Abstract
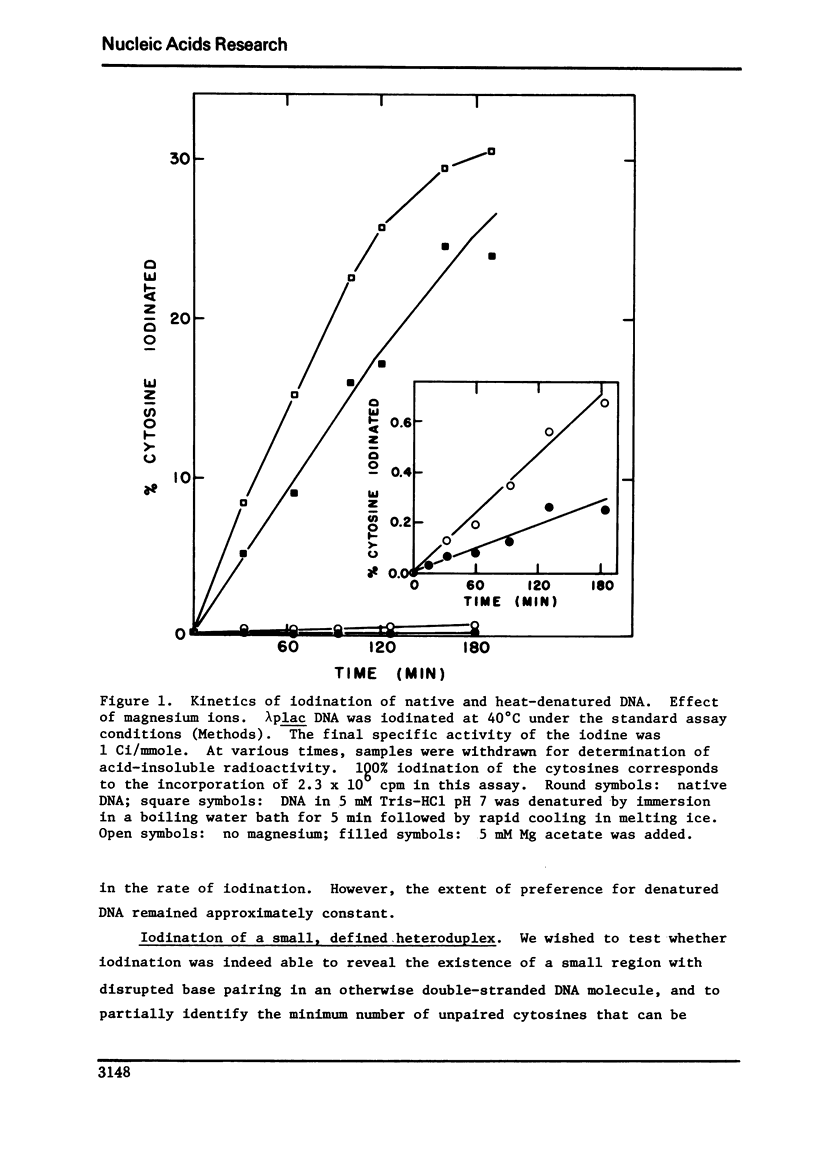
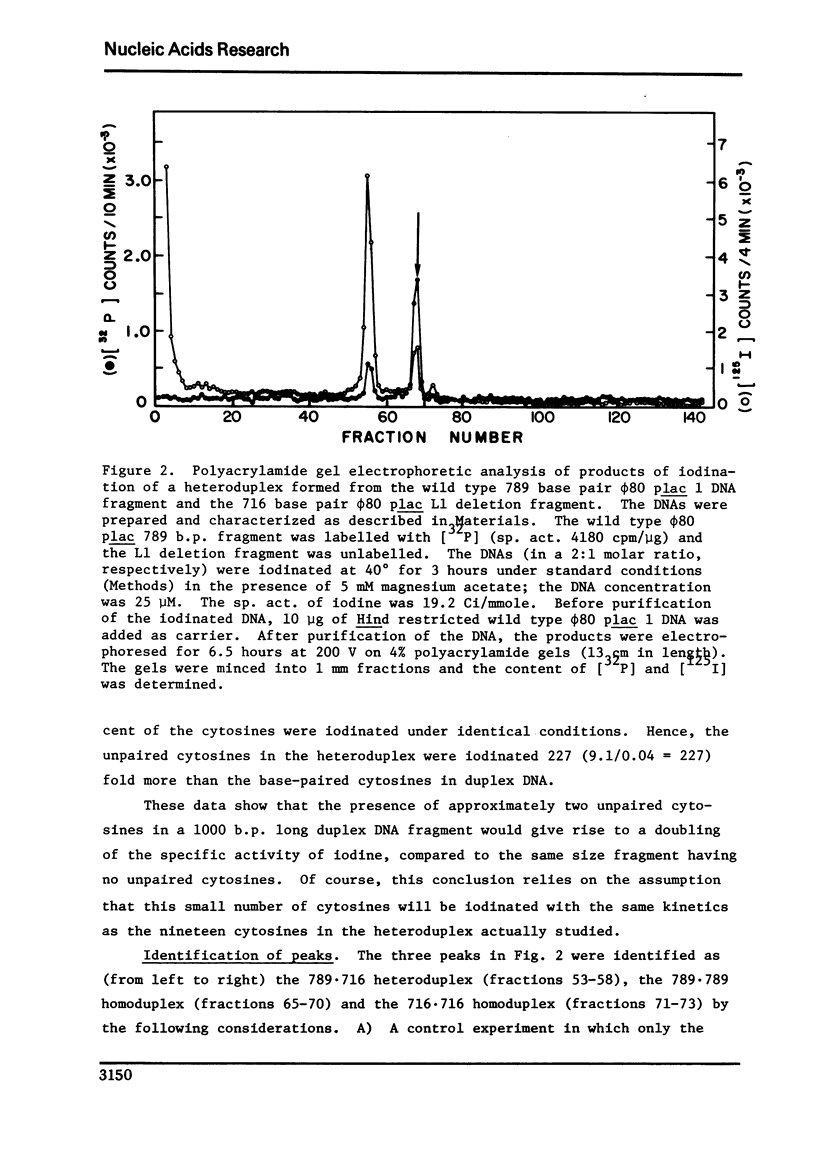
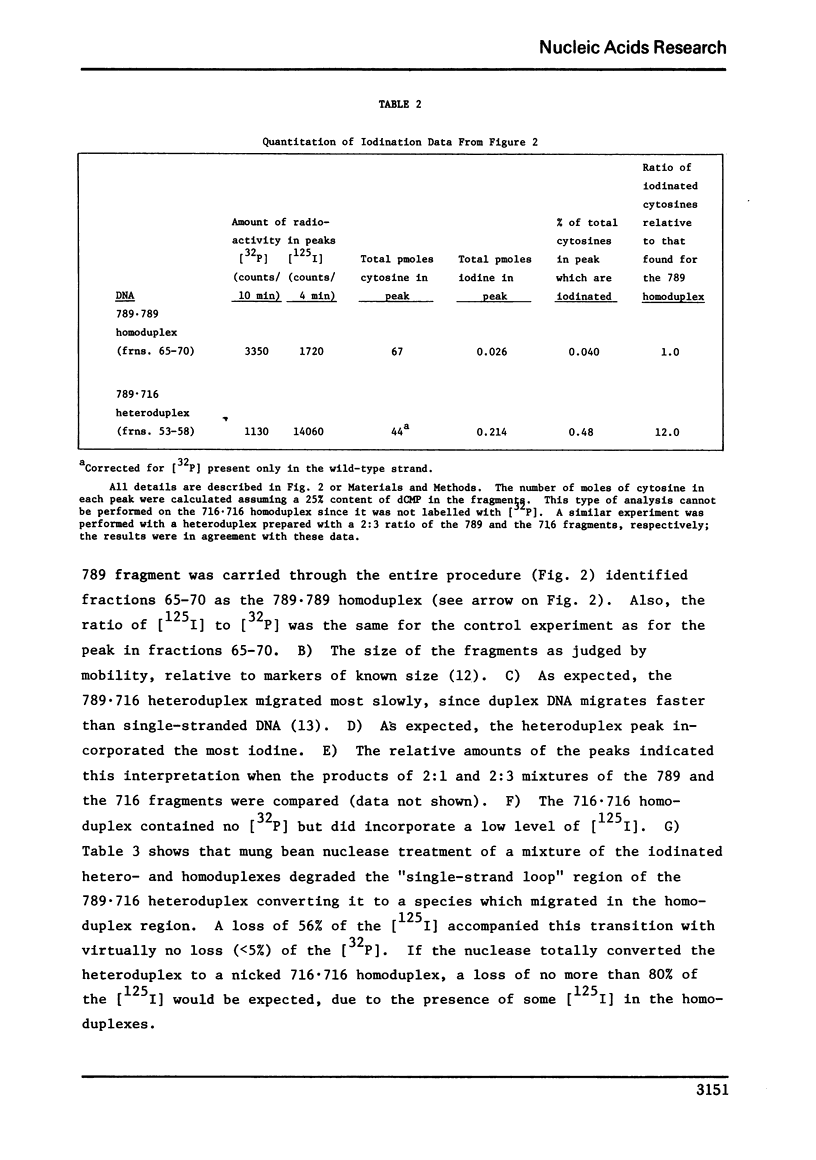
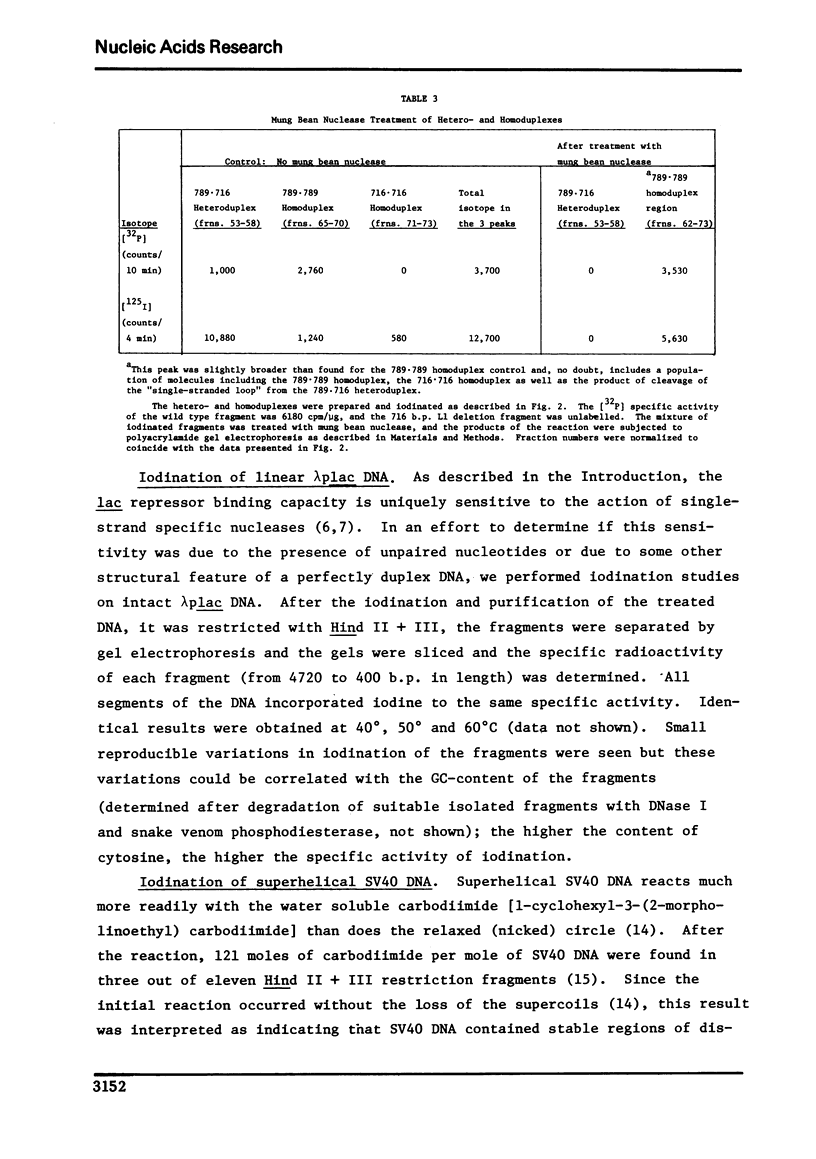
Conditions were established where the thallium-catalyzed iodination of random coil DNA proceeded 100-200 times faster than for native DNA. This reaction was explored as a probe for localized regions of disrupted base pairs in duplex DNA. A heteroduplex was constructed between DNA fragments produced by Hind II + III cleavage of phi80 plac DNA and phi80 plac DNA containing the Ll deletion (73 nucleotides in length). This heteroduplex incorporated twelve times as much iodine as the parent homoduplex fragments. Hence the technique could reveal the presence of a few (two or more) nonpaired cytosines, if they existed within an otherwise helical DNA fragment 789 base pairs long. Iodination studies were performed on superhelical SV40 DNA and on linear lambdaplac DNA. Analysis of the relative amount of iodine