Abstract
Cation channels of the Transient Receptor Potential Canonical (TRPC) group, which belong to the larger TRP superfamily of channel proteins, are critical players in cardiovascular disease. Recent studies underscored a role of TRPC3 in macrophage survival and efferocytosis, two critical events in atherosclerosis lesion development. Also, other members of the TRP channel superfamily are found expressed in monocytes/macrophages, where they participate in processes that might be of significance to atherogenesis. These observations set a framework for future studies aimed at defining the ultimate functions not only of TRPC3, but probably other TRP channels, in macrophage biology. The purpose of this manuscript is to provide a timely revision of existing evidence on the role of members of the TRP channel superfamily, in particular TRPCs, in macrophages and discuss it in the context of the macrophage’s function in atherogenesis.
Keywords: TRP channels, macrophage survival, macrophage apoptosis, atherosclerosis, efferocytosis, Ca2+ influx, cardiovascular disease
Introduction
Evolution of an atherosclerotic lesion is a complex process that involves participation of different cell types, a multitude of signaling molecules and an intricate interplay between factors intrinsic to the arterial wall and genetic and environmental variables. Despite the notable advance in our knowledge on the mechanisms underlying lesion formation, atherosclerosis remains one of the most devastating cardiovascular diseases in western societies and remains as the most salient vascular complication in several endocrine and metabolic diseases.1 While the earliest molecular and cellular events in atherogenesis are somewhat predictable responses that follow endothelial activation and subintima inflammation, progression of the lesion and its commitment toward more advanced stages can be envisaged as a maladaptive inflammatory response, characterized by persistent endothelial inflammatory signaling, sustained recruitment of inflammatory immune cells and a vicious interplay among cytokines and pro-inflammatory mediators in the lesion microenvironment that promote lesion growth and plaque instability. Within this context, the balance between survival and apoptosis of lesional macrophages, as well as their timely clearance from the lesion by resident phagocytes—efferocytosis—are now recognized as key determinants of lesion progression and fate.2 Indeed, a rapidly growing area of research in the field is focused on characterizing mechanisms that regulate macrophage survival, apoptosis and efferocytosis in atherosclerosis, with the hope of identifying novel targets to develop alternative strategies to manage the disease. In this regard, recent work from the author’s laboratory identified Transient Receptor Potential Canonical 3 (TRPC3), a member of the canonical TRP family of channel forming proteins (TRPC, see section below) as an obligatory component in endothelial inflammatory signaling associated to monocyte recruitment3,4 as well as in mechanisms mediating macrophage survival and efferocytic properties5 (discussed in detail later), suggesting that TRPC3 may be a contributing factor to lesion formation and progression. In addition, over recent years a number of reports provided experimental evidence regarding expression of several other members of the TRP channel superfamily in monocytes/macrophages, and in most instances their channel function exhibits potential physiopathological implication. It is thus timely for this field, which is at its very infancy, to revisit the existing knowledge on monocyte/macrophage TRP channels and to discuss it in the context of the role of these cells in the pathogenesis of atherosclerosis. Whereas emphasis is put on members of the TRPC group, TRP members for which evidence exists about their involvement in monocyte/macrophage function are also discussed. Altogether, the findings discussed here set a provocative framework from where additional in vitro and in vivo studies can be designed to define the ultimate roles of TRP channels in macrophage biology.
TRPC Channels: Classification and General Structural/Regulatory Aspects
Transient Receptor Potential Canonical (TRPC) channels belong to the larger superfamily of mammalian TRP channel forming proteins which includes other less related channel proteins: the TRPV family (TRPV1–6) named after its first group member, the vanilloid receptor; the TRPM family (TRPM1–8) named after its founding member, melastatin; and other more distantly related members originally identified as inherited disease-related genes such as the TRPMLs and TRPPs that include mucolipins and polycystins, respectively, and TRPA1, which is a nociceptive channel characterized by having about 14 ankyrin repeats.6-8 For detailed information on structural and regulatory aspects of members of the TRP superfamily other than TRPCs, and of TRPCs in particular, the reader is referred to previous reviews on the subject.8,9 By far, TRPCs are the most prominent non-voltage-gated, Ca2+-permeable cation channels in non-excitable cells.7,9 TRPC proteins can be grouped into four subfamilies: TRPC1, TRPC2 (a pseudogene in humans but a functional channel in rodents), TRPC3/6/7 and TRPC4/5.7,10 Physiologically, TRPCs function as multifunctional Ca2+-permeable non-selective cation channels activated downstream of phosphoinositide-specific phospholipase C (PI-PLC). Regardless of the activating signal for individual TRPC members, there is no question about the importance of TRPC-mediated Ca2+ influx in cardiovascular physiology and disease.11 Changes in intracellular