Abstract
Context:
The testicular phenotype in McCune-Albright syndrome (MAS) has not been well characterized. Boys present with a relatively low incidence of precocious puberty in comparison with girls. Radiographic and histological studies are limited to small series and case reports, which report testicular microlithiasis and Sertoli cell hyperplasia.
Objective:
Our objective was to characterize the biochemical, radiological, and histological spectrum and clinical management of testicular pathology in males with MAS.
Patients, Design, and Setting:
Fifty-four males with MAS participated in this prospective cohort study at a clinical research center.
Intervention:
Evaluation included testicular exam, pubertal staging, testicular ultrasound, measurement of LH, FSH, and testosterone. Orchiectomies were performed when considered clinically indicated.
Main Outcome Measure:
Prevalence and characterization of ultrasound lesions with correlation to histology were evaluated.
Results:
Of 54 males, 44 (81%) presented with ultrasound abnormalities including hyperechoic lesions (49%), hypoechoic lesions (30%), microlithiasis (30%), heterogeneity (47%), and focal calcifications (11%). Eight subjects underwent orchiectomy revealing large foci of Leydig cell hyperplasia, which could not be definitively distinguished from Leydig cell tumor. After no subjects developed clinical malignancy, a conservative approach was instituted, and subsequent subjects were followed with serial imaging. Testosterone and gonadotropins were normal in subjects without precocious puberty or pituitary disease. Eleven (21%) presented with precocious puberty, and a combination of aromatase inhibitors, androgen receptor blockers, and leuprolide resulted in improved predicted adult height. In addition, the first cases of testicular adrenal rest and bilateral germ cell tumors in association with MAS are presented.
Conclusions:
Contrary to prevailing thinking, the incidence of gonadal pathology in MAS is equal in males and females. The predominant histopathological finding was Leydig cell hyperplasia, which carries a low risk of malignant transformation and can be managed conservatively.
McCune-Albright syndrome (MAS) is a combination of café-au-lait macules, polyostotic fibrous dysplasia, and hyperfunctioning endocrinopathies, including precocious puberty (PP), hyperthyroidism, GH excess, fibroblast growth factor 23-mediated hypophosphatemia and Cushing's syndrome (CS) (1–3). MAS arises from postzygotic mutations of the α-subunit of the Gs stimulatory protein (Gsα), leading to ligand-independent receptor activation and inappropriate cAMP production (4, 5). Disease burden is determined by the stage of embryogenesis during which the mutation occurs and the locations to where mutated progenitors subsequently migrate (6). The resulting phenotype is mosaic with wide clinical variability.
PP is common in girls, affecting 83% of the National Institutes of Health (NIH) cohort. The clinical course has been relatively well described (7–10), and relatively effective treatment options are available (11, 12). In contrast, the impact of testicular Gsα mutations is inadequately described. MAS-associated PP in boys is uncommon and is generally considered to present later with a slower, progressive course (13). Characterizing this process is difficult, because the literature is limited to case reports and small series (14–16). Treatment is guided by studies in familial male-limited PP (FMPP), another form of PP arising from activating mutations of the LH receptor (LHR) (17, 18).
Testicular pathology has been observed in the absence of PP, including macro-orchidism and microlithiasis (16). Single-patient reports describe testicular biopsy findings, both in boys with PP (15, 19–22) and macro-orchidism alone (23–26). All report Sertoli cell hyperplasia (SCH) and a paucity of Leydig cells with one exception (21), where occasional mature Leydig cells were seen in a boy with PP and SCH. In most cases, biopsies were performed to evaluate for malignancy in the setting of testicular enlargement.
We performed a systematic evaluation in a large cohort of males with MAS to characterize the male gonadal phenotype and to define clinical management.
Subjects and Methods
Subjects
All 129 subjects with MAS in the NIH cohort were evaluated. Subjects or parents gave informed consent/assent. Fifty-four (44%) were male, ranging from 3–59 yr.
Subjects underwent physical examination, biochemical evaluation, and imaging.
Surgical procedures
Early in the study course, five of seven consecutive male subjects presented with extensive testicular ultrasound (TUS) abnormalities concerning for malignancy. Three subjects subsequently underwent unilateral total orchiectomy. No invasion of adjacent tissues was found intraoperatively or histologically, and computed tomography (CT) of the chest, abdomen and pelvis showed no metastases. Five subsequent subjects with similar TUS findings underwent testis-sparing partial orchiectomies in addition to surveillance CT. Again there was no clinical malignancy at presentation or with longitudinal follow-up. A more conservative approach was adopted for subsequent subjects, who were followed with serial imaging alone.
Three subjects underwent testicular biopsy and one underwent unilateral orchiectomy at outside institutions. Tissue was obtained from autopsy in one subject who died from polyostotic fibrous dysplasia-related scoliosis.
Longitudinal follow-up
Of subjects with TUS abnormalities, 37 were followed for up to 13 yr with serial US, and of these, 21 underwent CT of the chest, abdomen, and pelvis.
Adrenal rest
A 15-yr-old boy with neonatal CS treated with bilateral adrenalectomy presented with rapid enlargement of a hypoechoic lesion in the right testis over a 2-yr period. In light of his presumed chronic ACTH elevation, testicular adrenal rest was suspected, and gonadal vein sampling was performed. Samples for human chorionic gonadotropin (hCG), cortisol, dehydroepiandrosterone sulfate, estradiol, estrone, and testosterone were obtained from the bilateral testicular veins and the left femoral vein at baseline, 15 min, and 30 min after ACTH infusion.
