Abstract
Histone deacetylases (HDACs) compact chromatin structure and repress gene transcription. In schizophrenia, clinical studies demonstrate that HDAC inhibitors are efficacious when given in combination with atypical antipsychotics. However, the molecular mechanism that integrates a better response to antipsychotics with changes in chromatin structure remains unknown. Here we show that chronic atypical antipsychotics down-regulate the expression of mGlu2, an effect that is associated with decreased histone acetylation at its promoter in mouse and human frontal cortex. This epigenetic change occurs in concert with a 5-HT2A receptor-dependent up-regulation and increased binding of HDAC2 to the mGlu2 promoter. Viral-mediated over-expression of HDAC2 in frontal cortex decreases mGlu2 transcription and its electrophysiological properties, thereby increasing psychosis-like behavior. Conversely, HDAC inhibitors prevent the repressive histone modifications induced at the mGlu2 promoter by atypical antipsychotics, and augment their therapeutic-like effects. These observations support the view of HDAC2 as a promising new target to improve schizophrenia treatment.
Schizophrenia is a severe and persistent psychiatric condition that affects almost one percent of the world’s population1,2. In some patients with schizophrenia, typical and atypical antipsychotic drugs produce complete remission of psychotic symptoms. However, about 30% of the patients are considered treatment resistant, and will continue to experience psychotic and other symptoms despite the optimal use of available antipsychotic medications3,4. Over the last forty years, a variety of adjunctive treatments have been used to enhance the response to antipsychotic medications5. Among these, preclinical6–8 and clinical9–11 studies suggest that drugs such as valproate, one of whose functions is to act as a nonspecific histone deacetylase (HDAC) inhibitor12,13, are efficacious when given chronically in combination with atypical antipsychotic drugs, including clozapine, olanzapine and risperidone. HDACs remove acetyl groups from lysine residues in the amino-terminal tails of core histones, which shifts the balance toward chromatin condensation and thereby silences gene expression14,15. As yet, the molecular mechanism that integrates a better response to antipsychotics with pharmacological modulation of HDAC function remains unknown.
Monoaminergic neurotransmitters have been heavily involved in the pathophysiology of schizophrenia and other psychotic disorders. Atypical antipsychotic drugs all have in common a high affinity for the serotonin 5-HT2A receptor (5HT2A), and a modest affinity for the dopamine D2 receptor16,17. Hallucinogenic drugs, such as lysergic acid diethylamide (LSD), psilocybin, and mescaline, recruit specific 5HT2A-mediated signaling pathways to affect behavior in humans and rodents18,19. These findings are consistent with the implication of the 5HT2A receptor in the neurochemical abnormalities responsible for psychosis. Multiple lines of evidence also associate schizophrenia with dysfunction of glutamatergic transmission20. Indeed, recent preclinical assays in rodents suggest that drugs that activate the metabotropic glutamate 2 receptor (mGlu2) represent potentially new antipsychotic medications21–23, which is further underscored by some of the clinical measures24. Our previous findings convincingly demonstrate that chronic treatment with the atypical antipsychotic clozapine induces down-regulation in the level of expression of mGlu2 in mouse frontal cortex25—a brain region that plays an important role in cognition and perception, and has been implicated more recently in schizophrenia and antipsychotic responses17,19,25. Together with the antipsychotic properties of drugs that bind to and activate the mGlu2 receptor, these studies led us to hypothesize that down-regulation of mGlu2 expression might restrain the therapeutic effects of atypical antipsychotic drugs.
Here we show that chronic administration of atypical antipsychotic drugs selectively up-regulate the expression of HDAC2 in both mouse and human frontal cortex, an effect that is associated with a 5HT2A-dependent regulation of HDAC2 transcriptional activity and increased binding of HDAC2 to the promoter region of the mGlu2 gene. We also show that recruitment of HDAC2 leads to a decrease in histone acetylation at the mGlu2 promoter, and that prevention of this mark of transcriptional repression by HDAC inhibitors improves atypical antipsychotic responses. Together, these data suggest that HDAC2 may be a novel therapeutic target to augment the treatment of schizophrenia.
RESULTS
Histone modifications at mGlu2 by chronic antipsychotics
In schizophrenia patients, antipsychotic drugs are administered chronically (weeks to months of sustained drug treatment)26. We have previously shown that chronic treatment with clozapine down-regulates the level of expression of mGlu2 in mice25. We found here similar effects with chronic clozapine and risperidone, but not haloperidol—a first generation antipsychotic drug, in mouse frontal cortex (Figs. 1a–1d; see also Supplementary Fig. 1d for the effect of chronic haloperidol on dopamine D2 receptor binding in striatum, and Supplementary Fig. 1h for absence of effect of chronic clozapine on mGlu2 expression in thalamus and striatum). Previous work demonstrated that activation of cortical mGlu2 modulates the cellular and behavioral responses induced by hallucinogenic and antipsychotic 5HT2A ligands23,25. Recent observations also suggest chromatin remodeling in cortical neurons as a mechanism involved in the molecular responses to chronic treatment with antipsychotic drugs6–8. The notable regulation of mGlu2 expression by atypical antipsychotic drugs prompted us to investigate the effect of chronic antipsychotic treatments on the epigenetic status of the mGlu2 promoter in mouse and human frontal cortex.
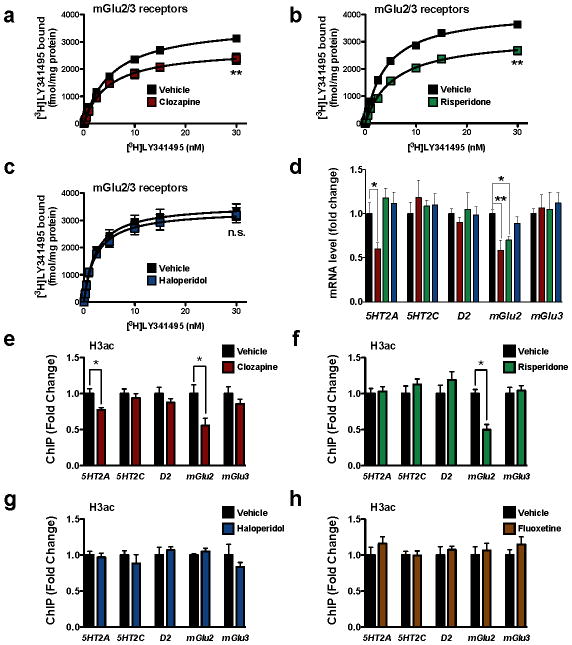
Figure 1. Decreased acetylation of histone H3 at the mGlu2 promoter by chronic treatment with atypical antipsychotic drugs in mouse frontal cortex.
(a–d) Chronic clozapine and risperidone, but not haloperidol, modulate the expression of mGlu2 in mouse frontal cortex. Mice were chronically (21 days) injected with vehicle (black), 10 mg/kg clozapine (red), 4 mg/kg risperidone (green), or 1 mg/kg haloperidol (blue), and sacrificed one day after the last injection. (a–c) [3H]LY341495 binding in mouse frontal cortex after vehicle or chronic clozapine (a), risperidone (b) or haloperidol (c). Effect of clozapine (n = 4 independent experiments performed in triplicate), F[2,95] = 65.34, P < 0.001; effect of risperidone (n = 6 independent experiments performed in triplicate), F[2,117] = 166.4, P < 0.001; effect of haloperidol (n = 6 independent experiments performed in triplicate), F[2,116] = 1.29, P > 0.05. Maximum number of binding sites (Bmax) for [3H]LY341495 obtained from individual saturation curves are decreased by chronic clozapine (t = 6.32; **P < 0.01), and risperidone (t = 6.53; **P < 0.01), but not haloperidol (t = 0.97; P > 0.05; n.s., not significant); two-tailed Student’s t-test. (d) Expression of 5HT2A, 5HT2C, dopamine D2, mGlu2, and mGlu3 mRNA in mouse frontal cortex assayed by qRT-PCR. Experiments were performed after vehicle or chronic clozapine, risperidone or haloperidol (n = 6 mice per group). Expression of 5HT2A, F[3,20] = 5.66, P < 0.01; Expression of 5HT2C, F[3,20] = 0.29, P > 0.05; Expression of D2, F[3,20] = 0.31, P > 0.05; Expression of mGlu2, F[3,20] = 6.62, P < 0.01; Expression of mGlu3, F[3,20] = 0.13, P > 0.05; one-way ANOVA. *P < 0.05; **P < 0.01; Bonferroni’s post hoc test of one-way ANOVA. (e–h) Decreased acetylation of histone H3 at the mGlu2 promoter by chronic clozapine (e) and risperidone (f), but not by haloperidol (g) or the antidepressant fluoxetine (h), in mouse frontal cortex. Mice were chronically (21 days) injected with clozapine (10 mg/kg), risperidone (4 mg/kg), haloperidol (1 mg/kg), fluoxetine (20 mg/kg), or vehicle, and sacrificed one day after the last injection. Fragmented chromatin was immunoprecipitated with antibody recognizing acetyl-histone H3 (H3ac), and the level of association of the 5HT2A, 5HT2C, dopamine D2, mGlu2, or mGlu3 promoters was measured by qPCR. Note also that acetylation of histone H3 at the 5HT2A promoter was decreased by chronic clozapine, but not by risperidone, haloperidol, or fluoxetine (n = 8 mice per group). H3ac at mGlu2 promoter: clozapine, t = 2.77; risperidone, t = 5.38; haloperidol t = 1.08. H3ac at 5HT2A promoter: clozapine, t = 2.96. The α value was corrected for multiple independent null hypotheses by using the Holm’s sequentially rejective Bonferroni method. *P < 0.011; two-tailed Student’s t-test. Error bars represent s.e.m.
We assayed the level of several post-translational histone modifications at the promoter region of the mGlu2 (Grm2) gene in mouse frontal cortex using a series of chromatin immunoprecipitation (ChIP) assays. Interestingly, histone H3 acetylation, which correlates with transcriptional activation14, was strongly decreased at the mGlu2 promoter by chronic clozapine and risperidone, but not haloperidol (Fig. 1e–1g). Earlier studies have also implicated monoaminergic and glutamatergic receptor genes, such as 5HT2A (Htr2a), 5HT2C (Htr2c), dopamine D2 (Drd2), and mGlu3 (Grm3) in psychosis and antipsychotic responses27–31. Wefound that only clozapine, and neither risperidone nor haloperidol, decreases histone H3 acetylation at the 5HT2A promoter in mouse frontal cortex (Fig. 1e–1g; see also Fig. 1d and Supplementary Fig. 1h for decreased 5HT2A mRNA expression, and Supplementary Fig. 1a–1c for decreased density of 5HT2A binding sites). No significant changes were found at the 5HT2C, D2, and mGlu3 promoters. Similar results were obtained with histone H4 acetylation (Supplementary Fig. 2a). Histone H3 methylation at lysine 4 (H3K4me1/2/3), another marker of gene activation, was not affected by any of the tested chronic antipsychotic treatments at these promoters (Supplementary Fig. 2a).
We next examined if histone modifications known to correlate with transcriptional repression are altered by chronic treatment with antipsychotics. Chronic clozapine or risperidone, but not haloperidol, elicited a significant increase in histone H3 tri-methylation at lysine 27 (H3K27me3), a repressive histone modification marker, at the mGlu2 promoter, with no apparent changes at the 5HT2A, 5HT2C, D2, and mGlu3 promoters (Supplementary Fig. 2a). Histone H3 tri-methylation at lysine 9 (H3K9me3), another histone modification that correlates with transcriptional repression, was not increased by any of the tested chronic treatments at these promoters (Supplementary Fig. 2a). In addition, the repressive histone modifications induced at the mGlu2 promoter by atypical antipsychotics were not detected after subchronic treatment with clozapine, risperidone or haloperidol (Supplementary Fig. 2b). Chronic treatment with the antidepressant fluoxetine did not affect the pattern of histone H3 acetylation or H3K27me3 modification at the promoter regions of the 5HT2A, 5HT2C, D2, mGlu2 and mGlu3 genes (Fig. 1h and Supplementary Fig. 2c).
Several reports have suggested that certain histone modifications facilitate the methylation of DNA at specific promoter regions6, and that atypical antipsychotics activate DNA demethylation in cortical inhibitory interneurons7. However, DNA methylation analysis revealed that chronic clozapine did not affect the methylation pattern of the tested CpG sites at the mGlu2 promoter (Supplementary Fig. 3). Therefore, DNA methylation does not seem to contribute to the repression of the mGlu2 gene.
