Abstract
Context:
Polycystic ovary syndrome (PCOS) is a heterogeneous common genetic disorder characterized by hyperandrogenemia and insulin resistance. Alterations in gene expression profiles of the ovary and adipose tissue identified the candidate gene FBJ murine osteosarcoma viral oncogene homolog (FOS) for further investigation of expression changes in metabolic tissues and genetic studies.
Objective:
The objective of the study was to confirm the underexpression of the FOS gene in sc adipose and determine whether variants in this gene are risk factors for PCOS.
Design:
RT-PCR was performed in sc fat from women with and without PCOS. Genotyping of single-nucleotide polymorphisms in the FOS locus was performed to test for association with PCOS.
Setting:
The study was conducted at a tertiary care academic institution.
Participants:
Twenty-two PCOS and 13 control subjects were recruited for gene expression studies. We assembled a discovery genotyping cohort of 354 cases and 161 controls and a replication cohort of 476 cases and 315 controls, all of whom were Caucasian.
Main Measurements:
Gene expression by quantitative real-time RT-PCR, FOS genotype, and PCOS status were measured.
Results:
FOS expression was confirmed to be reduced in PCOS adipose tissue. Three single-nucleotide polymorphisms were significantly associated with PCOS in the discovery cohort (rs8006998, P = 0.0031; rs8013918, P = 0.0006; rs8013942, P = 0.0087). rs8006998 was also associated with PCOS in the replication cohort (P = 0.013).
Conclusions:
Differential gene expression in sc fat and genetic association at the FOS locus in PCOS subjects implicates a role for this transcription factor in PCOS. FOS dysfunction may be a common factor between hyperandrogenism and insulin resistance.
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy of reproductive-aged women, with a prevalence of 5–7% (1). The main phenotypes of PCOS include hyperandrogenism, oligoovulation, and polycystic ovaries. Infertility, obesity, and insulin resistance as well as an increased risk of cardiovascular disease and the metabolic syndrome are commonly observed in patients with PCOS (2, 3).
PCOS is determined in part by inherited factors as a complex trait; however, the genetic basis of this complex disorder remains largely unknown (4). Gene expression studies from PCOS tissues have identified novel pathways and genes important in PCOS pathophysiology (5–8). Studies in sc adipocytes from PCOS women have revealed resistance to insulin stimulated glucose transport and inhibition of lipolysis (9, 10); thus, adipocyte dysfunction may have a role in the pathogenesis of the syndrome.
Several studies suggest that the activator protein-1 (AP-1) transcription factor FBJ murine osteosarcoma viral oncogene homolog (FOS) may have a role in the pathogenesis of PCOS. Microarray analysis performed on whole ovary (containing both stromal and cortical fractions) found decreased FOS expression in PCOS ovaries compared with normal ovaries (5). In vitro studies have demonstrated theca cell CYP17 expression and androgen synthesis were inhibited by FOS (11). Silencing of FOS expression in both theca and granulosa cells resulted in significant increases in CYP17 mRNA, implicating the FOS family in regulation of the production of androgens in the ovary (11, 12). Our microarray gene expression profiling of PCOS adipose tissue vs. controls found that the FOS gene was considerably underexpressed (−30.4-fold, P = 0.002) in PCOS sc adipose tissue (13).
The purpose of the present study was to confirm the underexpression of FOS in sc fat in PCOS patients as well as conduct a genetic association study of the FOS gene with PCOS. We hypothesized that FOS gene expression would be decreased in sc fat from PCOS subjects, similar to reported data from the PCOS ovary (5) and that genetic variation at this locus would be associated with PCOS.
Materials and Methods
Expression study
Subjects
For mRNA and protein analysis, sc lower abdominal adipose tissue was obtained from 22 white PCOS and 13 white control subjects (clinical characteristics are shown in Table 1); the protocol for acquiring and processing sc adipose tissue was previously described (14). These subjects are independent from those used in the whole-genome expression discovery study (13). Cases were premenopausal, were nonpregnant, were on no hormonal therapy, including oral contraceptives, for at least 3 months, and met 1990 National Institutes of Health criteria for PCOS (15). Parameters for defining hirsutism, hyperandrogenemia, ovulatory dysfunction, and exclusion of related disorders were previously reported (16). Controls were healthy women, with regular menstrual cycles and no evidence of hirsutism, acne, alopecia, or endocrine dysfunction and had not taken hormonal therapy (including oral contraceptives) for at least 3 months. Controls were recruited by word of mouth and advertisements calling for healthy women.
Table 1.
