Summary
The dynamic process of pathogen transmission by the bite of an insect vector combines several biological processes that have undergone extensive co-evolution. Whereas the host response to an insect bite is only occasionally confronted with the parasitic pathogens that competent vectors might transmit, the transmitted parasites will always be confronted with the acute, wound healing response that is initiated by the bite itself. Invariably, this response involves neutrophils. In the case of Leishmania, infection is initiated in the skin following the bite of an infected sand fly, suggesting that Leishmania must possess some means to survive their early encounter with recruited neutrophils at the bite site. Here, we review the literature regarding the impact of neutrophils on the outcome of infection with Leishmania, with special attention to the role of the sand fly bite.
Introduction
Neutrophils are a key component of innate immunity and are essential for protection from bacterial infections due to their ability to recognize, phagocytose, and ultimately destroy these pathogenic organisms (Segal, 2005, Nauseef, 2007, Xu et al., 2007, Weinrauch et al., 2002). The importance of neutrophils is evidenced by uncontrolled dermal infections caused by group A Streptocuccus or Staphylococcus aureus in humans when neutrophil functions are impaired (Hidalgo-Grass et al., 2006, Hidalgo-Grass et al., 2004, Gilad et al., 2006). The protective role of neutrophils is associated with rapid recruitment to sites of tissue damage and pathogen entry, and the subsequent clearance of these recruited neutrophils by macrophage/monocyte populations. Despite the neutrophil’s potent anti-microbial arsenal, Helicobacter pylori, Francisella tularensis, and Anaplasma phagocytophilum have all evolved mechanisms to resist direct killing following phagocytosis (Allen et al., 2007, Carlyon et al., 2006). In addition, persistent neutrophil recruitment is associated with immuno-pathology and adverse outcomes, as observed during chronic conditions such as arthritis and gout (Jakus et al., 2009, Eyles et al., 2006, Cronstein et al., 2006).
Recently, we reported on the dynamics of neutrophil recruitment to a site of Leishmania inoculation into the skin by the bite of an infected sand fly, the natural insect vector for this infectious, protozoan, parasite (Peters et al., 2008). Numerous species of Leishmania exist, all of which share a digenetic lifecycle, alternating between the promastigote form in the sand fly and the obligate intracellular amastigote form in the mammalian host. The clinical outcomes of infection with different Leishmania spp. is highly diverse, ranging from self-limiting, cutaneous lesions following infection with L. major, to fatal, systemic involvement of the liver spleen, and bone marrow following infection with L. donovani. Regardless of the Leishmania spp., parasite deposition in the skin coincides with tissue damage caused by the sand fly bite. The highly conserved, wound healing response involving neutrophils is therefore likely to influence the early establishment of infection, and both host protective and disease promoting roles for neutrophils have been reported (Ribeiro-Gomes et al., 2004, Ribeiro-Gomes et al., 2006, Tacchini-Cottier et al., 2000, Chen et al., 2005, van Zandbergen et al., 2004, McFarlane et al., 2008, Laskay et al., 2008, Peters et al., 2008). Here, we incorporate the influence of sand fly bite on current ideas regarding the role of neutrophils in Leishmaniasis.
Protective and Non-protective Roles for Neutrophils during in-vivo infection with Leishmania
Early experimental observations of Leishmania-neutrophil interactions during acute disease in vivo employed histological analysis of infected skin lesions and indicated both the early presence of neutrophils at sites of needle inoculation, and the presence of parasites inside neutrophil phagosomes. This was observed for L mexicana, L. donovani, and L. chagasi (Andrade et al., 1984, Grimaldi et al., 1984, Pimenta et al., 1987, Wilson et al., 1987, Laurenti et al., 1996). Although the presence of degraded parasites within neutrophils was interpreted to indicate neutrophil-mediated killing, this analysis was not able to discriminate between phagocytosis of damaged or dead parasites and direct killing of viable organisms, an interpretation further complicated by the use of large doses of heterogeneous parasites. Early in-vitro observations employing human neutrophils indicated that L. donovani promastigotes or amastigotes were killed within hours of phagocytosis in an oxygen free-radical dependent manner (Chang, 1981, Pearson et al., 1981). In contrast, acid phosphatases from L. donovani and L. major were shown to confer resistance to lysosome-derived toxic oxygen metabolites and to inhibit their production by neutrophils, suggesting these Leishmania strains have evolved mechanisms to avoid neutrophil killing (Remaley et al.,1984, el-On et al., 1990, al Tuwaijri et al., 1990).
