Abstract
Context:
Cognitive decline is prevalent in aging populations, and cognitive complaints are common during menopause. However, the extent of hormonal influence is unclear, particularly when considered independent of the aging process.
Objective:
We sought to determine differences in cognitive function attributable to menopause, hypothesizing that differences would be associated with reproductive rather than chronological age.
Design and Setting:
In this cross-sectional study at a university hospital, we combined neuropsychological measures with functional magnetic resonance imaging to comprehensively assess cognitive function.
Participants:
Sixty-seven menopausal women, aged 42–61 yr, recruited from a population-based menopause study, grouped into menopause stages based on hormonal and cycle criteria (premenopause, perimenopause, and postmenopause), participated in the study.
Main Outcome Measures:
Neuropsychological and functional magnetic resonance imaging measures of verbal, visual, and executive cognitive function.
Results:
We found age-independent menopause effects on verbal function. Menopause groups differed in phonemic verbal fluency (F = 3.58, P < 0.019) and regional brain activation (inferior frontal cortex: corrected P < 0.000 right, P < 0.036 left; left prefrontal cortex: P < 0.012); left temporal pole: P < 0.001). Verbal measures correlated with estradiol and FSH (phonemic fluency: R = 0.249, P < 0.047 estradiol, R = −0.275, P < 0.029 FSH; semantic fluency: R = 0.318, P < 0.011 estradiol, R = −0.321, P < 0.010 FSH; right inferior frontal cortex: R = 0.364, P < 0.008 FSH; left inferior frontal cortex: R = −0.431, P < 0.001 estradiol, left prefrontal cortex: R = 0.279, P < 0.045 FSH; left temporal pole: R = −0.310, P < 0.024 estradiol, R = 0.451, P < 0.001 FSH; left parahippocampal gyrus: R = −0.278, P < 0.044 estradiol; left parietal cortex: R = −0.326, P < 0.017 estradiol).
Conclusions:
Results suggest that verbal fluency mechanisms are vulnerable during the menopausal transition. Targeted intervention may preserve function of this critical cognitive domain.
Age-related declines in physical function and reproductive senescence are universal eventualities, and in women, the effects of aging and menopause are systemically intertwined. Changes in cognitive function are also associated with the aging process, with memory complaints increasing as women approach menopause (1, 2). Although it can be difficult to distinguish between effects of chronological vs. reproductive aging, the transitioning hormonal environment preceding menopause may have distinct effects on specific cognitive domains (3, 4). Considering the substantial variability (and controversies) regarding cognitive outcomes of menopausal hormone therapies (5–17), understanding the distinction between cognitive effects specifically associated with menopause and those more generally related to chronological aging will improve our understanding of which aspects of cognitive function are addressable during the menopausal transition.
Although there is no consensus on the cognitive impact of menopause, a pattern is emerging of differential effects across cognitive domains. Observational studies of cognition through the unmedicated menopause have fairly consistently demonstrated little change in executive function or episodic memory but provide evidence for reduced verbal fluency in postmenopausal women (4, 8, 12, 18–23). Many of the same studies found little association between cognitive measures and circulating estrogen levels (4, 12, 21, 22, 24–28). Functional magnetic resonance imaging (fMRI) provides an additional measure of neural pathways used during cognitive processing and allows detection of subtle changes in neural activity that may precede measureable behavioral changes (29, 30). fMRI has been used to demonstrate effects of postmenopausal hormone treatment during visual and verbal cognitive tasks (31–35) but has not previously been used to study activation patterns in unmedicated women during the menopause transition.
In the current study, we examined cognitive function in a cross-sectional sample of women spanning premenopause to postmenopause. We combined neuropsychological assessments of verbal, visual, and executive domains with fMRI detection of activation patterns during cognitive tasks, theoretically of greater sensitivity than traditional neuropsychological testing, to achieve a comprehensive assessment of cognitive function. We sought to determine differences in cognitive function attributable to menopause rather than chronological age by evaluating behavioral cognitive outcomes and neural activation patterns during verbal and visual tasks across the menopause stage groups. We expected to find that differences in verbal and visual cognition would be associated with reproductive rather than chronological age.
Materials and Methods
Subjects
Sixty-seven women (56 included in the fMRI imaging analysis), aged 42–61 yr, were recruited from an ongoing study of the menopausal transition in a well-characterized, population-based sample. Women were divided into three menopause status groups based primarily on hormones and menstrual cycle criteria: 1) premenopause (regular menstrual cycles and FSH < 11 IU/liter); 2) perimenopause (at least one cycle in the previous year and FSH between 11 and 45 IU/liter; and 3) postmenopause (no cycles in previous year and FSH > 40 IU/liter). Women with a previous hysterectomy but at least one intact ovary were categorized using hormonal criteria. Women were excluded for acute illness, uncorrected thyroid disease, diabetes, neurological or psychiatric illness, current or past substance abuse, claustrophobia, contraindications to magnetic resonance imaging (pacemakers, surgical clips, or metallic surgical devices), smoking within 3 yr, and hormone use within 3 months. Left-handed women were also excluded because potential hemispheric variability in cognitive function between right- and left-handed people, including differences in hemispheric lateralization particularly noted in women, can impede accurate comparisons of regional brain activation (36).
Study protocol
This was a cross-sectional study of women at three stages of the menopause transition. Women underwent a clinical evaluation including assessment of reproductive hormone levels, neuropsychological assessment of cognitive function, and fMRI to observe neural activation patterns during verbal and visual cognitive tasks. All procedures were approved by the University of Michigan Institutional Review Board, and written informed consent was obtained.
Clinical evaluation
Serum was collected before 1100 h after an 8-h fast, during follicular d 2–7 in cycling women. Estradiol concentrations were measured with a modified off-line ACS:180 E2-6 immunoassay (Bayer Diagnostics Corp., Norwood, MA). FSH concentrations were measured with a two-site chemiluminometric immunoassay using two monoclonal antibodies with specificity for intact FSH (Bayer Diagnostics). Sleep quality was assessed with a self-rated scale from 0 (no difficulty sleeping) to 6 (maximum difficulty sleeping). Data on subjective complaints related to cognitive function were not collected during this study. All clinical measures were assessed during the longitudinal study yearly visit.
