Abstract
Context/Objectives:
Pazopanib, an inhibitor of kinases including vascular endothelial growth factor receptor, demonstrated impressive activity in progressive metastatic differentiated thyroid cancer, prompting its evaluation in anaplastic thyroid cancer (ATC).
Design/Setting/Patients/Interventions/Outcome Measures:
Preclinical studies, followed by a multicenter single arm phase 2 trial of continuously administered 800 mg pazopanib daily by mouth (designed to provide 90% chance of detecting a response rate of >20% at the 0.10 significance level when the true response rate is >5%), were undertaken. The primary trial end point was Response Evaluation Criteria in Solid Tumors (RECIST) response.
Results:
Pazopanib displayed activity in the KTC2 ATC xenograft model, prompting clinical evaluation. Sixteen trial patients were enrolled; 15 were treated: 66.7% were female, median age was 66 yr (range 45–77 yr), and 11 of 15 had progressed through prior systemic therapy. Enrollment was halted, triggered by a stopping rule requiring more than one confirmed RECIST response among the first 14 of 33 potential patients. Four patients required one to two dose reductions; severe toxicities (National Cancer Institute Common Toxicity Criteria-Adverse Events version 3.0 grades >3) were hypertension (13%) and pharyngolaryngeal pain (13%). Treatment was discontinued because of the following: disease progression (12 patients), death due to a possibly treatment-related tumor hemorrhage (one patient), and intolerability (radiation recall tracheitis and uncontrolled hypertension, one patient each). Although transient disease regression was observed in several patients, there were no confirmed RECIST responses. Median time to progression was 62 d; median survival time was 111 d. Two patients are alive with disease 9.9 and 35 months after the registration; 13 died of disease.
Conclusions:
Despite preclinical in vivo activity in ATC, pazopanib has minimal single-agent clinical activity in advanced ATC.
Anaplastic thyroid cancer (ATC) is among the most deadly of all human malignancies, with a historical median overall survival, regardless of stage, of only about 5 months from diagnosis and less than 20% 1-yr survival (1, 2). Although antimicrotubule agents including taxanes (3, 4), combretastatin family members (5, 6), and anthracyclines (namely doxorubicin) (7) have some activity in advanced ATC, responses to cytotoxic chemotherapy are typically short lived (2, 3). More effective therapies for advanced ATC are sorely needed.
Within the last several years, kinase inhibitors have emerged as promising agents in the therapy of advanced medullary thyroid cancer and differentiated thyroid cancer (DTC) (8–13). For example, our recent multicenter phase 2 trial of pazopanib reported a Response Evaluation Criteria In Solid Tumors (RECIST) response rate of 49% in advanced DTC, with responses lasting more than 1 yr in about two thirds of all patients (10). Other kinase inhibitors have also shown promise in DTC (8, 9, 11, 12), and in 2011 vandetanib was approved for use in advanced symptomatic and progressive medullary thyroid cancer based on improved disease-free survival relative to placebo in a randomized international phase 3 trial (13).
Based on the results in our phase 2 trial of pazopanib in DTC, we consequently became interested in assessing whether pazopanib might also have a therapeutic role in advanced ATC. Noting encouraging in vitro antineoplastic effects of pazopanib in multiple DTC cell lines (14), we undertook the studies presented in this manuscript to better define the in vivo single-agent effects of pazopanib in advanced ATC.
