SUMMARY
Amyloid-beta (Aβ) oligomers are thought to trigger Alzheimer’s disease (AD) pathophysiology. Cellular Prion Protein (PrPC) selectively binds oligomeric Aβ and can mediate AD-related phenotypes. Here, we examined the specificity, distribution and signaling from Aβ/PrP complexes, seeking to explain how they might alter the function of NMDA receptors in neurons. PrPC is enriched in post-synaptic densities, and Aβ/PrPC interaction leads to Fyn kinase activation. Soluble Aβ assemblies derived from human AD brain interact with PrPC to activate Fyn. Aβ engagement of PrPC/Fyn signaling yields phosphorylation of the NR2B subunit of NMDA-receptors, which is coupled to an initial increase and then loss of surface NMDA-receptors. Aβ-induced LDH release and dendritic spine loss require both PrPC and Fyn, and human familial AD transgene-induced convulsive seizures do not occur in mice lacking PrPC. These results delineate an Aβ oligomer signal transduction pathway requiring PrPC and Fyn to alter synaptic function with relevance to AD.
INTRODUCTION
Genetic, pathological and biomarker studies of Alzheimer’s Disease (AD) provide support for Amyloid-Beta (Aβ) as a key factor in pathogenesis. More specifically, oligomeric species of Aβ peptide (Aβo) are thought to trigger synaptic dysfunction, neurodegeneration and dementia1–4. Key is delineation of the pathway(s) leading from Aβo to downstream pathogenic events. We identified cellular Prion Protein (PrPC) as an oligomer-specific, high-affinity binding site for Aβ5. Binding of Aβo to PrPC has been confirmed by in vivo and in vitro studies6–10.
The contribution of Aβo/PrPC complexes to AD models has been examined. Certain phenotypes occur in the absence of PrPC7,9,11,12. In other paradigms, PrPC is essential for Aβo-induced cell death and impaired synaptic plasticity, as well as AD transgene-induced spatial memory deficits, synapse loss, serotonin axon degeneration and early death5,10,13–20. Critically, human AD brain-derived extracts require PrPC to suppress hippocampal long-term potentiation (LTP)10,16. Treatment of aged APPswe/PSen1-M146L mice with anti-PrPC antibody reverses memory deficits and restores synaptic density14. Despite evidence that PrPC binds Aβo and contributes pathogenically, signal transduction downstream of Aβo/PrPC remains undefined.
Alterations in NMDA receptor (NMDA-R) function contribute to AD pathogenesis21–23. Therefore, Aβo/PrPC complexes are predicted to modify NMDA-R function. Here, we examined the connection of Aβ/PrPC signal transduction to NMDA-R dysfunction. We focused on Fyn kinase for several reasons. Both Fyn and PrPC localize to lipid rafts, and clustering of PrPC is reported to activate Fyn in cell lines24–26. Moreover, PrPC loss-of-function in zebrafish is mimicked by reduced Fyn and rescued by increasing Fyn activation27, while mutant PrPC-induced degeneration in worm requires Fyn28. With regard to synapses, Fyn is localized to the post-synaptic density (PSD)29, plays a critical role in LTP30, and phosphorylates the NMDA-R subunits, NR2A/B29,31. Of relevance to AD, transgenic models are exacerbated by Fyn overexpression and ameliorated by Fyn deletion32.
We show that PrPC is enriched in the PSD, that Aβo binding to PrPC activates Fyn, and that human AD brain-derived Aβ stimulates PrPC signaling. This Fyn pathway leads to NR2B phosphorylation and altered NMDA-R localization, with destabilization of dendritic spines. A PrPC-dependent pathway is required for seizures in AD transgenic mice, providing a basis for the rescue of survival by PrPC deletion.
ON-LINE METHODS
Mice
WT, Prnp−/−, APP/PSen and APP/PSen Prnp−/− mice on the C57B6/J background were as described13. Fyn−/− mice51 were obtained from Jackson Laboratories. Both males and females were used in approximately equal numbers, and none excluded. All experiments were approved by the Institutional Animal Care and Use Committee.
Aβ peptide
Aβ42 oligomer, monomer and fibrillary preparations have been characterized5. Concentrations are in monomer equivalents. Oligomeric preparations with 1 µM total Aβ42 peptide contain about 10 nM oligomeric species5.
Recombinant human PrP(23–111)
PrP(23–111) protein was produced by modification of previous procedures52. DNA encoding aa 23–111 of human PrPC was cloned into pRSETA vector with an N-terminal extension encoding a hexa-histidine tag and thrombin cleavage site. Plasmid-transformed BL21(DE3) E. coli (Agilent) were cultured overnight without induction, and then diluted 1:100 in ZYM-5052 auto-inducing medium and grown for 16 h at 37C. Bacteria were lysed in Buffer G (6M Guanidine HCl, 100 mM Na2HPO4, 10 mM Tris-HCl, pH8) and then centrifuged at 100,000 × g for 1 h. The supernatant was applied to Ni-NTA resin. To refold bound protein, a 20–100% stepwise gradient of Buffer B (100 mM Na2HPO4, 10 mM Tris-HCl, 10 mM Imidazole, pH 8) in Buffer G was applied. After washing the resin, bound protein was eluted with 100 mM Na2HPO4, 10 mM Tris-HCl, 500 mM Imidazole, pH 5.8 and dyalized against 10 mM Na2HPO4 pH 5.8 and then against water. Final yields were 30–40 mg of protein per L of culture. Protein was stable at 4C for >2 months.
Generation of PrPC and Fyn stable cell lines
PrPC-expressing CV1 cells were isolated by clonal selection of a co-transfected Neo resistance gene in G418. A clone with PrPC immunblot levels comparable to brain was propagated as SCA-7. Recombinant lentiviral particles expressing Fyn (pLEX-JRed, Open Biosystems) were transduced into CV-1 and SCA-7 cells. Cells stably expressing Fyn were selected with puromycin and confirmed by anti-Fyn immnuoblot. Cells were maintained in 25 µg/ml puromycin.
Cell line cultures and preparation of cell lysates
HEK293, CV-1 and Neuro-2A cells were rinsed with ice-cold PBS and solubilized in 20 mM Tris, pH 7.4, 1.0% Triton X-100, 0.1% SDS, 150 mM NaCl, 10% glycerol, complete protease inhibitor cocktail (Roche), phosphatase inhibitor (Roche). To separate cell lysates into soluble (S) and insoluble (I) fraction, material centrifuged at 20,000 × g for 20 min at 4C. Supernatants from the initial fractionation were saved as S fraction. The pellets were washed once with PBS before re-extraction with 2% SDS as I fraction.
Primary neuronal cultures
Rat E18 and mouse E17 cortical neurons were cultured for 21 days in Neurobasal-A media with B-27, 0.5 mM L-glutamine, penicillin and streptomycin (all from Invitrogen) on plates coated with poly-L-lysine.
Immunoblots
Protein was electrophoresed through 4–20% tris-glycine or 10–20% tris-tricine gels (Bio-Rad), transferred to nitrocellulose membranes (Bio-Rad) and blocked (Rockland MB-070-010). Membranes were incubated overnight 4C with primary antibodies: 6D11 (Covance 39810-500, 1:1000), anti-phospho-SFK (Cell Signaling Technology #2101, 1:1000), anti-Fyn (Cell Signaling Technology #4023, 1:1000), anti-Src (Cell Signaling Technology #2110, 1:1000), anti-Lyn (Santa Cruz Biotechnology sc-28790, 1:1000), anti-c-Yes (Santa Cruz Biotechnology sc-28883, 1:1000) anti-actin (Sigma-Aldrich A2066, 1:10000), anti-Myc (SIGMA C3956 1:1000), anti-GAPDH (Sigma-Aldrich G8795, 1:20000), anti-phospho-NR2B (pTyr1472) (SIGMA M2442, 1:1000), anti-NR2B (BD Transduction Laboratories #610416, 1:500), anti-PSD95 (Cell Signaling Technology #2507, 1:1000), anti-Synaptophysin (Cell Signaling Technology #4329, 1:1000), 6E10 (Covance SIG-39300 1:1000), 82E1 (IBL 1:100), anti-cleaved caspase-3 (Cell Signaling Technology #9661), anti-caspase-3 (Cell Signaling Technology #9665), anti-β amyloid (Cell Signaling Technology #2454, 1:1000). Secondary antibodies were then applied for 1 h at RT (Odyssey goat or donkey anti-mouse or anti-rabbit IRDye 680 or 800) and visualized with a Licor Odyssey system.
Immunoprecipitation
One µg of antibody was incubated overnight at 4C with 1 mg lysate protein. Thirty microliter of a 1:1 suspension of protein A-Sepharose (Amersham) was then incubated with sample for 2 h at 4C. The resin was washed, and immunocomplexes were resolved by SDS-PAGE and immunoblotted.
Immunocytochemistry
CV1-derived cells were fixed with formaldehyde and permeabilized with TritonX-100. Slides were blocked, and incubated at 4C with anti-phospho-SFK (1:100 dilution) and anti-PrPC (6D11, Covance, 1:300 dilution). Alexa-Fluor-488 goat anti-mouse or Alexa-Fluor-568 goat anti-rabbit antibodies were used to detect bound antibodies.
Dissociated hippocampal neurons were prepared from E17-E18 C57BL/6 mice. At 21DIV, cultures were fixed in paraformaldehyde, permeabilized and blocked. Immunostaining was performed with antibodies against PrPC (6D11, Covance, 1:100 dilution) and PSD-95 (Invitrogen #51–6900, 1:100 dilution) followed by Alexa-Fluor secondary antibodies. Images were acquired on a Zeiss (Oberkochen, Germany) LSM 510 META laser scanning confocal microscope using 63× water objective, with channels scanned separately and a pinhole set to 1.1 µm.
LDH release
LDH release into culture medium was measured with the Cytotoxicity Detection Kit (Roche). Total LDH was determined by lysing all cells with 2% TritonX-100, and experimental values expressed as percentage of total LDH. Absorbance was measured at 490 nm using a VictorX3 Multilabel Plate Reader (PerkinElmer).
MTT assay
Cell viability was monitored by the conversion of 3(4,5-dimethythiazozyl-2)-2,5 diphenyl tetrazolium bromide to colored formazan product (MTT; Roche). MTT labeling reagent (final concentration 0.5 mg/ml) was added to neurons and incubated for 4 h at 37C. Solubilization solution was added plates were incubated for 16 h at 37C. Colored formazan products were detected as absorbance at 550 nm in a VictorX3 Multilabel Plate Reader.
Cell surface biotinylation
Rat E18 and mouse E17 cortical neurons at 21DIV were untreated or treated with Aβo for various times, placed on ice and rinsed in cold PBS. Then, neurons were incubated with 1.5 mg/ml sulfo-NHS-LC-biotin (Thermo Scientific) in PBS for 30 min at 4C. Cells were rinsed to remove unbound biotin, and extracted with 1% TritonX-100, 0.1% SDS, complete protease inhibitor cocktail (Roche), phosphatase inhibitor (Roche). Biotinylated proteins were isolated with NeutrAvidin agarose (Pierce), separated by SDS-PAGE, and analyzed by immunoblotting.