Subsequent observations on the impact of Leishmania-neutrophil interactions have been interpreted within the context of strains of mice that are resistant or susceptible to infection with L. major. In BALB/c mice, which do not resolve parasitic lesions and eventually succumb to disseminated disease, neutrophils were found to dominate the early cellular infiltrate following s.c. needle inoculation of 2×107 stationary phase promastigotes, and importantly were maintained over the first 12 days of infection (Beil et al., 1992, Sunderkotter et al., 1993). Furthermore, depletion of neutrophils at the time of L. major challenge in BALB/c mice inhibited the IL-4 response and promoted partial resistance (Tacchini-Cottier et al., 2000). In contrast, neutrophil recruitment in resistant C57Bl/6 mice was shown to be short-lived, dropping to baseline levels by day 3 (Beil et al., 1992). Furthermore, the transient depletion of neutrophils at the time of s.c. footpad challenge with L. major in resistant mice enhanced early parasite growth and lesion pathology, though in each case these mice eventually healed (Lima et al., 1998, Tacchini-Cottier et al., 2000, Ribeiro-Gomes et al., 2004, Chen et al., 2005). Interestingly, the mouse strain differences in the kinetics of neutrophil recruitment and maintenance in the L. major injection site was also shown to occur within non-immune granulomas initiated using inert particles (Belkaid et al., 2000), suggesting that inherent differences in acute inflammation influence the subsequent Th1/Th2 balance that controls susceptibility and resistance to L. major. This notion has been extended to include intrinsic differences in the neutrophils themselves. Specifically, whereas co-injection of dead, syngeneic neutrophils exacerbated L. major growth in BALB/c mice, a similar injection of dead, syngeneic neutrophils into C57Bl/6 mice enhanced parasite killing (Ribeiro-Gomes et al., 2004). And whereas BALB/c neutrophils elicted the production of PGE2 and TGF-β by macrophages, C57Bl/6 macrophages were activated by syngeneic neutrophils to produce TNF-α. More recently, Charmoy et al. (Charmoy et al., 2007) reported distinct neutrophil phenotypes responding to i.p. injection of L. major in resistant and susceptible mice that differed in expression of cell surface integrins, TLRs, and secreted cytokines. The production of IL-12p70 by L. major activated neutrophils from C57Bl/6 mice, as compared to the secretion of inhibitory IL-12-p40 homodimers by L. major activated BALB/c neutrophils, seems an especially relevant finding that could account, at least in part, for the subsequent Th1 vs Th2 polarization that occurs in these mice.
As strong as these various associations between the innate neutrophilic response to L. major and infection outcome appear to be, these observations must contend with a number of reports in which quite opposite conclusions were drawn. Both Lima et al. (Lima et al., 1998) and Chen et al., (Chen et al., 2005) reported exacerbation of footpad lesion development in BALB/c mice treated with a neutrophil depleting antibody, and more recently, our own studies have documented a role for neutrophils in promoting sand fly transmitted infections in C57BL/6 mice (Peters et al., 2008). Differences in the specificity and regimen of the depleting antibody, the strain, dose, and inoculation site of the L. major parasites, might all account for these discrepant results, and addressing some of these differences in a series of parallel experiments will be needed to reconcile these prior findings.
