Abstract
Opiate abuse and HIV-1 have been described as interrelated epidemics, and even in the advent of combined anti-retroviral therapy, the additional abuse of opiates appears to result in greater neurologic and cognitive deficits. The central nervous system (CNS) is particularly vulnerable to interactive opiate-HIV-1 effects, in part because of the unique responses of microglia and astroglia. Although neurons are principally responsible for behavior and cognition, HIV-1 infection and replication in the brain is largely limited to microglia, while astroglia and perhaps glial progenitors can be latently infected. Thus, neuronal dysfunction and injury result from cellular and viral toxins originating from HIV-1 infected/exposed glia. Importantly, subsets of glial cells including oligodendrocytes, as well as neurons, express µ-opioid receptors and therefore can be direct targets for heroin and morphine (the major metabolite of heroin in the CNS), which preferentially activate µ-opioid receptors. This review highlights findings that neuroAIDS is a glially driven disease, and that opiate abuse may act at multiple glial-cell types to further compromise neuron function and survival. The ongoing, reactive cross-talk between opiate drug and HIV-1 co-exposed microglia and astroglia appears to exacerbate critical proinflammatory and excitotoxic events leading to neuron dysfunction, injury, and potentially death. Opiates enhance synaptodendritic damage and a loss of synaptic connectivity, which is viewed as the substrate of cognitive deficits. We especially emphasize that opioid signaling and interactions with HIV-1 are contextual, differing among cell types, and even within subsets of the same cell type. For example, astroglia even within a single brain region are heterogeneous in their expression of µ-, δ-, and κ-opioid receptors, as well as CXCR4 and CCR5, and Toll-like receptors. Thus, defining the distinct targets engaged by opiates in each cell type, and among brain regions, is critical to an understanding of how opiate abuse exacerbates neuroAIDS.
Keywords: HIV, SIV, opiate drug abuse, NeuroAIDS, µ-opioid receptors, HIV-associated neurocognitive disorders (HAND), CNS, neurotoxicity, microglia, astroglia, oligodendroglia, myelin, neuroimmunology.
INTRODUCTION
There are compelling reasons to investigate opioid and HIV-1 interactions and their role in accelerated neuropathogenesis. Studies in HIV-1-infected opiate abusers show severe neuropathology compared to infected non-drug users [1-4], with selective increases in microgliosis [2, 4, 5]. In this cohort, multinucleated giant cells and HIV-1 p24 immunoreactive glia were found over 3-fold more frequently in injection drug users than in non-drug using HIV-1-infected individuals [1]. These initial findings were supported by subsequent histopathological confirmation [2, 3] indicating that opioid use can exacerbate the central nervous system (CNS) complications of HIV-1 infection. Despite some initial controversies discussed below, current studies now describe diminished cognitive function in HIV-1-infected individuals who preferentially abuse opiates even in patients who are receiving combined antiretroviral therapy (cART) therapy [6-8]. Prolonged heroin use exaggerates deficits in recall and working memory beyond impairments seen with HIV-associated neurocognitive disorders (HAND) (for definitions see [9-11]) alone [8]. Earlier controversies questioning the extent that opiate drug use worsens simian immunodeficiency virus (SIV)/simian-human chimeric immunodeficiency virus (SHIV) infection and neuropathogenesis may partially relate to the fact that some of the SIV/SHIV strains employed were not especially neurotrophic and that many of the studies failed to examine CNS pathology beyond CSF viral loads. An authoritative review covering opioid-SIV interactions appeared recently [12].
In the present review, the term “opiate” is used to refer to alkaloids derived from the opium poppy Papaver somniferum and includes opium and heroin [13]. Opiates act by mimicking endogenous “opioid” ligands and bind “opioid” receptors [14-20]. In the case of opiate drugs with abuse liability, they principally act by triggering the μ-opioid receptor (MOR) [13], and to a lesser extent δ- (DOR) and κ- (KOR) opioid receptors. Heroin is quickly deacetylated to morphine in the CNS and morphine is the main bioactive product of heroin in the brain [21, 22].
HIV-1 enters the brain early in the disease and establishes latent reservoirs in perivascular macrophages prior to the onset of HIV encephalitis (HIVE) [23]. Interestingly, preferential opiate abuse may selectively affect the turnover of the perivascular macrophage pool [4] and/or release cells chronically infected with HIV-1 from latency as assessed by increased LTR transactivation in a neuroblastoma cell line [24]. Opiates increase viral loads and hasten the progression and neuropathology in SIV models [25-29] (reviewed in [12]). Novel findings in SIV models also indicate that chronic opiate exposure can shape viral evolution [30-32].
Post-cART era studies find diminished cognitive function in HIV-1-infected individuals who preferentially abused heroin that is partially attributable to opioid abuse [6-8]. A recent study, examining the relationship of substance use history to neurocognitive impairment in HAND, indicated that “lifetime heroin dosage” correlated significantly with “poor recall and working memory” [8]. Since the participants in this study were currently abstaining from drug use, the findings indicate that heroin use can potentially result in lasting deficits to cognitive function in HIV-1-infected individuals [8]. A separate consideration is polysubstance abuse and the concept that opiate drug use interacts uniquely with other abuse substances to exacerbate HAND. For example, a rare fulminant encephalopathy with extensive basal ganglia involvement is associated with combined cocaine and heroin, i.e., “speedball”, use in a small subset of HIV-1-infected individuals [33]. Heroin, by virtue of immune suppression and increased HIV-1 replication, may worsen the inherent neurotoxic effects of cocaine and vice versa in neuroAIDS [33]. Nonetheless, some clinical studies have reported minimal or no neurocognitive differences between HIV-1-infected and uninfected drug abusers [34-36]. Clinical inconsistencies may be partially attributable to our lack of understanding of the mechanisms underlying drug and HIV-1 interactions and the spectrum of resultant comorbid manifestations [37-39]. Genetic risks for opiate abuse [40] or neuroAIDS, such as familial predisposition to dementia [41], MOR polymorphisms [42-44] and/or epigenetic changes in MOR [45], as well as polymorphisms in comorbid factors such as CCR5 [46-48], CCL2/MCP-1 [49], apolipoprotein ε (ApoE) allelic variations [50] (the ApoE4 allele has been linked to HIV-1 dementia and neuropathy [51, 52]) and prodynorphin [53-58] may also contribute to the complexity. In addition, street drug impurities [38], variable pharmacokinetics [59] and usage/dosage regimen [38], as well as the timing and duration of opiate exposure during the course of HIV-1 infection [39, 60], also are likely to determine the nature and severity of the comorbidity.
THE CNS IS PREFERENTIALLY SUSCEPTIBLE TO OPIATE DRUG-HIV-1 INTERACTIONS
The heightened vulnerability to opiate drug actions in HIV-1-infected individuals [39, 61-65] results from a coordinated and exaggerated response of glia, and especially astroglia. Not only does morphine directly affect the response of neurons [66, 67] (including human neurons [50, 68, 69]), but opiates can directly affect MOR-expressing astroglia [70-79], microglia [61, 66, 80-82], oligodendroglia [83], and glial precursors [84, 85]. Morphine’s unique actions in HIV-1-exposed astroglia, in particular, appear to drive spiraling, intercellular feedback loops with microglia and perivascular macrophages that increase and sustain inflammation [39, 71]. In fact, unlike other HIV-1-infected "end organs" [target organs], which also contain subsets of MOR-expressing resident and newly recruited macrophages, the brain differs in the unique and exaggerated response of astroglia to opioids [39]. This is especially apparent in striatal astrocytes, which show far more pronounced morphine-HIV-1 protein-induced cytokine production [70, 72] compared to astrocytes isolated from the cerebral cortex, cerebellum, or spinal cord [86], and may contribute to the enhanced neuropathology seen in regions of the basal ganglia.
NEUROTOXICITY WITH CHRONIC OPIATE EXPOSURE
With more sensitive and sophisticated approaches for assessing neuronal injury, emerging evidence suggests that sustained morphine exposure may be intrinsically neurotoxic. While there has been sporadic experimental evidence especially using cell culture models that opiates can be cytotoxic to neurons, as well as other cell types, only a few clinical studies reported modest astrogliosis in opiate abusers [87]. Rarely, severe astrogliosis is reported with heroin abuse [87-89] or in individuals inhaling volatilized heroin vapor [89, 90]. By contrast, in another study, dopaminergic function, as assessed by tyrosine hydroxylase (TH) terminals, was significantly reduced in the nucleus accumbens; while an index of serotonergic (5-hydroxyindoleacetic acid) function and TH was variably affected in the putamen and caudate nucleus [91]. Reports of increased perivascular infiltrates of lymphocytes and macrophages were also noted [92]. However, the nondescript nature of the gliosis in the above instances was potentially attributable to the physical and psychological "side effects" of addiction, including poor nutrition, poor health and lack/avoidance of medical care, and/or a marginal life-style [89, 90]. More recently, hyperphosphorylated tau was described in hippocampal neurons of a carefully characterized cohort of preferential opiate abusers [93]. In this cohort, the authors were careful to control for risk factors such as age and neurodegenerative or infectious diseases that could confound the interpretation of the deleterious effects of chronic opiate abuse per se [93]. Similar neuropathology characteristic of the aged brain involving tau hyperphosphorylation and increased amyloid and amyloid precursor disposition was seen in opiate abusers less than 40 years of age [94].
METHODS TO MEASURE NEUROTOXICITY
Cell culture combined with computer-guided, time-lapse microscopy enables dramatic increases in throughput (see [95-97]) using well-established repeated measures designs to examine dynamic changes in the same neurons over time [98-104]. For example, this strategy reveals subtle, but significant, neurodegenerative effects after 60 h of sustained morphine exposure [95] that are even more evident after 72 h [60]. These otherwise subtle morphine-induced neuron losses and synaptodendritic injury (in preparation) were less apparent when examining average changes in populations of neurons. Depending on the outcome measure, the heterogeneity within the same class of neuron from a particular brain region [e.g., see 105] can preclude studying average changes in populations of neurons. This is particularly true for examining subcellular changes in the genesis and degeneration of individual synapses, which by necessity rely on repeated-measure design strategies [98-104]. This approach also enables meaningful analysis of small numbers of cells (e.g., rare samples of human neurons) or to discriminate among subsets of neurons (e.g., differentiating transfected versus non-transfected neurons in the same culture dish [106]).
The advantage of using repeated measures time-lapse microscopy is that individual neurons (within a treatment group) are compared to themselves prior to and during treatment (these designs are a “block randomized” or “repeated measure” design). This eliminates intersubject variability and differences each time new cell cultures are established, and is fundamentally more sensitive than examining cell populations. It is a common approach in electrophysiological studies and has been used to study neuron differentiation and death [e.g., see 101, 107]. The assay permits the use of repeated measures ANOVA, rather than regular multi-way ANOVA, and provides far greater statistical power with a small number of experiments [95, 97, 108, 109]. Moreover, although the strategy is used to assay neuronal cell death, it is equally valuable in structural and functional assessments of non-lethal synaptodendritic alterations in neurons and glia. It is important to note, in a repeated measure analysis, significance is not reflected in the error bars, which do not correspond to the error term used in the statistical analyses. Statistically, the response of an individual neuron can be correlated to itself over time, and the repeated measures design extracts the individual variation in the same neurons over time from the error term, increasing the power to detect treatment differences [110].
NEUROAIDS IS GLIALLY-MEDIATED
HIV-1 infection in the brain is almost exclusively limited to microglia, latently infected astroglia [111], and astroglial progenitors [112]. Neuron injury or death is mainly through bystander effects as neurons, which lack the CD4 receptor, are not infected by the virus. Moreover, although endothelial cells and perivascular macrophages can become infected with HIV-1, intervening glia (especially astroglia) are typically present between infected endothelia or perivascular macrophages and neurons. By virtue of harboring HIV-1 or through aggressive attempts to control the infection, glia become the sources of toxins, which cause bystander effects on neurons. Thus, neuronal injury in HIV-1-infected individuals is caused by intercellular (glia-to-neuron) transfer of neurotoxic signals [38, 113-117].
SUBLETHAL NEURON CHANGES UNDERLIE HIV-ASSOCIATED NEUROCOGNITIVE DISORDERS
The dendritic arborizations and synaptic connectivity of neurons are reduced in neuroAIDS [118] and the losses in connectivity are the likely substrates of behavioral and cognitive impairment in HAND [119-123]. Similarly, dendritic pruning and synaptic culling have been suggested as underlying comorbid opiate drug-HIV-1 interactive deficits in CNS function [2, 124]. Our recently published [124] and unpublished data suggest that synaptodendritic injury is highly correlated with (and likely underlies) the exaggerated neurobehavioral defects seen in opioid abuse-HIV-1 comorbidity. Although cumulative and sustained sublethal injury may ultimately result in reduced survival [124], the signaling events underlying opiate and HIV-1-induced sublethal injury may differ qualitatively from those regulating death, are potentially reversible, and more amenable to treatment and recovery.
If neuronal injury is associated with synaptodendritic injury, then why is neuron death typically described as an endpoint? Initial histopathological observations displayed evidence of neuronal and glial cell death at the end stages of neuroAIDS, especially in the pre-cART era. Early in vitro studies attempted to model the lethal changes. As noted, however, subsequent evidence increasingly supports the notion that neurobehavioral deficits are accompanied by sublethal synaptodendritic injury and a loss of neural circuitry [119-123]. While cumulative sublethal neuron injury may lead to neuronal death, ongoing studies from multiple laboratories clearly indicate that the extent of the damage is directly related to the concentration/dosage of HIV-1 proteins or HIV-1 titer. In fact, our published and unpublished findings indicate that synaptodendritic injury may be evident following exposure to concentrations of HIV-1 Tat that are 2-3 orders of magnitude less than required to induce rapid death of neurons (within 24-72 h) [60, 67, 95, 96, 106, 108, 109]. Lastly, even at high concentrations of HIV-1 proteins, sustained synaptodendritic injury precedes neuron death. We propose that as the concentrations of HIV-1 virions and HIV-1 Tat and gp120 proteins are reduced to levels normally seen in the HIV-1-infected CNS, the predominant neuronal alterations will be synaptic culling and pathophysiological changes in dendrites, without precipitous neuron death. Importantly, the sublethal changes are likely to be reversible and therefore highly amenable to therapeutic intervention.