Materials and Methods
Xenograft (in vivo) studies
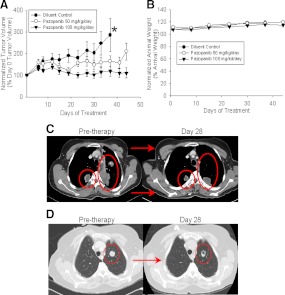
All mouse experiments followed institutional guidelines and were approved by the Mayo Clinic Institutional Animal Care and Use Committee. Briefly, KTC2 ATC cells (a gift from Dr. J. A. Copland, Mayo Clinic Florida, Jacksonville, FL; genotyped to assure identity with founder cells) were harvested, washed twice, and resuspended in PBS (7.5 million cells per 100 μl). Female athymic ν/ν mice (5–6 wk old; Harlan Laboratories, Inc., Indianapolis, IN) were anesthetized with isoflurane and injected sc in their flanks with 100 μl of KTC2 inoculum. After measurable (4–5 mm) tumors formed, mice were randomly assigned to a control diet (OpenStandard diet; 20 kcal% protein, 15 kcal% fat, and 65 kcal% carbohydrate; Research Diets Inc., NJ) or to one of two experimental group diets (575 mg pazopanib or 287.5 mg pazopanib per kilogram of diet, both compounded in the OpenStandard diet); all groups consisted of 10 animals each. Daily food intake was estimated to be 4.5 g/mouse and body weights were between 20 and 25 g for dosing calculations; as a consequence, estimated pazopanib doses approximately 100 or approximately 50 mg/kg·d were given to the two treatment groups. Selected dosage levels were based on available prior mouse pazopanib dosing information and on the requirement to avoid prohibitive mouse adverse effects (the tolerability of used dosages is illustrated in Fig. 1B). Note that dosing in mice and humans varies greatly due to differential interspecies drug metabolism and half-life. Tumors were measured with calipers two times weekly, with tumor volumes calculated using the formula: volume = A × B × B/2 (where A was the longest tumor dimension and B the smallest). Animal weights were monitored as a part of the close surveillance needed to assure tolerance of administered therapies. A two-sample t test was used to assess whether tumor volume (relative to baseline) differed between any of treatment doses and diluent.
Fig. 1.
Effects of single-agent pazopanib in mouse ATC xenografts and in ATC study patients. A, Effects of pazopanib (100 or 50 mg/kg·d in chow) on growth of sc implanted KTC2 nude mouse flank xenografts. *, Time of termination of control experiment due to the need to euthanize animals based on tumor progression; differences between control and treatment group results, P < 0.01 for both pazopanib doses; results are representative of duplicate in vivo experiments). B, Effects of pazopanib (100 or 50 mg/kg·d in chow) vs. control/untreated animals on animal weight (differences between control and treatment group results were not statistically significantly different from control for both pazopanib treatment groups; results are representative of duplicate in vivo experiments). In A and B, error bars represent 1 sem. Effects of pazopanib (after first 4 wk of study therapy) on lung/pleural-based metastases observed in a study patient (C) or alternatively on lung metastases observed after 4 wk of therapy in a different study patient (D) (note smaller tumor size and necrosis in response to therapy in D).
Clinical trial design and execution
A National Cancer Institute-sponsored multiinstitution, phase II, single-arm clinical trial was conducted to assess the antitumor activity and safely profile of pazopanib, 800 mg, administered daily orally without interruption and adjusted to tolerance until disease progression, in patients with advanced anaplastic thyroid cancer. The institutional review boards of all participating institutions approved this study before patient accrual, and all patients provided signed informed written consent before therapy and upon protocol amendments. The trial was also registered at www.clinicaltrials.gov before accrual began.
The trial enrolled patients 18 yr of age or older with histologically confirmed advanced/metastatic anaplastic thyroid cancer (central pathology review was not required). Eligibility criteria also included the following: at most two prior therapeutic regimens, disease progression within 6 months before enrollment, measurable disease per RECIST criteria 1.0 using computed tomography or magnetic resonance imaging scan; Eastern Cooperative Oncology Group performance status 0–2; adequate organ and marrow function; and systolic blood pressure (BP) less than 140 mm Hg and diastolic BP less than 90 mm Hg. Contradictions to study entry included the following: unsatisfactory hematological or blood chemistry profiles; brain metastases requiring ongoing therapy; receiving cytochrome P450-containing enzyme-interactive concomitant medications; QTc interval of 480 msec or greater or other significant electrocardiographic abnormalities; receiving concomitant medications known to affect the pharmacokinetics of pazopanib or associated with a risk of QTc prolongation and/or torsades de pointes; receiving antiretroviral therapy for HIV; discontinuation of other cancer therapy less than 4 wk before registration; and the inability to provide informed written consent. All women of child-bearing potential had a serum pregnancy test within 7 d of registration. Pregnant or lactating women were ineligible. Contraception use in fertile men and women was also required.
