Abstract
Context:
Type 2 diabetes mellitus (T2D) is associated with an increased risk of fractures and low bone formation. However, the mechanism for the low bone formation is not well understood. Recently, circulating osteogenic precursor (COP) cells, which contribute to bone formation, have been characterized in the peripheral circulation.
Objective:
Our objective was to characterize the number and maturity of COP cells in T2D.
Patients, Design, and Setting:
Eighteen postmenopausal women with T2D and 27 controls participated in this cross-sectional study at a clinical research center.
Main Outcome Measures:
COP cells were characterized using flow cytometry and antibodies against osteocalcin (OCN) and early stem cell markers. Histomorphometric (n = 9) and molecular (n=14) indices of bone turnover and oxidative stress were also measured.
Results:
The percentage of OCN+ cells in peripheral blood mononuclear cells was lower in T2D (0.8 ± 0.2 vs. 1.6 ± 0.4%; P < 0.0001), whereas the percentage of OCN+ cells coexpressing the early marker CD146 was increased (OCN+/CD146+: 33.3 ± 7 vs. 12.0 ± 4%; P < 0.0001). Reduced histomorphometric indices of bone formation were observed in T2D subjects, including mineralizing surface (2.65 ± 1.9 vs. 7.58 ± 2.4%, P = 0.02), bone formation rate (0.01 ± 0.1 vs. 0.05 ±0.2 μm3/um2 · d, P = 0.02), and osteoblast surface (1.23 ±0.9 vs. 4.60 ± 2.5%, P = 0.03). T2D subjects also had reduced molecular expression of the osteoblast regulator gene Runx2 but increased expression of the oxidative stress markers p66Shc and SOD2.
Conclusions:
Circulating OCN+ cells were decreased in T2D, whereas OCN+/CD146+ cells were increased. Histomorphometric indices of bone formation were decreased in T2D, as was molecular expression of osteoblastic activity. Stimulation of bone formation may have beneficial therapeutic skeletal consequences in T2D.
The skeleton has recently been recognized as another organ that is adversely affected in type 2 diabetes mellitus (T2D). Accumulating data show that in addition to the many target organs that are classically associated with dysfunction in T2D, the disorder is also associated with a 50–80% increased risk of hip (1–3) and extremity (1, 4) fractures. However, assessment of skeletal mass in T2D by bone mineral density (BMD) does not explain these findings. Despite the increased incidence of fragility fractures, BMD in T2D is similar to (5) or higher than (6–9) that in matched controls, even when normalized for larger body size in T2D (10). Nor do neurological, ophthalmological, or cardiovascular dysfunction, common in T2D, provide a satisfactory explanation for the increased risk of fractures (11). Another possibility for increased fracture risk in T2D is abnormal skeletal dynamics. Using biochemical markers of bone turnover in T2D, previous studies have suggested that bone formation might be reduced, although this has not been clearly established (12–16). The mechanism for possible low bone formation in the diabetic skeleton is not well understood.
A growing body of evidence has recently suggested that fluid-phase osteoblastic cells, known as circulating osteogenic precursor (COP) cells, are normally present in the peripheral circulation and that these circulating cells contribute to bone formation (17–20). COP cells have been associated with pubertal growth (21) and are known to be attracted to fracture sites (22). They can be detected in the peripheral blood by flow cytometry using antibodies specific for the osteoblast matrix protein osteocalcin (OCN) (23). Other cell surface markers can be used to characterize COP cells. CD34, for example, a marker of hematopoietic stem cells, identifies cells that give rise to functional osteoblasts that are capable of forming mineralized nodules and can heal fractures (24). CD146 is an early marker of bone marrow stromal cells, pericytes, and hematopoietic cells, which function as self-renewing, clonogenic skeletal progenitors (25). Cells that are positive for CD34 or CD146 are thought to be more immature than cells that are positive only for OCN, because the cells that are positive for CD34 and CD146 diminish when osteoblasts mature (26).
Characterization of COP cells in T2D could provide insight into a mechanism to account for reduced bone formation in this disease. Specifically, alterations in the number and maturity of COP cells would support abnormalities in osteoblast development. We hypothesized that low bone formation in T2D is associated with a delay in osteoblast development. To test this hypothesis, characteristics of COP cells in T2D subjects were analyzed, together with measurements of biochemical, histomorphometric, and molecular indices of bone formation. Because decreased osteoblastogenesis has also been strongly associated with increased oxidative stress (27), an important pathogenic factor in T2D (28), markers of oxidative stress in COP cells were also measured.
Subjects and Methods
Study population
All subjects, T2D and controls, were recruited by advertisement at Columbia University Medical Center. T2D and controls were women at least 5 yr postmenopause. The diagnosis of T2D had been established for at least 1 yr by use of an antiglycemic medication. Subjects with T2D were excluded as defined by a history of ketoacidosis, age of onset of diabetes before 25 yr, body mass index (BMI) below 20 kg/m2, and use of insulin without a concomitant oral hypoglycemic agent. Exclusion criteria for both T2D and controls included renal or hepatic abnormalities (glomerular filtration rate <60 ml/min · 1.73 m2 as calculated by the modification of diet in renal disease calculation or liver function tests above the upper limit of normal); therapy with rosiglitazone or pioglitazone; disorders of bone or mineral metabolism (primary hyperparathyroidism, Paget's disease, osteomalacia, or osteogenesis imperfecta); abnormal thyroid function tests; medical history or clinical findings consistent with Cushing's syndrome; continuous glucocorticoid use at any dose for greater than 3 months over the past 3 yr; history of intestinal disorders (celiac disease, exocrine pancreatic insufficiency, or inflammatory bowel disease); current use of anticonvulsants, anticoagulants, loop diuretics, methotrexate, or antidepressants; current use of estrogen preparations, raloxifene, calcitonin, denosumab, or teriparatide; and current or previous use of a bisphosphonate within 36 months before study. Smokers were also excluded.
Bone mineral density
BMD of the lumbar spine (L1–L4), femoral neck, total hip, and nondominant forearm was measured by dual-energy x-ray absorptiometry using the Hologic Discovery Series W densitometer (Hologic, Inc., Waltham, MA). Short-term, in vivo reproducibility was 0.68% for the spine, 1.36% for the proximal femur, and 0.70% for the radius.