SYNAPTODENDRITIC ORIGINS OF EXCITOTOXIC INJURY, METABOLIC COMPROMISE, AND [Ca2+]i OVERLOAD
HIV-1 gp120 and Tat overactivate glutamate receptors and are excitotoxic [125]. Tat [126-130] and gp120 [131-136] have been proposed to activate NMDA receptors (NMDARs) through direct and indirect mechanisms. In addition to the activation of NMDARs by Tat [126, 137], the virotoxin also interrupts mitochondrial function [138], ATP production [50, 139], and can cause focal, transient (“hit and run” [140]) disruptions to neuronal homeostasis through mechanisms responsible for dendrotoxicity and synaptic losses [140-142]. These may be compartmentalized to dendrites, axons, or perhaps specific synapses [143, 144]. Focal swelling [145] is accompanied by a disruption of ion homeostasis and ATP production [138, 146] causing a failure of neuritic transport [147-149]. Autophagosomes form at sites with collapsed cytoskeletal proteins and dendritic swelling [150], as do focal elevations in cleaved caspase-3 [151], respectively, suggesting roles for autophagy and “synaptic apoptosis” [152, 153]. Thus, Tat [154-156], gp120 [157, 158], and/or opiates [159] may trigger synaptodendritic injury through localized contributions from caspases, autophagic, and/or the ER-stress/unfolded protein response (UPR) effectors.
OPIOID EXPOSURE IS LIKELY TO EXACERBATE THE EXCITOTOXIC EFFECTS OF HIV-1 AT THE LEVEL OF THE SYNAPSE
Endogenous opioids [95, 160, 161], as well as opiate drugs with abuse liability [162-167], have long-been known to reduce both the complexity of dendrites and the density of dendritic spines in a variety of brain regions. Interrelated studies describe how morphine can cull spines in pyramidal neurons from the cerebral cortex through a series of events involving NeuroD, microRNA-190, and Ca2+/calmodulin-dependent kinase II (CaMKII) [103, 168-170]. The ability of opiate drugs to reduce synaptic interconnections is highly selective. MOR-dependent reductions in dendritic spines display "agonist selective" responses. The "biased agonism" [102, 103] is reliant on the differential coupling of MOR to Gα, Gβγ, and/or β-arrestin by different MOR agonists. For example, morphine readily causes spine retractions, while fentanyl, a MOR agonist with much higher selectivity for MOR than morphine, fails to reduce spine numbers [170].
Morphine may potentially act through several MOR-dependent mechanisms to potentiate the excitotoxic effects of HIV-1; each will be explored below. Initially, morphine via Gβγ increases the activity of G-protein-gated inwardly rectifying K+ (GIRK/Kir3) channels making the neuron less excitable [171]. However, with chronic activation accompanied by tolerance (and dependence), MORs uncouple from the inwardly rectifying K+ channels [16], resulting in increased neuron excitability despite the sustained presence of opioid drugs. Morphine may also indirectly increase neuron excitability through actions in astroglia. Morphine can augment [Ca2+]i via Gβγ [172, 173] (or perhaps uniquely via Gq/11-α in astroglia [76]) to drive increases in phospholipase C (PLC), further increasing excitation. The resultant IP3-dependent increases in [Ca2+]i potentiate Ca2+-induced Ca2+ release (or regenerative Ca2+) via ryanodine receptors in astroglia [76]. HIV-1 Tat can similarly increase [Ca2+]i in astroglia [174] and restrict glutamate uptake [95], but is also a potent activator of NF-κB [70, 175] resulting in cytokine and chemokine release by astroglia [70-72, 176, 177]. In combination, morphine can potentiate Tat-induced increases in astroglial [Ca2+]i [72], reactive oxygen species (ROS) production [95], and IL-6, RANTES, and MCP-1 release [72], while causing subadditive restrictions in glutamate uptake [95]. Gp120 can also increase [Ca2+]i [86, 178-180], alter cytokine expression [181], and limit glutamate uptake by astroglia [60, 179, 180, 182, 183], and likely interacts with opiate drugs through one or more of these mechanisms to increase neuron injury [60, 69]. By attenuating the presynaptic activity of inhibitory GABAergic interneurons, morphine can disinhibit postsynaptic target neurons thereby decreasing their excitotoxic threshold [184]. Morphine can cause significant increases in glutamate release by microglia [80], beyond that seen with Tat treatment alone [80]. Lastly, we speculate that the excessive astroglial response further exaggerates the intrinsic microglial response to HIV-1 [71], creating an opioid-driven, astroglial-to-microglial escalating feedback loop, which increases neuron injury [39, 61, 63, 80].
In cerebral cortical neurons, morphine decreases NeuroD phosphorylation, which increases CaMKII phosphorylation and maintains excitatory dendritic spines [170]. CaMKII is an important target in neuroAIDS [185] and potentially pivotal in convergent opiate drug and HIV-1 interactions. The loss of spines impedes glutamate signals through α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor (AMPAR) and NMDAR subtypes [102-104]. Excitotoxic injury reportedly originates from “extrasynaptic” NMDARs or via deleterious NMDAR subunit conformations [186, 187]. Alternatively, “synaptic” NMDAR signaling at excitatory PSD-95+ dendritic spines is reportedly neuroprotective [188, 188-190, 190, 191], and associated with specific subunit configurations, including NR3A [192] and NR2A [186] (through activation of phosphoinositide 3-kinase (PI3K)/Akt (protein kinase B or PKB), extracellular signal-regulated kinase (ERK), glycogen synthase kinase 3β, and/or FOXO [193-197]). Assuming chronic morphine exposure affects striatal neurons similarly, reductions in "favorable" spines are likely to be detrimental especially when confronted by an excitotoxic Tat or gp120 challenge (Fig. 1). This may reveal a potential mechanism by which opiate drugs would aggravate the negative consequences of HIV-1 in medium spiny neurons.
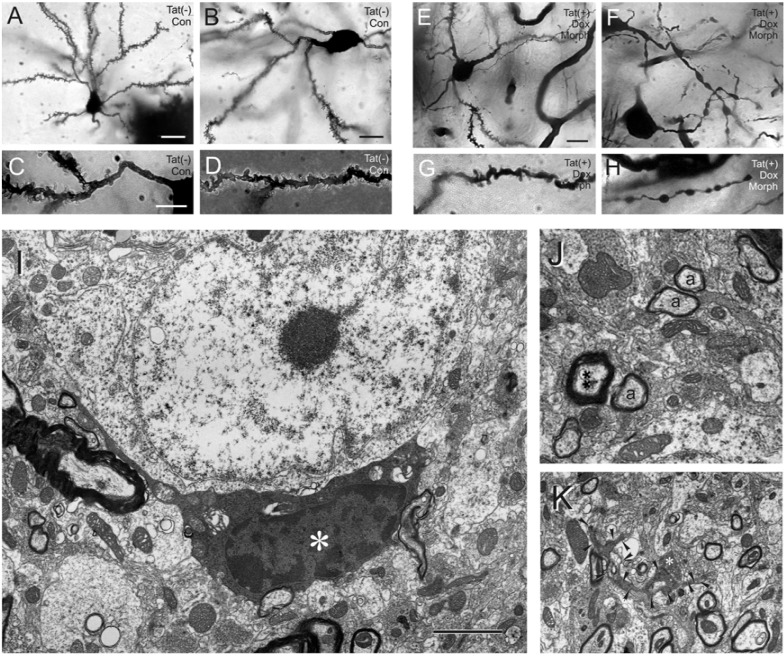
Fig. (1).
Golgi-impregnations of striatal neurons and dendrites from control Tat(-) (A-D) and Tat(+) (E-H) transgenic mice at 7 days following combined morphine exposure and Tat induction. The medium spiny neurons from Tat(-) mice are morphologically normal (A, B), and possess normal complements of spines on proximal (C) and distal (D) dendritic segments. By contrast, combined morphine exposure and Tat induction caused severe deficits in spine numbers and synaptodendritic injury (E-H), including severe dendritic varicosities and degeneration (F-H), that was worse than with morphine exposure or Tat induction alone (see reference [82] for detailed explanation); scale bar in A = 20 µm; the scale bars in B, C, E = 10 µm; C, D, F, G, H are the same magnification (note, figures E-H above correspond to figures R-U in ref [124]). Electron micrographs showing an abnormal oligodendrocyte (I) and myelin (J), and a degenerating dendrite in the striata of HIV-1 transgenic mice following 7 days of continuous Tat induction and co-exposure to morphine (I-K). An oligodendrocyte with abnormally dense cytoplasm with excessive vacuoles and condensed nucleoplasm (*) in close association with several myelinated axons (I); scale bar = 2 µm. Axons (**) that appear to be hypermyelinated compared to nearby axons of similar diameter (a) are quite common in the striata of Tat transgenic mice (J). Normally, myelin thickness is directly proportional to the diameter of the axon and is constant for axons of a particular diameter (J); the relationship is referred to as the g-ratio [306]. An electron dense dendrite (arrowheads) with a single presynaptic contact (*) appears to be degenerating (K); in all the above instances (E-K) morphine was continuously administered via a time-release pelleted implant (5 mg per day) (see [82]). (A-H): Reprinted from reference [124]; Copyright (2010), with permission from Elsevier and the American Society for Investigative Pathology.
Besides direct actions on the medium spiny neurons themselves, opioids can act by synergistically disrupting the function of MOR-expressing astroglia [70-72] and/or microglia [61, 80, 81]. In addition, morphine can excite dopamine neurons projecting from the ventral tegmental area (VTA) to striatal spiny neurons by hyperpolarizing inhibitory γ-aminobutyric acid (GABA)- expressing interneurons in the VTA [184]. Dopaminergic afferents into striatum from substantia nigra and VTA are markedly altered in neuroAIDS [65, 198-205], and are important targets for substance abuse [65, 200, 201, 206, 207]. Following chronic morphine exposure, GABA transporter function is disrupted leading to hyperexcitability of GABAergic neurons upon precipitated morphine withdrawal [208]. Assuming aspects of these findings can be generalized to striatal function, this would suggest that chronic opiate exposure causes sufficient maladaptive changes in both GABAergic and glutamatergic responsiveness (both presynaptically [209] and postsynaptically) to result in maladaptive neuron injury when confronted with HIV-1 Tat or gp120 [115, 124-126, 210].
Gp120 derived from CCR5 (R5)- or CXCR4 (X4)- tropic HIV-1 strains preferentially use CCR5 or CXCR4, respectively, as co-receptors for viral entry; dual-tropic strains can use either receptor. All strains are neurotoxic, but activate their receptors in specific ways that can induce effects similar to and/or different from those of the natural ligand [117]. This biased agonism can change the downstream signaling from beneficial or neutral to neurotoxic. For example, in the study cited above gp120 binding to CCR5 produces a neurotoxic response through activation of p38 MAPK even though CCR5’s natural β-chemokine ligands are neuroprotective. In comparison, CXCR4 activation by either gp120 or stromal cell-derived factor-1 (SDF-1 or CXCL12) leads to neuronal death. Others have reported that at a lower chemokine concentration the effects of SDF-1 and X4-tropic gp120 diverge, with SDF-1 phosphorylating both pro-survival (p-Akt) and pro-apoptotic c-Jun-terminal kinase (p-JNK) signals, whereas gp120 selectively activates pro-apoptotic signaling [211]. Strain differences have also been revealed in the production of brain-derived neurotrophic factor, which is suppressed by X4-tropic gp120 and enhanced by R5-tropic gp120 [212].
We found fundamental differences in the interaction of morphine with X4- compared to R5-tropic strains of HIV-1 in an infectious model of hepatitis C virus [213]. Prompted by these findings, we questioned whether morphine would interact with HIV-1 gp120 to cause neurotoxicity in a strain-dependent manner [60]. Interestingly, we found that morphine had no interactive cytotoxic effects with R5-tropic gp120ADA, while morphine caused a highly reproducible transient and coordinated acceleration of X4-tropic gp120IIIB neurotoxicity. By contrast, there was a sustained additive neurotoxicity with combined exposure to morphine and bitropic gp120MN [60]. While it is tempting to conclude that the coincident activation of both X4 and R5 co-receptors by gp120 imparts greater toxicity, it is equally reasonable to assume that gp120 displays agonist selective or biased agonism properties and that gp120 from each HIV-1 variant has a somewhat different effect. Lastly, although additional study is needed, in instances where morphine and gp120 co-exposure fails to show toxic interactions, a sound assumption may be that they are acting through a similar mechanism. This seems especially plausible considering the potential for interactions between MOR and CXCR4 or CCR5.
How opioids might directly (or indirectly through actions on glia) worsen the neurotoxic effects of gp120? One possibility is through overlapping actions at K+ channels. CXCR4-driven Gαq increases outwardly directed K+ currents [214]. Since chronic opioid exposure can lead to a loss in MOR coupling to inwardly directed K+ currents in neurons [16], and through mechanisms that may be partially dependent on glia [16], an exaggerated bias toward the outward movement of K+ may partially explain the interactive neurotoxicity seen with chronic opioid and gp120MN co-exposure [60]. Gp120 appears to induce apoptosis in most neuron types studied through a caspase-3-dependent process [109, 115, 214-219]. How outwardly directed K+ activates caspase-3 is less certain [214], but is likely to contribute to gp120-induced imbalances in CXCR4 or CCR5 signaling in neurons through reduced ERK activation [211, 220-222] with accompanying overactivation of p38 [69, 108, 117, 223], JNK [108, 220, 224], and/or other MAPKs. More recently, Haughey et al. [225] described gp120-induced clustering of NMDARs in the dendrites of hippocampal neurons and subsequent declines in the excitotoxic threshold through facilitated increases in [Ca2+]i. The effects of gp120 on membrane clustering were partially blocked by the irreversible antagonist of CXCR4, plerixafor hydrochloride or AMD3100, indicating the involvement of CXCR4 signaling in NMDAR clustering [225]. Considering the known interaction(s) between MOR and CXCR4 [226-228], as well as maladaptive DOR-CXCR4 interactions unmasked in MOR knockout glia [229], the convergent effects represent a potential site where opiate drugs and gp120 interface in neuroAIDS [60, 230-232].