NMDA-R trafficking
WT or Prnp−/− 14DIV cortical neurons were transfected with expression vector or NR2B-GFP (kindly provided by Dr. Yan, State University of New York39) using calcium-phosphate (Clontech). After 3 d, neurons were treated with 0–1 µM Aβo for 0–60 min. Neurons were placed on ice, and then incubated with Alexa Fluor 555-conjugated anti-GFP antibody (Molecular Probes, A31851) for 30 min. After three washes with PBS, the cells were fixed with 4% paraformaldehyde/4% sucrose in PBS for 15 m, and then washed 3 times with PBS. Images were acquired on a Zeiss LSM 510 META laser scanning confocal microscope with 63X objective, as above. The relative intensity (Surface/Total) normalized to the untreated condition was measured by MATLAB software. Values were collected from at least 4 fields in each culture condition.
Subcellular Fractionation of Brain Tissue
Rat forebrains were homogenized in ice-cold 5 mM NaHEPES, pH7.4, 1 mM MgCl2, 0.5 mM CaCl2, complete protease inhibitor cocktail (Roche), phosphatase inhibitor (Roche) with a glass/Teflon pestel. Extract was spun at 1400 × g for 10 min. Supernatant (S1) was centrifuged at 13,800 × g for 10 min to collect the pellet (P2). P2 was resuspended in Buffer B (0.32 M sucrose, 6 mM TrisHCl, pH 8.0, complete protease inhibitor cocktail (Roche), phosphatase inhibitor (Roche)). The P2 suspension was loaded onto a discontinuous sucrose gradient (0.85 M/ 1 M/ 1.15 M sucrose solution in 6 mM TrisHCl, pH 8.0), followed by centrifugation for 2 h at 82,500 × g. The synaptosome fraction between 1 M and 1.15 M sucrose was collected and adjusted to 4 ml with Buffer B. Equal volume of 6 mM TrisHCl, pH 8.1, and 1 % Triton X-100 was added and incubated for 15 min. The suspension was spun at 32,800 × g for 20 min. The resulting pellet was extracted again with 6 mM TrisHCl, pH 8.1 and 0.5 % Triton X-100 for 15 min, and spun again at 201,000 × g for 1h. Resulting pellet was analyzed as the PSD.
Human Brain Fractionation
Fresh-frozen post-mortem human pre-frontal cortex from the brains of AD patients or Control subjects were obtained from pre-existing collections, as approved by Yale Institutional Review Board. Clinical diagnoses were confirmed histologically by examination of adjacent tissue in paraffin sections for abundant Aβ plaques and neurofibrillary tangles, Braak stage VI. Demographic and case details are provided in Suppl. Fig. S5d. One g tissue was homogenized in 5 ml of 25 mM Tris pH 7.4; 100 mM NaCl; protease inhibitor cocktail, Roche Diagnostics 11836170001, using a polytron. Particulate components were removed after centrifugation at 175,000 × g for 30 min.
For PrP-Fc affinity resin absorption, TBS-soluble extracts were cleared overnight at 4°C with Protein A sepharose beads (GE 71-5002-73 AI), followed by 2 hours clearance with the same beads crosslinked to human Fc (Jackson Immunoresearch 009-000-008). Equal volumes of cleared extract were incubated 4 h at 4°C with the same beads covalently crosslinked with either human Fc or recombinant human PrP-Fc. The unbound material was utilized for activity assays.
Assay of PrPC-Interacting Aβ Species in Brain Samples
To prepare capture plates, 20 µl/well of 0 or 100 nM human recombinant PrP(23–111) in 30 mM Na2CO3, 80 mM NaHCO3, pH 9.6 was added to 384-well Black MaxiSorp ELISA plates (Thermo Scientific). After 2 h at 23C, plates were washed with PBS-T and blocked with 5% BSA in PBS-T (100 µl/well) overnight at 4C. After washing again, samples (50 µl) were applied and incubated for 1h at 23C. All the samples included added BSA at 0.5%. The plates were then washed, and 20 µl/well of rabbit anti-amyloid beta antibody (#2454, Cell Signaling Technology, 1:1000 dilution in PBS-T, 0.5%BSA) was added for 1h. After another wash, plates were incubated with 20 µl/well of biotinylated donkey anti-rabbit antibody (Jackson Immunoresearch, 1:1000 in PBS-T, 0.5%BSA) for 30 minutes, washed 3X with PBS-T and then incubated with 20 µl of Europium-conjugated streptavidin, 1:1000 in DELFIA Assay Buffer (Perkin Elmer). After a final 4X PBS-T wash, 20 µl of DELFIA Enhancement Solution (Perkin Elmer) was added to the wells and time-resolved Europium fluorescence was measured using Victor 3V plate reader (Perkin Elmer). Background fluorescence values from uncoated wells were subtracted from the corresponding values of PrPC-coated wells.
For PrP(23–111) depletion of Aβ from AD brain TBS-soluble extracts, samples were incubated with PrP(23–111)-coated or uncoated control wells in 96-well BSA-blocked MaxiSorp plates (Nunc 80040LE 0903) for 3 h at 23C. Unbound material was recovered for ELISA. The plates were washed 4 times with PBS-T, and bound Aβ was eluted from PrP(23–111) using 30 µl/well of 10M urea. The original and recovered extracts, as well as the urea elutions, were assayed for Aβ42 using a commercially available kit (Invitrogen KHB3441). Elution fractions were diluted to 1M urea prior to ELISA.
Imaging of Dendritic Spine Stability
Hippocampi were dissected from E17–19 mouse embryos and digested with papain (37C; 5% CO2 for 30 min), and then washed with 1X HBSS. The neurons were transfected with myristoyl-EGFP expression vector using an Amaxa Nucleofector, and plated on poly-D-lysine-coated (100 µg/ml) glass at 100,000 cells/well in 8 well plates (Lab-Tek Chambered Coverslip 155411). WT controls were plated on half of the 8-well dish and either Fyn−/− or Prnp−/− neurons on the other half of the 8-well dish. Culture medium was Neurobasal A supplemented with penicillin/streptomycin, 1 mM Na-pyruvate, 2 mM GlutaMax, and B27 supplement.
Between 19–23DIV, hippocampal neurons were observed with a 100X objective on a Nikon Eclipse Ti Spinning Disk Confocal Microscope using a 488 laser. Approximately 25 fixed locations per 8-well dish were imaged on an automated stage every 15 min for 6 h in a 10 µm Z-stack at 0.1 µm intervals. For the first h, neurons were untreated. After 1 h, neurons were treated with 500 nM Aβo or F12 vehicle and imaged for 5 h. In some experiments, 50 µM AP5 or vehicle were added immediately before Aβo.
Dendritic spine persistence or creation was assessed in consecutive images of the dendritic segments using ImageJ software without knowledge of genotype or treatment. For each embryo, at least 4 segments with >30 spines were monitored, and statistics were calculated based on variablility between separate embryo cultures. Rare images showed dendritic segments with widespread blebbing and retraction; these were excluded from the analysis.
Cellular Aβ oligomer binding assay
Aβo binding was performed as described5 with slight modifications. Briefly, transfected COS7 cells were treated with biotinylated Aβo at 4C for 1 h. After washing, cells were fixed, incubated at 65C for 2 h, and incubated with alkaline-phosphatase-conjugated neutravidin. Bound phosphatase was visualized by 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium reaction and quantified with ImageJ5.
Measurement of intracellular calcium
Cortical neurons (21DIV) from WT, Prnp−/− or Fyn−/− mouse embryos were incubated in 96 well plates and treated with 0–1 µM Aβo for 0–60 min plus a calcium dye (FLIPR Calcium 4 assay kit, Molecular Devices). Plate fluorescence (λex = 485 nm, λem = 535 nm) was monitored in a VictorX3 Multilabel Plate Reader (PerkinElmer) with or without 50 µM NMDA, 100 µM glutamate or 500 nM ionomycin.
EEG surgeries and analysis
We studied 9–10 month old mice of the following genotypes: WT (C57B6/J) (n=11), Prnp−/− (n=11), APP/PSen (n=10), and APP/PSen Prnp−/− (n=12). Mice were anesthetized with isoflurane, and mounted in a stereotaxic frame (Kopf). A midline incision was made, and 2 bilateral burr holes were drilled anterolateral and posterolateral to bregma. Four pre-soldered intracranial screw electrodes (Pinnacle Technology #8403) were inserted, and secured with dental cement (A-M Systems #526000). Electrode wires were soldered to a 6-pin surface mount connector (Pinnacle Technology #8235-SM). Mice were allowed to recover for 7 d prior to EEG.
Mice were recorded using video-EEG monitoring (Pinnacle Systems #8200-K1-SE3, #8236). EEGs were sampled at 400HZ with 100X preamplifier gain. Each mouse underwent 72 h of continuous recording. EEG traces were scored manually by an investigator unaware of genotype. A seizure was defined as the abrupt onset of evolving spike-and-wave discharge lasting >5 s, followed by a period of post-ictal attenuation of cerebral rhythms. The recorded seizures were each 20–60 s in duration. There was no requirement for bilateral involvement, but all recorded seizures were generalized. Each seizure was correlated with the video recording and each was accompanied by convulsive behavior. Four of ten APP-PSen had ≥1 seizure over 72 h. Two mice had 1 seizure in this period, the third mouse had 1 seizure per d, and the 4th mouse had an average of 1–2 seizures per d (a total of 5 in 3 d).
There were also single spikes and 3–5 s spike wave discharges that were not analyzed here. Subjectively, spike wave discharges were specific to APP/PSen mice, and greatly reduced by Prnp deletion.
Statistical analyses
Statistical comparisons included one-way ANOVA and Repeated Measures ANOVA with post-hoc Tukey pairwise comparisons, as specified in the Figure Legends, using SPSS or Prism statistical software. Survival data were analyzed with the logrank test (Mantel-Cox) with SPSS software.
RESULTS
Prion Protein is Enriched in Post-Synaptic Densities
Aβo binding sites show post-synaptic dendritic localization5,22,33. To the extent that PrPC is relevant to Aβo-driven pathology, it may be concentrated in similar regions. Our initial studies documented co-localization between Aβo binding and PrPC, but did not characterize sites of PrPC enrichment5.