Clinical characteristics of PCOS and control subjects in the expression study
| Control (n = 13) | PCOS (n = 22) | P valuea | |
|---|---|---|---|
| Age (yr) | 33.0 (13) | 28.0 (3.0) | 0.032 |
| BMI (kg/m2) | 25.5 (8.0) | 31.0 (7.6) | 0.23 |
| Insulin (μIU/ml) | 9.0 (6.5) | 7.0 (8.3) | 0.60 |
| Glucose (mg/dl) | 71.0 (21.3) | 88.0 (9.8) | 0.053 |
| HOMA-IR | 1.05 (0.31) | 0.76 (0.81) | 0.66 |
| HOMA-%B | 114.2 (75.3) | 88.6 (32.1) | 0.18 |
| Total testosterone (ng/dl) | 28.0 (4.5) | 32.5 (24.0) | 0.12 |
| DHEAS (ng/ml) | 1995.0 (1290.0) | 2995.0 (1280.0) | 0.030 |
Data are median (interquartile range). To convert glucose from milligrams per deciliter to millimoles per liter, multiply by 0.05551; to convert insulin from microinternational units per milliliter to picomoles per liter, multiply by 6; to convert total testosterone from nanograms per deciliter to nanomoles per liter, multiply by 0.03467; to convert DHEAS from nanograms per milliliter to micromoles per liter, multiply by 0.002714. HOMA values were calculated using the web tool at http://www.dtu.ox.ac.uk/homacalculator/index.php. HOMA-IR, Homeostasis model assessment of insulin resistance; HOMA-%B, homeostasis model assessment of β-cell function (insulin secretion); DHEAS, dehydroepiandrosterone sulfate.
P values are derived from the Mann-Whitney test.
Quantitative real-time PCR (qRT-PCR) gene expression analysis
Total RNA was isolated from sc fat tissue after rapid thaw with the AllPrep DNA/RNA/protein minikit (QIAGEN, Valencia, CA) and was quantified and checked for quality on a Nanodrop-1000 (Nanodrop, Wilmington, DE). cDNA was created using the Applied Biosystems high-capacity cDNA reverse transcription kit (Life Technologies, Carlsbad, CA). According to the manufacturer's protocol, 10 μl of RNA at 5 ng/μl was used to generate cDNA. A preamp protocol was then performed using the Taqman PreAmp master mix (Life Technologies) and a custom pool of primers for 30 gene expression targets following the manufacturer's directions. The genes consisted of FOS, two housekeeping genes (GAPDH, GUSB), plus genes for 13 adipose tissue factors (ADIPOQ, CRP, IL1B, IL6, IL8, LEP, LPIN1, NAMPT, PPARG, RETN, TGFB1, TNFA, VEGFA), five steroidogenic factors (CYP17A1, CYP19A1, FSHB, SF1, STAR), five factors involved in FOS transcription (ELK1, ERK1, ERK2, JNK, SRF), and four FOS cofactors (ATF4, JUN, JUNB, NCOA1) (gene names and assay numbers are listed in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org); the latter 27 genes were selected based on a literature search for factors that regulate or are regulated by FOS. Quantitative RT-PCR analysis was performed using the OpenArray platform and Taqman gene expression assays (Life Technologies). Samples were run in technical triplicate with a no template control carried forward from cDNA generation. The housekeeping genes GAPDH and GUSB were used for signal normalization. Relative mRNA expression levels of each transcript in PCOS tissues were compared by fold change to the control samples; average cycle threshold (Ct) values of each group were adjusted by subtracting the average Ct values of the housekeeping genes, yielding ΔCt (17). The ΔCt for PCOS vs. controls was subjected to a two-tailed t test. Logistic regression was used to adjust for age. The ΔΔCt for each transcript across groups was calculated as ΔΔCt = ΔCtPCOS − ΔCtcontrols. The fold change of PCOS relative to controls was calculated as 2(−ΔΔCt).
Western blot analysis
Western blot analysis was performed with the mini-Protean TGX gel electrophoresis system (Bio-Rad Laboratories, Hercules, CA). Samples were diluted 3:1 in Laemmli sample buffer and denatured for 5 min at 95 C. Proteins were separated on precast 4–20% Tris-glycine gels and electroblotted using the Trans-Blot Turbo onto 0.2 μm polyvinyl difluoride membranes (Bio-Rad Laboratories). Membranes were blocked for 1 h in 5% nonfat dry milk (Bio-Rad Laboratories) in Tris-buffered saline and 0.2% Tween 20 (TBST) buffer and then incubated overnight in anti-c-Fos (Cell Signaling Technology, Danvers, MA), antiphospho-c-Fos (Ser32) (Cell Signaling Technology), and antiglyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology, Santa Cruz, CA). Phosphorylation at Ser32 protects FOS from degradation and promotes its nuclear localization (18). After washing with TBST, membranes were incubated with the appropriate peroxidase-conjugated secondary antibody. Blots were washed again in TBST and immunoreactive bands were detected using the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ). Densitometric analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Genetic association study
Discovery cohort
We studied 354 unrelated white PCOS patients and 161 white control women recruited at two centers, the University of Alabama at Birmingham (242 PCOS and 146 controls) and Cedars-Sinai Medical Center (112 PCOS and 15 controls). The discovery cohort was recruited using the same protocol as that used for recruitment of sc fat biopsy subjects (described above). Table 2 presents clinical characteristics of both cohorts.
Table 2.
