Abstract
In cortical collecting ducts (CCDs) perfused in vitro, inhibiting the epithelial Na+ channel (ENaC) reduces Cl− absorption. Since ENaC does not transport Cl−, the purpose of this study was to determine how ENaC modulates Cl− absorption. Thus, Cl− absorption was measured in CCDs perfused in vitro that were taken from mice given aldosterone for 7 days. In wild-type mice, we observed no effect of luminal hydrochlorothiazide on either Cl− absorption or transepithelial voltage (VT). However, application of an ENaC inhibitor [benzamil (3 μM)] to the luminal fluid or application of a Na+-K+-ATPase inhibitor to the bath reduced Cl− absorption by ∼66–75% and nearly obliterated lumen-negative VT. In contrast, ENaC inhibition had no effect in CCDs from collecting duct-specific ENaC-null mice (Hoxb7:CRE, Scnn1aloxlox). Whereas benzamil-sensitive Cl− absorption did not depend on CFTR, application of a Na+-K+-2Cl− cotransport inhibitor (bumetanide) to the bath or ablation of the gene encoding Na+-K+-2Cl− cotransporter 1 (NKCC1) blunted benzamil-sensitive Cl− absorption, although the benzamil-sensitive component of VT was unaffected. In conclusion, first, in CCDs from aldosterone-treated mice, most Cl− absorption is benzamil sensitive, whereas thiazide-sensitive Cl− absorption is undetectable. Second, benzamil-sensitive Cl− absorption occurs by inhibition of ENaC, possibly due to elimination of lumen-negative VT. Finally, benzamil-sensitive Cl− flux occurs, at least in part, through transcellular transport through a pathway that depends on NKCC1.
Keywords: epithelial sodium channel, plendrin, chloride, amiloride
amiloride and thiazide analogs have been used as antihypertensives for many years. Thiazide analogs inhibit an electroneutral NaCl cotransporter, encoded by Slc12a3, which localizes to the distal convoluted tubule (18). However, Terada and Knepper (39) observed that thiazides inhibit transporters other than the NaCl cotransporter, since they reduce NaCl absorption in the cortical collecting duct (CCD), although the NaCl cotransporter is not expressed in that segment (18). More recently, Leviel et al. (26) demonstrated that thiazides inhibit Na+ and Cl− absorption in the mouse CCD by inhibiting an electroneutral Na+-dependent, Cl−/HCO3− exchanger encoded by Slc4a8.
The relative contribution of the thiazide-sensitive and amiloride-sensitive components of Cl− absorption have varied between studies. In CCDs from NaCl-restricted mice, virtually all Cl− absorption is thiazide sensitive, whereas amiloride-sensitive Cl− absorption is undetectable (26). However, in DOCA-treated rats, ∼50% of Cl− absorption is thiazide sensitive, whereas the other ∼50% is amiloride sensitive (39). Differences in the relative contribution of the thiazide- and amiloride-sensitive components of Cl− absorption may reflect species- or strain-specific differences. Alternatively, the expression and function of diuretic-sensitive transporters may differ after dietary NaCl restriction and aldosterone analog administration. If so, the relative action of these two diuretics might differ significantly in these two treatment models.
Amiloride analogs induce natriuresis by inhibiting the epithelial Na+ channel (ENaC), which localizes to the apical regions of principal cells (17). Since ENaC does not transport Cl−, the mechanism of the amiloride-sensitive component of Cl− absorption is unresolved. ENaC-mediated Na+ absorption generates a lumen-negative transepithelial voltage (VT), which should enhance the driving force for Cl− uptake through paracellular transport. However, other mechanisms are possible. Since the CCD is generally thought to be a “tight epithelium,” amiloride may alter transcellular rather than paracellular Cl− absorption. Moreover, since apical plasma membrane Cl− channels have been reported in rabbit intercalated cells (8, 27), eliminating the lumen-negative VT generated by ENaC should enhance the driving force for Cl− secretion mediated by such an apical anion channel. Finally, amiloride analogs may target Na+ transporters other than ENaC. For example, Na+-HCO3− cotransporter 3 (NBC3) is amiloride sensitive (34, 47) and localizes to the apical regions of intercalated cells in the rat (25) and mouse (21) CCD. Therefore, amiloride might directly inhibit NBC3 in intercalated cells, which reduces NaHCO3 uptake across the apical plasma membrane, thereby attenuating apical Cl−/HCO3− exchange due to reduced intracellular HCO3− concentration. To resolve these issues, the goals of this study were to 1) determine the relative contribution of the amiloride- and thiazide-sensitive components of Cl− absorption in the CCD of aldosterone-treated mice, 2) determine if amiloride-sensitive Cl− absorption in the mouse CCD occurs through an ENaC-dependent mechanism, and 3) characterize the amiloride-sensitive component of Cl− absorption.
METHODS
Animals and Treatments
Unless otherwise indicated, experiments were performed on male and female wild-type mice on a 129S6/SvEv Tac background (Taconic Farms). We studied Na+-K+-2Cl− cotransporter 1 (NKCC1)-null mice on a FVB/N background, which were produced by breeding NKCC1 heterozygotes as previously described (7). Collecting duct-specific α-subunit ENaC-null mice (Hoxb7:CRE, Scnn1aloxlox) were compared with CRE-negative littermates (37), which were on a 129B6 background. The following three treatments were performed.
Treatment 1.
Mice received aldosterone by minipump (250 μg·kg body wt−1·day−1) and ate a balanced diet (no. 53881300, Zeigler Brothers) prepared as a gel (0.6% agar, 74.6% water, and 24.8% mouse chow) supplemented with NaCl (∼0.8 meq NaCl/day) for 5–7 days before euthanization (19).
Treatment 2.
Mice received a gelled diet (0.8 meq/day NaCl) and received vehicle by minipump.
Treatment 3.
Mice ate the same gelled diet but supplemented with NaCl to receive 1.25 meq/day NaCl. In these experiments, mice did not receive minipumps or aldosterone.
The Institutional Animal Care and Use Committee of Emory University approved all treatment protocols.
In Vitro Perfusion of Isolated CCDs
CCDs were dissected from medullary rays and perfused at flow rates of 2–3 nl/min in the presence of a symmetric HCO3−-buffered physiological solution, which contained (in mM) 125 NaCl, 2.5 K2HPO4, 24 NaHCO3, 2 CaCl2, 1.2 MgSO4, and 5.5 glucose bubbled with 95% air-5% CO2 (36). Unless otherwise stated, ANG II (10 nM) was present in the bath solution in all experiments (31, 32). Tubules were equilibrated at 37°C for 30 min before the collections were started. A stock solution of benzamil hydrochloride (3 × 10−3 M) was prepared in water. A hydrochlorothiazide stock solution (10−1 M) and a CFTRinh 172 solution (1 × 10−2 M) solution were prepared in DMSO. A stock solution of bafilomycin (10−5 M) was prepared in absolute ethanol. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except CFTRinh 172, which was purchased from Calbiochem (La Jolla, CA).
Measurement of Net Transepithelial Cl− Flux
Cl− concentrations were measured in the perfusate and collected samples using a continuous-flow fluorimeter and the Cl−-sensitive fluorophore 6-methoxy-N-(3-sulfopropyl)quinolinium (Molecular Probes, Eugene, OR), as previously described (9, 42, 43). Transepithelial Cl− flux (JCl) was calculated according to the following equation: JCl = (CO − CL)Q/L, where CO and CL are perfusate and collected fluid Cl− concentrations, respectively, Q is the flow rate (in nl/min), and L is the tubule length. Net fluid transport was taken to be zero since net fluid flux has not been observed in CCDs when perfused in vitro in the presence of symmetric solutions and in the absence of vasopressin (22, 23). JCl was expressed as picomoles per millmeter per minuter.
Transepithelial Voltage
Transepithelial voltage (VT) was measured in the perfusion pipette connected to a high-impedance electrometer through an agar bridge saturated with 0.16 M NaCl and a calomel cell, as previously described (41). The reference was an agar bridge from the bath to a calomel cell.