Flow cytometry and cell sorting
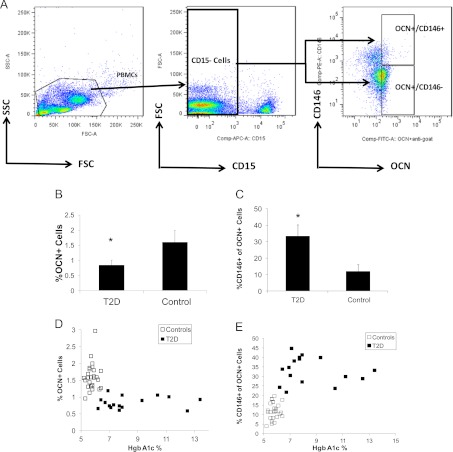
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation using Ficoll-Hypaque and were counted with Trypan blue for viability using a hemocytometer. PBMC were resuspended in flow-staining buffer (PBS plus 2% fetal bovine serum), and the primary antibodies were added. After 30 min incubation at 4 C, the cells were washed twice, and fluorochrome-conjugated primary and secondary antibodies were added. The cells were then incubated for an additional 30 min at 4 C and washed twice before flow cytometry analysis. The primary unconjugated antibody was a goat polyclonal antihuman OCN (Santa Cruz Biotechnology, Santa Cruz, CA) antibody (a control isotype antibody was used at the same concentrations); secondary conjugated antibodies included fluorescein isothiocyanate-conjugated AffinityPure IgG f(ab′)2 fragment donkey antigoat (Jackson ImmunoResearch, West Grove, PA) antibodies. Primary conjugated antibodies were allophycocyanin-conjugated anti-CD15, phycoerythrin (PE)-conjugated anti-CD146 and anti-PE-Cy7-conjugated CD34 (all from Becton Dickinson, San Diego, CA). Five-color flow cytometry acquisition was performed using a LSR II flow cytometer (Becton Dickinson) and analysis using FLO-JO software (Treestar, Inc.). Cells were gated for size, shape, and granularity using forward- and side-scatter parameters. The positive populations were identified as cells that expressed specific levels of fluorescence activity above the nonspecific autofluorescence of the isotype control. The region was set to encompass both the lymphocyte/monocyte-enriched area and the granulocyte-enriched area and to exclude dead cells. All CD15+ granulocytes were excluded before gating for specific populations to exclude contamination of isolated mononuclear cells with granulocytes. The sorting strategy that was employed is shown in Fig. 1A.
Fig. 1.
Cell populations in T2D and controls. A, Representative flow cytometry dot plots showing sorting strategy for OCN+/CD146+ and OCN+/CD146− cells. Total PBMC were first gated to exclude CD15+ cells. The OCN+/CD146+ and OCN+/CD146− cells were then sorted out of the CD15− populations (FSC, forward scatter; SSC, side scatter); B, the percentage of PBMC that was positive for OCN was lower in the T2D subjects (n = 18) compared with the controls (n = 27); C, the percentage of OCN+ cells that was also positive for CD146 was greater in the T2D subjects; D, HbA1c levels in the overall group (T2D plus controls combined) correlated inversely with the population of OCN+ cells (r = −0.49; P = 0.001), although within the T2D group alone, the correlation between HbA1c and OCN+ cells was not significant; E, HbA1c levels in the overall group (T2D plus controls combined) correlated with the subpopulations of OCN+ cells with the early stromal CD146 marker (OCN+/CD146+: r = 0.59; P < 0.0001), although within the T2D group alone, the correlation between HbA1c and OCN+/CD146+ cells was not significant. *, P < 0.05.
For flow sorting, PBMC were resuspended in flow staining buffer at 1 × 106/ml and labeled with polyclonal antihuman OCN. After 30 min incubation at 4 C, the cells were washed twice and the following fluorochrome-conjugated primary and secondary antibodies were added: fluorescein isothiocyanate-conjugated AffinityPure IgG f(ab′)2 fragment donkey antigoat antibody, allophycocyanin-conjugated anti-CD15 and PE-conjugated anti-CD146. After 30 min incubation, cells were washed twice using flow buffer. Flow sorting was performed using FACSAria (Becton Dickinson). Cells were sorted into the following populations: OCN+/CD146+ and OCN+ /CD146−. Sorted populations were then stored in RLT (guanidine thiocyanate) buffer at −80 C for extraction of RNA.
Expression analysis for molecular markers
Total RNA was isolated from cells using TRIZOL reagent followed by a clean-up step using RNeasy mini-purification kit. Only the RNA samples that gave A260/A280 of approximately 1.8–1.9 in the Nanodrop ND-1000 UV-VIS spectrophotometer were used. One microgram of total RNA was first treated with DNAase at room temperature, and RNA was then reverse transcribed using Superscript III reverse transcriptase at 42 C for 60 min. The resulting cDNA were used for real-time PCR analysis of various genes using the Stratagene (La Jolla, CA) quantitative PCR machine. Primers for the assays were obtained from Superarray Biosciences (Frederick, MD). Reactions were set up in the total volume of 25 μl with Bio-Rad (Hercules, CA) 2× quantitative PCR mix in triplicate for each sample and were measured against standard curves for respective genes. mRNA expression was normalized vs. the housekeeping gene β-actin and reported relative to control PBMC. Using real-time PCR, the expression of the osteoblast gene marker Runx2 (Runt-related transcription factor 2) was evaluated in sorted OCN+/CD146+ and OCN+/CD146− cells. Other molecular markers that were also measured included expression of the insulin receptor gene, the glucose transporter GLUT-1, the molecular marker of oxidative stress p66Shc, and the antioxidant defense enzyme SOD2 (superoxide dismutase 2).
Calciotropic indices and biochemical markers of bone remodeling
Biochemistries were measured by automated techniques. Intact PTH was measured by immunoradiometric assay (Scantibodies, Santee, CA; sensitivity, 1 pg/ml; inter-day, 6.80%; intra-day, 4.80%; reference range, 14–66 pg/ml) and 25-hydroxyvitamin D [25(OH)D] was measured by HPLC/tandem mass spectroscopy, validated against National Institute of Standards and Technology reference material [25(OH)D2 and 25(OH)D3 sensitivity, 1.25 ng/ml; interassay, 5.5% (D3), 2.0% (D2); intraassay, 3.5% (D3), 2.4% (D2); reference range, as sum of 25(OH)D2 and 25(OH)D3, deficiency, <10 ng/ml; insufficiency, 10–30 ng/ml; normal, >30 ng/ml]. All biochemical markers of bone turnover were measured in duplicate from a morning fasting serum specimen. Procollagen type I amino-terminal propeptide (P1NP) was measured by RIA. Intra- and interassay precision is 6.5 and 8.3%, respectively, sensitivity 2 ng/ml, and normal range 16–96 ng/ml for postmenopausal women (IDS Ltd., Scottsdale, AZ). Bone-specific alkaline phosphatase (BAP) was measured by enzyme immunoassay (Quidel Corp., San Diego, CA); intra- and interassay variability is 4 and 8%, respectively, sensitivity 0.7 U/liter, and normal range 14.2–42.7 U/liter in postmenopausal women. N-terminal mid-fragment OCN was measured by ELISA (IDS); intraassay and interassay variability is 2 and 4%, respectively, sensitivity 0.5 ng/ml, and normal range 12.8–55.0 ng/ml for postmenopausal women. Undercarboxylated OCN was measured by ELISA (Takara Bio Inc., Tokyo, Japan); intra- and interassay variability is 4.4 and 9.5%, respectively, sensitivity 0.25 ng/ml, and normal range 0.6–1.8 ng/ml. Carboxyl-terminal cross-linking telopeptide of bone collagen (CTx) was measured using ELISA (IDS); intra- and interassay variability is 3.0 and 10.9%, respectively, sensitivity 0.020 ng/ml, and normal range 0.142–1.351 ng/ml for postmenopausal women. Tartrate-resistant acid phosphatase (TRAP-5b) was measured by ELISA (IDS); intra- and interassay variability is 5 and 9%, respectively, sensitivity less than 1.0 U/liter, and normal range 1.49–4.89 U/liter for postmenopausal women.
