Abstract
Context:
Insulin resistance can be compensated by increased functional pancreatic β-cell mass; otherwise, diabetes ensues. Such compensation depends not only on environmental and genetic factors but also on the baseline β-cell mass from which the expansion originates.
Objective:
Little is known about assembly of a baseline β-cell mass in humans. Here, we examined formation of β-cell populations relative to other pancreatic islet cell types and associated neurons throughout the normal human lifespan.
Design and Methods:
Human pancreatic sections derived from normal cadavers aged 24 wk premature to 72 yr were examined by immunofluorescence. Insulin, glucagon, and somatostatin were used as markers for β-, α-, and δ-cells, respectively. Cytokeratin-19 marked ductal cells, Ki67 cell proliferation, and Tuj1 (neuronal class III β-tubulin) marked neurons.
Results:
Most β-cell neogenesis was observed preterm with a burst of β-cell proliferation peaking within the first 2 yr of life. Thereafter, little indication of β-cell growth was observed. Postnatal proliferation of α- and δ-cells was rarely seen, but a wave of ductal cell proliferation was found mostly associated with exocrine cell expansion. The β-cell to α-cell ratio doubled neonatally, reflecting increased growth of β-cells, but during childhood, there was a 7-fold change in the β-cell to δ-cell ratio, reflecting an additional loss of δ-cells. A close association of neurons to pancreatic islets was noted developmentally and retained throughout adulthood. Negligible neuronal association to exocrine pancreas was observed.
Conclusion:
Human baseline β-cell population and appropriate association with other islet cell types is established before 5 yr of age.
The onset of obesity-linked type 2 diabetes is marked by the loss of functional pancreatic β-cell mass that is no longer able to compensate for inherent insulin resistance (1). However, the plasticity of β-cell mass should be noted, especially because two thirds of obese subjects do not acquire type 2 diabetes. This is because the β-cell mass and insulin-secretory function can adapt to meet the increased metabolic demand (1–4). Another example is pregnancy, where a counterbalancing of the functional β-cell mass to avoid gestational diabetes occurs (5, 6). A question remains as to why certain subjects are susceptible to diabetes and their β-cells are not able to compensate for the metabolic need. There is a complex inherited genetic susceptibility that may reside at the level of the β-cell (7), but certain environmental influences also play a significant role (8). Another consideration is the concept of baseline β-cell mass, which is the critical starting β-cell population from which a compensatory β-cell expansion may occur (9). The extent of the human β-cell population in adult individuals is likely quite variable, and if one has an insufficient baseline β-cell mass from which to expand, an underlying susceptibility to obesity-linked and/or gestational diabetes would be present.
How does a baseline β-cell mass form? In rodents, it has been shown that pancreatic endocrine cells develop from the embryological branching epithelium, originally derived from endodermal cells (10, 11). It is presumed that a similar process takes place in human embryological pancreatic development, although there have been relatively few studies to support this notion. A certain number of differentiated β-cells are established by birth (12), but this does not determine the full baseline complement of β-cells. In rodents, there is also a burst of neonatal β-cell growth that is contributed to mostly by proliferation of existing β-cells (13, 14) and, to a lesser extent, by β-cell neogenesis (i.e. the formation of new β-cells from ductal epithelial progenitor cells) (15). A limited number of human studies have indicated a similar neonatal burst of β-cell proliferation, but thereafter, β-cell replication is rarely observed in normal subjects (9). Indeed, it has been estimated that adult human β-cells turn over very slowly, perhaps once every 25 yr (16). Notwithstanding, there is a need to substantiate the few human studies conducted to date as well as to better establish how a human baseline β-cell population forms. Moreover, the process is complex, and not all parameters of human pancreatic islet formation have been considered to date. For example, for pancreatic β-cells to have normal insulin-secretory function, they need to be in contact with the other pancreatic endocrine islet cell types (glucagon-producing α-cells; somatostatin-producing δ-cells, pancreatic polypeptide-producing γ-cells; and ghrelin-producing ε-cells) as well as endothelial cells that form the microcirculation within islets and neuronal cells that render neurological control to islet cell functions (17–19). In rodents, adult pancreatic β-cells are found at the core of an islet with the other endocrine cell types located on the islet periphery, but in humans, such islet architecture seems only to be observed developmentally, and it is as of yet unclear whether adult human islet and associated cells coordinate functionally in a similar manner to rodents (20, 21). Unfortunately, there is inherent variability among human autopsy specimens, and many more need to be examined so as to arrive at a consensus on how and when islets are formed to set a functional baseline β-cell population. To date, a few studies suggest this occurs in childhood (9, 12, 14), but these observations need substantiating and verification. Here, we have examined the formation of human pancreatic islet β-cells early on in normal life from premature to adolescent individuals relative to an established adult islet cell population. The alternative immunofluorescence analysis approach used for characterizing the early growth of β-cells and surrounding islet cell types indicates that the baseline β-cell population is established neonatally.
