Abstract
Temporalis fascia has long been regarded as the ideal graft material for tympanic membrane repair. However it often does not seem to withstand negative middle ear pressure in the post operative period. Tragal cartilage with perichondrium would appear to be a better graft material with good hearing outcome. It can be obtained easily with cosmetically acceptable incision. In the present study, we have compared the graft properties of temporalis fascia verses tragal cartilage perichondrium with respect to healing, hearing and rate of post operative retraction or reperforation. 132 patients of chronic otitis media with pure conductive hearing loss were posted for tympanoplasty. Temporalis fascia graft was used in 71 patients and cartilage perichondrium (composite graft) was used in 61 patients. Post operative healing, hearing and rate of retraction or reperforation was compared for both the graft materials. All the patients were followed up for 2 years. Patients where temporalis fascia graft was used, 60 (84.5%) showed a good neotympanum, 7(9.85%) had reperforation and 5(7.04%) had retraction pockets. Patients where tragal cartilage perichondrium was used, 60(98.36%) showed a healed tympanic membrane and only 1(1.63%) had reperforation. None of the patients showed retraction pocket or cholestetoma. Postoperative hearing was accessed 6 months after surgery. Patients with temporalis fascia graft showed an air bone gap of less than 10 dB in 49 (82%) patients and more than 10 dB in 11 (18%) patients. Air bone gap closure with tragal cartilage perichondrium was less than 10 dB in 45 (78%) patients and more than 10 dB in 13 patients (22%). Tragal cartilage perichondrium (<0.5 mm) seems to be an ideal graft material for tympanic membrane in terms of postoperative healing and acoustic properties. It can easily withstand negative middle ear pressure which may have contributed to the development of otitis media and significantly affect healing outcomes in postoperative period. Tragal cartilage being composed of collagen type II is also physiologically similar to the nature of the tympanic membrane.
Keywords: Tragal cartilage perichondrium, Temporalis fascia, Tympanoplasty, Collagen type II
Introduction
Temporalis fascia has long been considered as the ideal graft material for tympanic perforation due to its healing and acoustic properties. The first closure was achieved by pedicled skin flap by Mortiz [1] in 1950. Thereafter, various materials were used e.g. free skin graft, vein graft, dura etc. Heerman was first to consider temporalis fascia as a grafting material. Storrs successfully employed it thereafter. The concept of grafting tragal cartilage and perichondrium was introduced by Goodhill.
The ideal grafting material used for tympanic membrane closure should meet certain criteria namely, low rejection rate, sufficient quantity, good tensile strength, conductive properties similar to that of tympanic membrane and easy availability. Membranous grafts like temporalis fascia and perichondrium meet these criteria and result in closure of tympanic membrane perforation in 95% of ears with normal ventilation. However, in situations such as recurrent perforation, total perforation, and chronic mucosal dysfunction or severe atelactatic tympanic membrane, fascia and perichondrium may undergo atrophy and result in graft reperforation [2, 3]. Cartilage perichondrium would theoretically work well in these conditions, being tougher and easily neovascularised. The incorporated cartilage would give it the necessary stiffness and mechanical stability to avoid retraction. Also, it has a low metabolic rate and good acceptance in the middle ear [4, 5]. Concerns have been raised about stiff nature of cartilage, as it could reduce the vibratory properties of neotympanum. However, adequate thinning of the cartilage seems to overcome this problem.
In our study, we have compared the grafting and acoustic properties of temporal fascia with cartilage–perichondrium (composite graft) especially in ears with large perforation and an unfavourable eustachian tube function.
Aim
To compare the results of healing of temporalis fascia versus cartilage–perichondrium as a graft material in tympanoplasty.
To compare the results of post-operative hearing using temporalis fascia and cartilage–perichondrium graft.
To compare the rate of post-operative retraction or reperforations of neotympanum in both.
