Abstract
Context:
Whether menopause-related changes in sex steroids account for midlife weight gain in women or whether weight drives changes in sex steroids remains unanswered.
Objective:
The objective of the study was to characterize the potential reciprocal nature of the associations between sex hormones and their binding protein with waist circumference in midlife women.
Design, Setting, and Participants:
The study included 1528 women (mean age 46 yr) with 9 yr of follow-up across the menopause transition from the observational Study of Women's Health Across the Nation.
Main Outcome Measures:
Waist circumference, SHBG, testosterone, FSH, and estradiol were measured.
Results:
Current waist circumference predicted future SHBG, testosterone, and FSH but not vice versa. For each sd higher current waist circumference, at the subsequent visit SHBG was lower by 0.04–0.15 sd, testosterone was higher by 0.08–0.13 sd, and log2 FSH was lower by 0.15–0.26 sd. Estradiol results were distinct from those above, changing direction across the menopause transition. Estradiol and waist circumference were negatively associated in early menopausal transition stages and positively associated in later transition stages (for each sd higher current waist circumference, future estradiol was lower by 0.15 sd in pre- and early perimenopause and higher by 0.38 sd in late peri- and postmenopause; P for interaction <0.001). In addition, they appeared to be reciprocal, with current waist circumference associated with future estradiol and current estradiol associated with future waist circumference. However, associations in the direction of current waist circumference predicting future estradiol levels were of considerably larger magnitude than the reverse.
Conclusions:
These Study of Women's Health Across the Nation data suggest that the predominant temporal sequence is that weight gain leads to changes in sex steroids rather than vice versa.
In women, the prevalence of obesity increases during midlife. However, whether this increase is due to aging, the menopause transition, or both remains unclear (1). Additionally, it is unclear whether weight gain and/or development of abdominal obesity induces changes in reproductive hormones or vice versa.
Weight gain increases the incidence of diabetes, cancer, and cardiovascular disease. An improved understanding of the temporal associations between weight gain and changing hormone levels may lead to new strategies to curb weight gain during midlife. Therefore, we characterized the sequential nature of the longitudinal associations between changes in selected hormones and changes in waist circumference and body weight in a multiethnic cohort of women undergoing the menopause transition.
Materials and Methods
Participants
The Study of Women's Health Across the Nation (SWAN) is a multicenter, longitudinal study to characterize the biological and psychosocial changes with menopause in a community-based sample (2). From 1996 to 1997, 3302 women aged 42–52 yr were enrolled across seven recruitment sites (Boston, MA; Chicago, IL; Detroit, MI; Los Angeles, CA; Newark, NJ; Pittsburgh, PA; and Oakland, CA). SWAN eligibility criteria included an intact uterus, at least one menstrual period in the previous 3 months, at least one ovary, not being pregnant or breast-feeding, and no oral contraceptive or sex steroid hormone therapy use in the previous 3 months. The protocol was approved by each site's institutional review board, and all women provided written informed consent.
This report is based on data from the baseline visit as well as follow-up visits occurring 3, 6, and 9 yr after the baseline visit. From the inception cohort of 3302 women, we excluded 253 women who had a hysterectomy or bilateral oophorectomy; 795 who missed at least two of the three follow-up visits; 656 who reported current hormone use or were in the middle of a 6-month hormone washout period during any of the three follow-up visits; 19 whose hormone use information was unknown at baseline; two who were pregnant; and women from the Newark site (n = 49) due to the fact that Newark did not collect data at visit 9. This left 1528 women for the current analyses.
Physical measures
Height and weight were measured in light clothing without shoes using calibrated scales. Body mass index was calculated as weight (kilograms) divided by height (meters) squared. Waist circumference was measured in nonrestrictive undergarments or over light clothing, at the narrowest part of the torso as seen from the anterior aspect. For women in whom a waist narrowing was difficult to identify, the measure was taken at the smallest horizontal circumference in the area between the ribs and the iliac crest.
Questionnaire data
Menstrual cycle bleeding history over the prior year was assessed by interview and categorized as follows:
Premenopause: one or more periods within the previous 3 months and no perceived change in cycle interval.
Early perimenopause: one or more periods within the past 3 months, with a perceived change in cycle interval.
Late perimenopause: no bleeding in the previous 3 months but bleeding in the last 11 months.
Postmenopause: 12 or more consecutive months of amenorrhea.
Race-ethnicity was self-assigned. Highest grade level or educational degree attained and smoking history were assessed by questionnaire. Alcohol consumption was assessed by interview using a modified version of the Block Food Frequency Questionnaire (3).
Hormone measurement
Fasting blood draws were targeted to the follicular phase of the menstrual cycle (d 2–5) for menstruating women. Two attempts were made to obtain a follicular phase sample. When a follicular phase sample could not be obtained and in women no longer menstruating regularly, a random fasting sample was taken within 90 d of the anniversary date of the baseline visit. For the purposes of analysis, cycle day of blood draw was dichotomized as within d 2–5 of the menstrual cycle or outside d 2–5/unknown. Samples were maintained at 4 C until separated and frozen at −80 C until assays were performed.