Histomorphometry
All participants were invited to undergo bone biopsies; five T2D subjects and four controls agreed. Two tetracycline labels were administered (Sumycin 250 mg four times daily) using a standard 3 d on, 12 d off, 3 d on regimen before the biopsy. Biopsy specimens were processed and subjected to histomorphometric analysis as previously described in detail in our laboratory (29). Histomorphometry was performed using a digitizing image-analysis system, OsteoMeasure (OsteoMetrics, Inc., Atlanta, GA). Cancellous and cortical bone structure was assessed by measuring cancellous bone volume, trabecular width, trabecular number, trabecular separation, cortical width, and cortical porosity area. Bone remodeling activity was evaluated on cancellous, endocortical, and intracortical bone surfaces and expressed by the variables of osteoid surface/bone surface, osteoid thickness, mineralizing surface, mineral apposition rate, adjusted apposition rate, bone formation rate/bone surface, eroded surface, osteoblast surface, and osteoclast number. All indices are expressed according to the recommendations of the American Society of Bone and Mineral Research nomenclature committee (30).
Statistical analysis
All statistical analyses were performed using PASW Statistics version 18 for Windows (SPSS, Chicago, IL). All continuous data are presented as mean value ± sd. Student's t tests were used to assess differences between groups. Pearson's correlation coefficients were used to assess the relationship between variables. Linear regression was used to adjust for the effect of BMI. A value of P < 0.05 was considered significant.
The study was approved by the Institutional Review Boards of Columbia University Medical Center and Helen Hayes Hospital. All subjects gave written informed consent.
Results
Characteristics of population
Eighteen T2D subjects and 27 controls were studied (Table 1). T2D subjects had a trend toward higher BMI measurements and had higher total alkaline phosphatase levels. There was a trend toward lower 25(OH)D levels in T2D, and 25(OH)D was inversely associated with glycated hemoglobin (HbA1c), which identifies the average plasma glucose concentration over the previous few months, in the total group (T2D plus controls combined; r = −0.4; P = 0.02); this relationship persisted after adjusting for BMI. BMD by dual-energy x-ray absorptiometry was not different from controls after adjusting for BMI; unadjusted total hip BMD was significantly higher in T2D than controls.
Table 1.
Characteristics of cohort
| T2D (n = 18) | Controls (n = 27) | P value | |
|---|---|---|---|
| Age (yr) | 58 ± 6 | 57 ± 5 | 0.38 |
| Race | |||
| Hispanic White | 12 | 10 | |
| Caucasian | 4 | 13 | |
| Hispanic Black | 2 | 1 | |
| Asian | 0 | 2 | |
| Native American | 0 | 1 | |
| Duration postmenopausal (yr) | 12 ± 9 | 8 ± 4 | 0.05 |
| Number of fractures | 5 (1 elbow, 1 ankle, 1 arm, 1 toe, 1 wrist) | 5 (1 5th digit, 2 foot, 1 wrist, 1 toe) | |
| Height (in.) | 62 ± 2 | 63 ± 2 | 0.17 |
| Weight (lb) | 175 ± 42 | 158 ± 32 | 0.13 |
| BMI (kg/m2) | 32 ± 7 | 28 ± 6 | 0.06 |
| Duration T2D (yr) | 9 ± 6 | ||
| Number of diabetes medications used | |||
| Metformin | 15 | ||
| Glipizide | 5 | ||
| Insulin | 2 | ||
| Glimepiride | 1 | ||
| Sitagliptin | 1 | ||
| Serum calcium (mg/dl) | 9.4 ± 0.5 | 9.4 ± 0.3 | 0.75 |
| Serum albumin (mg/dl) | 4.3 ± 0.2 | 4.4 ± 0.2 | 0.65 |
| Serum creatinine (mg/dl) | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.40 |
| PTH (pg/ml) | 46 ± 26 | 40.9 ± 20 | 0.46 |
| Total alkaline phosphatase (U/liter) | 97 ± 23 | 79 ± 29 | 0.03a |
| 25(OH)D (ng/ml) | 21 ± 11 | 29 ± 14 | 0.67 |
| TSH (mIU/liter) | 2.9 ± 4 | 2.6 ± 3 | 0.80 |
| Glucose (mg/dl) | 169 ± 94 | 107 ± 8 | <0.001a |
| HbA1c (%) | 8.4 ± 2b | 5.8 ± 0.3 | <0.001a |
| Lumbar spine | |||
| g/cm2 | 0.967 ± 0.16 | 0.920 ± 0.10 | 0.24 |
| T score | −0.56 ± 1.5 | −1.1 ± 1.0 | 0.28 |
| Femoral neck | |||
| g/cm2 | 0.778 ± 0.15 | 0.708 ± 0.09 | 0.06 |
| T score | −0.8 ± 1.2 | −1.3 ± 0.7 | 0.08 |
| Total hip | |||
| g/cm2 | 0.935 ± 0.18 | 0.835 ± 0.11 | 0.03a,c |
| T score | −0.2 ± 1.3 | −0.9 ± 0.8 | 0.04a,c |
| 1/3 distal radius | |||
| g/cm2 | 0.672 ± 0.07 | 0.654 ± 0.06 | 0.35 |
| T score | −0.5 ± 1.2 | −0.7 ± 1.0 | 0.61 |
To convert calcium to millimoles per liter, multiply by 0.25; albumin to grams per liter, multiply by 10; creatinine to micromoles per liter, multiply by 88.4; PTH to nanograms per liter, multiply by 1; 25(OH)D to nanomoles per liter, multiply by 2.496; glucose to millimoles per liter, multiply by 0.056.
P < 0.05.
Six T2D had HbA1c higher than 7.9%.
No longer significant after adjusting for BMI.