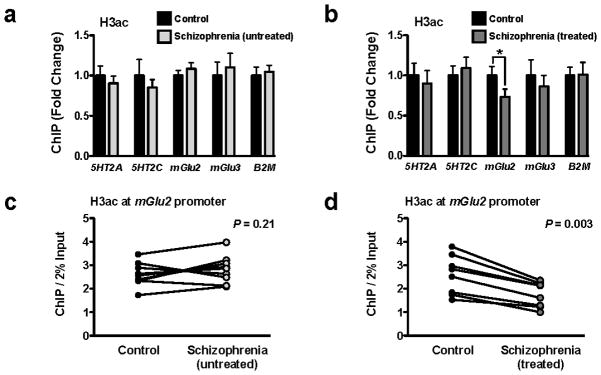
To further investigate the effect of chronic atypical antipsychotic drugs on the epigenetic modifications of histones at the mGlu2 promoter, we performed ChIP experiments in postmortem frontal cortex of schizophrenic subjects and controls. We compared two different groups of schizophrenic subjects: those either untreated or treated with atypical antipsychotics. Each of these two groups included individually matched control subjects (see Supplementary Table 1 for demographic information). Consistent with our findings in mice, a similar decrease in histone H3 acetylation at the mGlu2 promoter was observed in treated, but not in untreated, schizophrenic subjects (Figs. 2a and 2b). This epigenetic change at the mGlu2 promoter was found in each matched pair of treated schizophrenia and control subjects (Figs. 2c and 2d). Histone H3 acetylation was not significantly different at the 5HT2A, 5HT2C or mGlu3 promoters in either treated or untreated schizophrenic subjects compared to controls (Figs. 2a and 2b). β2-Microglobulin (B2M), a constitutively expressed gene in frontal cortex32, was used as an internal control (see also Supplementary Fig. 4a for assays including non-immune immunoglobulin [IgG] to control for the specificity of ChIP assays in postmortem human brain).
Figure 2. Decreased acetylation of histone H3 at the mGlu2 promoter in prefrontal cortex of treated, but not untreated, schizophrenic subjects.
(a,b) Digested chromatin was immunoprecipitated with antibody recognizing acetyl-histone H3 (H3ac), and the level of association of the 5HT2A, 5HT2C, mGlu2, or mGlu3 promoters was measured by qPCR. The promoter of β2-microglobulin (B2M) was included as internal control. Experiments were performed in frontal cortex of untreated schizophrenic subjects and matched controls (a), and in frontal cortex of atypical antipsychotic-treated schizophrenic subjects and matched controls (b). H3ac at mGlu2 promoter in treated schizophrenics: t = 3.96. The α value was corrected for multiple independent null hypotheses by using the Holm’s sequentially rejective Bonferroni method. *P < 0.011; two-tailed Student’s t-test. Error bars represent s.e.m. (c,d) Individual representation of histone H3 acetylation at the mGlu2 promoter in postmortem frontal cortex of untreated schizophrenic subjects and matched controls (c), and in postmortem frontal cortex of atypical antipsychotic-treated schizophrenic subjects and matched controls (d). Results are shown as enrichment values (bound/input). Each datapoint of schizophrenic subject is connected to the datapoint of the respective matched control.
Up-regulation of HDAC2 by chronic atypical antipsychotics
These changes in the histone code at the mGlu2 promoter led us to speculate that chronic atypical antipsychotic drugs might regulate the transcription and function of genes associated with histone post-translational modifications. HDACs are epigenetic regulators that repress gene transcription through the removal of acetyl groups from histone tails. Mammalian HDACs fall into four main classes, with class I and II HDACs receiving the most attention in the CNS8. We examined whether levels of expression of class I or class II Hdac mRNAs are altered in mouse frontal cortex by treatment with the atypical antipsychotic clozapine. Interestingly, only Hdac2 mRNA levels were significantly increased by chronic clozapine (Fig. 3a), but not haloperidol (data not shown). We next tested the effect of chronic clozapine on the level of HDAC2 protein expression in mouse frontal cortex. Expression of HDAC2 protein was increased by chronic clozapine as compared to controls (Figs. 3c and 3e). HDAC1 protein, another class I HDAC member8, and HDAC4 protein, which has been shown to be highly expressed in neocortical regions33, were unaffected (Figs. 3c and 3e).
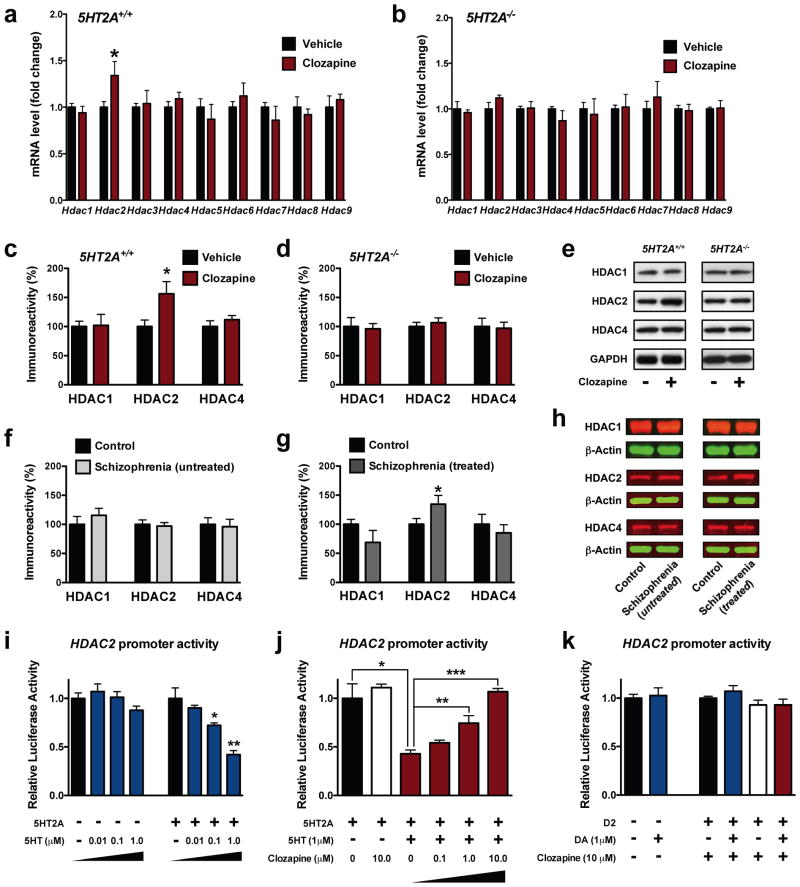
Figure 3. 5-HT2A-dependent increased expression of HDAC2 in frontal cortex by chronic atypical antipsychotics.
(a–b) Chronic clozapine modulates the expression of Hdac2 mRNA in mouse frontal cortex. 5HT2A+/+ (a) and 5HT2A−/− (b) mice were chronically (21 days) injected with vehicle (black) or 10 mg/kg clozapine (red), and sacrificed one day after the last injection (5HT2A+/+, n = 12; 5HT2A−/−, n = 6 mice per group). Expression of Hdac1, Hdac2, Hdac3, Hdac4, Hdac5, Hdac6, Hdac7, Hdac8, and Hdac9 mRNAs was assayed by qRT-PCR. Effect of chronic clozapine on Hdac2 mRNA expression in 5HT2A+/+ mice: t = 3.40. The α value was corrected for multiple independent null hypotheses by using the Holm’s sequentially rejective Bonferroni method. *P < 0.006; two-tailed Student’s t-test.
(c–e) Chronic clozapine up-regulates protein levels of HDAC2, and not HDAC1 or HDAC4, in mouse frontal cortex. 5HT2A+/+ (c) and 5HT2A−/− (d) mice were chronically (21 days) injected with vehicle (black) or 10 mg/kg clozapine (red) and sacrificed one day after the last injection (n = 6 mice per group). Effect of chronic clozapine on HDAC2 expression in 5HT2A+/+ mice: t = 3.17. The α value was corrected for multiple independent null hypotheses by using the Holm’s sequentially rejective Bonferroni method. *P < 0.017; two-tailed Student’s t-test. Representative immunoblots are shown (e).
(f–h) HDAC2, and not HDAC1 or HDAC4, is increased in postmortem human brain of atypical antipsychotic-treated, and not untreated, schizophrenic subjects. Western blots showed no change (f) of HDAC2 protein levels in frontal cortex of untreated schizophrenics compared to matched control subjects; and up-regulation (g) of HDAC2 protein levels in frontal cortex of atypical antipsychotic-treated schizophrenics compared to matched control subjects (t = 4.22). The α value was corrected for multiple independent null hypotheses by using the Holm’s sequentially rejective Bonferroni method. *P < 0.017; two-tailed Student’s t-test. Representative immunoblots are shown (h).
(i–j) Clozapine reverses the 5HT2A-dependent repression of HDAC2 promoter activity by serotonin. HEK293 cells were transfected with a HDAC2 promoter-luciferase construct and/or with a plasmid encoding the 5HT2A, treated with serotonin (5HT), clozapine, and/or vehicle, and then analyzed for luciferase activity (n = 3 independent experiments performed in triplicate). Concentration response of 5HT in mock-transfected cells (i), F[3,8] = 1.81, P > 0.05; concentration response of 5HT in 5HT2A-transfected cells (i), F[3,8] = 17.52, P < 0.001; one-way ANOVA. *P < 0.05; **P < 0.01; Bonferroni’s post hoc test of one-way ANOVA. Concentration response of clozapine in 5HT2A-transfected cells (j), F[5,12] = 15.01, P < 0.001; one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; Bonferroni’s post hoc test of one-way ANOVA.
(k) Dopamine D2-dependent signaling does not affect the promoter activity of HDAC2. HEK293 cells were transfected with a HDAC2 promoter-luciferase construct and/or with a plasmid encoding the D2 receptor, treated with dopamine (DA), clozapine, or vehicle, and then analyzed for luciferase activity (n = 6 independent experiments performed in triplicate). Effect of DA in mock-transfected cells (k), t = 0.30, P > 0.05; two-tailed Student’s t-test. Effect of DA in D2-transfected cells (k), F[3,8] = 1.77, P > 0.05; one-way ANOVA. Error bars represent s.e.m.
To study the relevance of the effects observed in mice, we examined HDAC1, HDAC2 and HDAC4 protein levels in postmortem frontal cortex of schizophrenic subjects untreated or treated with atypical antipsychotics, and controls (see Supplementary Table 1). Equivalent to the higher HDAC2 expression found in mouse frontal cortex by chronic treatment with clozapine, we observed that atypical antipsychotics induce increased expression of HDAC2, and not HDAC1 or HDAC4, in frontal cortex of schizophrenic subjects, with no effect seen in untreated schizophrenic subjects (Figs. 3f–3h).
5-HT2A-dependent modulation of HDAC2 promoter activity
Next, we explored the mechanism through which chronic antipsychotics up-regulate HDAC2 expression in mouse frontal cortex. The high affinity at the 5HT2A in conjunction with the dopamine D2 receptor16,17 prompted us to consider whether signaling pathways downstream of these receptors may act as modulators of HDAC2 promoter activity. Using promoter-reporter gene constructs, we examined the impact of 5HT2A- and/or D2-dependent signaling on the transcriptional activity of HDAC2 in tissue culture. We observed that activation of the 5HT2A receptor by the endogenous neurotransmitter serotonin resulted in inhibition of HDAC2 promoter activity (Fig. 3i) and, importantly, this effect was reversed by clozapine in a concentration-dependent manner (Fig. 3j). Neither dopamine nor clozapine affected the promoter activity of HDAC2 in cells expressing the D2 receptor (Fig. 3k). These data demonstrate that, in vitro in HEK293 cells, clozapine and serotonin differentially modulate HDAC2 promoter activity in a 5HT2A-dependent manner. However, the overall signal transduction amplification systems present in HEK293 cells and in mouse frontal cortex and the levels of receptor expressed per cell may differ34, which could consequently affect at different extent the promoter activity of the HDAC2 gene. We therefore assessed whether the ability of clozapine to enhance the transcriptional activity of HDAC2 in mouse frontal cortex may be 5HT2A-dependent.
