Abstract
Hepatocellular carcinoma (HCC) has a tendency for intravascular dissemination leading to a poor prognosis. The importance of the sinusoidal structure of the tumor vasculature in HCC has been implicated in the metastasis formation. To clarify the role of tumor angiogenesis in HCC metastasis, we morphologically investigated the interaction of HCC cells with blood vessels during the sequential process of metastasis. Autopsy specimens of 80 patients with HCC were examined with immunohistochemistry using a specific antibody against CD31, a marker for endothelial cells. The most frequent sites of metastasis were the liver (82.5%) and lung (43.8%). In most cases, the metastatic process was initiated by vascular involvement where tumor nests surrounded by sinusoidal vessels extend into the portal and hepatic veins. Subsequently, these endothelial-coated tumor emboli enter the circulation, embolize at distant organs, proliferate within the blood vessel and ultimately form metastatic foci. These steps are indicative of an invasion-independent pathway. Our findings in animal models and now in human cases suggest that sinusoidal angiogenesis may represent a novel target for therapeutic strategies to limit HCC metastasis. In combination with primary tumor treatment, perturbation of tumor emboli may reduce dissemination of disease.
Keywords: Hepatocellular carcinoma, Metastasis, Angiogenesis, Invasion-independent pathway, Autopsy
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, especially in Asia and Africa, and is the third leading cause of cancer mortality in Japan [1, 2]. Although recent progress in therapeutic modalities has improved the morbidity and mortality rates of this disease [3], the long-term prognosis remains far from satisfactory because of high recurrence rates and metastasis [4, 5]. HCC dissemination tends to involve blood vessels; portal, and hepatic veins leading to intrahepatic, and lung metastasis, respectively [6–9]. The current dogma would suggest that active vascular invasion by the cancer cells themselves is essential to the metastatic process; however, the predilection of HCC for vascular dissemination may not be only attributed to invasion of high tumor vascularity, but also to the interaction between tumor cells and the endothelial cells of nascent blood vessels [10, 11].
A recent study suggests that intravasation of HCC involves the invasion-independent metastasis pathway, where tumor nests are enveloped by blood vessels and enter into the bloodstream [12]. Experimental studies using animal models indicate that biological activities and gene expression profiles associated with this invasion-independent metastasis pathway are different from those associated with a pathway that depends on the invasive activity of tumor cells [13, 14]. An understanding of the common and differential mechanisms involved in the two pathways to metastasis should greatly facilitate the development of improved, combinatorial therapeutic strategies to limit this lethal aspect of cancer progression.
To better define the histopathological basis of HCC metastasis, we morphologically examined the multi-step process of HCC progression using autopsy cases. We present evidence that tumor cells from HCC can gain access into blood vessels by intravasation of tumor cell nests surrounded by vascular endothelial cells, followed by intravascular tumor growth in the lung, without penetration of the vascular wall during either process. The fact that sinusoidal tumor vessels appear to play an important role in both initiation and maintenance of the invasion-independant metastasis pathway in HCC, indicate that sinusoidal angiogenesis is a potential target for the prevention and treatment of HCC metastasis.
Materials and methods
Patients
Autopsy cases of hepatocellular carcinoma recorded between 1989 and 2003 were retrieved from the files of the Pathological Division at the Fukushima Medical University Hospital and studied in accordance with national and local human material investigative protocols. Tissue specimens from tumors with metastasis were selected for review. In each case, the clinical presentation, location of the tumor, and the outcome for the patient were studied. Details of the pathological examination, including the gross appearance and size of the tumors, were noted. Paraffin blocks of the primary and metastatic tumors were used for immunohistochemical studies.
Antibodies
A mouse monoclonal antibody for CD31 (clone JC70, Dako, Grostrup, Denmark) was used for endothelial cell staining. Other primary antibodies used were a mouse monoclonal antibody for lymphatic endothelial marker, D2-40 (Signet Laboratories, Inc., Dedham, MA), and a rabbit polyclonal antibody for basement membrane component laminin (ICN Biomedicals, Inc., Zoetermeer, the Netherlands).
Immunohistochemistry
Sections of primary and metastatic tumors were stained with haematoxylin and eosin and Elastica Masson stain to detect elastic layers of blood vessels. Immunohistochemical staining was performed using an indirect streptavidin-biotin immunoperoxidase method (SAB-PO (M) kit, Nichirei Corp., Tokyo, Japan). After blocking of endogenous peroxidase activity in 0.3% hydrogen peroxide in methanol for 30 min, slides were incubated with primary antibodies overnight at 4°C, washed with PBS, and then incubated with secondary biotin-labeled antibodies for 30 min at room temperature. Antibody localization was visualized with peroxidase-conjugated streptavidin for 30 min at room temperature, followed by the diaminobenzidine reaction. The slides were counterstained with hematoxylin.