Materials and Methods
Materials
The following antibodies were used for immunofluorescence: guinea pig antiinsulin (Millipore, Billerica, MA), which is used to mark pancreatic islet β-cells; mouse anti-glucagon (Sigma-Aldrich, St. Louis, MO), which is used to mark pancreatic islet α-cells; goat antisomatostatin (Santa Cruz Biotechnology Inc., Santa Cruz, CA), which is used to mark pancreatic islet δ-cells; mouse anti-cytokeratin-19 (CK-19), which is used as a marker of pancreatic ductal epithelial cells; rabbit anti-Ki67 (Fitzgerald Industries International, North Acton, MA), where Ki67 is a nuclear protein that is expressed only during the active phases of the cell cycle (i.e. G1, S, G2, and mitosis) but not in the resting G0 phase and as such is able to mark cells undergoing proliferation (6); mouse anti-cleaved caspase-3 (Cell Signaling Technology Inc., Danvers, MA), which recognizes only the proteolytically cleaved form of procaspase-3, indicating that cellular caspase activities have been activated and as such the cell is undergoing apoptosis (22); and rabbit anti-Tuj1 (neuronal class III β-tubulin; Covance Inc., Princeton, NJ), which specifically recognizes microtubules only expressed in neuronal cells, including axons, and as such is an excellent marker of neurons. Species-specific secondary antibodies labeled with fluorophores Cy5, Cy3, or Cy2 were from Jackson ImmunoResearch Laboratories (West Grove, PA). Unless otherwise stated, all other chemicals were from Sigma-Aldrich or Fisher Scientific (Pittsburgh, PA) and were of the highest purity grade available.
Pancreatic tissue specimens
Human pancreatic tissue, fixed in paraffin blocks, was obtained from individuals aged 24 wk premature to 72 yr (total n = 40) either at the time of autopsy from the University of Chicago Department of Pathology tissue bank or at the time of organ procurement by the Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes program (nPOD). The autopsy cases were identified by retrospective analysis of the University of Chicago Department of Pathology tissue bank and with an exemption from the Institutional Review Board. None of the individuals included in the study had diabetes or any other diseases affecting the pancreas; neither was there any apparent family history of type 2 diabetes in as far as it could be determined. For younger and premature subjects, the mothers did not have any history of gestational diabetes. Only normal subjects were examined, and obese individuals with a body mass index over 30 kg/m2 were excluded from these studies. Pancreatic tissue is notorious to undergo rapid autolysis unless fixed effectively. All pancreatic specimens were subjected to a previous pathological review, and there was no sign of autolysis in any of the sections examined for this study. Wherever possible, sections were taken from the head, midsection, and tail of the pancreata, but due to scarcity of material from some of the younger subjects, only midsection portions of the pancreas could be examined. To examine a proxy for fetal pancreatic morphology after 24 wk, pancreata from prematurely born infants were examined. The available characteristics of the cases and diagnoses leading to death are presented in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Pancreatic tissue processing
Pancreatic serial sections (5–10 μm) were deparaffinized and washed as described (23) and then subjected to heat-induced isotope retrieval using either sodium citrate buffer or 10 mm Tris with 1 mm EDTA (pH 9.0) and heating in the microwave at 1100 W for 12–15 min. Immunofluorescent analysis was carried out as described using an Olympus 1x81 DSU confocal microscope as previously described (24).
Morphometric analysis
The various cell counts and islet size was conducted on at least 25 representative sample images from each pancreatic section of a subject using NIH ImageJ software. Each image was analyzed on three separate occasions and blinded as to the identity of the subject. For the islet cell composition analysis, approximately 2000 insulin-positive β-cells were counted per sample, and the average ratio of β-cells to glucagon-positive α-cells or somatostatin-positive δ-cells was then calculated for each subject. Assessment of islet innervation was determined as the distance (micrometers) of Tuj1-positive neurons from a cluster of insulin-positive β-cells (where more than three adjacent insulin-positive cells was considered an islet cluster). Although not well defined, mostly due to inadequate markers for progenitor pancreatic β-cells, β-cell neogenesis was determined as insulin-expressing cells within clusters of, or proximal (<25 μm) to, pancreatic ductal CK-19-positive cells and/or insulin-expressing cells that coexpress the ductal cell marker CK-19 as previously outlined (15, 25). A minimum of 500 insulin-positive β-cells were counted per image, and the mean percentage of β-cell neogenesis was calculated. For determination of β-cell and ductal cell replication, the sample images marked for insulin, Ki67, and CK-19 were analyzed. An average of approximately 600 insulin-positive β-cells were counted per sample, and the percentage of Ki67 costaining was calculated for each subject. The incidence of β-cell apoptosis was determined as the percentage of insulin-positive cells costained for cleaved caspase-3, where an average of approximately 800 insulin-positive β-cells was counted per sample image. Average islet diameter (μm) was calculated in at least 25 islets per subject using the average of four distinct diameter measurements per islet.
Statistical analysis
The results are presented as individual subject data points and as mean ± sem of each age group category. Statistically significant differences between groups relative to the adult group were analyzed using one-way ANOVA where a P value <0.05 was considered to indicate a significant difference.