Hormone assays were performed at the University of Michigan Endocrine Laboratory on an ACS-180 automated analyzer (Bayer Diagnostics Corp., Tarrytown, NY) using a double-antibody chemiluminescent immunoassay with a solid phase anti-IgG immunoglobulin conjugated to paramagnetic particles, antiligand antibody, and competitive ligand labeled with dimethylacridinium ester. The FSH assay modified a manual assay kit (Bayer Diagnostics) using two monoclonal antibodies directed to different regions on the β-subunit, with a lower limit of detection (LLD) of 0.4–1.0 mIU/ml. The estradiol (E2) assay modified the rabbit anti-E2–6 ACS-180 immunoassay to increase sensitivity, with the visit-specific LLD ranging from 1.0–7.0 pg/ml. The testosterone (T) assay modified the rabbit polyclonal anti-T ACS-180 immunoassay, with a LLD of 2.0–2.2 ng/dl. The SHBG assay was developed on-site using rabbit anti-SHBG antibodies, with a LLD of 1.9–3.2 nm. Duplicate E2 assays were conducted with results reported as the arithmetic mean for each subject, with a coefficient of variation of 3–12%. All other assays were single determinations. Hormone values below the LLD were replaced with a random value between zero and the LLD. This occurred for at least one hormone in 3.5% (n = 53) of women; these women did not differ from those with values above the LLD in age, body weight, waist circumference, race-ethnicity, education levels, menopause status, cycle day of blood draw, or the prevalence of smoking or alcohol use.
Statistical analysis
Distributions of variables of interest were summarized using means and sd for normally distributed variables, medians, and interquartile ranges for skewed variables and frequency counts for categorical variables.
The dynamic sequential relationships between hormones and waist circumference were examined using structural equation modeling (SEM). Specifically these equations examined the following three longitudinal relationships: 1) the association of current hormone values and waist circumferences with their respective future values, 2) the associations of current waist circumference with future hormone values, and 3) the associations of current hormone values with future waist circumference values. Additionally, concurrent correlations of hormone levels and waist circumference as well as ethnicity, education, alcohol use, smoking status, height (all measured at baseline) and age, cycle day of hormone blood draw, and menopausal status (all time varying) were adjusted for. Waist circumference associations were additionally adjusted for body weight (time varying). Because findings for waist circumference and body weight were similar, only waist circumference results are presented here. Identical analyses for body weight are available in an appendix (Supplemental Figs. 1–4, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
To test whether the sequential relationships between hormones and waist circumference varied by menopausal status, we selected two subpopulations: 1) pre/early perimenopausal women (n = 655): women in this group remained premenopausal or early perimenopausal from baseline through visit 6; and 2) late perimenopausal/postmenopausal women (n = 334): women in this group had transitioned to late perimenopause or postmenopause by visit 3 and provided data through visit 9. Analyses were repeated in these subgroups. Effect modification of the waist circumference/hormone relationships by menopausal status was also tested using generalized estimation equation modeling with SAS Windows 9.2 (SAS Institute, Cary, NC), rather than SEM, to allow for testing of the overall interaction effect across the follow-up period (4). Interaction terms between prior menopausal status and prior hormone values were included to predict subsequent waist circumference. Conversely, interaction terms between prior menopausal status and prior waist circumference were included to predict subsequent hormone levels.
To examine whether analyses that used a shorter time between visits would result in similar conclusions, models based on visits 5, 6, and 7 were run (n = 1424 women with complete data for all three visits). To evaluate whether results were influenced by large changes in abdominal girth, we also ran models confined to the subgroup who had a greater than 10% increase or greater than 5% decrease in waist circumference between baseline and visit 9 or between any two adjacent visits.
The Mplus statistical package (1998–2008 version; Los Angeles, CA) was used for SEM modeling, using the maximum likelihood procedure with nonnormality robust se. Model fit was evaluated by root mean square error of approximation (good fit if values ≤0.05 or 95% confidence interval lower values close to 0 and upper values <0.08); Bentler's comparative fit index (good fit if values ≥0.95) (5); and standardized root mean square residual (good fit if values <0.05).
Results
Baseline mean age was 46 yr, and mean body mass index (BMI) was in the overweight range (Table 1). Most women had greater than a high school education, were never or former smokers, and reported minimal or no intake of alcoholic beverages. Table 1 also presents characteristics of the two menopausal status subsamples. As expected, the group of women who remained pre- or early perimenopausal through visit 6 were younger than the group that became late perimenopausal or postmenopausal by visit 3. Women who transitioned to late peri- or postmenopause by visit 3 also had higher BMI values, were more likely to be African-American, and were more often current smokers. Mean weight, waist circumference, and FSH increased across follow-up, whereas mean estradiol decreased (Table 2). After small initial declines, serum testosterone levels increased slightly, whereas SHBG levels remained stable.
Table 1.