Physical examination (including blood pressure), assessment of performance status, complete blood counts with differential, comprehensive metabolic panel, urinalysis for proteinuria, and tumor assessment by computed tomography or magnetic resonance imaging were required 7 d or less before registration. Additionally, before each 4-wk treatment cycle, patients underwent toxicity assessments (National Cancer Institute Common Toxicity Criteria-Adverse Events version 3.0), urinalysis, hematological and chemistry tests; formal tumor restaging with tumor measurements per RECIST criteria version 1.0 was required every 4 wk due to the aggressive nature of ATC.
The pazopanib dose was reduced by 200 mg for grade 2 hemorrhage/bleeding or by 400 mg for grade 3 or greater thrombocytopenia, neutropenia, or anemia. Antihypertensive medications were to be administered and the daily pazopanib dose decreased by 200 mg if systolic BP rose to 160–179 mm Hg and diastolic BP rose to 95–104 mm Hg or decreased by 400 mg if systolic BP rose above 179 mm Hg or diastolic BP above 104 mm Hg. In the event of persistent therapy-refractory hypertension, gastrointestinal perforation, diverticulitis/colitis/typhilitis, or grade 3 or greater bleeding, pazopanib was to be discontinued. All patients received standard supportive care, including antiemetics, antibiotics, transfusions, and growth factor support as clinically appropriate.
Statistical considerations
The primary end point for evaluating this regimen was overall response rate, defined by the number of patients whose tumor measurements met the RECIST criteria version 1.0 for partial response (PR) or complete response on two consecutive evaluations at least 4 wk apart, divided by the total number of eligible patients who began pazopanib treatment. A three-outcome (promising, inconclusive, not promising), one-stage modified Simon optimal phase II clinical trial study design with an interim analysis (15) was chosen so that there would be a 90% chance of detecting a tumor response rate of at least 20% when the true tumor response rate was at least 5% at a 0.10 significance level, deeming that a RECIST response rate of less than 20% would be of little clinical importance in ATC. A minimum of 14 or a maximum of 33 eligible patients were to be enrolled. Interim analysis was planned after 14 patients had been followed up for at least 3 months; if none of these 14 patients had a documented complete response or PR, enrollment would terminate and the regimen would be considered to have insufficient activity for further testing in advanced ATC. Otherwise, a total of 33 eligible patients were to be enrolled, with the regimen considered promising if four or more tumor responses were documented.
Results
Preclinical/xenograft studies
Having established preliminary preclinical rationale in support of the further assessment of pazopanib in ATC in the form of in vitro data indicating that pazopanib has single-agent activity in ATC (14), we sought to define whether in vitro pazopanib effects might also translate into in vivo efficacy. Xenograft studies using KTC2 ATC cells sc implanted into nude mice were therefore undertaken. Significantly reduced tumor growth relative to diluent was seen with higher pazopanib doses around d 18 and with the lower dose around d 32 (Fig. 1A, P < 0.01) without affecting animal weights (Fig. 1B), thereby providing rationale for the clinical assessment of pazopanib monotherapy in human ATC.
Clinical trial
Sixteen patients from four centers were enrolled onto this study from February 2008 through February 2011, when the study temporarily closed for interim analysis (data cutoff date May 15, 2011). One patient canceled participation before the start of treatment; none of the 15 remaining patients were found ineligible. Characteristics of the 15 eligible patients at registration are listed in Table 1. There were no tumor responses among the first 14 eligible patients enrolled; as such, trial enrollment was permanently closed due to the declaration of insufficient antitumor activity for further evaluation in accord with preset criteria.
Table 1.
