Summary
Background
Smoking has been linked to mucinous ovarian cancer, but its effects on other ovarian cancer subtypes and on overall ovarian cancer risk are unclear, and the findings from most studies with relevant data are unpublished. To assess these associations, we review the published and unpublished evidence.
Methods
Eligible epidemiological studies were identified by electronic searches, review articles, and discussions with colleagues. Individual participant data for 28 114 women with and 94 942 without ovarian cancer from 51 epidemiological studies were analysed centrally, yielding adjusted relative risks (RRs) of ovarian cancer in smokers compared with never smokers.
Findings
After exclusion of studies with hospital controls, in which smoking could have affected recruitment, overall ovarian cancer incidence was only slightly increased in current smokers compared with women who had never smoked (RR 1·06, 95% CI 1·01–1·11, p=0·01). Of 17 641 epithelial cancers with specified histology, 2314 (13%) were mucinous, 2360 (13%) endometrioid, 969 (5%) clear-cell, and 9086 (52%) serous. Smoking-related risks varied substantially across these subtypes (pheterogeneity<0·0001). For mucinous cancers, incidence was increased in current versus never smokers (1·79, 95% CI 1·60–2·00, p<0·0001), but the increase was mainly in borderline malignant rather than in fully malignant tumours (2·25, 95% CI 1·91–2·65 vs 1·49, 1·28–1·73; pheterogeneity=0·01; almost half the mucinous tumours were only borderline malignant). Both endometrioid (0·81, 95% CI 0·72–0·92, p=0·001) and clear-cell ovarian cancer risks (0·80, 95% CI 0·65–0·97, p=0·03) were reduced in current smokers, and there was no significant association for serous ovarian cancers (0·99, 95% CI 0·93–1·06, p=0·8). These associations did not vary significantly by 13 sociodemographic and personal characteristics of women including their body-mass index, parity, and use of alcohol, oral contraceptives, and menopausal hormone therapy.
Interpretation
The excess of mucinous ovarian cancers in smokers, which is mainly of tumours of borderline malignancy, is roughly counterbalanced by the deficit of endometrioid and clear-cell ovarian cancers. The substantial variation in smoking-related risks by tumour subtype is important for understanding ovarian carcinogenesis.
Funding
Cancer Research UK and MRC.
Introduction
Until recently, smoking was not thought to be a risk factor for ovarian cancer, but in 2009 the International Agency for Research on Cancer added mucinous ovarian tumours (which comprise about a tenth of all ovarian cancers) to their list of tobacco-related cancers.1 We identified 56 epidemiological studies of ovarian cancer that obtained information about women's smoking history. Some results have been published from 55 of the 56 studies,2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 but results on smoking-related risks have been published from only about a third of these studies.4, 5, 10, 15, 16, 18, 20, 23, 27, 32, 34, 35, 43, 44, 46, 50, 53, 54, 56 Almost all reported little or no association between smoking and overall risk of ovarian cancer; some, but not all, reported an increased risk of mucinous tumours in smokers, but not for other subtypes of ovarian cancer.
The Collaborative Group on Epidemiological Studies of Ovarian Cancer was set up to bring together and reanalyse the available epidemiological evidence, published and unpublished, on the association between various factors and ovarian cancer risk.57 To avoid selective emphasis on results from the few studies that have published their findings, this report sought data from all studies larger than a specific size that have obtained relevant information about the relation between ovarian cancer risk and women's smoking history, whether published or not.
Methods
Search strategy and selection criteria
This collaboration began in 1998, and since then potentially eligible epidemiological studies have been sought regularly by searches of review articles and from computer-aided literature searches in Medline, Embase, and PubMed, with combinations of the search terms “ovarian cancer”, “ovary cancer”, “smok*”, and “tobacco”. To be eligible for these analyses, studies needed to have obtained individual data for women's reproductive history, use of hormonal therapies, and smoking history and to have studied at least 200 women with ovarian cancer (before 2006, studies with less than 200 cases of ovarian cancer had been eligible, so there are fewer cases in some early studies). Studies that had obtained relevant data, but had not published on ovarian cancer and smoking, were sought by correspondence with colleagues, by discussions at collaborators meetings, and by electronic searches with additional terms “cohort”, “prospective”, “women”, and “cancer risk”.
We identified 56 eligible studies and invited principal investigators from each to participate in the collaboration. Investigators from two eligible studies52, 53 did not respond to our enquiries and those from three other eligible studies54, 55, 56 were unable to participate. Thus, data from 51 of the 56 eligible studies identified are analysed in this report, and implications of the slight incompleteness are discussed later.
Data extraction
Cases were women with malignant epithelial (borderline malignant or fully malignant) or with non-epithelial ovarian cancer and controls were women without ovarian cancer who had not undergone bilateral oophorectomy. Information sought from principal investigators about each individual case and control included their age, ethnic group, education, alcohol and tobacco use, height, weight or body-mass index (BMI), age at menarche, reproductive history, use of hormonal contraceptives, use of menopausal hormonal therapy, hysterectomy, and family history of ovarian or breast cancer. The information sought about these factors related to the time preceding the onset of symptoms for cases and to an equivalent time for controls. So that similar analytical methods could be used across studies, we incorporated cohort studies using a nested case–control design, in which up to four controls were selected at random, matched by age at cancer diagnosis and, where appropriate, by broad geographical region. In one cohort study,21 cases were women with fatal ovarian cancer, whereas in all other studies cases were women with incident disease.
Principal investigators of the 51 epidemiological studies (one of which is unpublished) in these analyses2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 provided individual information about smoking history for cases and controls. The analyses used information provided by principal investigators about a woman's smoking history before the onset of symptoms. For retrospective case–control studies, this was smoking history before the onset of symptoms for cases and at an equivalent time for controls. For prospective studies, principal investigators generally provided information about women's smoking history recorded at the time they were recruited into the cohort. The information provided was used to classify all women as ever or never smokers, and in all but three studies7, 14, 45 ever smokers could be classified as either current or past smokers. Information about amount smoked and timing of exposure was sought only for the few studies that joined the collaboration after 2009, so reliable analyses of these aspects of smoking could not be done. All data contributed by principal investigators were checked and collated centrally so that analyses could use definitions that were as similar as possible across studies. Apparent inconsistencies in the data were rectified, where possible, by correspondence with the investigators. After the records had been checked and corrected, investigators were sent summary tables and listings of the variables to be used in analyses for final confirmation that their data had been correctly interpreted.
Information about histological classification and malignant potential of the ovarian cancers was sought from principal investigators. Tumours were classified as epithelial, non-epithelial, or malignant not otherwise specified (NOS). The epithelial cancers were then classified as clear-cell, endometrioid, mucinous, serous, other, mixed, or NOS, and were further subdivided by their malignant potential as borderline malignant, fully malignant, and not known whether borderline or fully malignant. If investigators provided International Classification of Diseases for Oncology58 codes, these were used to classify tumours centrally. Information about ovarian cancer histology was provided by investigators of all but five9, 10, 12, 21, 36 of the 51 participating studies; not all studies had included non-epithelial and borderline malignant ovarian cancer.
Statistical analysis
We used conditional logistic regression to calculate the relative risk (RR) of ovarian cancer in relation to smoking history (ie, the incidence rate ratio among otherwise similar women of the same age, calculated as the ratio of the odds of smoking among cases to the odds of smoking among controls). To ensure that women in one study were compared directly only with otherwise similar women in the same study, all analyses were routinely stratified by study, by centre within study, by fine divisions of age (5-year age groups up to 85–89 years), ever use of menopausal hormonal therapy (yes, no), menopausal status or hysterectomy (premenopausal or perimenopausal, natural menopause before age 50 years, natural menopause at or after age 50 years, previous hysterectomy, other or unknown), and BMI (<25 kg/m2, ≥25 kg/m2) and were routinely adjusted by parity (0, 1–2, ≥3) and use of oral contraceptives (no or yes for durations of <5 years and ≥5 years). For other potential confounding factors (year of birth, ethnic origin, education, family history of ovarian or breast cancer, age at menarche, and alcohol use), we did sensitivity analyses comparing results before and after adjustment for each variable in turn and for all simultaneously. Unknowns for each stratification and adjustment variable were assigned to separate groups. We made comparisons across different subgroups of women using standard χ2 tests for heterogeneity, calculated from the change in log likelihood on adding extra terms. Significance tests for heterogeneity of the effect of smoking by tumour subtype were based on analyses within cases only, because controls provide no additional information. Smoking status was treated as a dichotomous outcome (current vs never) and the term for tumour subtype was treated as the variable of interest in a conditional logistic regression, stratified and adjusted as described previously.