Testicular cancer
A 28-yr-old man with MAS developed an enlarging left testicular mass. He underwent radical orchiectomy at an outside institution, which revealed poorly differentiated embryonal cell tumor. There were no metastases, and he received local radiation. Five years later, a right testicular seminoma developed, with extension into the tunica albuginea but no further local invasion or metastases. He was treated with radical orchiectomy and chemotherapy and after 20 yr has had no recurrence.
Results
Clinical characteristics
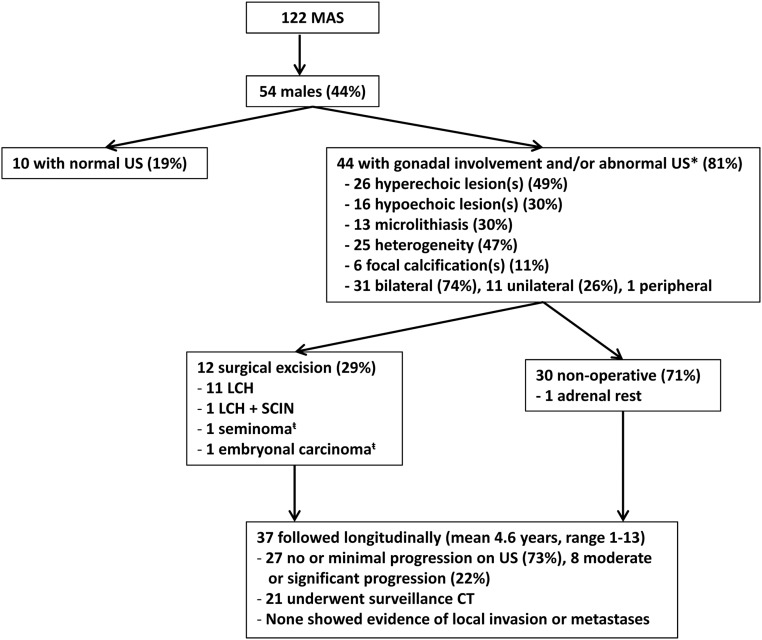
Figure 1 shows an outline of the study population. Twenty of 47 subjects with recorded testicular volumes had macro-orchidism (44%), including 13 of 26 children. Macro-orchidism was unilateral in four subjects (9%) and uniform in all cases without discrete palpable masses. All were asymptomatic.
Fig. 1.
Summary flow diagram describing the US abnormalities, pathological findings, and clinical management of MAS-associated testicular lesions. *, One patient with gonadal involvement on autopsy, US not available; t̄, patient with left-sided embryonal carcinoma resected, followed by subsequent development of right-sided seminoma.
Eleven subjects (21%) presented with PP. A summary of clinical characteristics and treatment responses is included in Table 1. Age at presentation ranged from 2–8 yr. Two subjects reported entering puberty by age 9 and reaching final height at a young age. Both showed evidence of premature epiphyseal fusion: one with extreme short stature and the other with a height sd score (SDS) equal to midparental height despite uncontrolled biochemical GH excess. Five subjects received a combination of aromatase inhibitors, androgen receptor blockers, and leuprolide initiated at the time of central PP (Table 1). All with longitudinal data available demonstrated improvement in bone age/chronological age and predicted adult height SDS with treatment. Two untreated adults with GH excess reached final heights greater than 0 SDS, whereas the untreated adult without GH excess had extreme short stature (SDS −5.13).
Table 1.
Summary of the clinical characteristics and response to treatment in subjects with PP
| Patient | Age at onset (yr) | Age at NIH presentation (yr) | BA/CA at presentation | Height at presentation (SDS) | PAH at presentation (SDS) | Current age (yr) | Current BA/CA | Current height (SDS) | Current PAH (SDS) | Other features of MAS | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 7.8 | 1.66 | 2.49 | −1.5 | 25 | 1a | PFD, CAL, GH, TH, PW | Flutamide, testolactone ages 5–12 | ||
| 2 | 7 | 30 | 39 | 1.57a | PFD, CAL, GH, TH, PW | None | |||||
| 3 | 9 | 42 | 47 | −5.13a | PFD, CAL, TH, PW | None | |||||
| 4 | 2.5 | 4.3 | 1.86 | −1.66 | −5.09 | 14.8 | 1.04 | −2.46 | −3.48 | PFD, CAL, TH, PW | Testolactone ages 3–6, spironolactone ages 3–13, leuprolide ages 8–13 |
| 5 | 2.5 | 5.8 | 1.65 | 0.65 | −2.37 | 13.8 | 0.9 | −1.32 | −0.1 | PFD, CAL, TH | Spironolactone ages 5–13, testolactone ages 5–8, letrozole ages 8–13, leuprolide ages 8–13 |
| 6 | 2 | 8.7 | 1.5 | 1.6 | −3.98 | 12.2 | 1.26 | 2.35 | −0.65 | PFD, CAL, TH | Testolactone ages 8–10, spironolactone ages 8 to current, letrozole ages 10 to current |
| 7 | 5.6 | 5.6 | 1.6 | 0.9 | −1.15 | 10.8 | 1.14 | 1.53 | 1.52 | PFD, CAL | None (progression mild) |
| 8 | 9 | 18 | 26 | 0.39a | PFD, CAL, GH, TH, PW | None | |||||
| 9 | 8 | 9.1 | 1.4 | 0.29 | −2.05 | 9.1b | 1.4b | 0.29b | −2.05b | PFD, CAL, TH | Letrozole, spironolactone ages 8 to current, leuprolide ages 9 to present |
GH indicates GH excess, and SDS is the number of sd from the age-matched mean. BA, Bone age; CA, chronological age; CAL, café-au-lait macule; PAH, predicted adult height, PFD, polyostotic fibrous dysplasia; PW, phosphate wasting; TH, hyperthyroidism.
