Abstract
Prior to the introduction of the International Network for Cancer Treatment and Research (INCTR) protocol INCTR 03-06, survival of patients with Burkitt lymphoma at 4 tertiary care centres in equatorial Africa was probably no more than 10–20%. The results reported here for 356 patients have demonstrated marked improvement in survival through the use of a uniform treatment protocol consisting of cyclophosphamide, methotrexate, vincristine, and intrathecal therapy, and the introduction of non-cross resistant second-line (salvage) therapy, consisting of ifosfamide, mesna, etoposide and cytarabine, when patients failed to achieve a complete response to first-line therapy or relapsed early. Overall survival rates of 67% and 62% were observed at 1 and 2 years (relapse is rare after one year). Of interest was the small impact of cerebrospinal fluid (CSF) and bone marrow involvement on outcome. However, the presence or absence of abdominal involvement clearly defined two prognostic groups. An additional finding was the association between CSF pleocytosis and orbital tumours, suggesting that spread of tumour cells to the central nervous system may occur via direct involvement of cranial nerves in the orbit. Survival rates may be increased in patients with abdominal involvement by combining first- and second-line therapy, but verification will require a further clinical study.
Keywords: Childhood haematological malignancies, clinical research, chemotherapy
Introduction
The clinical syndrome of African or endemic Burkitt lymphoma (BL) was discovered in 1958 by Denis Burkitt, an Irish surgeon working at the time in Mulago Hospital, Kampala, Uganda (Burkitt, 1958). Subsequently, Burkitt and others described the distribution of BL in Africa and New Guinea (Burkitt, 1962; Booth et al, 1967), and suggested that malaria may be a risk factor (Dalldorf et al, 1964; Morrow et al, 1976; Rainey et al, 2007). During this same time period, and prompted by the inadequacy of surgery and the unavailability of radiation therapy, investigators in equatorial Africa explored the efficacy of chemotherapy and demonstrated dramatic responses to a number of chemotherapeutic agents, sometimes producing long-term survival even with as little as a single dose of cyclophosphamide (CTX) (Burkitt, 1967a; Clifford et al, 1967; Ngu, 1965, Burkitt, 1967b). Of considerable interest was the subsequent observation that patients who did not achieve a complete remission with one or two doses of CTX, or who relapsed, could often achieve long term survival following either additional doses of CTX or, when CTX was no longer effective, the administration of other drugs, particularly methotrexate (MTX), vincristine (VCR) and cytarabine (Ara-C), indicating that these drugs were largely non-cross resistant with CTX (Ziegler, 1972, Olweny et al, 1980, Ziegler et al, 1972, Ziegler et al, 1979).
Following Burkitt’s departure from Uganda, a series of clinical trials was undertaken at the Uganda Cancer Institute in collaboration with the National Cancer Institute (USA) to try to define optimal therapy (Magrath, 2009), including the treatment or prevention of central nervous system (CNS) spread, which was sometimes present at the time of initial diagnosis and was a common site of recurrent disease (Ziegler & Bluming, 1971; Olweny et al, 1977). The last study conducted in Uganda in this era was a randomized comparison between CTX and a regimen consisting of CTX, VCR and MTX, designated as COM, but without intrathecal (IT) therapy (Olweny et al, 1976). Although a small study, it demonstrated that patients receiving COM were less likely to develop a systemic relapse, although in the absence of IT therapy relapse with isolated CNS recurrence was frequent and a survival advantage was not apparent. After longer follow-up, and presumably because CNS disease could often be effectively treated (Ziegler & Bluming 1971; Ziegler et al, 1979), patients who received combination therapy were shown to have significantly prolonged survival (Olweny et al, 1980). At that time, although it was known that IT therapy with either MTX or Ara-C could induce prolonged remission in patients with CNS involvement either at presentation or relapse (Ziegler et al, 1979), the optimal means of preventing CNS spread had not been defined (Magrath, 2012), although the available evidence strongly suggested that craniospinal radiation was not effective in this regard (Olweny et al, 1977).
In 2004, the International Network for Cancer Treatment and Research (INCTR) brought together a group of African physicians from three countries, subsequently referred to as INCTR’s “African BL strategy group,” to discuss their results in the treatment of BL and to design a joint treatment protocol. BL is particularly important in equatorial Africa, because it is generally the most common childhood cancer, accounting for 30–50% of all cases in children less than 15 years of age. The strategy group physicians were all using combinations of CTX, MTX, and VCR, but with varying doses of systemic agents and different regimens of IT therapy. Because follow-up was very poor, it was not possible to determine event-free survival (EFS) or overall survival (OS) rates. However, there was little doubt that these were very low, due to a variety of factors including treatment abandonment, the inability of families to afford therapy, failure to treat recurrent disease and/or poor supportive care. The group designed a standard regimen based on the three drugs they were all using in addition to IT therapy spread over multiple cycles. The objectives were to attempt to precisely define response and toxicity to this therapy in order to provide a solid baseline with which other regimens for the treatment of BL in equatorial Africa could be compared. Because not all centres were able to rapidly examine the cerebrospinal fluid (CSF) and bone marrow (BM) prior to therapy, it was decided that all patients –regardless of whether CSF and or BM examinations were performed – would be eligible for the study if all other eligibility criteria were met.
Although the INCTR strategy group felt that there was sufficient published information to suggest that combination therapy was superior to CTX alone [Traoré et al, 2011] they also agreed that more intensive therapy, as used in the USA and Europe, for example, would not be feasible in the low resource settings in which they worked. They felt that the cost of the three-drug regimen would be affordable in most equatorial African countries, and that this approach was superior to focusing on very low cost approaches likely to have a very poor outcome (e.g., CTX alone), as the latter would not provide a way forward, by demonstrating to policy makers what can be accomplished, but rather could be seen as acceptance of the status quo, with the emphasis on cheap rather than effective therapy.
In order to gain the maximal amount of information possible from the trial, it was proposed to test a new combination of drugs, ifosfamide, mesna, etoposide and Ara-C for patients who failed therapy. This combination was based on similar combinations shown to be effective and feasible (albeit with differences in drug doses) in the USA (Magrath et al, 1996; Adde et al, 1998), Egypt (Gad-el-Mawla et al, 1989) and India (Advani et al, 1997). If non-cross resistant and feasible, in terms of toxicity, in the African setting, the combination could be incorporated into first-line therapy in high risk patients in a future treatment regimen, with potentially improved survival rates.
This report describes the results of this study, INCTR 03-06, in which 356 patients were entered between August 2004 and December 31, 2009.
Patients and Methods
INCTR Clinical Study 03-06 was approved by INCTR’s ethical review committee after external scientific review, and was also approved by the ethical review committees of all participating centres. Patients were enrolled on this study at four participating institutions in three sub- Saharan African countries between August 2004 and December, 2009. These institutions included the Ocean Road Cancer Institute (ORCI) in Dar es Salaam, Tanzania, the Kenyatta National Hospital (KNH) in Nairobi, Kenya, and two in Nigeria, the Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC) in Ile-Ife and the University College Hospital (UCH) in Ibadan. All previously untreated patients with BL, B-cell intermediate between BL and diffuse large B-cell lymphoma (DLBCL) and DLBCL, between the ages of 2 to 59 years of age, were eligible for the protocol after informed consent was obtained and a consent form signed or otherwise affirmed. In view of the fact that many of the patients came to these treatment centres from distant or remote regions of their countries, only patients willing to remain in the hospital as long as necessary and to return to the hospital for planned treatments were eligible for entry onto treatment protocol INCTR 03-06.
Institutions varied with respect to the psychosocial support provided during hospitalization. At ORCI, care for children less than 5 years of age was technically free of charge, although funds were not always available. At KNH, costs of all tests, hospitalization, including food, and transportation were borne by the patient’s family. Care was not withheld at KNH when urgent problems arose, but discharge was delayed until such time as the hospital bill was fully paid or social support was obtained to cover costs. At the two Nigerian centres, OAUTHC and UCH, patients were required to bear all of the costs associated with treatment. At times, care was not provided and tests not performed if parents could not afford the associated costs. For the study, however, INCTR provided administrative support, including overall study coordination, data management, and drugs (initially only cytotoxic drugs, and subsequently antibiotics as well).
Diagnosis
The diagnosis of BL was made in the majority of cases by fine needle aspirate (FNA) of accessible tumour by local haematopathologists, which is the most usual method of diagnosing BL in Africa. Subsequent pathological review by a team of expert haematopathologists was carried out on 159 tumour aspirates and the diagnoses were considered to be consistent with BL in all but a small minority of cases, in which a diagnosis of aggressive B cell lymphoma not otherwise specified was made, or in which the available material was of poor quality (Naresh et al, 2011a; Naresh et al, 2011b]. These cases were included in the analysis. Six cases, however, were considered to have a diagnosis not eligible for treatment on protocol INCTR 03-06 and were therefore excluded from the study. Given that not all cases were reviewed, it is highly likely that a small fraction of the patients included in the study did not have BL. These cases may account for some of the therapy failures and could have resulted in a worse survival rate than would otherwise have been the case.