Ca2+ concentration that follow Ca2+ influx through TRPC channels modulate numerous Ca2+-dependent signaling pathways and this can be seen throughout the entire cardiovascular system, in smooth muscle and endothelial cells, cardiomyocytes, lymphocytes, monocytes and macrophages (see section below), among other cell types. TRPC members exhibit several common structural motifs: cytoplasmic N- and C-termini separated by six transmembrane domains (TM1-TM6); a channel pore between TM5 and TM6; ankyrin repeats and a caveolin binding site on the N-terminus; and a TRP signature motif (EWKFAR) and calmodulin-Ins(1,4,5)P3 receptor binding domain (CIRB) on the C-terminus (7 and refs. therein). Functional TRPC channels are made of homo- or hetero-tetrameric arrangements of four TRPC subunits. The mechanisms underlying activation and regulation of TRPC channels are diverse, ranging from store-depletion dependent activation to direct activation by diacylglycerol or conformational coupling to the Ins(1,4,5)P3 receptor, the latter being reminiscent to the excitation-contraction coupling between L-type voltage-gated channels and ryanodine receptors in skeletal muscle (discussed in7,10-12). Nevertheless, a great deal of our current knowledge on the roles of TRPCs in cardiovascular physiology and disease derives from the identification of cellular and molecular events that rely upon TRPC function for proper operation, rather than from a clear understanding of their activating mechanisms. Because TRPCs are ubiquitously expressed throughout the cardiovascular system and hematopoietic cells, it is not surprising that most TRPC members have been implicated in different pathogenetic mechanisms that lead to cardiovascular disease (reviewed in 11; see also 9) such as those associated to essential hypertension, ventricular hypertrophy and endothelial dysfunction, among others. Despite this, the potential role of TRPCs—and for that matter, of TRPs in general—in atherosclerosis remains largely unexplored. Recent studies underscored a role of endothelial TRPC3 in inflammatory signaling and monocycle recruitment to coronary endothelium,3,4 suggesting that this channel—and perhaps other members of the TRPC group—might play a signaling role in atherogenesis (discussed by us in 13)More recently, TRPC3 was also identified as a critical component in macrophage survival signaling and efferocytosis (see section below). A number of recent reports also provide experimental evidence regarding expression of other members of the TRP superfamily in monocytes/macrophages, in most instances with functions that can be of potential significance to the molecular and cellular events associated to atherogenesis. The following section briefly revises basic aspects of the macrophage’s role in atherogenesis with emphasis on the critical impact of macrophage apoptosis and efferocytosis in lesion progression. This will be followed by a discussion of findings from the author’s laboratory and those from other groups—although the latter not directly aimed at studying atherosclerosis—regarding the role of TRPC channels and other members of the TRP superfamily, in macrophage function in the context of atherosclerosis.
Role of Macrophages in Atherosclerosis
Accumulation of apo-B containing lipoproteins in the subintima is a major triggering factor of the immune response that initiates and maintains the atherogenic process. The most salient and critical feature of this immunological response is the recruitment of monocytes to the subintima and their differentiation to macrophages.13 Whereas macrophages are indeed the predominant phagocytic cells within the intima, recent studies also indicate the presence of neutrophils and dendritic cells in lesion sites,14,15 but the overall contribution of these other phagocyte cells to lesion development is still poorly understood. Macrophage uptake of modified, mostly oxidized apo-B containing lipoproteins (i.e., oxLDL) causes further differentiation into cells with a typical foamy appearance—foam cells—due to intracellular accumulation of cholesterol. Such increase in cell’s cholesterol content results mostly from upregulated expression of scavenger receptors and augmented lipoprotein uptake, rather than reduced cholesterol efflux.16 Importantly, other mechanisms have been recognized that do not require scavenger receptor-mediated uptake of lipoproteins but also contribute to foam-cell formation, such as macropinocytosis of native LDL, macropinocytosis and phagocytosis of oxLDL and internalization of aggregated LDL (17 and references therein). Lipid-laden macrophages exhibit a reduced rate of exit from the lesion—either back to circulation or to the lymphatics—with a resultant increased lifespan in the subintima that only contributes to exacerbate the inflammatory response, induce smooth muscle cell proliferation and maintain the endothelium in an activated state with additional recruitment of circulating monocytes. The continued migration of monocytes to the lesion area increases lesion cellularity and complexity. Therefore, the number of macrophages present in the lesion at a particular time is an important determinant of lesion progression. The number of lesional macrophages is thus directly influenced by monocyte influx, their transition to macrophage phenotype, macrophage apoptosis and efferocytosis. Depending on the stage of the lesion, macrophage apoptosis has differential consequences in terms of lesion progression and stability, and this depends directly upon apoptosis rate and the efficiency of efferocytosis. This is discussed in more detail in the next two sections.
