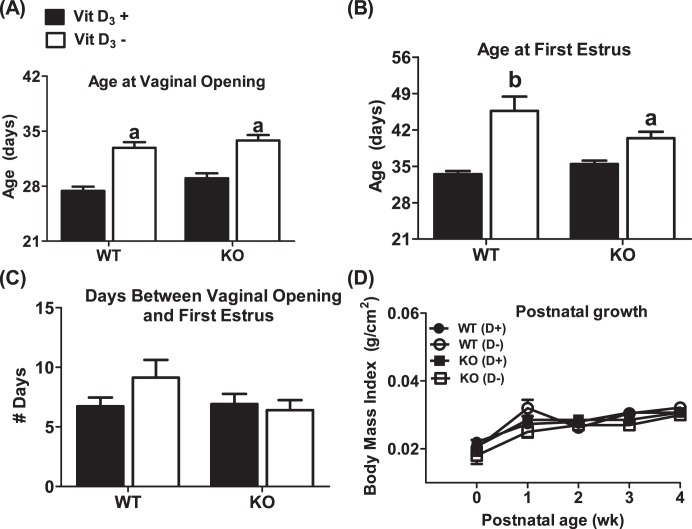
Multiple confounding environmental factors can affect puberty. Genetic background commonly alters the timing of puberty [50]. To determine whether deletion of the Cyp27b1 gene caused a delay in puberty independent of vitamin D3 status, we assessed the timing of puberty in Cyp27b1 null mice maintained on a Vit D3+ diet throughout gestation and lactation and then weaned onto a Vit D3+ diet. There were no differences between the onset of puberty in WT and that in Cyp27b1 null mice born from dams exposed to Vit D3+ during pregnancy and lactation and then weaned onto a Vit D3+ diet (n = 8–15) (Fig. 3, A–C). These data suggest that the delayed puberty was the result of Vit D3 deficiency and not an unanticipated effect of the deletion of the Cyp27b1 gene. Under- and overnutrition are also reported to delay and advance the pubertal transition, respectively [51]. To determine if the reproductive phenotype of mice exposed to peripubertal vitamin D3 deficiency reflected poor growth, we compared the body mass indexes of mice weaned onto a Vit D3+ or Vit D3− diet. There were no significant differences in postnatal and peripubertal growth curves across diet treatment (n = 8–15) (Fig. 3D). Moreover, regardless of diet, the weight of 4-wk-old mice, an age at which puberty in most Vit D3+ but not Vit D3− mice has already been initiated, was not significantly different (17.1 ± 0.6 [WT, Vit D3+] vs. 16.7 ± 1 [WT, Vit D3−] vs. 16.8 ± 0.4 [Cyp27b1,Vit D3+] vs. 16.2 ± 0.7 [Cyp27b1,Vit D3−], P > 0.05).
FIG. 3. .
Mice fed a vitamin D3-deficient diet during the prepubertal period have a delayed pubertal onset but normal developmental growth curves. A) Age at vaginal opening. B) Age at first estrus. C) Days between vaginal opening and first estrus. D) Developmental growth curves of mice. KO denotes Cyp27b1 null mice. WT denotes Cyp27b1+/+ mice. Vit D3+ or D+ denotes mice fed a Vit D3-sufficient diet before and after weaning. Vit D3− or D− denotes mice supplemented with calcium gluconate and fed a Vit D3-deficient diet after weaning (n = 8–15). aP < 0.01 vs. Vit D3+; bP < 0.0001 vs. Vit D3+
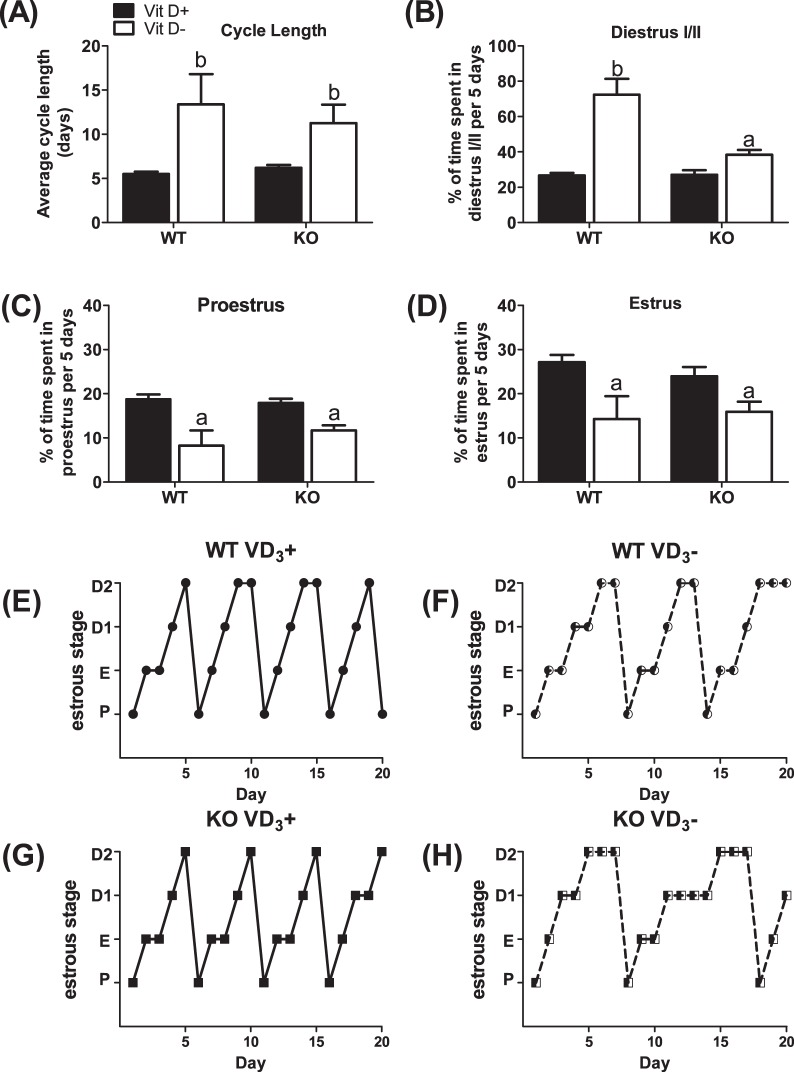
After completion of the pubertal transition, Vit D3+ females typically exhibited 5-day estrous cycles. To determine whether Vit D3 deficiency effects were sustained beyond puberty, estrous cycle length, and the amount of time spent in the various stages of estrous, we used daily vaginal smears to monitor estrous cycle length and the percentage of time spent in each stage of estrous over a 5-day interval (n = 8–15). The main effect of diet (F = 7.94; P = 0.009 vs. Vit D3+ diet) on estrous cycle length when Cyp27b1 null and WT mice were sustained on a Vit D3− diet was Vit D3-deficient females exhibited prolonged estrous cycles (Fig. 4A) characterized by extended periods of diestrus (F = 52.3, P < 0.001 vs. Vit D3+ diet) (Fig. 4B). Cyp27b1 null and WT mice maintained on a Vit D3− diet also spent significantly fewer days in proestrus (F = 23.07, P < 0.0001 vs. Vit D3+ diet) (Fig. 4C) and estrus (F = 10.7 P = 0.002 vs. Vit D3+ diet) (Fig. 4D). WT and Cyp27b1 null mice exposed to a Vit D3+ diet throughout gestation, lactation, and postweaning had equivalent estrous cycle lengths and spent similar amounts of time in each estrous stage (Fig. 4, A–D).