Materials and Methods
132 patients of chronic otitis media (Photo 1) with pure conductive hearing loss without any complications and without any other septic foci were selected between Jan 2004 and Jan 2008. 65(49.24%) were males and 67(50.78%) were females. The age group was from 12 to 60 years. Other medical conditions like diabetes mellitus, hypertension, bronchial asthma were managed medically simultaneously. All patients were subjected to examination under microscope, pure tone audiometry, routine investigations and X-rays. Patients with pure conductive hearing loss were selected. The pre-operative pure tone audiometry showed a hearing loss of 5–15 db in 32 (24.24%) patients, 15–25 db in 44 (33.33%) patients and >25 db loss in 56 (42.42%) patients. Patients with ossicular dysfunction, bilateral disease or external ear pathology were excluded from the study. Patients were posted for tympanoplasty, once their ear became dry for at least 6 weeks. They were divided into two groups:
Group A: Temporalis fascia graft (n = 71)
Group B: Tragal cartilage with perichondrium (n = 61)
Photo 1.
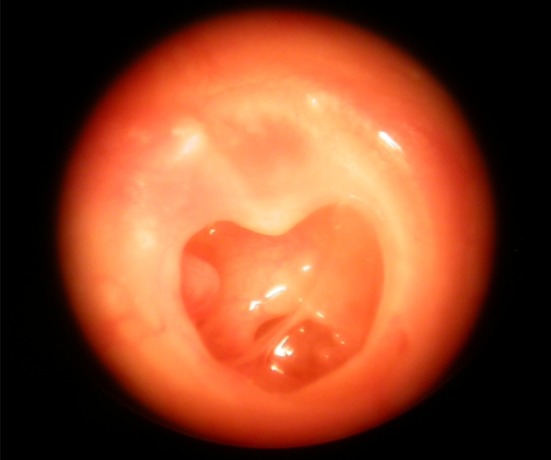
Right chronic otitis media
Local anaesthesia was used for 102 patients and general anaesthesia was used for 30 patients during surgery. Temporalis fascia or tragal cartilage with posterior layer of perichondrium was harvested. Anterior layer of perichondrium was not included in graft. The tragal cartilage was thinned to <0.5 mm and the perichondrium was spread out laterally giving it a “Fried egg appearance” (Photo 2). After exposing the middle ear and checking the ossicular integrity and mobility, the perforation was closed using the graft material. For large perforations or anterior perforations with small anterior rim, we used the “anterior tucking technique”. A small incision in the skin of anterior canal wall 2–3 mm lateral to the annulus with the help of an angled knife was made. Subsequently, a tunnel to the middle ear cavity was developed after limited lifting of the annulus. By using a cartilage plate with a strip of perichondrium still attached, the perichondrial part of the graft was pulled through this tunnel by using a small hook. This gives a mechanically stable reconstruction in this area. Underlay technique with perichondrium on lateral side (Photo 3) was used in all patients. Any patient requiring ossiculoplasty were excluded from the study.
Photo 2.
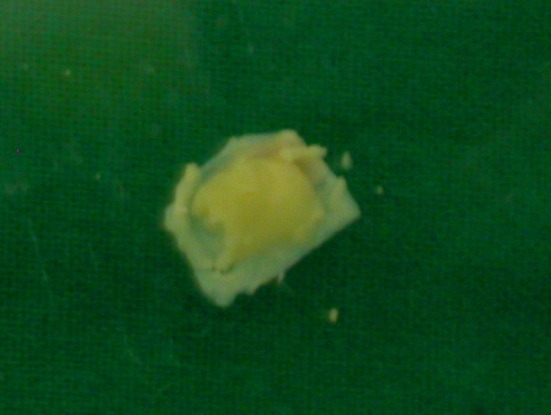
“Fried-egg” appearance of tragal cartilage–perichondrium
Photo 3.
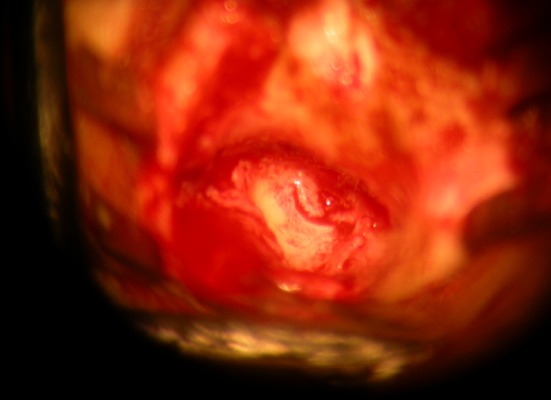
Tragal cartilage–perichondrium graft underlaid
Post-operatively antibiotics, anti-inflammatory, nasal decongestants and eardrops were used. Patients were followed up at 2 weeks, 6 weeks, 3 months, 6 months, 1 year and 2 years interval. Postoperative audiogram was done after 6 months of surgery.