Macrophage apoptosis and efferocytosis in the early lesion
Available experimental evidence predicts that macrophage apoptosis in the early stages of atherosclerotic lesions may have both beneficial and harmful effects. In most instances macrophage apoptosis is barely detectable in small, stable lesions probably due to low frequency of apoptosis that is rapidly buffered by efficient phagocyte-mediated clearance of apoptotic cells. Indeed, if mechanisms of apoptotic cell uptake are intact, then macrophage apoptosis is beneficial in that it reduces lesion cellularity and progression. In lethally-irradiated LDL receptor deficient (LDLR−/−) mice transplanted with bone marrow from mice deficient for the pro-apoptotic protein BAX—a BCL-2 family member—reduced macrophage apoptosis leads to increased lesion area.18 Two conclusions can be derived from these observations. First, that apoptosis plays a protective role in the early stages of lesion development by keeping a balance between influx of active macrophages and clearance of apoptotic ones. Second, that prolonged survival of functional macrophages in the lesion environment is detrimental. This notion is supported by studies showing that increased macrophage susceptibility to apoptosis results in reduction of lesion progression from early to advanced stages. For example, macrophage deficiency of apoptosis inhibitory factor (AIM), which normally promotes macrophage survival upon oxLDL uptake, reduces early atherosclerosis lesions in LDLR−/− mice.19 Furthermore, absence of the prostaglandin receptor EP4 in macrophages shows a similar effect on lesion development by compromising macrophage survival, increasing apoptosis and decreasing lesion area.20 Although in these studies apoptotic macrophages were found in significant numbers in aortic lesions, the decrease in lesion area and absence of necrotic cells suggests that efferocytosis is efficient. Indeed, when apoptotic cells are not effectively cleared from the lesion site they undergo secondary cellular necrosis, also called post-apoptotic necrosis, which results in release of intracellular content, debris accumulation and formation of a necrotic core.21 Therefore, in early lesions the combination of increased macrophage apoptosis and effective clearance of apoptotic cells seems to play a protective role. In a therapeutic context though, inducing macrophage apoptosis in the early stages would deplete the population of resident phagocytes as well, with the subsequent reduction in clearance of apoptotic cells—even if few—and lead to detrimental post-apoptotic necrosis. Nevertheless, although macrophage apoptosis is generally beneficial, in the atherosclerotic lesion setting macrophages survive and persist in the lesions, favoring its progression. Deficient efferocytosis in early lesion has not been reported, but it cannot be ruled out, as most studies focusing on early lesional stages do not examine efferocytic properties of resident macrophages.
Macrophage apoptosis and efferocytosis in the advanced lesion
During early stages of atherogenesis (stages I-III of the Stary classification22) macrophages efficiently handle lipoprotein-derived cholesterol and they manage to export most of the engulfed cholesterol through the concerted action of cholesterol transporters and extracellular carriers such as apoA-I and HDL.17 In advanced lesions (stages IV-VI of the Stary classification23), macrophages noticeably accumulate unesterified free cholesterol, which is a major triggering factor of endoplasmic reticulum (ER) stress. Persistent ER stress promotes apoptosis through the PERK, IRE-1α and ATF6 pathways.24,25 In contrast to the atheroprotective role of apoptosis in early lesions, macrophage apoptosis in advanced lesions is associated with plaque destabilization, mostly due to impaired efferocytosis.2,15 This is illustrated by the marked reduction in lesion area and number of apoptotic cells in transgenic mice overexpressing the anti-apoptotic protein BCL-2 in macrophages after 15 weeks on a high cholesterol diet.26 Sustained induction of apoptosis in CD11c-positive cells in advanced lesions caused an increase in newly recruited monocytes and accumulation of apoptotic cells, both contributing to increased lesion area. An acute induction of apoptosis in mice injected with Diphtheria toxin resulted in appearance of a significant number of TUNEL-positive cells in the lesions compared with the spleen, suggesting an impairment of efferocytosis in the lesion, as apoptotic cells in the spleen were cleared in a highly efficient manner.26 The MAPK member p38αMAPK seems to play an important role in protection from necrosis, as macrophage p38αMAPK deficiency results in necrotic core enlargement due to augmented macrophage apoptosis, and also reduction in collagen content and plaque cap thinning, compromising the stability of the lesion.27 In LDLR−/− mice transplanted with bone marrow cells deficient in signal transducer and activator of transcription-1 (STAT1), a critical component of ER stress-induced macrophage apoptosis, a significant decrease in lesional apoptosis correlated with reduced necrotic core area.28 Taken together, these studies suggest that macrophage apoptosis in advanced atherosclerotic lesions is detrimental in that it leads to perpetuation of an overlying and surrounding- activated endothelium, persistent monocyte recruitment and incoming of newly differentiated macrophages, accumulation of apoptotic cells which undergo secondary necrosis, necrotic core enlargement and plaque destabilization.
Expression and Function of TRPC Channels in Monocytes/Macrophages
Whereas some information exists regarding expression of TRPCs in mononuclear hematopoietic cells, their potential roles in monocyte/macrophage biology remain largely unexplored. In human THP-1 monocytes, 7-ketocholesterol, one of the predominant oxysterols in oxLDL, induces apoptosis through a mechanism that requires an increase in Ca2+ influx and Ca2+-dependent activation of the serine/threonine phosphatase calcineurin.29 Calcineurin dephosphorylates the pro-apoptotic BCL-2 family member BAD leading to increased apoptosis through the mitochondrial pathway.29 Based on experiments showing that treatment of THP-1 monocytes with 7-ketocholesterol induced translocation of TRPC1 into lipid raft domains, it was suggested that TRPC1 might contribute to oxysterol-induced Ca2+ influx.29 This is an interesting observation as newly recruited monocytes at the lesion site can also engulf oxLDL while transitioning toward a defined macrophage phenotype.30 As of this writing however, no experimental evidence has been provided linking TRPC1 function to 7-ketocholesterol-dependent apoptosis.
