1. Introduction
The main characteristic of the cornea is transparency that allows the light to pass through the lens into the retina, constituting the most powerful refractive tissue in the eye. Another important characteristic of this tissue is that it is densely innervated with sensory nerves mostly derived from the ophthalmic branch of the trigeminal ganglion [1, 2]. Recently, our laboratory has developed a modified method of immunofluorescence staining and imaging that provided, for the first time, a detailed map of the entire human corneal nerve architecture [3]. The nerves enter the cornea at the thickest layer, the stroma, in a radial pattern. To maintain its transparency, they lose their myelin. Subsequently, the nerve trunks divide into smaller branches to penetrate upwards into the epithelium and constitute the epithelial nerve network. There is intense innervation in the epithelium where the nerves reach as free nerve terminals (Fig 1).
Fig 1. Entire architecture of human epithelial corneal nerves.
Whole mount corneas were stained with neuro-β tubulin and images of the corneal epithelial nerves were acquired with a fluorescent microscope and merged together to obtain the complete architecture. In the right image there is a composed figure of the eye with the intense array of epithelial nerves.
Many conditions can alter corneal innervation; among the most common are surgery, diabetes, infections, chemical burns, prolonged use of contact lenses, Sjogren syndrome and multiple sclerosis.
One of the consequences of nerve damage is injury to the ocular surface. Damage to corneal nerves decreases sensitivity and blink reflex. Loss of efferent sensory input to the lacrimal gland leads to diminished lacrimal secretion reduced nutritional support and produces a dry ocular surface [4-6]. Recent studies have shown that 70% of diabetes patients have corneal disorders [7-8]. The patients experience a decrease in corneal sensitivity, recurrent corneal erosion, delayed corneal epithelial healing and persistent epithelial defect and neurotrophic corneal ulcers.
We were able to map the entire corneal architecture in a very recent study using human corneas from donors with insulin dependent diabetes. We found that in every age group there was a significant decrease in nerve density with diabetes [9]. We also found a major decrease of epithelial nerves in corneas of patients with 5 years or more of insulin dependent diabetes.
Refractive surgery is another frequently occurring source of nerve damage, due to the volume of these performed procedures. In a clinical study, recovery of corneal sub basal nerves was followed for 5 years in patients after refractive surgery with a confocal microscope. Dependent on the procedure used, it took between 2-5 years after surgery for the nerves to recover 80% of the original density [10].
2. PEDF and DHA combination stimulate corneal nerve regeneration
Recent experimental and clinical studies indicate that omega -3 fatty acids appear to have a beneficial effect in dry eye conditions [11-13]. One of the most significant studies, with a recruitment of 32,470 participants in the Women’s Health Study, shows a correlation of low dry eye syndrome in patients with higher intake of omega 3 fatty acids [14]. Another recent multicenter study shows that a nutritional supplementation for three months of enriched omega-3 eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids with a small proportion of omega-6 and antioxidants can reduce conjunctival inflammation in patients suffering from dry eye [15]. Resolvin E1 (RvE1), a mediator derived from EPA [16], increased tear production and decreased inflammation in a mouse model of dry eye [17]. However, evidence as to which fatty acid or what combination of fatty acids or derivatives is more efficient in the treatment of dry eye disease is still not clear [18]. In addition, in pathologic situations in which cornea innervation is compromised, treatment with omega-3 fatty acids, although successful in alleviating the symptoms of dry eye, do not treat the underlying condition of alteration in the innervation.
Pigment epithelial derived factor (PEDF) is a 50 KDa glycoprotein with neurotrophic and anti-angiogenic properties that is expressed in eye tissues, including the corneal epithelium [19-23]. PEDF, as a potent and broadly acting neurotrophic factor, induces cell differentiation and protects neurons in the brain, eye, and spinal cord against a wide range of neurodegenerative insults including ischemia, axotomy, glutamate excitotoxicity and oxidative stress [24-27]. In addition, in retinal pigment epithelial cells PEDF is a very potent inducer of the biosynthesis of neuroprotectin D1 (NPD1; 10R, 17S-dihydroxy-docosa-4Z, 7Z,11E,13E,15Z,19Z-hexaenoic acid) [28,29], a docosanoid biosynthesized from DHA with anti-inflammatory and neuroprotective actions [30-33].