Statistical analysis
Fisher’s exact test was used to assess the relationship between the presence of vascular involvements of HCC and intrahepatic and pulmonary metastases, and tumor emboli in the lung. A P-value < 0.05 was considered statistically significant.
Results
Distribution pattern of metastatic foci from human hepatocellular carcinoma
The clinicopathological data and organ distribution of metastasis from hepatocellular carcinoma (HCC) in 80 autopsy cases are summarized in Table 1. The preferential metastasis sites of HCC were the liver (82.5%) and lung (43.8%). Tumor emboli in the pulmonary arteries or arterioles were present in 35 cases (43.8%), in which tumor embolism with no extravascular growth was found in seven cases. The correlations of portal and hepatic vein involvements of HCC with intrahepatic and pulmonary metastases, and pulmonary tumor embolism are shown in Table 2. The incidence of metastases to both liver and lung, and of pulmonary tumor emboli were strongly related to portal vein involvement (P < 0.01). In contrast, the presence of hepatic vein involvement was significantly associated with pulmonary metastasis (P = 0.01) and tumor embolism (P = 0.01), but not correlated with intrahepatic metastasis (P = 0.73).
Table 1.
Clinicopathological features of 80 patients with primary HCC in autospy
| Characteristics | n = 80 (%) |
|---|---|
| Sex | |
| Male | 56 (70) |
| Female | 24 (30) |
| Age (y) | |
| Median | 65.3 |
| Range | 38–84 |
| Tumor size (cm) | |
| Median | 6.63 |
| Range | 1.0–21.0 |
| Macro type | |
| Nodular type | 58 (62.5) |
| Multinodular type | 19 (23.8) |
| Diffuse type | 3 (3.8) |
| Metastasis site | |
| Liver | 66 (82.5) |
| Lung | 35 (43.8) |
| Peritoneum | 16 (20) |
| Pleura | 4 (5) |
| Adrenal | 8 (10) |
| Bone | 13 (16.3) |
| Brain | 2 (2.5) |
| Lymph node | 14 (17.5) |
| Pulmonary tumor embolization | 35 (43.8) |
| Liver cirrhosis | 70 (87.5) |
| Survival (months) | 24.6 |
Table 2.
Correlation of vascular involvements of HCC with metastases
| Portal vein involvement | Hepatic vein involvement | |||||
|---|---|---|---|---|---|---|
| Present (n = 57) | Absent (n = 23) | P-value | Present (n = 26) | Absent (n = 54) | P-value | |
| Intrahepatic metastasis | 53 (93%) | 13 (57%) | 0.0001 | 22 (85%) | 44 (80%) | 0.7297 |
| Pulmonary metastasis | 32 (56%) | 3 (13%) | 0.0004 | 19 (73%) | 16 (29%) | 0.0014 |
| Pulmonary tumor emboli | 31 (54%) | 4 (17%) | 0.0025 | 19 (73%) | 16 (29%) | 0.0002 |