Most of our knowledge on the expression and potential roles of TRPCs in monocytes derives from the efforts of Martin Tepel’s laboratory. This group showed that TRPC3, by mechanisms not yet understood, was upregulated in monocytes from spontaneously hypertensive rats compared with normotensive animals31,32 and that was paralleled by increased Ca2+ influx through both store-operated and non-store operated mechanisms. This group also reported a similar increase in store-operated and diacylglycerol-regulated Ca2+ influx in circulating monocytes from patients with essential hypertension, and this had a direct correlation with augmented expression of TRPC3 and 5, but not TRPC6 which is also expressed in these cells.33,34 Of importance, increased TRPC3 expression was also associated with increased in vitro migration of monocytes from hypertensive patients, when challenged with the chemotactic peptide formyl-Met-Leu-Phe (fMLP)35; however, whether TRPC3 function was required for the signaling underlying fMLP-induced cell migration was not examined. Interestingly, mRNA levels of TRPC5 were found to be elevated in hemodialysis patients undergoing erythropoietin treatment, a therapeutic approach which often leads to development of hypertension.36 Notably, in monocytes from patients with essential hypertension TRPC3 mRNA exhibited a strong correlation with transcripts for the pro-inflammatory cytokines interleukin-1β and tumor necrosis factor α (TNFα).37 More recently, in studies aimed at characterizing the response of human monocytes exposed to high glucose and oxidative stress, protein levels of TRPC3 and 6 were found to be increased, and TRPC6 mRNA was also augmented in circulating monocytes from patients with type 2 diabetes.38 Whereas none of these studies explored whether increased TRPC levels had a direct impact on signaling events associated to monocyte activation, their endothelial transmigration and/or their ultimate differentiation into macrophages, the evidence provided is suggestive of a potential novel link between the expression/function of TRPCs and the monocyte’s role in vascular disease associated to hypertension and metabolic disorders.
The first direct attempt to address a role for TRPC channels in macrophages comes from studies by Finney-Hayward et al.39 using alveolar macrophages from patients with chronic obstructive pulmonary disease (COPD). Both alveolar macrophages and macrophages derived from lung tissue exhibited increased mRNA levels for TRPC6 when compared with monocytes and monocyte-derived macrophages. In addition, TRPC6 transcripts were markedly augmented in alveolar macrophages from COPD patients compared with control subjects.39 Interestingly, the authors found that TRPC6-like currents were active even under basal conditions. This suggests that some tonic receptor stimulation must exist as TRPC6 channels are not characterized by high levels of constitutive function.10 The authors speculated on the possibility that TRPC6 might be influential in modulating macrophage function in the lung in the context of COPD, but this awaits experimental evaluation. Nevertheless, although macrophage subsets and functional properties differ among tissues and under different inflammatory settings—v.g., alveolar vs. subintimal inflammation—, these findings are provocative in regards of potential roles of macrophage TRPC6 during atherogenesis.
Role of TRPC3 in macrophage survival and efferocytosis
In macrophages, as in other cell types, the phosphatidylinositol-3-kinase (PI3K)/AKT axis and the NFκB route represent canonical survival pathways. AKT-dependent survival can take place via AKT-mediated phosphorylation of the pro-apoptotic protein BAD, a member of the BCL-2 family, thus releasing the anti-apoptotic proteins BCL-2 and BCL-X from BAD and preventing activation of the mitochondrial apoptotic pathway.40 In addition, AKT-mediated phosphorylation of glycogen synthase kinase β3 prevents proteasome-dependent degradation of β-catenin41 which can then translocate to the nucleus and, similarly to NFκB, promote transcription of survival and anti-apoptotic genes. Using THP-1 derived macrophages (TDMs) we recently described operation of an alternative survival pathway involving AKT-dependent transactivation of NFkB, as shown in cell types other than macrophages.42-44 In TDMs the atherorelevant cytokine TNFα promotes activation of AKT and NFκB45 through a mechanism that exhibits an obligatory requirement for constitutive Ca2+ influx. Indeed, blocking constitutive Ca2+ entry or selective inhibition of calmodulin (CAM) and calmodulin-dependent kinase II (CAMKII), markedly reduced the phosphorylation of IκBα, AKT and BAD and resulted in increased number of apoptotic TDMs,45 suggesting that CAM/CAMKII couples constitutive Ca2+ influx to survival pathways. Most importantly, these observations indicate that Ca2+ permeable channels endowed with constitutive, non-regulated activity can provide a biologically relevant source of Ca2+ influx for proper operation of survival mechanisms in macrophages. It has been proposed that apoptosis is a default mechanism meant to be executed unless tonic inhibition exists; it is thus tempting to speculate that constitutive Ca2+ influx exerts a role in supporting survival signaling whenever regulated Ca2+ entry is not available.
Among all TRPC channels, TRPC3 is endowed with high constitutive activity.7,10 Bone-marrow derived macrophages from TRPC3-deficient mice (TRPC3−/−) exhibit a marked reduction in constitutive Ca2+ influx, increased susceptibility to apoptosis and impaired TNFα-induced survival signaling when compared with TRPC3+/+ cells.5 These findings suggest an obligatory role for TRPC3 in macrophage survival, likely acting as the major source of constitutive Ca2+ influx that supports survival signaling. TNFα actions are not associated to changes in cytosolic Ca2+ and it is thus not clear how constitutive TRPC3 activity modulates TNFα-dependent survival signaling. The speculation was made that any impact of constitutive channel function on TNFα actions should occur in the immediate channel’s vicinity, promoting localized changes that modulate proximal signaling molecules. TRPCs are able to function in signaling complexes46 and thus it is possible that in macrophages TRPC3 operates in signaling microdomains where highly localized constitutive influx of Ca2+ modulates CAM/CAMKII proximal to the channel. Interestingly, also a pro-apoptotic role of CAMKII has been reported in macrophages undergoing ER stress, where CAMKII couples CHOP (C/EBP-homologous protein)-induced ER oxidase-1α to mitochondrial-dependent apoptosis.47 It should be noted that the model above predicts that constitutive channel function affects only the pool of CAMKII in close proximity to the channel but not necessarily that in other intracellular locations, providing a plausible explanation for a dual role of CAMKII in survival and/or apoptosis based on signaling compartmentalization. Additional studies are required to identify which pro-apoptotic and anti-apoptotic targets are affected downstream TRPC3 constitutive function as well as the impact of TRPC3 activity in ER-stress induced macrophage apoptosis.