An important finding from our laboratory has been that topical application of PEDF in combination with DHA increases nerve regeneration after experimental surgery that damaged the stroma nerves in rabbits [34]. Application of PEDF or DHA alone to the corneas did not produce significant changes in nerve regeneration. The combination of PEDF and DHA induces the biosynthesis of NPD1. Moreover, the treatment restores nerve sensitivity, increases epithelial wound healing, and promotes the regeneration of calcitonin gene-related peptide (CGRP) positive nerves, one of the main neuropeptides expressed by the sensory corneal nerves [35]. The data demonstrate the functionality of the regenerated nerves after PEDF plus DHA treatment.
3. Release of PEDF after corneal epithelial injury
Injury to the cornea promotes the biosynthesis of several growth factors [36-42] but it is not known how PEDF is affected. In our analysis of corneas after lamellar keratectomy we observe increase in the biosynthesis of NPD1 when the rabbits were treated with DHA; we hypothesize that PEDF could be released from corneas after injury [34]. In this context we investigated whether injury to the corneal epithelium promotes the biosynthesis and secretion of PEDF.
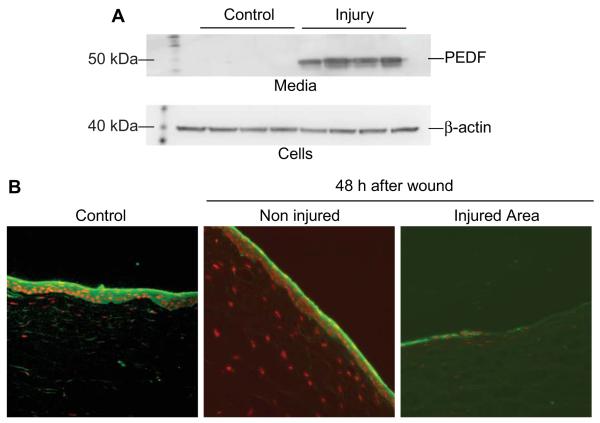
For these experiments we used rabbit corneas in organ culture. In this system we injured the epithelium, and when incubated in a media without growth factors the epithelial cells migrate and cover the wound in 48 h [43]. The media and epithelial cells of wounded and control corneas were collected and analyzed by Western blot. There was no release of PEDF to the media in control corneas; however a very significant release to the media occurred after injury (Fig 2A). Immunostaining of cornea sections showed that normal corneas have strong PEDF staining mainly in the epithelial basal layer; 48 h after the injury, the non-injured area showed similar PEDF expression, while in the injured area staining was found in the superficial layer (Fig 2B). The results suggest that there is release of PEDF after corneal epithelial injury. They also suggest that PEDF in the presence of DHA could synthesize NPD1, activating a PEDF receptor by an autocrine mechanism. A PEDF receptor has been identified containing a patatin-like phospholipase domain and four transmembrane domains. The receptor has been found in retina and retina pigmented epithelial cells [44]. Binding of PEDF to the receptor stimulates a phospholipase A2 activity that releases fatty acids [44, 45].
Fig 2. PEDF expression in corneas after epithelial injury.
Epithelial debridement wounds were made in a 7 mm area in rabbit corneas that were incubated for 48 h. A) Media was collected and analyzed by Western blot using a mouse monoclonal PEDF antibody from Santa Cruz (CA), cells were probed with β-actin antibody as a loading control. B) Corneas were fixed in 2% formaldehyde and cryostat sections were prepared. The sections were stained with the PEDF antibody and observed with a fluorescent microscope. DAPI was used to stain the nuclei.
There are very low amounts of DHA in the cornea [46]. Therefore, in order to stimulate the biosynthesis of NPD1 we postulate that DHA will need to be supplied. In fact, there is an increase of NPD1 biosynthesis in corneas treated with DHA in vivo, although we did not observe an increase in nerve regeneration with DHA alone [34]. We reasoned that nerve regeneration is not significantly increased when DHA is applied alone compared to treatment with both PEDF and DHA, because the proportion of NPD1 formed is much less than when both compounds are added to the cornea [34].