Clinical characteristics of PCOS and control subjects in the genetic association study
| Discovery |
Replication |
|||
|---|---|---|---|---|
| Control (n = 161) | PCOS (n = 354) | Control (n = 315) | PCOS (n = 476) | |
| Age (yr) | 33.0 (15.0) | 26.8 (10.2)a | 50.6 (23.4) | 28.0 (8.0)a |
| BMI (kg/m2) | 24.1 (5.6) | 31.2 (14.7)a | 25.8 (8.4) | 34.1 (13.3)a |
| Insulin (μIU/ml) | 7.1 (6.4) | 14.0 (16.2)a | 12.0 (7.4) | 20.0 (16.0)a |
| Glucose (mg/dl) | 85.0 (10.0) | 85.0 (11.0) | 90.2 (12.4) | 87.0 (11.0) |
| HOMA-IR | 0.98 (0.82) | 1.77 (1.91)a | 1.57 (0.95) | 2.49 (1.89)a |
| HOMA-%B | 106.7 (52.2) | 152.5 (100.8)a | 129.5 (59.3) | 193.7 (81.2)a |
| Total testosterone (ng/dl) | 40.0 (26.8) | 70.0 (40.0)a | 29.0 (14.8) | 69.0 (34.0)a |
| DHEAS (ng/ml) | 983.5 (733.0) | 2188.0 (1831.0)a | 1363.0 (720.5) | 2155.0 (1434.8)a |
Data are median (interquartile range). To convert glucose from milligrams per deciliter to millimoles per liter, multiply by 0.05551; to convert insulin from microinternational units per milliliter to picomoles per liter, multiply by 6; to convert total testosterone from nanograms per deciliter to nanomoles per liter, multiply by 0.03467; to convert DHEAS from nanograms per milliliter to micromoles per liter, multiply by 0.002714. HOMA values were calculated using the web tool at http://www.dtu.ox.ac.uk/homacalculator/index.php. HOMA-IR, Homeostasis model assessment of insulin resistance; HOMA-%B, homeostasis model assessment of β-cell function (insulin secretion); DHEAS, dehydroepiandrosterone sulfate.
P < 0.001 compared with control group. In the replication cohort, androgen measurements were not available for controls from the Cholesterol and Pharmacogenetics study.
Replication cohort
We assembled a cohort of 476 unrelated white PCOS patients and 315 white control women. The replication cohort was constituted from three sources: 403 PCOS subjects (all meeting 1990 National Institutes of Health criteria) and 71 healthy controls previously recruited by Legro et al. (19), 73 PCOS subjects and 11 healthy controls recruited at Cedars-Sinai Medical Center using the same criteria as those used in the discovery cohort; and 233 white control women derived from the Cholesterol and Pharmacogenetics study, a component of the Pharmacogenomics and Risk of Cardiovascular Disease Study (20).
Written informed consent was provided by all subjects at all study sites. The use of these samples was approved by institutional review boards at all study sites, including Cedars-Sinai Medical Center, where the genetic studies were undertaken.
Genotyping
Discovery genotyping was performed using 10 single-nucleotide polymorphisms (SNPs, listed in Table 3) across the FOS locus (chromosome 14q24) using Golden Gate technology from Illumina (Illumina, San Diego, CA). SNPs were selected using genotype data from the CEU [Centre d'Etude du Polymorphisme Humain (CEPH), Europe (E), and Utah (U), Utah residents with ancestry from northern and western Europe] population of the HapMap database (http://hapmap.ncbi.nlm.nih.gov/). These SNPs were selected because they are predicted to tag the common (minor allele frequency > 0.05) SNPs in the FOS gene, plus 50 kb upstream and 5 kb downstream. These SNPs capture 15 of 21 (71%) of the CEU HapMap SNPs at r2 greater than 0.8 for the region. Replication genotyping was performed on three of the discovery SNPs, rs8006998, rs8013918, and rs8013942, using Taqman Assays-on-Demand (Life Technologies) according to the manufacturer's instructions. In both the discovery and replication genotyping, duplicate genotyping of a subset of samples yielded a 100% concordance rate. Haploview (version 4.1, www.broad.mit.edu/mpg/haploview/) was used to calculate linkage disequilibrium (the D′ statistic) between each pair of SNPs. The solid spine of linkage disequilibrium algorithm in Haploview was used to determine haplotype blocks.
Table 3.
SNP information and association results for the FOS gene region in the discovery and replication cohorts
| Variant | Alleles (major/minor) | PCOS MAF | Control MAF | Overall MAF | Odds ratio | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| Discovery cohort | |||||||
| rs4899553 | C/T | 0.227 | 0.252 | 0.234 | 0.96 | 0.64–1.45 | 0.86 |
| rs887890 | T/G | 0.189 | 0.217 | 0.198 | 0.92 | 0.60–1.41 | 0.69 |
| rs4899554 | C/T | 0.182 | 0.212 | 0.192 | 0.73 | 0.48–1.13 | 0.16 |
| rs8006998 | C/T | 0.429 | 0.357 | 0.407 | 1.74 | 1.20–2.51 | 0.0031 |
| rs8013918 | T/C | 0.477 | 0.562 | 0.496 | 1.86 | 1.30–2.67 | 0.0006 |
| rs8013942 | C/T | 0.460 | 0.398 | 0.441 | 1.61 | 1.13–2.29 | 0.0087 |
| rs741847 | T/C | 0.225 | 0.258 | 0.235 | 0.72 | 0.49–1.08 | 0.11 |
| rs2205189 | T/A | 0.288 | 0.329 | 0.301 | 0.76 | 0.53–1.08 | 0.13 |
| rs1569328 | C/T | 0.165 | 0.189 | 0.173 | 0.67 | 0.43–1.06 | 0.084 |
| rs10133595 | C/T | 0.062 | 0.081 | 0.068 | 0.95 | 0.52–1.76 | 0.88 |
| Replication cohort | |||||||
| rs8006998 | C/T | 0.418 | 0.404 | 0.412 | 1.68 | 1.12–2.53 | 0.013 |
| rs8013942 | C/T | 0.497 | 0.492 | 0.495 | 1.39 | 0.92–2.10 | 0.12 |
P values are from additive logistic models adjusted for age, BMI, and recruitment site. Significant P values (corrected for multiple testing) are in bold. MAF, Minor allele frequency; CI, confidence interval.