Pre-Treatment Evaluation
A complete history and physical examination were performed in all patients. Biochemical profiles and complete blood counts were performed in the majority of patients prior to the start of treatment. Pre-treatment imaging studies included chest x-rays and ultrasound examinations of the abdomen and pelvis. The majority of patients enrolled at KNH, OAUTHC and UCH had a pre-treatment BM evaluation and a CSF examination at the time of the first IT therapy to detect the presence of malignant cells. BM examinations prior to the start of treatment, however, were not possible in the majority of patients treated at ORCI. In the early years of the study, ORCI patients were sent to the nearby university hospital for this procedure, which was both expensive for patients and often resulted in delays in the initiation of therapy in extremely ill patients for up to one month while patients waited for the procedure to be performed, so that subsequently, treatment was initiated even though a BM examination had not been performed. CSF examinations were also not routinely performed at ORCI due to the lack of staff trained in the preparation and interpretation of cytospins. For these reasons, 89.1% of patients at ORCI did not have pre-treatment BM examinations and 98.4% did not have CSF examinations, although IT therapy was given. At other institutions, BM aspirate needles were not always available and thus BM examinations were sometimes not performed. Patients with documented BM involvement and/or positive CSF examinations or other signs of CNS disease were not excluded from the protocol.
Patients who were known to be or who tested positive for human immunodeficiency virus (HIV) were eligible for the protocol.
Risk criteria and definitions
Patients were assigned to one of two treatment groups based upon the extent of disease at the time of presentation. Patients who had a single extra-abdominal tumour where the widest diameter was less than 10 cm were considered to be in the low-risk (LR) group while all other patients were considered to be in the high-risk (HR) group.
For purposes of analysis, patients were retrospectively staged according to a previously described staging system (Magrath et al, 1974) and based on survival in patients with BL at the Lymphoma Treatment Centre (LTC) in Uganda. In this system, referred to hereafter as the “LTC” system, Stage A was defined as a single extra-abdominal tumour, Stage B, multiple extra-abdominal tumours (including CNS involvement, i.e, CSF pleocytosis or cranial nerve palsies), Stage C, abdominal tumour with or without facial tumours and Stage D as abdominal tumour with tumour at any other site or sites, except the face alone. Patients were also retrospectively staged according to the St Jude Staging classification, which was derived from a staging system used for Hodgkin lymphoma and modified for children with non-Hodgkin lymphoma, initially in the USA (Murphy, 1980). This staging system is still widely used throughout the world
Specific therapy
Protocol INCTR 03-06 consisted of a first-line (FL) regimen for newly diagnosed, previously untreated patients and a second-line (SL) regimen for patients who failed to respond to FL or who had an early relapse (i.e., relapsed after achieving a complete response (CR) either during FL or within 6 weeks from the completion of FL). Late relapses were defined as those that occurred more than 6 weeks from the completion of FL. Patients who had late relapses were re-treated with FL therapy (repeat FL), but were eligible for SL therapy if they developed progressive disease (an increase of 25% in the diameter of the tumour) at any time after the initiation of repeat FL therapy. The FL (for both LR and HR patients, including those with CNS disease) and SL regimens are outlined in Table I. Treatment cycles subsequent to the first cycle were commenced when the absolute neutrophil count (ANC) was ≥ 1.0 × 109/l and the platelet count (PLT) was ≥ 75.0 × 109/l for both FL and SL therapy. For FL therapy, the intent was to initiate subsequent cycles every 15 days whenever possible and for SL therapy, every 21 days.
Table I.
Protocol INCTR 03-06 Chemotherapy Regimen.
| Regimen | Chemotherapy | Dose and schedule |
|---|---|---|
| First Line (FL)1 | Cyclophosphamide | 1200 mg/m2 i.v., day 1 |
| Vincristine2 | 1.4 mg/m2, i.v., day 1 | |
| Methotrexate | 75 mg/m2, i.v., day 1 | |
| Methotrexate | 12 mg, IT days 1 and 83 | |
| Cytarabine | 50 mg, IT, day 44 | |
| Second Line (SL)5 | Etoposide | 60 mg/m2, i.v., days 1–3 |
| Ifosfamide | 1500 mg/m2, i.v., days 1–3 | |
| Mesna | 300 mg/m2, i.v. with Ifosfamide, then × 3 doses every 3 hours post Ifosfamide, days 1–3 | |
| Cytarabine | 100 mg/m2, i.v., days 1–3 | |
| Cytarabine | 50 mg. IT, day 44 | |
| Methorexate | 12 mg, IT days 1 and 83 | |
| Repeat First Line (RFL) | Same as FL | Same as FL |
Each cycle subsequent to the first was given every 15 days and when the absolute neutrophil count (ANC) was ≥ 1.0 × 109/l and the platelet count (PLT) was ≥ 75.0 × 109/l. High-risk patients received a total of 6 cycles and low-risk, 3 cycles. IT therapy was given in all 6 cycles for patients with CNS disease.
The maximum dose of Vincristine given was 2.0 mg.
Dosages were age-adjusted, patients ≥ 3 years received 12 mg and patients ≥ 2 and < 3 years received 10 mg.
Dosages were age-adjusted, patients ≥ 3 years received 50 mg and patients ≥ 2 and < 3 years received 40 mg.
Each cycle subsequent to the first was given every 21 days and when the ANC was ≥ 1.0 × 109/l and the PLT was ≥ 75.0 × 109/l. Patients received a total of 4 cycles.
Supportive care
All patients were to be hospitalized and receive oral and intensive intravenous hydration and allopurinol prior to the initiation of treatment as described in the protocol. Prior to commencing treatment, patients were monitored carefully for adequate urine output. Due to the costs of measuring serum electrolytes and uric acid, some institutions were unable to perform these tests after treatment had been initiated.
Febrile neutropenic patients were treated according to institutional procedures and standard guidelines for managing such episodes that were provided in the protocol. Blood products were also given according to institutional guidelines.
The majority of patients treated at ORCI and KNH remained hospitalized or within the vicinity of the treating institution due to the generally long distances between the patients’ residence and the institution, and concern regarding poor access to emergency care whilst at home, e.g, for febrile neutropenia. In the two Nigerian centres, however, patients were often discharged between treatment cycles.
After therapy, follow-up visits were scheduled as out-patient visits at the treating hospital, but those who failed to attend for an appointment were visited at home or their families and neighbors were contacted by mobile phone to determine whether the patient remained well.
Evaluation of response
Physical examination of all clinically documented sites of disease was performed prior to the commencement of each cycle. Repeat ultrasounds of the abdomen and pelvis and chest X-rays (if abnormal at presentation) were performed after the second or third treatment cycle.
Patients were considered to have achieved a CR if clinically detected sites of disease were no longer present and/or if abnormalities detected by ultrasound or other imaging studies had resolved. In some cases, a determination of CR was delayed as some abnormalities (deformities or fibrous masses) caused by bony destruction from large jaw tumours, or abnormalities resulting from cranial nerve palsies or spinal cord compression or infarction at presentation, often resulted in prolonged or permanent abnormalities (e.g., bony deformities, facial paralysis and paraplegia often never resolved although patients may have no residual tumour). In such patients, the patient was not considered to be in CR, even if the patient was otherwise free of disease and residual abnormalities stable, until one year from the start of initial treatment (after which time, disease recurrence is very uncommon).
Partial response (PR) was defined as a greater than 50% reduction in tumour size at all measurable sites, but not otherwise meeting the definition of CR. Patients were considered to have had no response (NR) if they failed to respond or progressed (an increase in the largest tumour diameter of 25%) during the initial two cycles. Patients who died early in the course of treatment, who abandoned treatment or who were lost to follow up prior to the initial response assessment, were not evaluated (NE) for response.
Data Management
Each centre was provided with funding for a data manager and data managers received basic training in their tasks. Case report forms (CRFs) and a detailed protocol document that was agreed upon by all institutional principal investigators were provided by INCTR. A standard consent form that could be translated into the local language(s) was also provided by INCTR and copies of consent forms were (and are) kept on file locally. Data was entered into an on-line database, also provided by INCTR. CRFs were checked at participating centres prior to being submitted on-line to the INCTR central data base in Brussels, after which time, data could not be modified locally. Submitted data were again checked for possible errors and queries generated and sent to the centres when possible errors were identified. On-site monitoring for each centre was performed at least once to evaluate data management procedures and to verify data recorded and submitted with source documents.
Statistical methods
Survival
The OS and EFS were calculated using the Kaplan-Meier method. OS was calculated from the date of the start of the first cycle of FL therapy to the date of last follow up or date of death. EFS was calculated from the date of the start of the first cycle of FL therapy to the date of last follow up or date of an event. Events were defined as early deaths or failure of FL therapy – either due to PR or NR or to relapse or death during remission. In the analysis of salvage therapy with either SL or repeat FL therapy, OS was calculated from the first day of salvage therapy to the date of last follow up or date of death. Comparisons of EFS and OS among centres and between sites and stages of disease were calculated using the log rank and likelihood ratio tests of the Cox proportional hazards model. Corrections for multiple comparisons were made by the Hochberg method. Results were considered significant when the p value was <0.05.