Results
132 patients of chronic otitis media underwent tympanoplasty for eradication of the disease and repair of the tympanic membrane with temporalis fascia (n = 71) or tragal cartilage with perichondrium (n = 61). The patients were followed up regularly for 2 years.
6 months post operatively, in patients where temporalis fascia was used as graft material, 68 (95.77%) showed formation of a neotympanun (Photo 4), whereas 3 (4.22%) had reperforation. In patients where cartilage perichondrium was used, 60 (98.36%) showed a good neotympanum (Photo 5), while only 1 (1.63%) has reperforation. No cases of retraction were seen.
Photo 4.
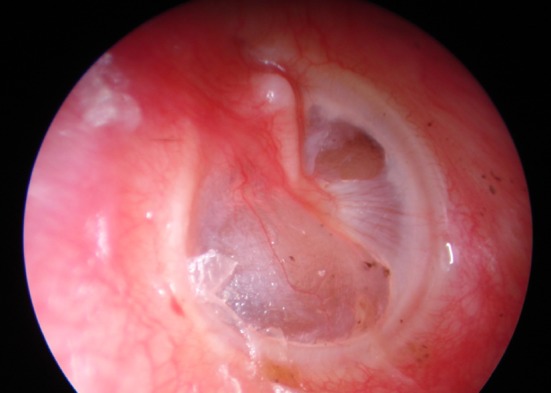
Formation of neotympanum after using temporalis fascia graft
Photo 5.
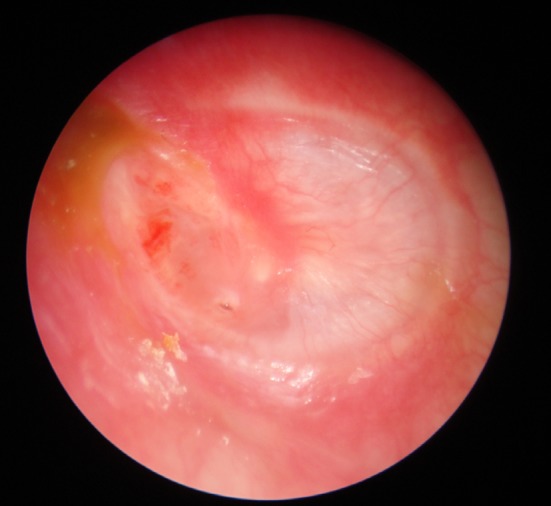
Formation of neotympanum after using tragal cartilage–perichondrium (<0.5 mm)
2 years post operatively, 60 (84.5%) patients with temporalis fascia graft showed a good neotympanum, 7 (9.85%) had reperforation and 5 (7.04%) had retraction pockets. Patients where tragal cartilage perichondrium was used, the results were consistent i.e. 60 (98.36%) showed a healed tympanic membrane and only 1 (1.63%) had reperforation. None of the patients showed retraction pocket or cholesteatoma (Table 1).
Table 1.
Graft take up
| Temporalis fascia (n = 71) | Tragal cartilage perichondrium (n = 61) | |||
|---|---|---|---|---|
| 6 month | 2 years | 6 month | 2 years | |
| Neotympanum | 68 (95.77%) | 60 (84.50%) | 60 (98.36%) | 60 (98.36%) |
| Reperforation | 3 (4.22%) | 7 (9.85%) | 1 (1.63%) | 1 (1.63%) |
| Retraction | 0 | 5 (7.04%) | 0 | 0 |
Postoperative hearing was accessed 6 months after surgery. Patients with temporalis fascia graft showed an air bone gap of less than 10 dB in 49 (82%) patients and more than 10 dB in 11 (18%) patients. Air bone gap with tragal cartilage perichondrium was less than 10 dB in 45 (78%) patients and more than 10 dB in 13 patients (22%) (Table 2).
Table 2.
