Abstract
We examined effects of fluence rate on the photobleaching of the photosensitizer Pc 4 during photodynamic therapy (PDT) and the relationship between photobleaching and tumor response to PDT. BALB/c mice with intradermal EMT6 tumors were given 0.03 mg/kg Pc 4 by intratumor injection and irradiated at 667 nm with an irradiance of 50 or 150 mW/cm2 to a fluence of 100 J/cm2. While no cures were attained, significant tumor growth delay was demonstrated at both irradiances compared to drug-only controls. There was no significant difference in tumor responses to these two irradiances (p = 0.857). Fluorescence spectroscopy was used to monitor the bleaching of Pc 4 during irradiation, with more rapid bleaching with respect to fluence shown at the higher irradiance. No significant correlation was found between fluorescence photobleaching and tumor regrowth for the data interpreted as a whole. Within each treatment group, weak associations between photobleaching and outcome were observed. In the 50 mW/cm2 group, enhanced photobleaching was associated with prolonged growth delay (p = 0.188), while at 150 mW/cm2 this trend was reversed (p = 0.308). Thus, it appears that Pc 4 photobleaching is not a strong predictor of individual tumor response to Pc4-PDT under these treatment conditions.
Introduction
One of a number of second-generation photosensitizers, the silicon phthalocyanine Pc 4 (HOSiPcOSi(CH3)3(CH2)3N(CH3)2) was developed in the laboratory of Malcolm Kenney at Case Western Reserve University. Its synthesis and structure have been reported previously (1). An interesting, succinct history of Pc 4 has been reviewed (2). It has been evaluated in humans to treat cutaneous T-cell lymphoma and a variety of other cutaneous neoplasms in phase 1 clinical trials at Case Western Reserve University (2–4). Our laboratory has previously demonstrated increased effectiveness of Pc 4-photodynamic therapy (PDT) in mice using intratumor vs. systemic injection of the photosensitizer with short drug-light intervals (5). This study also showed recovery of Pc 4 fluorescence 24 hours after PDT, which we interpreted as an indication of initially aggregated Pc 4 monomerizing following the bleaching of a fraction of the locally-injected sensitizer population. Thus it appeared that intratumor injection had resulted in excess Pc 4 under the conditions of those experiments.
Most photosensitizers undergo irreversible photobleaching in response to irradiation during PDT (6). Since photobleaching can be mediated by reactive oxygen species, it has often been speculated that the loss of photosensitizer fluorescence could be used to predict dose deposition and therefore the outcome of PDT. A number of studies have been carried out with various photosensitizers in order to examine this possibility. The most promising results have been reported for the prodrug aminolevulinic acid (ALA), which is metabolized into protoporphyrin IX (PpIX). A correlation between PpIX photobleaching and tumor response has been shown in a rat model of Barrett's esophagus (7), a rat model of ovarian cancer (8), and in humans with actinic keratoses, Bowen's disease, and basal cell carcinoma (9). In all cases, high PpIX bleaching was associated with a strong response to PDT. However, the ability of fluorescence photobleaching to predict tumor outcome at the level of an individual animal or patient was not explored in these studies.
In the case of Pc 4, work in tumor cell monolayers and very low fluences demonstrated an increase in sensitizer fluorescence in response to irradiation (10). However, this phenomenon has not been observed in vivo, where several studies have shown that Pc 4 does indeed bleach in response to irradiation (5, 11, 12). Whether a correlation between photobleaching dynamics and tumor outcome exists for Pc 4 has not been thoroughly examined. A link between Pc 4 concentration before irradiation, assessed using reflectance spectroscopy, and tumor outcome was shown in an interesting study by Bai et al (12). That work demonstrated that pre-PDT Pc 4 concentration at the tumor could be used as a predictor of outcome for individual animals. They also noted a correlation between total Pc 4 photobleaching and tumor response.
In this study, we explored the relationship between spectroscopy measurements made during PDT and tumor response in individual tumor-bearing mice. If established, such a relationship would allow prediction of treatment response in individual patients, thereby enabling earlier intervention in those cases where PDT is likely to fail. To do this, we examined tumor response to Pc 4-PDT at 100 J/cm2 using either 50 or 150 mW/cm2 and a whole-body photosensitizer concentration of 0.03 mg/kg injected intratumorally. Fluorescence and reflectance spectra were collected before, during, and after PDT in order to quantify the photobleaching of Pc 4 in response to irradiation. The relationship between photobleaching and tumor response was examined for individual animals within both treatment groups and as a pooled group. Correlation between photobleaching and tumor growth delay was found to be statistically insignificant in all cases.
Materials and Methods
Tumor Model
Intradermal (ID) mouse mammary EMT6 tumors were initiated on the backs of female BALB/c mice by ID injection of 106 cells. Tumor growth was monitored every 2–3 days. For a period of approximately 2 weeks prior to PDT and spectroscopic measurements, mice were fed a chlorophyll-free diet prepared using the recipe of Holmes et al (13). Tumors were treated when volumes reached approximately 100 mm3. All experiments were conducted according to the institutional guidelines of the University of Rochester Medical Center and approved by the University Committee on Animal Resources.