Analyses were done using STATA (version 11). Results in the figures are presented with squares and lines. The position of the square shows the value of the RR and its area is inversely proportional to the variance of the logarithm of the RR, thereby providing an indication of the amount of statistical information available for that particular estimate. When results from many studies, many tumour subtypes, or many subgroups are presented in the figures, the lines show 99% CIs (rather than 95% CIs) to help to allow for multiple testing. When the main results are given in the text, however, 95% CIs are used.
Role of the funding source
The funders had no role in study design, data collection, analysis or interpretation of data, preparation of the report, or the decision to publish. All members of the analysis and writing committee (VB, KG, CH, KM, RP, GR) had access to the raw data and are responsible for the final submission for publication.
Results
Table 1 shows details of the women in the 51 participating studies. The studies are grouped by their design and, within each type of design, are ordered by the median year when the ovarian cancers were diagnosed. All but eight of the studies were done in North America or Europe, and all but six in high-income countries. Overall, the studies contributed 28 114 women with ovarian cancer (cases) and 94 942 women without ovarian cancer (controls), with 10 362 (37%) cases from Europe and 12 817 (46%) from North America. 1423 (5%) women were younger than 35 years at diagnosis, 2696 (10%) were aged 35–44 years, 6467 (23%) were 45–54 years, 9206 (33%) were 55–64 years, and 8322 (30%) were 65 years or older. Similar percentages of cases and controls reported having ever smoked.
Table 1.
Studies and women included
| Number of cases/controls | Median year of diagnosis of cases | Median year of birth of cases | Mean age at diagnosis of cases (years) | ||
|---|---|---|---|---|---|
| 19 prospective studies | |||||
| Oxford/FPA (UK)12 | 49/196 | 1988 | 1937 | 48·1 | |
| BCDDP (USA)37 | 220/1184 | 1991 | 1925 | 65·3 | |
| Nurses' Health Study (USA)46 | 663/2707 | 1991 | 1930 | 58·7 | |
| RCGP (UK)14 | 176/704 | 1991 | 1936 | 52·8 | |
| IOWA Women's Health (USA)28 | 175/705 | 1991 | 1924 | 68·1 | |
| Radiation technologists (USA)36 | 45/177 | 1992 | 1945 | 47·6 | |
| Netherlands Cohort26 | 261/1805 | 1992 | 1923 | 67·9 | |
| CNBSS (Canada)27 | 483/1932 | 1993 | 1932 | 59·2 | |
| Norwegian Counties30 | 130/520 | 1993 | 1937 | 55·1 | |
| CPS-II Mortality (USA)21 | 2554/10 718 | 1994 | 1923 | 70·3 | |
| CPS-II Nutrition (USA)38 | 349/1399 | 1997 | 1929 | 67·7 | |
| Southern Swedish25 | 73/293 | 1997 | 1938 | 57·4 | |
| WLH (Norway/Sweden)43 | 106/427 | 1998 | 1947 | 48·8 | |
| NIH-AARP (USA)47 | 763/3052 | 1999 | 1932 | 65·9 | |
| EPIC (Europe)50 | 769/3099 | 2000 | 1939 | 59·8 | |
| NOWAC (Norway)29 | 78/326 | 2000 | 1937 | 61·4 | |
| PLCO (USA)49 | 202/807 | 2001 | 1933 | 68·2 | |
| Swedish mammography48 | 89/498 | 2001 | 1936 | 65·7 | |
| Million Women Study (UK)41 | 3608/14 341 | 2002 | 1941 | 61·0 | |
| All prospective studies | 10 793/44 890 | 1999 | 1934 | 63·5 | |
| 21 case–control studies with population controls | |||||
| Weiss (USA)6 | 298/1137 | 1977 | 1921 | 55·1 | |
| Cramer I (USA)3 | 248/238 | 1979 | 1926 | 51·5 | |
| CASH (USA)16 | 575/4233 | 1981 | 1937 | 41·9 | |
| Whittemore (USA)4 | 234/683 | 1984 | 1933 | 50·5 | |
| Shu/Brinton (China)7 | 228/229 | 1985 | 1933 | 48·4 | |
| Western New York (USA)24 | 117/686 | 1988 | 1930 | 58·3 | |
| Risch (Canada)11 | 450/564 | 1991 | 1934 | 56·7 | |
| Green/Purdie (Australia)18 | 793/854 | 1992 | 1935 | 55·2 | |
| Mosgaard (Denmark)13 | 907/1071 | 1992 | 1943 | 45·9 | |
| Cramer II (USA)15 | 563/525 | 1993 | 1942 | 51·1 | |
| Riman (Sweden)32 | 802/3361 | 1994 | 1932 | 61·6 | |
| German OCS22 | 281/533 | 1995 | 1937 | 55·1 | |
| Pike/Wu (USA)31 | 477/660 | 1995 | 1939 | 55·5 | |
| Goodman/Wu (USA)23 | 720/895 | 1996 | 1942 | 55·0 | |
| NISOC study (Israel)19 | 1342/2262 | 1996 | 1938 | 56·6 | |
| OVCARE (USA)40 | 378/1637 | 1996 | 1950 | 45·7 | |
| SHARE (USA)20 | 767/1367 | 1996 | 1943 | 51·6 | |
| Newcomb (Two States; USA)39 | 498/3163 | 1998 | 1942 | 55·0 | |
| Polish Study42 | 299/1994 | 2002 | 1947 | 55·4 | |
| AOCS (Australia)44 | 1426/1492 | 2004 | 1946 | 56·6 | |
| HOPE (USA)51 | 670/1551 | 2005 | 1948 | 57·1 | |
| All with population controls | 12 073/29 135 | 1995 | 1940 | 53·8 | |
| 11 case–control studies with hospital controls | |||||
| Newhouse (UK)2 | 293/597 | 1973 | 1918 | 54·2 | |
| Booth (UK)5 | 288/491 | 1980 | 1927 | 50·9 | |
| Tzonou/Tricopoulos (Greece)10 | 150/249 | 1980 | 1924 | 55·5 | |
| Rosenberg (USA)34 | 950/3808 | 1983 | 1935 | 49·5 | |
| Negri/Franceschi (Italy)9 | 972/2481 | 1986 | 1932 | 53·1 | |
| WHO (developing)8 | 177/6474 | 1986 | 1943 | 40·0 | |
| PEDS (USA)35 | 418/1765 | 1989 | 1933 | 54·7 | |
| Negri (Italy)17 | 1031/2408 | 1995 | 1939 | 54·9 | |
| Zhejiang-Curtin (China)33 | 287/652 | 1999 | 1952 | 46·3 | |
| Johannesburg (South Africa)45 | 182/1492 | 2001 | 1945 | 54·7 | |
| Guangzhou (China), unpublished | 500/500 | 2006 | 1947 | 58·6 | |
| All with hospital controls | 5248/20 917 | 1990 | 1937 | 52·7 | |
| All 51 studies | 28 114/94 942 | 1995 (1991–2000) | 1937 (1928–45) | 57·3 (12·4) | |
Data in parentheses are IQR or SD.