Neuropsychological assessment
Neuropsychological measures were administered by a trained neuropsychology technician and were selected to measure executive function (Trail Making Test) (37), perceptual processing speed (Letter and Pattern Comparison Task) (38), visual memory (Brief Visual Memory Test-revised) (39, 40), verbal memory (word list learning subtest from the Wechsler Memory Scale-Third Edition (41), and verbal fluency (Controlled Oral Word Association) (42). Women also completed a measure of general intelligence (Shipley Institute of Living Scale) (43).
fMRI cognitive tasks
Episodic verbal memory processes (verbal encoding and storage) were assessed during the fMRI scanning session using the levels of processing paradigm, in which the women were asked to judge words based on physical characteristics (shallow processing) or on word meanings (deep processing) (44). Sixteen lists of 12 words each were equated for letter length and frequency, with four counterbalanced lists presented in each of four scanning runs. Each list was preceded by 8 sec of instructions, and each word was presented for 1.5 sec with a 1.5-sec interstimulus interval. In the phonemic condition, subjects decided whether each word was presented in uppercase or lowercase letters (shallow processing) and in the semantic condition, whether each word represented an abstract or concrete concept (deep processing). Each list contained equal numbers of uppercase, lowercase, abstract, and concrete words. Responses were made by button-press with the right hand.
Visual working memory was evaluated during the same scanning session using a validated visual delayed-matching-to-sample task (45, 46). The visual stimuli consisted of 9 × 9 grids, with 40 squares darkened into a random pattern, presented under three conditions: 1) matching to sample, 2) 1-sec delayed matching to sample, or 3) 4-sec delayed matching to sample. In the matching condition, a target stimulus was presented simultaneously with two test items. Matches were indicated by button-press. Stimuli were presented for 3 sec, followed by a 7-sec fixation cross between items. The delay conditions assessed visual working memory: the target stimulus was presented alone for 1.5 sec, followed by a 1- or 4-sec delay. After the delay, test items were presented for 3 sec, during which time women indicated the match. Four blocked trials from each condition were counterbalanced over three 6-min runs, for 180 total scans with a 2-sec interscan interval. E-Prime software (Psychology Software Tools Inc., Pittsburgh, PA) controlled the stimulus presentation timing.
Visual and verbal tasks were presented on a computer monitor through radio frequency-shielded goggles mounted to a head coil (Resonance Technology Inc., Northridge, CA). To minimize performance differences, participants practiced tasks before the scanning session until they achieved at least 70% accuracy.
fMRI acquisition and processing
Scans were acquired using a 3T whole-body magnetic resonance imaging scanner (General Electric, Milwaukee, WI) equipped with a standard head coil. Anatomical images were acquired axially with a spoiled gradient recalled, three-dimensional, volumetric acquisition (repetition time = 9.6, echo time = 3.3, inversion recovery preparation = 200 msec, flip angle = 17°, bandwidth = 15.63, 24 cm field of view, 1.5 mm slice thickness, 106–110 slices, 256 × 256 matrix, two excitations). fMRI acquisition was sensitized for the blood oxygen level-dependent (BOLD) effect using a T2-weighted single-shot spiral pulse sequence with 32 oblique-axial slices prescribed to be parallel to the anterior commissure-posterior commissure line (spiral gradient echo, echo time = 25, repetition time = 2000, flip angle = 60°, 4 mm thick contiguous slices, 24 cm field of view, 64 × 64 image matrix). Image reconstruction included steps to remove distortions caused by magnetic field inhomogeneity and other sources of misalignment to the structural data (47). Slices were sinc interpolated in time to correct for the staggered slice-acquisition sequence (48). The first four volumes of each run were discarded to remove magnetic saturation effects, remaining images were realigned to eliminate movement artifacts, and realignment parameters were examined to ensure head movements did not exceed 2 mm (49). Anatomical and functional images were coregistered to each other through rigid body affine transformation using a mutual information algorithm (50). Each T1-weighted magnetic resonance imaging was spatially normalized via linear and nonlinear warping to a standardized template (International Consortium for Brain Mapping/Montreal Neurological Institute, Québec, Canada). The transformation matrix was applied to functional images, and a three-dimensional Gaussian smoothing kernel set at 8 mm full-width half maximum was applied to accommodate residual anatomical variability and improve signal to noise ratios.
fMRI data analysis
fMRI data analyses were conducted using the general linear model (GLM) in statistical parametric mapping imaging software (SPM, Department of Cognitive Neurology, Wellcome, London, UK). For the first-level analysis, contrast images were generated for each subject to assess activation differences between task conditions. For verbal processing task image analysis, initial contrast images (deep encoding-shallow encoding) were subtracted to isolate the deep encoding component. For visual memory task image analysis, contrast images were similarly generated to isolate the visual working memory component (4 sec delay − 1 sec delay). We assessed effects of menopause status, independent of age, using these final deep verbal encoding and visual working memory components. We performed initial one-sample t tests to evaluate the effects of the tasks in our study population. For regions significant at false discovery rate (FDR)-corrected P < 0.05, we calculated percent signal change using extracted beta coefficients and used these values for further analyses in SPSS (SPSS Inc., Chicago, IL). Initial effect-of-group analyses were performed, with and without controlling for age, using the GLM with menopause status as the independent variable and performed subsequent pairwise analyses between groups. Correlations between reproductive hormones and cognitive measures were determined using bivariate and partial (age covariate) correlation analyses.
Results
Study demographics
Participants' mean age was 52 yr, and groups had similar education, intelligence quotient, body mass index, sleep quality, testosterone, and SHBG. FSH and estradiol differed between groups (Table 1). Seven women had previous hysterectomy (three of 20 premenopause, one of 15 perimenopause, and three of 32 postmenopause), accompanied by single oophorectomy in one case (one of 32 postmenopause) and double oophorectomy in two cases (two of 32 postmenopause). Hysterectomies did not significantly differ between groups (Pearson χ2, P = 0.663).
