Abstract
Context:
Animal models and human studies suggest that osteocytes regulate the skeleton's response to mechanical unloading in part by an increase in sclerostin. However, few studies have reported changes in serum sclerostin in humans exposed to reduced mechanical loading.
Objective:
We determined changes in serum sclerostin and bone turnover markers in healthy adult men undergoing controlled bed rest.
Design, Setting, and Participants:
Seven healthy adult men (31 ± 3 yr old) underwent 90 d of 6° head down tilt bed rest at the University of Texas Medical Branch Institute for Translational Sciences-Clinical Research Center.
Outcomes:
Serum sclerostin, PTH, vitamin D, bone resorption and formation markers, urinary calcium and phosphorus excretion, and 24-h pooled urinary markers of bone resorption were evaluated before bed rest [baseline (BL)] and at bed rest d 28 (BR-28), d 60 (BR-60), and d 90 (BR-90). Bone mineral density was measured at BL, BR-60, and 5 d after the end of the study (BR+5). Data are reported as mean ± sd.
Results:
Consistent with prior reports, bone mineral density declined significantly (1–2% per month) at weight-bearing skeletal sites. Serum sclerostin was elevated above BL at BR-28 (+29 ± 20%; P = 0.003) and BR-60 (+42 ± 31%; P < 0.001), with a lesser increase at BR-90 (+22 ± 21%; P = 0.07). Serum PTH levels were reduced at BR-28 (−17 ± 16%; P = 0.02) and BR-60 (−24 ± 14%; P = 0.03) and remained lower than BL at BR-90 (−21 ± 21%; P = 0.14), but did not reach statistical significance. Serum bone turnover markers were unchanged; however, urinary bone resorption markers and calcium were significantly elevated at all time points after bed rest (P < 0.01).
Conclusions:
In healthy men subjected to controlled bed rest for 90 d, serum sclerostin increased, with a peak at 60, whereas serum PTH declined, and urinary calcium and bone resorption markers increased.
Reduced mechanical loading of the skeleton is invariably associated with muscle atrophy and bone loss. Bone loss is observed clinically after prolonged bed rest, immobilization, stroke, and spinal cord injury (1–6). In addition, microgravity leads to profound muscle atrophy and bone loss in astronauts (7). The precise mechanisms underlying disuse-induced bone loss are incompletely understood. Previous bed rest studies have reported that bone loss at weight-bearing skeletal sites is accompanied by decreased serum PTH, along with increased urinary calcium excretion and bone resorption markers; however, the precise mechanisms underlying these changes remain unknown.
Osteocytes play a key mechanosensing role, modulating bone modeling and remodeling by orchestrating the activity of osteoblasts and osteoclasts (8). At the molecular level, osteocytes regulate the bone's response to mechanical loading by at least two key molecules, sclerostin and receptor activation of nuclear factor-κβ ligand (RANKL) (8, 9). Mature osteocytes are one of the few cells that postnatally produce the protein sclerostin, a product of the gene SOST (10, 11). Sclerostin has been shown, both in vitro and in vivo, to inhibit bone formation, in part through competing with Wnt for the LRP4/5/6 receptor to reduce osteoblast proliferation and differentiation (12). Consistent with the negative effect of sclerostin on bone formation, transgenic mice overexpressing SOST are osteopenic (13), whereas SOST-null animals have high bone mass (14), similar to the human conditions of Van Buchem disease and sclerosteosis. Importantly, the SOST/sclerostin pathway has been implicated in the response of bone to mechanical loading in murine models because increased skeletal loading reduces SOST expression, whereas decreased mechanical loading increases it (15). Furthermore, SOST-null animals are resistant to disuse-induced bone loss (14). An alternate mechanism by which osteocytes respond to altered mechanical loading is suggested by the observation that osteocytes are a major source of the osteoclastogenic cytokine RANKL (8), and further, that mice lacking RANKL in osteocytes are protected from bone loss induced by hindlimb unloading (9).
Despite evidence of several molecular mechanisms by which osteocytes may regulate the response to mechanical loading in animal models, little is known about how osteocytes orchestrate skeletal adaptation to mechanical unloading in humans. In a cross-sectional study, older women who had suffered a stroke 10 months beforehand had 3-fold higher serum sclerostin levels than age-matched, fully ambulating controls (4), consistent with the notion that sclerostin levels increase in response to mechanical unloading. Yet, men with unloading due to chronic spinal cord injury (mean ± sd, 22.4 ± 11.2 yr) have lower sclerostin levels than ambulating control subjects (6). To address a gap in the literature with regard to the acute response to unloading, we evaluated the longitudinal changes in serum sclerostin levels in healthy men that participated in a 90-d, controlled bed rest study, with the hypothesis that acute unloading would lead to an increase in serum sclerostin.