Figure 1 shows study-specific results for ever versus never smokers, with studies grouped by design. The findings varied significantly by study design (pheterogeneity<0·0001) with a slightly increased risk of ovarian cancer in ever-smokers in prospective studies (RR 1·06, 95% CI 1·01–1·11, p=0·02) and in case–control studies with population controls (RR 1·08, 95% CI 1·03–1·14, p=0·003), but an apparent reduced risk in studies with hospital controls (RR 0·81, 95% CI 0·75–0·89, p<0·0001). We could not exclude the possibility that hospital controls were more likely to have conditions associated with smoking and thus not be representative of smoking habits in the general population and so we omitted studies with hospital controls from all subsequent analyses.
Figure 1.
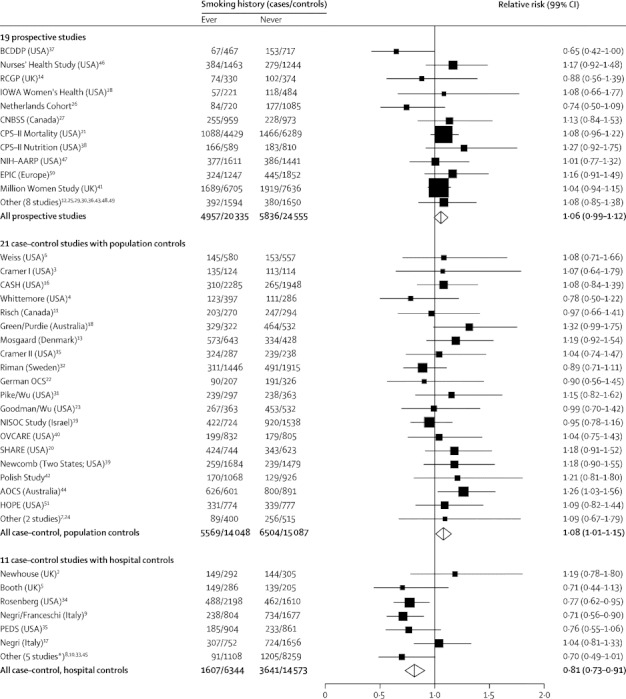
Relative risk of ovarian cancer in ever versus never smokers
Stratified by study, age at diagnosis, menopausal status or hysterectomy, body-mass index, and ever use of hormonal therapy and adjusted for parity and duration of oral contraceptive use. *Including one unpublished study (Guangzhou, China).
After excluding studies with hospital controls, we found a small increase in the risk of ovarian cancer in ever smokers compared with never smokers (RR 1·07, 95% CI 1·03–1·10, p<0·0001). There was no significant heterogeneity in the RR estimates between prospective studies and case–control studies with population controls, nor between studies within each of these designs (p>0·05 for all comparisons). All studies were of incident ovarian cancer except one (of fatal ovarian cancer21) and results were similar for both incident and fatal disease (figure 1).
After further exclusion of the studies unable to differentiate between current and past smokers, the RR of ovarian cancer in ever smokers was 1·06 (95% CI 1·03–1·09) and was similar in current (1·06, 95% CI 1·01–1·11, p=0·01) and in past smokers (1·06, 95% CI 1·02–1·11, p=0·003) (figure 2). Of the 22 462 cases of ovarian cancer in figure 2, almost 90% (19 814) were from studies that had recorded information about tumour histology, and the RR estimates for current and past smokers were similar when analyses were restricted to these women. Most cases in studies with recorded histology were epithelial; table 2 shows information about tumour subtype and malignant potential. There was a 10-year range in mean age at diagnosis by subtype, from mean 58·6 (SD 10·4) years in women with fully malignant serous tumours to 48·8 (13·5) years in those with borderline malignant serous tumours.
Figure 2.
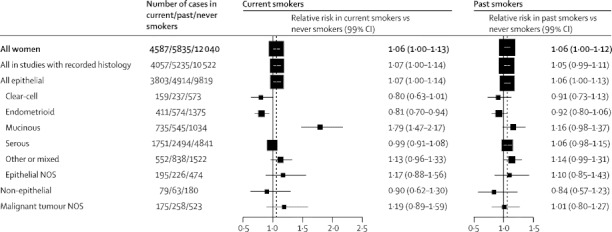
Relative risk of subtypes of ovarian cancer in current and past smokers compared with never smokers
Stratified by study, age at diagnosis, menopausal status or hysterectomy, body-mass index, and ever use of hormonal therapy and adjusted for parity and duration of oral contraceptive use. Case–control studies with hospital controls were excluded. The dotted line represents the overall result for all women. NOS=not otherwise specified.
Table 2.
Distribution and characteristics of subtypes of ovarian cancer in studies with recorded histology
| Number of cases | Age at diagnosis (years) | Year of diagnosis | ||
|---|---|---|---|---|
| Cases in studies with recorded histology | ||||
| All | 19 814 | 57·1 (11·6) | 1996 (7) | |
| Non-epithelial | 322 | 49·3 (16·2) | 1994 (8) | |
| Epithelial | 18 536 | 57·0 (11·5) | 1996 (7) | |
| Malignant NOS | 956 | 61·2 (10·8) | 1998 (7) | |
| Epithelial cases* | ||||
| Clear-cell | 969 | 56·7 (9·5) | 1996 (7) | |
| Endometrioid | 2360 | 56·8 (10·3) | 1996 (6) | |
| Mucinous | 2314 | 52·4 (13·1) | 1995 (7) | |
| Fully malignant | 1311 | 53·7 (12·9) | 1995 (7) | |
| Borderline malignant | 984 | 50·3 (13·1) | 1996 (7) | |
| Serous | 9086 | 57·3 (11·5) | 1996 (7) | |
| Fully malignant | 7498 | 58·6 (10·4) | 1996 (7) | |
| Borderline malignant | 1389 | 48·8 (13·5) | 1995 (7) | |
| Other | 2369 | 59·5 (10·3) | 1997 (6) | |
| Mixed | 543 | 57·8 (11·0) | 1999 (6) | |
| Epithelial NOS | 895 | 59·9 (10·9) | 1996 (4) | |
Data are n or mean (SD). Data from case–control studies with hospital controls and studies unable to differentiate between past and current smoking were excluded. NOS=not otherwise specified.
Fully malignant or borderline malignant status was not known for all cases; there were only two borderline malignant clear-cell, 36 borderline malignant endometrioid, and five borderline malignant mixed tumours.
In current versus never smokers, RRs varied substantially by histological subtype of the tumour (figure 2). For mucinous tumours, risk was significantly increased in current versus never smokers (RR 1·79, 95% CI 1·60–2·00, p<0·0001), but for endometrioid and for clear-cell tumours, there were significantly reduced risks (RR 0·81, 95% CI 0·72–0·92, p=0·001, and RR 0·80, 95% CI 0·65–0·97, p=0·03, respectively). Serous tumours were not significantly associated with current smoking (RR 0·99, 95% CI 0·93–1·06, p=0·8). The differences in smoking-related risk across these four specific subtypes of epithelial ovarian cancer were significant (heterogeneity p<0·0001).
The association between mucinous ovarian cancer and current smoking varied further when cancers were subdivided by their malignant potential (figure 3). The increased risk was much greater for borderline malignant (RR 2·25, 95% CI 1·91–2·65) than for fully malignant mucinous cancers (RR 1·49, 95% CI 1·28–1·73) (heterogeneity p=0·01). Neither the increased risk of borderline malignant nor that of fully malignant mucinous tumours in current smokers were driven by the findings in any one study or groups of studies (figure 4). For serous tumours, the difference in risk between borderline malignant and fully malignant cancers in current smokers was not significant (RR 1·15, 95% CI 0·99–1·33, and RR 0·96, 95% CI 0·89–1·04; heterogeneity p=0·4; figure 3). Only 36 borderline malignant endometrioid tumours were reported, which was too few to study reliably.
Figure 3.