Notably, we found that the up-regulation of HDAC2 expression by chronic clozapine observed in wild-type (5HT2A+/+) mice (see Figs. 3a, 3c and 3e above) was abolished in 5HT2A null mutant mice (5HT2A−/−) (Figs. 3b, 3d and 3e). No other HDAC tested demonstrated regulation by chronic clozapine in 5HT2A−/− mice (Figs. 3b, 3d and 3e). Together with the experiments in tissue culture (see Figs. 3i, 3j and 3k above), this work provides mechanistic insight into how chronic atypical antipsychotics lead to elevations in HDAC2 expression within the frontal cortex.
Clozapine up-regulates HDAC2 binding to mGlu2 promoter
The highly specific effect of chronic atypical antipsychotics on HDAC2 expression in frontal cortex, together with the repressive histone modifications induced at promoter region of the mGlu2 gene (see Fig. 1 and Fig. 2 above), led us to examine the role of HDAC2 in regulating the transcriptional activity of the mGlu2 promoter. We first tested the association of HDAC1, HDAC2 and HDAC4 with the promoter regions of 5HT2A, 5HT2C, mGlu2, and mGlu3 genes, including the β-actin (Actb) promoter as internal control. We found that HDAC2 was more enriched than HDAC1 or HDAC4 at the mGlu2 and mGlu3 promoters, whereas no detectable binding was seen at promoter regions of the 5HT2A and 5HT2C genes (Figs. 4a and 4b).
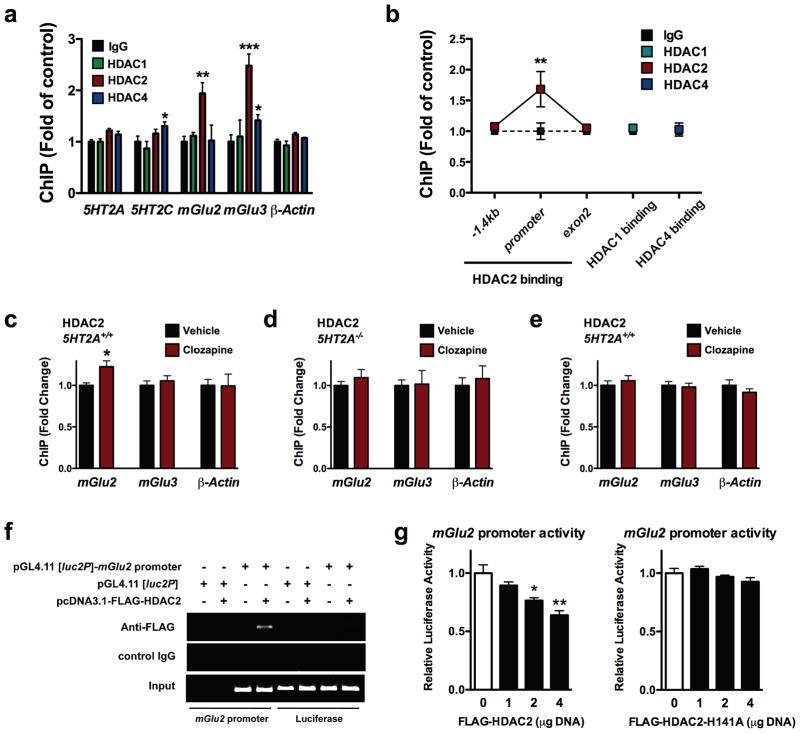
Figure 4. HDAC2 binds to the mGlu2 promoter and represses its function; an effect that is increased by chronic clozapine in mouse frontal cortex.
(a) Binding of HDAC2 to the mGlu2 and mGlu3 promoters in mouse frontal cortex. Fragmented chromatin was immunoprecipitated with antibody recognizing HDAC1, HDAC2, HDAC4, or control IgG, and the level of association of the 5HT2A (n = 12 mice per group), 5HT2C (n = 12 mice per group), mGlu2 (n = 15 mice per group), mGlu3 (n = 12 mice per group) and β-actin (n = 12 mice per group) promoters was measured by qPCR. Binding at the 5HT2A promoter, F[3,44] = 1.82, P > 0.05; binding at the 5HT2C promoter, F[3,44] = 3.22, P < 0.05; binding at the mGlu2 promoter, F[3,44] = 5.36, P < 0.01; binding at the mGlu3 promoter, F[3,44] = 9.83, P < 0.001; binding at the β-actin promoter, F[3,44] = 2.39, P > 0.05; one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; Bonferroni’s post hoc test of one-way ANOVA.
(b) HDAC2, and not HDAC1 or HDAC4, binds to the mGlu2 promoter in mouse frontal cortex. Fragmented chromatin was immunoprecipitated with antibody recognizing HDAC2, or control IgG, and the level of association of the -1.4 kb region, promoter region, and exon 2 region of the mGlu2 gene was measured by qPCR (n = 8 mice per group). Binding of HDAC2, F[2,42] = 3.71, P < 0.05; two-way ANOVA. **P < 0.01; Bonferroni’s post hoc test of two-way ANOVA. Association of HDAC1 and HDAC4 to the mGlu2 promoter was also measured (n = 8 mice per group). HDAC1 at mGlu2 promoter: t = 1.03, P > 0.05; HDAC4 at mGlu2 promoter: t = 0.24, P > 0.05; two-tailed Student’s t-test.
(c–e) Binding of HDAC2 to the mGlu2, and not mGlu3, promoter is increased by chronic clozapine treatment in mouse frontal cortex. 5HT2A+/+ (c,e) and 5HT2A−/− (d) mice were chronically (21 days) injected with vehicle (black) or 10 mg/kg clozapine (red), and sacrificed one day after the last injection (5HT2A+/+, n = 8; 5HT2A−/−, n = 6 mice per group). Fragmented chromatin was immunoprecipitated with antibody recognizing HDAC2 (c,d) or HDAC4 (e), and the level of association of the mGlu2, mGlu3 and β-actin promoters was measured by qPCR. Effect of chronic clozapine on HDAC2 binding to the mGlu2 promoter: t = 3.50. The α value was corrected for multiple independent null hypotheses by using the Holm’s sequentially rejective Bonferroni method. *P < 0.016; two-tailed Student’s t-test.
(f) HDAC2 binds to the mGlu2 promoter in HEK293 cells. ChIP assay was performed in HEK293 cells transfected with the pGL4.11 [luc2P] mouse mGlu2 promoter (−241/+97)-luciferase plasmid in combination with the pcDNA3.1-FLAG-HDAC2 plasmid. After incubation for 48 h, cells were cross-linked by formaline treatment. The lysates were immunoprecipitated with anti-FLAG antibody, or control IgG. The plasmid-derived mGlu2 promoter and a DNA fragment encoding luciferase were amplified by PCR using immunoprecipitated DNA (ChIP) and total DNA for control (Input). The results shown are representative of three independent experiments.
(g) Luciferase reporter assay. HEK293 cells were transfected with the mGlu2 promoter-luciferase construct in combination with pcDNA3.1-FLAG-HDAC2 (n = 6 independent experiments performed in triplicate), or with the deacetylase activity-deficient mutant pcDNA3.1-HDAC2-H141A (n = 3 independent experiments performed in triplicate), (1, 2 and 4 μg). After incubation for 24 h, luciferase activity in cell lysates was measured with a luminometer. Effect of FLAG-HDAC2 on mGlu2 promoter activity, F[3,8] = 12.11, P < 0.01; effect of FLAG-HDAC2-H141A on mGlu2 promoter activity, F[3,8] = 2.36, P > 0.05; one-way ANOVA. *P < 0.05; **P < 0.01; Bonferroni’s post hoc test of one-way ANOVA. Error bars represent s.e.m.
We next evaluated the effect of chronic clozapine on HDAC2 binding to the mGlu2 and mGlu3 promoters in mouse frontal cortex. Interestingly, chronic clozapine significantly increased HDAC2 binding to the mGlu2, and not to the mGlu3, promoter (Fig. 4c). This does not occur in 5HT2A−/− mice, which further supports the role of 5HT2A-dependent signaling as a modulator of expression and epigenetic function of HDAC2 (Fig. 4d; see also Supplementary Figs. 1e–1g for absence of effect of chronic atypical antipsychotics on mGlu2 expression in 5HT2A−/− mice, and Supplementary Fig. 2d for absence of effect of chronic atypical antipsychotics on the repressive histone modifications induced at the mGlu2 promoter in 5HT2A−/− mice). Binding of HDAC4 to the promoters of mGlu2, mGlu3, and β-actin genes was not affected by chronic clozapine (Fig. 4e).
The functional significance of this finding was further investigated in cell culture. We first tested whether HDAC2 is associated with the mGlu2 promoter in HEK293 cells. ChIP analysis revealed that HDAC2 binds to the mGlu2 promoter, since it was efficiently immunoprecipitated by anti-FLAG antibody in cells over-expressing FLAG-tagged HDAC2 (Fig. 4f). Next, the effect of HDAC2 on mGlu2 promoter activity was determined by promoter reporter assay. Over-expression of HDAC2 (but not the deacetylase activity-deficient mutant HDAC2-H141A) impaired mGlu2 promoter function in a concentration-dependent manner, which demonstrates that HDAC2 negatively regulates mGlu2 expression in cultured cells (Fig. 4g; see also Supplementary Fig. 5a for immunoblotting experiments showing over-expression of FLAG-HDAC2 and FLAG-HDAC2-H141A in HEK293 cells). These findings provide evidence that HDAC2 is critical for regulating transcriptional activation of the mGlu2 promoter. Taken together, our findings indicate that chronic atypical antipsychotic drugs up-regulate HDAC2 promoter activity and expression through an epigenetic mechanism that involves 5HT2A-dependent signaling, which consequently leads to a higher binding and negative regulation of HDAC2 to the mGlu2 promoter in mouse frontal cortex.
HDAC2 over-expression induces schizophrenia-like behavior
To further establish a causal link between changes in HDAC2 expression and transcriptional repression of the mGlu2 promoter, we over-expressed HDAC2 in mouse frontal cortex to examine whether this manipulation would regulate mGlu2 expression and its behavioral function. Mice received intra-frontal cortical injections of bicistronic herpes simplex 2 viral particles (HSV-2) expressing green fluorescent protein (GFP) and FLAG-HDAC2, or GFP alone. First, we confirmed that the virus over-expresses FLAG-HDAC2 in mouse frontal cortex (Fig. 5a and Supplementary Fig. 5b). Notably, such over-expression of HDAC2 decreases mRNA levels of mGlu2, but not 5HT2A, 5HT2C, mGlu3 or β-actin in this brain region (Fig. 5b). The absence of significant effect of HDAC2 over-expression on the transcription of the mGlu3 gene may be due to the predominant location of the mGlu3 receptor in cortical presynaptic terminals and glial cells17,35.
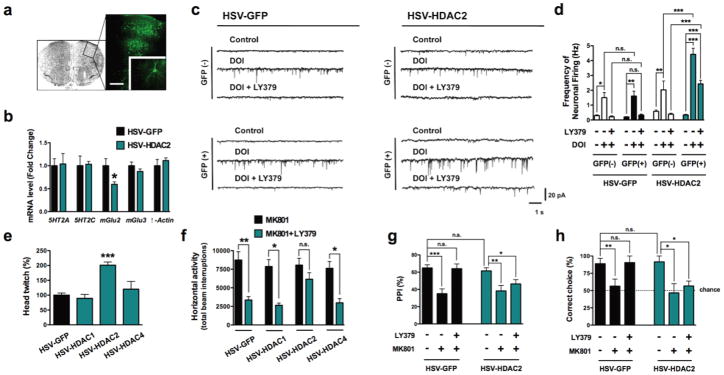
Figure 5. Viral over-expression of HDAC2 in frontal cortex decreases mGlu2-dependent behavioral function.
(a) Representative image of HSV-mediated transgene expression in frontal cortex. HSV-FLAG-HDAC2, which also expresses GFP, was injected into frontal cortex, and GFP expression was revealed by immunocytochemistry (Scale bar, 200 μm). Inset: GFP-expressing neuron.
(b) Viral-mediated over-expression of HDAC2 decreases expression of mGlu2 mRNA levels, and not 5HT2A, 5HT2C, mGlu3, or β-actin mRNA levels, in frontal cortex (n = 6 mice per group). Effect of HSV-HDAC2 on mGlu2 expression: t = 3.32. The α value was corrected for multiple independent null hypotheses by using the Holm’s sequentially rejective Bonferroni method. *P < 0.011; two-tailed Student’s t-test.