Comparative results of post operative hearing using temporalis fascia and tragal cartilage perichondrium
| Air-bone gap closure | Temporalis fascia (n = 60) | Percentage | Tragal cartilage perichondrium (n = 58) | Percentage |
|---|---|---|---|---|
| <10db | 49 | 82 | 45 | 78 |
| >10db | 11 | 18 | 13 | 22 |
Discussion
Chronic mucosal otitis media implies a permanent abnormality of the pars tensa. It is usually a result of earlier active otitis media or otitis media with effusion. Surgery is relevant in patient with active or inactive chronic otitis media. Wullstein coined the term Tympanoplasty in 1953. Tympanoplasty is “any operation where the tympanic membrane is reconstructed with or without reconstruction of the ossicular chain”. In tympanoplasty, the grafting of the tympanic membrane can be done with homologous, autologous or xeno graft material. Numerous graft materials have been tried to reconstruct the tympanic membrane. The most popular materials used are temporalis fascia and perichondrium, whereas cartilage and composite grafts have lately gain more acceptance in cases of recurrence or total perforation, severe atelectatic ears or cholestetoma cases [4].
The pre requisites for a successful tympanoplsty are:
Repair of defect so as to close the tympanic cavity. Thus, preventing recurrent middle ear infection.
Neotympanum should be able to resist middle ear pressure changes in eustachian tube dysfunction where the perforation is large.
The acoustic properties of the neotympanum should be similar to a healthy tympanic membrane.
The advantages of temporalis fascia are: -
Available from the same incision.
Sufficiently large graft can be taken up.
It is thin, relatively strong and withstands the rigors of the hearing process.
After surgery, when the middle ear cavity is ventilated adequately, inflammation decreases and a good neotympanum is formed. The growth rate of tympanic membrane is 1 mm/day. After tympanoplasty, the tympanic membrane repair starts after 12 h of surgery and the granulation tissue starts appearing after 36 h. The graft acts as a scaffold to support regenerating mucosa as medial aspect of ear drum and squamous epithelium on lateral side. The graft constitutes the connective tissue portion of the “new” drum. It however lacks the orderly collagen fibre arrangement of normal drum [6]. The success rate of tympanic membrane perforation closure is usually cited as 64–95% [7]. But this figure may be unrealistic as long term follow up shows recurrent perforation and retraction in some ears.
The eustachian tube is crucial factor in success of tympanoplasty or myringoplasty. However, with poor eustachian tube dysfunction, adhesive otitis media, tympanic fibrosis or total or subtotal perforation, the results are poor. The healing rate of the repaired tympanic membrane may be hampered by reperforation and retraction of neotympanum. Endarteritis and defective repair mechanism at the cellular level can further alter the hearing of the tympanic membrane [8].
The advantages of tragal cartilage perichondrium are:
Readily available in operative field.
Large graft can be harvested from tragus and concha.
It has its own blood supply.
Easily shaped.
No extra cost.
Low metabolic rate.
Low extrusion rate.
The nourishment of cartilage is by diffusion with the help of perichondrium. Hence for the viability of the cartilage, perichondrium on at least one side should be intact. There is alteration in shape and size of cartilage with time. It was seen that though the cartilage becomes non viable, it remains functional. There is gradual replacement of cartilage with fibrous tissue and deeper proliferation of blood vessels. Sufficient nourishment of the grafted material is ensured because cartilage and chondrocytes are bradytrophic tissues. This fact is supported by low metabolic rate of cartilage in general and the ability of receiving nutrients by diffusion [4]. This is extremely important in the early postoperative weeks in which nourishment is absent, and fibroblasts, like in fascia, which requires fresh blood circulation for survival, would become a vital.
Atelectasis is collapse of the drum on to the ossicular chain and on the promontory. An atelectatic drum demonstrates an absent or diminished fibrous layer. Many patients have undergone fascia grafting in an attempt to recreate fibrous layer and thereby strengthen the drum, but in patients with chronic eustachian tube dysfunction, this tympanic membrane retracts and adheres again to promontory, thus increasing the rate of cholesteatoma. In these cases, cartilage–perichondrium gives better results than temporalis fascia or perichondrium alone [9–11], because of its higher mechanical stability against middle ear pressure changes. Incidence of post operative retraction pocket are seen less frequently with cartilage [3, 12, 13].
The normal tympanic membrane combines stability against atmospheric pressure loads and acoustic sensitivity in its only 100 μm thin structure of three dimentionally arranged collagen and elastic fibres [14]. Additionally, this arrangement causes the outward curved shape of the tympanic membrane and provides the cante lever first proposed by Helmholtz [15, 16]. It is difficult to reconstruct this sophisticated design and post operative the acoustic characteristics of tympanic membrane may change [17]. The extent of this change is strongly influenced by material properties of the graft and by the surgical technique.