Table 1.
Group demographic, clinical, & cognitive characteristics
| Demographics | Whole sample (n = 67) Mean ± sd | Premenopause (n = 20) Mean ± sd | Perimenopause (n = 15) Mean ± sd | Postmenopause (n = 32) Mean ± sd | ANOVA P | Age covariate P |
|---|---|---|---|---|---|---|
| Age (yr) | 52 ± 4.10 | 47.49 ± 2.78 | 52.66 ± 3.43 | 54.38 ± 2.70 | 0.000 | |
| Education (yr) | 14 ± 2.60 | 15.16 ± 3.13 | 13.87 ± 2.33 | 13.31 ± 2.09 | 0.043 | |
| BMI | 27.7 ± 5.00 | 26.66 ± 5.30 | 30.03 ± 5.51 | 27.23 ± 4.24 | 0.108 | 0.214 |
| Sleep quality | 2.9 ± 2.09 | 2.58 ± 2.31 | 3.13 ± 2.33 | 2.97 ± 1.88 | 0.722 | 0.765 |
| Shipley IQ | 107.47 ± 6.74 | 108.33 ± 5.67 | 105.63 ± 9.70 | 0.519 | 0.209 | |
| Hormones | ||||||
| FSH (mIU/ml) | 50.5 ± 40.40 | 8.13 ± 3.04 | 29.99 ± 14.08 | 84.00 ± 28.20 | 0.000 | 0.000 |
| Estradiol (pg/ml) | 45.7 ± 49.10 | 86.84 ± 46.21 | 56.77 ± 63.98 | 16.40 ± 7.19 | 0.000 | 0.000 |
| Testosterone (ng/dl) | 32.7 ± 11.70 | 30.27 ± 10.70 | 36.65 ± 10.36 | 32.48 ± 12.74 | 0.303 | 0.317 |
| SHBG (nm) | 48.8 ± 22.20 | 54.75 ± 27.01 | 42.95 ± 19.44 | 47.74 ± 20.03 | 0.306 | 0.497 |
| Neuropsychology | ||||||
| Trail-making test A | 23.60 ± 6.41 | 20.42 ± 3.89 | 23.07 ± 5.23 | 25.81 ± 7.36 | 0.012 | 0.023 |
| Trail-making test B | 57.88 ± 22.43 | 49.53 ± 19.13 | 58.87 ± 23.09 | 62.38 ± 23.19 | 0.139 | 0.270 |
| Letter comparison | 9.83 ± 1.90 | 10.42 ± 1.69 | 9.40 ± 1.90 | 9.67 ± 1.99 | 0.247 | 0.412 |
| Pattern comparison | 17.85 ± 2.94 | 19.11 ± 3.01 | 17.53 ± 2.87 | 17.25 ± 2.79 | 0.082 | 0.128 |
| Phonemic fluency | 39.61 ± 11.97 | 44.63 ± 12.07 | 42.40 ± 11.93 | 35.31 ± 10.63 | 0.014 | 0.019 |
| Semantic fluency | 20.06 ± 5.55 | 20.95 ± 4.73 | 22.47 ± 8.18 | 18.41 ± 3.92 | 0.044 | 0.063 |
| Verbal recall | 7.44 ± 2.93 | 8.42 ± 2.17 | 6.67 ± 3.15 | 7.22 ± 3.13 | 0.187 | 0.299 |
| Verbal retention | 22.91 ± 1.32 | 23.05 ± 1.51 | 22.60 ± 1.45 | 22.97 ± 1.15 | 0.581 | 0.767 |
| Visual recall | 20.11 ± 5.93 | 22.84 ± 5.07 | 18.40 ± 6.15 | 19.28 ± 5.93 | 0.050 | 0.104 |
| Visual retention | 95.67 ± 9.01 | 98.11 ± 7.25 | 93.33 ± 13.23 | 95.31 ± 7.36 | 0.299 | 0.489 |
Bold values are statistically significant at P < 0.05.
BMI, Body mass index; IQ, intelligence quotient.
Neuropsychological assessment
Between-groups GLM ANOVA comparisons of neuropsychological cognitive assessments revealed differences in executive function (trail making test A) and verbal fluency (phonemic and semantic fluency) and in visual recall. After controlling for age, only differences in executive function and verbal fluency persisted (Table 1).
Neuroimaging task performance
Women were trained on the cognitive tasks before scanning and performed similarly on all tasks. For shallow verbal processing, premenopausal women averaged 98 ± 5% (sd) accuracy, perimenopause 99 ± 2%, and postmenopause 98 ± 4%; for deep verbal processing, premenopausal women averaged 87 ± 4%, perimenopause 86 ± 5%, and postmenopause 81 ± 8%. For the visual task, matching condition averages for premenopause were 88 ± 8%, perimenopause, 87 ± 10%, and postmenopause, 88 ± 5%; 1-sec delay averages were premenopause, 92 ± 7%, perimenopause, 92 ± 5%, and postmenopause, 90 ± 7%; and 4-sec delay averages for premenopause were 88 ± 9%, perimenopause, 88 ± 9%, and postmenopause, 87 ± 6%. Groups differed in deep verbal processing task accuracy (F = 4.297, P = 0.019), and this difference persisted after controlling for age (F = 2.882, P = 0.045).
Regional activity during deep verbal encoding and visual working memory tasks
We initially determined whole-sample task effects (one sample t test) for both cognitive tasks (Table 2 and Fig. 1). For the verbal processing task, we found fMRI BOLD effects in the right inferior frontal cortex [Montreal Neurological Institute coordinates x, y, z (millimeters) 38, 26, −4; Z = 6.20; PFDR = 0.000], the left inferior frontal cortex (−56, 24, −10; Z = Inf, PFDR = 0.000), the medial prefrontal cortex (−4, 26, 50; Z = Inf, PFDR = 0.000), the right prefrontal cortex (52, 22, 30; Z = 6.45, PFDR = 0.000), the left prefrontal cortex (−54, 22, 20; Z = 7.01, PFDR = 0.000), the left temporal pole (−38, 22, −40; Z = 3.24, PFDR = 0.008), the left parahippocampal cortex (−56, −16, −34; Z = 4.18, PFDR = 0.000), and the left parietal cortex (−34, −66, 44; Z = 4.08, PFDR = 0.000).