Patient Characteristics
Patient characteristics were summarized according to frequencies and expressed as percentages. The Fisher’s exact test and the Fisher-Freeman-Halton test were used to assess differences between categorical characteristics and the Wilcoxon rank sum test was used to compare quantitative characteristics.
Results
Patient Characteristics
A total of 356 patients were accrued on study between August 2004 and December 2009. The patient characteristics are shown in Table II. Patients ranged in age from 2 to 59 years with a median age of 7 years. The majority of patients (96.4%) were aged between 2 and 15 years. There were 230 males and 126 females. Most patients (90.9%) had multiple sites of disease at presentation. Jaw involvement was the number one site of involvement and was present in most patients (216, or 60.7%). This was followed by abdominal/pelvic involvement, which occurred in 198 (55.6%) patients. Some patients had involvement of both of these sites: approximately one third (75) of the 216 patients with jaw tumours (whether maxillary, mandibular or both) had abdominal involvement. Among the 198 patients with abdominal disease, 75 (37.9%) had jaw involvement, 47 involved the maxilla alone, 10 the mandible alone and 18 involved both the maxilla and mandible. Thus, a total of 75 (21.1% of the entire study population) patients had both jaw and abdominal disease and 17 (4.8%) had neither jaw nor abdominal disease. Orbital involvement was present in 74 patients (20.8%) and occurred approximately 3 times as often in patients with jaw tumours as in those without jaw tumours - 59 (27%) versus 15 (10.7%). This difference was statistically significantly different by Fisher’s exact test (p=0.0002). Orbital tumours were also associated with CNS disease. Baseline CSF examinations were performed in 171 patients (48%), of whom 14 (8.2%) were positive for malignant cells. One of these patients also had cranial nerve involvement. Five other patients had cranial nerve palsies, but did not have a diagnostic CSF examination. Among the 171 patients in whom CSF was examined, 10 of 34, or 29% of patients with orbital tumours had malignant CSF pleocytosis. In comparison, 9 (7%) of 137 patients without orbital disease had CSF involvement (p=0.0007 by Fisher’s exact test). This led to further exploration of the association between jaw tumours and orbital tumours. In these same 171 patients in whom CSF was examined, orbital tumours occurred in 20 out of 61, or 33% of patients with maxillary tumours versus 14 of 110 or 13% of patients without maxillary tumours (p=0.0025). The association between orbital and maxillary tumours was almost identical in patients in whom CSF was not examined. Orbital involvement was, however, equally common among patients with mandibular tumours (24 out of 121, or 20%) versus patients without mandibular tumours (10 out of 50, or 20%) in the 171 patients whose CSF was examined, and numbers were remarkably similar in those in whom CSF was not examined. Patients with jaw tumours were slightly younger than those without jaw tumours in all four centres. Although this did not reach significance when individual centres were considered alone, when all the data was included and stratified by centre, jaw tumours were significantly more common in younger patients (p=0.015 by the Wilcoxon rank sum test). Peripheral lymphadenopathy occurred in 72 patients (20.2%); among the 188 (52.8%) patients who had a BM examination prior to therapy, 27, or 14.4% were positive. Eleven patients had paraplegia at presentation, but this was not associated with CSF involvement.
Table II.
Presentation Features of 356 Previously Untreated Patients.
| Characteristics | Number | (%) |
|---|---|---|
| Age (in years) | ||
| Range | 2 – 59 | |
| Median | 7 | |
| ≥2 – ≤ 7 | 206 | 57.9 |
| >7– ≤ 15 | 137 | 38.5 |
| >15 – ≤ 21 | 6 | 1.7 |
| > 21 | 7 | 2.0 |
| Sex | ||
| Males | 230 | 64.6 |
| Females | 126 | 35.4 |
| Sites of Disease at Presentation | ||
| Jaw (120 maxilla, 52 mandible and 44 both) | 216 | 60.7 |
| Abdominal and/or pelvic | 198 | 55.6 |
| Orbit | 74 | 20.8 |
| Peripheral lymphadenopathy | 72 | 20.2 |
| Bone marrow1 | 27 | 14.4 |
| CSF2 | 14 | 8.2 |
| Paraplegia | 11 | 3.1 |
| Cranial nerve palsies | 11 | 3.1 |
| Bones | 9 | 2.5 |
| Chest | 9 | 2.5 |
| Other sites | 25 | 7.0 |
| Multiple Sites of Disease at Presentation3 | 299 | 90.9 |
| HIV Status4 | ||
| Negative | 310 | 95.4 |
| Positive | 15 | 4.6 |
Pre-treatment bone marrow examinations were not performed in 168 patients (47.2%)
Pre-treatment cerebrospinal fluid (CSF) examinations were not performed prior to treatment or at the time of the administration of the first intrathecal (IT) therapy in 185 patients (52%).
27 patients who had single sites of disease, but who did not have either a pre-treatment bone marrow examination and a pre-treatment CSF examination were considered unknown when calculating the percentage of patients with multiple sites of disease.
HIV testing was not performed in 31 patients (8.7%)
A total of 325 patients were tested for HIV and 15 (4.6%) were HIV-positive. Eleven of the patients were less than 11 years of age and 4 patients were above the age of 40. The presentation features of patients with HIV did not differ from those who were HIV-negative when compared using the Fisher’s exact test. However, the number of HIV-positive patients was small; a much larger study would be necessary to compare the tumour sites at presentation in HIV+ and HIV − patients.
Risk Criteria and Stage
Approximately 75% of enrolled cases underwent histological/cytological review and could be considered at least as aggressive B cell lymphomas, even if a further definition could not be agreed upon. According to the protocol-defined risk criteria, it was noted that 345 were HR, 6 were LR and another 5 could not be classified as either HR or LR (due to the lack of BM or CSF examination), although at the treatment sites, all patients except one were classified as HR. All but one of the 356 patients were treated according to the HR arm of the protocol. The single patient treated as LR was mistakenly classified.
Not all patients could be retrospectively staged because of the lack of BM or CSF examination. Among all patients, 172 were staged according to both the St Jude and LTC systems and another 80 were staged according to the LTC system only. The majority of patients who could not be staged according to either the LTC and the St Jude staging systems were patients from ORCI (Table III).
Table III.
Risk Stratification and Stage of 356 Patients.
| Risk Stratification1 | Number | (%) |
|---|---|---|
| High Risk | 345 | 96.9 |
| Low Risk | 6 | 1.7 |
| Unknown | 5 | 1.4 |
| LTC Stage | ||
| A | 20 | 5.6 |
| UA2 | 27 | 7.6 |
| B | 111 | 31.1 |
| C | 60 | 16.8 |
| UC3 | 77 | 21.6 |
| D | 61 | 17.1 |
| St Jude Stage4 | ||
| I | 19 | 11.0 |
| II | 33 | 19.2 |
| III | 75 | 43.6 |
| IV | 45 | 26.1 |
Retrospective “risk stratification” confirmed that the risk group was unknown for 5 patients (1.4%) because pre-treatment bone marrow (BM) and/or cerebrospinal fluid (CSF) examinations were not performed.
UA is defined as meeting the criteria for Stage A, but unknown with certainty to be stage A because a pre-treatment BM and/or CSF examination was not performed.
UC is defined as meeting the criteria for Stage C, but unknown with certainty to be stage C because a pre-treatment BM and/or CSF was not performed.
Because pre-treatment BM and/or CSF examinations were not performed, it was not possible to determine the St. Jude Stage for 184 patients (51.7%). The percentage was based upon the total number of patients who had these tests performed.
LTC, Lymphoma Treatment Centre
Response to Treatment and Subsequent Treatment
Of the 356 patients enrolled on the protocol, 270 achieved CR (75.8%). Forty-six were considered to have had PR (12.9%) and 10 had NR (2.8%). Thirty patients (8.4%) could not be evaluated for response due to early deaths (24) and abandonment of treatment with subsequent loss to follow up (6). Early deaths within the first cycle were due to tumour lysis syndrome (10), several of which may have been avoidable, infection (7), progressive disease (4) and sudden respiratory arrest (1). Two other patients who were not evaluated for response refused treatment after the first cycle and subsequently died, but the cause of death was unknown.