FIG. 4. .
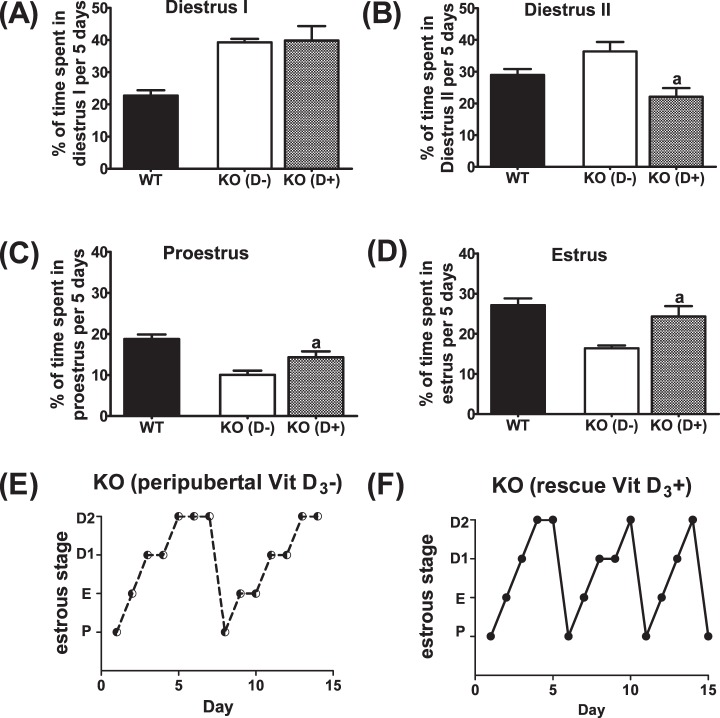
Vitamin D3 deficiency extends the estrous cycle by increasing time spent in diestrus. A) Average cycle length in WT and Cyp27b1 null mice fed a Vit D3+ or Vit D3− diet during the peripubertal period. Average percentage of time spent in diestrus I/II (B), proestrus (C), and estrus (D) in WT and Cyp27b1 null mice fed a Vit D3+ diet or Vit D3− diet during the prepubertal period and into early adulthood (n = 8–15); aP < 0.05 vs. Vit D3+ diet; bP < 0.001 vs. Vit D3+ diet. E) Representative estrous cycle of WT mouse fed Vit D3+ diet throughout gestation and lactation and after weaning. F) Representative estrous cycle of WT mouse fed Vit D3+ diet throughout gestation and lactation and weaned onto a Vit D3− diet. G) Representative estrous cycle of Cyp27b1 mouse fed Vit D3+ diet throughout gestation and lactation and after weaning. H) Representative estrous cycle of Cyp27b1 mouse fed Vit D3+ diet throughout gestation and lactation and weaned onto a Vit D3− diet. P, proestrus; E, estrus; D1, diestrus 1; D2, diestrus 2.
To determine whether the effect of Vit D3 deficiency on estrous cycle was reversible, we replaced the Vit D3− diet with a Vit D3+ diet in a subgroup of Cyp27b1 null mice (n = 6) (Fig. 5). After approximately 3 to 4 wk of a Vit D3+ diet, irregularly cycling Cyp27b1 null mice began to exhibit regular estrous cycle length (11.3 ± 2.1 vs. 5.4 ± 0.2; t = 1.97, P = 0.06) and patterns of estrous cycle staging. Cyp27b1 null mice transferred to a Vit D3+ diet also began to spend less time in diestrus II (t = 2.83, P = 0.03) and more time in estrus (t = 3.37, P = 0.019) and proestrus (t = 4.23, P = 0.008) (Fig. 5).
FIG. 5. .
Vitamin D3 deficiency reversibly disrupts the estrous cycle. Average percentage of time spent in diestrus I (A), diestrus II (B), proestrus (C), and estrus (D) when Vit D3− Cyp27b1 mice (KO [D-]) were switched to a Vit D3+ (D+) diet. WT (Vit D3+) mice described in Figure 4 were included for comparison. aP < 0.03 vs. WT and KO (D+) mice; n = 6. E) Representative estrous cycle of Cyp27b1 mouse fed Vit D3+ diet throughout gestation and lactation and weaned onto a Vit D3− diet. F) Representative estrous cycle of the same Cyp27b1 mouse transferred to a Vit D3+ diet for 4 wk. P, proestrus; E, estrus; D1, diestrus 1; D2, diestrus 2. (n = 6).