Table 2.
Regional effects of cognitive tasks across entire sample
| Region | Coordinates | Z | P(FDR corr) |
|---|---|---|---|
| Verbal task | |||
| Left inferior frontal cortex | −56, 24, −10 | Infinite | 0.000 |
| Right inferior frontal cortex | 38, 26, 04 | 6.2 | 0.000 |
| Medial prefrontal cortex | −4, 26, 50 | Infinite | 0.000 |
| Right prefrontal cortex | 52, 22, 30 | 6.45 | 0.000 |
| Left prefrontal cortex | −54, 22, 20 | 7.01 | 0.000 |
| Left temporal pole | −38, 22, −40 | 3.24 | 0.008 |
| Left parahippocampal gyrus | −56, −16, −34 | 4.18 | 0.000 |
| Left parietal cortex | −34, −66, 44 | 4.08 | 0.000 |
| Visual task | |||
| Medial prefrontal cortex | −2, 16, 52 | 4.73 | 0.012 |
| Left prefrontal cortex | −52, 10, 28 | 4.48 | 0.013 |
| Right superior parietal cortex | 26, −74, 56 | 3.79 | 0.026 |
| Left superior parietal cortex | −24, −74, 54 | 5.11 | 0.012 |
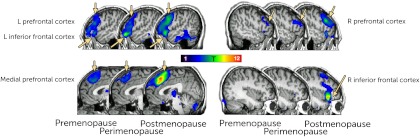
Fig. 1.
Regions activated during the verbal fMRI task in the entire sample (one sample t test). Images are presented in neurological orientation, with a visualization threshold of P = 0.01.
Task effects for the visual memory task were found in the medial prefrontal cortex (−2, 16, 52; Z = 4.73, PFDR = 0.012) and the left prefrontal cortex (−52, 10, 28; Z = 4.48, PFDR = 0.013) and bilaterally in the superior parietal cortex (right 26, −74, 56; Z = 3.79, PFDR = 0.026; left −24, −74, 54, Z = 5.11, PFDR = 0.012).
For regions significantly activated during the tasks, we used extracted beta coefficients to determine group differences. Only regions activated during the verbal task had significant differences in activation between the menopause status groups. Because age differed between groups, we also compared group differences in activation while controlling for age. All regions remained significantly different between groups except the prefrontal cortex (Table 3).
Table 3.
Regional activation during cognitive tasks differed between menopause status groups
| n (verbal fMRI data) | Coordinates | Premenopause (n = 20) (15) Mean ± sd | Perimenopause (n = 15) (12) Mean ± sd | Postmenopause (n = 32) (27) Mean ± sd | ANOVA P | Age covariate P |
|---|---|---|---|---|---|---|
| Verbal task regions | ||||||
| Right inferior frontal cortex | −56, 24, −10 | 0.06 ± 0.13 | 0.05 ± 0.11 | 0.20 ± 0.11 | 0.000 | 0.000 |
| Left inferior frontal cortex | 38, 26, 04 | −0.08 ± 0.25 | 0.17 ± 0.32 | 0.09 ± 0.18 | 0.022 | 0.036 |
| Medial prefrontal cortex | −4, 26, 50 | 0.22 ± 0.21 | 0.17 ± 0.18 | 0.27 ± 0.13 | 0.269 | 0.423 |
| Right prefrontal cortex | 52, 22, 30 | 0.15 ± 0.19 | 0.17 ± 0.14 | 0.25 ± 0.20 | 0.172 | 0.171 |
| Left prefrontal cortex | −54, 22, 20 | −0.03 ± 0.18 | −0.01 ± 0.09 | 0.11 ± 0.13 | 0.005 | 0.012 |
| Left temporal pole | −38, 22, −40 | 0.03 ± 0.16 | 0.04 ± 0.12 | 0.18 ± 0.08 | 0.000 | 0.001 |
| Left parahippocampal gyrus | −56, −16, −34 | −0.06 ± 0.39 | 0.12 ± 0.40 | 0.02 ± 0.37 | 0.484 | 0.694 |
| Left parietal cortex | −34, −66, 44 | −0.08 ± 0.22 | 0.18 ± 0.30 | 0.04 ± 0.20 | 0.292 | 0.052 |
| n (visual fMRI data) | Coordinates | (n = 20) (16) | (n = 15) (12) | (n = 32) (28) | ANOVA P | Age P |
|---|---|---|---|---|---|---|
| Visual task regions | ||||||
| Medial prefrontal cortex | −2, 16, 52 | 0.25 ± 0.10 | 0.15 ± 0.29 | 0.20 ± 0.08 | 0.816 | 0.935 |
| Lefteft prefrontal cortex | −52, 10, 28 | 0.19 ± 0.32 | 0.05 ± 0.31 | 0.24 ± 0.35 | 0.239 | 0.312 |
| Right superior parietal cortex | 26, −74, 56 | 0.52 ± 0.79 | 0.18 ± 0.49 | 0.57 ± 0.73 | 0.259 | 0.369 |
| Left superior parietal cortex | −24, −74, 54 | 0.43 ± 0.44 | 0.18 ± 0.39 | 0.41 ± 0.42 | 0.216 | 0.384 |
Bold values are statistically significant at P < 0.05.
Correlations between hormones and cognitive measures
Estradiol and FSH levels were associated with select neuropsychological and fMRI measures of cognition (Table 4). Controlling for age, increasing estradiol and decreasing FSH levels were correlated with measures of phonemic and semantic fluency, and estradiol was positively correlated with visual memory retention. Neither hormone was correlated with measures of executive function or processing speed. Although cognitive measures correlated with reproductive hormones for both verbal and visual cognitive domains, regional activation was correlated with levels of estradiol, FSH, or both only for the verbal task. This association between reproductive hormones and task-associated brain activity persisted after controlling for age.
Table 4.