Meucci and co-workers propose that the ferritin heavy chain subunit can influence opiate drug toxicity by negatively regulating CXCR4 signals via Akt (PKB) [231, 232]. They go on to offer that otherwise neuroprotective CXCL12/SDF-1-CXCR4 homeostatic mechanisms normally present can be disrupted by increased ferritin heavy chain subunit expression [231]. This has considerable implications for X4-tropic gp120 and morphine neurotoxic interactions [60], implying that the degree of neurotoxicity may be “tunable” or modifiable by environmental or metabolic factors affecting the expression or trafficking of the ferritin heavy chain subunit. In addition, this may also be of considerable importance for opiate and X4-tropic gp120 interactive toxicity in oligodendroglia (Zou and Knapp, unpublished), since oligodendroglia are exquisitely dependent on ferritin heavy chain subunit expression for iron regulation, myelination, and their survival [233-235].
OPIOID DRUGS EXACERBATE HIV-1-INDUCED NEURON DEATH THROUGH ACTIONS IN MOR-EXPRESSING GLIA
In considering the convergence of HIV-1 and drug abuse, we proposed several years ago that critical interfacing of abused substances with HIV-1 occurs in glia [39]. More recent examination of opiate drug interactions with HIV-1 Tat protein provides further support for this concept and additionally suggests that the deleterious effects of opiates are principally mediated through direct actions at MOR-expressing astroglia and microglia (Fig. 2) [95]. The near exclusive role of glia infers that the principal site(s) of opiate actions that exacerbate neuroAIDS occur directly on glia and not neurons. This crucial observation implies that key neurotoxic signals originate from opioid receptor-expressing glia. Lastly, despite the involvement of glia in interactive striatal neuron death [60, 95], it remains uncertain whether opiates and HIV-1 can directly affect more subtle forms of synaptodendritic injury. For example, morphine can directly affect spine reductions in cerebral cortical neurons [102, 103, 169, 236]; however, it is uncertain the extent to which morphine would similarly influence spine reductions in medium spiny neurons in the striatum or in pyramidal neurons within the CA1 region of the hippocampus. Defining the neural cell targets and aberrant glial-to-neuron signals (and vice versa) that mediate neuronal dysfunction and death are central toward understanding the pathogenesis of neuroAIDS in the context of opiate abuse.
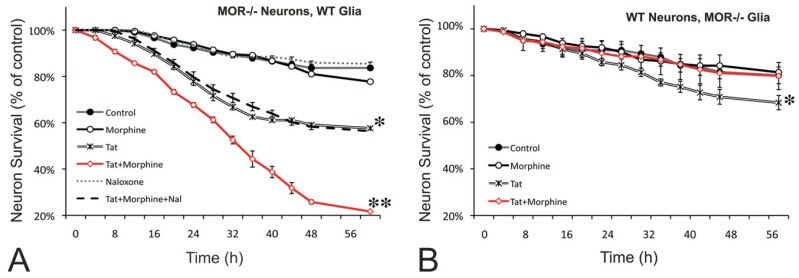
Fig. (2).
Morphine and HIV-1 Tat-induced interactive neurotoxicity is mediated by MOR-expressing glia. Synergistic HIV-1 Tat and morphine neurotoxicity was only evident when MOR-deficient striatal neurons were co-cultured with wild-type glia (**P < 0.01 versus all other groups, red line) (A), but not when wild-type neurons were co-cultured with MOR-deficient glia (B, red line). Co-cultures consisted of neurons and glia (~10:1 ratio of astroglia:microglia) derived from the striata of wild-type or MOR knockout mice. Note that HIV-1 Tat alone was neurotoxic to striatal neurons irrespective of MOR genotype (*P < 0.05 vs untreated controls, double lines) (see [95] for further explanation). Reprinted with permission from reference [95]; Copyright 2011 Oxford University Press.
Opioid signaling is highly contextual and fundamentally different in each cell type [237-239]. For this reason, defining the distinct and cell specific targets that are engaged by opiates to trigger CNS inflammation and neuronal injury is critical toward understanding how opiate abuse worsens the disease. A contributing factor in differential opioid signaling may be the vast amount of alternative splicing of MOR [239], and the variant splicing differences in different cell types is a largely unexplored area [45]. Given that subpopulations of astroglia and microglia (Fig. 3), as well as neurons can express MOR, future evaluation of the MOR variant expression profile in these individual cell types is of great importance. Furthermore, defining differential signaling cascades associated with each MOR variant, and determining whether HIV-1 affects expression or preferentially interacts with particular MOR variants in humans (Fig. 3), may shed additional light on the intracellular pathways in glia that trigger glia-mediated neurotoxicity in HIV-1-infected opiate abusers.
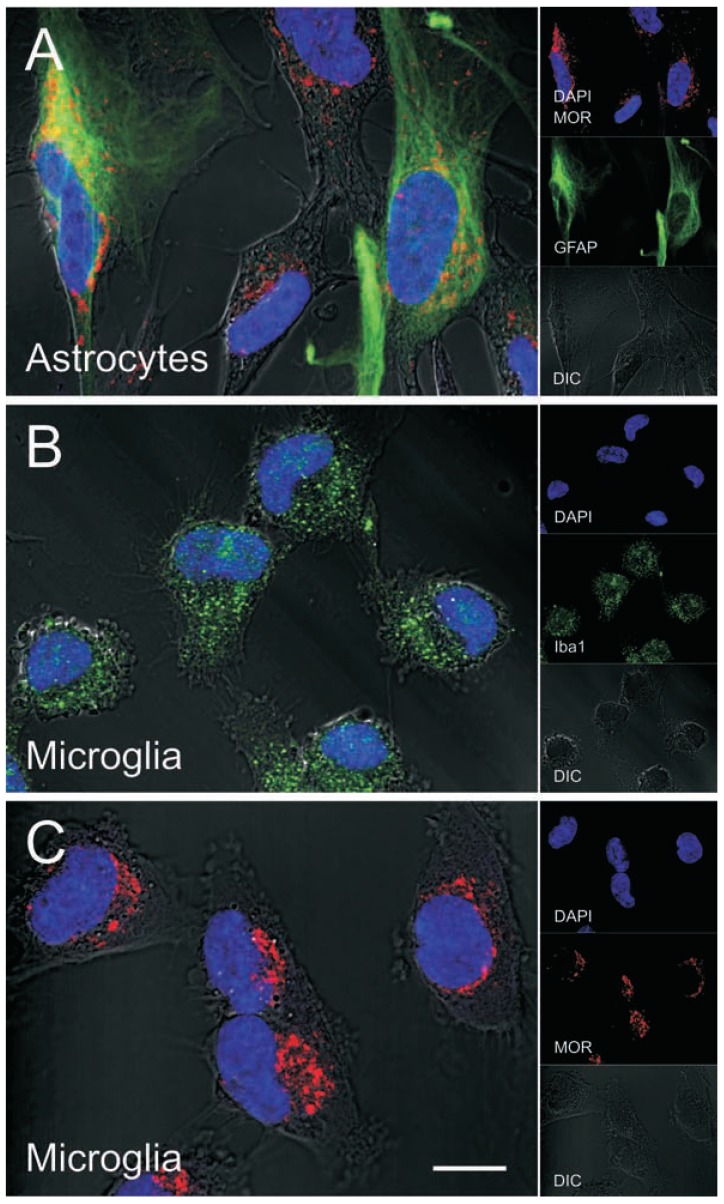
Fig. (3).
MOR immunofluorescence in human astrocytes and microglia. MOR and GFAP co-localization in subsets of primary human astrocytes (A). Astrocytes (catalog number 1800) were obtained from ScienCell Research Laboratories and cultured for 7-10 days according to the manufacturer's instructions. Iba-1 (B) and MOR (C) immunofluorescence in subpopulations of primary human microglia (ScienCell; catalog number 1900-f1) cultured as described for astrocytes. Cells were fixed with 3.7% paraformaldehyde, permeabilized with 0.5% Triton X-100, immuno-labeled, nuclei were stained with DAPI (blue), and images were enhanced by differential interference contrast (DIC) optics. Primary antibodies used were MOR (epitope within amino acids 1-15 of the N-terminus of human MOR) (Novus Biologicals, catalog number NBP1-31180), GFAP (Millipore, catalog number MAB360), and Iba-1 (Wako, catalog number 019-19741); all at a 1:200 dilution. Images were acquired using a Zeiss LSM 700 laser scanning confocal microscope at 63x (1.42 NA) magnification and ZEN 2010 software (Carl Zeiss Inc, Thornwood, NY), and edited using ZEN 2009 Light Edition (Zeiss) and Adobe Photoshop CS3 Extended 10.0 software (Adobe Systems, Inc.).
HOW MIGHT OPIATE-EXPOSED GLIA MEDIATE BYSTANDER TOXICITY IN NEURONS?
Critical Importance of Neurotoxic Intercellular Signals
If, as noted above, HIV-1 is a glia-specific disease and opiate drug abuse imparts significant neurotoxicity through actions in MOR-expressing astroglia and microglia, then identifying the specific, bidirectional intercellular glia-neuron signaling events driving bystander neuron injury and death is of critical importance for understanding the pathogenesis. What is the nature of the intercellular signals that might be affected by opiates? Many glially derived signals that are likely to affect neuron injury or the maintenance of synapses have been identified [39, 60, 95, 96, 151, 240-243]. Several factors have been assumed to be involved in opiate drug toxicity because they are known to be modified by opiate abuse and because of their established importance in experimental models or clinical studies of HIV-1 neuropathology, but without direct testing for an interaction. Prime examples of these are excessive extracellular glutamate, extracellular ROS, and reactive nitrogen species, long proposed as "known suspects" in HIV-1 neuropathogenesis [115, 116, 244-247]. When we directly tested whether these “presumed” interactions were in fact operative in HIV-1 Tat and opiate-mediated neurotoxicity, all appear to be complicit to varying degrees, but none actually drive the interactive pathogenesis.
Glutamate.
Glutamate has long been thought to contribute to the generalized excitotoxicity attributed to HIV-1-induced neuron injury and/or death. These effects are not limited to a particular viral protein since HIV-1 gp120, Tat, and intact virions can all contribute to excess glutamate in the extracellular milieu and hyperexcitability in neurons. Gp120 and intact virions restrict glutamate uptake by astroglia through inhibition of excitatory amino acid transporter 2 (EAAT2) [183]. EAAT1 is altered by HIVE and may respond to cART-induced reductions in encephalitis [248, 249]. The activation of the Na+/H+ exchanger is another potential target for HIV-1 [250].
Exposure to either morphine or HIV-1 Tat by itself inhibits buffering of a glutamate challenge, and this occurs largely through effects on astrocytes [60, 95]. Although combined Tat and morphine tended to further reduce glutamate-buffering ability, the interaction was not significant [95]. Therefore, reduced glutamate buffering did not correlate with increases in neuron death due to morphine and Tat co-exposure. Gp120 and morphine similarly failed to block glutamate uptake in an additive manner, although either treatment by itself did prevent enriched astroglial cultures from responding appropriately to a glutamate challenge [60].
In all of the above studies, excess glutamate was applied to cultures enriched in astroglia (90.2 ± 0.4%) and microglia (8.8 ± 0.6%). Under normal physiologic conditions, EAAT1 and EAAT2 are predominantly expressed by astroglia and minimally expressed by microglia [251, 252]. However, specific proinflammatory triggers, including infection with SIVmac251 in non-human primates, can increase the expression of EAAT1 and EAAT2 in macrophages or microglia [253]. Interestingly, the particular inflammatory mediators involved can differ among species including humans and mice (reviewed in [251]), and effects in murine microglia may not be generalizable to humans. Since astroglia outnumber microglia about 10:1 in our striatal mixed-glial cultures [95], astroglia were assumed to be the principal cell type involved. Moreover, since there were no net increases in extracellular glutamate in the presence of the EAAT1-5 inhibitor, DL-threo-b-benzyloxyaspartate (DL-TBOA), or the EAAT1-3 inhibitor, (2S, 3S)-3-[3-[4 (trifluoromethyl) benzoylamino] benzyloxy] aspartate (TFB-TBOA), it is assumed that the inability of glia to deplete the glutamate increases caused by Tat ± morphine exposure was due to restricted uptake [95]. Despite our efforts to distinguish between reduced uptake and enhanced release, the assay may be relatively insensitive to transient and subtle alterations in glutamate release [254]. For example, under appropriate conditions, the activation of CXCR4 can cause glutamate release from astroglia [254]. Moreover, this assay is obviously unable to discriminate synaptically versus extrasynaptically directed glutamate release.
Regarding disrupted astroglial function, a compelling question regards the consequences of opiate abuse and HIV-1 on the function of the “tripartite synapse” and “gliotransmission”. Evidence is clear that astroglia affect synaptic transmission and plasticity [255-258]. Glutamate and D-serine released from astrocytes at the synapse are essential for synaptic plasticity [259], as defined by changes in long-term potentiation in hippocampal neurons (reviewed in [256]). By restricting EAATs that remove extracellular glutamate [60, 95], HIV-1 proteins and opiates are likely to affect gliotransmission indirectly, as well as permit excess glutamate to accumulate at extrasynaptic sites. Moreover, chronic morphine exposure significantly depresses EAAT2 expression in the striatum and hippocampus [260], and EAAT1 and EAAT2 expression in the spinal cord [261], while opiate withdrawal dramatically increases EAAT2 transcripts in the striatum [260].