Among the 326 patients evaluable for response, those who had abdominal involvement had a worse CR rate compared to those who did not (138, or 78.5% versus 132, or 88.0%). There was no difference in response rate in patients who had jaw involvement compared to those who did not. Patients with BM involvement were slightly less likely to achieve CR than those without BM involvement (79% versus 84%) and slightly more likely to have NR (11% versus 4%), but these differences did not achieve statistical significance. A total of 125 patients did not achieve continuous CR. Fifty-six had a PR or NR to FL and 69 patients relapsed after achieving CR (25.5%). Of these 125 patients, 54 received SL due to early relapse or PR/NR, 19 patients received repeat FL for late relapse followed by SL treatment or for PR, 17 received repeat FL only for late relapse and 35 patients received no treatment at the time of relapse or progression of disease. Among the 73 patients who received SL treatment for failure of initial FL therapy or repeat FL therapy, there were 28 (38.3%) who achieved a CR. Of the 54 who received SL due to early relapse or PR/NR, 23 (42.6%) achieved a CR, 15 (25.9%) a PR and 15 (27.8%) had NR (two were not evaluable). There were 12 CRs among the 21 who originally achieved a CR, 10 among the 29 who originally achieved a PR and 1 among the 4 who originally had no response. Among the 17 patients who received repeat FL for late relapse, 10 (59%) achieved a CR (Table IV). Of the 18 who received SL after repeat FL, 3 patients achieved a CR and 4 a PR.
Table IV.
Response to Treatment
| Response | Number | (%) | |
|---|---|---|---|
| First-Line (FL) Treatment | CR | 270 | 75.8 |
| PR | 46 | 12.9 | |
| NR | 10 | 2.8 | |
| NE1 | 30 | 8.4 | |
| Total | 356 | ||
| Second-Line (SL) Treatment | CR | 27 | 37.0 |
| PR | 18 | 24.6 | |
| NR | 25 | 34.2 | |
| NE2 | 3 | 4.1 | |
| Total | 73 | ||
| Repeat FL Treatment3 | CR | 19 | 52.8 |
| PR | 5 | 13.9 | |
| NR | 8 | 22.2 | |
| NE4 | 4 | 11.1 | |
| Total | 36 |
Not evaluated for response to FL: 22 deaths during the first cycle (10, tumour lysis syndrome, 7, infection, 4, progressive disease, 1, respiratory arrest); 2 deaths due to unknown reasons following the first cycle, but prior to first evaluation; and 6 were lost to follow up before evaluation.
Not evaluated for response to SL: 2 deaths during the first cycle (1, infection and 1, hemorrhage) and 1 was lost to follow up before evaluation.
Patients received Repeat FL for late relapses.
Not evaluated for Repeat FL: 4 early deaths (3, infection and 1, progressive disease).
CR, complete response; PR, partial response; NR, no response; NE, not evaluated.
Sites of Relapse
Of the 270 patients who achieved a CR there were 69 relapses. The majority of relapses were in the abdomen/pelvis (32) of whom 28 patients had tumour in the abdomen or pelvic at presentation (9 were stage D, the remainder, stage C or UC [defined as meeting Stage C criteria but unconfirmed because pre-treatment BM and/or CSF examinations not performed]). Relapse in the jaw (maxilla, mandible or both) occurred in 20 patients and CNS (CSF + cranial nerve palsy and one “brain” recurrence) in 9 (3.3%). None of the patients who relapsed in the CSF had CNS disease at presentation, but 5 of 9 had a maxillary tumour at presentation, 3 of whom also had orbital involvement. There were 8 relapses in the orbit, 1 extradural (paraplegia), 6 relapsed with peripheral lymph node disease and 3 relapsed with chest involvement. A further 8 patients relapsed at miscellaneous sites, 3 of which were not specified.
Toxicity and Treatment Delays
The degree of myelosuppression, presence of culture-proven infections, hepatic and renal toxicity proved difficult to assess in the majority of patients. Patients could not afford the laboratory tests and frequently, equipment failed or reagents were out of stock, rendering measurements of routine laboratory tests difficult. In addition, not all institutions had the capability of performing routine bacteriological cultures and sensitivities in the presence of fever, although malarial smears were always available. For these reasons, delays in starting cycles subsequent to the first cycle proved to be a more useful indicator of significant toxicity. In cycles subsequent to the first cycle in both the FL regimen and the SL regimen there were no treatment delays in the majority of cycles, which were initiated within the time period specified by the protocol in 83.2% and 81.3% of cases, respectively. Myelosuppression was known to have caused a delay in treatment in 11.0% of FL cycles and 12.5% of SL treatment cycles. Other toxicities, including infections such as chicken pox, and other reasons for delays in treatment are detailed in Table V.
Table V.
Delays in Initiation of Cycles Subsequent to Cycle 1 of First-Line and Second-Line Treatment.
| First-Line Treatment | No. of Cycles | (%) |
|---|---|---|
| No delays in treatment | 1131 | 83.2 |
| Myelosuppression | 149 | 11.0 |
| Failure to keep appointment | 28 | 2.0 |
| Other toxicities | 14 | 1.0 |
| Chicken pox/infection | 12 | 0.9 |
| Drug supply problems | 9 | 0.7 |
| Other reasons1 | 17 | 1.2 |
| Total cycles2 | 1360 | |
| Second-Line Treatment | ||
| No delays in treatment | 117 | 81.3 |
| Myelosuppression | 18 | 12.5 |
| Failure to keep appointment | 3 | 2.1 |
| Chicken pox/infection | 2 | 1.4 |
| Drug supply problems | 1 | 0.7 |
| Other reasons3 | 3 | 2.1 |
| Total cycles4 | 144 |
Note: Treatment delays were defined as those that resulted in a greater than one week delay in starting treatment cycles subsequent to the first cycle for each regimen.
Other reasons included: financial constraints (13); delays in obtaining laboratory results (1); general strike (1); family given incorrect appointment date (1); and difficulty maintaining intravenous access (1).
Total cycles includes Cycles 2 to 6 for first-line treatment.
Other reasons included: financial constraints (1); surgery (1); and not stated (1).
Total cycles includes Cycles 2 to 4 for second-line treatment.
Survival
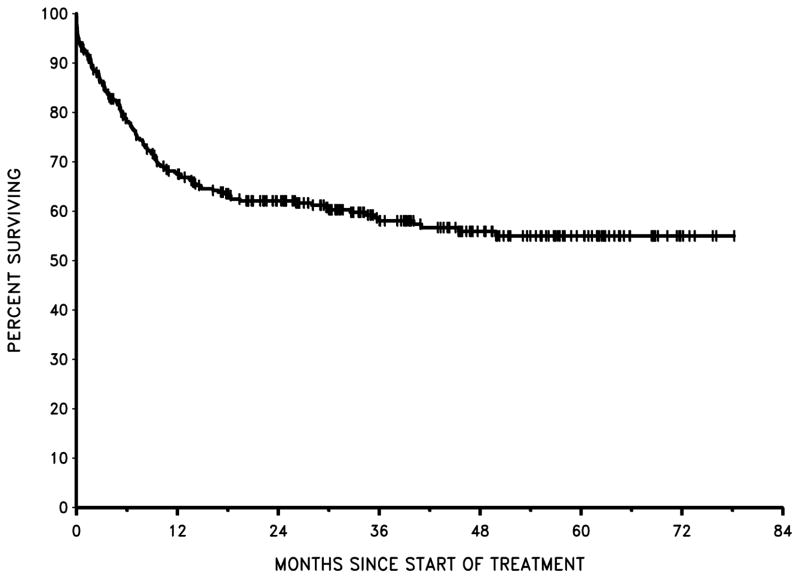
With all patients included in the analysis, EFS was 54% at 1 year and 52% at 2 years. OS was 67% at 1 year and 62% at 2 years (Figure 1). There was no significant difference in EFS among the four centres. The EFS probabilities at 2 years by centre were as follows: KNH, 59%; UCH, 43%; OAUTHC, 48%; and ORCI, 53%.
Figure 1.
Overall Survival: all patients
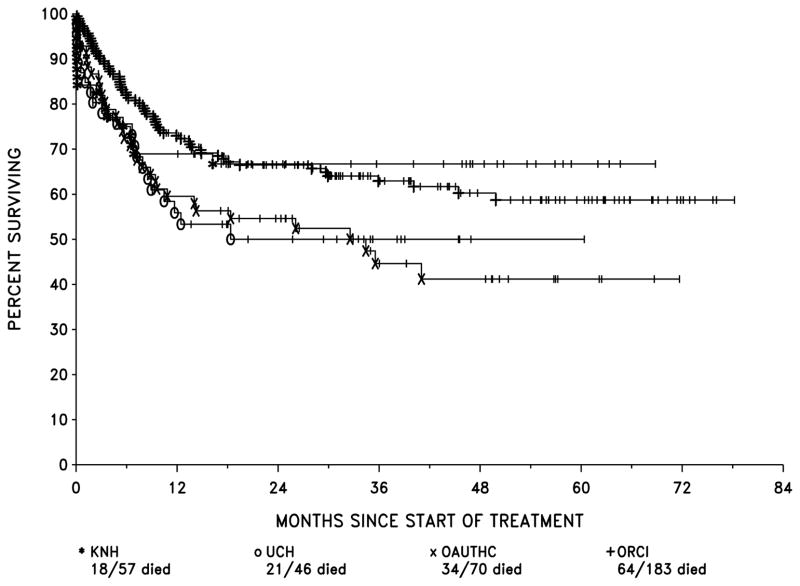
The OS probabilities by centre were: KNH, 69% at 1 year, 67% at 2 years; UCH, 56% at 1 year, 50% at 2 years; OAUTHC, 60% at 1 year, 55% at 2 years; and ORCI, 73% at 1 year and 66% at 2 years (Figure 2). Survival results for ORCI and KNH combined were significantly superior to those of the two Nigerian centres, UCH and OAUTHC, combined (p=0.0086). When corrected for the seven possible tests of every combination of centres against the others combined, p=0.06. In the Nigerian centres, 45 patients had PR/NR or relapse and 18 (40%) were not given any treatment after failure of primary therapy. In KNH and ORCI there were 80 such patients, of whom 17 were not treated (21.3%). This accounts for some part of the differences in OS between the Nigerian centres and ORCI and KNH considered together.