4. PEDF action on AA metabolites
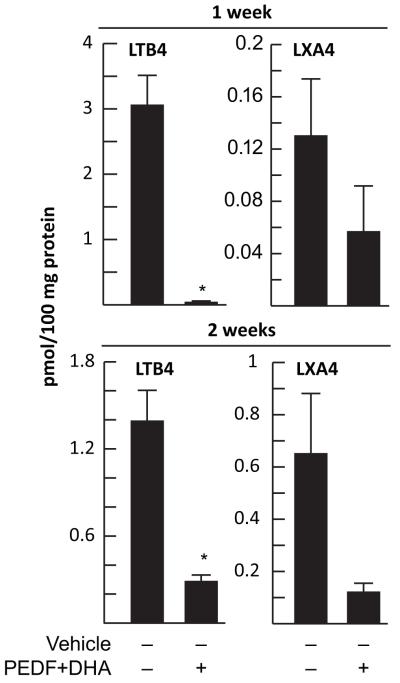
We also investigated if components of the arachidonic acid (AA) cascade were formed after injury and treatment with PEDF plus DHA. Previous work from our laboratory had established that corneal injury stimulates the biosynthesis of AA derivates such as platelet activating factor (PAF), prostaglandins, and the lipoxygenase metabolites 12- hydroxyeicosatetraenoic acid (12(S)-HETES) and 15- hydroxyeicosatetraenoic acid (15-(S) HETES) [47-52]. We found a significant decrease in leukotriene B4 (LTB4; 5S, 12R-dihydroxy-eicosa-6Z, 8E, 10E, 14Z-tetraenoic acid) biosynthesis after corneal surgery in rabbits and PEDF plus DHA treatment for 1 or 2 weeks (Fig 3). In contrast, the changes in lipoxin A4 (LXA4; 5S,6R,15S-trihydroxy-eicosa-7E,9E,11Z,13E-tetraenoic acid) were not significant. LTB4 is a chemotactic factor for PMNs in the cornea [53, 54]. The results suggest that PEDF plus DHA treatment, in addition to promoting nerve regeneration, has an anti-inflammatory action. In fact, corneas of rabbits stained with rose Bengal to determine if the epithelium and the conjunctiva were damage after lamellar keratectomy show no staining when treated with PEDF plus DHA compared to PEDF or DHA alone, [18] suggesting that the treatment reduces inflammation and produces a clear cornea.
Fig 3. Decreased biosynthesis of LTB4 in corneas treated with PEDF plus DHA.
Anesthetized rabbits underwent a lamellar keractectomy and were then treated with PEDF plus DHA for 1 and 2 weeks as previously described [32]. Lipids were extracted from a corneal strip from the surgical area and quantification of LTB4 and LXA4 was determined with a LC-MS/MS. Values are average ±SD from four samples/condition; *significant decrease after treatment.
5. PEDF action on DHA metabolites in corneal epithelial cells
As mentioned, previous studies in vivo have shown that treatment with PEDF plus DHA increase the biosynthesis of NPD1 [34]. In order to determine if biosynthesis of NPD1 would occur in the corneal epithelial cells, a human corneal epithelial cell (HCEC) line was use for the experiments [37]. The cells were incubated for 2 h with DHA to allow incorporation of the fatty acid in phospholipids, and afterwards stimulated with PEDF for 24 and 48h. Lipid extracts from cells and media were analyzed for NPD1 by LC-MS/MS. A significant increase of NPD1 biosynthesis in HCEC was observed after 24 and 48 h of PEDF stimulation (Fig 4). There was also increased release of NPD1 to the media, similar to previously reported data in retinal pigmented epithelial cells [28]. The results raise several possibilities for NPD1 function; such as an autocrine signal on epithelial cells that protects the cells after injury. In fact, NPD1 induces cell survival in RPE and neuronal cells by mechanisms that upregulate anti-apoptotic proteins of the Bcl-2 family [30-33, 55, 56]. Another possible action is through a paracrine mechanism involving other corneal cells (e.g. stroma cells). A receptor for NPD1 still needs to be uncovered, but stereospecific binding sites had been reported in retinal pigment epithelial cells and human neutrophils [57]. A third possibility is that NPD1 could bind to proteins that facilitate its transport to corneal neurons to exert its action. The mechanism of corneal nerve regeneration by PEDF plus DHA treatment is not known, but our results imply that NPD1 has a major role. Importantly, recent unpublished experiments show that corneas from rabbits treated with NPD1 after surgery will regenerate their nerves to similar densities of PEDF plus DHA treated corneas [58].