Results
Pancreatic islet formation
During human pancreatic fetal development (24–40 wk of gestation) and the neonatal period (≤1 month), scattered small clusters of endocrine hormone-expressing cells were spread throughout the pancreas, as previously observed (12, 26) (Supplemental Fig. 1, B and C). The preterm specimens showed a relative abundance of endocrine cells, scattered in clusters of various sizes throughout the premature/developing pancreas but with numerous single hormone-positive cells also seen. The developmental endocrine cell clusters had atypical human adult islet architecture, with bipolar [i.e. half glucagon/somatostatin-expressing cells at one end and insulin-expressing cells at the other (Fig. 1B)] and mantle structures (i.e. insulin-expressing cells in a core surrounded by a mantle of glucagon/somatostatin-expressing cells, reminiscent of rodent pancreatic islet architecture commonly observed (21) (Fig. 1, C and D). However, such immature pancreatic islet forms were rarely seen after 6 months of life. Thereafter, pancreatic islets were more typical of human adult islet architecture (Fig. 1, E–G, and Supplemental Fig. 1, D–G). During the neonatal period, islet size significantly increased to plateau at an average diameter of about 135 μm from approximately 2 yr old onward (P ≤ 0.01; Supplemental Fig. 1A), consistent with previous observations (12, 14).
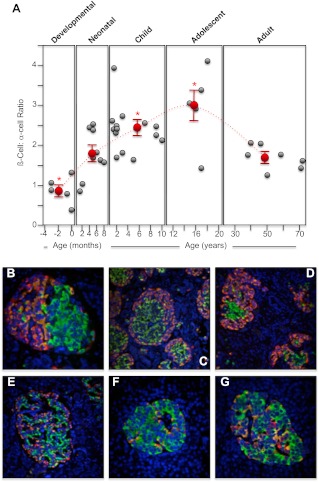
Fig. 1.
Change in human pancreatic islet β-cell to α-cell ratio from midgestation to adult. Human pancreatic sections were analyzed by immunofluorescence and confocal microscopy as outlined in Materials and Methods for insulin (green), glucagon (orange), somatostatin (red), and nuclei (4′,6-Diamidino-2-phenylindole; blue). The numbers of insulin-positive β-cells and somatostatin-positive α-cells were counted and the islet β-cell to δ-cell ratio calculated, with at least 10 islets analyzed per section. A, Individual β-cell to α-cell ratio for each human specimen during the developmental, neonatal, childhood, adolescent, and adult periods (gray circles). The mean ± semβ-cell to α-cell ratio for each period is shown as red circles. *, Significant difference (P ≤ 0.05) from the average β-cell to α-cell ratio found in adult islets. The dashed line indicates the trend in change of the average β-cell to α-cell ratios between the different life periods (note that the x-axis time scale is different for each period). B, Example image of a human 24-wk-old premature pancreas (×40 magnification); C, example image of a human 35-wk-old premature pancreas (×20 magnification); D, example image of a human full-term pancreas (×20 magnification); E, example image of a human 6-month-old pancreas (×20 magnification); F, example image of a human 18-yr-old pancreas (×40 magnification); G, example image of a human 39-yr-old pancreas (×40 magnification).
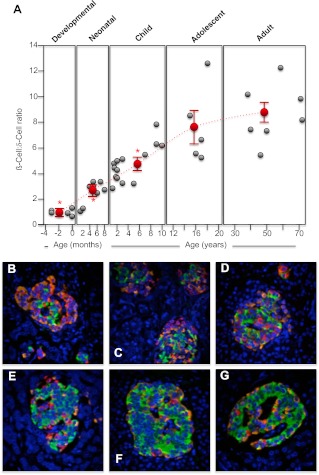
Glucagon-producing α-cells were significantly more abundant during the human premature/developmental preterm (P ≤ 0.05; Fig. 1, A–D), as previously noted (27). But during the first 2 yr after term, there is a doubling of the β- to α-cell ratio (Fig. 1A). This occurs as pancreatic islet cells form their normal architecture (Fig. 1, E and F) and during a burst of β-cell proliferation. Indeed, the α-cell population does not appreciably change during this period, and it is an increase in β-cell numbers that largely reflects the change in the β- to α-cell ratio (Fig. 1). Although variable, the average β- to α-cell ratio peaks in adolescence and then tapers off in adulthood (Fig. 1A). Somatostatin-expressing δ-cells are relatively common during human pancreatic premature/development (Fig. 2, B–D) (27). But during childhood, the β- to δ-cell ratio changes 6- to 7-fold (Fig. 2A) so that δ-cells are significantly less common in adolescence and adulthood (P ≤ 0.001; Fig. 2, F and G). This may, in part, be due to the neonatal burst of β-cell growth but also an apparent loss of δ-cells during childhood.
Fig. 2.