Baseline characteristics of the full analytic sample and subgroups used to examine menopausal status interactions
| Mean (sd) or n (%) |
|||
|---|---|---|---|
| Overall (visit 0) (n = 1528) | Premenoapusal/early perimenopausal (visit 0) (n = 655) | Late perimenopausal/ postmenopausal (visit 3) (n = 334) | |
| Age (yr) | 46.2 (2.6) | 44.7 (1.8) | 51.4 (2.4) |
| BMI (kg/m2) | 28.1 (7.3) | 27.5 (6.7) | 29.4 (8.1) |
| Body weight (kg) | 74.7 (21.2) | 73.6 (19.7) | 77.7 (22.6) |
| Waist circumference (cm) | 86.0 (16.4) | 84.6 (15.5) | 89.5 (18.0) |
| Race/ethnicity, n (%) | |||
| Caucasian | 674 (44.1) | 308 (47.0) | 136 (40.7) |
| African-American | 511 (33.4) | 200 (30.5) | 125 (37.4) |
| Chinese | 157 (10.3) | 64 (9.8) | 33 (9.9) |
| Japanese | 186 (12.2) | 83 (12.8) | 40 (12.0) |
| Menopause status, n (%) | |||
| Premenopausal | 884 (58.8) | 448 (69.8) | |
| Early menopausal | 620 (41.2) | 194 (30.2) | |
| Late perimenopausal | 162 (48.5) | ||
| Postmenopausal | 172 (51.5) | ||
| Education at V0, n (%) | |||
| High school or less | 306 (20.2) | 114 (17.5) | 74 (22.3) |
| High school | 499 (32.9) | 195 (29.9) | 126 (38.0) |
| College | 332 (21.9) | 158 (24.2) | 59 (17.8) |
| Postcollege | 381 (25.1) | 186 (28.5) | 73 (22.0) |
| Smoking status at V0, n (%) | |||
| Never | 902 (59.4) | 417 (64.4) | 179 (53.9) |
| Former | 383 (25.2) | 147 (22.7) | 96 (28.9) |
| Current | 233 (15.4) | 84 (13.0) | 57 (17.2) |
| Alcohol consumption at V0, n (%) | |||
| No | 798 (52.4) | 354 (54.4) | 175 (52.6) |
| One or fewer drinks/d | 637 (41.8) | 258 (39.6) | 133 (39.9) |
| More than drink/d | 88 (5.8) | 39 (6.0) | 25 (7.5) |
| Blood drawn in cycle d 2–5, n (%) | |||
| Yes | 1217 (79.9) | 559 (85.7) | 14 (4.7) |
| No/unknown | 307 (20.1) | 93 (14.3) | 285 (95.3) |
Visit 3 occurred 3 yr after baseline, and visit 6 occurred 6 yr after baseline. V, Visit.
Table 2.
Study characteristics across follow-up
| Visit 0 (baseline) | Visit 3 | Visit 6 | Visit 9 | |
|---|---|---|---|---|
| Body weight (kg) | 74.7 (21.2) | 75.7 (21.1) | 76.3 (21.1) | 76.5 (20.8 |
| Waist circumference (cm) | 86.0 (16.4) | 87.5 (16.8) | 89.0 (16.7) | 90.2 (16.8) |
| E2 (pg/ml) | 54.8 (33.3–85.2) | 34.6 (21.2–75.0) | 22.2 (12.8–47.1) | 20.3 (15.0–29.9) |
| FSH (mIU/ml) | 15.2 (11.0–24.9) | 26.0 (13.9–59.3) | 58.8 (21.2–94.3) | 89.5 (58.3–122.6) |
| T (ng/dl) | 42.0 (30.2–56.7) | 34.7 (24.6–48.6) | 36.9 (26.5–50.0) | 37.9 (26.5–51.9) |
| SHBG (nm) | 40.4 (27.4–57.6) | 39.1 (25.5–53.6) | 39.5 (26.1–56.3) | 39.4 (25.6–58.6) |
| Menopausal status, n (%) | ||||
| Premenopausal | 884 (58.8) | 213 (14.9) | 69 (4.6) | 14 (1.0) |
| Early perimenopausal | 620 (41.2) | 885 (61.8) | 586 (39.4) | 234 (16.6) |
| Late perimenopausal | 0 | 162 (11.3) | 203 (13.5) | 143 (10.1) |
| Postmenopausal | 0 | 172 (12.0) | 631 (42.4) | 1021 (72.3) |
Values are mean (sd), median (interquartile range), or n (%). Visit 3 occurred 3 yr after baseline; visit 6 occurred 6 yr after baseline; and visit 9 occurred 9 yr after baseline.