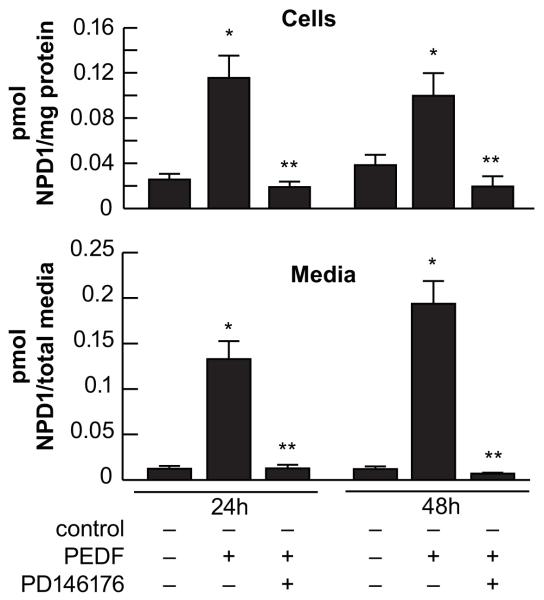
Fig 4. Biosynthesis and release of NPD1 from corneal epithelial cells.
HCEC were incubated with 5μM of a sodium solution of DHA for 2h and then stimulated with 40ng/ml of PEDF for 24 and 48h. Controls were cells without stimulation. Some samples were also treated with 10 μM of the 15-LOX-1 inhibitor PD146176 in the presence of PEDF. The media and the cells were extracted and processed for lipidomic analysis [32] using a liquid chromatograph-tandem mass spectrometer. Values are average ± SD of 6-9 samples/condition; *significant increase from control, ** significant decrease from PEDF treatment.
Biosynthesis of NPD1 occurs by the activation of 15-lipoxygenease-1 (15-LOX-1) [59]. To determine if in HCEC the biosynthesis requires 15-LOX-1, the cells were also incubated in the presence of the specific 15-LOX1 inhibitor (PD146146) when stimulated with PEDF, showing complete inhibition of NPD1 biosynthesis (Fig 4).
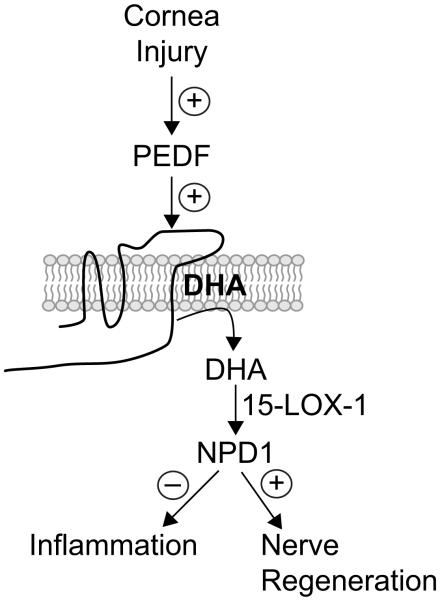
The data reveals that PEDF acts through its receptor, probably to increase the release of DHA, then is converted to NPD1 by the action of 15-LOX-1(Fig 5).
Fig 5. Proposed model of PEDF signaling in corneal epithelial cells.
Injury will increase release of PEDF from epithelial cells that by an autocrine mechanism will bind to its receptor. PEDF then stimulates the release of DHA. DHA appears in very low concentrations in the cornea, [44] therefore to induce its release in significant amounts requires DHA to be administered. Release of incorporated DHA from phospholipids is transformed by the 15-LOX-1 in NPD1. A proportion of NPD1 is also released from the epithelial cells to exert its anti-inflammatory and nerve regenerative properties.
6. Summary and Conclusions
Corneal sensory nerves are important to maintain tissue homeostasis. Several pathologies that damage the nerves induce a dry eye surface and can produce neurotrophic keratatis with consequences such as epithelial erosions, ulcerations, and in advances cases, corneal melting.