Change in human pancreatic islet β-cell to δ-cell ratio from midgestation to adult. Human pancreatic sections were analyzed by immunofluorescence and confocal microscopy as outlined in Materials and Methods for insulin (green), glucagon (orange), somatostatin (red), and nuclei (4′,6-Diamidino-2-phenylindole; blue). The numbers of insulin-positive β-cells and somatostatin-positive δ-cells were counted and the islet β-cell to δ-cell ratio calculated, with at least 10 islets analyzed per section. A, Individual β-cell to δ-cell ratio for each human specimen during the developmental, neonatal, childhood, adolescent, and adult periods (gray circles). The mean ± semβ-cell to δ-cell ratio for each period is shown as red circles. *, Significant difference (P ≤ 0.001) from the average β-cell to δ-cell ratio found in adult islets. The dashed line indicates the trend in change of the average β-cell to δ-cell ratios between the different life periods (note that the x-axis time scale is different for each period). B, Example image of a human 35-wk-old premature pancreas (×40 magnification); C, example image of a human full-term pancreas (×40 magnification); D, example image of a human 1-yr-old pancreas (×40 magnification); E, example image of a human 3-yr-old pancreas (×40 magnification); F, example image of a human 17-yr-old pancreas (×40 magnification); G, example image of a human 72-yr-old pancreas (×40 magnification).
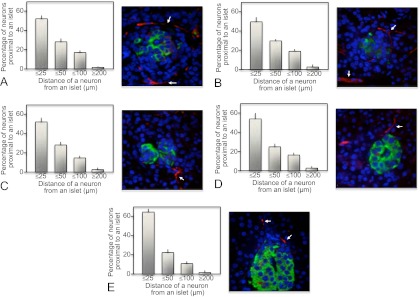
Other cell types are involved in normal pancreatic islet formation and function, in particular endothelial cells (17) and neuronal cell connections (19). Endothelial cells are required to establish normal pancreatic islet mass and function (28). However, when neuronal connections are made to human pancreatic islets has been unclear. Here, we found that an intimate association between neurons and islet β-cells is established developmentally (Fig. 3A). The close proximity of neurons to islets, marked by clusters of β-cells, persisted throughout life (Fig. 3). This remained apparent even as pancreatic islets occupy less of the total pancreatic area postnatally (Fig. 3, C–E). Innervation to the body of the exocrine pancreas was relatively rare by comparison.
Fig. 3.
The proximity of neuronal cells to human pancreatic islet β-cells from midgestation to adult. Human pancreatic sections were analyzed by immunofluorescence and confocal microscopy as outlined in Materials and Methods for insulin (green), the neuronal-specific marker Tuj1 (neuronal class III β-tubulin; red), and nuclei (4′,6-Diamidino-2-phenylindole; blue). The distance of Tuj1-positive neurons from clusters of islet β-cells was measured. The percentage of neurons in quartile group distances of 25 or less, 25–50, 50–100, and 200 μm or more from islet β-cell clusters was calculated and presented graphically as a mean ± sem. Arrows indicate neurons close to islet β-cell clusters in example images. A, Distance of neurons from β-cell clusters during human pancreatic development, with an example image of human 24-wk-old premature pancreas (×40 magnification); B, distance of neurons from β-cell clusters in human neonatal pancreata, with an example image of human 6-month-old pancreas (×40 magnification); C, distance of neurons from β-cell clusters in human childhood pancreata, with an example image of human 5-yr-old pancreas (×40 magnification); D, distance of neurons from β-cell clusters in human adolescent pancreata, with an example image of human 15-yr-old pancreas (×40 magnification); E, distance of neurons from β-cell clusters in human adult pancreata, with an example image of human 59-yr-old pancreas (×40 magnification).
Pancreatic β-cell neogenesis
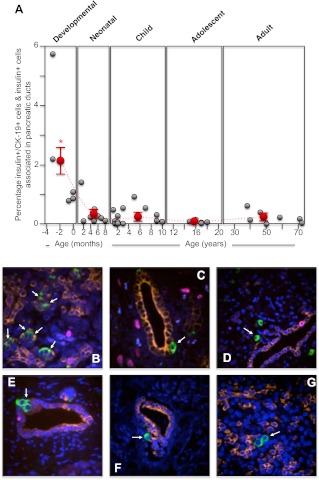
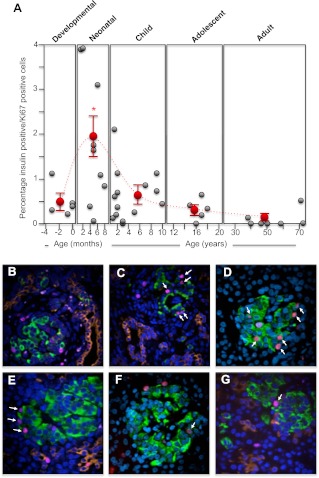
A significantly higher incidence of β-cell neogenesis was observed in the premature/developing human pancreas (P ≤ 0.001; Fig. 4, A–D). Postnatally, human β-cell neogenesis was observed at a very low rate of 0.5% or less in these normal human pancreatic samples (Fig. 4A). However, this is not to say that β-cell neogenesis does not occur after birth. Several rare instances of this process were observed in normal human pancreas, for example at 1 yr (Fig. 4F), 15 yr (Fig. 4F), and 39 yr (Fig. 4G).
Fig. 4.