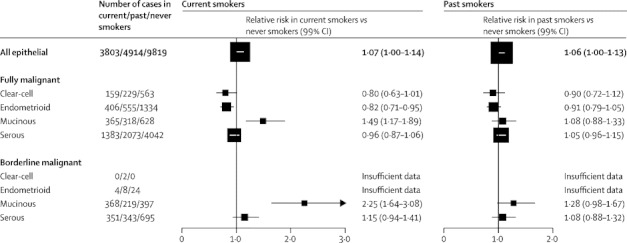
Relative risk of clear-cell, endometrioid, mucinous, and serous epithelial ovarian tumours by malignant potential and smoking history
Stratified by study, age at diagnosis, menopausal status or hysterectomy, body-mass index, and ever use of hormonal therapy and adjusted for parity and duration of oral contraceptive use. Case–control studies with hospital controls were excluded. The numbers do not always match those in figure 2 because of a few cases with missing information about malignant potential.
Figure 4.
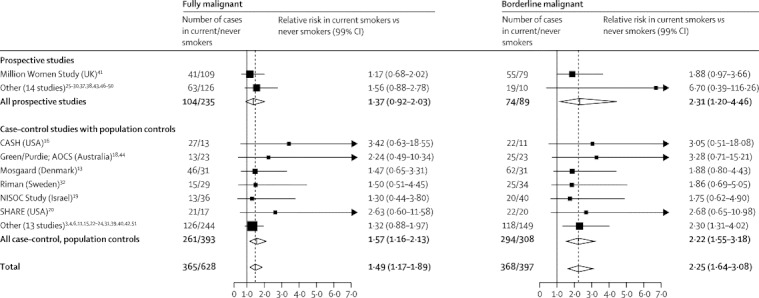
Relative risk of mucinous ovarian cancer in current versus never smokers by study
Stratified by study, age at diagnosis, menopausal status or hysterectomy, body-mass index, and ever use of hormonal therapy and adjusted for parity and duration of oral contraceptive use. Case–control studies with hospital controls were excluded. The dotted line represents the overall result for all women.
For past smokers compared with never smokers, there was a significant increase in the risk of borderline malignant mucinous cancers (RR 1·28, 95% CI 1·06–1·53, p=0·009), but not in the risk of fully malignant mucinous tumours (RR 1·08, 95% CI 0·93–1·26, p=0·3). There was no material increase or decrease in risk of other ovarian cancer subtypes in past versus never smokers (figure 3).
All analyses in Figure 2, Figure 3, Figure 4 were stratified by age, study, use of menopausal hormone therapy, menopausal status or hysterectomy, and BMI and adjusted by parity and duration of oral contraceptive use. Additional adjustment by year of birth, ethnic origin, education, family history of ovarian or breast cancer, age at menarche, and alcohol use changed the RR estimates by less than 2%. Furthermore, the observed associations between current smoking and overall risk of ovarian cancer, and the risk in the endometrioid, mucinous, and serous subtypes, did not vary substantially by year of birth, age at diagnosis, ethnic origin, education, alcohol use, BMI, parity, age at menarche, use of oral contraceptives, having a first-degree relative with ovarian or breast cancer, menopausal status, hysterectomy, or use of menopausal hormone therapy (table 3). There were too few clear-cell tumours to compare reliably the association with smoking between subgroups.
Table 3.
Relative risk of all and subtypes of ovarian cancer in current versus never smokers in various subgroups of women
|
All |
Endometrioid |
Mucinous |
Serous |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases in current/never smokers* | Relative risk (99% CI) | Cases in current/never smokers* | Relative risk (99% CI) | Cases in current/never smokers* | Relative risk (99% CI) | Cases in current/never smokers* | Relative risk (99% CI) | ||
| All women | 4587/12 040 | 1·06 (1·00–1·13) | 411/1375 | 0·81 (0·70–0·94) | 735/1034 | 1·79 (1·47–2·17) | 1751/4841 | 0·99 (0·91–1·08) | |
| Year of birth | |||||||||
| Before 1935 | 1533/5419 | 0·99 (0·90–1·09) | 130/523 | 0·87 (0·66–1·14) | 160/306 | 1·92 (1·27–2·91) | 482/1949 | 0·86 (0·74–1·00) | |
| 1935 or later | 3054/6621 | 1·11 (1·03–1·20) | 281/852 | 0·79 (0·66–0·95) | 575/728 | 1·75 (1·40–2·18) | 1269/2892 | 1·05 (0·94–1·17) | |
| Age at diagnosis | |||||||||
| <60 years | 2925/5568 | 1·09 (1·00–1·18) | 296/793 | 0·78 (0·66–0·93) | 569/644 | 1·77 (1·41–2·23) | 1226/2404 | 1·06 (0·94–1·19) | |
| ≥60 years | 1662/6472 | 1·01 (0·92–1·10) | 115/582 | 0·86 (0·66–1·13) | 166/390 | 1·82 (1·26–2·62) | 525/2437 | 0·88 (0·76–1·01) | |
| Ethnic origin | |||||||||
| White | 3239/7784 | 1·08 (1·00–1·16) | 304/927 | 0·84 (0·70–1·01) | 529/623 | 1·89 (1·47–2·42) | 1238/3087 | 1·04 (0·93–1·17) | |
| Other | 214/691 | 1·28 (0·89–1·85) | 8/80 | 0·48 (0·20–1·15) | 24/75 | 1·11 (0·42–2·95) | 87/258 | 1·26 (0·71–2·23) | |
| Years of education | |||||||||
| <13 years | 2902/6664 | 1·06 (0·98–1·15) | 202/569 | 0·84 (0·67–1·06) | 424/510 | 1·65 (1·27–2·15) | 1024/2324 | 1·00 (0·88–1·13) | |
| ≥13 years | 1249/4175 | 1·05 (0·93–1·18) | 128/515 | 0·89 (0·67–1·19) | 224/398 | 1·97 (1·36–2·85) | 550/1960 | 0·96 (0·82–1·12) | |
| Alcohol use | |||||||||
| Any | 2088/4950 | 1·10 (1·00–1·20) | 184/548 | 0·85 (0·67–1·08) | 310/448 | 1·79 (1·33–2·41) | 788/2104 | 1·02 (0·89–1·17) | |
| None | 919/3758 | 1·04 (0·91–1·19) | 81/508 | 0·71 (0·52–0·97) | 138/322 | 1·65 (1·08–2·51) | 369/1548 | 0·99 (0·81–1·21) | |
| Body-mass index | |||||||||
| <25 kg/m2 | 2553/5590 | 1·06 (0·98–1·15) | 224/604 | 0·83 (0·68–1·02) | 424/490 | 1·88 (1·43–2·47) | 971/2281 | 1·00 (0·89–1·13) | |
| ≥25 kg/m2 | 1815/5862 | 1·04 (0·95–1·14) | 171/718 | 0·78 (0·63–0·97) | 272/488 | 1·64 (1·23–2·19) | 683/2310 | 0·95 (0·83–1·08) | |
| Parity | |||||||||
| Parous | 3534/9176 | 1·06 (0·99–1·13) | 304/1013 | 0·78 (0·66–0·92) | 582/751 | 1·87 (1·49–2·35) | 1361/3769 | 0·99 (0·89–1·10) | |
| Nulliparous | 922/2374 | 1·07 (0·90–1·27) | 106/326 | 0·87 (0·58–1·30) | 148/261 | 1·57 (1·00–2·47) | 346/888 | 1·03 (0·80–1·32) | |
| Age at menarche | |||||||||
| <13 years | 1767/4507 | 1·09 (0·98–1·21) | 163/509 | 0·84 (0·65–1·08) | 246/349 | 1·81 (1·27–2·57) | 656/1797 | 0·98 (0·84–1·14) | |
| ≥13 years | 2612/7037 | 1·08 (1·00–1·17) | 230/808 | 0·85 (0·69–1·04) | 450/629 | 1·81 (1·40–2·35) | 1008/2843 | 1·01 (0·90–1·14) | |
| Oral contraceptives | |||||||||
| Ever-use | 2075/4163 | 1·08 (0·98–1·19) | 177/512 | 0·73 (0·59–0·91) | 427/463 | 1·87 (1·41–2·47) | 841/1806 | 1·05 (0·91–1·21) | |
| Never-use | 2202/7150 | 1·03 (0·94–1·12) | 207/792 | 0·86 (0·69–1·08) | 253/505 | 1·72 (1·25–2·36) | 777/2686 | 0·95 (0·83–1·08) | |
| Mother or sister with a history of ovarian or breast cancer | |||||||||
| Yes | 434/1356 | 0·98 (0·78–1·23) | 51/180 | 0·79 (0·47–1·33) | 56/97 | 1·51 (0·62–3·66) | 196/620 | 0·99 (0·71–1·39) | |
| No | 2178/6114 | 1·09 (0·99–1·19) | 207/811 | 0·75 (0·61–0·91) | 393/575 | 1·96 (1·48–2·59) | 914/2688 | 1·02 (0·90–1·16) | |
| Menopausal status† | |||||||||
| Premenopausal | 1333/2623 | 1·18 (1·04–1·33) | 114/364 | 0·69 (0·54–0·89) | 300/380 | 1·67 (1·25–2·24) | 546/1104 | 1·14 (0·96–1·35) | |
| Postmenopausal | 1590/4463 | 1·02 (0·93–1·12) | 137/507 | 0·75 (0·59–0·95) | 249/366 | 1·82 (1·32–2·50) | 593/1693 | 0·99 (0·86–1·14) | |
| Hysterectomy | |||||||||
| Yes | 602/1901 | 1·06 (0·89–1·26) | 35/181 | 0·68 (0·42–1·09) | 87/133 | 1·87 (1·01–3·45) | 235/771 | 0·97 (0·74–1·27) | |
| No | 3727/9652 | 1·06 (0·99–1·13) | 345/1123 | 0·80 (0·69–0·93) | 605/848 | 1·75 (1·43–2·14) | 1406/3846 | 1·01 (0·92–1·11) | |
| Menopausal hormone therapy‡ | |||||||||
| Ever-use | 888/2733 | 0·95 (0·83–1·08) | 98/304 | 0·91 (0·64–1·29) | 103/164 | 1·73 (1·04–2·88) | 348/1236 | 0·83 (0·69–1·00) | |
| Never-use | 1590/4463 | 1·03 (0·93–1·14) | 137/507 | 0·80 (0·62–1·03) | 249/366 | 1·91 (1·35–2·71) | 593/1693 | 0·97 (0·83–1·13) | |
In tests for heterogeneity between subgroups no p value implies <0·01. Relative risk (RR) estimates were stratified by study and age at diagnosis, and, where appropriate, menopausal status or hysterectomy, body-mass index, and ever use of menopausal hormone therapy, and adjusted by parity and duration of oral contraceptive use. Data from case–control studies with hospital controls were excluded.