Circulating osteogenic precursor cells
The percentage of total PBMC that expressed OCN on the surface (OCN+) was significantly lower in T2D subjects compared with controls (0.8 ± 0.2 vs. 1.6 ± 0.4%, P < 0.0001; Fig. 1B). The percentage of total PBMC that regardless of OCN status expressed the early marker CD146, which includes T cells, was greater in T2D subjects (14.2 ± 4 vs. 6.6 ± 2%, P < 0.0001). Within the pool of OCN+ cells, the percentage of cells that coexpressed the marrow stromal marker was also increased in T2D subjects. Specifically, OCN+ cells with CD146 (OCN+/CD146+) were greater in the T2D subjects (33.3 ± 7 vs. 12.0 ± 4%, P < 0.0001; Fig. 1C). Likewise, OCN+ cells with both early markers CD146 and CD34 (OCN+/CD34+/CD146+) were increased in T2D subjects (10.3 ± 4 vs. 4.8 ± 3%, P < 0.0001). HbA1c levels in the overall group (T2D plus controls combined) correlated inversely with the population of OCN+ cells (r = −0.49; P = 0.001; Fig. 1D), although within the T2D group alone, the correlation between HbA1c and OCN+ cells was not significant (r = 0.12; P = 0.66). HbA1c levels in the overall group (T2D plus controls combined) correlated positively with the subpopulations of OCN+ cells with the early stromal CD146 marker (OCN+/CD146+, r= 0.59; P < 0.0001; Fig. 1E), although within the T2D group alone, the correlation between HbA1c and OCN+/CD146+ cells was not significant (r = −0.13; P = 0.62). In T2D subjects alone, HbA1c levels correlated positively with the percentage of total PBMC that expressed the early marker CD146 (r = 0.86; P = 0.03) in those whose HbA1c was over 7.9% (n = 6).
Expression analysis for molecular markers in sorted cells
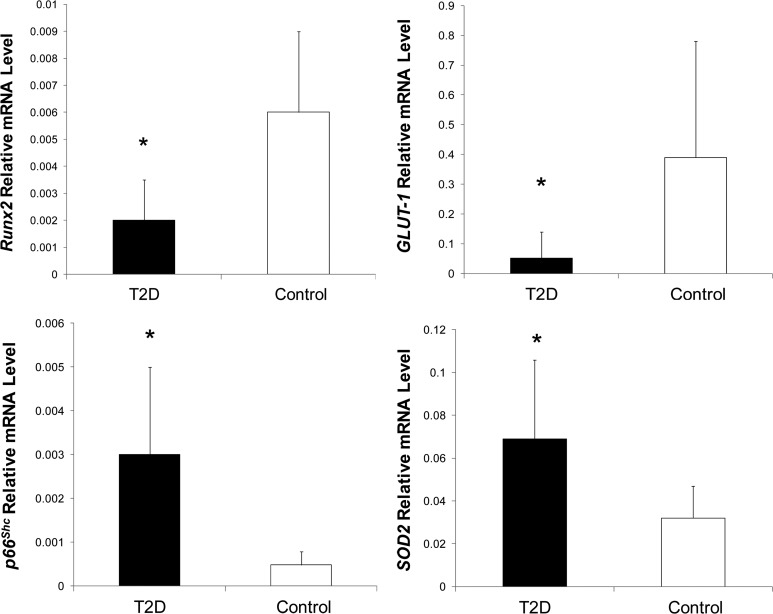
Expression profiling of sorted OCN+/CD146+ cell populations was evaluated in seven T2D subjects and seven controls using real-time PCR. These subjects and controls were selected because their blood samples yielded adequate numbers of PBMC so as to allow for cell sorting. The seven T2D subjects whose cells underwent the molecular analyses did not differ from the remaining 11 T2D subjects with regard to BMI, duration of T2D, number of previous fractures, fasting glucose, HbA1c, 25(OH)D, and BMD; likewise, the seven controls did not differ in these aspects from the remaining 20 controls. The expression profile of Runx2, the major regulator of osteoblast formation, was decreased in T2D. The magnitude of Runx2 expression in the control sorted OCN+/CD146+ cells was comparable to the magnitude of Runx2 expression in mature osteogenic cells (23). Runx2 expression was also measured in sorted OCN+/CD146− cells and was similarly lower in T2D (relative mRNA in T2D, 0.0014 ± 0.001 vs. in control, 0.0054 ± 0.004, P = 0.03). On the other hand, the expression of the oxidative stress marker p66Shc was increased in T2D (Fig. 2). Consistent with the increase in p66Shc, expression of the antioxidant defense enzyme SOD2 was also increased in T2D. Within the diabetics, a longer duration of T2D (in years) correlated positively with greater expression of p66Shc (r = 0.89; P = 0.02). Expression of the insulin receptor gene did not differ between the diabetics and controls, but expression of the glucose transporter GLUT-1 was decreased in T2D (Fig. 2).
Fig. 2.
Expression analysis for molecular markers in sorted OCN+/CD146+ cells. T2D subjects (n = 7) had decreased expression of the osteoblastic master gene Runx2 and the glucose transporter GLUT-1 compared with controls (n = 7) but greater expression of the oxidative stress markers p66Shc and SOD2. *, P < 0.05.
Biochemical markers of bone remodeling
Biochemical markers of bone remodeling were measured in all T2D and controls. The bone formation markers P1NP and OCN were significantly lower in T2D (Table 2). The bone formation marker BAP, however, was higher in T2D (Table 2). Higher BAP levels were associated with lower 25(OH)D levels (r = −0.36; P = 0.02). Undercarboxylated OCN tended to be lower in T2D (T2D, 2.2 ± 1.0 vs. control, 2.6 ± 1.0, P = 0.17), but when levels were corrected for total OCN (undercarboxylated OCN/total OCN), this trend disappeared. The biochemical marker of bone resorption serum CTx was lower in T2D, although the difference in TRAP-5b levels was not significant (Table 2). The differences in turnover markers correlated with the degree of hyperglycemia in the total group (T2D plus controls combined); HbA1c levels correlated inversely with P1NP, OCN, and serum CTx but positively with BAP (Table 3). In the control group alone, there was no relationship between HbA1c and biochemical markers of turnover (Table 3). In the T2D subjects alone, P1NP and OCN tended to be inversely correlated with HbA1c (Table 3). In T2D subjects whose HbA1c was over 7.9% (n = 6), HbA1c levels correlated inversely with P1NP (r = −0.96; P = 0.002).
Table 2.