Experiment 2: Prepubertal Vitamin D3 Deficiency Does Not Adversely Affect Ovarian Responsiveness to Superovulation with Exogenous Gonadotropins
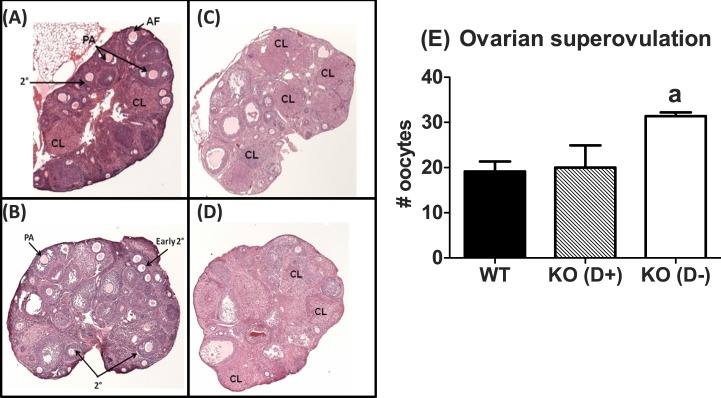
Studies in VDR null female mice suggest that vitamin D3 deficiency induces gonadotropin-resistant atrophic ovaries [28, 38]. To determine whether abnormal estrous cyclicity in Vit D3-deficient Cyp27b1 null mice resulted from ovarian insufficiency or reduced ovarian responsiveness to gonadotropins, we superovulated 7- to 9-wk-old WT and Cyp27b1 null mice weaned onto and maintained on a Vit D3+ or Vit D3− diet with exogenous gonadotropins or saline on diestrus (n = 4–6). Ovaries of saline-treated WT mice and Cyp27b1 null mice on a Vit D3+ diet exhibited follicular heterogeneity and multiple corpora lutea (Fig. 6A, ovaries of Cyp27b1 null mice fed Vit D3+ diets not shown). In contrast, Cyp27b1 null mice on a Vit D3− diet had few or no corpora lutea (CL) and exhibited arrested folliculogenesis, with most follicles in the preantral stage (Fig. 6B). Regardless of diet, superovulation with exogenous gonadotropins supported follicular development and ovulation in all mice (Fig. 6E). Interestingly, superovulated vitamin D3-deficient Cyp27b1 null mice deposited significantly more oocytes in the oviducts than the WT or Cyp27b1 null mice exposed to Vit D3+ diet throughout life (F = 6.4, P = 0.01).
FIG. 6. .
Diet-induced vitamin D3 deficiency is associated with a robust response to superovulation with exogenous gonadotropins. Representative photomicrograph (original magnification ×40) of WT (top) and Cyp27b1 null mice fed a Vit D3-deficient diet during the peripubertal period (bottom), injected with saline or superovulated with eCG plus hCG. A) WT mice injected with saline. B) Cyp27b1 null mice fed a Vit D3− diet, injected with saline. C) WT mice fed a Vit D3+ diet, injected with eCG and hCG. D) Cyp27b1 null mice fed a Vit D3− diet, injected with eCG and hCG. E) Number of oocytes deposited into the oviduct of WT and CYP27b1 null (KO) mice fed a Vit D3+ diet and Cyp27b1 null mice fed a Vit D3− diet after superovulation with eCG and hCG. AF, antral follicle; CL, corpus luteum; PA, early preantral; 2°, secondary. aP = 0.02 vs. WT and KO mice fed a Vit D3+ diet during the peripubertal transition (n = 4–6).
Experiment 3: Prepubertal Vitamin D3 Deficiency Does Not Affect Diestrus Gonadotropin Levels
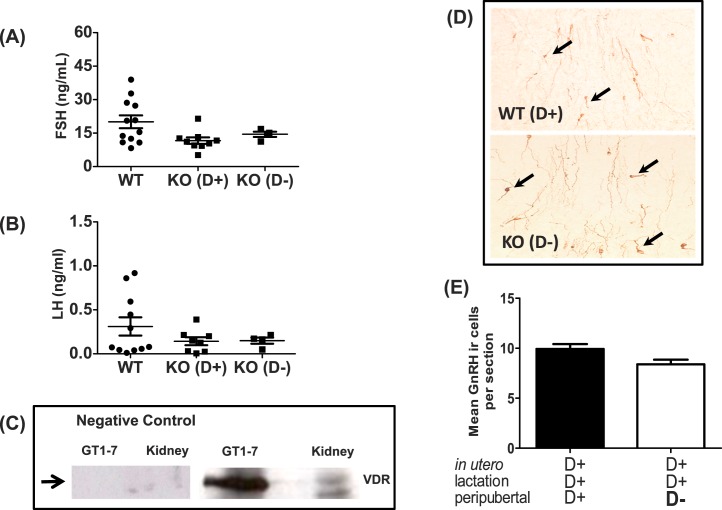
Studies in VDR KO female mice suggest that vitamin D3 deficiency induces hypergonadotropic hypogonadism [28]. To determine whether Vit D3 deficiency effects were sustained beyond the pubertal transition affected endogenous gonadotropin levels in Cyp27b1 null mice, we killed 9- to 11-wk-old female mice in diestrus and measured serum FSH and LH levels (Fig. 7, A and B). Diet did not affect FSH (F = 3.3, P > 0.05) or LH (F = 1.2, P > 0.05) levels in diestrus WT or Cyp27b1 null mice (n = 4–12).
FIG. 7. .
Effects of vitamin D3 on serum gonadotropin levels during mice in diestrus, VDR expression in GT1-7, and density of hypothalamic GnRH neurons. A) Serum FSH in reproduction-aged WT and Cyp27b1 null mice fed a vitamin D3-sufficient diet (KO [D+]) or a D3-deficient diet (KO [D−]) (n = 4–12). B) Serum LH in reproduction-aged WT and Cyp27b1 null mice fed a vitamin D3-sufficient diet (KO [D+]) or D3-deficient diet (KO [D−]) (n = 4–12). C) Western blot showing VDR in cell lysates from GT1-7 neurons and kidney. The positive control is WT mouse kidney, and negative control is GT1-7 and kidney lysate without primary VDR antibody. D) Representative sections of single-label immunohistochemistry (original magnification ×40) showing GnRH neurons (brown cytoplasm) in WT Vit D3+ and Cyp27b1 null Vit D3− mice. Arrows indicate GnRH immunoreactive neurons (n = 4). E) Average number of GnRH neuron numbers per hypothalamic section (means ± SEM) in WT Vit D3+ and Cyp27b1 null Vit D3− mice. Hypothalamic sections reviewed corresponded to plates 25–32 of the Paxinos and Watson mouse atlas [49] and the hypothalamic region between the organum vasculosum of lamina terminalis and the medial POA (n = 4).
Experiment 4: VDR Is Present in GT1-7 Neurons
Our data suggest that prepubertal Vit D3 deficiency, in part, disrupts hypothalamic-pituitary function. Cyp27b1 and VDR are highly expressed in the hypothalamus [29, 30], and hypothalamic VDR expression is 17-fold increased from birth to Postnatal Day 60 in male rats [30]. To determine whether GnRH neurons express VDR, we analyzed Western blots by using antibodies against VDR on lysates of immortalized GnRH neurons (GT1-7 cells) and found that GT1-7 neurons express VDR (Fig. 7C).