Statistical analysis for genetic association study
Unpaired t tests and χ2 tests were used to compare clinical characteristics between cases and controls; quantitative traits were log or square root transformed as appropriate to reduce nonnormality. Data are presented as median (interquartile range).
Genotypic association with PCOS status was evaluated using logistic regression, adjusting for recruitment site, body mass index (BMI), and age. Bonferroni correction for multiple testing was used; significance was taken as P < 0.01 in the discovery cohort (P = 0.05 divided by five haplotype blocks) and as P < 0.025 in the replication cohort (P = 0.05 divided by two SNPs successfully genotyped in the replication cohort). To limit multiple testing in the replication cohort, we carried forward only the SNPs that displayed statistically significant associations with PCOS in the discovery cohort.
Results
Validation of gene expression
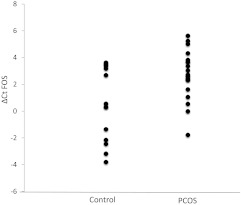
Cases and controls in the group used for gene expression studies were similar in BMI, whereas the cases were younger (Table 1). We confirmed our previously reported reduction in expression of FOS mRNA in the PCOS adipose tissue in this independent replication set of samples using qRT-PCR. Using GAPDH and GUSB expression in the control samples as a reference, FOS was underexpressed (ΔΔCt for PCOS samples, 2.4, compared with control samples) with a significant relative fold change (1.00 in control samples, 0.19 in PCOS samples; P = 0.0056) (Fig. 1). FOS underexpression remained significant after adjustment for age. FOS ΔCt levels were not correlated with any of the quantitative traits listed in Table 1 (data not shown).
Fig. 1.
Relative expression (ΔCT) of FOS in sc adipose tissue from 22 PCOS (mean ΔCT 2.71 ± 1.82 sd in PCOS cases) and 13 control subjects (mean ΔCT 0.35 ± 2.74 sd in healthy controls) detected by qRT-PCR (the higher cycles indicates lower expression).
To examine potential functional implications of FOS underexpression, we conducted exploratory analyses of mRNA levels of adipose tissue factors, steroidogenic factors, factors that regulate FOS transcription, and FOS cofactors/coactivators (ΔΔCt and fold change in PCOS vs. controls in Supplemental Table 1). Only the FOS binding factor JUNB was significantly differentially underexpressed in PCOS adipose tissue (ΔΔCT 1.1, fold change 0.48, P = 0.010). However, the ΔCt levels of several genes were significantly correlated with the ΔCt of FOS (Supplemental Table 2); these included the adipokines IL6, IL8, and NAMPT (visfatin); the FOS cofactors JUNB and NCOA1; and the steroidogenic factors CYP19A1 (aromatase) and STAR.
To determine the protein levels of FOS in adipose tissue, we performed Western blots in whole-cell protein lysates. There were no significant differences between PCOS patients and controls in protein levels of FOS (mean relative values 0.74 ± 0.37 and 0.75 ± 0.46, respectively; P = 0.90) or phosphorylated FOS (mean relative values 0.80 ± 0.65 and 0.85 ± 0.49, respectively; P = 0.81).