Sinusoidal development of tumor vasculature and cancer cell intravasation
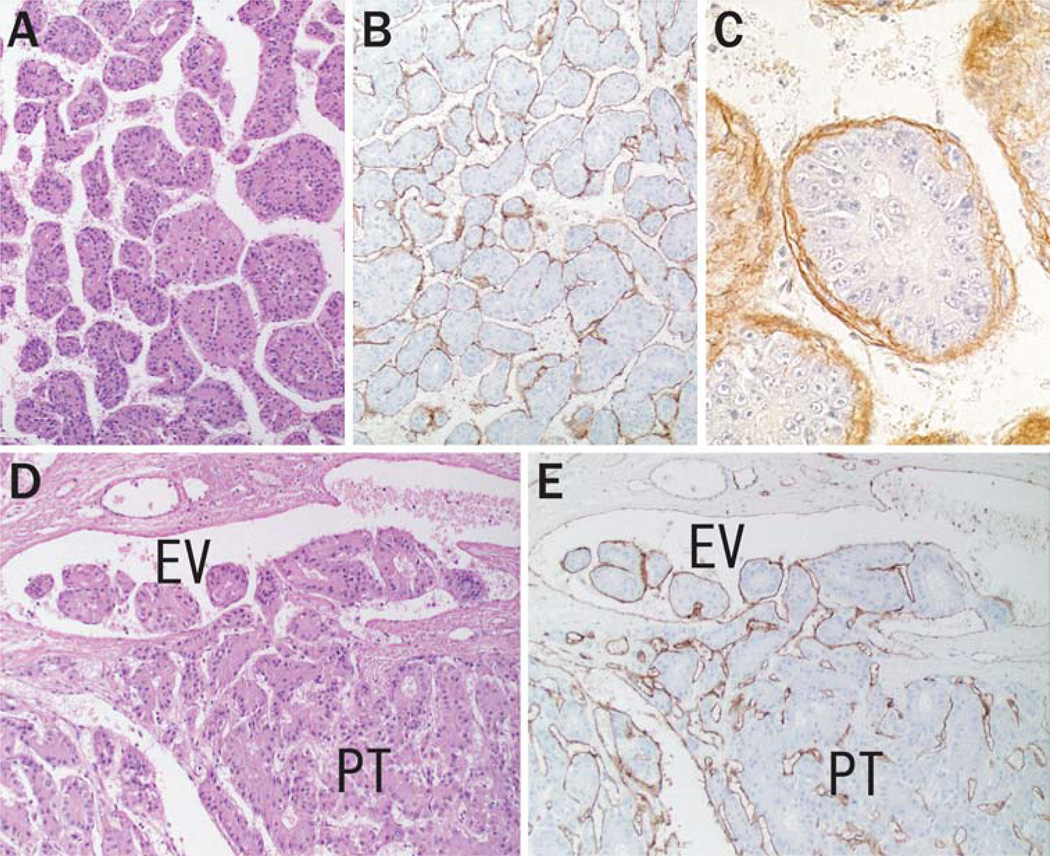
Most of the primary tumors of HCC formed an organized tissue pattern consisting of trabecular nests of cancer cells surrounded by sinusoidal tumor vessels and intervening duplicated basement membrane (Fig. 1a–c). Morphological observation revealed that intravasation was achieved by the well-organized tumor nests rather than by individual cancer cells invading through the vascular wall (Fig. 1d). Intravasated tumor cells expansively grew within the efferent vessels and subsequently extended to the larger portal and hepatic veins. A CD31 or CD34-positive endothelial cell lining was conserved on the tumor emboli in every step of the intravasation process (Fig. 1e). Tumor emboli in the main branches of portal and hepatic veins were observed in 25 (31%) and 12 (15%) cases, respectively.
Fig. 1.
Sinusoidal vascular architecture and cancer cell intravasation in primary liver tumor. (a) Basic structure of HCC forming thick trabecular pattern (hematoxylin-eosin stain, original magnification 100×). Immunostaining of CD31 (b) and laminin (c) shows sinusoidal tumor vasculature with basement membrane surrounding the tumor nests (original magnification, panel b, 100×; panel c, 400×). (d, e) Intravasation of HCC. (d) Multiple tumor nests move into an efferent vein (EV) from the primary tumor (PT) without destruction of the vascular wall (hematoxylin-eosin stain, original magnification 100×). (e) The tumor emboli conserve their trabecular architecture with endothelial coverage during the intravasation process (immunostaining of CD31, original magnification 100×)
Transportation of cancer cells in the blood
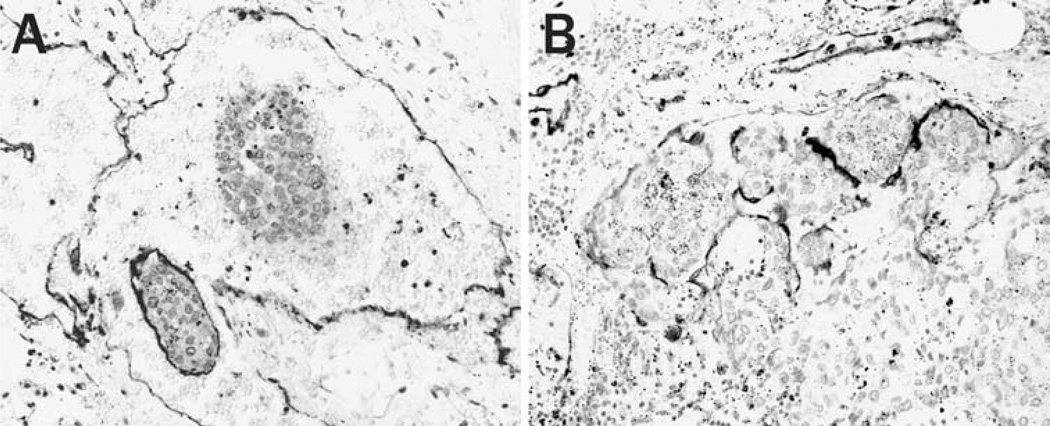
Free emboli were observed in the blood of three cases. In one case, a 2 mm-sized tumor mass was macroscopically found within a blood coagulation in the main trunk of the pulmonary artery (Fig. 2a, b). This embolus would most likely be transported via the inferior vena cava and right cardiac system. In another two cases, small emboli were observed in the esophageal varices (Fig. 2c) and small veins in the rectum (Fig. 2d), presumably transported via the collateral portal venous pathway. All emboli were composed of solitary or multiple tumor nests covered by vascular endothelial cells. These findings demonstrate that the tumor emboli conserve their tissue organization within the circulation.