Photosensitizer Administration and Light Treatment
Powdered Pc 4 was obtained from Dr. Malcolm Kenney at Case Western Reserve University, and was prepared as described previously (14). Pc 4 was dissolved in a 1:1 solution of ethanol and Cremophor (Cremophor® EL, Sigma-Aldrich, St. Louis, MO) to create a stock solution at a concentration of 2.1 mg/mL. This stock solution was diluted at a ratio of 1:9 in a 1:1 solution of ethanol and Cremophor, and then further diluted at a ratio of 1:9 in 0.9% saline. This series of dilutions yielded a Pc 4 concentration of 0.021 mg/mL, in a solution consisting of 90% saline, 5% ethanol, and 5% Cremophor. A fresh stock solution of Pc 4 was created on each day for which PDT was delivered.
Prior to light treatment, 35 μL of 0.021 mg/mL Pc 4 was delivered by IT injection at a single site using a 29 gauge needle. Given an average mass of 25 g for mice used in the study, this resulted in a whole body Pc 4 concentration of 0.03 mg/kg. In our experience with this injection vehicle (5, 15), this procedure results in relatively uniform distribution of photosensitizer throughout tumors of this size. Immediately after drug administration, 667 nm PDT treatment light from a diode laser (Power Technology, Alexander, AR) was delivered at either 50 or 150 mW/cm2. The fluence delivered was fixed at 100 J/cm2. Irradiation was interrupted at pre-defined points for approximately 3 seconds in order to perform spectroscopy as described below. Drug-only control animals were given the same Pc 4 injection but were not irradiated, while drug-free controls received neither Pc 4 nor irradiation.
Light Delivery and Spectroscopic Measurements
Treatment light, excitation light for fluorescence, and broadband white light for reflectance were delivered to the surface of the tumor using a custom, off-surface probe that has been described previously (15). During a treatment session, PDT irradiation was interrupted at pre-defined fluences with a computer controlled in-line shutter (Mikropack, Ostfildern, Germany) and fluorescence excitation and broadband light were routed sequentially via an optical switch (Piezosystem Jena, Hopedale, MA). Fluorescence excitation was performed with 639 nm light from a diode laser (Oz Optics, Ottawa, Ontario, Canada) filtered by a band-pass filter (Z635/20x, Chroma Technology, Bellows Falls, VT). The broadband source was a tungsten-halogen lamp (Avantes, Broomfield, CO). Fluorescence and reflectance spectra were acquired by dedicated, TE-cooled, 16 bit spectrometers (B&W Tek, Newark, DE) using integration times of 2 and 0.5 seconds, respectively. Spectra were captured before, during, and immediately after PDT, as well as 24 hours post-PDT. The fluorescence detection path included a long pass filter (HQ645LP, Chroma). The source and detector fibers on the probe interrogated an overlapping area at the center of the treatment field with a diameter of 3 mm. A custom LabVIEW program (National Instruments, Austin, TX) controlled the sequence of PDT irradiation and spectral acquisition.
Spectral Processing and Analysis
Spectra were corrected by background subtraction and division by a measured, wavelength-dependent system response. Background spectra were taken before treatment for each animal by integrating dark signals for the intervals described above. System responses were acquired by reflecting the light from a NIST-traceable lamp (Model # LS-1-CAL, Ocean Optics, Dunedin, FL) off of a diffuse reflectance standard (Part # WS-1, Ocean Optics) into the detection fiber on the probe. The measured spectrum was then background subtracted and divided by the known lamp spectrum to obtain the system response. Fluorescence spectra were further corrected for the effects of tissue optical properties by dividing the fluorescence by reflectance spectra measured in the same wavelength range and geometry (16). Effects of PDT-induced changes in optical properties at the fluorescence excitation wavelength were accounted for by dividing the emission spectrum by the measured reflectance at this wavelength raised to the power 0.88. This empirical, probe-specific correction factor was derived from measurements in tissue simulating phantoms as reported previously (17).
After fluorescence spectra were acquired and corrected, they were fit using a singular value decomposition (SVD) algorithm based on the work of Press et al (18). The basis spectrum used in fitting was acquired by measuring the fluorescence emission of 0.3 μM Pc 4 in a 5% ethanol, 5% Cremophor, 90% saline solution using a commercial fluorometer (Varian Eclipse, Palo Alto, CA). Fitting followed the SVD scheme found in MATLAB (Mathworks, Natick, MA). The Pc 4 basis was allowed to shift a few nanometers in either direction in order to account for environment-induced changes in fluorescence that were not corrected by the division by reflectance. A Fourier series was used to fit small contributions of unknown origin to the measured fluorescence spectra (17).
In order to quantify Pc 4 photobleaching, the magnitude of the Pc 4 basis returned by SVD fitting of fluorescence was plotted against fluence, normalized to the magnitude of the Pc 4 basis immediately prior to irradiation. A single exponential model of photobleaching was then fit to the degradation of fluorescence,
| (1) |
where F(d) is the fluorescence at a given fluence, d (J/cm2), F0 is the fluorescence prior to irradiation, k is the photobleaching decay constant, and FB is a term representing background fluorescence (19). Fitting was performed using the MATLAB curve fitting toolbox (Mathworks).