SEM results are summarized in the figures. After accounting for the associations between current waist circumference and future waist circumference as well as current hormone levels and future hormone levels (black arrows), the aims of the SEM models were to discern whether current waist circumference predicts future hormone level (red arrows) and whether current hormone level predicts future waist circumference (blue arrows). Current refers to the value at each study visit and future to the value at the next study visit. Statistically significant associations are demarcated with solid lines, with thicker arrows corresponding to a larger effect size. Dashed lines represent nonsignificant associations. Hormones (original values for SHBG and T, and log2 for FSH and estradiol) and waist size were quantified in units of 1 sd to enable comparison of the strength of association across hormones. Estimates equating to original values, rather than standardized units, are presented in Supplemental Table 1.
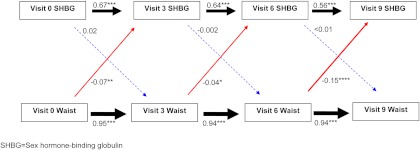
Figure 1 shows the SEM results for the relationships between SHBG and waist circumference. Current waist circumference predicted future SHBG but not vice versa. Specifically, for each sd increment in current waist circumference, SHBG was lower by 0.04–0.15 sd at the subsequent visit (Fig. 1, solid red arrows). Corresponding results using original units, rather than standardized units, indicated that for each 1-cm increment in waist circumference, SHBG was lower by 0.07–0.25 nm at the subsequent visit (Supplemental Table 1). At no time point did current SHBG predict future waist circumference (Fig. 1, dashed blue arrows).
Fig. 1.
Sequential relationships between waist circumference and SHBG using standardized estimates. Red arrows, Association between current waist circumference and future SHBG level; Blue arrows, association between current SHBG level and future waist circumference; black arrows, top association between current SHBG and future SHBG; bottom association between current waist circumference and future waist circumference. Solid lines indicate statistically significant associations, whereas dashed lines indicate nonsignificant associations. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The thickness of the arrow corresponds to the magnitude of the association (i.e. thicker arrows mean a larger effect size). Values provided are standardized regression estimates. Visit 3 occurred 3 yr after baseline; visit 6 occurred 6 yr after baseline; and visit 9 occurred 9 yr after baseline.
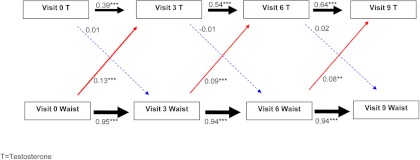
Figure 2 shows the SEM results for the relationships between T and waist circumference. As with SHBG, current waist circumference predicted future T (Fig. 2, solid red arrows) but not vice versa (Fig. 2, dashed blue arrows). Each sd higher current waist circumference was associated with a 0.08- to 0.13-sd increase in T at the subsequent visit (actual units: 0.08–0.16 mg/dl higher subsequent T for each centimeter higher current waist circumference; Supplemental Table 1). At no time point did current T predict future waist circumference (Fig. 2, dashed blue arrows).
Fig. 2.
Sequential relationships between waist circumference and T using standardized estimates. Red arrows, Association between current waist circumference and future SHBG level; blue arrows, association between current SHBG level and future waist circumference; black arrows, top association between current SHBG and future SHBG; bottom association between current waist circumference and future waist circumference. Solid lines indicate statistically significant associations, whereas dashed lines indicate nonsignificant associations. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The thickness of the arrow corresponds to the magnitude of the association (i.e. thicker arrows mean a larger effect size). Values provided are standardized regression estimates. Visit 3 occurred 3 yr after baseline; visit 6 occurred 6 yr after baseline; and visit 9 occurred 9 yr after baseline.
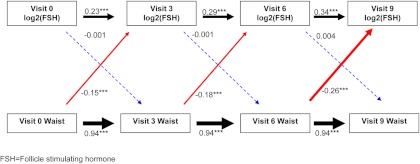
Similar results were again observed for relationships between waist circumference and FSH (Fig. 3). Current waist circumference was associated with future FSH (Fig. 3, solid red arrows) but not vice versa (Fig. 3, dashed blue arrows). For each centimeter increment in current waist circumference, subsequent FSH values were lower by 0.15–0.26 sd. Thus, for a woman with average FSH values at baseline (15.2 mIU/ml), each centimeter increment in baseline waist circumference was associated with a 0.11 mIU/ml lower FSH at visit 3, whereas for a woman with average FSH values at visit 6 (58.8 mIU/ml), each centimeter increment in visit 6 waist circumference was associated with a 0.82 mIU/ml lower FSH at visit 9 (Supplemental Table 1). At no time did current FSH predict future waist circumference (Fig. 3, dashed blue arrows).
Fig. 3.
Sequential relationships between waist circumference and FSH using standardized estimates. Red arrows, Association between current waist circumference and future SHBG level; blue arrows, association between current SHBG level and future waist circumference; black arrows, top association between current SHBG and future SHBG; bottom association between current waist circumference and future waist circumference. Solid lines indicate statistically significant associations, whereas dashed lines indicate nonsignificant associations. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The thickness of the arrow corresponds to the magnitude of the association (i.e. thicker arrows mean a larger effect size). Values provided are standardized regression estimates. Visit 3 occurred 3 yr after baseline; visit 6 occurred 6 yr after baseline; and visit 9 occurred 9 yr after baseline.