As discussed above, efficient clearance of apoptotic macrophages by resident phagocytes is a major factor in determining atherosclerotic lesion cellularity. In an in vitro efferocytosis assay using TRPC3+/+ and TRPC3−/− macrophages as either phagocytes or apoptotic cells, TRPC3−/− macrophages exhibited impaired efferocytic activity compared with TRPC3+/+ phagocytes. Notably, apoptotic TRPC3−/− cells were not good substrates for phagocytosis regardless of the TRPC3 expression status of the acting phagocyte.5 It remains to be determined whether the impact of lack of TRPC3 expression on efferocytosis reflects a potential role of this channel in the signaling associated to phagocytic activity and/or on cell-cell recognition processes that are critical for efficient efferocytosis. Elucidating whether the above in vitro findings are of relevance to lesion development in the setting of atherosclerosis requires generation of macrophage-specific TRPC3 knockout mouse models of the disease.
Potential role of other TRP superfamily members in monocyte/macrophage function
As mentioned above, in recent years a number of reports have provided evidence on the expression of other members of the TRP superfamily in monocytes/macrophages and although none of those studies were focused on atherosclerosis, some of the findings offer an interesting scenario on potential significance of the role of those TRP proteins in atherogenesis. For instance, Nagasawa et al.48 found that among all members of the TRPV family, only TRPV2 is expressed in macrophages. Using the mouse macrophage cell line TtT/M87 and an EGFP-tagged TRPV2 construct these authors showed that TRPV2 localized in the ER but rapidly trafficked to the plasma membrane in response to serum or fMLP. Notably, fMLP-induced translocation of TRPV2 was abolished by inhibition of PI3K or Gi/o trimeric G proteins, indicating that channel trafficking was dependent upon receptor dependent generation of PtdIns(3,4,5)P3; fMLP-induced TRPV2-mediated Ca2+ influx was also required for fMLP-induced macrophage migration.48 In more recent work, the same laboratory reported that fMLP promoted localization of TRPV2 to the podosome with concomitant increase in subplasmalemmal Ca2+ concentration.49 These observations point toward potential relevance of TRPV2 function in the context of macrophage movement in response to chemotactic substances during, for example, efferocytic activity. Nevertheless, these studies and those above were performed using transient transfection of the TtT/M87 cell line with EGFP or c-Myc-tagged TRPV2 constructs, and thus it remains to be determined if a similar behavior applies to native TRPV2 channels and whether such inducible trafficking has an impact on macrophage’s function in inflammation. That TRPV2 does have a role in the inflammatory response is clearly shown by the findings of Link et al.,50 who showed that macrophages deficient in TRPV2 exhibit impaired in vitro phagocytic activity –manifested by poor phagocytosis in response to zymosan, immunoglobulin G or complement- and this was in line with the observation that TRPV2-deficient mice were notoriously sensitive to Listeria monocytogenes infection. This suggests that TRPV2 may play a critical role in early phagocytosis and points to this channel as an interesting candidate to be explored regarding a potential modulatory role of efferocytic properties of macrophages in atherogenesis. At the subcellular level, TRPV2 recruitment to the early phagosome caused plasma membrane depolarization, synthesis of phosphatidylinositol-4,5-bisphosphate and actin depolymerization, a mandatory step for occupancy-elicited clustering of the phagocytic receptor.50
Toll-like receptors (TLR) are important players in ER-stress dependent apoptosis of macrophages and in cytokine production by these cells, two processes that have been shown to be modulated by increased cytoplasmic Ca2+.51, 52 Recent studies by Yamashiro et al.53 show that TRPV2, but not members of the TRPC or TRPM families, mediates the Ca2+ entry that is required for lipopolysaccharide (LPS)-induced TNFα and interleukin-6 production in the murine macrophage-like cell line Raw264.7. Because in these cells LPS acts through TLR4, it is tempting to speculate on a role of TRPV2 in modulating TLR4-dependent signaling during ER stress-induced apoptosis in macrophages. Another member of the TRPV family, TRPV1, was shown to be activated in response to lysophosphatidylcholine (LPC) in THP-1 monocytes.54 This stimulation occurred independently of GPCR or PI-PLC activation. Notably, pharmacological maneuvers showed that TRPV1-dependent, LPC-induced monocyte migration required simultaneous activation of the canonical TRP member TRPC6.54 Because LPC is a major component of oxLDL which promotes the recruitment of monocytes to atherosclerotic lesions, these authors suggested that TRPV1/TRPC6 inhibition may represent a novel strategy to reduce monocyte infiltration into the subintima and thus attenuate atherosclerotic lesion progression.