Immunohistochemistry
Kidneys were preserved by in vivo perfusion fixation and embedded in paraffin as previously described (20). We used an NKCC1 antibody (α-wNT) raised in rabbits against a 6xHis fusion protein corresponding to amino acids 3–202 of the rat NKCC1 sequence (5), which was a generous gift from Dr. R. James Turner. NKCC1 immunoreactivity was detected using immunoperoxidase procedures; blocking was done with 3% H2O2 in methanol for 30 min followed by protein blocking using 1% BSA, 0.2% gelatin, and 0.05% saponin solution. The anti-NKCC1 antibody was diluted 1:3,000 in PBS, and the secondary antibody was peroxidase-conjugated goat anti-rabbit IgG (DAKO). For double-labeling experiments, NKCC1 immunostaining was performed as described above and then labeled for both aquaporin 2 (AQP2; 1:1,000) (3) and pendrin (1:5,000) (36). In other experiments, NKCC1 labeling was followed by H+-ATPase labeling (1:500) (21). Sections that labeled NKCC1, as visualized by diaminobenzidine staining, were washed with distilled water and incubated in 0.5% H2O2 in absolute methanol for 30 min at room temperature to quench the remaining peroxidase activity. Sections were washed with distilled water and incubated in PBS supplemented with 1% BSA, 0.05% saponin, and 0.2% gelatin followed by an overnight incubation at 4°C with primary antibodies against H+-ATPase or against both AQP2 and pendrin. Sections were rinsed with PBS supplemented with 0.1% BSA, 0.05% saponin, and 0.2% gelatin, and labeling was visualized with horseradish peroxide-conjugated goat anti-rabbit secondary antibody (1:200, DAKO). For the detection of immunoreactivity, Vector SG (Vector laboratories) was used as the chromogen to produce a blue-gray label, which can be distinguished from the brown staining produced by diaminobenzidine, which labeled NKCC1.
Statistics
All data are presented as means ± SE. Each n value used in the statistical analysis represents data from separate mice. To test for statistical significance between two groups, a paired or an unpaired Student's t-test was used. The criterion for statistical significance was P < 0.05.
RESULTS
Benzamil Abolishes Cl− Absorption in the CCD of Aldosterone-Treated Mice
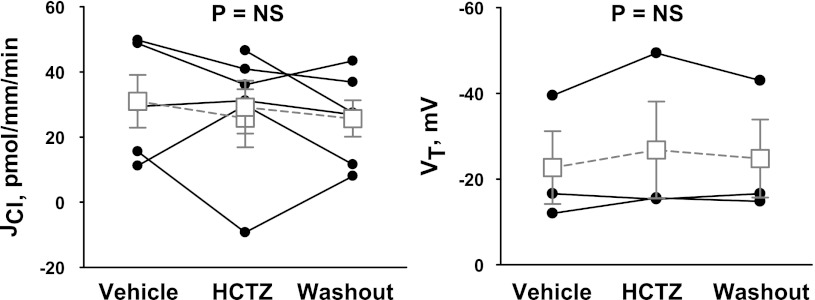
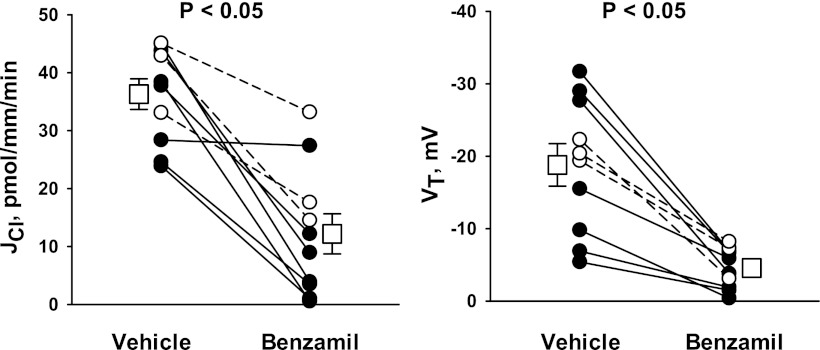
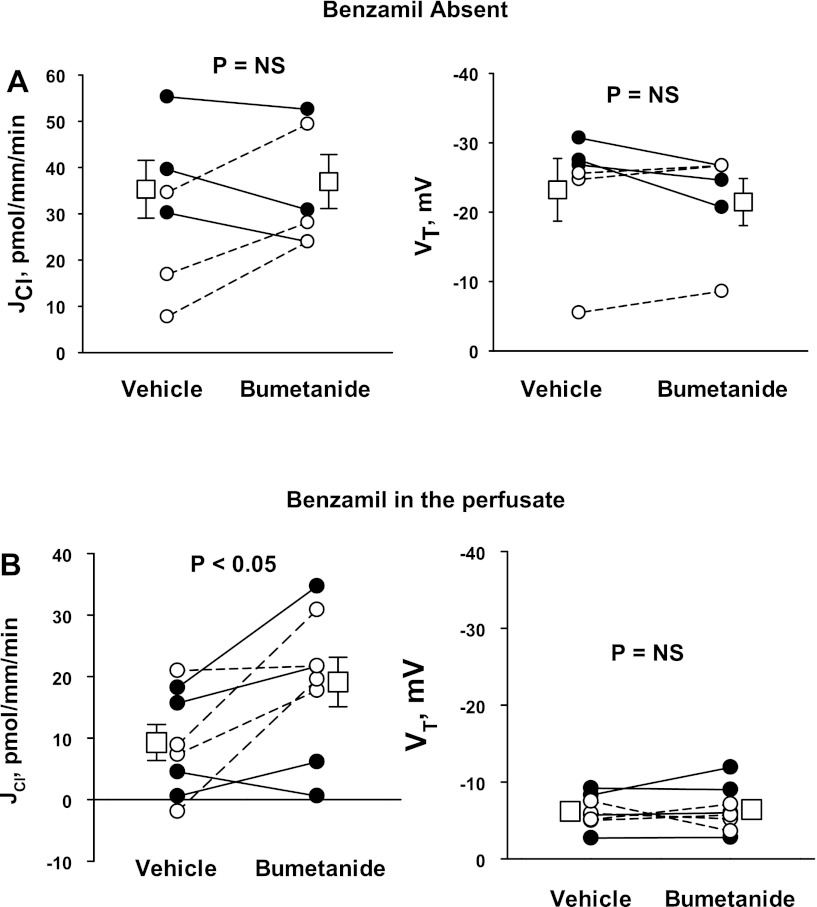
The magnitude of the thiazide- and amiloride-sensitive components of JCl and VT were measured in the CCD of aldosterone-treated mice (treatment 1). ANG II was applied to the perfusate to upregulate ENaC (33), pendrin (31), and H+-ATPase (32) in the CCD. To examine the contribution of the Na+-dependent Cl−/HCO3− exchanger Slc4a8, Cl− absorption was measured before and after the application of hydrochlorothiazide to the luminal fluid (26). As shown in Fig. 1, 100 μM hydrochlorothiazide had no measurable effect on either VT or JCl. Further experiments explored the effect of ENaC inhibition on Cl− absorption. Since benzamil is thought to be a more selective inhibitor of ENaC than amiloride (1), we studied the effect of benzamil (3 μM) on JCl and VT (Fig. 2). In contrast to thiazides, benzamil application reduced Cl− absorption by ∼66% and reduced lumen-negative VT by ∼75%. We conclude that in the CCD of aldosterone-treated mice, Cl− absorption occurs predominantly through a benzamil-sensitive, thiazide-insensitive pathway.
Fig. 1.
Hydrochlorothiazide (HCTZ) does not reduce Cl− absorption in the cortical collecting duct (CCD) of aldosterone (Aldo)-treated mice. Transepithelial Cl− flux (JCl; left) and transepithelial voltage (VT; right) were measured simultaneously in CCDs from Aldo-treated mice (treatment 1) in either the presence or absence of 100 μM HCTZ in the luminal fluid. ANG II (10−8 M) was present in the bath solution. Each solid line displays data from a single experiment. Open squares show means ± SE of all experiments. NS, not significant.
Fig. 2.
Benzamil reduces Cl− absorption in the CCD of Aldo-treated mice. JCl (left) and VT (right) were measured simultaneously in CCDs from Aldo-treated mice (treatment 1) in either the presence or absence of 3 μM benzamil in the luminal fluid. ANG II (10−8 M) was present in the bath. Solid lines indicate results from experiments where the vehicle was applied in the first period and benzamil was applied in the second period. Dashed lines indicate experiments where the order was reversed (i.e., benzamil was applied in the initial period and then removed in the second period). Open squares show means ± SE under each condition.