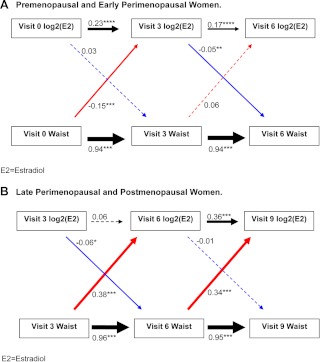
In contrast to SHBG, T, and FSH, the associations between estradiol levels and waist circumference were bidirectional and were different during the early menopause transition stages (Fig. 4A) than they were in later menopause transition stages (Fig. 4B) (P for interaction from generalized estimation equation modeling <0.001). Among premenopausal and early perimenopausal women (Fig. 4A), greater current waist circumference at baseline predicted lower future estradiol levels at visit 3; specifically, each centimeter increment in baseline waist circumference was associated with a lower visit 3 log2 estradiol of 0.15 sd [0.55 pg/ml for a woman of average estradiol levels (54.8 pg/ml) at baseline]. In contrast, during late perimenopause and postmenopause, greater current waist circumference predicted higher future estradiol levels (Fig. 4B): each centimeter increment in waist circumference was associated with a 0.34- to 0.38-sd higher future estradiol level [0.69 and 0.22 pg/ml for a woman with average estradiol values at visit 3 (34.6 pg/ml) and visit 6 (22.2 pg/ml), respectively]. In both the early transition stages (Fig. 4A) and later transition stages (Fig. 4B), there was a small, negative association between the current estradiol measured at visit 3 and future waist circumference measured at visit 6.
Fig. 4.
Sequential relationships between waist circumference and estradiol, stratified by menopausal status using standardized estimates. A, Premenopausal and early perimenopausal women. B, Late perimenopausal and postmenopausal women. Red arrows, Association between current waist circumference and future SHBG level; blue arrows, association between current SHBG level and future waist circumference; black arrows, top, association between current SHBG and future SHBG; bottom association between current waist circumference and future waist circumference. Solid lines indicate statistically significant associations, whereas dashed lines indicate nonsignificant associations. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The thickness of the arrow corresponds to the magnitude of the association (i.e. thicker arrows mean a larger effect size). Values provided are standardized regression estimates. Visit 3 occurred 3 yr after baseline; visit 6 occurred 6 yr after baseline; and visit 9 occurred 9 yr after baseline.
Adjustment of the preceding analyses for body weight had little effect on the results (data not shown). Additionally, substituting body weight for waist circumference in the analyses described above did not alter the basic patterns of the associations, although the associations were less consistent and weaker than those for waist circumference (Supplemental Figs. 1–4). The notable exception was the P value for the association between visit 3 T and visit 6 body weight, which was statistically significant, even though the standardized estimate was very similar to the corresponding estimate for waist circumference (−0.02 vs. −0.01, respectively).
Sensitivity analyses (i.e. subset with complete, consecutive data at visits 5, 6, and 7; those with >10% increase or >5% decrease in waist circumference) yielded results similar to those presented above (data not shown).
Discussion
We prospectively evaluated the potential reciprocal nature of associations between adiposity and endogenous sex hormones and SHBG. Our results suggest unidirectional relationships between waist circumference and body weight with SHBG, T, and FSH, such that the changes in adiposity precede the changes in SHBG, T, and FSH, rather than the converse. In contrast, the relationship between waist girth and estradiol appeared to be bidirectional, suggesting both that adiposity may lead to changes in estradiol levels and that estradiol levels may lead to changes in adiposity. Additionally, the relationship between estradiol and waist circumference (and body weight) varied according to menopause state such that larger waist circumference and body weight were associated with lower future estradiol levels early in the menopausal transition but with higher future estradiol levels later in the menopausal transition.
Current weight and waist circumference levels predicted lower future SHBG levels throughout the menopause transition. This finding agrees with cross-sectional findings and may be related to adiposity-induced hyperglycemia, which may suppress hepatic SHBG synthesis (6). In addition, hepatic triglyceride accumulation is associated with lower SHBG levels (7, 8). Because the prevalence of hyperinsulinemia, hyperglycemia, and fatty liver are higher with more abdominal fat, it is plausible that weight gain lowers SHBG by increasing insulin and glucose and/or by promoting hepatic fat accretion. Such a potential mechanism suggests that increased adiposity precedes reduced SHBG, in accordance with our current results and with prior interventional studies in which weight loss preceded increases in SHBG (10, 11). However, other longitudinal observational studies have not reported associations between SHBG and either BMI or waist circumference (12).
That greater girth and weight predict higher testosterone levels is in accordance with cross-sectional analyses and with the frequent co-occurrence of hyperandrogenemia and abdominal obesity observed in polycystic ovary syndrome (13). However, most prior studies have not been able to discern the sequential nature of the associations because measurements were made too infrequently and/or because the analyses were limited to concurrent repeated measurements of testosterone and adiposity, rather than lagged relationships (14, 15). Two prior studies tested for lagged relationships between testosterone and adiposity measures: one found inconsistent and weak associations, whereas the other found that higher baseline testosterone levels and greater increases in testosterone were associated with moderate increases in the risk of incident obesity, even after adjusting for baseline BMI (16).