While the net consequences of opiate drug and HIV-1-induced alterations in the response of astroglia to extracellular glutamate may be largely attributable to EAAT1 and EAAT2 function, the glial response may not be limited to altered EAAT function and appears to differ among glial types. By contrast to observations in cultures containing a large proportion of astrocytes, in cultures of isolated microglia, HIV-1 Tat exposure markedly increases glutamate secretion, while morphine co-exposure can significantly increase glutamate secretion above levels seen with Tat alone through actions involving the xc- cystine-glutamate antiporter [80]. Lastly, as noted, EAAT1 and EAAT2 are minimally functional in resting microglia, but are inducible with immune activation [251]. The extent that more protracted exposure to opiates or HIV-1 proteins upregulates or fails to upregulate EAAT1 and EAAT2 expression in microglia is uncertain but a potentially important consideration.
Cytokines and chemokines.
The role of cytokines, and especially chemokines, in intercellular signaling from infected microglia to neurons in neuroAIDS is well established and has been extensively reviewed elsewhere [20, 115, 262-271]. Based on these and other findings within the CNS, we tested whether opioids might affect the release of pro-inflammatory cytokines and chemokines by astroglia and microglia exposed to HIV-1 proteins. The findings indicated that opioids could accelerate and enhance the release of cytokines and chemokines caused by HIV-1 Tat [71, 72, 124], as well as gp120 exposure in some instances [86, 181, 272]. Much of this data has been reviewed previously [13, 39].
Elevated levels of fractalkine/CX3CR1 have been found in the brains and CSF of patients with HIVE, and have been localized to both neurons and astroglia [273, 274]. These and other findings suggest that elevations in fractalkine by neurons facilitate interactions with CNS immune cells and it was demonstrated that moderate levels of exogenously administered fractalkine were able to protect against Tat or gp120 mediated neurotoxicity [222, 274, 275]. We recently reported that exogenous fractalkine/CX3CL1 can be neuroprotective against the deleterious effects of morphine and HIV-1 Tat co-exposure [96]. Collectively, the above findings suggest a potential therapeutic course for fractalkine in neuroAIDS. Although the cellular mechanisms underlying the observed neuroprotection are not certain, findings that exogenous fractalkine reduces microglial motility and fails to protect neurons co-cultured with Cx3cr1-/- mixed glia suggest that fractalkine acts by interfering with toxic microglial-neuron interactions. In addition to its blockade of Tat and morphine mediated neurotoxicity, fractalkine and its receptor have more direct interactions with gp120 [222]. CX3CR1 acts as a co-receptor for viral entry [276], and thus fractalkine is able to displace gp120 on the fractalkine receptor to prevent microglial activation. Additionally, CX3CL1-CX3CR1 interactions can attenuate gp120-mediated neurotoxicity by preventing microglial activation and upregulation of pro-survival factors such as p-Akt [222].
Glial heterogeneity.
Astroglia and microglia are phenotypically diverse in terms of their expression of opioid system peptides and receptors. Subsets of astrocytes can express MOR, DOR, and/or KOR [73, 75, 76, 81, 277, 278], as well as the proenkephalin opioid gene and proenkephalin-derived peptides, including Met-enkephalin, Leu-enkephalin, and partially processed enkephalin precursors [79, 279-284]. The heterogeneity within astroglia is not limited to the opioid system, as other neurochemical systems similarly show a high degree of phenotypic diversity [285-289], a degree that is perhaps only rivaled by neurons. Glial heterogeneity exists even within a single brain region. For example, astroglia in the striatum vary individually in their expression of MOR, DOR, and KOR [73]. Heterogeneity in the expression of endothelin-1, and α1-adrenergic and muscarinic [290] receptors is present among astroglia in other brain regions. Astroglia also display significant regional differences in their functional response to HIV-1 Tat and gp120 (Fig. 4).
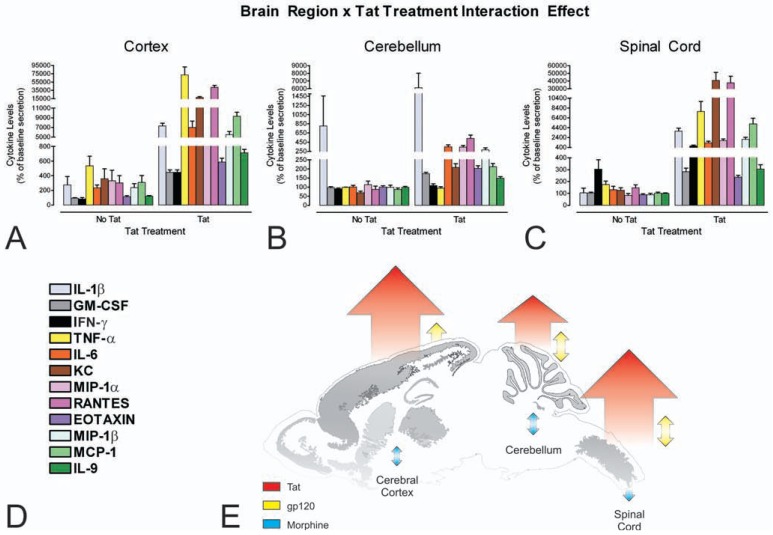
Fig. (4).
Astroglia isolated from the cerebral cortex (A), cerebellum (B), and spinal cord (C) display significant regional differences in the pattern of cytokine release in response to HIV-1 Tat in vitro (A-C). Interestingly, the pattern of cytokine release in response to Tat paralleled the incidence of HIV-1-related neuropathology in the brain and spinal cord (see reference [86]). Striatal astrocytes, analyzed as part of another study, show a far more dramatic interaction between HIV-1 Tat and morphine [72]. Cytokines and chemokines were analyzed simultaneously by multiplex suspension array assays [86]; legend provided in (D). Overall responses to HIV-1 Tat, gp120 and morphine across brain regions are summarized (E) (see text and reference [86] for further explanation). Reprinted with permission from reference [86]. Copyright 2010 American Chemical Society.
Unlike neurons, astroglial characteristics appear modifiable by adjacent neurons and perhaps other regional and extrinsic cues within the extracellular milieu [287, 291, 292] (Fig. 5). For instance, intrastriatal injection of Tat significantly increases the number of MOR immunoreactive astroglia at 48 h post-injection [72], while the proportion of MOR immunoreactive neurons remains unchanged (Hauser, unpublished). The diversity and plasticity of receptor expression by astrocytes is not limited to neurotransmitter receptors. Pattern-recognition receptors (PRRs), which recognize conserved microbial molecular motifs and are critical components of the innate immune system, show considerable diversity in astroglia. PRRs expressed by astrocytes include multiple members of the Toll-like receptor (TLR) family including TLR2, TLR3, TLR4, and TLR9 [106, 293]. Importantly, exposure to HIV-1 and/or opiates can alter the expression of TLR2 and TLR9, which are important in the host defense response to HIV-1. Chronic opiate exposure may predispose the CNS to HIV-1 infection by suppressing the innate immune response and by inhibiting TLR9 expression by astrocytes [293]. Morphine suppresses the response of alveolar macrophages against Streptococcus pneumoniae pathogenicity by altering TLR9-dependent NF-κB signaling [294]. Morphine reportedly can induce apoptosis in neurons via TLR2 [295]. Recently, morphine has been proposed to increase nociception and inflammation through a non-canonical mechanism involving direct interactions with TLR4 in glia [296, 297], although the detailed molecular mechanisms underlying the interactions remain to be elucidated. Collectively, the above results suggest that opiate drugs can affect the innate immune response through MOR-dependent alterations in one or more TLR signaling pathways and perhaps via novel MOR-independent actions at TLR4.
Fig. (5).
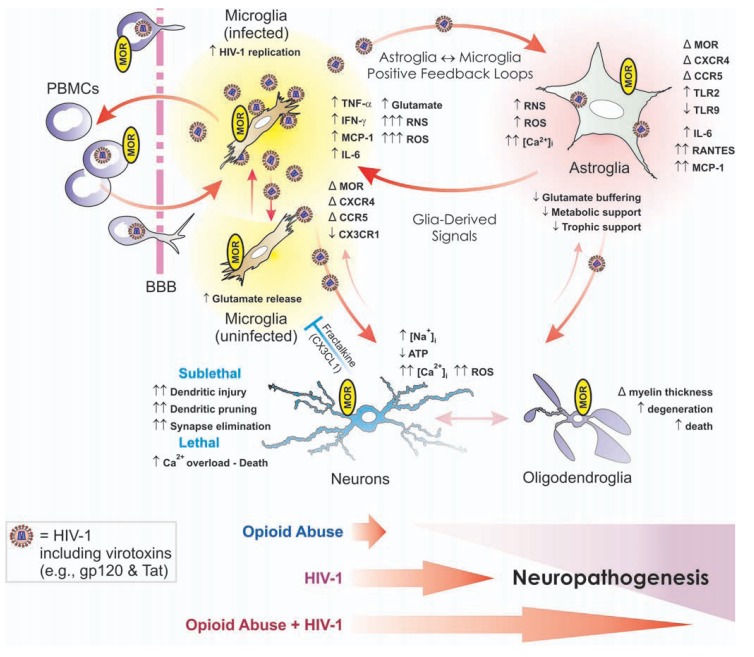
Opiate drugs exacerbate HIV-1 neuropathogenesis through direct actions on glia, especially microglia and astroglia, in addition to affecting neurons. In the CNS, HIV-1 infects microglia, and to a lesser extent astroglia, causing the production of reactive oxygen and nitrogen species (ROS and RNS, respectively), pro-inflammatory cytokines, and the release of HIV-1 proteins such as gp120 and Tat, which promote inflammation and cytotoxicity in bystander neurons and glia. Chronic opiate abuse by itself can cause some neuropathology (e.g., see [93]); however, in HIV-1-infected individuals opiates can potentiate many of the pathophysiological effects of the disease—especially in the central nervous system. Multiple neuronal and glial types can express the µ-opioid receptor (MOR). Many of the neurodegenerative effects of opioid-driven synergy arise through direct actions on microglia and astroglia. In fact, evidence suggests that reverberating inflammatory/cytotoxic positive feedback signaling between HIV-1-infected microglia and astroglia is exacerbated by opiate exposure—revealing novel targets for therapeutic intervention in opioid drug abuse and HIV-1 comorbidity. Abbreviations: α-chemokine “C-X-C” receptor 4 (CXCR4); altered or changed (Δ); β-chemokine “C-C” receptor 5 (CCR5); blood-brain barrier (BBB); decreased (↓); fractalkine (CX3CL1); fractalkine receptor (CX3CR1); increased (↑); interferon-γ (IFN-γ); interleukin-6 (IL-6); intracellular Ca2+ concentration ([Ca2+]i); intracellular sodium concentration ([Na+]i); monocyte chemoattractant protein-1 (MCP-1 [or CCL2]); peripheral blood mononuclear cells (PBMCs); regulated upon activation, normal T-cell expressed, and secreted (RANTES [or CCL5]); Toll-like receptor (TLR). Fractalkine released by neurons (and astroglia) can be neuroprotective by limiting the neurotoxic actions of microglia (blue “┴”); red arrows suggest pro-inflammatory/cytotoxic interactions. Modified and reprinted from reference [307], Copyright (2006), with permission from Springer.
Oligodendroglia and demyelination.
Oligodendroglia within the striatum are highly sensitive to the effects of morphine in HIV-1 Tat transgenic mice [83]. Striatal oligodendrocytes are the only cell type in the Tat transgenic mice to die, albeit at very low (~1.5%) rates, within 2 to 7 d of combined morphine exposure and Tat induction [83] (Fig. 1). Besides cell death per se, there is considerable evidence of cellular degeneration in Golgi silver-impregnated oligodendroglia, and a reduction in the number and extent of myelinating processes in a subset of cells [83]. The effects of Tat and morphine on oligodendrocytes are likely to be direct. Unlike astrocytes, oligodendrocytes can express NMDARs [298-300], a molecular target of HIV-1 Tat in neurons [126, 130, 137]. Furthermore, oligodendrocytes can express MOR and KOR [277, 279, 301], and immature oligodendrocytes appear to be particularly sensitive to the effects of MOR and KOR activation [302-305]. Perinatal exposure to buprenorphine, a partial MOR agonist and partial KOR antagonist, results in aberrant myelin g-ratios [303], indicating an inappropriate relationship between myelin thickness:axon diameter and inferring that intercellular neuron-to-oligodendroglia signaling is disrupted [306]. Taken together, the above findings suggest that oligodendrocytes are preferentially vulnerable to HIV-1 and opiate drug interactions [83] and this may be an additional mechanism by which opiate-HIV-1 interactions in glia result in neuronal dysfunction and injury (Fig. 5).
ACKNOWLEDGEMENTS
We would like to thank Dr. Douglas Wedell for his help with the discussion of statistics in this review. The support of grants, R01 DA018633, R21 DA028741, P01 DA019398, T32 DA007027, R01 DA024461, and K02 DA027374 from the NIH—National Institute on Drug Abuse, and R21 NS069216 from the National Institute on Neurological Diseases and Stroke, is gratefully acknowledged.
CONFLICT OF INTEREST
No conflict of interest is declared.