Biochemical indices of bone turnover in T2D and controls (±sd)
| T2D | Control | P value | |
|---|---|---|---|
| P1NP (ng/ml) | 42.9 ± 9 | 62.3 ± 29 | 0.01 |
| BAP (U/liter) | 43.6 ± 14 | 35.6 ± 12 | 0.05 |
| Osteocalcin (ng/ml) | 15.3 ± 6 | 20.3 ± 8 | 0.03 |
| TRAP-5b (U/liter) | 3.3 ± 0.8 | 3.6 ± 1.1 | 0.42 |
| Serum CTx (ng/ml) | 0.44 ± 0.2 | 0.68 ± 0.3 | 0.01 |
Table 3.
Correlations between biochemical markers of bone turnover and Hba1c in T2D and controls
| T2D |
Control |
T2D and control combined |
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| P1NP (ng/ml) | −0.34 | 0.17 | −0.14 | 0.49 | −0.34 | 0.03 |
| BAP (U/liter) | 0.30 | 0.24 | 0.19 | 0.33 | 0.34 | 0.02 |
| Osteocalcin (ng/ml) | −0.45 | 0.07 | −0.10 | 0.63 | −0.39 | 0.01 |
| TRAP-5b (U/liter) | −0.10 | 0.71 | −0.01 | 0.64 | −0.13 | 0.41 |
| Serum CTx (ng/ml) | −0.18 | 0.49 | −0.03 | 0.87 | −0.32 | 0.04 |
Histomorphometry
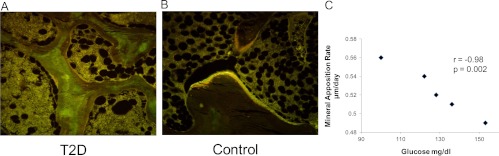
All T2D subjects and controls were invited to undergo tetracycline double-labeled percutaneous transiliac crest bone biopsies; only five T2D subjects and four controls agreed. The five T2D subjects who underwent the biopsy did not differ from the remaining 13 T2D subjects with regard to BMI, duration of T2D, number of previous fractures, fasting glucose, HbA1c, 25(OH)D, and BMD; likewise, the four biopsied controls did not differ in these aspects from the remaining 23 controls. Of the nine biopsied individuals, five were the same participants who also had the measurements of the molecular markers (three T2D subjects and two controls). Analysis of structural parameters showed that there was no difference between groups in trabecular parameters but that cortical width was significantly decreased in T2D subjects (420.0 ± 85 vs. 824.8 ± 208 μm, P = 0.005), as was cortical area (11.6 ± 2 vs. 28.9 ± 8%, P = 0.002). With regard to dynamic indices, significantly lower values were observed for mineralizing surface, bone formation rate, osteoid surface, and osteoblast surface in T2D in all three envelopes of bone (Table 4). A representative example is shown in Fig. 3. In the total group (T2D plus controls combined), HbA1c levels correlated inversely with the percentage of osteoid surface (r = −0.68; P = 0.045) and mineral apposition rate (r = −0.71; P = 0.049), and fasting glucose levels correlated inversely with mineralized surface (r= −0.75; P = 0.03), bone formation rate (r = −0.75; P = 0.03), and osteoid surface (r = −0.74; P = 0.02). In the T2D subjects alone, there was no correlation between HbA1c and dynamic indices, but fasting glucose levels correlated inversely with mineral apposition rate (r = −0.98; P = 0.002; Fig. 3C) and osteoclast number (r = −0.97; P = 0.006).
Table 4.
Histomorphometric variables of bone remodeling in T2D and controls (±sd)
| O.Th (μm) | MS/BS (%) | MAR (μm/d) | BFR/BS (μm3/um2 · d) | OS/BS (%) | ES/BS (%) | AjAR (μm/d) | Ob.S/BS (%) | OC.No/BS (no./mm) | |
|---|---|---|---|---|---|---|---|---|---|
| Cancellous | |||||||||
| T2D | 5.49 ± 0.9 | 2.65 ± 1.9 | 0.52 ± 0.1 | 0.01 ± 0.1 | 3.60 ± 1.6 | 2.99 ± 1.1 | 0.40 ± 0.2 | 1.23 ± 0.9 | 0.012 ± 0.01 |
| Control | 7.03 ± 1.8 | 7.58 ± 2.4 | 0.59 ± 0.1 | 0.05 ± 0.2 | 9.81 ± 2.8 | 2.84 ± 1.7 | 0.40 ± 0.1 | 4.60 ± 2.5 | 0.05 ± 0.04 |
| P value | NS | 0.02 | 0.08 | 0.02 | 0.004 | NS | NS | 0.03 | NS |
| Endocortical | |||||||||
| T2D | 6.02 ± 2.0 | 3.84 ± 2.4 | 0.48 ± 0.3 | 0.02 ± 0.2 | 7.51 ± 6.1 | 3.22 ± 0.6 | 0.30 ± 0.2 | 2.93 ± 1.8 | 0.032 ± 0.03 |
| Control | 6.16 ± 1.3 | 16.62 ± 4.6 | 0.57 ± 0.1 | 0.09 ± 0.1 | 18.06 ± 3.5 | 5.37 ± 3.4 | 0.40 ± 0.3 | 8.64 ± 4.2 | 0.10 ± 0.11 |
| P value | NS | 0.002 | NS | 0.002 | 0.02 | NS | NS | 0.03 | NS |
| Intracortical | |||||||||
| T2D | 5.05 ± 1.7 | 4.87 ± 4.0 | 0.42 ± 0.2 | 0.03 ± 0.1 | 3.44 ± 2.6 | 2.48 ± 1.2 | 0.68 ± 0.5 | 0.97 ± 0.6 | 0.03 ± 0.1 |
| Control | 7.23 ± 1.4 | 16.07 ± 4.5 | 0.74 ± 0.1 | 0.12 ± 0.1 | 11.90 ± 3.9 | 4.78 ± 1.5 | 0.700 ± 0.5 | 5.64 ± 3.4 | 0.10 ± 0.1 |
| P value | 0.03 | 0.01 | 0.08 | 0.01 | 0.006 | 0.04 | NS | 0.02 | NS |
AjAR, adjusted apposition rate; BFR/BS, bone formation rate; ES/BS, eroded surface; MAR, mineral apposition rate; MS/BS, mineralizing surface; NS, not significant; Ob.S/BS, osteoblast surface; Oc.No/BS, osteoclast number; OS/BS, osteoid surface; O.Th, osteoid thickness.
Fig. 3.
Histomorphometric changes in bone formation. A and B, Tetracycline double-labeled bone biopsies in a 58-yr-old T2D Caucasian woman (A) and a 57-yr-old Caucasian female control (B). Bone formation is decreased in T2D with reduced mineralizing surface. C, Higher glucose levels were associated with lower mineral apposition rate in the T2D subjects.