Tumor Response
Tumor dimensions along three axes were measured every 2–3 days following PDT. The tumor volume was computed assuming an ellipsoidal shape. Mice were removed from the study if the tumor grew to a volume twice that measured immediately before PDT.
Statistical Analysis
Comparison of photobleaching between the treatment groups was performed using a two-sample paired t-test at each measured fluence point (Origin 7, OriginLab, Northampton, MA). Statistical analysis of tumor doubling times was performed using the same test. The correlations between tumor doubling and photobleaching or reflectance were analyzed by calculating Spearman's rank correlation coefficients. The coefficients calculated were tested for significance using the test statistic,
| (2) |
where n is the sample size and r is the Spearman rank correlation coefficient. Statistical significance was determined by comparing the t value to a Student's t-distribution with n-2 degrees of freedom (18).
Results
Spectroscopy
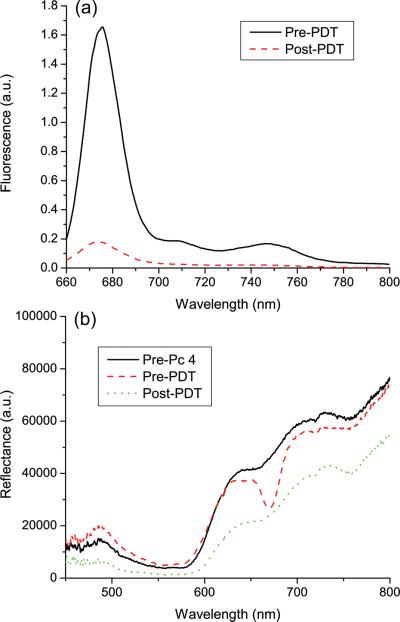
Fluorescence and reflectance were acquired from Pc 4-sensitized EMT6 tumors in vivo before, during, and immediately after irradiation. Typical fluorescence and reflectance spectra are shown in figure 1. The effect of Pc 4 absorption is evident in the reflectance spectra, with reflectance decreasing markedly around 667 nm after administration of the photosensitizer. Pc 4 photobleaching is visible in both fluorescence and reflectance spectra, with both the magnitude of Pc 4 fluorescence and the relative reduction of reflectance at 667 nm decreasing in response to irradiation. SVD fitting was performed on all fluorescence spectra, using a Pc 4 basis as described above. A typical SVD fit is shown in figure 2. The spectrum is fit well with the Pc 4 basis, as indicated by the relatively small magnitude of the Fourier terms in the overall fit.
Figure 1.
Representative (a) fluorescence and (b) reflectance spectra taken in vivo from the same EMT6 tumor illustrate photobleaching of Pc 4 in response to PDT. The pre-Pc 4 reflectance spectrum was taken before administration of Pc 4 and pre-PDT spectra were taken immediately after IT injection of 0.03 mg/kg Pc 4. Post-PDT spectra were taken immediately after conclusion of irradiation (100 J/cm2). Both sets of spectra were background subtracted and corrected for the wavelength-dependent system response. Fluorescence spectra were additionally divided by the corresponding reflectance spectra as described in the text.
Figure 2.
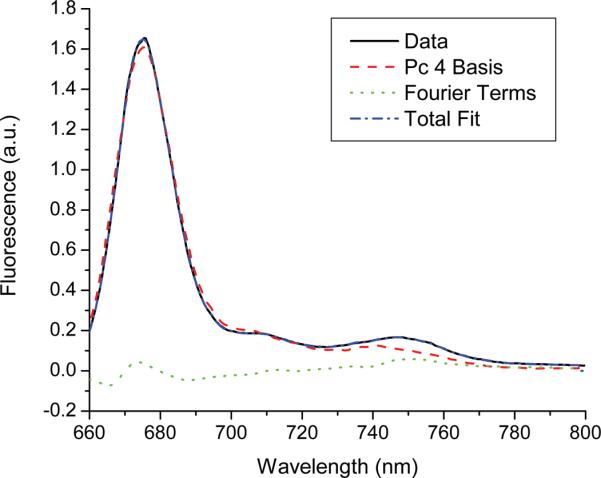
Results of SVD fitting of a representative fluorescence spectrum taken in vivo after IT injection of 0.03 mg/kg Pc 4, but before delivery of treatment light. The Pc 4 basis was created by measuring 0.3 μM Pc 4 in solution using a commercial fluorometer. A 61-term Fourier series was used to fit unknown contributions to the fluorescence.
Spectra were also acquired 24 hours after irradiation. The results of this are shown for a representative tumor in figure 3. As can be seen in the figure, the magnitude of Pc 4 fluorescence is essentially unchanged from immediately after to 24 hours after irradiation. The lack of fluorescence recovery, which we observed with a ten-fold higher injected Pc 4 concentration (5), suggests that a fluence of 100 J/cm2 effectively consumes an intratumorally-administered whole-body drug dose of 0.03 mg/kg.
Figure 3.