We would, in the meantime, argue that the role of neutrophils in the pathogenesis of cutaneous leishmaniasis and in modulation of host immunity is most appropriately studied in the context of infection in the skin initiated by the bite of an infected sand fly. In this setting, parasites deposited into the skin of neutrophil-depleted, C57BL/6 mice were only half as successful at establishing infection as the parasites transmitted to neutrophil sufficient mice. The enhanced host resistance was associated with an increase in pro-inflammatory cytokines (Peters et al., 2008). While both needle and sand fly inoculation of parasites induced acute neutrophil recruitment in C57BL/6 mice, an intriguing aspect of sand fly inoculation was the persistence of neutrophils at the bite site, and this occurred following exposure to both infected and uninfected flies (Peters, et. al. 2009 PLoS Pathogens accepted for publication, (Peters et al., 2008). Thus, the acute neutrophilic response following sand fly bite or needle inoculation is likely part of the host defense mechanism responsible for wound repair and sterilization. For example, a dynamic infiltration of neutrophils to localized sites of laser-induced brain injury has been observed (Kim et al., 2006), suggesting endogenous factors released during tissue damage can drive this response. In contrast, the mechanism(s) of neutrophil maintenance at bite sites is likely mediated by sand fly derived factors that either mimic a tissue damage signal or activate an additional chemokine/chemokine receptor pathway (Teixeira et al., 2005). Thus, the vigorous and neutrophilic host response to sand fly bite has likely influenced the evolution of Leishmania towards counter acting and even exploiting the presence of these cells.
The Influence of Apoptotic Neutrophils on the Initiation of Infection with Leishmania major
In protozoan infections, macrophage killing of Trypanosoma cruzi was shown to be inhibited by exposure to apoptotic, but not necrotic, T cells, and was one of the first studies to suggest that apoptotic cells might play an important role in promoting infection (Freire-de-Lima et al., 2000). The suppressive influence of apoptotic cells on macrophage function was extended to L. major following the observation that human neutrophils efficiently phagocytosed L. majorin vitro, and that the parasite was capable of surviving within these cells (Laufs et al., 2002, Aga et al., 2002). Moreover, the infected neutrophils acquired an apoptotic phenotype but remained intact (van Zandbergen et al., 2004). Additional in vitro observations gave rise to the “Trojan Horse” model of infection, whereby macrophages acquire L. major by phagocytosing infected, apoptotic, neutrophils (van Zandbergen et al., 2004, Laskay et al., 2003). Our own observations employing flow-cytometry and 2 photon-intra vital microscopy (2P-IVM) following needle or sand fly inoculation of RFP expressing L. major showed that the majority of parasites were phagocytosed by neutrophils during acute infection without being killed, followed by a transition of the RFP signal into macrophage/monocytes (Peters et al., 2008). While ourin vivo imaging failed to definitively capture this transition in real time, and in fact revealed release of parasites from stationary neutrophils that appeared to be undergoing apoptosis in the site, the clearance of these apoptotic bodies along with the large number of apoptotic, non-infected neutrophils in the localized bite site, would still be expected to modulate the function of infected macrophages in the site.
The cell contact-dependent induction of the non-inflammatory cytokines TGF-β and PGE2 by macrophages following interactions with apoptotic neutrophils potentially facilitates both the ‘silent entry’ of parasites following uptake of infected neutrophils, and the silencing of infected macrophages that co-ingest non-infected by-stander neutrophils (Lucas et al., 2006, van Zandbergen et al., 2007). Observations by Ribiero-Gomes et. al., involving cell-contact dependent interactions of macrophages with uninfected, thioglycolate elicited neutrophils containing a proportion of apoptotic cells, support a model in which Fas ligand-dependent neutrophil apoptosis drives acute infection in BALB/c mice (Ribeiro-Gomes et al., 2004, Ribeiro-Gomes et al., 2005, van Zandbergen etal., 2007). In contrast, the authors reported a cell-contact independent, protective, role for apoptotic neutrophils in C57BL/6 mice under the same conditions, which was later shown to be dependent upon TLR4 recognition of secreted neutrophil elastase (NE) (Ribeiro-Gomes et al., 2007). Recently, Afonso et. al. (Afonso et al., 2008)reported that induction of NE dependent killing of L. amazonensis by human macrophages occurred following exposure to necrotic, but not apoptotic, neutrophils. Therefore, one potential explanation for contact dependent enhancement of infection by neutrophils in BALB/c mice versus the contact independent reduction of infection in B6 mice is the propensity of thioglycolate neutrophils from these two strains to become necrotic under similar conditions. We would again argue that the most relevant population of neutrophils are those present in the dermis following transmission by sand fly bite, since these neutrophils will have undergone the natural exposure to the inflammatory mediators involved in their recruitment, extravasation and interstitial migration in the dermis, and may therefore be unique in their apoptotic vs necrotic cell death programs. Furthermore, a proportion of these neutrophils will have undergone a degree of modulation following parasite phagocytosis (Aga et al., 2002, van Zandbergen et al., 2004). The opposing outcomes of high-dose, sub-cutaneous infection of the footpad versus sand fly transmitted infection of the dermis in neutrophil depleted C57BL/6 mice may be the degree to which these two routes induce populations of either necrotic versus apoptotic neutrophils, or neutrophils secreting elastase.