Estradiol and FSH levels are associated with cognitive measures
| Estradiol R (P) | FSH R (P) | |
|---|---|---|
| Behavioral measures | ||
| Trail making test A | −0.099 (0.442) | 0.115 (0.374) |
| Trail making test B | −0.014 (0.911) | 0.061 (0.636) |
| Letter comparison | 0.070 (0.582) | −0.008 (0.953) |
| Pattern comparison | 0.166 (0.190) | −0.035 (0.788) |
| Phonemic fluency | 0.249 (0.047) | −0.275 (0.029) |
| Semantic fluency | 0.318 (0.011) | −0.321 (0.010) |
| Verbal recall | 0.089 (0.486) | −0.201 (0.115) |
| Verbal retention | 0.021 (0.872) | −0.040 (0.754) |
| Visual recall | −0.097 (0.448) | −0.234 (0.065) |
| Visual retention | 0.267 (0.033) | 0.049 (0.705) |
| Verbal fMRI regions | ||
| Right interior frontal cortex | −0.220 (0.114) | 0.364 (0.008) |
| Left interior frontal cortex | −0.431 (0.001) | 0.248 (0.077) |
| Medial prefrontal cortex | −0.217 (0.119) | 0.049 (0.73) |
| Right prefrontal cortex | −0.201 (0.149) | −0.008 (0.953) |
| Left prefrontal cortex | −0.149 (0.288) | 0.279 (0.045) |
| Left temporal pole | −0.310 (0.024) | 0.451 (0.001) |
| Left parahippocampal gyrus | −0.278 (0.044) | 0.160 (0.258) |
| Left parietal cortex | −0.326 (0.017) | 0.078 (0.585) |
Bold values are statistically significant at P < 0.05.
Discussion
Cognitive decline is prevalent in aging populations, and cognitive complaints are common during the menopause transition (1, 51). However, the extent of hormonal influence is unclear, particularly when considered independent of the aging process. In this cross-sectional study of women at three stages of the menopause transition, we found effects of menopause status, independent of age, on aspects of verbal but not visual or executive cognitive function. Menopause stage groups differed in neuropsychological measures and in functional imaging during cognitive task processing in regions involved in verbally based tasks, and verbal fluency and imaging measures correlated with reproductive hormone levels.
Menopause status affects verbal, but not visual, cognitive measures
Consistent with established patterns of cognitive aging (52, 53), we found that measures of executive function, processing speed, and verbal cognitive ability declined across the menopause status groups. Effects of cognitive aging tend to be most evident in domains with primarily prefrontal or frontal-striatal influence (54–56), and in this context, differences in executive function across the menopause stages reflects typical patterns of cognitive aging.
Similar to previous studies (19, 21, 23), after controlling for age effects, differences in verbal fluency, but not visual memory, remained between the groups, suggesting that declines in verbal ability are an effect of the menopausal transition independent of aging. This pattern persisted for differences in regional BOLD activation during the verbal cognitive task. Brain regions activated during the task in the entire sample were typical for those reported for verbal tasks, including cognitive association areas, with greater activation in left hemisphere regions (44, 57, 58). Group differences persisted after controlling for age. The effect of menopause stage on regional activation during this task may be due to estrogen-mediated changes in cognitive circuitry. Estrogens have been shown to have a stimulatory effect on prefrontal-hippocampal connectivity, enhance hippocampal synaptic plasticity, and may interface with cholinergic and serotonergic transmission to influence working memory and verbal cognition (59–64). This hypothesis is further supported by evidence that menopausal estradiol treatment selectively benefits prefrontal verbal cognitive processes (35).
Differences between menopause status groups in measures of cognitive processing and function did not extend to the visual domain after controlling for age. Visual memory recall differed between groups before controlling for age, but we did not detect significant differences in visual behavioral measures when age was considered in the analyses. Similarly, no regional BOLD effects of menopause status were obtained for the visual memory task, whether controlling or not for chronological age. This was contrary to our initial hypothesis of group differences in both visual and verbal activation patterns, based on previously detected differences between untreated and hormone-treated postmenopausal women (5, 31, 32). However, our results are consistent with those of other observational studies of unmedicated women transitioning through menopause. Such studies have largely found little cognitive difference between pre- and postmenopausal women (12, 18–20, 22), but those that included a measure of verbal fluency mostly found decrements in postmenopausal women (19, 21, 23). Furthermore, studies that included a perimenopausal group have detected cognitive decrements in some domains in perimenopausal women, compared with both pre- and postmenopausal women (8, 19). An exception is a study that found no differences in most cognitive domains, including verbal fluency, between early and late postmenopausal women but measured lower scores of executive function in late postmenopausal women (65). The reason for this discrepancy is unclear but could be due to differences in sensitivity of the cognitive measures used.
It has been suggested that somatic symptoms, particularly difficulty sleeping, may underlie cognitive changes during menopause, an idea circumstantially supported by studies that found more cognitive complaints during perimenopause than pre- or postmenopausally (8, 66). Although we detected a similar trend of lower cognitive performance during perimenopause for several measures, self-reported sleep difficulty did not differ between the menopause stages, suggesting that sleep quality does not majorly account for cognitive differences between groups. This is in agreement with results from the Study of Women's Health Across the Nation, which found that transient deficits in processing speed during late perimenopause were not associated with depression, sleep disturbance, or vasomotor symptoms (66), and with a separate study that found no cognitive differences in high vs. low symptom menopausal women (67).
Correlations between regional activation, cognitive measures, and hormonal environment
We detected significant associations between estradiol and FSH and measures of verbal fluency and visual retention but not executive function, independent of age. Regional brain activation during the verbal task was negatively correlated with estradiol, positively correlated with FSH, or both, whereas none of the visual regions were associated with either hormonal measure. These results could suggest that estradiol levels are associated with increased regional functional efficiency, consistent with neurotrophic effects of estrogens (68, 69). The associations we observed contrast previous studies that failed to show a direct relationship between circulating estrogens or FSH concentrations and cognitive function during or shortly after the menopausal transition, although one study found higher estradiol levels to be associated with semantic memory (12, 21, 22, 28, 70). Although differences between hormonal effects on cognitive domains may partially explain this discrepancy, even studies that included measures of verbal fluency failed to detect an association with estrogen levels (21, 28). Both FSH and estradiol levels fluctuate throughout the menopause transition, and the larger context of a changing reproductive environment likely carries more influence on cognitive function, a point supported by a longitudinal study in which women with the highest baseline estrogen levels were more likely to experience declines in verbal fluency over time (27) and by studies of older postmenopausal women, which more often detected significant correlations between hormone levels and cognitive measures, including verbal fluency (27, 71, 72). It is also likely that direct measures of brain regional function show stronger associations with endocrine variables likely to modulate brain function, compared with more distal neuropsychological testing measures.