Figure 2.
Overall Survival by centre
BM involvement in the 188 patients in whom the marrow was examined at presentation was associated with a somewhat worse survival. OS, which at 6 months was 53% with BM involvement versus 78% without it, and at 12 months 45% versus 66%, was significantly different (p=0.027)
There was no difference in EFS and OS in patients with CNS disease (although numbers were small) or those with HIV infection (although again, numbers were small).
Stage and Survival
St Jude Stage
Although it was not possible to stage 177 of the 183 patients from ORCI according to the St Jude staging system due to the inability to perform BM and/or CSF examinations (only 3% had both examinations), there were no significant differences in EFS and OS with respect to patients from any centre to whom a St Jude stage could be assigned. The numbers of patients in each stage were evenly distributed among the centres, the majority being Stage III and the least common, Stage I. The percentage of patients within each stage having an event suggested a trend towards fewer events in Stage I and II patients versus those in Stages III and IV, but this did not reach statistical significance. The same was true for OS.
LTC Stage
Prior to analysing differences among LTC stage, patients who were confirmed as Stage A by virtue of CSF and BM examinations were analysed separately from UA patients, who could not be confirmed as Stage A because of the lack of pre-treatment BM and CSF analyses). Similarly, Stage C and Stage UC (unknown if BM and CSF were positive) were compared with respect to differences in EFS and OS. There were no statistical differences between Stage A versus Stage UA patients or Stage C versus Stage UC. Therefore, for purposes of comparing differences among LTC stages, the Stage A and UA patients were combined as were the Stage C and UC patients.
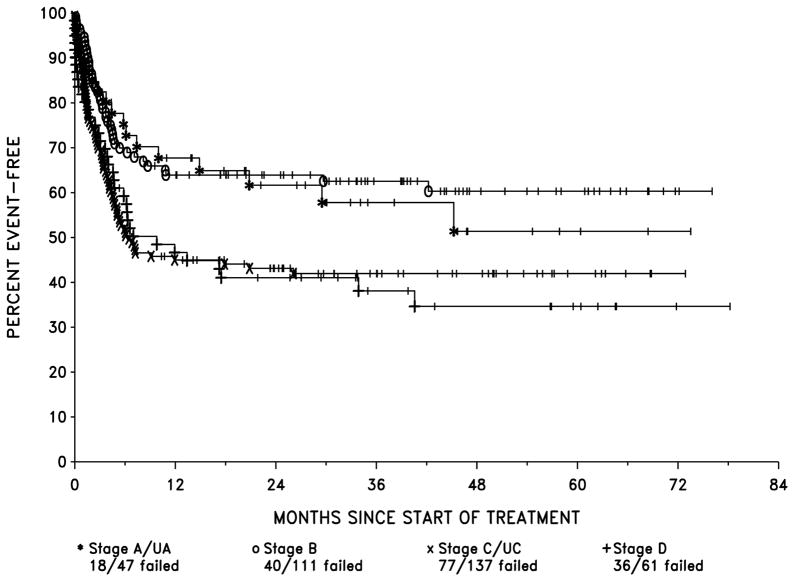
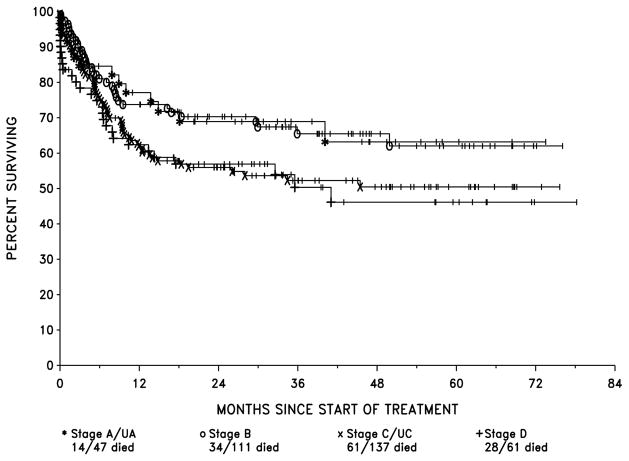
EFS probabilities by LTC stage were : stage A/UA, 68% and 62% at 1 and 2 years respectively, stage B, 64% at both 1 and 2 years, stage C/CU, 45% and 43% at 1 and 2 years and stage D, 47% and 41% at 1 and 2 years (Fig 3). OS by LTC stage for stage A/UA was 77% and 69% at 1 and 2 years respectively, stage B, 74% and 70% at 1 and 2 years, stage C/UC, 61% and 55% at 1 and 2 years and stage D, 62% and 57% at 1 and 2 years (Fig 4).
Figure 3.
EFS by LTC Stage
Figure 4.
Overall Survival by LTC Stage
There was a significant difference in EFS, between the four stages (p=0.005) by the likelihood ratio test of the proportional hazards model when corrected for multiple comparisons) although the difference among the stages with respect to OS was not significant (p=0.15). The latter, of course, is influenced by salvage therapy, which improves outcome in those patients in whom initial therapy fails.
Patients in stages A, UA and B differed only slightly with respect to EFS and OS. Similarly, patients in stages C, UC and D patients had similar EFS and OS. However, stages A/UA and B patients combined differed significantly from stages C/UC and D. In fact, combining stages in this way resulted in the creation of two groups, which differed only with respect to the presence or absence of abdominal involvement. These groups also differed significantly with respect to EFS (p=0.0002, estimated hazard ratio (HR) = 1.81, using the proportional hazards model) and OS (p=0.0092, HR = 1.59).
Differences in Stage among the 4 Centres
KNH, UCH and ORCI had roughly balanced proportions of patients in the two stage groups; Stages A/UA and B and Stages C/UC and D whereas 70% of OAUTHC patients were in the C/UC and D stage group. EFS did not differ among the centres however, and this remained true after the adjustment for LTC stage was made. The much higher proportion of stages C/UC and D patients at OAUTHC explains, at least in part, its lower OS compared to the other centres.
Salvage Therapy
Second-Line Treatment
Of the 73 patients who received SL treatment, the estimated OS from the time of the start of SL treatment was 44% at 6 months, 33% at one year and 27% at 2 years. One patient who received SL treatment suffered a “late relapse” and was re-treated with repeat FL and achieved a CR. For the other 72 patients, the median survival time was 5.1 months (95% confidence interval, 3.9 7.7 months). Three patients remain alive at 60, 64 and 71 months after the start of SL therapy. Patients with stages C/UC and D at the time of their original presentation tended to have better survival at 12 months (37% versus 19% in the Stage A/UA and B patients), but this difference was not significant p=0.20 by the log rank test.
Repeat First-line Treatment
Patients who relapsed “late” received repeat FL treatment. A total of 36 patients received repeat FL and 18 required additional treatment with SL therapy due to PR or NR to repeat FL (10 patients) or early relapse from repeat FL (8 patients). One patient had received SL treatment for an early relapse, but then subsequently relapsed “late” and received repeat FL.
Seventeen patients received repeat FL only. Three died early in the course of treatment prior to evaluation, but with evidence of disease, 4 had PR or NR to repeat FL and died, and 10 achieved a CR. Of the 10 who achieved a CR, two died in remission while one died following a subsequent late relapse of disease. The remaining 7 patients are alive and in remission with a range of survival times from 4 to 63 months. Thus, among the late relapsing patients, some achieved CR followed by up to 5 years of additional survival when treated with the same therapy used at presentation.
Causes of Death
A total of 138 of the 356 patients died (38.9%) (Table VI). Twenty-four patients (17.4%) died during the first or second cycles of treatment, 26 (18.8%) died while in remission and 88 (63.8%) died of progressive disease or other complications while still not in remission.
Table VI.
Causes of Death
| No. of Patients | % of Deaths | |
|---|---|---|
| Total deaths | 138 | |
| Deaths Prior to First Evaluation | 24 | 17.4 |
| Tumour lysis | 10 | |
| Infection | 6 | |
| Disease | 4 | |
| Infection and bleeding | 1 | |
| Respiratory arrest | 1 | |
| Unknown | 2 | |
| Remission deaths1 | 26 | 18.8 |
| Infection2 | 9 | |
| Bleeding | 1 | |
| Gastrointestinal complications | 1 | |
| Cardiomyopathy | 1 | |
| Central nervous system complications | 1 | |
| Multiple organ toxicity | 1 | |
| Unknown cause, but last seen in remission | 12 | |
| Treatment Failures | 88 | 63.8 |
| Disease | 61 | |
| Infection | 4 | |
| Bleeding | 1 | |
| GI complications | 1 | |
| Unknown, but no further treatment planned | 21 |
Four deaths in remission occurred following repeat first-line therapy (2) and second-line therapy (2).