Cartilage can be inserted in the form of several parallel, full thickness strips (palisade technique) or as a single plate of different sizes and shapes (island technique, book cover technique, etc.). Overbosch in1971 was first to describe a microslice technique to improve the acoustic properties of the reconstructed tympanic membrane. He cut the cartilage by a dermatome into plates with thicknesses of 0.2–1 mm [18]. The placement of the cartilage also determines the influence of its stiffness on the acoustic behaviour of the reconstructed tympanic membrane. If the cartilage strips or plates are suspended in between the osseous annular rim, the acoustic transfer characteristics of the tympanic membrane is determined by the stiffness. A small cartilage plate that is placed only on the top of a prosthesis, with a regular tympanic membrane surrounding it, influences the vibrational characteristics, mainly by its mass, because the stiffness of the membrane is determined by surrounding tympanic membrane remnant.
Cartilage slices <0.5 mm thick are similar to the tympanic membrane in their acoustic properties. In a normally ventilated middle ear, a cartilage plate of thickness <0.5 mm possesses sufficient mechanical stability and low acoustic transfer loss [19]. In aerated middle ear without any risk factors, thinner cartilage plates can be used (0.1–0.3 mm). However, in cases of tubal dysfunction, the stiffer cartilage plates of >0.5 mm layer thickness may be more advantageous from a mechanical point of view. Temporalis fascia and perichondrium alone often fails as graft material for tympanic membrane reconstructions because of their low mechanical stability and tendency to atrophy over the years [2, 3]. The “Take” rate for these materials ranges between 86 and 97% [21–24]. The advantages of cartilage is high mechanical stability and long lasting vitality ever years after surgery. The success rate of “take up” by using cartilage grafts is reported up to 100% [24–30]. In our study, all the patients were followed up for 2 years. Patients where temporalis fascia graft was used, 60 (84.5%) showed a good neotympanum, 7 (9.85%) had reperforation and 5 (7.04%) had retraction pockets. Patients where tragal cartilage perichondrium was used, 60 (98.36%) showed a healed tympanic membrane and only 1 (1.63%) had reperforation. None of the patients showed retraction pocket or cholestetoma.
Fascia and perichondrium display acoustic properties similar to those of tympanic membrane. This might be a reason for the good hearing results after myringoplasty with airbone gaps <30 dB in 90% of the cases, when these materials are used [18, 20]. But they may not withstand negative middle ear pressure in the postoperative period causing retraction and reperforation. For optimal acoustic transfer behaviour, the cartilage should be cut as thin as possible. The cartilage plates with the thickness of 0.5 mm show stiffness similar to that of healthy tympanic membrane and might be considered an acceptable compromise between satisfactory acoustic properties and sufficient mechanical stability [30]. In our study, Postoperative hearing was accessed 6 months after surgery. Patients with temporalis fascia graft showed an air bone gap of less than 10 dB in 49 (82%) patients and more than 10 dB in 11 (18%) patients. Air bone gap closure with tragal cartilage perichondrium was less than 10 dB in 45 (78%) patients and more than 10 dB in 13 patients (22%).
The tympanic membrane has to resist significant pressure gradients but at the same time be flexible and compliant with subtle pressure variations of sounds that are collected and transmitted to the malleus. Therefore, it needs to be strong in relation to its thickness. Thus, it seems reasonable that collagen Type II, with a higher tensile strength than Type III, makes up the major portion of tympanic membrane, that is, the radiating fibrous layer that covers the entire surface of the tympanic membrane. The circular fiber layer is thicker in the peripheral portions. It contains collagen Types I, II and III, with Type III being most common. Type III provides less strain resistance than Type II. Its role may therefore be mechanoacoustic, promoting the complex vibration patterns of the tympanic membrane that congregate in the umbo velocity [31]. Temporalis fascia consists primarily of collagen type I [32]. Postoperatively, the graft integrates with the remnant lamina propria of tympanic membrane. Since cartilage is formed mainly by type 2 collagen, which is also the main type in lamina propria, a thin cartilage graft would be a better option [33].