REFERENCES
- 1. Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–52. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- 2. Anthony IC, Arango JC, Stephens B, Simmonds P, Bell JE. The effects of illicit drugs on the HIV infected brain. Front Biosci. 2008; 13:1294–307. doi: 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- 3. Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–91. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- 4. Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol Appl Neurobiol . 2005;31:325–38. doi: 10.1111/j.1365-2990.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 5. Arango JC, Simmonds P, Brettle RP, Bell JE. Does drug abuse influence the microglial response in AIDS and HIV encephalitis? AIDS. 2004;18 Suppl 1:S69–S74. [PubMed] [Google Scholar]
- 6. Cohen RA. The Changing Face of HIV-Associated Cognitive and Neuropsychiatric Disturbance. In: Paul RH, Sacktor NC, Valcour V, Tashima KT, editors. HIV and the Brain: New Challenges in the Modern Era. 1. New York: Humana Press Inc; 2009. pp. 133–86. [Google Scholar]
- 7. Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: neurocognitive and neuroimaging features. Neuropsychol Rev. 2009;19:215–31. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Byrd DA, Fellows RP, Morgello S, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr . 2011;58:154–62. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69: 1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the Central Nervous System. Curr HIV/AIDS Rep. 2011; 8:54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 12. Banerjee A, Strazza M, Wigdahl B, Pirrone V, Meucci O, Nonnemacher MR. Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis. J Neurovirol. 2011;17:291–302. doi: 10.1007/s13365-011-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hauser KF, El-Hage N, Bruce-Keller A, Knapp PE. Opioids, astroglial chemokines, microglial reactivity, and neuronal injury in HIV-1 encephalitis. In: Meucci O, editor. Chemokine Receptors and NeuroAIDS: Beyond Co-Receptor Function and Links to Other Neuropathologies. 1. New York: Springer; 2010. pp. 353–78. [Google Scholar]
- 14. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Ann Rev Neurosci . 1984;7:223–55. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- 15. Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63: 772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154: 384–96. doi: 10.1038/bjp.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stefano GB, Kream R. Endogenous opiates, opioids, and immune function: evolutionary brokerage of defensive behaviors. Semin Cancer Biol. 2008;18:190–8. doi: 10.1016/j.semcancer.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 18. Bidlack JM, Khimich M, Parkhill AL, Sumagin S, Sun B, Tipton CM. Opioid receptors and signaling on cells from the immune system. J Neuroimmune Pharmacol. 2006;1:260–9. doi: 10.1007/s11481-006-9026-2. [DOI] [PubMed] [Google Scholar]
- 19. Evans CJ. Secrets of the opium poppy revealed. Neuropharmacology. 2004;47 Suppl 1:293–9. doi: 10.1016/j.neuropharm.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 20. McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62:111–23. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 21. Wright CI. The enzymatic deacetylation of heroin and related morphine derivatives by blood serum. J Pharmacol Exp Ther. 1941; 71:164–77. doi: 10.1126/science.92.2385.244-a. [DOI] [PubMed] [Google Scholar]
- 22. Jaffe JH, Martin WR. Opioid analgesics and antagonists. In: Gilman AG, Goodman LS, Rall TW, Murad F, editors. The Pharmacological Basis of Therapeutics. 7. New York: MacMillan Publishing Co; 1985. pp. 491–531. [Google Scholar]
- 23. Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179:1623–9. doi: 10.1016/j.ajpath.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Squinto SP, Mondal D, Block AL, Prakash O. Morphine-induced transactivation of HIV-1 LTR in human neuroblastoma cells. AIDS Res Hum Retroviruses. 1990;6:1163–8. doi: 10.1089/aid.1990.6.1163. [DOI] [PubMed] [Google Scholar]
- 25. Marcario JK, Riazi M, Adany I, et al. Effect of morphine on the neuropathogenesis of SIVmac infection in Indian Rhesus Macaques. J Neuroimmune Pharmacol. 2008;3:12–5. doi: 10.1007/s11481-007-9085-z. [DOI] [PubMed] [Google Scholar]
- 26. Bokhari SM, Hegde R, Callen S, et al. Morphine potentiates neuropathogenesis of SIV infection in Rhesus macaques. J Neuroimmune Pharmacol. 2011;6:626–39. doi: 10.1007/s11481-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perez-Casanova A, Husain K, Noel RJ, Jr, Rivera-Amill V, Kumar A. Interaction of SIV/SHIV infection and morphine on plasma oxidant/antioxidant balance in macaque. Mol Cell Biochem. 2008; 308:169–75. doi: 10.1007/s11010-007-9625-0. [DOI] [PubMed] [Google Scholar]
- 28. Perez-Casanova A, Noel RJ, Jr, Rivera-Amill V, Husain K, Kumar A. Morphine-mediated deterioration of oxidative stress leads to rapid disease progression in SIV/SHIV-infected macaques. AIDS Res Hum Retroviruses. 2007;23:1004–7. doi: 10.1089/aid.2006.0286. [DOI] [PubMed] [Google Scholar]
- 29. Kumar R, Orsoni S, Norman L, et al. Chronic morphine exposure causes pronounced virus replication in cerebral compartment and accelerated onset of AIDS in SIV/SHIV-infected Indian rhesus macaques. Virology. 2006;354:192–206. doi: 10.1016/j.virol.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 30. Noel RJ, Jr, Kumar A. SIV Vpr evolution is inversely related to disease progression in a morphine-dependent rhesus macaque model of AIDS. Virology. 2007;359:397–404. doi: 10.1016/j.virol.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rivera-Amill V, Noel RJ, Jr, Orsini S, et al. Variable region 4 of SIV envelope correlates with rapid disease progression in morphine-exposed macaques infected with SIV/SHIV. Virology . 2007;358:373–83. doi: 10.1016/j.virol.2006.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noel RJ, Jr, Marrero-Otero Z, Kumar R, Chompre-Gonzalez GS, Verma AS, Kumar A. Correlation between SIV Tat evolution and AIDS progression in cerebrospinal fluid of morphine-dependent and control macaques infected with SIV and SHIV. Virology. 2006; 349:440–52. doi: 10.1016/j.virol.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 33. Newsome SD, Johnson E, Pardo C, McArthur JC, Nath A. Fulminant encephalopathy with basal ganglia hyperintensities in HIV-infected drug users. Neurology. 2011;76:787–94. doi: 10.1212/WNL.0b013e31820e7b4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Royal W 3rd, Updike M, Selnes OA, et al. HIV-1 infection and nervous system abnormalities among a cohort of intravenous drug users. Neurology. 1991;41:1905–10. doi: 10.1212/wnl.41.12.1905. [DOI] [PubMed] [Google Scholar]
- 35. Martin EM, Pitrak DL, Rains N, et al. Delayed nonmatch-to-sample performance in HIV-seropositive and HIV-seronegative polydrug abusers. Neuropsychology. 2003;17:283–8. doi: 10.1037/0894-4105.17.2.283. [DOI] [PubMed] [Google Scholar]
- 36. Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998; 83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- 37. Everall IP. Interaction between HIV and intravenous heroin abuse? J Neuroimmunol. 2004;147:13–5. doi: 10.1016/j.jneuroim.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 38. Nath A. Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci. 2010;1187:122–8. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- 39. Hauser KF, El-Hage N, Stiene-Martin A, et al. HIV-1 neuropathogenesis: Glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–86. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8: 1450–7. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 41. Moore DJ, Arce M, Moseley S, et al. Family history of dementia predicts worse neuropsychological functioning among HIV-infected persons. J Neuropsychiatry Clin Neurosci. 2011;23:316–23. doi: 10.1176/appi.neuropsych.23.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crystal HA, Hamon S, Randesi M, et al. A C17T polymorphism in the mu opiate receptor is associated with quantitative measures of drug use in African American women. Addict Biol. 2012;17:181–91. doi: 10.1111/j.1369-1600.2010.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klepstad P, Rakvag TT, Kaasa S, et al. The 118 A > G polymorphism in the human mu-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol Scand. 2004;48:1232–9. doi: 10.1111/j.1399-6576.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 44. Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ. Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann N Y Acad Sci. 2010;1187:184–207. doi: 10.1111/j.1749-6632.2009.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Regan PM, Dave RS, Datta PK, Khalili K. Epigenetics of micro-opioid receptors: Intersection with HIV-1 infection of the central nervous system. J Cell Physiol. 2012;227:2832–41. doi: 10.1002/jcp.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Faure S, Meyer L, Costagliola D, et al. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274–7. doi: 10.1126/science.287.5461.2274. [DOI] [PubMed] [Google Scholar]
- 47. Hladik F, Liu H, Speelmon E, et al. Combined effect of CCR5-Delta32 heterozygosity and the CCR5 promoter polymorphism - 2459 A/G on CCR5 expression and resistance to human immunodeficiency virus type 1 transmission. J Virol. 2005;79: 11677–84. doi: 10.1128/JVI.79.18.11677-11684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 49. Gonzalez E, Rovin BH, Sen L, et al. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci USA. 2002;99:13795–800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turchan-Cholewo J, Liu Y, Gartner S, et al. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and L-deprenyl. Neurobiol Dis. 2006;23:109–19. doi: 10.1016/j.nbd.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 51. Corder EH, Robertson K, Lannfelt L, et al. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med. 1998;4:1182–4. doi: 10.1038/2677. [DOI] [PubMed] [Google Scholar]
- 52. Andres MA, Feger U, Nath A, Munsaka S, Jiang CS, Chang L. APOE epsilon 4 allele and CSF APOE on cognition in HIV-infected subjects. J Neuroimmune Pharmacol. 2011;6:389–98. doi: 10.1007/s11481-010-9254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–35. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clarke TK, Krause K, Li T, Schumann G. An association of prodynorphin polymorphisms and opioid dependence in females in a Chinese population. Addict Biol. 2009;14:366–70. doi: 10.1111/j.1369-1600.2009.00151.x. [DOI] [PubMed] [Google Scholar]
- 55. Wei SG, Zhu YS, Lai JH, Xue HX, Chai ZQ, Li SB. Association between heroin dependence and prodynorphin gene polymorphisms. Brain Res Bull. 2011;85:238–42. doi: 10.1016/j.brainresbull.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 56. Yuferov V, Ji F, Nielsen DA, et al. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology. 2009;34:1185–97. doi: 10.1038/npp.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drakenberg K, Nikoshkov A, Horvath MC, et al. Mu opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci USA. 2006;103:7883–8. doi: 10.1073/pnas.0600871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116: 306–21. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rook EJ, Huitema AD, van den Brink W, Van Ree JM, Beijnen JH. Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: review of the literature. Curr Clin Pharmacol. 2006;1: 109–18. doi: 10.2174/157488406775268219. [DOI] [PubMed] [Google Scholar]
- 60. Podhaizer EM, Zou S, Fitting S, et al. Morphine and gp120 toxic interactions in striatal neurons are dependent on HIV-1 strain. J Neuroimmune Pharmacol. 2012. in press, DOI: 10.1007/s11481-011-9326-z. [DOI] [PMC free article] [PubMed]
- 61. Turchan-Cholewo J, Dimayuga FO, Gupta S, et al. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. J Neurochem. 2009; 108:202–15. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hauser KF, El-Hage N, Buch S, et al. Impact of opiate-HIV-1 interactions on neurotoxic signaling. J Neuroimmune Pharmacol . 2006;1:98–105. doi: 10.1007/s11481-005-9000-4. [DOI] [PubMed] [Google Scholar]
- 63. Hauser KF, El-Hage N, Buch S, et al. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res. 2005;8:63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nath A, Hauser KF, Wojna V, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31 Suppl 2:S62–S9. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- 65. Nath A, Jones M, Maragos W, et al. Neurotoxicity and dysfunction of dopamine systems associated with AIDS dementia. Psychopharmacol. 2000;14:222–7. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- 66. Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56: 1414–27. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gurwell JA, Nath A, Sun Q, et al. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–63. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Malik S, Khalique H, Buch S, Seth P. A growth factor attenuates HIV-1 Tat and morphine induced damage to human neurons: implication in HIV/AIDS-drug abuse cases. PLoS One. 2011;6: e18116. doi: 10.1371/journal.pone.0018116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine potentiates HIV-1 gp120-induced neuronal apoptosis. J Infect Dis . 2005;191:886–9. doi: 10.1086/427830. [DOI] [PubMed] [Google Scholar]
- 70. El-Hage N, Bruce-Keller AJ, Yakovleva T, Bakalkin G, Knapp PE, Hauser KF. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca2+]i, NF-κB trafficking and transcription. PLoS One. 2008;3:e4093. doi: 10.1371/journal.pone.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. El-Hage N, Wu G, Wang J, et al. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53: 132–46. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in µ, δ, and κ-opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–59. [PMC free article] [PubMed] [Google Scholar]
- 74. Hauser KF, Harris-White ME, Jackson JA, Opanashuk LA, Carney JM. Opioids disrupt Ca2+ homeostasis and induce carbonyl oxyradical production in mouse astrocytes in vitro: transient increases and adaptation to sustained exposure. Exp Neurol. 1998; 151:70–6. doi: 10.1006/exnr.1998.6788. [DOI] [PubMed] [Google Scholar]
- 75. Gurwell JA, Duncan MJ, Maderspach K, Stiene-Martin A, Elde RP, Hauser KF. κ-Opioid receptor expression defines a phenotypically distinct subpopulation of astroglia: relationship to Ca2+ mobilization, development, and the antiproliferative effect of opioids. Brain Res. 1996;737:175–87. doi: 10.1016/0006-8993(96)00728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. µ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+-dependent mechanism. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stiene-Martin A, Mattson MP, Hauser KF. Opiates selectively increase intracellular calcium in developing type 1 astrocytes: Role of calcium in morphine-induced morphologic differentiation. Dev Brain Res. 1993;76:189–96. doi: 10.1016/0165-3806(93)90207-q. [DOI] [PubMed] [Google Scholar]