Strengths and limitations of study
The current study design had several strengths contributing to the ability to detect behavioral and functional differences between menopause stage groups and cognitive associations with reproductive hormone levels. Three distinct menopause stage groups, defined by a combination of hormonal environment and cycle pattern, allowed us to delineate not only differences between pre- and postmenopausal women but also to assess women within the transitional period itself. A wide age span that overlapped menopause stages additionally allowed us to differentiate the effects of chronological age from effects of menopause status and the hormonal environment.
There is evidence that regional activation patterns detected with functional imaging reflect a more sensitive measure of physiological events underlying behavioral phenomena and may be indicative of future behavioral outcomes (29, 30, 73, 74). In the work presented, menopause and hormonal effects on brain regional activity during the verbal and visual cognitive tasks were consistent with those observed in neuropsychological testing, and are complement by correlational associations between reproductive hormones and behavioral measures. Discrepancies between our results and those of previous studies, such as those related to associations between measures of cognitive function and hormonal measures, may be due to assay sensitivity but also differences in study design, including differences in participant characteristics, menopause status designation, specific hormones or cognitive domains evaluated, or choice of cognitive measures (4, 75, 76). The issues of age and menopause status designation are critical, and the current study design allowed effective separation of age effects from effects of menopause or hormonal environment. Although a cross-sectional study cannot detect changes over time in a given sample, the results from this study provide a strong background for future longitudinal examination of this sample of women as they continue the aging process.
Conclusions
The results of our study suggest that menopause status and hormonal environment directly influence aspects of verbal, but not visual, cognitive functioning, both at a behavioral and neuroanatomical level. This effect of menopause was independent of age, suggesting that declines in verbal fluency during the menopausal transition represent a target for intervention.
Although previous clinical trials of hormone therapy in midlife menopausal women have not demonstrated improved verbal fluency (6, 9), imaging studies have revealed alterations in neural activation patterns in response to hormone treatment (31–35), indicating a potential benefit of earlier or continued treatment. With the awareness that verbal fluency mechanisms are vulnerable during this transitioning time of life, pharmacological and nonpharmacological interventions may preserve function of this critical cognitive domain.
Acknowledgments
We thank the University of Michigan fMRI laboratory, Mary Crutchfield, and Anne Tkaczyk for study coordination, and especially the women who participated in our study.
This work was supported by the National Institute of Health Grants R01AG027675 and RO1AG17104, the Office of Women's Health, and for investigator support, by the National Institute of Health Grant R01AG027675-04S1, the University of Michigan Postdoctoral Translational Scholars Program, and the Phil F. Jenkins Research Fund.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BOLD
- Blood oxygen level dependent
- FDR
- false discovery rate
- fMRI
- functional magnetic resonance imaging
- GLM
- general linear model.
References
- 1. Sullivan Mitchell E, Fugate Woods N. 2001. Midlife women's attributions about perceived memory changes: observations from the Seattle Midlife Women's Health Study. J Womens Health Gend Based Med 10:351–362 [DOI] [PubMed] [Google Scholar]
- 2. Weber M, Mapstone M. 2009. Memory complaints and memory performance in the menopausal transition. Menopause 16:694–700 [DOI] [PubMed] [Google Scholar]
- 3. Will MA, Randolph JF. 2009. The influence of reproductive hormones on brain function in the menopausal transition. Minerva Ginecol 61:469–481 [PubMed] [Google Scholar]
- 4. Henderson VW, Popat RA. 2011. Effects of endogenous and exogenous estrogen exposures in midlife and late-life women on episodic memory and executive functions. Neuroscience 191:129–138 [DOI] [PubMed] [Google Scholar]
- 5. Smith YR, Giordani B, Lajiness-O'Neill R, Zubieta JK. 2001. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertil Steril 76:1101–1107 [DOI] [PubMed] [Google Scholar]
- 6. Kocoska-Maras L, Zethraeus N, Rådestad AF, Ellingsen T, von Schoultz B, Johannesson M, Hirschberg AL. 2011. A randomized trial of the effect of testosterone and estrogen on verbal fluency, verbal memory, and spatial ability in healthy postmenopausal women. Fertil Steril 95:152–157 [DOI] [PubMed] [Google Scholar]
- 7. Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Gaussoin S, Dailey M, Shumaker SA. 2010. Postmenopausal hormone therapy and cognitive outcomes: the Women's Health Initiative Memory Study (WHIMS). J Steroid Biochem Mol Biol 118:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS. 2009. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 72:1850–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. 2007. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology 69:1322–1330 [DOI] [PubMed] [Google Scholar]
- 10. Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. 2005. Estrogen therapy and risk of cognitive decline: results from the Women's Estrogen for Stroke Trial (WEST). Am J Obstet Gynecol 192:387–393 [DOI] [PubMed] [Google Scholar]
- 11. Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. 2002. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med 113:543–548 [DOI] [PubMed] [Google Scholar]
- 12. Henderson VW, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. 2003. Estrogen exposures and memory at midlife: a population-based study of women. Neurology 60:1369–1371 [DOI] [PubMed] [Google Scholar]