The modulation of macrophage functions by apoptotic neutrophils likely contributes to the establishment of the early, ‘silent,’ phase of disease (Belkaid et al., 2000), characterized by a remarkable avoidance of a host protective Th1 response during a prolonged phase (4–5 wks) of L. major amplification in the skin. As immature dendritic cells (DC) might also be expected to participate in the clearance ofapoptotic cells, their encounter with infected and/or uninfected neutrophils in the skin might have an especially important bearing on the delayed onset of T cell priming that is observed during L. major infection. There is ample evidence that the clearance of apoptotic cells by DC is critical for the maintenance of peripheral tolerance (Steinman et al., 2000). Immature DCs are capable of extensive phagocytosis, and DC maturation can be inhibited by the engulfment of apoptotic cells, resulting in reducede xpression of costimulatory molecules CD40, CD80, andCD86, impaired allogeneic T cell responses, and down-regulation of LPS-driven interleukin-12 production(Stuart et al., 2002, Clayton et al., 2003) Neutrophil elastase has been shown to convert human immature DCs into TGF-beta secreting cells that modulate their immunostimulatory capacity (Maffia et al., 2007). With respect to Leishmania, 2P-IVM confirmed that within minutes after injection, at least some parasites were directly incorporated into the phagocytic vacuoles of dermal DCs (Ng et al., 2008). Our own studies have indicated, nonetheless, that the majority of the infected DCs recovered from the skin after 24 hr contain granulocyte markers, suggesting that their early encounter with L. major occurs in the context of parasitized neutrophils (Ribeiro-Gomes, F.L., et al., unpublished). Furthermore, we have observed that the DCs recovered following their uptake of infected neutrophils in vivo or in vitro are incapable of cross-priming, or of activating Leishmania-specific CD4+ and CD8+ memory cells. The compromised function of DC with respect to the expression of secondary responses at the site of infected sand fly bite may be especially relevant to the failure to date of Leishmania vaccines to confer significant protection in humans against natural challenge. We have recently found that depletion of persistent neutrophils during an ongoing secondary response in vaccinated mice promoted the efficacy of a killed vaccine against L. major transmitted by sand fly bite (Peters, et. al. 2009 PLoS Pathogens accepted for publication). In this setting, neutrophil depletion was associated with an increase in the frequency of IFN-γ and TNF-α producing CD4+ T cells specific for L. major at the site of infection in the skin. These observations suggest that sand fly bite mediated neutrophil persistence at a site of parasite deposition inhibits effector function beyond the down-modulatory influence of neutrophils at the initial stage of infection.
It is important to note that neutrophils elicited in response to other infections, e.g. Toxoplasma gondii, BCG, and Candida albicans, have been reported to deliver activation signals to DC and to promote Th1 cell-mediated responses (Bennouna et al., 2003, Morel et al., 2008, Megiovanni et al., 2006). These organisms appear to possess stimuli, absent in Leishmania, that activate neutrophils to produce type I inflammatory cytokines and chemokines that instruct recruitment and activation of DC during infection. The cross talk between neutrophils and DC might be further influenced by differences in the apoptotic programs that are induced by these infections in distinct inflammatory settings. Live, non-apoptotic neutrophils appeared to be involved in the activation of DC capable of priming T cell responses to BCGand C. albicans. By contrast, the suppression of macrophage (van Zandbergen et al., 2004)and DC functions (Ribeiro-Gomes, F.L., et. al. unpublished) following L. major infection was clearly associated with apopotic neutrophils, which at least in the case of the infected neturophils recovered from the L. major loaded dermis, were accelerated in their apoptotic program.