in restriction endonuclease fragments of these DNA's revealed the absence of localized single-stranded regions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Bukhari A. I. Analysis of bacteriophage mu and lambda-mu hybrid DNAs by specific endonucleases. J Mol Biol. 1975 Mar 15;92(4):529–540. doi: 10.1016/0022-2836(75)90307-1. [DOI] [PubMed] [Google Scholar]
- Anderson D. M., Folk W. R. Iodination of DNA. Studies of the reaction and iodination of papovavirus DNA. Biochemistry. 1976 Mar 9;15(5):1022–1030. doi: 10.1021/bi00650a012. [DOI] [PubMed] [Google Scholar]
- Blakesley R. W., Wells R. D. 'Single-stranded' DNA from phiX174 and M13 is cleaved by certain restriction endonucleases. Nature. 1975 Oct 2;257(5525):421–422. doi: 10.1038/257421a0. [DOI] [PubMed] [Google Scholar]
- Chan H. W., Wells R. D. Structural uniqueness of lactose operator. Nature. 1974 Nov 15;252(5480):205–209. doi: 10.1038/252205a0. [DOI] [PubMed] [Google Scholar]
- Chen M., Lebowitz J., Salzman N. P. Hin D restriction mapping of upaired regions in simian virus 40 superhelical DNA I: considerations regarding structure-function relationships. J Virol. 1976 Apr;18(1):211–217. doi: 10.1128/jvi.18.1.211-217.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Coulter M., Flintoff W., Paetkau V., Pulleyblank D., Morgan A. R. In vitro synthesis and detection of deoxyribonucleic acids with covalently linked complementary sequences. Biochemistry. 1974 Apr 9;13(8):1603–1609. doi: 10.1021/bi00705a008. [DOI] [PubMed] [Google Scholar]
- Lebowitz J., Garon C. G., Chen M. C., Salzman N. P. Chemical modification of simian virus 40 DNA by reaction with a water-soluble carbodiimide. J Virol. 1976 Apr;18(1):205–210. doi: 10.1128/jvi.18.1.205-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Metz D. H., Brown G. L. The investigation of nucleic acid secondary structure by means of chemical modification with a carbodiimide reagent. I. The reaction between N-cyclohexyl-N'-beta-(4-methylmorpholinium)ethylcarbodiimide and model nucleotides. Biochemistry. 1969 Jun;8(6):2312–2328. doi: 10.1021/bi00834a012. [DOI] [PubMed] [Google Scholar]
- Orosz J. M., Wetmur J. G. In vitro iodination of DNA. Maximizing iodination while minimizing degradation; use of buoyant density shifts for DNA-DNA hybrid isolation. Biochemistry. 1974 Dec 31;13(27):5467–5473. doi: 10.1021/bi00724a003. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Morgan A. R. The sense of naturally occurring superhelices and the unwinding angle of intercalated ethidium. J Mol Biol. 1975 Jan 5;91(1):1–13. doi: 10.1016/0022-2836(75)90368-x. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Woodworth-Gutai M., Lebowitz J. Introduction of interrupted secondary structure in supercoiled DNA as a function of superhelix density: consideration of hairpin structures in superhelical DNA. J Virol. 1976 Apr;18(1):195–204. doi: 10.1128/jvi.18.1.195-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