Demographic and baseline characteristics
| Patient characteristics | n = 15 |
|---|---|
| Median age (yr) (range) | 66 (range 45–77) |
| Male | 33% |
| Race | |
| Caucasian | 80% |
| Asian | 13% |
| Not reported | 7% |
| Radiation therapy | 80% |
| Prior systemic therapies (some patients had more than one regimen) | |
| None | 27% |
| Paclitaxel + carboplatin | 40% |
| Doxorubicin | 13% |
| Paclitaxel +/− CS-7017 | 20% |
| Radioiodine | 7% |
| Eastern Cooperative Oncology Group performance status | |
| 0 | 33% |
| 1 | 53% |
| 2 | 13% |
| Preexisting grade 2 signs and symptoms | |
| Anorexia | 13% |
| Anemia | 20% |
| Baseline medications | |
| Narcotics | 20% |
| High BP medications | 33% |
| Sites of metastases | |
| Predominantly lung | 73% |
| Predominantly neck/locoregional | 13% |
| Node only | 7% |
| Liver and adrenal | 7% |
All patients have discontinued treatment; the median number of cycles administered was two (range 1–9, total 36). Four of the 10 patients not taking hypertensive mediation(s) at the time of registration required such medication during treatment.
Four patients had one to two dose reductions due to grade 3 hypertension (one patient), grade 3 radiation recall with neck pain (one patient), grade 3 pharyngolaryngeal pain (one patient), and grade 3 aminotransferase and aspartate aminotransferase (one patient). The most common severe toxicities (National Cancer Institute Common Toxicity Criteria-Adverse Events version 3.0 grade 3 or greater possibly, probably or definitely related to treatment) were hypertension (13%) and pharyngolaryngeal pain (13%). Other severe adverse events are presented in Table 2.
Table 2.
All study adverse events reported as possibly, probably, or definitely related to study treatment among the 15 eligible patients enrolled
| Toxicity | Grade 1–2 | Grade 3–5 |
|---|---|---|
| Agitation | 6.7% | 0 |
| Alanine aminotransferase increase | 0 | 6.7% |
| Anorexia | 53% | 0% |
| Aspartate aminotransferase increase | 20% | 6.7% |
| Atrial fibrillation | 0 | 6.7% |
| CD4 lymphocytes decreased | 0 | 6.7% |
| Constipation | 6.7% | 0 |
| Diarrhea | 47% | 0 |
| Dizziness | 6.7% | 0 |
| Fatigue | 73% | 0 |
| Hemoglobin decrease | 20% | 0 |
| Hot flashes | 6.7% | 0 |
| Hypertension | 40% | 13% |
| Joint pain | 6.7% | 0 |
| Leukocyte count decrease | 27% | 6.7% |
| Lymphocyte count decrease | 0 | 6.7% |
| Myalgia | 6.7% | 0 |
| Nausea | 40% | 0 |
| Neck pain | 0 | 6.7% |
| Neutrophil count decrease | 13% | 0 |
| Pharyngo laryngeal pain | 0 | 13% |
| Platelet count decrease | 6.7% | 0 |
| Protein urine positivity | 33% | 0 |
| Pruritus | 6.7% | 0 |
| Serum magnesium increase | 6.7% | 0 |
| Serum potassium increase | 6.7% | 0 |
| Serum sodium decrease | 0 | 6.7% |
| Skin hypopigmentation | 33% | 0 |
| Sweating | 6.7% | 0 |
| Taste alteration | 13% | 0 |
| Thrombosis | 0 | 6.7% |
| Vascular disorder | 0 | 6.7%a |
| Vomiting | 13% | 0 |
Grade 5 tumor-associated hemorrhage; this is the only grade 5 event observed in conjunction with the study.
Reasons for treatment discontinuation included the following: disease progression (12 patients), death on study in a single patient (due to out of hospital tumor hemorrhage deemed possibly related to treatment), and intolerability (radiation recall tracheitis or uncontrolled hypertension, one patient each).