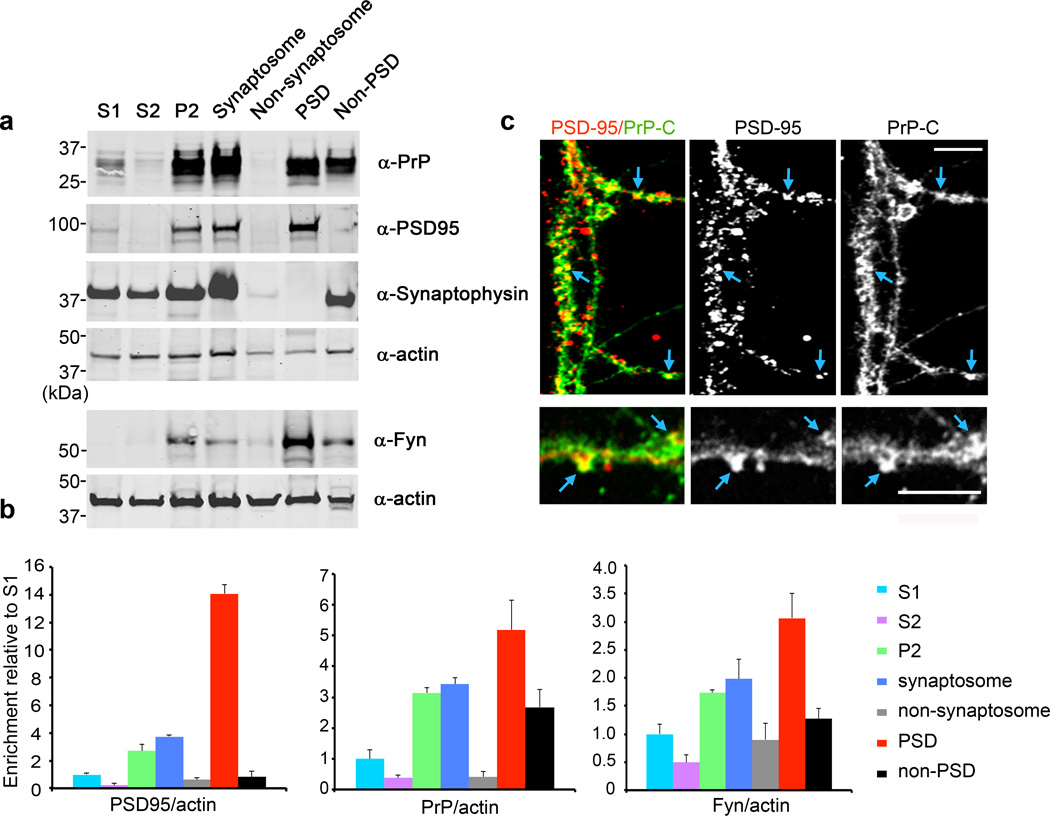
The PSD is distinct ultrastructurally and biochemically. Proteomic analyses of PSDs by mass spectrometry have revealed PrPC as a component34. To define PrPC enrichment in the PSD, we performed immunoblots on various subcellular fractions (Fig. 1a, b). PrPC is enriched in synaptosomal fractions. Isolation of the PSD from synaptosomes segregates the PSD-95 marker protein fully to the PSD and synaptophysin to the detergent extractable fraction. Substantial amounts of PrPC co-fractionate with PSD-95, yielding five-fold PrPC enrichment in PSD relative to initial homogenate. Fyn kinase is enriched three-fold in PSDs. We also observed substantial colocalization of PSD-95 with PrPC immunoreactivity in hippocampal cultures (Fig. 1c). Thus, PrPC is present in the PSD where it may mediate effects of Aβo.
Figure 1. Localization of Prion Protein to the Post-Synaptic Density.
a The indicated subcellular fractions (20 µg protein) were analyzed by immunoblot with anti-PrPC, anti-Fyn, anti-PSD95, anti-synaptophysin, and anti-actin antibodies. The lower two panels are from a separate preparation.
b Quantification of PSD-95, PrPC or Fyn levels normalized to actin. Data are mean ± s.e.m. for 3 independent fractionations from separate animals.
c Immunohistology of 21DIV hippocampal neurons stained with anti-PSD-95 (red in merge) and anti-PrPC (6D11, green in merge) antibodies. Colocalization indicated by arrows. Scale bars, 4 µm.
Aβo Binding to PrPC Generates Fyn Kinase Activation
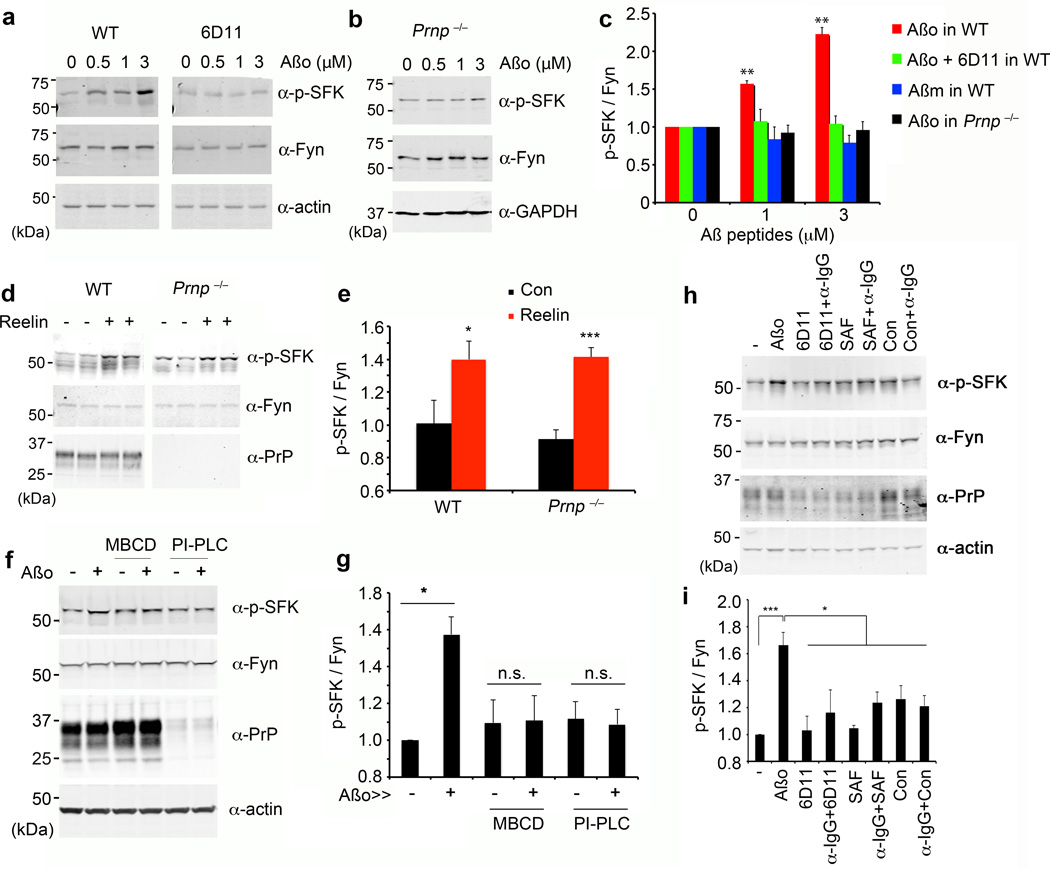
The non-receptor tyrosine kinase Fyn is a candidate mediator of signal transduction from an Aβo/PrPC complex for the reasons introduced above. It is possible to monitor the activation of Src family kinases (SFK) by phospho-specific epitopes. We examined cultures for SFK activation after exposure to Aβ species for 15 minutes (Fig. 2, Suppl. Figs. S1–4). Wild type cortical neurons at 21 days in vitro (DIV) increase pY416-Fyn (SFK) in response to Aβo (Fig. 2a, c). This antibody detects pY416 in several SFKs, but kinase-specific immunoprecipitations demonstrate that PrPC-dependent activation is Fyn-specific (Suppl. Fig. S1a). Fyn activation is oligomer-specific, not being observed with monomeric Aβ (Fig. 2c, Suppl. Fig. 1b) or fibrillary Aβ (Suppl. Fig. S1c,d).
Figure 2. Aβ Oligomers Activate Fyn Kinase.
a Mouse E17 WT cortical neurons at 21 DIV were treated with 0–3 µM Aβo (monomer equivalent concentration, estimated Aβo 0–30 nM) for 20 min in the absence (left panel) or presence (right panel) of 6D11 pre-incubation for 1 h. Whole cell lysates were analyzed by anti-phospho-SFK (Tyr 416) or anti-Fyn immunoblot. Actin served as a loading control.
b Cortical neurons from E17 Prnp−/− mice after 21 DIV were treated with 0–3 µM Aβo for 15 min. Lysates were analyzed by anti-phospho-SFK (Tyr 416) or anti-Fyn immunoblot. GAPDH served as a loading control.
c Quantification of phospho-SFK level in the lysate, normalized to Fyn immunoreactivity. WT, n = 4; Prnp−/−, n = 4. Mean ± s.e.m. **, P < 0.001; one-way ANOVA (F=8.83; df=11), with Tukey post-hoc pairwise comparisons. Data normalized to 0 µM; the phospho-SFK densities without normalization at 0 µM for Aβo, Aβo+6D11, Aβm (Aβ monomer) and Aβo+Prnp−/− are 0.82±0.22, 0.86±0.10, 0.99±0.21 and 0.94±0.02.
d Cortical neurons from WT or Prnp−/− mice after 21 DIV were treated with 5 nM reelin for 25 min. Lysates analyzed by anti-phospho-SFK (Tyr 416), anti-Fyn, or anti-PrPC immunoblot.
e Quantification of phospho-SFK level in the lysates (from d) normalized to Fyn immunoreactivity from 3 biologically independent experiments. Mean ± s.e.m. *, P < 0.05; ***, P < 0.001; Student’s two-tailed t test.
f Cortical neurons from WT after 21 DIV were treated with 0–1 µM Aβo. Prior to Aβo exposure, the indicated cultures were pre-treated with 5 mg/ml methyl-β-cyclodextrin (MBCD) for 1 h or 0.1 unit of PI-PLC for 10 min. Whole cell lysates were analyzed by anti-phospho-SFK (Tyr 416), anti-Fyn, or anti-PrPC immunoblot. Actin served as a loading control.
g Quantification of phospho-SFK level in the lysates (from f) normalized to Fyn immunoreactivity from 3 independent experiments. Mean ± s.e.m. *, P < 0.05; Student’s two-tailed t test.
h Cortical neurons from WT after 21 DIV were treated for with 1 µM Aβo, 10 µg/ml anti-PrPC antibodies (6D11 or SAF32) or control IgG (Con) antibodies, followed by adding 5 µg/ml mouse anti-IgG antibodies for crosslinking (α-IgG). Whole cell lysates were analyzed by anti-phospho-SFK (Tyr 416), anti-Fyn, or anti-PrPC immunoblot. Actin served as a loading control.
i Quantification of phospho-SFK level normalized to Fyn immunoreactivity from 3 independent experiments, as in h. Mean ± s.e.m. ***, P < 0.001; *, P < 0.05; one-way ANOVA (F=5.38; df=7), Tukey post-hoc comparisons.
We asked whether PrPC is required for the Aβo-induced Fyn activation. Previously, we showed that Aβo binding to PrPC is blocked by anti-PrPC 6D11 antibody5. This antibody also prevents Fyn activation by Aβo (Fig. 2a, c). Most importantly, in Prnp−/− cultures, activation of Fyn by Aβo is eliminated (Fig. 2b, c). Absence of Fyn signaling in Prnp−/− neurons is specific for Aβo, since Reelin-induced Fyn activation is preserved (Fig. 2d, e). Thus, while PrPC accounts for ~50% of Aβo binding sites5,33, it accounts fully for Fyn signaling.
We also verified Aβo-induced PrPC-dependent Fyn activation in cell lines. Stably transfected CV1 cells expressing PrPC (Sca-7), Fyn or both were generated (Suppl. Fig. S2a). PrPC protein in parental CV1 cells is <5% that in brain, and the level in PrPC expressors is 50–100% of brain (not shown). Aβo enhances pY-416-SFK level 2-fold exclusively in the PrPC/Fyn cells (Suppl. Fig. S2a, b). In N2A neuroblastoma, similar PrPC-dependent, Aβo-induced Fyn activation is observed in detergent-insoluble subcellular fractions (Suppl. Fig. S2c, d), though activation is not detected in HEK293T cells. We conclude that Aβo/PrPC complexes activate Fyn in many cells.