In human U937 monocytes TRPM2, a member of the TRPM family, mediates the Ca2+ influx necessary for production of interleukin-8 (CXCL8).55 Monocytes derived from TRPM2-deficient mice exhibited reduced Ca2+ influx and production of macrophage inflammatory protein-2 (CXCL2; the mouse CXCL8 functional homolog) in response to hydrogen peroxide (H2O2).55 Notably, in the mouse model of dextran sodium sulfate-induced inflammatory colitis, CXCL2 expression, neutrophil infiltration and ulcerative lesions were reduced upon TRPM2 knockdown. These findings point to a pro-inflammatory role of TRPM2, which seems to act as a reactive oxygen species (ROS) sensor to trigger the signaling cascade driving chemokine synthesis and inflammation. Similarly to TRPV2-deficient mice, it was recently shown that TRPM2-deficient animals are highly sensitive to infection with Listeria monocytogenes and exhibit an impaired innate immune response,56 supporting a role for TRPM2 in the phagocytic activity of macrophages. TRPM2 can be activated by H2O2, ADP-ribose—a metabolite formed under oxidative stress conditions—and other signaling molecules. It was recently shown that TRPM2-mediated Ca2+ influx promotes chemokine production in monocytes and macrophages, and this exacerbates neutrophil infiltration in mice (57 and references therein). However, the pathophysiological significance of ROS-induced TRPM2 channel activity in vivo remains to be examined. In addition, in human primary and THP-1 monocytes LPS induces upregulation of TRPM2 expression which is paralleled by increased ADP-ribose-induced membrane currents, and TRPM2 expression seems mandatory for LPS-dependent production of interleukins-6, -8, -10, and TNFα58. Altogether, these findings point to TRPM2 as a promising candidate target for pharmacological strategies aimed at manipulating monocyte function.
The nociceptor agonist icilin is a potent activator of TRPM8 channels. In Raw264.7 macrophages icilin stimulates TRPM8-like non-selective cation currents that result in membrane depolarization of the macrophage59 Whereas these studies were strictly focused on characterizing the electrophysiological properties of icilin-induced currents in Raw264.7 cells with no further examination of functional implications, it is intriguing whether TRPM8-dependent membrane depolarization, similarly to what has been described for TRPV2 (see above and 50) can also induce actin depolymerization and thus modulate macrophage’s efferocytic properties. In addition, TRPM8-mediated membrane depolarization can reduce the driving force for Ca2+, modulating the function of other Ca2+ permeable channels in the macrophage and thus indirectly influencing Ca2+ dependent processes related to either survival or apoptosis in these cells.
Concluding Remarks
Macrophage function, and particularly, the balance between macrophage apoptosis and efferocytosis, is now recognized as a key determinant of lesion progression in atherosclerosis. Exploring and fully characterizing the signaling mechanisms underlying such fundamental functions of the macrophage will definitely expand the repertoire of candidate molecular targets that can be used to design new therapeutic strategies for these diseases. As discussed in the sections above, several members of the TRP superfamily of channel forming proteins, including members from the TRPC, TRPV and TRPM groups, have been identified as components of critical properties of the monocyte/macrophage which can be of potential relevance to the molecular and cellular events of atherogenesis (Fig. 1). TRPC3 emerges as a key player in the signaling associated to macrophage survival, and TRPC3, TRPV2 and TRPM2 seem important for proper phagocytic activity and thus, might be of relevance during efferocytosis of lesional apoptotic cells. Whereas the existing evidence is not sufficient at this time to assign definitive roles to individual TRP channels in monocyte/macrophage biology, it warrants further in vitro and in vivo studies to examine their true impact on the many functions of these cells during atherogenesis.
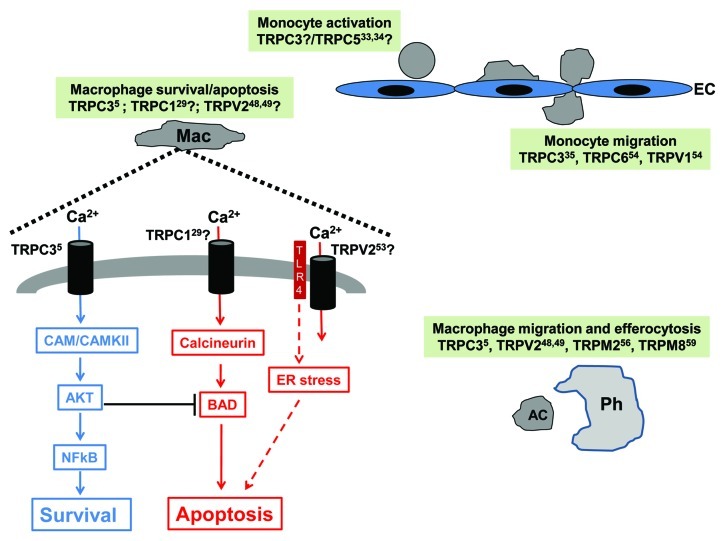
Figure 1. This figure summarizes the evidence discussed in the text regarding potential contribution of TRP channels to monocyte/macrophage function in the context of atherorelevant events. Members of the TRPC and TRPV groups emerge as potential players in processes related to monocyte activation and migration to the subintima, while members of the TRPC, TRPV and TRPM groups might participate in signaling associated to survival, apoptosis and/or efferocytosis (see text for details). Question marks indicate a potential, but not yet examined role for that particular TRP based on published evidence (corresponding reference indicated as a superscript number next to the channel’s name) on its role on related monocyte/macrophage processes. Mac: macrophage; CAM/CAMKII: calmodulin/calmodulin-dependent kinase II; TLR4: Toll-like receptor 4; AC: apoptotic cell; Ph: phagocyte; EC: endothelial cell.
