Abstract
Objective:
The aim was to provide a scholarly review of the published literature on biological, clinical, and nonclinical contributors to race/ethnic and sex disparities in endocrine disorders and to identify current gaps in knowledge as a focus for future research needs.
Participants in Development of Scientific Statement:
The Endocrine Society's Scientific Statement Task Force (SSTF) selected the leader of the statement development group (S.H.G.). She selected an eight-member writing group with expertise in endocrinology and health disparities, which was approved by the Society. All discussions regarding the scientific statement content occurred via teleconference or written correspondence. No funding was provided to any expert or peer reviewer, and all participants volunteered their time to prepare this Scientific Statement.
Evidence:
The primary sources of data on global disease prevalence are from the World Health Organization. A comprehensive literature search of PubMed identified U.S. population-based studies. Search strategies combining Medical Subject Headings terms and keyword terms and phrases defined two concepts: 1) racial, ethnic, and sex differences including specific populations; and 2) the specific endocrine disorder or condition. The search identified systematic reviews, meta-analyses, large cohort and population-based studies, and original studies focusing on the prevalence and determinants of disparities in endocrine disorders.
Consensus Process:
The writing group focused on population differences in the highly prevalent endocrine diseases of type 2 diabetes mellitus and related conditions (prediabetes and diabetic complications), gestational diabetes, metabolic syndrome with a focus on obesity and dyslipidemia, thyroid disorders, osteoporosis, and vitamin D deficiency. Authors reviewed and synthesized evidence in their areas of expertise. The final statement incorporated responses to several levels of review: 1) comments of the SSTF and the Advocacy and Public Outreach Core Committee; and 2) suggestions offered by the Council and members of The Endocrine Society.
Conclusions:
Several themes emerged in the statement, including a need for basic science, population-based, translational and health services studies to explore underlying mechanisms contributing to endocrine health disparities. Compared to non-Hispanic whites, non-Hispanic blacks have worse outcomes and higher mortality from certain disorders despite having a lower (e.g. macrovascular complications of diabetes mellitus and osteoporotic fractures) or similar (e.g. thyroid cancer) incidence of these disorders. Obesity is an important contributor to diabetes risk in minority populations and to sex disparities in thyroid cancer, suggesting that population interventions targeting weight loss may favorably impact a number of endocrine disorders. There are important implications regarding the definition of obesity in different race/ethnic groups, including potential underestimation of disease risk in Asian-Americans and overestimation in non-Hispanic black women. Ethnic-specific cut-points for central obesity should be determined so that clinicians can adequately assess metabolic risk. There is little evidence that genetic differences contribute significantly to race/ethnic disparities in the endocrine disorders examined. Multilevel interventions have reduced disparities in diabetes care, and these successes can be modeled to design similar interventions for other endocrine diseases.
Differences in the incidence, prevalence, mortality, and burden of diseases and other adverse health conditions among specific population groups exist throughout the world, in developed and developing nations alike. Such disparities in disease burden, comorbidities, and outcomes are a feature of many of the world's most prevalent disorders, including endocrine diseases. The underlying reasons for disparities vary depending on the condition in question and on the geographical region in which the disparities are identified. Nonetheless, regardless of region, there are a number of common factors contributing to these disparities. The global nature of health disparities makes this one of the most pressing issues facing science and medicine today.
This Scientific Statement will focus on population differences in adult endocrine disorders that, due to very high prevalence, the medical community has identified as significant public health burdens. These disorders include diabetes mellitus and related conditions (prediabetes and diabetic complications), gestational diabetes, metabolic syndrome with a focus on obesity and dyslipidemia, thyroid disorders, osteoporosis, and vitamin D deficiency (1). Because 95% of diabetes mellitus in the United States is characterized as type 2 diabetes (2) and type 2 diabetes presents a significant public health burden with race/ethnic disparities, in this statement we focus on type 2 diabetes.
Global prevalence data will be presented when it is available, but a comprehensive focus on worldwide disparities in these endocrine disorders is beyond the scope of this statement. Instead, we rely on information available on population differences in endocrine diseases within the United States. Nonetheless, global statistics on obesity will be included, but discussion of obesity will be in the context of type 2 diabetes and the metabolic syndrome. Health disparities in hormone-sensitive cancers and pediatric endocrine disorders are also beyond the scope of this statement. We will also not address health disparities in pituitary and reproductive endocrine disorders and endocrine hypertension because there are not yet sufficient population-based data to determine the presence of race/ethnic differences (1). Essential hypertension is an enormous public health concern; however, its management significantly overlaps with the fields of cardiology and nephrology. This statement will focus on disorders specifically managed by endocrinologists.
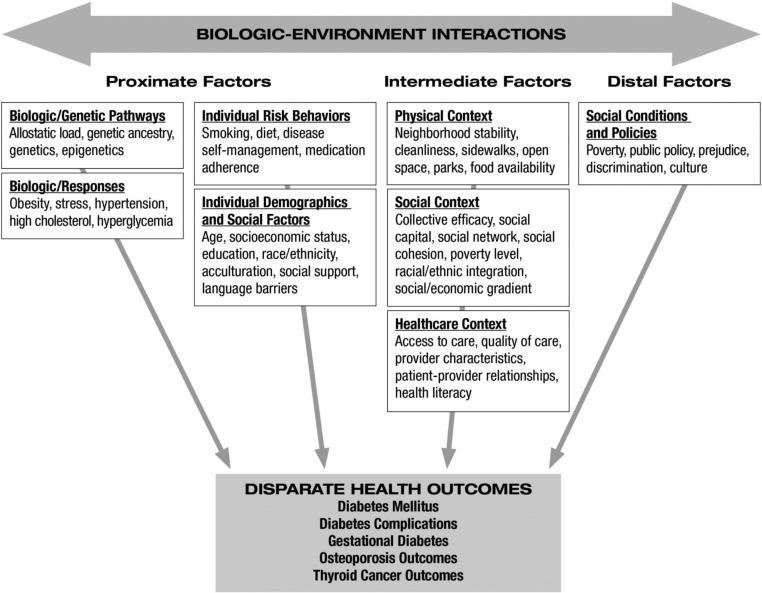
Even as we address biological differences related to race, ethnicity, or sex, we also discuss factors that contribute to health disparities and have been well documented for U.S. populations in reports from the Institute of Medicine. The Institute of Medicine report “Unequal Treatment” (3) published in 2002 remains the most comprehensive study of racial and ethnic disparities in health care in the United States. The report examined issues related to the health system, the provider, and the patient and assessed the extent of racial/ethnic differences in health care. For the 10 yr since its publication, racial and ethnic disparities in health and health care have persisted, especially type 2 diabetes and related complications and outcomes for thyroid cancers and metabolic bone disease. Data suggest that racial and/or ethnic minorities in the United States have less access to preventative care, treatment, and surgery, and as a result, they experience delayed diagnoses and more advanced disease at presentation. Combined, these factors contribute to disparate health outcomes (4).
In this statement, we also examine the impact of sex on endocrine disorders, particularly disorders that occur disproportionately in women (i.e. cardiovascular complications of diabetes, autoimmune thyroid disease, thyroid cancer, and osteoporosis). As we do this, we will use the term “sex” to describe health outcomes specific to being male or female according to reproductive organs and chromosomal complement, as opposed to “gender,” which refers to a person's self-representation as male or female (5). An Institute of Medicine report published in 2001, entitled “Exploring the Biological Contributions of Human Health: Does Sex Matter?” (5) highlighted the effect sex has on health care disparities. The report looked at the importance of promoting research on sex at the cellular level; determining how sex differences influence health, illness, and longevity across the lifespan; considering sex as a biological variable in all biomedical and health-related research; making sex-specific data more readily available; designing longitudinal studies so that sex-specific analyses can be conducted; and reducing the potential for discrimination based on sex difference (5).
Disparities due to income, education, geography, and other measures of socioeconomic status that may influence access to care will be discussed, where relevant, because these vulnerabilities often co-occur, making it difficult to disentangle potential biological contributions from other social influences on health disparities.
Definitions of Race and Ethnicity
We use Williams' definition of ethnicity as “a complex multidimensional construct reflecting the confluence of biological factors and geographical origins, culture, economic, political, and legal factors, as well as racism” (6). The concepts of “race” and “ethnicity” play important roles in understanding disparities in health and health care and are essential to the rigorous monitoring of progress toward eliminating these inequities (7, 8).
Overall, there was significant variation in the precise terms used to define specific race/ethnic groups in the literature reviewed. For consistency, we have used the terms “non-Hispanic black” to refer to individuals of African descent born and/or residing in the United States; “non-Hispanic white” for nonminority individuals; “Hispanic-American” for individuals of Mexican, South American, Cuban, or Puerto Rican descent born and/or residing in the United States; “Asian-American” for individuals of South Asian (e.g. Indian), East Asian (e.g. Japanese, Chinese, Korean), Southeast Asian (e.g. Cambodian, Vietnamese, Laotian, Thai), and Pacific Island (e.g. Filipino) descent born and/or residing in the United States; and “Native American” to refer to American Indians and Alaska Natives. We recognize that these categories may be arbitrary and sometimes contain heterogeneous groups. We have indicated in the text when studies were more specific in defining ethnic subgroups, particularly for Asian and Hispanic individuals. We also recognize that some individuals within every ethnic subgroup may be a mixture of native born and foreign born.
Literature Search Strategy
The primary sources of data on global disease prevalence are from the World Health Organization (WHO). In addition, we conducted a comprehensive literature search of PubMed to identify U.S. population-based studies. We used a combination of Medical Subject Headings (controlled vocabulary) terms and keyword terms and phrases to develop search strategies to define two concepts: the first was a search filter, defining racial, ethnic, and sex differences including specific populations; the second described a specific endocrine disorder or condition. We applied the filter to each separate disorder to optimize consistency. We identified systematic reviews, meta-analyses, large cohort and population-based studies, and original studies that focused on the prevalence and determinants of disparities in endocrine disorders. We limited all searches to the English language.
Diabetes Mellitus and Prediabetes
Disparities by race/ethnicity
Epidemiology
Diabetes mellitus.
More than 346 million people worldwide have diabetes mellitus, and it is predicted to become the seventh leading cause of death by the year 2030 (http://www.who.int/features/factfiles/diabetes/en/index.html). A 2010 comparison of 223 countries by the WHO showed that Nauru, the United Arab Emirates, and Saudi Arabia have the highest prevalence of diabetes, whereas Mongolia, Rwanda, and Iceland have the lowest (http://www.allcountries.org/ranks/diabetes_prevalence_country_ranks.html). Additional WHO analysis has shown that prevalence rates are similar across income groupings of countries (ranging from 8% in adults of both sexes 25 yr and older in the low-income countries to 10% in the same demographic in the upper middle-income countries) (http://www.who.int/nmh/publications/ncd_report_chapter1.pdf), but death from diabetes is higher in low- and middle-income countries, with roughly 80% of deaths from diabetes occurring in these countries (http://www.who.int/features/factfiles/diabetes/en/index.html).
In the United States, roughly 25.8 million individuals (8.3% of U.S. population) have diabetes mellitus (http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf). Although 18.8 million are diagnosed, another 7 million remain undiagnosed (http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf). These undiagnosed individuals have an elevated fasting plasma glucose but lack a prior physician diagnosis. The prevalence of diagnosed diabetes in adults over 20 yr of age (summarized in Table 1) is highest among non-Hispanic blacks (NHBs) (12.6%), Mexican-Americans (13.3%), Puerto Rican-Americans (13.8%), and Native Americans (16.1%) (http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf). The prevalence of diagnosed diabetes is similar among non-Hispanic whites (NHWs), Asian-Americans, Cuban-Americans, Central Americans, and South Americans. It is important to note that diabetes prevalence in Hispanic-Americans is influenced by the country of origin and primarily driven by the high prevalence in people of Mexican and Puerto Rican descent. Similarly, diabetes prevalence is lowest in Alaska Natives (5.5%) but very high in Native Americans (approaching 33%) (http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf). In the National Health and Nutrition Examination Survey (NHANES), over 40% of NHWs and Mexican-Americans with diabetes were undiagnosed, whereas 24% of NHBs were undiagnosed (13), suggesting race/ethnic bias in diagnoses based on fasting glucose vs. known physician diagnosis. Estimates based only on physician diagnosis may underestimate diabetes in certain race/ethnic groups. In addition, a recent study suggested that using glycosylated hemoglobin (HbA1c) to determine diabetes prevalence may lead to an underestimate in some race/ethnic groups (14) and an overestimate in others (15) (see Glycemic Control section under Race/Ethnic Differences in Biological Factors Contributing to Complications).
Table 1.
Age-adjusted prevalence of diagnosed diabetes mellitus in the United States by race/ethnicity in adults ≥20 yr of age
| Race/ethnic group | Age-adjusted prevalence (%) |
|---|---|
| NHWs | 7.1 |
| Asian-Americans | 8.4 |
| Hispanic-Americans overall | 11.8 |
| Cuban, Central, and South Americans | 7.6 |
| Mexican-Americans | 13.3 |
| Puerto Rican-Americans | 13.8 |
| NHBs | 12.6 |
| Native Americans and Alaska Natives | 16.1 |
Reproduced from National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the U.S., 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, 2011 (12), with permission.
As of 2010, approximately 1.9 million individuals over 20 yr of age in the United States developed incident diabetes (12). Compared with NHWs, the risk of incident diabetes was 18% higher in Asian-Americans, 66% higher in Hispanic-Americans, and 77% higher in NHBs (12). A significantly higher risk in Mexican-Americans (87%) and Puerto Rican-Americans (94%) drove the higher risk among Hispanic-Americans, compared with NHWs (12). The risk of developing diabetes is similar in Cuban-Americans, Central Americans, and South Americans, compared with NHWs (12).
The National Health Interview Survey contains the only estimates of the prevalence of type 2 diabetes in Asian-Americans in the United States. It revealed that there is significant ethnic variation in the prevalence of type 2 diabetes, which is highest among Asian-Indians (close to 15%) and Filipinos (close to 10%) and lowest and among Koreans, Vietnamese, and Chinese (3–7%) in the United States (16), consistent with the known higher diabetes prevalence in individuals living in India, South Asia, and the Philippines (10).
Prediabetes.
Prediabetes is an important risk factor for developing type 2 diabetes and cardiovascular disease (CVD). The age-adjusted prevalence of prediabetes in adults 20 yr of age or older in 2005–2008 (based on fasting glucose or HbA1c) was similar among NHWs, NHBs, and Mexican-Americans, at approximately 35% (12). Based on fasting glucose only, 20% of Native Americans 15 yr of age or older had prediabetes in 2001–2004 (12).
The Diabetes Prevention Program enrolled approximately 3000 multiethnic individuals with impaired glucose tolerance diagnosed using a 75-g oral glucose tolerance test. In this important study, the risk of incident diabetes (approximately 11%) was the same in NHBs, Asian-Americans and Pacific Islanders, NHWs, Hispanic-Americans, and Native Americans (17). These data suggest that once individuals progress from normal glucose tolerance to impaired glucose tolerance, the risk for further progression to diabetes is the same across ethnic groups. Thus, the progression from normal glucose tolerance to impaired glucose tolerance rather than from impaired glucose tolerance to diabetes appears to account for race/ethnic differences in type 2 diabetes risk.
Race/ethnic differences in biological factors
Obesity and fat distribution.
Obesity is one of the strongest risk factors for developing type 2 diabetes and is a major, and growing, public health problem (2). In 2008 worldwide prevalence statistics, the WHO estimated that 500 million adults 20 and older were obese, a figure representing more than one in 10 individuals worldwide (http://www.who.int/mediacentre/factsheets/fs311/en/).The 2010 WHO prevalence data show the highest rates of obesity in Nauru, Cook Islands, and Micronesia for men aged 15 yr and older, and in Nauru, Tonga, and Micronesia among women of the same age (https://apps.who.int/infobase/Comparisons.aspx?l=&NodeVal=WGIE_BMI_5_cd.0704&). The United States ranked fifth highest in male obesity, at 44.2%, and 12th in female obesity (tied with Jamaica), at 48.3%.
In the United States, obesity prevalence varies by race/ethnicity. Recent data from the 2009–2010 NHANES showed that the age-adjusted prevalence of obesity in adults 20 yr of age and older [body mass index (BMI) greater than or equal to 30 kg/m2] was highest among NHBs (49.5%), followed by Mexican-Americans (40.4%) and NHWs (34.3%) (20). Of note, the prevalence of grade 2 (BMI of at least 35 kg/m2) and grade 3 obesity (BMI greater than or equal to 40 kg/m2) was highest among NHBs (26% for grade 2, and 13.1% for grade 3), compared with NHWs (14.4% for grade 2, and 5.7% for grade 3) and Mexican-Americans (14.9% for grade 2, and 5.4% for grade 3) (20). In general, as discussed below (see Race/ethnic and sex differences in body fat distribution—implications for defining metabolic syndrome section under Metabolic Syndrome), NHB and Mexican-American women are more affected by obesity and have had a greater rise in obesity prevalence over the last 12 yr than NHB and Mexican-American men and NHW men and women. Race/ethnic differences in obesity contribute to the higher risk of type 2 diabetes in NHBs and Mexican-Americans; however, as discussed further below (see Race/ethnic and sex differences in body fat distribution—implications for defining metabolic syndrome section under Metabolic Syndrome), NHB women have greater sc fat compared with visceral fat than NHW women at a given BMI and waist circumference (WC) and may therefore develop type 2 diabetes at a higher BMI than NHW women. Although there are less data on Native Americans and Alaska Natives, data from the 2007 National Health Interview Survey showed that both groups have a higher prevalence of obesity (33.2%) than their NHW counterparts (24.8%) (21), which likely contributes to their higher risk of type 2 diabetes.
Among Asian-Americans, there is variation in the prevalence of overweight and obesity (Table 2). Although Asian Indians in the United States have one of the highest prevalence of type 2 diabetes, their prevalence of obesity is only 6%; however, they have a 30 to 35% prevalence of overweight. In contrast, Filipinos have a similarly high prevalence of type 2 diabetes, and also have a high prevalence of obesity. Compared with Chinese and Vietnamese individuals, Asian Indians and Filipinos in the United States have the highest rates of overweight (BMI, 25 to <30 kg/m2). These data suggest that body weight alone is not the only contributor to increased diabetes risk in Asian-Americans. Differences in body fat distribution are important contributors to diabetes risk in Japanese-Americans, a well-studied Asian population. Despite a lower average BMI in participants in the Japanese-American Community Diabetes Study, compared with the overall U.S. population, there was a higher incidence of type 2 diabetes in Japanese-Americans (16). This study linked larger visceral abdominal fat in this population with incident diabetes, independent of age, sex, and sc fat (16). Similarly, visceral adiposity is an important determinant of diabetes risk in Filipinos. In the University of San Diego Filipino Health Study, Filipino women had more visceral adipose at each level of WC and a higher prevalence of type 2 diabetes than NHWs (16).
Table 2.
Age-adjusted prevalence of overweight and obesity by Asian-American ethnicity from the National Health Interview Survey (16)
| Race/ethnic group | Age-adjusted overweight prevalence (%) | Age-adjusted obesity prevalence (%) |
|---|---|---|
| Chinese | 21.8 | 4.2 |
| Filipino | 33.0 | 14.1 |
| Asian Indian | 34.4 | 6.0 |
| Japanese | 25.9 | 8.7 |
| Vietnamese | 19.1 | 5.3 |
| Korean | 27.3 | 2.8 |
| Other Asian and Native Hawaiian or other Pacific Islander | 29.2 | 12.5 |
Glucose metabolism and insulin resistance.
The higher prevalence of type 2 diabetes in minorities compared with NHW populations is partially attributable to differences in glucose metabolism and homeostasis (22), as summarized in Table 3. Compared with their NHW counterparts and independent of adiposity, NHBs have greater hyperinsulinemia (23–25) and insulin resistance, assessed by the iv glucose tolerance test (23, 26–28), hyperglycemic clamp (29), or homeostasis model assessment of insulin resistance (30). The majority of studies that have assessed a measure of β-cell function indicate that, compared with NHWs, NHBs augment insulin secretion to compensate for insulin resistance (23, 26–31).
Table 3.
Features of glucose metabolism in minority populations relative to NHWs
| First author, year (Ref.) | Population | Method of assessing glucose metabolism | Findings relative to NHWs |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fasting glucose | 2-h post-load glucose | Fasting insulin | 2-h post-load insulin | Insulin-mediated glucose disposal or other measures of insulin sensitivity | AIR or other measure of β-cell function | Other | ||||||
| NHBs | ||||||||||||
| Haffner, 1996 (23) | IRAS; n = 288 NHBs, 435 NHWs nondiabetic | 2-h OGTT; insulin-modified frequently sampled iv glucose tolerance test | ↑ | ↑ | ↓ | ↑ | Differences persisted after adjustment for obesity, body fat distribution, and behavioral factors | |||||
| Donahue, 1997 (24) | Miami Community Health Study; n = 159 NHBs, 207 NHWs | 75-g OGTT (insulin area under the curve) | ↑ | Persisted after adjustment for percentage body fat | ||||||||
| Chiu, 2001 (29) | n = 28 NHBs, 45 NHWs | Hyperglycemic clamp | ↓ | ↑ | Persisted after adjustment for age, sex, BMI, WHR, and blood pressure | |||||||
| Carnethon, 2002 (25) | ARIC Study; n = 13,416 NHB and NHW nondiabetic men and women | Fasting insulin | ↑ | Fasting insulin was significantly higher among nonobese NHB compared to NHW women, but there was no difference among men | ||||||||
| Jensen, 2002 (31) | First-degree relatives of individuals with type 2 diabetes; n = 55 NHBs, 217 NHWs | 2-h OGTT; HOMA-IR estimated insulin resistance; ratio of incremental insulin to glucose responses over first 30 min (ΔI30/ΔG30) | ↑ | Adjustment for HOMA-IR explained difference in AIR between Hispanics and NHWs | ||||||||
| Torrens, 2004 (30) | SWAN study; nondiabetic pre- and perimenopausal women; n = 746 NHBs, 1,359 NHWs | HOMA %S (insulin sensitivity), HOMA %β (β-cell function) | ↓ | ↑ | Difference in HOMA %S and HOMA %β persisted after adjustment for WC, presence of IFG, TG, and site | |||||||
| Albu, 2005 (26) | n = 32 NHB women, 28 NHW women | Intravenous glucose tolerance test | ↓ | ↑ | Difference in insulin sensitivity persisted after adjustment for weight, height, and MRI-assessed intramuscular adipose tissue, but was attenuated after adjustment for MRI-assessed skeletal muscle volume. Differences in AIR persisted after multivariable adjustment | |||||||
| Rasouli, 2007 (27) | n = 55 NHBs, 85 NHWs; healthy, obese nondiabetic individuals | Intravenous glucose tolerance test | ↓ | ↑ | NHBs also had higher disposition index but lower maximum disposition index than age-, sex-, and BMI-matched NHWs | |||||||
| Chow, 2011 (28) | n = 17 NHB women, 17 NHW women; healthy, obese | Insulin-modified frequently sampled iv glucose tolerance test | ↓ | ↑ | NHB women had higher free fatty acid clearance after adjusting for fat mass. Additional adjustment for AIR attenuated the association | |||||||
| Hispanic-Americans | ||||||||||||
| Boyko, 1991 (32) | n = 464 Hispanic-Americans, 676 NHWs; normal glucose tolerance | Fasting, 1-h and 2-h glucose insulin from OGTT | ↑ | ↑ | Differences in fasting, 1-h, and 2-h insulin persisted after adjustment for age, sex, BMI, WHR, family history of diabetes, and glucose. ↑ Fasting and 1-h and 2-h glucose-stimulated C-peptide and C-peptide:insulin molar ratio greater in Hispanics than NHWs | |||||||
| Haffner, 1996 (23) | IRAS; n = 363 Hispanic-Americans, 435 NHWs; nondiabetic | 2-h OGTT; insulin-modified frequently sampled iv glucose tolerance test | ↑ | ↑ | ↓ | ↑ | Differences in 2-h insulin and AIR persisted after adjustment for obesity, body fat distribution, and behavioral factors, but differences in insulin sensitivity index did not persist | |||||
| Donahue, 1997 (24) | Miami Community Health Study; n = 128 Cuban-Americans, 207 NHWs | 75-g OGTT (insulin area under the curve) | ↑ | Persisted after adjustment for percentage body fat | ||||||||
| Chiu, 2001 (29) | n = 20 Mexican-Americans, 45 NHWs | Hyperglycemic clamp | ↓ | ↑ | Persisted after adjustment for age, sex, BMI, WHR, and blood pressure | |||||||
| Jensen, 2002 (31) | First-degree relatives of individuals with type 2 diabetes; n = 193 Hispanic-American, 217 NHWs | 2-h OGTT, HOMA-IR estimated insulin resistance, ratio of incremental insulin to glucose responses over first 30 min (ΔI30/ΔG30) | ↓ | ↑ | Difference in insulin resistance persisted after adjustment for BMI. Adjustment for HOMA-IR explained difference in AIR between Hispanics and NHWs | |||||||
| Ferrannini, 2003 (33) | n = 172 Mexican-Americans, 60 NHWs; NGT or diabetes | Insulin sensitivity of glucose uptake by clamp technique; endogenous glucose production by 3-[3H] glucose infusion; insulin secretory response to oral glucose | ↓ | No difference | Persisted after adjustment for BMI, age, diabetes, and family history of diabetes | |||||||
| Torrens, 2004 (30) | SWAN study; nondiabetic pre- and perimenopausal women; n = 218 non-Mexican-American Latinos, 1,359 NHWs | HOMA %S (insulin sensitivity); HOMA %β (β-cell function) | No difference | No difference | Lack of difference in HOMA %S persisted after adjustment for WC, presence of IFG, TG, and site | |||||||
| Asian-Americans and Pacific Islanders | ||||||||||||
| Chiu, 2001 (29) | n = 28 Asian-Americans, 45 NHWs | Hyperglycemic clamp | ↓ | ↑ | Persisted after adjustment for age, sex, BMI, WHR, and blood pressure | |||||||
| Jensen, 2002 (31) | First-degree relatives of individuals with type 2 diabetes; n = 66 Asian-Americans, 217 NHWs | 2-h OGTT; HOMA-IR estimated insulin resistance; ratio of incremental insulin to glucose responses over first 30 min (ΔI30/ΔG30) | ↑ | ↓ | Less insulin resistance for Asian-Americans compared to NHWs and other ethnic groups (NHB, Hispanic-American); persisted after adjustment for BMI. AIR was lower in Asian-Americans compared to other ethnic groups but was attenuated after adjustment for HOMA-IR | |||||||
| Torrens, 2004 (30) | SWAN study; nondiabetic pre- and perimenopausal women; n = 210 Chinese-Americans, 255 Japanese-Americans, 1,359 NHWs | HOMA %S (insulin sensitivity), HOMA %β (β-cell function) | ↓ | ↓ | Difference in HOMA %S persisted after adjustment for WC, presence of IFG, and site, but was attenuated after adjustment for TG. Differences in HOMA %β persisted after multivariable adjustment | |||||||
| Native Americans | ||||||||||||
| Resnick, 2002 (34) | Strong Heart Study; n = 61 Native Americans (compared to NHWs in IRAS) | Frequently sampled iv glucose tolerance test | ↓ | Mean insulin sensitivity index of nondiabetic Native Americans was lower than that of nondiabetic counterparts in IRAS (with exception of some Hispanic-Americans). The insulin sensitivity index of diabetic Native Americans was lower than that of all diabetic IRAS participants | ||||||||
AIR, Acute insulin response; ARIC, Atherosclerosis Risk in Communities Study; HOMA-IR, homeostatic model assessment of insulin resistance; IFG, impaired fasting glucose; IRAS, Insulin Resistance Atherosclerosis Study; MRI, magnetic resonance imaging; NGT, normal glucose tolerance; OGTT, oral glucose tolerance test; SWAN, Study of Women Across the Nation; WHR, waist-hip ratio;
, increased compared to NHWs;
, decreased compared to NHWs.
The majority of studies have shown that, compared with NHWs, Hispanic-Americans of Mexican descent also have greater hyperinsulinemia (23, 32) and insulin resistance, as assessed by the iv glucose tolerance test (23), hyperglycemic clamp (29), or homeostasis model assessment of insulin resistance (31), independent of adiposity. Although most studies show increased β-cell insulin secretion (23, 29, 31), one study found no difference between the two race groups (33). Studies in non-Mexican Hispanic-Americans have yielded conflicting results. A study of Cuban-Americans showed a greater insulin response to an oral glucose tolerance test, compared with NHWs, independent of percentage body fat (24); however, another study of non-Mexican Hispanic-Americans showed no difference in insulin sensitivity or β-cell function between the two groups (30).
Few studies have been conducted in Asian-Americans and Native Americans. With the exception of one study that found lower insulin resistance (31), research has indicated that Asian-Americans have greater insulin resistance than NHWs, assessed by the hyperglycemic clamp (29) and homeostatic model assessment insulin sensitivity (30), after adjustment for adiposity. One study showed a compensatory higher degree of β-cell insulin secretion (29); however, two studies showed lower β-cell insulin secretion compared with NHWs (30, 31). The reduced β-cell insulin response, in the setting of insulin resistance, likely contributes to the increased risk of diabetes seen in Asian-Americans. Finally, the Strong Heart Study assessed Native Americans using the iv glucose tolerance test and compared their insulin sensitivity to results from the Insulin Resistance Atherosclerosis Study of nondiabetic and diabetic NHWs, NHBs, and Hispanic-Americans (34). For Native Americans, the study showed reduced insulin sensitivity, relative to NHWs, in those without diabetes and reduced insulin sensitivity, relative to NHWs, NHBs, and Hispanic-Americans, in those with diabetes (34). Similarly, a separate study showed reduced insulin sensitivity for nondiabetic Pima Indians relative to NHWs (35).
Although beyond the scope of this statement, there are metabolic studies that have attempted to identify contributors to ethnic differences in insulin sensitivity by race/ethnicity, many of which have focused on differences between NHB and NHW women. Studies have shown that, compared with NHW women, NHB women who were fed a high-fat diet failed to shift metabolism to increase fat oxidation or decrease carbohydrate metabolism, despite increases in their insulin levels (36). This “metabolic inflexibility” may contribute to their higher risk of obesity and insulin resistance. Reduced fat oxidation can promote accumulation of lipids in skeletal muscle and development of insulin resistance (37). Compared with NHW women, NHB women have reduced muscle oxidative capacity associated with insulin resistance, suggesting the presence of skeletal muscle mitochondrial dysfunction as a potential contributor to insulin resistance (38, 39). A recent study suggested that lower levels of 25-hydroxyvitamin D in NHBs, compared with NHWs, explained differences in insulin sensitivity between the two races. Whether these hypothesized mechanisms are operative in other race/ethnic groups requires further research in clinical and population-based studies.
Genetics.
Recent reviews have chronicled the ongoing evolution of research on susceptibility genes for type 2 diabetes. Association studies initially revealed susceptibility genes in common coding variants in PPARG and KCNJ11; however, genome-wide association studies (GWAS) (the current systematic approach to identifying type 2 diabetes susceptibility genes) have been most successful (40). Table 4 summarizes results of studies that have linked gene variants with 15–20% increased risk of diabetes along with their proposed pathways (40). In 2010, there were approximately 40 confirmed loci for which variants increase risk of type 2 diabetes (40). The GWAS Catalog of the National Human Genome Research Institute (www.genome.gov) summarizes currently identified loci and their nearby genes.
Table 4.
Genes with GWAS-identified genetic loci associated with insulin secretion and resistance and conferring a 15–20% increased risk of type 2 diabetes
| Genes | |
|---|---|
| Genes associated with reduced insulin secretion | |
| Reduced β-cell mass | CDKAL1, CDKN2A, CDKN2B |
| β-cell dysfunction | MTNR1B, TCF7L2, KCNJ11 |
| Genes associated with insulin resistance | |
| Obesity | FTO |
| Insulin resistance unrelated to obesity | IRS1, PPARG |
Although most of the early studies focused on individuals of European descent, several recent studies have demonstrated that these susceptibility loci are also present and associated with increased type 2 diabetes risk in ethnic minority populations. To date, the limited amount of data in non-European ancestry populations does not suggest that the genetic architecture of type 2 diabetes differs across race/ethnic groups. Two large GWAS analyses in multiethnic populations have found that common variants discovered in European populations are also significantly associated with type 2 diabetes in ethnic minorities (41). In one study, 14 of 19 allelic variants, reproducibly associated with type 2 diabetes in European populations, had a significant link to type 2 diabetes in NHBs, Hispanic-Americans, Japanese-Americans, and Native Hawaiians (41). The other study (which pooled data from 39 multiethnic population-based studies, case-control studies, and clinical trials) showed a significant link between a composite genetic score of single nucleotide polymorphisms (SNP) from new and established type 2 diabetes signals and diabetes risk in NHB, Hispanic-American, and Asian-American populations (42). A study in NHANES III (examining 16 novel fasting glucose-associated SNP in multiple genes in NHWs, Mexican-Americans, and NHBs) showed that whereas allele frequency varied by race/ethnicity, there was a significant link between a weighted genetic score and increased fasting glucose and odds for impaired fasting glucose in all ethnicities (43). Together, these data suggest that common genetic variants contribute similarly to diabetes risk across race/ethnicities.
Until recently, there were no GWAS analyses in non-European populations; however, several studies have identified novel diabetes-associated SNP in specific race/ethnic groups (Table 5). In a study of individuals from Mexico City and Mexican-Americans from Texas, there were signals for type 2 diabetes corresponding to previously described regions; however, the study identified additional loci that were not replicated in the Diabetes Genetics Replication and Meta-Analysis Consortium, suggesting novel loci that may be linked to type 2 diabetes risk in Hispanic populations (44). As summarized in Table 4, recent GWAS analyses in South Asians, East Asians, and NHBs have identified new loci linked with type 2 diabetes in those race/ethnic groups (45–47).
Table 5.
Novel GWAS-identified loci associated with type 2 diabetes in non-European populations
| Population | Newly identified SNP | Chromosome | Associated gene(s) | Associated physiological function |
|---|---|---|---|---|
| South Asians (45) | rs3923113 | 2 | GRB14 | Associated with insulin sensitivity |
| rs16861329 | 3 | ST6GAL1 | Associated with pancreatic β-cell function | |
| rs1802295 | 10 | VPS26A | ||
| rs7178572 | 15 | HMC20A | ||
| rs2028299 | 15 | AP3S2 | ||
| rs4812829 | 20 | HNF4A | Associated with pancreatic β-cell function | |
| East Asians (46) | ||||
| Loci with strong associations | rs6815464 | 4 | MAEA | |
| rs7041847 | 9 | GLIS3 | Associated with pancreatic β-cell development, insulin gene expression, and glucose | |
| rs6017317 | 20 | FITM2-R3HDML-HNF4A | ||
| rs6467136 | 7 | GCC1-PAX4 | ||
| rs831581 | 3 | PSMD6 | ||
| rs9470794 | 6 | ZFAND3 | ||
| rs3786897 | 19 | PEPD | ||
| rs1535500 | 6 | KCNK16 | May regulate glucose-independent insulin secretion in the pancreas | |
| Loci with moderate associations | rs16955379 | 16 | CMIP | |
| rs17797882 | 16 | WWOX | ||
| NHBs (47) | ||||
| Loci with strong associations | Rs7560163 | 2 | RBM43, RND3 | |
| Loci with nominal associations | Rs7542900 | 1 | SLC44A3, F3 | |
| Rs4659485 | 2 | RYR2, MTR | ||
| Rs2722769 | 11 | GALNTL4, LOC729013 | ||
| Rs7107217 | 11 | TMEM45B, BARX2 |
Individual race/ethnic differences in nonbiological factors
Acculturation.
Acculturation is “the process by which immigrants adopt the attitudes, values, customs, beliefs, and behaviors of a new culture” and contributes to health behaviors, obesity, and type 2 diabetes in immigrant populations (48). In Hispanic-American immigrants, studies have linked acculturation with a change in lifestyle choices, including poorer nutrition and more tobacco use. However, studies have also linked acculturation with a higher socioeconomic position, more access to health care, and more leisure-time physical activity, which may have differential effects on health outcomes (48). Studies have shown that acculturation in Hispanic-American immigrants results in an increase in dietary habits that promote obesity, such as a higher consumption of sugar and sugar-sweetened beverages, higher solid fat intake, higher sugar intake, more eating out, and more fast food consumption. Studies have also shown higher sodium intake, higher salty snack consumption, and a lower fruit and vegetable intake (48). A study comparing obesity trends in U.S.-born vs. immigrant Hispanic-Americans in NHANES showed that from 1995 to 2005, obesity rates increased significantly more in age-matched U.S.-born individuals relative to immigrants (49). The association of acculturation with type 2 diabetes, however, is mixed and inconsistent. Although acculturation appears to promote obesity, its association with diabetes may be offset by its positive influence on physical activity and access to care, in that screening and intervention occur sooner (48). The Multi-Ethnic Study of Atherosclerosis links greater acculturation with a higher prevalence of diabetes among non-Mexican-origin Hispanic-Americans, but not among Mexican-origin Hispanic-Americans (50). The association in non-Mexican-origin Hispanic-Americans was independent of socioeconomic status and partially attenuated with adjustment for BMI and diet (50).