Conclusion
Examination of the earliest events following transmission of Leishmania major by infected sand flies reveals that neutrophils are recruited to the site of sand fly bite and phagocytose deposited parasites. This process appears to be critical for disease progression since parasites transmitted in the absence of neutrophils are less likely to establish infection. (Peters et al., 2008). In addition, the chronic maintenance of neutrophils appears to compromise the expression of adaptive immunity (Peters, et. al. 2009 PLoS Pathogens accepted for publication). These findings and others (van Zandbergen et al., 2007, Laskay et al., 2008), suggest that neutrophils play a prominent role in promoting the pathogenesis of cutaneous leishmaniasis, see Figure 1. While other studies suggest that in different settings, and under different experimental conditions, neutrophils are able to promote resistance, we would emphasize that those models which best replicate the natural route of infection are more likely to reveal the true nature, friend or foe, of the neutrophil in the clinical outcome of infection with Leishmania.
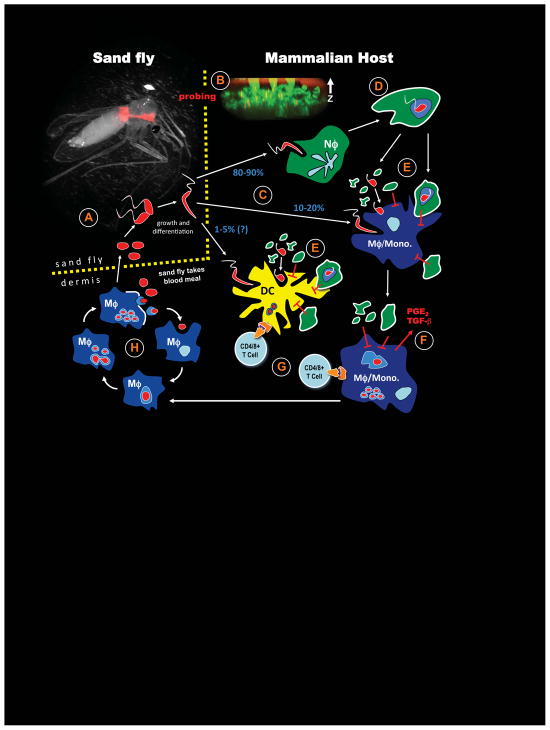
Figure 1.
Life cycle of L. major incorporating the influence of sand fly bite and the role of neutrophils in the pathogenesis of disease. (A) Phlebotomine spp. of sand flies take a blood meal from an infected individual, acquiring parasites in the process. Intracellular amastigotes undergo transformation, growth and differentiation in the sand fly midgut, eventually becoming metacyclic promastogites positioned in the anterior gut. The picture is of P. dubosqi infected with RFP-expressing L. major. (B) In search of a second blood meal, sand flies again probe the skin of a mammalian host, creating a hemorrhagic pool from which to feed while simultaneously depositing parasites into the dermis and epidermis. Tissue damage caused by sand fly probing induces highly localized neutrophil recruitment and the formation of neutrophil ‘plugs’ in the regions of skin punctured by the sand fly proboscis. The picture is of L.m.-RFP metacyclic promastigotes deposited among the Lys-GFPhi neutrophils, and neutrophil plugs extending up through the epidermis and stratum corneum. (C) Neutrophils dominate in sites of tissue damage following sand fly probing and phagocytose the majority (80–90%) of parasites. Smaller numbers of parasites also gain direct access to macrophages/monocytes (10–20%). A few parasites may gain direct access to resident dermal dendritic cells (1–5%). (D) Infected neutrophils in the skin fail to kill L.m., and the parasitized cells acquire apoptotic markers such as phosphatidylserine (indicated by a white cell surface). (E) At later time points (1–2 days), parasites begin to transition out of neutrophils into macrophage/monocytes and DCs. Parasite acquisition by these phagocytes can occur via uptake of released, viable organisms from infected neutrophils, or via uptake of parasitized neutrophils, i.e. the ‘Trojan Horse’ route. Infected macrophage/monocyte and DC populations are also exposed to apoptotic bodies and large numbers of uninfected, apoptotic neutrophils that will down-modulate both innate mechanisms of parasite killing and APC functions. (F) During the ‘silent’ phase of parasite growth, neutrophils are persistently recruited to and maintained in the bite site by sand fly derived factors, and parasite killing and APC functions will continue to be suppressed, associated with production of non-inflammatory cytokines PGE2 and TGF-β. (G) Parasites propagate in inflammatory, monocyte –derived cells in the skin, and increase to sufficient numbers so that they are eventually taken up by immunocompetent DCs in the skin or draining lymph node, initiating T cell priming and the healing phase of disease.