We have discovered that a combination of PEDF plus DHA induces a remarkable regeneration and return of functionality in corneal nerves after they are sectioned by experimental surgery. Here we discuss some of the most recent findings from our laboratory concerning the action of PEDF after corneal injury and the production of the DHA bioactive molecule NPD1 with anti-inflammatory and nerve regenerative properties. NPD1 could then constitute a new therapeutic agent to prevent serious consequences from corneal nerve damage. In the future it will be important to uncover the mechanisms by which NPD1 induces nerve repair. This will allow the designing of therapies that selectively manipulate its actions not only in corneal nerve repair, but in other disorders that affect the sensory peripheral nerves.
Acknowledgments
Supported by National Eye Institute Grant: NIH NEI 019465
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Commercial relationships policy: N
References
- 1.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, Content and function. Exp. Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 2.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal Innervation. Exp. Eye Res. 2010;90:478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 3.He J, Bazan NG, Bazan HE. Mapping the entire human corneal nerve architecture. Exp. Eye Res. 2010;91(4):513–523. doi: 10.1016/j.exer.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004;78:513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br. J. Ophthalmol. 2009;93:853–860. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest. Ophthalmol.Vis. Sci. 2000;41:2915–2921. [PubMed] [Google Scholar]
- 7.Su DH, Wong TY, Foster PJ, Tay WT, Saw SM, Aung T. Central corneal thickness and its associations with ocular and systemic factors: the Singapore Malay Eye Study. Arch Ophthalmol. 2008;26:981–985. doi: 10.1016/j.ajo.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Jeganathan VS, Wang JJ, Wong TY. Ocular associations of diabetes other than diabetic Retinopathy. Diabetes Care. 2008;31:1905–1912. doi: 10.2337/dc08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Bazan HE. Mapping the nerve architecture of diabetic human corneas. Ophthalmology. 2011 doi: 10.1016/j.ophtha.2011.10.036. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Jackson MA, Burrell K, Gaddie IB, Richardson SD. Efficacy of a new prescription-only medical food supplement in alleviating signs and symptoms of dry eye, with or without concomitant cyclosporine A. Clin Ophthalmol. 2011;5:1201–1206. doi: 10.2147/OPTH.S22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30:308–314. doi: 10.1097/ICO.0b013e3181f22e03. [DOI] [PubMed] [Google Scholar]
- 13.Viau S, Maire MA, Pasquis B, et al. Efficacy of a 2-month dietary supplementation with polyunsaturated fatty acids in dry eye induced by scopolamine in a rat model. Graefes Arch Clin Exp Ophthalmol. 2009;247:1039–1050. doi: 10.1007/s00417-009-1080-z. [DOI] [PubMed] [Google Scholar]
- 14.Miljanović B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–893. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brignole-Baudouin F, Baudouin C, Aragona P, et al. A multicentre, double masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmol. 2011;89:591–597. doi: 10.1111/j.1755-3768.2011.02196.x. [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Hong S, Gronert K, S P, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010;26:431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortina MS, Bazan HE. Docosahexaenoic acid, protectins and dry eye. Curr Opin Clin Nutr Metab Care. 2011;14:132–137. doi: 10.1097/MCO.0b013e328342bb1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becerra SP. Focus on Molecules: pigment epithelium-derived factor (PEDF) Exp. Eye. 2006;82:739–740. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Tombran-Tink J, Shivaram SM, Chader GJ, Johnson LV, Bok D. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J. Neurosci. 1995;15:4992–5003. doi: 10.1523/JNEUROSCI.15-07-04992.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ablonczy Z, Prakasam A, Fant J, Fauq A, Crosson C, Sambamurti K. Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through gamma-secretase. J. Biol. Chem. 2009;284:30177–30186. doi: 10.1074/jbc.M109.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He S, Kumar SR, Zhou P, et al. Soluble EphB4 inhibition of PDGF-induced RPE migration in vitro. Invest. Ophthalmol. Vis. Sci. 2010;51:543–552. doi: 10.1167/iovs.09-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat. Rev. Neurosci. 2003;4:628–636. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- 24.Cao W, Tombran-Tink J, Chen W, Mrazek D, Elias R, McGinnis JF. Pigment epithelium-derived factor protects cultured retinal neurons against hydrogen peroxide- induced cell death. J Neurosci Res. 1999;57:789–800. [PubMed] [Google Scholar]
- 25.DeCoster MA, Schabelman E, Tombran-Tink J, Bazan NG. Neuroprotection by pigment epithelial-derived factor against glutamate toxicity in developing primary hippocampal neurons. J Neurosci Res. 1999;56:604–610. doi: 10.1002/(SICI)1097-4547(19990615)56:6<604::AID-JNR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 26.Houenou LJ, D’Costa AP, Li L, et al. Pigment epithelium derived factor promotes the survival and differentiation of developing spinal motor neurons. J Comp Neurol. 1999;412:506–514. [PubMed] [Google Scholar]
- 27.Bilak MM, Becerra SP, Vincent AM, Moss BH, Aymerich MS, Kuncl RW. Identification of the neuroprotective molecular region of pigment epithelium-derived factor and its binding sites on motor neurons. J Neurosci. 2002;22:9378–9386. doi: 10.1523/JNEUROSCI.22-21-09378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A. 2007;104:13152–13157. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark DT, Bazan NG. Neuroprotectin D1 induces neuronal survival and down regulation of amyloidogenic processing in Alzheimer’s disease cellular models. Mol Neurobiol. 2011;43:131–138. doi: 10.1007/s12035-011-8174-4. [DOI] [PubMed] [Google Scholar]
- 31.Bazan NG, Musto AE, Knott EJ. Endogenous signaling by omega-3 Docosa hexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol Neurobiol. 2011;44:216–222. doi: 10.1007/s12035-011-8200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calandria JM, Bazan NG. Neuroprotectin D1 modulates the induction of pro- inflammatory signaling and promotes retinal pigment epithelial cell survival during oxidative stress. Adv Exp Med Biol. 2010;664:663–670. doi: 10.1007/978-1-4419-1399-9_76. [DOI] [PubMed] [Google Scholar]
- 34.Cortina MS, He J, Li N, Bazan NG, Bazan HE. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010;51:804–810. doi: 10.1167/iovs.09-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortina MS, He J, Li N, Bazan NG, Bazan HE. Recovery of corneal sensitivity, calcitonin gene-related Peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic Acid after experimental surgery. Arch Ophthalmol. 2012;130:76–83. doi: 10.1001/archophthalmol.2011.287. [DOI] [PubMed] [Google Scholar]
- 36.Wilson SE, Chen L, Mohan RR, Liang Q, Liu J. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res. 1999;68:377–397. doi: 10.1006/exer.1998.0603. [DOI] [PubMed] [Google Scholar]
- 37.Kakazu A, Chandrasekher G, Bazan HE. HGF protects corneal epithelial cells from apoptosis by the PI-3K/Akt-1/Bad- but not the ERK1/2-mediated signaling pathway. Invest Ophthalmol Vis Sci. 2004;45:3485–3492. doi: 10.1167/iovs.04-0372. [DOI] [PubMed] [Google Scholar]
- 38.Ottino P, Taheri F, Bazan HE. Growth factor-induced proliferation in corneal epithelial cells is mediated by 12(S)-HETE. Exp Eye Res. 2003;76:613–622. doi: 10.1016/s0014-4835(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 39.Chandrasekher G, Kakazu AH, Bazan HE. HGF and KGF-induced activation of PI-3K/p70 s6 kinase pathway in corneal epithelial cells: its relevance in wound Healing. Exp Eye Res. 2001;73:191–202. doi: 10.1006/exer.2001.1026. [DOI] [PubMed] [Google Scholar]
- 40.Lambiase A, Manni L, Bonini S, et al. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000;41:1063–1069. [PubMed] [Google Scholar]
- 41.Tuli SS, Liu R, Chen C, Blalock TD, Goldstein M, Schultz GS. Immunohistochemical localization of EGF, TGF-alpha, TGF-beta, and their receptors in rat corneas during healing of excimer laser ablation. Curr Eye Res. 2006;31:709–719. doi: 10.1080/02713680600837390. [DOI] [PubMed] [Google Scholar]