Subjects and Methods
Subjects
Seven healthy men were recruited by the National Aeronautics and Space Administration (NASA) Johnson Space Center (JSC) to participate in a 90-d bed rest experiment. Eligibility included physically fit men between the ages of 25 and 55 yr who were not taking prescribed medication that would interfere with physiological measurements. Mean (±sd) age of the participants was 31 ± 3 yr (range, 28–36 yr). Mean (±sd) height, weight, and body mass index of subjects were 183 ± 6 cm, 88 ± 12 kg, and 26 ± 2.8 kg/m2, respectively. Mean dietary calcium and vitamin D intake were fixed at 1674 ± 251 mg/d and 384 ± 72 IU/d, respectively. Subjects were continuously monitored via remote-controlled cameras and remained in 6° head down tilt for 90 d with controlled nutrition, water intake, day-night cycles, ambient room temperature, and in-bed hygiene. Daily vital sign measurements were collected. Additional details regarding the University of Texas Medical Branch (UTMB)-NASA bed rest protocol can be found in prior publications (1–3, 5). Although some data from these studies have been published, the data from these individuals are being reported herein for the first time. The institutional review boards of both NASA JSC and UTMB approved the study protocol, and all subjects gave written informed consent.
Serum sclerostin and bone turnover markers
Serum (fasting, 0630 h collection) and urine samples were collected at two pre-bed rest time points (10 and 3 d before bed rest) and at bed rest d 28 (BR-28), bed rest d 60 (BR-60), and bed rest d 90 (BR-90). For all serum and urine markers, the results from the two pre-bed rest measurements were averaged to provide a baseline (BL) value for each subject. In addition, urinary collections were normalized to a 24-h time period at each time point.
Serum sclerostin levels were assayed in duplicate using an ELISA kit (ALPCO/Biomedica, Salem, NH). All sclerostin samples were assayed with a single assay. The intraassay variability as reported by the manufacturer is 5%. We also measured markers of bone metabolism, including serum PTH, 25 (OH) vitamin D, and 1,25 (OH)2 vitamin D; serum markers of bone formation [bone-specific alkaline phosphatase (BSAP) and osteocalcin]; serum markers of osteoclast activity [soluble RANKL, osteoprotegerin (OPG)]; urinary calcium and phosphorus excretion; and urinary markers of bone resorption [N-terminal telopeptide (NTX), deoxypridinoline (DPD), pyridinium crosslinks (PYD)], using previously reported methods (2).
Bone mineral density (BMD)
Areal BMD (grams per square centimeter) was measured by dual-energy x-ray absorptiometry (DXA Hologic Discovery; Hologic Inc., Bedford, MA). BMD of the whole body, lumbar spine, averaged left and right hips, heel, and forearm was assessed at BL and repeated at BR-60 and 5 d after the end of the bed rest period (BR+5). One subject was lost to follow-up at BR+5. BMD measurements for each subject reported are the mean of triplicate scans.
Statistical analyses
All data are summarized by mean ± sd unless otherwise specified. All data were analyzed with repeated-measures ANOVA. Analyses were performed with SAS 9.2 (SAS Institute Inc., Cary, NC) using the proc mixed procedure with an autoregressive covariance structure. Given the small sample size, a two-sided α of 0.10 for the overall ANOVA model was accepted as significant to proceed to prespecified pairwise comparisons of specific time points vs. BL (BL vs. BR-28, BR-60, and BR-90), for which two-sided α of 0.05 was considered significant.
Results
Serum sclerostin and PTH
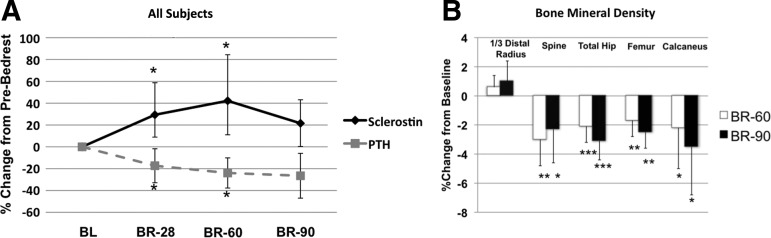
Serum sclerostin increased after bed rest in all subjects (Fig. 1). Specifically, serum sclerostin levels increased above BL at BR-28 (+29 ± 20%; P = 0.003) and appeared to plateau in most subjects at BR-60 (+42 ± 31%; P < 0.001). Sclerostin levels remained mildly elevated at BR-90, although this result did not reach statistical significance (+22 ± 21%; P = 0.07). In contrast, serum PTH levels declined at BR-28 (−17 ± 16%; P = 0.02) and BR-60 (−24 ± 14%; P = 0.03), remained reduced at BR-90 (−21 ± 21%; P = 0.14), but did not reach statistical significance.