Final adult height.
Same as initial presentation.
Imaging
Of 54 males with MAS, 44 (81%) had abnormalities on TUS (Fig. 1). Representative images are shown in Fig. 2. The most common findings were focal hyper- and hypoechoic lesions, seen in 49 and 30%, respectively. Lesions ranged from several millimeters to over four centimeters. Other findings included diffuse heterogeneity (47%), microlithiasis (30%), and focal calcifications (11%). Disease was bilateral in 74% and unilateral in 26%, and one subject had an extratesticular calcification in the scrotal sac.
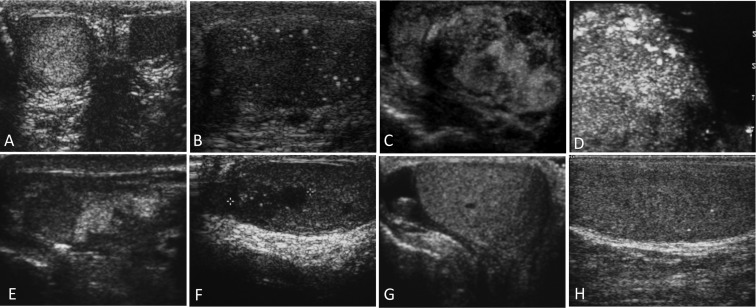
Fig. 2.
Representative US images of testes of males with MAS. A, Right and left testes demonstrating unilateral heterogeneic texture; B, multiple punctate calcifications consistent with testicular microlithiasis; C, multiple large, irregular hyper and hypoechoic lesions; D, heterogeneous hyperechoic lesion in a patient with seminoma; E, multiple hyper and hypoechoic lesions with associated focal calcifications; F, mediastinal hypoechoic lesion consistent with adrenal rest; G and H, a child with unilateral macro-orchidism, a small hypoechoic area with adjacent calcification in a normal-size testicle (G), and punctate calcifications in the enlarged testicle.
Histopathology
Of eight subjects who underwent surgical excision at the NIH, seven had Leydig cell hyperplasia (LCH) on pathological examination, and one had both LCH and Sertoli cell intraepithelial neoplasia (SCIN) (Fig. 3). Tissue obtained from autopsy showed extensive LCH. Tissue was available for one subject who underwent orchiectomy at an outside institution, which also revealed LCH. Both focal nodules and background diffuse LCH were noted in all cases. Tissue was unavailable for three subjects who underwent procedures at outside institutions; however, the pathology report for one identified LCH and in another both LCH and SCH. Subjects who underwent orchiectomies ranged in age from 14–54 yr. None showed local invasion or metastases. Both nodular and diffuse hyperplasias were composed of typical Leydig cells forming islands with occasional cellular pleomorphism; the stroma was scanty and well vascularized. Although surrounding tissues were not infiltrated, the proliferation compressed the normal testicular parenchyma, inducing extensive atrophy extending to the seminiferous tubules and rete testis. Leydig cells contained characteristic cytoplasmic brown pigment (lipofuscin) and lipid droplets. Reinke crystalloids were not identified.
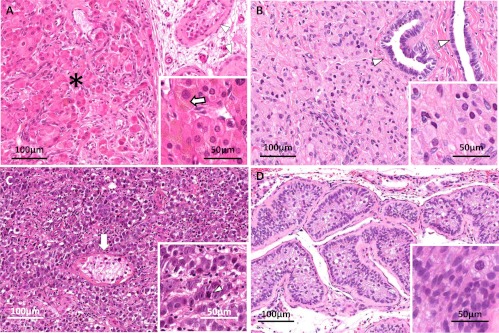
Fig. 3.
Representative pathology from testicular lesions in subjects with MAS. A, Leydig cell hyperplasia (LCH). Nests of proliferating cells (asterisk) are pushing aside the testicular parenchyma, where a few remaining atrophic seminiferous tubules can be observed (white arrowheads). The features of the proliferating Leydig cells containing characteristic brown pigment (lipofuscin) are seen in the inset (white arrows). B, LCH compromising the rete testis (arrowheads) and showing some pleomorphism. At a higher magnification (inset), the cytoplasm is seen to contain lipid droplets. C, Malignant germ cell tumor consistent with embryonal carcinoma. The tumor is composed of round to oval highly atypical cells with prominent dark nucleoli. The proliferation infiltrates and destroys the surrounding testicular parenchyma. A distorted and atrophic remaining seminiferous tubule is seen in the center of this picture (white arrow). The inset shows a higher magnification of the neoplastic cells; the white arrowhead points to an atypical mitosis. D, Sertoli cell intraepithelial neoplasia. The tubules are filled with atypical Sertoli cells and are surrounded by a thick basement membrane. The inset shows a higher magnification of the pleomorphic proliferating cells.
In one case, an intratubular SCIN was identified. Atypical Sertoli cells filled seminiferous tubules delimited by a thickened basement membrane. Diffuse cellular pleomorphism and karyopyknosis were noted, but few mitoses were observed. Cytoplasmic macro- and microvacuolization and nuclear cytoplasmic pseudoinclusions were also identified as well as occasional cells with dense eosinophilic or granular cytoplasm.