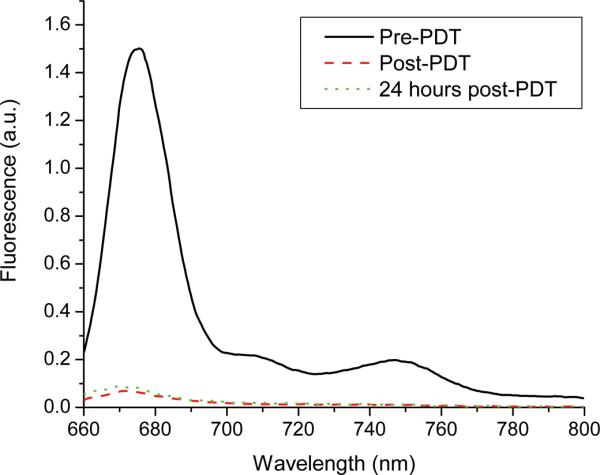
Representative fluorescence spectra collected from the same EMT6 tumor in vivo before, immediately after, and 24 hours after irradiation (100 J/cm2), as indicated in the legend. All spectra were collected under the same excitation and detection conditions, corrected for background and system response, and divided by the corresponding reflectance spectrum.
Pc 4 Photobleaching
Pc 4 photobleaching was quantified by examining the amplitude of the Pc 4 fluorescence basis returned by SVD fitting, normalized to its amplitude measured immediately before irradiation. A representative bleaching curve for one mouse is shown in figure 4. In this case, the open circles represent Pc 4 fluorescence measured during irradiation at 50 mW/cm2. The solid line shown is the result of fitting Equation (1) to the measured data. The quality of the fit is good, indicating that Equation (1) is an appropriate empirical model in this case.
Figure 4.
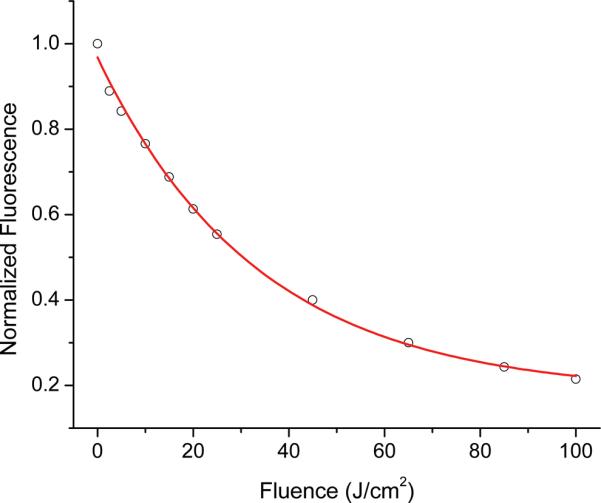
Photobleaching of Pc 4 fluorescence measured in vivo during delivery of 667 nm treatment light at 50 mW/cm2. Open circles represent the fit coefficient of the Pc 4 basis obtained from SVD fitting, normalized to the magnitude at the beginning of PDT. The solid line corresponds to a single exponential fit to the measured photobleaching, as shown in Equation (1).
Average photobleaching curves were also generated for the two treatment groups. The results of this are shown in figure 5. Both photobleaching curves are the result of averaging the data from the five animals in each group, and the error bars are the standard deviations. Qualitatively, Pc 4 appears to photobleach more rapidly as a function of fluence for the fluence rate of 150 vs. 50 mW/cm2. Photobleaching curves were compared on a point-by-point basis using a two-sample paired t-test, with the null hypothesis being that the two treatment groups had the same mean. All of the points, with the exception of the normalized pre-PDT value, were found to be significantly different at a significance level of p<0.1. At a significance level of p<0.05, only the points marked with an asterisk in figure 5 were shown to be significantly different.
Figure 5.
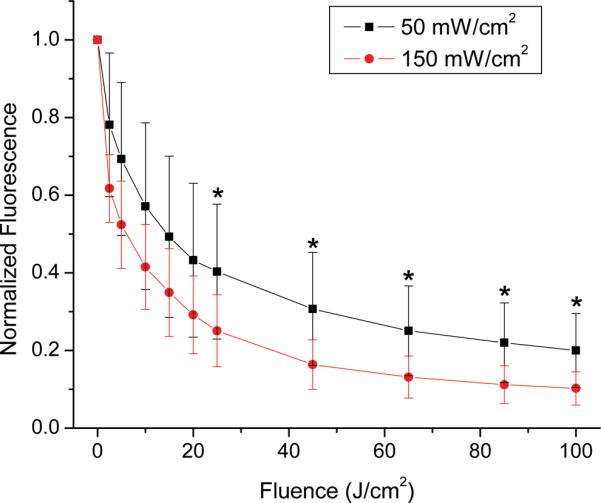
Degradation of Pc 4 fluorescence due to photobleaching of Pc 4 in response to irradiation at (■) 50 mW/cm2 (n=5) or (●) 150 mW/cm2 (n=5), normalized to pre-PDT fluorescence. The increased rate of photobleaching at 150 mW/cm2 was found to be significant with a significance level of p<0.1. The points marked with an asterisk (*) are fluences for which the difference between fluence rates was significant with a significance level of p<0.05. Error bars are standard deviations of measurements made in n=5 tumors for each group.