Of the 9 deaths in remission that were due to infection, 3 were attributed to malaria, 2 to sepsis, 2 to meningitis, 1 to measles and 1 to chest infection.
Treatment Compliance
First-line Treatment
Of the 356 patients, 227 patients (63.8%) completed planned FL treatment. Of the 129 patients who did not complete planned treatment, 24 died early during treatment, prior to evaluation for response, 38 (29.5%; 10.7% of the entire population) were changed to SL therapy due to PR or NR to FL or early relapse during FL treatment and an additional 21 patients died of progressive disease without receiving any treatment for relapse or PR/NR. Eleven patients died in CR during FL. Twenty-one patients abandoned therapy but were known to be alive and in CR at the time of last follow up. Of these 21 patients, one patient who did not complete FL had a late relapse which was treated with repeat FL therapy and 14 patients (3.9%) abandoned treatment and could not be followed, such that their outcome is unknown. The outcome of therapy is not known in 15 of the 227 who completed FL treatment. When last seen, these patients had a follow up time of less than one year. In summary, a total of 29 (8.1%) of the 356 patients were lost to follow up.
Second-Line Treatment
Of the 73 patients who required SL treatment for early relapse or PR/NR, 34 (46.6%) completed all 4 cycles of planned therapy. Of the 39 who did not complete treatment, 32 died without achieving CR, 3 died prior to evaluation, 1 died of toxicity, 1 was lost to follow-up and 2 are known to be alive and in CR. Of the 34 who completed all 4 cycles of therapy on SL, 15 remain alive and in CR, 14 died of disease, 1 patient died in CR and 4 were lost to follow up – 1 who achieved CR and 3 who failed to respond to SL. Therefore, a total of 5 of the 73 patients who received SL were lost to follow up (6.8%).
Discussion
This study, performed by the INCTR African BL Strategy Group, is the largest published treatment study of BL in equatorial Africa to date, and was conducted in several major hospitals in three countries. The hospitals, although tertiary care centres, varied with respect to the social assistance provided to patients and this is probably an important determinant of outcome, because it influences the quality of care (e.g., blood transfusions, antibiotics for febrile neutropenia, etc.) (Meremikwu et al, 2005). Outcome may also have been influenced by delays caused by the cost of transportation to the few tertiary care centres capable of diagnosing and treating cancer patients and the paucity of skilled pathologists and oncologists, even in these centres. In spite of major deficiencies in resources, however, many lives can be saved when patients are treated with a standardized protocol in a disciplined manner and data regarding the delivery and outcomes of therapy is collected and monitored in a central data centre. Participation in multicentre treatment protocols, such as the present one, lead to a greater understanding of research methodology and infrastructure, and stimulate improved diagnosis and clinical care due to both external and internal surveillance. Moreover, when the same treatment protocol is used over time, results may well improve because of increasing familiarity with toxic side effects and their management (Magrath et al, 2005). The participating centres in the present study had little or no information about the outcome of the treatment they were using prior to INCTR 03-06, because a high fraction of their patients never completed therapy, and those who did were not followed up. However, the use of mobile telephones and the introduction of mechanisms to trace patients who did not return for follow up in this study enabled an accurate picture of remission duration to be achieved and there can be no doubt that the survival rate, of 67% at one year and 62% at two years, achieved with INCTR 03-06 represents a remarkable improvement in the outcome of therapy in these centres.
A number of additional observations emerged from the characterization of patients enrolled on protocol INCTR 03-06. Firstly, the distribution of tumour sites in African, or endemic BL has not changed markedly over an almost 40-year period, particularly with respect to the frequency of jaw and abdominal tumour (Magrath, 1991). Secondly, there was a strong correlation between orbital and maxillary involvement, suggesting that at least some orbital tumours are likely to arise by extension from the maxilla. And thirdly, there was a significant association between the presence of malignant CSF pleocytosis and orbital tumours, suggesting that CSF involvement may sometimes arise by direct extension to the CNS via the orbit. Post mortem examinations performed many years ago (Wright, 1964) demonstrated involvement of cranial nerves by tumour cells, and involvement and passage along the leptomeningeal nerve sheath of the III, IV, and VI nerves (the most commonly involved in BL) that pass through the orbit is likely to be at least one pathway by which tumour cells spread to the CSF. The low incidence of relapse in the CSF in the present study suggests that the IT therapy specified in the protocol is sufficient to prevent CSF dissemination in the majority of cases, although it is of interest that among the few patients who developed CSF recurrence, several had presented with maxillary tumours.
The results obtained with INCTR 03-06 also suggested that patients were divisible into two major risk categories - with or without extensive abdominal disease. This is consistent with earlier African data (Magrath et al, 1974; Magrath, 2009). It would be of value to determine whether a simple objective test, such as the serum lactate dehydrogenase level, which is associated with total tumour burden, would add to this clinical separation (Magrath et al, 1980; Arthur et al, 2011). If this were the case, this test would mean that even simple imaging studies, such as abdominal ultrasound, (Marjerrison et al, 2012) would be unnecessary, avoiding further delays and the cost associated with the performance of imaging studies in Africa, where patients are many, and equipment and health professionals in short supply.
One difficulty encountered in analysing the results of the present study was the lack of data regarding CSF and BM involvement in patients treated at one of the centres. While this created difficulties in comparisons of clinical stage among centres, there was little evidence that the inability to perform these examinations had any impact on treatment outcome, consistent with data obtained in Uganda many years ago (Ziegler et al 1979). Although patients with known CSF malignant pleocytosis were treated with additional IT therapy, the therapeutic value of this has not been demonstrated in the context of protocol INCTR 03-06. Patients with BM involvement had a somewhat worse prognosis but the difference in outcome was not large, and the importance of BM involvement to outcome may well be protocol dependent. With respect to CSF examination, most hospitals in Africa do not have access to a cytocentrifuge machine, without which CSF cytology is of very poor quality. It is probably better to treat patients without this information rather than incur significant delay in the initiation of therapy, or additional hardship to poor families who may have to pay for these tests out of their own pocket. Thus, the design of protocols, such as that of Hesseling et al, (2012), in which the presence of CSF and BM involvement is a determinant of therapy, should be carefully considered in the African context. Similarly, the exclusion of patients with BM or CNS involvement on the grounds that they have a poor prognosis (extrapolated from results obtained in technologically advanced countries using different protocols) is probably not justified (Kazembe et al, 2003). The existing evidence is consistent with the probability that the most important risk factor for treatment outcome is the overall tumour burden rather than the presence or absence of CSF pleocytosis per se (Haddy et al, 1991).
While combination therapy of the type used in protocol INCTR 03-06 is more expensive than monotherapy with CTX, the overall survival of 67% at 1 year and 62% at 2 years (relapse is rare after disease-free survival of a year), especially in the context of a number of potentially avoidable deaths (e.g., due to poor management of tumour lysis syndrome in one centre) appears to be superior to the earlier results achieved with CTX alone (Kazembe et al, 2003; Hesseling et al, 2009). They are similar to recent results reported from Abidjan of therapy involving CTX, Ara-C and MTX (Koffi et al, 2012) and to results from Cameroon using higher dose CTX and IT MTX followed by stage and response-specific therapy (Hesseling et al, 2012). The published literature is consistent with the idea that combination therapy is superior in African BL to the use of CTX as the sole systemic drug (Magrath, 2012), although the randomized trial in which this was examined was carried out prior to the development of effective CNS prophylaxis and involved very small numbers (Olweny et al, 1976). CTX as the sole systemic agent with IT MTX is often used because it is more affordable for African patients. In studies conducted in Malawi, however, CTX monotherapy was found to be inferior for patients with St Jude stages III and IV disease (Kazembe et al, 2003, Hesseling et al, 2009). Similarly, in a recent multicentric, prospective study published by the French-African Paediatric Oncology Group, it was reported that CR with standard dose CTX monotherapy was 47% with 33% EFS, and the majority of patients (76/108 eligible patients) progressed rapidly and received second-line therapy with MTX, VCR, Ara-C, and hydrocortisone (Traoré et al, 2011). The CR rate after second line therapy was 35.7% and the toxic death rate 21%. The authors concluded that CTX may be recommended in stages I and II because of an optimal cost-benefit ratio, but more intensive therapy is required for stages III and IV (Traoré et al, 2011).