Acculturation and the duration of time in the United States might also influence the degree of obesity in Asian immigrants. Japanese individuals genetically predisposed to diabetes have a higher rate of incident diabetes in an obesity-promoting environment. This is evidenced by Japanese immigrants to the United States having a 3-fold higher rate of type 2 diabetes compared with their native Japanese counterparts (22). The Multi-Ethnic Study of Atherosclerosis, however, did not link acculturation with type 2 diabetes prevalence in Chinese-Americans (50). The role of acculturation in the development of obesity and type 2 diabetes in other Asian-American subgroups is unclear.
Health behaviors.
Physical activity is an important intervention for reducing diabetes risk in all race/ethnic groups (17). NHBs, Native Americans, and Alaska Natives report less leisure-time physical activity than NHWs (51). Mexican-American women report less leisure-time physical activity than NHW and NHB women (51). Thus, lower levels of physical activity in ethnic minority populations is an important contributor to their elevated risk of obesity and subsequent diabetes. There are sparse data currently on physical activity in Asian-American populations (16).
Smoking is a risk factor for development of type 2 diabetes (52). Native Americans and Alaska Natives have a higher prevalence of smoking that NHWs (51). NHBs and NHWs have similar smoking rates, whereas Mexican-Americans have significantly lower smoking rates. Thus, higher rates of smoking may contribute to the elevated diabetes risk seen in Native American populations. In Asian-Americans, there is variation in rates of smoking, with the highest prevalence among Korean men and the lowest prevalence among Asian Indian men (16). It is unclear whether smoking contributes to diabetes risk in other Asian-American subgroups.
Interface of environmental and clinical/biological factors: epigenetics and early life events
As recently reviewed (53), early life conditions, such as prenatal undernutrition and stress, maternal stress, or maternal obesity during pregnancy, can modify the developmental biology in offspring, leading to a future increased risk of developing obesity and type 2 diabetes. Overweight and diabetic women give birth to overweight, diabetes-prone offspring, likely through intrauterine influences on development, which might explain higher rates of diabetes in South Asian and Native American populations (53); however, additional work, summarized below, has shown low birth weight to be strongly associated with type 2 diabetes risk.
NHBs have lower birth weight than NHWs, which may be related to maternal conditions during pregnancy, including stressful life events, depressive and anxiety symptoms, economic inequality (e.g. lower income and education, less access to health care in NHBs), racial discrimination, residential segregation, and neighborhood-level poverty (53). Maternal hypertension, which is more prevalent in NHB women, can also lead to low birth weight (53). Low birth weight seems to be unique to NHBs because infants born to women of other minority races/ethnicities in similar socioeconomic circumstances are not small compared with NHWs (54).
In response to undernutrition, researchers believe that the fetus will slow its growth rate and modify the structure and function of organs and systems involved in metabolism, leading to metabolic dysfunction after birth (53). Animal studies have shown that restricting nutritional intake in pregnant rats, mice, or sheep or directly restricting blood flow to the fetus increases offspring blood pressure, cholesterol, abdominal fat deposition, and diabetes risk (53). Human studies have demonstrated similar findings, linking smaller birth weight to insulin resistance and diabetes, abdominal pattern of fat distribution, higher blood pressure, abnormal lipid profiles, and increased CVD risk (53). Studies have also linked low birth weight with elevated cortisol reactivity in childhood and adolescence (53). And chronic cortisol exposure can contribute to abdominal adiposity and insulin resistance (55).
Maternal psychological stress and fetal overexposure to cortisol lead to the same metabolic abnormalities as fetal undernutrition (53). Under normal conditions, placental 11-β hydroxysteroid dehydrogenase protects the fetus from maternal cortisol by converting cortisol to inactive cortisone. However, in a severely stressed mother, excessive stress hormones can reduce the effectiveness of this protection, exposing the fetus to maternal cortisol (53). This can lead to offspring with elevated blood pressure, stress reactivity, abdominal adiposity, insulin resistance, and other diabetes and CVD precursors (53).
Epigenetic changes in the pattern of cellular gene expression may influence how a fetus adapts to an adverse intrauterine environment (53). Epigenetic changes are those that “modify patterns of gene expression without changing the nucleotide sequence of its DNA” and may represent an adaptation to a significant prior stressor (53). Restricting the protein intake of pregnant rats resulted in decreased methylation of the promoter region of the glucocorticoid receptor in offspring liver cells, increased glucocorticoid gene expression, and increased hepatic glucocorticoid receptor expression. As a result, these offspring had a heightened hepatic response to stress hormones, causing increased gluconeogenesis in response to stress (53). The same model saw a decrease in methylation of the angiotensinogen receptor gene in the offspring adrenal glands, leading to increased systolic blood pressure (53). In human studies of holocaust survivors with posttraumatic stress disorders, their disease severity predicts the pattern of cortisol secretion in their offspring. Similarly, the offspring of women who were pregnant in Manhattan on September 11, 2001, show altered hypothalamic-pituitary-adrenal axis activity in childhood (53). In the future, studies in humans that examine genetic methylation patterns will help us determine how epigenetic changes contribute to the role of the fetal environment in race/ethnic differences in future risk of obesity and type 2 diabetes (56).
Summary, implications, and future research needs
The literature shows that insulin resistance is a key contributor to type 2 diabetes risk in NHB, Mexican-American, Asian-American, and Native American populations, indicating that diabetes prevention and treatment strategies should focus on improving insulin sensitivity. Basic science studies are needed to determine whether race/ethnic differences in insulin signaling contribute to the lower insulin sensitivity in minority populations. Because studies suggest that Asian-Americans have reduced β-cell function, this represents an additional prevention and treatment target for this population. The reduced β-cell function in Asian-Americans may also explain why they are at high risk for diabetes at lower levels of BMI (30). Finally, the available literature suggests that there may be differences in glucose metabolic features among Hispanic-Americans from different countries of origin. This may explain the higher prevalence of type 2 diabetes in Mexican-Americans compared with those of Cuban-American and South American descent; however, because few studies have included non-Mexican Hispanic-American populations or specified the countries of origin, additional research is needed to further evaluate the presence of and contributors to these metabolic differences.
Genetic studies suggest that susceptibility loci for type 2 diabetes discovered in European populations are also operative in ethnic minority populations and that race/ethnic differences are unlikely to be explained by genetic variation; however, recent GWAS analyses have revealed potential ethnic-specific loci. Future GWAS analyses should include Native American, Hispanic-American, and NHB populations. Also, because genetic admixture may mask differences attributable to race/ethnicity, future genetic studies should also use ancestral markers to account for admixture. Nonclinical factors, such as acculturation and health behaviors, also appear to be important contributors to obesity and diabetes risk in ethnic minority populations and warrant further study because they may be targets for future preventive interventions. Finally, low birth weight, fetal undernutrition, and maternal-fetal stress appear to be unique contributors to elevated diabetes and metabolic risk in NHBs and represent a potential early target for diabetes preventive interventions.
Disparities by sex
The prevalence of diabetes mellitus has increased over the past decade in both women and men (57). Factors that have driven this trend include increasing obesity [affecting 53% of the diabetic population in 2007 (58)] and sedentary lifestyle [affecting 33% of the diabetic population in 2007 (59)]. In 2009, 6.6% of men had diabetes compared with 5.9% of women (57), with a similar median age of onset of 60 to 70 yr. However, diabetes most commonly affects NHB women (60). We have already reviewed disparities by race/ethnicity.
Complications of Diabetes Mellitus
Disparities by race/ethnicity
Microvascular complications
Epidemiology.
Retinopathy.
Ethnic minority populations are more likely than NHWs to experience diabetic retinopathy and visual impairment. The presence of any diabetic retinopathy is 46% higher in NHBs and 84% higher in Mexican-Americans, compared with NHWs. NHBs and Mexican-Americans are also more likely to have moderate to severe retinopathy (61). Compared with NHWs, the risk of visual impairment secondary to retinopathy is 4-fold higher in NHBs; and Hispanic-Americans have higher rates of severe vision-threatening diabetic retinopathy than NHWs (62). Although both of these populations have high rates of diabetic retinopathy, there is some suggestion that the phenotype of disease may be different in NHBs and Hispanic-Americans. In one study, both groups had a similar prevalence of clinically significant macular edema and diabetic retinopathy grades; however, Hispanic-Americans had more prevalent intraretinal hemorrhages involving a greater area of the retina (63). Studies are inconsistent in determining whether the observed differences between NHBs and Hispanic-Americans, and NHWs can be explained by ethnic differences in risk factors such as diabetes duration, glycemic control, blood pressure, socioeconomic status, access to care, and health behaviors (64). Although fewer studies have been conducted in Native Americans, the Strong Heart Study showed a high (45.3%) prevalence of retinopathy among diabetic Native American individuals, indicating a similar or higher prevalence than in non-Native American populations (65).
Nephropathy.
Although the incidence of end-stage renal disease (ESRD) secondary to diabetes has declined or remained stable in most race/ethnic groups in the United States over the last 15 yr (66), ESRD continues to disproportionately affect minority populations. Data from the U.S. Renal Data System show that, compared with NHWs, age- and sex-adjusted incidence of ESRD secondary to diabetes is 3.8 times higher for NHBs, 3.5 times higher for Native Americans, and 1.5 times higher for Asian-Americans (67). The higher risk in Asian-Americans occurs over the age of 45 yr, whereas among the other race/ethnic groups the risk is higher compared with NHWs across all age groups (67). The difference in ESRD rates between NHB and Native American diabetic women, and nondiabetic NHW women, was greater than a similar comparison between men in these groups (67). Among Native Americans with type 2 diabetes, incident nephropathy and progression to ESRD are greater when compared with NHWs (67). The incidence of ESRD is also higher in Hispanic-Americans than in NHWs (67). Despite the higher incidence and prevalence of ESRD in NHBs, they surprisingly have lower mortality on dialysis compared with NHWs (22).
Neuropathy.
One prior comprehensive review did not find significant differences in the prevalence of neuropathy in NHBs and Hispanic-Americans compared with NHWs (64). This literature, however, is hampered by varying definitions of neuropathy and cultural and language barriers that can affect interpretation of the tests (68). We summarized the estimated prevalence of diabetic neuropathy by race/ethnic group in Table 6. Among Native Americans, there are regional differences in prevalence, with the highest rates being in those from Arizona (68). In the San Luis Valley Diabetes Study, Hispanic-Americans had a nonsignificantly lower rate of neuropathy than NHWs, although the incidence of neuropathy was similar among the two groups (68). In the National Health Interview Survey, rates were similar among all three race/ethnic groups (Table 6), although there was a nonsignificant trend for Mexican-Americans and NHBs to have a higher prevalence with a diabetes duration greater than or equal to 15 yr (68). In the Appropriate Blood Pressure Control in Diabetes trial, sensorimotor neuropathy prevalence was higher in NHWs than Hispanic-Americans and NHBs; however, autonomic neuropathy was slightly higher in Hispanic-Americans (68).
Table 6.
Prevalence of diabetic neuropathy by race/ethnicity (68)
| Population | Study | Neuropathy assessment | Prevalence |
|---|---|---|---|
| Native Americans: Hopi and Navajo Indians | Subjective symptoms, absent deep tendon reflexes, decreased sensory perception | 12% | |
| Native Americans | Strong Heart Study | Monofilament testing (5 of 10 areas incorrect) | |
| Arizona | 22% | ||
| Oklahoma | 8% | ||
| Dakotas | 9% | ||
| Hispanic Americans | San Luis Valley Diabetes Study | Symptoms, absent knee or ankle deep tendon reflexes, absent sensation to iced tuning fork | 23.3/100 persons (vs. 31.6/100 persons in NHWs) |
| Mexican Americans, NHBs and NHWs | National Health Interview Survey | Questionnaire | ∼25% in all groups |
| Hispanic-Americans | ABCD Trial | Neurological symptom score, neurological disability score, autonomic function testing, and quantitative testing of vibration and thermal perception | 35% (sensorimotor neuropathy); 51% (autonomic neuropathy) |
| NHBs | 37% (sensorimotor neuropathy); 45% (autonomic neuropathy) | ||
| NHWs | 47% (sensorimotor neuropathy); 44% (autonomic neuropathy) |
ABCD, Appropriate blood pressure control in non-insulin-dependent diabetes mellitus.
Macrovascular complications
Epidemiology.
Cardiovascular disease (coronary heart disease, stroke, and congestive heart failure).
In 2009, there was no significant difference in the age-adjusted prevalence of coronary heart disease or stroke between NHBs (34.4%) and NHWs (33.6%) who were over the age of 35 yr (66) and had diabetes mellitus. Compared with NHWs, however, Hispanic-Americans had the lowest prevalence of CVD (26.6%), and that trend has been present from 1997 through 2009 (66). In a comprehensive review, after adjustment for multiple confounders and CVD risk factors, Asian-Americans, Hispanic-Americans, and NHBs had a lower risk of CVD than NHWs (64). Despite their lower rate of disease, however, NHBs with diabetes have a higher CVD mortality rate (22), and Hispanic-Americans with diabetes and hyperglycemia have higher mortality after acute stroke (69). We were unable to identify good population-based data on CVD outcomes in Native Americans with diabetes. Overall, Native Americans have a 2-fold greater likelihood of coronary heart disease compared with NHWs (70).
Peripheral arterial disease/amputations.
Certain ethnic minorities with diabetes suffer from a higher burden of lower extremity amputations than NHWs, although one study found that race/ethnicity did not predict lower extremity amputation (71). Because the prevalence of neuropathy is similar among race/ethnic groups, we can assume that the higher rates of amputation are secondary to peripheral arterial disease; however, many studies do not make this distinction (72). In a comprehensive review, NHBs had higher rates of lower extremity amputation than NHWs; however, several studies pointed to differences in risk factors as the reason for this outcome (64). Native Americans also had a higher risk of lower extremity amputation than NHWs; this disparity persisted despite an adjustment for risk factors (64). In contrast, compared with NHWs, Asian-Americans had a lower risk of amputation in multivariable models (64). Data in Hispanic-Americans are mixed, with some studies showing an increased risk, some showing a lower risk, and others indicating no difference when compared with NHWs (64).
Mortality
According to data from the Centers for Disease Control, NHBs, Native Americans and Alaska Natives, and Hispanic-Americans are 2.3, 1.9, and 1.5 times more likely to die from diabetes than NHWs (70). And whereas Asian-Americans overall are 20% less likely to die from diabetes than NHWs, there are variations among subgroups. Native Hawaiians and Filipinos living in Hawaii are 5.7 and 3.0 times more likely to die from diabetes than NHWs living in Hawaii (70).
Race/ethnic differences in biological factors contributing to complications
Glycemic control.
Hyperglycemia is an important, modifiable risk factor in preventing all diabetic microvascular complications (73, 74). Several systematic reviews and meta-analyses have shown that ethnic minorities with type 2 diabetes have worse glycemic control than NHWs, which likely contributes to the higher risk of microvascular complications seen in these populations. In one review of 21 studies, three studies showed a mean HbA1c or glycated hemoglobin at least 1% higher among diabetic ethnic minorities than diabetic NHWs, and all but two studies showed a significantly higher HbA1c in diabetic ethnic minorities (75). Compared with diabetic NHWs, the HbA1c in diabetic NHBs was approximately 0.6% higher (76), and in diabetic Hispanic-Americans it was approximately 0.5% higher (77). The proportion of diabetic ethnic minorities with poor glycemic control, defined as glycemia above a specific threshold, was significantly higher among NHBs, Hispanic-Americans, Native Americans, and Asian-Americans and Pacific Islanders (75).
Treatment is also an important contributor to HbA1c levels. A recent study showed that intensive insulin therapy using three daily injections resulted in comparable HbA1c outcomes across race/ethnicity (NHWs, NHBs, Hispanic-Americans, and Asian-Americans), although Asian-Americans and Hispanic-Americans required higher weight-adjusted insulin doses (78). A study assessing the willingness of patients with type 2 diabetes on oral agents to initiate insulin therapy found that minorities were less willing to initiate insulin therapy compared with NHWs, a concept coined as “psychological insulin resistance” (79). We summarize several other factors related to health care access and delivery that may contribute to race/ethnic disparities in glycemic control in diabetes in the Conceptual Framework for Health and Health Care Disparities section.
Although discussed more in the context of using HbA1c as a diagnostic criterion in nondiabetic individuals, recent studies have suggested that nonglycemic factors may contribute to the higher HbA1c at similar levels of fasting glucose seen in NHBs, Hispanic-Americans, Asian-Americans, and Native Americans, compared with NHWs (15). Suggested factors include variations in erythrocyte membrane permeability to glucose, regulation of glucose transport across the erythrocyte membrane, glycolytic rates, differences in nonenzymatic glycosylation reactions, and deglycation (15). Although genetic factors may contribute to race/ethnic differences in HbA1c levels, the Atherosclerosis Risk in Communities Study showed that for NHBs the percentage of European ancestry explained less than 1% of the variance in HbA1c, and socioeconomic factors, and metabolic factors (including fasting glucose) accounted for a significantly larger percentage of the variance (80).
Two recent studies have examined the effect glycemia has on HbA1c differences in NHBs and NHWs with diabetes (81, 82). In one study, using data from NHANES III and the Screening for Impaired Glucose Tolerance Studies, NHBs with diabetes had significantly higher HbA1c than NHWs with diabetes, which persisted after adjustment for plasma glucose and other factors that correlate with HbA1c. This result challenged whether HbA1c should be used to indicate risk for complications, measure quality of care, and evaluate disparities in health (81). A subsequent study, using data from the Atherosclerosis Risk in Communities Study, however, came up with a different conclusion and supports HbA1c as an indicator of significantly higher levels of glycemia in minority populations (82). This study attributed the higher HbA1c in NHBs with diabetes (compared with NHWs with diabetes) to higher fasting glucose and other covariates (82). In addition, nontraditional glycemic markers, glycated albumin and fructosamine, which are not subject to the effects of erythrocyte turnover and hemoglobin glycation, were also significantly elevated in NHBs with diabetes, compared with NHWs with diabetes (82). Thus, these data support more hyperglycemia in NHBs with diabetes. In individuals with diabetes, true differences in glycemia are likely much more relevant than glucose-independent mechanisms in explaining HbA1c variations (83). Also, the fact that HbA1c is similarly associated with prevalence and risk of microvascular and macrovascular complications, and mortality, among NHBs and NHWs with diabetes (84–86) lends credence to true differences in glycemia between the two race/ethnic groups. Because conflicting evidence is evolving about the contribution of nonglycemic factors to HbA1c levels in minorities with diabetes, caution should be used in applying HbA1c as the only measure to assess either diabetes diagnosis or management.
Cardiovascular risk factors.
Blood pressure.
Hypertension is an important risk factor for diabetic nephropathy/ESRD and peripheral arterial disease, and likely contributes to the higher prevalence of these complications in certain ethnic groups. NHBs have a higher prevalence of hypertension than NHWs (51). And Mexican-American women have a higher prevalence of hypertension than NHW women; however, the prevalence of hypertension is similar among men in the two race/ethnic groups (51). In general, Native Americans and Alaska Natives have a lower prevalence of hypertension compared with NHWs and NHBs; however, there are regional differences, with Native Americans in Montana having a higher hypertension prevalence than non-Native American groups in Montana (51).
A previous systematic review assessed control of CVD risk factors, specifically among U.S. adults with type 2 diabetes, and found disparities by race/ethnicity (75). Ten studies showed significant blood pressure differences between ethnic minorities and NHWs, with NHBs having higher systolic and diastolic blood pressure and a higher percentage of individuals with hypertension (75). The average blood pressure exceeded the recommended target of 130/80 mm Hg for all ethnic groups in most instances, and the percentage of individuals with inadequate blood pressure control (>140/90 mm Hg) was higher in NHBs and Hispanic-Americans than NHWs (75). Unfortunately, NHBs and Hispanic-Americans with type 2 diabetes are less likely to have their blood pressure checked, which is a missed opportunity for preventive intervention (67). Studies of blood pressure control and hypertension prevalence comparing NHWs to Native Americans and Asian-Americans with diabetes are lacking. Research to date from clinical trials indicates that control of reversible risk factors, including hypertension, is equally effective in lowering the risk of nephropathy and CVD in minority and NHW populations (87).
Lipids.
In a prior systematic review comparing low-density lipoprotein cholesterol between minority populations and NHWs with type 2 diabetes, only two studies showed ethnic differences, with Hispanic-Americans having slightly lower low-density lipoprotein cholesterol than NHBs and NHWs. Overall, low-density lipoprotein cholesterol control was moderate to poor in all race/ethnic groups. In this review, there were no studies comparing lipid control in NHWs with Native Americans and Asian-Americans with type 2 diabetes (67). As summarized in Metabolic Syndrome, NHBs have lower triglycerides (TG) than NHWs (88), which may explain their lower risk of macrovascular disease; however, they generally have a higher prevalence of low high-density lipoprotein cholesterol, a strong CVD risk factor (88).
Genetics.
As noted in the prior section on genetics and type 2 diabetes risk, few GWAS analyses have been performed in ethnic minority populations. A GWAS for diabetic nephropathy genes in NHBs identified several candidate genes, including RPS 12, LIMK2, and SFI1 (89). A GWAS for diabetic nephropathy in Japanese individuals with type 2 diabetes identified the SLC12A3 and engulfment and cell motility 1 genes as candidate genes for susceptibility to diabetic nephropathy in this population (90). We are unaware of GWAS for other diabetic complications in ethnic minority populations. Therefore, this is a potential area for future research.
Epigenetics.
Just as studies have linked low birth weight with an increased risk of type 2 diabetes and hypertension in adulthood (67), they have also suggested that it is a potential contributor to diabetic nephropathy. NHBs have lower birth weight than NHWs, likely due to environmental and behavioral factors discussed previously (53). Animal studies have associated low birth weight with postpartum alterations in the anatomical structure and function of the kidneys and pancreas, which can predispose to renal damage (67). And human studies have associated low birth weight with increased odds of ESRD (67). This increased risk of kidney disease may be due to the smaller and reduced number of nephrons present in the kidneys of prenatally undernourished individuals (53).
Individual race/ethnic differences in nonbiological factors contributing to complications
Health behaviors.
Self-monitoring of blood glucose (SMBG).
The medical community recommends SMBG for patients using multiple daily insulin injections to achieve glycemic targets (2). Major clinical trials of insulin-treated patients, which have included SMBG as part of the multifactorial treatment interventions, have demonstrated significant reductions in diabetes complications (2). SMBG is important in preventing hypoglycemia and adjusting medications, nutrition therapy, and exercise regimens. A systematic review found that SMBG rates were generally poor among all patients with diabetes, although SMBG was more frequent in diabetic individuals taking insulin compared with those who were not (91). Although some studies have shown no differences in SMBG by race/ethnicity, several have shown lower rates among NHBs, Hispanic-Americans, and Asian-Americans, compared with NHWs. Two studies found no difference in SMBG frequency in Native Americans compared with NHWs (91). There are several barriers to SMBG, including inconvenience and intrusiveness, pain, lower socioeconomic position, education level, social class, and living in a high poverty area, many of which are disproportionately prevalent in ethnic minority groups (91). One study found that NHBs and Hispanic-Americans did not perform SMBG due to financial concerns (92). Addressing these barriers will be important in reducing disparities in diabetes self-management behaviors.
Physical activity and smoking.
As summarized earlier, ethnic minorities are less likely to engage in leisure-time physical activity, which can contribute to worse glycemic control and a propensity to developing microvascular complications (51). In addition, Native Americans and Alaska Natives have a higher prevalence of smoking than NHWs (51), which can contribute to their higher risk of peripheral arterial disease and amputations. NHBs and NHWs have similar smoking rates, whereas Mexican-Americans have significantly lower smoking rates. Therefore, smoking may not explain ethnic differences in peripheral arterial disease in these populations.
Depression.
Depression is a well-established comorbidity of type 2 diabetes that has an adverse effect on health behaviors, glycemic control, and complications (93–95). Among individuals with diabetes, those with depression have poor adherence to self-management behaviors compared with those without depression (96). Although there are few studies of the prevalence of depression in minorities with diabetes, one study found that depression was more prevalent in minorities with diabetes, particularly Native Americans and Alaska Natives (97). Although another study found similar rates of depressive symptoms among NHWs, NHBs, and Hispanic-Americans, NHBs were less likely to report physician diagnosis or treatment with pharmacotherapy, suggesting that the presence of depression may be underdiagnosed and undertreated in NHBs with diabetes (98).
Summary and future research
Overall, ethnic minorities appear to be disproportionately affected by microvascular complications and mortality associated with diabetes. This is likely related to poorer glycemic and CVD risk factor control; however, there are some notable paradoxes in NHBs. First, the reason for the lower incidence of CVD in NHBs with diabetes is unclear. Whether diabetes raises the risk of CVD in NHWs more than in NHBs or whether NHBs have higher CVD mortality in the prediabetic state before their diagnosis of diabetes requires further research. Second, although NHBs have a lower incidence of macrovascular complications, they are more likely to die once they have CVD. This may be related to poor CVD risk-factor control or lack of access to appropriate care, as discussed in the Conceptual Framework for Health and Health Care Disparities section. Further basic, translation, and clinical research studies are needed to elucidate the higher CVD mortality rate in NHBs with diabetes. Third, whereas NHBs have a higher rate of ESRD secondary to diabetic nephropathy, they are less likely to die on dialysis than NHWs. The reasons for this are unclear, but some hypotheses include: better overall health of NHBs who survive to ESRD and are offered or elect to initiate dialysis, compared with NHWs; more organ-limited kidney disease with less overall atherosclerosis in NHBs, compared with NHWs; and race/ethnic differences in handling of inflammation (99). We need additional studies to elucidate the mechanisms contributing to this survival difference.
Disparities by sex
Microvascular complications
Epidemiology
Retinopathy.
National survey data suggest that women with diabetes have visual impairment more often than men with diabetes, although this is not necessarily all attributable to retinopathy (100). Roy et al. (101) examined the prevalence of retinopathy among adults with type 1 diabetes. There were significant age-by-race-by-sex interactions. NHB women over 50 yr of age were the subgroup most likely to have severe retinopathy and self-reported blindness (101). In contrast, NHW women were more likely to have retinopathy than NHW men, but NHW men were more likely to have severe retinopathy (101). A pooled-analysis including adults with type 2 diabetes (102) did not observe differences by age and sex, although there were some modest differences by race/ethnicity. In an exposure unique to women, diabetic retinopathy can transiently worsen during pregnancy, particularly in women with rapid glucose changes preconception (103).
Nephropathy.
Women with diabetes appear similarly likely to undergo albuminuria screening as men (104). Overall, studies have not linked female sex with poorer outcomes; within race/ethnicity, men have slightly higher rates of ESRD than women (105). Men with diabetic kidney disease are also more likely to have elevated blood glucose levels than women (106).
Neuropathy.
Similar to the last report of diabetic neuropathy in the NHANES (1999–2002), which found no sex differences (107), recent reports on diabetic neuropathy have found no significant sex differences (108). However, Gregg et al. (109) noted that, regardless of diabetes diagnosis, peripheral neuropathy was more common in men than women and was more common among diabetic compared with nondiabetic adults (110). But the study did not note sex differences in peripheral neuropathy between diabetic men and diabetic women (109, 110). The QT interval on the electrocardiogram may be less sensitive for detecting autonomic neuropathy in women compared with men (111).
Macrovascular complications
Epidemiology.
Cardiovascular disease (coronary heart disease, stroke, and congestive heart failure).
Diabetes increases the risk of coronary heart disease in women more than in men, a finding that has been consistent across multiple studies (112–114) and holds true in national discharge data as of 2006 (115). Of note, absolute coronary disease mortality is also higher in diabetic women than in diabetic men, after adjustment for other CVD risk factors. Although sex differences were present in the early 1990s, Champney et al. (116) found recently that diabetic women and men had equivalent outcomes after percutaneous intervention in one large hospital-based series. In national hospital discharge data, rates of congestive heart failure appear similar in men and women after age adjustment (117). In the United States, hospital discharge data showed no difference in stroke hospitalizations between men and women between 1988 and 2006 (118). Two large prospective studies from the United Kingdom conflict about the risk of stroke in diabetic women compared with men (119, 120).
Peripheral arterial disease/amputations.
Men are more likely to undergo diabetes-related lower extremity amputations (121). The sex gap narrowed in the early 2000s, although diabetic men still had higher amputation rates than diabetic women in 2006 (122). Hospital discharge data suggest significantly higher rates of peripheral arterial disease in men than in women (123).
Gregg et al. (109, 110) linked male sex and diabetes with more frequent lower extremity disease (defined as ulcers, peripheral neuropathy, and peripheral arterial disease), although the study did not report sex differences in adults with diabetes. Dorsey et al. (124) also noted that diabetic men were significantly more likely than diabetic women to report lower extremity disease.
Mortality
Gregg et al. (125) examined mortality trends in men and women with diabetes from 1971–2000. All-cause mortality and CVD mortality both decreased in diabetic men. However, all-cause mortality increased in diabetic women with no significant change in CVD mortality (125); women with diabetes actually had higher overall mortality than men with diabetes (125).
Sex differences in biological factors contributing to complications
Glycemic control and cardiovascular risk factors.
Between 1988 and 2002, Saaddine et al. (126) found that the proportion of women with diabetes and elevated HbA1c levels (≥9.0%) declined to a significantly greater extent than that of men. The majority of women in this study had type 2 diabetes. The increased risk of CVD in women conferred by diabetes may be due to the presence of other CVD risk factors, including age, hypertension, and dyslipidemia (113). In particular, lipid abnormalities are more severe in women, particularly low-density lipoprotein cholesterol particle size and TG levels (113).
Sex differences in nonbiological factors contributing to complications
Over the past 20 yr, lipid measurement and elevated lipid levels, performance of eye exams, aspirin use, smoking cessation, and foot exams improved in both men and women in national studies (126, 127). However, women with type 2 diabetes are less likely to use aspirin than men (126, 128). Aspirin is beneficial in secondary prevention of CVD in individuals with diabetes, although its role in primary CVD prevention remains controversial (129). Reports conflict as to whether lipid control is worse among women with diabetes than men with diabetes. In a study of several managed care plans, Bird et al. (130) found that women with diabetes had higher lipid levels than men with diabetes. In contrast, Ferrara et al. (131) did not find differences in blood pressure control or lipid control or medication management between men and women with diabetes or the subgroup of diabetic adults with CVD. Kim et al. (132) also did not find sex differences in treatment of lipid abnormalities in adults with diabetes, although women were more likely to have elevated lipid levels. However, Wexler et al. did find that women were less likely to have adequate lipid or systolic blood pressure control (133). Some of these differences may explain the differences in CVD complications in diabetes by sex.
Summary and future research
The most marked sex differences in diabetic complications are for coronary disease and peripheral arterial disease. Although men with diabetes suffer from these conditions more frequently, the impact of diabetes is significantly higher for coronary disease and lower for peripheral arterial disease. Postulated mechanisms for sex differences include endothelial dysfunction and adipokine activity due to dimorphic sex hormone status. Less aggressive treatment with anticoagulants may also play a role. However, the reasons for these sex differences remain largely speculative. In particular, the reasons for differential effects of CVD risk factors on different arterial beds remain unknown. Future basic, translational, and clinical research studies are needed to elucidate the differential impact of diabetes on these two vascular beds.
Perhaps the most striking finding regarding sex differences in complications is the interactions with race/ethnicity. Most of the investigation into the reasons for disparities focuses not only upon sex, but also how its effects are modified by race/ethnicity. In particular, we need to investigate both the clinical and nonclinical factors by sex and race/ethnicity to better understand the reasons for disparities in either. Ultimately, these disparities can shed light on mechanisms in disadvantaged groups as well as pathophysiology in general.
Gestational Diabetes Mellitus (GDM)
Definition
The medical community broadly defines GDM as glucose intolerance, first recognized during pregnancy (134), that is likely due to a failure of pancreatic β-cells to compensate for the increased insulin resistance that occurs during pregnancy (135) and possibly to the antagonistic effects of progesterone on insulin action (136). In less than 10% of women, GDM probably represents type 2 diabetes that was unrecognized before pregnancy (137). The literature uses prevalence and incidence estimates interchangeably. The reasons for this are: 1) GDM is limited to pregnancy; 2) most women do not conceive twice in the same year; and 3) of those who do conceive more than once a year, most women are not diagnosed with GDM before their second trimester. However, for a small proportion of women who conceive twice in the same year and do not carry a full-term pregnancy, incidence estimates may be slightly higher than prevalence estimates. For the purposes of this statement, we refer to prevalence and incidence interchangeably.
Epidemiology of GDM
In the late 1990s to early 2000s, studies from New York and southern California reported the overall prevalence of GDM at approximately 5 to 8% (138–141). As with type 2 diabetes mellitus and obesity, the prevalence of GDM increased in the 1990s (138). However, GDM prevalence may have stabilized between 1999 and 2005; in a report from Kaiser Southern California, Lawrence et al. (139) found that GDM prevalence remained stable at 7.5 per 100 births in 1999 to 7.4 per 100 births in 2005. Simultaneously, preconception diabetes affected 10% of women in 1999 and increased to 21% of women in 2005 (139). The increase in preconception type 2 diabetes occurred in all racial/ethnic groups (139). The results are also concerning because they suggest that the severity of insulin resistance and β-cell failure may be occurring earlier in life than previously noted, leading to an earlier age of diagnosis of type 2 diabetes and more frequent diabetes in the reproductive years.
Population-based studies have consistently demonstrated that GDM prevalence is higher in Hispanics, Asians, and Native Americans compared with non-NHWs and non-NHBs (138–140, 142–147). Unlike type 2 diabetes, estimates of GDM have not usually been markedly elevated in NHBs, compared with NHWs (138–140, 142–147). This lack of difference does not seem to be due to different ages at delivery or to a different prevalence of preconception type 2 diabetes (143).
There is heterogeneity among subgroups of race/ethnicity. In an examination of birth certificates in New York City, Thorpe et al. (138) noted that Mexican women under 35 yr of age had an increased prevalence of GDM, when compared with women under 35 from Puerto Rico, the Dominican Republic, and other Central American or South American countries. However, among women 35 yr of age, Puerto Rican women had a higher prevalence of GDM (138). In addition, there is significant heterogeneity between subgroups of Asians. South Asians, including women from India and Pakistan, tend to have the highest prevalence of GDM (143, 145). However, reports vary regarding the risk among Southeast Asians (143, 145, 148, 149), including women from Cambodia, Vietnam, Laos, Thailand, or the Philippines, as well as East Asians such as Koreans, Japanese, and Chinese (143, 145, 147, 148). Japanese women consistently have a lower GDM prevalence than other Asians (143, 145, 147, 148).
Race/ethnic differences in clinical outcomes in GDM
Among women with GDM, there are racial/ethnic differences in pregnancy processes and outcomes. Berggren et al. (150) found that Hispanics were more likely to receive medical management (as opposed to lifestyle changes only) than NHBs or NHWs (150). Hispanic women were less likely to have gestational hypertension, preterm delivery, low birth weight infants, and neonatal intensive care unit admissions, but more likely to have shoulder dystocia than NHW or NHB women (150). The study noted that NHB and NHW women have similar perinatal processes and outcomes (150). Esakoff et al. (151) found that Hispanics and Asians with GDM actually had lower odds of cesarean delivery than NHW women, and Asians had lower odds of macrosomia than NHW women.