Discussion
Traditionally, osteoblasts are considered to arise from marrow stromal cells that do not cross the endothelium or are solid phase. However, it is now thought that there are also fluid-phase COP cells that access bone formation sites through blood vessels (17, 31) and function as bona fide osteoblasts (18–22, 32, 33). Such cells have been suggested to form osteoblast-like cells, express bone-related proteins, and deposit mineralized matrix (21). They also might participate in physiological functions, including long bone development, with levels being higher in pubertal boys than in men (21), as well as fracture healing, with mobilization to fracture sites and contribution to osteogenesis in the early stages of fracture healing (22). Our previous studies showed that measurements of COP cells were reflective of biochemical, histomorphometric, and molecular markers of osteoblast function (23). We now report that subjects with T2D display a significant decrease in OCN+ cells, suggesting fewer COP cells. This finding is consistent with the decreased serum OCN levels that we observed, as well as the reduced histomorphometric indices of bone formation in a subgroup, and suggests a reduction in the precursor pool of osteoblasts.
We further found that within the decreased pool of overall OCN+ cells, the T2D subjects had an increased subpopulation of immature OCN+ cells, i.e. cells that also had the early markers CD146 and CD34. This suggests that this smaller pool of COP cells was also more immature. In particular, there was an increase in the OCN+ cells that coexpressed CD146, a marker that characterizes a subpopulation that is phenotypically similar to classic marrow stromal cells (25). In contrast, we did not find that the diabetic subjects had an increase in the subpopulation that coexpressed CD34 alone, suggesting that the cells we identified did not overlap with hematopoietic or vascular lineage cells, which are generally CD34+ (17). Finally, higher HbA1c levels were associated with a higher proportion of immature (OCN+/CD146+) cells in T2D subjects with HbA1c higher than 7.9%. Although CD146 is also a marker of hematopoietic cells, this may suggest that the worse the glycemic control, the greater the delay of COP cell maturation.
Our data suggest that not only are alterations in circulating osteogenic cell number present in T2D but also that differences in circulating osteogenic cell function exist as well. In a subgroup, sorted OCN+/CD146+ cells from diabetic subjects had decreased molecular expression of Runx2. This transcription factor regulates the expression of the majority of genes expressed in osteoblasts (34), and its decreased expression suggests that osteoblast differentiation may be impaired in T2D. We also investigated whether an increase in oxidative stress was present in the osteogenic cells of the diabetic subjects. Increased oxidative stress is an important pathogenic factor in T2D and in mice leads directly to low bone formation (27). In the subgroup of sorted cells, we found that in the T2D subjects, there was an increase in the molecular expression of p66Shc, a protein that is activated by oxidative stress (35), correlating with a longer duration of T2D. We also observed an increase in the anti-oxidant defense enzyme SOD2, which is the target of the Fox01 transcription factor that is activated in response to oxidative stress (35). Taken together, these exploratory molecular expression data suggest the presence of increased oxidative stress in COP cells from diabetic subjects.
Our data are consistent with previous studies that have suggested that biochemical markers of bone turnover are lower in T2D (12–16). Serum levels of P1NP and OCN were decreased, consistent with reduced bone formation. BAP, however, was increased in T2D, as was total alkaline phosphatase, and was inversely correlated with 25(OH)D levels. BAP increases when mineralization processes are impaired, possibly in association with reduced levels of 25(OH)D; this discrepancy between BAP and other markers of bone formation is incompletely understood but has been noted in other reports of T2D (12, 16). Undercarboxylated OCN, which has been shown to promote insulin secretion and sensitivity (12, 36), tended to be lower in T2D, consistent with the presence of greater insulin resistance. Yet the observed difference was not significant, possibly because of our small sample size or alternatively because of our specific assay. It has been questioned whether the ELISA that we used recognizes the active form of undercarboxylated OCN, in which only the first of the three glutamic acid residues is decarboxylated (37).
The lower levels of P1NP and OCN are consistent with the reductions in dynamic histomorphometric indices of bone formation in the subgroup of biopsied diabetic subjects. Lower values for mineralizing surface, bone formation rate, osteoid surface, and osteoblast surface were found in all three envelopes of bone in the biopsied T2D subjects. Moreover, the extent to which the glucose was elevated correlated with a reduction in mineral apposition rate and osteoclast number within the diabetic group. Although we did not find a similar correlation between HbA1c and dynamic histomorphometric indices in the diabetics alone, this might be because a high serum glucose level can acutely suppress bone formation (38), perhaps to a greater extent than chronic intermittent hyperglycemia.
Despite our small sample size, these histomorphometry results are an important contribution to the body of knowledge regarding T2D and bone. To date, it has not been unequivocally established that T2D is characterized by lower bone formation in humans. Results for biochemical bone turnover markers are not invariably consistent (12–16) as demonstrated in the current report. Moreover, hardly any other histomorphometric data exist. A small study by Krakauer et al. (15) published in 1995 reported lower bone formation in T2D based on biopsy but had small numbers (n = 6 T2D patients; two female and two Black); patients were selected for biopsy based on low BMD, and the control group was premenopausal women. The only other published biopsy studies are at least 40 yr old (39, 40). Thus, our biopsy data provide a meaningful addition to the available literature.
These changes suggest that glucose might be directly associated with an imbalance in bone turnover. In states of high bone turnover, such as postmenopausal osteoporosis, bone loss is common because bone resorption exceeds bone formation. However, markedly reduced bone turnover, as occurs in male osteoporosis (41) or in glucocorticoid-induced osteoporosis (42), can also be associated with increased bone fragility. In such cases, low bone turnover is associated with an imbalance that is due to preferentially reduced bone formation. Similarly, our data suggest that bone resorption was reduced in T2D but perhaps not to the same extent as bone formation. Serum CTx was decreased in T2D, but in the biopsies, eroded surface, adjusted apposition rate, and osteoclast number were not lower in T2D, nor were serum TRAP-5b levels. Although these findings may simply be a function of our small sample size, it may also be that there is a preferential reduction of bone formation over resorption in T2D, a dynamic that could lead to accumulation of older bone of poorer quality. In particular, low bone formation in T2D is associated with accumulation of advanced glycation end products (43, 44), which make bone more brittle and further retard remodeling activity (45).
Our study has a number of limitations. Our sample size was small, and we did not have histomorphometric and molecular data on most of the cohort. Nevertheless, the exploratory histomorphometric and molecular data suggest directions for further investigation, particularly with regard to the mechanisms of low bone formation in T2D. Importantly, uncertainty exists about the role of circulating OCN+ cells in bone formation. Specifically, the choice of OCN to isolate COP cells could be questioned because OCN is a secreted protein and not a cell surface protein anchored in the membrane. Nevertheless, OCN does possess Gla residues (46), which may allow for at least temporary anchoring of the protein to the cell membrane as the protein is secreted, similar to the mechanisms by which Gla residues on clotting factors allow attachment of these proteins to cell membranes (47). Alternatively, OCN-producing cells may also possess an OCN receptor (48, 49), and because the protein is secreted, it may bind to this cell surface receptor.