Genetic association analysis
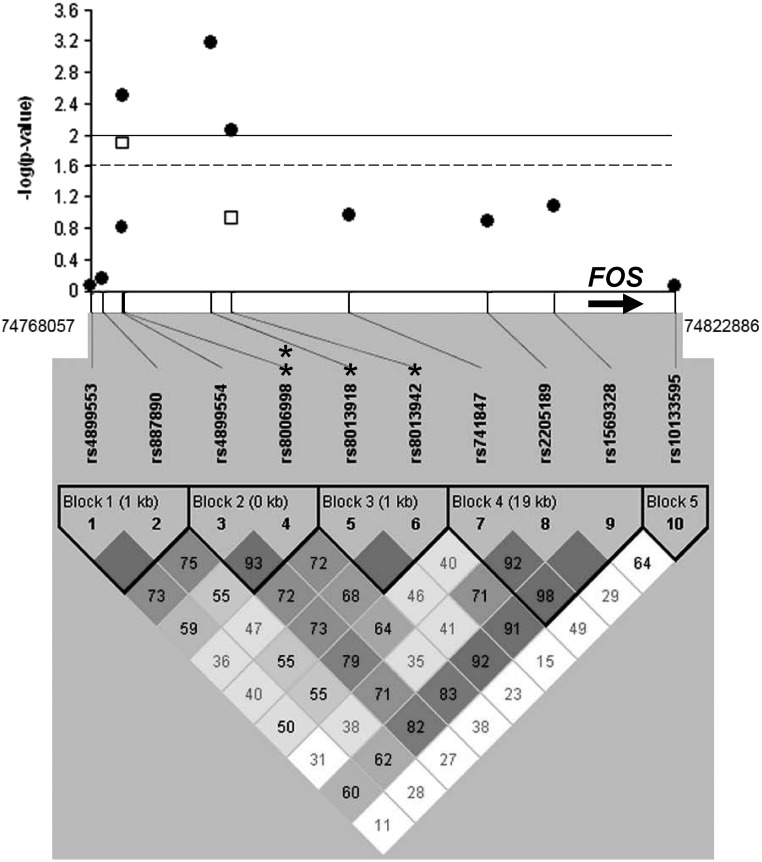
All 10 SNPs were successfully genotyped in the discovery cohort. Linkage disequilibrium was calculated between the 10 markers and is displayed in Fig. 2, combined with the results of the association analyses. We used an adjusted logistic regression model applying the additive model and found that three SNPs were associated with PCOS in the discovery cohort after correction for multiple testing (rs8006998, P = 0.0031; rs8013918, P = 0.0006; rs8013942, P = 0.0087). We selected these three SNPs for replication genotyping in our independent replication cohort; the assay for one of these, rs8013918, was a technical failure. Of the two SNPs successfully genotyped in the replication sample, rs8006998 was again associated with PCOS risk (P = 0.013), even after adjustment for multiple testing. No SNP associations with BMI were observed.
Fig. 2.
Regional association and linkage disequilibrium plot for the FOS locus. P values for association with PCOS for each SNP genotyped in the gene region (50 kb upstream/5 kb downstream) are plotted as −log 10 (P values). Filled circles represent results from the discovery cohort; open squares represent results from the replication cohort. The solid line indicates the significance level in the discovery cohort (P = 0.01), and the dotted line indicates the significance level in the replication cohort (P = 0.025). A single asterisk indicates SNPs associated with PCOS in the discovery cohort; the double asterisk indicates the SNP associated with PCOS in both cohorts. The heavy arrow indicates the FOS gene. Linkage disequilibrium is plotted and displays D′ (dark solid blocks indicate D′ = 100% with a logarithm of the odds score of 2 or greater for the corresponding pair of variants). The SNPs were grouped into five haplotype blocks as indicated.
Discussion
We have confirmed that FOS expression is reduced in sc adipose tissue in PCOS patients, and genetic variation in the upstream, potentially the promoter region, is associated with PCOS susceptibility in two independent case/control cohorts. FOS repressed CYP17 action in an ovarian tumor model (11), and its expression was reduced in PCOS ovarian tissue (5), making it a target for both gene expression studies in other tissues and for genetic studies. Our finding of reduced FOS expression in PCOS sc adipose in two independent sets of women suggests underexpression of FOS may be a pathogenic factor in PCOS adipose tissue. Our genome-wide expression microarray in adipose tissue aimed to identify novel factors that may explain metabolic abnormalities in PCOS and be important targets for genetic studies (13).
PCOS women often present with both reproductive and metabolic abnormalities; adipose tissue dysfunction may be a major factor contributing to insulin resistance in PCOS. Compared with BMI-matched controls, adipocytes from women with PCOS have increased size; this adipocyte hypertrophy is associated with insulin resistance (21). Lipolysis is reduced in sc adipose tissue from PCOS women (9, 22), possibly suppressed by elevated androgens (23). Adipose tissue secretes numerous adipokines that influence metabolism and insulin resistance; studies have documented altered production of several of these adipokines in PCOS, such as reduced adiponectin secretion (21, 24). Adipocytes from PCOS women have reduced insulin-dependent glucose uptake resulting from reduced insulin receptor activation and glucose transporter translocation (10, 25); however, these abnormalities are not observed in cultured PCOS preadipocytes (26). Whether intrinsic or resulting from the hyperandrogenic milieu (27), adipose tissue dysfunction is intimately linked to insulin resistance in PCOS.
What has remained unclear in gene expression studies focusing on reproductive tissue is the role genes differentially expressed in these PCOS tissues may play in key metabolic tissues such as adipose tissue. Genes under differential expression in both reproductive and metabolic tissues may represent key factors that disrupt both the steroidogenic and insulin signaling pathways that are dysfunctional in PCOS subjects. By confirming reduced expression of FOS in PCOS sc fat, we have identified such a factor.
The association of variants in the FOS region provides further suggestion that this locus may be important in the pathogenesis of PCOS. The SNP rs8006998 was significantly associated with PCOS risk in both our discovery and replication cohorts and is located upstream of the coding region of this leucine zipper. This marker is located in transcription factor binding sites identified by chromatin immunoprecipitation-sequencing as part of the ENCODE project (http://genome.ucsc.edu/ENCODE/), suggesting this region may be important in regulating FOS expression levels.