Other reports have found that NHB women with GDM have poorer outcomes than NHW women. Esakoff et al. (151) found that NHB women had higher odds of cesarean delivery and intrauterine fetal demise than NHW women. Hunt et al. (152) examined birth certificates, inpatient and outpatient records in South Carolina and found that GDM in NHBs was more likely to confer greater birth weight than GDM in NHWs. This racial/ethnic difference persisted after adjustments for age, education, prenatal care, smoking, and hypertension. Nicholson et al. (153) examined the association between NHB race/ethnicity and delivery procedures among GDM women. NHB women had a lower prevalence of episiotomy but a greater likelihood of cesarean delivery and low birth weight infants than NHWs (153). This finding was attenuated but persisted after adjustment for multiple factors including demographics, parity, payer source, hospital teaching, volume, teaching status, and other comorbid conditions (such as hypertension, macrosomia, and fetal heart abnormalities) (153). These analyses suggest that race/ethnicity is independently associated with the care GDM women receive at their delivery, either through local variations or other differences in disease severity.
The racial/ethnic differences reported in subgroups of Asians for risk of GDM may extend to risk of complications for GDM. Silva et al. (154) found that macrosomia was more common among Native Hawaiian/Pacific Islanders and Filipinas compared with Japanese, Chinese, and NHW mothers. Their analyses suggested that maternal weight conferred different risk for macrosomia, with similar maternal weights associated with greater odds of macrosomia in Filipinas, compared with NHWs. In a report from New York City, Mocarski and Savitz (155) found racial/ethnic differences in outcomes between subgroups of NHBs. Caribbean and sub-Saharan African women tended to show a larger impact of GDM upon perinatal events compared with North Africans. In the same report, these investigators noted that South Asians had the highest prevalence of GDM, while experiencing the smallest relative effects of GDM upon macrosomia and cesarean deliveries (155).
Women with GDM are at increased risk for future diabetes, and this risk may vary by race/ethnicity (156). In an analysis of pregnancies from southern California, GDM conferred greater risk for diabetes for NHB women than for other racial/ethnic groups, even after consideration of BMI. Because women of NHB race/ethnicity have a similar prevalence of GDM to NHW women, despite having a poorer diabetes risk factor profile (lower socioeconomic status, stronger family history for diabetes, greater BMI), it is possible that NHB women who actually develop GDM may be at high risk for diabetes (156). Self-rated health and quality of life are poorer in women with GDM compared with women without GDM (157, 158). However, studies have yet to report comparisons by race/ethnicity.
Biological factors contributing to racial/ethnic disparities in GDM
Genetics
SNP that confer increased risk for impaired insulin secretion and sensitivity may explain some of the racial/ethnic differences. No studies have reported on the contribution of the polymorphisms to racial/ethnic differences in GDM in U.S. populations. The BetaGene study has found a link between polymorphisms of GCK and G6PC2 and impaired insulin secretion among Mexican-American women with histories of GDM and Finns with a similar impairment (159). In addition, the BetaGene study found a link between polymorphisms in IGF2BP2 (160), TCF7L2 (161), PPARG2 (162), and HNF4A (162) and altered insulin sensitivity in women with histories of GDM.
Recently, international studies have linked specific polymorphisms to GDM incidence; these associations vary by race/ethnicity. The Hyperglycemia and Adverse Pregnancy Outcome Study linked GCK and TCF7L2 polymorphisms with increased glucose levels and increased risk of GDM in Thai women (163). The study also found an association between the GCK variant and greater birth weight, fat mass, skin-fold thicknesses in NHW Europeans, but not Thai people, and the study linked the TCF7L2 variant with these outcomes in Europeans, but not Thai people (163).
Caughey et al. (164) found that having a mother or father of Asian or Hispanic race/ethnicity increased the odds of GDM roughly 30% compared with having an NHW parent. In a similar population, a study by Nystrom et al. (165) suggested that having two Asian parents resulted in greater odds of GDM when compared with having one Asian parent or two NHW parents, which had the lowest odds of GDM.
Obesity
Obesity is a significant risk factor for GDM in all racial/ethnic groups. As with type 2 diabetes, the BMI cut-points for Asians may be lower than in other racial/ethnic groups. Hunsberger et al. found that Asians with both high and low body mass indices had greater odds of GDM (144). However, even after adjustment for BMI during pregnancy, Hedderson et al. found that the greater risk in Asians and the relatively low risk in NHBs persisted (143).
Nonbiological factors contributing to racial/ethnic disparities in GDM
Health behaviors
Although studies have linked dietary content and physical activity to GDM, studies have not examined their relative contribution by race/ethnicity. Reports from the Nurses' Health Study II have indicated that higher prepregnancy intake from animal fat and cholesterol (166), dietary heme iron (167), meat (168), low fiber and high glycemic load foods (169), and sugar-sweetened beverages (170) all increase risk of GDM, but the majority of the population was NHW, and investigation by race/ethnicity was not possible. Similarly, the same group has found that pregravid physical activity is a risk factor, but racial/ethnic comparisons were not possible (171).
Socioeconomic status
Studies have associated lower socioeconomic status with greater prevalence of GDM apart from race/ethnicity. Devlin et al. (146) in Minnesota found that a 7% annual increase in GDM among women with a high-school education compared with a 4% annual increase among those with a college education. Adjustment for socioeconomic status tends to reduce racial/ethnic differences. Hunsberger et al. (144) found that women with less than a high school education had 70% greater odds of having GDM than women with at least a high school education, and that being below the poverty limit also increased the odds of GDM apart from race/ethnicity. However, adjustment for both of these factors tended to reduce the differences in GDM prevalence seen between Hispanic vs. NHWs, and NHBs vs. NHWs (144). Of note, even in multivariable models, racial/ethnic differences persisted.
Country of nativity
Foreign-born women also have a greater prevalence of GDM than women born in the United States, and this disparity has increased from the 1990s to early 2000s (146, 172, 173). In two studies, most of these births occurred to Hispanic women, the majority of whom were from Mexico (146, 173). In New York City, Savitz et al. (172) found that foreign nativity increased the odds for GDM in women of most racial/ethnic groups and subgroups of Hispanics, as well as among Chinese and Filipinos. However, the risk conferred by nativity may differ between race/ethnic groups. Hedderson et al. (143) found a link between foreign nativity and a greater risk of GDM among NHB, South Asian, Filipino, Pacific Islander, Chinese, Mexican, and NHW women, but foreign born Japanese and Korean women actually had a lower risk of GDM than Japanese and Korean women born in the United States.
Geographic region
Within the United States, there is regional variation in the prevalence of GDM. In an analysis of the National Hospital Discharge Survey, which is based on ICD-9 codes, Getahun et al. (174) found that the prevalence of GDM increased markedly in the western, northeastern, and midwestern United States, primarily due to the increase in GDM births among NHBs. They speculate that these regional differences might represent differences in screening practices and prenatal care delivery and/or regional increases in obesity (174). On a smaller geographical scale, one study did not find an association between neighborhood food environment and GDM prevalence in New York City, although the study did not examine associations by race/ethnicity (175).
Health care access and delivery
There are race/ethnic differences in access to care among women with histories of GDM (176). Kim et al. (176) found that among women who had histories of GDM, Hispanic women were more likely than NHW women to lack health insurance and a primary care provider, and to report poor or only fair health. Such differences suggest that there could be differences in screening and prenatal counseling among women with GDM, with subsequent impact upon outcomes, as summarized above, although studies have not yet examined this question.
Summary, implications, and future research
The investigation of racial/ethnic disparities in GDM prevalence should focus upon the prevalence of significant SNP in the BetaGene study in other populations at high-risk for GDM (particularly Asians in the United States). Studies examining gene-environment interactions, specifically the contribution of clinical factors such as adiposity, activity, and diet in combination with polymorphisms, could shed additional light upon the racial/ethnic disparities in both prevalence and complications.
Almost no epidemiologic studies examine the relative contribution of social and economic factors to racial/ethnic disparities in GDM. These factors include traditional markers of socioeconomic status, as well as mental stress, the built environment, and the impact of intrauterine glucose intolerance. Further investigation of the contribution of these factors is clearly needed. Finally, future studies should examine the documented disparities in access to care among women with histories of GDM. This would help determine the impact of these disparities on screening for GDM, the quality of care women with GDM receive, and the additional impact upon outcomes and quality of life.
Metabolic Syndrome
The metabolic syndrome is considered to be an antecedent to CVD and type 2 diabetes; thus, the identification and treatment of the metabolic syndrome might delay or prevent the onset of both CVD and type 2 diabetes (177). However, the metabolic syndrome is a controversial construct. In 2005, the American Diabetes Association and the European Association for the Study of Diabetes declared in a joint statement that the widespread use of the metabolic syndrome was premature and that clinicians “should evaluate and treat all CVD risk factors without regard to whether a patient meets the criteria for diagnosis of the metabolic syndrome” (178). We are not entering into the debate on the utility of the metabolic syndrome as a clinical construct, but we will address whether the metabolic syndrome is an effective tool to identify minority individuals at risk for CVD or type 2 diabetes. We will focus primarily on data presented from NHANES, which includes NHB, NHW, and Mexican-American populations. Within NHANES datasets, members of the medical community have questioned the usefulness of the metabolic syndrome in identifying high-risk individuals most often in relation to NHBs (179–183). We will examine possible etiologies for why the metabolic syndrome may be a less successful screening program in NHBs than NHWs or Mexican-Americans.
Definition
The metabolic syndrome (originally called Syndrome X) is based on principles first set forth by Gerald Reaven's Banting Lecture in 1988, where he described a cluster of metabolic risk factors related to insulin resistance (184). Since that time, the medical community has given the syndrome different names and definitions. Without a uniform definition, researchers could not make comparisons across studies. To rectify this situation, the International Diabetes Federation; National Heart, Lung, and Blood Institute; American Heart Association; World Health Federation; International Atherosclerosis Society, and International Association for the Study of Obesity released a joint statement in 2009 titled, “Harmonizing the Metabolic Syndrome” (177). The six organizations agreed that: 1) insulin resistance was the underlying cause of metabolic syndrome, CVD, and type 2 diabetes; 2) the metabolic syndrome was an important tool to use to screen for future risk of CVD and type 2 diabetes; and 3) there needed to be a uniform, worldwide definition. Their definition of the metabolic syndrome requires any three of five factors, each of which are considered to be closely linked to insulin resistance: central obesity, low high-density lipoprotein cholesterol (HDL-C), hypertriglyceridemia, hypertension, and fasting hyperglycemia (Table 7). The Endocrine Society was not part of the 2009 joint statement for harmonizing the metabolic syndrome, but in 2008 The Endocrine Society issued a clinical practice guideline endorsing the use of the metabolic syndrome as a tool for the primary prevention of CVD and type 2 diabetes (185).
Table 7.
Criteria for clinical diagnosis of the metabolic syndrome (177)
| Measure | Categorical cut-points |
|---|---|
| Elevated WCa | Population-and country-specific definitions |
| Elevated TG (drug treatment for elevated TG is an alternate indicator)b | ≥150 mg/dl (1.7 mmol/liter) |
| Reduced HDL-C (drug treatment for reduced HDL-C is an alternate indicator)b | <40 mg/dl (1.0 mmol/liter) in males; <50 mg/dl (1.3 mmol/liter) in females |
| Elevated blood pressure (antihypertensive drug treatment in a patient with a history of hypertension is an alternate indicator) | Systolic ≥130 and/or diastolic ≥85 mm Hg |
| Elevated fasting glucosec (drug treatment of elevated glucose is an alternate indicator) | ≥100 mg/dl |
Reprinted from K. G. Alberti et al.: Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645, 2009 (177), with permission. © American Heart Association, Inc.
It is recommended that the International Diabetes Federation (IDF) cut-points be used for non-Europeans and either the IDF or American Heart Association/National Heart, Lung, and Blood Institute cut-points used for people of European origin until more data are available.
The most commonly used drugs for elevated TG and reduced HDL-C are fibrates and nicotinic acid. A patient taking one of these drugs can be presumed to have high TG and low HDL-C. High-dose ω-3 fatty acids presumes high TG.
Most patients with type 2 diabetes mellitus will have the metabolic syndrome by the proposed criteria.
Epidemiology
In two different NHANES datasets, separated by 15 yr (1988 to 1994, and 2003 to 2006), NHB men had a lower prevalence of the metabolic syndrome than NHW or Mexican-American men (183, 186). In the 1988 to 1994 survey, the prevalence of metabolic syndrome in NHB, NHW, and Mexican-American men was 14, 21, and 24%, respectively. In 2003 to 2006, the prevalence of metabolic syndrome rose in all three groups to 26, 38, and 34%, respectively, but still remained significantly lower in NHB men than NHW or Mexican-American men. Because the prevalence of type 2 diabetes and CVD is higher in NHBs than NHWs and Mexican-Americans (187), one would anticipate that the prevalence of the metabolic syndrome would also be higher. Even as there is much speculation as to why the prevalence of the metabolic syndrome is lower in NHB men than NHW or Mexican-American men, the overall consensus is that the metabolic syndrome is not reflective of metabolic risk in NHB men (181).
As with men, the prevalence of CVD and type 2 diabetes is higher in NHB women than NHW women (187). Yet in NHANES from 1988–1994 the prevalence of metabolic syndrome was similar in NHBs and NHWs and higher in Mexican-Americans (20.9, 22.9, and 27.2%, respectively) (183). In the 2003–2006 dataset, the prevalence of metabolic syndrome increased in each group to 38.2, 31.3, and 41.9%, respectively (186). It is possible that the high prevalence of metabolic syndrome in the later survey is affected by the confounding effect of obesity. Between these two NHANES surveys, NHB women had a relatively greater rise in the prevalence of obesity than NHW women (20).
Asian-Americans have a high prevalence of metabolic syndrome despite several subpopulations having a lower BMI, and the prevalence varies by the criteria used to define adiposity, as discussed below (see Race/ethnic and sex differences in body fat distribution—implications for defining metabolic syndrome section under Metabolic Syndrome). Using the 2002 National Cholesterol Education Program definition of metabolic syndrome, the prevalence among overweight individuals was higher in Asian-Americans (Asian Indians, Chinese, Filipino, Japanese, Korean, and Vietnamese) compared with NHWs (48 vs. 25%, respectively) (188). Among Asian subgroups, the prevalence of metabolic syndrome was highest among Asian Indians and Filipinos in every BMI category (188). Native Americans also have a very high prevalence of metabolic syndrome regardless of the definition used, ranging from 44 to 59% in men and 53 to 73% in women.
The metabolic syndrome has engendered major international support because studies in largely NHW cohorts have demonstrated that the syndrome carries twice the risk of CVD and five times the risk of type 2 diabetes (177, 181, 189). It is widely assumed that the prevalence of the metabolic syndrome will vary directly with the prevalence of CVD and type 2 diabetes in each population. However, as reviewed below (see Race/ethnic and sex differences in body fat distribution—implications for defining metabolic syndrome section under Metabolic Syndrome), this is not the case for NHBs because two of the metabolic syndrome components, WC and TG, are significantly different in NHBs than NHWs (180, 182, 190–193). The impact of these two components on defining metabolic syndrome in NHBs may lead to an over- or underdiagnosis of metabolic risk in this population.
Race/ethnic and sex differences in body fat distribution—implications for defining metabolic syndrome
Non-Hispanic Blacks
Accumulating evidence suggests that the relationship of BMI to health is not the same in NHB women as it is in NHW women (194–196). For example, Fontaine et al. (194), in a combined analysis of data from NHANES surveys performed from 1971 to 1992, showed that the BMI associated with years of life lost secondary to obesity in NHW women was 28 kg/m2, compared with 38 kg/m2 in NHB women.
Because WC is highly correlated with BMI, a greater rise in the prevalence of obesity could result in relatively more NHB than NHW women meeting the WC criteria of at least 88 cm. Yet it is unclear whether 88 cm is even the proper WC threshold to diagnose central obesity in NHB women. In Scottish women, a WC cutoff of 88 cm (which the U.S. medical community widely uses) predicts, by linear regression, a BMI of 30 kg/m2. Because visceral adipose tissue (VAT) is the fat depot most often associated with insulin resistance (197), the metabolic syndrome uses WC in its definition as an inexpensive visceral fat proxy. Although emerging evidence demonstrates that the WC of risk might be similar in NHB and NHW men, the WC of risk may be higher in NHB than NHW women (191, 192).
At similar WC, NHB women have less VAT than NHW women (190, 193, 198). In a study of NHB, the WC that predicted insulin resistance in NHB women was 98 cm, or a full 10 cm higher than the recommended threshold of 88 cm (193). In the same cohort, the WC that predicted insulin resistance in NHB men was 102 cm, or identical to the WC currently used for NHW men (193). In a cross-sectional evaluation of Pennington Center Longitudinal Study data of over 6000 NHB and NHW individuals, the optimal WC cutoff to predict cardiometabolic risk was 97 cm in NHB women and 92 cm in NHW women (192). For both NHB men and NHW men, the optimal WC threshold to predict cardiometabolic risk was 99 cm. Therefore, the major race difference in metabolic risk associated with WC appears to be a function of sex, with differences in women but not men. And whereas the medical community has not yet agreed upon the exact WC associated with metabolic risk in NHB women, it seems as though the WC that predicts VAT, insulin resistance, and cardiometabolic risk factors appears to be higher in NHB women than NHW women (191, 192).
Asian-Americans
As in NHBs, current BMI and WC criteria derived from NHWs may not adequately identify Asian-Americans at increased risk for metabolic disorders. In a study of 43,507 Asian-Americans, including Asian Indians, Chinese, Filipino, Japanese, Korean, and Vietnamese Americans, the prevalence of metabolic syndrome, according to 2002 National Cholesterol Education Program criteria, was significantly higher in Asian-Americans than NHWs at every BMI category (188). This higher prevalence of metabolic syndrome appeared to be driven by higher prevalence of hyperglycemia, diabetes, low HDL-C, and hypertriglyceridemia (188). In this study, a comparable metabolic syndrome prevalence for NHWs with BMI greater than or equal to 25 kg/m2 occurred at a lower BMI for Asian-Americans (19.6 kg/m2 for women and 19.9 kg/m2 for men), suggesting that the current BMI ranges for defining overweight/obesity underestimate metabolic risk in Asian populations (188). Similarly, studies suggest that metabolic risk occurs at lower WC in Asian-Americans. In a study of South Asians in Texas, more individuals were identified as overweight and obese when researchers used the International Diabetes Federation criteria (WC ≥90 cm in men and ≥80 cm in women), compared with the National Cholesterol Education Program criteria (WC ≥102 cm for men and ≥88 cm for women) (199). A recent study in Asian Indians suggested modified WC cut-points of 90 cm for men and 85 cm for women because these values predicted the presence of other metabolic syndrome components (i.e. dyslipidemia, dysglycemia, and hypertension) (200). A study of Japanese-Americans similarly suggests that lower WC cut-points are needed for this Asian subpopulation. They determined the accuracy of the International Diabetes Federation definition of metabolic syndrome in identifying at least two nonadipose syndrome components based on various WC cut-points (199). Their data suggested that more appropriate WC cut-points for Japanese-Americans were 87–90 cm for men and 80–90 cm for women (199). In addition, the optimal cut-points varied by age, particularly in women, with optimal cut-points of 80.8 and 89 cm in women age 56 or younger and women older than 57 yr, respectively, and 90 and 87.1 cm in men age 56 or younger and men older than 57 yr, respectively (199). More studies are necessary to examine how these alternative cut-points predict risk of type 2 diabetes and CVD in Asian-Americans.
Race/ethnic differences in lipoproteins—implications for defining metabolic syndrome
Hypertriglyceridemia
The medical community chose fasting TG levels as a metabolic syndrome component because of their strong, positive association with insulin resistance (184, 201). In NHWs, elevated TG is one of the most common features of the metabolic syndrome; however, in NHBs and West Africans, the most common features of the metabolic syndrome are low HDL-C, hypertension, and central obesity, not elevated TG (88). Use of hypertriglyceridemia as a diagnostic criterion for metabolic syndrome could potentially lead to underrecognition of metabolic risk in NHBs.
Increased VAT, hepatic fat accumulation, and hyperinsulinemia all contribute to the hypertriglyceridemia of insulin resistance (202, 203). However, studies show that TG levels are usually normal in insulin-resistant NHBs (180). The current belief is that low hepatic fat, low VAT, low apolipoprotein CIII levels, and high lipoprotein lipase all contribute to lower TG levels in NHBs (204–207). Hepatic fat is a major source of fatty acids that are ultimately reesterified into TG and secreted into the circulation as very low-density lipoprotein-TG. Because NHBs have lower hepatic fat than NHWs, it is likely that NHBs secrete less very low-density lipoprotein-TG. This requires further study. VAT is an important source of free fatty acids to the liver for TG synthesis. Because NHBs have less VAT than NHWs, low VAT contribute to low TG in NHBs. Lipoprotein lipase is the enzyme that clears TG from the circulation, and therefore high lipoprotein lipase activity could be a major factor leading to normal TG levels in insulin-resistant NHBs. As reviewed in the Diabetes Mellitus and Prediabetes section, NHBs have more insulin resistance than NHWs, despite low TG in NHBs. Further basic and translational research studies of the pathways that allow TG to be normal in the presence of insulin resistance could lead to the development of therapeutic interventions for populations that do not experience this benefit.
Low HDL-C
The other lipid component of the metabolic syndrome is low HDL-C. It is well known that on a population basis, HDL-C levels are higher in NHBs than NHWs; however, insulin-resistant NHBs often have low HDL-C levels, even when TG is not elevated (88, 208, 209). For example, NHBs and Africans, with insulin resistance and/or the metabolic syndrome, usually have normal TG levels but low HDL-C (88, 208). Presumably, in NHBs, hepatic lipase, the enzyme that clears HDL-C particles from the circulation, remains active in response to the hyperinsulinemia of insulin resistance. Therefore, low HDL-C may be a factor in development of type 2 diabetes or CVD in NHBs, even in the absence of elevated TG levels. This raises the possibility that in NHBs, shifting emphasis from elevated TG to low HDL-C in screening for metabolic risk is worthy of investigation.
Summary and implications
Compared with NHWs and Mexican-Americans, NHB men and women have a higher prevalence of type 2 diabetes and CVD, particularly hypertension, stroke, and congestive heart failure (187). Furthermore, in NHBs, the prevalence of CVD is higher than the prevalence of the metabolic syndrome (186, 187). This observation, combined with the doubts raised about the effectiveness of the metabolic syndrome in NHBs (181), means that the use of the metabolic syndrome to identify risk in NHBs is problematic. Similarly, current criteria used to define obesity and elevated WC associated with the metabolic syndrome may underestimate diabetes and CVD risk in Asian-Americans, which is why Alberti et al. (177) have suggested ethnic-specific WC criteria for consideration (Table 8).
Table 8.
Current recommended WC thresholds for abdominal obesity by organization
| Population | Organization (Ref.) | Recommended WC threshold for abdominal obesity (cm) |
|
|---|---|---|---|
| Men | Women | ||
| Europid | IDF (4) | ≥94 | ≥80 |
| Caucasian | WHO (7) | ≥94 (increased risk) | ≥80 (increased risk) |
| ≥102 (still higher risk) | ≥88 (still higher risk) | ||
| United States | AHA/NHLBI (ATP III) (5)a | ≥102 | ≥88 |
| Canada | Health Canada (8, 9) | ≥102 | ≥88 |
| European | European Cardiovascular Societies (10) | ≥102 | ≥88 |
| Asian (including Japanese) | IDF (4) | ≥90 | ≥80 |
| Asian | WHO (11) | ≥90 | ≥80 |
| Japanese | Japanese Obesity Society (12) | ≥85 | ≥90 |
| China | Cooperative Task Force (13) | ≥85 | ≥80 |
| Middle East, Mediterranean | IDF (4) | ≥94 | ≥80 |
| Sub-Saharan African | IDF (4) | ≥94 | ≥80 |
| Ethnic Central and South American | IDF (4) | ≥90 | ≥80 |
AHA/NHLBI, American Heart Association/National Heart, Lung, and Blood Institute; IDF, International Diabetes Federation. [Reprinted from K. G. Alberti et al.: Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645, 2009 (177), with permission. © American Heart Association, Inc.].
Recent AHA/NHLBI guidelines for metabolic syndrome recognize an increased risk for CVD and diabetes at WC thresholds of ≥94 cm in men and ≥80 cm in women and identify these as optional cut-points for individuals or populations with increased insulin resistance.
The Black Women's Health Study (http://www.bu.edu/bwhs/) and the Jackson Heart Study (www.jsums.edu/jhs/) collected data on metabolic risk in NHBs, analogous to data collected in NHWs in the Framingham Heart, Nurse's Health, and Physician's Health studies. Indeed, the Black Women's Health Study, initiated in 1995, has yielded critically important information on obesity, cancer, and CVD in NHB women; however, researchers did not design the study to specifically address the prevalence or predictive value of the metabolic syndrome in NHBs. The Jackson Heart Study, which began enrollment in 2000, is a longitudinal study of CVD risk in over 5000 NHBs from the tri-county region of Jackson, Mississippi. It is anticipated that the Jackson Heart Study will contribute to erasing the information gap on the efficacy of the metabolic syndrome in NHBs. Within this context one can use the Jackson Heart Study, as well as other smaller studies of NHBs, to determine whether ethnic-specific values for TG and the WC of risk can be identified. We need similar population-based studies of Asian-Americans to define WC cut-points that identify those at risk for type 2 diabetes and CVD. In the meantime, health care providers need to appreciate that in NHBs and Asian-Americans, the absence of the metabolic syndrome, as it is currently formulated, does not mean the absence of risk. To address type 2 diabetes and CVD health disparities in NHBs, the medical community should emphasize direct screening and intervention for hypertension and hyperglycemia.
Thyroid Disorders
Thyroid disorders are common in the United States (1), with up to 5% of the population reporting abnormalities in thyroid function or the development of nodules. In autopsy studies, 80% of patients had thyroid nodules (4). Given the potential public health implications of thyroid disease, studies assessing racial, ethnic, sex, and age differences among patients are important to inform health care providers of individual patient needs and to advise health care policies to ensure equal treatment.
Racial/ethnic differences in benign thyroid disorders
Epidemiology
Hypothyroidism and hyperthyroidism.
The NHANES measured serum TSH and total serum T4 to evaluate thyroid function and measured thyroid antibodies to gauge autoimmune thyroid disease (210). In more than 17,300 people over 12 yr of age, the study showed that 4.6% had hypothyroidism and 1.3% had hyperthyroidism. NHBs had significantly lower mean TSH concentrations than NHWs in all populations (P < 0.01). The percentage of NHBs with TSH greater than or equal to 4.5 mIU/liter (hypothyroidism) was significantly lower than in NHWs (P < 0.001), whereas the percentage with TSH less than 0.4 mIU/liter (hyperthyroidism) was significantly higher in NHBs than NHWs (P < 0.01). It has been debated whether the lower TSH levels are of clinical significance or whether they present simply a normal shift in biochemical values, with no untoward consequences for the individuals. This shift may, in fact, lead to overdiagnosis of subclinical hyperthyroidism and overtreatment of otherwise healthy and normal NHB individuals.
Autoimmune thyroid disease.
Positive antithyroid antibody levels were present in 11.5 to 13.0% of individuals. A higher percentage of NHWs had positive antibodies (14.3%) compared with NHBs (5.3%) and Hispanic-Americans (10.9%; all P < 0.001) (210). These data suggest that NHWs may be at higher risk of autoimmune thyroid disease, which a prior study indicated. Okayasu et al. (211) evaluated autopsy material from American subjects and found that over 41% of NHW females over 20 yr old and 20% of NHW males had chronic lymphocytic thyroiditis, compared with 17% of NHB females and 8% of NHB males. Asian-Americans had rates of thyroiditis similar to those in NHBs.
In the women in the Health and Retirement Study, Rogers et al. (212) examined the prevalence of autoimmune disease, including thyroiditis and Graves' disease, by age and race/ethnicity. They found that younger women were at higher risk of developing Graves' disease, whereas older women were more likely to have thyroiditis. They found no significant racial and ethnic differences among women in the prevalence of autoimmune disease, except for a 1.87 times higher likelihood in women of Jewish descent.
Biological factors contributing to race/ethnic disparities
Iodine intake.
Since 1971, NHANES has used urinary iodine concentrations to monitor dietary iodine in the United States because extremes in iodine intake are associated with the development of goiter (deficiency) and thyroid cancer (excess) (213, 214). The general U.S. population over 6 yr old had median urinary iodine concentrations of 164 mg/liter [95% confidence interval (CI), 154–174] in 2005–2006 and 164 mg/liter (95% CI, 154–173) in 2007–2008, which appeared unchanged from the surveys conducted since 2000. NHBs had persistently lower urinary iodine concentrations than NHWs and Hispanic-Americans. These differences among racial groups are likely due to variations in their dietary intake. These data suggest that NHB individuals may not achieve iodine sufficiency according to WHO criteria. The authors concluded that comprehensive assessments of iodine sufficiency in the general population should be continued because it would likely influence development of thyroid disease.
Genetics.
Studies have reported genetic susceptibility to Graves' disease associated with human leukocyte antigens (HLA) in NHWs (215), and studies have shown that specific loci differ by race. Chen et al. (216) studied a group of NHB patients with adult-onset Graves' disease and racially matched controls, seeking HLA and CTLA-4 associations. They included the major alleles of DRB1, DQB1, DQA1, the four subtypes of DRB3, and the presence of DRB4, and CTLA-4 gene polymorphisms. They studied 39 NHBs and 47 racially matched controls. They found that neither the DQB1*0301 allele nor DR3 specificity were present in NHB patients with Graves' disease but were present in NHWs. The discordance of the results between NHWs and NHBs in the United States may be attributable to the ethnic diversity of HLA genotypes or the complex interaction of HLA molecules with environmental factors, which may lead to pathogenic processes.
Thyroid cancer
Epidemiology
Thyroid cancer is the most common malignancy of the endocrine system worldwide. Kilfoy et al. (217) examined trends in the worldwide incidence of thyroid cancer from 1973–2002 and found that thyroid cancer incidence increased in all countries examined except Sweden, with an average increase of 48% among males and 67% among females. Data from the period of 1998–2002 showed a 5-fold global geographic variation among incidence rates for men and a 10-fold variation for women. The highest incidences were among U.S. whites for both men and women, and the lowest incidences were in Uganda for men and women (217).
The incidence of thyroid cancer in the United States has been increasing over the last 50 yr for women and men of every racial background, except Native Americans (218). Although the prevalence and incidence of thyroid cancer is similar by race/ethnicity, survival from thyroid cancer differs by race/ethnicity. Hollenbeak et al. (219) examined the Surveillance, Epidemiology and End Result (SEER) database to determine 5-yr survival rates for patients with thyroid cancer by race. They included 25,210 NHW and 1,692 NHB patients between 1992 and 2006. They found that NHB patients were 2.3 times more likely than NHWs to be diagnosed with anaplastic thyroid cancer, two times more likely to have tumors greater than 4 cm, and 1.8 times more likely to be diagnosed with follicular nodules (all P < 0.0001). NHB patients also had lower 5-yr survival compared with NHWs (96 vs. 97%; P < 0.006). They could not analyze surgeon volume data. The authors concluded that the differences observed likely stemmed from later disease presentation in NHB patients, and possibly genetic predisposition to more aggressive forms of thyroid cancer.
In a study of thyroid cancer incidence in Asian-American women from the California Cancer Registry, Horn-Ross et al. (220) found that age-adjusted incidence rates of papillary thyroid cancer among Filipino (13.7 per 100,000) and Vietnamese (12.7 per 100,000) women were more than double those of Japanese women (6.2 per 100,000). U.S.-born Chinese [incidence rate ratio (IRR) = 0.48; 95% CI, 0.40–0.59] and Filipino women (IRR = 0.74; 95% CI, 0.58–0.96) had significantly higher rates than those who were foreign-born, but the opposite was observed for Japanese women (IRR = 1.55; 95% CI, 1.17–2.08). The age-specific incidence among all foreign-born Asian women and U.S.-born Japanese women showed a steady increase in the incidence of thyroid cancer until age 70. However, among U.S.-born Asian women, elevated incidence rates during their reproductive years were evident (220).
In a study of the Indian Health Service patient database from 1999–2004, Weir et al. (221) examined various cancer rates in Native Americans compared with NHWs. Specifically related to thyroid cancer, they found that Native Americans had a lower relative risk (RR) of 0.71 when compared with NHWs, but these rates varied across Indian Health Service regions. The authors concluded that more studies are needed to address unique genetic, social, cultural, and environmental aspects of Native Americans.
Thyroidectomy is the main treatment for thyroid cancer. Sosa et al. (4) measured the effects of race and ethnicity on clinical and economic outcomes after thyroidectomy in patients with benign and malignant thyroid disease. This study used the Health Care Utilization Project National Inpatient Sample and included 16,878 patients who underwent thyroidectomy. They found that 71% of patients were NHWs, 14% NHB, 9% Hispanic-American, and 6% other. Mean length of stay was longer for NHBs (2.5 d) than for NHWs (1.8 d; P < 0.001). NHBs trended toward higher overall complication rates (4.9%), compared with NHWs (3.8%) and Hispanic-Americans (3.6%; P < 0.056). Mean total costs were significantly lower for NHWs, compared with those for NHBs and Hispanic-Americans.
Racial differences were noted not only in the extent of surgery, but also in administration of postoperative radioactive iodine. Famakinwa et al. (222) examined adherence to the American Thyroid Association (ATA) thyroid cancer treatment guidelines in the United States. Using the SEER database, the researchers assessed compliance with ATA guideline recommendations 26 and 27 (extent of surgery) and 32 (postoperative radioiodine administration) in a retrospective fashion. Among 52,964 patients with differentiated thyroid cancer, 71% of patients underwent treatment in compliance with the surgical recommendations, but only 62% had radioactive iodine treatment in compliance with recommendations (222). Compliance was lowest in patients over age 65 yr and among NHBs (P < 0.001) (222). The authors concluded that focus should be directed to dissemination of evidence-based recommendations and improved access for elderly and minority patients.
Biological factors contributing to race/ethnic disparities
Genetics.
Molecular studies point to several point mutations, which may play a role in papillary thyroid cancer. The most pertinent somatic mutation is BRAF V600E. Research has linked it with an aggressive tumor phenotype in papillary thyroid cancer, including larger tumors, more extrathyroidal extension, and local and distant metastases. Researchers have also linked the BRAF mutation with older age and higher recurrence rates (223). A meta-analysis of 1168 patients with papillary thyroid cancer showed no differences in BRAF status by race/ethnicity, suggesting that genetic factors do not contribute to race/ethnic differences in thyroid cancer survival (224).
Nonbiological factors contributing to race/ethnic disparities
Socioeconomic status and access to care.
Research has indicated disparities in stage at presentation and has associated this with socioeconomic and insurance status and ethnicity. Lim et al. (225) reported a retrospective review of a large public hospital and a university teaching hospital at New York University of 419 patients with differentiated thyroid cancer. The authors found that 96% of patients at the public hospital were ethnic minorities, mostly Asians and Hispanic-Americans, compared with 16% at the university hospital. Only 2% of patients had insurance at the public hospital, compared with 85% at the university hospital. Tumors were more advanced by stage, and extrathyroidal extension was more prevalent in patients at the public hospital. Public hospital patients were three times more likely to present with advanced disease than those at the university hospital. This poor access to adequate medical care contributes to the higher risk of complications and mortality from thyroid cancer in minority populations.
Minority populations also lack access to adequate surgical care for thyroid cancer. In one study, the majority of Hispanic-Americans (55%) and NHBs (52%) had surgery by the lowest-volume surgeons (one to nine cases per year), compared with only 44% of NHWs. Highest-volume surgeons (>100 cases per year) performed 5% of thyroidectomies, but 90% of their patients were white (P < 0.001) (4). These findings suggest that whereas health care professionals consider thyroidectomy safe, significant racial disparities exist in short-term clinical and economic outcomes. Inequities appear to result at least in part from racial differences in access to experienced surgeons, as discussed below.
Epstein et al. (226) identified access issues for minority patients in a study examining the use of high-volume hospitals and surgeons for 10 types of surgeries, including cardiovascular, cancer, and orthopedic procedures, in New York City. Among 133,821 patients examined, NHB patients were significantly less likely than NHW patients to be operated on by a high-volume surgeon at a high-volume hospital for nine of 10 procedures analyzed, whereas Hispanic and Asian-American patients had similar access to low-volume surgeons and hospitals for four and five of the procedures, respectively. Explanations for this access inequity could include systematic barriers, such as geographic location and financial reasons, as well as the known paucity of high-volume surgeons overall. NHW patients also may have access to better-informed referral networks. The literature has not extensively examined referral patterns; thus, we need future studies to inform policies aimed at addressing possible inequities based on patient race or ethnicity.