Histopathology of testicular cancer
The embryonal carcinoma developed solid masses and Indian files extensively infiltrating the surrounding parenchyma, trapping seminiferous tubules with epithelial atrophy and thickening the basement membrane. The neoplasia was composed of large atypical cells with clear cytoplasm and large oval, variably hyperchromatic nuclei containing one or more hypertrophic nucleoli. Mitotic figures were frequent. The stroma was scanty and focally edematous. Variable mononuclear infiltration was seen peripherally and within the tumor, mostly lymphocytes and plasma cells. Uncompromised testicular structures, tubules, and rete testis showed tumor compression, with atrophy and variable inflammation.
Multiple areas of lymphovascular invasion were identified within the tumor proper, and focal invasion of the epididymis and rete testis was noted. Areas of testis not involved with tumor were notable for LCH.
Longitudinal follow-up
Thirty-seven patients were followed longitudinally (mean 4.6 yr, range 1–13 yr) (Fig. 1). To date, 27 (73%) have had no or minimal progression in size of TUS lesions, and eight (22%) have had moderate or significant progression. None have developed local invasion or metastases.
Biochemical evaluation of testicular function
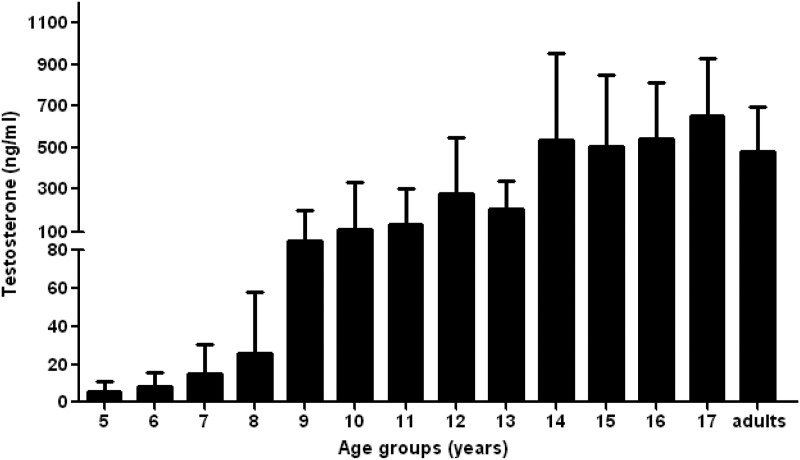
In subjects followed longitudinally, testosterone and gonadotropin levels increased appropriately with age (Fig. 4). Ten prepubertal boys without PP entered puberty at median age 11 with an SDS of 1.4 yr. Of 20 adult subjects, three had central hypogonadism related to GH- and prolactin-secreting pituitary adenomas, and the remainder maintained normal gonadotropin and testosterone levels.
Fig. 4.
Median testosterone values by age in subjects with MAS-associated testicular lesions without precocious puberty. Testosterone levels increase appropriately with age and reach normal adult levels.
Adrenal rest
Gonadal vein sampling results were consistent with adrenal rest. Cortisol levels from the right and left gonadal veins were greater than peripheral (9.4, 7.3, and 2 μg/dl, respectively), and increased with ACTH stimulation (72.3, 28.7, and 8.8 μg/dl, respectively). High testosterone values in gonadal vein samples confirmed appropriate catheter positioning. Testosterone levels from both testes increased slightly after ACTH stimulation (left more than right), whereas dehydroepiandrosterone sulfate, estradiol, estrone, and hCG were unchanged (data not shown). The subject was changed from hydrocortisone (10 mg/m2 · d) to dexamethasone (12 mg/m2 · d hydrocortisone equivalent) each evening, after which the adrenal rest tissue decreased significantly.
Discussion
Evaluation of this large cohort establishes that contrary to conventional thinking, MAS-associated gonadal involvement is not significantly more common in females than males. Gonadal abnormalities were seen in 81% of males, which is roughly equal to the 83% prevalence in our female cohort with MAS. This finding has several implications. First, as MAS is diagnosed clinically based on a constellation of phenotypic features, the presence of characteristic testicular abnormalities may be used as a diagnostic criterion in males. Second, these findings support the current hypothesis of the embryological development of MAS. By this model, a GNAS mutation arises early in embryogenesis and is distributed throughout the organism as the progeny of the mutated clone disperse. The prevalence of male and female gonadal involvement is thus expected to be equal, as was found here. Finally, although MAS-associated testicular lesions appear at present almost universally benign, one case of malignancy was seen. Until the propensity and risk factors for malignant transformation of testicular lesions are known, all males with MAS should undergo routine ultrasonographic screening and follow-up.
Testicular pathology in MAS
LCH was the predominant finding on pathological examination in our cohort. This represents a unique observation, because previous case reports universally describe SCH with absent or reduced number of Leydig cells. Extensive foci of LCH were seen in all orchiectomy specimens, with SCH/SCIN in one subject who also had widespread LCH. The source of the disparity between findings in this series vs. those in the literature is unclear. It is possible the discrepancy is due to the fact that previous cases were limited to testicular biopsies, whereas our series analyzed orchiectomy specimens, allowing for a more representative view of testicular histology. Another factor may be that subjects in this cohort were generally older than those in previous reports. Patients who underwent orchiectomy in our series were pubertal or postpubertal, ranging in age from 14–54 yr. In contrast, the age range in previous cases is 2–18 yr, with half presenting with PP and the remainder prepubertal. Our subject with SCH was 14 yr old and the only child to undergo orchiectomy. At age 6, this boy underwent testicular biopsy at another institution, which showed SCH and no LCH (20). These data suggest that SCH may underlie the macro-orchidism in boys with MAS, whereas LCH may develop at the time of puberty.