The survival (cure) rate of BL in the more developed countries is approximately 90% (Magrath et al, 1996; Adde et al, 1998; Patte et al, 2001, Magrath, 2009), although use of the intensive regimens for high risk patients in these protocols, as described, would be too toxic for use in African institutions (Hesseling et al, 2003). However, the efficacy of these protocols, all of which use at least 6 drugs systemically for patients with advanced disease, may depend as much on the number of drugs used as on their doses, and lower dose multidrug regimens could improve outcome without greatly increasing toxicity. The data presented on the SL salvage regimen used in poor responders and relapsed patients in the present study, which demonstrated that CR could be obtained by SL therapy even in patients with partial or no response during FL therapy, or relapse shortly after FL therapy, indicates that SL is non-cross reactive with FL. Further, data reported from other developing countries in which similar alternating regimens have been used (Gad-el-Malwa et al, 1989; Advani et al, 1997), as well as a similar approach used in the USA (Magrath et al, 1996) suggests that further improvement in the cure rate of African BL could be achieved by using SL therapy alternating with FL therapy in higher risk patients. The question of whether or not the addition of rituximab to chemotherapy would improve outcome is certainly worthy of consideration, but at present would be cost-prohibitive in equatorial Africa.
The present study indicates a number of areas where improvements can be made. Firstly, it may be time to abandon FNA as the accepted diagnostic approach to BL. Aspirates were sometimes poorly performed and the amount of material available, even with good aspirates does not permit additional studies, such as immunophenotyping, to be performed, thereby increasing the degree of uncertainty in the diagnosis. Unfortunately, immunophenotyping is rarely available in equatorial African countries because of the cost of reagents. INCTR is introducing immunophenotyping into several tertiary care centres so that in future it should be possible for at least basic immunophenotyping to be performed at major centres in all equatorial African countries (Naresh et al, 2011a; Naresh et al, 2011b).
Finally, differences in survival rates between the different centres participating in the present studies is worthy of comment. There were a number of explanations that could account for this: one was differences in the average extent of disease (the number with abdominal involvement) in each hospital series, but others included delayed institution or failure to provide SL therapy to a large fraction of patients - generally because of cost considerations. Even though INCTR provided the cost of the drugs, other cost items had to be met locally (such as the hospitalization itself). Sensitization of primary care health providers to the presenting signs of BL could well result in earlier diagnosis and improvement in outcome. Taking all of these considerations into account, and bearing in mind the higher toxicity encountered at a single centre during induction of remission, as well as the failure to provide relapse therapy at others, it seems likely that the optimal performance of this protocol has not yet been reached, although even so, it appears to be at least as effective a protocol for African (endemic) BL as any other published to date. Further studies, perhaps exploring the alternation of the non-cross reactive FL and SL regimens in high-risk patients, defined purely in terms of abdominal involvement in the first instance, would appear to be warranted.
Acknowledgments
This work was partly supported by a task order from the Office of HIV/AIDs Malignancy of the National Cancer Institute (NCI), National Institutes of Health (NIH), USA and from core funding provided via the Office of International Affairs, NCI, NIH, USA.
Cytocentrifuges and training in the use of these machines was supported by MundiPharma International Ltd., Cambridge, UK.
The INCTR UK Challenge Fund provided financial support for the purchase of chemotherapy and antibiotics through donations made to the Christopher Niblett Memorial Fund.
Georgetown University Hospital, Washington, DC, USA provided financial support for the purchase of chemotherapy and antibiotics through donations made by families and friends of paediatric cancer patients treated at the Lombardi Cancer Center.
INCTR would like to acknowledge the data managers at the participating institutions for their contributions made to this study: Seif Mkamba (OCRI), Devotha Kovaga (ORCI), Lukman Bashir (OAUTHC), Oliver Oruko (NKH), Tosin Oladejo (UCH) and it would also like to acknowledge former data managers of INCTR’s Clinical Trials Office staff, Lolita Lantican and Carol Falcon.
Our thanks are due to all hospital and laboratory staff that participated In the care of these patients.
Principal investigators, who were involved in the design and conduct of the study included Twalib Ngoma, Muheez Durosinmi, Jessie Githanga and Yetunde Aken’Ova. Other medical staff that played a major role in patient care included Jane Kaijage, Biobele Brown, Patricia Scanlan, O Adeodou and J Rajab. INCTR Pathologists who reviewed the diagnostic samples and provided training for African pathologists included Lorenzo Leoncini, Kikkeri Naresh, Martine Raphael and Nina Hurwitz. The study was coordinated by Melissa Adde, who wrote the protocol document, organized scientific and ethical review, provided annual reports to the INCTR ethical review committee, ensured that all participating enters undertook ethical review, performed monitoring visits as well as training of the data managers. Melissa Adde also provided queries in response to unclear entries in the CRFs, supervised all aspects of data collection, training of the data managers (continuing throughout the study) and played a major role in the analysis of the data and the writing of the manuscript. Ama Rohatiner assisted in training of PIs, development of the protocol and monitoring. Ian Magrath had a major role in the design of the protocol, provided advice and consultation on specific cases, and had a major role in the analysis of the data and the writing of the manuscript. David Venzon was the study statistician and was involved in the writing of the statistical section of the protocol, the analysis of the data, including choice and performance of statistical methods, and participated in the writing of the protocol.
References
- Adde M, Shad A, Venzon D, Arndt C, Gootenberg J, Neely J, Nieder M, Owen W, Seibel N, Wilson W, Horak ID, Magrath I. Additional chemotherapy agents improve treatment outcome for children and adults with advanced B-cell lymphomas. Seminars in Oncology. 1998;2(Suppl 4):33–39. [PubMed] [Google Scholar]
- Advani S, Pai S, Adde M, Vaidya S, Vats T, Naresh NK, Venzon D, Magrath I. Preliminary report of an intensified, short duration chemotherapy protocol for the treatment of pediatric non-Hodgkin’s lymphoma in India. Annals of Oncology. 1997;8:893–897. doi: 10.1023/a:1008228529397. [DOI] [PubMed] [Google Scholar]
- Arthur FK, Owusu L, Yeboah FA, Rettig T, Osei-Akoto A. Prognostic significance of biochemical markers in African Burkitt’s lymphoma. Clinical and Translational Oncology. 2011;13:731–6. doi: 10.1007/s12094-011-0724-8. [DOI] [PubMed] [Google Scholar]
- Booth K, Burkitt DP, Bassett DJ, Cooke RA, Biddulph J. Burkitt lymphoma in Papua New Guinea. British Journal of Cancer. 1967;21:657–664. doi: 10.1038/bjc.1967.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt D. A sarcoma involving the jaws in African children. British Journal of Surgery. 1958;46:218–223. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- Burkitt D. A children’s cancer dependent on climatic factors. Nature. 1962;194:232–234. doi: 10.1038/194232a0. [DOI] [PubMed] [Google Scholar]
- Burkitt D. Long-term remissions following one and two-dose chemotherapy for African lymphoma. Cancer. 1967a;20:756–759. doi: 10.1002/1097-0142(1967)20:5<756::aid-cncr2820200530>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Burkitt D. Treatment of Burkitt’s Tumour. Monograph series number 8, International Union Against Cancer. Springer; New York: 1967b. Chemotherapy of jaw tumours; pp. 94–104. [Google Scholar]
- Clifford P. Long term survival of patients with Burkitt’s lymphoma. An assessment of treatment and other factors which may relate to survival. Cancer Research. 1967;27:2578.b. [PubMed] [Google Scholar]
- Dalldorf G, Linsell CA, Barnhart FE, Martyn R. An epidemiological approach to the lymphomas of African children and Burkitt’s sarcoma of the jaws. Perspectives in Biological Medicine. 1964;7:435–449. doi: 10.1353/pbm.1964.0023. [DOI] [PubMed] [Google Scholar]