(c,d) Viral-mediated over-expression of HDAC2 attenuates the inhibition of 5HT2A receptor agonist DOI-induced sEPSC by LY379268. Sample traces (c). Whole-cell recordings from GFP-positive and GFP-negative neurons in acute cortical slices show that the increase in sEPSC frequency by the hallucinogenic 5HT2A agonist DOI (5 μM) is completely blocked by the mGlu2/3 agonist LY379268 (1 μM), while HSV-HDAC2 over-expression significantly decreases the effect of LY379268 on sEPSC frequency evoked by DOI in cortical pyramidal neurons (d). Effect of HSV-GFP (n = 2 mice per group, 10 neurons per mice) or HSV-HDAC2 (n = 3 mice per group, 10 neurons per mice), F[3,51] = 30.06, P < 0.001; effect of DOI and/or LY379, F[2,51] = 62.53, P < 0.001; two-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant; Bonferroni’s post hoc test of two-way ANOVA.
(e) Viral-mediated over-expression of HDAC2, but not HDAC1 or HDAC4, in frontal cortex significantly increases the head-twitch response induced by the hallucinogen DOI (0.5 mg/kg), (HSV-GFP, n = 10 mice; HSV-HDAC1, n = 5 mice; HSV-HDAC2, n = 5 mice; HSV-HDAC4, n = 5 mice). Effect of HSV-mediated over-expression, F[3,19] = 17.32, P < 0.001; one-way ANOVA. ***P < 0.001; Bonferroni’s post hoc test of one-way ANOVA.
(f) Viral-mediated over-expression of HDAC2, but not HDAC1 or HDAC4, in frontal cortex attenuates the inhibition of MK801-stimulated locomotor response by LY379268. Mice were administered LY379268 (8 mg/kg) or vehicle, followed by MK801 (0.5 mg/kg). The panel shows bar graph summary data of the total MK801-induced locomotor as a summation of horizontal activity from t = 15 to t = 120 min (n = 6-14 mice per group). Effect of HSV-mediated over-expression, F[3,61] = 2.76; P < 0.05; effect of MK801 and/or LY379, F[1,61] = 32.39, P < 0.001; two-way ANOVA. *P < 0.05; **P < 0.01; n.s., not significant; Bonferroni’s post hoc test of two-way ANOVA.
(g) Viral-mediated over-expression of HDAC2 attenuates the reversal of MK801-decreased prepulse inhibition (PPI) of startle by LY379268. Mice were administered LY379268 (5 mg/kg) or vehicle, followed by MK801 (0.1 mg/kg), (HSV-GFP, n = 25 mice per group; HSV-HDAC2, n = 35 mice per group). Effect of HSV-HDAC2, F[1,174] = 7.82, P < 0.01; Effect of LY379, F[2,174] = 35.26, P < 0.001; two-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant; Bonferroni’s post hoc test of two-way ANOVA.
(h) Viral-mediated over-expression of HDAC2 attenuates the reversal of MK801-impaired delayed alternation memory performance (percentage of correct trials at 10 s delay) by LY379268. Mice (HSV-GFP and HSV-HDAC2) made significantly fewer correct choices after the 40 s delay relative to the 10 s delay (see Supplementary Fig. 5h). Mice were administered LY379268 (5 mg/kg) or vehicle, followed by MK801 (0.5 mg/kg), (HSV-GFP, n = 10 mice per group; HSV-HDAC2, n = 16 mice per group). The dashed line indicates chance performance. Effect of HSV-HDAC2, F[1,67] = 5.28, P < 0.05; Effect of LY379, F[2,67] = 14.02, P < 0.001; two-way ANOVA. *P < 0.05; **P < 0.01; n.s., not significant; Bonferroni’s post hoc test of two-way ANOVA. Error bars represent s.e.m.
Previous findings demonstrate that activation of mGlu2 attenuates spontaneous excitatory postsynaptic currents (sEPSC) induced by 5HT2A agonists, including DOI, in cortical pyramidal neurons36–38. To determine whether over-expression of HDAC2 influences the functional responses mediated by mGlu2, we examined the inhibitory effects of the mGlu2/3 agonist LY379268 on the DOI-induced (5HT2A-dependent) sEPSC in cortical slices prepared from mice injected with HSV-HDAC2, and their HSV-GFP counterparts. As expected, activation of the 5HT2A receptor by DOI induced an increase in sEPSC, an effect that was reduced by LY379268 in animals over-expressing GFP alone as well as in uninfected control neurons (Figs. 5c and 5d, and Supplementary Fig. 5c). Further, and importantly, HDAC2 over-expression significantly decreased the inhibitory effect of LY379268, as compared to the reduction obtained in uninfected GFP-negative and HSV-GFP cortical neurons (Figs. 5c and 5d, and Supplementary Fig. 5c). Together, the reduction of mGlu2 expression (Fig. 5b) and the reduction in the efficacy of the mGlu2/3 agonist on 5HT2A-mediated sEPSC consistently support that HSV-HDAC2 negatively regulates mGlu2 transcription, which limits the functional antagonism between mGlu2 and 5HT2A receptors in mouse frontal cortex.
To gain further insight into these phenomena, we next examined whether this manipulation would affect the behavioral responses that require 5HT2A- and/or mGlu2-mediated signaling pathways. We have previously demonstrated that the head-twitch murine behavioral response is reliably and robustly elicited by hallucinogenic drugs such as psilocybin, mescaline and LSD, and is absent in 5HT2A−/− mice19. Remarkably, we found here that the head-twitch response induced by a low dose of the hallucinogenic drug DOI (0.5 mg/kg) was significantly increased in mice over-expressing HDAC2, but not HDAC1 or HDAC4, in frontal cortex compared to that seen in animals expressing GFP (Fig. 5e). Since activation of the mGlu2 receptor abolishes the head-twitch response induced by hallucinogens25,36, these findings correlate with the lower expression of mGlu2 in mice over-expressing HDAC2 in frontal cortex.
To complement the behavioral findings using hallucinogens, we next investigated the effects of HDAC2 over-expression in frontal cortex on the behavioral responses induced by the potent and selective non-competitive NMDA receptor antagonist MK801 (dizocilpine). MK801 and other non-competitive NMDA receptor antagonists evoke in normal humans psychotic and other symptoms resembling aspects of schizophrenia39. Previous findings demonstrate that activation of mGlu2, and not mGlu3, by the mGlu2/3 receptor agonist LY379268 reduces the locomotor hyperactivity induced by MK80122. We found that neither HSV-GFP nor HSV-HDAC2 affected the locomotor activity induced by MK801 (Fig. 5f and Supplementary Fig. 5d). In notable contrast, however, HDAC2 over-expression diminished the ability of LY379268 (8 mg/kg), the mGlu2/3 agonist, to reduce the MK801-dependent locomotor response. Over-expression of HDAC1 or HDAC4 in frontal cortex did not affect the locomotor behavioral effects of MK801 or LY379268 (Fig. 5f and Supplementary Fig. 5d). Spontaneous locomotor activity and time spent in the center of the arena (paradigms used in the evaluation of anxiety [see 40]) were comparable between mice that received intra-frontal cortex injections of HSV-2 vectors expressing HDAC1, HDAC2, HDAC4, and/or GFP (Supplementary Fig. 5e).
Deficits in the filtering or gating sensory and cognitive information have also been theorized to be core features of schizophrenia. Prepulse inhibition (PPI) is a measure of sensorimotor gating that refers to the reduction in the startle response produced by a low-intensity non-startling stimulus (the prepulse) presented shortly before the startle stimulus41. Patients with schizophrenia exhibit diminished PPI of the acoustic startle reflex42, which represents one operational measure of sensorimotor gating and cognitive impairment. We compared the effects of LY379268 on the impaired PPI response induced by MK801 in mice over-expressing HDAC2 and controls. The MK801-dependent deficit in the PPI test was not affected by viral-mediated over-expression of HDAC2 (Fig. 5g). Consistent with previous findings in rodents and healthy human subjects41, LY379268 prevented the PPI deficits evoked by MK801 in HSV-GFP control mice. Remarkably, the behavioral effects of LY379268 on the MK801-dependent PPI deficits were significantly reduced in mice over-expressing HCAC2 (Fig. 5g). These data suggest that HDAC2 negatively regulates sensorimotor gating of the startle reflex.
Schizophrenia patients also demonstrate impairments on a variety of working memory tests. Working memory requires the ability to form memory traces over a brief time period in order to manipulate this information to guide a goal-oriented behavior40. In rodents, working memory can be reliable assessed in a T-maze using a discrete-trial delayed spatial alternation task. We found that MK801 impaired choice accuracy in the T-maze delayed alternation task, an effect that was reversed by LY379268 in HSV-GFP control mice (Fig. 5h and Supplementary Fig. 5g). In contrast, the effect of LY379268 on MK801-induced impairment in the T-maze was reduced in mice over-expressing HDAC2 in frontal cortex, as measured by the percentage of entries into the correct arms (Fig. 5h and Supplementary Fig. 5g; see also Supplementary Fig. 5f for T-maze task in uninfected control mice). Taken together, our findings indicate that increased expression of HDAC2 in mouse frontal cortex results in behaviors that are associated with impaired mGlu2 function.
SAHA improves antipsychotic-like effects of clozapine
Recent preclinical and clinical results suggest that drugs that activate the mGlu2 receptor may represent a new approach to treat schizophrenia21–24. Our results thus far indicate that chronic atypical antipsychotics selectively up-regulate the expression of HDAC2 in frontal cortex, which precisely parallels the decreased acetylation at the promoter region of the mGlu2 gene in both mouse and human. These findings led us to hypothesize that prevention of this repressive histone modification at the mGlu2 promoter with HDAC inhibitors would augment the therapeutic-like behavioral responses induced in mice by atypical antipsychotic drugs.
To directly examine this possibility, we first investigated the effects of two HDAC inhibitors, SAHA and MS-275, which are selective inhibitors of class I/II HDACs and have been shown to modulate mGlu2 expression in mammalian CNS43. Mice were stereotactically injected in the frontal cortex with a single dose of SAHA, MS-275, or vehicle, and analyzed 24 h later. Both SAHA and MS-275 increased mGlu2 and mGlu3 mRNA expression, whereas 5HT2A and 5HT2C mRNA expression was unaffected (Supplementary Fig. 4b). ChIP assays showed that histone H3 acetylation was significantly increased at the promoter regions of the mGlu2 and mGlu3 genes by local administration of SAHA or MS-275 in frontal cortex (Supplementary Fig. 4c; see also Supplementary Figs. 4d and 4e for promoter assay and ChIP experiments with SAHA and MS-275, and another HDAC inhibitor trichostatin A in tissue culture). These data indicate that HDAC inhibitors stimulate mGlu2 promoter activity in vitro as well as in vivo in mouse frontal cortex.
Next, we directly compared the effects of chronic SAHA treatment with that of clozapine on the expression of mGlu2. We tested the effects of SAHA because MS-275 does not effectively penetrate the blood brain barrier when given systemically44, and our data validate the capability of chronic intraperitoneal administration of SAHA to increase histone H3 acetylation in frontal cortex, an effect that was not observed with chronic clozapine (Supplementary Fig. 4f and 4g). As stated above (see Fig. 1 and Supplementary Fig. 1), chronic clozapine down-regulates the expression of 5HT2A and mGlu2, but not 5HT2C and mGlu3, in mouse frontal cortex (Figs. 6a–6e). Strikingly, while chronic SAHA had no effect on 5HT2A (Figs. 6a, 6b and 6e), it increased the expression of mGlu2 and mGlu3, and, more importantly, reversed the downregulation of mGlu2 caused by chronic clozapine (Fig. 6c–6e). Such transcriptional regulation of the mGlu2 and mGlu3 genes, and the lack of regulation of the 5HT2A gene, was tightly correlated with active acetyl-histone H3 binding at their corresponding promoters (Fig. 6f). Together, these data show that chronic SAHA increases the expression of mGlu2 and mGlu3, and that adjunctive SAHA treatment prevents the repressive epigenetic changes induced at the mGlu2 promoter by chronic clozapine.