The incidence of human pancreatic islet β-cell neogenesis from midgestation to adult. Human pancreatic sections were analyzed by immunofluorescence and confocal microscopy as outlined in Materials and Methods for insulin (green), CK-19 for pancreatic ductal cells (orange), Ki67 for proliferating cells (purple), and nuclei (4′,6-diamidino-2-phenylindole; blue). The number of insulin-positive cells emerging and/or associated with CK-19-positive ductal cells as well as insulin-positive/CK-19-positive coexpressing cells were counted and calculated as a percentage of all insulin-positive cells in each section. A, Incidence of β-cell neogenesis for each human specimen during the developmental, neonatal, childhood, adolescent, and adult periods (gray circles). The mean ± sem incidence of β-cell neogenesis for each period is shown as red circles. *, Significant difference (P ≤ 0.001) from the average β-cell neogenesis found in adult islets. The dashed line indicates the trend in change of the average incidence of β-cell neogenesis between the different life periods (note that the x-axis time scale is different for each period); B, example image of a human 24-wk-old premature pancreas (×60 magnification); C, example image of a human 24-wk-old premature pancreas (×60 magnification); D, example image of a human full-term pancreas (×40 magnification); E, example image of a human 1-yr-old pancreas (×60 magnification); F, example image of a human 15-yr-old pancreas (×40 magnification); G, example image of a human 39-yr-old pancreas (×60 magnification).
Pancreatic β-cell proliferation
The β-cell proliferation rate in late human pancreas development was relatively infrequent, having a general incidence of about 0.5% (Fig. 5A). However, although variable, there was a significant increase in the average incidence of human β-cell proliferation neonatally (P ≤ 0.01; Fig. 5, A, C, and D). But by 2 yr, this burst of human β-cell proliferation had decreased to an incidence rate of 0.5–1% that was sustained throughout childhood (Fig. 5, A and F). Thereafter, during human adolescence and adulthood, pancreatic β-cell proliferation was observed quite rarely, with an incidence consistently below 0.5% (Fig. 5, A and G).
Fig. 5.
The incidence of human pancreatic islet β-cell proliferation from midgestation to adult. Human pancreatic sections were analyzed by immunofluorescence and confocal microscopy as outlined in Materials and Methods for insulin (green), CK-19 for pancreatic ductal cells (orange), Ki67 for proliferating cells (purple), and nuclei (4′,6-Diamidino-2-phenylindole; blue). The number of insulin-positive cells/Ki67-positive coexpressing cells were counted and calculated as a percentage of all insulin-positive cells in each section. A, Incidence of β-cell proliferation for each human specimen during the developmental, neonatal, childhood, adolescent, and adult periods (gray circles). The mean ± sem incidence of β-cell proliferation for each period is shown as red circles. *, Significant difference (P ≤ 0.01) from the average β-cell proliferation found in adult islets. The dashed line indicates the trend in change of the average incidence of β-cell proliferation between the different life periods (note that the x-axis time scale is different for each period); B, example image of a human 24-wk-old premature pancreas (×40 magnification); C, example image of a human full-term pancreas (×40 magnification); D, example image of a human 2-month-old pancreas (×60 magnification); E, example image of a human 6-month-old pancreas (×60 magnification); F, example image of a human 7-yr-old pancreas (×60 magnification); G, example image of a human 39-yr-old pancreas (×60 magnification).
Pancreatic ductal-cell proliferation
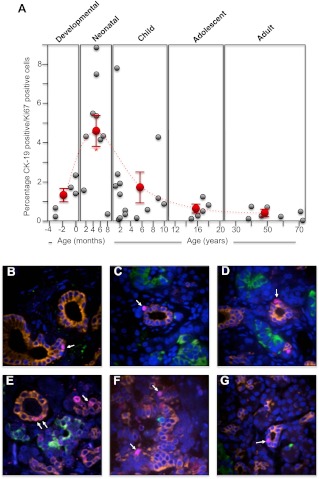
Human pancreatic ductal cell proliferation also significantly increased during the neonatal period and peaked at 4–6 months of age (P ≤ 0.001; Fig. 6, A–D). Up to 2 yr of age, ductal cell proliferation was still relatively high (Fig. 6, A and E) but during the rest of childhood tailed off to an incidence below 1% (Fig. 6, A and F). Thereafter, during human adolescence and adulthood, pancreatic ductal-cell proliferation was observed relatively rarely, with an incidence consistently below 1% (Fig. 6, A and G).
Fig. 6.
The incidence of human pancreatic ductal cell proliferation from midgestation to adult. Human pancreatic sections were analyzed by immunofluorescence and confocal microscopy as outlined in Materials and Methods for insulin (green), CK-19 for pancreatic ductal cells (orange), Ki67 for proliferating cells (purple), and nuclei (4′,6-Diamidino-2-phenylindole; blue). The number of insulin-positive cells/CK-19-positive coexpressing cells were counted and calculated as a percentage of all CK-19-positive cells in each section. A, The incidence of pancreatic ductal cell proliferation for each human specimen during the developmental, neonatal, childhood, adolescent, and adult periods (gray circles). The mean ± sem incidence of ductal cell proliferation for each period is shown in as red circles. *, Significant difference (P ≤ 0.01) from the average ductal cell proliferation found in adult islets. The dashed line indicates the trend in change of the average incidence of ductal cell proliferation between the different life periods (note that the x-axis time scale is different for each period); B, example image of a human 7-month-old pancreas (×40 magnification); C, example image of a human 1-yr-old pancreas (×60 magnification); D, example image of a human 1-yr-old pancreas (×40 magnification); E, example image of a human 17-month-old pancreas (×60 magnification); F, example image of a human 3-yr-old pancreas (×40 magnification); G, example image of a human 18-yr-old pancreas (×40 magnification).