A marker of Aβo-induced PrPC/Fyn signaling is physical proximity of the proteins. In untreated CV-1 or HEK cells, a proportion of Fyn co-immunoprecipitates with PrPC (Suppl. Fig. S3a, b). Association is increased by Aβo treatment. An Aβo-induced complex can also be observed immunohistologically (SCA-7/Fyn cells, Suppl. Fig. S3c). At baseline, CV-1 cells expressing PrPC and Fyn show plasma membrane PrPC and diffuse Fyn staining with limited overlap. After Aβo, PrPC immunoreactivity is concentrated at the cell periphery in ruffles together with bound Aβo, with total Fyn, and with activated Fyn.
Cell-bound Aβo26, PrPC and Fyn are known to localize to lipid rafts. When rafts are disrupted by pretreatment of cultures with methyl-β-cyclodextrin (MBCD), Aβo activation of Fyn is absent from primary neurons (Fig. 2f, g), from CV-1 cells (Suppl. Fig. S4a) and from neuroblastoma cells (Suppl. Fig. S4b). The Aβo induced-enhancement of PrPC/Fyn co-immunprecipitation is absent after MBCD preincubation (Suppl. Fig. S4c). Phosphoinositide-specific phospholipase C (PI-PLC) releases GPI-anchored PrPC into the medium, and no Aβo signaling occurs in neurons (Fig. 2f, g) or cell lines (Suppl. Fig. S4a, b). Thus, GPI-anchored PrPC and intact lipid rafts are required for Aβo signaling to Fyn.
Aβo may activate PrPC/Fyn signaling by altered PrPC clustering or by induced PrPC conformations or by differential association with other proteins. To examine PrPC clustering, we treated primary neurons with anti-PrPC antibodies (6D11 or SAF32), as described for other cells24,25,35. Neither bivalent nor clustered anti-PrPC activates Fyn in primary neurons (Fig. 2h, i). Thus, PrPC clustering alone is not sufficient for the Aβo activation of Fyn in neuronal lipid rafts.
Human AD Brain Contains PrPC Interacting Aβ Species
Aβo have been prepared from multiple sources including synthetic peptide, transgenic mouse brain, transfected cell lines and human AD brain1–4. It is critical to evaluate human AD brain-derived Aβo of greatest relevance for pathophysiology. Importantly, human AD brain-derived Aβo require PrPC to suppress LTP10,16. Therefore, we assessed PrPC-interacting Aβo from human AD tissue for Fyn activation (Fig. 3)
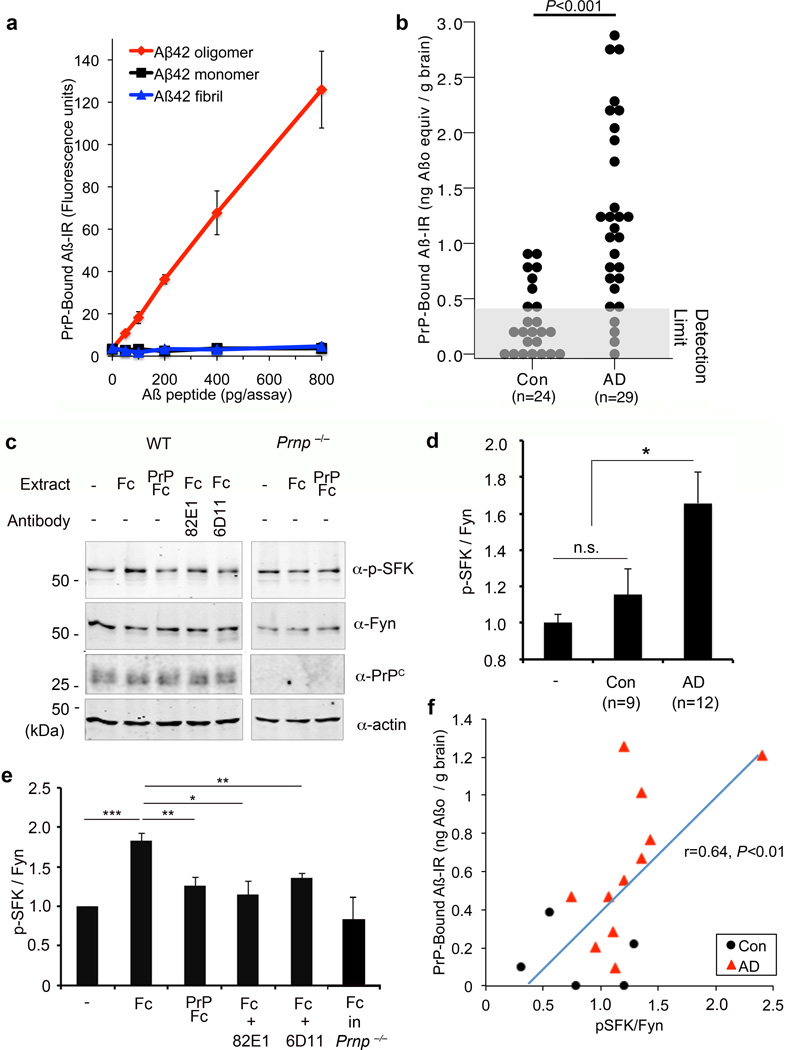
Figure 3. Aβ Species from Human AD Brain Associate with PrPC to Activate Fyn.
a Different amounts of monomer, oligomer and fibrillary Aβ were incubated with immobilized PrP(23–111). Bound Aβ was detected as time-resolved fluorescence derived from detection with anti-Aβ antibody and europium-tagged secondary reagents. Mean ± s.e.m. for 3 replicates, in one of 6 assays with similar results.
b Quantification of PrPC-interacting Aβ species level in TBS-soluble brain extract from age-matched human control brain (Con, n=24 individuals), or human AD brain (AD, n=29). Detection limit of the assay is shown as a gray box. P < 0.001; one-way ANOVA (F=20.70; df=1).
c E17 cortical neurons from WT or Prnp−/− mice after 21 DIV were treated with the indicated human AD brain extracts (6 µg total protein/ml) for 15 min. The AD brain extracts were pre-absorbed with Fc (control) or PrP-Fc resin, or incubated with 82E1 anti-Aβ antibody, or the cells were pretreated with 6D11 anti-PrPC antibody, as indicated. Whole cell lysates were analyzed by anti-phospho-SFK (Tyr 416) or anti-Fyn immunoblot.
d Quantification of phospho-SFK level in the lysate, normalized to Fyn immunoreactivity. Mean ± s.e.m., human Control brain (Con), n = 9; human AD brain, n =12. *, P < 0.05; one-way ANOVA (F=6.58; df=2), with Tukey post-hoc comparisons.
e Quantification of phospho-SFK level in the lysate, normalized to Fyn immunoreactivity. Mean ± s.e.m., WT, n = 5; Prnp−/−, n = 3 independent cultures. *, P < 0.05; **, P < 0.01; ***, P < 0.001; one-way ANOVA (F=8.26; df=5), with Tukey post-hoc comparisons.
f For samples analyzed for both PrPC-interacting Aβ species (as in c) and Fyn activation (as in e), the correlation between the assays is plotted. Each point is from a different brain sample. Pearson coefficient of linear correlation reported with two-tailed P.
We developed a sensitive assay for the detection of Aβ species interacting with PrPC (Fig. 3a). The 23–111 Aβ-interacting domain of PrPC was purified from recombinant E. coli and immobilized in microtiter plates. Specimens containing Aβo were added, and bound Aβ was detected with anti-Aβ antibodies. Detection of PrPC-bound Aβo was robust with N-terminally directed Aβ antibodies, polyclonal 2454 and monoclonal 82E1 (Suppl. Fig. S5a), or with NU-4 anti-oligomer antibody (not shown), but not with C-terminally directed antibodies, including AB5306 (Suppl. Fig. S5a). This may be due to inaccessibility of the Aβ C-terminus in oligomeric assemblies under native conditions. Using purified Aβo, the assay has a linear range from 20 pg to 14,000 pg (Fig. 3a). The specificity for oligomeric Aβ over monomeric Aβ or fibrillary Aβ exceeds 30-fold (Fig. 3a).
We assessed PrPC-interacting Aβ species in brain TBS homogenates cleared by ultracentrifugation. PrPC-interacting Aβ is detected in transgenic APP/PSen mouse brain, but not in WT brain (using synthetic Aβo as standard, 394±72 (transgenic, n=9 brains) versus <15 (WT, n=7) ng Aβ/g brain, mean ± sem, P<0.001, ANOVA, F=28.48; df=1). We measured PrPC-interacting Aβo in TBS-soluble cortical extracts from a cohort of autopsy-confirmed AD cases versus Controls. The average level in Control brains is below the detection limit, while AD samples contain 20.8+2.6 ng Aβ/g brain (Fig. 3b, mean ± sem, P<0.001, AD versus Control). This value suggests that a substantial proportion of Aβ in TBS-soluble extracts of AD brain interacts with PrPC. ELISA measurement demonstrates that ~50% of Aβ42 immunoreactivity in TBS-soluble AD extract absorbs to PrP-coated wells (Suppl. Fig. 5b). Urea elution recovers ELISA-detectable Aβ42 selectively from PrP(23–111) wells exposed to AD extracts (Suppl. Fig. 5c). Urea allows >100% recovery of initial Aβ42 ELISA signal (Suppl. Fig. 5b, 5c), likely due to denaturation of secondary structure in Aβ or associated proteins, which otherwise limit detection.
Having detected PrPC-interacting Aβ in human AD brain, we sought to determine whether these assemblies activate neuronal Fyn (Fig. 3c–e). AD brain extracts at 6 µg protein/ml stimulate Fyn activation in mouse cortical cultures, but Control brain extracts do not (Fig. 3c, d, P<0.05). To assess whether this actvation is due to PrP-interacting species, we preabsorbed human TBS brain extracts with PrPC-Fc affinity resin. PrPC-Fc resin, but not Fc control resin, prevents Fyn activation (Fig. 3c, e). The Aβ dependence of Fyn activation was assessed with anti-Aβ 82E1 antibody; abrogation is observed (Fig. 3c, e). As for synthetic Aβo stimulation of Fyn above, the signaling induced by AD TBS extract is absent in Prnp−/− cells, or in cells pretreated with 6D11 anti-PrPC antibody (Fig. 3c, e). Moreover, the level of PrPC-interacting Aβ species in human brain extracts correlates with the level of Fyn activation (Fig. 3f). Thus, TBS-soluble Aβ derived from human AD stimulates neuronal Fyn via PrPC.