Fig. 1.
A, Effect of bed rest on serum sclerostin and PTH. B, BMD (mean ± sd). *, P < 0.05; **, P < 0.005; ***, P < 0.0001, indicating significant change from BL.
Serum and urinary markers of bone turnover
As summarized in Table 1, urinary levels of bone resorption markers (NTX, DPD, PYD) increased significantly compared with BL at all time points. Urinary calcium excretion was also significantly increased throughout the study, whereas urinary phosphorus levels were elevated at BR-28 and BR-60 (P < 0.005 for both), with a return to BL at BR-90. Serum bone formation markers (serum BSAP, osteocalcin), serum RANKL, OPG, and the RANKL/OPG ratio did not change. 25 (OH) vitamin D was above BL at all time points (P < 0.005), whereas 1,25 (OH)2 vitamin D was significantly lower than BL at BR-28 (−13%; P < 0.05) and tended also to be lower at BR-60 (P = 0.06) and BR-90 (P = 0.07).
Table 1.
Serum and urinary measurements of bone turnover markers, urinary calcium, and phosphorous at BL, BR-28, BR-60, and BR-90
| BL | BR-28 | BR-60 | BR-90 | |
|---|---|---|---|---|
| Serum | ||||
| Sclerostin (pmol/liter) | 35.4 ± 7 | 45.3 ± 9.4b | 48.8 ± 4.8b | 42.1 ± 4.7 |
| PTH (pg/ml) | 31.5 ± 12 | 25.5 ± 10.5a | 24 ± 10.9a | 25.6 ± 13.6 |
| 25 (OH) vitamin D (ng/ml) | 13 ± 3 | 17 ± 3b | 19 ± 2c | 18 ± 3c |
| 1,25 (OH)2 vitamin D (pg/ml) | 38 ± 5 | 34 ± 7a | 34 ± 9 | 33 ± 7 |
| BSAP (U/liter) | 27 ± 6 | 29 ± 5 | 30 ± 5 | 32 ± 3 |
| Osteocalcin (ng/ml) | 13 ± 3 | 13 ± 2 | 14 ± 3 | 13 ± 4 |
| Soluble RANKL (pmol/liter) | 0.3 ± 0.2 | 0.3 ± 0.3 | 0.3 ± 0.2 | 0.3 ± 0.3 |
| OPG (pmol/liter) | 2.5 ± 0.9 | 2.4 ± 1 | 2.2 ± 1 | 2.2 ± 1 |
| RANKL/OPG (pmol/liter) | 0.12 ± 0.12 | 0.13 ± 0.11 | 0.14 ± 0.13 | 0.18 ± 0.18 |
| 24-h pooled urine | ||||
| NTX (nmol) | 482 ± 127 | 795 ± 182c | 735 ± 153b | 796 ± 165a |
| DPD (nmol) | 67 ± 16 | 108 ± 22c | 112 ± 25c | 116 ± 26c |
| PYD (nmol) | 221 ± 63 | 367 ± 119b | 380 ± 145b | 409 ± 171b |
| Calcium (mmol/d) | 5.9 ± 1.3 | 7.9 ± 0.6c | 7.4 ± 1.1a | 7.5 ± 1.2a |
| Phosphorus (mg/d) | 849 ± 117 | 1039 ± 200b | 1074 ± 160b | 946 ± 136 |
Data are expressed as mean ± sd. Assay manufacturers were: sclerostin, soluble RANKL, and OPG—ALPCO/Biomedica, Salem, NH; PTH—Scantibodies, Santee, CA; 25 (OH) vitamin D and 1,25 (OH)2 vitamin D—DiaSorin, Stillwater, MN; BSAP, DPD, and PYD—Quidel, San Diego, CA; osteocalcin—Biomedical Technologies, Stoughton, MA; NTX and phosphorus—Alere North American, Waltham, MA; and calcium—Atomic Absorption, Perkins Elmer Flame; PerkinElmer Inc., Waltham, MA.
P < 0.05;
P < 0.005;
P < 0.0001 compared to BL.