- Gad-el-Mawla N, Hussein MH, Abdel-Hadi S, el-Taneer O, Adde M, Magrath IT. Childhood non-Hodgkin’s lymphoma in Egypt: preliminary results of treatment with a new ifosfamide-containing regimen. Cancer Chemotherapy and Pharmacology. 1989;24(Suppl 1):S20–23. doi: 10.1007/BF00253233. [DOI] [PubMed] [Google Scholar]
- Haddy TB, Adde MA, Magrath IT. CNS involvement in small noncleaved-cell lymphoma: is CNS disease per se a poor prognostic sign? Journal of Clinical Oncology. 1991;9:1973–1982. doi: 10.1200/JCO.1991.9.11.1973. [DOI] [PubMed] [Google Scholar]
- Hesseling PB, Broadhead R, Molyneux E, Borgstein E, Schneider JW, Louw M, Mansvelt EP, Wessels G. Malawi pilot study of Burkitt lymphoma treatment. Medical and Pediatric Oncology. 2003;41:532–540. doi: 10.1002/mpo.10322. [DOI] [PubMed] [Google Scholar]
- Hesseling P, Molyneux E, Kamiza S, Israels T, Broadhead R. Endemic Burkitt lymphoma: a 28-day treatment schedule with cyclophosphamide and intrathecal methotrexate. Annals of Tropical Paediatrics. 2009;29:29–34. doi: 10.1179/146532809X402006. [DOI] [PubMed] [Google Scholar]
- Hesseling PB, Njume E, Kouya F, Katayi T, Wharin P, Tamannai M, Achu P, Kidd M, McCormick P. The Cameroon 2008 Burkitt lymphoma protocol: improved event-free survival with treatment adapted to disease stage and the response to induction therapy. Pediatric Hematology Oncology. 2012;9:119–129. doi: 10.3109/08880018.2011.644881. [DOI] [PubMed] [Google Scholar]
- Kazembe P, Hesseling PB, Griffin BE, Lampert I, Wessels G. Long term survival of children with Burkitt lymphoma in Malawi after cyclophosphamide monotherapy. Medical and Pediatric Oncology. 2003;40:23–25. doi: 10.1002/mpo.10190. [DOI] [PubMed] [Google Scholar]
- Koffi GK, Tolo A, Nanho DC, N’dathz E, Kouassi MY, N’Diaye FD, Kouakou B, Meité N, Ayemou R, Sekongo M, Kouehion P, Meité M, Tea ND, Sangaré A, Sanogo I. Results of treatment with CMA, a low intermediate regimen, in endemic Burkitt lymphomas in sub-Saharan Africa: experience of Côte d’Ivoire. International Journal of Hematology. 2010;91:838–843. doi: 10.1007/s12185-010-0591-z. [DOI] [PubMed] [Google Scholar]
- Magrath IT. African Burkitt’s lymphoma. History, biology, clinical features, and treatment. American Journal of Pediatric Hematology Oncology. 1991;13:222–246. [PubMed] [Google Scholar]
- Magrath I. Lessons from clinical trials in African Burkitt lymphoma. Current Opinions in Oncology. 2009;21:462–468. doi: 10.1097/CCO.0b013e32832f3dcd. [DOI] [PubMed] [Google Scholar]
- Magrath I. Towards curative therapy in Burkitt lymphoma: the role of early African studies in demonstrating the value of combination therapy and CNS prophylaxis. Advances in Hematology. 2012;2012:Article ID 130680. doi: 10.1155/2012/130680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrath I, Lee YJ, Anderson T, Henle W, Ziegler J, Simon R, Schein P. Prognostic factors in Burkitt’s lymphoma: importance of total tumor burden. Cancer. 1980;45:1507–1515. doi: 10.1002/1097-0142(19800315)45:6<1507::aid-cncr2820450634>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Magrath IT, Lwanga S, Carswell W, Harrison N. Surgical reduction of tumour bulk in the management of abdominal Burkitt’s lymphoma. British Medical Journal. 1974;2:308–312. doi: 10.1136/bmj.2.5914.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrath I, Adde M, Shad A, Venzon D, Seibel N, Gootenberg J, Neely J, Arndt C, Nieder M, Jaffe E, Wittes RA, Horak ID. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. Journal of Clinical Oncology. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- Magrath I, Shanta V, Advani S, Adde M, Arya LS, Banavali S, Bhargava M, Bhatia K, Gutiérrez M, Liewehr D, Pai S, Sagar TG, Venzon D, Raina V. Treatment of acute lymphoblastic leukaemia in countries with limited resources; lessons from use of a single protocol in India over a twenty year period [corrected] 2005 doi: 10.1155/2012/13068041,1570-1583. Erratum in: Eur J Cancer. 2007 Feb;43(3):632. Raina, V [added] [DOI] [PubMed] [Google Scholar]
- Marjerrison S, Fernandez CV, Price VE, Njume E, Hesseling P. The use of ultrasound in endemic Burkitt lymphoma in Cameroon. Pediatric Blood Cancer. 2012;58:352–355. doi: 10.1002/pbc.23050. [DOI] [PubMed] [Google Scholar]
- Meremikwu MM, Ehiri JE, Nkanga DG, Udoh EE, Ikpatt OF, Alaje EO. Socioeconomic constraints to effective management of Burkitt’s lymphoma in south-eastern Nigeria. Tropical Medicine International Health. 2005;10:92–98. doi: 10.1111/j.1365-3156.2004.01348.x. [DOI] [PubMed] [Google Scholar]
- Morrow RH, Kisuule A, Pike MC, Smith PG. Burkitt’s lymphoma in the Mengo districts of Uganda ; epidemiological features and their relationship to Malaria. Journal of the National Cancer Institute. 1976;56:479–483. doi: 10.1093/jnci/56.3.479. [DOI] [PubMed] [Google Scholar]
- Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Seminars in Oncology. 1980;7:332–339. [PubMed] [Google Scholar]
- Naresh KN, Raphael M, Ayers L, Hurwitz N, Calbi V, Rogena E, Sayed S, Sherman O, Ibrahim HA, Lazzi S, Mourmouras V, Rince P, Githang’a J, Byakika B, Moshi E, Durosinmi M, Olasode BJ, Oluwasola OA, Akang EE, Aken’Ova Y, Adde M, Magrath I, Leoncini L. Lymphomas in sub-Saharan Africa - what can we learn and how can we help in improving diagnosis, managing patients and fostering translational research? British Journal of Haematology. 2011a;154:696–703. doi: 10.1111/j.1365-2141.2011.08772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naresh KN, Ibrahim HA, Lazzi S, Rince P, Onorati M, Ambrosio MR, Bilhou-Nabera C, Amen F, Reid A, Mawanda M, Calbi V, Ogwang M, Rogena E, Byakika B, Sayed S, Moshi E, Mwakigonja A, Raphael M, Magrath I, Leoncini L. Diagnosis of Burkitt lymphoma using an algorithmic approach - applicable in both resource-poor and resource-rich countries. British Journal of Haematology. 2011b;154:770–776. doi: 10.1111/j.1365-2141.2011.08771.x. [DOI] [PubMed] [Google Scholar]
- Ngu VA. The African lymphoma (Burkitt tumour) survivals exceeding two years. British Journal of Cancer. 1965;19:101–107. doi: 10.1038/bjc.1965.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olweny CL, Katongole-Mbidde E, Kaddu-Mukasa A, Atine I, Owor R, Lwanga S, Carswell W, Magrath IT. Treatment of Burkitt’s lymphoma: randomized clinical trial of single-agent versus combination chemotherapy. International Journal of Cancer. 1976;17:436–440. doi: 10.1002/ijc.2910170404. [DOI] [PubMed] [Google Scholar]
- Olweny CL, Atine I, Kaddu-Mukasa A, Katongole-Mbidde E, Lwanga SK, Johansson B, Onyango J, Host H, Norin T, Willey B. Cerebrospinal irradiation of Burkitt’s lymphoma. Failure in preventing central nervous system relapse. Acta Radiologica Therapeutica Physics Biology. 1977;16:225–231. doi: 10.3109/02841867709133941. [DOI] [PubMed] [Google Scholar]
- Olweny CL, Katongole-Mbidde E, Otim D, Lwanga SK, Magrath IT, Ziegler JL. Long-term experience with Burkitt’s lymphoma in Uganda. International Journal of Cancer. 1980;26:261–266. doi: 10.1002/ijc.2910260302. [DOI] [PubMed] [Google Scholar]
- Patte C, Auperin A, Michon J, Behrendt H, Leverger G, Frappaz D, Lutz P, Coze C, Perel Y, Raphaël M, Terrier-Lacombe MJ on behalf of the Société Française d’Oncologie Pédiatrique. The Société Française d’Oncologie Pédiatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- Rainey JJ, Mwanda WO, Wairiumu P, Moormann AM, Wilson ML, Rochford R. Spatial distribution of Burkitt’s lymphoma in Kenya and association with malaria risk. Tropical Medicine and International Health. 2007;12:936–943. doi: 10.1111/j.1365-3156.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- Traoré F, Coze C, Atteby JJ, André N, Moreira C, Doumbe P, Ravelomanana N, Ye D, Patte C, Raquin MA, Raphaël M, Lemerle J. Cyclophosphamide monotherapy in children with Burkitt lymphoma: a study from the French-African Pediatric Oncology Group (GFAOP) Pediatric Blood Cancer. 2011;56:70–76. doi: 10.1002/pbc.22746. [DOI] [PubMed] [Google Scholar]
- Wright DH. Gross distribution and haematology. In: Burkitt PD, Wright, editors. Burkitt’s tumour. E. & S. Livingstone; Edinburgh and London: 1970. pp. 64–81. [Google Scholar]
- Ziegler JL. Chemotherapy of Burkitt’s lymphoma. Cancer. 1972;30:1534–1540. doi: 10.1002/1097-0142(197212)30:6<1534::aid-cncr2820300619>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ziegler JL, Bluming AZ. Intrathecal chemotherapy in Burkitt’s lymphoma. British Medical Journal. 1971;3:508–512. doi: 10.1136/bmj.3.5773.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JL, Bluming AZ, Fass L, Morrow RH. Relapse Patterns in Burkitt’s Lymphoma. Cancer Research. 1972;32:1267–1272. [PubMed] [Google Scholar]
- Ziegler JL, Magrath IT, Olweny CLM. Cure of Burkitt’s lymphoma: Ten-year follow-up of 157 Ugandan Patients. Lancet. 1979;ii:936–938. doi: 10.1016/s0140-6736(79)92627-8. [DOI] [PubMed] [Google Scholar]