Doubt about the role of circulating OCN+ cells could also arise from the point that osteoprogenitors are admittedly rare in the circulation. Moreover, abundant evidence using in vivo transplantation assays is lacking to show that circulating OCN+ cells are capable of true bone formation. Nevertheless, numerous transplantation studies exist that support the existence of circulating marrow stromal cells with capacity to contribute to bone remodeling (18, 19, 50). CD146+ cells when transplanted into immunocompromised mice have been shown to form ectopic bone (25). Bone marrow OCN+ cells have been shown to form colonies in vitro of osteoblast-like cells, express bone-related proteins, and deposit mineralized matrix (51, 52). Most relevantly, Eghbali-Fatourechi et al. (21) performed an in vivo transplantation assay in which peripheral OCN+ cells, OCN− cells, and unsorted PBMC were implanted sc into immunocompromised mice. In their study, radiography and quantitative computed tomography showed higher radiodensity and volumetric bone mineral density in the area of the OCN+ cell suspensions than in the OCN− cells. They also found that histological examination of the implanted cell suspensions showed evidence of mineralized bone formation by the OCN+ cells, which was lacking in the OCN− cells. These rigorous studies, although not definitive, suggest that OCN+ cells may play a role in bone formation.
Recently, Fadini and co-workers (53) described a population of circulating OCN+/BAP+ myeloid calcifying cells that was increased in T2D in contrast to the decreased total OCN+ cells that we observed. A possible explanation for this discrepancy is that Fadini et al.(53) studied a different cellular sub-population, selecting only OCN+ cells that coexpressed BAP and myeloid markers (22% of total OCN+ cells) and was clearly distinct from marrow stromal cells. In contrast, our population of total OCN+ cells likely reflected a composite of cells of lymphoid, myeloid, and marrow stromal origin. Moreover, the diabetic patient population studied by Fadini et al. (53) had multiple calcification risk factors (78% men, 26% smokers, 10% with nephropathy), whereas our diabetic subjects were less predisposed to have increased calcifying cells (all women who did not smoke or have nephropathy). Nevertheless, these contrasting data emphasize the important point that circulating OCN+ cells are heterogeneous and cannot be unambiguously identified with osteoblasts in bone. Although we have previously shown that OCN+ cell measurements correlate well with biochemical, histomorphometric, and molecular indices of bone formation as well as with formation of mineralized nodules (23), future work will further validate the value of this approach.
In conclusion, we found that circulating OCN+ cells were decreased in T2D, whereas OCN+/CD146+ cells were increased. Histomorphometric indices of bone formation were decreased in T2D, as was molecular expression of osteoblastic activity. Although questions exist about the role of circulating OCN+ cells in bone formation, evidence that osteoblast progenitor cell maturation is inhibited in T2D could lead to interventions targeting improved bone formation to enhance bone strength in the diabetic skeleton.
Acknowledgments
This work was supported by the National Institutes of Health Irving Institute for Clinical and Translational Research CTSA (Clinical and Translational Science Awards) pilot award.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- BAP
- Bone-specific alkaline phosphatase
- BMD
- bone mineral density
- BMI
- body mass index
- COP
- circulating osteogenic precursor
- CTx
- carboxyl-terminal cross-linking telopeptide of bone collagen
- HbA1c
- glycated hemoglobin
- OCN
- osteocalcin
- 25(OH)D
- 25-hydroxyvitamin D
- PBMC
- peripheral blood mononuclear cells
- PE
- phycoerythrin
- P1NP
- procollagen type I amino-terminal propeptide
- T2D
- type 2 diabetes mellitus
- TRAP-5b
- tartrate-resistant acid phosphatase.
References
- 1. Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. 2006. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J Clin Endocrinol Metab 91:3404–3410 [DOI] [PubMed] [Google Scholar]
- 2. Lipscombe LL, Jamal SA, Booth GL, Hawker GA. 2007. The risk of hip fractures in older individuals with diabetes: a population-based study. Diabetes Care 30:835–841 [DOI] [PubMed] [Google Scholar]
- 3. Janghorbani M, Van Dam RM, Willett WC, Hu FB. 2007. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166:495–505 [DOI] [PubMed] [Google Scholar]
- 4. Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR. 2001. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 86:32–38 [DOI] [PubMed] [Google Scholar]
- 5. Tuominen JT, Impivaara O, Puukka P, Rönnemaa T. 1999. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care 22:1196–1200 [DOI] [PubMed] [Google Scholar]
- 6. Futakuchi S, Ishiguro H, Naruse S, Ko SB, Fujiki K, Yamamoto A, Nakakuki M, Song Y, Steward MC, Kondo T, Goto H. 2009. High glucose inhibits HCO3(-) and fluid secretion in rat pancreatic ducts. Pflugers Arch 459:215–226 [DOI] [PubMed] [Google Scholar]
- 7. Vestergaard P. 2007. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a meta-analysis. Osteoporos Int 18:427–444 [DOI] [PubMed] [Google Scholar]
- 8. Melton LJ, 3rd, Riggs BL, Leibson CL, Achenbach SJ, Camp JJ, Bouxsein ML, Atkinson EJ, Robb RA, Khosla S. 2008. A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab 93:4804–4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Daele PL, Stolk RP, Burger H, Algra D, Grobbee DE, Hofman A, Birkenhäger JC, Pols HA. 1995. Bone density in non-insulin-dependent diabetes mellitus. The Rotterdam Study. Ann Intern Med 122:409–414 [DOI] [PubMed] [Google Scholar]
- 10. Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Zmuda JM, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB. 2004. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The Health, Aging, and Body Composition Study. J Bone Miner Res 19:1084–1091 [DOI] [PubMed] [Google Scholar]
- 11. Vestergaard P, Rejnmark L, Mosekilde L. 2009. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int 84:45–55 [DOI] [PubMed] [Google Scholar]
- 12. Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, Sugimoto T. 2009. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab 94:45–49 [DOI] [PubMed] [Google Scholar]
- 13. Dobnig H, Piswanger-Sölkner JC, Roth M, Obermayer-Pietsch B, Tiran A, Strele A, Maier E, Maritschnegg P, Sieberer C, Fahrleitner-Pammer A. 2006. Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, bone mass, and fracture risk. J Clin Endocrinol Metab 91:3355–3363 [DOI] [PubMed] [Google Scholar]
- 14. Okazaki R, Totsuka Y, Hamano K, Ajima M, Miura M, Hirota Y, Hata K, Fukumoto S, Matsumoto T. 1997. Metabolic improvement of poorly controlled noninsulin-dependent diabetes mellitus decreases bone turnover. J Clin Endocrinol Metab 82:2915–2920 [DOI] [PubMed] [Google Scholar]
- 15. Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. 1995. Bone loss and bone turnover in diabetes. Diabetes 44:775–782 [DOI] [PubMed] [Google Scholar]