Discussion
Here, we have investigated the formation of a normal human islet β-cell population in the first 20 yr of life relative to that in adults. Rare premature cadaver samples were used as a surrogate for pancreatic developmental specimens. Relatively few studies examining the normal formation of β-cell mass have been conducted, and more are needed, especially considering the variability between human specimens (9, 14, 29). As more studies are conducted, using a variety of different technical approaches, collectively, they should eventually lead to a solid consensus of how normal human β-cell growth occurs. In this study, we have used an immunofluorescence approach to examine the early establishment of a human β-cell population relative to that of other pancreatic islet cell types and associated neurons compared to that found in adult islets.
Normal formation of β-cell mass largely arises by replication of existing β-cells and neogenesis from pancreatic progenitor and/or ductal precursor cells (10, 11, 14, 15). Perhaps not surprisingly, β-cell neogenesis commonly occurred developmentally (10, 11). Thereafter, it was quite a rare occurrence but nonetheless was occasionally observed in adolescents (Fig. 4F) and adults (Fig. 4G). However, there is a proviso in that human β-cell neogenesis could differ between different pancreatic regions. Due to the scarcity of fetal tissue, not all regions of the pancreas could be examined in younger subjects, and as such, it is possible that β-cell neogenesis was not fully appreciated.
It has been shown that proliferation of insulin-expressing cells can occur at relatively high rates in early development, but later on, at 24 wk or more, tails off to a lower rate (12, 30), comparable to what was observed in the younger premature pancreatic specimens here. In this study, the highest rate of β-cell proliferation was observed neonatally, up to about 2 yr of age. Thereafter, rates of β-cell proliferation dropped to approximately 0.5%, consistent with a previous study (14). However, it should be noted that there was variability in rates of β-cell proliferation observed up to 2 yr old. Whether this is due to a window for the rapid burst of β-cell proliferation being missed in some specimens or reflective of the heterogeneity in the human population requires further analysis of many more human pancreatic specimens at this age. Nonetheless, the consensus to date suggests that a neonatal burst of β-cell proliferation is a major driving force that doubles human β-cell mass by 5 yr old (14). In examining net β-cell growth, it is also important to consider the rate of β-cell death (1). In this study, β-cell apoptosis was also measured, using activated caspase-3 as a marker (22). Although variable, the rate was relatively low (on average, ≤1.5%) and constant throughout the normal human pancreata examined (data not shown). As such, β-cell apoptosis does not apparently contribute to normal formation of human β-cell mass, consistent with previous observations (14).
Pancreatic ductal cell proliferation was also examined. It has previously been observed that there is marked pancreatic ductal epithelial cell proliferation early in human pancreatic development, which precedes and then accompanies the first appearance of β-cells (12). However, the highest rate of ductal cell proliferation was observed neonatally. This peak of ductal cell proliferation had a tendency to follow that for β-cell proliferation but did not correlate with any indicators for β-cell neogenesis. Rather, it correlated with the expansion of pancreatic exocrine tissue (as indicated in Supplemental Fig. 1, F and G, where pancreatic islet cells occupy less of the total pancreatic surface area in older subjects). This is consistent with previous ideas that both endocrine and exocrine cells can be derived from pancreatic ductal cell precursors (11).
A normal pancreatic β-cell population forms alongside with that of some other pancreatic islet endocrine cells (27). The α-cell/β-cell relationship is particularly important considering that normally insulin and glucagon work in partnership to keep circulating glucose levels within a narrow physiological range (31). In addition, normal human islet β-cell function requires an association with α-cells (18). In this study, pancreatic α-cell and δ-cell proliferation was not frequently observed. The apparent rapid increase in the β-cell to α-cell ratio in the neonatal period is mostly reflective of the increase in β-cell numbers, with the α-cell population staying relatively constant. This implies that the pancreatic α-cell population is set developmentally and does not appreciably change through the rest of human life. Intriguingly, there was a more dramatic early change in the β-cell to δ-cell ratio. During late development, there is an approximately equal number of β- and δ-cells (32). The neonatal increase in the β-cell to δ-cell ratio is most likely reflective of the burst in β-cell growth at that time but thereafter during childhood is probably due to loss of δ-cells, because β-cell numbers normally remain relatively constant from 5 yr of age and onward (9). The loss of δ-cells in the first 10 yr of human life is unlikely to compromise somatostatin production, because there should be adequate production from gut cells (33). However, the relatively large numbers of δ-cells found during development and in the early neonatal period may suggest that local production of somatostatin in the pancreas could play a role, perhaps in limiting insulin secretion during this time.