Benzamil Reduces Cl− Absorption by Inhibiting ENaC, Most Likely by Eliminating Lumen-Negative VT
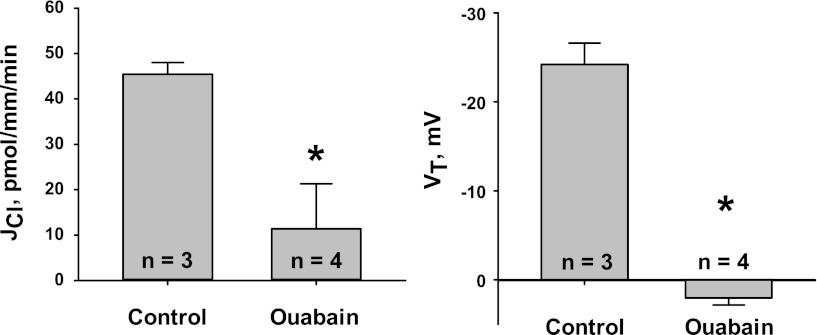
We hypothesized that if benzamil reduces Cl− absorption by inhibiting ENaC, then inhibiting ENaC by raising the intracellular Na+ concentration through Na+ pump blockade (38) should mimic the effect of benzamil on JCl and VT. Thus, we examined the effect of the Na+-K+-ATPase inhibitor ouabain on JCl and VT (Fig. 3). Ouabain reduced JCl by >75% and obliterated lumen-negative VT. Therefore, benzamil and ouabain may inhibit a common pathway for Cl− absorption, which likely involves elimination of lumen-negative VT generated by ENaC.
Fig. 3.
Na+-K+-ATPase inhibition reduces JCl and VT. CCDs from Aldo-treated mice (treatment 1) were perfused with ANG II (10−8 M) in the bath. JCl (left) and VT (right) were measured in the presence of vehicle. In separate tubules, JCl and VT were measured when the Na+-K+-ATPase inhibitor ouabain (2 mM) was added to the bath. *P < 0.05.
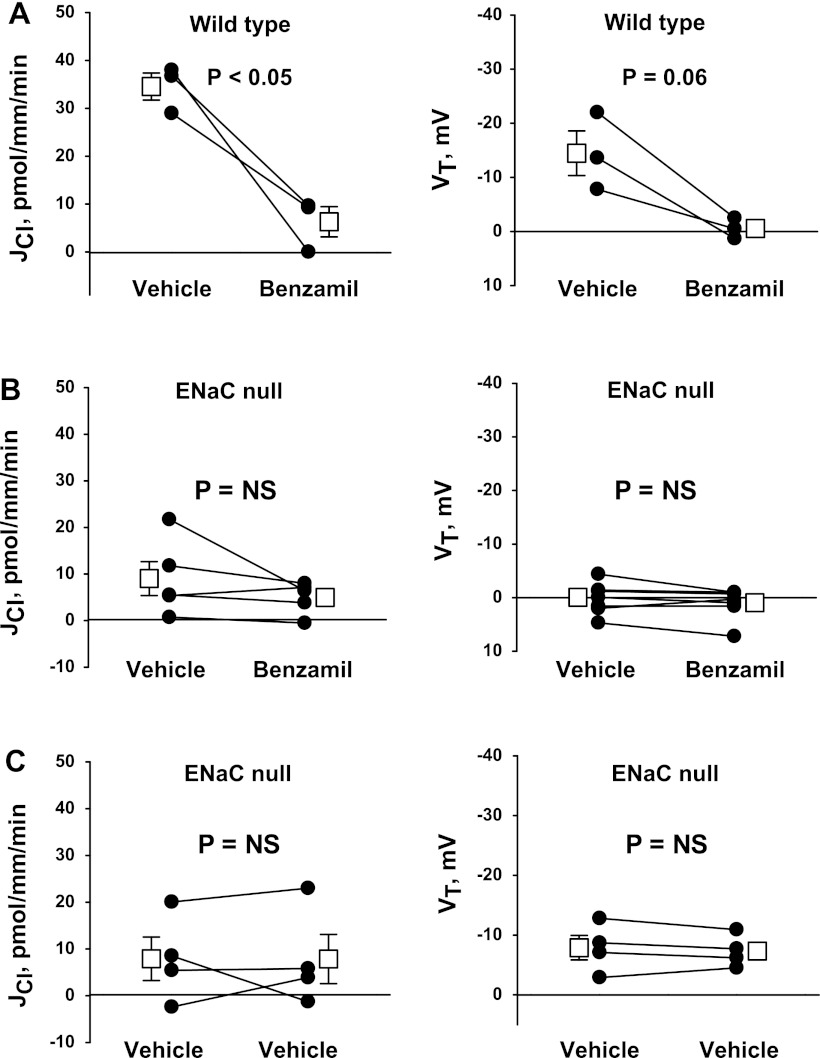
Benzamil may reduce Cl− absorption by directly inhibiting a transporter other than ENaC, such as NBC3, or another transport process coupled with Na+-K+-ATPase (34, 47). We hypothesized that if benzamil reduces JCl through an ENaC-dependent pathway, then the benzamil-sensitive component of Cl− absorption should be abolished in ENaC-null mice. To test this hypothesis, we examined the effect of benzamil on JCl and VT in collecting duct-specific ENaC-null mice (Hoxb7:CRE, Scnn1aloxlox) and in wild-type, CRE-negative littermates (Fig. 4). Application of benzamil to the lumen reduced JCl and VT in wild-type, CRE-negative mice but had no detectable effect on JCl and VT in collecting duct-specific ENaC-null mice. Moreover, in collecting duct-specific ENaC-null mice, the effect of benzamil (Fig. 4B) was no different from that of vehicle (Fig. 4C). We conclude that benzamil reduces JCl through a mechanism that is dependent on ENaC.
Fig. 4.
Ablation of the gene encoding the α-subunit of the epithelial Na+ channel (ENaC) eliminates benzamil-sensitive JCl and VT in the CCD. The effect of benzamil (3 μM) on JCl and VT was examined in CCDs taken from Aldo-treated collecting duct-specific ENaC-null mice (B; Hoxb7:CRE, Scnn1aloxlox) and wild-type, CRE-negative littermates (A) (treatment 1). C: time control experiments performed in collecting duct-specific ENaC-null mice. In all experiments, vehicle was applied in the first period and benzamil (or vehicle) was applied in the second period. ANG II was present in the bath in all experiments. The solid lines display data from a single experiment. Open squares show means ± SE of all experiments.
Benzamil Increases Cl− Secretion in the Mouse CCD
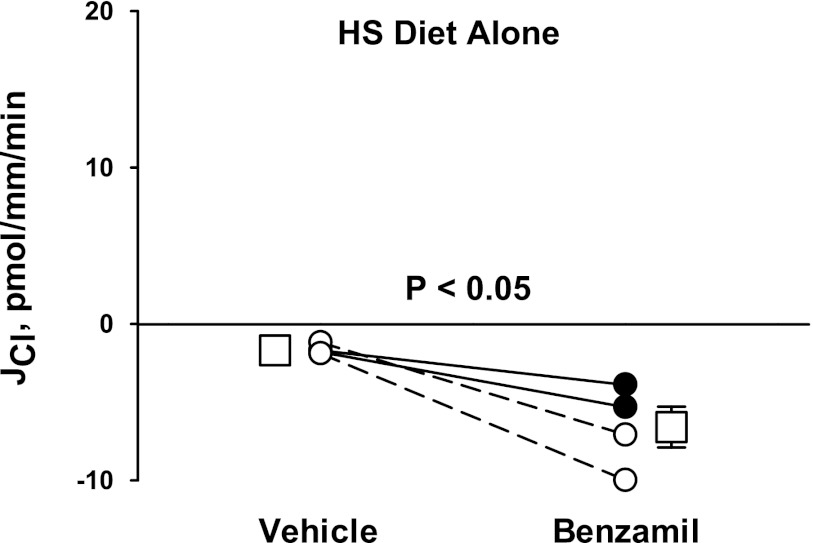
Under physiological conditions, the lumen-negative VT generated by ENaC drives electrogenic Cl− absorption (2). However, in cultured mouse CCD monolayers, conductive Cl− secretion is observed when VT is clamped to zero (“short circuit” conditions) (2, 30). Since the application of amiloride analogs reduces VT to nearly zero in CCDs perfused in vitro, amiloride effectively creates a short circuit condition in the perfused CCD. Therefore, we hypothesized that these diuretics increase the driving force for a conductive, Cl− secretory pathway or reduce the driving force for conductive Cl− absorption. To test this hypothesis, Cl− flux was measured in CCDs taken from mice that received a high-NaCl diet alone, without aldosterone administration in vivo or ANG II administration in vitro. Under these conditions, endogenous aldosterone and ANG II production should be low and transporters, such as pendrin, that mediate net Cl− absorption are downregulated (31, 40, 43). As shown in Fig. 5, in the absence of a diuretic, net Cl− flux was very low. However, with the application of benzamil to the perfusate, net Cl− secretion was observed. We conclude that benzamil application unmasks net Cl− secretion under some treatment conditions.
Fig. 5.
Benzamil increases Cl− secretion. CCDs taken from mice that received a high-NaCl [high-salt (HS)] diet (treatment 3) were perfused in vitro in the absence of ANG II. Under basal conditions (vehicle), JCl was very low. With the application of benzamil to the perfusate, Cl− secretion was observed. Solid lines display results from experiments in which the vehicle was applied in the first period and benzamil was applied in the second period. Dashed lines indicate experiments where the order was reversed (i.e., benzamil was applied in the initial period and then removed in the second period). Open squares show means ± SE under each condition.