FOS family members (e.g. FOS, FOSB) heterodimerize with JUN family members (e.g. JUN, JUNB, JUND) to constitute the transcription factor AP-1. The FOS family of proteins have been implicated as regulators of cell proliferation, differentiation, apoptotic cell death, and transformation (28). They are widely expressed and have been shown to down-regulate CYP17 expression in both theca and granulosa (29). Inhibition of FOS expression in granulosa has been shown to result in a significant increase in CYP17 expression and subsequent androstenedione production, even in this classically nonandrogenic cell type (29). Aberrant down-regulation of FOS expression in androgen-producing tissues is a potential mechanism by which excess androgen production may occur in PCOS. The production of androgens in adipose tissue has not been extensively characterized; however, along with the full isoform of CYP17, adipose-specific isoforms of CYP17 are expressed in the adipose of adult women, and these enzymes are capable of activity (30). In the current study, CYP17 mRNA levels were below sensitivity for detection.
In addition to its inhibitory effect on CYP17 in androgen-producing tissues (12, 29), FOS is important in regulating FSH levels. The AP-1 complex regulates expression of the gene for the FSH β-subunit (FSHB), via two low-affinity AP-1 sites present within the proximal FSHB promoter; overexpression of FOS was sufficient to induce FSHB (31). This is consistent with our finding of underexpression of FOS in PCOS patients, who typically have relatively low FSH levels. FOS is also implicated in p38MAPK/ERK1/2 signaling and can be induced by GnRH in the gonadotropes, providing evidence that GnRH regulation of FSHB gene expression may be via induction of FOS (32).
In addition to the steroidogenic and hypothalamic effects of FOS outlined above, this widely expressed transcriptional regulator has a potential role in insulin resistance. Binding of FOS at the peroxisome proliferator-activated receptor-γ (PPARG) promoter in adipose tissue in culture is necessary for gene transcription to occur (33). Recent studies suggest that effects of thiazolidinediones, which act via peroxisome proliferator-activated receptor-γ, may be dependent on the activity of FOS (34). With a range of functions in both reproductive and metabolic pathways, underexpression of FOS may act to increase androgen production in ovarian tissues and promote insulin resistance in adipose tissue.
To gain insight on the functional implications of FOS underexpression, we assessed mRNA levels of factors discussed above as well as other genes implicated as FOS regulators, cofactors, or targets and conducted exploratory analyses relating their expression levels with FOS expression levels. JUNB, one of the JUN family members that heterodimerizes with FOS, was also underexpressed, suggesting depressed AP-1 activity in PCOS adipose tissue. Consistent with depressed AP-1 function, we observed a trend for depressed JUN levels and have previously reported depressed FOSB expression in PCOS adipose tissue (13). AP-1 activity is necessary for adipose tissue development, as evidenced by absence of white fat in transgenic mice with adipose tissue-specific ablation of AP-1 function; these lipoatrophic mice were diabetic and had fatty livers (35). During adipocyte differentiation, AP-1 participates in activating adipose-tissue specific genes, such as adipocyte P2 (36). Perhaps depressed AP-1 function in PCOS adipose tissue reflects impairment in adipose tissue differentiation, proliferation, and/or function. This may contribute to metabolic abnormalities via the adipose tissue expandability hypothesis (37), wherein compromised ability of adipose tissue to store fat leads to ectopic fat deposition in other organs, resulting in insulin resistance. Fatty liver has recently been found to be common in women with PCOS (38).
FOS expression was positively correlated with expression of genes for several inflammatory adipokines, including IL-6, IL-8, and visfatin/nicotinamide phosphoribosyltransferase. Steroidogenic factors STAR and CYP19A1 mRNA levels were correlated with those of FOS, consistent with prior reports of AP-1 regulating these genes (39, 40). Taken together, these observations suggest FOS underexpression in PCOS may have antiinflammatory effects and uncertain consequences on steroidogenesis in adipose tissue. However, because IL6, IL8, NAMPT, STAR, and CYP19A1 were not significantly differentially expressed in PCOS, the relevance of the effect of FOS on these factors is uncertain, and these observations are hypothesis generating. Further exploration of the many factors upstream and downstream of FOS/AP-1 will be needed to fully understand the implications of FOS underexpression.
Despite confirmed reduction in mRNA levels, we did not observe a corresponding decrease in FOS protein levels. That the FOS protein is unstable and undergoes rapid turnover may have been a contributing factor (28). A limitation of our protein analysis is that we examined whole cells. Given the role of FOS as a transcription factor, protein analysis in the nuclear fractions would have been preferable. The antiphospho-Fos antibody that was used herein recognizes only one site (Ser32); however, phosphorylation at multiple other serine and threonine residues promotes FOS activity and nuclear retention (39). Thus, we cannot rule out the possibility of altered levels of nuclear FOS protein. The finding of unaltered FOS protein levels in the face of reduced mRNA may also reflect a compensatory mechanism in PCOS adipose tissue.