Summary, implications, and future research
There are a few notable differences in thyroid function in NHBs compared with NHWs, including lower TSH and iodine concentration in NHBs. We need additional studies to determine whether lower TSH levels have clinical implications for NHBs and whether the lower iodine concentrations increase their risk of goiter and hypothyroidism. The etiology of differences in thyroid cancer incidence in Asian-Americans based on their country of origin, and in Native Americans based on their region of residence in the United States, also requires further study. Finally, the medical community needs to develop policies to increase minority access to high-volume surgeons and earlier intervention in centers with appropriate endocrine and surgical expertise, so as to improve clinical outcomes and reduce mortality in minorities with thyroid cancer (especially NHBs).
Sex differences in benign thyroid disease
Epidemiology
Hypothyroidism and hyperthyroidism.
In NHANES, among individuals with no thyroid disease, mean TSH concentration and the percentage of individuals with TSH greater than or equal to 4.5 mIU/liter (hypothyroidism) were significantly higher in females than males (P < 0.01). The percentage of individuals with TSH less than 0.4 (hyperthyroidism) was significantly higher in females than males (P < 0.05).
Autoimmune thyroid disease.
The overall prevalence of positive antithyroid antibodies was significantly higher in females (17.0%) than males (8.7%) and increased with age. Females over 80 yr old had a 30.2% rate of positive antibodies compared with 12.3% of males over 80 yr old (210).
NHW females had a 55% increase in the prevalence of thyroiditis from the first decade onward, compared with minimal age effect in NHW males (211). The predominance of female patients presenting with autoimmune thyroid disease is significant. Jacobson et al. (227) reported that 95% of patients with thyroiditis and 88% of patients with Graves' disease are women. This may play a potential role in the development of thyroid cancer because Hashimoto's thyroiditis recently has been noted as a possible risk factor for harboring thyroid cancer (228).
Sex differences in thyroid cancer
Epidemiology
Thyroid cancer is the seventh most common malignancy in women, but it is not among the most common 15 cancers in men (229). It is 2.9 times more common in women than in men (female:male ratio, 16.3:5.7). Over the last 10 yr, localized disease had the greatest increase in incidence (5.2/100,000 in 1999 to 9.6/100,000 in 2008), but more advanced thyroid cancer also has increased in incidence (218). Differentiated thyroid cancer, including papillary and follicular histologies, accounts for 80% of the cases. There are sex differences in the incidence of specific histological subtypes of thyroid cancer. The rate of differentiated thyroid cancer in women is three times higher than in men. Age-specific incidence rises at the beginning of the reproductive years, with a peak incidence for women between ages 40 and 50 yr; in men, the peak is between ages 60 and 70 yr. By age 85 yr, the sex incidence equalizes. Women have an earlier age at diagnosis, but men have more aggressive disease, with a higher mortality rate (1.9 per 100,000 persons for women; 3.9 per 100,000 persons for men) (229). The more aggressive types of thyroid cancer, anaplastic thyroid cancer and medullary thyroid cancer, have similar rates of incidence in men and women.
Biological factors contributing to sex disparities in thyroid cancer
Genetics.
Sex association with BRAF mutation has been controversial and weak. Xu et al. (230) analyzed 80 tumor samples and observed that BRAF V600E mutation in men with papillary thyroid cancer was 64% compared with 27% in women. In another study of 203 patients with papillary thyroid cancer, BRAF V600E mutation was present in 91% of men (n = 35) compared with 70% of women (n = 168) (231). A meta-analysis of 12 studies with a total of 1168 patients (male:female ratio, 259:909) did not show a significant difference in mutation rate by sex, with 50% of the males and 49% of the females with papillary thyroid cancer having the BRAF V600E mutation. This suggests that genetics contributes little to the sex disparity in thyroid cancer (224).
Sex hormones.
Research indicates that sex hormones influence the higher incidence of differentiated thyroid cancer in women and may play a role in the observed sex disparity. Papillary thyroid cancer expresses estrogen receptors, mediating the effects of estrogen systemically. Lee et al. (232) investigated the effects of estradiol and testosterone on cell proliferation in papillary thyroid cancer cells. They showed that estradiol promotes cell proliferation, when compared with cells treated with testosterone or untreated cells, and that tamoxifen attenuated the growth-promoting effect (232). Researchers have made similar molecular observations in patients who are pregnant. Chorionic gonadotropin has a close homology with TSH, and evidence indicates that chorionic gonadotropin causes rapid growth of thyroid tumors (benign or malignant) during pregnancy (233)
Obesity.
Several studies examining possible environmental factors predisposing for thyroid cancer have focused on the impact of obesity, as well as patient height and anthropometric factors such as physical activity. BMI is particularly important as a possible risk factor because obesity has increased in the United States. BMI and weight gain vary widely among Americans of different sex, ethnic, socioeconomic, cultural, and educational backgrounds, as previously reviewed. In a study using NHANES data to measure BMI and body fat composition in the U.S. population, Heo et al. (234) noted that, on average, women had higher BMI than men, and that NHB and Hispanic women had significantly higher BMI and percentage of body fat than NHW women (BMI, 31.2 and 28.9 vs. 27.7 kg/m2, respectively; P < 0.0001), whereas there was no difference between men of different races.
A pooled analysis of five prospective studies reported a positive association of BMI and an increased risk of thyroid cancer in both men and women, whereas height was a risk factor in women but not in men (235). Studies also have examined weight in early adulthood and weight gain from early adulthood to middle age in relation to risk, finding a positive association between increasing body fat and cancer risk (236, 237).
Associations of obesity and physical activity with thyroid cancer risk are plausible and could contribute to sex differences. Studies have linked obesity with increased circulating hormone levels (including estrogens, androgens, insulin, and IGF-I), and studies link attained height with increased IGF-I. Estrogen may increase the risk of thyroid cancer through increased cell proliferation, whereas IGF-I is a potent mitogen and inhibitor of apoptosis, and its effects on thyroid growth are amplified by TSH (238, 239). Physical activity contributes to energy balance, may affect the levels of steroid hormones and insulin, and may improve insulin sensitivity, thereby modifying and reducing the risk of thyroid cancer (240, 241).
Summary, implications, and future research
Autoimmune thyroid disease and thyroid cancer are more common in women than in men; however, women are diagnosed at a younger age and have less aggressive disease and lower mortality from thyroid cancer than men. Genetics is likely not a significant contributor to this sex difference, but estrogen may play an important role in promoting proliferation of thyroid cancer cells. Finally, sex difference in obesity, which is more common in women, may also increase thyroid cancer risk through hormonal effects on thyroid cancer cells, and further basic science research is needed to elucidate these mechanisms. Preventing and treating obesity may also have a beneficial effect in lowering thyroid cancer risk in women.
Osteoporosis and Fracture Risk
Osteoporotic fractures are a major public health problem. Such fractures result in morbidity, disability, mortality and reduced quality of life. Understanding osteoporotic fractures is important for improving health care delivery and reducing fractures. Ethnicity and race are important factors that affect the incidence, risk factors, and treatment outcomes for these types of fractures. Understanding ethnic and racial influences on osteoporotic fractures is critical to decreasing the burden of such fractures on patients and society. Because there are sparse data on osteoporosis and fracture risk in men, and most data in men are summarized by race/ethnicity, we will also discuss sex differences in this section.
Given the current demographic trends leading to an increase in the number of persons older than 65 yr, the numbers of fractures will increase even if fracture incident rates remain stable. Moreover, improvements in life expectancy have been greater for minority populations. Given the increase in life expectancy among NHB populations and Hispanics, the number of fractures will increase in these groups. In 2005, 12% of all fractures occurred in minority populations. By 2025, this percentage will rise to 21% (242).
Epidemiology
Osteoporosis prevalence by bone mineral density (BMD)
The NHANES 2005–2006 estimated the prevalence of osteoporosis (T-score, ≥2.5) and osteopenia (T-score, 1 to less than −2.5) among U.S. adults age 50 and older (243). The study used 20- to 29-yr-old NHW women from NHANES III as the referent group for both men and women. The prevalence of osteopenia was 52, 36, and 38% among NHW, NHB, and Mexican-American women, respectively, and 33, 22, and 34% among NHW, NHB, and Mexican-American men. The prevalence of osteoporosis was similar in NHWs and Mexican-Americans, with lower rates among NHBs. For men, the prevalence of osteoporosis in NHW men was 2%. Insufficient data were available to estimate the prevalence of osteoporosis in NHB or Mexican-American men.
The National Osteoporosis Risk Assessment is an observational study of women carried out in the late 1990s. Primary care physicians identified women age 50 or older. Women underwent BMD testing using a variety of peripheral devices. The study calculated T-scores from the normative populations used by the manufacturers. This study is unique in that it includes multiple ethnicities. The prevalence of osteopenia and osteoporosis was lowest in NHB women. Despite lower fracture rates, the prevalence of osteopenia and osteoporosis was higher in Asian and Hispanic women compared with NHWs. Native American women also had higher prevalence than NHWs (Fig. 1).
Fig. 1.
Prevalence of osteopenia and osteoporosis by race/ethnicity. [Adapted from E. Barrett-Connor et al.: Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res 20:185–194, 2005 (446), with permission. © The American Society for Bone and Mineral Research.]
Fracture rates
Non-Hispanic Blacks.
There are marked differences in the sex-specific rates of hip and other fractures in the United States across race/ethnicity. Many publications have consistently shown lower rates of hip fracture in NHBs compared with NHWs: the rate of hip fracture in the United States is about 50–60% lower in NHB women compared with NHW women, and 30–40% lower in NHB men compared with NHW men. Recent data from Medicare (2000–2005) showed lower rates of all types of fractures in NHBs compared with NHWs, with some variability by fracture site (Fig. 2; reviewed in Ref. 244). Sex differences are greater for NHWs than NHBs. In both NHBs and NHWs, fracture incidence increases with age.
Fig. 2.
Fracture IRR among Medicare beneficiaries 2000–2005 by race/ethnicity. White: Referent group. [Adapted from A. J. Taylor et al.: Clinical and demographic factors associated with fractures among older Americans. Osteoporos Int 22:1263–1274, 2011 (447), with permission. © National Osteoporosis Foundation.]
Vertebral fractures are the hallmark of osteoporosis. Only 25 to 33% of all vertebral fractures come to medical attention (245, 246). As shown in Fig. 2, clinical vertebral fractures are about 75% lower in NHBs compared with NHWs. Less is known about radiographic vertebral fractures: a single study of older women reported a 33% lower prevalence among NHBs compared with NHWs (247).
Hispanic-Americans.
The ratio of hip fracture in Mexican-Americans living in California compared with NHWs living in California was 0.35 in women and 0.45 in men (248, 249). A similar ratio of hip fracture rates using the Medicare data and assigning ethnicity by surname was considerably higher (0.72 in women and 0.77 in men) (250). Zingmond et al. (249) updated their earlier paper and showed that hip fracture rates doubled in California Hispanic-Americans from 1983 to 2000. Results from a later study of hip fracture hospitalizations in New York City reported a rate ratio of 0.34 in Hispanic-American women and 0.25 in Hispanic-American men compared with NHWs (251). Some diversity within Hispanic-American subgroups is evident, with higher rates in Mexican-Americans compared with Cuban-Americans and Puerto Rican-Americans (250). In the most recent data from Medicare, the rate of hip and spine fracture was about 31% lower among Hispanics compared with NHWs. However, the incidence of distal radius and tibia/fibula fracture was more similar in the two ethnic groups. In the National Osteoporosis Risk Assessment (NORA) study, multivariate models, including adjustment for BMD T-score, yielded a hazard ratio (HR) of fracture of 0.91 (95% CI, 0.72–1.16) in Hispanics vs. NHWs, indicating a similar risk (252).
Asian-Americans.
Hip fracture rates in Asian-Americans are lower compared with NHWs, but the rate ratio varies markedly across studies and within different subgroups of the Asian population. Hip fracture incidence data are available for Asian-Americans in California (10), the Medicare population (250, 253), Japanese in Hawaii (254), and from New York City hospital discharge data (251).
There are limited data on non-hip fractures in Asian-Americans. In the Women's Health Initiative (WHI), the incidence of all clinical fractures in Asian-Americans was 1,200 per 100,000, compared with 2,000 per 100,000 in NHWs. The relative hazard of fracture in Asian-Americans compared with NHWs was 0.59 (95% CI, 0.53–0.65) (255). In NORA, the relative hazard of fractures in Asian-American vs. NHW women was 0.41 (95% CI, 0.21–1.79) in multivariate models, including BMD T-score (252). In a later publication, holding HR for T-score constant for all ethnic groups, Asian-Americans had a significantly lower HR for fracture (HR, 0.32; 95% CI, 0.15–0.66) (256). Using the recent Medicare data, the incidence of hip and distal radius fracture was about 35% lower in Asians compared with NHWs. The data showed smaller differences for clinical spine fractures (Fig. 2).
Native Americans.
In the WHI, during a mean of 7.6 yr, five of 417 (0.4%) Native American women experienced a hip fracture. To our knowledge, there is no other information on hip fractures in Native Americans. There are limited data on non-hip fracture rates in Native Americans. In the WHI, the incidence of fracture was similar in Native Americans compared with NHWs. The age-adjusted relative hazard of fracture in Native Americans was 1.03 (95% CI, 0.85–1.25) compared with NHWs (255). In NORA, the multivariable-adjusted (including BMD) relative hazard of fractures comparing Native Americans to NHW women was 0.89 (95% CI, 0.59–1.34) (252). To our knowledge there are no data on Native American men or within different Native American tribes.
Mortality and disability after osteoporotic fractures
Studies have linked both hip and vertebral fractures with an increased risk of mortality (257), but mortality after hip fracture is higher among NHB women than among NHW women (258). The factors contributing to the higher mortality rate among NHB women after a hip fracture are not completely understood, but could reflect their older age at the time of fracture, greater comorbidity, or disparities in health care. Indeed, the average number of diagnoses at admission to the hospital at the time of hip fracture is greater among NHB women than NHW women (259).
Hip fractures have serious consequences regarding mobility and independent living. About 50% of women are unable to achieve their prefracture walking ability after their hip fracture (260). Most of the research on the consequences of hip fracture has been carried out in NHW patients, but one small study suggests that the percentage of patients with hip fracture who were nonambulatory at the time of their discharge was six times higher among NHB women than among NHW women (259). We need more data on the consequences of osteoporosis in populations that are not NHW.
Biological and clinical factors contributing to race/ethnic disparities
Race/ethnic differences in BMD
BMD is a significant predictor of fracture risk and could account for at least some of the ethnic differences in fractures observed in population studies. Age-adjusted mean femoral neck BMD is approximately 10% higher in NHB women and 6.5% higher in Mexican-American women compared with NHW women (243). NHB men have a 10.7% higher femoral neck BMD, but there was no difference in BMD in NHW men or Mexican-American men (243). Unadjusted lumbar spine and femoral neck BMD are higher in premenopausal and early perimenopausal NHB women, next highest in NHW women, and lowest in Chinese- and Japanese-American women (261). When studies adjust BMD for ethnic differences in anthropometric and lifestyle factors that affect BMD, with careful adjustment for body weight, lumbar spine BMD is similar in NHB, Chinese-American, and Japanese-American women, all of whom have higher spine BMD than NHW women (261). For femoral neck BMD, adjustment for these same factors (most importantly body weight) reduces the advantage of NHB women, compared with other groups, and eliminates differences among Chinese-American, Japanese-American, and NHW women (261). Lifestyle and anthropometric factors account for some of the ethnic variation in BMD, although additional ethnic variation in BMD remains unexplained.
Skeletal risk factors for fracture
Bone mineral density.
For NHBs, at least 27 to 36% of their lower fracture risk is due to their higher BMD (247). For Hispanic-Americans, based on WHI data, BMD explains at least 17% of their lower fracture risk. There are no data to address this in Asian-Americans. The Study of Osteoporotic Fractures did a follow-up for 6.1 yr of 7334 NHW women (age, 67–99 yr) and 636 NHB women (age, 65–94 yr) (262). At every level of BMD, the fracture rate was lower in NHB than NHW women. In age-adjusted models, NHBs had a 64% lower risk of fracture compared with NHWs. The addition of femoral neck BMD to the model attenuated the effect such that the risk of fracture was 51% lower in NHBs. This suggests that approximately one third of the lower fracture risk in NHBs could be explained by BMD; however, NHB women still had a lower fracture risk even after adjusting for BMD and other risk factors. In this same study, the prevalence of morphometric vertebral fractures was 67% lower in NHB compared with NHW women, which was attenuated but still significantly lower after adjustment for femoral neck BMD (247).
In the NORA Study, the relative hazard of fracture was significantly lower in NHB (48% lower) and Asian-American (68% lower) women, compared with NHW women, independent of BMD, T-score, body weight, and other fracture risk factors (248, 250). In the WHI, the risk of fracture was significantly lower in NHB (49%), Asian-American (34%), and Hispanic-American (33%) women compared with NHW women (263). The addition of fracture risk factors, including body weight, to the models had little effect, suggesting that these risk factors could not explain the race/ethnic differences in fracture. In a subset of women with BMD measurements, adding BMD to the models did attenuate the risk reduction by about 33% in NHBs and 17% in Hispanic-Americans; however, fracture risk remained significantly lower in NHBs and Hispanic-Americans, even after adjusting for BMD (263).
Finally, it should be noted that although BMD is higher in NHB than NHW women, low BMD is still a risk factor for fracture in NHBs. In the Study of Osteoporotic Fractures, lower BMD resulted in a greater likelihood of prevalent vertebral fracture in both NHW and NHB women (247). The study linked a 1 sd decrease in femoral neck BMD with a 47% increased odds of prevalent vertebral fracture in NHB women and an 80% increased odds of prevalent vertebral fracture in NHW women (P value = 0.14 for interaction) (247).
Other skeletal traits—bone geometry and structure.
Race/ethnic differences in fracture rates appear independent of BMD. Asian women have lower fracture rates than NHWs, despite their similar areal BMD. Several investigators have described additional differences in bone size and geometry that could also contribute to ethnic differences in fracture rates. Studies have linked hip axis length, measured by dual-energy x-ray absorptiometry (DXA) scanners as the distance from the greater trochanter through the femoral neck to the inner pelvic brim near the acetabulum for the femoral head, with hip fractures. Women with shorter hip axis length have a lower risk of fracture (256). The mean hip axis length was also significantly shorter in NHB and Asian-American women and may also contribute to their lower fracture rates (264).
Hip structural analysis, developed by Beck et al. (265), estimates multiple dimensional and geometric variables using DXA. Results suggested some important race/ethnic variation. NHB women have longer, narrower femurs on average, with smaller medullary cavities than NHW women (266). The thicker cortices in NHB women could also contribute to greater mechanical strength. NHB women also had greater section modulus, an index of strength suggesting that the spatial distribution of bone is such that their bones can resist greater loads compared with NHW women.
Studies have also described geometric differences for Mexican-American and Native American women (267). Higher BMD in Mexican-American women was due to a smaller size of bone, not greater bone mineral content. NHBs, Mexican-Americans, and Native Americans all had greater section modulus than NHWs, which could contribute to their lower fracture rates.
More recently, composite indices of femoral neck strength, which integrate body size and bone size with bone density, may capture the major structural contributions to bone strength relative to bone loads applied to bone during falls (268). NHB, Chinese-American, and Japanese-American women all had significantly higher composite strength indices than NHW women (268). Studies observed these differences despite the lower areal BMD in Japanese-American and Chinese-American women and the higher body weight in NHB compared with NHW women. These indices may contribute to ethnic differences in fracture.
Studies have also described ethnic differences in rates of bone turnover (269), although these patterns do not necessarily parallel ethnic differences in BMD.
Menopause.
The most important risk factor for bone loss in women in midlife is the menopause. The Study of Women's Health Across the Nation (SWAN) enrolled 1902 NHB, NHW, Chinese-American, and Japanese-American women into a longitudinal study of BMD changes during the menopause (270). BMD loss accelerated markedly in the late perimenopause in each race/ethnic group. Lumbar spine BMD loss was most rapid in Japanese-American and Chinese-American women, intermediate in NHW women, and slowest in NHB women (P < 0.001). However, the definition of menopausal status was imprecise, and the time in which bone loss decelerates after the final menstrual period could not be determined. Therefore, a follow-up report from the SWAN described changes in BMD in relationship to the final menstrual period in a subset of women for whom researchers could determine the final menstrual period. Cumulative, transmenopausal changes were greatest for Chinese-American and Japanese-American women and lowest for NHB women (271). However, the absolute difference in bone loss by race/ethnicity was statistically significant but small—1 to 2%. Therefore, it is unlikely that this amount of bone loss at menopause could explain racial/ethnic differences in fracture rates. Nevertheless, the age at menopause may differ across race/ethnicity. In some studies, an earlier age of menopause was observed among NHB women (272). In SWAN, there was no difference in age at menopause for NHB women, but a much greater proportion of NHB women underwent a surgical menopause, which might influence osteoporosis risk (273). If this is true, then it could mean that NHB women are entering menopause at an earlier age, which could increase their risk for osteoporosis over time.
Nonskeletal risk factors for fracture
There are limited data on nonskeletal risk factors for fracture in multiethnic women. Most of the information comes from a single paper from the WHI that focused on all clinical fractures in women only, and BMD was not measured in all women (255). To our knowledge, there is essentially no information on risk factors for fracture in non-white men, other than a small study of 39 NHB men, nine of whom had a fracture. The only risk factor that was significant was older age among the NHB men who experienced fractures (274). The data we summarize below are from the WHI, unless otherwise indicated.
Body weight and height.
The WHI did not link weight to any clinical fractures in any race/ethnic group; however, WHI women were on average overweight or obese, so there was not much variability in body weight. In another study, however, low body weight was a risk factor for nonvertebral fractures (275). The association between weight and fracture may be strongest for hip fractures; in two case-control studies in NHB women, those who were underweight (276) and in the lowest quintile of BMI (277) had a significantly increased risk of hip fracture. The Hispanic Established Populations for Epidemiologic Studies of the Elderly study did not link BMI to hip fracture (253). The underlying mechanism for this association could reflect additional fat at the hip resulting in weaker impact and greater absorption of the force of the fall in more obese women. In addition, obesity is associated with higher endogenous estradiol levels and lower SHBG (278), both of which have been linked to hip fracture risk (279).
The WHI showed a significant association between height greater than 162 cm and an increased risk of all clinical fractures in NHWs and Hispanic-Americans (263). There was a similar trend in Asian-American women, although not statistically significant. There was no association between height and fracture risk in NHB or Native American women (Table 9).
Table 9.
Nonskeletal risk factors for fracture risk by race/ethnicity from the Women's Health Initiative and other studies
| Risk factor | NHWs | NHBs | Hispanic- Americans | Asian- Americans | Native Americans |
|---|---|---|---|---|---|
| Clinical risk factors | |||||
| Low body weight/BMI | ± | ± | ± | ± | ± |
| Low BMI | |||||
| OR = 13.5 (4.2, 43.3) | |||||
| Increased height | ↑ | − | ↑ | ± | − |
| HR = 1.14 (1.10, 1.18) | HR = 1.56 (1.11, 2.17) | HR = 1.46 (0.85, 2.52) | |||
| Previous fracture | ↑ | ↑ | ↑ | ↑ | ↑ |
| HR = 1.55 (1.49, 1.61) | HR = 1.74 (1.38, 2.20) | HR = 1.86 (1.39, 2.50) | HR = 1.50 (1.10, 2.05) | HR = 2.89 (1.47, 5.66) | |
| Parental fracture history | ↑ | ± | ↑ | ± | ± |
| HR = 1.18 (1.15, 1.22) | HR = 1.14 (0.96, 1.36) | HR = 1.28 (1.04, 1.58) | HR = 1.21 (0.96, 1.53) | HR = 1.58 (0.94, 2.67) | |
| Corticosteroid use | ↑ | ± | ↑ | − | ± |
| HR = 1.43 (1.22, 1.68) | HR = 1.54 (0.82, 2.90) | HR = 3.88 (1.89, 7.99) | HR = 0.73 (0.10, 5.27) | HR = 2.75 (0.26, 28.9) | |
| Current hormone therapy use | − | ± | − | ↓ | ↓ |
| OR = 0.1 (<0.1, 0.5) | HR = 0.7 (0.5, 0.8) | HR = 0.5 (0.3, 0.9) | |||
| Parity (>5 pregnancies) | − | − | − | ↑ | − |
| HR = 1.8 (1.1, 3.0) | |||||
| Nonclinical risk factors | |||||
| Sociodemographic factors | |||||
| Age | ↑ | ↑ | ↑ | ↑ | ↑ |
| Education (at least high school) | − | ↑ | |||
| HR = 1.22 (1.0, 1.5) | − | − | − | ||
| Health behaviors | |||||
| Smoking | − | − | − | − | − |
| Alcohol use | − | ± | − | − | − |
| History of at least two falls | ↑ | ↑ | ↑ | ↑ | − |
| HR = 1.27 (1.22, 2.32) | HR = 1.7 (1.9, 2.0) | HR = 1.8 (1.4, 2.3) | HR = 1.4 (1.1, 1.9) | HR = 1.38 (0.75, 2.55) |
Data on risk are indicated where available. Data in parentheses represent 95% CI.
, Data inconsistent regarding risk;
, no association with fracture risk;
, associated with increased fracture risk;
, associated with decreased fracture risk.
Prior fracture history.
The WHI linked prior fracture history with an increased fracture risk in all race/ethnic groups (Table 9) (263).
Parental history of fracture and ancestry.
The WHI linked parental history of any fracture with an increased risk of fracture in all race/ethnic groups, although it was not consistently statistically significant, perhaps reflecting the low power in some groups (Table 9) (263).
Individuals will self-identify with one race/ethnic group despite differences in admixture. In one study, the distribution of European ancestry among U.S. NHBs ranged from 0.1 to 89.95%, with a median of 18.7% (280). Using ancestry informative markers, studies have linked a greater amount of European admixture with lower BMD and weaker hip geometry in NHBs (280, 281). In the future, greater emphasis of these ancestral markers may improve our understanding of the impact of race and ethnic variability.
Medication use.
The WHI linked corticosteroid use for more than 2 yr with an increased risk of fracture in all race/ethnic groups, except Asian-Americans, although it was not statistically significant in NHBs or Native Americans (263). This analysis did not evaluate the risk by race/ethnic group associated with shorter courses of corticosteroids. Recent guidelines indicate that fracture risk is increased with 3 months or more of daily use of at least 5 mg of prednisolone or its equivalent (282); however, future studies are needed to determine whether this shorter exposure similarly predicts fracture risk in minority and NHW women. In a case-control study of NHB women, postmenopausal estrogen therapy for at least 1 yr was protective against fractures in members of this population under 75 yr of age (277). The WHI linked current hormone therapy use with a lower risk of fracture in Asian-American and Native American women (263).
Medical comorbidities.
In NHB women, the WHI linked a history of stroke with a 3-fold increased risk of fracture (277). In Hispanic-American women, the WHI linked the presence of diabetes with an increased fracture risk (283).
Parity.
In Asian-American women, the WHI linked having at least five pregnancies with a significantly higher risk of fracture (263).
Treatment efficacy
The majority of women enrolled in randomized controlled trials of osteoporosis medication efficacy have been NHW, largely due to enrollment criteria based on BMD or prevalent fracture status. However, there is no evidence that treatment efficacy varies by race, although the “evidence” is by far incomplete. A single trial of alendronate given to 65 NHB women with T-scores less than −3.0 showed that alendronate treatment increases BMD at all sites. The magnitude of the effect was similar to trials of NHW women (284). Vitamin D3 supplement of 2000 IU/d was sufficient to raise 25-hydroxyvitamin D [25(OH)D] levels to greater than 50 nmol/liter in almost all postmenopausal NHB women (285). Finally, as part of the WHI Hormone Trials, there was no evidence that the beneficial effect of hormone therapy on fracture reduction differed by race/ethnicity (286, 287).
Nonbiological factors contributing to race/ethnic disparities
Sociodemographic factors
Fracture rates in the WHI increased with age in all race/ethnic groups; however, in NHB women, rates did not increase until after 75 yr of age (263). In NHB women, the WHI linked having at least a high school education with a higher risk of fracture (263).
Health behaviors
The WHI did not link smoking with fracture in any ethnic group, but the prevalence of smoking was low in the WHI women, and thus they had low power (263). Alcohol consumption was unrelated to fracture risk in all race/ethnic groups in the WHI, but the study stratified intake at one or more drinks per day, and not at the FRAX level of three or more drinks per day (263). In two case-control studies in NHB women, higher alcohol consumption was associated with an increased risk of fracture (276, 277).
History of falls
The WHI linked a history of falls with a significantly higher risk of fracture in all race/ethnic groups, but the association was much stronger in NHB and Hispanic-American women (Table 9) (263).
Disparities in screening and treatment
Congress passed the Bone Mass Measurement Act in 1997 providing DXA reimbursement for qualified Medicare beneficiaries. Yet, despite almost universal access for women, at least for BMD testing (an approved indication is estrogen deficiency), few older Americans take advantage of this screening test. Using Medicare claims data from 2000–2005, Curtis et al. (288) showed that the proportion of 65-yr-old women who underwent central DXA increased from 8.4% in 1999 to 12.9% in 2005. Corresponding proportions of men were 0.6 to 1.7%, respectively. Nevertheless, a much lower proportion of NHBs underwent testing: for all years, 31.3% of NHW women vs. 15.3% of NHB women; and 4.6% of NHW men vs. 1.9% of NHB men. A retrospective chart review also reported that significantly fewer NHB women than NHW women were referred for DXA, although they had similar risk estimates (289). Physicians were also less likely to mention osteoporosis in the medical records of the NHB women or to recommend calcium or vitamin D supplementation, compared with NHW women.
NHB women with a history of fracture are also less likely to receive a diagnosis of osteoporosis. Using Medicare claims data (2000–2005), among women with a history of fracture, 33% of NHB women had an osteoporosis claim, compared with 59% of NHWs, 74% of Asian-Americans, and 58% of Hispanic-Americans (290). The data showed similar race/ethnic differences for men, but the overall proportion of men with an osteoporosis diagnosis was much lower than women. These results suggest suboptimal recognition and management of osteoporosis among NHB women and men.
NHBs were also substantially less likely to receive prescription osteoporosis medications than NHWs, even after adjusting for socioeconomic factors or clinical risk factors for fractures (291). In a large population-based cohort of almost 25,000 participants, the odds of NHBs receiving osteoporosis medication were 56% lower than that of NHWs. These results are similar to smaller studies that also showed disparity in screening and counseling in NHBs compared with NHWs (292, 293).
New treatment guidelines from the National Osteoporosis Foundation suggest that more than 40% of NHB women and more than 60% of Hispanic-American women older than 80 yr meet the guidelines for treatment (294), yet treatment disparities are evident. New treatment guidelines suggest that women with a 10-yr probability of hip fracture of 3% or greater should be recommended for therapy. However, roughly 20% of NHW women and less than 12% of NHB women who meet this guideline receive treatment. At every level of predicted hip fracture risk, the percentage of women receiving osteoporosis therapies is lower among NHB women (288).
Summary, implications, and future research
Although there are race/ethnic differences in osteoporosis and osteopenia as well as fracture risk, fracture risk factors common to all race/ethnicities include increasing age, prior fracture history, and history of falls, indicating that these factors identify all women at increased risk. Fracture risk also increases with the number of risk factors in women of every race/ethnic group (247, 255) and in older men (295). Thus, targeting older men and women with multiple risk factors for interventions is warranted to reduce fractures. Sex differences in fracture risk are greater for NHWs; however, there are little data on osteoporosis and fracture risk in minority men—a clear gap in the literature and an important area for future research.
With the exception of Asian-American women, minority women have higher BMD than NHW women, which translates into a reduced fracture risk in minority women. Asian-American women also have lower fracture risk than NHWs, despite the lower BMD. Studies suggest that differences in non-BMD skeletal traits may be protective in minority women. We need future research to determine whether these novel traits—bone size, bone geometry, hip axis length—differentially predict fracture risk in NHW and minority women, compared with BMD. In addition, the medical community needs to develop intervention strategies to preserve BMD in NHW women and protect them from the osteoporotic fractures. Genetic studies may shed light on whether certain genetic polymorphisms contribute to race/ethnic differences in BMD. In a prior review of studies examining racial contributors to differences in BMD, 15 of 40 articles suggested that genetic or inherited factors contributed, but studies did not include genetic data (296). Future studies should also determine whether race/ethnic differences in social or behavioral factors contribute to race/ethnic differences in BMD because these factors have been infrequently examined (296).
Despite the lower relative risk of fractures in minority women, their absolute risk of fracture is still substantial. In addition, NHB women suffer higher mortality and disability after fracture than NHW women. We need further research to determine the contributors to this morbidity difference. Fractures are more common in minority women than the combined number of myocardial infarctions, coronary heart disease deaths, and breast cancers (297). Using annualized incidence rates for all fractures, breast cancer, myocardial infarction, and coronary heart disease deaths observed in the Observational Study of the WHI, more NHB, Asian-American, Hispanic-American, and Native American women experience a fracture in 1 yr than the combined number of those who experience breast cancer and a composite CVD end-point. Thus, we need interventions to increase osteoporosis screening and treatment in minority women to prevent fractures and their associated morbidities.
Vitamin D deficiency
Epidemiology
Vitamin D is important for bone health as well as other metabolic disorders (298). The Institute of Medicine released new dietary reference intakes for calcium and vitamin D in November 2010. The Institute defined four categories of vitamin D status based on serum 25(OH)D levels: risk of deficiency (<30 nmol/liter), risk of inadequacy (30–49 nmol/liter), sufficiency (50–125 nmol/liter), and possibly harmful (>125 nmol/liter). In the United States from 2001–2006, 8% of persons age 1 yr and over were at risk of 25(OH)D deficiency, 24% were at risk of inadequacy, 67% were sufficient, and 1% had possibly harmful levels (299).
The season-adjusted prevalence of risk of deficiency, by age, ranged from 1 to 8% in men and 1 to 12% in women. The season-adjusted prevalence of risk of inadequacy, by age, ranged from 9 to 28% in men and 11 to 28% in women. Men were less likely to be deficient than women. The prevalence of risk of deficiency varied by race/ethnicity and was 3, 32, and 9% for NHWs, NHBs, and Mexican-Americans, respectively. There were also race/ethnic differences in the prevalence of risk for inadequacy: 18, 41, and 33% for NHWs, NHBs, and Mexican-Americans.
NHANES III (1988–1994) and NHANES (2001–2004) assessed serum 25(OH)D status of the U.S. population, and the prevalence of risk of deficiency and inadequacy increased from the earlier to the later study. Age-adjusted mean 25(OH)D levels were lower in NHBs compared with NHWs at every level of percentage body fat (300). Differences between NHWs and NHBs were greater among those ages 12–29 yr than among those age 70 yr and older, primarily due to lower mean levels among NHWs age 70 yr and older. The NHANES finding of lower 25(OH)D among NHBs is consistent with most other studies (301).
Higher 25(OH)D levels have been linked to a decreased risk of fracture in NHW women. In multivariable models, compared with 25(OH)D levels of less than 20 ng/ml, higher levels were associated with a lower risk of fracture in NHW women—for 25(OH)D levels of 20 to 30 ng/ml, odds ratio (OR) = 0.82 (95% CI, 0.58 to 1.16), and for 25(OH)D levels greater than 30 ng/ml, OR = 0.56 (95% CI, 0.35 to 0.90). In contrast, higher 25(OH)D levels (greater than 20 ng/ml) compared with levels of less than 20 ng/ml were associated with a higher risk of fracture in NHB women (OR = 1.45; 95% CI, 0.99 to 7.80; P trend = 0.04). These results suggest divergent associations between 25(OH)D for skeletal health by race/ethnicity and warrant further investigation in future studies to confirm or refute these findings. The optimal level of 25(OH)D for skeletal health may differ in NHW and NHB women (302).
Biological contributors to race/ethnic disparities
The etiology of lower vitamin D levels in NHBs compared with NHWs is likely multifactorial. NHBs are at higher risk for vitamin D deficiency because the high melanin concentration in their skin reduces the capacity to synthesize vitamin D (303). Studies have associated obesity with vitamin D deficiency, which researchers believe is caused by deposition of vitamin D into adipose tissue, decreasing the vitamin's bioavailability (303). Studies examining whether differences in adiposity contribute to race/ethnic differences in vitamin D deficiency have been conflicting. A study in women of childbearing age linked lower BMI with hypovitaminosis D in NHB women (303); however, it also linked obesity with hypovitaminosis D in both NHB and NHW older women (304) and NHB men (305). Genetic factors might also determine vitamin D levels. A systematic review identified several genetic variants that influence serum vitamin D levels—two SNP in vitamin D binding protein group-specific component (rs4588, rs7041), one SNP in the vitamin D receptor (rs10735810), and one SNP in a cytochrome P450 enzyme CYP27B1 (rs10877012) (306). Although this study did not look specifically at variation by race/ethnicity, a recent study examined the association of vitamin D levels with 94 SNP in five vitamin D pathway genes in a case-control study of NHBs and NHWs (307). They found statistically significant associations with three SNP, only in NHBs (rs2298849 and rs2282679 in the vitamin D binding protein group-specific component, and rs10877012 in the CYP27B1 gene) (307). The study associated a higher genetic score with lower vitamin D levels and a greater odds of vitamin D insufficiency; however, overall, these three SNP only accounted for 4.6% of the variation in serum vitamin D levels in NHBs, indicating the importance of nongenetic factors (307).