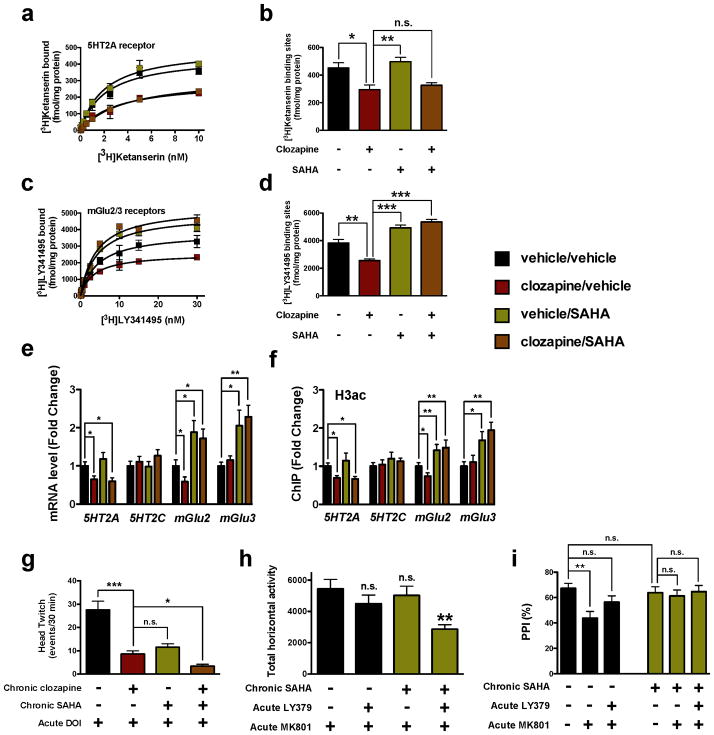
Figure 6. Chronic treatment with SAHA, an HDAC inhibitor, prevents the repressive histone modifications induced at the mGlu2 promoter by chronic clozapine.
(a,b) Chronic clozapine affects the expression of 5HT2A in mouse frontal cortex, and chronic SAHA does not. Animals were chronically (21 days) injected with clozapine (10 mg/kg), and/or SAHA (20 mg/kg), or vehicle, and sacrificed one day after the last injection. Effect of clozapine and/or SAHA on binding saturation curves (a) for the 5HT2A antagonist [3H]ketanserin (n = 6 independent experiments performed in triplicate), F[6,136] = 43.59, P < 0.001. Maximum number of binding sites (Bmax) obtained from individual saturation curves (b), F[3,12] = 9.55, P < 0.01; one-way ANOVA. *P < 0.05; **P < 0.01; n.s., not significant; Bonferroni’s post hoc test of one-way ANOVA.
(c,d) Chronic clozapine affects the expression of mGlu2/3 in mouse frontal cortex, an effect that is reversed by co-administration of chronic SAHA. Animals were chronically (21 days) injected with clozapine (10 mg/kg), and/or SAHA (20 mg/kg), or vehicle, and sacrificed one day after the last injection. Effect of clozapine and/or SAHA on binding saturation curves (c) for the mGlu2/3 antagonist [3H]LY341495 (n = 6 independent experiments performed in triplicate), F[6,168] = 69.34, P < 0.001. Maximum number of binding sites (Bmax) obtained from individual saturation curves (d), F[3,12] = 9.55, P < 0.01; one-way ANOVA. **P < 0.01; ***P < 0.001; Bonferroni’s post hoc test of one-way ANOVA.
(e) Expression of 5HT2A, 5HT2C, mGlu2, and mGlu3 mRNA in mouse frontal cortex assayed by qRT-PCR after chronic clozapine and/or chronic SAHA, or vehicle (n = 6 mice per group). Expression of 5HT2A, F[3,20] = 8.58, P < 0.001; Expression of 5HT2C, F[3,20] = 0.85, P > 0.05; Expression of mGlu2, F[3,20] = 12.71, P < 0.001; Expression of mGlu3, F[3,20] = 5.81, P < 0.01; one-way ANOVA. *P < 0.05; **P < 0.01; Bonferroni’s post hoc test of one-way ANOVA.
(f) Co-administration of SAHA reverses the repressive histone H3 modifications induced at the mGlu2 promoter by chronic clozapine. Fragmented chromatin was immunoprecipitated with antibody recognizing acetyl-histone H3 (H3ac), and the level of association of the 5HT2A, 5HT2C, mGlu2, or mGlu3 promoters was measured by qPCR (n = 8 mice per group). Binding at the 5HT2A promoter, F[3,28] = 7.40; P < 0.001; binding at the 5HT2C promoter, F[3,28] = 0.53; P > 0.05; binding at the mGlu2 promoter, F[3,28] = 18.79; P < 0.001; binding at the mGlu3 promoter, F[3,28] = 5.81; P < 0.01; one-way ANOVA. *P < 0.05; **P < 0.01; Bonferroni’s post hoc test of one-way ANOVA.
(g) Chronic SAHA decreases the head-twitch behavioral response, and potentiates the antipsychotic-like behavioral effect of clozapine. Animals were chronically (21 days) treated with clozapine (10 mg/kg) and/or SAHA (20 mg/kg), or vehicle, and the head-twitch response induced by the hallucinogen DOI (2 mg/kg) was determined one day after the last injection (vehicle, n = 6; clozapine, n = 5; SAHA, n = 5; clozapine + SAHA, n = 6 mice per group). DOI-dependent head-twitch response, F[3,16] = 35.74, P < 0.001; one-way ANOVA. *P < 0.05; ***P < 0.001; n.s., not significant; Bonferroni’s post hoc test of one-way ANOVA.
(h) Co-administration of chronic SAHA and acute LY379268 inhibits the MK801-stimulated locomotor activity, an effect not seen with administration of either compound alone. Animals were chronically (21 days) treated SAHA (20 mg/kg), or vehicle, and the effect of LY379268 (1.5 mg/kg) on the MK801-stimulated locomotor activity was determined one day after the last injection (vehicle, n = 12; clozapine, n =8; SAHA, n = 11; clozapine + SAHA, n = 12 mice per group). Effect of chronic SAHA, F[3,39] = 5.30, P < 0.01; one-way ANOVA. **P < 0.01; n.s., not significant; Bonferroni’s post hoc test of one-way ANOVA.
(i) Chronic SAHA attenuates the MK801-decreased prepulse inhibition (PPI) of startle. Animals were chronically (21 days) treated SAHA (20 mg/kg), or vehicle, and the effect of LY379268 (3 mg/kg) on the MK801-decreased prepulse inhibition (PPI) of startle was determined one day after the last injection (vehicle + vehicle, n = 17; vehicle + MK801, n = 24; vehicle + MK801 + LY379268, n = 24; SAHA + vehicle, n = 17; SAHA + MK801, n = 17; SAHA + MK801 + LY379268, n = 23 mice per group). Effect of chronic SAHA, F[1,115] = 4.43, P < 0.05; Effect of LY379268, F[2,115] = 4.75, P < 0.05; two-way ANOVA. **P < 0.01; n.s., not significant; Bonferroni’s post hoc test of two-way ANOVA. Error bars represent s.e.m.
To examine the therapeutic-like relevance of our findings, we next investigated whether abolition by SAHA of the clozapine-induced histone modifications at the mGlu2 promoter in frontal cortex is associated with alterations in behavioral responses induced by antipsychotic drugs. We treated mice with clozapine, risperidone, haloperidol, or vehicle chronically (21 days) or subchronically (2 days) before assessing the head-twitch response to the hallucinogenic drug DOI one day after the last injection. Importantly, the head-twitch response induced by DOI (2 mg/kg) was decreased by chronic, and not subchronic, clozapine or risperidone (Supplementary Figs. 6a and 6b). Chronic haloperidol did not affect the head-twitch response induced by DOI (Supplementary Fig. 6a). We also observed that chronic SAHA diminished the head-twitch response induced by DOI to a similar extent to that found after chronic clozapine (Fig. 6g). Furthermore, chronic SAHA potentiates this antipsychotic-like effect of chronic clozapine (Fig. 6g), a lasting behavior that was even further accentuated five days after the last chronic injection (Supplementary Fig. 6c).
We next compared the effects of chronic SAHA on the behavioral response induced by the psychotomimetic drug MK801. We found that neither a low dose of LY379268 (1.5 mg/kg) alone nor chronic SAHA alone influenced the locomotor response induced by MK801 (Fig. 6h and Supplementary Fig. 6e). However, the MK801-dependent locomotor hyperactivity was attenuated significantly in mice that received both chronic SAHA and acute LY379268 (Fig. 6h and Supplementary Fig. 6e; see also Supplementary Fig. 6d for selection of a low dose of LY379268 that by itself does not affect the locomotor activity induced by MK801). We also compared the effects of chronic SAHA on the impaired PPI response induced by MK801. As shown in Fig. 5f above, MK801 induced PPI deficits in control mice, an effect that was reversed by LY379268 (Fig. 6i). Notably, we also found that chronic treatment with SAHA prevented the PPI deficits evoked by MK801, an effect that was similar to that induced by LY379268 (Fig. 6i). Together, these findings demonstrate that chronic treatment with the HDAC inhibitor SAHA enhances the behavioral effects induced by LY379268 using three different mouse models. Recent pathophysiological studies highlight the role of altered brain connectivity and synaptic plasticity in schizophrenia2. Our data do not exclude the possibility that HDAC2-dependent alterations in the expression of other genes, each of which has previously been implicated in cognitive function and synaptic plasticity45,46, also affect the behavioral responses induced by chronic treatment with SAHA (Supplementary Fig. 4h).
DISCUSSION
Chromatin remodeling has been implicated in the neurochemical mechanisms by which antipsychotic drugs achieve their clinical efficacy8,47. Although these findings provide compelling evidence for the involvement of epigenetic processes in mediating the molecular events associated with the treatment of schizophrenia, they do not exclude the possibility of compensatory mechanisms that may emerge in response to chronic antipsychotic drug exposure and ultimately restrain their therapeutic effects. Compensatory mechanisms are seen as negative correlations between processes that are pivotal for system function48. Within an individual neuron or individual animal, alterations in one parameter may produce minor changes in the state of the system if there are mechanisms that cause adjustments in another parameter to compensate for the first change. Here, we demonstrate that chronic treatment with atypical antipsychotic drugs dramatically decreases the density of 5HT2A binding sites in mouse frontal cortex, which leads to repressive histone modifications at the promoter region of the mGlu2 gene. Furthermore, our data reveal a critical role for HDAC2 in mediating these 5HT2A-dependent repressive epigenetic modifications at the mGlu2 promoter in mouse and human frontal cortex. We show that chronic administration of atypical antipsychotic drugs selectively up-regulate HDAC2 expression and binding to the mGlu2 promoter, an effect that is associated with the regulation of HDAC2 promoter transcriptional function by activation of the 5HT2A receptor. We then demonstrate that such over-expression of HDAC2 in frontal cortex is sufficient to down-regulate mGlu2 expression and its physiological inhibitory effects, which exacerbates schizophrenia-like behavior. Activation of mGlu2 is well known to repress cellular, electrophysiological and behavioral responses that require the 5HT2A receptor20. Similarly, drugs that activate the mGlu2 receptor have potential for the treatment of schizophrenia, whereas 5HT2A agonists, such as hallucinogenic compounds, result in the opposite effect. Taken together, these data suggest that the decreased density of 5HT2A binding sites by chronic treatment with atypical antipsychotic drugs results in a compensatory mechanism of repressive chromatin structure at the promoter region of the mGlu2 gene, with consequently less inhibitory effects of the mGlu2 receptor on 5HT2A-regulated pathways and behaviors.
We also observed that adjunctive SAHA abolishes the repressive histone modifications induced at the mGlu2 promoter by chronic atypical antipsychotics, which augments the behavioral effects induced by atypical and glutamate antipsychotics. Overall, our findings support the hypothesis that compensatory epigenetic events at the mGlu2 promoter may be responsible for the high incidence of patients that do not benefit from conventional therapy with atypical antipsychotic drugs, and provide a biochemical explanation for the clinical association of pharmacological inhibition of HDACs with improved schizophrenia treatment (Supplementary Fig. 8).
Previous studies from our laboratory have shown that 5HT2A and mGlu2 interact through specific transmembrane domains to form a G protein-coupled receptor (GPCR) heterocomplex in mouse and human frontal cortex25. The signaling properties of this receptor heterocomplex have been proposed to be necessary for therapeutic-like effects of atypical antipsychotic drugs23. We show here that chronic atypical antipsychotic drugs induce 5HT2A-dependent repressive histone modifications at the mGlu2 promoter in frontal cortex, which is consistent with previous reports describing effects of long-term treatment with 5HT2A agonists on behavioral responses that require the mGlu2 receptor36,37. Further work will be directed to assess the relative contribution of these epigenetic factors to the modulation of expression of 5HT2A and mGlu2 as a GPCR heterocomplex that may balance the response to ligand inputs in cortical pyramidal neurons.