In conclusion, underexpression of FOS was demonstrated and confirmed in sc adipose tissue in two independent sets of PCOS cases vs. weight-matched controls. This replicates previous reports of FOS underexpression in PCOS ovarian tissue. Three SNPs from the predicted transcription factor binding region upstream of FOS were significantly associated with PCOS in the discovery cohort, and this association was independently replicated for rs8006998, confirming that genetic variation from this region may be important in PCOS risk in Caucasian populations. Impaired FOS activity may result in both reproductive and metabolic dysfunction in PCOS. The expression, activity, and function of FOS in additional tissues deserve further investigation.
Supplementary Material
Acknowledgments
We thank Vincent Funari and his associates in the Cedars-Sinai Genomics Core for excellent technical assistance.
This work was supported by National Institutes of Health Grants R01-HD29364 and K24-HD01346 (to R.A.), Grant R01-DK79888 (to M.O.G.), Grant U54-HD034449 (to R.S.L.), Grant R01-HL069757 (to R.M.K.); National Center for Research Resources Grant M01-RR00425 (to the Cedars-Sinai General Clinical Research Center), Grant U54 RR026071 (to Penn State Clinical and Translational Science Institute), and Grant UL1-RR033176 (to the University of California, Los Angeles, Clinical and Translational Science Institute, now at the National Center for Advancing Translational Sciences, Grant UL1-TR000124); the Winnick Clinical Scholars Award (to M.O.G.); and an endowment from the Helping Hand of Los Angeles, Inc.
Present address for Y.-H.C. and R.A.: Department of Obstetrics and Gynecology and Department of Medicine, Georgia Health Sciences University, Augusta, Georgia 30912.
Disclosure Summary: M.R.J., G.C., N.X., A.K.C., T.E., E.M., Y.-H.C., J.-M.L., M.P., X.L., Y.-D.I.C., K.D.T., R.M., R.M.K., J.I.R., R.S.L., R.A., and M.O.G. have nothing to declare.
Footnotes
- AP-1
- Activator protein-1
- BMI
- body mass index
- Ct
- cycle threshold
- FOS
- FBJ murine osteosarcoma viral oncogene homolog
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- PCOS
- polycystic ovary syndrome
- qRT-PCR
- quantitative real-time PCR
- SNP
- single-nucleotide polymorphism
- TBST
- Tris-buffered saline and 0.2% Tween 20.
References
- 1. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. 2011. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 7:219–231 [DOI] [PubMed] [Google Scholar]
- 2. Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. 2006. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 91:48–53 [DOI] [PubMed] [Google Scholar]
- 3. Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ. 2008. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health-National Heart, Lung, and Blood Institute sponsored Women's Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 93:1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Goodarzi MO. 2008. Looking for polycystic ovary syndrome genes: rational and best strategy. Semin Reprod Med 26:5–13 [DOI] [PubMed] [Google Scholar]
- 5. Jansen E, Laven JS, Dommerholt HB, Polman J, van Rijt C, van den Hurk C, Westland J, Mosselman S, Fauser BC. 2004. Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol Endocrinol 18:3050–3063 [DOI] [PubMed] [Google Scholar]
- 6. Jones MR, Chua A, Chen YD, Li X, Krauss RM, Rotter JI, Legro RS, Azziz R, Goodarzi MO. 2011. Harnessing expression data to identify novel candidate genes in polycystic ovary syndrome. PLoS One 6:e20120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kenigsberg S, Bentov Y, Chalifa-Caspi V, Potashnik G, Ofir R, Birk OS. 2009. Gene expression microarray profiles of cumulus cells in lean and overweight-obese polycystic ovary syndrome patients. Mol Hum Reprod 15:89–103 [DOI] [PubMed] [Google Scholar]
- 8. Wood JR, Dumesic DA, Abbott DH, Strauss JF., 3rd 2007. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab 92:705–713 [DOI] [PubMed] [Google Scholar]
- 9. Ciaraldi TP. 2000. Molecular defects of insulin action in the polycystic ovary syndrome: possible tissue specificity. J Pediatr Endocrinol Metab 13(Suppl 5):1291–1293 [PubMed] [Google Scholar]
- 10. Ciaraldi TP, Aroda V, Mudaliar S, Chang RJ, Henry RR. 2009. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J Clin Endocrinol Metab 94:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beshay VE, Havelock JC, Sirianni R, Ye P, Suzuki T, Rainey WE, Carr BR. 2007. The mechanism for protein kinase C inhibition of androgen production and 17α-hydroxylase expression in a theca cell tumor model. J Clin Endocrinol Metab 92:4802–4809 [DOI] [PubMed] [Google Scholar]
- 12. Patel SS, Beshay VE, Escobar JC, Suzuki T, Carr BR. 2009. Molecular mechanism for repression of 17α-hydroxylase expression and androstenedione production in granulosa cells. J Clin Endocrinol Metab 94:5163–5168 [DOI] [PubMed] [Google Scholar]
- 13. Chazenbalk G, Chen YH, Heneidi S, Lee JM, Pall M, Chen YDI, Azziz R. 2012. Abnormal expression of genes involved in inflammation, lipid metabolism, and Wnt signaling in the adipose tissue of polycystic ovary syndrome. J Clin Endocrinol Metab 97:E765–E770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodarzi MO, Antoine HJ, Pall M, Cui J, Guo X, Azziz R. 2007. Preliminary evidence of glycogen synthase kinase 3β as a genetic determinant of polycystic ovary syndrome. Fertil Steril 87:1473–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zawadzki J, Dunaif A. 1992. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, eds. Polycystic ovary syndrome. Boston: Blackwell Scientific; 377–384 [Google Scholar]