Fig. 2.
Circulating HCC cells. (a, b) A free embolus in the main trunk of the pulmonary artery. (a) The tumor embolus within blood coagulation is composed of multiple tumor nests. (b) The embolus keeps their tissue organization with sinusoidal tumor vasculature. (c, d) Tumor emboli in the esophageal varix. Well-organized tumor nests with endothelial lining are embolized in dilated veins. [(a, c) Hematoxylin eosin stain, original magnification 40×, (b, d) immunostaining of CD31, original magnification 100×]
Tumor embolization to the target organs
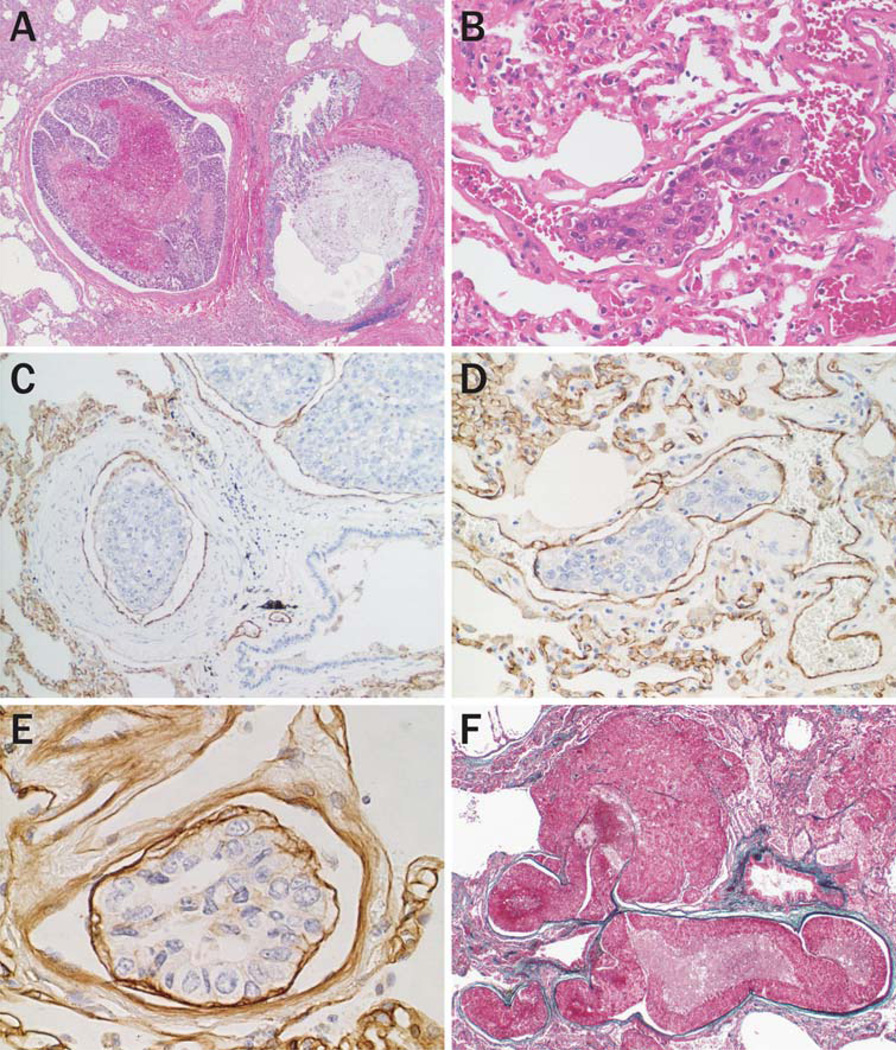
Of 35 cases with lung metastasis, 28 (80%) were associated with tumor emboli in pulmonary arteries (Fig. 3a) or arterioles (Fig. 3b). Most of the tumor emboli microscopically conserved the tissue structure composed of tumor nests surrounded by vascular endothelial cells (Fig. 3c, d) intervened by basement membrane (Fig. 3e).
Fig. 3.