Clozapine is the only antipsychotic drug with proven superior efficacy in treatment-resistant schizophrenic patients4. We found that both clozapine and risperidone, but not haloperidol, decreased the density of 5HT2A binding sites in mouse frontal cortex. Similar findings on 5HT2A receptor binding have been reported in postmortem human brain of treated schizophrenic subjects25,49. These results suggest that the 5HT2A receptor is involved in treating the psychotic symptoms of schizophrenia, and that down-regulation of 5HT2A receptor binding sites by chronic atypical antipsychotic drugs may be one of the mechanisms underlying their therapeutic effects. Remarkably, only chronic clozapine induces changes in histone acetylation at the 5HT2A promoter that correlate with its transcriptional repression. Although further investigation is needed to understand the mechanisms and consequences of this interesting finding, it is tempting to speculate that these histone modifications at the promoter of the 5HT2A gene may account for the enhanced antipsychotic properties of clozapine. Further work is also needed to determine the molecular mechanisms responsible for down-regulation of 5HT2A mRNA expression in subcortical regions by chronic treatment with clozapine.
A notable finding of the current study is that chronic treatment with clozapine up-regulates HDAC2 in mouse frontal cortex. In concordance with the effects of clozapine in murine models, the expression of HDAC2 is increased in postmortem frontal cortex of schizophrenic subjects treated with atypical antipsychotic drugs. Since no alteration is detected in untreated schizophrenic subjects, these results suggest that dysregulation of HDAC2 expression represents a consequence of antipsychotic drug medication, and not a biochemical marker of schizophrenia in postmortem human brain. This is an intriguing observation because viral-mediated over-expression of HDAC2 in mouse frontal cortex induces behavioral alterations that replicate psychotic symptoms and cognitive impairments in schizophrenia patients. Notably, clinical studies indicate that deficits in attention and memory function, cognitive impairments that represent core features of schizophrenia1,2, are exacerbated by treatment with atypical antipsychotic drugs50. Together, these findings suggest that the 5HT2A-dependent up-regulation of HDAC2 and repressive histone modifications at the mGlu2 promoter might be related to the negative modulation of cognitive processes by chronic treatment with atypical antipsychotic drugs.
The ultimate goal of understanding the pathophysiology of schizophrenia is to develop therapeutic strategies that improve or restore normal brain activity and, ultimately, the associated deficits in sensory processing, perception and memory function. The functions of HDAC2 described here involve important implications for the molecular basis of the currently limited response to treatment with atypical antipsychotics. We propose that atypical antipsychotic drugs induce a selective up-regulation of HDAC2 in frontal cortex of individuals with schizophrenia, which alters the chromatin state of the mGlu2 promoter and thereby limits the therapeutic effects of these agents. Identification of the epigenetic mechanisms through which administration of HDAC inhibitors potentiates the biochemical and behavioral responses to atypical antipsychotics will help in discovering more effective treatments to improve the clinical efficacy of the currently available antipsychotic medications. Specifically, our findings encourage the development and testing of HDAC2-selective inhibitors for schizophrenia.
METHODS
Materials and Drug Administration
1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), (5R,10S)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (dizocilpine, (+)-MK-801), serotonin (5HT), dopamine (DA), and picrotoxin were purchased from Sigma-Aldrich. Clozapine, risperidone, haloperidol, fluoxetine, (1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY379268), and trichostatin A (TSA) were obtained from Tocris Cookson Inc. Suberoylanilide hydroxamic acid (vorinostat; SAHA) was purchased from Cayman Chemical. N-(2-Aminophenyl)-4-[N-(pyridine-3-ylmethoxy-carbonyl)aminomethyl]benzamide (MS-275) was obtained from Enzo Life Sciences. The injected doses (i.p.) were clozapine, 10 mg/kg; risperidone, 4 mg/kg; haloperidol, 1 mg/kg; fluoxetine, 20 mg/kg; and SAHA, 20 mg/kg; unless otherwise indicated. DOI and LY379268 were dissolved in saline. MK-801, SAHA, risperidone, haloperidol and fluoxetine were injected after suspension in a minimal amount of DMSO and made up to volume with saline. Clozapine was dissolved in DMSO supplemented with a minimal amount of acetic acid and suspended in saline. SAHA and MS-275 were administered locally in frontal cortex after suspension in a minimal amount of DMSO and made up to volume with saline. [3H]Ketanserin and [3H]raclopride were purchased from PerkinElmer Life and Analytical Sciences, Inc. [3H]-2S-2-amino-2-(1S,2S-2-carboxycyclopropan-1-yl)-3-(xanth-9-yl)-propionic acid ([3H]LY341495) was purchased from American Radiolabeled Chemicals, Inc. All other chemicals were obtained from standard sources.
Transient Transfection of HEK293 cells
Human embryonic kidney (HEK293) cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) foetal bovine serum at 37°C in a 5% CO2 humidified atmosphere. Transfection was performed using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer’s instructions.
Plasmid Construction
All PCR reactions were performed using Pfu Ultra Hotstart DNA polymerase (Stratagene) in a Mastercycler Ep Gradient Auto thermal cycler (Eppendorf). All the constructs were confirmed by DNA sequencing. For the HDAC2 promoter construct, mouse HDAC2 promoter (−481 to +141 bp) was PCR amplified from mouse genomic DNA (Clontech) using the following primers: 5′-TGAAACACGTGGGAATATCG-3′ and 5′-GCCAGACAAGAGGGCTGAC-3′. The amplicon was inserted into pCR-blunt vector (Invitrogen), and then sequenced. The product was re-amplified using the primers 5′-GATctcgagTGAAACACGTGGGAATATCG-3′ and 5′-CTTaagcttGCCAGACAAGAGGGCTGAC-3′, and then digested with XhoI and HindIII and subcloned into the XhoI and HindIII sites of pGL4.11 [luc2P] plasmid (Promega). For the mGlu2 promoter construct, mouse mGlu2 promoter (−241 to +97 bp) was PCR amplified from mouse genomic DNA (Clontech) using the following primers: 5′-CACACTGTGGACAGCTCCAG-3′ and 5′-CACCAAGGGCTAACCCTACC-3′. The amplicon was inserted into pCR-blunt vector (Invitrogen), and then sequenced. The product was re-amplified using the primers 5′-GATctcgagCACACTGTGGACAGCTCCAG-3′ and 5′-CTTaagcttCACCAAGGGCTAACCCTACC-3′, and then digested with XhoI and HindIII and subcloned into the XhoI and HindIII sites of pGL4.11 [luc2P] plasmid (Promega). For the HSV-HDAC2 construct, mouse HDAC2 cDNA (gift of Dr. Patrizia Casaccia) along with the FLAG tag was PCR amplified using the following primers: 5′-AGTggatccATGGACTACAAGGACGACGA-3′ and 5′-CCGCGctcgagAGTTCACTT-3′. The PCR product was inserted into the BamHI and XhoI sites of pcDNA3.1 (+) plasmid (Invitrogen) and the bicistronic p1005+ HSV plasmid expressing GFP under the control of the CMV promoter51, and then sequenced. The catalytically inactive mutant HDAC2-H141A (see [52]) was constructed using site-directed mutagenesis according to the manufacturer’s protocol (Stratagene).
Luciferase Reporter Assay
Luciferase activity was measured with a luminometer (TD-20/20; Turner Biosystems) using the Dual-Luciferase reporter assay system (Promega) according to the manufacturer’s instructions. HEK293 cells were plated at a density of 1 × 105 in six-well dishes, cultured for 24 h, and transfected with the corresponding plasmids. For the HDAC2 promoter assay, cells were transfected with the pGL4.11 [luc2P] plasmid (Promega) containing the mouse HDAC2 promoter (−481 to +141 bp; 1.0 μg), and the pcDNA3.1-c-Myc-5HT2A plasmid25 (1.0 μg) or the pcDNA3.1-HA-D2 plasmid (Missouri S&T cDNA Resource Center; 1.0 μg). Serotonin (0.01, 0.1, and 1.0 μM), dopamine (1.0 μM), clozapine (0.1, 1.0, and 10 μM), or vehicle was added to the medium without serum 5 h after transfection. For the mGlu2 promoter assay, cells were transfected with the pGL4.11 [luc2P] plasmid (Promega) containing the mouse mGlu2 promoter (−241 to +97 bp; 1.5 μg), and the pcDNA3.1-FLAG-HDAC2 plasmid (1.0, 2.0, and 4.0 μg) or the pcDNA3.1-FLAG-HDAC2-H141A plasmid (1.0, 2.0, and 4.0 μg). TSA (0.1, 1.0, and 10 μM), SAHA (0.1, 1.0, and 10 μM), MS-275 (1.0, 10, and 100 μM), or vehicle was added to the medium 5 h after transfection. Transfected cells were incubated for 24 h, and the luciferase activity was measured with a luminometer (TD-20/20; Turner Biosystems) using the Dual-Luciferase reporter assay system (Promega) according to the manufacturer’s instructions. Transfection efficiency was normalized with coexpressed pGL4.75 [hRluc/CMV] (Promega) (0.02 μg).
Experimental Animals
Experiments were performed on adult (8–12 weeks old) male 129S6/SvEv mice. 5HT2A−/− mice, developed in 129S6/SvEv strain, have been previously described19,34. For experiments involving 5HT2A−/− mice, 5HT2A+/+ littermates were used as controls. Animals were housed at 12 h light/dark cycle (lights on, 8:00 to 20:00) at 23°C with food and water ad libitum. For the working memory test (see below), experiments were performed on adult (8–14 weeks) male C57BL/6 mice because 129S6/SvEv mice are best avoided for this protocol53. The Institutional Animal Use and Care Committee at Mount Sinai School of Medicine approved all experimental procedures.
Mouse brain samples
The day of the experiment, mice were sacrificed by cervical dislocation, and bilateral frontal cortex (bregma 1.90 to 1.40 mm) was dissected and frozen at −80°C, or immediately processed for RNA extraction, chromatin immunoprecipitation and/or biochemical assays. The coordinates were taken according to a published atlas of the 129S6/Sv mouse strain54.
Postmortem Human Brain Tissue Samples
Human brains were obtained at autopsies performed in the Basque Institute of Legal Medicine, Bilbao, Spain, in compliance with policies of research and ethical boards for postmortem brain studies. Deaths were subjected to retrospective searching for previous medical diagnosis and treatment using examiner’s information and records of hospitals and mental health centers. After searching of antemortem information was fulfilled, 21 subjects who had met criteria of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)55 were selected. A toxicological screening for antipsychotics, other drugs and ethanol was performed on blood, urine, liver and gastric contents samples. All subjects who were drug-free before death (as revealed by the absence of prescriptions in medical histories) also gave negative results in the toxicological screening. The toxicological assays were performed at the National Institute of Toxicology, Madrid, Spain, using a variety of standard procedures including radioimmunoassay, enzymatic immunoassay, high-performance liquid chromatography and gas chromatography-mass spectrometry. Controls for the present study were chosen among the collected brains on the basis, whenever possible, of the following cumulative criteria: (1) negative medical information on the presence of neuropsychiatric disorders or drug abuse; (2) appropriate gender, age, postmortem delay (time between death and autopsy), and freezing storage time to match each subject in the schizophrenia group; (3) sudden and unexpected death (motor vehicle accidents); and (4) toxicological screening for psychotropic drugs with negative results except for ethanol. Specimens of prefrontal cortex (Brodmann’s area 9) were dissected at autopsy (0.5–1 g tissue) on an ice-cooled surface and immediately stored at −80°C until use. The definitive pairs of antipsychotic-untreated schizophrenics and respective matched controls, and the definitive pairs of atypical antipsychotic-treated schizophrenics and respective matched controls are shown in Supplementary Table 1. Pairs of schizophrenia and matched controls were processed simultaneously and under the same experimental conditions. Tissue pH values56 were within a relatively narrow range (control subjects: 6.33 ±0.05; schizophrenic subjects: 6.23 ±0.07). All brain samples were assayed for RNA integrity number (RIN)56 using the Agilent 2100 Bioanalyzer (Applied Biosystems) (see Supplementary Table 1).