- 78. Stiene-Martin A, Gurwell JA, Hauser KF. Morphine alters astrocyte growth in primary cultures of mouse glial cells: Evidence for a direct effect of opiates on neural maturation. Dev Brain Res . 1991;60:1–7. doi: 10.1016/0165-3806(91)90149-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hauser KF, Osborne JG, Stiene-Martin A, Melner MH. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990;522:347–53. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gupta S, Knight AG, Gupta S, et al. HIV-Tat elicits microglial glutamate release: role of NAPDH oxidase and the cystine-glutamate antiporter. Neurosci Lett. 2010;485:233–6. doi: 10.1016/j.neulet.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Turchan-Cholewo J, Dimayuga FO, Ding Q, et al. Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia. J Neurosci Res. 2008;86:2100–10. doi: 10.1002/jnr.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. El-Hage N, Bruce-Keller AJ, Knapp PE, Hauser KF. CCL5/RANTES gene deletion attenuates opioid-induced increases in glial CCL2/MCP-1 immunoreactivity and activation in HIV-1 Tat exposed mice. J Neuroimmune Pharmacol. 2008;3:275–85. doi: 10.1007/s11481-008-9127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hauser KF, Hahn YK, Adjan VV, et al. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57:194–206. doi: 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Buch SK, Khurdayan VK, Lutz SE, Knapp PE, El-Hage N, Hauser KF. Glial-restricted precursors: Patterns of expression of opioid receptors and relationship to HIV-1 Tat and morphine susceptibility in vitro. Neuroscience. 2007;146:1546–54. doi: 10.1016/j.neuroscience.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Khurdayan VK, Buch S, El-Hage N, et al. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci. 2004;19:3171–82. doi: 10.1111/j.0953-816X.2004.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fitting S, Zou S, Chen W, Vo P, Hauser KF, Knapp PE. Regional Heterogeneity and diversity in cytokine and chemokine production by astroglia: differential responses to HIV-1 Tat, gp120 and morphine revealed by multiplex analysis. J Proteome Res. 2010;9: 1795–804. doi: 10.1021/pr900926n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Oehmichen M, Meissner C, Reiter A, Birkholz M. Neuropathology in non-human immunodeficiency virus-infected drug addicts: hypoxic brain damage after chronic intravenous drug abuse. Acta Neuropathol (Berl) 1996;91:642–6. doi: 10.1007/s004010050478. [DOI] [PubMed] [Google Scholar]
- 88. Rizzuto N, Morbin M, Ferrari S, et al. Delayed spongiform leukoencephalopathy after heroin abuse. Acta Neuropathol (Berl) . 1997;94:87–90. doi: 10.1007/s004010050676. [DOI] [PubMed] [Google Scholar]
- 89. Buttner A, Mall G, Penning R, Weis S. The neuropathology of heroin abuse. Forensic Sci Int. 2000;113:435–42. doi: 10.1016/s0379-0738(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 90. Buttner A. The Neuropathology of Drug Abuse. Neuropathol Appl Neurobiol. 2011;37:118–34. doi: 10.1111/j.1365-2990.2010.01131.x. [DOI] [PubMed] [Google Scholar]
- 91. Kish SJ, Kalasinsky KS, Derkach P, et al. Striatal dopaminergic and serotonergic markers in human heroin users. Neuropsychopharmacology. 2001;24:561–7. doi: 10.1016/S0893-133X(00)00209-8. [DOI] [PubMed] [Google Scholar]
- 92. Makrigeorgi-Butera M, Hagel C, Laas R, Puschel K, Stavrou D. Comparative brain pathology of HIV-seronegative and HIV-infected drug addicts. Clin Neuropathol. 1996;15:324–9. [PubMed] [Google Scholar]
- 93. Anthony IC, Norrby KE, Dingwall T, et al. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133: 3685–98. doi: 10.1093/brain/awq263. [DOI] [PubMed] [Google Scholar]
- 94. Ramage SN, Anthony IC, Carnie FW, Busuttil A, Robertson R, Bell JE. Hyperphosphorylated tau and amyloid precursor protein deposition is increased in the brains of young drug abusers. Neuropathol Appl Neurobiol. 2005;31:439–48. doi: 10.1111/j.1365-2990.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 95. Zou S, Fitting S, Hahn YK, et al. Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at µ-opioid receptor-expressing glia. Brain. 2011;134:3613–28. doi: 10.1093/brain/awr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Suzuki M, El-Hage N, Zou S, et al. Fractalkine/CX3CL1 protects striatal neurons from synergistic morphine and HIV-1 Tat-induced dendritic losses and death. Mol Neurodegener. 2011;6:78. doi: 10.1186/1750-1326-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bakalkin G, Watanabe H, Jezierska J, et al. Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23. Am J Hum Genet. 2010;87:593–603. doi: 10.1016/j.ajhg.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Purves D, Lichtman JW. Elimination of synapses in the developing nervous system. Science. 1980;210:153–7. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- 99. Mattson MP, Kater SB. Excitatory and inhibitory neurotransmitters in the generation and degeneration of hippocampal neuroarchitecture. Brain Res. 1989;478:337–48. doi: 10.1016/0006-8993(89)91514-x. [DOI] [PubMed] [Google Scholar]
- 100. Mattson MP, Dou P, Kater SB. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci . 1988;8:2087–100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mattson MP, Kater SB. Calcium regulation of neurite elongation and growth cone motility. J Neurosci. 1987;7:4034–43. doi: 10.1523/JNEUROSCI.07-12-04034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY. Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci. 2007;35:456–69. doi: 10.1016/j.mcn.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liao D, Grigoriants OO, Loh HH, Law PY. Agonist-dependent postsynaptic effects of opioids on miniature excitatory postsynaptic currents in cultured hippocampal neurons. J Neurophysiol. 2007; 97:1485–94. doi: 10.1152/jn.00790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liao D, Lin H, Law PY, Loh HH. Mu-opioid receptors modulate the stability of dendritic spines. Proc Natl Acad Sci USA. 2005; 102:1725–30. doi: 10.1073/pnas.0406797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lichtman JW, Denk W. The big and the small: challenges of imaging the brain's circuits. Science. 2011;334:618–23. doi: 10.1126/science.1209168. [DOI] [PubMed] [Google Scholar]
- 106. Zou S, El-Hage N, Podhaizer EM, Knapp PE, Hauser KF. PTEN gene silencing prevents HIV-1 gp120IIIB-induced degeneration of striatal neurons. J Neurovirol. 2011;17:41–9. doi: 10.1007/s13365-010-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. McCobb DP, Cohan CS, Connor JA, Kater SB. Interactive effects of serotonin and acetylcholine on neurite elongation. Neuron. 1988; 1:377–85. doi: 10.1016/0896-6273(88)90187-0. [DOI] [PubMed] [Google Scholar]
- 108. Singh IN, El-Hage N, Campbell ME, et al. Differential involvement of p38 and JNK MAP kinases in HIV-1 Tat and gp120-induced apoptosis and neurite degeneration in striatal neurons. Neuroscience. 2005;135:781–90. doi: 10.1016/j.neuroscience.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Singh IN, Goody RJ, Dean C, et al. Apoptotic death of striatal neurons induced by HIV-1 Tat and gp120: differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–51. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Keppel G. Design and Analysis: A Rresearcher's Handbook. 3. Upper Saddle River, NJ: Prentice Hall; 1991. [Google Scholar]
- 111. Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005; 111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 112. Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78:7319–28. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li J, Bentsman G, Potash MJ, Volsky DJ. Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC Neurosci. 2007;8:31. doi: 10.1186/1471-2202-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;19:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 116. Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 117. Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- 118. Masliah E, Ge N, Morey M, DeTeresa R, Terry RD, Wiley CA. Cortical dendritic pathology in human deficiency virus encephalitis. Laboratory Investigation. 1992;66:285–91. [PubMed] [Google Scholar]
- 119. Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–72. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 120. Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol (Berl) . 2004;107:97–110. doi: 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- 121. Everall IP. Neuronal damage - recent issues and implications for therapy. J Neurovirol. 2000;6 Suppl 1:S103–S5. [PubMed] [Google Scholar]
- 122. Everall IP, Heaton RK, Marcotte TD, et al. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–17. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8: 33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- 124. Fitting S, Xu R, Bull CM, et al. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: Chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177:1397–410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12:893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- 126. Li W, Huang Y, Reid R, et al. NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci. 2008;28:12190–8. doi: 10.1523/JNEUROSCI.3019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bursztajn S, Rutkowski MD, DeLeo JA. The role of the N-methyl-D-aspartate receptor NR1 subunit in peripheral nerve injury-induced mechanical allodynia, glial activation and chemokine expression in the mouse. Neuroscience. 2004;125:269–75. doi: 10.1016/j.neuroscience.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 128. Adam F, Dufour E, Le Bars D. The glycine site-specific NMDA antagonist (+)-HA966 enhances the effect of morphine and reverses morphine tolerance via a spinal mechanism. Neuropharmacology . 2008;54:588–96. doi: 10.1016/j.neuropharm.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 129. Song L, Nath A, Geiger JD, Moore A, Hochman S. Human immunodeficiency virus type 1 Tat protein directly activates neuronal N-methyl-D-aspartate receptors at an allosteric zinc-sensitive site. J Neurovirol. 2003;9:399–403. doi: 10.1080/13550280390201704. [DOI] [PubMed] [Google Scholar]
- 130. Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–67. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 131. Chen W, Tang Z, Fortina P, et al. Ethanol potentiates HIV-1 gp120-induced apoptosis in human neurons via both the death receptor and NMDA receptor pathways. Virology. 2005;334:59–73. doi: 10.1016/j.virol.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 132. Bennett BA, Rusyniak DE, Hollingsworth CK. HIV-1 gp120-induced neurotoxicity to midbrain dopamine cultures. Brain Res . 1995;705:168–76. doi: 10.1016/0006-8993(95)01166-8. [DOI] [PubMed] [Google Scholar]
- 133. Meucci O, Miller RJ. gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. J Neurosci. 1996;16:4080–8. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lannuzel A, Lledo PM, Lamghitnia HO, Vincent JD, Tardieu M. HIV-1 envelope proteins gp120 and gp160 potentiate NMDA-induced [Ca2+]i increase, alter [Ca2+]i homeostasis and induce neurotoxicity in human embryonic neurons. Eur J Neurosci. 1995; 7:2285–93. doi: 10.1111/j.1460-9568.1995.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 135. Lipton SA. Memantine prevents HIV coat protein-induced neuronal injury in vitro. Neurology. 1992;42:1403–5. doi: 10.1212/wnl.42.7.1403. [DOI] [PubMed] [Google Scholar]
- 136. Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–8. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- 137. Eugenin EA, King JE, Nath A, et al. HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci USA. 2007;104: 3438–43. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Perry SW, Norman JP, Gelbard HA. Adjunctive therapies for HIV-1 associated neurologic disease. Neurotox Res. 2005;8:161–6. doi: 10.1007/BF03033827. [DOI] [PubMed] [Google Scholar]
- 139. Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA. HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J Immunol. 2007;178:869–76. doi: 10.4049/jimmunol.178.2.869. [DOI] [PubMed] [Google Scholar]
- 140. Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes: A hit and run phenomenon. J Biol Chem. 1999;274: 17098–102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- 141. Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73: 578–86. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- 142. Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186 Suppl 2:S193–8. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 143. Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–66. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 145. Oliva AA Jr, Lam TT, Swann JW. Distally directed dendrotoxicity induced by kainic acid in hippocampal interneurons of green fluorescent protein-expressing transgenic mice. J Neurosci . 2002;22:8052–62. doi: 10.1523/JNEUROSCI.22-18-08052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Greenwood SM, Connolly CN. Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology . 2007;53:891–8. doi: 10.1016/j.neuropharm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 147. Yang Y, Fukui K, Koike T, Zheng X. Induction of autophagy in neurite degeneration of mouse superior cervical ganglion neurons. Eur J Neurosci. 2007;26:2979–88. doi: 10.1111/j.1460-9568.2007.05914.x. [DOI] [PubMed] [Google Scholar]
- 148. Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol Dis. 1996;3:215–27. doi: 10.1006/nbdi.1996.0022. [DOI] [PubMed] [Google Scholar]
- 149. Greenwood SM, Mizielinska SM, Frenguelli BG, Harvey J, Connolly CN. Mitochondrial dysfunction and dendritic beading during neuronal toxicity. J Biol Chem. 2007;282:26235–44. doi: 10.1074/jbc.M704488200. [DOI] [PubMed] [Google Scholar]
- 150. Yang Y, Xu K, Koike T, Zheng X. Transport of autophagosomes in neurites of PC12 cells during serum deprivation. Autophagy. 2008; 4:243–5. doi: 10.4161/auto.5431. [DOI] [PubMed] [Google Scholar]
- 151. Bellizzi MJ, Lu SM, Masliah E, Gelbard HA. Synaptic activity becomes excitotoxic in neurons exposed to elevated levels of platelet-activating factor. J Clin Invest. 2005;115:3185–92. doi: 10.1172/JCI25444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mattson MP, Duan W. "Apoptotic" biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:152–66. [PubMed] [Google Scholar]
- 153. Mattson MP, Keller JN, Begley JG. Evidence for synaptic apoptosis. Exp Neurol. 1998;153:35–48. doi: 10.1006/exnr.1998.6863. [DOI] [PubMed] [Google Scholar]
- 154. Van Grol J, Subauste C, Andrade RM, Fujinaga K, Nelson J, Subauste CS. HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3. PLoS One. 2010;5:e11733. doi: 10.1371/journal.pone.0011733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Norman JP, Perry SW, Reynolds HM, et al. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS One . 2008;3:e3731. doi: 10.1371/journal.pone.0003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Li JC, Au KY, Fang JW, et al. HIV-1 trans-activator protein dysregulates IFN-γ signaling and contributes to the suppression of autophagy induction. AIDS. 2011;25:15–25. doi: 10.1097/QAD.0b013e328340fd61. [DOI] [PubMed] [Google Scholar]