Pulmonary tumor emboli of HCC. (a) Large tumor mass composed of multiple tumor nests, and with central necrosis, are embolized in a pulmonary artery (hematoxylineosin stain, original magnification 20×). (b) A single tumor nest is arrested in an arteriole (hematoxylin-eosin stain, original magnification 200×). (c, d) Immunostaining of CD31 highlights tumor-associated blood vessels surrounding tumor emboli both in the pulmonary artery (c) and arterioles (d) (original magnification (c) 100×; (d) 200×). (e) The embolus conserves the trabecular architecture with the basement membrane (Immunostaining of laminin, original magnification 200×). (f) Intravascular growth and extravasation of HCC cells embolized in pulmonary artery (Elastica Masson stain, original magnification 20×)
Intravascular growth and metastasis
Embolized tumor cells proliferated within the pulmonary arteries or arterioles with vascular expansion (Fig. 3f). Intravascular tumors induced a vasculature with a sinusoidal structure similar to that of the primary tumor. Most of the metastatic nodules detected in the lung contained collapsed arterioles with a rupture, indicative of an origin from a pulmonary embolism. Similar tumor emboli in the portal vein and subsequent metastatic nodules were observed in the liver.
Lymphatic metastasis of HCC
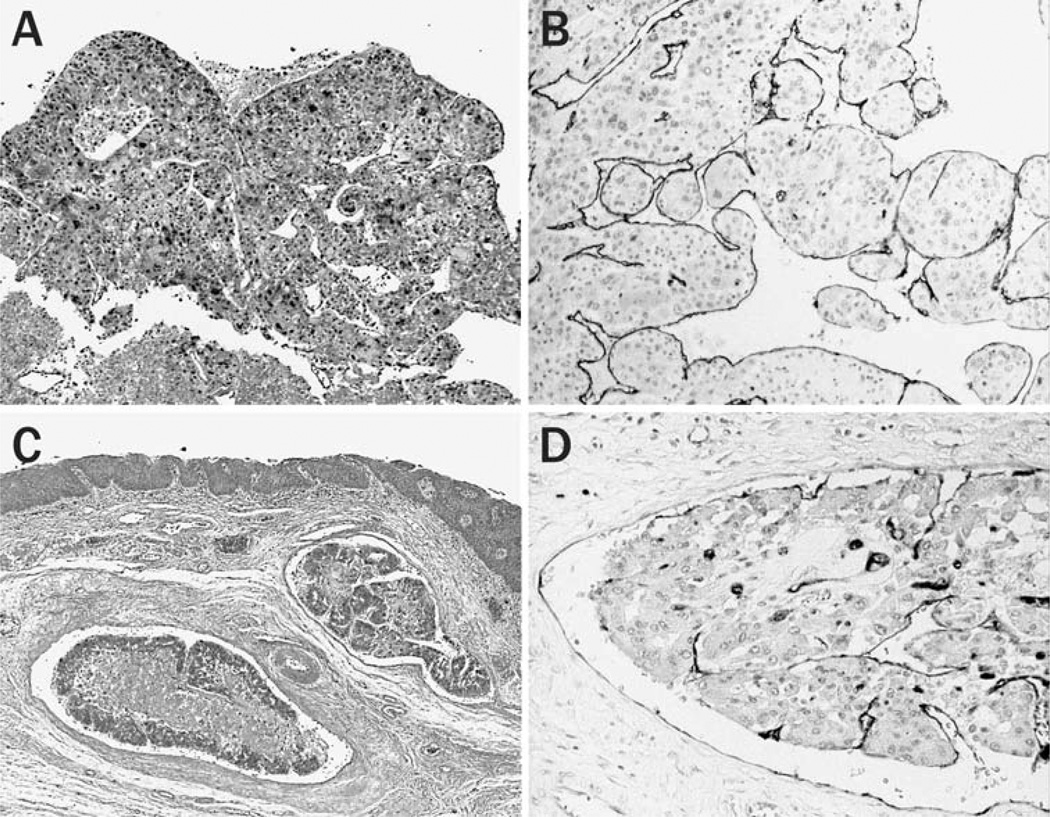
Lymph node metastasis was observed in 14 cases (17.5%), commensurate with the observation that lymphatic involvement of HCC was not observed as frequently as that of hematogenous vascular involvement. Some of the tumor emboli identified within lymph vessels and within a marginal sinus of the lymph node were composed of well-organized tumor nests associated with endothelial covering. This was confirmed by immunohistochemical staining using an antibody, D2-40, a specific marker for lymphatic endothelial cells (Fig. 4a, b).
Fig. 4.