Experiment 5: Postweaning Vitamin D3 Deficiency Does Not Affect the Number of Immunoreactive GnRH Neurons Found in the Hypothalamus
To determine if the reproductive phenotype observed with peripubertal Vit D3 deficiency reflected a reduced quantity of GnRH-ir neurons [52], we quantified the average number of GnRH-ir neurons in five 30-μm-thick hypothalamic sections (between the level of the organum vasculosum of lamina terminalis through the medial POA) collected from WT mice maintained on a Vit D3+ diet and Cyp27b1 null mice exposed to peripubertal Vit D3−. Peripubertal Vit D3 deficiency did not significantly affect the number of hypothalamic GnRH neurons found in young adult females (Fig. 7, D and E).
DISCUSSION
The present study demonstrates that peripubertal vitamin D3 deficiency delays puberty and causes prolonged estrous cycles characterized by extended periods of diestrus and reduced frequency of proestrus and estrus. These phenotypes are observed in the absence of obesity or delayed weight gain. Moreover, estrous cycles can be normalized in young adults by correcting the Vit D3 deficiency. In addition, the ovaries of Vit D3-deficient Cyp27b1 null mice respond robustly to exogenous gonadotropins. These findings suggest the delayed pubertal transition in Vit D3-deficient Cyp27b1 null mice does not result from primary ovarian insufficiency or primary ovarian or pituitary failure. Vitamin D3-deficient Cyp27b1 null mice also have equivalent levels of LH and FSH during diestrus as those in Vit D3-sufficient mice, suggesting that estrogen-negative feedback may be intact. However, additional experiments that confirm these data and assess progesterone and prolactin levels, other known modifiers of gonadotropin secretion, are needed. To our knowledge, we also provide the first evidence showing that GT1-7 neurons, immortalized GnRH neurons, express VDR. Together, these data suggest peripubertal Vit D3 deficiency delays pubertal transition and disrupts estrous cyclicity by disrupting hypothalamic-pituitary axis physiology.
Peripubertal Vitamin D3 Deficiency Delays Puberty
The largest growing populations with vitamin D3 deficiency are peripubertal children and reproduction-aged adult females [14, 18, 53, 54]. We demonstrated that the effect of vitamin D3 deficiency on puberty, delayed vaginal opening and first estrus, was restricted to the peripubertal period. When mice were deficient in vitamin D3 in utero, during lactation, and prior to weaning, pubertal timing was normal. These finding suggest that the vitamin D3-sensitive physiological events critical to the pubertal transition occur peripubertally. Puberty depends upon coordinated interactions among all components of the hypothalamic-pituitary-gonadal axis. The onset of puberty is driven by nongonadal events characterized by dynamic changes in glial-neuron interactions [55, 56] and trans-synaptic changes in afferent glutamatergic, kisspeptinergic, and GABAergic input onto GnRH neurons [57–65]. These changes are hypothesized to induce sustained GnRH peptide release, activation of the pituitary-gonadal axis, an estrogen surge, and vaginal opening. It is possible that peripubertal vitamin D3 deficiency delays puberty by compromising the trans-synaptic excitatory and/or inhibitory afferent input required for peripubertal activation of GnRH neurons. The mechanism by which vitamin D3 deficiency might disrupt peripubertal GnRH neuron activation is unclear. Several recent studies suggest that vitamin D3 regulates expression of L-type voltage-sensitive calcium channels and nerve growth factor release in the brain [66–68]. It is possible that vitamin D3 deficiency disrupts L-type voltage-sensitive calcium channel expression systems critical for peripubertal GnRH neuronal activation [69]. More studies are needed in females to determine whether hypothalamic VDR expression changes during the pubertal transition and to determine why the hypothalamic-pituitary axis of peripubertal females is susceptible to the adverse effects of vitamin D3. Interestingly, a recent prospective cohort study that assessed the pubertal transition of Columbian girls suggested that vitamin D3 deficiency is associated with early puberty (11.8 ± 0.2 vs. 12.6 ± 0.2 years) [70]. Unfortunately this study did not control for other nutritional deficiencies, environmental exposures, hyperparathyroidism, or calcium homeostasis. Moreover, the authors only determined vitamin D3 levels at one time point. Consequently, it is unclear how long the girls were vitamin D3 deficient or what their levels were at the time of the pubertal transition. Thus, it is difficult to compare our findings to that observational study.
Whereas the initiation of puberty begins with activation of the hypothalamic-pituitary axis, the completion of the pubertal transition and attainment of reproductive competence depend upon functional and gonadotropin-responsive gonads. Within a week of vaginal opening, a second estrogen surge occurs, which is followed by ovulation and first estrus [71]. Vaginal opening and first estrus signify the completion of puberty and the potential to reproduce. Vit D3 deficiency significantly delayed first estrus. However, regardless of the peripubertal Vit D3 dietary status, the time between vaginal opening and first estrus was not delayed. These data are consistent with the hypothesis that delayed first estrus results from delayed vaginal opening. Moreover, these data suggest that the delayed puberty observed in Vit D3-deficient females may reflect primary neuroendocrine dysfunction rather than primary ovarian event such as ovarian failure or primary ovarian resistance to gonadotropins.
Delayed puberty could reflect an unanticipated effect of total body KO of the Cyp27b1 gene. However, compared to WT mice, the Cyp27b1 null littermates weaned onto a Vit D3+ diet did not have delayed puberty. Moreover, puberty was delayed in WT females also exposed to peripubertal Vit D3 deficiency. These data argue against a main effect of Cyp27b1 gene deletion on timing of puberty. Delayed puberty can also be seen in states of malnourishment and/or caloric restriction. To ensure that the reproductive phenotype did not reflect malnourishment, we assessed growth curves of WT and Cyp27b1null mice weaned onto a Vit D3+ or Vit D3− diet. Neither Cyp27b1 deletion nor peripubertal Vit D3 deficiency affected postnatal growth curves or weight gain proximal to the time of vaginal opening.