Tumor Response
The results of Pc 4 PDT on tumor regrowth are shown in figure 6. As described in the Methods section, animals were removed from the study when their tumor grew to twice its pre-PDT volume. All mice, with the exception of drug-free controls, received the same intratumor injection of 35 μL of 0.021 mg/mL Pc 4 in a 5% ethanol, 5% Cremophor, 90% saline vehicle, corresponding to a whole-body dose of 0.03 mg/kg. As can be seen, no tumors were cured as a result of Pc 4-PDT with this drug concentration, although there was significant tumor growth delay compared to the control cases. Using a paired t-test, the effect of PDT treatment was shown to be statistically significant compared to animals that received Pc 4 without irradiation (50 mW/cm2, p = 0.019; 150 mW/cm2, p = 0.036; combined, p = 0.014). Pc 4 administration alone had a modest effect on tumor growth compared to drug-free controls (p = 0.063). Irradiance (50 vs. 150 mW/cm2) had no significant effect on tumor growth delay (p = 0.857).
Figure 6.
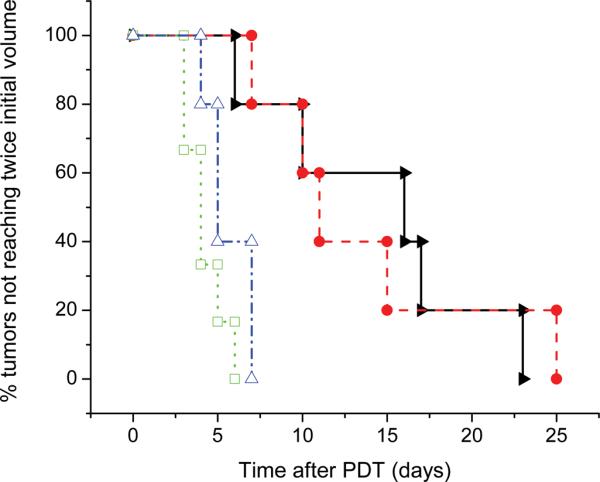
Kaplan-Meier curves illustrating the effects of fluence rate on response of EMT6 tumors to Pc 4-PDT in vivo. Curves shown are (◻) drug and irradiation free control (n=6); (Δ) 0.03 mg kg−1 Pc 4, drug only control (n=5); (▶) 0.03 mg kg−1 Pc 4, 50 mW/cm2, 100 J/cm2 (n=5); (●) 0.03 mg kg−1 Pc 4, 150 mW/cm2, 100 J/cm2 (n=5). The effect of PDT on tumor growth delay was significant when compared to the effect of drug alone (50 mW/cm2, p = 0.019; 150 mW/cm2, p = 0.036; combined, p = 0.014). The effect of fluence rate on tumor growth delay between treatment groups was not found to be significant (p = 0.857).
Correlation Between Tumor Response and Spectroscopy
Pc 4 photobleaching curves were fit using Equation (1). In order to examine the relation between photobleaching and tumor growth delay, the reciprocal of the fitted k value from Equation (1) was plotted against tumor doubling time for each animal. The results of this are shown in figure 7. The relationship between doubling time and the fluence at which Pc 4 photobleaches to 1/e of its initial value was examined using Spearman's rank correlation coefficient. Probability values were calculated using Equation (2), and these were then converted to p values by treating the calculated values as coming from a Student's t-distribution with n-2 degrees of freedom. When all PDT-treated mice were combined into one data set, no correlation was found between tumor volume doubling time and the 1/e bleaching fluence, assuming 8 degrees of freedom for the underlying Student's t-distribution (p = 0.977). When the mice were grouped by treatment irradiance, interesting but relatively weak associations were identified. The Spearman coefficients were r = −0.7 for the 50 mW/cm2 treatment group and r = 0.6 for the 150 mW/cm2 treatment group. The negative value of the Spearman coefficient for the 50 mW/cm2 group indicates that there is a decreasing monotonic trend between tumor doubling and the 1/e bleaching fluence, while the positive value for the 150 mW/cm2 group indicates an increasing monotonic trend. These trends were not statistically significant at a p<0.05 level, but exhibited p-values of 0.188 and 0.308 for the 50 and 150 mW/cm2 treatment groups, respectively.
Figure 7.
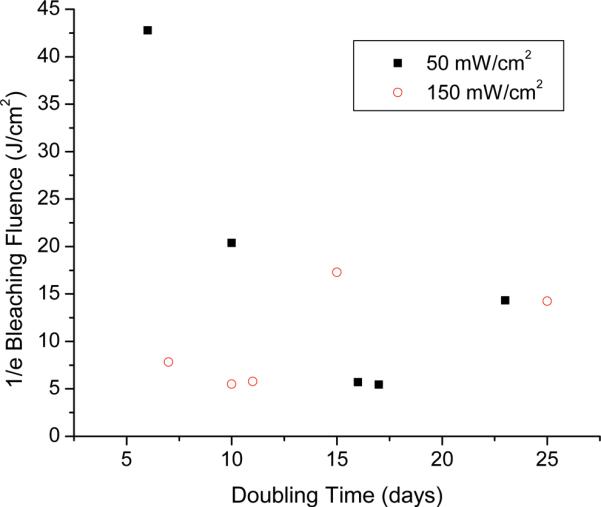
Relationship between tumor doubling time and the fluence at which Pc 4 fluorescence photobleaches to 1/e of its pre-PDT value for irradiation at fluence rates of (■) 50 mW/cm2 and (◯) 150 mW/cm2. Both treatment groups received 100 J/cm2 of irradiation. For the treatment groups taken individually, no significant correlation was found between doubling and bleaching (50 mW/cm2, p=0.188; 150 mW/cm2, p=0.308). For the data taken as a whole, there was also no significant correlation found between doubling and bleaching (p=0.977).