Lymphatic dissemination of HCC. Immunostaining with D2-40 antibody shows multicellular tumor emboli covered by lymphatic endothelial cells in a lymph vessel (a) and the marginal sinus in a lymph node (b) (original magnification 200×)
Discussion
To metastasize, a tumor cell must gain access to the vasculature from the primary tumor, survive the circulation, escape immune surveillance, localize in the vasculature of the target organ, escape from the organ vasculature, and thrive and proliferate in the secondary site. The tumor cell may be the driving force in the progression of disease, but other host reactive cells, especially endothelial cells, are critical in facilitating tumor growth and spread. While many studies have shown that intratumoral microvessel density correlates with tumor aggressiveness, this correlation is likely due not only to the resulting increased access of tumor cells to the bloodstream, but also to specific dissemination mechanisms that result from the increased interaction of nascent endothelial cells with tumor cells.
Results of this study have provided morphological evidence for a non-invasive mechanism of blood-borne metastasis in HCC, in which cancer cells do not individually invade the vascular walls but rather disseminate as multicellular tumor emboli that conserve a tissue architecture reminiscent of the primary tumor. In the primary tumor, the metastatic process is initiated by intravasation of tumor nests surrounded by tumor vessels of sinusoidal origin. Subsequently, the tumor emboli associated with endothelial cells disseminate via the portal and hepatic veins and form metastatic foci in the liver and lung, respectively. The terminal feature of metastasis was not only extravasation but also intravascular growth of embolized HCC cells, a phenomenon previously proposed by Al-Mehdi et al. [15]. These findings suggest that the ability of HCC cells to actively invade the wall of the vasculature is not necessarily a prerequisite to achieve blood-borne liver and lung metastases, and support our hypothesis that an invasion-independent pathway of metastasis is operational in human HCC.
The statistical evaluation of our data on HCC metastases (Table 2) reveals similarities with previous reports that have investigated surgical and autopsy cases [6–9]. The characteristics of the dissemination pattern of HCC are summarized as follows. (1) HCC frequently involves the portal and hepatic veins, which is sometimes associated with direct extension of a tumor. (2) Intrahepatic metastases via portal vein dissemination are common. (3) While extrahepatic metastases appear to be a late event, blood-borne lung metastasis is the most frequent site, followed by the regional lymph nodes at autopsy. Most of these unique characteristics can be explained by our morphological findings.
Our current and previous works add support for a new paradigm for intravasation of tumor cells into vascular space. To date, circulating tumor cells have been thought to persist as single cells or small cell clusters. Several experimental studies indicate that circulating clusters have a greater chance of generating a secondary tumor than do single cells [16–18]. In contrast, tumor emboli of HCC are composed of multicellular tumor nests, which are strikingly large enough to get arrested in the target organ where they subsequently can thrive and create expansive secondary tumors. In addition to the size, the shape of the intravascular tumor cell emboli may also increase the metastatic efficiency of HCC. The majority of single or clustered tumor cells are thought to expire in the circulation and fail to metastasize because of either anoikis (programmed cell death resulting from the loss of cell–matrix interactions) [19, 20], mechanical trauma [21, 22] or attack and clearance by the host defense system [23, 24]. Tumor emboli of HCC, however, conserve elements of their primary tumor tissue organization, being associated with basement membrane and vascular endothelial cells on the surface. This architecture can provide an integrated ecosystem that may protect the tumor cells from anoikis and direct immunological engagement during dissemination. This situation would greatly increase the metastatic efficiency of HCC, because the probability that tumor cells released into the blood stream will achieve the entire multistep process of metastasis is enhanced. This hypothesis is supported by our data showing that portal and hepatic vein involvement is strongly correlated with the incidence of liver and lung metastasis, respectively.
This alternative intravasation mechanism may also help us to understand previously observed behavior peculiar to HCC, such as intravascular extension and growth. There are many case reports of HCC continuously extending into the inferior vena cava and right cardiac system [25, 26], and the gastric and esophageal varices [27, 28]. The ability of intravasated HCC cells to conserve their tissue structure will protect them from shearing forces, and may enable them to maintain a stable metabolic environment such that they can grow vigorously in the bloodstream. Furthermore, in the well-organized tissue, cell–cell, and cell–matrix adhesion may be sufficiently strong to allow continuous extension into the large vessels.