Numbers of current or never smokers do not always add to the total because of some missing values.
Restricted to never users of menopausal hormone therapy.
Analyses relating to use of menopausal hormone therapy were restricted to postmenopausal women.
There was no significant heterogeneity between prospective studies and case–control studies with population controls in the association between current smoking and ovarian cancer risk overall (heterogeneity p=0·2) or when analyses were restricted to mucinous, endometrioid, and serous tumour subtypes (heterogeneity: mucinous, p=0·2; endometrioid, p=0·4; serous, p=0·07).
Discussion
This collaboration has brought together and reanalysed individual participant data for about 28 000 women with ovarian cancer from 51 studies of the effect of smoking on ovarian cancer incidence. These studies provide almost all the available epidemiological evidence worldwide on the topic. Although current smoking was associated with an excess of mucinous ovarian cancer, as had been reported previously,1 we found that the increase was mainly in tumours of borderline malignancy rather than in fully malignant tumours. Furthermore, current smoking was associated with deficits in two other subtypes of ovarian cancer—endometrioid and clear-cell tumours. Hence, smoking had little net effect on the overall ovarian cancer incidence. The significant adverse and favourable effects of current smoking were attenuated in past smokers, so past smoking had little net effect on ovarian cancer incidence.
Results of case–control studies that used hospital controls differed qualitatively from those of studies that used other designs. These differences are unlikely to be due merely to selectively inaccurate retrospective reporting of smoking, since the results differ substantially between the retrospective studies using hospital controls and the retrospective studies using population controls. Since smoking is associated with various diseases that could lead to hospital admission it is plausible that, on average, the hospital controls were more likely to smoke than were women in the general population. This difference would dilute, and could even reverse, an association between smoking and ovarian cancer risk, as suggested by figure 1. For this reason, we omitted studies with hospital controls from the main analyses. Nevertheless, to ensure that all the epidemiological information is published, details of those studies are included in table 1 and in figure 1.
Even in case–control studies with population controls there might have been some differential participation by smoking history and the retrospective reporting of smoking might have been differentially affected by the cases' knowledge that they had ovarian cancer. Although these possibilities cannot be excluded, the similarity of the findings in case–control studies with population controls and in studies with prospective recording of smoking suggests that they might not be a serious issue here.
An advantage of seeking to review all epidemiological studies of ovarian cancer with information on smoking, published and unpublished, is that this helps to avoid unduly selective emphasis on published results or on just some studies. Only a third of eligible studies have published on the association between smoking and risk of ovarian cancer, so reviews based solely on published studies could have been susceptible to publication bias. Eligible studies that did not contribute data to this collaboration, but had published on ovarian cancer risk associated with smoking,53, 54, 56 together contain fewer than a tenth as many cases as are included in the present analyses. Failure to include these studies would not have substantially changed the associations reported here, because their published findings are broadly similar to our findings. Despite extensive efforts to identify all studies with unpublished results, a guarantee that others do not exist is clearly impossible. Furthermore, to have completely up-to-date information from continuing prospective studies that are accumulating data beyond the time when information was contributed to this collaboration is not possible. Ongoing prospective studies will continue to accrue women with ovarian cancer, but there is no good reason to expect that these additional data will materially change the evidence that is already available.
A further advantage of bringing together worldwide evidence on the association between ovarian cancer and smoking is that large numbers of cases are needed to assess reliably whether the association varies by tumour subtype. The histological classifications used were those provided by investigators for each study. The classification of ovarian cancers by histology and by malignant potential might have varied between studies and possibly also over time. Misclassification of tumour subtype would tend to dilute RR estimates, and blur differences between them, yet sharp differences in the smoking-related risks were found (similar differences by tumour subtype were not found for other factors such as oral contraceptive use and adiposity57, 59).
The findings for different tumour subtypes were not driven by the results from any one study or group of studies and are unlikely to be due to confounding. All analyses were routinely stratified by age, study, use of menopausal hormone therapy, menopausal status, and BMI and were adjusted by parity and oral contraceptive use; additional adjustment for six other factors hardly changed the RR estimates.
The large proportional increase in risk of mucinous ovarian tumours associated with current smoking, and the differences between the proportional increases in fully malignant and in borderline malignant mucinous tumours, are both definite findings and could reflect a real effect of smoking. Moreover, the excess risk of mucinous tumours was far greater in current than in past smokers. Although borderline malignant mucinous tumours are less aggressive than fully malignant mucinous tumours, they can have microinvasive or invasive components.60 The proportional reductions in smoking-related risks of clear-cell and endometrioid tumours, although not as great as the proportional increase in mucinous tumours, could also be a real effect of smoking. Since information about the amount smoked and the timing of exposure was not sought systematically for this collaboration, little could be done to examine these associations further.
Smoking is known to affect the ovaries, in that smokers have an earlier menopause than do non-smokers,61 but this effect does not necessarily imply that smoking would affect ovarian cancer incidence or have different effects on different tumour subtypes. The findings here support the view that different subtypes of ovarian cancer might originate in different types of cells. In particular, endometrial cancer risk is reduced in smokers62 and our finding of a reduced risk of endometrioid tumours in current smokers is consistent with the hypothesis that endometrioid ovarian cancers might have their origin in endometrial cells. No equivalent analogy exists for clear-cell tumours.