Other cell types associated with pancreatic islets are also important for their formation and function, including endothelial cells (17, 18, 28) and neurons (19, 34–36). In mice, islet innervation occurs late in gestation and in early life (35). Here, in humans, it was found that pancreatic β-cells also have a close association with neurons beginning in late development, which is then retained throughout life. Recently, it has been suggested that sympathetic neuronal connections to human pancreatic islets, unlike that in rodents where neurons contact endocrine islets, are preferentially associated with blood vessel endothelial cells and parasympathetic neuronal connections are few (37). This is mostly based on an immunofluorescent analysis, and in our study, the resolution was not sufficient to make such conclusions. Notwithstanding, supplementary central control of both insulin and glucagon secretion is known in humans (34, 38), but additional complementary functional studies are required to better understand the mechanism. In particular, the specific areas of the brain/central nervous system that mediate this additional control of the endocrine pancreas remain relatively undefined.
In adult humans, pancreatic β-cell mass has a degree of plasticity and can expand, mostly by controlled hyperplasia and hypertrophy, to compensate for insulin resistance (1). A failure of the functional β-cell mass to adequately compensate in these states results in the onset of diabetes (1). The extent of baseline β-cell mass is a contributing factor to susceptibility for diabetes (9). In this study, complementary evidence is presented that human baseline β-cell mass is established before 5 yr of age. However, it is a functional β-cell mass that is effective in compensating for insulin resistance, and normal β-cell function is influenced by other cell types in a pancreatic islet, including endothelial cells (17), α-cells (18), and associated neurons (34, 38). Here we find that the human pancreatic islet association with neurons could be set even earlier, during pancreatic development. Moreover, although the final baseline β-cell population is contributed to by a neonatal burst of β-cell proliferation (with little input from β-cell neogenesis), the magnitude of this early β-cell growth is reliant on the numbers of β-cells that were formed developmentally. As such, genetic factors and the maternal intrauterine environment could be an influence on the degree of human pancreatic islet formation and size of functional baseline β-cell mass. If this is small, as perhaps in neonates of low birth weight, then a susceptibility to diabetes can be set very early on in life (39, 40). A final consideration, also emerging from this study, is that some preterm human pancreatic specimens were obtained from prematurely born neonates. These pancreata were fetal in their morphometric characteristics rather than neonatal. Currently, approximately one in eight births in the United States are premature, and it will be important to monitor whether these children are also more susceptible to diabetes later on in life as a consequence of inadequate formation of pancreatic islet β-cells outside of the uterus.
Supplementary Material
Acknowledgments
We gratefully acknowledge the assistance of Dr. Vytas Bindokas in the University of Chicago light microscope core facility. A special note of thanks goes to those families who kindly donate human tissues for research purposes.
This research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International (JDRF). Organ Procurement Organizations partnering with nPOD to provide research resources are listed at www.jdrfnpod.org/our-partners.php. This work was supported by National Institutes of Health Grant DK55267 (to C.J.R.), the Diabetes Research Center at the University of Chicago (DK20595), a Lawson Wilkins Pediatric Endocrinology Society Research Scholarship (to B.E.G.), the JDRF-sponsored Network for Pancreatic Organ Donors with Diabetes (JDRF nPOD) (to M.A.A.), and the Brehm Coalition (to C.J.R. and M.A.A.).
Disclosure Summary: The authors have nothing to disclose; there is no conflict of interest.
Footnotes
- CK-19
- Cytokeratin-19
- Tuj1
- Neuronal class III β-tubulin.
References
- 1. Rhodes CJ. 2005. Type-2 diabetes; a matter of β-cell life and death? Science 307:380–384 [DOI] [PubMed] [Google Scholar]
- 2. Kahn SE, Zraika S, Utzschneider KM, Hull RL. 2009. The β-cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 52:1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leahy JL. 2005. Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36:197–209 [DOI] [PubMed] [Google Scholar]
- 4. Ogilvie RF. 1933. The islands of Langerhans in 19 cases of obesity. J Pathol 37:473–481 [Google Scholar]
- 5. Sorenson RL, Brelje TC. 1997. Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 29:301–307 [DOI] [PubMed] [Google Scholar]
- 6. Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. 2010. Adaptive changes in pancreatic β-cell fractional area and β-cell turnover in human pregnancy. Diabetologia 53:2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonnefond A, Froguel P, Vaxillaire M. 2010. The emerging genetics of type 2 diabetes. Trends Mol Med 16:407–416 [DOI] [PubMed] [Google Scholar]
- 8. Romao I, Roth J. 2008. Genetic and environmental interactions in obesity and type 2 diabetes. J Am Diet Assoc 108:S24–S28 [DOI] [PubMed] [Google Scholar]
- 9. Butler PC, Meier JJ, Butler AE, Bhushan A. 2007. The replication of β-cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab 3:758–768 [DOI] [PubMed] [Google Scholar]