NKCC1 Localizes to the Basolateral Regions of Type A Intercalated Cells in the Mouse CCD
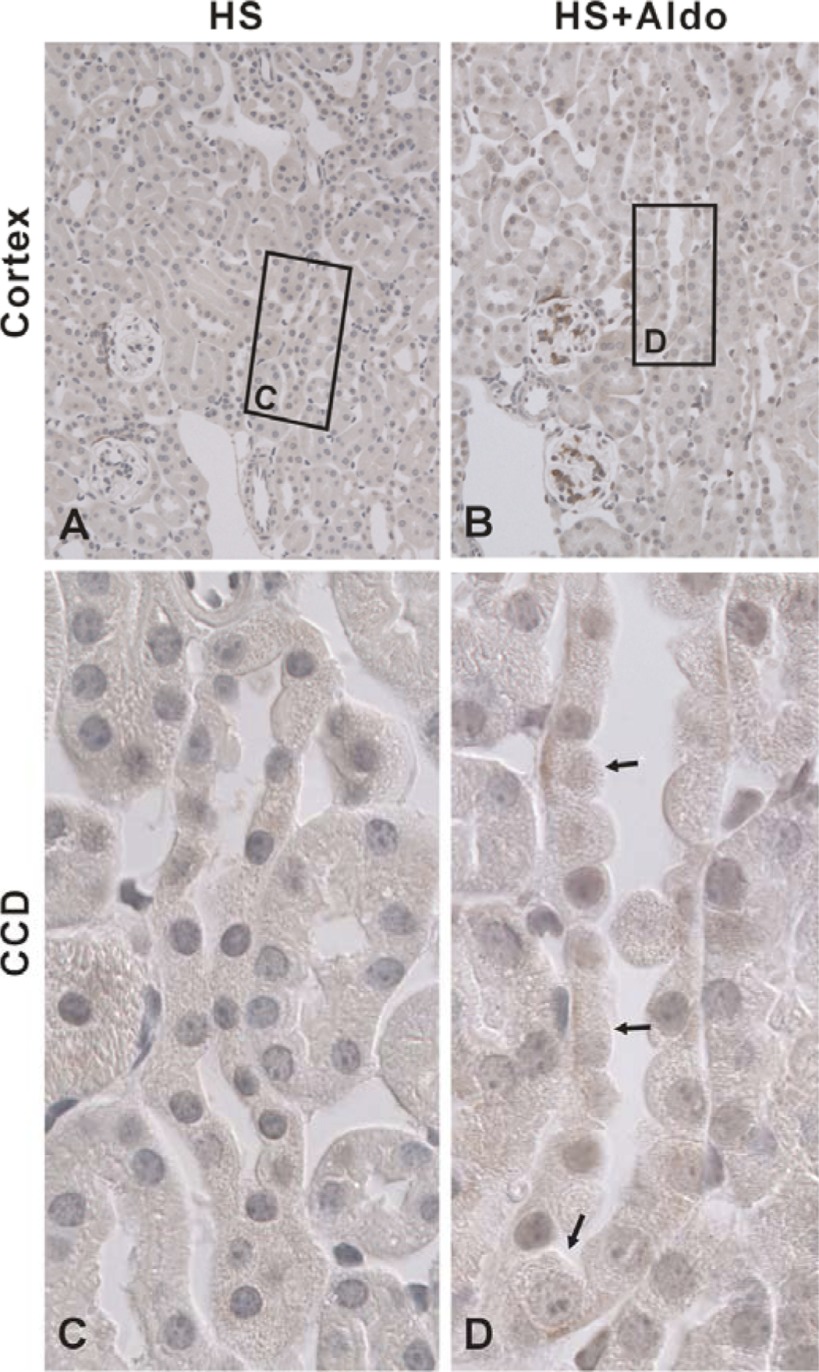
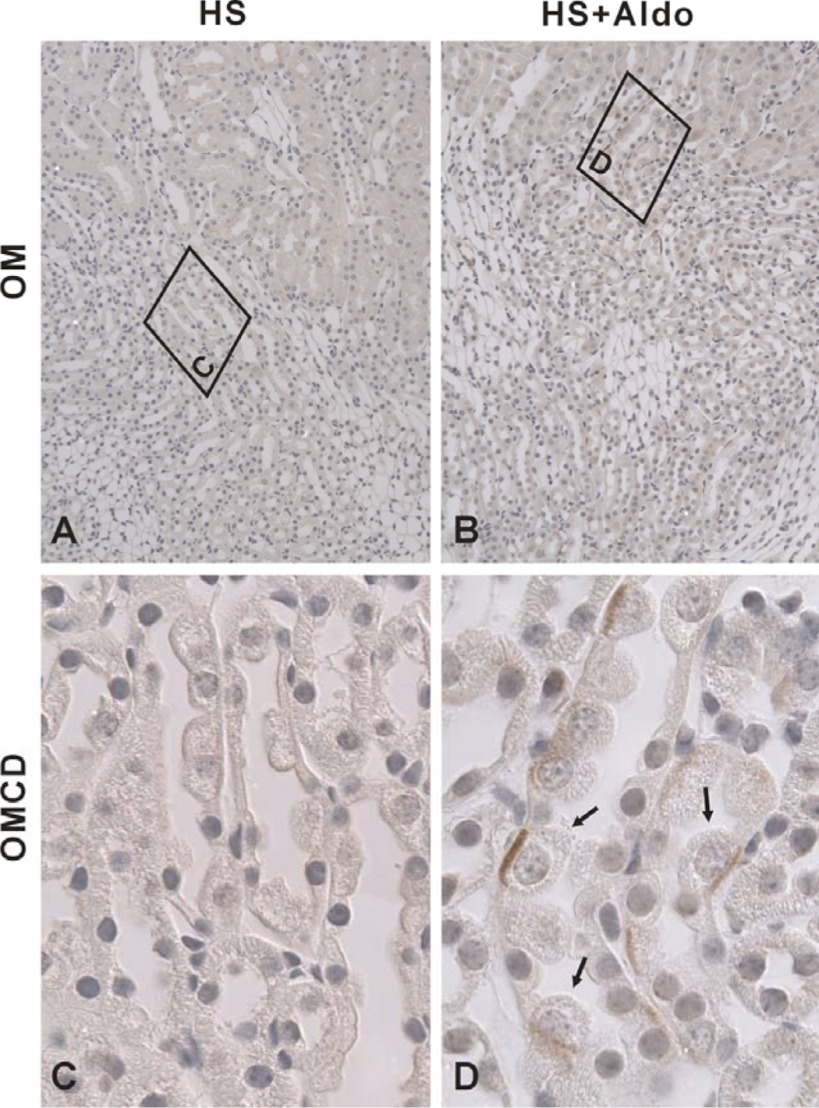
During short circuit conditions, cultured CCD cells secrete Cl− through NKCC1-mediated Cl− uptake across the basolateral membrane, which acts in series with CFTR-mediated Cl− secretion across the apical plasma membrane (2, 30). Since NKCC1 mRNA is expressed in the mouse CCD (13) and since NKCC1 protein is expressed in the basolateral regions in principal and intercalated cells in the rabbit CCD (28), we asked if NKCC1 protein is also expressed in the mouse CCD and if NKCC1 modulates transepithelial Cl− transport in that segment. Figure 6 shows NKCC1 labeling in cortical sections taken from mice that received aldosterone or vehicle. Under basal conditions, no NKCC1 labeling was detected in the CCD. However, in cortical sections from aldosterone-treated mice, NKCC1 was observed in the basolateral regions of a minority cell type. Similarly, NKCC1 labeling was observed in the basolateral regions of a minority cell type in the outer medullary collecting duct (OMCD) of aldosterone-treated mice but not from vehicle-treated mice (Fig. 7). We conclude that NKCC1 protein can be detected in intercalated cells in CCDs and OMCDs from aldosterone-treated mice but not from vehicle-treated mice.
Fig. 6.
Na+-K+-2Cl− cotransporter 1 (NKCC1) localizes to the basolateral regions of intercalated cells in CCDs from Aldo-treated mice but not from vehicle-treated mice. Shown are micrographs of cortical sections labeled for NKCC1 that were taken from mice given Aldo or vehicle (treatments 1 or 2). No NKCC1 labeling was noted in CCDs taken from vehicle-treated mice (A and C). However, in mice given Aldo, NKCC1 label was observed in the basolateral regions of a minority subtype within the CCD (B and D, arrows). C and D are higher-magnifications of the boxed regions in A and B, respectively.