- 42.Kenchegowda S, Bazan NG, Bazan HE. EGF stimulates lipoxin A4 synthesis and modulates repair in corneal epithelial cells through ERK and p38 activation. Invest Ophthalmol Vis Sci. 2011;52:2240–2249. doi: 10.1167/iovs.10-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandrasekher G, Bazan HE. Corneal epithelial wound healing increases the expression but not long lasting activation of the p85alpha subunit of phosphatidylinositol-3 kinase. Curr Eye Res. 1999;18:168–176. doi: 10.1076/ceyr.18.3.168.5372. [DOI] [PubMed] [Google Scholar]
- 44.Notari L, Baladron V, Aroca-Aguilar JD, et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch J, Johnson CL, Nelius T, Kennedy R, Riese W, Filleur S. PEDF inhibits IL8 production in prostate cancer cells through PEDF receptor/phospholipase A2 and regulation of NFκB and PPARγ. Cytokine. 2011;55:202–210. doi: 10.1016/j.cyto.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Bazan HE, Bazan NG. Composition of phospholipids and free fatty acids and incorporation of labeled arachidonic acid in rabbit cornea. Comparison of epithelium, stroma and endothelium. Curr Eye Res. 1984;3:1313–1319. doi: 10.3109/02713688409007418. [DOI] [PubMed] [Google Scholar]
- 47.Bazan HE, Reddy ST, Lin N. Platelet-activating factor (PAF) accumulation correlates with injury in the cornea. Exp Eye Res. 1991;52:481–491. doi: 10.1016/0014-4835(91)90046-h. [DOI] [PubMed] [Google Scholar]
- 48.Hurst JS, Balazy M, Bazan HE, Bazan NG. The epithelium, endothelium, and stroma of the rabbit cornea generate (12S)-hydroxyeicosatetraenoic acid as the main lipoxygenase metabolite in response to injury. J Biol Chem. 1991;266:6726–6730. [PubMed] [Google Scholar]
- 49.Lin N, Hurst J, Bazan HE. Increased synthesis of specific eicosanoids in rejected corneal grafts. Curr Eye Res. 1996;15:1208–1212. doi: 10.3109/02713689608995157. [DOI] [PubMed] [Google Scholar]
- 50.Bazan HE, Birkle DL, Beuerman R, Bazan NG. Cryogenic lesion alters the metabolism of arachidonic acid in rabbit cornea layers. Invest Ophthalmol Vis Sci. 1985;26:474–480. [PubMed] [Google Scholar]
- 51.Bazan HE. Corneal injury alters eicosanoid formation in the rabbit anterior segment in vivo. Invest Ophthalmol Vis Sci. 1987;28:314–319. [PubMed] [Google Scholar]
- 52.Bazan HE, Tao Y, DeCoster MA, Bazan NG. Platelet-activating factor induces cyclooxygenase-2 gene expression in corneal epithelium. Requirement of calcium in the signal transduction pathway. Invest Ophthalmol Vis Sci. 1997;38:2492–2501. [PubMed] [Google Scholar]
- 53.Pfister RR, Haddox JL, Sommers CI. Injection of chemoattractants into normal cornea: a model of inflammation after alkali injury. Invest Ophthalmol Vis Sci. 1998;39:1744–1750. [PubMed] [Google Scholar]
- 54.Kernacki KA, Berk RS. Characterization of arachidonic acid metabolism and the polymorphonuclear leukocyte response in mice infected intracorneally with Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 1995;36:16–23. [PubMed] [Google Scholar]
- 55.Calandria JM, Bazan NG. Neuroprotectin D1 modulates the induction of pro- inflammatory signaling and promotes retinal pigment epithelial cell survival during oxidative stress. Adv Exp Med Biol. 2010;664:663–670. doi: 10.1007/978-1-4419-1399-9_76. [DOI] [PubMed] [Google Scholar]
- 56.Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010;90:718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marcheselli VL, Mukherjee PK, Arita M, et al. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2010;82:27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cortina MS, He J, Russ T, Bazan NG, Bazan HE. Topical treatment with Neuroprotectin D1 increases corneal nerve regeneration after experimental surgery. ARVO Poster. 2011:982–D963. [Google Scholar]
- 59.Calandria JM, Marcheselli VL, Mukherjee PK, et al. Selective survival rescue in 15- lipoxygenase-1-deficient pigment epithelial cells by the novel docosahexaenoic acid derived mediator, neuroprotectin D1. J Biol Chem. 2009;284:17877–17882. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]