NMDA-R Subunits Phosphorylated by Aβ/PrP/Fyn Signaling
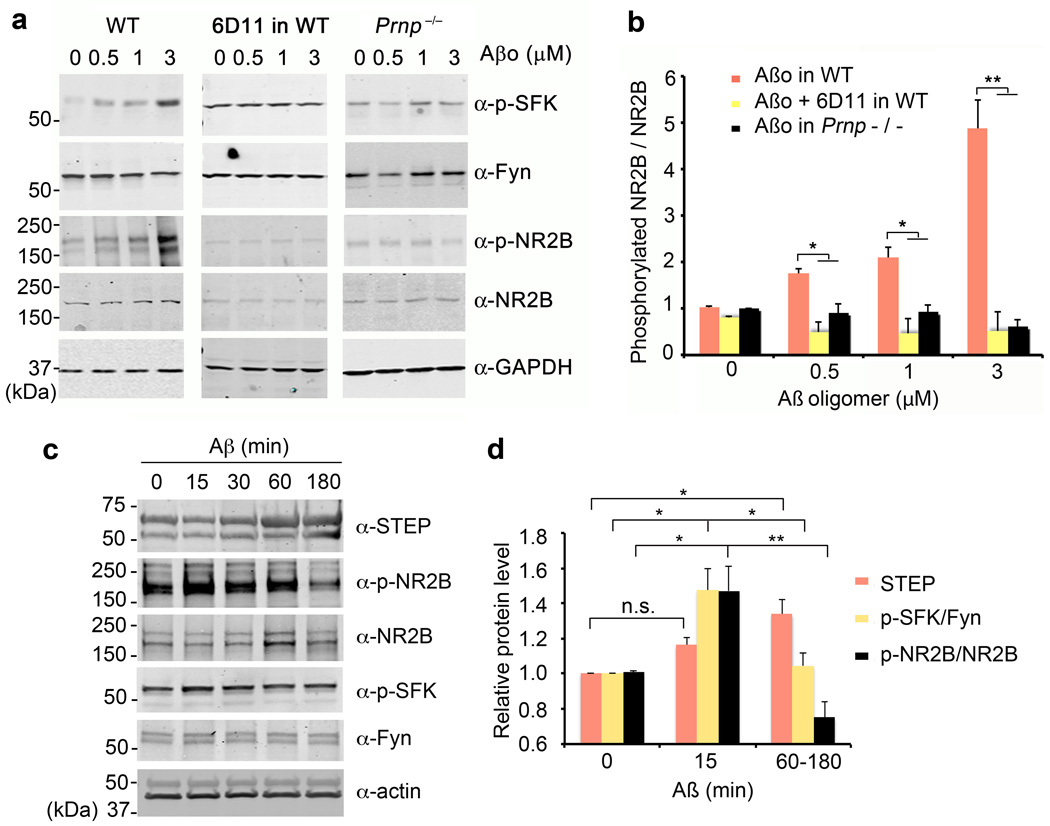
NMDA-Rs play a key role in synaptic plasticity and AD. Intracellular segments of NR2A and NR2B subunits contain tyrosine residues phosphorylated by SFKs36. Thus, Aβo/PrPC-mediated Fyn activation may be directed to NMDA-R. We examined total and pY-1472 NR2B levels in neuroblastoma cells expressing Fyn, PrPC and NR2B after exposure to Aβo (Suppl. Fig. S6a). Selectively in Fyn-overexpressing cells, Aβo increases pY-1472 NR2B. This increase is blocked by 6D11 anti-PrPC antibody. Next, we studied endogenous proteins in cortical cultures exposed to Aβo (Fig. 4a, b). Over 20 minutes, Aβo induces a dose-dependent 5-fold increase in pY-1472 NR2B. This effect is specific for the oligomeric Aβ, since fresh Aβ has no effect (not shown). Aβo-induced NR2B phosphorylation is eliminated in Prnp−/− cultures (Fig. 4a, b), in Fyn−/− cultures (Suppl. Fig. S7b), and by 6D11 anti-PrPC antibody (Fig. 4a, b). Thus, Aβo-induced phosphorylation of Y1472 in NR2B is a PrPC/Fyn-mediated signaling event. Moreover, the roles of PrPC and Fyn are gene-dose-dependent, being reduced in heterozygous neurons (Suppl. Fig. S7a, S7c).
Figure 4. Aβo Increase NR2B Phosphorylation Transiently via PrPC and Fyn.
a Cortical neurons from E17 wild type or Prnp−/− mice after 21 DIV were treated with 0–3 µM Aβo for 20 min. Prior to Aβo exposure, the indicated cultures were pre-incubated with 10 µg/ml of 6D11 antibody for 1 h. Whole cell lysates were analyzed by anti-phospho-SFK (Tyr 416), anti-Fyn, anti-phospho-NR2B (Tyr 1472) or anti-NR2B immunoblot. GAPDH served as a loading control.
b Quantification of phospho-NR2B level in the lysate (from b) normalized to NR2B immunoreactivity. WT, n = 4; Prnp−/−, n = 4. Mean ± s.e.m. *, P < 0.05; **, P < 0.01; one-way ANOVA (F=36.98; df=11), with Tukey post-hoc comparisons.
c Cortical neurons from E17 wild type after 21 DIV were treated with 1 µM Aβo for 0–180 min. Whole cell lysates were analyzed by anti-STEP, anti-phospho-NR2B (Tyr 1472), anti-NR2B, anti-phospho-SFK (Tyr 416), or anti-Fyn immunoblot. Actin served as a loading control.
d Quantification of STEP level, phospho-SFK level normalized to Fyn immunoreactivity or phospho-NR2B level in the lysate (from c) normalized to NR2B immunoreactivity. Mean ± s.e.m. for 3 biologically independent replicates. n.s., not significant; *, P < 0.05; **, P < 0.01; one-way ANOVA (F=13.15; df=2), with Tukey post-hoc comparisons.
During the first 15 minutes with Aβo, NR2B phosphorylation is enhanced, but after 1–3 hours phosphorylation is suppressed (Fig. 4c, d). Aβo is known to increase STEP tyrosine phosphatase23,37. Since STEP and Fyn counteract one another, we compared the time course of these events relative to NR2B phosphorylation (Fig. 4c, d). At the onset of Aβo action, Fyn is activated and pY-1472-NR2B is increased with no change in STEP. Later, STEP increases while Fyn returns to baseline with a net decrease in NR2B phosphorylation. Thus, a biphasic effect of Aβo on NR2B is triggered by PrPC engagement.
Surface NMDA-R and Calcium Signaling Induced by Aβ/PrP/Fyn
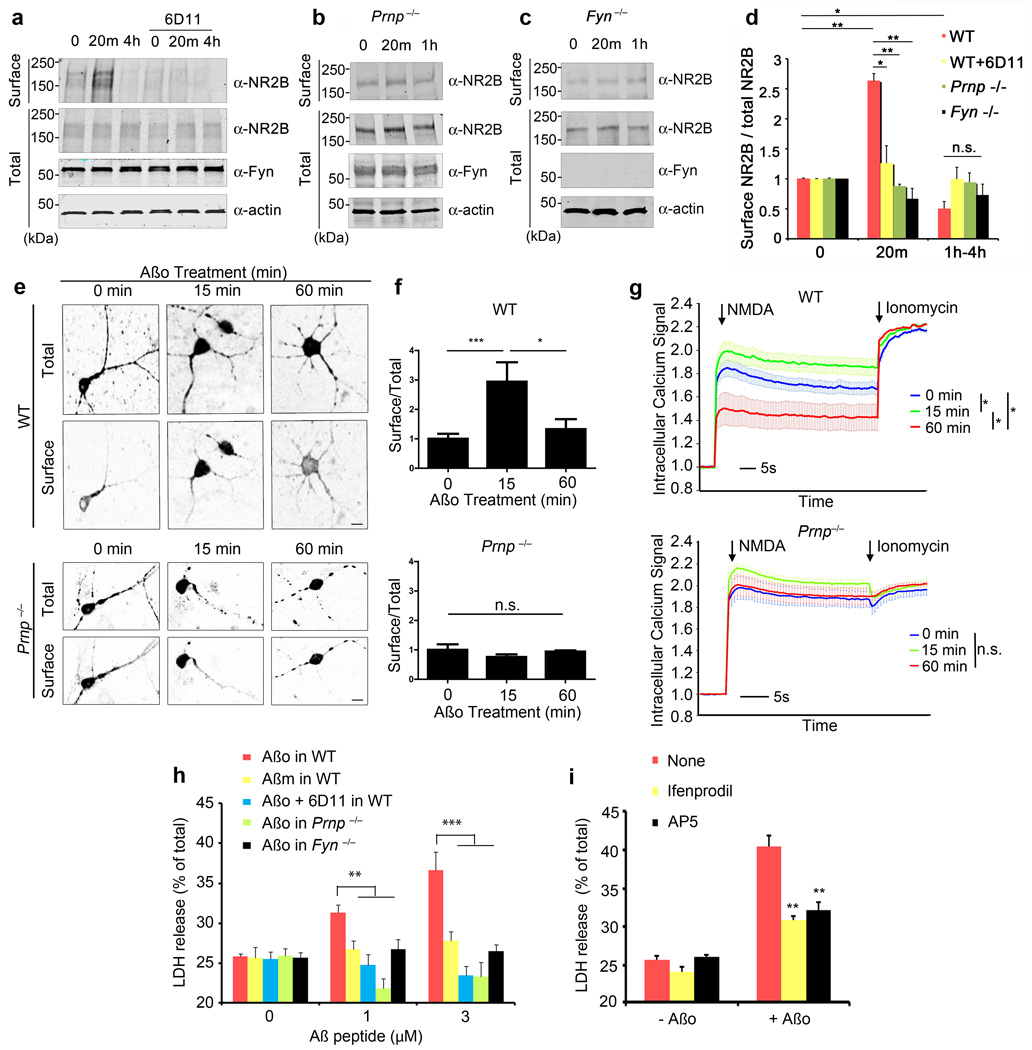
Phosphorylation of NR2B at Y1472 is known to reduce AP-2 mediated endocytosis38. We examined the extent to which NR2B is accessible at the cell surface versus being sequestered intracellularly, using cell surface biotinylation (Fig. 5a–d). In concert with the NR2B-pY-1472 increase, biotinylated NR2B increases shortly after Aβo exposure (Fig. 5a, d). There is no change in total NR2B. The increase is transient, and is followed by suppression of surface NR2B below baseline, matching the late decrease in pY-1472-NR2B. Both the early and late surface NR2B response to Aβo require PrPC and Fyn, as demonstrated by lack of response in Prnp−/−, Fyn−/− and 6D11-treated cultures (Fig. 5a–d). The levels of PrPC are limiting, with a substantial reduction in short-term Aβo-induced NR2B relocalization in Prnp+/− neurons (Suppl. Fig. S7d). The biochemical changes can be imaged using an extracellularly GFP-tagged NR2B39. The ratio of surface:total NR2B protein is detected by assessing the anti-GFP staining without permeablization versus the intrinsic GFP fluorescence (Fig. 5e, f). In WT neurons, Aβo triples this ratio at 15 minutes with a return to baseline by 60 minutes. There is no change in neurons lacking PrPC.