Smoking has a wide range of adverse effects resulting in large increases in mortality from many specific causes.63 Although the excess of mucinous tumours in smokers is definite, it seems to be counterbalanced by a small deficit in clear-cell and endometrioid tumours. Hence, the overall increase in incidence of ovarian cancer in smokers is small and, even in this extensive dataset, barely significant. This study could not address survival, but since about half the mucinous tumours in smokers were of borderline malignancy, smoking is likely to have little net effect on mortality from ovarian cancer.
Acknowledgments
Acknowledgments
Funding for this collaborative reanalysis of original data was provided by Cancer Research UK and MRC. Funding for the contributing studies is described in the publications of those studies.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51
Contributors
VB, KG, CH, KM, RP, and GR analysed the data, had full access to the pooled data, wrote the first draft of the report, and had final responsibility for the decision to submit for publication; all are guarantors. Collaborators were responsible for the design of one or more of the 51 contributing epidemiological studies and had the opportunity to comment on this report.
Analysis and writing committee
V Beral, K Gaitskell, C Hermon, K Moser, G Reeves (Secretariat, Cancer Epidemiology Unit, University of Oxford, Oxford, UK); R Peto (CR-UK/MRC/BHF Clinical Trial Service Unit and Epidemiological Studies Unit, University of Oxford, Oxford, UK).
Steering committee
L Brinton (National Cancer Institute, Bethesda, MD, USA), P Marchbanks (Centers for Disease Control and Prevention, Atlanta, GA, USA), E Negri (Istituto di Ricerche Farmacologiche Mario Negri, University of Milan, Milan, Italy), R Ness (School of Public Health, University of Texas, Houston, TX, USA), P H M Peeters (University Medical Center Utrecht, Utrecht, Netherlands), M Vessey (Department of Public Health, University of Oxford, Oxford, UK).
Collaborators (in alphabetical order of institution, study name, or location)
American Cancer Society, Atlanta, GA, USA E E Calle*, S M Gapstur, A V Patel; Aviano Cancer Center, Pordenone, Italy L Dal Maso, R Talamini; Cancer and Radiation Epidemiology Unit, the Gertner Institute, Tel Hashomer, Israel A Chetrit, G Hirsh-Yechezkel, F Lubin, S Sadetzki, for the National Israeli Study on Ovarian Cancer (NISOC) group; Cancer Epidemiology Unit, Oxford, UK (Secretariat) E Banks, V Beral, D Bull, K Callaghan, B Crossley, K Gaitskell, A Goodill, J Green, C Hermon, T Key, K Moser, G Reeves; Cancer Research Division, Cancer Council New South Wales, Australia F Sitas; Cancer Research UK/MRC/BHF Clinical Trial Service Unit and Epidemiological Studies Unit, Oxford, UK R Collins, R Doll*, R Peto; Catalan Institute of Oncology, Barcelona, Spain C A Gonzalez; Centers for Disease Control and Prevention, Atlanta, GA, USA N Lee, P Marchbanks, H W Ory, H B Peterson, P A Wingo; Chiang Mai University, Chiang Mai, Thailand N Martin, T Pardthaisong*, S Silpisornkosol, C Theetranont; Chulalongkorn University, Bangkok, Thailand B Boosiri, S Chutivongse, P Jimakorn, P Virutamasen, C Wongsrichanalai; Danish Cancer Society, Institute of Cancer Epidemiology, Copenhagen, Denmark A Tjonneland; Dartmouth Medical School, Hanover, NH, USA L Titus-Ernstoff; Department of Epidemiology, Colorado School of Public Health, Denver, CO, USA T Byers; Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA T Rohan; Department of Gynaecology and Obstetrics, Herlev University Hospital, Copenhagen, Denmark B J Mosgaard; Department of Public Health, Oxford, UK M Vessey, D Yeates; Department of Social and Preventive Medicine, University at Buffalo, State University of New York, Buffalo, NY, USA J L Freudenheim; Division of Cancer Epidemiology, German Cancer Research Center (DKFZ), Heidelberg, Germany J Chang-Claude, R Kaaks; Division of Epidemiology, University of Minnesota School of Public Health Minneapolis, MN, USA K E Anderson, A Folsom, K Robien; Fred Hutchinson Cancer Research Center, University of Washington, Seattle, WA, USA J Hampton, P A Newcomb, M A Rossing, D B Thomas, N S Weiss; Imperial College London, London, UK E Riboli; Inserm U1018 and Paris South University, UMRS 1018, Institut de Cancérologie Gustave-Roussy, Villejuif, France F Clavel-Chapelon; Harvard Medical School, Cambridge, MA, USA D Cramer (Brigham and Women's Hospital), S E Hankinson, S S Tworoger (Channing Division of Network Medicine, Brigham and Women's Hospital), for the Nurses' Health Study; International Agency for Research on Cancer, Lyon, France S Franceschi; Istituto di Ricerche Farmacologiche Mario Negri, University of Milan, Milan, Italy C La Vecchia, E Negri; Karolinska Institutet, Stockholm, Sweden H O Adami, C Magnusson, T Riman, E Weiderpass, A Wolk; Maastricht University, Maastricht, Netherlands L J Schouten, P A van den Brandt; Mahidol University, Bangkok, Thailand N Chantarakul, S Koetsawang, D Rachawat; Molecular and Nutritional Epidemiology Unit, Cancer Research and Prevention Institute, Florence, Italy D Palli; National Cancer Institute, Bethesda, MD, USA A Black, L A Brinton, D M Freedman, P Hartge, A W Hsing, J V Lacey Jr, R N Hoover, C Schairer; NHLS/MRC Cancer Epidemiology Research Group, National Health Laboratory Service, Johannesburg, South Africa M Urban; Norwegian Institute of Public Health, Oslo, Norway S Graff-Iversen, R Selmer; Queensland Institute of Medical Research and University of Queensland, Brisbane, QLD, Australia C J Bain, A C Green, D M Purdie, V Siskind, P M Webb; Roswell Park Cancer Institute, Buffalo, NY, USA K Moysich, S E McCann; Royal College of General Practitioners' Oral Contraception Study, London, UK P Hannaford, C Kay; School of Public Health, Curtin University of Technology, Perth, WA, Australia C W Binns, A H Lee, M Zhang; School of Public Health, University of Texas, Houston, TX, USA R B Ness; School of Public Health and Health Sciences, University of Massachusetts, Boston, MA, USA P Nasca; Slone Epidemiology Center, Boston University, Boston, MA, USA P F Coogan, J R Palmer, L Rosenberg; Stanford University, Stanford, CA, USA J Kelsey, R Paffenbarger*, A Whittemore; University of Athens Medical School, Athens, Greece K Katsouyanni, A Trichopoulou, D Trichopoulos, A Tzonou; University of Chile, Santiago, Chile A Dabancens, L Martinez, R Molina, O Salas; University of Hawaii, Honolulu, HI, USA M T Goodman, G Lurie, M E Carney, L R Wilkens; University Hospital, Lund, Sweden L Hartman, J Manjer, H Olsson; University of Pennsylvania, Philadelphia, PA, USA J A Grisso, M Morgan, J E Wheeler; University of Pittsburgh, Pittsburgh, PA, USA C H Bunker, R P Edwards, F Modugno; University Medical Center Utrecht, Utrecht, Netherlands P H M Peeters; University of Southern California, Los Angeles, CA, USA J Casagrande, M C Pike, R K Ross*, A H Wu; University of Toronto, Toronto, ON, Canada A B Miller; University of Tromso, Tromso, Norway M Kumle, I T Gram, E Lund; George Washington University, Washington, DC, USA L McGowan; Vanderbilt University, Nashville, TN, USA X O Shu, W Zheng; World Health Organization, UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction, Geneva, Switzerland T M M Farley, S Holck, O Meirik; Yale School of Public Health, Yale, CT, USA H A Risch. *Deceased.