Acknowledgments
Work at Dr. G. Vazquez’s lab is supported by University of Toledo College of Medicine and National Heart, Lung and Blood Institute (1R01HL111877–01 to GV).
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/20292
References
- 1.Association AH. Heart Disease and Stroke Statistics. Update, Dallas TX, AHA 2008. [Google Scholar]
- 2.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smedlund K, Tano J-Y, Vazquez G. The constitutive function of native TRPC3 channels modulates vascular cell adhesion molecule-1 expression in coronary endothelial cells through nuclear factor kappaB signaling. Circ Res. 2010;106:1479–88. doi: 10.1161/CIRCRESAHA.109.213314. [DOI] [PubMed] [Google Scholar]
- 4.Smedlund K, Vazquez G. Involvement of native TRPC3 proteins in ATP-dependent expression of VCAM-1 and monocyte adherence in coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:2049–55. doi: 10.1161/ATVBAHA.108.175356. [DOI] [PubMed] [Google Scholar]
- 5.Tano J-Y, Smedlund K, Lee R, Abramowitz J, Birnbaumer L, Vazquez G. Impairment of survival signaling and efferocytosis in TRPC3-deficient macrophages. Biochem Biophys Res Commun. 2011;410:643–7. doi: 10.1016/j.bbrc.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 6.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–8. doi: 10.1016/S0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 7.Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney JW., Jr. The mammalian TRPC cation channels. Biochim Biophys Acta. 2004;1742:21–36. doi: 10.1016/j.bbamcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trebak M, Vazquez G, Bird GS, Putney JW., Jr. The TRPC3/6/7 subfamily of cation channels. Cell Calcium. 2003;33:451–61. doi: 10.1016/S0143-4160(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 11.Tano JY, Smedlund K, Vazquez G. Endothelial TRPC3/6/7 proteins at the edge of cardiovascular disease. Cardiovasc Hematol Agents Med Chem. 2010;8:76–86. doi: 10.2174/187152510790796138. [DOI] [PubMed] [Google Scholar]
- 12.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, et al. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta. 2006;1763:1147–60. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 13.Vazquez G. TRPC channels as prospective targets in atherosclerosis: terra incognita. Front Biosci (Schol Ed) 2012;4:157–66. doi: 10.2741/258. [DOI] [PubMed] [Google Scholar]
- 14.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–88. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 15.Thorp E, Subramanian M, Tabas I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. Eur J Immunol. 2011;41:2515–8. doi: 10.1002/eji.201141719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh J, Riek AE, Weng S, Petty M, Kim D, Colonna M, et al. Endoplasmic reticulum stress controls m2 macrophage differentiation and foam cell formation. J Biol Chem. 2012;287:11629–41. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaren JE, Michael DR, Ashlin TG, Ramji DP. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog Lipid Res. 2011;50:331–47. doi: 10.1016/j.plipres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–9. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, et al. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–13. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, et al. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab. 2008;8:492–501. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe-/- mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–8. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr., Rosenfeld ME, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1994;14:840–56. doi: 10.1161/01.ATV.14.5.840. [DOI] [PubMed] [Google Scholar]
- 23.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr., et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–31. doi: 10.1161/01.ATV.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 24.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–50. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, et al. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 27.Seimon TA, Wang Y, Han S, Senokuchi T, Schrijvers DM, Kuriakose G, et al. Macrophage deficiency of p38alpha MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J Clin Invest. 2009;119:886–98. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim W-S, Timmins JM, Seimon TA, Sadler A, Kolodgie FD, Virmani R, et al. Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–51. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berthier A, Lemaire-Ewing S, Prunet C, Monier S, Athias A, Bessède G, et al. Involvement of a calcium-dependent dephosphorylation of BAD associated with the localization of Trpc-1 within lipid rafts in 7-ketocholesterol-induced THP-1 cell apoptosis. Cell Death Differ. 2004;11:897–905. doi: 10.1038/sj.cdd.4401434. [DOI] [PubMed] [Google Scholar]
- 30.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–16. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu D, Scholze A, Zhu Z, Kreutz R, Wehland-von-Trebra M, Zidek W, et al. Increased transient receptor potential channel TRPC3 expression in spontaneously hypertensive rats. Am J Hypertens. 2005;18:1503–7. doi: 10.1016/j.amjhyper.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 32.Liu DY, Scholze A, Kreutz R, Wehland-von-Trebra M, Zidek W, Zhu ZM, et al. Monocytes from spontaneously hypertensive rats show increased store-operated and second messenger-operated calcium influx mediated by transient receptor potential canonical Type 3 channels. Am J Hypertens. 2007;20:1111–8. doi: 10.1016/j.amjhyper.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Liu D, Scholze A, Zhu Z, Krueger K, Thilo F, Burkert A, et al. Transient receptor potential channels in essential hypertension. J Hypertens. 2006;24:1105–14. doi: 10.1097/01.hjh.0000226201.73065.14. [DOI] [PubMed] [Google Scholar]