The mechanism of MAS-associated LCH is presumably related to excess cAMP signaling (27). In normal testicular development, LH or hCG stimulation results in formation of immature Leydig cells, which persist throughout childhood and mature into adult Leydig cells at puberty (28). Gsα mutations in MAS may disrupt Leydig cell development, which in some patients results in PP with autonomous Leydig cell maturation and excess testosterone production in childhood. Abnormal Leydig cell proliferation in MAS may also manifest after the onset of central puberty, potentially due to increasing pituitary LH production or other hormonal changes. Although conventional thinking is that elevated cAMP in MAS results from LHR ligand-independent activation alone, it is possible that pubertal LH increases lead to more pronounced cAMP production in mutation-bearing cells, resulting in elevated cAMP and Leydig cell proliferation. This phenomenon is seen in cultured mutation-bearing bone marrow stromal cells from patients with MAS (Collins, M. T., personal observation). To investigate the causative role of Gsα mutation in testicular pathology, mutation analysis was performed by PCR amplification of formalin-fixed, paraffin-embedded, microdissected surgical specimens. In only one subject with typical LCH was there unequivocal evidence of an R201 mutation (R201H) (data not shown). The absence of mutation detection in the other specimens does not exclude the possibility that disease was Gsα mutation driven. In fact, it is our supposition that testicular pathology is Gsα mutation driven, and the lack of mutation detection is a false-negative finding. Mutation detection in mosaic conditions is technically difficult due to both normal and wild-type alleles in each genome. Additionally, DNA extracted from formalin-fixed, paraffin-embedded blocks is often of low quality, particularly if samples are many years old, as was the case here. A more sensitive protein nucleic acid clamping technique was used in an effort to overcome these intrinsic and technical hurdles (29) but was unsuccessful in all but one case. All subjects in our cohort carried a definitive clinical diagnosis of MAS, and we therefore hypothesize the testicular phenotype is related to an underlying Gsα mutation, despite our inability to demonstrate tissue-specific mutations in most subjects.
Distinguishing between nodular LCH, benign LCT, and malignant LCT presents a diagnostic challenge, because each share similar histological features. LCH is characterized by a lack of cytological atypia; however, it is not uncommon for LCT to lack this feature as well. Additionally the relationship between LCH and LCT is not well defined, and reports of LCH in the setting of LCT have prompted conjecture that LCH may be a potentially preneoplastic process (30, 31). Two large series of LCT report characteristics more frequently associated with clinical malignancy (32, 33), including diameter greater than 5 cm, lymphatic or vascular invasion, infiltrative margins, and increased mitotic activity. Excluding the single case of malignancy in this series, none of these features were found in our cases of LCH. Although these features may be useful in predicting potential malignant behavior, the diagnosis of malignant LCT is only definitively made on clinical grounds.
Activating LHR mutations have been previously shown to play a role in Leydig cell tumorigenesis, presumably through chronic Leydig cell stimulation. R201C Gsα mutations were detected in four individuals in a series of six non-MAS patients with LCT (34). Somatic LHR mutations were found in three boys with Leydig cell adenomas (35), and nodular LCH has been reported in FMPP (36). There is one report of a seminoma in FMPP (37), suggesting these mutations may also be a predisposing factor for germ cell tumorigenesis. This association is further strengthened by the first report of MAS-associated germ cell tumors presented here.
Whether FSH receptor (FSHR) signaling plays a role in LCH is uncertain. Although nodular LCH has been reported in FMPP, its incidence appears to be significantly lower than in MAS, with a series of 32 individuals identifying only one case (36). The presence of both autonomous FSHR and LHR activation in MAS may thus lead to a greater degree of Leydig cell overgrowth than that seen in LHR activation alone. Several reports provide additional evidence that FSH may play at least a minor role in regulation of Leydig cell function. An activating FSHR mutation was diagnosed in a man with higher than castrate testosterone levels in the absence of gonadotropins after hypophysectomy (38). Haywood et al. (39) created transgenic mice with GnRH deficiency and the same FSHR mutation and demonstrated elevated testosterone compared with mice with GnRH deficiency alone. These findings suggest testosterone production by Leydig cells is affected by FSH signaling, which may underlie the high prevalence of LCH in MAS compared with conditions with isolated LHR mutations.
Both Sertoli and Leydig cell tumors have been reported in Carney complex, a syndrome arising from PRKAR1A mutations, which, similar to GNAS mutations in MAS, lead to dysregulation of cAMP (40). The association of similar testicular tumors with both MAS and Carney complex supports the hypothesis that MAS-associated testicular disease arises from autonomous receptor signaling and dysregulated cAMP production.
Management of testicular lesions in MAS
Management of testicular pathology in our cohort has evolved over time. TUS were initially performed as part of a study characterizing features of MAS, and shortly thereafter, we encountered extensive, previously unreported TUS lesions with features concerning for malignancy. On pathological examination, LCT with potential for malignancy could not be definitively excluded. When no patients developed clinical malignancy after several years of observation, we altered our management to reflect a lower likelihood of malignant transformation. Total orchiectomies thus gave way to testis-sparing enucleation procedures and eventually to nonsurgical management.