- 157. Espert L, Varbanov M, Robert-Hebmann V, et al. Differential role of autophagy in CD4 T cells and macrophages during X4 and R5 HIV-1 infection. PLoS One. 2009;4:e5787. doi: 10.1371/journal.pone.0005787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Espert L, Denizot M, Grimaldi M, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161–72. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhao L, Zhu Y, Wang D, et al. Morphine induces Beclin 1- and ATG5-dependent autophagy in human neuroblastoma SH-SY5Y cells and in the rat hippocampus. Autophagy. 2010;6:386–94. doi: 10.4161/auto.6.3.11289. [DOI] [PubMed] [Google Scholar]
- 160. Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioid systems and the regulation of dendritic growth and spine formation. J Comp Neurol. 1989;281:13–22. doi: 10.1002/cne.902810103. [DOI] [PubMed] [Google Scholar]
- 161. Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioids regulate dendritic growth and spine formation in developing rat brain. Brain Res. 1987;416:157–61. doi: 10.1016/0006-8993(87)91509-5. [DOI] [PubMed] [Google Scholar]
- 162. Hauser KF, Gurwell JA, Turbek CS. Morphine inhibits Purkinje cell survival and dendritic differentiation in organotypic cultures of the mouse cerebellum. Exp Neurol. 1994;130:95–105. doi: 10.1006/exnr.1994.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 Suppl 1: 33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 164. Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33: 160–2. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 165. Seatriz JV, Hammer RP., Jr Effects of opiates on neuronal development in the rat cerebral cortex. Brain Res Bull. 1993;30: 523–7. doi: 10.1016/0361-9230(93)90078-p. [DOI] [PubMed] [Google Scholar]
- 166. Hammer RP, Jr, Hauser KF. Consequences of early exposure to opioids on cell proliferation and neuronal morphogenesis. In: Miller MW, editor. Development of the Central Nervous System: Effects of Alcohol and Opiates. New York: Wiley-Liss; 1992. pp. 319–39. [Google Scholar]
- 167. Ricalde AA, Hammer RP., Jr Perinatal opiate treatment delays growth of cortical dendrites. Neurosci Lett. 1991;115:137–43. doi: 10.1016/0304-3940(90)90444-e. [DOI] [PubMed] [Google Scholar]
- 168. Zheng H, Chu J, Zeng Y, Loh HH, Law PY. Yin Yang 1 phosphorylation contributes to the differential effects of mu-opioid receptor agonists on microRNA-190 expression. J Biol Chem. 2010; 285:21994–2002. doi: 10.1074/jbc.M110.112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zheng H, Chu J, Zhang Y, Loh HH, Law PY. Modulating µ-opioid receptor phosphorylation switches agonist-dependent signaling as reflected in PKCεactivation and dendritic spine stability. J Biol Chem. 2011;286:12724–33. doi: 10.1074/jbc.M110.177089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Zheng H, Zeng Y, Chu J, Kam AY, Loh HH, Law PY. Modulations of NeuroD activity contribute to the differential effects of morphine and fentanyl on dendritic spine stability. J Neurosci. 2010;30:8102–10. doi: 10.1523/JNEUROSCI.6069-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Luscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–15. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Mathews JL, Smrcka AV, Bidlack JM. A novel Gbetagamma-subunit inhibitor selectively modulates mu-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. J Neurosci. 2008;28: 12183–9. doi: 10.1523/JNEUROSCI.2326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Bonacci TM, Mathews JL, Yuan C, et al. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science . 2006;312:443–6. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 174. Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 Protein Tat. J Neurochem. 1999;73:1363–74. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- 175. Conant K, Ma M, Nath A, Major EO. Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-kappa B binding and protein kinase C activity in primary human astrocytes. J Virol. 1996;70:1384–9. doi: 10.1128/jvi.70.3.1384-1389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74:9214–21. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Conant K, Garzino-Demo A, Nath A, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95: 3117–21. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Benos DJ, Hahn BH, Shaw GM, Bubien JK, Benveniste EN. gp120-mediated alterations in astrocyte ion transport. Adv Neuroimmunol. 1994;4:175–9. doi: 10.1016/s0960-5428(06)80254-8. [DOI] [PubMed] [Google Scholar]
- 179. Benos DJ, McPherson S, Hahn BH, Chaikin MA, Benveniste EN. Cytokines and HIV envelope glycoprotein gp120 stimulate Na+/H+ exchange in astrocytes. J Biol Chem. 1994;269:13811–6. [PubMed] [Google Scholar]
- 180. Holden CP, Nath A, Haughey NJ, Geiger JD. Involvement of Na+/H+ exchangers, Ca2+ channels, and excitatory amino acid receptors in intracellular Ca2+ responses to HIV-1 gp120 in cultured human fetal brain cells. Neuroscience. 1999;91:1369–78. doi: 10.1016/s0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- 181. Mahajan SD, Aalinkeel R, Reynolds JL, et al. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr HIV Res. 2005;3:277–88. doi: 10.2174/1570162054368048. [DOI] [PubMed] [Google Scholar]
- 182. Vesce S, Bezzi P, Rossi D, Meldolesi J, Volterra A. HIV-1 gp120 glycoprotein affects the astrocyte control of extracellular glutamate by both inhibiting the uptake and stimulating the release of the amino acid. FEBS Lett. 1997;411:107–9. doi: 10.1016/s0014-5793(97)00674-1. [DOI] [PubMed] [Google Scholar]
- 183. Wang Z, Pekarskaya O, Bencheikh M, et al. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology . 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- 184. Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Gupta RG, Kelly KM, Helke KL, et al. HIV and SIV induce alterations in CNS CaMKII expression and activation: a potential mechanism for cognitive impairment. Am J Pathol. 2010;176: 2776–84. doi: 10.2353/ajpath.2010.090809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Paul S, Connor JA. NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J Neurochem. 2010;114: 1107–18. doi: 10.1111/j.1471-4159.2010.06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Leveille F, El GF, Gouix E, et al. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22:4258–71. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- 188. Leveille F, Papadia S, Fricker M, et al. Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci. 2010;30:2623–35. doi: 10.1523/JNEUROSCI.5115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–25. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Papadia S, Soriano FX, Leveille F, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008; 11:476–87. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Soriano FX, Hardingham GE. Compartmentalized NMDA receptor signalling to survival and death. J Physiol. 2007;584:381–7. doi: 10.1113/jphysiol.2007.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Balazs R. Trophic effect of glutamate. Curr Top Med Chem. 2006; 6:961–8. doi: 10.2174/156802606777323700. [DOI] [PubMed] [Google Scholar]
- 194. Hetman M, Kharebava G. Survival signaling pathways activated by NMDA receptors. Curr Top Med Chem. 2006;6:787–99. doi: 10.2174/156802606777057553. [DOI] [PubMed] [Google Scholar]
- 195. Sutton G, Chandler LJ. Activity-dependent NMDA receptor-mediated activation of protein kinase B/Akt in cortical neuronal cultures. J Neurochem. 2002;82:1097–105. doi: 10.1046/j.1471-4159.2002.01031.x. [DOI] [PubMed] [Google Scholar]
- 196. Friguls B, Petegnief V, Justicia C, Pallas M, Planas AM. Activation of ERK and Akt signaling in focal cerebral ischemia: modulation by TGF-alpha and involvement of NMDA receptor. Neurobiol Dis . 2002;11:443–56. doi: 10.1006/nbdi.2002.0553. [DOI] [PubMed] [Google Scholar]
- 197. Schmitt HP. Pouring oil into the fire? On the conundrum of the beneficial effects of NMDA receptor antagonists in Alzheimer disease. Psychopharmacology (Berl) 2005;179:151–3. doi: 10.1007/s00213-004-2110-5. [DOI] [PubMed] [Google Scholar]
- 198. Gelman BB, Spencer JA, Holzer CE, III, Soukup VM. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. J Neuroimmune Pharmacol. 2006;1:410–20. doi: 10.1007/s11481-006-9030-6. [DOI] [PubMed] [Google Scholar]
- 199. Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- 200. Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. Hyperdopaminergic tone in HIV-1 protein treated rats and cocaine sensitization. J Neurochem. 2010;115:885–96. doi: 10.1111/j.1471-4159.2010.06968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Scheller C, Sopper S, Jenuwein M, et al. Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J Neurochem. 2005;95:377–87. doi: 10.1111/j.1471-4159.2005.03373.x. [DOI] [PubMed] [Google Scholar]
- 203. Marcario JK, Manaye KF, SantaCruz KS, Mouton PR, Berman NE, Cheney PD. Severe subcortical degeneration in macaques infected with neurovirulent simian immunodeficiency virus. J Neurovirol . 2004;10:387–99. doi: 10.1080/13550280490521131. [DOI] [PubMed] [Google Scholar]
- 204. Czub S, Czub M, Koutsilieri E, et al. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta Neuropathol (Berl) 2004;107:216–26. doi: 10.1007/s00401-003-0801-3. [DOI] [PubMed] [Google Scholar]
- 205. Czub S, Koutsilieri E, Sopper S, et al. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol (Berl) 2001; 101:85–91. doi: 10.1007/s004010000313. [DOI] [PubMed] [Google Scholar]
- 206. Theodore S, Cass WA, Maragos WF. Methamphetamine and human immunodeficiency virus protein Tat synergize to destroy dopaminergic terminals in the rat striatum. Neuroscience. 2006; 137:925–35. doi: 10.1016/j.neuroscience.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 207. Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res . 2003;984:133–42. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- 208. Bagley EE, Hacker J, Chefer VI, et al. Drug-induced GABA transporter currents enhance GABA release to induce opioid withdrawal behaviors. Nat Neurosci. 2011;14:1548–54. doi: 10.1038/nn.2940. [DOI] [PubMed] [Google Scholar]
- 209. Jiang ZG, North RA. Pre- and postsynaptic inhibition by opioids in rat striatum. J Neurosci. 1992;12:356–61. doi: 10.1523/JNEUROSCI.12-01-00356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31 Suppl 2:S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- 211. Khan MZ, Brandimarti R, Patel JP, et al. Apoptotic and antiapoptotic effects of CXCR4: is it a matter of intrinsic efficacy? Implications for HIV neuropathogenesis. AIDS Res Hum Retroviruses. 2004;20:1063–71. doi: 10.1089/aid.2004.20.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Bachis A, Cruz MI, Mocchetti I. M-tropic HIV envelope protein gp120 exhibits a different neuropathological profile than T-tropic gp120 in rat striatum. Eur J Neurosci. 2010;32:570–8. doi: 10.1111/j.1460-9568.2010.07325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. El-Hage N, Dever SM, Fitting S, Ahmed T, Hauser KF. HIV-1 coinfection and morphine co-exposure severely dysregulate HCV-induced hepatic pro-inflammatory cytokine release and free radical production: increased pathogenesis coincides with uncoordinated host-defenses. J Virol. 2011;85:11601–14. doi: 10.1128/JVI.05239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Chen L, Liu J, Xu C, Keblesh J, Zang W, Xiong H. HIV-1gp120 induces neuronal apoptosis through enhancement of 4-aminopyridine-senstive outward K currents. PLoS One. 2011;6: e259940. doi: 10.1371/journal.pone.0025994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Garden GA, Budd SL, Tsai E, et al. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci . 2002;22:4015–24. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Bachis A, Biggio F, Major EO, Mocchetti I. M- and T-tropic HIVs promote apoptosis in rat neurons. J Neuroimmune Pharmacol. 2009; 4:150–60. doi: 10.1007/s11481-008-9141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Peng F, Dhillon N, Callen S, et al. Platelet-derived growth factor protects neurons against gp120-mediated toxicity. J Neurovirol . 2008;14:62–72. doi: 10.1080/13550280701809084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Acquas E, Bachis A, Nosheny RL, Cernak I, Mocchetti I. Human immunodeficiency virus type 1 protein gp120 causes neuronal cell death in the rat brain by activating caspases. Neurotox Res. 2004;5: 605–15. doi: 10.1007/BF03033180. [DOI] [PubMed] [Google Scholar]
- 219. Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–22. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Lannuzel A, Barnier JV, Hery C, et al. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–56. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- 221. Lazarini F, Casanova P, Tham TN, et al. Differential signalling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes. Eur J Neurosci. 2000;12:117–25. doi: 10.1046/j.1460-9568.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- 222. Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA. 1998;95:14500–5. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Medders KE, Sejbuk NE, Maung R, Desai MK, Kaul M. Activation of p38 MAPK is required in monocytic and neuronal cells for HIV glycoprotein 120-induced neurotoxicity. J Immunol. 2010;185: 4883–95. doi: 10.4049/jimmunol.0902535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Bodner A, Toth PT, Miller RJ. Activation of c-Jun N-terminal kinase mediates gp120IIIB- and nucleoside analogue-induced sensory neuron toxicity. Exp Neurol. 2004;188:246–53. doi: 10.1016/j.expneurol.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 225. Xu H, Bae M, Tovar YRL, et al. The human immunodeficiency virus coat protein gp120 promotes forward trafficking and surface clustering of NMDA receptors in membrane microdomains. J Neurosci. 2011;31:17074–90. doi: 10.1523/JNEUROSCI.4072-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003; 24:116–21. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 227. Happel C, Steele AD, Finley MJ, Kutzler MA, Rogers TJ. DAMGO-induced expression of chemokines and chemokine receptors: the role of TGF-beta1. J Leukoc Biol. 2008;83:956–63. doi: 10.1189/jlb.1007685. [DOI] [PubMed] [Google Scholar]
- 228. Patel JP, Sengupta R, Bardi G, Khan MZ, Mullen-Przeworski A, Meucci O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J Neurovirol. 2006;12:492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Burbassi S, Sengupta R, Meucci O. Alterations of CXCR4 function in mu-opioid receptor-deficient glia. Eur J Neurosci. 2010;32: 1278–88. doi: 10.1111/j.1460-9568.2010.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Pitcher J, Shimizu S, Burbassi S, Meucci O. Disruption of neuronal CXCR4 function by opioids: preliminary evidence of ferritin heavy chain as a potential etiological agent in neuroAIDS. J Neuroimmunol. 2010;224:66–71. doi: 10.1016/j.jneuroim.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Abt AC, Meucci O. Regulation of neuronal ferritin heavy chain, a new player in opiate-induced chemokine dysfunction. J Neuroimmune Pharmacol. 2011;6:466–76. doi: 10.1007/s11481-011-9278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Sengupta R, Burbassi S, Shimizu S, et al. Morphine increases brain levels of ferritin heavy chain leading to inhibition of CXCR4-mediated survival signaling in neurons. J Neurosci. 2009;29:2534–44. doi: 10.1523/JNEUROSCI.5865-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Todorich B, Zhang X, Connor JR. H-ferritin is the major source of iron for oligodendrocytes. Glia. 2011;59:927–35. doi: 10.1002/glia.21164. [DOI] [PubMed] [Google Scholar]