Vitamin D3 Deficiency Acts on the Neuroendocrine Axis to Disrupt Ovarian Physiology
Young adult Cyp27b1 null mice maintained on a Vit D3− diet after puberty had prolonged estrous cycles that were characterized by extended periods of diestrus and fewer episodes of proestrus and estrus. Ovaries collected from these mice exhibited reduced follicular heterogeneity with few to no corpora lutea. Additionally, most ovarian follicles in ovaries of peripubertal Vit D3-deficient females were arrested in the preantral stage. The reproductive phenotype of prolonged estrous cycles and arrested follicular development could reflect hypothalamic, pituitary, or ovarian dysfunction. We sought to determine whether abnormal estrous cyclicity reflected a primary ovarian failure or insufficiency, because both VDR and Cyp27b1 are expressed in the ovary [38, 39]. Additionally, studies with VDR null mice suggest that the ovaries are resistant to gonadotropins [28, 38]. We treated WT and Cyp27b1 null mice weaned and maintained on a Vit D3+ diet and Cyp27b1 null mice weaned and maintained on a Vit D3− diet with doses of equine chorionic gonadotropin (eCG) and hCG, typically used for superovulation. Regardless of dietary vitamin D3 status, superovulation stimulated ovarian follicular development in all mice. Thus, it is unlikely that Vit D3 deficiency caused primary ovarian insufficiency, which is associated with reduced numbers of oocytes after superovulation, or primary ovarian gonadotropin resistance, which would be associated with the failure of the mice to respond to exogenous gonadotropins. Instead, vitamin D3-deficient Cyp27b1 null mice ovulated significantly more oocytes into the oviducts following superovulation than WT or Cyp27b1 null mice maintained on a Vit D3+ diet. It is unlikely that the increased number of oocytes in the peripubertal Vit D3− mice reflect multiple ova per follicle because the initial pool of ovarian follicles is established before weaning [72, 73]. These data suggest that arrested ovarian follicular development most likely reflects suboptimal endogenous gonadotropin secretion. Additionally, the paucity of corpora lutea in vitamin D3− Cyp27b1 null mice ovaries could reflect abnormal or absent estradiol-positive feedback. Nonetheless, these data do not completely rule out the possibility of reduced ovarian responsiveness to gonadotropin. Studies that assess the dose responsiveness of the ovaries of Vit D3-deficient mice to exogenous gonadotropin are needed to investigate this possibility.
The reproductive phenotype of vitamin D3-deficient Cyp27b1 null mice is dissimilar to that of the transgenic VDR null female mice, which display hypergonadotropic hypogonadism, reduced ovarian aromatase expression, and gonadotropin-resistant and atrophic ovaries [28, 38]. Differences between the reproductive phenotypes observed in VDR null and Cyp27b1 null mice could result from the deletion of VDR rather than Vit D3 deficiency. It is well established that binding of 1,25-(OH)2D3 to VDR causes recruitment and heterodimerization with the RXR receptor [11]. Because VDR/RXR heterodimers regulate the transcription of a number of tissue-specific genes, it is possible that the inability to form VDR/RXR heterodimers may result in phenotypes and pathologies that are independent of those specific to Vit D3 deficiency [74]. Consistent with this hypothesis, VDR null mice have physical and behavioral abnormalities suggestive of cognitive dysfunction, premature alopecia, and other findings consistent with an early aging phenotype [74]. A premature aging phenotype is not seen in Cyp27b1 null mice or in comparably aged, diet-induced Vit D3-deficient rats [23, 39]. Cyp27b1 null mice have functional VDR but lack the ability to convert Vit D3 to its most metabolically active form. Thus, Cyp27b1 null mice have pathological findings that are more consistent with nutritional Vit D3 deficiency and therefore are a more suitable model with which to investigate the pathophysiological consequences of diet-induced Vit D3 deficiency in female reproductive physiology.
Vit D3-deficient Cyp27b1 null mice also exhibited irregular estrous cycles. To determine if the effect of postweaning Vit D3 deficiency on estrous cyclicity was reversible, we monitored daily vaginal smears after replacing the Vit D3− diet with a Vit D3+ diet. Within 4 wk of introducing a Vit D3+ diet, mice with previously prolonged estrous cycles began to exhibit normal estrous cycles lengths and staging patterns. These data suggest that the pathophysiological consequences of peripubertal Vit D3 deficiency on the estrous cycle are reversible.
Pituitary Gonadotrophs
VDRs are found in the pituitary. Therefore, it is possible that Vit D3 directly regulates gonadotropin synthesis or release and consequently estrous cyclicity. Consistent with this hypothesis, VDR null mice failed to suppress gonadotropins when estradiol levels were increased, thereby suggesting abnormalities in estradiol-negative feedback [28]. In contrast to VDR null mice, Vit D3-deficient Cyp27b1 null females had gonadotropin levels during diestrus that were as low as those of Vit D3-sufficient WT and Cyp27b1 null mice, suggesting estradiol-negative feedback was intact. In contrast, if Vit D3 deficiency reduced the responsiveness of the hypothalamic-pituitary axis to estradiol-negative feedback similar to the effect of VDR KO [28], then we would have expected hypergonadotropism rather than eugonadotropism. Moreover, because estradiol levels are typically low in diestrus, it is unlikely that the effect of vitamin D3 deficiency on gonadotropin release was masked or minimized by persistently elevated estradiol. Consistent with this hypothesis, we have preliminary data that demonstrate gonadectomized females exposed to vitamin D3 deficiency and primed with estradiol and progesterone have equally low levels of gonadotropins (data not shown). Nonetheless, it is still possible that Vit D3 deficiency alters hypothalamic-pituitary responsiveness to estrogen positive feedback.
GnRH Neurons Express VDRs
Our studies suggest, in part, that peripubertal vitamin D3 deficiency disrupts hypothalamic-pituitary physiology, resulting in suboptimal exposure to endogenous gonadotropins, arrested follicular development, and estrous cycle irregularities. In situ hybridization studies localize VDR, Cyp27b1, and vitamin D binding protein in the hypothalamus [30, 33, 75]. Additionally, a recent study by Walker et al. [40] reported that VDR mRNA expression increases in the medial preoptic area between the peripubertal period until 60 days of age in male rats. The presence of VDR and Cyp27b1 in the preoptic area of the hypothalamus raises the possibility that Vit D3 regulates the activity of GnRH neurons or other hypothalamic neurons important for reproduction. We used Western blots of GT1-7 neuron extracts to determine whether immortalized GnRH neurons express VDR and demonstrated the presence VDR protein in GT1-7 neurons. Although there are differences between adult GnRH neurons and GT1-7 neurons [76], the presence of VDR on immortalized GnRH neurons raises the possibility that vitamin D3 has direct regulatory effects on GnRH neuronal physiology. Nonetheless, our studies do not rule out the possibility that Vit D3 may regulate afferent input to GnRH neurons [77].