Currently, we advocate a conservative approach to management of MAS-associated testicular lesions, with emphasis on testicular preservation, close observation, and serial imaging. Screening exam and TUS should be performed in all males suspected of MAS. If abnormalities are noted without evidence of local invasion, we recommend serial exam and TUS rather than testicular biopsy. Typical MAS-associated testicular lesions present with uniform macro-orchidism and a palpable mass should alert the practitioner to a higher likelihood of malignancy. Patients with palpable masses and large and/or progressive lesions should undergo surveillance imaging to assess for distant metastases. We recommend surgical evaluation for lesions that are palpable, rapidly progressive, or locally invasive. Patients should be counseled on the importance of testicular self-examination to monitor for the development of palpable lesions. All individuals evaluated for MAS should be screened for GH excess; however, this is particularly important in the setting of testicular lesions, because uncontrolled GH excess may increase the risk of tumorigenesis in MAS (1).
Testicular function in MAS
Testicular lesions did not impair testosterone production, with normal testosterone and gonadotropins in subjects without pituitary disease. The effect of MAS-associated testicular disease on reproductive function is unclear. In our series, there was histopathological evidence of impaired spermatogenesis in areas of the testes adjacent to the tumors, likely secondary to compression atrophy. Impaired spermatogenesis was reported in one individual with unilateral macro-orchidism and a history of PP; however, this patient had histological findings different from our cohort, including Sertoli cell-only and scarcity of Leydig cells (22). The fertility effects of MAS-associated testicular disease are unknown, and additional studies including semen analyses are needed to investigate reproductive function.
There are multiple reports of spermatogenesis in biopsies of children with MAS-associated PP and SCH (19–21, 23); however, this has not been reported in prepubertal children with MAS-associated SCH. Leydig cell activation thus appears to be required for spermatogenesis (41). Autonomous Leydig cell activation alone does not necessitate the development of LCH, because LCH has not been observed in young children with PP.
Despite the high prevalence of testicular pathology, only 21% of our male cohort presented with PP, consistent with previous observations that MAS-associated PP is more common in girls. We have demonstrated that the higher incidence of MAS-associated PP in girls is not likely due to differences in mutation burden but instead to inherent differences in ovarian and testicular pathophysiology. In our series, treatment of four subjects with aromatase inhibitors, androgen receptor blockers, and leuprolide resulted in improvement in bone age advancement and predicted height. These findings are similar to those in studies of FMPP (17, 18) and in previous case reports of patients with MAS (14, 15). This is the largest reported series describing treatment of boys with MAS-associated PP; the previous literature is limited to two single-patient reports (14, 15). Additional studies following subjects to final height are needed to establish the effectiveness of these treatments.
Adrenal rest
We report the first case of testicular adrenal rest in a patient with MAS treated with bilateral adrenalectomy. In this subject, gonadal vein sampling was useful to distinguish adrenal rest from typical MAS-associated testicular lesions. As has been reported in patients with congenital adrenal hyperplasia, testicular adrenal rest improved with higher-dose glucocorticoids (42). Adrenal rest tissue should thus be included in the differential diagnosis of testicular lesions in patients with MAS who have undergone treatment for CS.
Testicular cancer
We also include for the first time testicular cancer in the context of MAS. A single patient developed both an embryonal carcinoma and a seminoma. Presumably, the activating Gsα mutation was contributory, but other involved molecular events are unknown. The patient had no known predisposing exposures or family history of malignancy. This finding leads us to conclude that MAS may be a predisposing factor for testicular malignancy.
Conclusions and future directions
Gonadal pathology is a hallmark feature of MAS and occurs with equal incidence in males and females. LCH is the predominant testicular phenotype in postpubertal males, whereas SCH may be more common in childhood. Available evidence suggests a low risk of malignant transformation, and we advocate conservative management with serial imaging and surgical intervention only for lesions that are rapidly enlarging, palpable, or locally invasive. We report the first case of testicular germ cell tumors in association with MAS, for which these patients may carry a slight predisposition. Despite a high prevalence of testicular pathology, testosterone production does not appear to be impaired in MAS; however, additional research is required to determine the impact on reproductive function. In contrast to girls, MAS-associated PP presents in a minority of boys with a prevalence of 21% in our cohort. A combination of aromatase inhibition, testosterone receptor blockade, and GnRH inhibition is effective short-term management; however, longitudinal studies are needed to follow patients to final height. The spectrum of MAS-associated testicular lesions should include adrenal rest as a complication of CS therapy.
Acknowledgments
This research was supported by the National Institute of Dental and Craniofacial Research, and the National Institute of Child Health and Development, National Institutes of Health.
Disclosure Summary: All authors state that they have no conflicts of interest.
Footnotes
- CS
- Cushing's syndrome
- CT
- computed tomography
- FMPP
- familial male-limited PP
- FSHR
- FSH receptor
- hCG
- human chorionic gonadotropin
- LCH
- Leydig cell hyperplasia
- LHR
- LH receptor
- MAS
- McCune-Albright syndrome
- PP
- precocious puberty
- SCH
- Sertoli cell hyperplasia
- SCIN
- Sertoli cell intraepithelial neoplasia
- SDS
- sd score
- TUS
- testicular ultrasound.