Bone mineral density
Subjects had normal BMD because BL Z-scores at the distal radius, lumbar spine, total hip, and femoral neck were 0.2 ± 0.7, −0.6 ± 1.0, 0.2 ± 1.0, and 0.0 ± 1.3, respectively. BMD declined significantly at BR-60 and BR+5 at all weight-bearing skeletal sites, including the lumbar spine, hip, femoral neck, and calcaneus (Fig. 1B; P < 0.05 for all). There was no change in forearm BMD.
Discussion
We found that in healthy men exposed to bed rest, serum sclerostin levels increased significantly by as early as 1 month and remained elevated for another month. Consistent with prior bed rest studies (3), serum PTH declined, urinary calcium and bone resorption markers increased, and BMD decreased at weight-bearing sites. There were no detectable changes in serum markers of bone formation.
Our observation of increased serum sclerostin after bed rest is consistent with previous reports of elevated sclerostin levels in animal and human models of disuse (4, 15). A prior cross-sectional study reported that 10 months after suffering a stroke, postmenopausal women (mean age = 80 yr) had 3-fold higher serum sclerostin levels than age-matched healthy controls (4). In comparison, in the current longitudinal study (subject mean age = 36), the maximum increase in serum sclerostin levels was +42% vs. BL at BR-60. Several differences between these two studies may have contributed to the different magnitudes of sclerostin increases after disuse, including: 1) we assessed the longitudinal response to acute mechanical unloading, whereas the study of stroke patients was a cross-sectional study of long-term disuse; 2) we enrolled healthy young men, whereas the stroke study examined elderly postmenopausal women; and 3) we studied strictly controlled bed rest, whereas activity levels of the stroke patients were more variable. Our data also differ from a previous cross-sectional study in middle-aged men with chronic spinal cord injury, in whom sclerostin levels were lower than normally ambulating age-matched controls (6). However, the increase in serum sclerostin that we observed supports a conceptual model where serum sclerostin rises acutely and then is suppressed in the chronic bone-wasting phase (6). Clearly, additional human studies are needed to better define the time course of changes in serum sclerostin in response to disuse, to address whether there is a neurological component to its regulation, and to test the efficacy of sclerostin antibody treatment in the setting of acute-onset, disuse-induced bone loss.
Decreased levels of serum PTH accompanied the increases in serum sclerostin after bed rest, presumably driven by a transient increase of serum calcium levels by bone resorption and a measured increase in urinary calcium. In animal models, PTH decreases sclerostin expression via activation of the PTH receptor expressed on osteocytes (16). Furthermore, there is an inverse correlation between PTH and sclerostin in male hypothyroid subjects (17), and PTH infusion in healthy men induces a decline in serum sclerostin levels (18). However, we cannot determine whether the reduction in PTH levels is driving the observed increase in sclerostin or whether sclerostin increases in disuse due to non-PTH-mediated mechanisms. To answer this question, future bed rest studies could employ more frequent measures of serum calcium, sclerostin, and PTH to more precisely define the time course of changes in each. Also, blocking the increase in serum calcium, perhaps by administration of an antiresorptive agent, might prevent the decrease in serum PTH and allow one to determine whether the increased sclerostin in bed rest is independent of serum PTH.
Although RANKL has been implicated in the mechanism of osteocytic regulation of mechanical unloading (8), we observed no change in the circulating serum levels of RANKL, OPG, or the RANKL/OPG ratio. However, the RANKL-OPG pathway primarily exerts effects at the cellular level (8), which may not be adequately reflected in the serum.
Several limitations of this study merit mention. We studied only young men, and thus the results cannot be generalized to women or to older men. Our study relies on measurements of circulating sclerostin, whereas sclerostin is thought to exert primarily paracrine effects. Although circulating sclerostin levels are strongly correlated with marrow plasma levels of sclerostin (18, 19), it is still possible that peripheral measurements of sclerostin do not reflect changes in the bone tissue.
Despite these limitations, the strengths of this study include the longitudinal assessment of sclerostin at multiple time points, controlled exposure to bed rest, controlled diet, and uniform study population.
Conclusion
In conclusion, serum sclerostin levels increased significantly in healthy young men exposed to 90 d of head down tilt bed rest. Bed rest was also associated with a decrease in serum PTH, an increase in bone resorption markers, and a decrease in BMD at weight-bearing sites. These are the first data to show the acute, longitudinal changes in serum sclerostin in response to bed rest. Given the key role that sclerostin plays in mediating bone metabolism and formation, additional studies exploring the regulation of sclerostin in disuse are warranted, particularly given the emergence of anti-sclerostin pharmacological therapies.