Quantitative Real-Time PCR
Quatitative real-time PCR (qRT-PCR) assays were carried out in quadruplicate as previously described with minor modifications19,25,34. See Supplementary Table 2 for primer pair sequences.
Chromatin Immunoprecipitation Assay in Mouse Frontal Cortex
Chromatin immunoprecipitation (ChIP) experiments were performedusing the EZ-Magna ChIP Kit (Millipore) according to the manufacturer’s instructions. Primary antibodies were added to diluted lysates, and incubated at 4°C for 12 h with 20 μl of fully suspended protein A or G magnetic beads (Millipore). The following primary antibodies were used: acetyl-histone H3 (Millipore 06-599, 1:200), acetyl-histone H4 (Millipore 06-866, 1:200), mono/di/tri-methyl-histone H3 (Lys4) (Millipore 05-791,1:300), tri-methyl-histone H3 (Lys 9) (Millipore 07-442, 1:250), tri-methyl-histone H3 (Lys27) (Millipore 07-449, 1:300), HDAC1 (Abcam 31263, 1:200), HDAC2 (Abcam 12169, 1:200), HDAC4 (Abcam 1437, 1:200) and FLAG (Sigma F7245, 1:200). Input and immunoprecipitated DNAs were subjected to quantitative real-time PCR19 (see Supplementary Table 2 for primer pair sequences).
Immunoprecipitation of Native Chromatin in Postmortem Human Brain
Postmortem tissue samples (50 mg) were homogenized with douncing buffer (4 mM MgCl2, 1 mM CaCl2, and 10 mM Tris-HCl; pH 7.5) and then incubated with 2 U/mL of micrococcal nuclease (MNase; Sigma-Aldrich) for 10 min at 37°C. The MNase activity was stopped by adding EDTA to a final concentration of 10 mM. The lysate was diluted by adding 10-fold volume excess of hypotonic lysis buffer (0.1 mM benzamidine, 0.1 mM phenylmethylsulfonylfluoride [PMSF],1.5 mM 1,4-dithio-DL-threitol [DTT], and 0.2 mM EDTA; pH 8.0) and then incubated on ice for 1 h. The lysate was centrifuged at 3000 x g to remove insoluble material. The soluble chromatin was diluted by adding 10-fold volume excess of incubation buffer (50 mM EDTA, 500 mM NaCl, and 200 mM Tris-HCl; pH 7.5). Immunoprecipitation and DNA purification was performed as described above for the ChIP assay in mouse frontal cortex (see Supplementary Table 2 for primer pair sequences).
DNA Methylation Assay
DNA methylation assay was performed using the Non-Organic DNA Extraction Kit (Millipore) according to the manufacturer’s instructions. Genomic DNA was isolated from mouse brain frontal cortex and liver tissue samples using the Non-Organic DNA Extraction Kit (Millipore) according to the manufacturer’s instructions, and subjected to bisulfite modification to convert all non-methylated cytosines into thymidines. For sodium bisulfite treatment, the CpGenomie Fast DNA Modification Kit (Millipore) was used according to the manufacturer’s instructions. Modified DNA was then amplified by PCR using bisulfite-treated DNA specific primer sets 5′-GGTATTAAGGGTTAATTTTATTTGG-3′ and 5′-ACACTATAAACAACTCCAAACCCTAC-3′; and 5′-TGAGAGGTTGAGATAAAGATAGAGATATAG-3′ and 5′-CCAAATAAAATTAACCCTTAATACC-3′, which did not include any CpG sites where possible methylation could be present. The PCR products were cloned using TOPO-TA cloning kit (Invitrogen) and transformed into TOP10 competent cells. 9–12 different colonies from each DNA amplification reaction were then analyzed for possible methylated CpG sites using direct sequencing from the TOPO plasmids containing the insert.
Radioligand Binding
[3H]Ketanserin and [3H]LY341495 binding in mouse frontal cortex was performed as previously reported with minor modifications25. [3H]Raclopride binding to dopamine D2 receptors was performed in mouse striatum membrane preparations. [3H]Raclopride binding (0.0625-20 nM; ten concentrations) was measured at equilibrium in 500 μl aliquots (50 mM Tris-HCl; pH 7.6, supplemented with 1 mM EDTA, 0.01% ascorbic acid, and 5 mM MgCl2) of membrane preparations (20–40 μg protein per tube) which were incubated at room temperature for 60 min. Non-specific binding was determined in the presence of 10 μM (S)-(-)-sulpiride (Tocris).
Immunoblot Assays
Western blot experiments in mouse and postmortem human brain samples were performed as previously reported with minor modifications25. The following primary antibodies were used: HDAC1 (mouse brain: Cell Signaling 5356, 1:1000; postmortem human brain: Santa Cruz sc-6299, 1:200), HDAC2 (mouse brain: Abcam 12169, 1:400; postmortem human brain: Abcam 32117, 1:2000), HDAC4 (mouse brain: Abcam 1437, 1:400; postmortem human brain: Santa Cruz sc-5245, 1:200), acetyl-histone H3 (Millipore 06-599B, 1:400), FLAG (Sigma F7425, 1:1000), β-actin (Sigma A1978, 1:200000), GAPDH (Cell Signaling 14C10, 1:1000), α-tubulin (Cell Signaling 11H10, 1:10000), and histone H1 (Santa Cruz sc-10806, 1:200). Incubation with the secondary antibody (1:5000) coupled to peroxidase (Amersham Biosciences) was performed at room temperature for 90 min, followed by repeated washing with TBST. Immunoreactive proteins were visualized with enhanced chemiluminiscence (Thermo Scientific) according to the manufacturer’s instructions. Western blot experiments in mouse brain samples were quantified by densitometry using the NIH image 1.62 software. Nuclear-cytoplasmic fractionation was performed using NE-PER extraction kit (Thermo Scientific), following manufacturer’s instructions (see Supplementary Fig. 4g). Western blot experiments in postmortem human brain samples were analyzed with the Odyssey infrared imaging system (LI-COR Biosciences). The membranes were incubated with secondary antibodies (Alexa 680 Fluor goat anti-rabbit [1:1500], Alexa 680 Fluor donkey anti-goat [1:2500], and IRDye 800 donkey anti-mouse [1:10000]). The membranes were then scanned on an Odyssey infrared imaging system, and images were acquired and analyzed according to manufacturer’s instructions.
Viral-mediated Gene Transfer
HDAC1, HDAC2 and HDAC4 cDNAs were subcloned into a published bicistronic HSV-GFP virus vector (see above)51, and viral particles were then packaged as described before51. HSV-HDAC1, HSV-HDAC2, HSV-HDAC4, or control HSV-GFP was injected into the frontal cortex by stereotaxic surgery according to standard methods51. Mice were anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) during the surgery. The virus was delivered bilaterally with a Hamilton syringe at a rate of 0.1 μl/min for a total volume of 0.5 μl on each side. The following coordinates were used: +1.6 mm rostral-caudal, −2.4 mm dorsal-ventral, +2.6 mm medial-lateral from bregma (relative to dura) with a 10° lateral angle. The coordinates were taken according to a published atlas of the 129/Sv mouse strain54. The Nissl staining of the coronal brain slice was taken from the mouse brain atlas with author’s permission (Figure 5a)54. All experiments were performed 3–4 days after the viral infection when transgene expression is maximal51. Viral-mediated HDAC1, HDAC2 and HDAC3 over-expression levels in frontal cortex were confirmed by Western blotting and quantitative real-time PCR (Supplementary Fig. 5b and data not shown).
Immunohistochemistry
Immunohistochemistry experiments were performed as previously reported23. GFP immunoreactivity was assayed by using a polyclonal antibody (Santa Cruz sc-8334; 1:100) and Alexa 488 dye-conjugated anti-rabbit antibody (1:500).
Electrophysiology
Whole-cell recordings were obtained from deep layer neurons of the frontal cortex in acute brain slices from 129S6/Sv mice that had been stereotaxically injected into the frontal cortex with HSV-GFP or HSV-HDAC2 (see viral-mediated gene transfer above). The preparation of acute brain slices of the frontal cortex and recordings from the frontal cortex were performed as described previously57. Briefly, the artificial cerebrospinal fluid (aCSF) contained the following: 128 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 10 mM D-glucose, 24 mM NaHCO3, 2 mM CaCl2, and 2 mM MgSO4; oxygenated with 95% O2 and 5% CO2 (pH 7.35, 295–305 mOsm). Sucrose aCSF was derived by fully replacing NaCl with 254 mM sucrose in aCSF, and used during the slice cutting. Patch pipettes (3–5 MΩ) for whole-cell voltage clamp recordings were filled with an internal solution containing the following: 115 mM potassium gluconate, 20 mM KCl, 1.5 mM MgCl2, 10 mM phosphocreatine, 10 mM HEPES, 2 mM Mg-ATP, and 0.5 mM GTP (pH 7.2, 285 mOsm). The experiments were carried out at 30°C. GFP-positive cells were visualized with an upright fluorescence microscope using infrared differential interference contrast illumination and identified by their pyramidal shape and size and prominent apical dendrite in the frontal cortex. The resting membrane potential and spontaneous EPSCs were recorded in voltage clamp mode, holding −70 mV using an amplifier Multiclamp 700B (Molecular Devices). Data acquisition was acquired with pClamp 10. Series resistance was monitored throughout experiments that were terminated whenever significant changes (> 20%) in series resistance occurred. DOI and LY379268 were kept as concentrated stocks and diluted in aCSF immediately prior to experiments. After 2 minutes of stable baseline recording, DOI (5 μM) was applied for 2–4 minutes and subsequently DOI (5 μM) and LY379268 (1 μM) were applied for 2–4 minutes. These concentrations were chosen according to previous findings38. The frequency and amplitude of spontaneous EPSCs were measured for >2 minutes. All experiments were performed in the presence of picrotoxin (50 μM). Responses were obtained from 10 neurons from each animal, with means per animal then used to generate group means and perform statistical analyses (Origin 7.0). Data were analyzed by use of Clampfit 10.
Drug Administration in Mouse Frontal Cortex
Stereotaxic surgery was performed as described above. SAHA (10 μM), MS-275 (10 μM), or vehicle was delivered bilaterally with a Hamilton syringe at a rate of 0.1 μl/min for a total volume of 0.5 μl on each side. ChIP and quantitative real-time PCR experiments were performed 24 h after local administration of the tested drug in frontal cortex.
Head-twitch Behavioral Response
Head-twitch behavioral response (rapid lateral movements of the head similar to the pinna reflex) is reliably elicited by a variety of hallucinogenic drugs, such as DOI, DOM, DOB, mescaline, LSD and psilocin, and is absent in 5HT2A−/− mice19,34. Dissociative drugs, such as ketamine, PCP and MK801, induce head-weaving behavioral response (slow, side to side lateral head movement) in rodents17. Repetitive shaking of the body (wet-dog shake behavior) is commonly observed in rodents during morphine withdrawal58. We investigated the head-twitch behavior induced by DOI as a mouse behavior model of hallucinogenic 5HT2A agonist action. Experiments were carried out as previously29. Videotapes were scored for head-twitches by an experienced observer blind to drug treatment and/or viral-mediated gene transfer.
Locomotor Activity
Motor function (locomotor activity) was assessed as previously reported29.
Prepulse Inhibition of Startle (PPI)
Mice were placed in acoustically isolated startle chambers (MED Associates). The test started with a 10 min acclimation followed by three sessions of trials. Background noise was 70 dB throughout the acclimation and trial periods. Sessions 1 and 3 included 10 trials of startle stimuli (120 dB; 40 ms). Session 2 consisted of 56 trials in which startle response magnitude, peak latency, and onset latency to each stimulus were obtained for trials in which the startle stimulus was presented alone or preceded by 100 ms with a 15 ms prepulse. The prepulse amplitude was 2, 6, or 8 dB above background. The startle response was defined as changes in force on the floor (i.e., “displacement”) between 30 and 70 ms after the onset of the startle stimulus. Animals that had a peak response in less than 20 ms or after 80 ms of the presentation of the stimuli were excluded. PPI was calculated as 100 × (PPR/SR), where PPR is the average latency of startle response across trials presenting prepulse, and SR is the average latency of startle response from trials in which the startle was presented alone. Mice were injected with vehicle or MK801 (0.1 mg/kg) 15 min after injection with LY379268 (5 mg/kg), and 15 minutes before the start of the PPI experiment.