Conflicts of interest
All members of the analysis and writing committee declare that they have no conflicts of interest.
References
- 1.Secretan B, Straif K, Baan R, on behalf of the WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 2.Newhouse ML, Pearson RM, Fullerton JM, Boesen EAM, Shannon HS. A case-control study of carcinoma of the ovary. Br J Prev Soc Med. 1977;31:148–153. doi: 10.1136/jech.31.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer DW, Welch WR, Scully RE, Wojciechowski CA. Ovarian cancer and talc: a case-control study. Cancer. 1982;50:372–376. doi: 10.1002/1097-0142(19820715)50:2<372::aid-cncr2820500235>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Whittemore A, Wu M, Paffenbarger R. Personal and environmental characteristics related to epithelial ovarian cancer. II. Exposures to talcum powder, tobacco, alcohol and coffee. Am J Epidemiol. 1988;128:1228–1240. doi: 10.1093/oxfordjournals.aje.a115077. [DOI] [PubMed] [Google Scholar]
- 5.Booth M, Beral V, Smith P. Risk factors for ovarian cancer: a case-control study. Br J Cancer. 1989;60:592–598. doi: 10.1038/bjc.1989.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrow DC, Weiss NS, Lyon JL, Daling JR. Association of obesity and ovarian cancer in a case-control study. Am J Epidemiol. 1989;129:1300–1304. doi: 10.1093/oxfordjournals.aje.a115249. [DOI] [PubMed] [Google Scholar]
- 7.Shu XO, Brinton LA, Gao YT, Yuan JM. Population-based case-control study of ovarian cancer in Shanghai. Cancer Res. 1989;49:3670–3674. [PubMed] [Google Scholar]
- 8.The WHO Collaborative Study of Neoplasia and Steroid Contraceptives Epithelial ovarian cancer and combined oral contraceptives. Int J Epidemiol. 1989;18:538–545. [PubMed] [Google Scholar]
- 9.Parazzini F, La Vecchia C, Negri E, Bocciolone L, Fedele L, Franceschi S. Oral contraceptive use and the risk of ovarian cancer: an Italian case-control study. Eur J Cancer. 1991;27:594–598. doi: 10.1016/0277-5379(91)90226-4. [DOI] [PubMed] [Google Scholar]
- 10.Polychronopoulou A, Tzonou A, Hsieh CC. Reproductive variables, tobacco, ethanol, coffee and somatometry as risk factors for ovarian cancer. Int J Cancer. 1993;55:402–407. doi: 10.1002/ijc.2910550312. [DOI] [PubMed] [Google Scholar]
- 11.Risch HA, Marrett LD, Howe GR. Parity, contraception, infertility and the risk of epithelial ovarian cancer. Am J Epidemiol. 1994;140:585–597. doi: 10.1093/oxfordjournals.aje.a117296. [DOI] [PubMed] [Google Scholar]
- 12.Vessey M, Painter R. Endometrial and ovarian cancer and oral contraceptives—findings in a large cohort study. Br J Cancer. 1995;71:1340–1342. doi: 10.1038/bjc.1995.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosgaard B, Lidegaard O, Kjaer S, Schou G, Andersen A. Infertility, fertility drugs, and invasive ovarian cancer: a case-control study. Fertil Steril. 1997;67:1005–1012. doi: 10.1016/s0015-0282(97)81431-8. [DOI] [PubMed] [Google Scholar]
- 14.Beral V, Hermon C, Kay C, Hannaford P, Darby S, Reeves G. Mortality associated with oral contraceptive use: 25 year follow-up of cohort of 46000 women from the Royal College of General Practitioners' Oral Contraceptive Study. BMJ. 1999;318:96–100. doi: 10.1136/bmj.318.7176.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuper H, Titus-Ernstoff L, Harlow BL, Cramer DW. Population based study of coffee, alcohol and tobacco use and risk of ovarian cancer. Int J Cancer. 2000;88:313–318. doi: 10.1002/1097-0215(20001015)88:2<313::aid-ijc26>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Marchbanks PA, Wilson H, Bastos E, Cramer DW, Schildkraut JM, Peterson HB. Cigarette smoking and epithelial ovarian cancer by histologic type. Obstet Gynecol. 2000;95:255–260. doi: 10.1016/s0029-7844(99)00531-1. [DOI] [PubMed] [Google Scholar]
- 17.Chiaffarino F, Pelucchi C, Parazzini F. Reproductive and hormonal factors and ovarian cancer. Ann Oncology. 2001;12:337–341. doi: 10.1023/a:1011128408146. [DOI] [PubMed] [Google Scholar]
- 18.Green A, Purdie D, Bain C, Siskind V, Webb PM. Cigarette smoking and risk of epithelial ovarian cancer (Australia) Cancer Causes Control. 2001;12:713–719. doi: 10.1023/a:1011297403819. [DOI] [PubMed] [Google Scholar]
- 19.Modan B, Hartge P, Hirsh-Yechezkel G. Parity, oral contraceptives, and the risk of ovarian cancer among carriers and noncarriers of a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:235–240. doi: 10.1056/NEJM200107263450401. [DOI] [PubMed] [Google Scholar]
- 20.Modugno F, Ness RB, Cottreau CM. Cigarette smoking and the risk of mucinous and nonmucinous epithelial ovarian cancer. Epidemiology. 2002;13:467–471. doi: 10.1097/00001648-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez C, Calle EE, Fakhrabadi-Shokoohi D, Jacobs EJ, Thun MJ. Body mass index, height, and the risk of ovarian cancer mortality in a prospective cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:822–828. [PubMed] [Google Scholar]
- 22.Royer J, Becher H, Chang-Claude J. Low dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2002;95:370–374. doi: 10.1002/1097-0215(20011120)95:6<370::aid-ijc1065>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Goodman MT, Tung KH. Active and passive tobacco smoking and the risk of borderline and invasive ovarian cancer (United States) Cancer Causes Control. 2003;14:569–577. doi: 10.1023/a:1024828309874. [DOI] [PubMed] [Google Scholar]
- 24.McCann SE, Freudenheim JL, Marshall JR, Graham S. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J Nutr. 2003;133:1937–1942. doi: 10.1093/jn/133.6.1937. [DOI] [PubMed] [Google Scholar]
- 25.Olsson HL, Ingvar C, Bladstrom A. Hormone replacement therapy containing progestins and given continuously increases breast carcinoma risk in Sweden. Cancer. 2003;97:1387–1392. doi: 10.1002/cncr.11205. [DOI] [PubMed] [Google Scholar]
- 26.Schouten LJ, Goldbohm RA, van den Brandt PA. Height, weight, weight change, and ovarian cancer risk in the Netherlands cohort study on diet and cancer. Am J Epidemiol. 2003;157:424–433. doi: 10.1093/aje/kwf224. [DOI] [PubMed] [Google Scholar]
- 27.Terry PD, Miller AB, Jones JG, Rohan TE. Cigarette smoking and the risk of invasive epithelial ovarian cancer in a prospective cohort study. Eur J Cancer. 2003;39:1157–1164. doi: 10.1016/s0959-8049(03)00195-3. [DOI] [PubMed] [Google Scholar]
- 28.Anderson JP, Ross JA, Folsom AR. Anthropometric variables, physical activity, and incidence of ovarian cancer: The Iowa Women's Health Study. Cancer. 2004;100:1515–1521. doi: 10.1002/cncr.20146. [DOI] [PubMed] [Google Scholar]
- 29.Bakken K, Alsaker E, Eggen AE, Lund E. Hormone replacement therapy and incidence of hormone-dependent cancers in the Norwegian women and cancer study. Int J Cancer. 2004;112:130–134. doi: 10.1002/ijc.20389. [DOI] [PubMed] [Google Scholar]
- 30.Graff-Iversen S, Hammar N, Thelle DS, Tonstad S. Hormone therapy and mortality during 14-year follow-up of 14324 Norwegian women. J Intern Med. 2004;256:1–9. doi: 10.1111/j.1365-2796.2004.01396.x. [DOI] [PubMed] [Google Scholar]