Fig. 7.
NKCC1 localizes to the basolateral regions of intercalated cells in the outer medullary collecting duct (OMCD) in Aldo-treated mice but not in vehicle-treated mice. Shown are sections of the outer medulla from mice given Aldo or vehicle (treatments 1 or 2) that were labeled for NKCC1. Areas in the boxes of A and B are shown at higher power in C and D. No NKCC1 labeling was noted in OMCDs taken from vehicle-treated mice (A and C). However, in mice given Aldo, NKCC1 label was observed in the basolateral regions of a minority cell type within the OMCD (B and D, arrows).
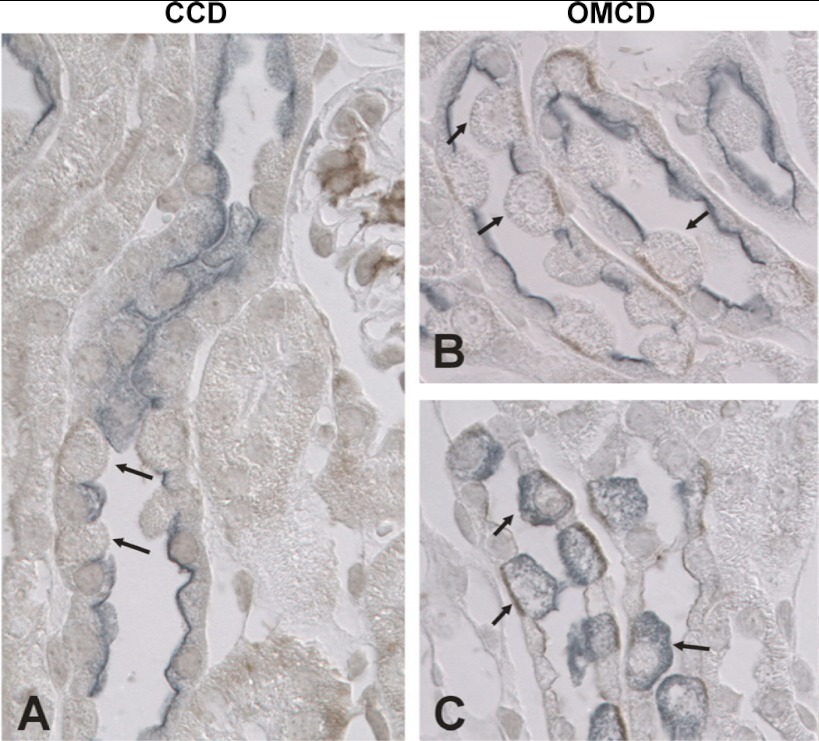
Further experiments explored which cell type expresses NKCC1 in the mouse OMCD and CCD. To do so, we used H+-ATPase as a marker of all intercalated cell subtypes, whereas pendrin was used as a marker of type B and non-A, non-B intercalated cells. AQP2 was used as a marker of principal cells. As shown in Fig. 8, A and B, in both the CCD and OMCD, cells that express NKCC1 do not express either AQP2 or pendrin. Thus, NKCC1 is not expressed in principal cells or in type B or non-A, non-B intercalated cells. In the mouse CCD and OMCD, the only cell type that is both pendrin and AQP2 negative is the type A intercalated cell. Therefore, we hypothesized that NKCC1 is expressed in type A intercalated cells. If so, NKCC1-positive cells should also be H+-ATPase positive. As shown in Fig. 8C, in the mouse OMCD, H+-ATPase labeling was detected in all cells that express NKCC1, consistent with NKCC1 expression in type A intercalated cells. We conclude that NKCC1 is expressed in the basolateral regions of type A intercalated cells in the mouse CCD and OMCD.
Fig. 8.
NKCC1 localizes to the basolateral regions of type A intercalated cells in the mouse CCD and OMCD. A and B: sections of the cortex (A) and outer medulla (B) from Aldo-treated mice (treatment 1) were labeled for NKCC1 (brown) and both pendrin (Pds) and aquaporin 2 (AQP2; blue-gray). NKCC1 labeling (arrows) was observed in the basolateral regions of cells within the CCD and OMCD that did not label for either pendrin or APQ2. C: NKCC1 labeling (brown) and H+-ATPase labeling (blue-gray) in sections of the outer medulla from Aldo-treated mice. NKCC1 labeling was observed in cells that express H+-ATPase (arrows). Thus, NKCC1 localizes to type A intercalated cells in the CCD and OMCD of Aldo-treated mice.
Benzamil-Sensitive Cl− Secretion Is Dependent on NKCC1
The OMCD of aldosterone-treated rats (42) secretes Cl− through transepithelial transport across type A intercalated cells, by way of Cl− uptake across the basolateral membrane through NKCC1. Since NKCC1 also localizes to the basolateral regions of type A cells in the mouse CCD, we asked if transepithelial Cl− transport occurs through an NKCC1-dependent pathway and if NKCC1 inhibitors modulate ENaC-dependent Cl− transport. We hypothesized that type A intercalated cells mediate Cl− secretion through NKCC1-mediated Cl− uptake across the basolateral plasma membrane (Fig. 9), similar to the rat OMCD (30, 42). We further hypothesized that amiloride analogs stimulate this Cl− secretory pathway. To examine the role of NKCC1 in transepithelial Cl− transport, Cl− absorption was measured in CCDs perfused in the presence and absence of an NKCC1 inhibitor [bumetanide (100 μM)] in the bath solution (Fig. 10A) (42, 45). In the absence of benzamil, inhibition of NKCC1 had no effect on either JCl or VT. However, with benzamil present in the perfusate (Fig. 10B), JCl more than doubled with bumetanide application to the bath, although VT was unaffected. Therefore, after ENaC blockade, bumetanide most likely alters Cl− flux by inhibiting Cl− secretion across type A intercalated cells.
Fig. 9.
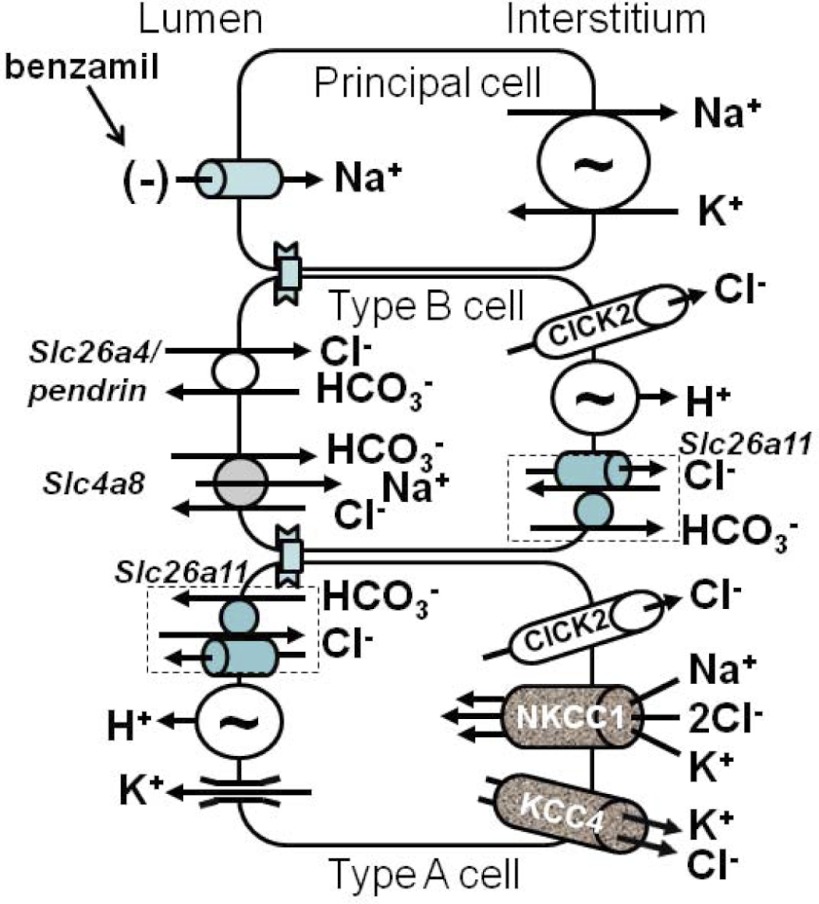
Possible role of NKCC1 in Cl− transport in the mouse CCD. The transporters present in the CCD are shown (4, 11, 14, 46). In the CCD of Aldo-treated mice, Cl− secretion may be through NKCC1-mediated Cl− uptake across the basolateral membrane of type A intercalated cells in tandem with an apical Cl− conductive pathway. This apical conductive pathway should be stimulated when the lumen-negative voltage is abolished with amiloride analogs, such as benzamil.