Conclusion
It would be worthwhile therefore to consider tragal perichondrium cartilage as suitable alternative to temporalis fascia. The key seems to be the use of cartilage of appropriate thickness. This would not hamper conduction of sound while protect from retraction or reperforation of the neo tympanum. Perichondrium is a tough graft material showing good revascularization. The incorporation of cartilage in perichondrium as a composite would counteract negative middle ear pressure. This is of paramount importance in poor eustachian tube function and in ears with a large perforation.
Summary
Cartilage perichondrium appears to be ideal graft material for ears where postoperative retraction, reperforation and eustachian tube dysfunction are of concern.
Cartilage thickness of <0.5 mm is seen to have similar acoustic properties as the tympanic membrane.
Cartilage–perichondrium is easy to harvest and readily available at site of surgery.
Cartilage consists of collagen type II and is therefore physiological more suitable for repair of tympanic membrane.
References
- 1.Glasscock ME, Shambaugh GE Surgery of the ear 5th edition pathology and clinical course of inflammatory disease of the middle ear. 21, pp 428–429
- 2.Buckingham RA. Fascia and perichondrium atrophy in tympanoplasty and recurrent middle ear atelectasis. Ann Otol Rhinol Laryngol. 1992;101:755–758. doi: 10.1177/000348949210100907. [DOI] [PubMed] [Google Scholar]
- 3.Milewski C. Composite graft tympanoplasty in the treatment of ears with advanced middle ear pathology. Laryngoscope. 1993;103:1352–1356. doi: 10.1288/00005537-199312000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Yung M. Cartilage tympanoplasty: literature review. J Laryngol Otol. 2008;122:663–672. doi: 10.1017/S0022215108001813. [DOI] [PubMed] [Google Scholar]
- 5.Dornhoffer JL. Cartilage tympanoplasty. Otolaryngol Clin North Am. 2006;39:1161–1176. doi: 10.1016/j.otc.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Schuknecht HF (1993) Pathology of the ear, 2nd edn. Lea and Febiger, Philadelphia, pp 191–253
- 7.Browning GG, Merchant SN, Kelly G et al (2008) Chronic otitis media. In: Gleeson M (ed) Scott-Brown’s Otorhinolaryngology, head and neck surgery, vol 3, 7th edn. 3423 pp
- 8.Browning GG, Merchant SN, Kelly G et al (2008) Chronic otitis media. In: Gleeson M (ed) Scott-Brown’s otorhinolaryngology, head and neck surgery, vol 3, 7th edn. 3420 pp
- 9.Goodhill V. Tragal Perichondrium and cartilage in tympanoplasty. Arch Otolaryngol. 1967;85:480–491. doi: 10.1001/archotol.1967.00760040482004. [DOI] [PubMed] [Google Scholar]
- 10.Heerman HJ, Heerman H, Kopstein E. Faszia and cartilage palisade tympanoplasty; nine years experience. Arch Otolaryngol. 1970;91:229–240. doi: 10.1001/archotol.1970.00770040334004. [DOI] [PubMed] [Google Scholar]
- 11.Hildman H, Luckhaupt H, Schmelzer A. Die verwendung von Knorpel in der Mittelohrchirurgie. HNO. 1996;44:597–603. doi: 10.1007/s001060050062. [DOI] [PubMed] [Google Scholar]
- 12.Adkins WY. Composite autograft for tympanoplasty and tympanomastoid surgery. Laryngoscope. 1990;100:244–247. doi: 10.1288/00005537-199003000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Gibb AG, Pang YT. Surgical treatment of tympanosclerose. Eur Arch Otolaryngol. 1995;252:1–10. doi: 10.1007/BF00171431. [DOI] [PubMed] [Google Scholar]
- 14.Lim DJ. Human Tympanic membrane: ultrastructural observation. Acta Otolaryngol. 1970;70:176–186. doi: 10.3109/00016487009181875. [DOI] [PubMed] [Google Scholar]
- 15.Helmholtz HLF. Die Mechanik der Gehorknochelchen und des Trommelfells. Pfliigers Arch Ges Physiol. 1868;1:50–53. doi: 10.1007/BF01640310. [DOI] [Google Scholar]