Our observations suggest that the intravasation capabilities of HCC are attributed to a remodeling of the tissue architecture in the primary tumor. An excessive development of sinusoidal tumor vessels appears to produce tumor islands enveloped by endothelial cells. We previously reported that endothelial-coated tumor emboli within the drainage veins were observed not only in HCC but also in most cases of renal cell carcinomas and follicular thyroid carcinomas [12]. The presence of such tumor emboli was closely associated with sinusoidal vascular development, but not with vascular density in the primary tumor. This observation is in line with our previous experimental study where we demonstrated that a specific factor can induce this particular mode of tumor cell/endothelial cell association. In a murine model, the activity of the secretory leukocyte protease inhibitor (SLPI) protein induces sinusoidal angiogenesis and consequently promotes intravasation of endothelial-coated tumor emboli and lung metastasis, whereas it inhibits vascular migration and does not affect endothelial cell proliferation [14]. Tissue remodeling of HCC organized by sinusoidal angiogenesis may be a prerequisite to intravasation and high rates of HCC metastasis.
The invasion-independant metastasis pathway has not been reported in lymphatic dissemination, we have previously only observed blood-borne metastasis [12–14]. In this study, however, tumor emboli surrounded by D2-40 positive cells were also observed in the lymphatic vasculature. This finding suggests that the same pathway may be operational in lymphatic metastasis. We could not determine the exact origin of the tumor-associated lymphatic endothelial cells, because lymphatic involvement or lymph node metastasis is rarely seen in HCC. Further studies including other cancers with a predilection for lymph node metastasis are required to elucidate whether cancer cells can invoke the invasion-independent pathway in lymphatic metastasis.
In conclusion, this study indicates an important role for the development of sinusoidal tumor vessels in blood-borne metastasis of human HCC. Our morphological studies using autopsy cases demonstrated that the tumor cells are able to recruit sinusoidal endothelial cells, and to produce tumor emboli capable of dissemination from the primary tumor as an independent entity. The conservation of the tissue organization of endothelial-coated tumor emboli throughout the metastatic process could facilitate the survival and growth of circulating tumor cells. The data show that HCC intravasation is amplified by the sinusoidal remodeling of nascent tumor vasculature and expand the current paradigms regarding primary HCC tumor extension and dissemination. The combination of our findings in cell line and animal models, and now a cohort of human cases provide momentum for considering alternative anti-angiogenic models in screening and for the development of new therapeutic agents for liver cancer metastasis. The detection of significant tumor cell/endothelial cell embolus structures in primary tumor histopathology evaluation could indicate a higher risk of disease dissemination and thus inform specific postsurgical treatment options. Furthermore, the development of therapies that could perturb the embolus structures would potentially diminish the risk for the establishment of HCC metastases.
Abbreviations
- HCC
Hepatocellular carcinoma
Contributor Information
Takashi Sugino, Email: sugino@fmu.ac.jp, Department of Pathology, Fukushima Medical University School of Medicine, Hikariga-oka 1, Fukushima 960-1295, Japan.
Tomiko Yamaguchi, Department of Pathology, Fukushima Medical University School of Medicine, Hikariga-oka 1, Fukushima 960-1295, Japan.
Nobuo Hoshi, Department of Pathology, Fukushima Medical University School of Medicine, Hikariga-oka 1, Fukushima 960-1295, Japan.
Takashi Kusakabe, Department of Pathology, Fukushima Medical University School of Medicine, Hikariga-oka 1, Fukushima 960-1295, Japan.
Go Ogura, Department of Pathology, Fukushima Medical University School of Medicine, Hikariga-oka 1, Fukushima 960-1295, Japan.
Steve Goodison, Department of Surgery, University of Florida, Jacksonville, FL, USA.