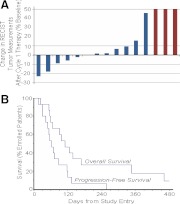
No confirmed RECIST tumor responses were documented among the 15 eligible patients. However, several impressive, but transient, tumor responses were observed (illustrated in two cases in Fig. 1, C and D, with RECIST response data after the first therapy cycle shown in Fig. 2A). The median time to progression was 62 d, and the median survival time was 111 d (Fig. 2B). Two patients were alive with progressive disease 9.9 months and 2.9 yr after registration. The remaining 13 patients have died of disease.
Fig. 2.
Best RECIST response (A) and progression-free and overall survival (B) in study patients (n = 15).
Discussion
Discouragingly, despite displaying evidence of in vivo antitumor activity (Fig. 1A), pazopanib demonstrated only minimal activity in human ATC within the context of the present clinical trial. Several transient responses were observed (e.g. Figs. 1, C and D, and 2A), but there were no confirmed RECIST responses, leading us to deem pazopanib of insufficient single-agent efficacy to justify further monotherapy study in advanced ATC.
There is as of yet no clear explanation for why the observed promising preclinical activity of pazopanib (Fig. 1A) did not translate into promising clinical activity, especially given that pazopanib therapy resulted in a 49% RECIST response rate in DTC (10). In general, however, like pazopanib, other kinase inhibitors studied to date in advanced ATC have also displayed little clinical activity. In particular, albeit that sorafenib produced two PR among 16 treated patients in a recent phase 2 trial, median disease free and overall survival were quite disappointing (1.5 and 3.5 months, respectively) (16), results quite similar to those reported in the present study (2.0 and 3.6 months, respectively; Fig. 2). Imatinib therapy, however, resulted in two of eight patients attaining partial responses, with four of eight imatinib-treated patients surviving 6 months from study enrollment (17); long-term survival has additionally been reported in an ATC patient treated with sunitinib (18). Whether imatinub or sunitinib may truly have more promising clinical activity than other kinase inhibitors, however, remains to be further clarified.
Overall, it nonetheless appears that durable responses in ATC to monotherapy using currently available kinase inhibitors are unusual. This situation is perhaps expected because the durations of responses even to cytotoxic chemotherapy has most often been brief (2, 3). In particular, the criteria for maintenance of response in the CATCHIT trial of paclitaxel were shortened to 2 wk (decreased from the usual 4 wk required to meet RECIST response criteria) to achieve the reported 53% response rate (3). Clearly, progress has been slow with regard to the development of improved therapies for patients afflicted with metastatic ATC.
Nonetheless, there is at least some room for optimism about the progress in the treatment of ATC in that recent data suggest benefit from the earlier implementation of systemic therapy, especially when combined with locoregional radiation therapy. In particular, docetaxel and doxorubicin in combination with intensity-modulated radiation therapy for stages 4A and 4B ATC have produced outcomes much superior to historical data in our practice (19), leading to ongoing efforts to further explore novel approaches not only to treating metastatic, but also to treating lower-stage, ATC in active pursuit of improved outcomes in a disease with historically miserable prognosis. Most recently we have also found that pazopanib enhances the cytotoxic effects of paclitaxel, an agent with established clinical activity in ATC, in vitro and in vivo in preclinical ATC models. Current clinical examination of pazopanib in ATC therefore now takes the form of a randomized, double-blind clinical trial intended to define whether pazopanib might enhance the clinical activity of paclitaxel therapy when administered in conjunction with locoregional intensity modulated radiotherapy (www.clinicaltrials.gov identifier NCT01236549).
Acknowledgments
We are deeply indebted to the patients participating in this trial as well as to all enrolling/treating clinicians and to participating institutions. The kind administrative assistance of Ms. Candace Kostelec is additionally much appreciated.
This work was supported by National Cancer Institute Grants CA15083 and CM62205.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATC
- Anaplastic thyroid cancer
- BP
- blood pressure
- DTC
- differentiated thyroid cancer
- PR
- partial response
- RECIST
- Response Evaluation Criteria In Solid Tumors.