Fig. 10.
When ENaC is inhibited, Na+-K+-2Cl− cotransport blockade increases Cl− absorption. CCDs taken from Aldo-treated mice (treatment 1) were perfused with ANG II (10−8 M) in the bath. A and B: JCl and VT were measured in the absence (A) or presence (B) of benzamil (3 μM) in the luminal fluid. Solid lines display results from experiments where the vehicle was applied in the first period and benzamil was applied in the second period. Dashed lines indicate experiments where the order was reversed (i.e., benzamil was applied in the initial period and then removed in the second period). Open squares show means ± SE of data under each condition.
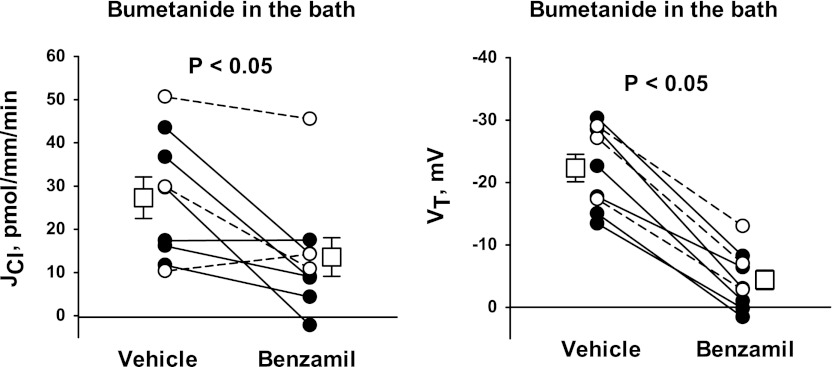
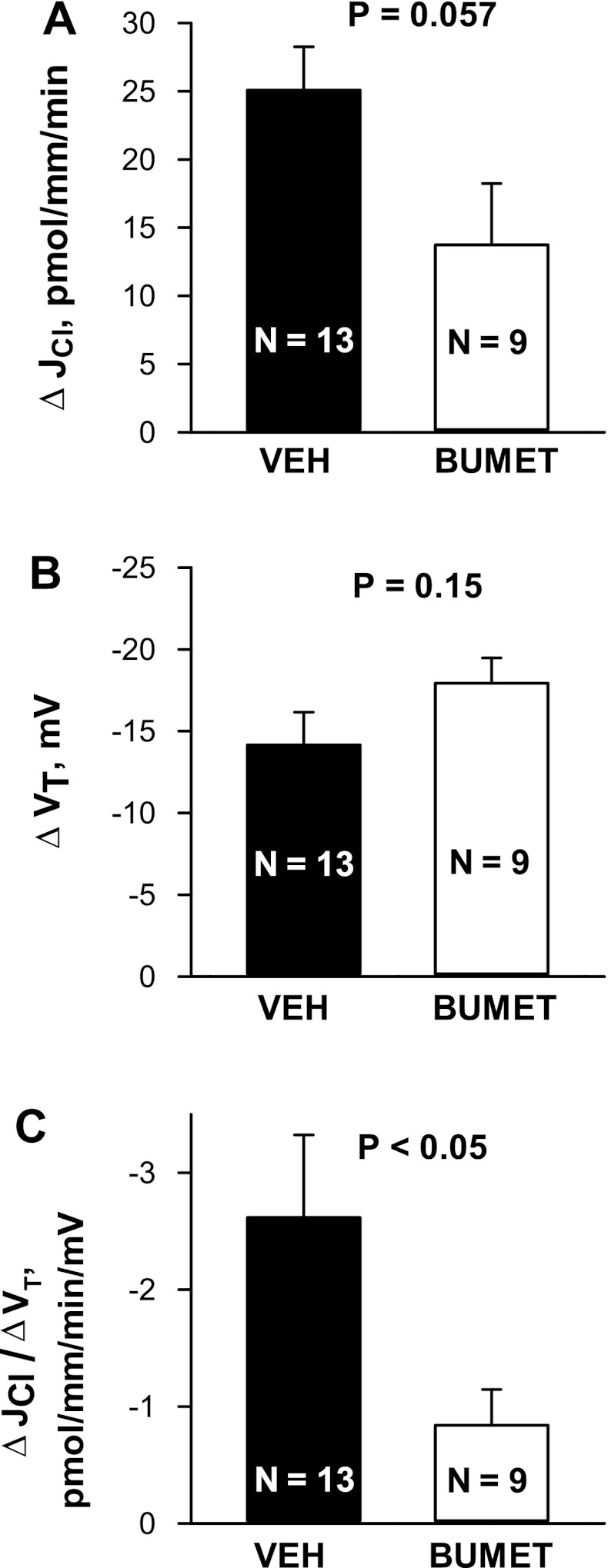
Further experiments tested whether chemical inhibitors of NKCC1 (bumetanide) blunt the benzamil-sensitive component of Cl− absorption (Fig. 11). To answer this question, we measured the benzamil-sensitive change in JCl and VT when bumetanide was applied to the bath. Benzamil reduced Cl− absorption and VT in the presence as well as in the absence of bumetanide. We reasoned that chemical inhibitors of NKCC1 blunt, but do not eliminate, the benzamil-sensitive component of JCl. Figure 12A shows that benzamil-sensitive JCl might be reduced with bumetanide application (P = 0.057), although bumetanide did not attenuate benzamil-sensitive VT (Fig. 12B). Most notably, the ratio of benzamil-sensitive Cl− absorption relative to benzamil-sensitive VT was lower in CCDs with bumetanide present in the bath relative to vehicle-treated tubules (Fig. 12C).
Fig. 11.
Effect of bumetanide on benzamil-sensitive JCl and VT. The effects of benzamil (3 μM) on JCl (left) and VT (right) were measured in CCDs from Aldo-treated mice (treatment 1) that were perfused with bumetanide (100 μM) with ANG II (10−8 M) present in the bath solution. Solid lines display results from experiments where the vehicle was applied in the first period and benzamil was applied in the second period. Dashed lines indicate experiments where the order was reversed (i.e., benzamil was applied in the initial period and then removed in the second period). Open squares show means ± SE of data under each condition.
Fig. 12.
Effect of bumetanide (Bumet) on benzamil-sensitive JCl and VT. A and B: benzamil-sensitive changes in JCl (A) and VT (B) taken from Aldo-treated mice (treatment 1) are shown. C: ratio of benzamil-sensitive JCl to benzamil-sensitive VT (ΔJCl/ΔVT). Data from Figs. 2 (n = 10), 4A (n = 3), and 9 (n = 9) were included.
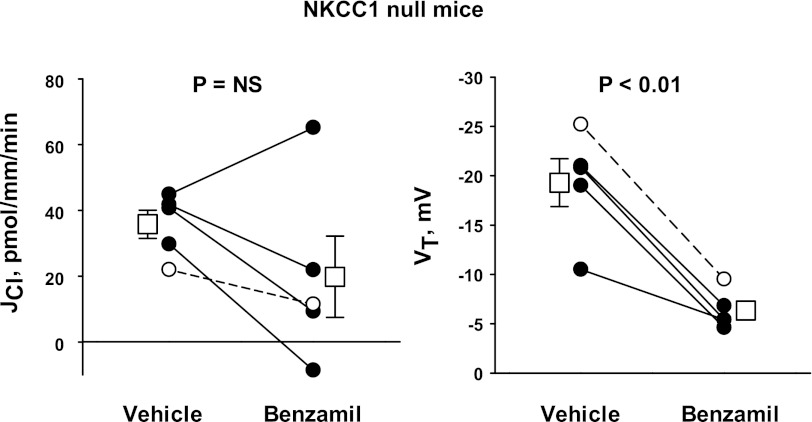
The role of NKCC1 in the benzamil-sensitive transepithelial transport of Cl− was explored further by testing the effect of benzamil on Cl− absorption in aldosterone-treated NKCC1-null mice (Fig. 13). In these mutant mice, benzamil produced a substantial fall in VT and decreased Cl− absorption in four of five tubules studied. However, in the five tubules studied collectively, benzamil did not produce a statistically significant change in Cl− absorption. In NKCC1-null mice, the benzamil-sensitive component of JCl was 16.0 ± 10.2 pmol·mm−1·min−1, which compares with 24.1 ± 3.9 pmol·mm−1·min−1 measured in CCDs from wild-type mice in the absence of bumetanide.
Fig. 13.
Benzamil-sensitive JCl is blunted in NKCC1-null mice. JCl (left) and VT (right) were measured in CCDs from Aldo-treated NKCC1-null mice (treatment 1) before and after the application of benzamil (3 μM) to the luminal fluid. ANG II (10−8 M) was present in the bath. Solid lines display results from experiments where the vehicle was applied in the first period and benzamil was applied in the second period. The dashed line indicates an experiment where the order was reversed (i.e., benzamil was applied in the initial period and then removed in the second period). Open squares show means ± SE of data under each condition.