- 16. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. 2004. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- 17. Yuan JS, Reed A, Chen F, Stewart CN., Jr 2006. Statistical analysis of real-time PCR data. BMC Bioinformatics 7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sasaki T, Kojima H, Kishimoto R, Ikeda A, Kunimoto H, Nakajima K. 2006. Spatiotemporal regulation of c-Fos by ERK5 and the E3 ubiquitin ligase UBR1, and its biological role. Mol Cell 24:63–75 [DOI] [PubMed] [Google Scholar]
- 19. Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. 1998. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA 95:14956–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simon JA, Lin F, Hulley SB, Blanche PJ, Waters D, Shiboski S, Rotter JI, Nickerson DA, Yang H, Saad M, Krauss RM. 2006. Phenotypic predictors of response to simvastatin therapy among African-Americans and Caucasians: the Cholesterol and Pharmacogenetics (CAP) Study. Am J Cardiol 97:843–850 [DOI] [PubMed] [Google Scholar]
- 21. Mannerås-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, Hellström M, Lönn L, Olivecrona G, Stener-Victorin E, Lönn M. 2011. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab 96:E304–E311 [DOI] [PubMed] [Google Scholar]
- 22. Faulds G, Rydén M, Ek I, Wahrenberg H, Arner P. 2003. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab 88:2269–2273 [DOI] [PubMed] [Google Scholar]
- 23. Dicker A, Rydén M, Näslund E, Muehlen IE, Wirén M, Lafontan M, Arner P. 2004. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia 47:420–428 [DOI] [PubMed] [Google Scholar]
- 24. Toulis KA, Goulis DG, Farmakiotis D, Georgopoulos NA, Katsikis I, Tarlatzis BC, Papadimas I, Panidis D. 2009. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update 15:297–307 [DOI] [PubMed] [Google Scholar]
- 25. Rosenbaum D, Haber RS, Dunaif A. 1993. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Physiol 264:E197–E202 [DOI] [PubMed] [Google Scholar]
- 26. Corbould A, Dunaif A. 2007. The adipose cell lineage is not intrinsically insulin resistant in polycystic ovary syndrome. Metabolism 56:716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corbould A. 2007. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol 192:585–594 [DOI] [PubMed] [Google Scholar]
- 28. Durchdewald M, Angel P, Hess J. 2009. The transcription factor Fos: a Janus-type regulator in health and disease. Histol Histopathol 24:1451–1461 [DOI] [PubMed] [Google Scholar]
- 29. Patel SS, Beshay VE, Escobar JC, Carr BR. 2010. 17α-Hydroxylase (CYP17) expression and subsequent androstenedione production in the human ovary. Reprod Sci 17:978–986 [DOI] [PubMed] [Google Scholar]
- 30. Puche C, José M, Cabero A, Meseguer A. 2002. Expression and enzymatic activity of the P450c17 gene in human adipose tissue. Eur J Endocrinol 146:223–229 [DOI] [PubMed] [Google Scholar]
- 31. Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, Bernard DJ. 2008. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology 149:5577–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ely HA, Mellon PL, Coss D. 2011. GnRH induces the c-Fos gene via phosphorylation of SRF by the calcium/calmodulin kinase II pathway. Mol Endocrinol 25:669–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao H, Leblanc SE, Wu Q, Konda S, Salma N, Marfella CG, Ohkawa Y, Imbalzano AN. 2011. Chromatin accessibility and transcription factor binding at the PPARγ2 promoter during adipogenesis is protein kinase A-dependent. J Cell Physiol 226:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma S, Sharma PM, Mistry DS, Chang RJ, Olefsky JM, Mellon PL, Webster NJ. 2011. PPARG regulates gonadotropin-releasing hormone signaling in LβT2 cells in vitro and pituitary gonadotroph function in vivo in mice. Biol Reprod 84:466–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. 1998. Life without white fat: a transgenic mouse. Genes Dev 12:3168–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herrera R, Ro HS, Robinson GS, Xanthopoulos KG, Spiegelman BM. 1989. A direct role for C/EBP and the AP-I-binding site in gene expression linked to adipocyte differentiation. Mol Cell Biol 9:5331–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Zegher F, Lopez-Bermejo A, Ibáñez L. 2009. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab 20:418–423 [DOI] [PubMed] [Google Scholar]
- 38. Setji TL, Holland ND, Sanders LL, Pereira KC, Diehl AM, Brown AJ. 2006. Nonalcoholic steatohepatitis and nonalcoholic fatty liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 91:1741–1747 [DOI] [PubMed] [Google Scholar]
- 39. Manna PR, Dyson MT, Stocco DM. 2009. Role of basic leucine zipper proteins in transcriptional regulation of the steroidogenic acute regulatory protein gene. Mol Cell Endocrinol 302:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao Y, Nichols JE, Valdez R, Mendelson CR, Simpson ER. 1996. Tumor necrosis factor-α stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol Endocrinol 10:1350–1357 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.