References
- Afonso L, Borges VM, Cruz H, Ribeiro-Gomes FL, DosReis GA, Dutra AN, et al. Interactions with apoptotic but notwith necrotic neutrophils increase parasite burden in human macrophages infected with Leishmania amazonensis. J Leukoc Biol. 2008;84:389–396. doi: 10.1189/jlb.0108018. [DOI] [PubMed] [Google Scholar]
- Aga E, Katschinski DM, van Zandbergen G, Laufs H, Hansen B, Muller K, et al. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J Immunol. 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- al Tuwaijri AS, al Mofleh IA, Mahmoud AA. Effect of Leishmania major on human polymorphonuclear leucocyte function in vitro. J Med Microbiol. 1990;32:189–193. doi: 10.1099/00222615-32-3-189. [DOI] [PubMed] [Google Scholar]
- Allen LA, McCaffrey RL. To activate or not to activate: distinct strategies used by Helicobacter pylori and Francisella tularensis to modulate the NADPH oxidase and survive in human neutrophils. Immunol Rev. 2007;219:103–117. doi: 10.1111/j.1600-065X.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Andrade ZA, Reed SG, Roters SB, Sadigursky M. Immunopathology of experimental cutaneous leishmaniasis. Am J Pathol. 1984;114:137–148. [PMC free article] [PubMed] [Google Scholar]
- Beil WJ, Meinardus-Hager G, Neugebauer DC, Sorg C. Differences in theonset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. J Leukoc Biol. 1992;52:135–142. doi: 10.1002/jlb.52.2.135. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000;165:969–977. doi: 10.4049/jimmunol.165.2.969. [DOI] [PubMed] [Google Scholar]
- Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052–6058. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- Carlyon JA, Fikrig E. Mechanisms of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr Opin Hematol. 2006;13:28–33. doi: 10.1097/01.moh.0000190109.00532.56. [DOI] [PubMed] [Google Scholar]
- Chang KP. Leishmanicidal mechanisms of human polymorphonuclear phagocytes. Am J Trop Med Hyg. 1981;30:322–333. doi: 10.4269/ajtmh.1981.30.322. [DOI] [PubMed] [Google Scholar]
- Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, et al. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J Leukoc Biol. 2007;82:288–299. doi: 10.1189/jlb.0706440. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang ZH, Watanabe T, Yamashita T, Kobayakawa T, Kaneko A, et al. The involvement of neutrophils in the resistance to Leishmania major infection in susceptible but not in resistant mice. Parasitol Int. 2005;54:109–118. doi: 10.1016/j.parint.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Clayton AR, Prue RL, Harper L, Drayson MT, Savage CO. Dendritic cell uptake of human apoptotic and necrotic neutrophils inhibits CD40, CD80, and CD86 expression and reduces allogeneic T cell responses: relevance to systemic vasculitis. Arthritis Rheum. 2003;48:2362–2374. doi: 10.1002/art.11130. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther. 2006;8(Suppl 1):S3. doi: 10.1186/ar1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-On J, Zvillich M, Sarov I. Leishmania major: inhibition of the chemiluminescent response of human polymorphonuclear leukocytes by promastigotes and their excreted factors. Parasite Immunol. 1990;12:285–295. doi: 10.1111/j.1365-3024.1990.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Eyles JL, Roberts AW, Metcalf D, Wicks IP. Granulocyte colony-stimulating factor and neutrophils--forgotten mediators of inflammatory disease. Nat Clin Pract Rheumatol. 2006;2:500–510. doi: 10.1038/ncprheum0291. [DOI] [PubMed] [Google Scholar]
- Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, et al. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- Gilad J, Borer A, Smolyakov R, Riesenberg K, Schlaeffer F, Levy R. Impaired neutrophil functions in the pathogenesis of an outbreak of recurrent furunculosis caused by methicillin-resistant Staphylococcus aureus among mentally retarded adults. Microbes Infect. 2006;8:1801–1805. doi: 10.1016/j.micinf.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Jr, Soares MJ, Moriearty PL. Tissue eosinophilia and Leishmania mexicana mexicana eosinophil interactions in murine cutaneous leishmaniasis. Parasite Immunol. 1984;6:397–408. doi: 10.1111/j.1365-3024.1984.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Grass C, Dan-Goor M, Maly A, Eran Y, Kwinn LA, Nizet V, et al. Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections. Lancet. 2004;363:696–703. doi: 10.1016/S0140-6736(04)15643-2. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Grass C, Mishalian I, Dan-Goor M, Belotserkovsky I, Eran Y, Nizet V, et al. A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues. Embo J. 2006;25:4628–4637. doi: 10.1038/sj.emboj.7601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus Z, Simon E, Frommhold D, Sperandio M, Mocsai A. Critical role of phospholipase Cgamma2 in integrin and Fc receptor-mediated neutrophil functions and the effector phase of autoimmune arthritis. J Exp Med. 2009;206:577–593. doi: 10.1084/jem.20081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JV, Dustin ML. Innate response to focal necrotic injury inside the blood-brain barrier. J Immunol. 2006;177:5269–5277. doi: 10.4049/jimmunol.177.8.5269. [DOI] [PubMed] [Google Scholar]
- Laskay T, van Zandbergen G, Solbach W. Neutrophil granulocytes--Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003;11:210–214. doi: 10.1016/s0966-842x(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Laskay T, van Zandbergen G, Solbach W. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: apoptosis as infection-promoting factor. Immunobiology. 2008;213:183–191. doi: 10.1016/j.imbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Laufs H, Muller K, Fleischer J, Reiling N, Jahnke N, Jensenius JC, et al. Intracellular Survival of Leishmania major in Neutrophil Granulocytes after Uptake in the Absence of Heat-Labile Serum Factors. Infect Immun. 2002;70:826–835. doi: 10.1128/iai.70.2.826-835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenti MD, Corbett CE, Sotto MN, Sinhorini IL, Goto H. The role of complement in the acute inflammatory process in the skin and in host-parasite interaction in hamsters inoculated with Leishmania (Leishmania) chagasi. Int J Exp Pathol. 1996;77:15–24. doi: 10.1046/j.1365-2613.1996.958096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima GM, Vallochi AL, Silva UR, Bevilacqua EM, Kiffer MM, Abrahamsohn IA. The role of polymorphonuclear leukocytes in the resistance to cutaneous Leishmaniasis. Immunol Lett. 1998;64:145–151. doi: 10.1016/s0165-2478(98)00099-6. [DOI] [PubMed] [Google Scholar]
- Lucas M, Stuart LM, Zhang A, Hodivala-Dilke K, Febbraio M, Silverstein R, et al. Requirements for apoptotic cell contact in regulation of macrophage responses. J Immunol. 2006;177:4047–4054. doi: 10.4049/jimmunol.177.6.4047. [DOI] [PubMed] [Google Scholar]
- Maffia PC, Zittermann SE, Scimone ML, Tateosian N, Amiano N, Guerrieri D, et al. Neutrophil elastase converts human immature dendritic cells into transforming growth factor-beta1-secreting cells and reduces allostimulatory ability. Am J Pathol. 2007;171:928–937. doi: 10.2353/ajpath.2007.061043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane E, Perez C, Charmoy M, Allenbach C, Carter KC, Alexander J, Tacchini-Cottier F. Neutrophils contribute to development of a protective immune response during onset of infection with Leishmania donovani. Infect Immun. 2008;76:532–541. doi: 10.1128/IAI.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megiovanni AM, Sanchez F, Robledo-Sarmiento M, Morel C, Gluckman JC, Boudaly S. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J Leukoc Biol. 2006;79:977–988. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- Morel C, Badell E, Abadie V, Robledo M, Setterblad N, Gluckman JC, et al. Mycobacterium bovis BCG-infected neutrophils and dendritic cells cooperate to induce specific T cell responses in humans and mice. Eur J Immunol. 2008;38:437–447. doi: 10.1002/eji.200737905. [DOI] [PubMed] [Google Scholar]