- 10. Bensley RR. 1911. Studies on the pancreas of the guinea pig. Am J Anat 12:297–388 [Google Scholar]
- 11. Seymour PA, Sander M. 2011. Historical perspective: beginnings of the β-cell: current perspectives in β-cell development. Diabetes 60:364–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meier JJ, Köhler CU, Alkhatib B, Sergi C, Junker T, Klein HH, Schmidt WE, Fritsch H. 2010. β-Cell development and turnover during prenatal life in humans. Eur J Endocrinol 162:559–568 [DOI] [PubMed] [Google Scholar]
- 13. Dhawan S, Georgia S, Bhushan A. 2007. Formation and regeneration of the endocrine pancreas. Curr Opin Cell Biol 19:634–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. 2008. β-cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. 2010. β-Cell growth and regeneration: replication is only part of the story. Diabetes 59:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, Kirby M, Pechhold S, Liu EH, Harlan DM, Tisdale JF. 2010. Significant human β-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab 95:E234–E239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC. 2006. Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes 55:2974–2985 [DOI] [PubMed] [Google Scholar]
- 18. Pipeleers DG, Schuit FC, in't Veld PA, Maes E, Hooghe-Peters EL, Van de Winkel M, Gepts W. 1985. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology 117:824–833 [DOI] [PubMed] [Google Scholar]
- 19. Thorens B. 2011. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes Metab 13(Suppl 1):82–88 [DOI] [PubMed] [Google Scholar]
- 20. Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. 2005. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53:1087–1097 [DOI] [PubMed] [Google Scholar]
- 21. Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. 2009. Islet architecture: a comparative study. Islets 1:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reddy S, Bradley J, Ginn S, Pathipati P, Ross JM. 2003. Immunohistochemical study of caspase-3-expressing cells within the pancreas of non-obese diabetic mice during cyclophosphamide-accelerated diabetes. Histochem Cell Biol 119:451–461 [DOI] [PubMed] [Google Scholar]
- 23. Guest PC, Abdel-Halim SM, Gross DJ, Clark A, Poitout V, Amaria R, Ostenson CG, Hutton JC. 2002. Proinsulin processing in the diabetic Goto-Kakizaki rat. J Endocrinol 175:637–647 [DOI] [PubMed] [Google Scholar]
- 24. Lingohr MK, Briaud I, Dickson LM, McCuaig JF, Alárcon C, Wicksteed BL, Rhodes CJ. 2006. Specific regulation of IRS-2 expression by glucose in rat primary pancreatic islet β-cells. J Biol Chem 281:15884–15892 [DOI] [PubMed] [Google Scholar]
- 25. Aye T, Toschi E, Sharma A, Sgroi D, Bonner-Weir S. 2010. Identification of markers for newly formed β-cells in the perinatal period: a time of recognized β-cell immaturity. J Histochem Cytochem 58:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falin LI. 1967. The development and cytodifferentiation of the islets of Langerhans in human embryos and foetuses. Acta Anat (Basel) 68:147–168 [DOI] [PubMed] [Google Scholar]
- 27. Stefan Y, Grasso S, Perrelet A, Orci L. 1983. A quantitative immunofluorescent study of the endocrine cell populations in the developing human pancreas. Diabetes 32:293–301 [DOI] [PubMed] [Google Scholar]
- 28. Villasenor A, Chong DC, Henkemeyer M, Cleaver O. 2010. Epithelial dynamics of pancreatic branching morphogenesis. Development 137:4295–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. 2000. β-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49:1325–1333 [DOI] [PubMed] [Google Scholar]
- 30. Bouwens L, Lu WG, De Krijger R. 1997. Proliferation and differentiation in the human fetal endocrine pancreas. Diabetologia 40:398–404 [DOI] [PubMed] [Google Scholar]
- 31. Unger RH, Orci L. 2010. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA 107:16009–16012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rahier J, Wallon J, Henquin JC. 1980. Abundance of somatostatin cells in the human neonatal pancreas. Diabetologia 18:251–254 [DOI] [PubMed] [Google Scholar]
- 33. Polak JM, Bloom SR. 1986. Somatostatin localization in tissues. Scand J Gastroenterol Suppl 119:11–21 [DOI] [PubMed] [Google Scholar]
- 34. Boden G, Chen X, DeSantis R, Kolaczynski J, Morris M. 1993. Evidence that suppression of insulin secretion by insulin itself is neurally mediated. Metabolism 42:786–789 [DOI] [PubMed] [Google Scholar]
- 35. Ohta Y, Kosaka Y, Kishimoto N, Wang J, Smith SB, Honig G, Kim H, Gasa RM, Neubauer N, Liou A, Tecott LH, Deneris ES, German MS. 2011. Convergence of the insulin and serotonin programs in the pancreatic β-cell. Diabetes 60:3208–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Imai J, Katagiri H, Yamada T, Ishigaki Y, Suzuki T, Kudo H, Uno K, Hasegawa Y, Gao J, Kaneko K, Ishihara H, Niijima A, Nakazato M, Asano T, Minokoshi Y, Oka Y. 2008. Regulation of pancreatic β-cell mass by neuronal signals from the liver. Science 322:2311–2320 [DOI] [PubMed] [Google Scholar]
- 37. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. 2011. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 14:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kendall DM, Teuscher AU, Robertson RP. 1997. Defective glucagon secretion during sustained hypoglycemia following successful islet allo- and autotransplantation in humans. Diabetes 46:23–27 [DOI] [PubMed] [Google Scholar]
- 39. Hales CN, Ozanne SE. 2003. The dangerous road of catch-up growth. J Physiol 547:5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simmons RA, Templeton LJ, Gertz SJ. 2001. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50:2279–2286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.