- 16. Shu A, Yin MT, Stein E, Cremers S, Dworakowski E, Ives R, Rubin MR. 2012. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int 23:635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pignolo RJ, Kassem M. 2011. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res 26:1685–1693 [DOI] [PubMed] [Google Scholar]
- 18. Dominici M, Pritchard C, Garlits JE, Hofmann TJ, Persons DA, Horwitz EM. 2004. Hematopoietic cells and osteoblasts are derived from a common marrow progenitor after bone marrow transplantation. Proc Natl Acad Sci USA 101:11761–11766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. 2007. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun 354:453–458 [DOI] [PubMed] [Google Scholar]
- 20. Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. 2002. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA 99:8932–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. 2005. Circulating osteoblast-lineage cells in humans. N Engl J Med 352:1959–1966 [DOI] [PubMed] [Google Scholar]
- 22. Alm JJ, Koivu HM, Heino TJ, Hentunen TA, Laitinen S, Aro HT. 2010. Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. J Orthop Res 28:1634–1642 [DOI] [PubMed] [Google Scholar]
- 23. Rubin MR, Manavalan JS, Dempster DW, Shah J, Cremers S, Kousteni S, Zhou H, McMahon DJ, Kode A, Sliney J, Shane E, Silverberg SJ, Bilezikian JP. 2011. Parathyroid hormone stimulates circulating osteogenic cells in hypoparathyroidism. J Clin Endocrinol Metab 96:176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, Miwa M, Horii M, Hayashi S, Oyamada A, Nishimura H, Murasawa S, Doita M, Kurosaka M, Asahara T. 2006. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol 169:1440–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. 2007. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131:324–336 [DOI] [PubMed] [Google Scholar]
- 26. Eghbali-Fatourechi GZ, Mödder UI, Charatcharoenwitthaya N, Sanyal A, Undale AH, Clowes JA, Tarara JE, Khosla S. 2007. Characterization of circulating osteoblast lineage cells in humans. Bone 40:1370–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, Depinho RA, Kousteni S. 2010. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab 11:147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamada Y, Kitazawa S, Kitazawa R, Fujii H, Kasuga M, Fukagawa M. 2007. Histomorphometric analysis of diabetic osteopenia in streptozotocin-induced diabetic mice: a possible role of oxidative stress. Bone 40:1408–1414 [DOI] [PubMed] [Google Scholar]
- 29. Dempster DW, Parisien M, Silverberg SJ, Liang XG, Schnitzer M, Shen V, Shane E, Kimmel DB, Recker R, Lindsay R, Bilezikian JP. 1999. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab 84:1562–1566 [DOI] [PubMed] [Google Scholar]
- 30. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- 31. Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. 2001. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res 16:1575–1582 [DOI] [PubMed] [Google Scholar]
- 32. Kumagai K, Vasanji A, Drazba JA, Butler RS, Muschler GF. 2008. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res 26:165–175 [DOI] [PubMed] [Google Scholar]
- 33. Egan KP, Kim JH, Mohler ER, 3rd, Pignolo RJ. 2011. Role for circulating osteogenic precursor cells in aortic valvular disease. Arterioscler Thromb Vasc Biol 31:2965–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. 1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754 [DOI] [PubMed] [Google Scholar]
- 35. Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. 2007. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. 2007. Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferron M, Wei J, Yoshizawa T, Ducy P, Karsenty G. 2010. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem Biophys Res Commun 397:691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clowes JA, Allen HC, Prentis DM, Eastell R, Blumsohn A. 2003. Octreotide abolishes the acute decrease in bone turnover in response to oral glucose. J Clin Endocrinol Metab 88:4867–4873 [DOI] [PubMed] [Google Scholar]
- 39. Wu K, Schubeck KE, Frost HM, Villanueva A. 1970. Haversian bone formation rates determined by a new method in a mastodon, and in human diabetes mellitus and osteoporosis. Calcif Tissue Res 6:204–219 [DOI] [PubMed] [Google Scholar]
- 40. Klein M, Wu K, Frost H. 1964. The numbers of bone resorption and formation foci in rib in diabetes mellitus. Henry Ford Hosp Med Bull 12:527–536 [Google Scholar]
- 41. Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. 2000. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076 [DOI] [PubMed] [Google Scholar]
- 42. Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, Dalsky GP, Marcus R. 2007. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357:2028–2039 [DOI] [PubMed] [Google Scholar]
- 43. Monnier VM, Kohn RR, Cerami A. 1984. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci USA 81:583–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwartz AV. 2003. Diabetes mellitus: does it affect bone? Calcif Tissue Int 73:515–519 [DOI] [PubMed] [Google Scholar]
- 45. Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. 2009. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int 20:887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Price PA, Williamson MK, Lothringer JW. 1981. Origin of the vitamin K-dependent bone protein found in plasma and its clearance by kidney and bone. J Biol Chem 256:12760–12766 [PubMed] [Google Scholar]
- 47. Huang M, Rigby AC, Morelli X, Grant MA, Huang G, Furie B, Seaton B, Furie BC. 2003. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat Struct Biol 10:751–756 [DOI] [PubMed] [Google Scholar]
- 48. Bodine PV, Komm BS. 1999. Evidence that conditionally immortalized human osteoblasts express an osteocalcin receptor. Bone 25:535–543 [DOI] [PubMed] [Google Scholar]
- 49. Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD. 2005. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280:40201–40209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. 2001. Circulating skeletal stem cells. J Cell Biol 153:1133–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Long MW, Williams JL, Mann KG. 1990. Expression of human bone-related proteins in the hematopoietic microenvironment. J Clin Invest 86:1387–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Long MW, Robinson JA, Ashcraft EA, Mann KG. 1995. Regulation of human bone marrow-derived osteoprogenitor cells by osteogenic growth factors. J Clin Invest 95:881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fadini GP, Albiero M, Menegazzo L, Boscaro E, Vigili de Kreutzenberg S, Agostini C, Cabrelle A, Binotto G, Rattazzi M, Bertacco E, Bertorelle R, Biasini L, Mion M, Plebani M, Ceolotto G, Angelini A, Castellani C, Menegolo M, Grego F, Dimmeler S, Seeger F, Zeiher A, Tiengo A, Avogaro A. 2011. Widespread increase in myeloid calcifying cells contributes to ectopic vascular calcification in type 2 diabetes. Circ Res 108:1112–1121 [DOI] [PubMed] [Google Scholar]