GnRH Neuron Density and Peripubertal Vitamin D3 Deficiency
Reduced numbers of GnRH neurons can adversely affect puberty and adult female reproductive physiology [52]. Additionally, developmental Vit D3 deficiency is hypothesized to adversely affect neurodevelopment [78]. To assess the possibility that our reproductive phenotype resulted from abnormal development of GnRH neurons, we counted GnRH neurons in hypothalami of females exposed to peripubertal Vit D3 deficiency and compared them to the numbers in those exposed to Vit D3 sufficiency. No significant differences were found in the average number of GnRH-ir neurons per hypothalamic section nor in the total number of GnRH neurons located between the organum vasculosum of lamina terminalis and the medial POA of the hypothalamus (data not shown). These data imply that neither the delayed pubertal transition nor the irregular estrous cycling observed in females exposed to peripubertal Vit D3 deficiency resulted from reduced numbers of GnRH neurons available to drive normal female reproductive physiology [52]. However, we have not ruled out the possibility that peripuberteral Vit D3 deficiency affects the ability of GnRH neurons to respond appropriately to hormonal cues.
Calcium Dysregulation and Vitamin D3 Deficiency
Hypocalcemia caused by Vit D3 deficiency induces male infertility by direct effects on sperm [23, 28, 37]. The role of calcium in Vit D3 regulation of female reproductive physiology is less clear. A recent study that used Cyp27b1 null mice reported that Vit D3-related hypocalcemia caused gonadotropin-resistant ovaries with arrested follicular development and irregular estrous cycles characterized by extended periods of diestrus. These abnormalities were restored by calcium-phosphate supplementation [26]. We documented similar changes in the reproductive phenotype of Cyp27b1 mice weaned onto a Vit D3-deficient diet but supplemented with calcium, suggesting that suboptimal calcium homeostasis and/or hypocalcemia does not cause the reproductive abnormalities. The main difference between the present study and that by Sun et al. [26] is that our mice received a diet completely devoid of Vit D3 but supplemented with calcium. Sun et al. [26] fed mice chow that contained 25-(OH)D3 and a calcium supplement for several weeks. High serum levels of 25-(OH)D3 can bind and activate VDR [79, 80]. Thus, it is possible that calcium supplementation on a background of elevated 25-(OH)D3 in Cyp27b1 null mice [39, 41] afforded normal VDR receptor physiology and rescued the abnormal reproductive phenotype [26]. Consistent with this hypothesis, Lou et al. [79] recently demonstrated that Cyp27b1 null mice but not VDR null mice responded robustly to 25-(OH)D3 [79]. Our approach of feeding a Vit D3-deficient diet eliminated this confounding factor. Moreover, our results are consistent with previous studies demonstrating that calcium supplementation and dietary Vit D3 but not calcium supplementation alone maintained Vit D3-regulated physiology in Cyp27b1 null mice [80].
In conclusion, this study provides compelling evidence that peripubertal Vit D3 is critical for the appropriately timed pubertal transition and the establishment of regular estrous cycles. Vit D3 deficiency occurring after weaning delays the onset of the pubertal transition, arrests ovarian follicular development, reduces ovulatory events, and reversibly disrupts estrous cyclicity. The presence of a eugonadotropic state as well as normal ovarian responsiveness to exogenous gonadotropins in Vit D3-deficient Cyp27b1 null mice suggests that vitamin D3 deficiency does not cause a primary ovarian insufficiency state or frank ovarian failure. We also demonstrated that GnRH neurons express VDR protein, raising the possibility that Vit D3 directly regulates GnRH neurons. Therefore, we propose that Vit D3 deficiency most likely impairs female reproductive function by inducing hypothalamic dysfunction, which secondarily affects pituitary and ovarian physiology. Future studies focused on the neuroendocrine axis are necessary to further define the mechanisms by which Vit D3 influences female reproduction.
ACKNOWLEDGMENT
We thank Dr. Anne Etgen for critical review and thoughtful comments. We also thank Drs. Marie Menke, Edward Nejat, and Jessica Santollo for their assistance with mouse care. Thanks also to Dr. Rani Sellers for assistance with histologic examination of ovaries, Dr. Ken Chen and the transgenic facility for their expertise with superovulation and oocytes harvesting, and Robin Sgueglia in the hormone assay core for performing the gonadotropin assay. We also acknowledge and thank Dr. David Goltzman for the generous donation of the Cyp27b1 mice and Pamela Mellon for providing GT1-7 cells.
Footnotes
Supported by National Institutes of Health grant R21 HD066355, the Zondek Award for Female Reproductive Research, and Department of Obstetrics and Gynecology and Women's Health, Montefiore Medical Center and Albert Einstein College of Medicine.
REFERENCES
- Christakos S, Barletta F, Huening M, Dhawan P, Liu Y, Porta A, Peng X. Vitamin D target proteins: function and regulation. J Cell Biochem 2003; 88 (2): 238 244 [DOI] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem 2003; 88 (4): 695 705 [DOI] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Peng X, Obukhov AG, Nowycky MC, Benn BS, Zhong Y, Liu Y, Shen Q. New insights into the function and regulation of vitamin D target proteins. J Steroid Biochem Mol Biol 2007; 103 (3–5): 405 410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D, Almeras L, Benech P, Patatian A, Mackay-Sim A, McGrath J, Feron F. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol 2007; 103 (3–5): 538 545 [DOI] [PubMed] [Google Scholar]
- Hakim I, Bar-Shavit Z. Modulation of TNF-alpha expression in bone marrow macrophages: involvement of vitamin D response element. J Cell Biochem 2003; 88 (5): 986 998 [DOI] [PubMed] [Google Scholar]
- Lips P. Vitamin D physiology. Prog Biophys Mol Biol 2006; 92 (1): 4 8 [DOI] [PubMed] [Google Scholar]
- Wecksler WR, Norman AW. A kinetic and equilibrium binding study of 1 alpha, 25-dihydroxyvitamin D3 with its cytosol receptor from chick intestinal mucosa. J Biol Chem 1980; 255 (8): 3571 3574 [PubMed] [Google Scholar]