Nonbiological contributors to race/ethnic disparities
In both NHBs and NHWs, studies have associated lack of vitamin D supplementation with lower vitamin D levels (303, 304); even among NHB men and women taking vitamin D supplements, the prevalence of vitamin D deficiency is higher than in NHWs (303, 305). Studies have linked levels of dairy intake with vitamin D deficiency in NHBs, with less than three servings of milk or cereal each week being associated with deficiency in NHB women and higher milk consumption being associated with less deficiency in NHB men (303, 305). In NHB men, higher levels of intentional physical activity were associated with a lower likelihood of vitamin D deficiency (305). Finally, the environment can also be an important determinant of vitamin D levels. Among NHB women, studies associated urban residence with vitamin D deficiency, which may be related to less outdoor activity and sun exposure or more exposure to environmental pollutants that decrease the skin's synthesis of vitamin D (303).
Summary, implications, and future research
Vitamin D deficiency is a common endocrine disorder, particularly among NHBs. The etiology of the race/ethnic differences is multifactorial, with biological, behavioral, and environmental contributors. Genetic factors likely only play a minor role in race/ethnic differences in vitamin D levels. We need intervention strategies to increase vitamin D supplementation use among NHBs, and we need additional research to determine why vitamin D deficiency still occurs among NHBs taking vitamin D supplementation. Basic science research is necessary to determine molecular contributors to differences in vitamin D synthesis and metabolism by race/ethnicity. Finally, we need future population-based studies to determine the prevalence of vitamin D deficiency in other race/ethnic groups, including Asian-Americans and Native Americans.
Conceptual Framework for Health and Health Care Disparities
Our conceptual framework, adapted from Warnecke et al. (308), combines a population health framework with a quality of care framework to elucidate the causes of disparities in endocrine conditions and to guide our approach to understanding and eliminating these disparities (308, 309). In this section, we will focus on how our framework explains race/ethnic disparities in diabetes and its complications because that is where much work has been focused; however, the framework is also relevant to other endocrine conditions, and we will highlight its application to those disorders where appropriate.
This framework recognizes population-level determinants of health outcomes as distinct from, yet intertwined with, individual-level determinants of health (Fig. 3). The model identifies proximate, intermediate, and distal factors that influence health. Proximate determinants of health include biological and genetic pathways, biological responses, individual health behaviors, and individual demographic and social factors. Genetic ancestry, epigenetic changes induced by environmental stress, and allostatic load (reviewed below under Proximate factor: biological pathways and responses), induce biological responses that may increase disease risk. It is important to note, however, that to date there is little evidence of genetic factors that explain disparities in rates of type 2 diabetes (310) or thyroid disease, as we already reviewed. Biological responses include poor glycemic control, obesity, hyperlipidemia, poor blood pressure control, and longer-term complications of diabetes, including CVD, renal disease, and stroke. Among the individual behaviors that influence disease risk or outcomes are diet, tobacco and alcohol use, medication adherence, and disease self-management. Individual demographic characteristics affect the individual's capacity to respond to environmental challenges and include factors such as age, race/ethnicity, language, literacy, cultural beliefs, education, income, social support, and acculturation.
Fig. 3.
Conceptual framework model for disparities in endocrine disorders. [Adapted from R. B. Warnecke et al.: Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health 98:1608–1615, 2008 (308), with permission. © American Public Health Association.
The intermediate determinants of health include neighborhood- or community-level physical and social environments, as well as the health care delivery environment. The physical context includes factors such as neighborhood disorder and accessible recreational facilities and healthy foods. The social context includes factors that may mitigate or intensify distal effects, such as community-level poverty, resources, or social interactions; and the health care delivery context includes access to care, quality of care, provider characteristics, and aspects of the patient-provider relationship. We hypothesize these intermediate determinants to be the mechanism through which the distal social and policy factors influence individual behavior. Finally, the distal factors include population social conditions (e.g. poverty, discrimination, prejudice), policies that affect social conditions, and the organizations and institutions that determine or influence such policies.
Our disease-specific sections have focused on biological pathways and responses, genetics, and certain individual demographic and health behaviors and their role in race/ethnic disparities in diabetes, thyroid disease, and osteoporosis and fracture risk. In the sections below, we begin with the individual level most proximal factors we have not previously addressed and then discuss the role of intermediate and distal factors in contributing to endocrine disparities, using diabetes as the exemplar condition.
Proximate factor: biological pathways and responses
The concept of allostatic load—impact of chronic stress on biological systems
Seyle first suggested that chronic stress might have a cumulative damaging effect on human biological systems (311, 448). Researchers introduced the concept of allostasis to describe the capacity of an organism to adapt to a changing environment or stressful challenge to support homeostatic systems essential to life (311, 312). Allostatic load summarizes the cumulative impact of physiological wear and tear related to adaptations that predispose individuals to disease (311). McEwen (313) described four maladaptive stress patterns, which can occur alone or in combination: 1) excessive and repeated stimulation over time; 2) inability to habituate to stressful stimuli; 3) inability to sufficiently recover from heightened, activated responses; and 4) inadequate response of a primary system, resulting in compensatory mechanisms. The biological systems involved in adaptation that mediate the link between stress and physiological functions are the hypothalamic-pituitary-adrenal axis, autonomic nervous system, and immune system (311, 314). Measuring mediators and/or outcomes related to alterations in these systems reflects potential indices of physiological dysfunction (311, 314). Researchers developed allostatic load scores to estimate stress-induced physiological dysfunction by summing the number of parameters for which individuals fall into the highest-risk quartile (311). Examples of physiological parameters include cardiovascular (systolic and diastolic blood pressure, cholesterol measures), metabolic (WC, HbA1c), hypothalamic-pituitary-adrenal axis (dehydroepiandrosterone sulfate, cortisol), autonomic function (heart rate, urinary catecholamines), and immune function (cytokines, albumin) (311). In the MacArthur studies, high allostatic load scores predicted incident CVD and all-cause mortality, independent of socioeconomic status and baseline morbidity (449, 450).
Several studies have examined race/ethnic and sex differences in allostatic load using data from NHANES. NHB populations have significantly higher allostatic load than NHW populations, after adjusting for socioeconomic status, with NHB women being most adversely affected (315–317). In one study, NHB women 40–49 yr old had allostatic load scores 1.14 times greater than NHW women 50–59 yr old, suggesting adverse physiological effects in NHB women at a younger age (315). Allostatic load in Hispanic-Americans depends on their country of nativity. U.S.-born Mexican-Americans have higher allostatic load scores than those born in Mexico who subsequently immigrate to the United States (315, 318, 319), suggesting that acculturation may be associated with maladaptation to stressors; however, in one study that measured differences in acculturation, these differences did not explain this disparate association. Recent results from the Boston Puerto Rican Health Study significantly associated increasing categories of allostatic load with several endocrine disorders, including abdominal obesity and diabetes (320). Thus, differences in stress exposure and subsequent higher allostatic load in minorities, related to socioeconomic status, acculturation, physical or social environment, or chronic discrimination, might contribute to disparities in development of and outcomes for endocrine disorders and requires further study.
Proximate factors: individual demographic characteristics
Individual risk behaviors
Most agree that racial/ethnic and socioeconomic differences in health behaviors and self-care behaviors are a main reason for disparities in health among adults with diabetes (321). A complex range of self-care behaviors is required to manage diabetes, among them dietary restrictions, regular exercise, adherence to and management of multiple medications and dosing schedules, SMBG, periodic examination of the feet, and maintaining glycemic control during intercurrent illness. Among adults with diabetes, studies associate minority race/ethnicity, low income, less education, and living in a poverty area with higher rates of smoking (322–325), lower rates of SMBG (326–329), less vigorous exercise (322, 324, 330, 331), poorer adherence to medications (332), and underuse of recommended preventive health screening (333, 334).
Although one study (332) suggested that an important mediator of the association between minority status and poorer glycemic control was poorer adherence to medication among NHBs and Hispanic-Americans, other research did not find this association between race and adherence (335). The lack of consistent findings suggests that more work is needed to understand the causal pathways underlying disparities in diabetes control.
Socioeconomic status
The well-known socioeconomic gradient in health certainly applies to diabetes on the individual and aggregate level. Diabetes incidence data using the NHANES I Epidemiologic Follow-Up Study (1971–1992), showed a strong inverse relationship with income, education, and occupational status (336). Women who had more than 16 yr of education had a much lower risk for incident diabetes compared with women who had less than 9 yr of education (HR, 0.26; 95% CI, 0.13–0.54). Among men, these trends were evident but much less consistent. A meta-analysis of 21 studies showed that there is an increased risk of type 2 diabetes when comparing the lowest to the highest groups in terms of education (RR = 1.41), occupation (RR = 1.31), and income (RR = 1.40) (337). Likewise, national data from the National Health Interview Survey showed that, among people with diabetes, CVD prevalence was higher, and all-cause mortality was 28% higher for those with the lowest educational levels, compared with the highest (338, 339). As already reviewed, lower socioeconomic status is also associated with a higher prevalence of gestational diabetes (144). Socioeconomic status is also associated with poor metabolic control in non-U.S. countries. In Germany, the United Kingdom, and Spain, individuals of lower socioeconomic status had higher HbA1c, worse dyslipidemia, and higher rates of obesity (340). Even independent of race, sex, and lifestyle factors, allostatic load is negatively associated with education and income, suggesting one potential mechanism through which socioeconomic stress may increase metabolic disease risk (341).
Language barriers
Spanish-speaking Hispanics in the United States are less likely than those who speak English to have a regular source of care. They also receive less screening, report lower rates of use of preventive services (342–344), and are less satisfied with their health care (345), when compared with either NHWs or English-speaking Latinos. In a study of a managed care population, patients with type 2 diabetes who reported difficulty with English monitored their blood sugars less frequently than persons with no language barriers (328).
Social support and competing demands for time
Evidence is strong for a relationship between having supportive social ties and better physical and mental health (346) and, conversely, between social isolation and greater morbidity and mortality (347). In the aggregate, among persons with diabetes, studies have associated higher levels of social support with better self-management, including adherence to recommended diet and exercise regimens, better glycemic control (348), and less vulnerability to social stressors (349). Social support may be particularly important for minority or low-income patients with diabetes. For example, NHBs with diabetes rely more heavily on informal social networks to meet their disease management needs (348), and poorer persons are more likely to be socially isolated and to have fewer supportive social ties (350). Although empiric data are sparse on how social support influences health outcomes for persons with diabetes, better self-management skills and improved access (through the health-seeking behaviors of the persons themselves or their social network) and quality (because of better communication with physicians) likely play a role. On the other hand, social ties may result in detrimental effects, as in the case of obligations that result in financial expenditures, demands on time, and criticism from social contacts (351). In addition, the health care needs of persons who are caregivers for disabled elders can compete with the physical and emotional needs of their dependents, and these caregivers often fail to obtain adequate health care for themselves (352). These effects are likely accentuated among the poor, NHBs, and possibly other ethnic minorities, among whom the caregiver role is common (353).
Health beliefs and perceptions
Race/ethnic differences in self-perception may lead to disparities in endocrine disorders. This has been studied most in the area of obesity and how it relates to body image. In NHBs, there is less stigma associated with overweight and obesity, and prior studies show that NHB women accept a body image considered overweight and larger than that preferred by NHW women (354, 355). Despite the higher prevalence of overweight and obesity in NHB women, they report higher levels of body image satisfaction than NHW women (356, 357). This comfort with a larger body size may increase the risk for obesity-related disorders and create a disconnect between patients and physicians regarding an ideal body weight.
Intermediate factors: physical and social contexts
Neighborhood socioeconomic and physical characteristics
The medical community increasingly recognizes the contribution of factors above and beyond individual behavior as major contributors to health. An explosion of research within the field of public health has investigated how these upstream factors might contribute to diabetes and related conditions. For social inequalities on a macro-level, there is solid evidence that energy-dense foods cost less, and that healthier diets, consumed more by affluent persons, cost more (358). For example, the energy cost of fresh produce is 10 times as much as that of vegetable oils and sugar. Studies have consistently shown that minority and disadvantaged populations live in inferior neighborhood environments with respect to food stores, places to exercise, esthetic problems (such as vacant houses), and traffic or crime-related safety. Studies have shown that these inferior neighborhood environments are independently associated with individuals' diet quality and with walking and exercise behaviors (359, 360).
Studies directly associate disadvantaged and minority neighborhoods with poor health outcomes through a number of different pathways, including aspects of the physical environment such as lack of healthy food stores and places to exercise, and aspects of the social environment including psychosocial stressors related to crime and limited social cohesion (359, 360). Studies have also associated lower neighborhood socioeconomic status with worse allostatic load. One study showed how this association is stronger in NHBs than NHWs and Mexican-Americans. Overall, communities with lower socioeconomic status have fewer resources available that promote healthy behaviors and facilitate good health. The last 15 yr of research on neighborhood environments has focused mostly on obesity or behaviors that influence obesity (diet and physical activity) (361). Overall, studies show consistent findings for the food environment, demonstrating that poor access to supermarkets was associated with higher BMI (362). For physical activity, studies associated greater neighborhood walkability and better access to recreational resources with lower BMI (363). Review articles done specifically for NHB (359) and disadvantaged (360) populations show consistent results with the general population, but comment that more research is needed. These review articles also provide evidence for intervention targets that might be useful for reducing health disparities in these populations. Although sparse, there is some evidence linking the neighborhood environment directly to clinical outcome such as diabetes. Two studies from the Multi-Ethnic Study of Atherosclerosis associated better neighborhood access to physical activity and food resources with lower insulin resistance (364) and type 2 diabetes (365). There are also studies that link the perception of neighborhood problems among people with diabetes with more smoking, physical inactivity, and poorer control of blood pressure, after adjusting for individual factors (366).
Poor persons, particularly those with a chronic condition like diabetes, are more likely to experience multiple dimensions of neighborhood disadvantage, and management of a chronic disease may be much more difficult in low socioeconomic status areas (367). Many believe that several community-level characteristics contribute to disparities in diabetes management. Price disincentives to eating healthy food are greater in poorer than in wealthier neighborhoods (368), and low-income communities have fewer supermarkets than more affluent neighborhoods (369, 370). Moreover, access to foods recommended for adults with diabetes (e.g. fresh fruits and vegetables, whole grains, diet sodas, and low-fat milk), is limited in low-income and predominantly minority areas (371, 372). Ease of access to parks and recreational facilities and the safety of the neighborhood may influence the ability or willingness of persons with diabetes to engage in regular exercise, another important strategy in diabetes management. Transportation may be an underappreciated element in managing diabetes and other endocrine disorders because lack of transportation is an important barrier to receiving appropriate health services (373) and may also influence other environmental factors, such as access to food, health care, and social networks (374).
Social relationships within communities
Much of the research conducted on the social environment has demonstrated the benefits of social cohesion and social capital in neighborhoods. For example, collective efficacy is the willingness of people to intervene for the good of the community, and the linkage of mutual trust within a community (375). Studies have linked collective efficacy to better neighborhood outcomes including lower crime, as well as better health outcomes such as rates of overweight and obesity and overall mortality (375). Another study by Christakis and Fowler (376) looked at social networks and found not only that obese persons were in discernible clusters, but also that a person's chance of becoming obese was 57% greater if he or she had a friend who became obese in a given time interval. Furthermore, an experimental study that randomized public housing residents to receive vouchers to move to lower poverty areas found that these respondents reported less neighborhood poverty, more collective efficacy, and lower rates of obesity and diabetes (377).
Intermediate factors: health care system context
Characteristics of the health care system
Because racial/ethnic minorities may be clustered in certain health care systems (378) or within a few facilities associated with a health care network, the kinds of systems in which minority patients receive their care might explain disparities in care and outcomes for diabetes and endocrine disorders (379, 380).
Health care organizations can promote access (for example, by reducing financial barriers to care), and many features of health care organizations can improve diabetes outcomes and/or reduce diabetes disparities (381–383) (see Interventions Shown to Be Effective in Reducing Disparities in Clinical Processes and Outcomes: Diabetes as an Exemplar Condition). Health system factors, however, also have the potential to negatively affect access, process, and health behaviors and to worsen health outcomes, particularly for minority or socioeconomically disadvantaged patients. Management of specialty referrals through a gatekeeper may lead to more appropriate referral patterns (384, 385), but restrictions on referrals to specialists that are differentially applied to patients who are poorer, less-educated, or have lower literacy levels may adversely influence health. Furthermore, although health care organizations may promote intensive diabetes management, data from two recent studies found little evidence that obtaining care in health care systems with more intensive diabetes management (e.g. through referral systems, diabetes registries, or provider feedback) or through intensive quality improvement efforts, reduced racial/ethnic or socioeconomic disparities in quality of care. This suggests that the reduction of many disparities requires more tailored approaches (386, 387).
Some financial and organizational arrangements may pose greater obstacles for persons of lower socioeconomic status. Many studies have focused on patient copayments, and low-income persons might be particularly sensitive to even modest changes in cost sharing because they spend a higher proportion of their income on out-of-pocket expenses than do higher-income enrollees (388). Specifically, studies have associated the introduction of new or higher copayments for glucometer strips with lower rates of SMBG among lower income patients (325, 328).
Financial incentives for physicians and physician organizations may affect clinical outcomes, and these effects may be more pronounced for low socioeconomic status patients. For example, more-educated patients and those who do not face language barriers may be more effective at negotiating with clinicians or health plans to have their needs met in the face of potential disincentives to provide care (389); and wealthier patients have a greater ability to purchase alternate health care. Recent evidence also suggests that physician organizations located in lower socioeconomic status communities may receive lower pay-for-performance incentive bonuses than those in wealthier communities, even after adjusting for other characteristics of the organization (390).
Health care access and health insurance
Inadequate access to care (e.g. due to a lack of health insurance) may be an important contributor to the current state of disparities in outcomes for endocrine disorders. Diabetic adults with only a primary or secondary education have more contacts with their general practitioner and dieticians but fewer visits with specialists (e.g. endocrinologists) or diabetes nurses, and they undergo fewer assessments of their body weight and receive fewer influenza vaccinations (391).
For persons with diabetes, access to health insurance is important for the receipt of high-quality care (326, 392, 393). Although over 90% of patients with diabetes have some form of health insurance (394, 395), a much greater proportion of minorities than NHWs with diabetes lacked insurance (394, 395). Compared to those with insurance, uninsured adults with diabetes receive fewer recommended processes of care, including dilated eye examinations, foot examinations, and other preventive health care services (326); have poorer intermediate and longer-term outcomes, such as poorer glycemic control (396); and have higher odds of developing diabetic eye disease (397). Among Latinos with diabetes, studies have shown that lack of insurance results in higher rates of microvascular complications (398). Similarly, thyroid cancer patients seen at a public hospital were significantly less likely to have health insurance and more likely to present with advanced thyroid cancer compared with those seen at a university hospital (225).
The persistence of some disparities, even in managed care settings (378) and in countries with universal health insurance coverage (329, 331, 399–401), suggests that neither access to care nor having insurance is sufficient to improve diabetes outcomes. In a meta-analysis of studies conducted in the Organization for Economic Cooperation and Development countries with universal health care systems, certain race/ethnic groups were not adequately controlled (340). In New Zealand, Europeans had better glycemic control than other ethnic groups, and in the United Kingdom, non-European women with gestational diabetes had worse glycemic control than their European counterparts (340).
Quality of care
Studies have associated race and ethnicity (378, 395, 402, 403), income (404–407), and education (326, 408) with gradients in processes of diabetes care. In one national study, minorities with diabetes were less likely to have a lipid test and a dilated ophthalmological examination than were NHWs (409). In contrast, another study found that ethnic disparities in quality and access to care can be substantially reduced, if not eliminated, within health care organizations that provide uniform care to large populations (403). Still, although there have been exceptions, reports from diverse settings indicate that the quality of diabetes care for socially disadvantaged persons is inferior to that of more affluent persons (326, 407, 410). Even in countries with universal health care, race/ethnic inequalities exist in the quality of care that patients with diabetes receive. Diabetic individuals in the United Kingdom living in high immigrant areas were less likely to be prescribed oral diabetic agents (340). In New Zealand, “other Asians” were less likely to be prescribed aspirin, antihypertensives, or insulin compared with Europeans, and Maoris and Pacific Islanders were less likely treated with diet changes and statins compared with Europeans (340). Studies have been mixed regarding the use of preventive services among minority populations in the Organization for Economic Cooperation and Development countries, with some showing great use of ophthalmological screening and preventive services (e.g. black Africans and Afro-Caribbeans vs. Europeans in the United Kingdom, and aboriginals vs. non-aboriginals in Canada), and others showing less use in minority populations (e.g. minority populations vs. Europeans in the United Kingdom) (340).
Similarly, minority patients with other endocrine disorders receive lower quality of care. Minority patients with thyroid cancer receive inadequate surgical care (4) and are less likely to be operated on by a high-volume, experienced surgeon (226), contributing to poorer clinical outcomes. Among women who meet DXA screening criteria for osteoporosis, NHB women are significantly less likely to be referred for screening, less likely to have osteoporosis mentioned in their medical record, and less likely to have their physicians recommend calcium or vitamin D supplementation (289). NHB women are also less likely to be prescribed osteoporosis medications (288).
Provider characteristics and patient-provider relationships
Patient-provider communication.
Effective communication between patients and providers (411, 412) and shared decision-making (413, 414) positively influence health behaviors and the process and outcomes of care for persons with diabetes. NHB patients experience lower quality patient-provider communication. Although evidence links patients' ratings of providers' general and diabetes-specific communication with self-care for diabetes (415, 416), communication is more likely to be suboptimal for lower socioeconomic status patients. For example, physicians are more likely to adopt a more directive, less participatory approach with less-educated patients, who are then less likely to have their expectations met (344).
Language barriers and health literacy and numeracy.
Language barriers appear to influence diabetes self-care behaviors and outcomes, and a provider's ability to successfully communicate with patients may counter the effects of inadequate English proficiency and poor comprehension of verbal instructions (417). Health literacy is “the ability to read and comprehend prescription bottles, appointment slips, and the other essential health-related materials required to successfully function as a patient” (418). Persons with type 2 diabetes who have low functional health literacy are less likely to know the symptoms of hypoglycemia, have more difficulty understanding physicians' explanations, have lower rates of shared decision-making, and have poorer glycemic control (389, 419–421). Although low health literacy poses risks for health outcomes for patients with diabetes, there is recent evidence to suggest that using strategies such as the “teach-back” technique, in which a patient repeats in his or her own words what needs to be done once he or she leaves the clinician's office, can increase patients' understanding of the medical information conveyed despite their low literacy (422).
Poor health numeracy is also an important contributor to race/ethnic disparities in glycemic control. Diabetes-related numeracy assesses calculation, interpretation of tables, graphs, or figures, and application of numeracy skills to solve problems and perform diabetes self-management care (423). Low diabetes-related numeracy is associated with NHB race and poor glycemic control, regardless of race. Adjusting for diabetes-related numeracy explained the difference in poorer glycemic control in NHBs compared with NHWs. Interventions to improve numeracy may reduce race/ethnic disparities in diabetes outcomes (423).
Discrimination and trust in health care providers.
Stereotypes about the behaviors or health of low socioeconomic status or disadvantaged minority patients and provider bias against these patients, even when unintentional or subtle, may influence the provider's willingness or ability to provide counseling on healthy behaviors and disease management, reinforce the tendency of patients of low socioeconomic status not to engage in these behaviors, lead to differential treatment of minority patients, and affect patient health through internalized racism (3, 424–426). These attitudes not only interfere with the provision of care but may also reduce a patient's confidence and self-efficacy, further undermining treatment, behavior, and adherence (427).
In one study, 8% of minority patients with diabetes reported discrimination by their doctors or the office staff due to their race, education, or income, and patients who reported racial discrimination in health care settings also reported worse health and glycemic control (428). In another study, however, researchers eliminated the association between discrimination and poor glycemic control after adjustment for health status and sociodemographic factors such as race/ethnicity and insurance. Studies have linked the provision of culturally competent care with better rapport, trust, and exchange of information (417). Patients' and providers' attitudes may influence each other reciprocally; if patients convey mistrust, demonstrate poor adherence, and refuse treatment, providers are likely to become less engaged in the treatment process and less clinically aggressive (3).
Distal factors: policies and social conditions
A broad range of federal, state, health care system, and clinical policies affect patients with diabetes, their clinicians, the hospitals and clinics where they seek care, their insurers, and the public health systems that implement diabetes prevention and control (429). For example, at the clinic level, a policy that required office staff to request that patients with diabetes remove their shoes in the examination room increased rates of foot exams by 60% (430). At the health plan or provider group level, implementation of the six integrated elements of the “Chronic Care Model” (self-management support, delivery system design, decision support, clinical information systems, structured health care organization, and community interactions) has improved processes of care, outcomes, and provider satisfaction (431–433). The most widely studied policies are related to reimbursement. Federal policies, such as the Balanced Budget Act in 1997 and the Medicare Modernization Act in 2003, expanded coverage for medications and for care outside the hospital setting. For patients with diabetes, policies such as these resulted in enhanced reimbursement for diabetes self-management training, and higher levels of coverage for prescription medications, diabetes supplies, and prescription medications. As discussed in the Osteoporosis and Fracture Risk section, the Bone Mass Measurement Act passed by Congress in 1997 provided DXA reimbursement for qualified Medicare beneficiaries; however, only a small proportion of eligible patients take advantage of osteoporosis screening, especially NHBs (291). This suggests that having policies in place may not be entirely adequate to change practice.
Health care is implementing a range of policies that may have direct or indirect impact on diabetes outcomes. For example, New York City's “A1c Registry” requires mandatory reporting of HbA1c test results. We will need to closely monitor the effect of this population-based registry on provider and patient behavior and levels of glycemic control (434). The Affordable Care Act, by improving support for the patient-centered medical home model (http://www.nachc.com/client/documents/CHCs%20ROI%20final%2011%2015%20v.pdf and http://www.pcpcc.net/joint-principles), has the potential to improve the quality of primary care and patient and provider experiences (437). The impact of the patient-centered medical home model on diabetes processes and outcomes requires further study, particularly in safety net settings, such as community clinics (http://www.fqhcmedicalhome.com/demooverview.aspx and Ref. 439). Policies that support the integration of community and health care system interventions to address both the medical and nonmedical factors that contribute to diabetes risk and outcomes might also be a promising approach to addressing health disparities (440). We need additional studies, however, to understand the impact of these new and emerging policies on diabetes disparities.
Finally, social conditions can have a significant impact on population-wide metabolic outcomes. Between 1989 and 1995, Cuba experienced a prolonged economic crisis secondary to a significant reduction in imports, local fuel supply, agricultural products, and many food items (441). These changes led to decreased per capita daily energy intake (from 2899 to 1863 calories) and increased walking and cycling due to lack of public transportation (441). During this period, there was a significant increase in the percentage of physically active individuals and normal-weight individuals and a significant decline in the percentage of overweight individuals (441). In addition, there were significant declines in the deaths secondary to diabetes and CVD as well as all-cause mortality (441). These data highlight the impact of social conditions on metabolic disorders and suggest that population-wide approaches to reducing caloric intake and increasing physical activity might have beneficial effects.
Interventions Shown to Be Effective in Reducing Disparities in Clinical Processes and Outcomes: Diabetes as an Exemplar Condition
A growing body of evidence identifies interventions that reduce disparities in health care or improve the outcomes of minority populations. Here, we summarize the literature on health care interventions to improve the care or outcomes of racial/ethnic minorities with diabetes. We chose diabetes because this condition captures the diverse set of factors previously described in the conceptual model for disparities; because many interventions have attempted to reduce diabetes disparities; and finally because most lessons from the diabetes literature are likely transferable to other endocrine conditions.
Health care can implement disparity interventions at one or more levels of influence: patients, providers, microsystems (immediate health care environment and delivery team), health care organization, community, and policy (442). A key maxim of implementation science is that the local context is critical. Although it is possible to draw general lessons from the literature, the translation of these findings into real-world practice requires adaptation to local culture, resources, incentives, patient preferences, and provider buy-in.
Lessons from the diabetes disparities intervention literature
Peek et al. (383) reviewed the diabetes disparities intervention literature between 1985 and 2006, and Baig et al. (443) updated that review between 2006 and 2009. These reviews summarize each individual study and have found themes of successful interventions that target different levels of influence.
Patient
Successful interventions tended to leverage interpersonal connections such as face-to-face encounters, social networks, family, peer support groups, and community health workers, rather than technological, computer-based ties. Culturally tailored interventions were more effective than generic patient self-management education and have resulted in improved glycemic control and diabetes-related knowledge (444).
Provider
Similarly to patients, interventions incorporating in-person feedback were more effective than computerized decision-support reminders in changing provider behavior and improving diabetes outcomes. Cultural competency training for providers, combined with diabetes performance reports stratified by patient race/ethnicity, have improved provider awareness of disparities, but not diabetes process and outcome measures (445).
Microsystem/health care organization
Care management interventions were effective. Key components of these programs include patient education that tackles barriers to adherence, ancillary services such as laboratory testing or linkage to home health, and addressing fundamental problems like transportation to the clinic. Also, when nurse and community health workers managed patients with treatment algorithms or had physician support, they were more effective in improving processes and outcomes than if they served solely as care managers. Disease management has improved diabetes care and outcomes. Components of disease management include: 1) identification of the diabetes population, for example through patient registries; 2) practice guidelines; 3) use of health information technology to track and monitor patients; and 4) care management. Although not specifically focused on disparities or minority populations, the Centers for Disease Control and Prevention's systematic reviews of diabetes case management and disease management from 2002 also support the use of these two interventions (http://www.thecommunityguide.org/diabetes/casemgmt.html and http://www.thecommunityguide.org/diabetes/diseasemgmt.html).
Community/Health Care System
A Centers for Disease Control and Prevention REACH 2010 initiative in Charleston and Georgetown, South Carolina, that integrated community and health care interventions, is one of the few efforts that not only improved minority health care, but also reduced racial and ethnic disparities in care (9). This ambitious initiative included patient education and empowerment, community coalition building and advocacy, community health workers, provider audit and feedback, quality improvement, and case management. An intervention on the south side of Chicago incorporating culturally tailored patient education and shared decision-making, a quality improvement collaborative, provider training in behavioral change, and community partnerships shows early signs of improved diabetes care and outcomes (440). Such combined community health care system approaches have been relatively uncommon, but are likely to increase with the emergence of policies incentivizing global payment approaches, including accountable care organizations and bundled episodes of payment.
Policy
Studies are lacking that examine policies to reduce diabetes disparities such as financial incentives (10). A pay-for-performance scheme introduced into United Kingdom primary care practices improved outcomes for all ethnic groups, but widened some disparities (11). However, it is difficult to make global conclusions about pay-for-performance because schemes can vary by factors such as the amount of the incentive, whether the individual provider or group is incentivized, whether payment is based upon attaining an absolute threshold of performance or relative improvement, and whether any safeguards are implemented such as rules to discourage cherry picking of well-insured, relatively healthy, advantaged patients.
Lessons from the general disparities intervention literature
The Robert Wood Johnson Foundation Finding Answers: Disparities Research for Change Program found six cross-cutting themes of successful disparity interventions based on 12 systematic reviews of conditions such as CVD, depression, diabetes, and asthma (12). These themes are: 1) target multiple barriers a patient faces rather than rely on a single solution; 2) culturally tailor the intervention; 3) use multidisciplinary teams; 4) employ interactive, skills-based patient training rather than passive learning approaches; 5) use patient navigators who can help patients work their way through the medical system; and 6) involve family and community. These themes emphasize a comprehensive, tailored approach to addressing the multiple factors impacting disparities.
A roadmap for reducing racial and ethnic disparities in health care
For a disparity intervention to be successfully implemented, health care providers, staff, and administrators must view the intervention within a wider roadmap of the process for improving quality of care and reducing disparities (12). The roadmap draws upon key principles of implementation science and translational research:
Recognize disparities and commit to reducing them. Create readiness to change on the part of the individual provider and organization. Raise awareness using cultural competency training and individual provider performance data stratified by patient race/ethnicity.
Implement a basic quality improvement structure and process.
Make equity an integral component of quality improvement efforts. Do not marginalize disparity intervention efforts.
Design the intervention(s). Adapt the intervention(s) to the local environment.
Implement, evaluate, and adjust the intervention(s). Revise interventions after pilot testing.
Sustain the intervention(s). Institutionalize the intervention(s) into standard practice, and align incentives appropriately.
Conclusion
We summarized important areas for future research in areas of race/ethnic and sex disparities in endocrine disorders based on our literature review for this statement in Table 10. We also summarize in Table 11 areas for future research to address race/ethnic disparities in diabetes generated from the 2011 Strategic Planning Report of the Diabetes Mellitus Interagency Coordinating Committee (18, 19) that supplement those highlighted by our review. Several important themes emerged in examining our current understanding of disparities in endocrine disorders. Compared with NHW populations, there are certain disorders for which NHB populations have a lower incidence (e.g. macrovascular complications of diabetes mellitus and osteoporotic fractures) or a similar incidence (e.g. thyroid cancer); however, once they have the disorder, NHBs have worse outcomes and higher mortality. This is likely related to inadequate access to high-quality health care among minority populations. In contrast, NHBs with diabetic nephropathy have lower mortality on dialysis than NHWs, despite a higher incidence of ESRD. Understanding the factors contributing to better survival will be beneficial in extending dialysis survival for other race/ethnic groups. Although osteoporotic fractures are more common in NHW women, preventive interventions should target all race/ethnic groups to reduce the morbidity and mortality associated with fractures. In addition to exploring the etiology for these disparities in clinical, population-based, and health services research studies, basic science and translational studies are also needed to explore underlying molecular mechanisms that may contribute to disparities.
Table 10.