Reflectance spectra were also examined for correlation with tumor doubling times. The method followed was the same as that used in the photobleaching analysis, but the metric used was the total measured reflectance at 667 nm before irradiation. This wavelength was chosen because it corresponds to the peak absorption for Pc 4. The results of this analysis are shown in figure 8. Since the metric being used was reflectance prior to irradiation, the data from both treatment groups were combined. The Spearman coefficient was calculated to be r = −0.3283. Again using the test statistic given in Equation (2) and assuming a Student's t-distribution with 8 degrees of freedom, no significant correlation was shown between tumor doubling time and measured reflectance at 667 nm prior to irradiation (p = 0.354).
Figure 8.
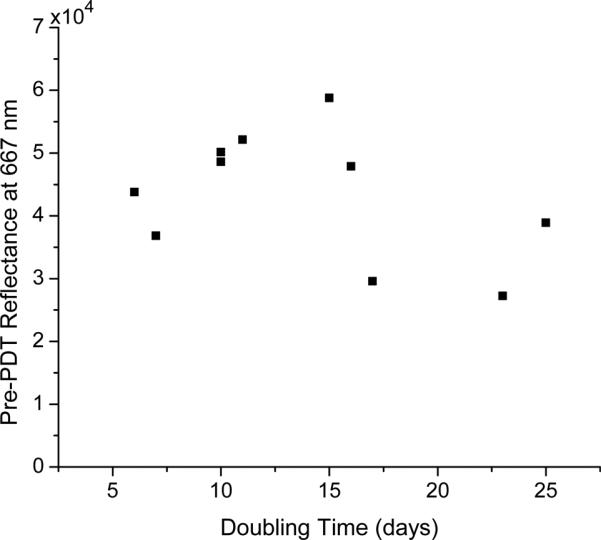
Relationship between tumor doubling time and pre-PDT reflectance at 667 nm for all treated animals. Treatment groups were combined because the reflectance measurements being examined were made prior to irradiation. No significant correlation was found between doubling and pre-PDT reflectance at 667 nm (p=0.354).
Discussion
Our experiments have shown that Pc 4 bleaches to a significant extent in response to irradiation with a fluence of 100 J/cm2. For both 50 and 150 mW/cm2 fluence rates, approximately 80–90% of Pc 4 photobleached during irradiation. Previous studies done in clinical trials of Pc 4-PDT for cutaneous T-cell lymphoma by our research group showed only 30% bleaching at 100 J/cm2, with a fluence of 200 J/cm2 increasing bleaching to approximately 50% (11). This study was done at a different photosensitizer concentration than the current study, and Pc 4 was applied topically rather than by IT injection. We therefore conclude that the increased degree of Pc 4 photobleaching observed in the current study is a function of the photosensitizer concentration and the intralesion distribution. Compared to other clinically relevant photosensitizers, particularly PpIX and Photofrin, however, Pc 4 is relatively photostable. For example, Finlay et al demonstrated approximately 80% bleaching of initial Photofrin fluorescence in vivo in rat skin in response to only 9 J/cm2 of irradiation at 514 nm (20), and for ALA-PpIX in the same system, a similar 80% bleaching fluence was reported (17). Boere et al showed approximately 70% bleaching of PpIX for those animals that responded to PDT with a fluence of 50 J/cm2 and approximately 40% bleaching for those that did not (7).
Under the conditions of our experiments, Pc 4 bleached more rapidly with respect to fluence at an irradiance of 150 vs. 50 mW/cm2. To the best of our knowledge, this is the first such observation with any PDT photosensitizer. Lower irradiances have generally been associated with more efficient photobleaching of PpIX (17, 21–23), which has been hypothesized to result from a reduced consumption of molecular oxygen. The bleaching of Photofrin in vivo exhibited no irradiance dependence (20). Thus, with these current results, all possible irradiance dependencies have been observed experimentally, once again underscoring the complexity of fluorescence-based PDT dosimetry and the need to evaluate each photosensitizer separately.
In our experiments, no improvement in tumor outcome was shown for the 150 mW/cm2treatment group, even though bleaching was more efficient at this irradiance. When the tumor responses from both protocols were pooled, neither did we find any significant correlation between Pc 4 bleaching and time to tumor doubling. We conclude that monitoring of Pc 4 photobleaching is unlikely to be a useful predictor of individual tumor response to Pc 4-PDT in vivo. When the 50 and 150 mW/cm2 treatment groups were analyzed separately, weak but intriguing associations were suggested. At the lower irradiance, there was a trend toward improved tumor response with increased photobleaching. On the other hand, tumor growth delays in response to irradiation at 150 mW/cm2 tended to be increased with decreased loss of Pc 4 fluorescence. One may speculate that these trends, although not statistically significant, suggest interesting differences in photochemistry in vivo under these different treatment conditions.