- Wecksler WR, Norman AW. Structural aspects of the binding of 1 alpha, 25-dihydroxyvitamin D3 to its receptor system in chick intestine. Methods Enzymol 1980; 67: 494 500 [DOI] [PubMed] [Google Scholar]
- Wecksler WR, Norman AW. Measurement of kinetic rate constants for the binding of 1 alpha, 25-dihydroxyvitamin D3 to its chick intestinal mucosa receptor using a hydroxyapatite batch assay. Methods Enzymol 1980; 67: 488 494 [DOI] [PubMed] [Google Scholar]
- Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev 1995; 16 (2): 200 257 [DOI] [PubMed] [Google Scholar]
- Christakos S, Raval-Pandya M, Wernyj RP, Yang W. Genomic mechanisms involved in the pleiotropic actions of 1, 25-dihydroxyvitamin D3. Biochem J 1996; 316 (Pt 2): 361 371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizwicki MT, Norman AW. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal 2009; 2 (75): re4 [DOI] [PubMed] [Google Scholar]
- Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 2008; 88 (6): 1519 1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 2002; 76 (1): 187 192 [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Catov JM, Roberts JM, Simhan HN. Prepregnancy obesity predicts poor vitamin D status in mothers and their neonates. J Nutr 2007; 137 (11): 2437 2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 2007; 137 (2): 447 452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. Am J Clin Nutr 2008; 88 (2): 520S 528S [DOI] [PubMed] [Google Scholar]
- Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism 2008; 57 (2): 183 191 [DOI] [PubMed] [Google Scholar]
- McCullough ML. Vitamin D deficiency in pregnancy: bringing the issues to light. J Nutr 2007; 137 (2): 305 306 [DOI] [PubMed] [Google Scholar]
- Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, Kimmig R, Mann K, Janssen OE. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes 2006; 114 (10): 577 583 [DOI] [PubMed] [Google Scholar]
- Halloran BP, DeLuca HF. Effect of vitamin D deficiency on fertility and reproductive capacity in the female rat. J Nutr 1980; 110 (8): 1573 1580 [DOI] [PubMed] [Google Scholar]
- Kwiecinksi GG, Petrie GI, DeLuca HF. 1, 25-Dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am J Physiol 1989; 256 (4 Pt 1): E483 487 [DOI] [PubMed] [Google Scholar]
- Kwiecinski GG, Petrie GI, DeLuca HF. Vitamin D is necessary for reproductive functions of the male rat. J Nutr 1989; 119 (5): 741 744 [DOI] [PubMed] [Google Scholar]
- Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril 2009; 94 (5): 1314 1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi T. Genetic variation in the vitamin D receptor and polycystic ovary syndrome risk. Fertil Steril 2009; 92 (4): 1381 1383 [DOI] [PubMed] [Google Scholar]
- Sun W, Xie H, Ji J, Zhou X, Goltzman D, Miao D. Defective female reproductive function in 1, 25(OH)2D-deficient mice results from indirect effect mediated by extracellular calcium and/or phosphorus. Am J Physiol Endocrinol Metab 2011; 299 (6): E928 935 [DOI] [PubMed] [Google Scholar]
- Johnson LE, DeLuca HF. Reproductive defects are corrected in vitamin d-deficient female rats fed a high calcium, phosphorus and lactose diet. J Nutr 2002; 132 (8): 2270 2273 [DOI] [PubMed] [Google Scholar]
- Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 2000; 141 (4): 1317 1324 [DOI] [PubMed] [Google Scholar]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 2005; 29 (1): 21 30 [DOI] [PubMed] [Google Scholar]
- Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1, 25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat 1999; 16 (2): 135 145 [DOI] [PubMed] [Google Scholar]
- Stumpf WE, O'Brien LP. 1, 25 (OH)2 vitamin D3 sites of action in the brain. An autoradiographic study. Histochemistry 1987; 87 (5): 393 406 [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M, O'Brien LP. Vitamin D sites of action in the pituitary studied by combined autoradiography-immunohistochemistry. Histochemistry 1987; 88 (1): 11 16 [DOI] [PubMed] [Google Scholar]
- Walbert T, Jirikowski GF, Prufer K. Distribution of 1, 25-dihydroxyvitamin D3 receptor immunoreactivity in the limbic system of the rat. Horm Metab Res 2001; 33 (9): 525 531 [DOI] [PubMed] [Google Scholar]
- Dokoh S, Donaldson CA, Marion SL, Pike JW, Haussler MR. The ovary: a target organ for 1, 25-dihydroxyvitamin D3. Endocrinology 1983; 112 (1): 200 206 [DOI] [PubMed] [Google Scholar]
- Corbett ST, Hill O, Nangia AK. Vitamin D receptor found in human sperm. Urology 2006; 68 (6): 1345 1349 [DOI] [PubMed] [Google Scholar]
- Johnson LE, DeLuca HF. Vitamin D receptor null mutant mice fed high levels of calcium are fertile. J Nutr 2001; 131 (6): 1787 1791 [DOI] [PubMed] [Google Scholar]
- Uhland AM, Kwiecinski GG, DeLuca HF. Normalization of serum calcium restores fertility in vitamin D-deficient male rats. J Nutr 1992; 122 (6): 1338 1344 [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 1997; 16 (4): 391 396 [DOI] [PubMed] [Google Scholar]
- Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A 2001; 98 (13): 7498 7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Juenger TE, Gore AC. Developmental profiles of neuroendocrine gene expression in the preoptic area of male rats. Endocrinology 2009; 150 (5): 2308 2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem 2004; 279 (16): 16754 16766 [DOI] [PubMed] [Google Scholar]
- Cohen PE, Zhu L, Nishimura K, Pollard JW. Colony-stimulating factor 1 regulation of neuroendocrine pathways that control gonadal function in mice. Endocrinology 2002; 143 (4): 1413 1422 [DOI] [PubMed] [Google Scholar]
- Cohen PE, Zhu L, Pollard JW. Absence of colony stimulating factor-1 in osteopetrotic (csfmop/csfmop) mice disrupts estrous cycles and ovulation. Biol Reprod 1997; 56 (1): 110 118 [DOI] [PubMed] [Google Scholar]
- Wu S, Divall S, Hoffman GE, Le WW, Wagner KU, Wolfe A. Jak2 is necessary for neuroendocrine control of female reproduction. J Neurosci 31 (1): 184 192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HP, Sarkar G, Etgen AM. Estradiol and progesterone modulate the nitric oxide/cyclic gmp pathway in the hypothalamus of female rats and in GT1-1 cells. Endocrine 2004; 24 (2): 177 184 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Le WW. Just cool it! Cryoprotectant anti-freeze in immunocytochemistry and in situ hybridization. Peptides 2004; 25 (3): 425 431 [DOI] [PubMed] [Google Scholar]