Summary recommendations for future research
| Race/ethnic disparities | Sex disparities |
|---|---|
| Diabetes mellitus | |
| Future research needs: biological and clinical factors | Future research needs |
| Inclusion of specific ethnic subgroups in studies of diabetes epidemiology and physiology. | Determine whether the risk of type 2 diabetes occurs at different BMI and WC thresholds in NHB vs. NHW women. |
| Elucidate reasons for differences in diabetes prevalence and incidence and glucose metabolism in Cuban, Central, and South Americans compared to Mexican- and Puerto Rican-Americans. | |
| Elucidate reasons for differences in diabetes prevalence and incidence among different Asian-American subgroups and the contribution of acculturation and immigrant status. | |
| Elucidate other nonobesity contributors to the higher insulin resistance seen in minority populations compared to NHWs because these differences are independent of adiposity. | |
| Basic science investigations needed to determine whether race/ethnic differences in insulin signaling contribute to greater insulin resistance in minority populations. | |
| GWAS needed in minority populations | |
| Design studies targeting perinatal prevention of fetal undernutrition and fetal-maternal stress as a diabetes preventive strategy in NHBs. | |
| Future research needs: nonbiological factors | |
| Determination of the contribution of acculturation to obesity and diabetes risk in Asian- and Hispanic-Americans. | |
| Elucidation of diet, physical activity, and health behavior differences in minority populations compared to NHWs and their contributions to ethnic differences in diabetes risk. | |
| Diabetes complications | |
| Future research needs: epidemiology of complications | Future research needs |
| Epidemiological data summarizing prevalence and incidence of lower extremity amputation and arterial disease in Asian-Americans. | Elucidation of the etiology for higher coronary heart disease risk and lower peripheral arterial disease risk in women compared to men with diabetes. |
| Exploration of etiology of regional differences in diabetes and diabetes complication rates in Native Americans. | Determination of reason for sex differences on the effects of CVD risk factors in different arterial beds through basic science and clinical research studies. |
| Exploration of the etiology of heterogeneity in diabetes complications among Asian-American subgroups. | Studies of clinical and nonclinical contributors to diabetic complications stratified by sex and race/ethnicity, as suggested above. |
| Exploration of contributors to higher diabetes mortality in minority populations compared to NHWs. | |
| Future research needs: biological and clinical factors | |
| Studies of prevalence and control of glycemic and cardiovascular risk factors in minorities with diabetes mellitus. | |
| Basic science, clinical, and population-based research studies to evaluate multifaceted (glycemic and nonglycemic) causes of ethnic variations in HbA1c, especially in Asian, Hispanic, and Native American populations (analogous to studies comparing NHBs and NHWs). | |
| Basic science and clinical research studies to examine the contribution of low birth weight, fetal undernutrition, and fetal-maternal stress to microvascular complications in NHBs with diabetes mellitus. | |
| Basic science, clinical, and translation research studies to determine the etiology of the survival advantage of NHBs on dialysis for diabetic nephropathy, which may lead to better understanding of how to improve survival in dialysis patients of other race/ethnicities. | |
| Basic, clinical, and population-based research studies to determine the contributors to the higher risk of microvascular but lower risk of macrovascular complications among NHBs with diabetes. | |
| Future research needs: nonbiological factors | |
| Development of policies and interventions to remove barriers to self-monitoring of blood glucose in ethnic minorities with diabetes mellitus. | |
| GDM | |
| Future research needs: biological factors | |
| Determine the prevalence of significant polymorphisms in the BetaGene Study in other high-risk populations for GDM, especially Asian-Americans. | |
| Examine gene-environment interactions with clinical factors—adiposity, physical activity, and diet. | |
| Future research needs: nonbiological factors | |
| Determine contribution of social and economic factors to race/ethnic disparities in GDM: socioeconomic status, mental stress, built environment. | |
| Examine the contribution of documented disparities in access to care in women with prior GDM on screening for GDM and type 2 diabetes, quality of care, and quality of life. | |
| Metabolic syndrome | |
| Future research needs | Future research needs |
| Determine whether current metabolic syndrome criteria similarly predict type 2 diabetes and CVD in NHBs and NHWs. | Determine whether race/ethnic-specific cutoffs for WC in the metabolic syndrome definition better identify NHB women at risk for metabolic disorders. |
| Determine whether race/ethnic-specific cutoffs for TG in the metabolic syndrome definition better identify NHBs at risk for metabolic disorders. | |
| Determine whether race/ethnic-specific cutoffs for WC in the metabolic syndrome definition better identify Asian-Americans at risk for metabolic disorders. | |
| Thyroid disorders | |
| Future research needs | Future research needs |
| Determine whether the lower TSH in NHBs translates into bone and cardiac effects compared to NHWs. | Determine the etiology of the higher prevalence and incidence of autoimmune thyroid disease in women compared to men through basic science and clinical research studies. |
| Determine whether the lower iodine concentration in NHBs is clinically significant and contributes to development of goiter and hypothyroidism. | Determine whether lifestyle interventions to promote weight loss and physical activity in obese women will lower the risk of thyroid cancer. |
| Determine the etiology of differences in thyroid cancer incidence in Asian-Americans based on country of origin and in Native Americans based on region of residence in the U.S. | Determine the etiology of compromised thyroid cancer survival in men as targets for future interventions. |
| Develop interventions and policies to increase minority access to high volume surgeons and earlier intervention in centers with endocrine and surgical expertise. | |
| Assess molecular pathways for thyroid cancer at a mutational level to determine whether there are differences by race, which may suggest different prognoses and targeted therapies. | |
| Osteoporosis/metabolic bone disease | |
| Future research needs | Future research needs |
| Determine non-BMD contributors to lower fracture rates in minority women compared to NHWs. | Generate data on fracture rates, risk factors for fracture, and incidence of disability and morbidity associated with fracture in minority men. |
| Vitamin D deficiency | |
| Determine whether there are genetic factors or differences in cell signaling that contribute to the higher BMD in certain minority populations compared to NHBs. | |
| Determine the etiology of the lower fracture risk in Asian-American women compared to NHW women despite lower BMD. | |
| Determine whether novel skeletal traits—bone size, bone geometry, hip axis length—differentially predict fracture risk in NHW and minority women compared to BMD. | |
| Develop intervention strategies to preserve BMD in NHW women and protect them from the osteoporotic fractures. | |
| Determine why NHB women suffer higher mortality and disability after fracture than NHW women. | |
| Develop interventions strategies to increase osteoporosis screening, diagnosis, and treatment in minority women to prevent fractures and their associated morbidities. | |
| Develop intervention strategies to increase vitamin D supplementation in NHBs. | |
| Conduct basic and translation science studies to determine whether race/ethnic differences in molecular signaling or vitamin D metabolism contribute to lower levels in NHBs. | |
| Determine the prevalence of vitamin D deficiency in minority populations, particularly Asian and Native Americans. |
Table 11.
Research needs related to reducing disparities in diabetes and diabetes complications from the 2011 Diabetes Mellitus Interagency Coordinating Committee
| Diabetes mellitus |
| Identify racial and ethnic differences in the interactions among antenatal care, diet, gestational glycemic burden, and other aspects of the intrauterine environment, and the risk of diabetes and obesity in childhood, adolescence, and adulthood. |
| Determine what behavioral strategies work well to promote and sustain effective lifestyle change in minority individuals at high risk for developing diabetes. |
| Determine what behavioral strategies are effective in promoting and sustaining adherence to diabetes treatment regimens in individuals belonging to racial and ethnic minority groups, to improve health outcomes. |
| Determine how the outcomes of the Diabetes Prevention Program can be translated in diverse settings and populations to prevent type 2 diabetes in youth and adults. |
| Determine the best approaches to optimize cardiometabolic risk reduction in diverse populations with prediabetes or type 2 diabetes. |
| Determine health care interventions that are effective at reducing disparities in diabetes outcomes. |
| Determine when culturally tailored interventions are necessary and more effective, as opposed to using more general interventions, in reducing health disparities in diabetes outcomes. |
| Determine how health communication science can be harnessed for the reduction of health disparities in the prevention and control of diabetes. |
| Determine how multilevel interventions, combining policy/marketing, community, organization, delivery system, provider, and patient/family components, can be implemented and sustained to improve diabetes care and outcomes. |
| Diabetes complications |
| Identify nontraditional biological risk factors that contribute to underlying race/ethnic disparities in the development of diabetes and its complications. |
| Determine the “nonbiological” (i.e. environmental, social, cultural, and economic) factors across the lifespan that affect diabetes prevalence and progression to complications in different ethnic and racial groups. |
| Develop novel interventions to address the racial/ethnic disparities in diabetes incidence, complication rates, and mortality, and that take into account pharmacogenomics, environmental, social, cultural, and economic factors. |
Reproduced from two chapters in Complete Diabetes Research Strategic Plan (http://www2.niddk.nih.gov/AboutNIDDK/ReportsAndStrategicPlanning/DiabetesPlan/PlanPosting.htm): Special Needs for Special Populations (18) and Clinical Research to Practice: Translational Research (19).
Obesity is an important contributor, not only to diabetes risk in minority populations, but also to sex disparities in thyroid cancer, which is more common in women. This suggests that population interventions targeting weight loss may have a favorable impact, even on non-diabetes-related endocrine disorders. Our statement also highlights important implications regarding the definition of obesity in different race/ethnic groups. Fat distribution is an important determinant of risk of metabolic disorders such as type 2 diabetes and CVD, and there is evidence that current cut-points for WC may underestimate disease risk in Asian-Americans and overestimate disease risk on NHB women. We need future studies to define ethnic-specific cut-points for central obesity. Although we did not address obesity as a stand-alone disorder in this statement, there is growing understanding of the neuroendocrine regulation of appetite and energy expenditure that contribute to obesity (435); however, basic science and translational studies are needed to determine whether these mechanisms differ by race/ethnicity.
Sex disparities exist in certain endocrine disorders, with autoimmune thyroid disease and cardiovascular complications of diabetes being more common in women than men. The etiology of these sex differences remains unclear and warrants further basic and clinical research. NHW females suffer osteoporotic fractures more frequently than their male counterparts; however, data on the prevalence and incidence of osteoporotic fractures in minority men are lacking, suggesting a need for further investigation given the aging of our population.
A major gap in our current understanding of race/ethnic disparities in endocrine disorders is a failure of most studies to specify Hispanic-American and Asian-American subgroups. In this statement, we have attempted to specify ethnic subgroups where they were identified in certain studies; however, the majority of studies did not clearly define subgroups. Where these data are available, there are clear ethnic-specific differences within Hispanic and Asian subgroups, particularly related to diabetes and obesity risk. Thus, it is imperative that future studies accurately identify ethnic subgroups. In general, except for diabetes, there are few data on endocrine disorders in Native Americans and Alaska Natives, an important population for future research as well.
For the endocrine disorders discussed in this statement, there is little evidence that genetic differences are a significant contributor to race/ethnic disparities; however, there is likely significant heterogeneity and admixture among populations, which may mask genetic differences in GWAS studies. As recently reviewed, however, there are emerging techniques to overcome these challenges in performing GWAS studies in minority populations (436). Future genetic studies should incorporate ancestral markers to account for admixture and more specifically address the contribution of race/ethnicity to disease risk and disparities. There is a paucity of data on race/ethnic differences in responses to various treatments for endocrine disorders, and determining whether these differences exist will allow us to implement more patient-specific treatment regimens.
Our improved awareness and knowledge of interventions regarding disparities in diabetes needs to be expanded to other endocrine disorders for which race/ethnic disparities exist. Specifically, it is important to study whether social determinants of disease presented in our health disparities framework, specifically the physical and community context, are important contributors to disparities in vitamin D deficiency and poor outcomes after thyroid cancer diagnosis and osteoporotic fractures in minority populations. In the field of diabetes, multilevel interventions targeting the patient, provider, health care organization, community and health care system, and policy have reduced disparities in diabetes care and improved quality of care. These successes can be used as a roadmap for designing similar interventions to improve quality of care for endocrine disorders where there is currently a gap—specifically reducing mortality and improving outcomes after thyroid cancer treatment and osteoporotic fractures. Understanding the biological, clinical, and nonclinical contributors to disparities in endocrine disorders will allow the design of effective interventions to reduce these disparities and improve public health.
Finally, according to a 2010 American Association of Medical Colleges survey, just under 27% of our current endocrine workforce is represented by minority physicians—16.9% Asian, 5.7% Hispanic, 3.6% NHB, and 0.3% Native American or Alaska Native—despite several endocrine disorders having a higher burden in these populations (438). Increasing the representation of ethnic minorities in both the clinical and research sectors of endocrinology and diabetes will help to raise awareness and prioritize research funding and policy development to reduce endocrine disparities.
Acknowledgments
We thank Jose Florez, M.D., and James Meigs, M.D., MPH, for assistance in identifying appropriate literature for the diabetes genetics section. We thank Eric Vohr for his technical writing and organization expertise in finalizing our statement. We thank Ivy Garner and Claire Twose for their expertise in information management and referencing assistance. We thank The Endocrine Society and Loretta Doan, Ph.D., for their support and guidance in preparing this statement.
S.H.G. was supported by a Program Project Grant for the Johns Hopkins Center to Eliminate Cardiovascular Health Disparities from the National Heart, Lung, and Blood Institute (P50 HL0105187). A.B. was supported by the National Center for Research Resources (Grant UL1RR033176), which is now at the National Center for Advancing Translational Sciences (Grant UL1TR000124). M.H.C. is supported by a Midcareer Investigator Award in Patient-Oriented Research (K24 DK071933) from the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) and the Chicago Center for Diabetes Translation Research (P30 DK092949). C.K. is supported by Grants R01DK083297 and K23DK071552 through the NIDDK. A.E.S. is supported by the intramural program of NIDDK, National Institutes of Health (NIH).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure Summary: S.H.G., A.B., T.L.G.-W., C.K., A.E.S., and B.A. have nothing to declare. J.A.C. receives consultant honorarium from Merck and Company, Inc., and Novartis. M.H.C. receives grant funding from Merck Company Foundation. J.A.S. received honorarium from Veracyte for a one time visiting speaker engagement.
Footnotes
- BMD
- Bone mineral density
- BMI
- body mass index
- CI
- confidence interval
- CVD
- cardiovascular disease
- DXA
- dual-energy x-ray absorptiometry
- ESRD
- end-stage renal disease
- GDM
- gestational diabetes mellitus
- GWAS
- genome-wide association study
- HbA1c
- glycosylated hemoglobin
- HDL-C
- high-density lipoprotein cholesterol
- HLA
- human leukocyte antigen
- HR
- hazard ratio
- IRR
- incidence rate ratio
- NHB
- non-Hispanic black
- NHW
- non-Hispanic white
- 25(OH)D
- 25-hydroxyvitamin D
- OR
- odds ratio
- RR
- relative risk
- SMBG
- self-monitoring of blood glucose
- SNP
- single nucleotide polymorphism
- TG
- triglyceride
- VAT
- visceral adipose tissue
- WC
- waist circumference.
References
- 1. Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. 2009. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab 94:1853–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association 2012. Standards of medical care in diabetes–2012. Diabetes Care 35(Suppl 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smedley BD, Stith AY, Nelson AR. 2002. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: The National Academies Press; [PMC free article] [PubMed] [Google Scholar]
- 4. Sosa JA, Mehta PJ, Wang TS, Yeo HL, Roman SA. 2007. Racial disparities in clinical and economic outcomes from thyroidectomy. Ann Surg 246:1083–1091 [DOI] [PubMed] [Google Scholar]
- 5. Committee on Understanding the Biology of Sex and Gender Differences, Board on Health Sciences Policy 2001. Exploring the biological contributions to human health: does sex matter? Wizemann TJ, Pardue ML, eds. Washington, DC: The National Academies Press; [PubMed] [Google Scholar]
- 6. Williams DR. 1997. Race and health: basic questions, emerging directions. Ann Epidemiol 7:322–333 [DOI] [PubMed] [Google Scholar]
- 7. Karter AJ. 2003. Commentary: race, genetics, and disease—in search of a middle ground. Int J Epidemiol 32:26–28 [DOI] [PubMed] [Google Scholar]
- 8. Karter AJ. 2003. Race and ethnicity: vital constructs for diabetes research. Diabetes Care 26:2189–2193 [DOI] [PubMed] [Google Scholar]
- 9. Jenkins C, McNary S, Carlson BA, King MG, Hossler CL, Magwood G, Zheng D, Hendrix K, Beck LS, Linnen F, Thomas V, Powell S, Ma'at I. 2004. Reducing disparities for African Americans with diabetes: progress made by the REACH 2010 Charleston and Georgetown Diabetes Coalition. Public Health Rep 119:322–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chien AT, Chin MH, Davis AM, Casalino LP. 2007. Pay for performance, public reporting, and racial disparities in health care: how are programs being designed? Med Care Res Rev 64:283S–304S [DOI] [PubMed] [Google Scholar]
- 11. Millett C, Netuveli G, Saxena S, Majeed A. 2009. Impact of pay for performance on ethnic disparities in intermediate outcomes for diabetes: a longitudinal study. Diabetes Care 32:404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chin MH, Clarke AR, Nocon RS, et al. 2012. A Roadmap and Best Practices for Organizations to Reduce Racial and Ethnic Disparities in Health Care. J Gen Intern Med 10.1007/s11606-012-2082-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. 2009. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care 32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christensen DL, Witte DR, Kaduka L, Jørgensen ME, Borch-Johnsen K, Mohan V, Shaw JE, Tabák AG, Vistisen D. 2010. Moving to an A1C-based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care 33:580–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dagogo-Jack S. 2010. Pitfalls in the use of HbA (c) as a diagnostic test: the ethnic conundrum. Nat Rev Endocrinol 6:589–593 [DOI] [PubMed] [Google Scholar]
- 16. Narayan KM, Aviles-Santa L, Oza-Frank R, Pandey M, Curb JD, McNeely M, Araneta MR, Palaniappan L, Rajpathak S, Barrett-Connor E. 2010. Report of a National Heart, Lung, and Blood Institute Workshop: heterogeneity in cardiometabolic risk in Asian Americans in the U.S. Opportunities for research. J Am Coll Cardiol 55:966–973 [DOI] [PubMed] [Google Scholar]
- 17. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diabetes Mellitus Interagency Coordinating Committee 2011. Special needs for special populations. Advances and emerging opportunities in diabetes research: a strategic planning report of the Diabetes Mellitus Interagency Coordinating Committee. Available at: http://www2.niddk.nih.gov/NR/rdonlyres/9F57E99F-4EA1-49DA-BA1B-EAA2DF0C546F/0/DSP2011_10_SpecialNeeds_508.pdf
- 19. Diabetes Mellitus Interagency Coordinating Committee 2011. Clinical research to practice: translational research. Advances and emerging opportunities in diabetes research: a strategic planning report of the Diabetes Mellitus Interagency Coordinating Committee. Available at: http://www2.niddk.nih.gov/NR/rdonlyres/C040182E-CDF2-4C2A-86BB-2A6500542BCB/0/DSP2011_12_TranslationalResearch_508.pdf
- 20. Flegal KM, Carroll MD, Kit BK, Ogden CL. 2012. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307:491–497 [DOI] [PubMed] [Google Scholar]
- 21. Pleis J, Lucas J. 2009. Summary health statistics for U.S. adults: National Health Interview Survey, 2007. National Center for Health Statistics. Vital and Health Statistics, series 10, no. 240. Available at: http://www.cdc.gov/nchs/data/series/sr_10/sr10_240.pdf [PubMed]
- 22. Dagogo-Jack S. 2003. Ethnic disparities in type 2 diabetes: pathophysiology and implications for prevention and management. J Natl Med Assoc 95:774, 779–789 [PMC free article] [PubMed] [Google Scholar]
- 23. Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkänen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE. 1996. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 45:742–748 [DOI] [PubMed] [Google Scholar]
- 24. Donahue RP, Bean JA, Donahue RA, Goldberg RB, Prineas RJ. 1997. Insulin response in a triethnic population: effects of sex, ethnic origin, and body fat. Miami Community Health Study. Diabetes Care 20:1670–1676 [DOI] [PubMed] [Google Scholar]
- 25. Carnethon MR, Palaniappan LP, Burchfiel CM, Brancati FL, Fortmann SP. 2002. Serum insulin, obesity, and the incidence of type 2 diabetes in black and white adults: the Atherosclerosis Risk in Communities Study: 1987–1998. Diabetes Care 25:1358–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, Burkey B, Heshka S, Gallagher D. 2005. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 82:1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rasouli N, Spencer HJ, Rashidi AA, Elbein SC. 2007. Impact of family history of diabetes and ethnicity on -cell function in obese, glucose-tolerant individuals. J Clin Endocrinol Metab 92:4656–4663 [DOI] [PubMed] [Google Scholar]
- 28. Chow CC, Periwal V, Csako G, Ricks M, Courville AB, Miller BV, 3rd, Vega GL, Sumner AE. 2011. Higher acute insulin response to glucose may determine greater free fatty acid clearance in African-American women. J Clin Endocrinol Metab 96:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiu KC, Chuang LM, Yoon C. 2001. Comparison of measured and estimated indices of insulin sensitivity and β cell function: impact of ethnicity on insulin sensitivity and β cell function in glucose-tolerant and normotensive subjects. J Clin Endocrinol Metab 86:1620–1625 [DOI] [PubMed] [Google Scholar]
- 30. Torréns JI, Skurnick J, Davidow AL, Korenman SG, Santoro N, Soto-Greene M, Lasser N, Weiss G. 2004. Ethnic differences in insulin sensitivity and β-cell function in premenopausal or early perimenopausal women without diabetes: the Study of Women's Health Across the Nation (SWAN). Diabetes Care 27:354–361 [DOI] [PubMed] [Google Scholar]
- 31. Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. 2002. β-Cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 51:2170–2178 [DOI] [PubMed] [Google Scholar]
- 32. Boyko EJ, Keane EM, Marshall JA, Hamman RF. 1991. Higher insulin and C-peptide concentrations in Hispanic population at high risk for NIDDM. San Luis Valley Diabetes Study. Diabetes 40:509–515 [DOI] [PubMed] [Google Scholar]
- 33. Ferrannini E, Gastaldelli A, Matsuda M, Miyazaki Y, Pettiti M, Glass L, DeFronzo RA. 2003. Influence of ethnicity and familial diabetes on glucose tolerance and insulin action: a physiological analysis. J Clin Endocrinol Metab 88:3251–3257 [DOI] [PubMed] [Google Scholar]
- 34. Resnick HE, Bergman RN, Henderson JA, Nez-Henderson P, Howard BV. 2002. Utility of a surrogate measure of insulin resistance in American Indians: the Strong Heart Study. Ethn Dis 12:523–529 [PubMed] [Google Scholar]
- 35. Nagulesparan M, Savage PJ, Knowler WC, Johnson GC, Bennett PH. 1982. Increased in vivo insulin resistance in nondiabetic Pima Indians compared with Caucasians. Diabetes 31:952–956 [DOI] [PubMed] [Google Scholar]
- 36. Berk ES, Kovera AJ, Boozer CN, Pi-Sunyer FX, Albu JB. 2006. Metabolic inflexibility in substrate use is present in African-American but not Caucasian healthy, premenopausal, nondiabetic women. J Clin Endocrinol Metab 91:4099–4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Russell AP. 2004. Lipotoxicity: the obese and endurance-trained paradox. Int J Obes Relat Metab Disord 28(Suppl 4):S66–S71 [DOI] [PubMed] [Google Scholar]
- 38. Sirikul B, Gower BA, Hunter GR, Larson-Meyer DE, Newcomer BR. 2006. Relationship between insulin sensitivity and in vivo mitochondrial function in skeletal muscle. Am J Physiol Endocrinol Metab 291:E724–E728 [DOI] [PubMed] [Google Scholar]
- 39. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. 2004. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCarthy MI. 2010. Genomics, type 2 diabetes, and obesity. N Engl J Med 363:2339–2350 [DOI] [PubMed] [Google Scholar]
- 41. Waters KM, Stram DO, Hassanein MT, Le Marchand L, Wilkens LR, Maskarinec G, Monroe KR, Kolonel LN, Altshuler D, Henderson BE, Haiman CA. 2010. Consistent association of type 2 diabetes risk variants found in Europeans in diverse racial and ethnic groups. PLoS Genet 6:e1001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saxena R, Elbers CC, Guo Y, Peter I, Gaunt TR, Mega JL, Lanktree MB, Tare A, Castillo BA, Li YR, Johnson T, Bruinenberg M, Gilbert-Diamond D, Rajagopalan R, Voight BF, Balasubramanyam A, Barnard J, Bauer F, Baumert J, Bhangale T, Böhm BO, Braund PS, Burton PR, Chandrupatla HR, Clarke R, Cooper-DeHoff RM, et al. 2012. Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci. Am J Hum Genet 90:410–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Q, Liu T, Shrader P, Yesupriya A, Chang MH, Dowling NF, Ned RM, Dupuis J, Florez JC, Khoury MJ, Meigs JB. 2010. Racial/ethnic differences in association of fasting glucose-associated genomic loci with fasting glucose, HOMA-B, and impaired fasting glucose in the U.S. adult population. Diabetes Care 33:2370–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parra EJ, Below JE, Krithika S, Valladares A, Barta JL, Cox NJ, Hanis CL, Wacher N, Garcia-Mena J, Hu P, Shriver MD, Kumate J, McKeigue PM, Escobedo J, Cruz M. 2011. Genome-wide association study of type 2 diabetes in a sample from Mexico City and a meta-analysis of a Mexican-American sample from Starr County, Texas. Diabetologia 54:2038–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kooner JS, Saleheen D, Sim X, Sehmi J, Zhang W, Frossard P, Been LF, Chia KS, Dimas AS, Hassanali N, Jafar T, Jowett JB, Li X, Radha V, Rees SD, Takeuchi F, Young R, Aung T, Basit A, Chidambaram M, Das D, Grundberg E, Hedman AK, Hydrie ZI, Islam M, Khor CC, Kowlessur S, Kristensen MM, Liju S, Lim WY, Matthews DR, Liu J, Morris AP, Nica AC, Pinidiyapathirage JM, Prokopenko I, Rasheed A, et al. 2011. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet 43:984–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cho YS, Chen CH, Hu C, Long J, Ong RT, Sim X, Takeuchi F, Wu Y, Go MJ, Yamauchi T, Chang YC, Kwak SH, Ma RC, Yamamoto K, Adair LS, Aung T, Cai Q, Chang LC, Chen YT, Gao Y, Hu FB, Kim HL, Kim S, Kim YJ, Lee JJ, Lee NR, Li Y, Liu JJ, Lu W, Nakamura J, Nakashima E, et al. 2012. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet 44:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Palmer ND, McDonough CW, Hicks PJ, Roh BH, Wing MR, An SS, Hester JM, Cooke JN, Bostrom MA, Rudock ME, Talbert ME, Lewis JP, Ferrara A, Lu L, Ziegler JT, Sale MM, Divers J, Shriner D, Adeyemo A, Rotimi CN, Ng MC, Langefeld CD, Freedman BI, Bowden DW, Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, et al. 2012. A genome-wide association search for type 2 diabetes genes in African Americans. PLoS One 7:e29202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pérez-Escamilla R. 2011. Acculturation, nutrition, and health disparities in Latinos. Am J Clin Nutr 93:1163S–1167S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park J, Myers D, Kao D, Min S. 2009. Immigrant obesity and unhealthy assimilation: alternative estimates of convergence or divergence, 1995–2005. Soc Sci Med 69:1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kandula NR, Diez-Roux AV, Chan C, Daviglus ML, Jackson SA, Ni H, Schreiner PJ. 2008. Association of acculturation levels and prevalence of diabetes in the multi-ethnic study of atherosclerosis (MESA). Diabetes Care 31:1621–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kurian AK, Cardarelli KM. 2007. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis 17:143–152 [PubMed] [Google Scholar]
- 52. Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. 2010. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med 152:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kuzawa CW, Sweet E. 2009. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am J Hum Biol 21:2–15 [DOI] [PubMed] [Google Scholar]
- 54. Hauck FR, Tanabe KO, Moon RY. 2011. Racial and ethnic disparities in infant mortality. Semin Perinatol 35:209–220 [DOI] [PubMed] [Google Scholar]
- 55. Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. 2009. Clinical review: the pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94:2692–2701 [DOI] [PubMed] [Google Scholar]
- 56. Slomko H, Heo HJ, Einstein FH. 2012. Minireview: epigenetics of obesity and diabetes in humans. Endocrinology 153:1025–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Centers for Disease Control and Prevention 2011. Age-adjusted percentage of civilian, noninstitutionalized population with diagnosed diabetes, by sex, United States, 1980–2010. Available at: http://www.cdc.gov/diabetes/statistics/prev/national/figbysex.htm
- 58. Centers for Disease Control and Prevention 2011. Age-adjusted percentage of obesity for adults with diabetes, United States, 1994–2007. Available at: http://www.cdc.gov/diabetes/statistics/comp/fig7_obesity.htm
- 59. Centers for Disease Control and Prevention 2011. Age-adjusted percentage of physical inactivity for adults with diabetes, United States, 1994–2007. Available at: http://www.cdc.gov/diabetes/statistics/comp/fig9.htm
- 60. Centers for Disease Control and Prevention 2011. Age-specific percentage of civilian, noninstitutionalized population with diagnosed diabetes, by age, race and sex, United States, 2010. Available at: http://www.cdc.gov/diabetes/statistics/prev/national/fig2004.htm
- 61. Harris MI, Klein R, Cowie CC, Rowland M, Byrd-Holt DD. 1998. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A U.S. population study. Diabetes Care 21:1230–1235 [DOI] [PubMed] [Google Scholar]
- 62. Nsiah-Kumi P, Ortmeier SR, Brown AE. 2009. Disparities in diabetic retinopathy screening and disease for racial and ethnic minority populations—a literature review. J Natl Med Assoc 101:430–437 [DOI] [PubMed] [Google Scholar]
- 63. Chen JL, Luviano DM, Chen JC, Yu F, Sarraf D. 2009. Comparison of diabetic retinopathy phenotype between Latinos and Blacks. J Diabetes Complications 23:371–375 [DOI] [PubMed] [Google Scholar]
- 64. Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH. 2005. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care 28:2280–2288 [DOI] [PubMed] [Google Scholar]
- 65. Berinstein DM, Stahn RM, Welty TK, Leonardson GR, Herlihy JJ. 1997. The prevalence of diabetic retinopathy and associated risk factors among Sioux Indians. Diabetes Care 20:757–759 [DOI] [PubMed] [Google Scholar]
- 66. Centers for Disease Control and Prevention 2012. Diabetes complications. Available at: http://www.cdc.gov/diabetes/statistics/complications_national.htm
- 67. Lopes AA. 2009. End-stage renal disease due to diabetes in racial/ethnic minorities and disadvantaged populations. Ethn Dis 19(1 Suppl 1):S1–47–51 [PubMed] [Google Scholar]
- 68. Sosenko JM. 2009. The prevalence of diabetic neuropathy according to ethnicity. Curr Diab Rep 9:435–439 [DOI] [PubMed] [Google Scholar]
- 69. Gentile NT, Seftchick MW. 2008. Poor outcomes in Hispanic and African American patients after acute ischemic stroke: influence of diabetes and hyperglycemia. Ethn Dis 18:330–335 [PubMed] [Google Scholar]
- 70. Office of Minority Health 2012. Office of Minority Health. Available at: http://www.minorityhealth.hhs.gov/templates/content.aspx?lvl=2&lvlID=52&ID=3025
- 71. Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. 2002. Ethnic disparities in diabetic complications in an insured population. JAMA 287:2519–2527 [DOI] [PubMed] [Google Scholar]
- 72. Lefebvre KM, Lavery LA. 2011. Disparities in amputations in minorities. Clin Orthop Relat Res 469:1941–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. UK Prospective Diabetes Study (UKPDS) Group 1998. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853 [PubMed] [Google Scholar]
- 74. Diabetes Control and Complications Trial Research Group 1993. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329:977–986 [DOI] [PubMed] [Google Scholar]
- 75. Kirk JK, Bell RA, Bertoni AG, Arcury TA, Quandt SA, Goff DC, Jr, Narayan KM. 2005. Ethnic disparities: control of glycemia, blood pressure, and LDL cholesterol among US adults with type 2 diabetes. Ann Pharmacother 39:1489–1501 [DOI] [PubMed] [Google Scholar]
- 76. Kirk JK, D'Agostino RB, Jr, Bell RA, Passmore LV, Bonds DE, Karter AJ, Narayan KM. 2006. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care 29:2130–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kirk JK, Passmore LV, Bell RA, Narayan KM, D'Agostino RB, Jr, Arcury TA, Quandt SA. 2008. Disparities in A1C levels between Hispanic and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care 31:240–246 [DOI] [PubMed] [Google Scholar]
- 78. Davidson JA, Lacaya LB, Jiang H, Heilmann CR, Scism-Bacon JL, Gates JR, Jackson JA. 2010. Impact of race/ethnicity on the efficacy and safety of commonly used insulin regimens: a post hoc analysis of clinical trials in type 2 diabetes mellitus. Endocr Pract 16:818–828 [DOI] [PubMed] [Google Scholar]
- 79. Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. 2005. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care 28:2543–2545 [DOI] [PubMed] [Google Scholar]
- 80. Maruthur NM, Kao WH, Clark JM, Brancati FL, Cheng CY, Pankow JS, Selvin E. 2011. Does genetic ancestry explain higher values of glycated hemoglobin in African Americans? Diabetes 60:2434–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, Narayan KM, Koch DD, Phillips LS. 2010. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med 152:770–777 [DOI] [PubMed] [Google Scholar]
- 82. Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. 2011. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med 154:303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Selvin E, Brancati FL. 2011. A conundrum addressed: the prognostic value of HbA1c. Nat Rev Endocrinol 7:c1. [DOI] [PubMed] [Google Scholar]
- 84. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. 2010. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Selvin E, Ning Y, Steffes MW, Bash LD, Klein R, Wong TY, Astor BC, Sharrett AR, Brancati FL, Coresh J. 2011. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes 60:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Matsushita K, Blecker S, Pazin-Filho A, Bertoni A, Chang PP, Coresh J, Selvin E. 2010. The association of hemoglobin a1c with incident heart failure among people without diabetes: the Atherosclerosis Risk in Communities Study. Diabetes 59:2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lakkis J, Weir MR. 2004. Pharmacological strategies for kidney function preservation: are there differences by ethnicity? Adv Ren Replace Ther 11:24–40 [DOI] [PubMed] [Google Scholar]