Toshimitsu Suzuki, Department of Pathology, Fukushima Medical University School of Medicine, Hikariga-oka 1, Fukushima 960-1295, Japan.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.The Research Group for Population-based Cancer Registration in Japan. Cancer incidence and incidence rates in Japan in 1995: estimates based on data from nine population-based cancer registries. Jpn J Clin Oncol. 2000;30:318–321. doi: 10.1093/jjco/hyd081. [DOI] [PubMed] [Google Scholar]
- 3.Ikai I, Itai Y, Okita K, et al. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28:21–29. doi: 10.1016/j.hepres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 5.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakashima T, Okuda K, Kojiro M, et al. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer. 1983;51:863–877. doi: 10.1002/1097-0142(19830301)51:5<863::aid-cncr2820510520>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Mitsunobu M, Toyosaka A, Oriyama T, et al. Intrahepatic metastases in hepatocellular carcinoma: the role of the portal vein as an efferent vessel. Clin Exp Metastasis. 1996;14:520–529. doi: 10.1007/BF00115112. [DOI] [PubMed] [Google Scholar]
- 8.Ishak KG, Goodman ZD, Stocker JT. Hepatocellular carcinoma. In: Rosai J, Sobin LH, editors. Atlas of tumor pathology, tumors of the liver and intrahepatic bile ducts. 3rd edn. vol 31. Washington, DC: Armed Forces Institute of Pathology; 2001. p. 227. [Google Scholar]
- 9.Hirohashi S, Blum HE, Ishak KG, et al. Hepatocellular carcinoma. In: Hamilton SR, Aaltonen LA, editors. World health organization classification of tumours, pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2000. pp. 159–172. [Google Scholar]
- 10.El-Assal ON, Yamanoi A, Soda Y, et al. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554–1562. doi: 10.1002/hep.510270613. [DOI] [PubMed] [Google Scholar]
- 11.Tanigawa N, Lu C, Mitsui T, et al. Quantitation of sinusoid-like vessels in hepatocellular carcinoma: its clinical and prognostic significance. Hepatology. 1997;26:1216–1223. doi: 10.1053/jhep.1997.v26.pm0009362365. [DOI] [PubMed] [Google Scholar]
- 12.Sugino T, Yamaguchi T, Ogura G, et al. Morphological evidence for an invasion-independent metastasis pathway exists in multiple human cancers. BMC Med. 2004;2:9. doi: 10.1186/1741-7015-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugino T, Kusakabe T, Hoshi N, et al. An invasion-independent pathway of blood-borne metastasis: a new murine mammary tumor model. Am J Pathol. 2002;160:1973–1980. doi: 10.1016/S0002-9440(10)61147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugino T, Yamaguchi T, Ogura G, et al. The secretory leukocyte protease inhibitor (SLPI) suppresses cancer cell invasion but promotes blood-borne metastasis via an invasion-independent pathway. J Pathol. 2007;212:152–160. doi: 10.1002/path.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Mehdi AB, Tozawa K, Fisher AB, et al. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 16.Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973;9:223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- 17.Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889–894. [PubMed] [Google Scholar]
- 18.Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer. 1983;48:665–673. doi: 10.1038/bjc.1983.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Z, Sanchez-Sweatman O, Huang X, et al. Anoikis and metastatic potential of cloudman S91 melanoma cells. Cancer Res. 2001;61:1707–1716. [PubMed] [Google Scholar]
- 20.Berezovskaya O, Schimmer AD, Glinskii AB, et al. Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Res. 2005;65:2378–2386. doi: 10.1158/0008-5472.CAN-04-2649. [DOI] [PubMed] [Google Scholar]
- 21.Weiss L, Orr FW, Honn KV. Interactions between cancer cells and the microvasculature: a rate-regulator for metastasis. Clin Exp Metastasis. 1989;7:127–167. doi: 10.1007/BF01787020. [DOI] [PubMed] [Google Scholar]
- 22.Albertsson PA, Nannmark U, Johansson BR. Melanoma cell destruction in the microvasculature of perfused hearts is reduced by pretreatment with vitamin E. Clin Exp Metastasis. 1995;13:269–276. doi: 10.1007/BF00133482. [DOI] [PubMed] [Google Scholar]
- 23.Qiu H, Orr FW, Jensen D, et al. Arrest of B16 melanoma cells in the mouse pulmonary microcirculation induces endothelial nitric oxide synthase-dependent nitric oxide release that is cytotoxic to the tumor cells. Am J Pathol. 2003;162:403–412. doi: 10.1016/S0002-9440(10)63835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HH, McIntosh AR, Hasinoff BB, et al. B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res. 2000;60:5862–5869. [PubMed] [Google Scholar]
- 25.Kojiro M, Nakahara H, Sugihara S, et al. Hepatocellular carcinoma with intra-atrial tumor growth. A clinicopathologic study of 18 autopsy cases. Arch Pathol Lab Med. 1984;108:989–992. [PubMed] [Google Scholar]
- 26.Oviedo J, Cerda S. Vascular invasion by hepatocellular carcinoma. Arch Pathol Lab Med. 2001;125:454–455. doi: 10.5858/2001-125-0454-VIBHC. [DOI] [PubMed] [Google Scholar]
- 27.Arakawa M, Kage M, Matsumoto S, et al. Frequency and significance of tumor thrombi in esophageal varices in hepatocellular carcinoma associated with cirrhosis. Hepatology. 1986;6:419–422. doi: 10.1002/hep.1840060316. [DOI] [PubMed] [Google Scholar]
- 28.Hiraoka T, Iwai K, Yamashita R, et al. Metastases from hepatocellular carcinoma in sclerosed oesophageal varices in cirrhotic patients. Br J Surg. 1986;73:932. doi: 10.1002/bjs.1800731134. [DOI] [PubMed] [Google Scholar]