Figure 5. Aβo Induces NR2B Surface Localization, Calcium Signaling and Toxicity via PrPC and Fyn.
a–c WT, Prnp−/− or Fyn−/− E17 cortical neurons after 21 DIV were pre-incubated with or without 10 µg/ml of 6D11 antibody for 1 h, and then treated with 0–1 µM Aβo (monomer equivalent, ~10 nM oligomer) for 20 min or 4 h. Biotinylated cell surface proteins and total lysate proteins were assessed by anti-NR2B, anti-Fyn or anti-Actin immunoblot.
d Quantification of surface expression of NR2B normalized to total NR2B level. Mean ± s.e.m., WT, n = 5; Prnp−/−, n = 3; Fyn−/−, n = 3. *, P < 0.05; **, P < 0.01; one-way ANOVA (F=8.02; df=11), Tukey post-hoc comparisons. Data are normalized to 0 min values; values for biotinylated NR2B in WT, WT+6D11, Prnp−/− and Fyn−/− cultures are 9.6±2.8, 9.1±2.4, 9.9±1.6 and 8.5±2.8, respectively, as a percentage of total NR2B.
e, f WT or Prnp−/− cortical neurons were transfected with expression vector encoding GFP-NR2B, and then treated with 0–1 µM Aβo for 0–60 min. Surface and total receptor were visualized. Scale bars, 10 µm. The graphs in f show the ratio of surface to total receptor as a function of time and genotype. Mean ± s.e.m., n=3 independent embryos per genotype. *, P < 0.05, ***, P < 0.001; one-way ANOVA(F=4.83; df=2), Tukey post-hoc comparisons.
g WT or Prnp−/− cortical neurons were treated with 0–1 µM Aβo for 15 or 60 min. The intracellular calcium response to NMDA (50 µM) or calcium ionophore ionomycin (500 nM) was monitored with FLIPR Calcium 4. Mean ± s.e.m., WT, n = 15 independent wells from 5 embryos; Prnp−/−, n=9 independent wells from 3 embryos. *, P < 0.05 by Repeated Measures ANOVA (F=11.75; df=2) after NMDA addition, Tukey post-hoc comparisons. Data are normalized to pre-NMDA fluorescence; the pre-NMDA values without normalization for WT Aβo 15 m, WT Aβo 60 m, Prnp−/− Aβo 15 min and Prnp−/− Aβo 60 min are 1.05±0.11, 1.06±0.14, 1.11±0.04 and 1.13±0.12, respectively.
h WT, Prnp−/− or Fyn−/− cortical neurons at 21 DIV were treated with 0–2 µM Aβo for 1.5 h, prior to measurement of LDH release. The indicated cultures were pre-incubated with 10 µg/ml of 6D11 antibody for 1 h prior to Aβo, or were treated with Aβ monomer (Aβm) in place of Aβo. Data are mean ± s.e.m of three independent experiments. *, P < 0.05; **, P < 0.01; one-way ANOVA (F=5.73; df=14), Tukey post-hoc comparisons.
i WT neurons were pretreated with 3 µM ifenprodil or 50 µM APV for 1 h, and then Aβo was added at 1 µM. Cell toxicity after 90 min was determined by LDH release. Data represent mean ± s.e.m. from 4 independent experiments. **, P < 0.01; one-way ANOVA (F=70.52; df=5), Tukey post-hoc comparisons relative to no drug.
Changing surface levels of NMDA-R are expected to mediate alterations in NMDA-induced calcium levels. We utilized a calcium-sensitive fluorescent dye to monitor intracellular calcium in cortical neurons. NMDA produces increased fluorescence signal microscopically (Supplemental Movie S1) or in microtiter wells (Fig. 5g). Pretreatment with Aβo for 15 minutes generates significantly increased NMDA-induced signal, as predicted. By 60 minutes, when NR2B receptors are dephosphorylated and internalized, NMDA-induced calcium signals are suppressed (Fig. 5g). In cortical neurons lacking PrPC, Aβo does not alter NMDA responsiveness (Fig. 5g). The Aβ effect was oligomer-specific, since neither Aβ monomers nor Aβ fibrils alter NMDA responses (Suppl. Fig. S8d). Bath application of glutamate produces elevations of intracellular calcium that are mediated by NMDA receptors and by other channels. One hour pretreatment with Aβo suppresses glutamate responses in WT, but not in Prnp−/− or Fyn−/− neurons (Suppl. Fig. S8a–c). Thus, Aβo-induced, PrPC-mediated alterations in NMDA-R create transient increases and then decreases in neuronal calcium.
We considered whether the transient increase in surface NR2B might lead to a brief period of excitotoxicity. We first examined N2A cells with and without overexpression of Fyn and PrPC. Combined expression of Fyn plus PrPC substantially increases the release of cellular LDH induced by 90-minute exposure to Aβo (Suppl. Fig. S6b). To explore a role for endogenous PrPC and Fyn in Aβo neuronal toxicity, we used primary cortical cultures. Brief exposure to Aβo reduces cell viability, with a release of 10% of cellular LDH (Fig. 5h, Suppl. Fig. S8e). A decrease in MTT reduction is not detectable, being within the range of variability of the assay (Suppl. Fig. S9a). When Aβo exposure is extended to 72 hours, no further LDH release is observed (Suppl. Fig. S9b), indicating that this cell toxicity occurs largely during the initial 90 min of Aβo exposure, when surface NR2B is increased.
To determine whether the correlation of acute Aβo cell toxicity with surface NR2B and Aβ/PrP/Fyn signaling is functional, we examined the effects of antibody and genetic blockade. Anti-PrPC 6D11 antibody pretreatment prevents the Aβo-induced LDH release (Fig. 5h). A requirement for PrPC in Aβo-induced neuronal cell death matches recent reports15,20. Genetic deletion of either Fyn or PrPC expression rescues neurons from Aβo (Fig. 5h). Heterozygosity for null alleles of Prnp or Fyn significantly reduces LDH release (Suppl. Fig. S7e). For transheterozygous Prnp+/−, Fyn+/− neurons, there is no detectable Aβo-induced LDH release during 90 minutes (Suppl. Fig. S7e). The toxicity depends primarily on NMDA-R since it is reduced by AP5, and on NR2B-containing receptors since ifenprodil suppresses LDH release (Fig. 5i). Consistent with an excitoxic, non-apoptotic cell toxicity, there was no increase in activated caspase 3 (Suppl. Fig. S9c, S9d). Thus, Aβo requires PrPC to induce Fyn activation and subsequent NR2B phosphorylation. This phosphorylation is associated with transient increase in NR2B at the cell surface with consequent excitotoxicity.
Aβo Destabilization of Dendritic Spines Requires PrP/Fyn
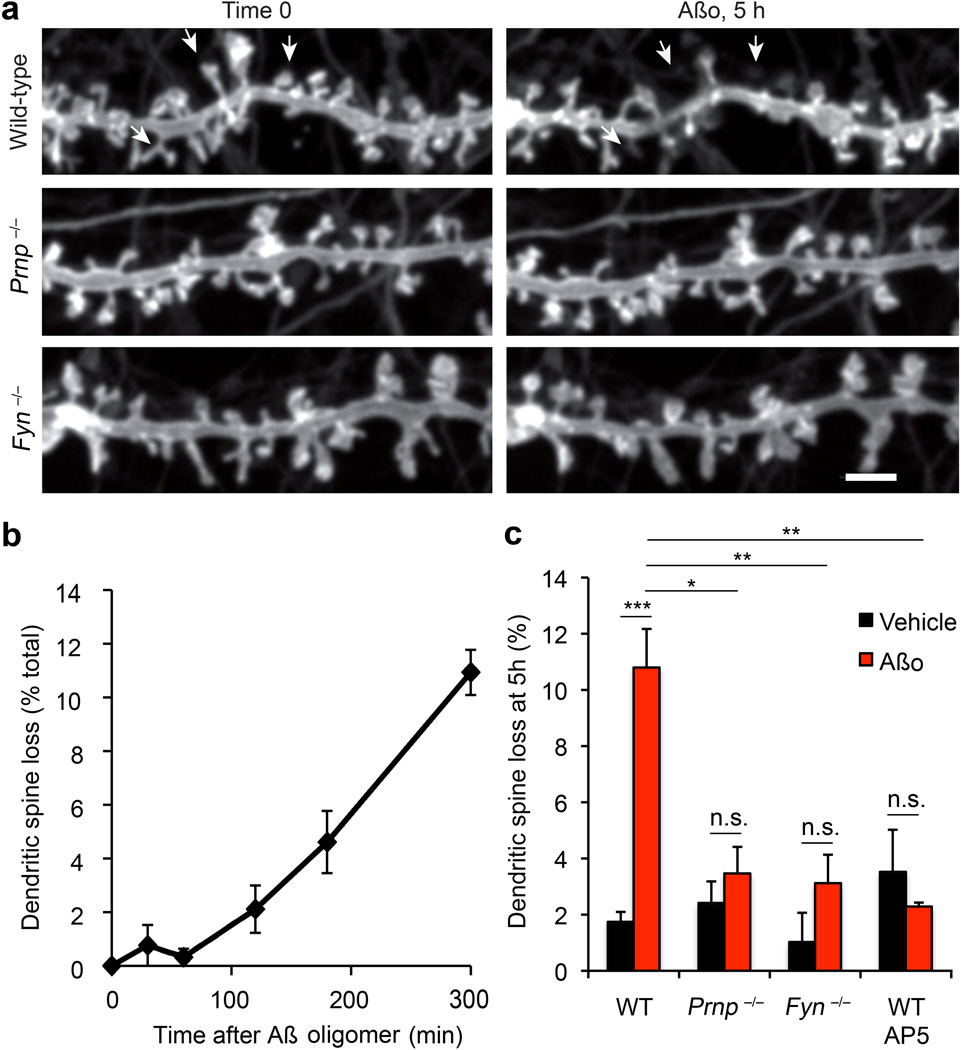
A hallmark of AD is synaptic loss40. In vitro studies have described dendritic spine loss after acute Aβo exposure3,21,22. We sought to assess whether PrPC and Fyn are involved. To provide a robust measure for fractional loss, we repeatedly imaged the same dendritic segments expressing membrane-tethered EGFP over 6 hours (Fig. 6a, Supplemental Movie S2). Under control conditions, dendritic spines are stable, with <2% gains or losses. Aβo increases spine loss, without significant alteration of spine gain. Over 5 hours, 10–15% of spines are lost with Aβo, while adjacent spines are morphologically stable (Fig. 6a, c). The time course is gradual after an initial lag (Fig. 6b). Although loss is increased five-fold by Aβo, loss remains minor compared to the variability of spine density, emphasizing the need for time-lapse imaging.
Figure 6. PrPC and Fyn are Required for Aβ-Induced Dendritic Spine Loss.
a Hippocampal neurons of the indicated genotypes were transfected with a Myr-EGFP expression vector and then cultured for 21 d prior to live imaging. After 1 h, 500 nM Aβo (monomer equivalent, 5 nM estimated oligomer) or vehicle was added and observations were continued for 5 h. Lost dendritic spines after Aβo addition in the WT neurons are indicated with arrowheads. Scale bar, 1 µm. A typical three-dimensional image of such spines is provided in Supplemental Movie 2.
b Dendritic spines were observed at 15 min intervals as described in a. The percentage of spines lost at the indicated times after 500 nM Aβo addition is indicated for a wild type culture. Data are mean ± sem from n = 3 separate cultures.
c Dendritic spine loss over 5 h is plotted as a function of Aβo addition and genotype. The indicated samples were incubated with 50 µM AP5 during the Aβ exposure. The data are mean ± sem from n = 12 WT, n = 3 Prnp−/−, n = 3 Fyn−/−, and n = 3 AP5 independent cultures from separate embryos for each genotype or drug. ***, P < 0.001; **, P < 0.01; *, P < 0.05; one-way ANOVA (F=8.94; df=7), Tukey post-hoc comparisons.