References
- 1. Dumitrescu CE, Collins MT. 2008. McCune-Albright syndrome. Orphanet J Rare Dis 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCune DJ. 1936. Osteitis fibrosa cystica; the case of a nine year old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism. Am J Dis Child 52:743–744 [Google Scholar]
- 3. Albright F, Butler AM, Hampton AO, Smith PH. 1937. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females, report of five cases. N Engl J Med 216:727–746 [Google Scholar]
- 4. Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. 1991. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 325:1688–1695 [DOI] [PubMed] [Google Scholar]
- 5. Schwindinger WF, Francomano CA, Levine MA. 1992. Identification of a mutation in the gene encoding the α-subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci USA 89:5152–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robey PG, Kuznetsov S, Riminucci M, Bianco P. 2007. The role of stem cells in fibrous dysplasia of bone and the Mccune-Albright syndrome. Pediatr Endocrinol Rev 4(Suppl 4):386–394 [PubMed] [Google Scholar]
- 7. Foster CM, Feuillan P, Padmanabhan V, Pescovitz OH, Beitins IZ, Comite F, Shawker TH, Loriaux DL, Cutler GB., Jr 1986. Ovarian function in girls with McCune-Albright syndrome. Pediatr Res 20:859–863 [DOI] [PubMed] [Google Scholar]
- 8. Feuillan PP. 1997. McCune-Albright syndrome. Curr Ther Endocrinol Metab 6:235–239 [PubMed] [Google Scholar]
- 9. de Sanctis C, Lala R, Matarazzo P, Balsamo A, Bergamaschi R, Cappa M, Cisternino M, de Sanctis V, Lucci M, Franzese A, Ghizzoni L, Pasquino AM, Segni M, Rigon F, Saggese G, Bertelloni S, Buzi F. 1999. McCune-Albright syndrome: a longitudinal clinical study of 32 patients. J Pediatr Endocrinol Metab 12:817–826 [DOI] [PubMed] [Google Scholar]
- 10. Chanson P, Salenave S, Young J. 2010. Ovarian dysfunction by activating mutation of GS alpha: McCune-Albright syndrome as a model. Ann Endocrinol 71:210–213 [DOI] [PubMed] [Google Scholar]
- 11. Feuillan P, Calis K, Hill S, Shawker T, Robey PG, Collins MT. 2007. Letrozole treatment of precocious puberty in girls with the McCune-Albright syndrome: a pilot study. J Clin Endocrinol Metab 92:2100–2106 [DOI] [PubMed] [Google Scholar]
- 12. Eugster EA, Rubin SD, Reiter EO, Plourde P, Jou HC, Pescovitz OH. 2003. Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: a multicenter trial. J Pediatr 143:60–66 [DOI] [PubMed] [Google Scholar]
- 13. Wasniewska M, Matarazzo P, Weber G, Russo G, Zampolli M, Salzano G, Zirilli G, Bertelloni S. 2006. Clinical presentation of McCune-Albright syndrome in males. J Pediatr Endocrinol Metab 19(Suppl 2):619–622 [DOI] [PubMed] [Google Scholar]
- 14. Zacharin M. 2005. Paediatric management of endocrine complications in McCune-Albright syndrome. J Pediatr Endocrinol Metab 18:33–41 [DOI] [PubMed] [Google Scholar]
- 15. Messina MF, Arrigo T, Wasniewska M, Lombardo F, Crisafulli G, Salzano G, De Luca F. 2008. Combined treatment with ketoconazole and cyproterone acetate in a boy with McCune-Albright syndrome and peripheral precocious puberty. J Endocrinol Invest 31:839–840 [DOI] [PubMed] [Google Scholar]
- 16. Wasniewska M, De Luca F, Bertelloni S, Matarazzo P, Weber G, Crisafulli G, Valenzise M, Lala R. 2004. Testicular microlithiasis: an unreported feature of McCune-Albright syndrome in males. J Pediatr 145:670–672 [DOI] [PubMed] [Google Scholar]
- 17. Leschek EW, Jones J, Barnes KM, Hill SC, Cutler GB., Jr 1999. Six-year results of spironolactone and testolactone treatment of familial male-limited precocious puberty with addition of deslorelin after central puberty onset. J Clin Endocrinol Metab 84:175–178 [DOI] [PubMed] [Google Scholar]
- 18. Lenz AM, Shulman D, Eugster EA, Rahhal S, Fuqua JS, Pescovitz OH, Lewis KA. 2010. Bicalutamide and third-generation aromatase inhibitors in testotoxicosis. Pediatrics 126:e728–e733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giovannelli G, Bernasconi S, Banchini G. 1978. McCune-Albright syndrome in a male child: a clinical and endocrinologic enigma. J Pediatr 92:220–226 [DOI] [PubMed] [Google Scholar]
- 20. 1993. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 7-1993. A six-year-old boy with multiple bone lesions, repeated fractures, and sexual precocity. N Engl J Med 328:496–502 [DOI] [PubMed] [Google Scholar]
- 21. Benedict PH. 1966. Sex precocity and polyostotic fibrous dysplasia. Report of a case in a boy with testicular biopsy. Am J Dis Child 111:426–429 [DOI] [PubMed] [Google Scholar]
- 22. De Luca F, Mitchell V, Wasniewska M, Arrigo T, Messina MF, Valenzise M, de Sanctis L, Lahlou N. 2008. Regulation of spermatogenesis in McCune-Albright syndrome: lessons from a 15-year follow-up. Eur J Endocrinol 158:921–927 [DOI] [PubMed] [Google Scholar]