In conclusion, the benzamil-sensitive component of Cl− absorption does not always change in parallel with benzamil-sensitive VT and therefore cannot be explained fully by changes in paracellular transport. Moreover, chemical inhibitors of NKCC1 or ablation of the gene encoding NKCC1 blunt benzamil-sensitive Cl− absorption in the CCD. Thus, the benzamil-sensitive component of transepithelial Cl− transport occurs, at least in part, through an NKCC1-dependent pathway.
Conductive Cl− Secretion Does Not Occur Through CFTR
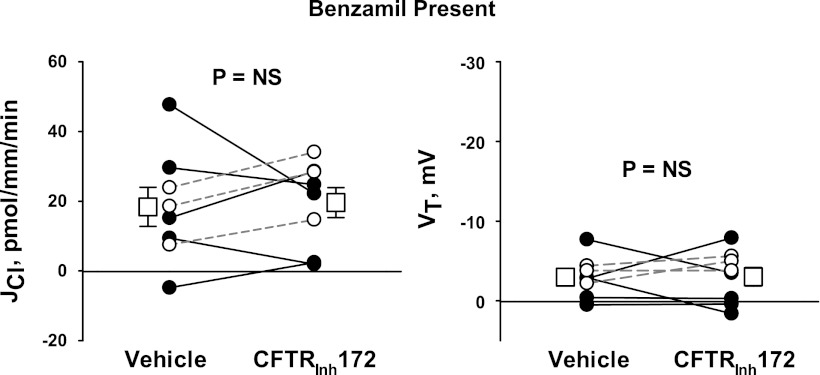
In cultured mouse CCD cells, Cl− secretion is accomplished through conductive Cl− secretion across the apical plasma membrane mediated by CFTR (30). Since CFTR-mediated Cl− channels have been observed in the native mouse CCD and in cultured mouse CCD cells (29, 30), we asked if CFTR modulates Cl− flux either in the presence or absence of benzamil in the luminal fluid. We hypothesized that if benzamil stimulates CFTR-mediated Cl− secretion, then inhibiting CFTR should increase Cl− absorption. To test this hypothesis, CCDs were perfused with benzamil present in the luminal fluid. Cl− flux was then measured before and after the application of a CFTR inhibitor (CFTRinh172). As shown in Fig. 14, inhibition of CFTR did not alter Cl− absorption or VT. Moreover, in two separate CCDs perfused in the absence of benzamil, we did not detect an effect of this CFTR inhibitor on Cl− absorption (26.0 pmol·mm−1·min−1 with vehicle and 26.1 pmol·mm−1·min−1 with CFTRinh172, n = 2). We conclude that CFTR does not represent a major pathway for Cl− transport in the CCD of aldosterone-treated mice. Thus, conductive apical Cl− secretion in the mouse CCD occurs through a pathway other than CFTR.
Fig. 14.
CFTR inhibition does not significantly alter JCl. CCDs from Aldo-treated mice (treatment 1) were perfused with benzamil (3 μM) present in the perfusate with ANG II (10−8 M) present in the bath. JCl (left) and VT (right) were measured before and after the application of CFTR inhibitor [CTFRihn 172 (5 μM)] to the perfusate. The dashed lines show means ± SE.
DISCUSSION
While amiloride has been available as an antihypertensive for many years, its current clinical use is limited since it is thought to be a mild diuretic. In patients with essential hypertension without hyperaldosteronism, 40 mg amiloride given once a day reduces systolic blood pressure by ∼ 12 mmHg (24). However, in patients with hypertension due to primary hyperaldosteronism, the same dose of amiloride reduces systolic blood pressure by 33–40 mmHg (6, 24). Therefore, amiloride is probably a more effective antihypertensive in high than in low aldosterone states (10) and is still used for the medical management of hypertension in primary aldosteronism (35). The utility of amiloride in the treatment of the hypertension observed in hyperaldosteronism is likely due to the upregulation of ENaC that follows increased aldosterone production as well as the greater amiloride-sensitive component of Cl− absorption.
The present study and previous studies have demonstrated that inhibiting ENaC with amiloride analogs produces a substantial reduction in Cl− absorption in CCDs from mice given a NaCl-replete diet and an aldosterone infusion, which could be considered to be a treatment model of primary hyperaldosteronism. This is in contrast to the absence of an amiloride-sensitive change in Cl− absorption in CCDs taken from mice after dietary NaCl restriction (26), where an appropriate physiological increase in aldosterone is observed. Therefore, amiloride might be a more effective antihypertensive during primary aldosteronism, due, in part, to the greater effect of ENaC on Cl− transport in this treatment model. However, the different results of these two studies may occur because in our study Cl− absorption was measured with ANG II present in the bath, whereas ANG II was absent in the study by Leviel et al. (26).
The mechanism of benzamil-sensitive Cl− absorption has been thought to occur through paracellular Cl− transport driven by the lumen-negative VT generated by ENaC (12). However, the present study demonstrates that benzamil-sensitive changes in Cl− absorption can be dissociated from benzamil-sensitive changes in VT since chemical inhibitors of NKCC1 or ablation of the gene encoding NKCC1 blunt benzamil-sensitive JCl, although the benzamil-sensitive change in VT is not reduced. The weaker effect of benzamil on JCl observed in CCDs from NKCC1-null mice does not occur because ENaC is downregulated in these mutant mice. Instead, we have previously observed that ENaC subunit abundance is the same or increased in kidneys from NKCC1-null mice relative to wild-type mice (44).
In the mouse kidney (16), NKCC1 is localized to the basolateral regions of the inner medullary collecting duct, papillary surface epithelium, the juxtaglomerular afferent arteriole, and extraglomerular mesangium. In the rabbit kidney, Liu et al. (28) observed NKCC1 labeling in the basolateral regions of both principal and intercalated cells in the CCD. However, NKCC1 labeling has not been previously reported in the mouse CCD or OMCD (16). While the present study did not detect NKCC1 labeling in the CCD or OMCD of mice under basal conditions (i.e., after a NaCl-replete diet), we observed NKCC1 labeling in the basolateral regions of type A intercalated cells in both the CCD and OMCD after aldosterone administration. These data demonstrate that in the kidney, NKCC1 is regulated by aldosterone and are consistent with a previous study (15) in the rat aorta that observed aldosterone-induced stimulation of NKCC1-mediated transport and consistent with a study (30) of cultured mouse CCD cells that observed increased NKCC1 mRNA after the application of aldosterone.
In the OMCD from rats given an aldosterone analog, lumen-positive VT and net Cl− secretion are observed (42). This lumen-positive VT should enhance Cl− secretion mediated by a conductive pathway. In the rat OMCD, Cl− secretion falls by ∼70% with the application of NKCC1 inhibitors, although VT is unchanged (42). Therefore, Cl− secretion in this segment is dependent on NKCC1-mediated Cl− uptake across the basolateral plasma membrane. Since NKCC1 is also expressed in basolateral regions of type A intercalated cells in the mouse CCD, one possible explanation for these findings is that in type A cells of the mouse CCD, Cl− is taken up across the basolateral plasma membrane through NKCC1 and is then secreted through a Cl− transport pathway that localizes to the apical plasma membrane (Fig. 9). With the application of benzamil to the luminal fluid, this Cl− secretory pathway is upregulated, which reduces the net Cl− absorption that is measured.
In the CCD, the driving force for Cl− secretion mediated by an apical plasma membrane Cl− channel should be enhanced when the lumen-negative voltage is eliminated, such as with benzamil application. While the nature of the conductive pathway on the apical plasma membrane is unknown, the present study demonstrates that CFTR is not a major mechanism of Cl− transport in the CCD of aldosterone-treated mice. However, a stilbene-sensitive, apical anion conductance has been observed in intercalated cells from rabbit CCDs perfused in vitro (8), which may represent a Cl− channel. Moreover, Slc26a11 localizes to the apical regions of type A intercalated cells and can act as a Cl− channel or a Cl−/HCO3− exchanger (Fig. 9) (46). Whether benzamil increases Cl− secretion through an apical Cl− channel or anion exchanger remains to be determined.
The Cl− secretion observed with ENaC blockade should be accompanied by cotransport of a cation, such as K+ or H+, or countertransport of an anion, such as HCO3−. Since inhibition of NKCC1 reduces flow-stimulated K+ secretion (28), NKCC1 may mediate KCl secretion by type A intercalated cells (Fig. 9). Alternatively, since H+ and Cl− transport occur in tandem in many cell types (46), type A intercalated cells may mediate net HCl secretion through apical plasma membrane H+-ATPase and this amiloride-sensitive Cl− secretory pathway acting in parallel (Fig. 9). The cosecreted cation or counteranion that accompanies amiloride-sensitive changes in Cl− transport remains to be determined.