- Le WW, Wise PM, Murphy AZ, Coolen LM, Hoffman GE. Parallel declines in Fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology 2001; 142 (11): 4976 4982 [DOI] [PubMed] [Google Scholar]
- Sun Y, Todd BJ, Thornton K, Etgen AM, Neal-Perry G. Differential effects of hypothalamic IGF-I on gonadotropin releasing hormone neuronal activation during steroid-induced LH surges in young and middle-aged female rats. Endocrinology 2011; 152 (11): 4276 4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 6th ed. San Diego, CA: Academic Press; 2007 [Google Scholar]
- Gajdos ZK, Hirschhorn JN, Palmert MR. What controls the timing of puberty? An update on progress from genetic investigation. Curr Opin Endocrinol Diabetes Obes 2009; 16 (1): 16 24 [DOI] [PubMed] [Google Scholar]
- Martos-Moreno GA, Chowen JA, Argente J. Metabolic signals in human puberty: effects of over and undernutrition. Mol Cell Endocrinol 324 (1–2): 70 81 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 2008; 149 (2): 597 604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradlee ML, Singer MR, Qureshi MM, Moore LL. Food group intake and central obesity among children and adolescents in the Third National Health and Nutrition Examination Survey (NHANES III). Public Health Nutr 2009; 1 9 [DOI] [PubMed]
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr 2008; 88 (2): 558S 564S [DOI] [PubMed] [Google Scholar]
- Ma YJ, Hill DF, Creswick KE, Costa ME, Cornea A, Lioubin MN, Plowman GD, Ojeda SR. Neuregulins signaling via a glial erbB-2-erbB-4 receptor complex contribute to the neuroendocrine control of mammalian sexual development. J Neurosci 1999; 19 (22): 9913 9927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YJ, Hill DF, Junier MP, Costa ME, Felder SE, Ojeda SR. Expression of epidermal growth factor receptor changes in the hypothalamus during the onset of female puberty. Mol Cell Neurosci 1994; 5 (3): 246 262 [DOI] [PubMed] [Google Scholar]
- Kasuya E, Nyberg CL, Mogi K, Terasawa E. A role of gamma-amino butyric acid (GABA) and glutamate in control of puberty in female rhesus monkeys: effect of an antisense oligodeoxynucleotide for GAD67 messenger ribonucleic acid and MK801 on luteinizing hormone-releasing hormone release. Endocrinology 1999; 140 (2): 705 712 [DOI] [PubMed] [Google Scholar]
- Terasawa E, Luchansky LL, Kasuya E, Nyberg CL. An increase in glutamate release follows a decrease in gamma aminobutyric acid and the pubertal increase in luteinizing hormone releasing hormone release in the female rhesus monkeys. J Neuroendocrinol 1999; 11 (4): 275 282 [DOI] [PubMed] [Google Scholar]
- Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 2002; 143 (4): 1459 1466 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol 2011; 23 (7): 557 569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci U S A 2010; 107 (52): 22693 22698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 2005; 25 (49): 11349 11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth CL, McCormack AL, Lomniczi A, Mungenast AE, Ojeda SR. Quantitative proteomics identifies a change in glial glutamate metabolism at the time of female puberty. Mol Cell Endocrinol 2006; 254–255: 51 59 [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Sandau U. Contribution of glial-neuronal interactions to the neuroendocrine control of female puberty. Eur J Neurosci 2010; 32 (12): 2003 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Loche A, Matagne V, Kaidar G, Sandau US, Dissen GA. The transcriptional control of female puberty. Brain Res 2010; 1364: 164 174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 2001; 21 (1): 98 108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gezen-Ak D, Dursun E, Yilmazer S. The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLoS One 2011; 6 (3): e17553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli A, Squecco R, Failli P, Filippi S, Vignozzi L, Chavalmane AK, Fibbi B, Mancina R, Luciani G, Gacci M, et al. The vitamin D receptor agonist elocalcitol upregulates L-type calcium channel activity in human and rat bladder. Am J Physiol Cell Physiol 2008; 294 (5): C1206 C1214 [DOI] [PubMed] [Google Scholar]
- Malinina E, Druzin M, Johansson S. Differential control of spontaneous and evoked GABA release by presynaptic L-type Ca(2+) channels in the rat medial preoptic nucleus. J Neurophysiol 104 (1): 200 209 [DOI] [PubMed] [Google Scholar]
- Villamor E, Marin C, Mora-Plazas M, Baylin A. Vitamin D deficiency and age at menarche: a prospective study. Am J Clin Nutr 2011; 94 (4): 1020 1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safranski TJ, Lamberson WR, Keisler DH. Correlations among three measures of puberty in mice and relationships with estradiol concentration and ovulation. Biol Reprod 1993; 48 (3): 669 673 [DOI] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol 2006; 298 (1): 132 148 [DOI] [PubMed] [Google Scholar]
- Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod 2009; 15 (12): 795 803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keisala T, Minasyan A, Lou YR, Zou J, Kalueff AV, Pyykko I, Tuohimaa P. Premature aging in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol 2009; 115 (3–5): 91 97 [DOI] [PubMed] [Google Scholar]
- Jirikowski GF, Kaunzner UW. Dief Ael E, Caldwell JD. Distribution of vitamin D binding protein expressing neurons in the rat hypothalamus. Histochem Cell Biol 2009; 131 (3): 365 370 [DOI] [PubMed] [Google Scholar]
- Selmanoff M. Commentary on the use of immortalized neuroendocrine cell lines for physiological research. Endocrine 1997; 6 (1): 1 3 [DOI] [PubMed] [Google Scholar]
- Baksi SN, Hughes MJ. Chronic vitamin D deficiency in the weanling rat alters catecholamine metabolism in the cortex. Brain Res 1982; 242 (2): 387 390 [DOI] [PubMed] [Google Scholar]
- Eyles DW, Feron F, Cui X, Kesby JP, Harms LH, Ko P, McGrath JJ, Burne TH. Developmental vitamin D deficiency causes abnormal brain development.Psychoneuroendocrinology 2009; 34 (suppl 1): S247 257 [DOI] [PubMed] [Google Scholar]
- Lou YR, Molnar F, Perakyla M, Qiao S, Kalueff AV, St-Arnaud R, Carlberg C, Tuohimaa P. 25-Hydroxyvitamin D(3) is an agonistic vitamin D receptor ligand. J Steroid Biochem Mol Biol 2010; 118 (3): 162 170 [DOI] [PubMed] [Google Scholar]
- Rowling MJ, Gliniak C, Welsh J, Fleet JC. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr 2007; 137 (12): 2608 2615 [DOI] [PMC free article] [PubMed] [Google Scholar]