We found no correlation between the total reflectance at 667 nm measured immediately before irradiation and tumor doubling time. This is in apparent disagreement with results reported by Bai et al (12), who demonstrated a significant relationship (p < 0.001) between pre-irradiation Pc 4 concentration and tumor growth delay using a calibrated measurement to convert the area under a measured absorbance curve to Pc 4 concentration. A number of differences exist between the current study and that reported by Bai et al, which may account for this disagreement. Most importantly, Bai et al administered Pc 4 via tail-vein injection, whereas we used an intratumor injection, almost certainly resulting in different Pc 4 concentrations and intratumor distributions. We note that the tumors studied by Bai et al were also larger than those used in the current study, with a mean tumor volume of 315.3 mm3 versus 115.3 mm3.
We did not achieve any cures in either treatment group. In contrast, a previous study by our group showed a >70% cure rate for Pc 4-PDT using IT injection of a 10-fold higher (0.3 mg/kg) sensitizer dose (5). In that previous study, we noted that Pc 4 fluorescence at the treated site recovered completely 24 hours after irradiation, suggesting to us that aggregated Pc 4 was monomerizing after the bleaching of a fraction of the initial concentration. It therefore seemed reasonable to evaluate a lower injected drug concentration. From figure 3, it can be seen that there is no recovery of Pc 4 fluorescence after irradiation in the current study, indicating that aggregation is not taking place and that the drug is being nearly completely consumed. However, the tumor responses indicate that the 10-fold reduction was too much and that an intermediate IT concentration is likely optimal.
There are a number of possible interpretations for why Pc 4 photobleaching does not predict tumor growth delay in individual animals. More generally, given these data it may be useful to consider the nature of the problem we are asking spectroscopy to address. We offer the following simple picture. We begin by asking, for a typical response to PDT, what is the volume of tumor spared by treatment relative to that sampled by spectroscopy? Assuming only that the tumor cells surviving PDT replicate at their pre-treatment rate, we may express the number of these cells as,
| (3) |
where N is the tumor volume in mm3, t is time in days, t0 is day of treatment, and kG is an empirically-determined growth rate constant. In our case, kG is 0.1663 days−1 based on control tumor growth data. Using this growth rate, the average pre-irradiation tumor volume of 115 mm3, and an average tumor doubling time of 14 days after PDT, we can estimate that approximately 80% of the tumor volume was destroyed by PDT. For the longest observed doubling time of 25 days, this figure increases to 97% of tumor volume destroyed. In these cases, in order for spectroscopy to predict outcome it would be necessary for it to discern differences in fluorescence signals originating from small (20 – 3% in these examples) volumes of tissue. For longer doubling times corresponding to more effective treatments, the fraction of the tumor that spectroscopy would need to report grows even smaller. Because these small populations of surviving cells are likely distributed throughout the tumor volume as microscopic foci, the prediction of tumor response based on volume-averaged fluorescence spectroscopy measurements appears daunting.
Acknowledgements
This work was supported by NIH grants CA68409 and CA122093 awarded by the National Cancer Institute. The authors would like to thank Malcolm Kenney for providing the Pc 4 used in the study and Andrea Baran for suggestions and guidance on statistical analysis methods.
References
- 1.Oleinick NL, Antunez AR, Clay ME, Rihter BD, Kenney ME. New phthalocyanine photosensitizers for photodynamic therapy. Photochem Photobiol. 1993;57:242–247. doi: 10.1111/j.1751-1097.1993.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 2.Miller JD, Baron ED, Scull H, Hsia A, Berlin JC, McCormick T, Colussi V, Kenney ME, Cooper KD, Oleinick NL. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: the case experience with preclinical mechanistic and early clinical-translational studies. Toxicol Appl Pharmacol. 2007;224:290–299. doi: 10.1016/j.taap.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron ED, Malbasa CL, Santo-Domingo D, Fu P, Miller JD, Hanneman KK, Hsia AH, Oleinick NL, Colussi VC, Cooper KD. Silicon phthalocyanine (Pc 4) photodynamic therapy is a safe modality for cutaneous neoplasms: results of a phase 1 clinical trial. Lasers Surg Med. 2010;42:728–735. doi: 10.1002/lsm.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinsella TJ, Baron ED, Colussi VC, Cooper KD, Hoppel CL, Ingalls ST, Kenney ME, Li X, Oleinick NL, Stevens SR, Remick SC. Preliminary clinical and pharmacologic investigation of photodynamic therapy with the silicon phthalocyanine photosensitizer Pc 4 for primary or metastatic cutaneous cancers. Front Oncol. 2011;1:14. doi: 10.3389/fonc.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster TH, Giesselman BR, Hu R, Kenney ME, Mitra S. Intratumor administration of the photosensitizer Pc 4 affords photodynamic therapy efficacy and selectivity at short drug-light intervals. Transl Oncol. 2010;3:135–141. doi: 10.1593/tlo.09295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnett R, Martínez G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron. 2001;57:9516–9547. [Google Scholar]