To assess the roles of PrPC and Fyn in Aβo-induced spine loss, neurons were cultured from embryos homozygous for null alleles (Fig. 6a, c). Spine destabilization by Aβo is eliminated in Prnp−/− and Fyn−/− neurons. Thus, Aβo-induced spine loss requires both PrPC and Fyn. We also tested whether Aβo-induced spine loss requires NMDA-R. As shown in previous studies with human AD brain derived species3, the NMDA antagonist AP5 blocks dendritic spine loss by Aβo (Fig. 6c).
Seizures in AD Transgenic Mice are Prevented by Prnp Loss
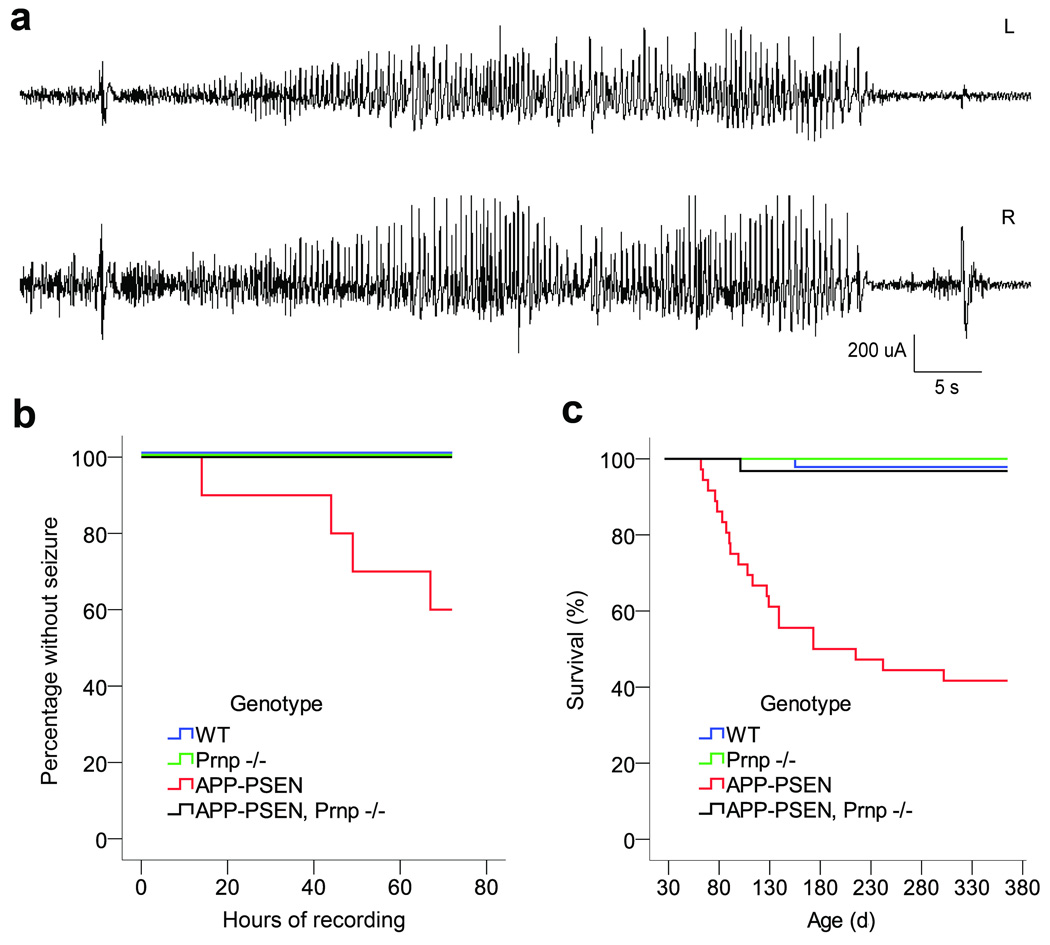
Epileptiform discharges have been observed in transgenic mouse models of AD and seizures are more common in AD41. We hypothesized that network instability may derive from Aβ/PrP/Fyn-induced alterations in synapses. We examined epileptiform discharges of >5 sec in APPswe/PSen1ΔE9 transgenic mice with or without PrPC expression at 9–10 months of age (Fig. 7a, b). After implantation of intracranial electrodes, continuous video plus EEG records were monitored for 72 hours. Consistent with previous studies42, 40% of APP/PSen transgenic mice had at least one electrographic seizure (Fig. 7b). The majority included an initiating spike, a 20s–60s run of high amplitude epileptiform activity and were followed by a post-ictal attenuation of cerebral rhythyms (Fig. 7a). At the video level, each of the electrographic seizures was accompanied by tonic posturing and myoclonus (Supplemental Movies 3, 4). The ictal period commonly includes locomotor hyperactivity, and the postictal period is marked by hypoactivity. None of the 12 APP/PSen transgenic lacking PrPC exhibited an electrographic or behavioral seizure during 72 hours (Fig. 7b). Neither the WT nor Prnp−/− mice had electrographic abnormalities during similar monitoring, though single spikes were occasionally observed in all genotypes. Thus, the electrographic phenotype of this AD model requires PrPC.
Figure 7. Seizures in Transgenic AD Mice Require PrPC.
a Chronic video-EEG recordings were obtained from freely moving WT, Prnp−/−, APP-PSen, and APP-PSen Prnp−/− mice. Each mouse was monitored continuously for 72 h. A spontaneous seizure recorded from a transgenic Alzheimer mouse (APP-PSen). This seizure is typical in its initiation by a spike-wave discharge, and by the postictal attenuation of cerebral rhythms. L, left and R, right hemisphere signal.
b Kaplan-Meyer curve showing the latency to first seizure during 72 h of continuous EEG. Forty percent of the APP-PSen mice with normal PrPC expression had at least 1 spontaneous generalized seizure during the recording session. The lack of PrPC completely rescues this phenotype in transgenic APP-PSen mice (P = 0.017, Log Rank test). No seizures were recorded in WT and Prnp−/− mice. For WT n=11, Prnp−/− n=11, APP-PSen n=10, APP-Psen Prnp−/− n=12.
c Kaplan-Meyer survival curve of the mouse cohort undergoing EEG. More than 50% of transgenic mice die by 12 months age, and this phenotype is fully reversed by the lack of PrPC expression (P < 0.001, Log Rank test). For WT n=46, Prnp−/− n=23, APP-PSen n=36, APP-Psen Prnp−/− n=31.
The seizure phenotype may account for the reduced survival of APP/PSen via status epilepticus, or Sudden Unexpected Death in Epilepsy (SUDEP). In the cohort studied here, reduced survival for the APP/PSen genotype is fully rescued by PrPC loss (Fig. 7c), as in our previous separate analysis13. De facto, mice monitored by EEG are selected from the two-thirds of transgenic mice that escaped early death. Considering both phenotypes together, 70% of the APP/PSen mice suffered either early death or seizures, while <4% of the APP/PSen, Prnp−/− mice showed one of these phenotypes.
DISCUSSION
We delineate a Fyn signaling pathway activated by Aβo/PrPC complexes. Pathophysiological relevance is supported by the binding of human AD-derived Aβo to PrPC, the enrichment of PrPC in PSDs, the PrPC-dependence of Fyn activation by Aβo in neurons, and the requirement of both Fyn and PrPC for Aβo-induced changes in NR2B. Short-term activation of this pathway increases in surface NMDA-R and excitotoxicity, followed by dendritic spine and surface receptor loss. The complete rescue of AD transgene mortality by PrPC deletion is dramatic, and may be secondary to the PrPC-dependence of epileptiform activity detected by EEG recording. Together, these data document a biochemical pathway downstream of Aβo/PrPC complexes that has potential contributions to AD pathogenesis (Suppl. Fig. S10).
Different Aβ Oligomer Species and Mechanisms
Aβo derived from synthetic peptide, cell culture, transgenic brain and human AD brain have been analyzed in various functional and biochemical assays. The Aβ peptide can assume different oligomeric states, each distinct from monomer or fibrillary peptide. The resolution of oligomeric Aβ forms is not defined biophysically, though molecular weight and/or valency clearly differ between preparations. Variable outcomes in functional experiments with different Aβo preparations may derive from uncharacterized variation between preparations10. Here, we demonstrate that Aβo from human AD brain interacts with PrPC, confirming the pathological relevance of the studies. PrPC-dependence for human AD brain extract suppression of LTP has been reported recently10,16.
The ability of Aβo species to interact with PrP(23–111) distinguishes a subset of peptide with deleterious actions on neurons and synapses. The level of PrPC-interacting Aβ species in AD brain TBS-soluble extracts is within the range of values from previous studies43–45. Measurements of Aβ after immunopreciptitation/immunblot of AD brain TBS-soluble extracts yielded values43 very similar to the values reported here for PrPC-interacting species. This raises the possibility that a substantial fraction of Aβ in human AD brain is capable of PrP(23–111) interaction. Further analysis of human samples for PrPC-interacting Aβ species may provide a useful tool to follow disease status and/or treatment efficacy.
Post-Synaptic Action of Aβo/PrPC Complexes
Synapse loss is amongst the most prominent and consistent aspects of AD40. Here, immunohistochemical localization and subcellular fractionation studies demonstrate that PrPC is concentrated at synapses and enriched in PSD. These findings are consistent with several unbiased proteomic studies documenting PrPC in PSD fractions46,47 and PrPC is included in a “Consensus PSD” data set34. PSD localization fits with a role for PrPC in mediating local effects of Aβo on synaptic plasticity, dendritic spine retraction and synaptic loss. Indeed, our genetic analysis demonstrates that both PrPC and Fyn are required for dendritic spine loss. Previous studies have demonstrated that chronic loss of synaptic markers in transgenic AD mice requires PrPC13,14. However, another study found that Aβ-induced loss of dendritic spines in culture was PrPC-independent12. The PrPC-negative result may reflect differences in the Aβ preparation. In this regard, the observation that PrPC-Fc recognizes AD-derived Aβ species, supports a disease relevance for PrPC-dependent pathways.
Engagement of an Aβo/PrPC/Fyn pathway increases NMDA-R phosphorylation and alters receptor localization. The biphasic effect on surface NMDA-R is coupled with short-term cell toxicity followed by a loss of surface receptor. Both acute augmentation and chronic suppression of NMDA responses by Aβo have been reported in various studies18,21,48. Future studies of GluR trafficking will delineate the sites of receptor relocalization within the neuron relative to synapes, as well as the timing of Aβo/PrPC/Fyn induced changes in neurotransmission.
Fyn Activation by PrPC
While PrPC and Fyn are associated physically and Aβ engagement of PrPC leads to Fyn activation, the two proteins are on different faces of the plasma membrane. PrPC is extracellular while Fyn is intracellular, so that the two polypeptides cannot be in direct physical contact. Lipid rafts are crucial for signal transduction and cell-bound Aβo is known to localize to rafts in a Fyn-dependent manner26. It is possible that the coalescence of Aβo/PrPC and Fyn in lipid rafts allows signaling without a transmembrane polypeptide partner. However, it seems more plausible that there are one or more membrane-spanning partners that link the two proteins.