- 13. Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. 2004. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials (London, England) 1:440–450 [DOI] [PubMed] [Google Scholar]
- 14. Wroolie TE, Kenna HA, Williams KE, Powers BN, Holcomb M, Khaylis A, Rasgon NL. 2011. Differences in verbal memory performance in postmenopausal women receiving hormone therapy: 17β-Estradiol versus conjugated equine estrogens. Am J Geriatr Psychiatry 19:792–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sherwin BB, Grigorova M. 2011. Differential effects of estrogen and micronized progesterone or medroxyprogesterone acetate on cognition in postmenopausal women. Fertil Steril 96:399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sherwin BB. 2005. Estrogen and memory in women: how can we reconcile the findings? Horm Behav 47:371–375 [DOI] [PubMed] [Google Scholar]
- 17. Sherwin BB. 2007. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol 19:77–81 [DOI] [PubMed] [Google Scholar]
- 18. Meyer PM, Powell LH, Wilson RS, Everson-Rose SA, Kravitz HM, Luborsky JL, Madden T, Pandey D, Evans DA. 2003. A population-based longitudinal study of cognitive functioning in the menopausal transition. Neurology 61:801–806 [DOI] [PubMed] [Google Scholar]
- 19. Fuh JL, Wang SJ, Lee SJ, Lu SR, Juang KD. 2006. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas 53:447–453 [DOI] [PubMed] [Google Scholar]
- 20. Kok HS, Kuh D, Cooper R, van der Schouw YT, Grobbee DE, Wadsworth ME, Richards M. 2006. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause 13:19–27 [DOI] [PubMed] [Google Scholar]
- 21. Herlitz A, Thilers PP, Habib R. 2007. Endogenous estrogen is not associated with cognitive performance before, during, or after menopause. Menopause 14:425–431 [DOI] [PubMed] [Google Scholar]
- 22. Luetters C, Huang MH, Seeman T, Buckwalter G, Meyer PM, Avis NE, Sternfeld B, Johnston JM, Greendale GA. 2007. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women's health across the nation (SWAN). J Womens Health (Larchmnt) 16:331–344 [DOI] [PubMed] [Google Scholar]
- 23. Thilers PP, Macdonald SW, Nilsson LG, Herlitz A. 2010. Accelerated postmenopausal cognitive decline is restricted to women with normal BMI: longitudinal evidence from the Betula project. Psychoneuroendocrinology 35:516–524 [DOI] [PubMed] [Google Scholar]
- 24. Yaffe K, Grady D, Pressman A, Cummings S. 1998. Serum estrogen levels, cognitive performance, and risk of cognitive decline in older community women. J Am Geriatr Soc 46:816–821 [DOI] [PubMed] [Google Scholar]
- 25. Barrett-Connor E, Goodman-Gruen D. 1999. Cognitive function and endogenous sex hormones in older women. J Am Geriatr Soc 47:1289–1293 [DOI] [PubMed] [Google Scholar]
- 26. den Heijer T, Geerlings MI, Hofman A, de Jong FH, Launer LJ, Pols HA, Breteler MM. 2003. Higher estrogen levels are not associated with larger hippocampi and better memory performance. Arch Neurol 60:213–220 [DOI] [PubMed] [Google Scholar]
- 27. Laughlin GA, Kritz-Silverstein D, Barrett-Connor E. 2010. Endogenous oestrogens predict 4-year decline in verbal fluency in postmenopausal women: the Rancho Bernardo Study. Clin Endocrinol (Oxf) 72:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ryan J, Stanczyk FZ, Dennerstein L, Mack WJ, Clark MS, Szoeke C, Henderson VW. 2011. Executive functions in recently postmenopausal women: absence of strong association with serum gonadal steroids. Brain Res 1379:199–205 [DOI] [PubMed] [Google Scholar]
- 29. Woodard JL, Seidenberg M, Nielson KA, Smith JC, Antuono P, Durgerian S, Guidotti L, Zhang Q, Butts A, Hantke N, Lancaster M, Rao SM. 2010. Prediction of cognitive decline in healthy older adults using fMRI. J Alzheimers Dis 21:871–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wierenga CE, Bondi MW. 2007. Use of functional magnetic resonance imaging in the early identification of Alzheimer's disease. Neuropsychol Rev 17:127–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith YR, Love T, Persad CC, Tkaczyk A, Nichols TE, Zubieta JK. 2006. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. J Clin Endocrinol Metab 91:4476–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berent-Spillson A, Persad CC, Love T, Tkaczyk A, Wang H, Reame NK, Frey KA, Zubieta JK, Smith YR. 2010. Early menopausal hormone use influences brain regions used for visual working memory. Menopause 17:692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dumas JA, McDonald BC, Saykin AJ, McAllister TW, Hynes ML, West JD, Newhouse PA. 2010. Cholinergic modulation of hippocampal activity during episodic memory encoding in postmenopausal women: a pilot study. Menopause 17:852–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gleason CE, Schmitz TW, Hess T, Koscik RL, Trivedi MA, Ries ML, Carlsson CM, Sager MA, Asthana S, Johnson SC. 2006. Hormone effects on fMRI and cognitive measures of encoding: importance of hormone preparation. Neurology 67:2039–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. 2006. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 13:411–422 [DOI] [PubMed] [Google Scholar]
- 36. van der Kallen BF, Morris GL, Yetkin FZ, van Erning LJ, Thijssen HO, Haughton VM. 1998. Hemispheric language dominance studied with functional MR: preliminary study in healthy volunteers and patients with epilepsy. AJNR Am J Neuroradiol 19:73–77 [PMC free article] [PubMed] [Google Scholar]
- 37. Lezak DL. 1995. Neuropsychological Assessment. 3rd ed New York: Oxford University Press [Google Scholar]
- 38. Salthouse TA. 1994. Aging associations: influence of speed on adult age differences in associative learning. J Exp Psychol Learn Mem Cogn 20:1486–1503 [DOI] [PubMed] [Google Scholar]
- 39. Benton AL. 1974. Revised visual retention test. New York: The Psychological Corp [Google Scholar]
- 40. Benton AL, Hamsher K, Varney N, Spreen O. 1983. Contributions to neuropsychological assessment. New York: Oxford University Press [Google Scholar]
- 41. Wechsler D. 1997. Manual for the Wechsler Adult Intelligence Scale-III. San Antonio, TX: The Psychological Corp [Google Scholar]
- 42. Benton AL, Hamsher KD. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates [Google Scholar]