- 88. Sumner AE, Zhou J, Doumatey A, Imoisili OE, Amoah A, Acheampong J, Oli J, Johnson T, Adebamowo C, Rotimi CN. 2010. Low HDL-cholesterol with normal triglyceride levels is the most common lipid pattern in West Africans and African Americans with metabolic syndrome: implications for cardiovascular disease prevention. CVD Prev Control 5:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McDonough CW, Palmer ND, Hicks PJ, Roh BH, An SS, Cooke JN, Hester JM, Wing MR, Bostrom MA, Rudock ME, Lewis JP, Talbert ME, Blevins RA, Lu L, Ng MC, Sale MM, Divers J, Langefeld CD, Freedman BI, Bowden DW. 2011. A genome-wide association study for diabetic nephropathy genes in African Americans. Kidney Int 79:563–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Maeda S, Osawa N, Hayashi T, Tsukada S, Kobayashi M, Kikkawa R. 2007. Genetic variations associated with diabetic nephropathy and type II diabetes in a Japanese population. Kidney Int Suppl 106:S43–S48 [DOI] [PubMed] [Google Scholar]
- 91. Kirk JK, Graves DE, Bell RA, Hildebrandt CA, Narayan KM. 2007. Racial and ethnic disparities in self-monitoring of blood glucose among US adults: a qualitative review. Ethn Dis 17:135–142 [PubMed] [Google Scholar]
- 92. Horowitz CR, Williams L, Bickell NA. 2003. A community-centered approach to diabetes in East Harlem. J Gen Intern Med 18:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. 2001. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 24:1069–1078 [DOI] [PubMed] [Google Scholar]
- 94. de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. 2001. Association of depression and diabetes complications: a meta-analysis. Psychosom Med 63:619–630 [DOI] [PubMed] [Google Scholar]
- 95. Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. 2000. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 23:934–942 [DOI] [PubMed] [Google Scholar]
- 96. Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, Safren SA. 2008. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care 31:2398–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li C, Ford ES, Strine TW, Mokdad AH. 2008. Prevalence of depression among U.S. adults with diabetes: findings from the 2006 behavioral risk factor surveillance system. Diabetes Care 31:105–107 [DOI] [PubMed] [Google Scholar]
- 98. Wagner J, Tsimikas J, Abbott G, de Groot M, Heapy A. 2007. Racial and ethnic differences in diabetic patient-reported depression symptoms, diagnosis, and treatment. Diabetes Res Clin Pract 75:119–122 [DOI] [PubMed] [Google Scholar]
- 99. Crews DC, Sozio SM, Liu Y, Coresh J, Powe NR. 2011. Inflammation and the paradox of racial differences in dialysis survival. J Am Soc Nephrol 22:2279–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Centers for Disease Control and Prevention 2011. Age-adjusted percentage of adults with diabetes reporting visual impairment, by sex, United States, 1997–2009. Available at: http://www.cdc.gov/diabetes/statistics/visual/fig4.htm
- 101. Roy MS, Klein R, O'Colmain BJ, Klein BE, Moss SE, Kempen JH. 2004. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol 122:546–551 [DOI] [PubMed] [Google Scholar]
- 102. Kempen JH, O'Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, Taylor HR, Hamman RF. 2004. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 122:552–563 [DOI] [PubMed] [Google Scholar]
- 103. Diabetes Control and Complications Trial Research Group 2000. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care 23:1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ferrara A, Williamson DF, Karter AJ, Thompson TJ, Kim C. 2004. Sex differences in quality of health care related to ischemic heart disease prevention in patients with diabetes: the translating research into action for Diabetes (TRIAD) study, 2000–2001. Diabetes Care 27:2974–2976 [DOI] [PubMed] [Google Scholar]
- 105. Centers for Disease Control and Prevention 2012. Age-adjusted incidence of end-stage renal disease related to diabetes mellitus (ESRD-DM) per 100,000 diabetic population, by race, ethnicity, and sex, United States, 1980–2008. Available at: http://www.cdc.gov/diabetes/statistics/esrd/fig5.htm
- 106. Whaley-Connell A, Sowers JR, McCullough PA, Roberts T, McFarlane SI, Chen SC, Li S, Wang C, Collins AJ, Bakris GL. 2009. Diabetes mellitus and CKD awareness: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES). Am J Kidney Dis 53(4 Suppl 4):S11–S21 [DOI] [PubMed] [Google Scholar]
- 107. Cheng YJ, Gregg EW, Kahn HS, Williams DE, De Rekeneire N, Narayan KM. 2006. Peripheral insensate neuropathy—a tall problem for US adults? Am J Epidemiol 164:873–880 [DOI] [PubMed] [Google Scholar]
- 108. Centers for Disease Control and Prevention 2011. Age-adjusted hospital discharge rates for neuropathy as first-listed diagnosis per 1,000 diabetic population, by sex, United States, 1988–2007. Available at: http://www.cdc.gov/diabetes/statistics/hosplea/diabetes_complications/fig4_neuro.htm
- 109. Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L. 2004. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 National Health and Nutrition Examination Survey. Diabetes Care 27:1591–1597 [DOI] [PubMed] [Google Scholar]
- 110. Gregg EW, Gu Q, Williams D, de Rekeneire N, Cheng YJ, Geiss L, Engelgau M. 2007. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabetes Res Clin Pract 77:485–488 [DOI] [PubMed] [Google Scholar]
- 111. Whitsel EA, Boyko EJ, Siscovick DS. 2000. Reassessing the role of QTc in the diagnosis of autonomic failure among patients with diabetes: a meta-analysis. Diabetes Care 23:241–247 [DOI] [PubMed] [Google Scholar]
- 112. Huxley R, Barzi F, Woodward M. 2006. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 332:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kanaya AM, Grady D, Barrett-Connor E. 2002. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 162:1737–1745 [DOI] [PubMed] [Google Scholar]
- 114. Natarajan S, Liao Y, Cao G, Lipsitz SR, McGee DL. 2003. Sex differences in risk for coronary heart disease mortality associated with diabetes and established coronary heart disease. Arch Intern Med 163:1735–1740 [DOI] [PubMed] [Google Scholar]
- 115. Centers for Disease Control and Prevention 2011. Age-adjusted hospital discharge rates for ischemic heart disease as first-listed diagnosis per 1,000 diabetic population, United States, by sex, 1988–2006. Available at: http://www.cdc.gov/diabetes/statistics/cvdhosp/ihd/fig5.htm
- 116. Champney KP, Veledar E, Klein M, Samady H, Anderson D, Parashar S, Wenger N, Vaccarino V. 2007. Sex-specific effects of diabetes on adverse outcomes after percutaneous coronary intervention: trends over time. Am Heart J 153:970–978 [DOI] [PubMed] [Google Scholar]
- 117. Centers for Disease Control and Prevention 2011. Age-adjusted hospital discharge rates for heart failure as first-listed diagnosis per 1,000 diabetic population, United States, by sex, 1988–2006. Available at: http://www.cdc.gov/diabetes/statistics/cvdhosp/hf/fig5.htm
- 118. Centers for Disease Control and Prevention 2011. Crude and age-adjusted hospital discharge rates for stroke as first-listed diagnosis per 1,000 diabetic population, United States, 1988–2006. Available at: http://www.cdc.gov/diabetes/statistics/cvdhosp/stroke/fig3.htm
- 119. Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA, De Vries CS. 2006. Risk of stroke in people with type 2 diabetes in the UK: a study using the General Practice Research Database. Diabetologia 49:2859–2865 [DOI] [PubMed] [Google Scholar]
- 120. Kothari V, Stevens RJ, Adler AI, Stratton IM, Manley SE, Neil HA, Holman RR. 2002. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke 33:1776–1781 [DOI] [PubMed] [Google Scholar]
- 121. Kuo S, Fleming BB, Gittings NS, Han LF, Geiss LS, Engelgau MM, Roman SH. 2005. Trends in care practices and outcomes among Medicare beneficiaries with diabetes. Am J Prev Med 29:396–403 [DOI] [PubMed] [Google Scholar]
- 122. Centers for Disease Control and Prevention 2011. Age-adjusted hospital discharge rates for non-traumatic lower extremity amputation per 1,000 diabetic population, by sex, United States, 1988–2006. Available at: http://www.cdc.gov/diabetes/statistics/lea/fig5.htm
- 123. Centers for Disease Control and Prevention 2011. Age-adjusted hospital discharge rates for peripheral arterial disease (PAD) as first-listed diagnosis per 1,000 diabetic population, by sex, United States, 1988–2007. Available at: http://www.cdc.gov/diabetes/statistics/hosplea/diabetes_complications/fig4_pad.htm
- 124. Dorsey RR, Eberhardt MS, Gregg EW, Geiss LS. 2009. Control of risk factors among people with diagnosed diabetes, by lower extremity disease status. Prev Chronic Dis 6:A114. [PMC free article] [PubMed] [Google Scholar]
- 125. Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. 2007. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 147:149–155 [DOI] [PubMed] [Google Scholar]
- 126. Saaddine JB, Cadwell B, Gregg EW, Engelgau MM, Vinicor F, Imperatore G, Narayan KM. 2006. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med 144:465–474 [DOI] [PubMed] [Google Scholar]
- 127. Imperatore G, Cadwell BL, Geiss L, Saadinne JB, Williams DE, Ford ES, Thompson TJ, Narayan KM, Gregg EW. 2004. Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: National Health and Nutrition Examination Surveys, 1971–2000. Am J Epidemiol 160:531–539 [DOI] [PubMed] [Google Scholar]
- 128. Persell SD, Baker DW. 2004. Aspirin use among adults with diabetes: recent trends and emerging sex disparities. Arch Intern Med 164:2492–2499 [DOI] [PubMed] [Google Scholar]
- 129. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. 2009. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373:1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bird CE, Fremont AM, Bierman AS, Wickstrom S, Shah M, Rector T, Horstman T, Escarce JJ. 2007. Does quality of care for cardiovascular disease and diabetes differ by gender for enrollees in managed care plans? Womens Health Issues 17:131–138 [DOI] [PubMed] [Google Scholar]
- 131. Ferrara A, Mangione CM, Kim C, Marrero DG, Curb D, Stevens M, Selby JV. 2008. Sex disparities in control and treatment of modifiable cardiovascular disease risk factors among patients with diabetes: Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 31:69–74 [DOI] [PubMed] [Google Scholar]
- 132. Kim C, Kerr EA, Bernstein SJ, Krein SL. 2006. Gender disparities in lipid management: the presence of disparities depends on the quality measure. Am J Manag Care 12:133–136 [PubMed] [Google Scholar]
- 133. Wexler DJ, Grant RW, Meigs JB, Nathan DM, Cagliero E. 2005. Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care 28:514–520 [DOI] [PubMed] [Google Scholar]
- 134. American Diabetes Association 2011. Summary of revisions to the 2011 clinical practice recommendations. Diabetes Care 34(Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Buchanan TA, Catalano PM. 1995. The pathogenesis of GDM: implications for diabetes after pregnancy. Diabetes Rev 3:584–601 [Google Scholar]
- 136. Brãni°teanu DD, Mathieu C. 2003. Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol Metab 14:54–56 [DOI] [PubMed] [Google Scholar]
- 137. Buchanan TA, Xiang AH, Kjos SL, Trigo E, Lee WP, Peters RK. 1999. Antepartum predictors of the development of type 2 diabetes in Latino women 11–26 months after pregnancies complicated by gestational diabetes. Diabetes 48:2430–2436 [DOI] [PubMed] [Google Scholar]
- 138. Thorpe LE, Berger D, Ellis JA, Bettegowda VR, Brown G, Matte T, Bassett M, Frieden TR. 2005. Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990–2001. Am J Public Health 95:1536–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lawrence JM, Contreras R, Chen W, Sacks DA. 2008. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care 31:899–904 [DOI] [PubMed] [Google Scholar]
- 140. Baraban E, McCoy L, Simon P. 2008. Increasing prevalence of gestational diabetes and pregnancy-related hypertension in Los Angeles County, California, 1991–2003. Prev Chronic Dis 5:A77. [PMC free article] [PubMed] [Google Scholar]
- 141. Shen JJ, Tymkow C, MacMullen N. 2005. Disparities in maternal outcomes among four ethnic populations. Ethn Dis 15:492–497 [PubMed] [Google Scholar]
- 142. Cabacungan ET, Ngui EM, McGinley EL. 21 November 2011. Racial/ethnic disparities in maternal morbidities: a statewide study of labor and delivery hospitalizations in Wisconsin. Matern Child Health J doi: 10.1007/s10995-011-0914-6 [DOI] [PubMed] [Google Scholar]
- 143. Hedderson MM, Darbinian JA, Ferrara A. 2010. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol 24:441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hunsberger M, Rosenberg KD, Donatelle RJ. 2010. Racial/ethnic disparities in gestational diabetes mellitus: findings from a population-based survey. Womens Health Issues 20:323–328 [DOI] [PubMed] [Google Scholar]
- 145. Chu SY, Abe K, Hall LR, Kim SY, Njoroge T, Qin C. 2009. Gestational diabetes mellitus: all Asians are not alike. Prev Med 49:265–268 [DOI] [PubMed] [Google Scholar]
- 146. Devlin HM, Desai J, Holzman GS, Gilbertson DT. 2008. Trends and disparities among diabetes-complicated births in Minnesota, 1993–2003. Am J Public Health 98:59–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rao AK, Daniels K, El-Sayed YY, Moshesh MK, Caughey AB. 2006. Perinatal outcomes among Asian American and Pacific Islander women. Am J Obstet Gynecol 195:834–838 [DOI] [PubMed] [Google Scholar]
- 148. Pedula KL, Hillier TA, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. 2009. Ethnic differences in gestational oral glucose screening in a large US population. Ethn Dis 19:414–419 [PubMed] [Google Scholar]
- 149. Cripe SM, O'Brien W, Gelaye B, Williams MA. 2011. Maternal morbidity and perinatal outcomes among foreign-born Cambodian, Laotian, and Vietnamese Americans in Washington State, 1993–2006. J Immigr Minor Health 13:417–425 [DOI] [PubMed] [Google Scholar]
- 150. Berggren EK, Boggess KA, Funk MJ, Stuebe AM. 2012. Racial disparities in perinatal outcomes among women with gestational diabetes. J Womens Health (Larchmt) 21:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Esakoff TF, Caughey AB, Block-Kurbisch I, Inturrisi M, Cheng YW. 2011. Perinatal outcomes in patients with gestational diabetes mellitus by race/ethnicity. J Matern Fetal Neonatal Med 24:422–426 [DOI] [PubMed] [Google Scholar]
- 152. Hunt KJ, Marlow NM, Gebregziabher M, Ellerbe CN, Mauldin J, Mayorga ME, Korte JE. 2012. Impact of maternal diabetes on birthweight is greater in non-Hispanic blacks than in non-Hispanic whites. Diabetologia 55:971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Nicholson WK, Fox HE, Cooper LA, Strobino D, Witter F, Powe NR. 2006. Maternal race, procedures, and infant birth weight in type 2 and gestational diabetes. Obstet Gynecol 108:626–634 [DOI] [PubMed] [Google Scholar]
- 154. Silva JK, Kaholokula JK, Ratner R, Mau M. 2006. Ethnic differences in perinatal outcome of gestational diabetes mellitus. Diabetes Care 29:2058–2063 [DOI] [PubMed] [Google Scholar]
- 155. Mocarski M, Savitz DA. 2012. Ethnic differences in the association between gestational diabetes and pregnancy outcome. Matern Child Health J 16:364–373 [DOI] [PubMed] [Google Scholar]
- 156. Xiang AH, Li BH, Black MH, Sacks DA, Buchanan TA, Jacobsen SJ, Lawrence JM. 2011. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia 54:3016–3021 [DOI] [PubMed] [Google Scholar]
- 157. Haas JS, Jackson RA, Fuentes-Afflick E, Stewart AL, Dean ML, Brawarsky P, Escobar GJ. 2005. Changes in the health status of women during and after pregnancy. J Gen Intern Med 20:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kim C, Vahratian A. 2010. Self-rated health and health care use among women with histories of gestational diabetes mellitus. Diabetes Care 33:41–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Li X, Shu YH, Xiang AH, Trigo E, Kuusisto J, Hartiala J, Swift AJ, Kawakubo M, Stringham HM, Bonnycastle LL, Lawrence JM, Laakso M, Allayee H, Buchanan TA, Watanabe RM. 2009. Additive effects of genetic variation in GCK and G6PC2 on insulin secretion and fasting glucose. Diabetes 58:2946–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Li X, Allayee H, Xiang AH, Trigo E, Hartiala J, Lawrence JM, Buchanan TA, Watanabe RM. 2009. Variation in IGF2BP2 interacts with adiposity to alter insulin sensitivity in Mexican Americans. Obesity (Silver Spring) 17:729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Watanabe RM, Allayee H, Xiang AH, Trigo E, Hartiala J, Lawrence JM, Buchanan TA. 2007. Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes 56:1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Black MH, Fingerlin TE, Allayee H, Zhang W, Xiang AH, Trigo E, Hartiala J, Lehtinen AB, Haffner SM, Bergman RN, McEachin RC, Kjos SL, Lawrence JM, Buchanan TA, Watanabe RM. 2008. Evidence of interaction between PPARG2 and HNF4A contributing to variation in insulin sensitivity in Mexican Americans. Diabetes 57:1048–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Freathy RM, Hayes MG, Urbanek M, Lowe LP, Lee H, Ackerman C, Frayling TM, Cox NJ, Dunger DB, Dyer AR, Hattersley AT, Metzger BE, Lowe WL., Jr 2010. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: common genetic variants in GCK and TCF7L2 are associated with fasting and postchallenge glucose levels in pregnancy and with the new consensus definition of gestational diabetes mellitus from the International Association of Diabetes and Pregnancy Study Groups. Diabetes 59:2682–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Caughey AB, Cheng YW, Stotland NE, Washington AE, Escobar GJ. 2010. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol 202:616.e1–5 [DOI] [PubMed] [Google Scholar]
- 165. Nystrom MJ, Caughey AB, Lyell DJ, Druzin ML, El-Sayed YY. 2008. Perinatal outcomes among Asian-white interracial couples. Am J Obstet Gynecol 199:385.e1–5 [DOI] [PubMed] [Google Scholar]
- 166. Bowers K, Tobias DK, Yeung E, Hu FB, Zhang C. 2012. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am J Clin Nutr 95:446–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bowers K, Yeung E, Williams MA, Qi L, Tobias DK, Hu FB, Zhang C. 2011. A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care 34:1557–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zhang C, Schulze MB, Solomon CG, Hu FB. 2006. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 49:2604–2613 [DOI] [PubMed] [Google Scholar]
- 169. Zhang C, Liu S, Solomon CG, Hu FB. 2006. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 29:2223–2230 [DOI] [PubMed] [Google Scholar]
- 170. Chen L, Hu FB, Yeung E, Willett W, Zhang C. 2009. Prospective study of pre-gravid sugar-sweetened beverage consumption and the risk of gestational diabetes mellitus. Diabetes Care 32:2236–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zhang C, Solomon CG, Manson JE, Hu FB. 2006. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Arch Intern Med 166:543–548 [DOI] [PubMed] [Google Scholar]
- 172. Savitz DA, Janevic TM, Engel SM, Kaufman JS, Herring AH. 2008. Ethnicity and gestational diabetes in New York City, 1995–2003. BJOG 115:969–978 [DOI] [PubMed] [Google Scholar]
- 173. Ramadhani TA, Canfield MA, Farag NH, Royle M, Correa A, Waller DK, Scheuerle A. 2011. Do foreign- and U.S.-born mothers across racial/ethnic groups have a similar risk profile for selected sociodemographic and periconceptional factors? Birth Defects Res A Clin Mol Teratol 91:823–830 [DOI] [PubMed] [Google Scholar]
- 174. Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC. 2008. Gestational diabetes in the United States: temporal trends 1989 through 2004. Am J Obstet Gynecol 198:525.e1–5 [DOI] [PubMed] [Google Scholar]
- 175. Janevic T, Borrell LN, Savitz DA, Herring AH, Rundle A. 2010. Neighbourhood food environment and gestational diabetes in New York City. Paediatr Perinat Epidemiol 24:249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kim C, Sinco B, Kieffer EA. 2007. Racial and ethnic variation in access to health care, provision of health care services, and ratings of health among women with histories of gestational diabetes mellitus. Diabetes Care 30:1459–1465 [DOI] [PubMed] [Google Scholar]
- 177. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr 2009. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 178. Kahn R, Buse J, Ferrannini E, Stern M. 2005. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 28:2289–2304 [DOI] [PubMed] [Google Scholar]
- 179. Deboer MD. 2010. Underdiagnosis of metabolic syndrome in non-Hispanic black adolescents: a call for ethnic-specific criteria. Curr Cardiovasc Risk Rep 4:302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Sumner AE, Cowie CC. 2008. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis 196:696–703 [DOI] [PubMed] [Google Scholar]
- 181. Grundy SM. 2008. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 28:629–636 [DOI] [PubMed] [Google Scholar]
- 182. Giannini E, Testa R. 2003. The metabolic syndrome: all criteria are equal, but some criteria are more equal than others. Arch Intern Med 163:2787–2788 [DOI] [PubMed] [Google Scholar]
- 183. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. 2003. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 163:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Reaven GM. 1988. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 185. Rosenzweig JL, Ferrannini E, Grundy SM, Haffner SM, Heine RJ, Horton ES, Kawamori R. 2008. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93:3671–3689 [DOI] [PubMed] [Google Scholar]
- 186. Ford ES, Li C, Zhao G. 2010. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes 2:180–193 [DOI] [PubMed] [Google Scholar]
- 187. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. 2012. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125:188–197 [DOI] [PubMed] [Google Scholar]
- 188. Palaniappan LP, Wong EC, Shin JJ, Fortmann SP, Lauderdale DS. 2011. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond) 35:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. 2007. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 49:403–414 [DOI] [PubMed] [Google Scholar]
- 190. Conway JM, Yanovski SZ, Avila NA, Hubbard VS. 1995. Visceral adipose tissue differences in black and white women. Am J Clin Nutr 61:765–771 [DOI] [PubMed] [Google Scholar]
- 191. Sumner AE, Sen S, Ricks M, Frempong BA, Sebring NG, Kushner H. 2008. Determining the waist circumference in African Americans which best predicts insulin resistance. Obesity (Silver Spring) 16:841–846 [DOI] [PubMed] [Google Scholar]
- 192. Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, Ryan DH, Bouchard C. 2011. Ethnic-specific BMI and waist circumference thresholds. Obesity (Silver Spring) 19:1272–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Sumner AE, Micklesfield LK, Ricks M, Tambay AV, Avila NA, Thomas F, Lambert EV, Levitt NS, Evans J, Rotimi CN, Tulloch-Reid MK, Goedecke JH. 2011. Waist circumference, BMI, and visceral adipose tissue in white women and women of African descent. Obesity (Silver Spring) 19:671–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. 2003. Years of life lost due to obesity. JAMA 289:187–193 [DOI] [PubMed] [Google Scholar]
- 195. Stevens J, Plankey MW, Williamson DF, Thun MJ, Rust PF, Palesch Y, O'Neil PM. 1998. The body mass index-mortality relationship in white and African American women. Obes Res 6:268–277 [DOI] [PubMed] [Google Scholar]
- 196. Sanchez AM, Reed DR, Price RA. 2000. Reduced mortality associated with body mass index (BMI) in African Americans relative to Caucasians. Ethn Dis 10:24–30 [PubMed] [Google Scholar]
- 197. Després JP, Lemieux I. 2006. Abdominal obesity and metabolic syndrome. Nature 444:881–887 [DOI] [PubMed] [Google Scholar]
- 198. Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. 2005. Comparison of visceral adipose tissue mass in adult African Americans and whites. Obes Res 13:66–74 [DOI] [PubMed] [Google Scholar]
- 199. Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. 2007. Minimum waist and visceral fat values for identifying Japanese Americans at risk for the metabolic syndrome. Diabetes Care 30:120–127 [DOI] [PubMed] [Google Scholar]
- 200. Pratyush DD, Tiwari S, Singh S, Singh SK. 2012. Waist circumference cutoff and its importance for diagnosis of metabolic syndrome in Asian Indians: a preliminary study. Indian J Endocrinol Metab 16:112–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. 2003. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med 139:802–809 [DOI] [PubMed] [Google Scholar]
- 202. Choi SH, Ginsberg HN. 2011. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 22:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Adiels M, Olofsson SO, Taskinen MR, Borén J. 2008. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 28:1225–1236 [DOI] [PubMed] [Google Scholar]
- 204. Guerrero R, Vega GL, Grundy SM, Browning JD. 2009. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology 49:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Florez H, Mendez A, Casanova-Romero P, Larreal-Urdaneta C, Castillo-Florez S, Lee D, Goldberg R. 2006. Increased apolipoprotein C-III levels associated with insulin resistance contribute to dyslipidemia in normoglycemic and diabetic subjects from a triethnic population. Atherosclerosis 188:134–141 [DOI] [PubMed] [Google Scholar]
- 206. Després JP, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. 2000. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol 20:1932–1938 [DOI] [PubMed] [Google Scholar]
- 207. Sumner AE, Vega GL, Genovese DJ, Finley KB, Bergman RN, Boston RC. 2005. Normal triglyceride levels despite insulin resistance in African Americans: role of lipoprotein lipase. Metabolism 54:902–909 [DOI] [PubMed] [Google Scholar]
- 208. Goedecke JH, Utzschneider K, Faulenbach MV, Rizzo M, Berneis K, Spinas GA, Dave JA, Levitt NS, Lambert EV, Olsson T, Kahn SE. 2010. Ethnic differences in serum lipoproteins and their determinants in South African women. Metabolism 59:1341–1350 [DOI] [PubMed] [Google Scholar]
- 209. Sumner AE. 2009. “Half the dsylipidemia of insulin resistance” is the dyslipidemia [corrected] of insulin-resistant blacks. Ethn Dis 19:462–465 [PMC free article] [PubMed] [Google Scholar]
- 210. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 211. Okayasu I, Hara Y, Nakamura K, Rose NR. 1994. Racial and age-related differences in incidence and severity of focal autoimmune thyroiditis. Am J Clin Pathol 101:698–702 [DOI] [PubMed] [Google Scholar]
- 212. Rogers MA, Levine DA, Blumberg N, Fisher GG, Kabeto M, Langa KM. 2012. Antigenic challenge in the etiology of autoimmune disease in women. J Autoimmun 38:J97–J102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. 2011. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 21:419–427 [DOI] [PubMed] [Google Scholar]
- 214. Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, Zhang Y, Shan Z, Teng W, Xing M. 2009. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab 94:1612–1617 [DOI] [PubMed] [Google Scholar]
- 215. Chen QY, Huang W, She JX, Baxter F, Volpe R, Maclaren NK. 1999. HLA-DRB1*08, DRB1*03/DRB3*0101, and DRB3*0202 are susceptibility genes for Graves' disease in North American Caucasians, whereas DRB1*07 is protective. J Clin Endocrinol Metab 84:3182–3186 [DOI] [PubMed] [Google Scholar]
- 216. Chen QY, Nadell D, Zhang XY, Kukreja A, Huang YJ, Wise J, Svec F, Richards R, Friday KE, Vargas A, Gomez R, Chalew S, Lan MS, Tomer Y, Maclaren NK. 2000. The human leukocyte antigen HLA DRB3*020/DQA1*0501 haplotype is associated with Graves' disease in African Americans. J Clin Endocrinol Metab 85:1545–1549 [DOI] [PubMed] [Google Scholar]
- 217. Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N, Zhang Y. 2009. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 20:525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Simard EP, Ward EM, Siegel R, Jemal A. 4 January 2012. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin doi: 10.3322/caac.20141 [DOI] [PubMed] [Google Scholar]
- 219. Hollenbeak CS, Wang L, Schneider P, Goldenberg D. 2011. Outcomes of thyroid cancer in African Americans. Ethn Dis 21:210–215 [PubMed] [Google Scholar]
- 220. Horn-Ross PL, McClure LA, Chang ET, Clarke CA, Keegan TH, Rull RP, Quach T, Gomez SL. 2011. Papillary thyroid cancer incidence rates vary significantly by birthplace in Asian American women. Cancer Causes Control 22:479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Weir HK, Jim MA, Marrett LD, Fairley T. 2008. Cancer in American Indian and Alaska Native young adults (ages 20–44 years): US, 1999–2004. Cancer 113:1153–1167 [DOI] [PubMed] [Google Scholar]
- 222. Famakinwa OM, Roman SA, Wang TS, Sosa JA. 2010. ATA practice guidelines for the treatment of differentiated thyroid cancer: were they followed in the United States? Am J Surg 199:189–198 [DOI] [PubMed] [Google Scholar]
- 223. Garnett MJ, Marais R. 2004. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 6:313–319 [DOI] [PubMed] [Google Scholar]
- 224. Lee JH, Lee ES, Kim YS. 2007. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer 110:38–46 [DOI] [PubMed] [Google Scholar]
- 225. Lim II, Hochman T, Blumberg SN, Patel KN, Heller KS, Ogilvie JB. 2012. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid 22:269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Epstein AJ, Gray BH, Schlesinger M. 2010. Racial and ethnic differences in the use of high-volume hospitals and surgeons. Arch Surg 145:179–186 [DOI] [PubMed] [Google Scholar]
- 227. Jacobson DL, Gange SJ, Rose NR, Graham NM. 1997. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84:223–243 [DOI] [PubMed] [Google Scholar]
- 228. Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, Evers BM. 2007. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg 204:764–773; discussion 773–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Rahbari R, Zhang L, Kebebew E. 2010. Thyroid cancer gender disparity. Future Oncol 6:1771–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. 2003. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res 63:4561–4567 [PubMed] [Google Scholar]
- 231. Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G, Lee S, Kim SY, Kim SC, Hong SJ, Shong YK. 2006. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 65:364–368 [DOI] [PubMed] [Google Scholar]
- 232. Lee ML, Chen GG, Vlantis AC, Tse GM, Leung BC, van Hasselt CA. 2005. Induction of thyroid papillary carcinoma cell proliferation by estrogen is associated with an altered expression of Bcl-xL. Cancer J 11:113–121 [DOI] [PubMed] [Google Scholar]
- 233. Knudsen N, Laurberg P, Perrild H, Bülow I, Ovesen L, Jørgensen T. 2002. Risk factors for goiter and thyroid nodules. Thyroid 12:879–888 [DOI] [PubMed] [Google Scholar]
- 234. Heo M, Faith MS, Pietrobelli A, Heymsfield SB. 2012. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999–2004. Am J Clin Nutr 95:594–602 [DOI] [PubMed] [Google Scholar]
- 235. Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, Schairer C, Schatzkin A, Shikany JM, Berrington de González A. 2011. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev 20:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Goodman MT, Kolonel LN, Wilkens LR. 1992. The association of body size, reproductive factors and thyroid cancer. Br J Cancer 66:1180–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. 2009. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control 20:75–86 [DOI] [PubMed] [Google Scholar]
- 238. Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M. 2001. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab 86:1072–1077 [DOI] [PubMed] [Google Scholar]
- 239. Ciampolillo A, De Tullio C, Perlino E, Maiorano E. 2007. The IGF-I axis in thyroid carcinoma. Curr Pharm Des 13:729–735 [DOI] [PubMed] [Google Scholar]
- 240. Balkau B, Mhamdi L, Oppert JM, Nolan J, Golay A, Porcellati F, Laakso M, Ferrannini E. 2008. Physical activity and insulin sensitivity: the RISC study. Diabetes 57:2613–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Kabat GC, Kim MY, Thomson CA, Luo J, Wactawski-Wende J, Rohan TE. 2012. Anthropometric factors and physical activity and risk of thyroid cancer in postmenopausal women. Cancer Causes Control 23:421–430 [DOI] [PubMed] [Google Scholar]
- 242. Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. 1999. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 353:878–882 [DOI] [PubMed] [Google Scholar]
- 243. Looker AC, Dawson-Hughes B, Tosteson AN, Johansson H, Kanis JA, Melton LJ., 3rd 2011. Hip fracture risk in older US adults by treatment eligibility status based on new National Osteoporosis Foundation guidance. Osteoporos Int 22:541–549 [DOI] [PubMed] [Google Scholar]
- 244. Cauley J, LaCroix AZ, Wu C, Lewis B, Wactawski-Wende J, Masaki K, Johnson K, O'Sullivan MJ, Jackson R, Hendrix S, Robbins J, FRAX: does fracture prediction differ by race/ethnicity. Proc 32nd Annual Meeting of the American Society for Bone and Mineral Research, Toronto, 2010 (Abstract A10005323) [Google Scholar]
- 245. Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ., 3rd 1992. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 7:221–227 [DOI] [PubMed] [Google Scholar]
- 246. Fink HA, Ensrud KE, Nelson DB, Kerani RP, Schreiner PJ, Zhao Y, Cummings SR, Nevitt MC. 2003. Disability after clinical fracture in postmenopausal women with low bone density: the fracture intervention trial (FIT). Osteoporos Int 14:69–76 [DOI] [PubMed] [Google Scholar]
- 247. Cauley JA, Palermo L, Vogt M, Ensrud KE, Ewing S, Hochberg M, Nevitt MC, Black DM. 2008. Prevalent vertebral fractures in black women and white women. J Bone Miner Res 23:1458–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Silverman SL, Madison RE. 1988. Decreased incidence of hip fracture in Hispanics, Asians, and blacks: California hospital discharge data. Am J Public Health 78:1482–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Zingmond DS, Melton LJ, 3rd, Silverman SL. 2004. Increasing hip fracture incidence in California Hispanics, 1983 to 2000. Osteoporos Int 15:603–610 [DOI] [PubMed] [Google Scholar]
- 250. Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J. 1998. Hip fracture incidence among elderly Hispanics. Am J Public Health 88:1245–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Fang J, Freeman R, Jeganathan R, Alderman MH. 2004. Variations in hip fracture hospitalization rates among different race/ethnicity groups in New York City. Ethn Dis 14:280–284 [PubMed] [Google Scholar]
- 252. Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. 2001. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 286:2815–2822 [DOI] [PubMed] [Google Scholar]
- 253. Lauderdale DS, Jacobsen SJ, Furner SE, Levy PS, Brody JA, Goldberg J. 1997. Hip fracture incidence among elderly Asian-American populations. Am J Epidemiol 146:502–509 [DOI] [PubMed] [Google Scholar]
- 254. Ross PD, Norimatsu H, Davis JW, Yano K, Wasnich RD, Fujiwara S, Hosoda Y, Melton LJ., 3rd 1991. A comparison of hip fracture incidence among native Japanese, Japanese Americans, and American Caucasians. Am J Epidemiol 133:801–809 [DOI] [PubMed] [Google Scholar]
- 255. Cauley JA, Wu L, Wampler NS, Barnhart JM, Allison M, Chen Z, Jackson R, Robbins J. 2007. Clinical risk factors for fractures in multi-ethnic women: the Women's Health Initiative. J Bone Miner Res 22:1816–1826 [DOI] [PubMed] [Google Scholar]
- 256. Faulkner KG, Cummings SR, Black D, Palermo L, Glüer CC, Genant HK. 1993. Simple measurement of femoral geometry predicts hip fracture: the study of osteoporotic fractures. J Bone Miner Res 8:1211–1217 [DOI] [PubMed] [Google Scholar]
- 257. Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. 2000. Risk of mortality following clinical fractures. Osteoporos Int 11:556–561 [DOI] [PubMed] [Google Scholar]
- 258. Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. 1992. Race and sex differences in mortality following fracture of the hip. Am J Public Health 82:1147–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Furstenberg AL, Mezey MD. 1987. Differences in outcome between black and white elderly hip fracture patients. J Chronic Dis 40:931–938 [DOI] [PubMed] [Google Scholar]
- 260. Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. 1990. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol 45:M101–M107 [DOI] [PubMed] [Google Scholar]
- 261. Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang MH, Greendale GA. 2002. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab 87:3057–3067 [DOI] [PubMed] [Google Scholar]
- 262. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. 2007. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475 [DOI] [PubMed] [Google Scholar]
- 263. Griffin MR, Ray WA, Fought RL, Melton LJ., 3rd 1992. Black-white differences in fracture rates. Am J Epidemiol 136:1378–1385 [DOI] [PubMed] [Google Scholar]
- 264. Cummings SR, Cauley JA, Palermo L, Ross PD, Wasnich RD, Black D, Faulkner KG. 1994. Racial differences in hip axis lengths might explain racial differences in rates of hip fracture. Study of Osteoporotic Fractures Research Group. Osteoporos Int 4:226–229 [DOI] [PubMed] [Google Scholar]
- 265. Beck TJ, Ruff CB, Warden KE, Scott WW, Jr, Rao GU. 1990. Predicting femoral neck strength from bone mineral data. A structural approach. Invest Radiol 25:6–18 [DOI] [PubMed] [Google Scholar]
- 266. Nelson DA, Barondess DA, Hendrix SL, Beck TJ. 2000. Cross-sectional geometry, bone strength, and bone mass in the proximal femur in black and white postmenopausal women. J Bone Miner Res 15:1992–1997 [DOI] [PubMed] [Google Scholar]
- 267. Nelson DA, Beck TJ, Wu G, Lewis CE, Bassford T, Cauley JA, LeBoff MS, Going SB, Chen Z. 2011. Ethnic differences in femur geometry in the Women's Health Initiative observational study. Osteoporos Int 22:1377–1388 [DOI] [PubMed] [Google Scholar]
- 268. Ishii S, Cauley JA, Greendale GA, Danielson ME, Safaei Nili N, Karlamangla A. 2012. Ethnic differences in composite indices of femoral neck strength. Osteoporos Int 23:1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Finkelstein JS, Sowers M, Greendale GA, Lee ML, Neer RM, Cauley JA, Ettinger B. 2002. Ethnic variation in bone turnover in pre- and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab 87:3051–3056 [DOI] [PubMed] [Google Scholar]
- 270. Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM. 2008. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 93:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Greendale GA, Sowers M, Han W, Huang MH, Finkelstein JS, Crandall CJ, Lee JS, Karlamangla AS. 4 October 2011. Bone mineral density loss in relation to the final menstrual period in a multi-ethic cohort: results from the Study of Women's Health Across the Nation (SWAN). J Bone Miner Res doi: 10.1002/jbmr.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. 1997. Prospective study of the determinants of age at menopause. Am J Epidemiol 145:124–133 [DOI] [PubMed] [Google Scholar]