References
- 1. McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, van Heerden JA, Goellner JR. 2001. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery 130:1028–1034 [DOI] [PubMed] [Google Scholar]
- 2. Smallridge RC, Copland JA. 2010. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol) 22:486–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ain KB, Egorin MJ, DeSimone PA. 2000. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid 10:587–594 [DOI] [PubMed] [Google Scholar]
- 4. Kawada K, Kitagawa K, Kamei S, Inada M, Mitsuma A, Sawaki M, Kikumori T, Fujimoto Y, Arima H, Imai T, Ando Y. 2010. The feasibility study of docetaxel in patients with anaplastic thyroid cancer. Jpn J Clin Oncol 40:596–599 [DOI] [PubMed] [Google Scholar]
- 5. Dowlati A, Robertson K, Cooney M, Petros WP, Stratford M, Jesberger J, Rafie N, Overmoyer B, Makkar V, Stambler B, Taylor A, Waas J, Lewin JS, McCrae KR, Remick SC. 2002. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res 62:3408–3416 [PubMed] [Google Scholar]
- 6. Mooney CJ, Nagaiah G, Fu P, Wasman JK, Cooney MM, Savvides PS, Bokar JA, Dowlati A, Wang D, Agarwala SS, Flick SM, Hartman PH, Ortiz JD, Lavertu PN, Remick SC. 2009. A phase II trial of fosbretabulin in advanced anaplastic thyroid carcinoma and correlation of baseline serum-soluble intracellular adhesion molecule-1 with outcome. Thyroid 19:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R. 1985. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer 56:2155–2160 [DOI] [PubMed] [Google Scholar]
- 8. Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O'Dwyer PJ, Brose MS. 2008. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 26:4714–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE, Jr, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH. 2009. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 27:1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Sideras K, Morris JC, 3rd, McIver B, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Goh BC, Tang H, Ivy SP, Erlichman C; Endocrine Malignancies Disease Oriented Group; Mayo Clinic Cancer Center; Mayo Phase 2 Consortium 2010. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 11:962–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB. 2008. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 26:4708–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG. 2010. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res 16:5260–5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. 2012. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind Phase III trial. J Clin Oncol 30:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isham CR, Negron V, Lingle WL, Fisher KE, Kumar R, Bible KC. Pazopanib potentiates paclitaxel-induced mitotic catastrophe in association with inhibition of aurora kinases. Proceedings of the Proc 102nd Annual Meeting of the American Association for Cancer Research, Orlando, Florida, 2011 (Abstract 3597) [Google Scholar]
- 15. Sargent DJ, Chan V, Goldberg RM. 2001. A three outcome design for phase II clinical trials. Controlled Clinical Trials 22:117–125 [DOI] [PubMed] [Google Scholar]
- 16. Nagaiah G, Fu P, Wasman JK, Cooney MM, Mooney C, Afshin D, Lavertu P, Bokar J, Savvides P, Remick SC. 2009. Phase II trial of sorafenib (bay 43-9006) in patients with advanced anaplastic carcinoma of the thyroid (ATC). J Clin Oncol 27:15 (Suppl abstr 6058) [Google Scholar]
- 17. Ha HT, Lee JS, Urba S, Koenig RJ, Sisson J, Giordano T, Worden FP. 2010. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid 20:975–980 [DOI] [PubMed] [Google Scholar]
- 18. Koussis H, Maruzzo M, Scola A, Ide EC, Fassina A, Marioni G, Lora O, Corti L, Karachontziti P, Jirillo A. 2010. A case of anaplastic thyroid cancer with long-term survival. Anticancer Res 30:1273–1278 [PubMed] [Google Scholar]
- 19. Foote RL, Molina JR, Kasperbauer JL, Lloyd RV, McIver B, Morris JC, Grant CS, Thompson GB, Richards ML, Hay ID, Smallridge RC, Bible KC. 2011. Enhanced survival in locoregionally confined anaplastic thyroid carcinoma: a single institution experience using aggressive multimodal therapy. Thyroid 21:25–30 [DOI] [PubMed] [Google Scholar]