- 43. Shipley WC. 1946. Institute of Living Scale. Los Angeles: Western Psychological Services [Google Scholar]
- 44. Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, Vaidya CJ, Glover GH. 1996. Functional magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychol Sci 7:278–283 [Google Scholar]
- 45. Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, Lieberman JA. 2003. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Arch Gen Psychiatry 60:238–243 [DOI] [PubMed] [Google Scholar]
- 46. Phillips W. 1974. On the distinction between sensory storage and short-term visual memory. Percept Psychophys 16:283–290 [Google Scholar]
- 47. Noll D, Stenger V, Vasquez A, Peltier S. 1999. Spiral scanning in functional MRI. Heidelberg: Springer-Verlag [Google Scholar]
- 48. Acquirre C, D'Esposito M. 1999. Experimental design for brain fMRI. Heidelberg: Springer-Verlag [Google Scholar]
- 49. Friston K, Ashburner J, Frith C, Poline JB, Heather J, Frackowiak R. 1995. Spatial registration and normalization of images. Hum Brain Mapp 2:165–189 [Google Scholar]
- 50. Meyer CR, Boes JL, Kim B, Bland PH, Zasadny KR, Kison PV, Koral K, Frey KA, Wahl RL. 1997. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Med Image Anal 1:195–206 [DOI] [PubMed] [Google Scholar]
- 51. Xu J, Bartoces M, Neale AV, Dailey RK, Northrup J, Schwartz KL. 2005. Natural history of menopause symptoms in primary care patients: a MetroNet study. J Am Board Fam Pract 18:374–382 [DOI] [PubMed] [Google Scholar]
- 52. Salthouse TA. 1996. The processing-speed theory of adult age differences in cognition. Psychol Rev 103:403–428 [DOI] [PubMed] [Google Scholar]
- 53. Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. 2007. Age changes in processing speed as a leading indicator of cognitive aging. Psychol Aging 22:558–568 [DOI] [PubMed] [Google Scholar]
- 54. Buckner RL. 2004. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 44:195–208 [DOI] [PubMed] [Google Scholar]
- 55. Drag LL, Bieliauskas LA. 2010. Contemporary review 2009: cognitive aging. J Geriatr Psychiatry Neurol 23:75–93 [DOI] [PubMed] [Google Scholar]
- 56. Glisky EL. 2007. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain aging: models, methods, and mechanisms. Chap 1 Boca Raton, FL: CRC Press; [PubMed] [Google Scholar]
- 57. Gabrieli JD, Poldrack RA, Desmond JE. 1998. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA 95:906–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gernsbacher MA, Kaschak MP. 2003. Neuroimaging studies of language production and comprehension. Annu Rev Psychol 54:91–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ottowitz WE, Siedlecki KL, Lindquist MA, Dougherty DD, Fischman AJ, Hall JE. 2008. Evaluation of prefrontal-hippocampal effective connectivity following 24 hours of estrogen infusion: an FDG-PET study. Psychoneuroendocrinology 33:1419–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Epperson CN, Amin Z, Ruparel K, Gur R, Loughead J. 2012. Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology 37:372–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maki PM, Dumas J. 2009. Mechanisms of action of estrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med 27:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Osterlund MK, Hurd YL. 2001. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol 64:251–267 [DOI] [PubMed] [Google Scholar]
- 63. Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. 2008. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 29:219–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smith YR, Bowen L, Love TM, Berent-Spillson A, Frey KA, Persad CC, Reame NK, Koeppe RA, Zubieta JK. 2011. Early initiation of hormone therapy in menopausal women is associated with increased hippocampal and posterior cingulate cholinergic activity. J Clin Endocrinol Metab 96:E1761–E1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Elsabagh S, Hartley DE, File SE. 2007. Cognitive function in late versus early postmenopausal stage. Maturitas 56:84–93 [DOI] [PubMed] [Google Scholar]
- 66. Greendale GA, Wight RG, Huang MH, Avis N, Gold EB, Joffe H, Seeman T, Vuge M, Karlamangla AS. 2010. Menopause-associated symptoms and cognitive performance: results from the study of women's health across the nation. Am J Epidemiol 171:1214–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. LeBlanc ES, Neiss MB, Carello PE, Samuels MH, Janowsky JS. 2007. Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause 14:191–202 [DOI] [PubMed] [Google Scholar]
- 68. Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. 2007. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids 72:381–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Behl C. 2002. Oestrogen as a neuroprotective hormone. Nat Rev 3:433–442 [DOI] [PubMed] [Google Scholar]
- 70. Ryan J, Stanczyk FZ, Dennerstein L, Mack WJ, Clark MS, Szoeke C, Kildea D, Henderson VW. 2012. Hormone levels and cognitive function in postmenopausal midlife women. Neurobiol Aging 33:617.e11–22 [DOI] [PubMed] [Google Scholar]
- 71. Drake EB, Henderson VW, Stanczyk FZ, McCleary CA, Brown WS, Smith CA, Rizzo AA, Murdock GA, Buckwalter JG. 2000. Associations between circulating sex steroid hormones and cognition in normal elderly women. Neurology 54:599–603 [DOI] [PubMed] [Google Scholar]
- 72. Wolf OT, Kirschbaum C. 2002. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm Behav 41:259–266 [DOI] [PubMed] [Google Scholar]
- 73. Kochan NA, Breakspear M, Valenzuela M, Slavin MJ, Brodaty H, Wen W, Trollor JN, Turner A, Crawford JD, Sachdev PS. 2011. Cortical responses to a graded working memory challenge predict functional decline in mild cognitive impairment. Biol Psychiatry 70:123–130 [DOI] [PubMed] [Google Scholar]
- 74. Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. 2008. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry 79:630–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rice K, Morse C. 2003. Measuring cognition in menopause research: a review of test use. Climacteric 6:2–22 [PubMed] [Google Scholar]
- 76. Gracia CR, Sammel MD, Freeman EW, Lin H, Langan E, Kapoor S, Nelson DB. 2005. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause 12:128–135 [DOI] [PubMed] [Google Scholar]