Discrete-Trials Rewarded Delayed Spatial Alternation
Working memory was tested using a T-maze alternation task. The experiments were performed in a T-maze constructed of wood and painted black. The walls were 15 cm high, and the alleys were 10.5 cm wide. The length of the mail alley was 28 cm, and the length of the side alleys was 22.5 cm. The side alleys were closed off from the main alley by movable doors. A week prior to habituation, all animals were partially food-deprived (each animal received 2 g of food/day) and remained that way throughout the remaining part of the experiment. This maintained each animal above 85% of its free-feeding body weight. A video camera was situated ~1 m above the T-maze to videotape the test session. The T-maze was cleaned between different animals but not between different trials. The food reward was a 14 mg salt pellet (Bio-Serv F05684). The full experiment consisted of three parts: habituation, training, and testing. During habituation, all animals were placed on the T-maze until they ate two pieces of food or 90 s had elapsed. This was repeated three times a day for 5 days. During training, all animals received six trials a day per day. Each trial consisted of two runs, a forced run and a free run. On the forced run, mice were forced to obtain a piece of food from one goal arm of the T-maze with the other goal arm blocked by its door. Animals were then placed back into the start arm for 10 s delay period. At the beginning of the free run, the mice were allowed to choose either goal arm. If the mice chose the arm opposite to the one they had been forced into during the forced run, they received the food reward. If the mice chose the same arm that they had been force into, they received no food reward. The training period ended after control animals made >70% correct choices on two consecutive days. Animals took 7–12 d to reach the criterion. Animals that did not reach the criterion by 14 d were rejected from the study. In this study, ~5% of mice belonged to this latter group. Mice were then tested for their performance at 10 or 40 s delay periods. Mice were given three 10 s delay and three 40 s delay trials during the day of testing. For HSV-mediated transgene expression in frontal cortex, drug testing was performed 3–4 days after the viral infection. The sequence of delays and forced run food locations (left or right) were randomized, with the stipulation that the same delay or the same forced arm location could not be used for three trials in a row. Goal entries were defined as placing four paws in the arm.
Statistical Analysis
Radioligand binding data were analyzed using a nonlinear curve fitting software (GraphPad Prism). An extra-sum-of-squares (F-test) was used to determine statistical difference for simultaneous analysis of binding saturation curves. For all ChIP and mRNA data, fold changes relative to controls were determined using the corrected Ct method34. In immunoblot assays in postmortem human brain, the theoretical amount of protein in the each sample was obtained by interpolation of the integrated optical density in the standard curve and compared with the real amount of protein loaded into the gel well59. Statistical significance of head-twitch experiments was assessed by normalizing (square root) the number of events34. Statistical significance of qRT-PCR, ChIP, and Western blot experiments was assessed by normalizing (log) the fold changes34. Statistical significance of experiments involving three or more groups and two or more experimental conditions was assessed by two-way ANOVA followed by Bonferroni’s post hoc test. Statistical significance of experiments involving three or more experimental conditions was assessed by one-way ANOVA followed by Bonferroni’s post hoc test. Statistical significance of experiments involving two experimental conditions was assessed by Student’s t-test. The effects on mRNA expression and histone modifications of multiple genes was assessed by correcting the α value for multiple independent null hypothesis. This was performed by using the Holm’s sequentially rejective Bonferroni method as follows34,60: α′ = 1 − (1 − α)(1/N), where α = 0.05 and N = the number of independent null hypotheses (e.g., number of modulated genes). The number of individual replicates has been estimated by combinational calculations of the number of variables according to personal experience with similar protocols. The level of significance was chosen at P = 0.05. All data are presented as mean ± s.e.m.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Andrew Chess, Yasmin Hurd (Mount Sinai School of Medicine), Cristina Alberini (New York University), Paula Bos (Memorial Sloan-Kettering Cancer Center), and John Greally (Albert Einstein College of Medicine) for their critical review of the manuscript; Dr. Matthew Shapiro for his help in working memory experiments; Dr. Susan Morgello and the Manhattan HIV Brain Bank for providing control brain cortex; Dr. Patrizia Casaccia for insightful discussions, and for providing the sequence of mouse HDAC2; Dr. Jay Gingrich for his gift of 5HT2A−/− mice; Vinayak Rayannavar for assistance with biochemical and behavioral assays; and the staff members of the Basque Institute of Legal Medicine for their cooperation in the study. NIH R01 MH084894 (J.G.M.), Dainippon Sumitomo Pharma (J.G.M.), NARSAD (J.G.M.), the Maltz Family Foundation (J.G.M.), NIH R01 MH090264 (S.J.R.), MICINN SAF2009-084609 (J.J.M.), the Basque Government IT-199-07 (J.J.M.), NIH P50 MH090963 (E.J.N.), NIH R01 MH092306 (M.H.H.), and NIH P50 MH066392 (J.D.B.) participated in the funding of this study. A.K.F. was supported by NIH grant F32 MH096464. A.G.B., J.L.M, and G.M. were recipients of predoctoral and postdoctoral fellowships from Basque Government, Ministerio de Ciencia e Innovación, and CSIC, Spain.
Footnotes
AUTHOR CONTRIBUTIONS
M.K. and J.G.M. designed experiments, analyzed data and wrote the manuscript. M.K. performed experiments. J.G.M. supervised the research. T.H. performed behaviour experiments. A.G.B. performed experiments in postmortem schizophrenia brain. A.K. assisted with cloning and performed promoter assay experiments. J.L.M. performed radioligand binding experiments. M.H. assisted with ChIP and DNA methylation assays. G.M., A.M.G., J.H., A.U., and A.L.G. assisted with experiments. R.L.N. performed viral packaging. L.S. performed biostatistical analyses. L.F.C. and J.J.M. obtained and classified postmortem human brain samples. S.G. supervised by S.J.R. helped with stereotaxic surgery. P.J.K. and D.M.D. supervised by E.J.N. helped with viral over-expression. A.K.F. supervised by M.H.H. performed electrophysiological studies. N.T. supervised by J.D.B. helped with prepulse inhibition test. All authors discussed the results and commented on the manuscript.
References
- 1.Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- 2.Dobbs D. Schizophrenia: The making of a troubled mind. Nature. 2010;468:154–156. doi: 10.1038/468154a. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman JA, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Sant SP, Buckley PF. Pharmacotherapy for treatment-refractory schizophrenia. Expert Opin Pharmacother. 2011;12:411–434. doi: 10.1517/14656566.2011.528200. [DOI] [PubMed] [Google Scholar]
- 6.Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc Natl Acad Sci U S A. 2007;104:4676–4681. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong E, Nelson M, Grayson DR, Costa E, Guidotti A. Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc Natl Acad Sci U S A. 2008;105:13614–13619. doi: 10.1073/pnas.0805493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citrome L, et al. Adjunctive divalproex and hostility among patients with schizophrenia receiving olanzapine or risperidone. Psychiatr Serv. 2004;55:290–294. doi: 10.1176/appi.ps.55.3.290. [DOI] [PubMed] [Google Scholar]
- 10.Kelly DL, et al. Adjunct divalproex or lithium to clozapine in treatment-resistant schizophrenia. Psychiatr Q. 2006;77:81–95. doi: 10.1007/s11126-006-7963-9. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, et al. Augmentation of atypical antipsychotics with valproic acid. An open-label study for most difficult patients with schizophrenia. Hum Psychopharmacol. 2009;24:628–638. doi: 10.1002/hup.1073. [DOI] [PubMed] [Google Scholar]
- 12.Loscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31–59. doi: 10.1016/s0301-0082(98)00075-6. [DOI] [PubMed] [Google Scholar]
- 13.Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64:1079–1086. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
- 14.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11:384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225–232. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Maeso J, et al. Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Moreno JL, Sealfon SC, Gonzalez-Maeso J. Group II metabotropic glutamate receptors and schizophrenia. Cell Mol Life Sci. 2009;66:3777–3785. doi: 10.1007/s00018-009-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039) J Pharmacol Exp Ther. 2008;326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- 22.Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- 23.Fribourg M, et al. Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil ST, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Maeso J, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60:1228–1235. doi: 10.1001/archpsyc.60.12.1228. [DOI] [PubMed] [Google Scholar]
- 27.Egan MF, et al. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A. 2004;101:12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, et al. Pharmacogenetic analysis of the mGlu2/3 agonist LY2140023 monohydrate in the treatment of schizophrenia. Pharmacogenomics J. 2010;12:246–254. doi: 10.1038/tpj.2010.90. [DOI] [PubMed] [Google Scholar]
- 29.Moreno JL, et al. Maternal Influenza Viral Infection Causes Schizophrenia-Like Alterations of 5-HT2A and mGlu2 Receptors in the Adult Offspring. J Neurosci. 2011;31:1863–1872. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdolmaleky HM, et al. Epigenetic dysregulation of HTR2A in the brain of patients with schizophrenia and bipolar disorder. Schizophr Res. 2011;129:183–190. doi: 10.1016/j.schres.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Matrisciano F, Tueting P, Maccari S, Nicoletti F, Guidotti A. Pharmacological activation of group-II metabotropic glutamate receptors corrects a schizophrenia-like phenotype induced by prenatal stress in mice. Neuropsychopharmacology. 2012;37:929–938. doi: 10.1038/npp.2011.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang HS, Matevossian A, Jiang Y, Akbarian S. Chromatin immunoprecipitation in postmortem brain. J Neurosci Methods. 2006;156:284–292. doi: 10.1016/j.jneumeth.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Darcy MJ, Calvin K, Cavnar K, Ouimet CC. Regional and subcellular distribution of HDAC4 in mouse brain. J Comp Neurol. 2010;518:722–740. doi: 10.1002/cne.22241. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Maeso J, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 36.Benneyworth MA, et al. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- 37.Aghajanian GK. Modeling “psychosis” in vitro by inducing disordered neuronal network activity in cortical brain slices. Psychopharmacology (Berl) 2009;206:575–585. doi: 10.1007/s00213-009-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhai Y, et al. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology. 2003;28:45–52. doi: 10.1038/sj.npp.1300013. [DOI] [PubMed] [Google Scholar]
- 39.Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Curr Opin Pharmacol. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- 43.Chiechio S, et al. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol Pharmacol. 2009;75:1014–1020. doi: 10.1124/mol.108.054346. [DOI] [PubMed] [Google Scholar]
- 44.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 45.Guan JS, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graff J, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483:222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peter CJ, Akbarian S. Balancing histone methylation activities in psychiatric disorders. Trends Mol Med. 2011;17:372–379. doi: 10.1016/j.molmed.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marder E, Taylor AL. Multiple models to capture the variability in biological neurons and networks. Nat Neurosci. 2011;14:133–138. doi: 10.1038/nn.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dean B. The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J Neurochem. 2003;85:1–13. doi: 10.1046/j.1471-4159.2003.01693.x. [DOI] [PubMed] [Google Scholar]
- 50.Goldberg TE, et al. The effect of clozapine on cognition and psychiatric symptoms in patients with schizophrenia. Br J Psychiatry. 1993;162:43–48. doi: 10.1192/bjp.162.1.43. [DOI] [PubMed] [Google Scholar]
- 51.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 52.Humphrey GW, et al. Complementary roles for histone deacetylases 1, 2, and 3 in differentiation of pluripotent stem cells. Differentiation. 2008;76:348–356. doi: 10.1111/j.1432-0436.2007.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- 54.Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative cytoarchitectonic atlas of the C57BL/6 and 129/Sv mouse brains. Elsevier; Amsterdam: 2000. [Google Scholar]
- 55.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4. Washington, DC: 1994. [Google Scholar]
- 56.Stan AD, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao JL, et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin WR, Wikler A, Eades CG, Pescor FT. Tolerance to and Physical Dependence on Morphine in Rats. Psychopharmacologia. 1963;4:247–260. doi: 10.1007/BF00408180. [DOI] [PubMed] [Google Scholar]
- 59.Gonzalez-Maeso J, Rodriguez-Puertas R, Meana JJ, Garcia-Sevilla JA, Guimon J. Neurotransmitter receptor-mediated activation of G-proteins in brains of suicide victims with mood disorders: selective supersensitivity of alpha(2A)-adrenoceptors. Mol Psychiatry. 2002;7:755–767. doi: 10.1038/sj.mp.4001067. [DOI] [PubMed] [Google Scholar]
- 60.Shaffer JP. Multiple hypothesis testing. Annu Rev Psychol. 1995;46:561–584. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.