We found a statistically significant negative association between testosterone at visit 3 and body weight at visit 6, suggesting a possible bidirectional relationship between body weight and T (see Supplemental Fig. 2). However, given that this association was weak and is not consistent with prior SWAN data (16), it is possible that this association is spurious. Taken together, the current SWAN results suggest that the predominant sequence is that increases in adiposity precede increases in T rather than the reverse. Consistent with this are data from murine models showing that knockout of the androgen receptor has no influence on fat mass; reported body weight reductions were due solely to decreases in lean mass (17). However, data in nonmidlife, female-to-male transsexuals suggests that testosterone administration may increase abdominal visceral fat. In these studies, supraphysiological, exogenous testosterone administration increased visceral fat but decreased sc fat, with no net change in overall BMI or total fat mass (18, 19). However, because participants received doses of testosterone that were massively supraphysiological, it is difficult to extrapolate these findings to normal women.
Our observed negative associations between weight or waist circumference with FSH are consistent with other studies of midlife women showing that obese women have lower FSH than nonobese women (20–22). Given the negative feedback between estradiol and FSH, higher postmenopausal estradiol levels among obese women may explain their lower FSH levels.
In contrast to the overarching pattern that greater girth and weight drive changes in levels of SHBG, T, and FSH, for estradiol we found not only that current waist circumference was associated with future estradiol levels but also the reverse, although the effect sizes were much larger in the former direction. The negative relation between current estradiol and future waist circumference was observed only in the interval between SWAN visits 3 and 6, reflecting probable heterogeneity in the transition from early to late perimenopause because many women transitioned from early to late perimenopause between these visits. These relative magnitudes are consistent with most studies examining weight changes in midlife, suggesting that weight gain is primarily due to aging rather than to menopause-related estrogen deficiency, (23–26) and with experimental data showing that exogenous estrogen administration in postmenopausal women does not prevent weight gain (27). However, although the effect sizes were larger for adiposity's effects on estradiol than for the reverse, murine models suggest the estrogen protects against fat accumulation; abdominal fat in particular is markedly increased in mice with null mutations of either their estrogen receptor-α gene or their aromatase gene (28, 29). This pattern of fat accumulation is consistent with some human epidemiological studies, suggesting that although menopause may not be associated with overall weight gain, it may lead to focused fat accumulation in the abdominal region (30–33) and with our finding that estradiol's associations were stronger for waist size than for body weight. Although these murine models are compelling, the effects of life-long estrogen deficiency, as in these animals, may differ from estrogen deficiency that develops in midlife as occurs with the menopause.
In accordance with our results, others have also found that the relation between waist circumference (or weight) and estradiol levels differ in early compared with later menopause transition stages, with a negative association early and a positive association later in the transition (20, 21, 34–36). One postulate for this finding is that low SHBG levels in obese women result in greater estradiol clearance and therefore lower circulating estradiol levels (21), which is potentially overwhelmed later in the transition, when estradiol levels are substantially lower, by peripheral estradiol production via androgen aromatization within adipose tissue. However, our data do not support this hypothesis because when we adjusted our estradiol results for SHBG levels, the negative associations between weight or waist circumference and estradiol in premenopausal women persisted. Alternatively, murine models document anorectic and activity-promoting effects of estradiol (37, 38), raising the possibility that before menopause, when estradiol levels are higher, the negative association between waist circumference and estradiol may result from estradiol-induced decreases in meal size and increases in physical activity.
The results of this study must be viewed within the context of its limitations. Waist circumference represents both abdominal visceral and sc fat. However, existing studies of computed tomography or magnetic resonance imaging measures of adiposity in midlife women do not include the necessary number of repeated measurements or the large sample of women required to address questions of bidirectionality. Estradiol levels in premenopausal women vary from hour to hour and day to day; standardization of blood sampling by time of day and day of the menstrual cycle was implemented to reduce measurement error. Hormone measurements were quantified by immunoassay rather than by mass spectrometry. Therefore, despite the careful assay development, measurement error at extremely low sex steroid cannot be ruled out. However, prior cohorts have found similar results and immunoassay is less prone to measurement error when estradiol levels are high, as in premenopausal women, which constituted an appreciable proportion of our sample. Furthermore, both the estradiol and testosterone assays have been validated against celite chromatography (9). Strengths of the current study include 9 yr of observation on a well-characterized, race-ethnically diverse cohort, and the use of SEM methodology, a methodology not used in previous longitudinal investigations, and particularly well suited to exploration of bidirectional, or reciprocal, associations.
In conclusion, both current body weight and waist circumference were strongly associated with future endogenous sex hormones and SHBG, whereas current hormones were either not found to be associated with future adiposity measures or, in the case of estradiol, were considerably more weakly associated with future adiposity measures. Therefore, it is likely that the more predominant temporal sequence among midlife women is that adiposity initiates changes in sex hormones, rather than vice versa.