Acknowledgments
We thank the National Aeronautics and Space Administration (NASA) Human Research Program, the Human Health and Countermeasures Element, and the Flight Analogs Project. We thank Dr. Robert Ploutz-Snyder (Universities Space Research Association) for advice on the statistical methods, and the staffs at the University of Texas Medical Branch in Galveston's Institute for Translational Sciences-Clinical Research Center. We also thank the NASA Nutritional Biochemistry Laboratory personnel, who were responsible for protocol coordination, sample collection and processing, and most of the analyses presented herein.
The authors acknowledge NASA's Human Research Program (NASA NNX10AE39G), the National Institutes of Health (R21 AR057522, UH2AR059655), Harvard-Massachusetts Institute of Technology Division of Health Sciences and Technology Bioastronautics Ph.D. Program, and Northrop Grumman Aerospace Systems Ph.D. Training Fellowship for providing support for this work.
Disclosure Summary: The authors report no disclosures.
Footnotes
- BL
- Baseline
- BMD
- bone mineral density
- BR-28
- bed rest d 28
- BR-60
- bed rest d 60
- BR-90
- bed rest d 90
- BR+5
- 5 d after the end of the bed rest period
- BSAP
- bone-specific alkaline phosphatase
- DPD
- deoxypridinoline
- NTX
- N-terminal telopeptide
- OPG
- osteoprotegerin
- PYD
- pyridinium crosslinks
- RANKL
- receptor activation of nuclear factor-κβ ligand.
References
- 1. Zwart SR, Crawford GE, Gillman PL, Kala G, Rodgers AS, Rogers A, Inniss AM, Rice BL, Ericson K, Coburn S, Bourbeau Y, Hudson E, Mathew G, Dekerlegand DE, Sams CF, Heer MA, Paloski WH, Smith SM. 2009. Effects of 21 days of bed rest, with or without artificial gravity, on nutritional status of humans. J Appl Physiol 107:54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zwart SR, Oliver SA, Fesperman JV, Kala G, Krauhs J, Ericson K, Smith SM. 2009. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat Space Environ Med 80(5 Suppl):A15–A22 [DOI] [PubMed] [Google Scholar]
- 3. Inniss AM, Rice BL, Smith SM. 2009. Dietary support of long-duration head-down bed rest. Aviat Space Environ Med 80(5 Suppl):A9–A14 [DOI] [PubMed] [Google Scholar]
- 4. Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE. 2010. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab 95:2248–2253 [DOI] [PubMed] [Google Scholar]
- 5. Spector ER, Smith SM, Sibonga JD. 2009. Skeletal effects of long-duration head-down bed rest. Aviat Space Environ Med 80(5 Suppl):A23–A28 [DOI] [PubMed] [Google Scholar]
- 6. Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, Garshick E, Battaglino RA. 2012. Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res 27:352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. 2007. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact 7:33–47 [PubMed] [Google Scholar]
- 8. Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. 2011. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231–1234 [DOI] [PubMed] [Google Scholar]
- 9. Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. 2011. Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. 2002. Identification of a 52 kb deletion downstream of the SOST gene in patients with Van Buchem disease. J Med Genet 39:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Bezooijen RL, ten Dijke P, Papapoulos SE, Löwik CW. 2005. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 16:319–327 [DOI] [PubMed] [Google Scholar]
- 12. Semënov M, Tamai K, He X. 2005. SOST is a ligand for LRP5/LRP6 and a wnt signaling inhibitor. J Biol Chem 280:26770–26775 [DOI] [PubMed] [Google Scholar]
- 13. Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. 2003. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. 2009. Sclerostin mediates bone response to mechanical unloading through antagonizing wnt/β-catenin signaling. J Bone Miner Res 24:1651–1661 [DOI] [PubMed] [Google Scholar]
- 15. Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. 2008. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875 [DOI] [PubMed] [Google Scholar]
- 16. O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. 2008. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One 3:e2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costa AG, Cremers S, Rubin MR, McMahon DJ, Sliney J, Jr, Lazaretti-Castro M, Silverberg SJ, Bilezikian JP. 2011. Circulating sclerostin in disorders of parathyroid gland function. J Clin Endocrinol Metab 96:3804–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu EW, Kumbhani R, Siwila-Sackman E, Leder BZ. 2011. Acute decline in serum sclerostin in response to PTH infusion in healthy men. J Clin Endocrinol Metab 96:E1848–E1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drake MT, Srinivasan B, Mödder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. 2010. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]