Previous reports have described PrPC-dependent signaling in certain cells after antibody cross-linking17,24,25. We find that clustering of PrPC with anti-PrP antibodies is not sufficient to induce Fyn activation as observed with Aβo in primary neurons. This suggests that PrPC conformational changes occur when Aβo binds. The Aβo binding domain of PrPC, aa 23–111, is thought to be natively disordered, so Aβo may stabilize a specific conformation of the protein leading to signal transduction through Fyn.
Although acute engagement of PrPC by Aβo activates Fyn, in vivo levels of Fyn are similar in WT and AD transgenic mice32. This is likely related to secondary changes in the chronic state. The biphasic effect of Aβo on NMDA-R is consistent with compensatory dysregulation. In this regard, it is notable that the STEP phosphatase counteracts Fyn activation and STEP levels are induced in transgenic AD models and AD32,37. In culture, early PrPC-dependent Fyn activation is required for the later loss of surface NMDA-R, which is correlated with STEP expression. Such compensation may play a crucial role in chronic AD pathophysiology after acute Aβo/PrPC/Fyn engagement.
Fyn in PrPC-Dependent and AD-Related Pathways
These studies suggest that Fyn plays a central role in coupling Aβo and PrPC to changes in neuronal function. There are multiple lines of evidence linking Fyn kinase function to synapse plasticity and dysfunction in AD. Fyn is enriched in the PSD, and is known to phosphorylate NR2A and NR2B29. Mice with loss of Fyn function exhibit reduced LTP, while gain-of-function leads to enhanced LTP and seizures30,49. When Fyn mutants are crossed with APP transgenic mice, Fyn gain-of-function enhances AD-related phenotypes while Fyn loss-of-function ameliorates AD-related phenotypes32. Fyn is known to associate with Tau and recent data indicate that aberrant Fyn-Tau interactions sensitize synapses to glutamate excitoxicity50. PrPC/Fyn signaling may couple Aβ and Tau pathologies.
The data here provide evidence that PrPC and Fyn are essential for certain Aβo induced biochemistry and AD transgene-dependent phenotypes. Other signaling pathways participating in Aβ action include calcineurin, insulin receptors, autophagy, glutamate reuptake and Tau-directed kinases. The interdependence and epistatic relationships between these multiple pathways require further investigation.
Seizures and AD
Mice carrying human AD transgenes exhibit altered network activity and epileptiform discharges41. We confirmed a similar finding in APPswe/PSen1ΔE9 mice42 and show that this phenotype is PrPC-dependent and includes convulsive seizures. Single spikes without behavioral changes were not a reliable distinguisher between WT and APP/PSen transgenic phenotypes in our studies. Since Fyn gain-of-function reduces seizure thresholds49, Aβo/PrPC/Fyn signaling may explain abnormal EEG patterns. It has been suggested that epileptiform discharges explain early death in AD transgenic mice. The PrPC-dependence of both epileptiform activity and early death provides indirect support for this hypothesis.
While seizure incidence has been reported to increase in AD, sudden death early in the disease is not typical. The relevance of EEG abnormalities to cognitive dysfunction in AD requires clinical correlation.
Conclusions
Previous work has demonstrated that Aβo bind to PrPC5–10, and in a number of scenarios PrPC is essential for the deleterious actions of Aβo5,10,13–20. Here, we provide evidence for Fyn kinase signaling that links an Aβo/PrPC complex in PSDs to changes in GluR function and dendritic spine anatomy. Importantly, Aβo from AD brain interact with PrPC, so this complex may play a pathological role in human AD. Furthermore, Fyn provides a molecular link to Tau pathology and to epileptiform phenotypes. Future studies will examine the Aβo/PrPC/Fyn pathway in multiple models of AD to validate its relevance for pathophysiology in different disease stages and manifestations.
Supplementary Material
ACKNOWLEDGMENTS
H.B.N. is an Ellison Medical Foundation AFAR Postdoctoral Fellow and S.M.S. is a member of the Kavli Institute for Neuroscience at Yale University. We acknowledge support from the National Institutes of Health (R01AG034924, R37NS033020, R01NS074319, P30DA018343), the Falk Medical Research Trust, and the Alzheimer’s Association to S.M.S., and from the National Institutes of Health (R01NS47433, R01NS073502) to T.W.
Footnotes
AUTHOR CONTRIBUTIONS: J.U., H.B.N., J.K.H., M.A.K., M.S., A.V., E.C.G. and S.M.S. designed various aspects of the research. J.U., H.B.N., J.K.H., H.T., M.S., and E.C.G. performed the research. J.U., H.B.N., J.K.H., H.T., M.S., T.W., E.C.G. and S.M.S. analyzed various parts of the data and contributed to the writing of the paper.
DISCLOSURE: S.M.S. is a co-founder of Axerion Therapeutics, seeking to develop NgR-and PrP-based therapeutics.
REFERENCES
- 1.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 3.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesne S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 5.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou WQ, et al. Amyloid-{beta}42 Interacts Mainly with Insoluble Prion Protein in the Alzheimer Brain. J Biol Chem. 2011;286:15095–15105. doi: 10.1074/jbc.M110.199356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balducci C, et al. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci U S A. 2010;107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Yadav SP, Surewicz WK. Interaction between human prion protein and amyloid-beta (Abeta) oligomers: role OF N-terminal residues. J Biol Chem. 2010;285:26377–26383. doi: 10.1074/jbc.M110.145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calella AM, et al. Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol Med. 2010;2:306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freir DB, et al. Interaction between prion protein and toxic amyloid beta assemblies can be therapeutically targeted at multiple sites. Nat Commun. 2011;2:336. doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cisse M, et al. Ablation of cellular prion protein does not ameliorate abnormal neural network activity or cognitive dysfunction in the J20 line of human amyloid precursor protein transgenic mice. J Neurosci. 2011;31:10427–10431. doi: 10.1523/JNEUROSCI.1459-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessels HW, Nguyen LN, Nabavi S, Malinow R. The prion protein as a receptor for amyloid-beta. Nature. 2010;466:E3–E4. doi: 10.1038/nature09217. discussion E4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimbel DA, et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung E, et al. Anti-PrPC monoclonal antibody infusion as a novel treatment for cognitive deficits in an Alzheimer's disease model mouse. BMC Neurosci. 2010;11:130. doi: 10.1186/1471-2202-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resenberger UK, et al. The cellular prion protein mediates neurotoxic signalling of beta-sheet-rich conformers independent of prion replication. Embo J. 2011;30:2057–2070. doi: 10.1038/emboj.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry AE, et al. Alzheimer's disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J Neurosci. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bate C, Williams A. Amyloid-beta-induced synapse damage is mediated via cross-linkage of cellular prion proteins. J Biol Chem. 2011;286:37955–37963. doi: 10.1074/jbc.M111.248724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You H, et al. Abeta neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 2012;109:1737–1742. doi: 10.1073/pnas.1110789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alier K, Ma L, Yang J, Westaway D, Jhamandas JH. Abeta inhibition of ionic conductance in mouse basal forebrain neurons is dependent upon the cellular prion protein PrPC. J Neurosci. 2011;31:16292–16297. doi: 10.1523/JNEUROSCI.4367-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo W, et al. Cellular prion protein is essential for oligomeric amyloid-beta-induced neuronal cell death. Hum Mol Genet. 2012;21:1138–1144. doi: 10.1093/hmg/ddr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 24.Pantera B, et al. PrPc activation induces neurite outgrowth and differentiation in PC12 cells: role for caveolin-1 in the signal transduction pathway. Journal of neurochemistry. 2009;110:194–207. doi: 10.1111/j.1471-4159.2009.06123.x. [DOI] [PubMed] [Google Scholar]
- 25.Mouillet-Richard S, et al. Cellular prion protein signaling in serotonergic neuronal cells. Ann N Y Acad Sci. 2007;1096:106–119. doi: 10.1196/annals.1397.076. [DOI] [PubMed] [Google Scholar]
- 26.Williamson R, Usardi A, Hanger DP, Anderton BH. Membrane-bound beta-amyloid oligomers are recruited into lipid rafts by a fyn-dependent mechanism. FASEB J. 2008;22:1552–1559. doi: 10.1096/fj.07-9766com. [DOI] [PubMed] [Google Scholar]
- 27.Malaga-Trillo E, et al. Regulation of embryonic cell adhesion by the prion protein. PLoS Biol. 2009;7:e55. doi: 10.1371/journal.pbio.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bizat N, et al. Neuron dysfunction is induced by prion protein with an insertional mutation via a Fyn kinase and reversed by sirtuin activation in Caenorhabditis elegans. J Neurosci. 2010;30:5394–5403. doi: 10.1523/JNEUROSCI.5831-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki T, Okumura-Noji K. NMDA receptor subunits epsilon 1 (NR2A) and epsilon 2 (NR2B) are substrates for Fyn in the postsynaptic density fraction isolated from the rat brain. Biochem Biophys Res Commun. 1995;216:582–588. doi: 10.1006/bbrc.1995.2662. [DOI] [PubMed] [Google Scholar]
- 30.Grant SG, et al. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 31.Nakazawa T, et al. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- 32.Chin J, et al. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renner M, et al. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66:739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins MO, et al. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. Journal of neurochemistry. 2006;97(Suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- 35.Stuermer CA, et al. PrPc capping in T cells promotes its association with the lipid raft proteins reggie-1 and reggie-2 and leads to signal transduction. FASEB J. 2004;18:1731–1733. doi: 10.1096/fj.04-2150fje. [DOI] [PubMed] [Google Scholar]
- 36.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, et al. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A. 2010;107:19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prybylowski K, et al. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen P, Gu Z, Liu W, Yan Z. Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Mol Pharmacol. 2007;72:40–51. doi: 10.1124/mol.107.034942. [DOI] [PubMed] [Google Scholar]
- 40.Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- 41.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minkeviciene R, et al. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mc Donald JM, et al. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133:1328–1341. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinerman JR, et al. Distinct pools of beta-amyloid in Alzheimer disease-affected brain: a clinicopathologic study. Arch Neurol. 2008;65:906–912. doi: 10.1001/archneur.65.7.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo YM, et al. Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996;271:4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- 46.Peng J, et al. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimura Y, et al. Molecular constituents of the postsynaptic density fraction revealed by proteomic analysis using multidimensional liquid chromatography-tandem mass spectrometry. Journal of neurochemistry. 2004;88:759–768. doi: 10.1046/j.1471-4159.2003.02136.x. [DOI] [PubMed] [Google Scholar]
- 48.Li S, et al. Soluble A{beta} Oligomers Inhibit Long-Term Potentiation through a Mechanism Involving Excessive Activation of Extrasynaptic NR2B-Containing NMDA Receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kojima N, Ishibashi H, Obata K, Kandel ER. Higher seizure susceptibility and enhanced tyrosine phosphorylation of N-methyl-D-aspartate receptor subunit 2B in fyn transgenic mice. Learn Mem. 1998;5:429–445. [PMC free article] [PubMed] [Google Scholar]
- 50.Ittner LM, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 51.Stein PL, Lee HM, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 52.Zahn R, von Schroetter C, Wuthrich K. Human prion proteins expressed in Escherichia coli and purified by high-affinity column refolding. FEBS letters. 1997;417:400–404. doi: 10.1016/s0014-5793(97)01330-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.