- 34.Liu DY, Thilo F, Scholze A, Wittstock A, Zhao ZG, Harteneck C, et al. Increased store-operated and 1-oleoyl-2-acetyl-sn-glycerol-induced calcium influx in monocytes is mediated by transient receptor potential canonical channels in human essential hypertension. J Hypertens. 2007;25:799–808. doi: 10.1097/HJH.0b013e32803cae2b. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Z, Ni Y, Chen J, Zhong J, Yu H, Xu X, et al. Increased migration of monocytes in essential hypertension is associated with increased transient receptor potential channel canonical type 3 channels. PLoS One. 2012;7:e32628. doi: 10.1371/journal.pone.0032628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Xu Y, Thilo F, Friis UG, Jensen BL, Scholze A, et al. Erythropoietin increases expression and function of transient receptor potential canonical 5 channels. Hypertension. 2011;58:317–24. doi: 10.1161/HYPERTENSIONAHA.111.173690. [DOI] [PubMed] [Google Scholar]
- 37.Thilo F, Scholze A, Liu DY, Zidek W, Tepel M. Association of transient receptor potential canonical type 3 (TRPC3) channel transcripts with proinflammatory cytokines. Arch Biochem Biophys. 2008;471:57–62. doi: 10.1016/j.abb.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Wuensch T, Thilo F, Krueger K, Scholze A, Ristow M, Tepel M. High glucose-induced oxidative stress increases transient receptor potential channel expression in human monocytes. Diabetes. 2010;59:844–9. doi: 10.2337/db09-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finney-Hayward TK, Popa MO, Bahra P, Li S, Poll CT, Gosling M, et al. Expression of transient receptor potential C6 channels in human lung macrophages. Am J Respir Cell Mol Biol. 2010;43:296–304. doi: 10.1165/rcmb.2008-0373OC. [DOI] [PubMed] [Google Scholar]
- 40.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 41.Deguchi JO, Yamazaki H, Aikawa E, Aikawa M. Chronic hypoxia activates the Akt and β-catenin pathways in human macrophages. Arterioscler Thromb Vasc Biol. 2009;29:1664–70. doi: 10.1161/ATVBAHA.109.194043. [DOI] [PubMed] [Google Scholar]
- 42.Bai D, Ueno L, Vogt PK. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer. 2009;125:2863–70. doi: 10.1002/ijc.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madrid LV, Wang C-Y, Guttridge DC, Schottelius AJG, Baldwin AS, Jr., Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–38. doi: 10.1128/MCB.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 45.Tano J-Y, Vazquez G. Requirement for non-regulated, constitutive calcium influx in macrophage survival signaling. Biochem Biophys Res Commun. 2011;407:432–7. doi: 10.1016/j.bbrc.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 46.Ambudkar IS, Ong HL. Organization and function of TRPC channelosomes. Pflugers Arch. 2007;455:187–200. doi: 10.1007/s00424-007-0252-0. [DOI] [PubMed] [Google Scholar]
- 47.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–41. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagasawa M, Nakagawa Y, Tanaka S, Kojima I. Chemotactic peptide fMetLeuPhe induces translocation of the TRPV2 channel in macrophages. J Cell Physiol. 2007;210:692–702. doi: 10.1002/jcp.20883. [DOI] [PubMed] [Google Scholar]
- 49.Nagasawa M, Kojima I. Translocation of calcium-permeable TRPV2 channel to the podosome: Its role in the regulation of podosome assembly. Cell Calcium. 2012;51:186–93. doi: 10.1016/j.ceca.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11:232–9. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buczynski MW, Stephens DL, Bowers-Gentry RC, Grkovich A, Deems RA, Dennis EA. TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. J Biol Chem. 2007;282:22834–47. doi: 10.1074/jbc.M701831200. [DOI] [PubMed] [Google Scholar]
- 52.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A. 2006;103:19794–9. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamashiro K, Sasano T, Tojo K, Namekata I, Kurokawa J, Sawada N, et al. Role of transient receptor potential vanilloid 2 in LPS-induced cytokine production in macrophages. Biochem Biophys Res Commun. 2010;398:284–9. doi: 10.1016/j.bbrc.2010.06.082. [DOI] [PubMed] [Google Scholar]
- 54.Schilling T, Eder C. Non-selective cation channel activity is required for lysophosphatidylcholine-induced monocyte migration. J Cell Physiol. 2009;221:325–34. doi: 10.1002/jcp.21857. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med. 2008;14:738–47. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knowles H, Heizer JW, Li Y, Chapman K, Ogden CA, Andreasen K, et al. Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proceedings of the National Academy of Sciences; 108:11578-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi N, Kozai D, Kobayashi R, Ebert M, Mori Y. Roles of TRPM2 in oxidative stress. Cell Calcium. 2011;50:279–87. doi: 10.1016/j.ceca.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Wehrhahn J, Kraft R, Harteneck C, Hauschildt S. Transient receptor potential melastatin 2 is required for lipopolysaccharide-induced cytokine production in human monocytes. J Immunol. 2010;184:2386–93. doi: 10.4049/jimmunol.0902474. [DOI] [PubMed] [Google Scholar]
- 59.Wu S-N, Wu P-Y, Tsai M-L. Characterization of TRPM8-like channels activated by the cooling agent icilin in the macrophage cell line RAW 264.7. J Membr Biol. 2011;241:11–20. doi: 10.1007/s00232-011-9358-6. [DOI] [PubMed] [Google Scholar]