Supplementary Material
Acknowledgments
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, National Institute of Nursing Research, Office of Research on Women's Health, or the National Institutes of Health. Clinical centers included the following: University of Michigan, Ann Arbor, Siobán Harlow, principal investigator (PI) 2011, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA, Joel Finkelstein, PI 1999 to present; Robert Neer, PI 1994 to 1999; Rush University, Rush University Medical Center, Chicago, IL, Howard Kravitz, PI 2009 to present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser Permanente, Ellen Gold, PI; University of California, Los Angeles, Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY, Carol Derby, PI 2011, Rachel Wildman, PI 2010–2011, Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry, New Jersey Medical School, Newark, NJ, Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA, Karen Matthews, PI. National Institutes of Health Program Office: National Institute on Aging, Bethesda, MD, Sherry Sherman, 1994 to present; Marcia Ory, 1994–2001; and National Institute of Nursing Research, Bethesda, MD, program officers. The central laboratory was the University of Michigan, Ann Arbor, Daniel McConnell (Central Ligand Assay Satellite Services). The coordinating centers were the following: University of Pittsburgh, Pittsburgh, PA, Kim Sutton-Tyrrell, PI 2001 to present; New England Research Institutes, Watertown, MA, Sonja McKinlay, PI 1995–2001. The steering committee: Susan Johnson, current chair; and Chris Gallagher, former chair. We thank the study staff at each site and all the women who participated in SWAN.
The Study of Women's Health Across the Nation has grant support from the National Institutes of Health, Department of Health and Human Services through the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Health Office of Research on Women's Health (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- E2
- estradiol
- LLD
- lower limit of detection
- SEM
- structural equation modeling
- SWAN
- Study of Women's Health Across the Nation
- T
- testosterone.
References
- 1. Wildman RP, Sowers MR. 2011. Adiposity and the menopausal transition. Obstet Gynceol Clin North Am 38:441–454 [DOI] [PubMed] [Google Scholar]
- 2. Sowers MR. 2000. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopause transition. In: Lobo RA, Kelsey J, Marcus J, eds. Menopause: biology and pathobiology. San Diego: Academic Press; 175–188 [Google Scholar]
- 3. Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. 1992. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc 92:686–693 [PubMed] [Google Scholar]
- 4. Zeger SL, Liang KY, Albert PS. 1988. Models for longitudinal data: a generalized estimating equation approach. Biometrics 44:1049–1060 [PubMed] [Google Scholar]
- 5. Bentler PM. 1990. Comparative fit indexes in structural models. Psycholog Bull 107:238–246 [DOI] [PubMed] [Google Scholar]
- 6. Selva DM, Hogeveen KN, Innis SM, Hammond GL. 2007. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest 117:3979–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stefan N, Schick F, Häring HU. 2009. Sex hormone-binding globulin and risk of type 2 diabetes. N Engl J Med 361:2675–2676; author reply 2677–2678 [DOI] [PubMed] [Google Scholar]
- 8. Shin JY, Kim SK, Lee MY, Kim HS, Ye BI, Shin YG, Baik SK, Chung CH. 2011. Serum sex hormone-binding globulin levels are independently associated with nonalcoholic fatty liver disease in people with type 2 diabetes. Diabetes Res Clin Pract 94:156–162 [DOI] [PubMed] [Google Scholar]
- 9. Anderson DC, Lasley BL, Risher RA, Shepherd JH, Newman L, Hendrickx AG. 1976. Transplacental gradients of sex-hormone-binding globulin in human and simian pregnancy. Clin Endocrinol (Oxf) 5:657–669 [DOI] [PubMed] [Google Scholar]
- 10. Tolino A, Gambardella V, Caccavale C, D'Ettore A, Giannotti F, D'Antò V, De Falco CL. 2005. Evaluation of ovarian functionality after a dietary treatment in obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 119:87–93 [DOI] [PubMed] [Google Scholar]
- 11. Kopp HP, Krzyzanowska K, Schernthaner GH, Kriwanek S, Schernthaner G. 2006. Relationship of androgens to insulin resistance and chronic inflammation in morbidly obese premenopausal women: studies before and after vertical banded gastroplasty. Obes Surg 16:1214–1220 [DOI] [PubMed] [Google Scholar]
- 12. Sternfeld B, Liu K, Quesenberry CP, Jr, Wang H, Jiang SF, Daviglus M, Fornage M, Lewis CE, Mahan J, Schreiner PJ, Schwartz SM, Sidney S, Williams OD, Siscovick DS. 2008. Changes over 14 years in androgenicity and body mass index in a biracial cohort of reproductive-age women. J Clin Endocrinol Metab 93:2158–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pasquali R. 2006. Obesity and androgens: facts and perspectives. Fertil Steril 85:1319–1340 [DOI] [PubMed] [Google Scholar]