In conclusion, in aldosterone-treated mice, the majority of Cl− absorption occurs through a benzamil-sensitive, thiazide-insensitive pathway. Inhibition of ENaC with benzamil reduces Cl− absorption, possibly from the reduced lumen-negative voltage, which should increase conductive Cl− secretion or reduce conductive Cl− absorption. However, other signaling mechanisms are possible. Benzamil-sensitive changes in Cl− absorption cannot be fully explained by changes in paracellular Cl− absorption. Instead, benzamil-sensitive Cl− absorption occurs, in part, through transcellular transport that depends on NKCC1.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-52935 (to S. M. Wall) and by American Society of Nephrology Career Development Grant 145596 (to V. Pech).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.P. conception and design of research; V.P., M.T., Y.H.K., D.A., and M.N. performed experiments; V.P., Y.H.K., A.M.W., and S.M.W. analyzed data; V.P., Y.H.K., A.M.W., and S.M.W. interpreted results of experiments; V.P. and Y.H.K. prepared figures; V.P. and S.M.W. drafted manuscript; V.P., A.M.W., and S.M.W. edited and revised manuscript; V.P., M.T., Y.H.K., D.A., E.H., B.C.R., A.M.W., M.N., and S.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Gary Shull for NKCC1-null mice, Dr. R. James Turner for rabbit anti-rat NKCC1 antibody, and Dr. Mark Knepper for AQP2 antibody. The authors also thank Dr. Steve Sansom for helpful suggestions.
REFERENCES
- 1. Chalfant ML, Peterson-Yantorno K, O'Brien TG, Civan MM. Regulation of epithelial Na+ channels from M-1 cortical collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 271: F861–F870, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Chang CT, Bens M, Hummler E, Boulkroun S, Schild L, Teulon J, Rossier BC, Vandewalle A. Vasopressin-stimulated CFTR Cl− currents are increased in the renal collecting duct cells of a mouse model of Liddle's syndrome. J Physiol 562.1: 271–284, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA 91: 8984–8988, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eladari D, Teulon J. A new regulator of the vacuolar H+-ATPase in the kidney. Kidney Int 80: 907–909, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE. Severe impairment of salivation in Na+-K+-2Cl− cotransporter (NKCC1)-deficient mice. J Biol Chem 275: 26720–26726, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Ferriss JB, Beevers DG, Boddy K, Brown JJ, Davies DL, Fraser R, Kremer D, Lever AF, Robertson JI. The treatment of low-renin (“primary”) hyperaldosteronism. Am Heart J 96: 97–109, 1978 [DOI] [PubMed] [Google Scholar]
- 7. Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem 274: 26946–26955, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Furuya H, Breyer MD, Jacobson HR. Functional characterization of α- and β- intercalated cell types in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 261: F377–F385, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Garcia NH, Plato CF, Garvin JL. Fluorescent determination of chloride in nanoliter samples. Kidney Int 55: 321–325, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Hoefnagels WHL, Drayer JIM, Smals AGH, Kloppenborg PWC. Spironolactone and amiloride in hypertensive patients with and without aldosterone excess. Clin Pharmacol Ther 27: 317–323, 1980 [DOI] [PubMed] [Google Scholar]
- 11. Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-α/β4 channels modulate sodium and potassium handling during potassium adaptation. J Am Soc Nephrol 21: 634–645, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci USA 107: 18010–18015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ikebe M, Nonoguchi H, Nakayama Y, Tashima Y, Tomita K. Upregulation of the secretory-type Na+/K+/2Cl− cotransporter in kidney by metabolic acidosis and dehydration in rats. J Am Soc Nephrol 12: 423–430, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Jentsch TJ. Chloride transport in the kidney: lessons from human disease and knockout mice. J Am Soc Nephrol 16: 1549–1561, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Jiang G, Cobbs S, Klein JD, O'Neill WC. Aldosterone regulates the Na-K-2Cl cotransporter in vascular smooth muscle. Hypertension 41: 1131–1135, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium and the glomerular afferent arteriole. J Clin Invest 98: 723–730, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin WK, Verlander JW, Sutliff RL, Wall SM. Reduced ENaC expression contributes to the lower blood pressure observed in pendrin null mice. Am J Physiol Renal Physiol 293: F1314–F1324, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Kim YH, Pham TD, Zheng W, Hong S, Baylis C, Pech V, Beierwaltes WH, Farley DB, Braverman LE, Verlander JW, Wall SM. Role of pendrin in iodide balance: going with the flow. Am J Physiol Renal Physiol 297: F1069–F1079, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim YH, Verlander JW, Matthews SW, Kurtz I, Shin WK, Weiner ID, Everett LA, Green ED, Nielsen S, Wall SM. Intercalated cell H+/OH− transporter expression is reduced in Slc26a4 null mice. Am J Physiol Renal Physiol 289: F1262–F1272, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Knepper MA, Good DW, Burg MB. Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am J Physiol Renal Fluid Electrolyte Physiol 247: F729–F738, 1984 [DOI] [PubMed] [Google Scholar]
- 23. Knepper MA, Good DW, Burg MB. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 249: F870–F877, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Kremer D, Boddy K, Brown JJ, Davies DL, Fraser R, Lever AF, Morton JJ, Robertson JIS. Amiloride in the treatment of primary hyperaldosteronism and essential hypertension. Clin Endocrinol 7: 151–157, 1977 [DOI] [PubMed] [Google Scholar]
- 25. Kwon TH, Pushkin A, Abuladze N, Nielsen S, Kurtz I. Immunoelectron microscopic localization of NBC3 sodium-bicarbonate cotransporter in rat kidney. Am J Physiol Renal Physiol 278: F327–F336, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hatim H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. Identification of a novel electroneutral Na+ reabsorption process in the distal nephron mediated by the Na+-dependent chloride-bicarbonate exchanger Slc4A8. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Light DB, Schwiebert EM, Fejes-Toth G, Naray-Fejes-Toth A, Karlson KH, McCann FV, Stanton BA. Chloride channels in the apical membrane of cortical collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 258: F273–F280, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Liu W, Schreck C, Coleman RA, Wade JB, Henandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol 301: F1088–F1097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu M, Dong K, Egan ME, Giebisch GH, Boulpaep EL, Hebert SC. Mouse cystic fibrosis transmembrane conductance regulator forms cAMP-PKA-regulated apical chloride channels in cortical collecting duct. Proc Natl Acad Sci USA 107: 6082–6087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Montesano R, Ghzili H, Carrozzino F, Rossier BC, Feraille E. cAMP-dependent chloride secretion mediates tubule enlargment and cyst formation by cultured mammalian collecting duct cells. Am J Physiol Renal Physiol 296: F446–F457, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells in mouse cortical collecting duct. J Am Soc Nephrol 19: 84–91, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. J Am Soc Nephrol 13: 1131–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem 274: 16569–16575, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Quinkler M, Stewart PM. Treatment of primary aldosteronism. Best Pract Res Clin Endocrinol Metab 24: 923–932, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC. Collecting duct-specific gene inactivation of aENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest 112: 554–565, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Terada Y, Knepper MA. Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 259: F519–F528, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorticosterone upregulates Pds (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Wall SM. NH4+ augments net acid secretion by a ouabain-sensitive mechanism in isolated perfused inner medullary collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 270: F432–F439, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Wall SM, Fischer MP, Mehta P, Hassell KA, Park SJ. Contribution of the Na+-K+-2Cl− cotransporter (NKCC1) to Cl− secretion in rat outer medullary collecting duct. Am J Physiol Renal Physiol 280: F913–F921, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension 44: 1–6, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin WK, Pham TD, Meyer JW, Lorenz JN, Beierwaltes WH, Dietz JR, Shull GE, Kim YH. Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol 290: F409–F416, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Wall SM, Trinh HN, Woodward KE. Heterogeneity of NH4+ transport in mouse inner medullary collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 269: F536–F544, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Xu J, Barone S, Li H, Holiday S, Zahedi K, Soleimani M. Slc26a11, a chloride transporter, localizes with the vacuolar H+-ATPase of A-intercalated cells of the kidney. Kidney Int 80: 926–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yip KP, Tsuruoka S, Schwartz GJ, Kurtz I. Apical H+/base transporters mediating bicarbonate absorption and pHi regulation in the OMCD. Am J Physiol Renal Physiol 283: F1098–F1104, 2002 [DOI] [PubMed] [Google Scholar]