- 23. Arrigo T, Pirazzoli P, De Sanctis L, Leone O, Wasniewska M, Messina MF, De Luca F. 2006. McCune-Albright syndrome in a boy may present with a monolateral macroorchidism as an early and isolated clinical manifestation. Horm Res 65:114–119 [DOI] [PubMed] [Google Scholar]
- 24. Khanna G, Kantawala K, Shinawi M, Sarwate S, Dehner LP. 2010. McCune-Albright syndrome presenting with unilateral macroorchidism and bilateral testicular masses. Pediatr Radiol 40(Suppl 1):S16–S20 [DOI] [PubMed] [Google Scholar]
- 25. Coutant R, Lumbroso S, Rey R, Lahlou N, Venara M, Rouleau S, Sultan C, Limal JM. 2001. Macroorchidism due to autonomous hyperfunction of Sertoli cells and Gsα gene mutation: an unusual expression of McCune-Albright syndrome in a prepubertal boy. J Clin Endocrinol Metab 86:1778–1781 [DOI] [PubMed] [Google Scholar]
- 26. Rey RA, Venara M, Coutant R, Trabut JB, Rouleau S, Lahlou N, Sultan C, Limal JM, Picard JY, Lumbroso S. 2006. Unexpected mosaicism of R201H-GNAS1 mutant-bearing cells in the testes underlie macro-orchidism without sexual precocity in McCune-Albright syndrome. Hum Mol Genet 15:3538–3543 [DOI] [PubMed] [Google Scholar]
- 27. Colecchia M, Nistal M, Gonzalez-Peramato P, Carmignani L, Salvioni R, Nicolai N, Regadera J. 2007. Leydig cell tumor and hyperplasia: a review. Anal Quant Cytol Histol 29:139–147 [PubMed] [Google Scholar]
- 28. Svechnikov K, Landreh L, Weisser J, Izzo G, Colón E, Svechnikova I, Söder O. 2010. Origin, development and regulation of human Leydig cells. Horm Res Paediatr 73:93–101 [DOI] [PubMed] [Google Scholar]
- 29. Bianco P, Riminucci M, Majolagbe A, Kuznetsov SA, Collins MT, Mankani MH, Corsi A, Bone HG, Wientroub S, Spiegel AM, Fisher LW, Robey PG. 2000. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res 15:120–128 [DOI] [PubMed] [Google Scholar]
- 30. Valensi P, Coussieu C, Kemeny JL, Attali JR, Amouroux J, Sebaoun J. 1987. Endocrine investigations in two cases of feminizing Leydig cell tumour. Acta Endocrinol 115:365–372 [DOI] [PubMed] [Google Scholar]
- 31. Castle WN, Richardson JR., Jr 1986. Leydig cell tumor and metachronous Leydig cell hyperplasia: a case associated with gynecomastia and elevated urinary estrogens. J Urol 136:1307–1308 [DOI] [PubMed] [Google Scholar]
- 32. Kim I, Young RH, Scully RE. 1985. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol 9:177–192 [DOI] [PubMed] [Google Scholar]
- 33. Cheville JC, Sebo TJ, Lager DJ, Bostwick DG, Farrow GM. 1998. Leydig cell tumor of the testis: a clinicopathologic, DNA content, and MIB-1 comparison of nonmetastasizing and metastasizing tumors. Am J Surg Pathol 22:1361–1367 [DOI] [PubMed] [Google Scholar]
- 34. Fragoso MC, Latronico AC, Carvalho FM, Zerbini MC, Marcondes JA, Araujo LM, Lando VS, Frazzatto ET, Mendonca BB, Villares SM. 1998. Activating mutation of the stimulatory G protein (gsp) as a putative cause of ovarian and testicular human stromal Leydig cell tumors. J Clin Endocrinol Metab 83:2074–2078 [DOI] [PubMed] [Google Scholar]
- 35. Liu G, Duranteau L, Carel JC, Monroe J, Doyle DA, Shenker A. 1999. Leydig-cell tumors caused by an activating mutation of the gene encoding the luteinizing hormone receptor. N Engl J Med 341:1731–1736 [DOI] [PubMed] [Google Scholar]
- 36. Leschek EW, Chan WY, Diamond DA, Kaefer M, Jones J, Barnes KM, Cutler GB., Jr 2001. Nodular Leydig cell hyperplasia in a boy with familial male-limited precocious puberty. J Pediatr 138:949–951 [DOI] [PubMed] [Google Scholar]
- 37. Martin MM, Wu SM, Martin AL, Rennert OM, Chan WY. 1998. Testicular seminoma in a patient with a constitutively activating mutation of the luteinizing hormone/chorionic gonadotropin receptor. Eur J Endocrinol 139:101–106 [DOI] [PubMed] [Google Scholar]
- 38. Gromoll J, Simoni M, Nordhoff V, Behre HM, De Geyter C, Nieschlag E. 1996. Functional and clinical consequences of mutations in the FSH receptor. Mol Cell Endocrinol 125:177–182 [DOI] [PubMed] [Google Scholar]
- 39. Haywood M, Tymchenko N, Spaliviero J, Koch A, Jimenez M, Gromoll J, Simoni M, Nordhoff V, Handelsman DJ, Allan CM. 2002. An activated human follicle-stimulating hormone (FSH) receptor stimulates FSH-like activity in gonadotropin-deficient transgenic mice. Mol Endocrinol 16:2582–2591 [DOI] [PubMed] [Google Scholar]
- 40. Stratakis CA, Horvath A. 1993. Carney Complex. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; [PubMed] [Google Scholar]
- 41. Ochsenkühn R, De Kretser DM. 2003. The contributions of deficient androgen action in spermatogenic disorders. Int J Androl 26:195–201 [DOI] [PubMed] [Google Scholar]
- 42. Merke DP, Bornstein SR, Avila NA, Chrousos GP. 2002. NIH conference. Future directions in the study and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Ann Int Med 136:320–334 [DOI] [PubMed] [Google Scholar]