- 16.Khanna SM, Tonndorf J. Tympanic membrane vibrations in cats studied by time averaged holography. J Acoustic Soc Am. 1972;51:1904–1920. doi: 10.1121/1.1913050. [DOI] [PubMed] [Google Scholar]
- 17.Williams KR, Blayney AW, Lesser THJ (1997) Mode shapes of a damaged and repaired tympanic membrane as analyzed by the finite element method. Clin Otolaryngol 126–131 [DOI] [PubMed]
- 18.Overbosch HC. Homograft myringoplasty with microsliced septal cartilage. Proc Otolaryngol. 1971;33:356–357. [PubMed] [Google Scholar]
- 19.Zahnert T, Huttenbrink KB, Murbe D, Bornitz M. Experimental investigations of the use of cartilage in tympanic membrane reconstruction. Am J Otol. 2000;21:322–328. doi: 10.1016/S0196-0709(00)80039-3. [DOI] [PubMed] [Google Scholar]
- 20.Huttenbrink KB (1997) Mechanical aspects of middle ear reconstruction. In: Huttenbrink KB (ed) Middle ear mechanics in research and otosurgery. Department of Oto-Rhino-Laryngology Press, Dresden, pp 165–169
- 21.Djalilian HR. Revision tympanoplasty using scar tissue graft. Otol Neurotol. 2006;27:131–135. doi: 10.1097/01.mao.0000190462.50755.f2. [DOI] [PubMed] [Google Scholar]
- 22.Kaylie DM, Gardner EK, Jackson CG. Revision chronic ear surgery. Otolaryngol Head Neck Surg. 2006;134:443–450. doi: 10.1016/j.otohns.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 23.Veldman JE, Braunius WW. Revision surgery for chronic otitis media: a learning experience. Report on 389 cases with a long term follow-up. Ann Otol Rhinol Laryngol. 1998;107:486–491. doi: 10.1177/000348949810700606. [DOI] [PubMed] [Google Scholar]
- 24.Ben GO, Mbarek C, Khammassi K, et al. Cartilage graft in type 1 tympanoplasty: audiological and otological outcome. EUR-Arch Otorhinolaryngol. 2008;265:739–742. doi: 10.1007/s00405-008-0645-5. [DOI] [PubMed] [Google Scholar]
- 25.Boone RT, Gardner EK, Dornhoffer JL. Success of cartilage grafting in revision tympanoplasty without mastoidectomy. Otol Neurotol. 2004;25:678–681. doi: 10.1097/00129492-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Moore GF (2002) Candidate’s thesis: revision tympanoplasty utilizing fossa triangularis. Laryngoscope 112:1543–1554 [DOI] [PubMed]
- 27.Sismanis A, Dodson K, Kyrodimos E. Cartilage “shield” grafts in revision tympanoplasty. Otol Neurotol. 2008;29:330–333. doi: 10.1097/MAO.0b013e318161aae1. [DOI] [PubMed] [Google Scholar]
- 28.Aidonis I, Robertson TC, Sismanis A. Cartilage shield tympanoplasty: a reliable technique. Otol Neurotol. 2005;26:838–841. doi: 10.1097/01.mao.0000185046.38900.1f. [DOI] [PubMed] [Google Scholar]
- 29.Bernal-Sprekelsen M, Romaguera L, Sanz Gonzalo JJ. Cartilage palisades in type 3 tympanoplasty: anatomic and functional long term results. Otol Neurotol. 2003;24:38–42. doi: 10.1097/00129492-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Ghanem MA, Monroy A, Alizade FS, Nicolau Y, Eavey RD. Butterfly cartilage graft inlay tympanoplasty for large perforations. Laryngoscope. 2006;116:1813–1816. doi: 10.1097/01.mlg.0000231742.11048.ed. [DOI] [PubMed] [Google Scholar]
- 31.Knutsson J, Bagger-Sjöbäck D, Von Unge M (2009) Collagen type distribution in the healthy human tympanic membrane. Otol Neurotol 30(8):1225–1229 [DOI] [PubMed]
- 32.Ross MH, Romrell LJ (1989) Connective tissue. In: Histology: a text and atlas, vol 89, 2nd edn. Williams and Wilkins, Baltimore
- 33.Szabo LZ. How can an under laid fascia graft from the middle layer of a reconstructed tympanic membrane? Laryngoscope. 2006;116:1674–1677. doi: 10.1097/01.mlg.0000233616.43643.f2. [DOI] [PubMed] [Google Scholar]