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Ng LG, Hsu A, Mandell MA, Roediger B, Hoeller C, Mrass P, et al. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 2008;4:e1000222. doi: 10.1371/journal.ppat.1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RD, Steigbigel RT. Phagocytosis and killing of the protozoan Leishmania donovani by human polymorphonuclear leukocytes. J Immunol. 1981;127:1438–1443. [PubMed] [Google Scholar]
- Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta PF, Dos Santos MA, De Souza W. Fine structure and cytochemistry of the interaction between Leishmania mexicana amazonensis and rat neutrophils and eosinophils. J Submicrosc Cytol. 1987;19:387–395. [PubMed] [Google Scholar]
- Remaley AT, Kuhns DB, Basford RE, Glew RH, Kaplan SS. Leishmanial phosphatase blocks neutrophil O-2 production. J Biol Chem. 1984;259:11173–11175. [PubMed] [Google Scholar]
- Ribeiro-Gomes FL, Moniz-de-Souza MC, Alexandre-Moreira MS, Dias WB, Lopes MF, Nunes MP, et al. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J Immunol. 2007;179:3988–3994. doi: 10.4049/jimmunol.179.6.3988. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Gomes FL, Moniz-de-Souza MC, Borges VM, Nunes MP, Mantuano-Barradas M, D’Avila H, et al. Turnover of neutrophils mediated by Fas ligand drives Leishmania major infection. J Infect Dis. 2005;192:1127–1134. doi: 10.1086/432764. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Gomes FL, Otero AC, Gomes NA, Moniz-De-Souza MC, Cysne-Finkelstein L, Arnholdt AC, et al. Macrophage interactions with neutrophils regulate Leishmania major infection. J Immunol. 2004;172:4454–4462. doi: 10.4049/jimmunol.172.7.4454. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Gomes FL, Silva MT, Dosreis GA. Neutrophils, apoptosis and phagocytic clearance: an innate sequence of cellular responses regulating intramacrophagic parasite infections. Parasitology. 2006;132(Suppl):S61–68. doi: 10.1017/S0031182006000862. [DOI] [PubMed] [Google Scholar]
- Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Kunz M, Steinbrink K, Meinardus-Hager G, Goebeler M, Bildau H, Sorg C. Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J Immunol. 1993;151:4891–4901. [PubMed] [Google Scholar]
- Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P, et al. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol. 2000;165:2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- Teixeira CR, Teixeira MJ, Gomes RB, Santos CS, Andrade BB, Raffaele-Netto I, et al. Saliva from Lutzomyia longipalpis induces CC chemokine ligand 2/monocyte chemoattractant protein-1 expression and macrophage recruitment. J Immunol. 2005;175:8346–8353. doi: 10.4049/jimmunol.175.12.8346. [DOI] [PubMed] [Google Scholar]
- van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- van Zandbergen G, Solbach W, Laskay T. Apoptosis driven infection. Autoimmunity. 2007;40:349–352. doi: 10.1080/08916930701356960. [DOI] [PubMed] [Google Scholar]
- Weinrauch Y, Drujan D, Shapiro SD, Weiss J, Zychlinsky A. Neutrophil elastase targets virulence factors of enterobacteria. Nature. 2002;417:91–94. doi: 10.1038/417091a. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Innes DJ, Sousa AD, Pearson RD. Early histopathology of experimental infection with Leishmania donovani in hamsters. J Parasitol. 1987;73:55–63. [PubMed] [Google Scholar]
- Xu Q, Seemanapalli SV, Reif KE, Brown CR, Liang FT. Increasing the recruitment of neutrophils to the site of infection dramatically attenuates Borrelia burgdorferi infectivity. J Immunol. 2007;178:5109–5115. doi: 10.4049/jimmunol.178.8.5109. [DOI] [PubMed] [Google Scholar]