- 14. Guthrie JR, Dennerstein L, Taffe JR, Ebeling PR, Randolph JF, Burger HG, Wark JD. 2003. Central abdominal fat and endogenous hormones during the menopausal transition. Fertil Steril 79:1335–1340 [DOI] [PubMed] [Google Scholar]
- 15. Sowers MF, Beebe JL, McConnell D, Randolph J, Jannausch M. 2001. Testosterone concentrations in women aged 25–50 years: associations with lifestyle, body composition, and ovarian status. Am J Epidemiol 153:256–264 [DOI] [PubMed] [Google Scholar]
- 16. Sutton-Tyrrell K, Zhao X, Santoro N, Lasley B, Sowers M, Johnston J, Mackey R, Matthews K. 2010. Reproductive hormones and obesity: 9 years of observation from the Study of Women's Health Across the Nation. Am J Epidemiol 171:1203–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Callewaert F, Venken K, Ophoff J, De Gendt K, Torcasio A, van Lenthe GH, Van Oosterwyck H, Boonen S, Bouillon R, Verhoeven G, Vanderschueren D. 2009. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-α. FASEB J 23:232–240 [DOI] [PubMed] [Google Scholar]
- 18. Elbers JM, Giltay EJ, Teerlink T, Scheffer PG, Asscheman H, Seidell JC, Gooren LJ. 2003. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf) 58:562–571 [DOI] [PubMed] [Google Scholar]
- 19. Giltay EJ, Elbers JM, Gooren LJ, Emeis JJ, Kooistra T, Asscheman H, Stehouwer CD. 1998. Visceral fat accumulation is an important determinant of PAI-1 levels in young, nonobese men and women: modulation by cross-sex hormone administration. Arterioscler Thromb Vasc Biol 18:1716–1722 [DOI] [PubMed] [Google Scholar]
- 20. Freeman EW, Sammel MD, Gracia CR, Kapoor S, Lin H, Liu L, Nelson DB. 2005. Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril 83:383–392 [DOI] [PubMed] [Google Scholar]
- 21. Freeman EW, Sammel MD, Lin H, Gracia CR. 2010. Obesity and reproductive hormone levels in the transition to menopause. Menopause 17:718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. 1999. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 84:4025–4030 [DOI] [PubMed] [Google Scholar]
- 23. Crawford SL, Casey VA, Avis NE, McKinlay SM. 2000. A longitudinal study of weight and the menopause transition: results from the Massachusetts Women's Health Study. Menopause 7:96–104 [DOI] [PubMed] [Google Scholar]
- 24. Davies KM, Heaney RP, Recker RR, Barger-Lux MJ, Lappe JM. 2001. Hormones, weight change and menopause. Int J Obes Relat Metab Disord 25:874–879 [DOI] [PubMed] [Google Scholar]
- 25. Guthrie JR, Dennerstein L, Dudley EC. 1999. Weight gain and the menopause: a 5-year prospective study. Climacteric 2:205–211 [DOI] [PubMed] [Google Scholar]
- 26. Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. 1991. Weight gain at the time of menopause. Arch Intern Med 151:97–102 [PubMed] [Google Scholar]
- 27. Kongnyuy EJN, RJ, Flight HKI, Rees MC. 2007. Oestrogen and progestogen hormone replacement therapy for peri-menopausal and post-menopausal women: weight and body fat distribution. Cochrane Database Syst Rev 7. [DOI] [PubMed] [Google Scholar]
- 28. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. 2000. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. 2000. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 97:12735–12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toth MJ, Tchernof A, Sites CK, Poehlman ET. 2000. Menopause-related changes in body fat distribution. Ann NY Acad Sci 904:502–506 [DOI] [PubMed] [Google Scholar]
- 31. Donato GB, Fuchs SC, Oppermann K, Bastos C, Spritzer PM. 2006. Association between menopause status and central adiposity measured at different cutoffs of waist circumference and waist-to-hip ratio. Menopause 13:280–285 [DOI] [PubMed] [Google Scholar]
- 32. Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. 2008. Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch Intern Med 168:1568–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pasquali R, Casimirri F, Pascal G, Tortelli O, Morselli Labate A, Bertazzo D, Vicennati V, Gaddi A. 1997. Influence of menopause on blood cholesterol levels in women: the role of body composition, fat distribution and hormonal milieu. Virgilio Menopause Health Group. J Intern Med 241:195–203 [DOI] [PubMed] [Google Scholar]
- 34. Randolph JF, Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. 2004. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 89:1555–1561 [DOI] [PubMed] [Google Scholar]
- 35. Randolph JF, Jr, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL. 2003. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 88:1516–1522 [DOI] [PubMed] [Google Scholar]
- 36. Randolph JF, Jr, Zheng H, Sowers MR, Crandall C, Crawford S, Gold EB, Vuga M. 2011. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab 96:746–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eckel LA. 2004. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav 82:35–41 [DOI] [PubMed] [Google Scholar]
- 38. Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. 2007. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc 39:248–256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.