- 234. Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57: 467–78. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 235. Connor JR, Menzies SL. Cellular management of iron in the brain. J Neurol Sci. 1995;134 Suppl:33–44. doi: 10.1016/0022-510x(95)00206-h. [DOI] [PubMed] [Google Scholar]
- 236. Kam AY, Liao D, Loh HH, Law PY. Morphine induces AMPA receptor internalization in primary hippocampal neurons via calcineurin-dependent dephosphorylation of GluR1 subunits. J Neurosci. 2010;30:15304–16. doi: 10.1523/JNEUROSCI.4255-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development: novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Dev Neurobiol. 1998;5:437–49. [PubMed] [Google Scholar]
- 238. Evans CJ. Diversity among the opioid receptors. In: Korenman SG, Barchas DJ, editors. Biological Basis of Substance Abuse. 1. New York: Oxford University Press; 1993. pp. 31–48. [Google Scholar]
- 239. Pan YX. Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol . 2005;24:736–50. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- 240. Ikeda H, Miyatake M, Koshikawa N, et al. Morphine modulation of thrombospondin levels in astrocytes and its implications for neurite outgrowth and synapse formation. J Biol Chem. 2010;285:38415–27. doi: 10.1074/jbc.M110.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Rusnati M, Taraboletti G, Urbinati C, et al. Thrombospondin-1/HIV-1 tat protein interaction: modulation of the biological activity of extracellular Tat. Faseb J. 2000;14:1917–30. doi: 10.1096/fj.99-0902com. [DOI] [PubMed] [Google Scholar]
- 242. Persidsky Y, Limoges J, Rasmussen J, Zheng J, Gearing A, Gendelman HE. Reduction in glial immunity and neuropathology by a PAF antagonist and an MMP and TNFalpha inhibitor in SCID mice with HIV-1 encephalitis. J Neuroimmunol. 2001;114:57–68. doi: 10.1016/s0165-5728(00)00454-9. [DOI] [PubMed] [Google Scholar]
- 243. Koning N, Swaab DF, Hoek RM, Huitinga I. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol . 2009;68:159–67. doi: 10.1097/NEN.0b013e3181964113. [DOI] [PubMed] [Google Scholar]
- 244. Kaul M, Lipton SA. Experimental and potential future therapeutic approaches for HIV-1 associated dementia targeting receptors for chemokines, glutamate and erythropoietin. Neurotox Res. 2005; 8:67–86. doi: 10.1007/BF03033828. [DOI] [PubMed] [Google Scholar]
- 245. Nath A. Pathobiology of human immunodeficiency virus dementia. Semin Neurol. 1999;19:113–27. doi: 10.1055/s-2008-1040830. [DOI] [PubMed] [Google Scholar]
- 246. Gendelman HE, Persidsky Y, Ghorpade A, et al. The neuropathogenesis of the AIDS dementia complex. AIDS. 1997;11: 35–45. [PubMed] [Google Scholar]
- 247. Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res. 2009;16:205–20. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- 248. Vallat-Decouvelaere AV, Chretien F, Gras G, Le Pavec G, Dormont D, Gray F. Expression of excitatory amino acid transporter-1 in brain macrophages and microglia of HIV-infected patients. A neuroprotective role for activated microglia? J Neuropathol Exp Neurol. 2003;62:475–85. doi: 10.1093/jnen/62.5.475. [DOI] [PubMed] [Google Scholar]
- 249. Gras G, Chretien F, Vallat-Decouvelaere AV, et al. Regulated expression of sodium-dependent glutamate transporters and synthetase: a neuroprotective role for activated microglia and macrophages in HIV infection? Brain Pathol. 2003;13:211–22. doi: 10.1111/j.1750-3639.2003.tb00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Patton HK, Zhou ZH, Bubien JK, Benveniste EN, Benos DJ. gp120-induced alterations of human astrocyte function: Na(+)/H(+) exchange, K(+) conductance, and glutamate flux. Am J Physiol Cell Physiol. 2000;279:C700–C8. doi: 10.1152/ajpcell.2000.279.3.C700. [DOI] [PubMed] [Google Scholar]
- 251. Gras G, Samah B, Hubert A, Leone C, Porcheray F, Rimaniol AC. EAAT expression by macrophages and microglia: still more questions than answers. Amino Acids. 2012;42:221–9. doi: 10.1007/s00726-011-0866-6. [DOI] [PubMed] [Google Scholar]
- 252. Gras G, Porcheray F, Samah B, Leone C. The glutamate-glutamine cycle as an inducible, protective face of macrophage activation. J Leukoc Biol. 2006;80:1067–75. doi: 10.1189/jlb.0306153. [DOI] [PubMed] [Google Scholar]
- 253. Chretien F, Vallat-Decouvelaere AV, Bossuet C, et al. Expression of excitatory amino acid transporter-2 (EAAT-2) and glutamine synthetase (GS) in brain macrophages and microglia of SIVmac251-infected macaques. Neuropathol Appl Neurobiol. 2002; 28:410–7. doi: 10.1046/j.1365-2990.2002.00426.x. [DOI] [PubMed] [Google Scholar]
- 254. Cali C, Bezzi P. CXCR4-mediated glutamate exocytosis from astrocytes. J Neuroimmunol. 2010;224:13–21. doi: 10.1016/j.jneuroim.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 255. Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86: 1009–31. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 256. Ho VM, Lee JA, Martin KC. The cell biology of synaptic plasticity. Science. 2011;334:623–8. doi: 10.1126/science.1209236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999; 22:208–15. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 258. Parpura V, Basarsky TA, Liu F, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369: 744–7. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 259. Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature . 2010;463:232–6. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Ozawa T, Nakagawa T, Shige K, Minami M, Satoh M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Res. 2001;905:254–8. doi: 10.1016/s0006-8993(01)02536-7. [DOI] [PubMed] [Google Scholar]
- 261. Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002; 22:8312–23. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Becker Y. The spreading of HIV-1 infection in the human organism is caused by fractalkine trafficking of the infected lymphocytes--a review, hypothesis and implications for treatment. Virus Genes. 2007;34:93–109. doi: 10.1007/s11262-006-0056-x. [DOI] [PubMed] [Google Scholar]
- 263. Persidsky Y, Poluektova L. Immune privilege and HIV-1 persistence in the CNS. Immunol Rev. 2006;213:180–94. doi: 10.1111/j.1600-065X.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- 264. Schols D. HIV co-receptors as targets for antiviral therapy. Curr Top Med Chem. 2004;4:883–93. doi: 10.2174/1568026043388501. [DOI] [PubMed] [Google Scholar]
- 265. Glass WG, Rosenberg HF, Murphy PM. Chemokine regulation of inflammation during acute viral infection. Curr Opin Allergy Clin Immunol. 2003;3:467–73. doi: 10.1097/00130832-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 266. Cotter R, Williams C, Ryan L, et al. Fractalkine (CX3CL1) and brain inflammation: Implications for HIV-1-associated dementia. J Neurovirol. 2002;8:585–98. doi: 10.1080/13550280290100950. [DOI] [PubMed] [Google Scholar]
- 267. Gabuzda D, Wang J. Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. J Neurovirol. 2000;6 Suppl 1: S24–S32. [PubMed] [Google Scholar]
- 268. Petito CK, Kerza-Kwiatecki AP, Gendelman HE, et al. Review: neuronal injury in HIV infection. J Neurovirol. 1999;5:327–41. doi: 10.3109/13550289909029474. [DOI] [PubMed] [Google Scholar]
- 269. Wesselingh SL, Thompson KA. Immunopathogenesis of HIV-associated dementia. Curr Opin Neurol. 2001;14:375–9. doi: 10.1097/00019052-200106000-00018. [DOI] [PubMed] [Google Scholar]
- 270. Lipton SA. Neuronal injury associated with HIV-1: approaches to treatment. Annu Rev Pharmacol Toxicol. 1998;38:159–77. doi: 10.1146/annurev.pharmtox.38.1.159. 159-77. [DOI] [PubMed] [Google Scholar]
- 271. Gendelman HE, Genis P, Jett M, Zhai QH, Nottet HS. An experimental model system for HIV-1-induced brain injury. Adv Neuroimmunol. 1994;4:189–93. doi: 10.1016/s0960-5428(06)80256-1. [DOI] [PubMed] [Google Scholar]
- 272. Avdoshina V, Biggio F, Palchik G, Campbell LA, Mocchetti I. Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120. Glia . 2010;58:1630–9. doi: 10.1002/glia.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Erichsen D, Lopez AL, Peng H, et al. Neuronal injury regulates fractalkine: relevance for HIV-1 associated dementia. J Neuroimmunol. 2003;138:144–55. doi: 10.1016/s0165-5728(03)00117-6. [DOI] [PubMed] [Google Scholar]
- 274. Tong N, Perry SW, Zhang Q, et al. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J Immunol. 2000; 164:1333–9. doi: 10.4049/jimmunol.164.3.1333. [DOI] [PubMed] [Google Scholar]
- 275. Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA. 2000;97:8075–80. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Combadiere C, Salzwedel K, Smith ED, Tiffany HL, Berger EA, Murphy PM. Identification of CX3CR1. A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV-1. J Biol Chem. 1998;273:23799–804. doi: 10.1074/jbc.273.37.23799. [DOI] [PubMed] [Google Scholar]
- 277. Stiene-Martin A, Knapp PE, Martin KM, et al. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia. 2001;36:78–88. [PMC free article] [PubMed] [Google Scholar]
- 278. Stiene-Martin A, Hauser KF. Glial growth is regulated by agonists selective for multiple opioid receptor types in vitro. J Neurosci Res . 1991;29:538–48. doi: 10.1002/jnr.490290415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Knapp PE, Itkis OS, Zhang L, Spruce BA, Bakalkin G, Hauser KF. Endogenous opioids and oligodendroglial function: possible autocrine/paracrine effects on cell survival and development. Glia . 2001;35:156–65. doi: 10.1002/glia.1080. [DOI] [PubMed] [Google Scholar]
- 280. Spruce BA, Curtis R, Wilkin GP, Glover DM. A neuropeptide precursor in cerebellum: Proenkephalin exists in subpopulations of both neurons and astrocytes. EMBO J. 1990;9:1787–95. doi: 10.1002/j.1460-2075.1990.tb08303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Low KG, Allen RG, Melner MH. Differential regulation of proenkephalin expression in astrocytes by cytokines. Endocrinology. 1992;131:1908–14. doi: 10.1210/endo.131.4.1396335. [DOI] [PubMed] [Google Scholar]
- 282. Melner MH, Low KG, Allen RG, Nielson CP, Young SL, Saneto RP. The regulation of proenkephalin expression in a distinct population of glial cells. EMBO J. 1990;9:791–6. doi: 10.1002/j.1460-2075.1990.tb08175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Shinoda H, Marini AM, Cosi C, Schwartz JP. Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes. Science. 1989;245:415–7. doi: 10.1126/science.2569236. [DOI] [PubMed] [Google Scholar]
- 284. Ruzicka BB, Akil H. The interleukin-1beta-mediated regulation of proenkephalin and opioid receptor messenger RNA in primary astrocyte-enriched cultures. Neuroscience. 1997;79:517–24. doi: 10.1016/s0306-4522(96)00669-0. [DOI] [PubMed] [Google Scholar]
- 285. McCarthy KD, Enkvist K, Shao Y. Astroglial adrenergic receptors: expression and function. In: Kettenmann H, Ransom BR, editors. Neuroglia. 1. New York: Oxford Univ. Press; 1995. pp. 354–66. [Google Scholar]
- 286. Emsley JG, Macklis JD. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2006;2:175–86. doi: 10.1017/S1740925X06000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–94. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 288. Shao Y, Porter JT, McCarthy KD. Neuroligand receptor heterogeneity among astroglia. Perspect Dev Neurobiol. 1994;2: 205–15. [PubMed] [Google Scholar]
- 289. Kimelberg HK. Receptors on astrocytes--what possible functions? Neurochem Int. 1995;26:27–40. doi: 10.1016/0197-0186(94)00118-e. [DOI] [PubMed] [Google Scholar]
- 290. Venance L, Premont J, Glowinski J, Giaume C. Gap junctional communication and pharmacological heterogeneity in astrocytes cultured from the rat striatum. J Physiol. 1998;510( Pt 2):429–40. doi: 10.1111/j.1469-7793.1998.429bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Bachoo RM, Kim RS, Ligon KL, et al. Molecular diversity of astrocytes with implications for neurological disorders. Proc Natl Acad Sci USA. 2004;101:8384–9. doi: 10.1073/pnas.0402140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- 293. El-Hage N, Podhaizer EM, Sturgill J, Hauser KF. Toll-like receptor expression and activation in astroglia: differential regulation by HIV-1 Tat, gp120, and morphine. Immunol Invest. 2011;40:498–522. doi: 10.3109/08820139.2011.561904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174: 426–34. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- 295. Li Y, Li H, Zhang Y, et al. Toll-like receptor 2 is required for opioids-induced neuronal apoptosis. Biochem Biophys Res Commun. 2010;391:426–30. doi: 10.1016/j.bbrc.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Hutchinson MR, Lewis SS, Coats BD, et al. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience. 2010;167:880–93. doi: 10.1016/j.neuroscience.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Hutchinson MR, Zhang Y, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–6. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Micu I, Jiang Q, Coderre E, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature . 2006;439:988–92. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 300. Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438: 1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 301. Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: µ and κ opioid receptors mediate differential mitogenic and growth responses. Glia . 1998;22:189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 302. Hahn JW, Jagwani S, Kim E, et al. Mu and kappa opioids modulate mouse embryonic stem cell-derived neural progenitor differentiation via MAP kinases. J Neurochem. 2010;112:1431–41. doi: 10.1111/j.1471-4159.2009.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia . 2008;56:1017–27. doi: 10.1002/glia.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Knapp PE, Adjan VV, Hauser KF. Cell-specific loss of κ opioid receptors in oligodendrocytes of the dysmyelinating jimpy mouse. Neurosci Lett. 2009;451:114–8. doi: 10.1016/j.neulet.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Tryoen-Toth P, Gaveriaux-Ruff C, Labourdette G. Down-regulation of mu-opioid receptor expression in rat oligodendrocytes during their development in vitro. J Neurosci Res. 2000;60:10–20. doi: 10.1002/(SICI)1097-4547(20000401)60:1<10::AID-JNR2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 306. Chomiak T, Hu B. What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS One. 2009;4:e7754. doi: 10.1371/journal.pone.0007754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Berman JW, Carson MJ, Chang L, et al. NeuroAIDS, drug abuse, and inflammation: building collaborative research activities. J Neuroimmune Pharmacol. 2006;1:351–99. doi: 10.1007/s11481-006-9048-9. [DOI] [PubMed] [Google Scholar]