- 31.Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. 2004;82:186–195. doi: 10.1016/j.fertnstert.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Riman T, Dickman PW, Nilsson S, Nordlinder H, Magnusson CM, Persson IR. Some life-style factors and the risk of invasive epithelial ovarian cancer in Swedish women. Eur J Epidemiol. 2004;19:1011–1019. doi: 10.1007/s10654-004-1633-8. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Lee AH, Binns CW. Reproductive and dietary risk factors for epithelial ovarian cancer in China. Gynecol Oncology. 2004;93:320–326. doi: 10.1016/j.ygyno.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Coogan PF, Palmer JR, Strom BL, Rosenberg L. Cigarette smoking and increased risk of mucinous epithelial ovarian cancer. Am J Epidemiol. 2004;159:133–139. doi: 10.1093/aje/kwh015. [DOI] [PubMed] [Google Scholar]
- 35.Baker JA, Odunuga OO, Rodabaugh KJ, Reid ME, Menezes RJ, Moysich KB. Active and passive smoking and risk of ovarian cancer. Int J Gynecol Cancer. 2006;16(suppl 1):211–218. doi: 10.1111/j.1525-1438.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 36.Doody MM, Freedman DM, Alexander BH. Breast cancer in US radiologic technologists. Cancer. 2006;106:2707–2715. doi: 10.1002/cncr.21876. [DOI] [PubMed] [Google Scholar]
- 37.Lacey JV, Jr, Leitzmann M, Brinton LA. Weight, height, and body mass index and risk for ovarian cancer in a cohort study. Ann Epidemiol. 2006;16:869–876. doi: 10.1016/j.annepidem.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Patel AV, Rodriguez C, Pavluck AL, Thun MJ, Calle EE. Recreational physical activity and sedentary behaviour in relation to ovarian cancer risk in a large cohort of US women. Am J Epidemiol. 2006;163:706–716. doi: 10.1093/aje/kwj098. [DOI] [PubMed] [Google Scholar]
- 39.Peterson NB, Trentham-Dietz A, Newcomb PA. Relation of anthropometric measurements to ovarian cancer risk in a population-based case-control study (United States) Cancer Causes Control. 2006;17:459–467. doi: 10.1007/s10552-005-0416-1. [DOI] [PubMed] [Google Scholar]
- 40.Rossing MA, Tang MT, Flagg EW, Weiss LK, Wicklund KG, Weiss NS. Body size and risk of epithelial ovarian cancer (United States) Cancer Causes Control. 2006;17:713–720. doi: 10.1007/s10552-006-0010-1. [DOI] [PubMed] [Google Scholar]
- 41.Million Women Study Collaborators Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369:1703–1710. doi: 10.1016/S0140-6736(07)60534-0. [DOI] [PubMed] [Google Scholar]
- 42.Garcıa-Closas M, Brinton L, Lissowska J. Ovarian cancer risk and common variation in the sex hormone-binding globulin gene: a population-based case–control study. BMC Cancer. 2007;7:60. doi: 10.1186/1471-2407-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gram IT, Braaten T, Adami HO, Lund E, Weiderpass E. Cigarette smoking and risk of borderline and invasive epithelial ovarian cancer. Int J Cancer. 2008;122:647–652. doi: 10.1002/ijc.23108. [DOI] [PubMed] [Google Scholar]
- 44.Olsen CM, Nagle CM, Whiteman DC. Australian Cancer Study (Ovarian Cancer), Australian Ovarian Cancer Study Group. Body size and risk of epithelial ovarian and related cancers: a population-based case-control study. Int J Cancer. 2008;123:450–456. doi: 10.1002/ijc.23509. [DOI] [PubMed] [Google Scholar]
- 45.Stein L, Urban MI, Weber M. Effects of tobacco smoking on cancer and cardiovascular disease in urban black South Africans. Br J Cancer. 2008;98:1586–1592. doi: 10.1038/sj.bjc.6604303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tworoger SS, Gertig DM, Gates MA, Hecht JL, Hankinson SE. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer. 2008;112:1169–1177. doi: 10.1002/cncr.23275. [DOI] [PubMed] [Google Scholar]
- 47.Leitzmann MF, Koebnick C, Danforth KN. Body mass index and risk of ovarian cancer. Cancer. 2009;115:812–822. doi: 10.1002/cncr.24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson SC, Akesson A, Wolk A. Long-term dietary acrylamide intake and risk of epithelial ovarian cancer in a prospective cohort of Swedish women. Cancer Epidemiol Biomarkers Prev. 2009;18:994–997. doi: 10.1158/1055-9965.EPI-08-0868. [DOI] [PubMed] [Google Scholar]
- 49.Buys SS, Partridge E, Black A. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 50.Gram IT, Lukanova A, Brill I. Cigarette smoking and risk of histological subtypes of epithelial ovarian cancer in the EPIC cohort study. Int J Cancer. 2012;130:2204–2210. doi: 10.1002/ijc.26235. [DOI] [PubMed] [Google Scholar]
- 51.Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB. Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology. 2012;23:311–319. doi: 10.1097/EDE.0b013e3182456ad3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mills PK, Riordan DG, Cress RD. Epithelial ovarian cancer risk by invasiveness and cell type in the Central Valley of California. Gynecol Oncol. 2004;95:215–225. doi: 10.1016/j.ygyno.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Soegaard M, Jensen A, Høgdall E. Different risk factor profiles for mucinous and nonmucinous ovarian cancer: results from the Danish MALOVA study. Cancer Epidemiol Biomarkers Prev. 2007;16:1160–1166. doi: 10.1158/1055-9965.EPI-07-0089. [DOI] [PubMed] [Google Scholar]
- 54.Pan SY, Ugnat AM, Mao Y, Wen SW, Johnson KC, Canadian Cancer Registries Epidemiology Research Group Association of cigarette smoking with the risk of ovarian cancer. Int J Cancer. 2004;111:124–130. doi: 10.1002/ijc.20242. [DOI] [PubMed] [Google Scholar]
- 55.Hoyo C, Berchuck A, Halabi S. Anthropometric measurements and epithelial ovarian cancer risk in African-American and White women. Cancer Causes Control. 2005;16:955–963. doi: 10.1007/s10552-005-3205-y. [DOI] [PubMed] [Google Scholar]
- 56.Rossing MA, Cushing-Haugen KL, Wicklund KG, Weiss NS. Cigarette smoking and risk of epithelial ovarian cancer. Cancer Causes Control. 2008;19:413–420. doi: 10.1007/s10552-007-9103-8. [DOI] [PubMed] [Google Scholar]
- 57.Collaborative Group on Epidemiological Studies of Ovarian Cancer Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23 257 women with ovarian cancer and 87 303 controls. Lancet. 2008;371:303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 58.Fritz AG, Percy C, Jack A, editors. International Classification of Diseases for Oncology (ICD-0) 3rd edn. World Health Organization; Geneva: 2000. [Google Scholar]
- 59.Collaborative Group on Epidemiological Studies of Ovarian Cancer Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLOS Med. 2012;9:e1001200. doi: 10.1371/journal.pmed.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tavassoli FA, Devilee P, editors. World Health Organization Classification of Tumours. Pathology and genetics: tumours of the breast and female genital organs. IARC Press; Lyon: 2003. [Google Scholar]
- 61.Collaborative Group on Hormonal Factors in Breast Cancer Alcohol tobacco and breast cancer—collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Health Organization International Agency for Research on Cancer . Monographs on the evaluation of carcinogenic risks to humans. Vol 83: Tobacco smoke and involuntary smoking. IARC Press; Lyon: 2004. [PMC free article] [PubMed] [Google Scholar]
- 63.Peto R, Lopez A, Boreham J, Thun M. Mortality from smoking in developed countries 1950–2000. 2nd edn. 2006. http://www.ctsu.ox.ac.uk/deathsfromsmoking/publications.html (accessed May 10, 2012).