- 273. Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. 2001. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 153:865–874 [DOI] [PubMed] [Google Scholar]
- 274. Thomas-John M, Codd MB, Manne S, Watts NB, Mongey AB. 2009. Risk factors for the development of osteoporosis and osteoporotic fractures among older men. J Rheumatol 36:1947–1952 [DOI] [PubMed] [Google Scholar]
- 275. Bohannon AD, Hanlon JT, Landerman R, Gold DT. 1999. Association of race and other potential risk factors with nonvertebral fractures in community-dwelling elderly women. Am J Epidemiol 149:1002–1009 [DOI] [PubMed] [Google Scholar]
- 276. Kato I, Toniolo P, Zeleniuch-Jacquotte A, Shore RE, Koenig KL, Akhmedkhanov A, Riboli E. 2000. Diet, smoking and anthropometric indices and postmenopausal bone fractures: a prospective study. Int J Epidemiol 29:85–92 [DOI] [PubMed] [Google Scholar]
- 277. Grisso JA, Kelsey JL, Strom BL, O'Brien LA, Maislin G, LaPann K, Samelson L, Hoffman S. 1994. Risk factors for hip fracture in black women. The Northeast Hip Fracture Study Group. N Engl J Med 330:1555–1559 [DOI] [PubMed] [Google Scholar]
- 278. Rinaldi S, Key TJ, Peeters PH, Lahmann PH, Lukanova A, Dossus L, Biessy C, Vineis P, Sacerdote C, Berrino F, Panico S, Tumino R, Palli D, Nagel G, Linseisen J, Boeing H, Roddam A, Bingham S, Khaw KT, Chloptios J, Trichopoulou A, Trichopoulos D, Tehard B, Clavel-Chapelon F, Gonzalez CA, Larrañaga N, Barricarte A, Quirós JR, Chirlaque MD, Martinez C, Monninkhof E, Grobbee DE, Bueno-de-Mesquita HB, Ferrari P, Slimani N, Riboli E, Kaaks R. 2006. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int J Cancer 118:2832–2839 [DOI] [PubMed] [Google Scholar]
- 279. Lee JS, LaCroix AZ, Wu L, Cauley JA, Jackson RD, Kooperberg C, Leboff MS, Robbins J, Lewis CE, Bauer DC, Cummings SR. 2008. Associations of serum sex hormone-binding globulin and sex hormone concentrations with hip fracture risk in postmenopausal women. J Clin Endocrinol Metab 93:1796–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Shaffer JR, Kammerer CM, Reich D, McDonald G, Patterson N, Goodpaster B, Bauer DC, Li J, Newman AB, Cauley JA, Harris TB, Tylavsky F, Ferrell RE, Zmuda JM. 2007. Genetic markers for ancestry are correlated with body composition traits in older African Americans. Osteoporos Int 18:733–741 [DOI] [PubMed] [Google Scholar]
- 281. Chen Z, Qi L, Beck TJ, Robbins J, Wu G, Lewis CE, Cauley JA, Wright NC, Seldin MF. 2011. Stronger bone correlates with African admixture in African-American women. J Bone Miner Res 26:2307–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Leib ES, Saag KG, Adachi JD, Geusens PP, Binkley N, McCloskey EV, Hans DB. 2011. Official positions for FRAX clinical regarding glucocorticoids: the impact of the use of glucocorticoids on the estimate by FRAX of the 10 year risk of fracture from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX. J Clin Densitom 14:212–219 [DOI] [PubMed] [Google Scholar]
- 283. Ottenbacher KJ, Ostir GV, Peek MK, Goodwin JS, Markides KS. 2002. Diabetes mellitus as a risk factor for hip fracture in Mexican American older adults. J Gerontol A Biol Sci Med Sci 57:M648–M653 [DOI] [PubMed] [Google Scholar]
- 284. Bell NH, Bilezikian JP, Bone HG, 3rd, Kaur A, Maragoto A, Santora AC. 2002. Alendronate increases bone mass and reduces bone markers in postmenopausal African-American women. J Clin Endocrinol Metab 87:2792–2797 [DOI] [PubMed] [Google Scholar]
- 285. Talwar SA, Aloia JF, Pollack S, Yeh JK. 2007. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr 86:1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. 2003. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA 290:1729–1738 [DOI] [PubMed] [Google Scholar]
- 287. Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, Robbins JA, Lewis CE, Beresford SA, Ko MG, Naughton MJ, Satterfield S, Bassford T. 2006. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the Women's Health Initiative randomized trial. J Bone Miner Res 21:817–828 [DOI] [PubMed] [Google Scholar]
- 288. Curtis JR, Carbone L, Cheng H, Hayes B, Laster A, Matthews R, Saag KG, Sepanski R, Tanner SB, Delzell E. 2008. Longitudinal trends in use of bone mass measurement among older Americans, 1999–2005. J Bone Miner Res 23:1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Miller RG, Ashar BH, Cohen J, Camp M, Coombs C, Johnson E, Schneyer CR. 2005. Disparities in osteoporosis screening between at-risk African-American and white women. J Gen Intern Med 20:847–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Cheng H, Gary LC, Curtis JR, Saag KG, Kilgore ML, Morrisey MA, Matthews R, Smith W, Yun H, Delzell E. 2009. Estimated prevalence and patterns of presumed osteoporosis among older Americans based on Medicare data. Osteoporos Int 20:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Curtis JR, McClure LA, Delzell E, Howard VJ, Orwoll E, Saag KG, Safford M, Howard G. 2009. Population-based fracture risk assessment and osteoporosis treatment disparities by race and gender. J Gen Intern Med 24:956–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Gourlay ML, Callahan LF, Preisser JS, Sloane PD. 2007. Osteoporosis preventive care in white and black women in community family medicine settings. South Med J 100:677–682 [DOI] [PubMed] [Google Scholar]
- 293. Mudano AS, Casebeer L, Patino F, Allison JJ, Weissman NW, Kiefe CI, Person S, Gilbert D, Saag KG. 2003. Racial disparities in osteoporosis prevention in a managed care population. South Med J 96:445–451 [DOI] [PubMed] [Google Scholar]
- 294. Dawson-Hughes B, Looker AC, Tosteson AN, Johansson H, Kanis JA, Melton LJ., 3rd 2010. The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporos Int 21:41–52 [DOI] [PubMed] [Google Scholar]
- 295. Lewis CE, Ewing SK, Taylor BC, Shikany JM, Fink HA, Ensrud KE, Barrett-Connor E, Cummings SR, Orwoll E. 2007. Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res 22:211–219 [DOI] [PubMed] [Google Scholar]
- 296. Megyesi MS, Hunt LM, Brody H. 2011. A critical review of racial/ethnic variables in osteoporosis and bone density research. Osteoporos Int 22:1669–1679 [DOI] [PubMed] [Google Scholar]
- 297. Cauley JA, Wampler NS, Barnhart JM, Wu L, Allison M, Chen Z, Hendrix S, Robbins J, Jackson RD. 2008. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women's Health Initiative Observational Study. Osteoporos Int 19:1717–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. 2012. The nonskeletal effects of vitamin D: an Endocrine Society Scientific Statement. Endocr Rev 33:456–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. 2011. Vitamin D status: United States, 2001–2006. NCHS Data Brief 59:1–8 [PubMed] [Google Scholar]
- 300. Looker AC. 2005. Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab 90:635–640 [DOI] [PubMed] [Google Scholar]
- 301. Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. 2002. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30:771–777 [DOI] [PubMed] [Google Scholar]
- 302. Cauley JA, Danielson ME, Boudreau R, Barbour KE, Horwitz MJ, Bauer DC, Ensrud KE, Manson JE, Wactawski-Wende J, Shikany JM, Jackson RD. 2011. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women's Health Initiative (WHI). J Bone Miner Res 26:2378–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. 2002. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 76:187–192 [DOI] [PubMed] [Google Scholar]
- 304. Shea MK, Houston DK, Tooze JA, Davis CC, Johnson MA, Hausman DB, Cauley JA, Bauer DC, Tylavsky F, Harris TB, Kritchevsky SB. 2011. Correlates and prevalence of insufficient 25-hydroxyvitamin D status in black and white older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc 59:1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Tseng M, Giri V, Bruner DW, Giovannucci E. 2009. Prevalence and correlates of vitamin D status in African American men. BMC Public Health 9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. McGrath JJ, Saha S, Burne TH, Eyles DW. 2010. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol 121:471–477 [DOI] [PubMed] [Google Scholar]
- 307. Signorello LB, Shi J, Cai Q, Zheng W, Williams SM, Long J, Cohen SS, Li G, Hollis BW, Smith JR, Blot WJ. 2011. Common variation in vitamin D pathway genes predicts circulating 25-hydroxyvitamin D levels among African Americans. PLoS One 6:e28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, Lurie N, Rebbeck T, Goodwin J, Flack J, Srinivasan S, Kerner J, Heurtin-Roberts S, Abeles R, Tyson FL, Patmios G, Hiatt RA. 2008. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health 98:1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Elders MJ. 2009. Role of endocrinologists in eliminating health care disparities. Endocr Pract 15:612–623 [DOI] [PubMed] [Google Scholar]
- 310. Dagogo-Jack S. 2012. Predicting diabetes: our relentless quest for genomic nuggets. Diabetes Care 35:193–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Carlson ED, Chamberlain RM. 2005. Allostatic load and health disparities: a theoretical orientation. Res Nurs Health 28:306–315 [DOI] [PubMed] [Google Scholar]
- 312. McEwen BS, Wingfield JC. 2003. The concept of allostasis in biology and biomedicine. Horm Behav 43:2–15 [DOI] [PubMed] [Google Scholar]
- 313. McEwen BS. 2002. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging 23:921–939 [DOI] [PubMed] [Google Scholar]
- 314. Chrousos GP. 2009. Stress and disorders of the stress system. Nat Rev Endocrinol 5:374–381 [DOI] [PubMed] [Google Scholar]
- 315. Chyu L, Upchurch DM. 2011. Racial and ethnic patterns of allostatic load among adult women in the United States: findings from the National Health and Nutrition Examination Survey 1999–2004. J Womens Health (Larchmt) 20:575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Bird CE, Seeman T, Escarce JJ, Basurto-Dávila R, Finch BK, Dubowitz T, Heron M, Hale L, Merkin SS, Weden M, Lurie N. 2010. Neighbourhood socioeconomic status and biological ‘wear and tear’ in a nationally representative sample of US adults. J Epidemiol Community Health 64:860–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Geronimus AT, Hicken M, Keene D, Bound J. 2006. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 96:826–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Kaestner R, Pearson JA, Keene D, Geronimus AT. 2009. Stress, allostatic load and health of Mexican immigrants. Soc Sci Q 90:1089–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Peek MK, Cutchin MP, Salinas JJ, Sheffield KM, Eschbach K, Stowe RP, Goodwin JS. 2010. Allostatic load among non-Hispanic whites, non-Hispanic blacks, and people of Mexican origin: effects of ethnicity, nativity, and acculturation. Am J Public Health 100:940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Mattei J, Demissie S, Falcon LM, Ordovas JM, Tucker K. 2010. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Soc Sci Med 70:1988–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Everson SA, Maty SC, Lynch JW, Kaplan GA. 2002. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res 53:891–895 [DOI] [PubMed] [Google Scholar]
- 322. Caddick SL, McKinnon M, Payne N, Ward TJ, Thornton-Jones H, Kells J, Ward JD. 1994. Hospital admissions and social deprivation of patients with diabetes mellitus. Diabet Med 11:981–983 [DOI] [PubMed] [Google Scholar]
- 323. Connolly V, Kesson CM. 1996. Socio-economic status and membership of the British Diabetic Association in Scotland. Diabet Med 13:898–901 [DOI] [PubMed] [Google Scholar]
- 324. Cubbin C, Hadden WC, Winkleby MA. 2001. Neighborhood context and cardiovascular disease risk factors: the contribution of material deprivation. Ethn Dis 11:687–700 [PubMed] [Google Scholar]
- 325. Karter AJ, Parker MM, Moffet HH, Ahmed AT, Chan J, Spence MM, Selby JV, Ettner SL. 2007. Effect of cost-sharing changes on self-monitoring of blood glucose. Am J Manag Care 13:408–416 [PMC free article] [PubMed] [Google Scholar]
- 326. Beckles GL, Engelgau MM, Narayan KM, Herman WH, Aubert RE, Williamson DF. 1998. Population-based assessment of the level of care among adults with diabetes in the U.S. Diabetes Care 21:1432–1438 [DOI] [PubMed] [Google Scholar]
- 327. Harris MI, Cowie CC, Howie LJ. 1993. Self-monitoring of blood glucose by adults with diabetes in the United States population. Diabetes Care 16:1116–1123 [DOI] [PubMed] [Google Scholar]
- 328. Karter AJ, Ferrara A, Darbinian JA, Ackerson LM, Selby JV. 2000. Self-monitoring of blood glucose: language and financial barriers in a managed care population with diabetes. Diabetes Care 23:477–483 [DOI] [PubMed] [Google Scholar]
- 329. Mühlhauser I, Overmann H, Bender R, Bott U, Jörgens V, Trautner C, Siegrist J, Berger M. 1998. Social status and the quality of care for adult people with type I (insulin-dependent) diabetes mellitus—a population-based study. Diabetologia 41:1139–1150 [DOI] [PubMed] [Google Scholar]
- 330. Chaturvedi N, Stephenson JM, Fuller JH. 1996. The relationship between socioeconomic status and diabetes control and complications in the EURODIAB IDDM Complications Study. Diabetes Care 19:423–430 [DOI] [PubMed] [Google Scholar]
- 331. Chaturvedi N, Jarrett J, Shipley MJ, Fuller JH. 1998. Socioeconomic gradient in morbidity and mortality in people with diabetes: cohort study findings from the Whitehall Study and the WHO Multinational Study of Vascular Disease in Diabetes. BMJ 316:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. 2007. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the Health and Retirement Study. Arch Intern Med 167:1853–1860 [DOI] [PubMed] [Google Scholar]
- 333. Witkin SR, Klein R. 1984. Ophthalmologic care for persons with diabetes. JAMA 251:2534–2537 [PubMed] [Google Scholar]
- 334. Karter AJ, Stevens MR, Brown AF, Duru OK, Gregg EW, Gary TL, Beckles GL, Tseng CW, Marrero DG, Waitzfelder B, Herman WH, Piette JD, Safford MM, Ettner SL. 2007. Educational disparities in health behaviors among patients with diabetes: the Translating Research Into Action for Diabetes (TRIAD) Study. BMC Public Health 7:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Adams AS, Trinacty CM, Zhang F, Kleinman K, Grant RW, Meigs JB, Soumerai SB, Ross-Degnan D. 2008. Medication adherence and racial differences in A1C control. Diabetes Care 31:916–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Robbins JM, Vaccarino V, Zhang H, Kasl SV. 2005. Socioeconomic status and diagnosed diabetes incidence. Diabetes Res Clin Pract 68:230–236 [DOI] [PubMed] [Google Scholar]
- 337. Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. 2011. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol 40:804–818 [DOI] [PubMed] [Google Scholar]
- 338. Dray-Spira R, Gary-Webb TL, Brancati FL. 2010. Educational disparities in mortality among adults with diabetes in the U.S. Diabetes Care 33:1200–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Dray-Spira R, Gary TL, Brancati FL. 2008. Socioeconomic position and cardiovascular disease in adults with and without diabetes: United States trends, 1997–2005. J Gen Intern Med 23:1634–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Ricci-Cabello I, Ruiz-Pérez I, Olry de Labry-Lima A, Márquez-Calderón S. 2010. Do social inequalities exist in terms of the prevention, diagnosis, treatment, control and monitoring of diabetes? A systematic review. Health Soc Care Community 18:572–587 [DOI] [PubMed] [Google Scholar]
- 341. Seeman T, Merkin SS, Crimmins E, Koretz B, Charette S, Karlamangla A. 2008. Education, income and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988–1994). Soc Sci Med 66:72–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Hu DJ, Covell RM. 1986. Health care usage by Hispanic outpatients as function of primary language. West J Med 144:490–493 [PMC free article] [PubMed] [Google Scholar]
- 343. Schur CL, Albers LA. 1996. Language, sociodemographics, and health care use of Hispanic adults. J Health Care Poor Underserved 7:140–158 [DOI] [PubMed] [Google Scholar]
- 344. Fiscella K, Franks P, Doescher MP, Saver BG. 2002. Disparities in health care by race, ethnicity, and language among the insured: findings from a national sample. Med Care 40:52–59 [DOI] [PubMed] [Google Scholar]
- 345. Morales LS, Cunningham WE, Brown JA, Liu H, Hays RD. 1999. Are Latinos less satisfied with communication by health care providers? J Gen Intern Med 14:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Glasgow RE, Strycker LA, Toobert DJ, Eakin E. 2000. A social-ecologic approach to assessing support for disease self-management: the Chronic Illness Resources Survey. J Behav Med 23:559–583 [DOI] [PubMed] [Google Scholar]
- 347. Berkman LF, Syme SL. 1979. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol 109:186–204 [DOI] [PubMed] [Google Scholar]
- 348. Ford ME, Tilley BC, McDonald PE. 1998. Social support among African-American adults with diabetes, Part 2: A review. J Natl Med Assoc 90:425–432 [PMC free article] [PubMed] [Google Scholar]
- 349. Griffith LS, Field BJ, Lustman PJ. 1990. Life stress and social support in diabetes: association with glycemic control. Int J Psychiatry Med 20:365–372 [DOI] [PubMed] [Google Scholar]
- 350. Oakley A, Rajan L. 1991. Social class and social support: the same or different. Sociology 25:31–59 [Google Scholar]
- 351. Finch JF, Okun MA, Barrera M, Jr, Zautra AJ, Reich JW. 1989. Positive and negative social ties among older adults: measurement models and the prediction of psychological distress and well-being. Am J Community Psychol 17:585–605 [DOI] [PubMed] [Google Scholar]
- 352. Brown AF, Ettner SL, Piette J, Weinberger M, Gregg E, Shapiro MF, Karter AJ, Safford M, Waitzfelder B, Prata PA, Beckles GL. 2004. Socioeconomic position and health among persons with diabetes mellitus: a conceptual framework and review of the literature. Epidemiol Rev 26:63–77 [DOI] [PubMed] [Google Scholar]
- 353. Batts ML, Gary TL, Huss K, Hill MN, Bone L, Brancati FL. 2001. Patient priorities and needs for diabetes care among urban African American adults. Diabetes Educ 27:405–412 [DOI] [PubMed] [Google Scholar]
- 354. Becker DM, Yanek LR, Koffman DM, Bronner YC. 1999. Body image preferences among urban African Americans and whites from low income communities. Ethn Dis 9:377–386 [PubMed] [Google Scholar]
- 355. Resnicow K, Baranowski T, Ahluwalia JS, Braithwaite RL. 1999. Cultural sensitivity in public health: defined and demystified. Ethn Dis 9:10–21 [PubMed] [Google Scholar]
- 356. Altabe M. 1998. Ethnicity and body image: quantitative and qualitative analysis. Int J Eat Disord 23:153–159 [DOI] [PubMed] [Google Scholar]
- 357. Rucker CE, III, Cash TF. 1992. Body images, body-size perceptions, and eating behaviors among African-American and white college women. Int J Eating Disord 12:291–299 [Google Scholar]
- 358. Drewnowski A. 2009. Obesity, diets, and social inequalities. Nutr Rev 67(Suppl 1):S36–S39 [DOI] [PubMed] [Google Scholar]
- 359. Casagrande SS, Whitt-Glover MC, Lancaster KJ, Odoms-Young AM, Gary TL. 2009. Built environment and health behaviors among African Americans: a systematic review. Am J Prev Med 36:174–181 [DOI] [PubMed] [Google Scholar]
- 360. Lovasi GS, Hutson MA, Guerra M, Neckerman KM. 2009. Built environments and obesity in disadvantaged populations. Epidemiol Rev 31:7–20 [DOI] [PubMed] [Google Scholar]
- 361. Diez Roux AV, Mair C. 2010. Neighborhoods and health. Ann NY Acad Sci 1186:125–145 [DOI] [PubMed] [Google Scholar]
- 362. Black JL, Macinko J. 2008. Neighborhoods and obesity. Nutr Rev 66:2–20 [DOI] [PubMed] [Google Scholar]
- 363. Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC. 2007. The built environment and obesity. Epidemiol Rev 29:129–143 [DOI] [PubMed] [Google Scholar]
- 364. Auchincloss AH, Diez Roux AV, Brown DG, O'Meara ES, Raghunathan TE. 2007. Association of insulin resistance with distance to wealthy areas: the multi-ethnic study of atherosclerosis. Am J Epidemiol 165:389–397 [DOI] [PubMed] [Google Scholar]
- 365. Auchincloss AH, Diez Roux AV, Mujahid MS, Shen M, Bertoni AG, Carnethon MR. 2009. Neighborhood resources for physical activity and healthy foods and incidence of type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med 169:1698–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Gary TL, Safford MM, Gerzoff RB, Ettner SL, Karter AJ, Beckles GL, Brown AF. 2008. Perception of neighborhood problems, health behaviors, and diabetes outcomes among adults with diabetes in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care 31:273–278 [DOI] [PubMed] [Google Scholar]
- 367. Brown AF, Ang A, Pebley AR. 2007. The relationship between neighborhood characteristics and self-rated health for adults with chronic conditions. Am J Public Health 97:926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Sooman A, Macintyre S, Anderson A. 1993. Scotland's health—a more difficult challenge for some? The price and availability of healthy foods in socially contrasting localities in the west of Scotland. Health Bull (Edinb) 51:276–284 [PubMed] [Google Scholar]
- 369. Morland K, Wing S, Diez Roux A. 2002. The contextual effect of the local food environment on residents' diets: the Atherosclerosis Risk in Communities Study. Am J Public Health 92:1761–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Morland K, Diez Roux AV, Wing S. 2006. Supermarkets, other food stores, and obesity: the Atherosclerosis Risk in Communities Study. Am J Prev Med 30:333–339 [DOI] [PubMed] [Google Scholar]
- 371. Horowitz CR, Colson KA, Hebert PL, Lancaster K. 2004. Barriers to buying healthy foods for people with diabetes: evidence of environmental disparities. Am J Public Health 94:1549–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Zenk SN, Schulz AJ, Israel BA, James SA, Bao S, Wilson ML. 2006. Fruit and vegetable access differs by community racial composition and socioeconomic position in Detroit, Michigan. Ethn Dis 16:275–280 [PubMed] [Google Scholar]
- 373. Rittner B, Kirk AB. 1995. Health care and public transportation use by poor and frail elderly people. Soc Work 40:365–373 [PubMed] [Google Scholar]
- 374. Bostock L. 2001. Pathways of disadvantage? Walking as a mode of transport among low-income mothers. Health Soc Care Community 9:11–18 [DOI] [PubMed] [Google Scholar]
- 375. Cohen DA, Finch BK, Bower A, Sastry N. 2006. Collective efficacy and obesity: the potential influence of social factors on health. Soc Sci Med 62:769–778 [DOI] [PubMed] [Google Scholar]
- 376. Christakis NA, Fowler JH. 2007. The spread of obesity in a large social network over 32 years. N Engl J Med 357:370–379 [DOI] [PubMed] [Google Scholar]
- 377. Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, Kessler RC, Kling JR, Lindau ST, Whitaker RC, McDade TW. 2011. Neighborhoods, obesity, and diabetes—a randomized social experiment. N Engl J Med 365:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Schneider EC, Zaslavsky AM, Epstein AM. 2002. Racial disparities in the quality of care for enrollees in Medicare managed care. JAMA 287:1288–1294 [DOI] [PubMed] [Google Scholar]
- 379. Bynum JP, Fisher ES, Song Y, Skinner J, Chandra A. 2010. Measuring racial disparities in the quality of ambulatory diabetes care. Med Care 48:1057–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Trivedi AN, Grebla RC, Wright SM, Washington DL. 2011. Despite improved quality of care in the Veterans Affairs health system, racial disparity persists for important clinical outcomes. Health Aff (Millwood) 30:707–715 [DOI] [PubMed] [Google Scholar]
- 381. Norris SL, Engelgau MM, Narayan KM. 2001. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 24:561–587 [DOI] [PubMed] [Google Scholar]
- 382. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. 2002. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 25:1159–1171 [DOI] [PubMed] [Google Scholar]
- 383. Peek ME, Cargill A, Huang ES. 2007. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev 64:101S–156S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Martin DP, Diehr P, Price KF, Richardson WC. 1989. Effect of a gatekeeper plan on health services use and charges: a randomized trial. Am J Public Health 79:1628–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Moore SH, Martin DP, Richardson WC. 1983. Does the primary-care gatekeeper control the costs of health care? Lessons from the SAFECO experience. N Engl J Med 309:1400–1404 [DOI] [PubMed] [Google Scholar]
- 386. Duru OK, Mangione CM, Steers NW, Herman WH, Karter AJ, Kountz D, Marrero DG, Safford MM, Waitzfelder B, Gerzoff RB, Huh S, Brown AF. 2006. The association between clinical care strategies and the attenuation of racial/ethnic disparities in diabetes care: the Translating Research Into Action for Diabetes (TRIAD) Study. Med Care 44:1121–1128 [DOI] [PubMed] [Google Scholar]
- 387. Sequist TD, Marshall R, Lampert S, Buechler EJ, Lee TH. 2006. Missed opportunities in the primary care management of early acute ischemic heart disease. Arch Intern Med 166:2237–2243 [DOI] [PubMed] [Google Scholar]
- 388. Karter AJ, Stevens MR, Herman WH, Ettner S, Marrero DG, Safford MM, Engelgau MM, Curb JD, Brown AF. 2003. Out-of-pocket costs and diabetes preventive services: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care 26:2294–2299 [DOI] [PubMed] [Google Scholar]
- 389. Schillinger D, Grumbach K, Piette J, Wang F, Osmond D, Daher C, Palacios J, Sullivan GD, Bindman AB. 2002. Association of health literacy with diabetes outcomes. JAMA 288:475–482 [DOI] [PubMed] [Google Scholar]
- 390. Chien AT, Wroblewski K, Damberg C, Williams TR, Yanagihara D, Yakunina Y, Casalino LP. 2012. Do physician organizations located in lower socioeconomic status areas score lower on pay-for-performance measures? J Gen Intern Med 27:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Chin MH, Zhang JX, Merrell K. 2000. Specialty differences in the care of older patients with diabetes. Med Care 38:131–140 [DOI] [PubMed] [Google Scholar]
- 392. Burge MR, Lucero S, Rassam AG, Schade DS. 2000. What are the barriers to medical care for patients with newly diagnosed diabetes mellitus? Diabetes Obes Metab 2:351–354 [DOI] [PubMed] [Google Scholar]
- 393. Piette JD. 2000. Perceived access problems among patients with diabetes in two public systems of care. J Gen Intern Med 15:797–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Harris MI, Cowie CC, Eastman R. 1994. Health-insurance coverage for adults with diabetes in the U.S. population. Diabetes Care 17:585–591 [DOI] [PubMed] [Google Scholar]
- 395. Harris MI. 1999. Racial and ethnic differences in health insurance coverage for adults with diabetes. Diabetes Care 22:1679–1682 [DOI] [PubMed] [Google Scholar]
- 396. Gregg EW, Geiss LS, Saaddine J, Fagot-Campagna A, Beckles G, Parker C, Visscher W, Hartwell T, Liburd L, Narayan KM, Engelgau MM. 2001. Use of diabetes preventive care and complications risk in two African-American communities. Am J Prev Med 21:197–202 [DOI] [PubMed] [Google Scholar]
- 397. Baker RS, Watkins NL, Wilson MR, Bazargan M, Flowers CW., Jr 1998. Demographic and clinical characteristics of patients with diabetes presenting to an urban public hospital ophthalmology clinic. Ophthalmology 105:1373–1379 [DOI] [PubMed] [Google Scholar]
- 398. Pugh JA, Tuley MR, Hazuda HP, Stern MP. 1992. The influence of outpatient insurance coverage on the microvascular complications of non-insulin-dependent diabetes in Mexican Americans. J Diabetes Complications 6:236–241 [DOI] [PubMed] [Google Scholar]
- 399. Larsson D, Lager I, Nilsson PM. 1999. Socio-economic characteristics and quality of life in diabetes mellitus—relation to metabolic control. Scand J Public Health 27:101–105 [PubMed] [Google Scholar]
- 400. Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM. 2001. Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ 322:1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. van der Meer JB, Mackenbach JP. 1999. The care and course of diabetes: differences according to level of education. Health Policy 46:127–141 [DOI] [PubMed] [Google Scholar]
- 402. Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA. 2003. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care 41:1221–1232 [DOI] [PubMed] [Google Scholar]
- 403. Brown AF, Gregg EW, Stevens MR, Karter AJ, Weinberger M, Safford MM, Gary TL, Caputo DA, Waitzfelder B, Kim C, Beckles GL. 2005. Race, ethnicity, socioeconomic position, and quality of care for adults with diabetes enrolled in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care 28:2864–2870 [DOI] [PubMed] [Google Scholar]
- 404. Connolly V, Unwin N, Sherriff P, Bilous R, Kelly W. 2000. Diabetes prevalence and socioeconomic status: a population based study showing increased prevalence of type 2 diabetes mellitus in deprived areas. J Epidemiol Community Health 54:173–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Kelly WF, Mahmood R, Kelly MJ, Turner S, Elliott K. 1993. Influence of social deprivation on illness in diabetic patients. BMJ 307:1115–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Kelly WF, Mahmood R, Turner S, Elliott K. 1994. Geographical mapping of diabetic patients from the deprived inner city shows less insulin therapy and more hyperglycaemia. Diabet Med 11:344–348 [DOI] [PubMed] [Google Scholar]
- 407. Peters AL, Legorreta AP, Ossorio RC, Davidson MB. 1996. Quality of outpatient care provided to diabetic patients. A health maintenance organization experience. Diabetes Care 19:601–606 [DOI] [PubMed] [Google Scholar]
- 408. Chin MH, Zhang JX, Merrell K. 1998. Diabetes in the African-American Medicare population. Morbidity, quality of care, and resource utilization. Diabetes Care 21:1090–1095 [DOI] [PubMed] [Google Scholar]
- 409. Thackeray R, Merrill RM, Neiger BL. 2004. Disparities in diabetes management practice between racial and ethnic groups in the United States. Diabetes Educ 30:665–675 [DOI] [PubMed] [Google Scholar]
- 410. Kirk JK, Bell RA, Bertoni AG, Arcury TA, Quandt SA, Goff DC, Jr, Narayan KM. 2005. A qualitative review of studies of diabetes preventive care among minority patients in the United States, 1993–2003. Am J Manag Care 11:349–360 [PubMed] [Google Scholar]
- 411. Greenfield S, Kaplan SH, Ware JE, Jr, Yano EM, Frank HJ. 1988. Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med 3:448–457 [DOI] [PubMed] [Google Scholar]
- 412. Kaplan SH, Greenfield S, Ware JE., Jr 1989. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care 27:S110–S127 [DOI] [PubMed] [Google Scholar]
- 413. Berger M, Mühlhauser I. 1999. Diabetes care and patient-oriented outcomes. JAMA 281:1676–1678 [DOI] [PubMed] [Google Scholar]
- 414. Olivarius NF, Beck-Nielsen H, Andreasen AH, Hørder M, Pedersen PA. 2001. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. BMJ 323:970–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Piette JD, Schillinger D, Potter MB, Heisler M. 2003. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med 18:624–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. 2002. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med 17:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Fernandez A, Schillinger D, Grumbach K, Rosenthal A, Stewart AL, Wang F, Pérez-Stable EJ. 2004. Physician language ability and cultural competence. An exploratory study of communication with Spanish-speaking patients. J Gen Intern Med 19:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs 1999. Health literacy: report of the Council on Scientific Affairs. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. JAMA 281:552–557 [PubMed] [Google Scholar]
- 419. Williams MV, Baker DW, Parker RM, Nurss JR. 1998. Relationship of functional health literacy to patients' knowledge of their chronic disease. A study of patients with hypertension and diabetes. Arch Intern Med 158:166–172 [DOI] [PubMed] [Google Scholar]
- 420. Schillinger D, Bindman A, Wang F, Stewart A, Piette J. 2004. Functional health literacy and the quality of physician-patient communication among diabetes patients. Patient Educ Couns 52:315–323 [DOI] [PubMed] [Google Scholar]
- 421. Schillinger D, Barton LR, Karter AJ, Wang F, Adler N. 2006. Does literacy mediate the relationship between education and health outcomes? A study of a low-income population with diabetes. Public Health Rep 121:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, Daher C, Leong-Grotz K, Castro C, Bindman AB. 2003. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 163:83–90 [DOI] [PubMed] [Google Scholar]
- 423. Osborn CY, Cavanaugh K, Wallston KA, White RO, Rothman RL. 2009. Diabetes numeracy: an overlooked factor in understanding racial disparities in glycemic control. Diabetes Care 32:1614–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. van Ryn M, Burke J. 2000. The effect of patient race and socio-economic status on physicians' perceptions of patients. Soc Sci Med 50:813–828 [DOI] [PubMed] [Google Scholar]
- 425. Krieger N, Sidney S, Coakley E. 1998. Racial discrimination and skin color in the CARDIA study: implications for public health research. Coronary Artery Risk Development in Young Adults. Am J Public Health 88:1308–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Tull ES, Chambers EC. 2001. Internalized racism is associated with glucose intolerance among Black Americans in the U.S. Virgin Islands. Diabetes Care 24:1498. [DOI] [PubMed] [Google Scholar]
- 427. Sherbourne CD, Hays RD, Ordway L, DiMatteo MR, Kravitz RL. 1992. Antecedents of adherence to medical recommendations: results from the Medical Outcomes Study. J Behav Med 15:447–468 [DOI] [PubMed] [Google Scholar]
- 428. Piette JD, Bibbins-Domingo K, Schillinger D. 2006. Health care discrimination, processes of care, and diabetes patients' health status. Patient Educ Couns 60:41–48 [DOI] [PubMed] [Google Scholar]
- 429. Narayan KMV, Williams D, Gregg EW, Cowie CC. 2011. Diabetes public health from data to policy. New York; Oxford University Press [Google Scholar]
- 430. Cohen SJ. 1983. Potential barriers to diabetes care. Diabetes Care 6:499–500 [DOI] [PubMed] [Google Scholar]
- 431. Heisler M, Wagner EH. 2004. Improving diabetes treatment quality in managed care organizations: some progress, many challenges. Am J Manag Care 10:115–117 [PubMed] [Google Scholar]
- 432. Landon BE, Hicks LS, O'Malley AJ, Lieu TA, Keegan T, McNeil BJ, Guadagnoli E. 2007. Improving the management of chronic disease at community health centers. N Engl J Med 356:921–934 [DOI] [PubMed] [Google Scholar]
- 433. Piatt GA, Orchard TJ, Emerson S, Simmons D, Songer TJ, Brooks MM, Korytkowski M, Siminerio LM, Ahmad U, Zgibor JC. 2006. Translating the chronic care model into the community: results from a randomized controlled trial of a multifaceted diabetes care intervention. Diabetes Care 29:811–817 [DOI] [PubMed] [Google Scholar]
- 434. Chamany S, Silver LD, Bassett MT, Driver CR, Berger DK, Neuhaus CE, Kumar N, Frieden TR. 2009. Tracking diabetes: New York City's A1C Registry. Milbank Q 87:547–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435. Valassi E, Scacchi M, Cavagnini F. 2008. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis 18:158–168 [DOI] [PubMed] [Google Scholar]
- 436. Rosenberg NA, Huang L, Jewett EM, Szpiech ZA, Jankovic I, Boehnke M. 2010. Genome-wide association studies in diverse populations. Nat Rev Genet 11:356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Jaén CR, Ferrer RL, Miller WL, Palmer RF, Wood R, Davila M, Stewart EE, Crabtree BF, Nutting PA, Stange KC. 2010. Patient outcomes at 26 months in the patient-centered medical home National Demonstration Project. Ann Fam Med 8(Suppl 1):S57–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Castillo-Page L. 2012. Diversity in the physician workforce: facts and figures 2010. Washington, DC: Association of American Medical Colleges [Google Scholar]
- 439. Clarke RM, Tseng CH, Brook RH, Brown AF. 2012. Tool used to assess how well community health centers function as medical homes may be flawed. Health Aff (Millwood) 31:627–635 [DOI] [PubMed] [Google Scholar]
- 440. Peek ME, Wilkes AE, Roberson TS, Goddu AP, Nocon RS, Tang H, Quinn MT, Bordenave KK, Huang ES, Chin MH. 2012. Early lessons from an initiative on Chicago's South Side to reduce disparities in diabetes care and outcomes. Health Aff (Millwood) 31:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Franco M, Orduñez P, Caballero B, Tapia Granados JA, Lazo M, Bernal JL, Guallar E, Cooper RS. 2007. Impact of energy intake, physical activity, and population-wide weight loss on cardiovascular disease and diabetes mortality in Cuba, 1980–2005. Am J Epidemiol 166:1374–1380 [DOI] [PubMed] [Google Scholar]
- 442. Chin MH, Goldmann D. 2011. Meaningful disparities reduction through research and translation programs. JAMA 305:404–405 [DOI] [PubMed] [Google Scholar]
- 443. Baig AA, Wilkes AE, Davis AM, Peek ME, Huang ES, Bell DS, Chin MH. 2010. The use of quality improvement and health information technology approaches to improve diabetes outcomes in African American and Hispanic patients. Med Care Res Rev 67:163S–197S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Hawthorne K, Robles Y, Cannings-John R, Edwards AG. 2008. Culturally appropriate health education for type 2 diabetes mellitus in ethnic minority groups. Cochrane Database Syst Rev 3:CD006424. [DOI] [PubMed] [Google Scholar]
- 445. Sequist TD, Fitzmaurice GM, Marshall R, Shaykevich S, Marston A, Safran DG, Ayanian JZ. 2010. Cultural competency training and performance reports to improve diabetes care for black patients: a cluster randomized, controlled trial. Ann Intern Med 152:40–46 [DOI] [PubMed] [Google Scholar]
- 446. Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. 2005. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res 20:185–194 [DOI] [PubMed] [Google Scholar]
- 447. Taylor AJ, Gary LC, Arora T, Becker DJ, Curtis JR, Kilgore ML, Morrisey MA, Saag KG, Matthews R, Yun H, Smith W, Delzell E. 2011. Clinical and demographic factors associated with fractures among older Americans. Osteoporos Int 22:1263–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Seyle H. 1950. The physiology and pathology of exposure to stress. Montreal, Quebec: Acta [Google Scholar]
- 449. Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. 1997. Price of adaptation—allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med 157:2259–2268 [PubMed] [Google Scholar]
- 450. Seeman TE, McEwen BS, Rowe JW, Singer BH. 2001. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A 98:4770–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]