- 7.Boere IA, Robinson DJ, Bruijn H. S. d., Kluin J, Tilanus HW, Sterenborg HJCM, Bruin R. W. F. d. Protoporphyrin IX fluorescence photobleaching and the response of rat Barrett's esophagus following 5-aminolevulinic acid photodynamic therapy. Photochem Photobiol. 2006;82:1638–1644. doi: 10.1562/2006-01-03-RA-763. [DOI] [PubMed] [Google Scholar]
- 8.Ascencio M, Collinet P, Farine MO, Mordon S. Protoporphyrin IX fluorescence photobleaching is a useful tool to predict the response of rat ovarian cancer following hexaminolevulinate photodynamic therapy. Lasers Surg Med. 2008;40:332–341. doi: 10.1002/lsm.20629. [DOI] [PubMed] [Google Scholar]
- 9.Tyrrell JS, Campbell SM, Curnow A. The relationship between protoporphyrin IX photobleaching during real-time dermatological methyl-aminolevulinate photodynamic therapy (MAL-PDT) and subsequent clinical outcome. Lasers Surg Med. 2010;42:613–619. doi: 10.1002/lsm.20943. [DOI] [PubMed] [Google Scholar]
- 10.Wang KK-H, Wilson JD, Kenney ME, Mitra S, Foster TH. Irradiation-induced enhancement of Pc 4 fluorescence and changes in light scattering are potential dosimeters for Pc 4-PDT. Photochem Photobiol. 2007;83:1056–1062. doi: 10.1111/j.1751-1097.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee TK, Baron ED, Foster TH. Monitoring Pc 4 photodynamic therapy in clinical trials of cutaneous T-cell lymphoma using noninvasive spectroscopy. J Biomed Opt. 2008;13:030507. doi: 10.1117/1.2939068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai L, Guo J, B. FA, III, Eiseman JL. The relationship of phthalocyanine (Pc 4) concentrations measured noninvasively to outcome of Pc 4 photodynamic therapy in mice. Photochem Photobiol. 2009;85:1011–1019. doi: 10.1111/j.1751-1097.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 13.Holmes H, Kennedy JC, Pottier R, Rossi F, Weagle G. A recipe for the preparation of a rodent food that eliminates chlorophyll-based tissue fluorescence. J Photochem Photobiol B. 1995;29:199. doi: 10.1016/1011-1344(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 14.Anula HM, Berlin JC, Wu H, Li Y-S, Peng X, Kenney ME, Rodgers MAJ. Synthesis and photophysical properties of silicon phthalocyanines with axial siloxy ligands bearing alkylamine termini. J Phys Chem A. 2006;110:5215–5223. doi: 10.1021/jp056279t. [DOI] [PubMed] [Google Scholar]
- 15.Baran TM, Giesselman BR, Hu R, Biel MA, Foster TH. Factors influencing tumor response to photodynamic therapy sensitized by intratumor administration of methylene blue. Lasers Surg Med. 2010;42:728–735. doi: 10.1002/lsm.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J, Feld MS, Rava RP. Analytical model for extracting intrinsic fluorescence in turbid media. Appl Opt. 1993;32:3585–3595. doi: 10.1364/AO.32.003585. [DOI] [PubMed] [Google Scholar]
- 17.Finlay JC, Conover DL, Hull EL, Foster TH. Porphyrin bleaching and PDT-induced spectral changes are irradiance dependent in ALA-sensitized normal rat skin in vivo. Photochem Photobiol. 2001;73:54–63. doi: 10.1562/0031-8655(2001)073<0054:pbapis>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C: The Art of Scientific Computing. Cambridge University Press; New York, NY: 1992. [Google Scholar]
- 19.Sheng C, Hoopes PJ, Hasan T, Pogue BW. Photobleaching-based dosimetry predicts deposited dose in ALA-PpIX PDT of rodent esophagus. Photochem Photobiol. 2007;83:738–748. doi: 10.1562/2006-09-07-RA-1033. [DOI] [PubMed] [Google Scholar]
- 20.Finlay JC, Mitra S, Patterson MS, Foster TH. Photobleaching kinetics of Photofrin in vivo and in multicell tumour spheroids indicate two simultaneous bleaching mechanisms. Phys Med Biol. 2004;49:4837–4860. doi: 10.1088/0031-9155/49/21/001. [DOI] [PubMed] [Google Scholar]
- 21.Cottrell WJ, Paquette AD, Keymel KR, Foster TH, Oseroff AR. Irradiance-dependent photobleaching and pain in δ-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas. Clin Cancer Res. 2008;14:4475–4483. doi: 10.1158/1078-0432.CCR-07-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson DJ, Bruijn H. S. d., Veen N. v. d., Stringer MR, Brown SB, Star WM. Fluorescence photobleaching of ALA-induced protoporphyrin IX during photodynamic therapy of normal hairless mouse skin: the effect of light dose and irradiance and the resulting biological effect. Photochem Photobiol. 1998;67:140–149. [PubMed] [Google Scholar]
- 23.Ericson MB, Sandberg C, Stenquist B, Gudmundson F, Karlsson M, Rosén A-MRA, Larkö O, Wennberg A-M, Rosdahl I. Photodynamic therapy of actinic keratosis at varying fluence rates: assessment of photobleaching, pain and primary clinical outcome. Br J Dermatol. 2004;151:1204–1212. doi: 10.1111/j.1365-2133.2004.06211.x. [DOI] [PubMed] [Google Scholar]